Abstract
Liver cancer is a high morbidity and low survival disease all over the world. Chromosomal instability is hallmark of liver cancer. Microtubule-associated serine and threonine kinase 2 (MAST2), as a microtubule associated protein, may involve in tumorous chromosomal instability and plays important roles in cell proliferation and survival. The role of MAST2 in liver cancer has not been well elucidated, which is the aim of our study. In this study, The Cancer Genome Atlas database was used to study the MAST2 mRNA expression in liver cancer, and Chi-squared tests were performed to test the correlation between clinical features and MAST2 expression. ROC curve was performed to examined the diagnostic capacity. The prognostic value of MAST2 in liver cancer was assessed through Kaplan–Meier curves as well as Cox analysis. Our results showed MAST2 was upregulated in liver cancer, and the area under the curve (AUC) was 0.925 and indicated powerful diagnostic capability. High MAST2 expression was associated with advanced clinical status such as histological type (p = 0.0059), histologic grade (p = 0.0142), stage (p = 0.0008), T classification (p = 0.0028), N classification (p = 0.0107), survival status (p = 0.0062), and poor prognosis of patients. Importantly, MAST2 was an independent risk factor for patients’ prognosis after adjusting for other risk factors including stage, T classification, and residual tumor. In total, MAST2 is a potential diagnostic and prognostic biomarker of liver cancer.
Subject terms: Diagnostic markers, Prognostic markers
Introduction
Cancer is a major problem in public health in the world. Liver cancer, a highly fatal cancer, is estimated to account for about 42030 new cancer cases and 31780 cancer deaths in the United States in 20191. Liver cancer is one of the lowest survival cancers, which is predominantly due to the fact that diagnosis is often made late or inaccurate2. Therefore, to identify a new biomarker for ea--rly and accurate diagnosis has great clinical significance.
Chromosomal instability is a hallmark for carcinoma. As a novel gene family which may involve in chromosomal instability, MAST functions in normal cell division. Its alterations lead to a few mitotic abnormalities, such as spindle malformation, chromosome missegregation, centrosome amplification, and failure of cytokinesis3. Furthermore, overexpression of MAST2 gene has a proliferative effect both in vitro and in vivo4. Microtubule-associated serine and threonine kinase 2 (MAST2) is a 205 kD protein that is associated with microtubules5. MAST2 interacts with the carboxyl-terminal of phosphatase and tensin homolog (PTEN) through its PDZ (PSD-95, Dlg1, Zo-1) domain6. They are crucial for cell division, survival and tumorigenesis7. However, until now, little is known about MAST gene family. The specific role of MAST2 in liver cancer needs more elucidation.
