Abstract
Due to the aging world population and increasing trend in clinical practice to treat patients with multiple drugs, adverse events (AEs) are becoming a major challenge in drug discovery and public health. In particular, identifying AEs caused by drug combinations remains a challenging task. Clinical trials typically focus on individual drugs rather than drug combinations and animal models are unreliable. An added difficulty is the combinatorial explosion in the number of possible combinations that can be made using the increasingly large set of FDA approved chemicals. We present a statistical and computational technique for identifying AEs caused by two-drug combinations. Taking advantage of the large and increasing data deposited in FDA’s postmarketing reports, we demonstrate that the task of predicting AEs for 2-drug combinations is amenable to the Likelihood Ratio Test (LRT). Our pAERS database constructed with LRT contains almost 77 thousand associations between pairs of drugs and corresponding AEs caused solely by drug-drug interactions (DDIs). The DDIs stored in pAERS complement the existing data sets. Due to our stringent statistical test, we expect many of the associations in pAERS to be unrecorded or poorly documented in the literature.
Subject terms: Drug safety, Molecular medicine
Introduction
An adverse event (AE) is an injury caused by a drug. Adverse events account for more than 100,000 deaths per year in the United States1, representing the 4th leading cause of death, after heart disease, cancer and stroke, but well ahead of diabetes, pulmonary diseases and AIDS.
Due to limited duration and small patient population, clinical trials lack the necessary statistical power to identify severe AEs. To address this limitation, postmarketing safety reporting was established (FAERS - https://open.fda.gov/data/faers). The assumption is that spontaneous reporting by health-care professionals, as well as consumers, family members and lawyers provides a more complete and accurate data on drug-AE associations. Nevertheless, the data from postmarketing safety surveillance is neither clean nor reliable. Part of the problem is that the adverse events in FAERS are reported cumulatively for all drugs taken by a patient, making it difficult to tell which drug, among multiple ones given to the patient, gave rise to the reported side-effects.
AEs arising from drug combinations
While limited information on AEs of individual drugs is available in the drug package inserts and lately in electronic forms2,3, there is an utter lack of data on side-effects arising from drug-drug interactions. On the other hand, there is an increasing trend in clinical practice to treat patients with drug combinations. Almost 80% of Americans over the age of 60 are taking multiple drugs4, and the number of drugs taken by a patient is the most reliable predictor of the occurrence and intensity of an adverse event5.
There are two reasons behind the sparseness of data on side-effects arising from drug combinations. First, clinical trials typically focus on a single compound and rarely on drug combinations. Additionally, a combinatorial explosion occurs in the number of possible drug therapies that can be made using the current market drugs. For instance, the existing set of ~1,500 FDA approved drugs yields more than one million 2-drug combinations. Such an enormous number of combined therapies makes the task of studying, organizing and storing AEs computationally challenging.
Due to the above difficulties, the existing AE resources are scarce. Among noteworthy exceptions are SIDER2,3 and the OFFSIDES6 database of AEs for individual drugs, as well as DrugBank7 and the TWOSIDES6 databases of AEs for pairs of drugs. While drug-drug interactions (DDIs) in DrugBank mainly come from manual curation of literature, the statistical significance of associations in TWOSIDES is computed using Propensity Score Matching (PSM)8,9. In addition to statistical techniques, network-based multi-scale modeling may provide critical mechanistic insights into DDIs10,11.
Here we demonstrate that the problem of detecting adverse events caused by DDIs is amenable to Likelihood Ratio Test (LRT) statistics12. LRT has been originally developed to address problems in fields as diverse as astronomy, forestry, zoology and epidemiology. The theory has been successfully applied to the study of the sudden infant death syndrome (SIDS)12 as well as in cancer surveillance to find clusters of high disease incidence and mortality13. In a study most relevant to ours, Huang et al., have demonstrated that LRT can lower the type I error in detecting AEs associated with one-drug therapies14.
Methods
We constructed two groups of two different AE databases. The first group contains associations between AEs and individual drugs, while the second group contains associations between AEs and drug combinations. In order to allow for more diverse user queries, we developed two databases for each group, one consisting of MedDRA Preferred Terms (PT)15 and one where AEs represent MedDRA High Level Terms (HLT).
Normalizing drug and AE names stored in FAERS reports
Drug lexicon
We use FDA’s Orange Book (https://www.accessdata.fda.gov/scripts/cder/ob/) to compile a set of representative drugs. To remove duplicate drugs (those that contain the same ingredient but in different pharmaceutical salt forms) we use RxNorm16- a suite of databases and tools that provides normalized names for clinical drugs. As of this writing, applying those filters yields a set of 1,309 unique chemicals. In order to boost the statistical power in extracting AEs for highly similar drugs and their combinations, we use the Complete-linkage clustering (Statistical Machine Intelligence and Learning Engine, http://haifengl.github.io/smile/) to group 1,309 drugs into 1,178 clusters. We define the dissimilarity of (i.e., the distance between) compounds c1 and c2 as the “inverse” Tanimoto metric17, namely, . We note that the Complete-linkage requires no parameters and is preferable to both Single-linkage and Average-linkage clustering since it is capable of finding compact clusters of approximately equal diameters18.
