Abstract
Cold atmospheric plasma (CAP) is known as the versatile tool in different biological, and medical applications. In this study, we investigated the effect of cold plasma on diabetes via in vitro and in vivo assessments. We performed the in vitro assay to evaluate the impact of CAP on glycated glutathione peroxidase (GPx) through enzyme activity measurement as a function index and far- and near-UV circular dichroism (CD) and fluorescence analysis as structure indices. The result of in vitro assessment showed that the exposure of glycated GPx to plasma causes a considerable increase in enzyme activity up to 30%. Also, the evaluation of far- and near-UV CD and fluorescence analysis indicated a modification in the protein structure. According to obtained result from in vitro assessment, in vivo assay evaluated the effect of CAP on diabetic mice through analyzing of blood glucose level (BGL), advanced glycation end products (AGEs), antioxidant activity, oxidative stress biomarkers such as malondialdehyde (MDA), advanced oxidation protein products (AOPP), and oxidized low-density lipoprotein (oxLDL), and inflammation factors including tumor necrosis factor (TNF-α), interleukin-1 (IL-1), and interleukin-6 (IL-6). The result of in vivo experiment also showed a 20% increase in antioxidant activity. Also, the reduction in AGEs, oxidative stress biomarkers, and inflammatory cytokines concentrations was observed. The result of this study revealed that CAP could be useful in diabetes treatment and can be utilized as a complementary method for diabetes therapy.
Subject terms: Applied physics, Plasma physics
Introduction
Plasma is a gas-like system, and considered as the fourth state of the material1. It is a partially or wholly ionized gas, which contains neutrals, free electrons, positively and negatively ions, free radicals, active species, molecules and atoms with or without excitation, and UV photons2. It can be classified into equilibrium- and non-equilibrium plasma. Also, it may categorize as high-temperature and low-temperature plasma termed as thermal and non-thermal plasma. In the non-thermal plasma, the species are in nonthermal equilibrium. Despite the existence of high-temperature electrons, the overall temperature of plasma, neutrals, ions, and radicals remain constant and close to room temperature; therefore, they are known as cold plasmas, with hindered macro heating of material that they contact with3. So, this feature makes the cold plasma suitable for many applications.
Lately, the application of none-thermal atmospheric-pressure plasma or cold atmospheric plasma (CAP) has attracted enormous interest in the medicine, and biology which lead to opening a new approach as the plasma medicine field4. Plasma medicine concentrates on increasing the collection of reactive oxygen (ROS) and nitrogen species (RNS), charged particles, UV radiation, and neutral species to cure various pathological conditions. So, reactive oxygen and nitrogen species (RONS) generated by plasmas can play the central roles5. Indeed, they can react with cells, tissues, and biological fluids containing proteins, lipids, and carbohydrates6. CAP has been widely studied for different applications of plasma medicine, such as various types of cancers, skin diseases, inflammatory disorders, infectious tissues, and production activated liquids7.
Diabetes mellitus is a class of chronic metabolic disorders that distinguished by hyperglycemia that is arising from insufficiency of insulin secretion or lack of response to insulin8. The control of diabetes, along with induction of oxidative stress, inflammation, and glycation of macromolecules is the leading health challenge and its pervasiveness is increasing in all the world, especially in the developing countries. Currently, more than 400 million people all over the world are suffering from diabetes and this number is expected to reach up to 550 million by 20309. Chronic hyperglycemia by elevating oxidative stress, along with inflammation, may cause to macrovascular and microvascular complications10.
Oxidative stress, which has been considered to be a general pathogenic factor of diabetic complications, is related to state which the production of oxidants increased and overcame the endogenous antioxidant capacity, secondary to a loss of the balance between them11. Free radical species as oxidants are unstable and highly reactive oxidizing agents because of the existence of single electrons in the outer layer of their orbitals12. Free radicals can attack the cellular structure through direct binding with macromolecules such as amino acids, lipids, and nucleic acids which cause oxidative damage to the cell or leading to cell death13. Among various types of free radicals, oxygen free radicals are the most common in aerobic organisms, which often known as ROS14. The cellular redox balance can be examined by the analysis of both antioxidant and oxidant biomarkers15. ROS have a very short lifetime (10−6–10−9 seconds), so it is not easy to detect their presence16. Among the oxidant biomarkers, hydrogen peroxide (H2O2) as a ROS molecule can be measured in body fluids due to their long half-life14. So oxidative stress can be evaluated by measuring the final products of ROS as biomarkers of oxidative stress, including oxidized products of lipid, protein, and low-density lipoprotein (LDL) such as the malondialdehyde (MDA), advanced oxidation protein products (AOPP), and oxidized low-density lipoprotein (oxLDL), respectively17.
Hyperglycemia not only leads to excessive free radicals but also reduces antioxidant activity through glycation and conformation change of antioxidant enzymes18. Under normal physiological conditions, the cellular redox balance depends on several antioxidant systems13. Glutathione peroxidase (GPx), as a protein, is an essential intracellular antioxidant enzyme decomposing H2O2 into the water, mainly in mitochondria and sometimes in the cytosol19. So, glycation of GPx denatures its structure and consequently, decreases its enzymatic activity, reduces H2O2 decomposition, which leads to an accumulation of hydrogen peroxide, and ultimately increase oxidative stress. In general view, the elevation of blood glucose level (BGL) results in long-term modification of lipids, proteins, and nucleic acids, which causees forming advanced glycation end-products (AGEs)20. AGEs are the final products of a sequence of chemical reactions which are triggered due to the bonding of the carbonyl group in the carbohydrate like glucose with the free amino group in a biomolecule such as protein, without enzyme intervention14,21. The formation of AGEs, and oxidative stress are intertwined; the increasing AGEs may lead to oxidative stress, and vice versa; ROS may facilitate AGEs formation20.
Also Suggested that the induction of oxidative stress and AGEs, due to hyperglycemia, leads to inducing inflammatory cytokines like tumor necrosis factor (TNF-α), interleukin-1 (IL-1), and interleukin-6 (IL-6) that can investigate as the biomarkers of hyperglycemia22.
Several studies have been conducted on using the cold plasma in diabetes mellitus complications treatment, directly, such as wound healing in case of diabetic rats, and indirectly such as nano-sized clinoptilolite production by the cold plasma, and its effect on reducing hyperglycemia in rats2,5,23. Also, in recently promising study has been reported after diabetic wound treatment by the cold plasma, the levels of superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase (CAT) elevated, and the result of CAP on wound healing revealed the plasma role in upregulating cytokines secretion (as an important part of inflammatory system) such as IL-1, and IL-65,24. In literature, yet there is no exhaustive and cumulative investigation into the effect of CAP on oxidative stress, AGEs, and irregular inflammatory system.