In this study, we compared MAST2 expression in liver cancer patients and then evaluated its diagnostic value. We also analyzed the relationship between clinical variables of patients and MAST2 expression, and further explored the prognostic value of MAST2 in patients’ overall survival (OS) and relapse-free survival (RFS). Our study demonstrated that MAST2 could become a novel diagnostic and prognostic biomarker for liver cancer patients.
Results
High MAST2 expression in liver cancer
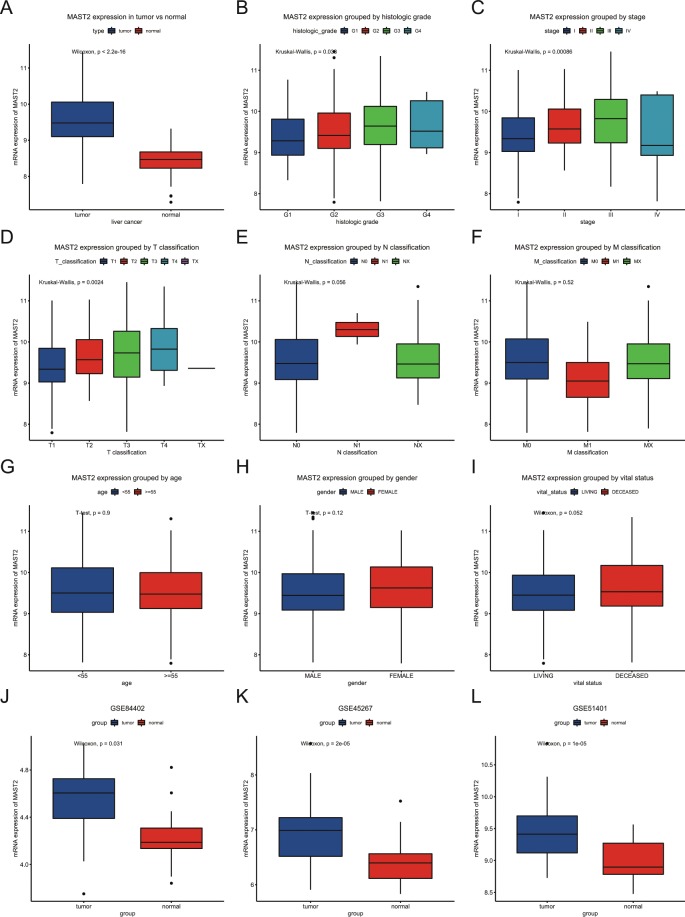
A total of 373 liver cancer patients were included. The detailed characteristics, including age, gender, stage, classifications, were shown in Table 1. Boxplots showed the differences in MAST2 expression by tumor vs adjacent normal tissue (Fig. 1A). The results in Fig. 1A demonstrated MAST2 expression was higher in tumors (p < 22e-16), which were also verified by GEO datasets including GSE84402, GSE45267, GSE51401 (Fig. 1J–L). Moreover, the expression of MAST2 was also distinct in subgroups of histologic grade (p = 0.03), stage (p = 0.00086), T classification (p = 0.0024). Higher histological grades (except G4), higher stages (except stage IV) and T classification have higher MAST2 expression. However, there were no significant differences in MAST2 expression between subgroups divided by N classification, M classification, age, gender and vital status (Fig. 1D–I).
Table 1.
Clinical characteristics.
| characteristics | Number | % |
|---|---|---|
| age | ||
| <55 | 117 | 31.45 |
| >=55 | 255 | 68.55 |
| not appplicable | 1 | 0.00 |
| gender | ||
| FEMALE | 121 | 32.44 |
| MALE | 252 | 67.56 |
| Histological_type | ||
| Fibrolamellar_Carcinoma | 3 | 0.8 |
| Hepatocellular_Carcinoma | 363 | 97.32 |
| Hepatocholangiocarcinoma | 7 | 1.88 |
| Histologic_grade | ||
| Grade_1 | 55 | 14.75 |
| Grade_2 | 178 | 47.72 |
| Grade_3 | 123 | 32.98 |
| Grade_4 | 12 | 3.22 |
| not appplicable | 5 | 1.34 |
| clinical_stage | ||
| stage_I | 172 | 46.11 |
| stage_II | 87 | 23.32 |
| stage_III | 85 | 22.79 |
| stage_IV | 5 | 1.34 |
| not appplicable | 24 | 6.43 |
| T_classification | ||
| T1 | 182 | 48.79 |
| T2 | 95 | 25.47 |
| T3 | 80 | 21.45 |
| T4 | 13 | 3.49 |
| Tx | 1 | 0.27 |
| not appplicable | 2 | 0.54 |
| N_classification | ||
| N0 | 253 | 67.83 |
| N1 | 4 | 1.07 |
| Nx | 115 | 30.83 |
| not appplicable | 1 | 0.27 |
| M_classification | ||
| M0 | 267 | 71.58 |
| M1 | 4 | 1.07 |
| Mx | 102 | 27.35 |
| Radiation_therapy | ||
| NO | 340 | 91.15 |
| YES | 8 | 2.14 |
| not appplicable | 25 | 6.7 |
| Residual_tumor | ||
| R0 | 326 | 87.4 |
| R1 | 17 | 4.56 |
| R2 | 1 | 0.27 |
| Rx | 22 | 5.9 |
| not appplicable | 7 | 1.88 |
| survival_status | ||
| DECEASED | 130 | 34.85 |
| LIVING | 243 | 65.15 |
| relapse | ||
| NO | 179 | 55.94 |
| YES | 141 | 44.06 |
| MAST2 | ||
| high | 110 | 29.49 |
| low | 263 | 70.51 |
Figure 1.
MAST2 expression in liver cancer. MAST2 expression was compared between normal tissues and liver cancer tissues. Subgroup analysis for histologic grade, stage, T classification, N classification, M classification, age, gender and vital status. The expression of MAST2 was verified by GEO datasets including GSE84402, GSE45267, GSE51401.