AE vocabulary
In contrast to normalizing drugs, normalizing AE names is a relatively simple task, because, by default, FAERS reports use MedDRA “preferred terms” (PTs) for adverse events that appear under the “reactionmeddrapt” field. Since FAERS safety reports always employ the most up-to-date release of MedDRA for ADR lexicon, the ADR normalization task amounts to mapping “preferred terms” (PTs) that appear in older safety reports (in the “reactionmeddrapt” field) to corresponding PT terms from the latest MedDRA dictionary. We configured our parser to adopt to frequent (twice a year) releases of MedDRA in order to take advantage of data from as many safety reports as possible.
What makes our task somewhat easier is the fact that MedDRA updates follow a set of well-defined rules. Namely, each MedDRA release either preserves the “preferred terms” (PTs) or, less frequently, demotes them to “lowest level terms” (LLTs). Hence, mapping an old to new PT boils down to searching for the “demoted” PT in the set of LLTs from the most recent MedDRA database. Once such an LLT is found, a new name for the old PT is assigned by moving up one edge in MedDRA hierarchy. Following this approach, our algorithm is able to map 99.8% (34,520 out of 34,599) of all unique AE strings from the current FAERS set of patient reports to the corresponding AE terms in the most recent MedDRA dictionary (as of this writing, MedDRA 21.0). The remaining 79 strings are simply discarded as most of them represent arbitrary and often meaningless user entries.
Extracting the set of representative FAERS reports
We downloaded a comprehensive set of 822 safety report files (all files available at FAERS as of March. 2019) containing over 9 million patient safety reports (Table 1). While LRT statistics reduces the selection bias by a large degree, it is important to preprocess raw data in order to dampen the effect of confounding variables, such as therapeutic indications (TIs). Our goal is to discard any drug-AE pair (or, in case of drug combinations, any therapy-AE pair) in which the AE represents a known therapeutic indication for the drug (respectively, one of the two drugs from the combination therapy). For instance, deep vein thrombosis (DVT) often appears as an AE in a safety report for a patient that takes an anticoagulant for DVT or related disease or condition (e.g., pulmonary embolism). However, DVT represents the condition/disease rather than an AE in majority of cases where the list of drugs taken by the patient includes an anticoagulant. For this reason, we discard all therapy-AE pairs where the AE represents a therapeutic indication for one or both drugs comprising the therapy.
Table 1.
Preprocessing patient reports.
| Action | # reports | |
|---|---|---|
| download 822 FAERS files | 9,487,279 | |
| normalize drugs, AEs & remove TIs | 4,848,332 (PT) | 3,304,130 (HLT) |
| removing duplicate reports | 3,701,413 (PT) | 2,323,193 (HLT) |
Instead of parsing the drug package inserts, as done in SIDER, we compile a comprehensive set of indications for each of 1,309 drugs from our list by parsing the FAERS’ “drugindication” field. This process results in a set of drug-TI pairs along with the total number of occurrences of each pair in FAERS reports. For increased specificity, we discard extremely rare drug-TI pairs, those that appear less than a predefined number of times (default = 5) in the FAERS database. We enrich the remaining set of therapeutic indications using MedDRA’s PTs and LLTs. For instance, if a therapeutic indication for a particular drug represents a PT, the list of indications for that drug is expanded to include all LLTs found below that PT in MedDRA hierarchy. If a therapeutic indication represents a LLT, the drug indication set is expanded to include the “parent” PT. Finally, we remove all drug-AE pairs that represent drug-TI pairs.
We also implement a simple procedure for removing duplicate reports. We consider two reports the same if they list the same set of medications (specified in “medicinalproduct” field), the same set of adverse events (“reactionmeddrapt” field) and the same “patientonsetageunit”, ”patientonsetage”, “occurcountry”, “patientsex” and ”patientweight”.
Compiling raw therapy-AE counts
A given safety report might list any number of medications and any number of adverse events. This data is given cumulatively for each patient without any reference to possible associations between drugs and AEs. While defining observed drug-AE counts is straightforward for individual drugs, it is less clear how to do this for drug combinations. Hypothetically, if the number of drugs taken by a patient was exactly two, it would make sense to say that such a drug combination contributes a single count for every AE in the report. A typical report, however, lists more than two drugs, making it difficult to tell which one of k = C(m, 2) combinations of two out of m drugs gave rise to each AE reported by the patient.
We adopt a simple approach to defining therapy-AE counts for 2-drug therapies. Assuming that each 2-drug combination is equally likely to cause each AE, a patient report that lists m drugs contributes 1/C(m,2) to every possible 2-drug therapy and each AE that appears in the report. Under this simple scheme, for instance, a report that contains exactly two drugs contributes one count to each AE listed in the report. If a report contains three drugs, the corresponding contribution is 1/3, etc. We use the sparse matrix representation (Linear Algebra for Java, http://la4j.org/) to circumvent large memory requirement arising from the enormous number of drug combinations and accompanying adverse events.
