Abstract
In everyday life, we continuously search for and classify objects in the environment around us. This kind of visual search is extremely important when performed by radiologists in cancer image interpretation and officers in airport security screening. During these tasks, observers often examine large numbers of uncorrelated images (tumor x-rays, checkpoint x-rays, etc.) one after another. An underlying assumption of such tasks is that search and recognition are independent of our past experience. Here, we simulated a visual search task reminiscent of medical image search and found that shape classification performance was strongly impaired by recent visual experience, biasing classification errors 7% more towards the previous image content. This perceptual attraction exhibited the three main tuning characteristics of Continuity Fields: serial dependence extended over 12 seconds back in time (temporal tuning), it occurred only between similar tumor-like shapes (feature tuning), and only within a limited spatial region (spatial tuning). Taken together, these results demonstrate that serial dependence influences shape perception and occurs in visual search tasks. They also raise the possibility of a detrimental impact of serial dependence in clinical and practically relevant settings, such as medical image perception.
Subject terms: Object vision, Pattern vision
Introduction
In everyday life, we continuously search for objects in the environment around us and classify them. We look for our wallet on a messy desk, for a friend in a crowd of people, or for a pair of socks in our room. Visual search can be literally life-saving in clinical and practically relevant settings. For example, in a medical screening setting, radiologists repeatedly search for signs of tumors in blurry radiological scan images, classifying them, judging their size, class, position and so on. Likewise, in airport security, transportation security officers need to identify prohibited items in carry-on luggage, in the midst of all possible kinds of personal belongings. The “human search engine” of radiologists and security officers is, like that of all humans, highly unreliable and fallible1,2; for example, more than 30–35% of mistakes in radiological screening are considered to reflect interpretation errors3–5.
An underlying assumption about visual search is that our current perceptual experience is independent of our previous perceptual experience. Recent results question this assumption, however. Our visual processing is characterized by serial dependencies, where what was seen previously influences (captures) what is seen and reported at this moment6. Serial dependencies occur for a variety of features and objects, including orientation6,7, position8,9, faces10,11, numerosity12–14 and other dimensions15. Such serial dependence is characterized by three types of selectivity. First, temporal tuning: serial dependence gradually decays over time6,9,10,16. Second, feature tuning: serial dependence occurs only between similar features, and not between dissimilar ones6,9,17. Third, spatial tuning: serial dependence occurs only within a limited spatial region and it is strongest when previous and current objects are presented at the same location6,8,9,12,13.
Based on these results, it was recently proposed that perception occurs through Continuity Fields (6,10, see also18,19): temporally and spatially tuned operators that bias our percepts towards previous ones through serial dependence. Continuity fields were proposed to be a beneficial mechanism that promotes perceptual stability in our ever-changing visual environment6,7,10,20. Over time, retinal images constantly change because our eyes, head and body move; because of many sources of external and internal noise; and because of discontinuities introduced by eye blinks. By recycling previously perceived features and objects, serial dependence helps us to represent a continuous and stable world despite noise and change.
In the autocorrelated world we live in, it is useful for our visual system to trade accuracy for increased perceptual stability. In artificially uncorrelated situations, however, serial dependencies can be detrimental for object recognition. The visual search tasks performed by radiologists in cancer image interpretation and officers in airport security screening are two pertinent examples. During these kinds of visual search tasks, observers often examine large numbers of uncorrelated images (tumor x-rays, checkpoint x-rays, etc.) one after another. We hypothesized that because of serial dependence, visual search performance on any given current x-ray image will be biased towards the previous x-ray, systematically altering image interpretation. To preview our results, we simulated a medical screening setting and found that simulated tumor shape classification was strongly impaired by an observer’s past visual experience. Classification errors increased and were pulled towards the previous x-ray content.
Results
Experiment 1
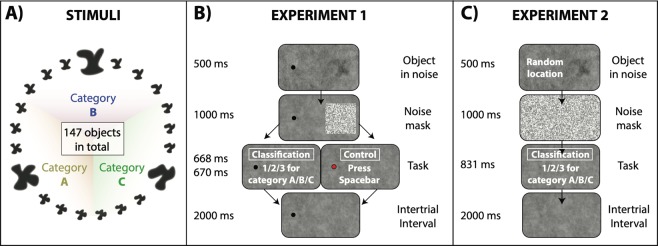
In Experiment 1, we tested whether serial dependence influences recognition of visual stimuli in a classification task. In order to simulate the medical screening performed by radiologists, we created three objects with random shapes (simulated tumors A, B, and C) and generated 48 morph objects in between each pair (147 objects in total; Fig. 1A). On each trial, subjects viewed a random tumor-like object superimposed on a noisy background (simulated x-ray), followed by a mask of black and white pixels. Observers were asked to continuously fixate a black dot and the random object was presented 15° away from fixation in the right peripheral field (Fig. 1B). Observers were then asked to classify the object as belonging to category A, B or C (simulated tumor classification task; 66% of the trials). If the fixation dot turned red after mask appearance, observers were asked to simply press the spacebar (control task; 33% of trials).
Figure 1.
(A) We created three objects with random shapes (prototypes A-B-C, shown in a bigger size) and generated 48 morph objects in between each pair (147 objects in total). Each shape category was defined as the prototype A/B/C −/+24 morph units (49 morph units in total). (B) In Experiment 1, observers were asked to continuously fixate a black dot on the left part of the screen. On the right part of the screen, they were presented with an object hidden in noise, followed by a noise mask. Depending on the color of the fixation dot, they were then asked to classify the object as belonging to category A–C (black dot, 60% of the trials) or press the spacebar (red dot, 40% of the trials). After a 2000 ms inter-trial interval, the next trial started. (C) Experiment 2 was very similar to Experiment 1, except that there was no fixation dot (unconstrained free viewing), and the object was presented on each trial in a random location. On each trial, observers were asked to find the object on the screen (visual search task) and to classify the object (classification task) as belonging to category A–C.
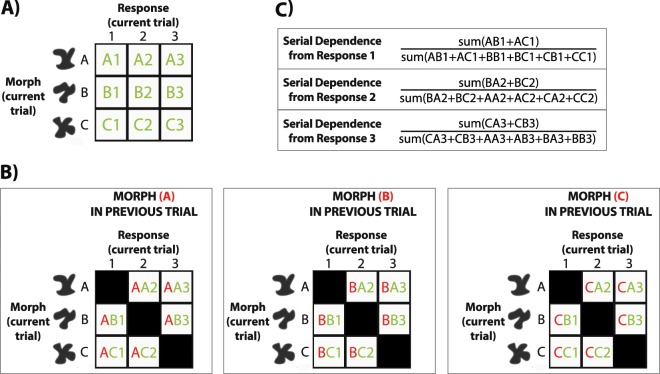
Object classification accuracy, defined as correspondence between stimulus category (−/+24 morph units around the prototype shape A/B/C) and response, was 75.6 ± 6.3% (N = 12). As a categorical measure of serial dependence, we investigated whether errors in object classification were biased more towards the object category on the previous trial compared to other previous object categories. We computed the percentage of mistakes towards the shape category in n-back trials, and normalized the index by subtracting 33.33% (chance percentage level) from each percentage index (see Fig. 2 for an in-depth explanation). We then bootstrapped each subject’s data with 5000 iterations and reported the mean bootstrapped percentage as a metric of serial dependence (Fig. 3A).