So, in this study, both in vitro and in vivo assessments helped us to evaluate the effect of CAP, as a noninvasive and safe method, on hyperglycemia, glycated protein, oxidative stress, and inflammatory system. For in vitro assay, the glycated GPx exposed to argon plasma jet, and function and structure of the enzyme analyzed. Also, the effect of the cold plasma on diabetic mice was investigated through an in vivo assessment with analyzing antioxidant activity, oxidant parameters, inflammation factors, and BGL.
Results
In vitro
OES
The emission spectrum of argon plasma jets in the air medium has been shown in Fig. 1. The spectrum line of argon plasma jet indicates the existence of hydroxyl radical, nitrogen, argon, and oxygen species.
Figure 1.
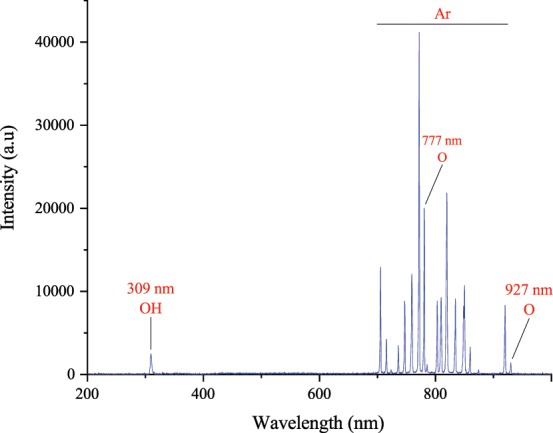
The emission spectrum of Ar plasma in air.
Effect of the cold plasma on enzyme activity
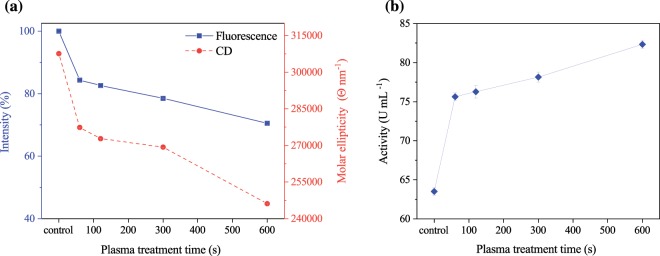
The activity of the enzyme (as a protein) is affected by protein conformational changes such as glycation. So, for studying the impact of CAP on protein conformation, enzyme activity can be analyzed. Figure 2 shows the effect of the argon plasma jet treatment on GPx activity for different plasma exposure times. As inferred from Fig. 2, enzyme activity is a function of the cold plasma treatment time. Such that, after 600 seconds of treatment, enzyme activity increased from 63.51 (for 100% glycated GPx) to 82.33 U mL−1.
Figure 2.
(a) Fluorimetry intensity, dichroism spectrum, and (b) activity of plasma treated glycated GPx for different plasma treatment times (60–600 seconds) (p < 0.001). Error bars stand for standard deviation.
Fluorimetry analyze
The effect of CAP on glycated GPx structure was investigated by fluorimetry analyze (Fig. 2). According to results, the improvement of protein denaturation, which is a reverse process of enzyme glycation has been occurred through receiving plasma treatment. Figure 2 shows that with the enhancement of plasma exposure time from 60 to 600 seconds, the naturation of GPx increased so that the 600-second imposing argon plasma jet to the solution leads to about 30% naturation.
Circular Dichroism (CD) analyze
The ellipticity measurement is the most popular method used for monitoring protein structural changes due to destabilizing agents such as glycation. The results of molar ellipticity analysis show a significant change after the cold plasma treatment for different plasma exposure times (Fig. 2); such that, by increasing treatment time, the molar ellipticity decrease, and after 600 seconds of treatment, its amount reduced from 307617 to 246139 Θ nm−1.
In vivo
Effect of CAP on BGL, AGEs, antioxidant activity, and H2O2 concentration
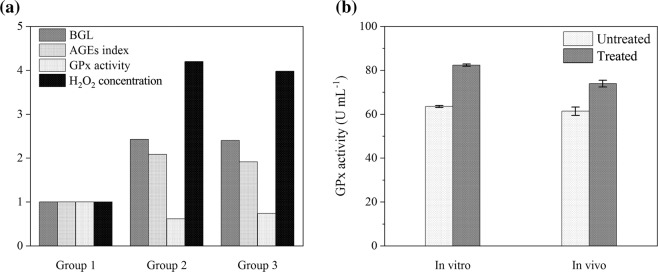
An increase in BGL leads to producing AGEs due to direct exposure of lipid or protein to sugar. Moreover rising glucose level results in enzyme glycation, a decrease in antioxidant activity, and oxidants accumulation. Table 1 illustrates the mean values and standard divisions of BGL, AGEs index, GPx activity, and H2O2 concentration for normal mice (group 1), diabetic mice (group 2), and CAP treated diabetic mice (group 3). Figure 2 also shows the mentioned indices in each group, normalized to that in group 1. As indicated in Table 1 and Fig. 3, BGL of plasma treated diabetic mice is lower than diabetic mice, and its amount is reduced from 391.5 to 388.1 mg dL−1 for 600 seconds applying argon plasma. Also, by comparing the AGEs index of Group 2, and 3, it is clear that the plasma has a positive effect on the reduction of this index. Moreover, the obtained data revealed that CAP is effective in antioxidant activity enhancement. Such that the plasma treatment leads to increasing GPx enzyme activity, and decreasing H2O2 concentration. On average, a 20% increase in enzyme activity was observed at 600 seconds plasma exposure time that this result was in accordance with in vitro ones (Fig. 3).
Table 1.
Mean values and standard deviations of BGL, AGEs index, GPx activity, and H2O2 concentration (p < 0.001) for normal mice (group 1), diabetic mice (group 2), and CAP treated diabetic mice (group 3).
| Variable | Group 1 | Group 2 | Group 3 | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| BGL (mg dL−1) | 161.40 | 3.85 | 391.53 | 23.19 | 388.13 | 19.10 |
| AGEs (mL−1) | 3.89 | 0.22 | 8.11 | 0.21 | 7.46 | 0.16 |
| GPx activity (U mL−1) | 130.37 | 2.28 | 79.97466 | 1.90 | 96.38 | 1.58 |
| H2O2 (mmol L−1) | 30.64 | 2.33 | 128.67 | 3.81 | 121.96 | 2.48 |
Figure 3.
