Abstract
Tomato bacterial wilt (BW) caused by Ralstonia solanacearum seriously restricts tomato production and no effective control measures are available. A microbial restoration substrate (MRS) had been proved to be effective control of tomato BW in a greenhouse cultivation. In this study, MRS was combined with an avirulent Ralstonia solanacearum (aRS) strain to control the disease under an open field condition. In the two consecutive year (2017 and 2018) trials, the combined use of aRS and MRS resulted in better disease control compared with either aRS or MRS alone. Moreover, the combined treatment was more effective than expected and suggesting a synergistic control effect. Compared with control (CK, non-aRS or MRS), the application of aRS and MRS treatments alone or in combination could all promote plant growth, increase root activity and yield (e.g. the yield for the treatment of aRS + MRS increased by 463.64% in 2017). Soil nutrients, including soil organic carbon, total nitrogen, total phosphorus and total potassium contents were also significantly increased by the application of aRS and MRS treatments alone or in combination (P < 0.05). The application of MRS or in combination with aRS changed the soil from acidic to neutral, which is one of the key factors for controlling BW. The soil enzymatic activities were notably influenced by the combined use of aRS and MRS, which increased urease (87.37% in 2017 and 60.89% in 2018), catalase (93.67% in 2017 and 279.37% in 2018) and alkaline phosphatase activities (193.77% in 2017 and 455.73% in 2018). These results suggest that the combination of MRS and aRS could effectively control tomato BW and thus represents a promising new tool to control this disease.
Subject terms: Applied microbiology, Plant development
Introduction
The tomato (Solanum Lycopersicum L.) is one of the world’s main vegetable crops, and it is cultivated worldwide for fresh vegetable consumption or for processing1. A large portion of tomato crop has been grown by continuous cropping for many years in the world. This practice has led to the outbreak of the destructive soilborne disease ‘bacterial wilt (BW, caused by virulent Ralstonia solanacearum)’, which can eventually causes a complete yield loss2. Many measures have been studied to control the disease, either by inhibiting pathogen growth within the rhizosphere or by inducing host plant resistance3–6, but limited success has been achieved due to the high surviving capacity of R. solanacearum in complex environments7.
Currently, biocontrol emerges as an environmentally friendly strategy and popular method to suppress tomato BW disease8,9, and several biocontrol agents have been isolated from the rhizosphere soil or plant tissues, such as Bacillus spp.10, Streptomyces spp.11 and avirulent mutants of R. solanacearum12–14, which have shown antagonistic effects against pathogenic R. solanacearum. Many literatures reported that the avirulent mutants of R. solanacearum can effectively reduce bacterial wilt severity15–17. A high density of avirulent R. solanacearum within plant tissues might influence host’s response to pathogen and prevent its wilting15. In our previous studies, the avirulent R. solanacearum strain FJAT-1458 was isolated from a healthy tomato plant with high purity and proved to have high biocontrol efficiency against BW of 100 and 77.45% under pot and greenhouse cultivations, respectively18,19.
Successful control of soilborne diseases by soil amendments is well documented5,10,20,21. Mathre et al. (1999) reported that soil remediation with organic matter brought soil-sanitization effects and then suppressed soilborne diseases20. Ding et al.5 and Yuan et al.10 combined the use of organic fertilizers and bacterial antagonists to control potato (Solanum tuberosum L.) and tobacco (Nicotiana tabacum L.) BW, respectively, and successfully decreased bacterial disease incidences. In our previous study, a type of microbial restoration substrate (MRS) was used to amend 7-year continuous cropping tomato soil under a greenhouse cultivation and proved to be effective in reducing the severity of BW22. Soil pH, rhizobacterial community and soil nutrient composition were all changed by MRS application.
Due to variable environmental conditions and the complexity of plant and soil systems, it is difficult to achieve satisfactory disease control using a single control method23. Guo et al.24 found that the suppression efficacy of antagonist inoculation against BW was inconsistent across field trials and was influenced by environmental conditions. Wu et al.25 reported that the application of biocontrol agents with organic fertilizers was more effective in controlling tobacco BW than the sole application of biocontrol agents. Peng et al.26 combined the use of Bacillus subtilis and bactericide Saisentong (N,N-methylene-bis-(2-amino-5-sulfhydryl-1,3,4-thiadiazole) copper) to control tomato BW, and this resulted in better disease control compared with either agent alone. Therefore, integrating different methods to manage tomato BW should be a feasible approach under field conditions.
Based on previous studies under greenhouse cultivation, we further investigated a combination of the biocontrol agent R. solanacearum FJAT-1458 with the soil amendment MRS in control of tomato BW under open field cultivation. The objectives of this study were (i) to evaluate the potential biocontrol abilities of the avirulent strain FJAT-1458 in combination with the microbial restoration substrate against BW, and (ii) to investigate their mode of action and effect on properties of tomato rhizosphere soils.
Materials and Methods
Bacterial culture
The avirulent strain of R. solanacearum FJAT-1458 used in this study was grown at 30 °C for 48 h on 2,3,5-triphenyltetrazolium chloride (TTC) medium (1% peptone, 0.5% glucose, 0.1% trypticas, 1.8% agar, and 0.05% 2,3,5-triphenyltetrazolium chloride)27. Then, a single colony was suspended in liquid sucrose peptone (SP) medium, which contained 20 g·L−1 sucrose, 5 g·L−1 peptone, 0.5 g·L−1 K2HPO4, and 0.25 g·L−1 MgSO4, at a pH between 7.2 and 7.4, and cultured in a shaker at 30 °C, 200 rpm for 48 h. The concentration of the cultured bacteria reached to 109 CFU mL−1 and bacterial cells density was diluted to 107 CFU mL−1 by water, when being used in the field.
Field experiments
The field experiment was conducted for two consecutive years, from August to December, in 2017 and 2018 at Licheng district, Putian City, Fujian Province, in southeastern of China (25°45′N, 118°32′E). Putian City is located in a subtropical monsoon climate (it has an annual rainfall of 1,500 mm, and the annual average temperature is 20 °C). A tomato field undergoing 15 years of continuous tomato cropping, which exhibited severe BW in previous years (disease incidence was over 50%), was selected for the experiment. The tomato (cv. Beiying) seeds were sown in 32-hole plugs on August 20 and were grown in a greenhouse at 25–30 °C at 85–100% relative humidity. The seedlings were transplanted into an open field after 1 month. Prior to the tomato planting, 160 kg hm−2 of nitogen (N) fertilizer,120 kg hm−2 of phosphate (P) fertilizer and 320 kg hm−2 of potassium (K) fertilizer were applied as base fertizer. Then, 100 kg ha−1 of K fertilizer was applied at fruit setting.
