Abstract
Helicobacter pylori (H. pylori) eradication using standard triple therapy (STT) with proton pump inhibitors (PPI), amoxicillin and clarithromycin (CLA) has been the standard in Latin America. However, CLA resistance is a rising problem affecting eradication rates. Genetic polymorphisms of CYP2C19, a PPI metabolizer may also affect eradication. The primary aims of this study were to evaluate the effect of clarithromycin resistance on H. pylori eradication in a population from Santiago, and to establish the pooled clarithromycin resistance in Santiago, Chile. Symptomatic adult patients attending a tertiary hospital in Santiago were recruited for this study. CLA resistance and the polymorphisms of CYP2C19 were determined on DNA extracted from gastric biopsies, using PCR. The STT was indicated for 14 days and eradication was determined by a urea breath test 4–6 weeks after therapy. A meta-analysis of CLA resistance studies among adult residents in Santiago was performed. Seventy-three out of 121 consecutive patients had positive rapid urease test (RUT) and received STT. Sixty-nine patients (95%) completed the study. The H. pylori eradication rate was 63% and the prevalence of CLA resistance was 26%. According to the CYP2C19 polymorphisms, 79.5% of the RUT-positive patients were extensive metabolizers. Multivariable analyses showed that only CLA resistance was significantly and inversely associated with failure of eradication (OR: 0.13; 95% confidence interval [95% CI], 0.04–0.49). A meta-analysis of two previous studies and our sample set (combined n = 194) yielded to a pooled prevalence of CLA resistance of 31.3% (95% CI 23.9–38.7). Our study shows that CLA resistance is associated with failure of H. pylori eradication. Given the high pooled prevalence of CLA resistance, consideration of CLA free therapies in Santiago is warranted. We could recommend bismuth quadruple therapy or high-dose dual therapy, according to bismuth availability. Further studies need to evaluate the best therapy.
Subject terms: Bacterial genes, Stomach diseases
Introduction
Helicobacter pylori (H. pylori) infection affects approximately 50% of the population worldwide. Prevalence in developing countries ranges between 70% to 90%1,2. In Chile, more than 70% of adults are infected by this bacterium3. H. pylori infection plays an important role in the development of duodenal ulcers, gastric cancer (GC) and mucosa associated lymphoid tissue lymphoma. The International Agency for Research on Cancer classified H. pylori as one of the primary risk factors for the development of noncardia GC4. In Chile, GC is the first cause of cancer death for men and the third in women5,6.
H. pylori eradication reduces the risk of development of the above mentioned clinical outcomes. Standard triple therapy (STT) with clarithromycin (CLA) and amoxicillin for 7 to 14 days is recommended as treatment for low CLA resistance regions with expected eradication success rates up to 85%7. The standard of care in Chile, as recommended by nationwide clinical guidelines is triple therapy with CLA8.
During the last decades, an increase in CLA resistance rates and a parallel decrease in eradication efficacy has been observed globally9. CLA is a bacteriostatic antibiotic that binds in a reversible manner to the peptidyl transferase located in dominion V of the 23S rRNA gene, inhibiting protein synthesis in H. pylori10. Single nucleotide mutations in A2142G and A2143G positions are the most common variations described11–13. A systematic review of Latin American studies by Camargo et al., reported a 12% pooled prevalence of resistance for CLA, 53% for metronidazole, 4% for amoxicillin, 6% for tetracycline, 15% for fluoroquinolones and 8% for dual CLA and metronidazole14. In two recently studies, Hooi JKY et al. and Savoldi A et al., analysed the information available worldwide describing an increasing antibiotic resistance in most regions. Resistance rates to CLA, metronidazole, and levofloxacin were ≥15%, which have a great effect on efficacy of CLA-containing regimens9,15,16.
In Chile, recent studies showed an increasing frequency of CLA resistance over 20%17–19. The Latin American, Toronto and Maastricht V consensus recommend not to use STT in regions with CLA resistance rates >15%. Alternatively, they recommend CLA-free regimes such as bismuth quadruple therapy (BQT)7,20–22. According to the meta-analysis by Fischbach et al., eradication success is 66% in the presence of CLA resistance23.