Likelihood ratio test statistics (LRT) for therapy-AE associations
We follow the LRT procedure in Huang et al.14 to assign p-values to associations between individual (single) drugs and AEs. The same procedure is extended to assign p-values to raw therapy-AE counts for therapies consisting of two drugs. LRT allows us to detect adverse and safety signals from groups of ADRs or groups of drugs (or drug combinations) simultaneously, thereby adjusting for the effects of the key confounding variables on the fly. We employ the LRT statistics to solve a two-fold problem: (A) Given a 2-drug therapy, find AEs that have high reporting rates, compared to other AEs for the same 2-drug therapy, and (B) Given an AE, find all 2-drug therapies that have high reporting rates for the AE, compared to reporting rates of other 2-drug therapies. What this means in practice is that LRT is applied once for every 2-drug therapy and once for every AE under consideration. For every therapy-AE pair, the larger of the two p-values (therapy-specific and AE-specific) represents the statistical significance of the therapy-AE association. Below, we describe the algorithm for computing the therapy-specific p-values.
Since each AE is a rare event, we assume that the number of times a drug combination i* causes an AE j follows the Poisson distribution , where denotes the reporting rate of drug combination i* for AE j and is the marginal total for AE j i.e., (Fig. 1). We also assume that the remaining AE counts for therapy i* are Poisson distributed i.e.,
Figure 1.
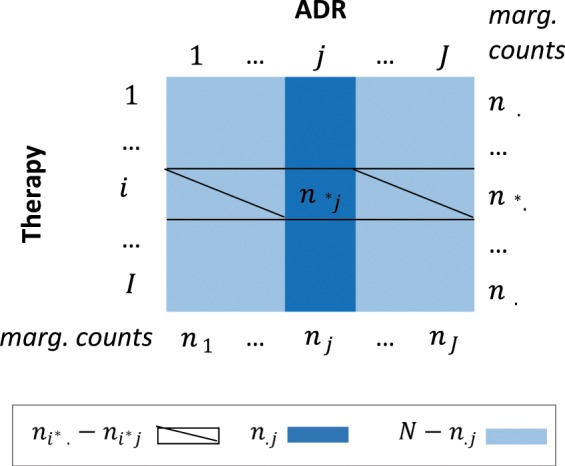
The components of LRT statistics. The matrix of raw therapy-AE counts.
, where , represents the reporting rate of drug combination i* for all other AEs (excluding j-th AE), and N is the total number of observations, . The null hypothesis is that the reporting rates for all AEs are the same i.e., , for all j. The two-sided alternative is that , for at least one AE j. Following Huang et al.14, we define the test statistics as , where represents the likelihood ratio for drug combination and AE , namely
where , and are the maximum likelihood estimates of , , and , respectively.
Since the joint distribution of under the null hypothesis is multinomial14
the background distribution of for can be obtained by simulating a number of datasets from the above distribution. We use parallel algorithm implementations to deal with computational expense in generating the background distributions. We reject the null hypothesis at α level if the MLR from the observed raw counts exceeds a predefined percentage (e.g. 99%, corresponding to α = 0.01) of MLRs calculated from Monte Carlo simulations. The alpha level balances the likelihood of Type I and Type II errors. The commonly accepted alpha levels are between 0.01 and 0.05. In our case, a more conservative α = 0.01 is selected to address the potentially high rates of false positives that are due to differential reporting in FAERS. Once the strongest AE signal for the 2-drug combination i* is detected, other strong AE signals for the same therapy are found by moving down to the second largest value of the likelihood ratio test statistics and testing the null hypothesis again. The p-values assigned to each MLR score represents the rank of that score in the set of all background MLR scores calculated from Monte Carlo simulations14.
For drug combination i*, the above procedure will accomplish the first task (task A), namely, it will detect ADRs that have high reporting rates for i* compared to other ADRs. The task of finding ADRs that have a high reporting rate for a given drug combination compared to other drug combinations (task B) is accomplished by reversing the roles ADRs and drug combinations e.g., by swapping rows and columns of the count matrix (nij).
Results
Database statistics
Table 2 and Figs. 2 and 3 show the summary statistics for our pAERS databases. To view this data in context, we note that the total number of PT and HLT terms in MedDRA 21.0 is 23,088 and 1,737 respectively, while the number of possible therapies (2-drug combinations) that can be made out of 1,178 drug clusters is 693,253.
Table 2.
pAERS statistics. Data on AEs unique to drug interactions in parentheses.
| Individual drugs | Drug combinations | |||
|---|---|---|---|---|
| PT | HLT | PT | HLT | |
| # therapies | 1,074 | 1,074 | 38,842 (30,872) | 16,754 (9,586) |
| #AEs | 8.167 | 1,389 | 7,451 (6,736) | 1,238 (1,107) |
| # entries | 78,686 | 44,743 | 144,335 (76,902) | 38,254 (13,074) |
| sparsity | 8.5E-3 | 3.0E-2 | 5.0E-4 (3.7E-4) | 1.8E-3 (1.2E-3) |
Figure 2.