(a) BGL, AGEs index, GPx activity, and H2O2 concentration for each group (group1: normal mice, group 2: diabetic mice, group 3: plasma treated diabetic mice) normalized to that in group 1 (p < 0.001). (b) Accordance of in vitro and in vivo assessments.
Effect of CAP on oxidative stress
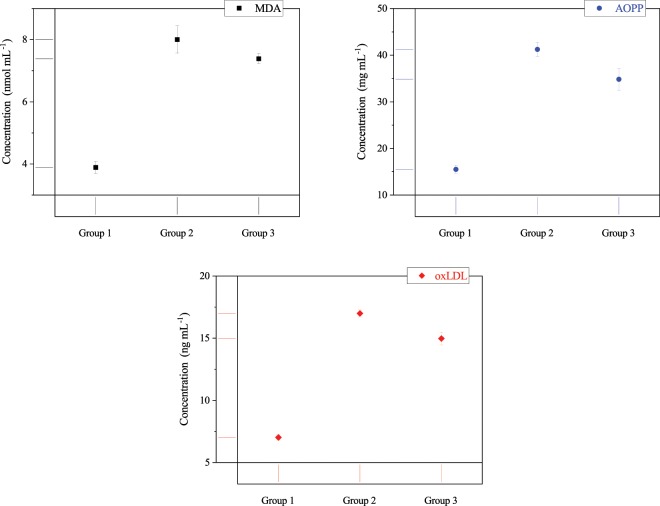
MDA, AOPP, and oxLDL indices, as the oxidant biomarkers of oxidative stress, are shown in Fig. 4. It indicates that the MDA concentration is reduced by more than 0.6 nmol mL−1 after using the cold plasma. Also, it is notable that the cold plasma has decreased AOPP concentration by more than 15%. Furthermore, the impact of CAP treatment on oxLDL can be distinguished as a reduction of 2.0 ng mL−1 in group 3. Overall, these results indicate that the cold plasma treatment plays an important role in the reduction of oxidative stress and related oxidant biomarkers.
Figure 4.
MDA, AOPP, and oxLDL concentrations (p < 0.001) as biomarkers of oxidative stress (group 1: normal mice, group 2: diabetic mice, group 3: plasma treated diabetic mice). Error bars stand for standard deviation.
Effect of CAP on inflammation factors
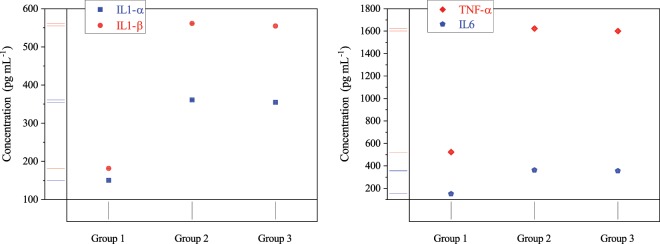
CAP impact on IL-1 (α, and β), TNF-α, and IL-6 concentrations were studied (Fig. 5). The result shows that the inflammatory factors of plasma treated group are closer to the normal ones (group one) relative to untreated mice and its reduction amounts for all indices was about 1%. These results confirm the cold plasma influence on the reduction of inflammation.
Figure 5.
Inflammation factors concentrations (p < 0.001) for normal mice (group 1), diabetic mice (group 2), and plasma treated mice (group 3). Error bars stand for standard deviation.
The overall effect of the cold plasma treatment on diabetic mice
The ANOVA test is a general analyze for studying and comparing mean vectors of the three groups. So, for the exact determination of pairwise differences for each variable based on the ANOVA test, post-hoc tests can be considered (Tables 2–4). Obtained results from Tukey-HSD test with 95% family-wise confidence level for BGL, AGEs index, GPx activity, and H2O2 concentration has been shown in Table 2, oxidant biomarkers concentrations (oxidative stress) has been illustrated in Table 3, and inflammation factors indices has been presented in Table 4. According to the confidence interval resulting from Tukey’s post-test, the possibility of comparing variable mean between two groups has been provided that this is a criterion to discuss about significant differences in three groups. For all factors, the means difference between group one and two (1–2: group 1 minus group 2) are negative (except to GPx) due to enhancing variable after STZ injection. So, the diabetes-induced process was carried out successfully. Also, for groups two and three, all mean difference (3–2) is negative except to GPx, as a variable was descended after CAP treatment. Therefore, this method is effective for complications arising from diabetes. By comparing the means difference (1–2), and (1–3) together, the same results can be obtained.
Table 3.
Obtained result from Tukey-HSD test for oxidative stress biomarkers concentrations with 95% family wise confidence level (p < 0.001).
| Variable | Groups | Difference | 95% family-wise confidence level | |
|---|---|---|---|---|
| Lower bound | Upper bound | |||
| MDA | 3–2 | −0.62 | −0.88 | −0.36 |
| 1–2 | −4.12 | −4.37 | −3.86 | |
| 1–3 | −3.50 | −3.76 | −3.24 | |
| AOPP | 3–2 | −6.44 | −7.95 | −4.92 |
| 1–2 | −25.76 | −27.27 | −24.24 | |
| 1–3 | −19.32 | −20.83 | −17.81 | |
| oxLDL | 3–2 | −2.02 | −2.34 | −1.70 |
| 1–2 | −9.97 | −10.28 | −9.65 | |
| 1–3 | −7.95 | −8.27 | −7.63 | |
Table 2.
Obtained result from Tukey-HSD test for BGL, AGEs index, GPx activity, and H2O2 concentration with 95% family wise confidence level (p < 0.001).
| Variable | Groups | Difference | 95% family-wise confidence level | |
|---|---|---|---|---|
| Lower bound | Upper bound | |||
| BGL | 3–2 | −3.40 | −18.91 | 12.11 |
| 1–2 | −230.13 | −245.64 | −214.62 | |
| 1–3 | −226.73 | −242.25 | −211.22 | |
| AGEs | 3–2 | −0.66 | −0.83 | −0.48 |
| 1–2 | −4.22 | −4.40 | −4.05 | |
| 1–3 | −3.57 | −3.74 | −3.39 | |
| GPx activity | 3–2 | 16.41 | 14.69 | 18.13 |
| 1–2 | 50.39 | 48.67 | 52.11 | |
| 1–3 | 33.98 | 32.26 | 35.70 | |
| H2O2 | 3–2 | −6.71 | −9.33 | −4.09 |
| 1–2 | −98.03 | −100.65 | −95.41 | |
| 1–3 | −91.32 | −93.94 | −88.70 | |
Table 4.