Proton pump inhibitors (PPIs) are used to maintain an alkaline pH and avoid antibiotic inactivation, particularly for CLA. In addition, elevated pH drives H. pylori into a replicative state contributing to increased antibiotic sensitivity24. The main isoenzyme involved in PPI metabolism is P4502C19 cytochrome (CYP2C19). The CYP2C19 gene is extensively polymorphic with 34 allelic variants composed of Single Nucleotide Polymorphisms (SNPs). The main allelic variants described are *2, *3 and *1725. CYP2C19*2 is an allele that is produced by substitution of a single base (rs4244285, 681G <A) in exon 5 causing a change into the alternative splicing site dramatically reducing drug metabolism. Defective allele CYP2C19*3 (rs4986893, 636G >A) produces a stop codon generating an abnormal form of the enzyme. Finally, variant *17 (rs12248560, −806C >T), increases enzymatic activity driving expression to an ultra-rapid metabolism25.
Several phenotypes have been identified for CYP2C19. The extensive or normal metabolizer (EM) characterized by wild type homozygous alleles *1/*1 or *1/*17 haplotype; the intermediate metabolizer (IM) carriers of *1/*2, *1/*3 and *2/*17 haplotypes; the poor metabolizer (PM) characterized by *2/*2, *2/*3, *3/*3 haplotypes and the ultra-fast metabolizer (UM) which is a *17 (*17/*17) homozygous carrier. Phenotypes EM and UM metabolize PPIs at a fast rate, so higher doses of these agents are required to achieve the same effectiveness that in IM and PM phenotypes26.
CYP2C19 polymorphisms show high population variability. Eradication rates vary according to the metabolizer phenotype. Higher eradication rates are achieved in PM (80%) in comparison to EM (60%)27,28. However, therapies that use PPIs such as rabeprazole and esomeprazole show less sensitivity CYP2C19 genetic polymorphisms29.
The primary aims of this study were: (i) to evaluate the effect of CLA resistance on H. pylori eradication success with STT based on Omeprazole-Amoxicillin-CLA for 14 days in a population from Santiago, (ii) to conduct a meta-analysis of the CLA resistance studies to calculate the pooled prevalence of CLA resistance in adult residents in Santiago, Chile. The secondary aims were: (i) to determine the effect of phenotypes of CYP2C19 polymorphisms on H. pylori eradication, (ii) to determine the H. pylori eradication rate using CLA-free quadruple therapy with Esomeprazole-Tetracycline-Metronidazole-Bismuth (ETMB) for 14 days as a second line treatment.
Methods
Study design
Prospective cohort study, we consecutively recruited symptomatic adult patients (18–75 years old), who had requested an endoscopy by their attending physician, between June 2017 and February 2018. Recruitment was performed in two endoscopic centers of the Healthcare network (Red de Salud UC-CHRISTUS) at the Pontificia Universidad Católica in Santiago. All patients signed an informed consent prior to upper gastrointestinal endoscopy. The project was approved by the Ethics Committee, School of Medicine, Pontificia Universidad Católica de Chile (project ID: 161205012) and was conducted according to the Helsinki declaration and Good Clinical Practices.
All patients completed a sociodemographic and clinical questionnaire. Exclusion criteria included individuals with partial gastrectomy for GC, GC treated by endoscopic resection, bariatric surgery, recent digestive bleeding, pregnancy or nursing period, PPI use for 7 days before endoscopy, antibiotic use 4 weeks before endoscopy, previous H. pylori eradication treatment and previous known intolerance or allergic reaction to the antibiotics used, and other malignancies.
H. pylori diagnosis and treatment
Rapid urease test (RUT) (Gastrex, Gilly les Citeaux, France) was performed during endoscopy to all patients. Positivity within 30 minutes resulted in an H. pylori diagnosis. RUT positive patients received STT consisting of 500 mg of CLA, 1 gr of amoxicillin and 20 mg of omeprazole every 12 hours for 14 days. RUT negative patients did not receive any antibiotic treatment and were excluded from the analysis. Follow-up for adverse effects was performed via telephone 7 days after treatment. Four to six weeks following the completion of the treatment. Urease breath test (UBT) (Heliforce, Beijing, China) was performed 4–6 weeks post therapy to determine eradication efficacy.