The diagnostic potential of MAST2
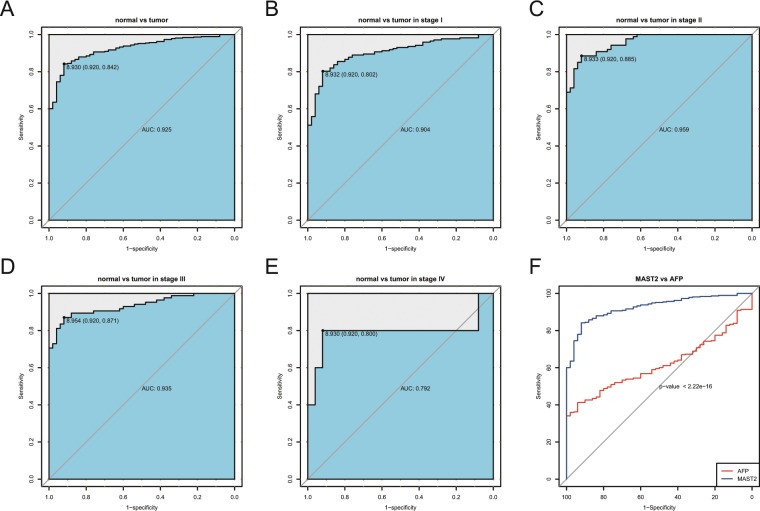
ROC showed the diagnostic capability of MAST2 (Fig. 2). The area under the curve (AUC) was 0.925 between tumor and normal tissues, which represented a powerful diagnostic capability (Fig. 2A). We further performed ROC analysis in subgroup of different stage, which also showed moderate to high diagnostic capability (stage I: 0.904; stage α: 0.959; stage III: 0.935; stage IV: 0.792; Fig. 2B–E). In addition, we compared the diagnostic value of MAST2 and AFP through ROC curve and found MAST2 had more diagnostic value (Fig. 2F).
Figure 2.
Diagnostic value of MAST2 expression in liver cancer. ROC for expression of MAST2 in normal tissues and liver cancer. Subgroup analysis for stage I, II, III and IV. ROC for MAST2 vs AFP.
The relationship between characteristics of patients and MAST2 expression
Table 2 summarized the association between clinical variables and MAST2 expression. Results showed MAST2 expression was significantly associated with histological type (p = 0.0059), histologic grade (p = 0.0142), stage (p = 0.0008), T classification (p = 0.0028), N classification (p = 0.0107), and survival status (p = 0.0062).
Table 2.
Relationship between clinical variables and MAST2 expression.
| Characteristics | Variable | Number | MAST2 expression | χ2 | p-value | |||
|---|---|---|---|---|---|---|---|---|
| High | % | Low | % | |||||
| age | <55 | 117 | 38 | 34.55 | 79 | 30.15 | 0.5046 | 0.4775 |
| >=55 | 255 | 72 | 65.45 | 183 | 69.85 | |||
| gender | FEMALE | 121 | 42 | 38.18 | 79 | 30.04 | 1.9902 | 0.1583 |
| MALE | 252 | 68 | 61.82 | 184 | 69.96 | |||
| histological_type | Fibrolamellar Carcinoma | 3 | 3 | 2.73 | 0 | 0 | 9.9642 | 0.0069 |
| Hepatocellular Carcinoma | 363 | 103 | 93.64 | 260 | 98.86 | |||
| Hepatocholangiocarcinoma (Mixed) | 7 | 4 | 3.64 | 3 | 1.14 | |||
| histologic_grade | Grade_1 | 55 | 9 | 8.18 | 46 | 17.83 | 10.1341 | 0.0142 |
| Grade_2 | 178 | 49 | 44.55 | 129 | 50 | |||
| Grade_3 | 123 | 47 | 42.73 | 76 | 29.46 | |||