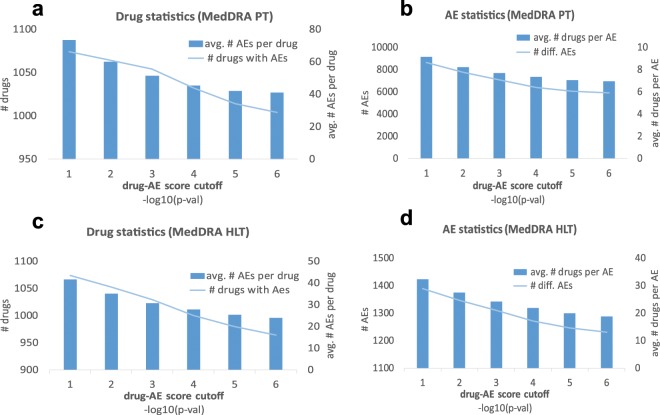
Cumulative and average statistics for AEs associated with single drug therapies. For each drug-AE p-value cutoff, we show the cumulative number of drugs and AEs (left axes in a,c and b,d respectively). We also give the average number of AEs per drug and the average number of drugs per AE (right axes in a,c and b,d, respectively).
Figure 3.
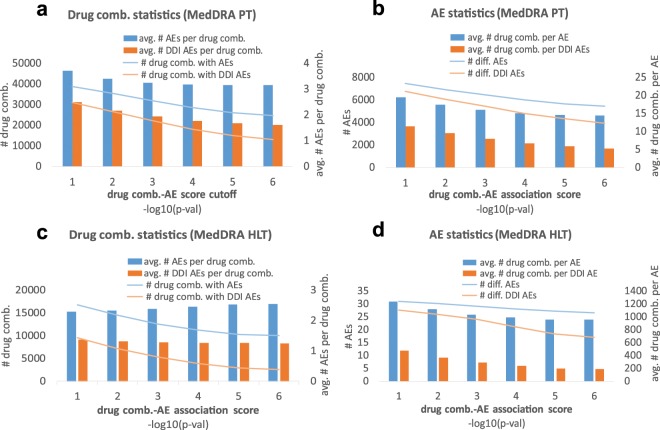
Cumulative and average statistics of therapy-AE associations for 2-drug therapies. For each therapy-AE p-value cutoff, we show the cumulative number of two-drug therapies and AEs (left axes in a,c and b,d respectively). We also give the average number of AEs per therapy and the average number of therapies per AE (right axes in subgraphs a,c and b,d, respectively). The DDI statistics (computed after removing AEs associated with either drug from the therapy) is shown in red.
Limitations and advantages of pAERS
Methods for detecting AE signals from postmarketing safety reports, such as LRT, face some common obstacles. If a drug (or one or both drugs from a 2-drug therapy) is relatively new, the data on said drug (or drug combination) reported in FAERS will be sparse, thereby reducing the statistical power of LRT. The same is true for drug combinations that are known to cause harmful AEs. Those combinations are rarely prescribed by physicians and, in turn, are poorly documented in FAERS. Taking all the above into account, the statistically insignificant therapy-AE associations in pAERS should be viewed as missing (rather than negative) data. The missing entries in pAERS can be recovered using techniques such as compressed sensing19,20 and collaborative filtering21,22 but those methodologies are outside of the scope of present study.
On the flipside (as we demonstrate later on the cyclosporine interactions) we expect many associations recorded in pAERS to be poorly documented in the literature. In order for LRT to declare a therapy-AE association “significant”, the association must pass two tests: the therapy must have a high reporting rate for AE and vice versa. Even innocuous and very frequent AEs (e.g., nausea or dizziness) will not show up in our database unless their reporting rates exceed the background reporting rates. Due to such stringent requirements, the LRT statistics have been shown to lower the Type I error in detecting AE signals significantly more than alternative approaches14.
The low false discovery rate of LRT explains why the average numbers of AEs per therapy (Figs. 2 and 3) are below the corresponding figures in SIDER2,3, OFFSIDES6 and TWOSIDES6. Even the maximum number of AEs per drug in pAERS are “only” 484 and 365 for PT and HLT lexicon, respectively, while the corresponding figures for drug combinations are 378 and 117 (125 and 29 for AEs caused by DDIs).
We note that some false positives in pAERS are difficult to eliminate. For instance, pAERS reports a high rate of rheumatoid arthritis and related “AEs” resulting from the therapy consisting of anticoagulants and tylenol. This therapy is given to patients suffering from both blood coagulative disorders and rheumatoid arthritis, since acetaminophen represents a relatively safe way to managing pain in patients who are taking warfarin or other anticoagulants. The false positives of this type “sneak through” our algorithm in part because they cannot be removed as therapeutic indications (in our example, rheumatoid arthritis is not the indication for either type of drug).