Four treatments were established as follows: (1) aRS, which was the avirulent strain FJAT-1458 inoculation alone; (2) MRS, which was soil amendment with microbial restoration substrate alone; (3) aRS + MRS, which was the combination of aRS and MRS; and (4) CK, in which neither soil amendment nor avirulent strain inoculation were used. Randomized block design and triplicate plots were created for the experiment. Each plot consisted of five 12 m-long rows, corresponding to a total 60 m2 plot area, and 216 plants per plot. The distance between adjacent plots was 0.4 m (Fig. 1). For the soil amendment, 1,500 kg ha−1 microbial restoration substrate was furrow applied two days (d) before tomato plant transplanting. For the avirulent strain FJAT-1458 inoculation, the plants were root drenching inoculated with FJAT-1458 (107 CFU mL−1, 500 mL per plant) at transplanting, and the root drenching inoculation was repeated one month later. The MRS was jointly produced by the Fujian Academy of Agriculture Sciences (FAAS) and Xiamen Jiang Ping Biological Co., Ltd., China (XJPBC), using a microbial fermentation bed28. The pig manure nitrogen was continuously added to achieve aerobic fermentation in medium temperature. The fully manufacturing processes of MRS were as follows: the litters consisting of 33% chaff, 33% coir and 34% rice straw were added onto the pig microbial fermentation bed; the aerobic fermentation was conducted for 20 days by ploughing the litters mixing with pig manures one time per day; then, the upper 20 cm litters were removed to produce MRS by drying, crushing, screening and packaging. Illumina-MiSeq sequencing of the MRS, showed that the dominant bacterial genera were Granulicella (3.31%), Acidothermus (3.20%) and Rhodanobacter (1.27%) (NCBI accession number: SRP144025). The physiochemical characteristics of MRS were as follows: pH 7.82, the soil organic carbon (SOC) 145.12 g kg−1, total nitrogen (TN) 4.62 g kg−1, total phosphorus (TP) 3.18 g kg−1, total potassium (TK) 16.51 g kg−1, and exchangeable calcium 28.54 g kg−1.
Figure 1.
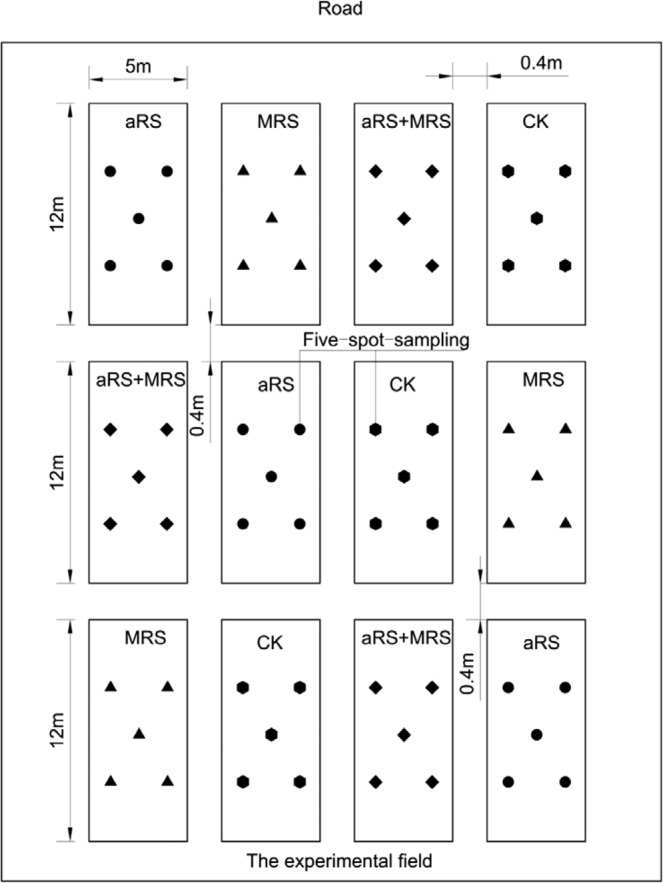
Diagram of different treatment plots in the field. aRS, avirulent Ralstonia solanacearum strain FJAT-1458 inoculation alone; MRS, soil amendment with microbial restoration substrate alone; aRS + MRS, combination of aRS and MRS; CK, non-(aRS or MRS) was used as control. Five solid circles in each plot of aRS, five solid triangles in each plot of MRS, five solid rhombus in each plot of aRS + MRS, five solid hexagon in each plot of CK represent of five-spot-sampling of rhizosphere soils for aRS, MRS, aRS + MRS and CK, respectively.
Soil sampling and disease investigation
Rhizosphere soil samples (attached to the root surface) were collected at the reproductive stage of tomato plants (90 d after transplanting). Each soil sample was taken from 25 plants in each block by the five-spot-sampling method (Fig. 1) and was partitioned into three subsamples, one for the enzyme assay, one for the biochemical property tests, and one for R. solanacearum detection.
The severity of tomato BW was investigated at the reproductive stage (90 d after transplanting). Based on the observations of leaf wilt symptoms, tomato bacterial wilt severity was empirically categorized into five grades as follows: 0, no wilting; 1, 1% to 25% wilting; 2, 26% to 50% wilting; 3, 51% to 75% wilting; and 4, 76% to 100% wilting or death of the entire plant29. The disease incidence, control efficiency, and disease severity index (DSI) were calculated as follows:
Disease incidence = ∑ (number of diseased plants/total number of plants investigated)
Control efficiency = [(disease incidence of CK - disease incidence of treatment aRS or MRS or “aRS + MRS”)/disease incidence of CK] × 100%
DSI = (4 A + 3B + 2 C + 1D)/N × 100, where A represents the number of plants in grade 4, B represents the number of plants in grade 3, C represents the number of plants in grade 2, D represents the number of plants in grade 1, and N represents the total number of plants30.
Ralstonia solanacearum detection
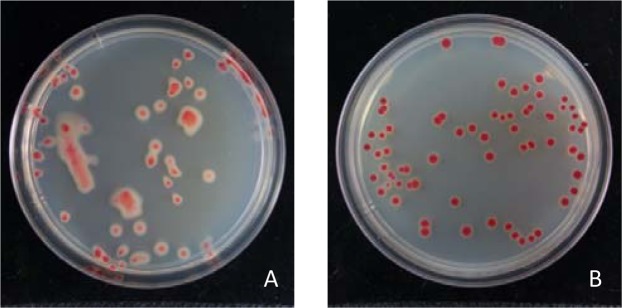
The quantification of R. solanacearum inoculum in the rhizosphere soil of tomato plants was determined by adding 10 g soil to 90 mL distilled water and shaking thoroughly. The suspension was subjected to a serial dilution, placed onto TTC medium to isolate R. solanacearum, and cultured at 30 °C for 48 h. The isolated R. solanacearum strains were confirmed by PCR with primers pehA#6/pehA#3 according to Gillings et al.31. Based on the colony morphology on TTC medium, the viable colonies were counted for each sample. According to Kelman27 and Liu et al.32, the colony of a virulent R. solanacearum strain is irregular, highly mobile, humid and displays a pink spot in the middle of the colony and a large white edge (Fig. 2A), while the colony of an avirulent R. solanacearum strain is round, immobile, dry and displays a dark red spot in the middle of colony and a narrow or no white edge (Fig. 2B).
