Abstract
Microbial endocrinology has demonstrated for more than two decades, that eukaryotic substances (hormones, neurotransmitters, molecules of the immune system) can modulate the physiological behavior of bacteria. Among them, the hormones/neurotransmitters, epinephrine (Epi) and norepinephrine (NE), released in case of stress, physical effort or used in medical treatment, were shown to be able to modify biofilm formation in various bacterial species. In the present study, we have evaluated the effect of Epi on motility, adhesion, biofilm formation and virulence of Pseudomonas aeruginosa, a bacterium linked to many hospital-acquired infections, and responsible for chronic infection in immunocompromised patients including persons suffering from cystic fibrosis. The results showed that Epi increased adhesion and biofilm formation of P. aeruginosa, as well as its virulence towards the Galleria mellonella larvae in vivo model. Deciphering the sensor of this molecule in P. aeruginosa and the molecular mechanisms involved may help to find new strategies of treatment to fight against this bacterium.
Subject terms: Biofilms, Pathogens
Introduction
Pseudomonas aeruginosa is a Gram-negative opportunistic pathogen known for its implication in many hospital-acquired infections and in immunocompromised patients suffering from cystic fibrosis1. Furthermore, it is a pathogen of concern, since it was classed as critical by the World Health Organization in 2017, due to its antibiotic resistance. The pathogenicity of this bacterium is associated with its capacity to induce chronic infections (strong biofilms, which are difficult to treat) and acute infections where many virulence factors can be secreted. P. aeruginosa possesses a large genome (6.3 million base pairs) including many regulatory two-component systems and transcriptional regulators which contribute to its remarkable environmental adaptability2,3.
During host infection, bacteria are exposed to many eukaryotic substances such as immune system molecules (interleukin), peptides hormones (CNP, substance P) and neurotransmitters (GABA)4. Molecules released under host stress can influence host-pathogens interactions and bacterial pathogenicity5. Stress, trauma6,7, physical effort and therapy utilization of inotropes leads to the presence of catecholamines in the human body, which gather three endogenous molecules: dopamine, norepinephrine (NE) and epinephrine (Epi). These substances were found to be able to modulate the growth of Gram-negative pathogens like Escherichia coli8, Vibrio cholerae9, Helicobacter pylori10, Salmonella enterica and Yersinia enterocolitica11. Furthermore, some bacteria increased their virulence when exposed to catecholamines such as Vibrio harveyi, which was more virulent towards brine shrimp larvae, following a treatment with NE or dopamine12. The motility and capacity to form biofilm of Escherichia coli O157:H7 and V. harveyi were also found to be enhanced in presence of catecholamines12,13. In P. aeruginosa PA14, NE modulates the virulence and motility of the bacterium, and the las quorum-sensing pathway may be involved in the response towards this molecule14. In another study conducted on P. aeruginosa, strain PAO1, the authors showed that NE was able to repress the production of siderophore and the expression of toxA coding an important virulence factor exotoxin A15, rather than enhancing virulence production as seen with other bacteria.
Contrary to NE, prior to this study, very little information was available concerning the behavior of P. aeruginosa exposed to Epi. In this context, the aim of our study was to investigate the effect of this molecule on P. aeruginosa motility, adhesion, biofilm formation and virulence.
Results
Motility of P. aeruginosa H103
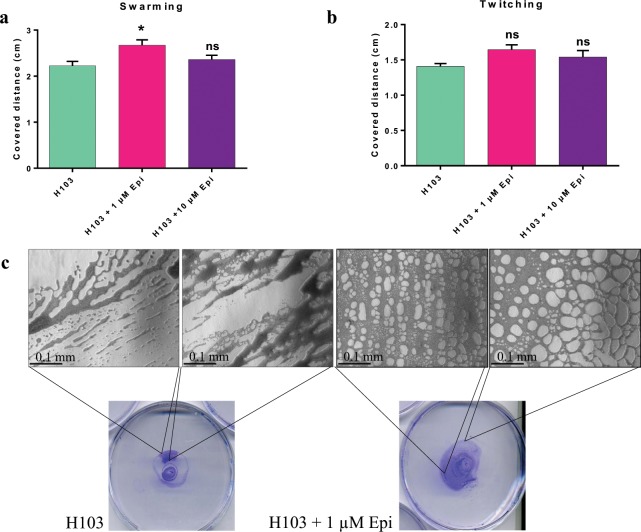
As motility was found to be modified in a large amount of Gram-negative bacteria after exposure to NE or Epi13,16–19, we decided to study this effect on P. aeruginosa H103 (Fig. 1). The results obtained showed that the radial growth of swarming phenotype was increased in presence of 1 µM Epi (Fig. 1a) and the twitching motility was qualitatively modulated (Fig. 1b). Indeed, the microscopic observation revealed that in presence of Epi, the twitching of P. aeruginosa H103 seemed to form a more well-organized network, compared to the untreated bacteria (Fig. 1c). As type IV pili can be involved in swarming and twitching of P. aeruginosa H10320,21, we then completed this study by using PO4 phage, which effect on P. aeruginosa H103 is highly dependent on type IV pili.
Figure 1.