Obtained result from Tukey-HSD test for inflammations factors indices with 95% family wise confidence level (p < 0.001).
| Variable | Groups | Difference | 95% family-wise confidence level | |
|---|---|---|---|---|
| Lower bound | Upper bound | |||
| IL-1α | 3–2 | −6.23 | −8.64 | −3.83 |
| 1–2 | −210.53 | −212.93 | −208.12 | |
| 1–3 | −204.29 | −206.70 | −201.89 | |
| IL-1β | 3–2 | −6.96 | −9.63 | −4.29 |
| 1–2 | −380.01 | −382.68 | −377.37 | |
| 1–3 | −373.05 | −375.72 | −370.38 | |
| TNF-α | 3–2 | −21.63 | −24.98 | −18.27 |
| 1–2 | −1100.38 | −1103.73 | −1097.03 | |
| 1–3 | −1078.75 | −1082.10 | −1075.40 | |
| IL-6 | 3–2 | −7.12 | −9.47 | −4.77 |
| 1–2 | −210.73 | −213.079 | −208.39 | |
| 1–3 | −203.61 | −205.96 | −201.27 | |
Discussion
In this study, the effect of CAP on glycated GPx as in vitro and diabetic mice as in vivo is investigated. To the best of our knowledge, we pioneered to introduce a basic nonclinical research for evaluating CAP impact either on glycated protein or diabetes.
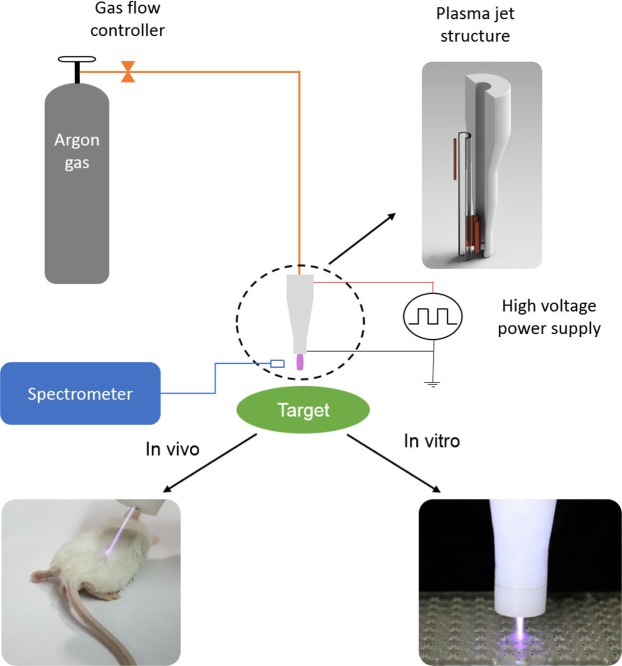
As illustrated in Fig. 6, the argon plasma jet is containing both hydroxyl, and singlet oxygen radicals. RONS has two-edged sword nature in biology, as it could be either beneficial or harmful to biological systems depending on plasma properties (such as plasma source type, mode of energy dissipation into the system, feeding gas, plasma plum, and types and concentrations of reactive species), type of organism, and exposure time3,6. However, in the present study, the physiological characteristic of plasma is used as a positive induction to modify denatured protein structure and enzymatic activity. The results of the in vitro assessment showed that plasma treatment led to a modification of protein conformation and enzymatic activity improvement (Fig. 2). It is clear that these factors strongly correlated; such that the improvement of protein conformation directly affects the enzyme activity. Also, these effects have been augmented over CAP treatment time.
Figure 6.
The schematic of in vitro and in vivo assessments.
Many studies have been focused on the interaction of plasma with various proteins. Attri et al. have shown that CAP could lead to a modification in protein oxidation, dimerization, nitration, and dehydrogenation of amino acids25. On the other hand, Takaei et al. reported that nitration, hydroxylation, sulfonation, amidation, and disulfide linkage formation in amino acids could occur during the cold plasma treatment26. For example, Choi et al. concluded that the reduction in the fluorescence intensity of the plasma-treated lysozyme was due to the modification of the Trp group or Trp surroundings27. Altogether, quenching of intrinsic fluorescence and CD spectra intensity of denatured GPx after exposure to CAP could be the result of modifications of amino acids and variations in the secondary and tertiary structures in the vicinity of the modified amino acids. Such that these modifications shifted the denatured GPx toward its native structure and subsequently enzymatic activity enhanced.
Also, in vivo result is consistent with data obtained from in vitro assay, which this accordance is notable (Fig. 3). The slight difference observed in enzymatic activity between in vitro and in vivo assessments could be due to the direct treatment of glycated GPx in exposure to plasma plum and short-lived reactive species. In the gas phase, plasma generates different types of ions and radicals that can interact with an aqueous solution to produce biodiverse reactive species with short/long lifetime in a liquid phase. Some of the short-lived RONS such as hydroxyl (OH·), superoxide (), and singlet oxygen (O·) have been measured in an aqueous solution28. These species decay to produce long-lived species such as ozone (O3), hydrogen peroxide (H2O2), nitrite (), and nitrate ions ()28. So, in direct treatment, the glycated GPx is affected by either short-lived species or long one, whereas glycated GPx in the bloodstream could receive only the effect of the long-lived species.
Reduction of H2O2 concentration in the blood of affected mice shows an increase in GPx activity, which implies a modification in protein structure, due to the fact that activity and structure of a protein are correlated to each other. As a result of this phenomenon, the oxidative stress reduced and inclined the body to the balanced state between oxidants and antioxidants. The decrease in the oxidative stress led to the reduction in the oxidation of proteins, lipids, and lipoproteins. Therefore, the oxidative stress biomarkers, including MDA, AOPP, and oxLDL diminished after plasma treatment.
Also, It is widely proved that low-density lipoprotein (LDL) particles are extremely susceptible to oxidative damage29. In other words, ROS could attack to LDLs and oxidize the lipids and protein components of LDL particles30. oxLDL plays a vital role in the initiation, development, and progression of atherosclerosis, and causes endothelial inflammation, and endothelial dysfunction31,32. Uptake of oxLDL by lectin-like oxLDL receptor-1 (LOX1), the receptor on endothelial cells, and macrophages via the scavenger receptors leads to the promotion of foam cell formation and increases the release of inflammatory cytokines including IL-1, IL-6, and TNF-α30,31,33,34. So, in our work, the decrease in oxLDL due to the reducing of either oxidant or oxidative stress can be related to the declined expression of inflammatory cytokines such as IL-1, IL-6, and TNF-α.
It is well known that the presence of RONS, as the essential cellular second messengers, are necessary for the physiological performance of insulin but, it turns out to that RONS is also associated with resistant of body cells to insulin35. That is (to say), they can have a physiological or pathological effect on insulin functions6. It seems the extension of RONS along with induction of oxidative stress alters the structure of the protein that acts as insulin signaling molecules or otherwise affects insulin signaling pathways in a harmful way35. Therefore, the decrease in oxidative stress could reduce the resistance of cell and body to insulin and improve insulin performance. One of the results in the following of these phenomena could be the reduction in the BGL.