Figure 2.
Colony morphology of virulent (A) and avirulent (B) Ralstonia solanacearum strains on TTC medium. The isolated R. solanacearum from the test samples showed two different colony morphologies on TTC medium. The colony of virulent R. solanacearum was irregular, highly mobile, humid and displayed a pink spot in the middle of the colony and a large white edge (A); while the colony of avirulent R. solanacearum was round, immobile, dry and displayed a dark red spot in the middle of colony and a narrow or no white edge (B).
Biological investigations
At vegetative stage (30 d after transplanting), some biological characteristics were investigated, including plant height, stem diameter (basal part of stem), and root activity. At harvest stage, the yield was also measured. For each treatment, 30 plants were selected using five-spot-sampling method to measure the plant height and stem diameter. The tomato roots of different treatments were collected (10 g) and their activity was measured using 2,3,5-triphenyl tetrazolium chloride (TTC) reduction method33. The tomato fruits of each treatment were weighed separately at per harvest time and the yields were calculated.
Enzyme assay
The soil samples were air-dried at room temperature for 48 h and passed through a 2 mm sieve for the enzyme activity analyses. All enzyme activities were assayed in triplicate with one control. The activities of urease, catalase, alkaline phosphatase and sucrase that are generally used to evaluate fertility and quality of soil were determined according to Guan34 with some minor modifications.
For urease activity assay, 5 g of soil was mixed with 5 mL of toluene, 10 mL of 10% urea solution, and 20 mL of distilled water, shaken and incubated at 37 °C for 24 h. Then, the soil suspension was centrifuged at 8,000 rpm for 5 min. A 0.5 mL of supernatant was treated with 4 mL of a mixed reagent (100 mL of a 6.6 M phenol solution and 100 mL of 6.8 M NaOH) and 3 mL of a sodium hypochlorite solution. The volume was adjusted to 20 mL with distilled water. The urease activity was quantified colorimetrically at 578 nm under UV-visible spectrophotometer (UV-2550, Shimadzu, Japan) and was expressed as μg NH4-N ·g−1 soil· 24 h−1.
For catalase activity assay, 3 g of soil was mixed with 5 mL of 0.3% H2O2 and 40 mL distilled water. The soil slurry was shaken thoroughly for 20 min. Subsequently, 5 mL of 3 N H2SO4 was added to remain peroxide stability and the suspension was then centrifuged at 8,000 rpm for 5 min. The catalase activity was determined by titration with 0.1 M KMnO4 and expressed as mL KMnO4·g−1 soil·20 min−1.
For alkaline phosphatase activity assay, 2 g of soil was mixed with 2 mL of toluene and 20 mL of borate saline buffer (pH 9.6). The soil slurry was incubated at 37 °C for 24 h. Then 40 mL of a 3% aluminum sulfate solution was added, and it was centrifuged at 8,000 rpm for 5 min. After centrifugation, 3 mL of supernatant was mixed with four drops of 2,6-dibromoquinone-4-chloroimide and was then diluted to 50 mL. The alkaline phosphatase activity was quantified colorimetrically at 660 nm under UV-visible spectrophotometer (UV-2550, Shimadzu, Japan) and was expressed as μg phenol·g−1 soil·24 h−1.
For sucrase activity assay, 5 g of soil was mixed with 5 mL of toluene, 15 mL of an 8% sucrose solution and 5 mL of phosphate buffer (pH 5.5). Then the soil slurry was inoculated at 37 °C for 24 h and was then centrifuged at 8000 rpm for 5 min. The suspension (1 mL) was added with 3 mL of salicylic acid and was incubated at 100 °C for 5 min, cooled for 3 min with flowing tap water and was then added with distilled water to make up 50 mL. The sucrase activity was measured under UV-visible spectrophotometer (UV-2550, Shimadzu, Japan) at an absorbance of 508 nm and was expressed as mg ·glucose ·g−1 soil·24 h−1.
Physicochemical assay
The soil samples were air-dried at room temperature and passed through a 2 mm sieve for physicochemical analyses. The soil organic carbon (SOC) was measured by the Walkley-Black method35, the total nitrogen (TN) was determined by the Kjeldahl method36 the total potassium (TK) was determined using an atomic absorption spectrophotometer after wet digestion of soil sample with NaOH37, the total phosphorus (TP) was determined by Vanado-Molybdate phosphoric yellow colorimetric procedure and the exchangeable calcium was determined using atomic absorption spectrophotometry after extracting with ammonium acetate38. The soil pH was measured using a 1:2.5 (w:v) soil: water ratio.
Data analysis
All statistical analyses were performed using the SPSS 13.0 software (SPSS Inc, Chicago, IL). Differences of the control efficiency, R. solanacearum population, soil physicochemical property and enzymatic activity among the treatments and years were assessed with two-way analysis of variance (ANOVA). The mean comparisons were made using ANOVA and a least significant difference (LSD) test (P < 0.05). The correlation analysis between the disease incidence and the soil chemical properties was conducted by a Pearson’s correlation. The synergistic responses of the MRS and aRS in the control efficiency were calculated using the method introduced by Colby39: E = If + Ib − If Ib/100, where If is the observed control efficiency of MRS, Ib is the observed control efficiency of aRS, and E is the expected control efficiency of the combination MRS and aRS. When the observed response was greater than expected, the combination was considered synergistic, and vice versa.
Results
Control of tomato BW by different treatments
The effect of MRS combined with aRS on tomato BW control was evaluated in a field experiment. The disease incidence of the aRS + MRS treatment was the lowest among the four treatments for the two consecutive years (Table 1). The combined use of aRS and MRS yielded better control of tomato bacterial wilt. The control efficiency of treatment aRS + MRS was 80.79% in 2017 and 85.79% in 2018, which was significantly higher than aRS (63.06% in 2017 and 63.79% in 2018) (p-value = 0.0001) and MRS (42.86% in 2017 and 52.64% in 2018) (p-value = 0.0001). Moreover, the observed control efficiency of the combined use of aRS and MRS was higher than the expected control efficiency, suggesting a synergistic control effect. Two-way ANOVA showed that the difference of control efficiency among different treatments reaching to significant level, but it was not for different years (except for MRS, p-value = 0.0021).
Table 1.