Comparison to TWOSIDES database
Several methods have been published that aim to predict DDI induced adverse events23,24. Our method is different in spirit, as it seeks to extract (rather than predict with machine learning) data on adverse events. Previously, an algorithm based on Propensity Score Matching (PSM)8,9, had been used to develop the TWOSIDES database of AEs associated with pairs of drugs6. The authors of TWOSIDES demonstrate that PSM is capable of filtering out about half of synthetic associations between drugs and AEs.
With 6,602 unique AEs reported for all 2-drug therapies combined, pAERS_PT database is richer than TWOSIDES in the number of adverse events reported overall, but the number of AEs in pAERS_HLT tails 1,301 AEs in TWOSIDES. On the other hand, TWOSIDES is almost twice as large in the total number of pairs of drugs (59,220), while the number of reported associations in TWOSIDES (868,221) far exceeds the corresponding figure in pAERS.
A theoretical advantage of the PSM method used in TWOSIDES is that it controls for all variables that have effects on treatment outcome. However, the critiques of PSM approach argue that, by excessively pruning important data, PSM can, in fact, increase data imbalance and bias, leading to the so-called “PSM paradox”25. In contrast, the Likelihood Ratio Test has a provable low false discovery rate12,14 and it places all database p-values on a uniform ground. Just like PSM, LRT can efficiently correct for the selection bias and sampling variance. Moreover, LRT can take gender and age into account with the potential of personalized AE stratification. It should also be noted that pAERS provides for much better coverage of FDA approved drugs (66% more individual drugs than in TWOSIDES).
Illustrative example - Cyclosporine interactions
We demonstrate the accuracy of our data extraction algorithm on the example of an immunosuppressant drug cyclosporine (neoral, sandimmune), which is commonly used to prevent rejection of liver, heart, lung, pancreas or bone marrow transplants.
In each of the examples below, the association between the drug pair and AE is due to DDIs, as evidenced by the LRT p-value ≤10−6 and a complete lack of association between the AE and any individual drug from the drug pair (p-value > 0.1).
Cyclosporine and azole antifungals
Immunocompromised patients, such as those receiving organ transplants, are affected by mycotic disease. In order to prevent infection and transplant rejection, cyclosporine is routinely co-administered with antifungal azoles26. However, interactions with azoles alter the concentrations of cyclosporine, increasing the risk of transplant failure. As seen in Table 3, LRT is able to detect statistically significant interactions between cyclosporine and different azoles, including itraconazole, voriconazole, pantoprazole and secnidazole.
Table 3.
Cyclosporine interactions in pAERS.
| Interacting Drug | Adverse Reaction |
|---|---|
| Itraconazole | Cardiac murmur |
| Pantoprazole | Osteomyelitis |
| Voriconazole | Tremor |
| Secnidazole | Atrial flutter, Palmar-Plantar erythrodysaesthesia |
| Rapamycin | Globulins decreased, Jacod syndrome |
| Prednisolone, Medrol | Colectomy, Peliosis hepatis (HLT) |
| Rosuvastatin | Hyperlipasaemia |
| Tecfidera or Methotrexate | Malignant melanoma stage I |
| Diprivan | Hyporeflexia, Mitochondrial myopathy acquired |
| Etoposide | Ocular vascular disorders NEX (HLT) |
Cyclosporine and rapamycin
When co-administered with antifungal antibiotic rapamycin, cyclosporine is known to cause nephrotoxic injuries. We observe significantly decreased globulin level with cyclosporine/rapamycin therapy, demonstrating additional evidence of the reduction in renal function27,28 due to interaction of these two drugs.
Cyclosporine with prednisolone or medrol
Cyclosporine interactions with corticosteroids have long been recognized and recorded with numerous case studies documented in the literature29,30. In our database, these interactions manifest as severe AEs resulting from the interaction of cyclosporine with prednisolone. There is also a strong association of Peliosis hepatis (PH) with cyclosporine/medrol therapy in pAERS, confirming previous findings that the combination of these two drugs is the main cause of PH after liver transplantation31.
Cyclosporine and rosuvastatin
We identify a link between cyclosporine/rosuvastatin therapy and high lipid levels, confirming the relatively recent findings on interactions between the two drugs32. We observe no individual, significant cyclosporine-hyperlipasaemia or rosuvastatin- hyperlipasaemia associations.
Cyclosporine and tecfidera or methotrexate
Cyclosporine is known to cause skin cancer when used with other immunosuppressive agents33. We find malignant melanoma to be strongly associated with the cyclosporine/methotrexate therapy. The same adverse event appears in co-administration of cyclosporine with tecfidera, which has been known as a “safer” immunosuppressant agent.
Cyclosporine and diprivan
Enhanced toxic reactions are documented in patients taking cyclosporine with propofol34. In our database this interaction is manifested by high incidence of hyporeflexia and mitochondrial myopathy.
Cyclosporine and etoposide
The effect of cyclosporine treatment on etoposide PK has been previously reported in both adults35 and children36. We note statistically significant link between cyclosporine/etoposide therapy and the ocular vascular disorders.