CLA resistance
Single nucleotide mutations in 23S rRNA gene (A2142G and A2143G) were determined to infer H. pylori resistance to CLA. DNA was extracted from four antral mucosal biospies obtained during endoscopy, using the QIAamp DNA mini kit (QIAGEN, Hilden, Germany) according to manufacturer instructions. PCR based amplification of 23S rRNA gene was performed using GoTaq Flexi DNA Polymerase (Promega, Madison, WI) in a PTC-100 Thermal Cycler (MJ Research, Waltham, MA). 23S-rRNA gene mutations associated with CLA resistance were determined as previously described24. Briefly, 10 μL of 23S-rRNA PCR products (267-bp) were digested with the restriction enzymes BbsI (5 U), or BsaI (5 U) (New England Biolabs, Ipswich, MA) in a final volume of 20 μL, according to the manufacturer’s instructions. Both 23S-rRNA amplicons and digested products were separated on 1.5% or 3% agarose gels respectively (SeaKem LE agarose; Lonza, Rockland, ME). DNA samples extracted from H. pylori strains (n = 2) with CLA point mutation A2142G and A2143G were used as internal positive controls.
Genetic polymorphisms of the CYP2C19 gene
Genotyping was performed from the DNA extracted from the above mentioned four gastric tissue samples using TaqMan assays from the Drug Metabolism Genotyping Assay (Thermofisher Scientific, USA) to detect *2 (c.681G >A; rs 4244285), *3 (c.636G >A; rs 4986893) and *17 (−806C >T; rs12248560) variants.
Second line treatment
In those patients who did not achieve H. pylori eradication with STT, ETMB was used as a second line treatment a quadruple therapy consisting in bismuth subsalicylate (262 mg every 6 hours), Tetracycline (500 mg every 6 hours), Metronidazole (500 mg every 6 hours) and Esomeprazole (20 mg every 6 hours) for 14 days. Successful H. pylori eradication was confirmed by UBT, 4–6 weeks post therapy.
Statistical analysis
For categorical variables, we compared relative frequencies using a Fisher’s exact test or chi-squared test. Continuous variables where analyzed using t-Test. Univariate logistic regression models were built to assess the association between H. pylori eradication and: CLA resistance, phenotypes of CYP2C19 polymorphisms, and demographic variables (age and gender). A multivariate logistic regression model was applied to test for the association of H. pylori eradication with CLA resistance and phenotypes of CYP2C19 polymorphisms, adjusting for demographic variables.
For all analyses, a two-sided statistical significance was considered at 5% level. All statistical analyses were performed in STATA 16 (StataCorp. LP, College Station, TX).
Sample size
The sample size was calculated considering the proportion of CLA resistance described in a previous similar study by Garrido L., et al.16. Thus, using this proportion (20%) with an alpha level = 0.05, and a precision of 0.1 (half width of confidence interval), the sample size required was 62 participants. Considering an eventual loss of 10%, we proposed to recruit 69 people. In addition, the number of events per variable rule was considered to calculate the sample size necessary to perform the multivariate logistic model30. Simulation studies31 have established that for logistic regression models to analyze dichotomous data, it is recommended to include one predictor variable per 10 events in the sample (i.e. H. pylori eradication success). Thus, in this current study, in order to include four variables in the multivariate logistic model (CLA resistance, phenotypes of CYP2C19 polymorphisms, age and gender), it was necessary to have 40 events. The final sample was comprised by 44 events (i.e. patients who eradicated H. pylori successfully).
Meta-analysis
Literature search and clinical eligibility criteria
Two reviewers independently searched in the following electronic databases: databases PubMed (United States National Library of Medicine, Bethesda, MD), Gastroenterología Latinoamericana Journal; http://www.gastrolat.org) and SciELO (Scientific Electronic Library Online; http://www.scielo.org), which conduct searches for systematic reviews in several other databases following PRISMA statement32. To identify studies in PubMed, the following search strategy was used: “Helicobacter pylori” [Mesh] AND “Clarithromycin” [Mesh] AND “Resistance” [Mesh] AND Chile.
The following information was abstracted from each selected article: first author, year of publication, study location (city), year of sample collection, participant age (range or mean), number of patients, indication for endoscopic examination, prevalence (% and confidence interval) of CLA resistance, and method of resistance assessment.
The following criteria were used to exclude publications: Children population; non-Chilean (i.e., other Latin American population), studies published before 2010, duplicated sample, populations from regions outside of Santiago, and review papers. There was no language restriction on publications. Discordance about study inclusion between the two reviewers was resolved through discussion until 100% agreement was reached on the final interpretation of the data.