Control of tomato bacterial wilt by an avirulent Ralstonia solanacearum strain and a microbial restoration substrate.
| Treatment | 2017 | 2018 | ||||||
|---|---|---|---|---|---|---|---|---|
| Disease severiy index | Observed control (%) | Expected control (%) | Difference | Disease severiy index | Observed control (%) | Expected control (%) | Difference | |
| CK | 43.18 ± 2.12 a | — | — | — | 53.10 ± 4.18 a | — | — | — |
| aRS | 14.49 ± 0.67c | 63.06 ± 0.91 b | — | — | 14.52 ± 1.58 b | 63.79 ± 1.14 c | — | — |
| MRS | 19.24 ± 1.91 b | 42.86 ± 1.18 c | — | — | 16.32 ± 1.65 b | 52.64 ± 1.99 b | — | — |
| aRS + MRS | 8.59 ± 1.06 d | 80.79 ± 3.87 a | 78.89 ± 0.08 | +1.90 | 6.13 ± 0.87 c | 85.79 ± 1.83 a | 82.86 ± 0.92 | +2.93 |
Data are means ± standard error (n = 3); Values within a row followed by the same letter are not significantly different at P ≤ 0.05.
aReduction in disease incidence relative to the control.
bExpected control is the cotrol resulting from the combination as predicted by the equation of Colby.
dDifference = % reduction observed-% reduction expected, with a plus sign indicating a synergistic
effect.
Quantification of R. solanacearum in the tomato rhizosphere soils after the different treatments
Compared to CK, the application of aRS, MRS and aRS + MRS treatments significantly reduced the population of virulent R. solanacearum by 96.64, 81.56 and 99.72% in 2017 (p-value = 0.0001), and by 97.62, 98.95 and 99.77% in 2018 (p-value = 0.0001) (Table 2). However, The avirulent R. solanacearum was detected only in the treatments of aRS and aRS + MRS. The population of avirulent R. solanacearum in the treatment of aRS + MRS was significantly higher than in the treatment of aRS (p-value = 0.0007).
Table 2.
Quantification of Ralstonia solanacearum in the tomato rhizosphere soil (×105 cfu g−1).
| Treatments | Experiment time (year) | Avirulent strain (×105 cfu g−1) | Virulent strain (×105 cfu g−1) |
|---|---|---|---|
| CK | 2017 | 0 d | 53.62 ± 7.51 b |
| aRS | 0.59 ± 0.03 c | 1.80 ± 0.21 d | |
| MRS | 0 d | 9.89 ± 0.94 c | |
| aRS + MRS | 2.57 ± 0.07 b | 0.15 ± 0.04 d | |
| CK | 2018 | 0 d | 60.00 ± 6.82 a |
| aRS | 0.53 ± 0.12 c | 1.43 ± 0.17 d | |
| MRS | 0 d | 0.63 ± 0.11 d | |
| aRS + MRS | 3.07 ± 0.45 a | 0.14 ± 0.01 d |
Data are means ± standard error (n = 3); Values within a row followed by the same letter are not significantly different at P ≤ 0.05.
Biological characteristics of tomato plants after the different treatments
The application of MRS, aRS alone or in combination could all increase plant growth, root activity and yield in comparison to CK (Table 3). Plants of treatment aRS + MRS had greater plant heights than those subjected to the other treatments. The stem diameters for treatments aRS + MRS and MRS were significantly greater than aRS (p-value = 0.0001) and CK (p-value = 0.0001). In addition, the root activities for treatments aRS + MRS and MRS were also significantly higher than aRS (p-value = 0.0011, 0.0088, respectively) and CK (both of p-values were 0.0001). The yields achieved following treatments aRS + MRS, aRS, and MRS were all significantly higher than CK (p-value = 0.0001), and the treatment of aRS + MRS had the highest yield of 28611.67 kg ha−1 in 2017 and 31667.33 kg ha−1 in 2018. Two-way ANOVA showed that the differences of plant height, root activity and yield among different treatments (all of p-values were 0.0001) and years (p-value = 0.0038, 0.0373, 0.0003, respectively) were all reaching to significant level.
Table 3.
Biological characteristics of tomato plant after different treatments.
| Treatment | Experiment time (year) | Plant height (cm) | Stem diameter (cm) | Root activity (µg g−1 h−1) | Yield (kg ha−1) |
|---|---|---|---|---|---|
| CK | 2017 | 32.27 ± 1.70 c | 0.53 ± 0.05 c | 23.12 ± 0.66 d | 6218.67 ± 621.67 e |
| aRS | 33.33 ± 3.38 c | 0.58 ± 0.00 c | 26.88 ± 1.43 c | 21765.67 ± 822.51 c | |
| MRS | 35.33 ± 1.29 bc | 0.74 ± 0.11 b | 30.04 ± 1.66 b | 15236.33 ± 822.58 d | |
| aRS + MRS | 40.40 ± 1.22 a | 0.84 ± 0.03 a | 31.10 ± 1.15 b | 28611.67 ± 619.18 b | |
| CK | 2018 | 34.59 ± 1.39 bc | 0.53 ± 0.02 c | 20.12 ± 1.81 e | 5618.33 ± 518.76 e |
| aRS | 36.49 ± 0.86 b | 0.58 ± 0.02 c | 26.15 ± 0.77 c | 21677.67 ± 535.72 c | |
| MRS | 39.93 ± 1.21 a | 0.82 ± 0.04 ab | 34.20 ± 1.70 a | 20575.33 ± 158.68 c | |
| aRS + MRS | 40.27 ± 2.11 a | 0.83 ± 0.02 a | 35.50 ± 0.43 a | 31667.33 ± 582.78 a |
Data are means ± standard error (n = 3); Values within a row followed by the same letter are not significantly different at P ≤ 0.05. Plant height, stem diameter and root activity were measured at vegetative stage.
Physicochemical properties of tomato rhizosphere soils after the different treatments
The soil chemical parameters were influenced by the different treatments (Table 4). Compared with CK, the application of MRS alone or in combination with aRS significantly increased the soil pH, by changing it from acidic (CK, pH 4.5 in 2017 and 4.4 in 2018) to nearly neutral (pH 6.57 in 2017 and 7.07 in 2018 for the treatment of MRS + aRS). The MRS and aRS + MRS treatments also significantly increased the soil organic carbon (SOC), the soil total nitrogen (TN), the total phosphorus (TP) and the total potassium (TK) contents compared with CK (all of p-values were 0.0001). For example, the SOC contents aRS + MRS treatment were 63.49 g kg−1 in 2017 and 82.90 g kg−1 in 2018, which were approximately 3-fold or 4-fold higher than that of CK (21.47 g kg−1 in 2017 and 19.09 g kg−1 in 2018). Moreover, the SOC, TN, TP, TK and exchangeable calcium contents of MRS and aRS + MRS treatments in 2018 were significantly higher than those in 2017 (all of p-values were 0.0001).
Table 4.