Figure 2.
Temporal tuning analysis in Experiments 1 and 2. (A) We divided observers’ response frequency into a 3 × 3 matrix based on their responses 1/2/3 and the actual object category (A/B/C) on a current trial. (B) We then divided observers’ mistakes into three matrixes depending on whether the previous objects category was (A/B/C). First letter (in red) indicates morph category on the previous trial, second letter indicates morph category on the current trial (in green), and the number indicates classification response on a current trial (in green). Only mistakes were considered and hits were not taken into account (black squares on the diagonal line). (C) For a given response (1, for example), we summed mistakes when response and previous category were the same (AB1,AC1), and divided them by the sum of all the mistakes (AB1,AC1,BB1,BC1,CB1,CC1). This ratio yielded an index of serial dependence for a given responses (1 in this example). We then averaged the indexes across the three responses 1/2/3 and subtracted the chance baseline (33%).
Figure 3.
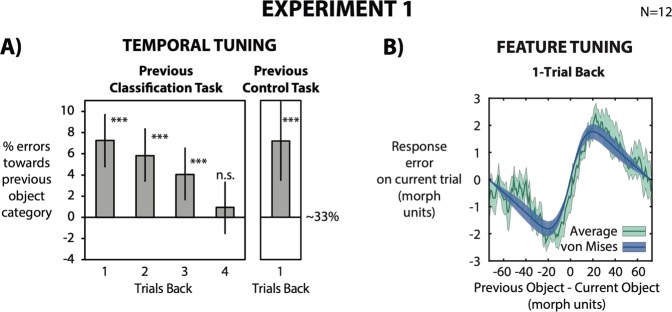
(A) We computed the percentage of errors towards the object category in the previous trial, normalized for the 33% chance level (chance baseline was confirmed by permuted null distribution). A 0% value indicates no bias from the previous object category, with a 33% chance of classifying the object as A/B/C. Higher and lower values than 0% indicate that a classification response was biased towards the previous object category (higher than 33%) or biased away (lower than 33%), respectively. For each observer we obtained a mean bootstrapped percentage by resampling the data with replacement 5000 times. Error bars are bootstrapped 95% confidence intervals, and p-value is based on the group bootstrapped distribution. Observers’ responses were strongly biased towards the previous object category up to 3 trials back (12 seconds back in time). Similar results were found when observers were asked to simply press the response bar in the 1-back trial, indicating that our results are not due to a response bias. (B) Response errors were computed as the distance between the response (corresponding to prototypes 1-A, 50-B or 99-C ) and current morph. Error plot as a function of the relative morph difference of the previous trial (1-back). The x-axis represents the difference between the previous and current object in morph units. The y-axis represents the error in the classification task (difference between response and object morph on current trial). The average error (green line) shows more negative response errors for a negative relative morph difference and more positive errors for a positive relative morph difference. Green shaded error bars indicate standard error across observers. The dark blue line indicates the average von Mises distribution across observers. Green shaded error bars indicate standard error across observers. Morph classification was attracted toward the morph seen on the previous trial. Importantly, it was tuned for similarity between previous and current morph (feature tuning).
Observers misclassified the object on a current trial as the shape in 1-back trials 7% more often than other shape categories. The misclassification lasted up to 3 trials back (1-back: p < 0.01; 2-back: p < 0.01; 3-back: p < 0.01; 4-back: p = 0.21; Fig. 3A, left panel). Average response time (RT) across subjects was 730 ± 200 ms; the 1-back object was presented on average ~4200 ms prior to the current trial object. The perceived object was therefore strongly attracted toward a random object seen more than 12 seconds prior, similar to the temporal tuning of serial dependence reported in the literature6,9,11,16,17,21.
In order to rule out a response bias, we analyzed the previous trials where observers gave an unrelated response (33% of the trials; control task) and the current trials where observers classified the object (66% of the trials; classification task). When observers were asked to withhold their classification responses in the previous trial, giving an unrelated “spacebar” response, serial dependence nevertheless occurred on the following trial (around 7%; Fig. 3A, right panel). In order to further control for unrelated biases and potential artifacts in our analysis that might manifest as spurious serial dependence22, we also computed serial dependence from future objects for 1, 2 and 3 trials forward. As expected, object classification responses were not significantly biased towards future objects (1-forward: 0.41%, p = 0.45; 2-forward: −0.39%, p = 0.52; 3-forward: 0.21%, p = 0.42). This confirms that neither systematic response biases, nor artifacts can explain the serial dependence effect.
Serial dependence did not simply occur at the response level (i.e., between responses 1/2/3), but was tuned for object similarity between the current and previous stimulus. We calculated perceptual error as the shortest distance along the morph wheel between the response categories (corresponding to 1, 50 and 99 morph units) and target objects (1–147 morph units). Each subject’s error on the current trial was compared to the difference in object morphs between the current and previous trial (Fig. 3B). We first computed the moving average of the response error as a function of the relative morph difference and averaged across observers (Fig. 3B, green line). In order to quantify feature tuning, we then fit a derivative-of-von Mises distribution to each subject’s running average data (Fig. 3B, blue line). When measuring the peak-to-trough amplitude and width of the derivative-of-von Mises distribution, response error was maximally biased with an average peak of 2.06 morph units for a relative morph difference of ±20 morph units, and gradually decreased with increasing morph difference (Fig. 3B; feature tuning).
Taken together, our results show that object classification is strongly biased towards previously presented objects up to 12 seconds in the past. In a task that mimics situations where observers assess target characteristics and classify them, such as in a medical screening setting, we show that serial dependence can have a harmful effect on object recognition, by biasing classification errors towards the previous x-ray content. As our visual system strongly expects constancy from one moment to the next, it tends to make our perception inaccurate in uncorrelated situations where extremely fine discriminations and accurate object recognition are required, such as during radiological screening or x-ray screening at airports.