Effect of Epi on P. aeruginosa H103 motility. (a) Covered distance by swarming motility on LB 0.6% agar, after 24 h, (b) Covered distance by twitching motility on LB 1% agar, (c) Macroscopic and microscopic observations of twitching. Micrographs were taken with an Axio Vert A.1 inverted microscope, scale bar: 0.1 mm. The error bars indicate the standard error of the mean (SEM). Two-tailed paired t-test was used, (n = 6 and n = 3 for swarming and twitching assays respectively), *p = 0.0125, ns: not significant.
PO4 phage sensitivity of P. aeruginosa H103
Bacteriophages require a specific component of bacteria for host infection, like lipopolysaccharides for the phage PaP122 or proteins on the bacterial surface. PO4 bacteriophages bind to the type IV pili of P. aeruginosa. To test the hypothesis of modulation of type IV pili in presence of Epi, a double agar overlay plaque assay was carried out. The results of this experiment, presented on Fig. 2, showed that P. aeruginosa H103 was more sensitive to the PO4 bacteriophage in presence of Epi. Indeed, the number of lysis plaque was higher when treated with 1 and 10 µM Epi, leading to 3.27-fold increase ± 0.5, and 1.24 ± 0.05, respectively. This means that when P. aeruginosa H103 was in contact with Epi, its sensitivity towards the PO4 phage was enhanced. This result reinforces the hypothesis that Epi may modify the type IV pili structure resulting in the modification of motility observed above.
Figure 2.
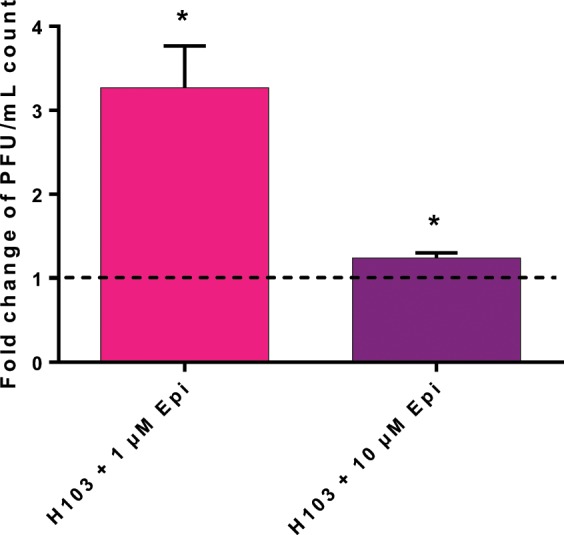
Effect of Epi on phage PO4 sensitivity. Phage PO4 sensitivity towards P. aeruginosa H103 was tested in presence of 1 and 10 µM of Epi after 24 h. Plaque lysis were enumerated and expressed in PFU/mL, fold change is expressed for each condition. The error bars indicate the standard error of the mean (SEM). Two-tailed paired t-test was used (n = 4), *p = 0.025.
Adhesion of P. aeruginosa H103
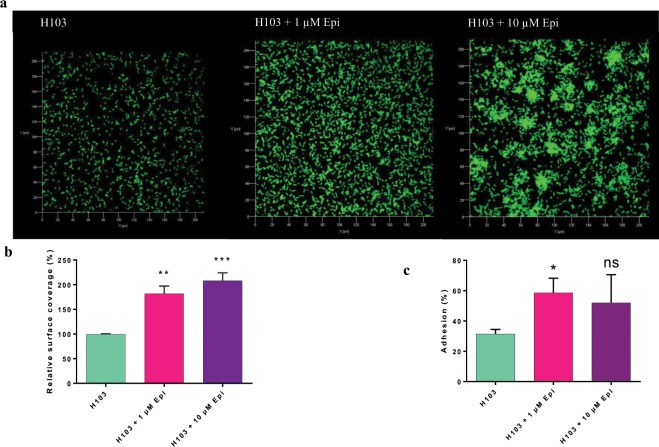
Adhesion to a surface is the first step which allows bacteria to colonize an environment and thereafter, to form biofilm. Bacteria possess many components that help them to adhere on surfaces, like adhesin or the type IV pili. In this work, we investigated the effect of 1 and 10 µM Epi on adhesion of P. aeruginosa H103 to abiotic and biotic surfaces. The results obtained are presented on Fig. 3. A COMSTAT analysis showed that P. aeruginosa H103 displayed an increased surface coverage (181% ± 15%) when treated with 1 µM of Epi, compared to control (Fig. 3b). A higher concentration (10 µM of Epi) also increased the covered surface of P. aeruginosa H103 (207% ± 16%) (Fig. 3b). Moreover, P. aeruginosa H103 seems to form aggregates when treated with 10 µM of Epi (Fig. 3a).
Figure 3.
Effect of Epi on adhesion of P. aeruginosa H103. (a) Top view of P. aeruginosa H103-GFP adhesion observed by CLSM with two different concentrations of Epi (1 and 10 µM), after 2 h. (b) Image analysis by COMSTAT. Data were normalized according to the untreated control set at 100%. (c) Adhesion assay on Caco-2/TC7 cells, *p = 0.0418. The error bars indicate the standard error of the mean (SEM). Two-tailed paired t-test was used (n = 5), **p = 0.001222, ***p = 0.000413, ns: not significant.
The capacity of P. aeruginosa H103 to adhere on biotic surface was then evaluated on Caco-2/TC7 cell line after 2 h of contact (Fig. 3c). The results showed that 1 µM of Epi significantly enhanced the capacity of the bacteria to adhere to these cells (58% ± 9.6%) compared to control (31% ± 3%).