Properties of tomato rhizosphere soils after the different treatments.
| Treatments | Experiment time (year) | Items | |||||
|---|---|---|---|---|---|---|---|
| pH | #SOC (g kg−1) | TN (g kg−1) | TP (g kg−1) | TK (g kg−1) | Exchangeable calcium (cmol kg−1) | ||
| CK | 2017 | 4.50 ± 0.10 e | 21.47 ± 1.30 e | 1.63 ± 0.02 d | 1.14 ± 0.05 d | 13.25 ± 0.16 c | 6.20 ± 0.02 d |
| aRS | 4.87 ± 0.06 d | 27.55 ± 0.68 d | 1.73 ± 0.02 c | 1.89 ± 0.01 c | 15.34 ± 0.09 b | 6.13 ± 0.03 d | |
| MRS | 6.17 ± 0.06 c | 54.69 ± 2.13 b | 1.83 ± 0.03 b | 2.21 ± 0.02 b | 15.90 ± 0.07 b | 6.63 ± 0.06 cd | |
| aRS + MRS | 6.57 ± 0.06 b | 63.49 ± 2.30 c | 1.81 ± 0.01 b | 2.14 ± 0.06 b | 15.93 ± 0.15 b | 7.47 ± 0.05 c | |
| CK | 2018 | 4.40 ± 0.10 e | 19.09 ± 1.04 e | 1.61 ± 0.01 d | 1.03 ± 0.12 d | 11.85 ± 0.14 d | 6.28 ± 0.14 d |
| aRS | 4.77 ± 0.15 d | 25.98 ± 2.97 d | 1.82 ± 0.03 b | 1.92 ± 0.04 c | 16.06 ± 0.45 b | 6.08 ± 0.07 d | |
| MRS | 7.00 ± 0.20 a | 77.61 ± 4.12 a | 1.96 ± 0.02 a | 3.00 ± 0.05 a | 18.30 ± 0.21 a | 14.57 ± 1.05 b | |
| aRS + MRS | 7.07 ± 0.23 a | 82.90 ± 2.72 a | 1.97 ± 0.01 a | 3.02 ± 0.04 a | 18.40 ± 0.22 a | 16.67 ± 1.40 a | |
Data are means ± standard error (n = 3); Values within a row followed by the same letter are not significantly different at P ≤ 0.05. #Soil organic carbon (SOC), total nitrogen (TN), total phosphorus (TP), total potassium (TK) and Exchangeable calcium.
Enzymatic activity in the tomato rhizosphere soils after the different treatments
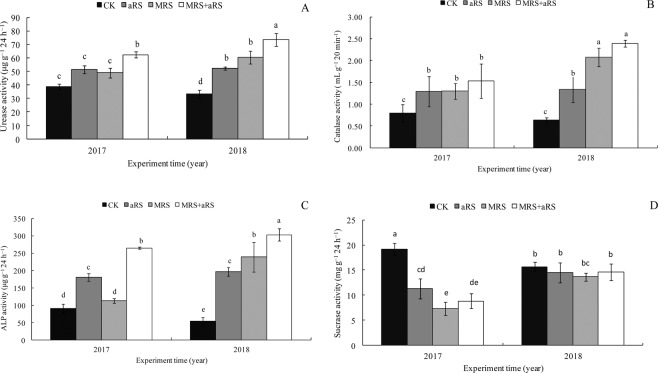
The urease, catalase and alkaline phosphatase (ALP) activities for aRS, MRS and aRS + MRS treatments were all significantly higher than CK both in 2017 and 2018 experiments (P < 0.05) (Fig. 3A–C). The aRS + MRS treatment showed maximum urease, catalase and ALP activities in the two consecutive years (Fig. 3A–C). Compared to CK, the application of aRS or MRS alone or in combination significantly decreased sucrase activity by 41.25%, 62.18% and 54.06%, respectively in 2017 (all of p-values were 0.0001) (Fig. 3D).
Figure 3.
Effect of different treatments on the soil enzymatic activity. A urase, B catalase, C alkaline phosphatase, and D sucrase. aRS, avirulent Ralstonia solanacearum strain FJAT-1458 inoculation alone; MRS, soil amendment with microbial restoration substrate alone; aRS + MRS, combination of aRS and MRS; CK, non-(aRS or MRS) was used as control. Bars represent the means of three replicates for each treatment with error bars denoting the SE. Different lower-case letters above the bars indicate statistically significantly differences according to LSD test.
Key soil factors taking part in BW control
A correlation analysis was conducted between BW disease incidence and soil properties in order to obtain the key soil factors involved in the process of BW control in tomato. The results showed that disease incidence was negatively correlated with soil pH and with SOC, TN, TP, TK and the exchangeable calcium contents, with correlation coefficients of −0.54, −0.62, −0.42, −0.71, −0.84 and −012, respectively (Table 5), which indicated that high soil pH and high SOC, TN, TP, TK and exchangeable calcium contents are beneficial for plant health.
Table 5.
Pearson correlation analysis between disease incidence and soil properties.
| pH | SOC | TN | TP | TK | Exchangeable calcium | |
|---|---|---|---|---|---|---|
| r | −0.54 | −0.62 | −0.42 | −0.71 | −0.84 | −0.12 |
r indicates correlation coefficient; SOC, Soil organic carbon; TN, total nitrogen; TP, total phosphorus; TK, total potassium.
Discussion
Tomato BW results in huge economic losses each year in many tropical and subtropical areas24. Various approaches including bactericide application40, crop rotation7, anaerobic soil disinfestation41,42, biological agents43 have been attempted to arrest the disease so far. The use of chemical bactericide is associated toxic residues and environmental pollution. Biological control is a non-hazardous alternative for integrated pest management. However, it is difficult to achieve satisfactory disease control using biological agent alone. Many studies report that the combination of biocontrol agents and soil amendment enhances the control efficiency of soilborne disease compared with either treatment alone44–46. Our study revealed that the application of aRS + MRS in combination had significantly higher control efficiency than the application of aRS and MRS alone. This may be attributed to the additive effects of aRS and MRS in promoting each other establishment in the rhizosphere, because aRS and MRS act through different mechanisms to combat disease. Similar results were reported by Singh et al.44, showing that combining biocontrol agents and soil amendments improved the control of Macrophomina phaseolina- and Fusarium- induced diseases in legume and spice crops.
The ability of biocontrol bacteria to efficiently colonize the rhizosphere is a key factor in their successful improvement of plant health and suppression of plant pathogens47,48. Kempe and Sequeira (1983) had reported that a high density of avirulent R. solanacearum within plant tissues prevented or delayed pathogen entry into the host13. In this study, the number of avirulent R. solanacearum in the tomato rhizosphere soil was significantly higher for the combined application of aRS and MRS than the application of aRS alone, which was consistent with a lower DSI value for aRS + MRS than for aRS. This could be because MRS improved the soil physicochemical characteristics, such as soil pH and nutrients’ availability, which increased the colonization ability of aRS.