Outcome measure
The included outcome in the analysis was prevalence (% and confidence interval) of CLA resistance.
Assessment of risk of bias in included studies
Risk of bias in the included studies was assessed by two independent reviewers using the Scale Newcastle Ottawa.
Data extraction and analysis
Data extraction and analysis was performed by two independent reviewers. To calculate a pooled prevalence of CLA resistance, we performed a random effects meta-analysis of proportions using the metaprop command developed by Nyaga et al.33 and the meta command incorporated in the STATA software version 16 (StataCorp. LP, College Station, TX). Between-study heterogeneity was measured by I2 which (i.e., percentage of the variability in effect estimates that it is due to heterogeneity rather than sampling error), and Cochran’s Q statistics (provides a method for testing the differences between three or more sets of proportions). Also, a sensitivity analysis was performed by exploring the variation in proportion estimates for different values of the between study heterogeneity I2 statistic.
Results
Sociodemographic, clinical and endoscopic variables
One hundred twenty-one patients were recruited for this study, mean age 47.2 ± 13.7 years, 60% were females and 60% (n = 73) were RUT positive. Tobacco use was reported by 29% of patients, and family history of GC by 12.3%.
Follow up and adverse effects
During the follow-up, four of the 73 RUT positive patients discontinued their participation in the study because did not accept to adhere to the treatment. Of the 69 remaining patients that initiated STT, only two interrupted treatment before 7 days because of intolerance to the treatment. We performed UBT regardless of that observation. In the telephonic interview, 80% of patients referred at least one adverse event during therapy. The main referred symptoms included altered taste (48%), followed by abdominal pain (40.6%), diarrhea (34.8%), nausea (20.3), and vomiting (5.8%). No serious adverse effects were reported.
CLA resistance prevalence
Nineteen (26%) of the 69 RUT positive patients were CLA resistant. The predominant mutation was A2143G with 17 (89.5%) patients, while A2142G was found in only 2 (10.5%) patients.
Polymorphism of the CYP2C19 gene
We obtained the following genotype frequencies: 64.4% of *1/*1, 11% of *1/*2, 4.1% of *2/*17: 16.4% of *1/*17 and 4.1% of *17/*17. We analyzed metabolizer phenotypes frequencies and found 79.5% (*1/*1, *1/*17) of EM, 16.4% of IM, 0% of PM (*2/*2, *3/*3, *2/*3) and 4.1% of UM (*17/*17).
Analysis of eradication rates
From the 69 treated RUT positive patients, 44 (63.8%) eradicated H. pylori. We analyzed the variables associated with H. pylori eradication, only CLA resistance was significant (p < 0.001). No clinical variables were associated (Table 1).
Table 1.
Demographic and clinical characteristics by H. pylori eradication groups (n = 69a).
| Variable | Eradication success n = 44 | Eradication failure n = 25 | p-value |
|---|---|---|---|
| Age, mean (±SD) | 46.4 (±14.0) | 49.2 (±14.1) | 0.429 |
| Gender female | 26 (59%) | 18 (72%) | 0.284 |
| University education | 31 (70.5%) | 20 (80%) | 0.385 |
| Alcohol consumption | 13 (29.5%) | 4 (16%) | 0.256 |
| Tobacco use | 11 (25%) | 4 (16%) | 0.290 |
| Pyrosis (+) | 14 (31.8%) | 10 (40%) | 0.493 |
| Dyspepsia (+) | 22 (50%) | 13 (52%) | 0.873 |
| Epigastralgia (+) | 17 (38.6%) | 11 (44%) | 0.663 |
| Esophagus findings | 6 (13.6%) | 6 (24%) | 0.275 |
| Gastric fundus findings: Congestion (+) | 14 (31.8%) | 9 (36%) | 0.723 |
| Gastric corpus findings: Congestion (+) | 20 (45.5%) | 10 (40%) | 0.660 |
| Antral findings: Congestion and/or nodular gastropathy (+)b | 18 (40.9%) | 13 (52%) | 0.373 |
| Duodenal findings: Erosions and ulcers (+) | 12 (27.3%) | 6 (24%) | 0.766 |
| CLA resistance | 5 (11.4%) | 13 (52.0%) | <0.001 |
| Phenotype EM | 34 (61.8%) | 21 (38.2%) | 0.235 |
| Phenotype UM | 1 (33.3%) | 2 (66.7%) | |
| Phenotype IM | 9 (81.8%) | 2 (18.2%) |
aDuring the follow-up four patients dropped their participation in the study.
bNodular gastropathy and congestion were pulled together for analysis. All nodular patients were H. pylori positive by rapid urease test.