Biofilm formation of P. aeruginosa H103
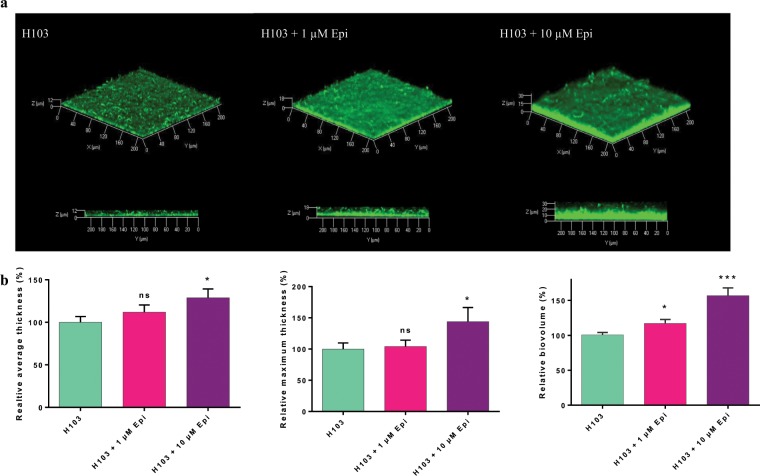
A flow-cell device with glass surface was used to investigate the biofilm formation of P. aeruginosa H103 in dynamic conditions to mimic in vivo conditions (Fig. 4). For both concentrations of Epi (1 and 10 µM) administrated continuously during biofilm formation, no modifications of biofilm architecture were observed compared to control (Fig. 4a). However, images analysis using COMSTAT software showed that average and maximum thicknesses were significantly increased in presence of 10 µM Epi compared to untreated biofilm, with +29% ± 10% (p = 0.0450) and +44% ± 22% (p = 0.0140), respectively. No significant difference of the average and maximum thicknesses were observed with 1 µM of Epi, but the biovolume reflecting the bacterial biomass was increased with +18% ± 5.7% (p = 0.0205) when exposed to 1 µM of this molecule and by +57% ± 11.4% (p < 0.0001) for 10 µM Epi (Fig. 4b).
Figure 4.
Effect of Epi on P. aeruginosa H103 biofilm formation. (a) Three dimensional (3D) and side views of P. aeruginosa H103-GFP 24 h biofilm observed by CLSM, in presence of 1 or 10 µM of Epi. (b) Images analysis by COMSTAT. Data were normalized according to the untreated control set at 100%. The error bars indicate the standard error of the mean (SEM) of at least three independent experiments. Two-tailed paired t-test was used (n = 4), *p < 0.05, ***p < 0.001, ns: not significant.
Virulence of P. aeruginosa H103 towards G. mellonella
The pathogenicity of P. aeruginosa H103 treated or not with 1 or 10 µM Epi was assessed with the in vivo model G. mellonella, which has been developed in recent years, as an alternative to murine or other vertebrate models of infection to contribute to the 3Rs (reduction, replacement, and refinement) of animal use in scientific research. The results of this experiment, presented on Fig. 5, showed that the mortality of the larvae was accelerated when bacteria were treated and co-injected with 10 µM Epi. Indeed, only 31% of the larvae remained alive at 13 h post-infection in the 10 µM Epi treated conditions compared to 58% of survival for infection with the pathogen alone. On the contrary, a treatment with 1 µM Epi had no effect on pathogenicity of P. aeruginosa H103. A control negative was also used to be sure that Epi had no direct effect on larvae mortality.
Figure 5.
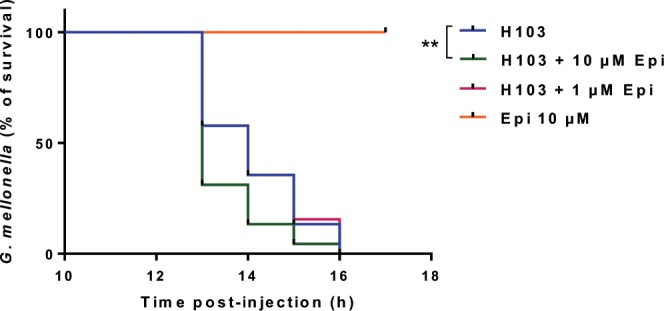
Virulence assay on G. mellonella. The virulence of P. aeruginosa H103 was tested towards the caterpillar G. mellonella larvae, during 17 h. Log-rank (Mantel-Cox) test was used, **p = 0.0086.
Discussion
Bacteria can be exposed to a multitude of host cell molecules such as catecholamines7 that could enhance the growth of many gram-negative bacteria8,9,12,17,18,23–26 as well as their virulence19,27. Till now, only few studies were conducted on the effect of these molecules on P. aeruginosa, which is an opportunistic pathogen, often found in hospital-acquired infections. Freestone and collaborators28 have shown that inotropes (dopamine, Epi and NE) are able to increase the biofilm formation of P. aeruginosa. Hedge et al.14 showed that NE increases P. aeruginosa PA14 virulence through the las quorum-sensing pathway by inducing, among others, the expression of lasA and lasB. Li et al.15 demonstrated that NE decreased siderophore production and toxA expression in PAO1.