| Grade_4 | 12 | 5 | 4.55 | 7 | 2.71 | |||
| clincial_stage | stage_I | 172 | 36 | 34.62 | 136 | 55.51 | 15.9814 | 0.0008 |
| stage_II | 87 | 28 | 26.92 | 59 | 24.08 | |||
| stage_III | 85 | 38 | 36.54 | 47 | 19.18 | |||
| stage_IV | 5 | 2 | 1.92 | 3 | 1.22 | |||
| T_classification | T1 | 182 | 39 | 35.45 | 143 | 54.79 | 14.7546 | 0.0028 |
| T2 | 95 | 31 | 28.18 | 64 | 24.52 | |||
| T3 | 80 | 34 | 30.91 | 46 | 17.62 | |||
| T4 | 13 | 6 | 5.45 | 7 | 2.68 | |||
| Tx | 1 | 0 | 0 | 1 | 0.38 | |||
| N_classification | N0 | 253 | 75 | 68.81 | 178 | 67.68 | 10.2393 | 0.0107 |
| N1 | 4 | 4 | 3.67 | 0 | 0 | |||
| Nx | 115 | 30 | 27.52 | 85 | 32.32 | |||
| M_classification | M0 | 267 | 82 | 74.55 | 185 | 70.34 | 0.6776 | 0.7702 |
| M1 | 4 | 1 | 0.91 | 3 | 1.14 | |||
| Mx | 102 | 27 | 24.55 | 75 | 28.52 | |||
| radiation_therapy | NO | 340 | 100 | 98.04 | 240 | 97.56 | 0 | 1 |
| YES | 8 | 2 | 1.96 | 6 | 2.44 | |||
| residual_tumor | R0 | 326 | 94 | 86.24 | 232 | 90.27 | 3.1493 | 0.3858 |
| R1 | 17 | 5 | 4.59 | 12 | 4.67 | |||
| R2 | 1 | 0 | 0 | 1 | 0.39 | |||
| Rx | 22 | 10 | 9.17 | 12 | 4.67 | |||
| survival_status | DECEASED | 130 | 50 | 45.45 | 80 | 30.42 | 7.075 | 0.0078 |
| LIVING | 243 | 60 | 54.55 | 183 | 69.58 | |||
MAST2 expression is associated with OS
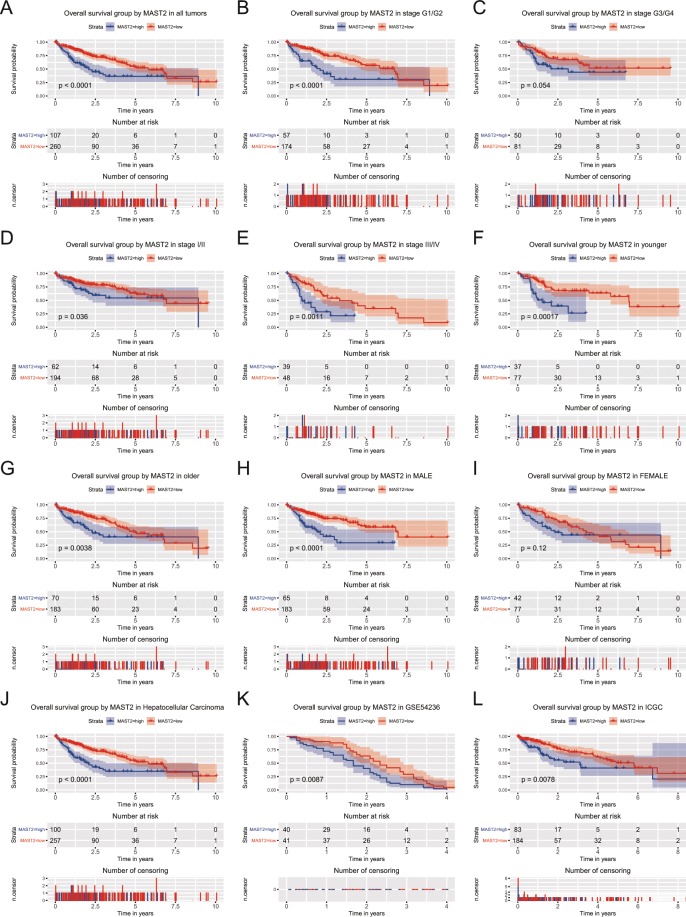
Proper threshold from ROC curve was cutoff to divided patients into two groups (high and low MAST2 expression). Kaplan-Meier curves were used to estimate the prognostic role of MAST2 in patients with liver cancer (Fig. 3). Results showed patients in MAST2 high expression group had worse OS (p < 0.0001; Fig. 3A). Subgroup analysis further indicated expression of MAST2 significantly decreased the OS of patients in stage G1/G2 (p < 0.0001), stage I/II (p = 0.036), stage III/IV (p = 0.0011), age of young (p = 0.00017) and old (p = 0.0038) and male (p < 0.0001). Since there is data on a large number of HCC samples, we performed a subgroup analysis among HCC tumors only and found the same results, which were also verified by GSE54236 and ICGC database (Fig. 3J–L).