In a multivariable analysis, CLA resistance was inversely and significantly associated with failure of eradication (OR 0.13; 95% CI, 0.04–0.49) while CYP2C19 phenotypes were not associated (Phenotype UM: OR 0.41, 95% CI 0.02–7.83; Phenotype IM: OR 1.65, 95% CI, 0.28–9.71) (Table 2).
Table 2.
Univariate and multivariable analyses associated to H. pylori eradication.
| Variable | Univariate OR (95% confidence interval) | Multivariable ORb (95% confidence interval) |
|---|---|---|
| CLA resistance | 0.12 (0.04–0.40) | 0.13 (0.04–0.49) |
| Phenotype EMa | 1.0 (Referent) | 1.0 (Referent) |
| Phenotype UMa | 0.31 (0.03–3.62) | 0.41 (0.02–7.83) |
| Phenotype IMa | 2.78 (0.55–14.13) | 1.65 (0.28–9.71) |
| Age | 0.99 (0.95–1.02) | 1.00 (0.96–1.05) |
| Gender female | 0.56 (0.19–1.62) | 0.49 (0.14–1.65) |
aPhenotypes of CYP2C19 polymorphisms.
bAge, sex, resistance to CLA, UM and EM phenotypes were included in the same model.
Second line quadruple therapy with bismuth
Twenty of the 25 patients (80%) who did not eradicate H. pylori by STT, received a second line of treatment. Seventeen (85%) patients eradicated H. pylori with ETMB. In the telephonic interview, 86% of patients referred at least one adverse event during therapy. Adverse effects reported were nausea (70%), abdominal pain (40%), vomiting (15%), muscular weakness (15%), diarrhea (10%), and taste alterations (10%). Only one case of tongue black pigmentation related to bismuth use was reported. No serious adverse effects were reported.
Meta-analysis
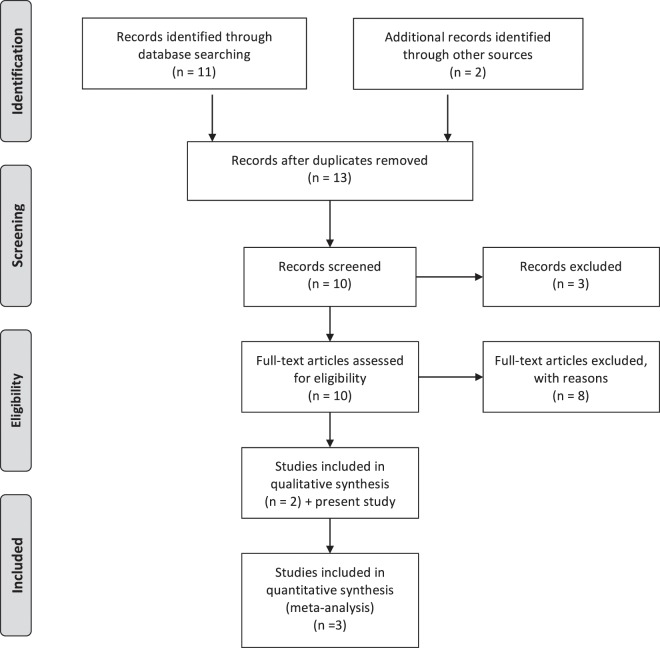
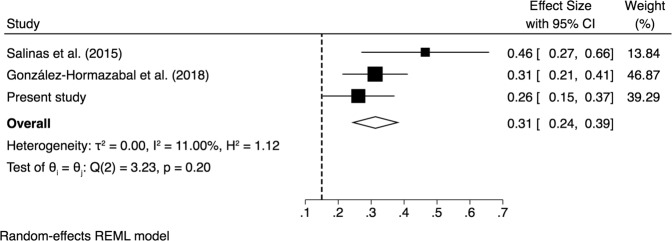
The literature searches identified a total of 11 studies and one meeting abstract17. Following exclusion criteria, nine studies were excluded14,16,19,34–40. The specific reasons were: Pediatric population19; Other Latin American population14,34,35; study published before 201016,37–39; reviews14,35,38; duplicated sample and population from regions outside Santiago36,37,39,40. Two studies met the inclusion criteria17,18 and were analyzed with our study, the combined sample size was 194 patients. PRISMA flow diagram is shown in Fig. 1. The characteristics of the studies are shown in Table 3. The pooled prevalence CLA resistance was 31.3% (95% CI 23.9–38.7) (Fig. 2). Heterogeneity was low (I2 = 11%; Q = Chi2 = 3.23; p = 0.20), and sensitivity analysis showed that there were minimal variations in proportion estimates for different values of the between study heterogeneity statistic I2. The overall risk of bias in each included study and the PRISMA checklist are shown in Supplementary Tables S1–S2.