Experiment 2
It might be argued that in visual search, observers do not only have to identify and classify objects, but they must also scan the environment to find targets (for example, radiologists searching within x-ray images). For this reason, in Experiment 2 we randomized the position of the objects on each trial. Hence, observers were asked to first find the target1 and then classify it2. Our purpose was twofold. First, we aimed at mimicking the standard visual search tasks that radiologists typically perform on radiological scans; they are required to find tumors or tumor-like structures (if present) in several consecutive radiological scans, and to classify them (e.g., as malignant, benign, type, etc). Second, we investigated whether serial dependence in a visual search setting is affected by the spatial distance between current and previous objects, as shown by previous results6,8,9,12,13.
The procedure for Experiment 2 was identical to that of Experiment 1, except for the following changes. Observers’ fixation was unconstrained (free viewing). Object position was randomized on each trial within a 25° spatial window, and each object was further blurred (from 20 to 30 blur pixel radius). Observers were presented with an object in a random location, followed by a noise mask covering the entire screen. As in Experiment 1, they were asked on all trials to classify the object as belonging to category A/B/C (Fig. 1C).
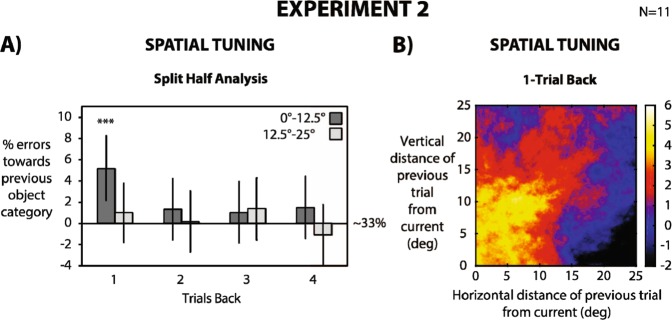
Mean Accuracy was 65.7% ± 9.5% (N = 11). For each subject, we binned the trials into two groups based on the relative object location between current and previous trial: 0°–12.5° and 12.5°–25°. We then analyzed the influence of the previously presented object on a subsequent trial in these two groups of relative distances for 1–4 trials back. For a relative distance between 0° and 12.5°, serial dependence occurred for 1 trial back, biasing classification responses up to 5% (1-back: p < 0.01; 2-back: p = 0.18; 3-back: p = 0.24; 4-back: p = 0.16; group bootstrapped distribution), whereas for a relative distance between 12.5° and 25°, no serial dependence occurred (1-back: p = 0.22; 2-back: p = 0.45; 3-back: p = 0.17 4-back: p = 0.77; group bootstrapped distribution). Serial dependence was weaker compared to Experiment 1, with 5% of errors towards previous object category (compared to 7% in Experiment 1) and no effect for 2–3 trials back. This difference is strength may be due to the change in blur of the stimulus (see Method) or a shift from peripheral (Experiment 1) to foveal vision (Experiment 2), in accordance with evidence showing that serial dependence strongly depends on stimulus noise and attention6,13,20.
In order to further characterize the spatial tuning of the effect, we computed serial dependence between morph tumors at different locations within a two-dimensional rolling window, over the relative positions of the previous and current stimuli (Fig. 4B; see Methods section): serial dependence gradually decreased with increasing relative spatial distance between the current and previous object. Taken together, our results show that serial dependence also occurs in a visual search setting reminiscent of a radiologist’s (visual search and classification), and that serial dependence strongly depends on the relative location of the target relative to the preceding ones.
Figure 4.
Stimuli and experimental procedures in Experiment 2 were the same as in Experiment 1, with the main difference that on each trial the tumor morph was presented at a random position. (A) We computed the percentage of errors towards the object category in the previous trial, normalized for the 33% chance level. Chance baseline was confirmed by a permuted null distribution. A 0% value indicates no bias from previous object category, with a 33% chance of classifying the object as (A–C). Higher and lower values than 0% indicate that classification response was biased towards the previous object category (higher than 33%) or biased away (lower than 33%), respectively. For each observer we obtained a mean bootstrapped percentage by resampling the data with replacement 5000 times. Error bars are bootstrapped 95% confidence intervals, and p-value is based on group bootstrapped distribution. For each subject, trials were divided into two groups based on the relative spatial distance between current and n-back trials (0–12.5° and 12.5°–25°). Observers’ responses were biased towards the previous object category up to 1 trial back, only when presented within a spatial window within 0 and 12.5° (dark bars). (B) This percentage of responses was computed between morph tumors at different locations within a two-dimensional rolling window over the relative positions of the previous and current stimuli. Color-coding shows the amplitude (in percentage) of biased responses computed at each window location. Observers’ responses were maximally biased toward previous stimuli in a relative spatial range of 10–12°.
Discussion
Visual search in a simulated medical screening paradigm is serially dependent; misclassifications were biased towards the object category in the previous trial by up to 7%. Serial dependence could therefore be a substantial source of error in several critical visual search situations, like for example cancer image interpretation1 and screening at airport checkpoints23. Importantly, our results show that this source of errors is not unavoidable. Serial dependence did not indiscriminately occur across time, features, and space. It lasted up to 12 seconds (Figs. 3A and 4A), occurred only between similar objects (Fig. 4B) and within a spatial window of 10–12.5° (Fig. 4B).
Our results are not due to a mere response bias for three main reasons. First, serial dependence on a current trial occurred also when, in the previous trial, observers were asked to give a “spacebar” response completely unrelated to the stimulus (Control Task, Fig. 1B). Hence, a previous classification response (or a perceptual decision) per se is not necessary for serial dependence to occur. Second, serial dependence did not simply occur between responses (sequence 1/2/3) but it was specifically tuned to stimulus characteristics. It impacted classification performance depending on object similarity on the current and previous trials, with a peak for an object morph difference of 20 morph units (Fig. 3B; feature tuning). Third, a response bias would indiscriminately occur across the entire visual field, but we showed in Experiment 2 that serial dependence is spatially tuned (Fig. 4) in accordance with previous results6,8,9,12,13.
Previous studies have investigated the impact of previous visual experience on visual search, but they mostly involved reaction time and eye movements measurements24,25. For example, search speed for a target is faster if previous and current targets share the same features26–33 or location27,34–38. Between-trial repetition of features and locations also improves recognition of briefly presented masked items39–41. Additionally, Chetverikov, Campana et Kristjánsson42,43 showed that observers can learn the shapes of distributions of items to be ignored if the distractors come from the same distribution on consecutive trials. Interestingly, eye movements are substantially influenced by memory for past fixation locations, although this was found in a detection task paradigm25.