Decreasing virulent R. solanacearum population in the soil is important for soil suppression of BW9. Soil amendment with compost or manure49,50 or pig slurry51 reduced the populations of soilborne pathogens, including R. solanacearum. Ghosh et al.52 reported that the integration of organic and inorganic soil amendments indeed suppressed the growth of R. solanacearum52. In this study, the applications of aRS and MRS alone or in combination directly suppressed the growth of R. solanacearum in the tomato rhizosphere, especially for the combination using aRS and MRS. Similarly, Khan et al.53 reported that the population of the R. solanacearum virulent strain was reduced in rhizosphere after the application of R. solanacearum avirulent strain53. The avirulent strain exhibited antagonism toward BW pathogen under culture, glasshouse and field conditions12–14,54.
Compared to CK, the SOC, TN, TP, TK, and exchangeable calcium contents and the soil pH were significantly increased with increasing application of MRS, SOC and TK contents increased and exchangeable calcium concentration decreased with increasing application of aRS. A Pearson correlation analysis indicated that soil pH, and the SOC, TN, TP, TK and exchageable calcium contents were negatively related to BW disease incidence, which was in agreement with previous reports9,55,56. For example, the results of Liu et al. (2015) revealed that the SOC, TN, TP and TK contents played a key role in preventing the tomato plant from being infected by the wilt pathogen9. Yamazaki57 reported that an increase in the Ca concentration in tomato plants decreased the incidence of BW as well as R. solanacearum population57. Soil acidification is closely related to BW outbreak. The average soil pH in fields infected by BW was much lower than that in non-disease fields58. Thus, it is especially important for controlling BW by improving soil pH59.
The mechanism of action of nonpesticide chemicals to suppress BW is considered to involve either inducing systemic resistance or antibacterial activity2. Li and Dong (2013) found that the application of a commercial organic fertilizer, combined with rock dust soil amendment, increased the activities of alkaline phosphatase, urease, catalase and sucrase to a greater extent in the soil60. In the present study, the application of aRS and MRS alone or in combination significantly enhanced the activities of soil urease, catalase and ALP enzymes. This result is in agreement with a previous study61, suggesting that some plant defense enzyme activities were induced by the biocontrol agent.
In this study, we found that continuously use MRS resulted in a better crop yield and BW control. The control efficiencies, root activities, yields and soil properties from treatments of MRS and aRS + MRS in 2018 were all significantly higher than those in 2017. This indicates there are cumulative effects of MRS and aRS + MRS in the experiment of 2018. Moreover, the combined use of aRS and MRS reaching to a synergistic control effect, for its observed control efficiency higher than the expected control efficiency. Thus, the combination of MRS and aRS represents a promising new tool to control BW.
Conclusions
The combination of MRS and aRS could significantly suppress virulent R. solanacearum survival, reduce tomato bacterial wilt disease severity, and increase root activities and tomato yield. The outcomes might mainly attributed to the “MRS + aRS” being able to change soil pH from acidic to nearly neutral, improve soil nutrients, and enhance some soil enzyme activities. In conclusion, the combined application of MRS and aRS is beneficial to soil nutrient cycling and plant health, indicating a promising new tool to control tomato bacterial wilt.
Acknowledgements
This work was supported by the National Key R & D Program of China (2017YFD0201100), National Natural Science Foundation of China (31701835) and its Extension Research Project from Fujian Academy of Agricultural Sciences (AB2017-6), the Fujian Special Fund for Public Interest Research (2018R1017-1), and the Science and Technology Innovation Team Program of Fujian Academy of Agricultural Sciences (STIT 2017-1-11).
Author contributions
Bo Liu and Xuefang Zheng designed the research and wrote the manuscript. Xuefang Zheng, Yujing Zhu and Jieping Wang performed the field experiments. Zirang Wan performed soil enzyme analysis. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Li Y, Niu WQ, Dyck M, Wang JW, Zou XY. Yields and nutritional of greenhouse tomato in response to different soil aeration volume at two depths of subsurface drip irrigation. Sci. Rep. 2016;6:39307. doi: 10.1038/srep39307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nion YA, Toyata K. Recent trends in control methods for bacterial wilt diseases caused by Ralstonia solanacearum. Microbes Environ. 2015;30:1–11. doi: 10.1264/jsme2.ME14144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramesh R, Joshi AA, Ghanekar MP. Pseudomonads: major antagonistic endophytic bacteria to suppress bacterial wilt pathogen, Ralstonia solanacearum in the eggplant (Solanum melongena L.) World J. Microb. Biot. 2009;1:47–55. doi: 10.1007/s11274-008-9859-3. [DOI] [Google Scholar]
- 4.Fujiwara A, et al. Biocontrol of Ralstonia solanacearum by treatment with lytic bacteriophages. Appl. Environ. Microb. 2011;7:4155–62. doi: 10.1128/AEM.02847-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ding CY, Shen QR, Zhang RF, Chen W. Evaluation of rhizosphere bacteria and derived bio-organic fertilizers as potential biocontrol agents against bacterial wilt (Ralstonia solanacearum) of potato. Plant Soil. 2013;366:453–466. doi: 10.1007/s11104-012-1425-y. [DOI] [Google Scholar]
- 6.Jogaiah S, Abdelrahman M, Tran LSP, Shin-ichi I. Characterization of rhizosphere fungi that mediate resistance in tomato against bacterial wilt disease. J. Exp. Bot. 2013;64:3829–3842. doi: 10.1093/jxb/ert212. [DOI] [PubMed] [Google Scholar]
- 7.King SR, Davis AR, Liu W, Levi A. Grafting for disease resistance. HortSci. 2008;43:1673–1676. doi: 10.21273/HORTSCI.43.6.1673. [DOI] [Google Scholar]
- 8.Bhunchoth A, et al. Isolation of Ralstonia solanacearum-infecting bacteriophages from tomato fields in Chiang Mai, Thailand, and their experimental use as biocontrol agents. J. Appl. Microbiol. 2015;118:1023–1033. doi: 10.1111/jam.12763. [DOI] [PubMed] [Google Scholar]