The examples above demonstrate the robustness of LRT, compared to the statistical procedure used in TWOSIDES. In TWOSIDES, cyclosporine interacts with 60% of all other drugs, yielding a total of 27,671 adverse events combined (or 72 AEs per drug pair). In general, one in every three (30.4%) 2-drug therapies in TWOSIDES gives rise to at least one AE (with 73 AEs per therapy on average). This is, to say the least, unrealistic, in particular given that 94% of all p-values in TWOSIDES are below 1E-05.
While assessing the accuracy of our platform on a large scale is computationally prohibitive, studying individual drug pairs provides useful insight into the value added by our method. In another illustrative example, we searched for drugs interacting with bosentan, a dual endothelin receptor antagonist, commonly prescribed in the treatment of pulmonary artery hypertension. We found a significant probability of interactions between bosentan and sildenafil, warfarin, norgestrel, ranolazine and clorazepate, resulting in adverse events that cannot be contributed to any single drug from the combination. These findings confirm previously recorded interactions. For instance, multiple studies have been published documenting pharmacokinetic interaction between sildenafil and bosentan that may influence the dosage of each drug when co-prescribed in pulmonary hypertension37,38. Co-prescribing warfarin with bosentan, on the other hand, leads to increased risks to changes in International Normalized Ratios (INR), as demonstrated in different studies and individual case reports39,40. Furthermore, we observed an elevated risk of neonatal respiratory distress syndrome, in cases where bosentan was taken along with norgestrel, thereby confirming the interactions of bosentan with oral contraceptives recorded in the drug’s package insert and described in the literature41. Furthermore, we noted adverse events associated with co-prescriptions with CYP3A inhibitors and substrates, which are well known to decrease the excretion rate and serum concentration of the drugs co-prescribed with bosentan.
With the exception of clorazepate, the above interactions are also present in DrugBank7, but it should be noted that DrugBank is not informative enough to allow for a direct comparison. In DrugBank, the combined therapies with bosentan are only quoted to increase or decrease the serum level of the interacting drugs. A similar observation can be made for cyclosporine interactions in our first example. Moreover, the search with DrugBank’s Drug Interaction Lookup yields 1,135 and 2,192 drugs that can potentially interact with bosentan and cyclosporine, respectively. Such an enormous number of interacting drugs might be indicative of low specificity of DrugBank data.
Finally, we note that some associations in pAERS have not been previously observed and thus are difficult to assess and classify. For instance, it is difficult to say whether the supposed bosentan’s interactions with orenitram and pregabalin, stored in pAERS, represent novel discoveries or simply the Type I errors.
Discussion and Conclusion
Nearly 4 out of 5 Americans over the age of 60 are taking multiple drugs4, while nearly one in three US adults are taking five or more medications42. Since the probability of an adverse event increases with the number of medications taken by a patient, predicting AEs is emerging as one of the most important tasks in drug discovery and biomedical research.
We present a statistical and computational method for accurately detecting adverse events arising from single- and two-drug therapies. While the therapy-AE associations stored in our databases need to be validated using epidemiological and clinical methods, the LRT technique adopted here has already been shown to reduce Type I error in detecting AE signals associated with single drug therapies.
The present study shares some common limitations with other predictive methods. The lack of statistical power of clinical trials makes it impossible to validate any individual association, let alone the millions of possible associations between all drug combinations and all adverse events. We are, however, aware of at least two limitations of our methodology. First, the LRT procedure lacks the statistical power in detecting interactions of new drugs due to insufficient postmarketing data on recently approved drugs. Second, our Monte Carlo simulation procedure used in estimating the association p-values is computationally expensive, due to the enormous number of drug combination and adverse events.
Better understanding of side-effects arising from drug interactions is becoming increasingly important, given a growing percentage of people who are prescribed multiple drugs and numerous scientific studies that show that the number of drugs taken by an individual is the most reliable predictor of an adverse event. Advances in computational prediction of adverse events arising from drug combinations will fuel the progress in drug repurposing and research in computational prediction of therapy-AE associations. We believe that our study is an important contributing step in this direction.
Acknowledgements
This research was supported by the National Institute of General Medical Sciences, NIH under the award number R01GM122845 (L.X.), The National Institute on Aging, NIH under the award number R01AG057555 (L.X.) and University of Northern Iowa Professional Development Award (A.P.).
Author contributions
A.P. and L.X. conceived and designed the method. A.P. implemented the algorithm. A.P. and L.X. analyzed the data. A.P. wrote the paper. All authors reviewed the manuscript.
Data availability
The datasets generated during this study are available at https://gpubox.cs.uni.edu.
Competing interests
The authors have no competing interests as defined by Nature Research, or other interests that might be perceived to influence the results and/or discussion reported in this paper.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Aleksandar Poleksic, Email: poleksic@cs.uni.edu.
Lei Xie, Email: lxie@iscb.org.