On the other hand, rising BGL content leads to non-enzymatic glycation process or Millard reaction involves unstable Schiff’s bases formation through the attachment of carbonyl group of reducing sugar (such as glucose) to free amino group in proteins. As a result, rearrangement to stable ketoamine products, or Amadori compounds occur36. These products can undergo further reactions, such as cyclization, and condensation, to form AGEs37. It is well known that reduction of BGL diminishes non-enzymatic glycation process of protein or lipid as a result of exposure to glucose. These phenomena result in decreasing of AGEs concentration of the plasma-treated diabetic mice.
Moreover, the effect of the cold plasma light also should be considered. The mitochondria rotary motor that is known as ATP synthase is the most efficient molecular motor38. It consists of two parts: a largely hydrophilic and a hydrophobic part39. So, a local change in nanoscopic interfacial water viscosity alters the performance and the dynamics of the nanomotor system38.
Mitochondria produces both ROS and ATP compounds that ROS can affect the intramitochondrial space. These species can improve hydrophilicity and thus the viscosity of the interfacial water layers bound to exposed surfaces of intramitochondrial. So oxidative stress situation leads to an increase in interfacial viscosity, along with an increase in viscous friction, which contributes to the drop in ATP production related to performance reduction of the rotary motor38.
It has been shown that the nanoscopic interfacial water layers structure can be modified by different wavelengths of laser lights such as 633 and 670 nm38,40,41. The modification involves expansion of the volume and reduction of viscosity, particularly on hydrophilic surfaces, that are known as promoting high viscosities42,43. As illustrated in Fig. 1, CAP spectrum includes different emissions; in ultraviolet, infrared, and visible regions. These emissions might be effective in modulation of interfacial water, promotion of mitochondrial ATP synthase and procession of glucose metabolism. So, the reduction in BGL can be the result of emitted light-spectra by CAP. This reduction might contribute directly to reduced GPx glycation (enhancement of antioxidant activity) and AGEs index, and might have an indirect outcome in the favorable change of MDA, AOPP, oxLDL, and inflammation factors indices.
Metformin is primarily utilized for Type 2 diabetes treatment as well as oral anti-diabetes agent44. Although, it has considerable benefits, including low risk of hyperglycemia, weight neutrality, cost-effective, and possible cardiovascular advantages, up to 25% of patients treated with metformin suffer metformin-associated gastrointestinal (GI) side-effects, and 5% are unable to tolerate metformin due to the severity of these side effects45.
As a conclusion, the result of this study revealed that the CAP could be effective in the modification of glycated GPx (as denatured protein) and improvement of its enzymatic activity. Also, the obtained data showed that the cold plasma treatment has a positive effect on the reduction of oxidative stress, AGEs, and inflammatory cytokines concentrations in diabetic mice. So, the cold plasma treatment, as a novel and complementary method, can be utilized with metformin for diabetes therapy to lower metformin dosage and reduce metformin’s side effects.
Materials and Methods
Materials
Atmospheric Pressure Plasma Jet (APPJ) device and instrumentation
As illustrated schematically in Fig. 6, the APPJ consisted of a dielectric, powered, and ground electrodes, and a high voltage power supply. A Pyrex tube (L: 150 mm, ID: 4 mm, OD: 6 mm) utilized, as the dielectric barrier and the nozzle. A copper rod (L: 30 mm, D: 1 mm) and a thin copper cylindrical tube (L: 15 mm) used as the powered and ground electrodes, respectively. The powered electrode was inserted in the tube from one end, while the other tube end was surrounded by the ground electrode, such that the distance between the nozzle tip and the lower edge of the ground electrode was 5 mm. The plasma was generated by a 10 kHz pulsed DC power supply with an amplitude up to 10.0 kV. Also, argon gas with a purity of 99.999%, and 3 l min−1 flow rate used as feeding gas.
Reagents and filter
GPx (9013-66-5), phosphate buffered saline (PBS) (P5368), and glucose (G7021) were obtained from Sigma-Aldrich Co (USA). 0.22 µm filter was supplied from Millipore Corporation, Billerica, MA (USA).
Assay kits
Activity assay kit of GPx (D-89075) was purchased from Biocore Diagnosik Ulm GmbH Co (Germany). Quantity assay kits of IL-1β (MBS175967), AGEs (MBS704846), AOPP (MBS263319), MDA (MBS264973), and oxLDL (MBS2512757) were acquired from MyBioSource Co (USA). Quantity assay kits of IL-1α (BMS611), IL-6 (LMC0061), and TNF-α (BMC607-3) were obtained from Termo Fisher Chem Co (USA). Also, quantity assay kits of H2O2 (E-BC-K102) and glucose (81692) were supplied from Elabscience and Crystal Chem companies (USA), respectively. Streptozotocin (STZ) (S0130) and nicotinamide (NA) (N0636) were purchased from Sigma-Aldrich Co (USA).
Methods
In vitro
Optical Emission Spectroscopy (OES)
CAP applications mostly are based on the generation capability of sufficient amounts of various reactive species. So, optical emission spectroscopy (OES) was used to analyze the existence, and intensity of any species. For this purpose, an ocean optic HR 2000 spectrometer was employed. The spectral range was chosen from 200 to 11000 nm with an optical resolution of 0.5 nm. Plasma optical emissions recorded at 20 mm distance from plasma stream. According to the atomic spectra database lines, the recorded spectrum was analyzed and different species were determined.
Preparation of glycated GPx solution
A pure solution of GPx (10 mg mL−1) was prepared by combining the protein with phosphate buffered saline (PBS) at pH 7.4, and a 50 mM l−1 glucose solution was made by mixing pure glucose with PBS. An aliquot of the prepared GPx solution was combined with glucose solution and named glycated GPx. Then, pure GPx and glycated GPx samples were filtered under the sterilized condition and incubated for 16 weeks at 37 °C46,47. At the end of 16th week, Glycated GPx was treated by cold plasma, and an aliquot of each of the three solutions (pure GPx, glycated GPx, and treated glycated GPx) was taken, and kept at −80 °C until could be analyzed by three methods: Circular Dichroism (CD), fluorometry, and activity assay48.
CAP treatment
CAP treatment was performed by direct exposing the prepared Glycated GPx to the plasma jet stream (Fig. 6). Two mL of the glycated enzyme solutions loaded in a 2 mL 96-well plate, and the the cold plasma applied at the 15 mm distance from the the solution surface with four different durations (60, 120, 300, and 600 seconds). Also, the untreated enzyme solution considered as the control sample.