Epi and catecholamines in general, can promote bacterial motility. In the present study, we showed that the swarming seemed to be slightly affected only at 1 µM Epi; moreover, the twitching motility displayed modifications in microscopic phenotype. In previous work, we have already observed that the swarming of P. fluorescens can also be affected by exposure to 1 µM Epi29. Twitching motility of P. aeruginosa PA14 strain was increased by Epi28. The present work showed that the stimulation of the twitching and the sensitivity to the PO4 bacteriophage are connected. Indeed, the PO4 phage needs the type IV pili to infect our strain30, and we saw that Epi enhance the intensity of the twitching, which indirectly measure the type IV pili function31. Production of rhamnolipids (another structure that can be involved in motility) has been quantified (Supplementary data 1) and no differences between treated and control conditions have been observed, reinforcing the hypothesis that the effect of Epi on motility may come from the type IV pili or the flagellum.
We demonstrated that upon exposure to Epi (1 and 10 µM), P. aeruginosa H103 adhesion on abiotic surface was increased and a concentration of 10 µM of Epi led to bacterial cell-cell attachment and microcolony formation. Lyte et al. (2003) demonstrated that adhesion of Staphylococcus epidermidis on polystyrene and silicon was increased upon addition to catecholamines inotropes (dopamine and NE)32. These authors also found the presence of bacterial aggregates following a microscopic evaluation of the biofilm. An enhancement of a specific biofilm exopolysaccharides production was proposed as an explanation for these phenomena. Attachment to surface was studied with P. aeruginosa PA14 on endotracheal tubes and the presence of cell-cell attachment was described as well28.
Biofilms enhance bacterial growth and survival by providing access to nutrients and provide protection from host immune system molecules and antimicrobial compounds. Furthermore, pathogenicity is often closely related to bacterial biofilm formation33–35. In the present study, we have examined the biofilm formation of P. aeruginosa under a continuous exposure (24 h) of Epi in dynamic conditions (i.e. flow cell system). We have observed an increased biovolume or thickness in presence of 1 or 10 µM of this molecule. None of these concentrations affected the growth of P. aeruginosa (Supplementary data 2). Freestone and collaborators (2012) also described an increased capacity of P. aeruginosa to form biofilm on eukaryotic cells and on endotracheal tube28 when treated with Epi on serum-SAPI medium, which mimics more closely the in vivo conditions. Other authors also showed an increase of biofilm formation when treated with catecholamines inotropes12,13,32,36.
The mechanism by which Epi could increase biofilm formation remains unclear. We can hypothesize that the impact of Epi on the type IV pili could contribute to the increase of adhesion and then biofilm formation. The type IV pili play an important role in the establishment of bacterial adhesion on abiotic surface (twitching motility) as well as in the development of microcolony and is required for biofilm formation by P. aeruginosa37–40.
The increasing ability of P. aeruginosa H103 to form biofilm when exposed to Epi suggested a role of this stress hormone in virulence. In our case, Epi at 10 µM significantly increased P. aeruginosa virulence towards G. mellonella. Similarly, Yang et al., (2014) demonstrated that catecholamines could enhance the virulence of pathogenic bacteria (Vibrio harveyi) towards a gnotobiotic brine shrimp larvae model12. Other authors studied the virulence of Vibrio campbellii BB120 toward giant freshwater prawn larvae, and showed that NE and dopamine were able to modulate the virulence of this bacterium17. In the prawn larvae model, the virulence can be increased by the production of extracellular protease41 and by the utilization of the type III secretion system42.
The interaction between bacteria and the host depends on their capacity to communicate. The production of catecholamines, which can occur after a trauma, stress or a physical effort, influences the bacterial pathogenicity6. Bacteria have adapted to their environment by developing various mechanisms, and two-component system is one of them. In E. coli O157:H7, two different systems, QseC/QseB and QseE/QseF have been described for their implication in sensing the presence of Epi or NE43–45. Other alternative adrenergic receptors have been found in organisms such as S. Typhimurium (BasS/R) or in S. enterica serovar Typhi46 (CpxA/R). To our knowledge, no adrenergic sensors have been identified in P. aeruginosa up to now. Investigations regarding the presence of homologous sequences to QseC in P. aeruginosa H103 have been performed in our laboratory. Some putative catecholamines receptors are now considered for further research. Deciphering the mechanism by which pathogenic bacteria are able to respond to host stress hormones could help understand how the signal is perceived and transduced, and the pathways impacted by these molecules. Proteomic analysis of P. aeruginosa H103 treated with Epi will also help us to discover how Epi acts on bacteria. P. aeruginosa can be present and responsible for skin, lung or intestine infections, where many eukaryotic substances are secreted; it is necessary to develop a way to prevent these phenomena to happen. Finding new therapeutic strategies could help to block bacterial capacity to perceive these eukaryotic signals and may reduce virulence.
Materials and Methods
Bacterial strains and growth conditions
The bacteria used in this study were P. aeruginosa H103 (PAO1 derivative strain)47 and the H103-GFP strain tagged with the green fluorescent protein (GFP)-encoding pHC60 plasmid (gfp, tetR)48. They were routinely grown at 37 °C under a rotary shaker (180 rpm) in Luria Bertani (LB) medium (containing 5 g/L NaCl). Depending on the assay, cultures were exposed to Epi (Sigma-Aldrich) from 1 to 10 µM. Tetracycline was used at 125 µg/mL for the culture of H103-GFP strain.