Previous visual experience can determine image recognition also in the medical screening domain. Kompaniez et al.44 adapted normal observers for 60 seconds to image samples of dense or fatty tissue, and they asked them to judge the appearance of intermediate images in a texture matching task. Long exposure to dense images caused an intermediate image to appear more fatty (and vice versa), thus leading to a negative aftereffect. In a similar paradigm, adaptation to radiological images was also found to modulate RT45. Whereas these two results show that long adaptation to previous history (60 seconds) can affect recognition and discrimination away from the past, our current results show for the first time that a much shorter exposure (500 ms) can strongly bias object recognition and discrimination in a visual search task towards the past. Future research will have to determine under which specific conditions these two opposing biases in visual perception determine recognition performance.
Our results are in accordance with the idea of Continuity Fields, spatiotemporally tuned operators where similar features and objects are integrated over time6,7,10. Our serial dependence effects exhibited the three main defining criteria of Continuity Fields: temporal tuning, feature tuning, and spatial tuning. The main purpose of Continuity Fields is to favor object stability by merging similar information over time (see also 18, 19); they are therefore a beneficial mechanism in the autocorrelated world in which we live. Here, we show another side of the same coin: in non-correlated situations, such as visual search for tumors in radiological scans, Continuity Fields can be detrimental, impairing classification performance toward previous x-ray content.
Our results cannot clearly disentangle whether serial dependence occurs on a perceptual6,9,12,20,46,47 or decisional/memory level8,17,48. According to the first account, serial dependence changes stimulus appearance, biasing the current stimulus towards the previous one6,12,20, whereas under the second account serial dependence biases our internal decision or memory towards the previous one8,17,49. Nevertheless, our, results lean toward the perceptual hypothesis, because serial dependence is selective to the characteristics of the stimulus (featural tuning) and its location in the visual field (spatial tuning). In addition, even when observers do not withhold decisions on the previous trial (as in the control task condition; Fig. 3A), serial dependence still biased the next stimulus (as opposed to Pascucci et al.50). However, it must be mentioned that these accounts are not necessarily mutually exclusive. Different mechanisms may interact with one another on several levels49; decision and memory may change target appearance and the other way around. More importantly, independent of the underlying mechanism(s) of serial dependence, our results carry a much broader and more pragmatic message: that a considerable percentage of errors in medical visual search may be due to sequential effects from previous perceptual experiences.
Previous research has shown that visual search in a clinical setting is determined by various factors. Attentional guidance plays a crucial role: by focusing their attention on spotting tumors in x-ray images, radiologists were shown to miss objects as salient as gorillas51–54. Different search strategies can be more effective than others: drilling through 3D images in depth (drillers strategy) can be more accurate than scanning each image before moving to the next one (scanners strategy, 55). Target prevalence and distribution also play a crucial role in visual search; target misses strongly increase with decreasing target prevalence56–61.
Serial dependence may play an important role in all these factors. Serial dependence depends on attention6,12, and should particularly impair the tumor shapes radiologists are looking for, independently of the background image content. Regarding search strategy, drilling through 3D images should keep the image more autocorrelated across time, and hence the Continuity Field should have a beneficial impact in stabilizing our percept. Conversely, scanning random locations in each image before going to the next one will lead to potentially less autocorrelated information and the Continuity Field should have a detrimental effect, biasing perception towards previous parts of the same radiological scan. Consistent with this, radiologists make more mistakes with the scanning than the drilling strategy55. Regarding target prevalence, in our experiments each object category was equally likely to be presented on the screen (33%), but under certain visual search situations targets are extremely rare. For example, malignant tumors for radiologists or threats in carry-on luggage for transportation security officers are rarely encountered. When searching through several consecutive empty scans, serial dependencies should continuously bias our percept towards more frequent objects, making it even more difficult to recognize rare targets for what they actually are.
One concern could be that our stimuli were not realistic. Indeed, our stimuli and task were intentionally controlled for the sake of measuring potential sequential dependencies. Our stimulus and task, however, are reminiscent of those that radiologists routinely encounter. Radiologists are usually asked to look at radiological images1, search for tumors2, and classify them as malignant or benign depending on their shape and location3. Accordingly, we adapted standard serial dependence paradigms to mimic a noisy background1, a target similar to a tumor shape2 and a classification task3. Hence, we consider our results as “proof of concept” that serial dependence can detrimentally affect clinically and practically relevant visual search settings. Of course, whether any particular visual search task (e.g., mammography, cytology, etc) or individual subject group (radiologist, resident, ultrasound technician) suffers from serial dependence remains an open and important question for further investigation.
Future research is needed to show effects of serial dependence in actual radiological screening settings. First, having context-related stimuli is crucial. Future research will present sequences of actual x-ray with actual tumors, instead of simulated ones. Second, in our experiments the simulated tumor was presented for a relatively short duration (500 ms). Radiologists may fixate radiological scans for a much longer period before they make a decision. However, there is psychophysical evidence that radiologists are capable of extracting valuable information from an image at first glance, without lengthy examination of it62–67. Tumor recognition is well above chance with short duration stimuli, but it increases to nearly perfect with unlimited viewing. Accordingly, future research will test whether sequential effects also occur with longer x-ray presentations. Of course, as mentioned above, long exposure may lead to negative perceptual aftereffects, leading to opposing results44,45. Third, there are well-known target frequency effects on visual search59,61,68–72. In our experiments, each shape category was equally likely to appear on any trial (33%), whereas in radiological screening the chance of encountering a radiological scan with a tumor is very low, around 1–2%. Future research will test whether having a less frequent target will increase or decrease serial dependence. On one hand, having a rare target may lead to the well-known phenomenon of satisfaction of search, i.e. failure to detect subsequent abnormalities after identifying an initial one73–75. This phenomenon would work against serial dependence, thus leading to a “no tumor” response when the previous x-ray contained one. On the other hand, such a low tumor frequency means also higher exposure to non-tumor stimuli (e.g., benign masses, cysts, etc.), and may therefore boost serial dependence strength. That is, benign tumors and non-tumor structures could generate serial dependence as well.
In addition to the potential importance for clinically and practically relevant settings, our results also provide new insights into the mechanism(s) underlying serial dependence. They show that serial dependence can bias perceptual decisions for simple stimuli like shapes, thus making dissimilar shapes appear more similar than they actually are. This shows that serial dependence can occur with low-level6,76,77 and high-level stimuli10,13,78. In the recent literature, serial dependence has been assessed with three main task types: stimulus adjustment and matching6,10,17, two-alternative forced choice tasks6,12,17, as well as rating scales and magnitude estimation15,79,80. Here, we show that serial dependence can also be investigated through a three-alternative forced-choice classification task, which has the advantage of not presenting any visual stimulus during the response. Finally, our results highlight the importance of serial dependence in the domain of visual search81–83. They show that serial dependence can strongly bias subsequent search for items, but only if the items are similar and are presented within a limited temporal and spatial window.