Function analyze
Measurement of GPx Function was performed by related activity assay kit, enzymatic colorimetric method, and Tecan’s Sunrise absorbance microplate reader (Switzerland). The enzyme activity was measured as U mL−1.
Fluorescence spectroscopy
Each sample with a concentration of 0.5 mg mL−1 was analyzed by Shimadzu spectrofluorometer RF-5000 (Japan, Kyoto). Wavelengths of 350, and 440 nm were considered as excitation, and emission wavelengths, respectively. The results have been presented as percentage46,49.
CD analyze
A CD Spectropolarimeter (JASCO-810, Jasco, Tokyo, Japan) was applied to study the structure of the samples with a concentration of 0.1 mg mL−1. The spectra were modulated and obtained, as units of mean residue molar ellipticity [Θ] (mdeg cm2 dmol−1), based on the average weight of the amino acids (112.4). The equation [Θ] λ = (Θ× 112.4)× cl−1 indicated the molar ellipticity (calculations were performed at 25 °C)50,51.
In vivo
Subjects
Sixty male BALB/c type mice aged 6 weeks with an average body weight of 30 g (bought from Pasteur Institute of Iran) were housed in a vivarium under controlled condition (a temperature of 23 ± 3 °C, and a relative humidity of 50 ± 10%) with a 12:12 h light-dark cycle, and had free access to rat chow and water ad libitum. After one week of acclimatization to these conditions, forty-five mice which had showed favorable growth were selected and randomly allotted into three groups (n = 15): (1) Nondiabetic control (Normal ones without any interference), (2) Diabetic control (Diabetic ones involved by STZ), (3) Affected diabetics (Diabetic ones involved by STZ, and affected by cold plasma). Diabetes was induced in mice (groups 2, and 3) by intraperitoneal (i.p.) injection of a single dose of STZ (50 mg kg−1) that after 15 min followed up by administration of nicotinamide (NA) (120 mg kg−1). One week after injection, serum glucose ≥200 g L−1 (checked by glucose assay kit (Crystal Chem Co, USA)) was considered as diabetes52. The animal ethics review committee (the Biological Research Institute of Cognitive, and Brain Sciences, Shahid Beheshti University) approved the study protocol under the international guidelines for the care and use of laboratory animals53.
CAP treatment
In vivo assay involved treatment of 15 diabetic mice (group 3) by APPJ. According to the information obtained from the analysis of in vitro processes, each mouse treated for 600 seconds. The back skin of the mice was chosen for treatment (Fig. 6).
Sampling
One week after exposure to plasma, blood samples of mice were obtained from the vein of their orbits. Related serum samples of each group were prepared to detect the biochemical parameters. Recent preparation was accomplished during 15 min centrifugation of blood at 5000 × g, clot separation, and storage at 70 °C for further studies.
Oxidants analyze
Oxidant parameters, including AGEs, AOPP, MDA, and oxLDL were measured by enzyme-linked immunosorbent assay (ELISA) techniques according to their assay kits and ELISA reader apparatus (MR-96A, Mindary Co) (Germany). H2O2 was detected by the colorimetric method, quantity assay kit, and Tecan’s Sunrise absorbance microplate reader (Switzerland).
Antioxidant analyze
The function of GPx was determined according to the method described in in vitro section.
Inflammatory factors analyze
Inflammatory parameters, including IL-1α, IL-1β, IL-6, and TNF-α, were assayed by ELISA method, detection kits, and ELISA reader apparatus (MR-96A, Mindary Co, Germany).
Glucose analyze
The enzymatic colorimetric method, related kit, and Tecan’s Sunrise absorbance microplate reader (Switzerland) were used to determine serum glucose of mice.
Data processing
Statistical analyze
All data are expressed as the mean value of 45 mice blood analyze results. In this study, 11 variables were investigated for each of the groups. For this purpose, statistical analysis were performed through the ANOVA test with 0.001 significant level (p < 0.001) by the dplry package of R software (R-3.5.2 version). Also, based on the ANOVA test, the posthoc test (Tukey-HSD test) was applied for pairwise comparison of the three groups means.
Author contributions
Alireza Rezaeinezhad was responsible for preparation of the main body of manuscripts, figures, conducting experiments, collecting data, and interpretation of data and also contributed to the conception, and design of the work. Pegah Eslami assisted in experiments. Hossein mirmiranpour contributed to the conception, and design of the work, preparation of the main body of manuscripts, and performing the analysis and also, supervised the project. Hamid Ghomi contributed to the conception and design of the work and also, supervised the project. All authors discussed the results and contributed to the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wende K, et al. Atmospheric pressure plasma jet treatment evokes transient oxidative stress in hacat keratinocytes and influences cell physiology. Cell biology international. 2014;38:412–425. doi: 10.1002/cbin.10200. [DOI] [PubMed] [Google Scholar]
- 2.Fathollah F, et al. Investigation on the effects of the atmospheric pressure plasma on wound healing in diabetic rats. Scientific reports. 2016;6:19144. doi: 10.1038/srep19144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farasat M, Arjmand S, Ranaei Siadat SO, Sefidbakht Y, Ghomi H. The effect of non-thermal atmospheric plasma on the production and activity of recombinant phytase enzyme. Scientific reports. 2018;8:16647. doi: 10.1038/s41598-018-34239-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nie Lea. Effect of tissue thickness and liquid composition on the penetration of long-lifetime reactive oxygen and nitrogen species (rons) generated by a plasma jet. Journal of Physics D: Applied Physics. 2018;51:345204. doi: 10.1088/1361-6463/aad427. [DOI] [Google Scholar]
- 5.Cheng K-Y, et al. Wound healing in streptozotocin-induced diabetic rats using atmospheric-pressure argon plasma jet. Scientific reports. 2018;8:12214. doi: 10.1038/s41598-018-30597-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graves DB. The emerging role of reactive oxygen and nitrogen species in redox biology and some implications for plasma applications to medicine and biology. Journal of Physics D: Applied Physics. 2012;45:263001. doi: 10.1088/0022-3727/45/26/263001/meta. [DOI] [Google Scholar]