Figure 3.
Kaplan-Meier curves for OS in liver cancer. Kaplan-Meier curves for OS in liver cancer for all patients, and patients in subgroup of stage G1/G2, stage G3/G4, stage I/II, stage III/IV, younger, older, male, female and HCC. The verification in GSE54236 and ICGC.
Univariate analysis selected several variables correlated with OS, including stage (p = 0.001), T classification (p < 0.001), residual tumor (p = 0.003) and expression of MAST2 (p < 0.001). Together with T classification (p < 0.001) and residual tumor (p = 0.006), MAST2 expression (HR = 2.110, 95%CI: 1.467–3.035, p = 0.000) was independent risk factor for OS in liver cancer patients (Table 3) after adjusting the other variables correlated with OS (stage, T classification, and residual tumor).
Table 3.
Univariate and multivariate analysis of overall survival.
| Characteristics | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95%CI (lower-upper) | p-value | Hazard Ratio | 95%CI (lower-upper) | p-value | |
| age (≥55/<55) | 0.999 | 0.689–1.449 | 0.997 | |||
| gender (male/female) | 0.801 | 0.562–1.142 | 0.220 | |||
| histological_type (hepatocholangiocarcinoma/hepatocellular/fibrolamellar) | 0.989 | 0.267–3.665 | 0.986 | |||
| histologic_grade (G4/G3/G2/G1) | 1.044 | 0.839–1.299 | 0.698 | |||
| clincial_stage (IV/III/II/I) | 1.381 | 1.148–1.660 | 0.001 | 0.838 | 0.672–1.044 | 0.116 |
| T_classification (T4/T3/T2/T1/NX) | 1.662 | 1.387–1.990 | 0.000 | 1.844 | 1.459–2.331 | 0.000 |
| N_classification (N1/N0/NX) | 0.727 | 0.506–1.046 | 0.086 | |||
| M_ classification (M1/M0/MX) | 0.716 | 0.495–1.037 | 0.077 | |||
| radiation_therapy (yes/no) | 0.515 | 0.258–1.028 | 0.060 | |||
| residual_ tumor (RX/R2/R1/R0) | 1.424 | 1.126–1.801 | 0.003 | 1.411 | 1.105–1.802 | 0.006 |
| MAST2 (high/low) | 2.248 | 1.572–3.215 | 0.000 | 2.110 | 1.467–3.035 | 0.000 |
Expression of MAST2 is associated with RFS
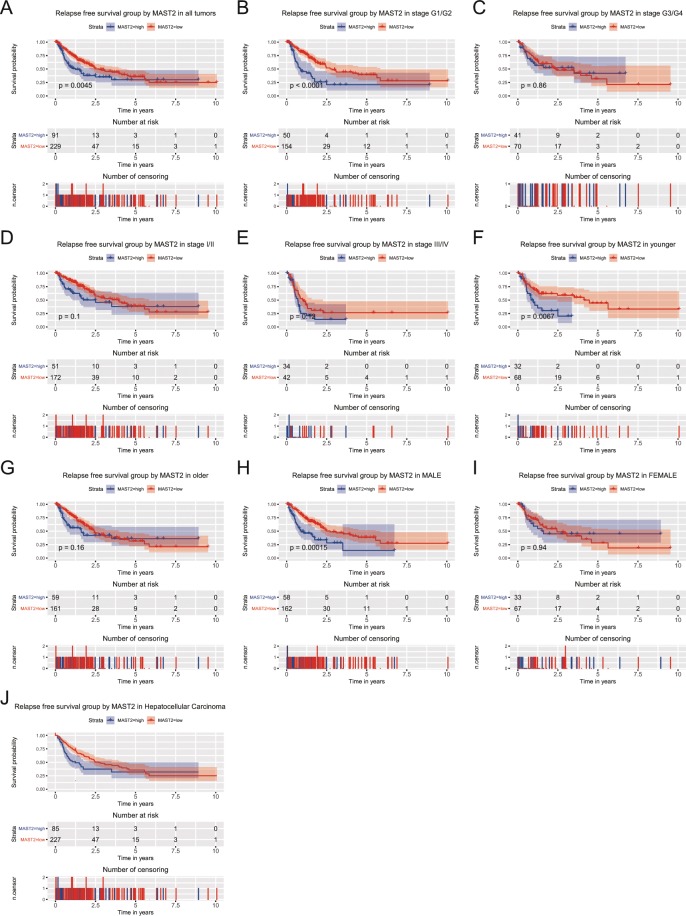
Kaplan-Meier curves indicated patients in group of high MAST2 expression exhibited worse RFS (p = 0.0045; Fig. 4). Moreover, patients in stage G1/G2 (p < 0.0001), younger (p = 0.0067) and male (p = 0.00015) were more sensitive to the poor prognostic effects of MAST2 high expression (Fig. 4). Subgroup analysis among HCC tumors only and found the same results (Fig. 4J). Univariate analysis selected that stage (p < 0.001), T classification (p < 0.001), residual tumor (p = 0.042) and expression of MAST2 (p = 0.005) were associated with RFS. In addition, multivariate analysis indicated MAST2 expression was an independent risk factor for RFS in liver cancer patients (HR = 1.517, 95%CI: 1.059–2.172, p = 0.023; Table 4).