Conclusion
Our results show 1) that visual search is serially dependent, and 2) that this occurs in a simulated clinically relevant setting; past experience can affect recognition, biasing classification errors towards previous simulated x-ray content. Serial dependence impairs classification performance within defined temporal, featural, and spatial boundaries in line with the defining characteristics of Continuity Fields. Importantly, the limits of these three types of tuning open the door to potential strategies which may mitigate their detrimental effects.
Method
All experimental procedures were approved by and conducted in accordance with the guidelines and regulations of the UC Berkeley Institutional Review Board. Participants were affiliates of UC Berkeley and provided informed consent in accordance with the IRB guidelines of the University of California at Berkeley. All participants had normal or corrected-to-normal vision, and were all naïve to the purpose of the experiment. Twelve subjects (6 females; age = 20–31 years) participated in Experiment 1. Eleven subjects (7 females; age = 19–28 years) participated in Experiment 2. Stimuli were generated on a Macintosh computer running Matlab PsychToolbox84 and presented on a gamma-corrected CRT Sony Multiscan G500 monitor. The refresh rate of the display was 100 Hz and the resolution 1024 × 768 pixels. Stimuli were viewed from a distance of 57 cm. Subjects used a keyboard for all responses (“1–3” keys for the classification task, and spacebar for the control task).
Stimuli
The stimuli consisted of dark-gray shapes based on 3 original prototype shapes (A/B/C; Fig. 1A). A set of 48 shape morphs was created between these prototypes, resulting in a morph continuum of 147 objects. Approximate width and height were 4°. Each object was blurred in Photoshop by using a gaussian blur filter. Blur pixel radius was 20 in Experiment 1 and 30 in Experiment 2. On each trial, a random object was inserted on a random brownian noise background (1/f2 spatial noise) with a 50% transparency level. The object embedded in the noisy background was presented for 500 ms, followed by a 1000 ms noise square mask (6° size) of black and white pixels (to reduce afterimages). Subjects were then asked to classify the object as belonging to category A/B/C by pressing 1/2/3 on the keyboard (classification task). After a 2000 ms delay, the next trial started. In a preliminary session, observers completed a practice block of 108 trials (Experiment 1) and 54 trials (Experiment 2), where the three prototype stimuli were shown on the screen when observers were asked to make a response. In addition, observers were continuously familiarized with the three prototype stimuli by seeing them on the screen for 10 seconds at the beginning of each block.
Experiment 1
Observers were asked to continuously fixate a black dot (0.2° radius) and on each trial the objects were presented at 15° eccentricity in the right peripheral field. On 66% of the trials, the fixation dot was black and observers were asked to perform the classification task (Classification task). On 33% of the trials, the fixation dot turned red after the mask stimulus and observers were asked to simply press the spacebar (Control task). Observers performed 20 blocks of 54 trials each (Fig. 1B). Mean reaction time was 668 ± 15 ms in the Classification Task and 670 ± 9 ms in the Control Task.
Experiment 2
There were no fixation constraints and the location of the object was changed randomly from trial to trial within a spatial square window of 25°. On each trial, observers were asked to find the object within the noisy background and classify whether it belonged to category A, B, or C. Observers performed 10 blocks of 108 trials each (Fig. 1C). Mean reaction time was 831 ± 346 ms.
Temporal tuning
As a measure of performance on the classification task, we computed the accuracy for each observer, defined as the correspondence between response 1/2/3 and morph category A/B/C. Trials were considered lapses and excluded if response times were longer than 5 seconds. On average, less than 5% of data was excluded. Observers were removed from the experiment if their overall classification accuracy was lower than 50% or higher than 85% (two observers removed). Even including these two observers in the analysis did not significantly change the overall pattern of results. If observers classified all objects more than 60% of the times with a specific category for the first 2 blocks, thus showing a strong bias towards a specific object or response, the experiment was immediately terminated (two observers removed).
As a measure of serial dependence, we computed the percentage of errors towards the previous object category on an n-back trial (Fig. 3A). First, we computed the number of erroneous responses A/B/C given object categories A/B/C on an n-back trial. Second, for each response 1/2/3 we summed up the number of erroneous responses where previous object category and current response were the same, and divided it by the sum of erroneous responses for all previous object categories. The ratio was computed for each response 1/2/3. For example, the number of erroneous responses A when the previous Object Category was A was divided by the number of erroneous responses A when the previous Object Category was A + B + C. Third, we averaged the three obtained percentages across the three response categories 1–3 and subtracted the chance baseline 33.3% to normalize our index (Fig. 2).
The final index denotes by how much the categorical responses on a current trial are biased by the previous stimulus category. Positive values indicate a positive serial dependence, negative values indicate a repulsion, and zero indicates no bias. For each subject’s data, we generated confidence intervals by calculating a bootstrapped distribution of the model-fitting parameter values by resampling the data with replacement 5000 times. On each iteration, we recalculated the error percentage to obtain a bootstrapped percentage index for each subject. P-values were calculated by computing the proportion of percentages in each subject’s distribution (standard index – bootstrapped distribution) that were greater than or equal to zero. In order to empirically confirm the 33.3% chancel level, we also generated a null distribution of percentage errors for each subject using a permutation analysis. We randomly shuffled the order of the previous n-back trial and recalculated the error percentage for each iteration of the shuffled data. We ran this procedure for 5000 iterations. The average of the null distribution across observers was 33.32% (s.d. 0.07) in Experiment 1 and 33.33% (s.d. 0.04) in Experiment 2.
Feature tuning
Relative morph difference (x-axis) was computed as the difference in morph units between the previous and current object (previous object morph–current object morph). Response error (y-axis) was computed as the difference between the response in the classification task, corresponding to morph 1 (prototype A), 50 (prototype B) and 99 (prototype C), and the current morph (current response – current object morph). For each observer, we computed the running circular average within a 15 morph units window and averaged the moving averages across all observers (Fig. 3B). We quantified feature tuning by fitting a von Mises distribution to each subject’s running average data. Peak-to-trough amplitude of the von Mises distribution across observers was significantly higher than zero (average across observers 2.06 morph units, s.d. 0.97; t11 = 7.34, p < 0.01) for an average width of ±19 morph units.
Spatial tuning
In order to measure the spatial tuning of serial dependence, we binned trials according to the distance between the current and previous object locations (Fig. 4A). Distance between successive object locations was computed as:
We divided trials into two main groups: 0°–12.5° (Fig. 4A; dark bars), and 12.5°–25° (Fig. 4A; bright bars) for n-back trials. For each subject, we extracted the first 400 trials in both groups and computed the bootstrapped percentage of serial dependence within each group (5000 iterations; see Temporal tuning section).