References
- 1.Bonn D. Adverse drug reactions remain a major cause of death. Lancet. 1998;351:1183. doi: 10.1016/S0140-6736(98)23016-9. [DOI] [PubMed] [Google Scholar]
- 2.Kuhn M, Letunic I. The SIDER database of drugs and side effects. Nucleic Acids Res. 2016;44:D1075–1079. doi: 10.1093/nar/gkv1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuhn M, Campillos M, Letunic I, Jensen LJ, Bork P. A side effect resource to capture phenotypic effects of drugs. Mol. Syst. Biol. 2010;6:343. doi: 10.1038/msb.2009.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan Y, et al. Improving drug safety: From adverse drug reaction knowledge discovery to clinical implementation. Methods. 2016;110:14–25. doi: 10.1016/j.ymeth.2016.07.023. [DOI] [PubMed] [Google Scholar]
- 5.Alomar MJ. Factors affecting the development of adverse drug reactions. Saudi Pharm. J. 2014;22:83–94. doi: 10.1016/j.jsps.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tatonetti NP, Ye PP, Daneshjou R, Altman RB. Data-driven prediction of drug effects and interactions. Sci. Transl. Med. 2012;4:125ra131. doi: 10.1126/scitranslmed.3003377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wishart DS, et al. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2018;46:D1074–D1082. doi: 10.1093/nar/gkx1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang J, Donnan PT. Propensity score methods in drug safety studies: practice, strengths and limitations. Pharmacoepidemiol. Drug Saf. 2001;10:341–344. doi: 10.1002/pds.656. [DOI] [PubMed] [Google Scholar]
- 9.Kuss O, Legler T, Börgermann J. Treatments effects from randomized trials and propensity score analyses were similar in similar populations in an example from cardiac surgery. J. Clin. Epidemiol. 2011;64:1076–1084. doi: 10.1016/j.jclinepi.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Guo Y, et al. Network-based combinatorial CRISPR-Cas9 screens identify synergistic modules in human cells. ACS Synth. Biol. 2019;8:482–490. doi: 10.1021/acssynbio.8b00237. [DOI] [PubMed] [Google Scholar]
- 11.Guo Y, et al. Multiscale modeling of inflammation-induced tumorigenesis reveals competing oncogenic and oncoprotective roles for inflammation. Cancer Res. 2017;77:6429–6441. doi: 10.1158/0008-5472.CAN-17-1662. [DOI] [PubMed] [Google Scholar]
- 12.Kulldorff M. A spatial scan statistic. Commun. Stat. - Theory Methods. 1997;26:1481–1496. doi: 10.1080/03610929708831995. [DOI] [Google Scholar]
- 13.Waller, L. A. & Gotway, C. A. Applied spatial statistics for public health data. (Hoboken, NJ: John Wiley & Sons, Inc., 2004).
- 14.Huang L, Zalkikar J, Tiwari RC. A likelihood ratio test based method for signal detection with application to FDA’s drug safety data. J. Am. Stat. Assoc. 2011;106:1230–1241. doi: 10.1198/jasa.2011.ap10243. [DOI] [Google Scholar]
- 15.Brown EG, Wood L, Wood S. The medical dictionary for regulatory activities (MedDRA) Drug safety. 1999;20:109–117. doi: 10.2165/00002018-199920020-00002. [DOI] [PubMed] [Google Scholar]
- 16.Nelson SJ, Zeng K, Kilbourne J, Powell T, Moore R. Normalized names for clinical drugs: RxNorm at 6 years. J. Am. Med. Inform. Assoc. 2011;18:441–448. doi: 10.1136/amiajnl-2011-000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rogers DJ, Tanimoto TT. A computer program for classifying plants. Science. 1960;132:1115–1118. doi: 10.1126/science.132.3434.1115. [DOI] [PubMed] [Google Scholar]
- 18.Everitt, B., Landau, S. & Leese, M. Cluster analysis. (A Hodder Arnold Publication, Willey, London, 2001).
- 19.Donoho DL. Compressed sensing. IEEE Trans. Inf. Theory. 2006;52:1289–1306. doi: 10.1109/TIT.2006.871582. [DOI] [Google Scholar]
- 20.Candès EJ. Compressive sampling. ICM Proc. 2006;3:1433–1452. [Google Scholar]
- 21.Lim H, Gray P, Xie L, Poleksic A. Improved genome-scale multi-target virtual screening via a novel collaborative filtering approach to cold-start problem. Sci. Rep. 2016;6:38860. doi: 10.1038/srep38860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim H, et al. Large-scale off-target identification using fast and accurate dual regularized one-class collaborative filtering and its application to Drug Repurposing. PLoS Comput. Biol. 2016;12:e1005135. doi: 10.1371/journal.pcbi.1005135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng Y, et al. Predicting adverse drug reactions of combined medication from heterogeneous pharmacologic databases. BMC Bioinf. 2018;19:517. doi: 10.1186/s12859-018-2520-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi JY, Shang XQ, Gao K, Zhang SW, Yiu SM. An integrated local classification model of predicting drug-drug interactions via Dempster-Shafer theory of evidence. Sci. Rep. 2018;8:11829. doi: 10.1038/s41598-018-30189-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.King, G. & Nielsen, R. Why propensity scores should not be used for matching. Political Anal. 1–20 (2016).