- 9.Liu LJ, et al. Bioorganic fertilizer enhances soil suppressive capacity against bacterial wilt of tomato. PLoS. ONE. 2015;10:e0121304. doi: 10.1371/journal.pone.0121304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuan S, et al. Evaluation of Bacillus-fortified organic fertilizer for controlling tobacco bacterial wilt in green house and field experiments. Appl. Soil. Ecol. 2014;75:86–94. doi: 10.1016/j.apsoil.2013.11.004. [DOI] [Google Scholar]
- 11.Boukaew S, Chuenchit S, Petcharat V. Evaluation of Streptomyces spp. for biological control of Sclerotium root and stem rot and Ralstonia wilt of chili pepper. Biocontrol. 2011;56:365–374. doi: 10.1007/s10526-010-9336-4. [DOI] [Google Scholar]
- 12.Arwiyanto T, Goto M, Tsuyumy T, Takikawa Y. Biological control of bacterial wilt of tomato by an avirulent strain of Pseudomonas solanacearum isolated from strelitzia reginae. Ann. Phytopathol. Soc. Japan. 1994;60:421–430. doi: 10.3186/jjphytopath.60.421. [DOI] [Google Scholar]
- 13.Frey P, et al. Hrp- mutants of Pseudomonas solanacearum as potentiall biocontrol agents of tomato bacterial wilt. Appl. Environ. Microbiol. 1994;60:3175–3181. doi: 10.1128/aem.60.9.3175-3181.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grimault V, Prior P. Invasiveness of avirulent strains of Pseudomonas solanacearum in tomato cultivars, resistant or susceptible to bacterial wilt. J. Phytopathol. 2008;141:195–201. doi: 10.1111/j.1439-0434.1994.tb01461.x. [DOI] [Google Scholar]
- 15.Kempe J, Sequeira J. Biological control of bacterial wilt of potatoes: attempts to induce resistance by treating tubers with bacteria. Plant. Dis. 1983;67:499–503. doi: 10.1094/PD-67-499. [DOI] [Google Scholar]
- 16.Frey P, et al. Hrp- mutants of Pseudomonas solanacearum as potential biocontrol agents of tomato bacterial wilt. Appl. Environ. Microb. 1994;60:3175–81. doi: 10.1128/aem.60.9.3175-3181.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spadaro D, Gullino ML. Improving the efficacy of biocontrol agents against soilborne pathogens. Crop. Prot. 2005;24:601–613. doi: 10.1016/j.cropro.2004.11.003. [DOI] [Google Scholar]
- 18.Zheng XF, Zhu YJ, Liu B, Yu Q, Lin NQ. Rapid differentiation of Ralstonia solanacearum avirulent and virulent strains by cell fractioning of an isolate using high performance liquid chromatography. Microb. Pathogenesis. 2016;90:84–92. doi: 10.1016/j.micpath.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 19.Zheng XF, Zhu YJ, Liu B, Ge XB. Preparation of colloidal suspension agent used as plant vaccine against tomato bacterial wilt disease and its control efficacy. Plant Prot. 2017;43:208–211. [Google Scholar]
- 20.Mathre DE, Cook RJ, Calla NW. From discovery to use: traversing the world of commercializing biocontrol agents for plant disease control. Plant Dis. 1999;83:972–983. doi: 10.1094/PDIS.1999.83.11.972. [DOI] [PubMed] [Google Scholar]
- 21.Giuliano B, Vincenzo A, Manuela C, Felice S. Identifying the characteristics of organic soil amendments that suppress soilborne plant diseases. Soil. Biol. Biochem. 2010;42:136–144. doi: 10.1016/j.soilbio.2009.10.012. [DOI] [Google Scholar]
- 22.Zheng XF, et al. Soil restoration for continuous cropping obstacles in tomato greenhouse field and the control effect against bacterial wilt disease. Chinese J. Biol. Control. 2018;34:117–123. [Google Scholar]
- 23.Prior, P. et al. Contribution to integrated control against bacterial wilt in different pedoclimatic situations: Guadeloupe experience. ACIAR Proceedings-Australian Centre for International Agricultural Research (Australia) (1993).
- 24.Guo JH, et al. Biocontrol of tomato wilt by plant growth promoting rhizobacterial. Biol Control. 2004;29:66–72. doi: 10.1016/S1049-9644(03)00124-5. [DOI] [Google Scholar]
- 25.Wu K, et al. Effects of bio-organic fertilizer plus soil amendment on the control of tobacco bacterial wilt and composition of soil bacterial communities. Biol. Fert. Soils. 2014;50:961–971. doi: 10.1007/s00374-014-0916-9. [DOI] [Google Scholar]
- 26.Peng D, et al. Combined use of Bacillus subtilis strain B-001 and bactericide for the control of tomato bacterial wilt. Pest. Manag. Sci. 2017;73:1253–1257. doi: 10.1002/ps.4453. [DOI] [PubMed] [Google Scholar]
- 27.Kelman A. The relationship of pathogenicity in Pseudomonas solanacearum to colony appearance on a tetrazolium medium. Phytopathol. 1954;44:693–695. [Google Scholar]
- 28.Chen QQ, Liu B, Wang JP, Che JM, Liu GH. Diversity and dynamics of the bacterial community involved in pig manure biodegradation in a microbial fermentation bed system. Ann. Microbiol. 2017;67:491–500. doi: 10.1007/s13213-017-1278-y. [DOI] [Google Scholar]
- 29.Roberts DP, Denny TP, Schell MA. Cloning of the egl gene of Pseudomonas solanacearum and analysis of its role in phytopathogenicity. J. Bacteriol. 1988;170:1445–1451. doi: 10.1128/jb.170.4.1445-1451.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li B, et al. Inhibitory activity of Paenibacillus macerans and Paenibacillus polymyxa against Ralstonia solanacearum. Afr. J. Microbiol. Res. 2010;19:2048–2054. [Google Scholar]
- 31.Gillings M, Fahy P, Davies C. Restriction analysis of an amplified polygalacturonase gene fragment differentiates strains of the phytopathogenic bacterium Pseudomonas solanacearum. Lett. Appl. Microbiol. 1993;17:44–48. doi: 10.1111/j.1472-765X.1993.tb01432.x. [DOI] [PubMed] [Google Scholar]
- 32.Liu, B., Lin, Y. Z., Zhu, Y. J., Ge, C. B. & Cao, Y. Attenuation characteristics of bacterial wilt disease biocontrol strain Anti-8098A (Bacillus cereus) to Ralstonia solanacearum. J. Agr. Biotechnol. 12, 322–329, (in Chinese with English abstract) (2004).
- 33.Xiao, L. T. & Wang, S. G. Experimental techniques of plant physiology, China Agricultural Press, Beijing, China (2005).
- 34.Guan, S. Y. Soil Enzymes and its Methodology (in Chinese). Chinese Agricultural Press, Beijing, pp 309–313 (1986).
- 35.Walkley A, Black TA. An examination of the Degtijareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934;37:29–38. doi: 10.1097/00010694-193401000-00003. [DOI] [Google Scholar]
- 36.Bremner J, Breitenbeck GA. A simple method for determination of ammonium in semimicro-Kjeldahl analysis of soils and plant materials using a block digester. Commun. Soil. Sci. Plant. Anal. 1983;14:905–913. doi: 10.1080/00103628309367418. [DOI] [Google Scholar]
- 37.Bao, S. D. Soil and Agricultural Chemistry Analysis. China Agriculture Press, Beijing, China (2000).