We repeated the analysis using the two-dimensional spatial separation between successive trials (i.e., considering x distance and y distance separately; Fig. 4B). The rolling window was a circle with a starting radius of 8° that parametrically increased with increasing diagonal distance until 16° in order to collect a similar amount of data at each point. From each subject, we extracted the first 800 trials and collapsed all the data into a “super subject” (9600 trials in total). We then computed the bootstrapped percentage of serial dependence (1000 iterations) at each spatial separation between the current and previous trial. In Fig. 4B, a value of 0 on both axes (relative vertical and relative horizontal distance) indicates that the objects were presented at the same location on the current and the previous trial. A value of 25° on both axes indicates that the objects were presented at 25°of horizontal and vertical distance on both the current and previous trial (35° of diagonal distance). In order to increase the number of available trials, we collapsed left-right and up-down quadrants into a unique quadrant.
Acknowledgements
We would like to thank Allison Yamanashi, Yuki Murai, and Zhimin Chen for helpful comments on data analysis and preliminary drafts of the manuscript. This work was supported in part by the Swiss National Science Foundation fellowship P2ELP3_158876 (M.M.).
Author contributions
M.M., A.K. and D.W. designed the study. M.M. conducted the experiments, analyzed the data and wrote the first draft of the manuscript, and A.K. and D.W. edited the manuscript.
Data availability
All relevant data are available from the authors.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wolfe J, Evans KK, Drew T, Aizenman A, Josephs E. How do radiologists use the human search engine? Radiation protection dosimetry. 2016;169(1–4):24–31. doi: 10.1093/rpd/ncv501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krupinski EA. The role of perception in imaging: past and future. Semin Nucl Med. 2011;41(6):392–400. doi: 10.1053/j.semnuclmed.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Gale AG. Medical image perception. Advances in psychology. 1984;22:295–6. doi: 10.1016/S0166-4115(08)61845-2. [DOI] [Google Scholar]
- 4.Kundel HL. Reader error, object recognition, and visual search. Medical Imaging 2004: Image. Perception, Observer Performance, and Technology Assessment. 2004;5372:1–12. [Google Scholar]
- 5.Manning DJ, Gale A, Krupinski EA. Perception research in medical imaging. Br J Radiol. 2005;78(932):683–5. doi: 10.1259/bjr/72087985. [DOI] [PubMed] [Google Scholar]
- 6.Fischer J, Whitney D. Serial dependence in visual perception. Nat Neurosci. 2014;17(5):738–43. doi: 10.1038/nn.3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manassi M, Liberman A, Chaney W, Whitney D. The perceived stability of scenes: serial dependence in ensemble representations. Sci Rep. 2017;7(1):1971. doi: 10.1038/s41598-017-02201-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bliss DP, Sun JJ, D’Esposito M. Serial dependence is absent at the time of perception but increases in visual working memory. Sci Rep. 2017;7(1):14739. doi: 10.1038/s41598-017-15199-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manassi M, Liberman A, Kosovicheva A, Zhang K, Whitney D. Serial dependence in position occurs at the time of perception. Psychon Bull Rev. 2018;25(6):2245–53. doi: 10.3758/s13423-018-1454-5. [DOI] [PubMed] [Google Scholar]
- 10.Liberman A, Fischer J, Whitney D. Serial dependence in the perception of faces. Curr Biol. 2014;24(21):2569–74. doi: 10.1016/j.cub.2014.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taubert J, Alais D, Burr D. Different coding strategies for the perception of stable and changeable facial attributes. Sci Rep. 2016;6:32239. doi: 10.1038/srep32239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cicchini GM, Mikellidou K, Burr D. Serial dependencies act directly on perception. J Vis. 2017;17(14):6. doi: 10.1167/17.14.6. [DOI] [PubMed] [Google Scholar]
- 13.Fornaciai M, Park J. Serial dependence in numerosity perception. J Vis. 2018;18(9):15. doi: 10.1167/18.9.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corbett JE, Fischer J, Whitney D. Facilitating stable representations: serial dependence in vision. PLoS One. 2011;6(1):e16701. doi: 10.1371/journal.pone.0016701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xia Y, Leib AY, Whitney D. Serial dependence in the perception of attractiveness. J Vis. 2016;16(15):28. doi: 10.1167/16.15.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wexler M, Duyck M, Mamassian P. Persistent states in vision break universality and time invariance. Proc Natl Acad Sci USA. 2015;112(48):14990–5. doi: 10.1073/pnas.1508847112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fritsche M, Mostert P, de Lange FP. Opposite Effects of Recent History on Perception and Decision. Curr Biol. 2017;27(4):590–5. doi: 10.1016/j.cub.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Burr D, Cicchini GM. Vision: efficient adaptive coding. Curr Biol. 2014;24(22):R1096–8. doi: 10.1016/j.cub.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cicchini GM, Anobile G, Burr DC. Compressive mapping of number to space reflects dynamic encoding mechanisms, not static logarithmic transform. Proc Natl Acad Sci USA. 2014;111(21):7867–72. doi: 10.1073/pnas.1402785111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cicchini GM, Mikellidou K, Burr DC. The functional role of serial dependence. Proc Biol Sci. 2018;285(1890):20181722. doi: 10.1098/rspb.2018.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moors P, Stein T, Wagemans J, van Ee R. Serial correlations in Continuous Flash Suppression. Neurosci Conscious. 2015;2015(1):niv010. doi: 10.1093/nc/niv010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maus GW, Chaney W, Liberman A, Whitney D. The challenge of measuring long-term positive aftereffects. Curr Biol. 2013;23(10):R438–9. doi: 10.1016/j.cub.2013.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCarley JS, Kramer AF, Wickens CD, Vidoni ED, Boot WR. Visual skills in airport-security screening. Psychol Sci. 2004;15(5):302–6. doi: 10.1111/j.0956-7976.2004.00673.x. [DOI] [PubMed] [Google Scholar]