- 26.Saad AH, DePestel DD, Carver PL. Factors influencing the magnitude and clinical significance of drug interactions between azole antifungals and select immunosuppressants. Pharmacotherapy. 2006;26:1730–1744. doi: 10.1592/phco.26.12.1730. [DOI] [PubMed] [Google Scholar]
- 27.Andoh TF, Lindsley J, Franceschini N, Bennett WM. Synergistic effects of cyclosporine and rapamycin in a chronic nephrotoxicity model1. Transplantation. 1996;62:311–316. doi: 10.1097/00007890-199608150-00002. [DOI] [PubMed] [Google Scholar]
- 28.Napoli KL, Wang ME, Stepkowski SM, Kahan BD. Relative tissue distributions of cyclosporine and sirolimus after concomitant peroral administration to the rat: evidence for pharmacokinetic interactions. Ther. Drug Monit. 1998;20:123–133. doi: 10.1097/00007691-199804000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Lam S, Partovi N, Ting LS, Ensom MH. Corticosteroid interactions with cyclosporine, tacrolimus, mycophenolate, and sirolimus: fact or fiction? Ann. Pharmacother. 2008;42:1037–1047. doi: 10.1345/aph.1K628. [DOI] [PubMed] [Google Scholar]
- 30.Öst L. Effects of cyclosporin on prednisolone metabolism. Lancet. 1984;1:8374. doi: 10.1016/s0140-6736(84)91778-1. [DOI] [PubMed] [Google Scholar]
- 31.Lui WH, et al. Peliosis hepatis in a kidney transplant recipient with manifestation as massive ascites and liver dysfunction: case report. Transplant. Proc. 2014;46:630–633. doi: 10.1016/j.transproceed.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 32.Launay-Vacher V, Izzedine H, Deray G. Statins’ dosage in patients with renal failure and cyclosporine drug–drug interactions in transplant recipient patients. Int. J. Cardiol. 2005;101:9–17. doi: 10.1016/j.ijcard.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 33.Muellenhoff MW, Koo JY. Cyclosporine and skin cancer: an international dermatologic perspective over 25 years of experience. A comprehensive review and pursuit to define safe use of cyclosporine in dermatology. J. Dermatol. Treat. 2012;23:290–304. doi: 10.3109/09546634.2011.590792. [DOI] [PubMed] [Google Scholar]
- 34.Steib A. Diprivan: drug interactions. Ann. Fr. Anesth. 1994;13:471–475. doi: 10.1016/S0750-7658(05)80674-4. [DOI] [PubMed] [Google Scholar]
- 35.Lum BL, et al. Alteration of etoposide pharmacokinetics and pharmacodynamics by cyclosporine in a phase I trial to modulate multidrug resistance. J. Clin. Oncol. 1992;10:1635–1642. doi: 10.1200/JCO.1992.10.10.1635. [DOI] [PubMed] [Google Scholar]
- 36.Bisogno G, et al. High-dose cyclosporin with etoposide-toxicity and pharmacokinetic interaction in children with solid tumours. Br. J. Cancer. 1998;77:2304–2309. doi: 10.1038/bjc.1998.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paul GA, Gibbs JSR, Boobis AR, Abbas A, Wilkins MR. Bosentan decreases the plasma concentration of sildenafil when coprescribed in pulmonary hypertension. Br. J. Clin. Pharmacol. 2005;60:107–112. doi: 10.1111/j.1365-2125.2005.02383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burgess G, Hoogkamer H, Collings L, Dingemanse J. Mutual pharmacokinetic interactions between steady-state bosentan and sildenafil. Eur. J. Clin. Pharmacol. 2008;64:43–50. doi: 10.1007/s00228-007-0408-z. [DOI] [PubMed] [Google Scholar]
- 39.Murphey LM, Hood EH. Bosentan and warfarin interaction. Ann. Pharmacother. 2003;37:1028–1031. doi: 10.1345/aph.1C398. [DOI] [PubMed] [Google Scholar]
- 40.Spangler ML, Saxena S. Warfarin and bosentan interaction in a patient with pulmonary hypertension secondary to bilateral pulmonary emboli. Clin. Ther. 2010;32:53–56. doi: 10.1016/j.clinthera.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 41.Van Giersbergen PLM, Halabi A, Dingemanse J. Pharmacokinetic interaction between bosentan and the oral contraceptives norethisterone and ethinyl estradiol. International J. Clin. Pharm. Ther. 2006;44:3. doi: 10.5414/CPP44113. [DOI] [PubMed] [Google Scholar]
- 42.Center, S. E. Patterns of medication use in the United States, 2006: a report from the Slone Survey. Boston: Boston University, 3 (2006).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during this study are available at https://gpubox.cs.uni.edu.