- 7.Weltmann K-D, von Woedtke T. Plasma medicine–current state of research and medical application. Plasma Physics and Controlled Fusion. 2016;59:14031. doi: 10.1088/0741-3335/59/1/014031/meta. [DOI] [Google Scholar]
- 8.Selim F, Wael A, KeithE J. Diabetes-induced reactive oxygen species: Mechanism of their generation and role in renal injury. Journal of Diabetes Research. 2017;2017:1–30. doi: 10.1155/2017/8379327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuan T, et al. New insights into oxidative stress and inflammation during diabetes mellitus-accelerated atherosclerosis. Redox Biology. 2019;20:247–260. doi: 10.1016/j.redox.2018.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maleki Vahid, Jafari-Vayghan Hamed, Saleh-Ghadimi Sevda, Adibian Mahsa, Kheirouri Sorayya, Alizadeh Mohammad. Effects of Royal jelly on metabolic variables in diabetes mellitus: A systematic review. Complementary Therapies in Medicine. 2019;43:20–27. doi: 10.1016/j.ctim.2018.12.022. [DOI] [PubMed] [Google Scholar]
- 11.Neerja A, Swati H. Oxidative stress in diabetes mellitus patients: A study of malondialdehyde (mda) and ischemia modified albumin (ima) as indicators of oxidative stress. Global Journal for Research Analysis. 2018;7:29–31. [Google Scholar]
- 12.Yaribeygi H, Butler AE, Barreto GE, Sahebkar A. Antioxidative potential of antidiabetic agents: A possible protective mechanism against vascular complications in diabetic patients. Journal of Cellular Physiology. 2019;234:2436–2446. doi: 10.1002/jcp.27278. [DOI] [PubMed] [Google Scholar]
- 13.Bhowmick D, Srivastava S, D’Silva P, Mugesh G. Highly efficient glutathione peroxidase and peroxiredoxin mimetics protect mammalian cells against oxidative damage. Angewandte Chemie International Edition. 2015;54:8449–8453. doi: 10.1002/anie.201502430. [DOI] [PubMed] [Google Scholar]
- 14.Meng Q, Chen F, Xiao T, Zhang L. Inhibitory effects of polysaccharide from diaphragma juglandis fructus on α-amylase and α-d-glucosidase activity, streptozotocin-induced hyperglycemia model, advanced glycation end-products formation, and h2o2-induced oxidative damage. International Journal of Biological Macromolecules. 2019;124:1080–1089. doi: 10.1016/j.ijbiomac.2018. [DOI] [PubMed] [Google Scholar]
- 15.Marrocco Ilaria, Altieri Fabio, Peluso Ilaria. Measurement and Clinical Significance of Biomarkers of Oxidative Stress in Humans. Oxidative Medicine and Cellular Longevity. 2017;2017:1–32. doi: 10.1155/2017/6501046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trivedi S, et al. Evaluation of antioxidant enzymes activity and malondialdehyde levels in patients with chronic periodontitis and diabetes mellitus. Journal of Periodontology. 2014;85:713–720. doi: 10.1902/jop.2013.130066. [DOI] [PubMed] [Google Scholar]
- 17.Rubio CP, et al. Biomarkers of oxidative stress in saliva of sheep: Analytical performance and changes after an experimentally induced stress. Research in Veterinary Science. 2018;123:71–76. doi: 10.1016/j.rvsc.2018.12.015. [DOI] [PubMed] [Google Scholar]
- 18.Palsamy P, Subramanian SP. Resveratrol protects diabetic kidney by attenuating hyperglycemia-mediated oxidative stress and renal inflammatory cytokines via nrf2–keap1 signaling. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2011;1812:719–731. doi: 10.1016/j.bbadis.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 19.Ighodaro OM, Akinloye OA. First line defence antioxidants-superoxide dismutase (sod), catalase (cat) and glutathione peroxidase (gpx): Their fundamental role in the entire antioxidant defence grid. Diabetes Care. 2018;54:287–293. doi: 10.1016/j.ajme.2017.09.001. [DOI] [Google Scholar]
- 20.Koska J, et al. Advanced glycation end products, oxidation products, and incident cardiovascular events in patients with type 2 diabetes. Diabetes Care. 2018;41:570–576. doi: 10.2337/dc17-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al-Malki AL. Shikimic acid from artemisia absinthium inhibits protein glycation in diabetic rats. International Journal of Biological Macromolecules. 2018;122:1212–1216. doi: 10.1016/j.ijbiomac.2018.09.072. [DOI] [PubMed] [Google Scholar]
- 22.Elmarakby AA, Sullivan JC. Relationship between oxidative stress and inflammatory cytokines in diabetic nephropathy. Cardiovascular Therapeutics. 2012;30:49–59. doi: 10.1111/j.1755-5922.2010.00218.x. [DOI] [PubMed] [Google Scholar]
- 23.Hossein Nia B, Khorram S, Rezazadeh H, Safaiyan A, Tarighat-Esfanjani A. The effects of natural clinoptilolite and nano-sized clinoptilolite supplementation on glucose levels and oxidative stress in rats with type 1 diabetes. Canadian Journal of Diabetes. 2018;42:31–35. doi: 10.1016/j.jcjd.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 24.Barton Annemarie, Wende Kristian, Bundscherer Lena, Hasse Sybille, Schmidt Anke, Bekeschus Sander, Weltmann Klaus-Dieter, Lindequist Ulrike, Masur Kai. Nonthermal Plasma Increases Expression of Wound Healing Related Genes in a Keratinocyte Cell Line. Plasma Medicine. 2013;3(1-2):125–136. doi: 10.1615/PlasmaMed.2014008540. [DOI] [Google Scholar]
- 25.Attri P, et al. Influence of reactive species on the modification of biomolecules generated from the soft plasma. Scientific reports. 2015;5:8221. doi: 10.1038/srep08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takai E, et al. Chemical modification of amino acids by atmospheric-pressure cold plasma in aqueous solution. Journal of Physics D: Applied Physics. 2014;47:285403. doi: 10.1088/0022-3727/47/28/285403. [DOI] [Google Scholar]
- 27.Choi S, et al. Structural and functional analysis of lysozyme after treatment with dielectric barrier discharge plasma and atmospheric pressure plasma jet. Scientific reports. 2017;7:1027. doi: 10.1038/s41598-017-01030-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szili EJ, Hong S-H, Oh J-S, Gaur N, Short RD. Tracking the penetration of plasma reactive species in tissue models. Trends in biotechnology. 2018;36:594–602. doi: 10.1016/j.tibtech.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 29.Banerjee J, Mishra N, Damle G, Dhas Y. Beyond ldl-c: The importance of serum oxidized ldl in predicting risk for type 2 diabetes in the middle-aged asian indians. Diabetes and Metabolic Syndrome: Clinical Research and Reviews. 2019;13:206–213. doi: 10.1016/j.dsx.2018.08.036. [DOI] [PubMed] [Google Scholar]