- 24.Kristjansson A, Asgeirsson AG. Attentional priming: recent insights and current controversies. Curr Opin Psychol. 2018;29:71–5. doi: 10.1016/j.copsyc.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 25.Najemnik J, Geisler WS. Optimal eye movement strategies in visual search. Nature. 2005;434(7031):387. doi: 10.1038/nature03390. [DOI] [PubMed] [Google Scholar]
- 26.Treisman A. Perceiving and re-perceiving objects. Am Psychol. 1992;47(7):862–75. doi: 10.1037/0003-066X.47.7.862. [DOI] [PubMed] [Google Scholar]
- 27.Maljkovic V, Nakayama K. Priming of pop-out: II. The role of position. Percept Psychophys. 1996;58(7):977–91. doi: 10.3758/BF03206826. [DOI] [PubMed] [Google Scholar]
- 28.Maljkovic V, Nakayama K. Priming of pop-out: I. Role of features. Mem Cognit. 1994;22(6):657–72. doi: 10.3758/BF03209251. [DOI] [PubMed] [Google Scholar]
- 29.Goolsby BA, Suzuki S. Understanding priming of color-singleton search: Roles of attention at encoding and “retrieval”. Perception & Psychophysics. 2001;63(6):929–44. doi: 10.3758/BF03194513. [DOI] [PubMed] [Google Scholar]
- 30.Fecteau JH. Priming of pop-out depends upon the current goals of observers. J Vis. 2007;7(6):1. doi: 10.1167/7.6.1. [DOI] [PubMed] [Google Scholar]
- 31.Becker SI. The mechanism of priming: episodic retrieval or priming of pop-out? Acta Psychol (Amst). 2008;127(2):324–39. doi: 10.1016/j.actpsy.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Campana G, Pavan A, Casco C. Priming of first- and second-order motion: mechanisms and neural substrates. Neuropsychologia. 2008;46(2):393–8. doi: 10.1016/j.neuropsychologia.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 33.Brascamp JW, Blake R, Kristjansson A. Deciding where to attend: priming of pop-out drives target selection. J Exp Psychol Hum Percept Perform. 2011;37(6):1700–7. doi: 10.1037/a0025636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kristjansson A, Vuilleumier P, Malhotra P, Husain M, Driver J. Priming of color and position during visual search in unilateral spatial neglect. J Cogn Neurosci. 2005;17(6):859–73. doi: 10.1162/0898929054021148. [DOI] [PubMed] [Google Scholar]
- 35.Geng JJ, et al. On-line attentional selection from competing stimuli in opposite visual fields: effects on human visual cortex and control processes. J Neurophysiol. 2006;96(5):2601–12. doi: 10.1152/jn.01245.2005. [DOI] [PubMed] [Google Scholar]
- 36.Campana G, Casco C. Repetition effects of features and spatial position: evidence for dissociable mechanisms. Spat Vis. 2009;22(4):325–38. doi: 10.1163/156856809788746318. [DOI] [PubMed] [Google Scholar]
- 37.Campana G, Cowey A, Casco C, Oudsen I, Walsh V. Left frontal eye field remembers “where” but not “what”. Neuropsychologia. 2007;45(10):2340–5. doi: 10.1016/j.neuropsychologia.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 38.Geyer T, Muller HJ, Krummenacher J. Cross-trial priming of element positions in visual pop-out search is dependent on stimulus arrangement. J Exp Psychol Hum Percept Perform. 2007;33(4):788–97. doi: 10.1037/0096-1523.33.4.788. [DOI] [PubMed] [Google Scholar]
- 39.Lamy D, Antebi C, Aviani N, Carmel T. Priming of Pop-out provides reliable measures of target activation and distractor inhibition in selective attention. Vision Res. 2008;48(1):30–41. doi: 10.1016/j.visres.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 40.Sigurdardottir HM, Kristjansson A, Driver J. Repetition streaks increase perceptual sensitivity in visual search of brief displays. Vis Cogn. 2008;16(5):643–58.. doi: 10.1080/13506280701218364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Asgeirsson AG, Kristjànsson A, Bundesen C. Independent priming of location and color in identification of briefly presented letters. Atten Percept Psychophys. 2014;76(1):40–8. doi: 10.3758/s13414-013-0546-6. [DOI] [PubMed] [Google Scholar]
- 42.Chetverikov A, Campana G, Kristjánsson Á. Building ensemble representations: How the shape of preceding distractor distributions affects visual search. Cognition. 2016;153:196–210. doi: 10.1016/j.cognition.2016.04.018. [DOI] [PubMed] [Google Scholar]
- 43.Chetverikov A, Campana G, Kristjànsson A. Rapid learning of visual ensembles. J Vis. 2017;17(2):21. doi: 10.1167/17.2.21. [DOI] [PubMed] [Google Scholar]
- 44.Kompaniez E, Abbey CK, Boone JM, Webster MA. Adaptation aftereffects in the perception of radiological images. PLoS One. 2013;8(10):e76175. doi: 10.1371/journal.pone.0076175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kompaniez-Dunigan E, Abbey CK, Boone JM, Webster MA. Adaptation and visual search in mammographic images. Atten Percept Psychophys. 2015;77(4):1081–7. doi: 10.3758/s13414-015-0841-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liberman A, Zhang K, Whitney D. Serial dependence promotes object stability during occlusion. J Vis. 2016;16(15):16. doi: 10.1167/16.15.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fornaciai M, Park J. Attractive Serial Dependence in the Absence of an Explicit Task. Psychol Sci. 2018;29(3):437–46. doi: 10.1177/0956797617737385. [DOI] [PubMed] [Google Scholar]
- 48.Czoschke, S., Fischer, C., Beitner, J., Kaiser, J., Bledowski, C. Two types of serial dependence in visual working memory. Br J Psychol. (2018). [DOI] [PubMed]
- 49.Kiyonaga A, Scimeca JM, Bliss DP, Whitney D. Serial Dependence across Perception, Attention, and Memory. Trends Cogn Sci. 2017;21(7):493–7. doi: 10.1016/j.tics.2017.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pascucci, D. et al. Laws of concatenated perception: Vision goes for novelty, Decisions for perseverance. bioRxiv. (2017). [DOI] [PMC free article] [PubMed]
- 51.Mack, A & Rock, I. Inattentional blindness: MIT press Cambridge, MA (1998).