Figure 1.
PRISMA flow diagram.
Table 3.
Sample characteristics and prevalence of CLA resistance reported in molecular studies among residents in Santiago, Chile.
| Study | Gender female | Median age | Indication for endoscopic examination | Sample size | Prevalence | 95% confidence interval |
|---|---|---|---|---|---|---|
| Salinas et al.17 | ___ | ___ | ___ | 28 | 0.46 | 0.27–0.66 |
| Gonzalez-Hormazabal et al.18 | 65% | 43 | Symptomatic | 93 | 0.31 | 0.21–0.41 |
| Present study | 60% | 47 | Symptomatic | 73 | 0.26 | 0.15–0.37 |
| Random effects pooled | 194 | 0.31 | 0.24–0.39 |
Figure 2.
Forest plot of the proportion of CLA resistance from studies conducted in adult populations, Santiago, Chile. Symbols: ■ single studies included in the meta – analysis; − confidence interval (CI); ◇ overall pool estimated; and a reference dashed vertical line was added at 0.15 (15%) to represent the value recommended by the consensus of Maastricht V/Florence on the management of H. pylori.
Discussion
In 2007, Graham et al.41 suggested that a first line treatment regime for H. pylori was acceptable if eradication rates were superior to 85%. In Latin America, a randomized multicentric eradication trial in 7 countries, including Chile (Santiago), was performed in 2009–2010 to compare STT with sequential therapy for 5 days and sequential therapy for 10 days. STT was the most successful with eradication rates over 85%42. In this study, we showed that a 14-day treatment with SST led to a lower eradication rate of 63.4%.
In our study, 26% of the RUT positive patients had 23S mutations compatible with CLA resistance. A2143G mutation was the more frequent, consistent with other international studies11–13. In addition, in our meta-analysis a pooled CLA resistance of 31.3% (95% CI 23.9–38.7) was observed, reaffirming a high resistance in Santiago city. On the other hand, Serrano et al.19 reported high CLA resistance (21%) in pediatric population in Santiago, but this article was excluded from the meta-analysis, because the pooled prevalence was restricted to adult populations. Our multivariable analysis showed that only CLA resistance was significantly and inversely associated with failure of eradication. Several authors have also described lower eradication rates in presence of CLA resistant strains7,20,43,44. All the available international guidelines recommend not to use CLA in eradication therapies due to suboptimal eradication rates if CLA resistance is >15%. However, this recommendation should not be generalized for the entire country, due to a heterogeneous CLA resistance reported in other cities of Chile. Supporting this statement, Otth et al.36 reported in Valdivia (South of Chile) only 9.1% CLA resistance in 2011. A low CLA resistance (6%) was also observed by our group in Curanilahue, a small town in the South of Chile (unpublished data). Nevertheless, a recent study from Temuco (South of Chile) showed a CLA resistance of 40% using agar dilution as antibiotic susceptibility method40, which could be related to greater exposure to antibiotics in recent years.
Based on our results, it is necessary to extend the surveillance to antibiotics to other urban and rural populations in our country. A limitation of this study is the low number of subjects, so to give greater consistency to our work, a meta-analysis was performed, but few studies managed to be included not being possible to assess the existence of publication bias. However, the results are consistent with an urban area constantly exposed to the use of antibiotics, which may not be the reality of other regions or rural areas in Chile.