Motility assays
Swarming and twitching motility were tested on LB with 0.6% and 1% (w/v) agar, respectively, and containing 1 µM or 10 µM as previously described49,50. Swarming plates were inoculated using a bacterial suspension spotted on the surface and incubated for 24 h at 37 °C. For twitching assay, the suspension was spotted underneath the agar layer. After 24 h at 37 °C, agar was removed, and cells attached to the Petri dish bottom were stained with crystal violet 0.4%. Micrographs were taken using a Zeiss Axio Vert A.1 inverted microscope (x40) (Zeiss, Germany) with a QImaging QIClick camera and Q-capture pro 7 software (QImaging, Canada). For both motility assays, each experiment was conducted three times with at least three biological replicates by test.
PO4 phage sensitivity assay
Sensitivity of PO4 bacteriophage towards P. aeruginosa H103 was tested in 1.5% LB agar plate, with or without Epi. The double agar overlay method was used for the first step of phage isolation and later for precise titration of phage suspension51. Dilutions of phage suspension were mixed with 100 µl of P. aeruginosa H103 (108 CFU/mL) in molted agar (supplemented or not with 1 µM or 10 µM Epi) and then poured on the top of LB agar plate. The plates were incubated at 37 °C for 24 h. Lysis plaques were then counted, and results were expressed in PFU/mL. Data were obtained from four independent experiments.
Biofilm culture
Biofilms grown under hydrodynamic conditions were performed in a three channel flow cell (1 × 40 × 44 mm; Biocentrum, DTU, Danemark)52. The flow system was assembled, prepared and sterilized as described by Tolker-Nielsen and Sternberg53. The substratum consisted of a microscope glass coverslip (24 × 50 st1 [Knittel Glasser, Germany]). Each channel was inoculated with 250 µl of an overnight culture of P. aeruginosa H103 diluted to an OD580 of 0.1 (108 CFU/mL) in physiological water (NaCl 0.9%). A two-hour attachment step was performed without any flow. A flow (2.5 mL/h) of LB medium with or without Epi (1 and 10 µM) was then applied for 24 h using a Watson Marlow 205U peristaltic pump (Watson Marlow, UK). The attached bacteria or biofilms were observed by confocal laser scanning microscopy (CLSM) as described below.
Confocal laser scanning microscopy (CLSM)
Biofilm observations were performed with a Zeiss LSM710 (Zeiss, Germany) using a 40x oil immersion objective. Bacteria were detected by monitoring the GFP fluorescence. GFP was excited at 488 nm and fluorescence emission was detected between 500 and 550 nm. Images were taken every micrometer throughout the whole biofilm depth. For visualization and processing of three-dimensional (3D) image data, the Zen 2.1 software (Zeiss, Germany) was used. Quantitative analyses of image stacks were performed using the COMSTAT software (http://www.imageanalysis.dk/)54. For bacterial adhesion, the percentage of covered surface was determined using ImageJ software55. At least three image stacks from each of three independent experiments (nine stacks in total) were used for each analysis.
Galleria mellonella virulence assay
P. aeruginosa H103 strain, used for infection of G. mellonella, were grown in LB supplemented with 1 or 10 µM Epi, for 6 h (late exponential phase). After centrifugation, bacterial cells were washed once in physiological water (NaCl 0.9%) and resuspended to a final concentration of 105 CFU/mL. The size of the inoculum was confirmed by numeration on solid LB. Fifteen larvae were infected with 10 μL of a cell suspension, supplemented or not with Epi, into the hemocoel using a microinjector (KDS100 Legacy, Fisher Scientific) with a sterilized microsyringe56,57. Larva were incubated at 37 °C and survival was monitored during 17 h post-infections.
Cells cultures
The human colon adenocarcinoma Caco-2/TC7 cells were grown in DMEM (Dulbecco’s Modified Eagle Medium) containing 15% of heat-inactivated fetal bovine serum (FBS), and penicillin/streptomycin (100 µg/mL). Cells were cultivated at 37 °C, in 5% CO2 - 95% air atmosphere and the medium was regularly changed. For adhesion assays, cells were seeded in 24 wells plates treated for tissue culture and used at confluence.
Adhesion
Adhesion of P. aeruginosa H103 on intestinal Caco-2/TC7 cells was investigated according to Baccouri et al.56 including some modifications. Bacteria were grown for 6 h in LB supplemented with 1 or 10 µM Epi (late exponential phase), then collected by centrifugation at 8000 x g for 10 min. The pellets were resuspended in DMEM without FBS and antibiotics, added on the confluent intestinal cells at a MOI of 100 bacteria per cell, and allowed to adhere. After 2 h of adhesion, Caco-2/TC7 cells were washed twice with PBS (Phosphate Buffer Saline) to remove planktonic bacteria, and then disrupted with 0.1% triton 100x. The lysates were then diluted and plated on LB agar, to determine the number of adherent bacteria.
Statistical analysis
Statistical analysis was performed using GraphPad Prism (GraphPad Prism 8.1.2; GraphPad Software, San Diego, California, USA). For the G. mellonella killing assay, larvae survival was calculated by the Kaplan-Meier method, and survival differences were tested for significance using the log rank test. In all other assays, t-test were used to compare the means within the same set of experiments. Statistical significance was determined at p < 0.05.