- 52.Simons DJ, Chabris CF. Gorillas in our midst: sustained inattentional blindness for dynamic events. Perception. 1999;28(9):1059–74. doi: 10.1068/p281059. [DOI] [PubMed] [Google Scholar]
- 53.Most SB, Scholl BJ, Clifford ER, Simons DJ. What you see is what you set: sustained inattentional blindness and the capture of awareness. Psychol Rev. 2005;112(1):217–42. doi: 10.1037/0033-295X.112.1.217. [DOI] [PubMed] [Google Scholar]
- 54.Drew T, Vo ML, Wolfe JM. The invisible gorilla strikes again: sustained inattentional blindness in expert observers. Psychol Sci. 2013;24(9):1848–53. doi: 10.1177/0956797613479386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Drew T, et al. Scanners and drillers: characterizing expert visual search through volumetric images. J Vis. 2013;13(10):1–13. doi: 10.1167/13.10.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wolfe JM, Horowitz TS, Kenner NM. Cognitive psychology: rare items often missed in visual searches. Nature. 2005;435(7041):439–40. doi: 10.1038/435439a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wolfe JM, Van Wert MJ. Varying target prevalence reveals two dissociable decision criteria in visual search. Curr Biol. 2010;20(2):121–4. doi: 10.1016/j.cub.2009.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wolfe JM, et al. Low target prevalence is a stubborn source of errors in visual search tasks. J Exp Psychol Gen. 2007;136(4):623–38. doi: 10.1037/0096-3445.136.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Evans KK, Birdwell RL, Wolfe JM. If you don’t find it often, you often don’t find it: why some cancers are missed in breast cancer screening. PLoS One. 2013;8(5):e64366. doi: 10.1371/journal.pone.0064366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Evans KK, Tambouret RH, Evered A, Wilbur DC, Wolfe JM. Prevalence of abnormalities influences cytologists’ error rates in screening for cervical cancer. Arch Pathol Lab Med. 2011;135(12):1557–60. doi: 10.5858/arpa.2010-0739-OA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mitroff SR, Biggs AT. The ultra-rare-item effect: visual search for exceedingly rare items is highly susceptible to error. Psychol Sci. 2014;25(1):284–9. doi: 10.1177/0956797613504221. [DOI] [PubMed] [Google Scholar]
- 62.Carmody DP, Nodine CF, Kundel HL. Finding lung nodules with and without comparative visual scanning. Perception & psychophysics. 1981;29(6):594–8. doi: 10.3758/BF03207377. [DOI] [PubMed] [Google Scholar]
- 63.Drew T, Evans K, Vo ML, Jacobson FL, Wolfe JM. Informatics in radiology: what can you see in a single glance and how might this guide visual search in medical images? Radiographics. 2013;33(1):263–74. doi: 10.1148/rg.331125023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kundel HL, Nodine CF. Interpreting chest radiographs without visual search. Radiology. 1975;116(3):527–32. doi: 10.1148/116.3.527. [DOI] [PubMed] [Google Scholar]
- 65.Mugglestone, M. D., Gale, A. G., Cowley, H. C., Wilson, A. editors. Diagnostic performance on briefly presented mammographic images. Medical Imaging 1995: Image Perception;: International Society for Optics and Photonics (1995).
- 66.Oestmann J, et al. Lung lesions: correlation between viewing time and detection. Radiology. 1988;166(2):451–3. doi: 10.1148/radiology.166.2.3336720. [DOI] [PubMed] [Google Scholar]
- 67.Evans KK, Haygood TM, Cooper J, Culpan AM, Wolfe JM. A half-second glimpse often lets radiologists identify breast cancer cases even when viewing the mammogram of the opposite breast. Proc Natl Acad Sci USA. 2016;113(37):10292–7. doi: 10.1073/pnas.1606187113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Horowitz TS. Prevalence in Visual Search: From the Clinic to the Lab and Back Again. Japanese Psychological Research. 2017;59(2):65–108. doi: 10.1111/jpr.12153. [DOI] [Google Scholar]
- 69.Wolfe J, Brunelli DN, Rubinstein J, Horowitz TS. Prevalence effects in newly trained airport checkpoint screeners: trained observers miss rare targets, too. J Vis. 2013;13(3):33. doi: 10.1167/13.3.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Menneer T, Donnelly N, Godwin HJ, Cave KR. High or low target prevalence increases the dual-target cost in visual search. J Exp Psychol Appl. 2010;16(2):133–44. doi: 10.1037/a0019569. [DOI] [PubMed] [Google Scholar]
- 71.Rich AN, et al. Why do we miss rare targets? Exploring the boundaries of the low prevalence effect. J Vis. 2008;8(15):15 1-7. doi: 10.1167/8.15.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wolfe J, Horowitz TS, Kenner NM. Cognitive psychology: rare items often missed in visual searches. Nature. 2005;435(7041):439. doi: 10.1038/435439a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Berbaum KS, et al. Satisfaction of search in diagnostic radiology. Invest Radiol. 1990;25(2):133–40. doi: 10.1097/00004424-199002000-00006. [DOI] [PubMed] [Google Scholar]
- 74.Berbaum KS, et al. Role of faulty visual search in the satisfaction of search effect in chest radiography. Academic radiology. 1998;5(1):9–19. doi: 10.1016/S1076-6332(98)80006-8. [DOI] [PubMed] [Google Scholar]
- 75.Fleck MS, Samei E, Mitroff SR. Generalized “satisfaction of search”: Adverse influences on dual-target search accuracy. Journal of Experimental Psychology: Applied. 2010;16(1):60. doi: 10.1037/a0018629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liberman A, Kosovicheva A, Whitney D. Serial Dependence of Position Perception. Journal of Vision. 2014;14(10):1186. doi: 10.1167/14.10.1186. [DOI] [Google Scholar]
- 77.Alais D, Leung J, Van, der Burg E. Linear Summation of Repulsive and Attractive Serial Dependencies: Orientation and Motion Dependencies Sum in Motion Perception. J Neurosci. 2017;37(16):4381–90.. doi: 10.1523/JNEUROSCI.4601-15.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Suárez-Pinilla, M., Seth, A., Roseboom, W. Serial Dependence In Visual Variance. PsyArXiv. (2018). [DOI] [PMC free article] [PubMed]
- 79.Kok R, Taubert J, Van der Burg E, Rhodes G, Alais D. Face familiarity promotes stable identity recognition: exploring face perception using serial dependence. Royal Society open science. 2017;4(3):160685. doi: 10.1098/rsos.160685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Taubert J, Alais D. Serial dependence in face attractiveness judgements tolerates rotations around the yaw axis but not the roll axis. Visual Cognition. 2016;24(2):103–14. doi: 10.1080/13506285.2016.1196803. [DOI] [Google Scholar]
- 81.Eckstein MP. Visual search: a retrospective. J Vis. 2011;11(5):14. doi: 10.1167/11.5.14. [DOI] [PubMed] [Google Scholar]
- 82.Beutter BR, Eckstein MP, Stone LS. Saccadic and perceptual performance in visual search tasks. I. Contrast detection and discrimination. JOSA A. 2003;20(7):1341–55. doi: 10.1364/JOSAA.20.001341. [DOI] [PubMed] [Google Scholar]
- 83.Rajashekar U, Bovik AC, Cormack LK. Visual search in noise: Revealing the influence of structural cues by gaze-contingent classification image analysis. Journal of Vision. 2006;6(4):7. doi: 10.1167/6.4.7. [DOI] [PubMed] [Google Scholar]
- 84.Brainard DH. The Psychophysics Toolbox. Spat Vis. 1997;10(4):433–6. doi: 10.1163/156856897X00357. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are available from the authors.