- 38.Jackson, M. L. Soil Chemical Analysis - Advanced Course (2nd edition). Department of Soil Science, University of Wisconsin, Madison, WI (1969).
- 39.Colby SR. Calculating synergistic and antagonistic responses of herbicide combinations. Weeds. 1967;15:20–22. doi: 10.2307/4041058. [DOI] [Google Scholar]
- 40.Li P, et al. Antibacterial activities against rice bacterial leaf blight and tomato bacterial wilt of 2-mercapto-5-substituted-1,3,4-oxadiazole/thiadiazole derivatives. Bioorg. Med. Chem. Lett. 2015;25:481–484. doi: 10.1016/j.bmcl.2014.12.038. [DOI] [PubMed] [Google Scholar]
- 41.Strauss SL, Kluepfel DA. Anaerobic soil disinfestation: A chemical –independent approach to pre-plant control of plant pathogens. J. Integr. Agr. 2015;14:2309–2318. doi: 10.1016/S2095-3119(15)61118-2. [DOI] [Google Scholar]
- 42.Momma N, Kobara Y, Uematsu S, Kita N, Shinmura N. Development of biological soil disinfestations in Japan. Appl. Microbiol. Biot. 2013;97:3801–3809. doi: 10.1007/s00253-013-4826-9. [DOI] [PubMed] [Google Scholar]
- 43.Kurabachew H, Wydra K. Characterization of plant growth promoting rhizobacteria and their potential as bioprotectant against tomato bacterial wilt caused by Ralstonia solanacearum. Biol. Control. 2013;67:75–83. doi: 10.1016/j.biocontrol.2013.07.004. [DOI] [Google Scholar]
- 44.Singh V, Mawar R, Lodha S. Combined effects of biocontrol agents and soil amendments on soil microbial populations, plant growth and incidence of charcoal rot of cowpea and wilt of cumin. Phytopathol Mediterr. 2012;51:307–316. [Google Scholar]
- 45.Garkoti A, Kumar V, Tripathi HS. Control of wilt disease of lentil through biocontol agents and organic amendments in Tarai region of Uttarakhand, India. J. Environ. Biol. 2014;35:1067–1070. [PubMed] [Google Scholar]
- 46.Ruano-Rosa, D. & Mercado-Blanco, J. Combining biocontrol agents and organics amendments to manage soil-borne phytopathogens. Pages 457–478 in: Organic Amendments and Soil Suppressiveness in Plant Disease Management. M. Meghvansi and A. Varma, eds. Springer International Press, Cham (2015).
- 47.Zhang N, et al. New bioorganic fertilizer can effectively control banana wilt by strong colonization with Bacillus substilis N11. Plant Soil. 2011;344:87–97. doi: 10.1007/s11104-011-0729-7. [DOI] [Google Scholar]
- 48.Chowdhury SP, et al. Effects of Bacillus amyloliquefaciens FZB42 on lettuce growth and health under pathogen pressure and its impact on the rhizoshpere bacterial community. PLoS. ONE. 2013;8:e68818. doi: 10.1371/journal.pone.0068818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schönfeld J, et al. Effects of compost addition and simulated solarisation on the fate of Ralstonia solanacearum biovar 2 and indigenous bacterial in soil. FEMS Microbiol. Ecol. 2003;43:63–74. doi: 10.1111/j.1574-6941.2003.tb01046.x. [DOI] [PubMed] [Google Scholar]
- 50.Yadessa GB, van Bruggen AHC, Ocho FL. Effects of different soil amendments on bacterial wilt caused by Ralstonia solanacearum and on the yield of tomato. J. Plant. Pathol. 2010;92:439–450. [Google Scholar]
- 51.Gorissen A, van Overbeekm LS, van Elsas JD. Pig slurry reduces the survival of Ralstonia solanacearum biovar 2 in soil. Cana J. Micorbiol. 2004;50:587–593. doi: 10.1139/w04-042. [DOI] [PubMed] [Google Scholar]
- 52.Ghosh PP, Dutta S, Chattopadhyay A. Integration of organic and inorganic amendments with native bioagents for bio-intensive management of vascular bacterial wilt on eggplant (Solanum melongena) Indian Phytopathol. 2015;68:32–38. [Google Scholar]
- 53.Khan, A. N. A., Karuna, K. & Ravikumar, M. R. Potential biocontrol agents in the management of bacterial wilt of tomato caused by Ralstonial solanacearum. 3rd International Bacterial Wilt Symposium. Held at Gaudeloupe form June, 23–27 (1997).
- 54.Hara H, Ono K. Effect of weakly-virulent bacteriocin-producing strain of Pseudomonas solanacearum on the protection of tobacco plant from bacterial wilt. Ann. Phytopathol. Soc. Japan. 1991;57:24–31. doi: 10.3186/jjphytopath.57.24. [DOI] [Google Scholar]
- 55.Höper H, Alabouvette C. Importance of physical and chemical soil properties in the suppressiveness of soils to plant disease. Eur. J. Soil. Biol. 1996;32:41–58. [Google Scholar]
- 56.Davis J, Huisman O, Everson D, Schneider A. Verticillium wilt of potato: a model of key factors related to disease severity and tuber yield in southeastern Idaho. American J. Potato Research. 2001;78:291–300. doi: 10.1007/BF02875694. [DOI] [Google Scholar]
- 57.Yamazaki H, Kikuchi S, Hoshina T, Kimura T. Calcium uptake and resistance to bacterial wilt of mutually grafted tomato seedlings. Soil. Sci. Plant. Nutr. 2000;46:529–534. [Google Scholar]
- 58.Li S, et al. Soil acidification aggravates the occurrence of bacterial wilt in South China. Front. Microbiol. 2017;8:703. doi: 10.3389/fmicb.2017.00703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang C, et al. Tobacco bacterial wilt suppression with biochar soil addition associates to improved soil physiochemical properties and increased rhizosphere bacteria abundance. Appl. Soil. Eol. 2016;112:90–96. doi: 10.1016/j.apsoil.2016.12.005. [DOI] [Google Scholar]
- 60.Li JG, Dong YH. Effect of a rock dust amendment on disease severity of tomato bacterial wilt. Antonie. Van. Leeuwenhoek. 2013;103:11–22. doi: 10.1007/s10482-012-9781-4. [DOI] [PubMed] [Google Scholar]
- 61.Anand T, et al. Association of some plant defense enzyme activities with systemic resistance to early leaf blight and leaf spot induced in tomato plants by azoxystrobin and Pseudomonas fluorescens. J. Plant. Interact. 2007;4:233–244. doi: 10.1080/17429140701708985. [DOI] [Google Scholar]