Supplementary information
Acknowledgements
We wish to thank Rikki Knowlton, native English speaker for editing our manuscript. Mélyssa Cambronel was recipient of a doctoral fellowship from the French Ministry of Research (MRT). This work was supported by grants from the Evreux Portes de Normandie Agglomeration, the Région Normandie and European Union (FEDER).
Author contributions
S.R., J.H., M.F., N.C., conceived and designed the experiments. M.C., S.R. and N.C. carried out most of the experiments and analyzed the data. M.C. realized and analyzed bacterial motility experiments. M.C. and D.T. performed phage experiments. S.R. helped for adhesion and biofilm experiments with flow-cell device. M.C., K.B., N.C. performed adhesion on eukaryotic cells. M.C., S.R., L.T. and K.R. extracted and analyzed rhamnolipdids. I.R., C.M., M.C., S.R. and D.T. performed the virulence test on G. mellonella model. O.M. contributed to technical assistance. M.C., S.R. and N.C. wrote the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-019-56666-7.
References
- 1.Ramsey DM, Wozniak DJ. Understanding the control of Pseudomonas aeruginosa alginate synthesis and the prospects for management of chronic infections in cystic fibrosis. Mol. Microbiol. 2005;56:309–322. doi: 10.1111/j.1365-2958.2005.04552.x. [DOI] [PubMed] [Google Scholar]
- 2.Chevalier S, et al. Extracytoplasmic function sigma factors in Pseudomonas aeruginosa. Biochim. Biophys. Acta - Gene Regul. Mech. 2019;1862:706–721. doi: 10.1016/j.bbagrm.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Rodrigue A, Quentin Y, Lazdunski A, Méjean V, Foglino M. Two-component systems in Pseudomonas aeruginosa: why so many? Trends Microbiol. 2000;8:498–504. doi: 10.1016/S0966-842X(00)01833-3. [DOI] [PubMed] [Google Scholar]
- 4.Lesouhaitier O, et al. Gram-negative bacterial sensors for eukaryotic signal molecules. Sensors (Basel) 2009;9:6967–6990. doi: 10.3390/s90906967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freestone PPE, Sandrini SM, Haigh RD, Lyte M. Microbial endocrinology: how stress influences susceptibility to infection. Trends Microbiol. 2008;16:55–64. doi: 10.1016/j.tim.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Crum R, Borrow B, Shackford S, Hansbrough J, Brown MR. The neurohumoral response to burn injury in patient resuscitated with hypertonic saline. J. Trauma. 1988;28:1181–1187. doi: 10.1097/00005373-198808000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Udupa KN. The neurohumoral response to trauma. Am. J. Surg. 1978;135:403–405. doi: 10.1016/0002-9610(78)90074-0. [DOI] [PubMed] [Google Scholar]
- 8.Lyte M, Ernst S. Catecholamine induced growth of gram negative bacteria. Life Sci. 1992;50:203–212. doi: 10.1016/0024-3205(92)90273-R. [DOI] [PubMed] [Google Scholar]
- 9.Halang P, et al. Response of Vibrio cholerae to the catecholamine hormones. J. Bacteriol. 2015;197:3769–3778. doi: 10.1128/JB.00345-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doherty NC, Tobias A, Watson S, Atherton JC. The effect of the human gut-signalling hormone, norepinephrine, on the growth of the gastric pathogen Helicobacter pylori. Helicobacter. 2009;14:223–230. doi: 10.1111/j.1523-5378.2009.00682.x. [DOI] [PubMed] [Google Scholar]
- 11.Freestone PPE, Haigh RD, Lyte M. Blockade of catecholamine-induced growth by adrenergic and dopaminergic receptor antagonists in Escherichia coli O157:H7, Salmonella enterica and Yersinia enterocolitica. BMC Microbiol. 2007;7:1–13. doi: 10.1186/1471-2180-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang Q, Anh NDQ, Bossier P, Defoirdt T. Norepinephrine and dopamine increase motility, biofilm formation, and virulence of Vibrio harveyi. Front. Microbiol. 2014;5:1–12. doi: 10.3389/fmicb.2014.00584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bansal T, et al. Differential effects of epinephrine, norepinephrine, and indole on Escherichia coli O157:H7 chemotaxis, colonization, and gene expression. Infect. Immun. 2007;75:4597–4607. doi: 10.1128/IAI.00630-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hegde M, Wood TK, Jayaraman A. The neuroendocrine hormone norepinephrine increases Pseudomonas aeruginosa PA14 virulence through the las quorum-sensing pathway. Appl. Microbiol. Biotechnol. 2009;84:763–776. doi: 10.1007/s00253-009-2045-1. [DOI] [PubMed] [Google Scholar]
- 15.Li W, et al. Norepinephrine represses the expression of toxA and the siderophore genes in Pseudomonas aeruginosa. FEMS Microbiol. Lett. 2009;71:3831–3840. doi: 10.1111/j.1574-6968.2009.01739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bearson BL, Bearson SMD. The role of the QseC quorum-sensing sensor kinase in colonization and norepinephrine-enhanced motility of Salmonella enterica serovar Typhimurium. Microb. Pathog. 2008;44:271–278. doi: 10.1016/j.micpath.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Pande GSJ, Suong NT, Bossier P, Defoirdt T. The catecholamine stress hormones norepinephrine and dopamine increase the virulence of pathogenic Vibrio anguillarum and Vibrio campbellii. FEMS Microbiol. Ecol. 2014;90:761–769. doi: 10.1111/1574-6941.12432. [DOI] [PubMed] [Google Scholar]