Figure 4.
Kaplan-Meier curves for RFS in liver cancer. Kaplan-Meier curves for RFS in liver cancer for all patients, and patients in subgroup of stage G1/G2, stage G3/G4, stage I/II, stage III/IV, younger, older, male, female and HCC.
Table 4.
Univariate and multivariate analysis of relapse-free survival.
| Characteristics | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95%CI (lower-upper) | p-value | Hazard Ratio | 95%CI (lower-upper) | p-value | |
| age (≥55/<55) | 0.898 | 0.631–1.278 | 0.550 | |||
| gender (male/female) | 0.992 | 0.696–1.415 | 0.966 | |||
| histological_type (hepatocholangiocarcinoma/hepatocellular/fibrolamellar) | 2.024 | 0.656–6.24 | 0.220 | |||
| histologic_grade (G4/G3/G2/G1) | 0.985 | 0.801–1.21 | 0.883 | |||
| clincial_stage (IV/III/II/I) | 1.656 | 1.379–1.988 | 0.000 | 1.114 | 0.862–1.439 | 0.410 |
| T_classification (T4/T3/T2/T1/NX) | 1.778 | 1.494–2.117 | 0.000 | 1.635 | 1.255–2.13 | 0.000 |
| N_classification (N1/N0/NX) | 0.971 | 0.674–1.399 | 0.874 | |||
| M_ classification (M1/M0/MX) | 1.172 | 0.789–1.742 | 0.432 | |||
| radiation_therapy (yes/no) | 0.742 | 0.256–2.156 | 0.584 | |||
| residual_ tumor (RX/R2/R1/R0) | 1.275 | 1.009–1.612 | 0.042 | 1.335 | 1.054–1.692 | 0.017 |
| MAST2 (high/low) | 1.663 | 1.166–2.372 | 0.005 | 1.517 | 1.059–2.172 | 0.023 |
Discussion
Liver cancer malignant tumor with poor prognosis, which is predominantly due to the fact that diagnosis is often made late or inaccurate2. To identify a new biomarker for early and accurate diagnosis has great clinical significance, many researchers have been working on developing novel biomarkers in liver cancer8–11. In this study, we explored the diagnostic and prognostic role of MAST2 in liver cancer patients. We found that MAST2 highly expressed in liver cancer and thus, may have diagnostic value for this cancer, and its expression was correlated with histological type, histologic grade, stage, T classification, N classification, and survival status. Moreover, high MAST2 expression was associated with poor OS and RFS in patients, which suggested the prognostic role of MAST2 in liver cancer.
MAST2, as a microtubule associated kinase, plays important roles in a wide range of life activities. Previous studies have reported the role of MAST2 in evolution12, marfan syndrome13, neurodegeneration14, rabies virus infection15, nonobstructive azoospermia16, experimental autoimmune encephalomyelitis17, chronic myeloid leukemia18 and breast cancer4. Our studies showed the abnormal expression and prognostic effects of MAST2 in liver cancer, which broadened the field of scientific research on MAST2.
The upregulation of MAST2 has been reported in several tumors, including esophageal cancer, pancreatic cancer, sarcomas5, chronic myeloid leukemia18 and breast cancer4. Our results showed the overexpression of MAST2 in liver cancer. It is consistent with previous reports. We also found that the upregulation of MAST2 was distinct in different clinical features of liver cancer, such as histologic grade, stage and T classification. Moreover, the AUC of MAST2 suggest a potentially important value in tumor diagnosis and prognosis.