- 30.Omar NN, Hefnawy MHEL, Soda MFEL, Heider NM, Hamed HI. Assessment of oxldl, anti-oxldl antibodies and lipoprotein-associated phospholipase a2 as cardiovascular risk markers in obese adolescents with and without t1dm. Bulletin of Faculty of Pharmacy, Cairo University. 2017;55:325–33155. doi: 10.1016/j.bfopcu.2017.05.002. [DOI] [Google Scholar]
- 31.Furukawa S, et al. Malondialdehyde-modified ldl-related variables are associated with diabetic kidney disease in type 2 diabetes. Diabetes Research and Clinical Practice. 2018;141:237–243. doi: 10.1016/j.diabres.2018.05.019. [DOI] [PubMed] [Google Scholar]
- 32.Xie L, Lin H, Wang C. Elevation of serum oxldl/β2-gpi complexes was correlated with diabetic microvascular complications in type 2 diabetes mellitus patients. Journal of Clinical Laboratory Analysis. 2019;33:e22676. doi: 10.1002/jcla.22676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hofmann A, Brunssen C, Morawietz H. Contribution of lectin-like oxidized low-density lipoprotein receptor-1 and lox-1 modulating compounds to vascular diseases. Vascular Pharmacology. 2017;107:1–11. doi: 10.1016/j.vph.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 34.Mortazavi-Jahromi, S. S., Alizadeh, S., Javanbakht, M. H. & Mirshafiey, A. Cardioprotective effect of β-d-mannuronic acid (m2000) as a novel nsaid on gene expression of oxldl scavenger receptors in the experimental diabetic model. Immunopharmacology and Immunotoxicology40. [DOI] [PubMed]
- 35.Bashan N, Kovsan J, Kachko I, Ovadia H, Rudich A. Positive and negative regulation of insulin signaling by reactive oxygen and nitrogen species. Physiological reviews. 2009;89:27–71. doi: 10.1152/physrev.00014.2008. [DOI] [PubMed] [Google Scholar]
- 36.Wu X, Monnier VM. Enzymatic deglycation of proteins. Archives of biochemistry and biophysics. 2003;419:16–24. doi: 10.1016/j.abb.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 37.Lin Z, Zheng J. Occurrence, characteristics, and applications of fructosyl amine oxidases (amadoriases) Applied microbiology and biotechnology. 2010;86:1613–1619. doi: 10.1007/s00253-010-2523-5. [DOI] [PubMed] [Google Scholar]
- 38.Sommer AP, Haddad MK, Fecht H-J. Light effect on water viscosity: Implication for atp biosynthesis. Scientific reports. 2015;5:12029. doi: 10.1038/srep12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hutcheon ML, Duncan TM, Ngai H, Cross RL. Energy-driven subunit rotation at the interface between subunit a and the c oligomer in the fo sector of escherichia coli atp synthase. Proceedings of the National Academy of Sciences. 2001;98:8519–8524. doi: 10.1073/pnas.151236798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sommer AP, Caron A, Fecht H-J. Tuning nanoscopic water layers on hydrophobic and hydrophilic surfaces with laser light. Langmuir. 2008;24:635–636. doi: 10.1021/la7032737. [DOI] [PubMed] [Google Scholar]
- 41.Sommer AP, Zhu D, Horst-Dieter F, Scharnweber T, Welle A. Crystalline water at room temperature− under water and in air. Crystal Growth and Design. 2008;8:2620–2622. doi: 10.1021/cg800382x. [DOI] [Google Scholar]
- 42.Sommer AP, et al. Breathing volume into interfacial water with laser light. The Journal of Physical Chemistry Letters. 2011;2:562–565. doi: 10.1021/jz2001503. [DOI] [Google Scholar]
- 43.Goertz MP, Houston JE, Zhu X-Y. Hydrophilicity and the viscosity of interfacial water. Langmuir. 2007;23:5491–5497. doi: 10.1021/la062299q. [DOI] [PubMed] [Google Scholar]
- 44.Dujic T, et al. Organic cation transporter 1 variants and gastrointestinal side effects of metformin in patients with type 2 diabetes. Diabetic Medicine. 2016;33:511–514. doi: 10.1111/dme.13040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCreight LJ, Bailey CJ, Pearson ER. Metformin and the gastrointestinal tract. Diabetologia. 2016;59:426–435. doi: 10.1007/s00125-015-3844-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gopalkrishnapillai B, Nadanathangam V, Karmakar N, Anand S, Misra A. Evaluation of autofluorescent property of hemoglobin-advanced glycation end product as a long-term glycemic index of diabetes. Diabetes. 2003;52:1041–1046. doi: 10.2337/diabetes.52.4.1041. [DOI] [PubMed] [Google Scholar]
- 47.Bathaie SZ, Nobakht BBF, Mirmiranpour H, Jafarnejad A, Moosavi-Nejad SZ. Effect of chemical chaperones on glucose-induced lysozyme modifications. The protein journal. 2011;30:480–489. doi: 10.1007/s10930-011-9353-x. [DOI] [PubMed] [Google Scholar]
- 48.Shaklai, N., Garlick, R. L. & Bunn, H. F. Nonenzymatic glycosylation of human serum albumin alters its conformation and function. Journal of Biological Chemistry6, 3812–3817 https://doi.org/ (1984). [PubMed]
- 49.Ogino T, Okada S. Oxidative damage of bovine serum albumin and other enzyme proteins by iron-chelate complexes. Biochimica et Biophysica Acta (BBA)-General Subjects. 1995;1245:359–365. doi: 10.1016/0304-4165(95)00101-8. [DOI] [PubMed] [Google Scholar]
- 50.Jafarnejad A, Bathaie SZ, Nakhjavani M, Hassan MZ, Banasadegh S. The improvement effect of l-lys as a chemical chaperone on stz-induced diabetic rats, protein structure and function. Diabetes/metabolism research and reviews. 2008;24:64–73. doi: 10.1002/dmrr.769. [DOI] [PubMed] [Google Scholar]
- 51.Jackson DY, King DS, Chmielewski J, Singh S, Schultz PG. General approach to the synthesis of short. alpha.-helical peptides. Journal of the American Chemical Society. 1991;113:9391–9392. doi: 10.1021/ja00024a067. [DOI] [Google Scholar]
- 52.Ahangarpour A, Shabani R, Farbood Y. The effect of betulinic acid on leptin, adiponectin, hepatic enzyme levels and lipid profiles in streptozotocin-nicotinamide-induced diabetic mice. Research in pharmaceutical sciences. 2018;13:142. doi: 10.4103/1735-5362.223796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Council, N. R. Guide for the care and use of laboratory animals. International series of monographs on physics. (National Academies Press, 2010).