- 18.Intarak N, Muangsombut V, Stevens MP, Korbsrisate S, Vattanaviboon P. Growth, motility and resistance to oxidative stress of the melioidosis pathogen Burkholderia pseudomallei are enhanced by epinephrine. Pathog. Dis. 2014;72:24–31. doi: 10.1111/2049-632X.12181. [DOI] [PubMed] [Google Scholar]
- 19.Torabi Delshad S, Soltanian S, Sharifiyazdi H, Bossier P. Effect of catecholamine stress hormones (dopamine and norepinephrine) on growth, swimming motility, biofilm formation and virulence factors of Yersinia ruckeri in vitro and an in vivo evaluation in rainbow trout. J. Fish Dis. 2019;42:477–487. doi: 10.1111/jfd.12934. [DOI] [PubMed] [Google Scholar]
- 20.Burrows LL. Pseudomonas aeruginosa twitching motility: type IV pili in action. Annu. Rev. Microbiol. 2012;66:493–520. doi: 10.1146/annurev-micro-092611-150055. [DOI] [PubMed] [Google Scholar]
- 21.Talà L, Fineberg A, Kukura P, Persat A. Pseudomonas aeruginosa orchestrates twitching motility by sequential control of type IV pili movements. Nat. Microbiol. 2019;4:774–780. doi: 10.1038/s41564-019-0378-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li G, et al. Adaptation of Pseudomonas aeruginosa to phage PaP1 predation via O-antigen polymerase mutation. Front. Microbiol. 2018;9:1–10. doi: 10.3389/fmicb.2018.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakano M, et al. Catecholamine-induced stimulation of growth in Vibrio species. Lett. Appl. Microbiol. 2007;44:649–653. doi: 10.1111/j.1472-765X.2007.02136.x. [DOI] [PubMed] [Google Scholar]
- 24.Freestone PP, Haigh RD, Williams PH, Lyte M. Stimulation of bacterial growth by heat-stable, norepinephrine-induced autoinducers. FEMS Microbiol. Lett. 1999;172:53–60. doi: 10.1111/j.1574-6968.1999.tb13449.x. [DOI] [PubMed] [Google Scholar]
- 25.Williams PH, et al. Stress and the periodontal diseases: effects of catecholamines on the growth of periodontal bacteria in vitro. Oral Microbiol. Immunol. 2003;17:296–303. doi: 10.1034/j.1399-302x.2002.170506.x. [DOI] [PubMed] [Google Scholar]
- 26.Li L, et al. Catecholamines promote Actinobacillus pleuropneumoniae growth by regulating iron metabolism. PLoS One. 2015;10:e0121887. doi: 10.1371/journal.pone.0121887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao J, et al. The stress hormone norepinephrine increases the growth and virulence of Aeromonas hydrophila. Microbiologyopen. 2019;8:e00664. doi: 10.1002/mbo3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freestone PP, et al. Pseudomonas aeruginosa -catecholamine inotrope interactions: a contributory factor in the development of ventilator-associated pneumonia? Chest. 2012;142:1200–1210. doi: 10.1378/chest.11-2614. [DOI] [PubMed] [Google Scholar]
- 29.Biaggini K, et al. The pathogenic potential of Pseudomonas fluorescens MFN1032 on enterocytes can be modulated by serotonin, substance P and epinephrine. Arch. Microbiol. 2015;3:983–990. doi: 10.1007/s00203-015-1135-y. [DOI] [PubMed] [Google Scholar]
- 30.Bradley DE. Basic characterization of a Pseudomonas aeruginosa pilus-dependent bacteriophage with a long noncontractile tail. J. Virol. 1973;12:1139–1148. doi: 10.1128/jvi.12.5.1139-1148.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCutcheon JG, Peters DL, Dennis JJ. Identification and characterization of type IV Pili as the cellular receptor of broad host range Stenotrophomonas maltophilia bacteriophages DLP1 and DLP2. Viruses. 2018;10:E338. doi: 10.3390/v10060338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lyte M, et al. Stimulation of Staphylococcus epidermidis growth and biofilm formation by catecholamine inotropes. Lancet. 2003;361:130–135. doi: 10.1016/S0140-6736(03)12231-3. [DOI] [PubMed] [Google Scholar]
- 33.Parsek MR, Singh PK. Bacterial biofilms: an emerging link to disease pathogenesis. Annu. Rev. Microbiol. 2003;57:677–701. doi: 10.1146/annurev.micro.57.030502.090720. [DOI] [PubMed] [Google Scholar]
- 34.Hassett DJ, et al. Pseudomonas aeruginosa biofilm infections in cystic fibrosis: insights into pathogenic processes and treatment strategies. Expert Opin. Ther. Targets. 2010;14:117–130. doi: 10.1517/14728220903454988. [DOI] [PubMed] [Google Scholar]
- 35.Bjarnsholt T. The role of bacterial biofilms in chronic infections. APMIS. 2013;121:1–58. doi: 10.1111/apm.12099. [DOI] [PubMed] [Google Scholar]