The effect of MAST2 in promoting tumor cell proliferation has been reported in glioblastoma. Eissmann et al. used lentiviral shRNA transduction in U87 cell line not only resulted in significantly increased apoptosis and decreased cell proliferation, but also delayed tumor growth5. The tumor promoting effects of MAST2 may provide a reasonable explanation for the phenomenon in our research that patients with advanced stage and worse status showed high MAST2 expression.
MAST2 plays its role through binding the C-terminal of PTEN with its PDZ domain. PTEN regulates multiple cellular processes, including polarity, migration, proliferation and metabolism19. PTEN, also as a tumor suppressor gene, its aberrant expression is associated with tumorigenesis and progression20. In our study, the poor prognosis of patients with high MAST2 expression might due to the aberrant function of PTEN.
This study firstly demonstrates the potentially diagnostic and prognostic significance of MAST2 in liver cancer patients. Moreover, the distinct expression of MAST2 and prognosis in subgroups by clinical features also provided multiple guidelines of precision therapy. However, the lower expression and AUC of MAST2 in stage IV might result from the limited sample size of stage IV patients, further studies are needed to verify these findings.
In conclusion, our study found upregulation of MAST2 in liver cancer, which corresponded with tumor progression and poor prognosis. Our findings suggest MAST2 could be a novel diagnostic and prognostic biomarker for liver cancer patients.
Material and Methods
Data mining
The characteristics and gene expression in patients with liver cancer were downloaded from TCGA database (https://cancergenome.nih.gov/), GEO database (https://www.ncbi.nlm.nih.gov/gds/) and ICGC database (https://icgc.org/). All data were analyzed by R (version 3.5.3)21.
Statistical analysis
Boxplots were used to illustrate the gene expression differences between different groups and subgroups through ggplot222. ROC curve was applied to examine the diagnostic capability of MAST2 in liver cancer23. Chi-square and Fisher test were used to explore the association between patients’ characteristics and MAST2 expression. Survival curves were applied to explore OS and RFS of patients in different MAST2 expression group through Survival package24. Univariate analysis was used to select variables relating to outcomes. Multivariate analysis was applied to investigate the influence of MAST2 expression on OS and RFS of patients with liver cancer. The methodological is partly similarity with previous publications8–11.
Acknowledgements
The results shown here are partly based upon data generated by the TCGA Research Network: https://www.cancer.gov/tcga. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
Y.J. collected and analyzed the data. P.J. made the figures. Y. Li made the tables. Z.F. wrote the manuscript. Y. Liu designed the study. All authors revised the manuscript and approved the final version to be published.
Data availability
All data generated or analyzed during this study are included in this published article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zhuo Fu, Email: fzwork@126.com.
Yahui Liu, Email: liuyh_2008@yeah.net.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Tsuchiya N, et al. Biomarkers for the early diagnosis of hepatocellular carcinoma. World J Gastroenterol. 2015;21:10573–10583. doi: 10.3748/wjg.v21.i37.10573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang X, et al. Association of genetic variation in mitotic kinases with breast cancer risk. Breast Cancer Res Treat. 2010;119:453–462. doi: 10.1007/s10549-009-0404-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robinson DR, et al. Functionally recurrent rearrangements of the MAST kinase and Notch gene families in breast cancer. Nat Med. 2011;17:1646–1651. doi: 10.1038/nm.2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eissmann M, et al. A functional yeast survival screen of tumor-derived cDNA libraries designed to identify anti-apoptotic mammalian oncogenes. PLoS One. 2013;8:e64873. doi: 10.1371/journal.pone.0064873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delhommel F, et al. Deciphering the unconventional peptide binding to the PDZ domain of MAST2. Biochemical Journal. 2015;469:159–168. doi: 10.1042/bj20141198. [DOI] [PubMed] [Google Scholar]