- 36.Sandrini S, Alghofaili F, Freestone P, Yesilkaya H. Host stress hormone norepinephrine stimulates pneumococcal growth, biofilm formation and virulence gene expression. BMC Microbiol. 2014;14:1–12. doi: 10.1186/1471-2180-14-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klausen M, et al. Biofilm formation by Pseudomonas aeruginosa wild type, flagella and type IV pili mutants. Mol. Microbiol. 2003;48:1511–1524. doi: 10.1046/j.1365-2958.2003.03525.x. [DOI] [PubMed] [Google Scholar]
- 38.O’Toole GA, Kolter R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 1998;30:295–304. doi: 10.1046/j.1365-2958.1998.01062.x. [DOI] [PubMed] [Google Scholar]
- 39.Jenkins ATA, Buckling A, McGhee M, ffrench-Constant RH. Surface plasmon resonance shows that type IV pili are important in surface attachment by Pseudomonas aeruginosa. J. R. Soc. Interface. 2005;2:255–259. doi: 10.1098/rsif.2005.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Craig L, Pique ME, Tainer JA. Type IV pilus structure and bacterial pathogenicity. Nat. Rev. Microbiol. 2004;2:363–378. doi: 10.1038/nrmicro885. [DOI] [PubMed] [Google Scholar]
- 41.Andrejko M, Zdybicka-Barabas A, Cytryńska M. Diverse effects of Galleria mellonella infection with entomopathogenic and clinical strains of Pseudomonas aeruginosa. J. Invertebr. Pathol. 2014;115:14–25. doi: 10.1016/j.jip.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 42.Miyata S, Casey M, Frank DW, Ausubel FM, Drenkard E. Use of the Galleria mellonella caterpillar as a model host to study the role of the type III secretion system in Pseudomonas aeruginosa pathogenesis. Infect. Immun. 2003;71:2404–2413. doi: 10.1128/IAI.71.5.2404-2413.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu C, Sperandio V, Boedeker EC, Hughes DT, Clarke MB. The QseC sensor kinase: a bacterial adrenergic receptor. Proc. Natl. Acad. Sci. 2006;103:10420–10425. doi: 10.1073/pnas.0604343103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rasko DA, et al. Targeting QseC signaling and virulence for antibiotic development. Science. 2008;321:1078–1080. doi: 10.1126/science.1160354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reading NC, et al. A novel two-component signaling system that activates transcription of an enterohemorrhagic Escherichia coli effector involved in remodeling of host actin. J. Bacteriol. 2007;189:2468–2476. doi: 10.1128/JB.01848-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karavolos MH, Winzer K, Williams P, Khan CMA. Pathogen espionage: multiple bacterial adrenergic sensors eavesdrop on host communication systems. Mol. Microbiol. 2013;87:455–465. doi: 10.1111/mmi.12110. [DOI] [PubMed] [Google Scholar]
- 47.Hancock RE, Carey AM. Outer membrane of Pseudomonas aeruginosa: heat- 2-mercaptoethanol-modifiable proteins. J. Bacteriol. 1979;140:902–910. doi: 10.1128/jb.140.3.902-910.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheng HP, Walker GC. Succinoglycan is required for initiation and elongation of infection threads during nodulation of alfalfa by Rhizobium meliloti. J. Bacteriol. 1998;180:5183–5191. doi: 10.1128/jb.180.19.5183-5191.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Turnbull L, Whitchurch CB. Motility assay: twitching motility. Methods Mol. Biol. 2014;1149:73–86. doi: 10.1007/978-1-4939-0473-0_9. [DOI] [PubMed] [Google Scholar]
- 50.Ha D-G, Kuchma SL, O’Toole GA. Plate-based assay for swarming motility in Pseudomonas aeruginosa. Methods Mol. Biol. 2014;1149:67–72. doi: 10.1007/978-1-4939-0473-0_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kropinski AM, Mazzocco A, Waddell TE, Lingohr E, Johnson RP. Enumeration of bacteriophages by double agar overlay plaque assay. Methods. Mol. Biol. 2009;501:69–76. doi: 10.1007/978-1-60327-164-6_7. [DOI] [PubMed] [Google Scholar]
- 52.Pamp SJ, Sternberg C, Tolker-Nielsen T. Insight into the microbial multicellular lifestyle via flow-cell technology and confocal microscopy. Cytom. Part A. 2009;75A:90–103. doi: 10.1002/cyto.a.20685. [DOI] [PubMed] [Google Scholar]
- 53.Tolker-Nielsen T, Sternberg C. Growing and analyzing biofilms in flow chambers. Curr. Protoc. Microbiol. 2011;21:1B.2.1–1B.2.17. doi: 10.1002/9780471729259.mc01b02s21. [DOI] [PubMed] [Google Scholar]
- 54.Heydorn A, et al. Quantification of biofilm structures by the novel computer program comstat. Microbiology. 2015;146:2395–2407. doi: 10.1099/00221287-146-10-2395. [DOI] [PubMed] [Google Scholar]
- 55.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baccouri O, et al. Probiotic potential and safety evaluation of Enterococcus faecalis OB14 and OB15, isolated from traditional tunisian testouri cheese and rigouta, using physiological and genomic analysis. Front. Microbiol. 2019;10:881. doi: 10.3389/fmicb.2019.00881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koch G, Nadal-Jimenez P, Cool RH, Quax WJ. Assessing Pseudomonas virulence with nonmammalian host: Galleria mellonella. Methods Mol. Biol. 2014;1149:681–688. doi: 10.1007/978-1-4939-0473-0_52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.