- 7.Terrien E., Chaffotte A., Lafage M., Khan Z., Prehaud C., Cordier F., Simenel C., Delepierre M., Buc H., Lafon M., Wolff N. Interference with the PTEN-MAST2 Interaction by a Viral Protein Leads to Cellular Relocalization of PTEN. Science Signaling. 2012;5(237):ra58–ra58. doi: 10.1126/scisignal.2002941. [DOI] [PubMed] [Google Scholar]
- 8.Jiao Y, Fu Z, Li Y, Meng L, Liu Y. High EIF2B5 mRNA expression and its prognostic significance in liver cancer: a study based on the TCGA and GEO database. Cancer Manag Res. 2018;10:6003–6014. doi: 10.2147/CMAR.S185459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiao Y, Li Y, Jiang P, Han W, Liu Y. PGM5: a novel diagnostic and prognostic biomarker for liver cancer. PeerJ. 2019;7:e7070. doi: 10.7717/peerj.7070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiao Y, Li Y, Lu Z, Liu Y. High Trophinin-Associated Protein Expression Is an Independent Predictor of Poor Survival in Liver Cancer. Digestive diseases and sciences. 2019;64:137–143. doi: 10.1007/s10620-018-5315-x. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, et al. High miR-454-3p expression predicts poor prognosis in hepatocellular carcinoma. Cancer management and research. 2019;11:2795–2802. doi: 10.2147/CMAR.S196655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Damert A. Phylogenomic analysis reveals splicing as a mechanism of parallel evolution of non-canonical SVAs in hominine primates. Mob DNA. 2018;9:30. doi: 10.1186/s13100-018-0135-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brett M, Korovesis G, Lai AHM, Lim ECP, Tan EC. Intragenic multi-exon deletion in the FBN1 gene in a child with mildly dilated aortic sinus: a retrotransposal event. J Hum Genet. 2017;62:711–715. doi: 10.1038/jhg.2017.32. [DOI] [PubMed] [Google Scholar]
- 14.Loh SH, Francescut L, Lingor P, Bahr M, Nicotera P. Identification of new kinase clusters required for neurite outgrowth and retraction by a loss-of-function RNA interference screen. Cell Death Differ. 2008;15:283–298. doi: 10.1038/sj.cdd.4402258. [DOI] [PubMed] [Google Scholar]
- 15.Prehaud C., Wolff N., Terrien E., Lafage M., Megret F., Babault N., Cordier F., Tan G. S., Maitrepierre E., Menager P., Chopy D., Hoos S., England P., Delepierre M., Schnell M. J., Buc H., Lafon M. Attenuation of Rabies Virulence: Takeover by the Cytoplasmic Domain of Its Envelope Protein. Science Signaling. 2010;3(105):ra5–ra5. doi: 10.1126/scisignal.2000510. [DOI] [PubMed] [Google Scholar]
- 16.Huang N, et al. A Screen for Genomic Disorders of Infertility Identifies MAST2 Duplications Associated with Nonobstructive Azoospermia in Humans. Biol Reprod. 2015;93:61. doi: 10.1095/biolreprod.115.131185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hasan M, et al. Novel genes in brain tissues of EAE-induced normal and obese mice: Upregulation of metal ion-binding protein genes in obese-EAE mice. Neuroscience. 2017;343:322–336. doi: 10.1016/j.neuroscience.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Riva E, et al. A novel e8a2 BCR-ABL1 fusion with insertion of MAST2 exon 2 in a four-way translocation t (1;17;9;22) (p35;q24;q44;q11) in a patient with chronic myeloid leukemia. Leuk Lymphoma. 2016;57:203–205. doi: 10.3109/10428194.2015.1043549. [DOI] [PubMed] [Google Scholar]
- 19.Worby CA, Dixon JE. Pten. Annu Rev Biochem. 2014;83:641–669. doi: 10.1146/annurev-biochem-082411-113907. [DOI] [PubMed] [Google Scholar]
- 20.Lee YR, Chen M, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor: new modes and prospects. Nat Rev Mol Cell Biol. 2018;19:547–562. doi: 10.1038/s41580-018-0015-0. [DOI] [PubMed] [Google Scholar]
- 21.Team RDCJC. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2009;14:12–21. [Google Scholar]
- 22.Wickham H. Ggplot2:elegant graphics for data analysis. Journal of the Royal Statistical Society. 2011;174:245–246. doi: 10.1111/j.1467-985X.2010.00676_9.x. [DOI] [Google Scholar]
- 23.Robin X, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:77. doi: 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Therneau, T. M. & Grambsch, P. M. Modeling Survival Data: Extending the Cox Model. Vol. 97 (Springer, 2000).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.