In our study, an eradication rate of 85% was achieved with our second line treatment. Although this treatment showed high prevalence of adverse effects, none of them were severe. A recent meta-analysis by Muñoz N et al.45, showed that eradication therapy second line treatment achieves over 90% of success with BQT, representing a viable option in high CLA resistance regions like Santiago. As we had described previously, the international guidelines recommend CLA-free regimes such as quadruple therapy with bismuth as the first line, in regions with CLA resistance rates >15%. Other studies have reinforced this recommendation, Zagari RM, et al.46 in a retrospective multicentre observational study reported “three-in-one” formulation of BQT (capsule containing bismuth subcitrate, tetracycline, and metronidazole) is highly effective and well tolerated.
Nevertheless, in many countries bismuth is not available, so other studies have showed high efficacy with different eradication regimens without bismuth in areas of high CLA resistance. Federico A, et al.47 reported that a 5-day levofloxacin containing quadruple concomitant therapy is effective and safe. Molina-Infante J, et al.48 in a multicenter trial showed efficacy of empiric optimized 14-day non-bismuth quadruple therapies (hybrid and concomitant). Tai WC, et al.49 a randomized controlled study from Taiwan, found a 14-day esomeprazole and amoxicillin containing high-dose dual therapy achieves a high eradication rate as first-line, comparable to that with 7-day non-bismuth quadruple therapy. A recent systematic review and meta-analysis found similar eradication rates for high-dose dual therapy compared to for BQT50.
On the other hand, phenotypes derived from the CYP2C19 polymorphisms found in this study are similar from the data reported by Roco A et al. 2012 for healthy Chilean population51. Regarding other Latin American populations, Saldaña-Cruz AM et al.52, reported a proportion of EM of 83%, IM 16.5% and PM 0.2% in a Mexican population, estimates that are similar to other populations in the region, Brazil, Colombia and Bolivia.
In our study, phenotypes of CYP2C19 polymorphisms were not effect on H. pylori eradication. This result could be explained by the low number of participants, but also most of the studies that support the association between CYP2C19 polymorphisms and H. pylori eradication rates are based on Asian populations, with a greater proportion of poor metabolizers. Nevertheless, large population-based studies should be conducted to determine the clinical impact of CYP2C19 polymorphisms in H. pylori eradication in Chile and other high GC risk Latin-American populations.
In conclusion, although our study does not allow a definitive recommendation about first line therapy for H. pylori infection in Chile as a country, we found a significant high CLA resistance prevalence associated with H. pylori eradication failure in residents in Santiago. Based on these findings and guidelines, we suggest changing STT for CLA free therapies in the urban area of Santiago city. We could recommend BQT or high-dose dual therapy, according to bismuth availability. Further studies need to evaluate the best therapy.
Supplementary information
Acknowledgements
This project was financed by Residents' project grant PUC, ID 161205012 (A.A.), PREVECAN project (A.R.) and research grant from Abbott-Recalcine Laboratories (A.R.).
Author contributions
Participated in research design: Arenas A., Serrano C., and Riquelme A. Patients recruitment: Arenas A., Sepúlveda R., Maquilón S., Echeverría A., Ríos C., Jorquera A. Conducted experiments: Serrano C., Quiñones L., Sandoval M., Lavanderos M., Pizarro M. Performed data analysis: Fuentes-López E., Harris P., Rojas L. and Camargo M.C. Performed tables and figure: Fuentes-López E. and Arenas A. Wrote the manuscript: Arenas A., Serrano C., Riquelme A., Harris P., Quiñones L., Pizarro M. and Camargo M.C. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-019-56399-7.
References
- 1.Ortega JP, et al. Helicobacter pylori infection in symptomatic patients with benign gastroduodenal diseases: analysis of 5.664 cases. RevMedChil May. 2010;138(5):529–35. [PubMed] [Google Scholar]
- 2.McColl KE. Clinical practice. Helicobacter pylori infection. N Engl J Med. 2010;362(17):1597–604. doi: 10.1056/NEJMcp1001110. [DOI] [PubMed] [Google Scholar]
- 3.Ferreccio C, et al. Gastric cancer is related to early Helicobacter pylori infection in a high-prevalence country. CancerEpidemiolBiomarkersPrev. 2007;16:662–667. doi: 10.1158/1055-9965.EPI-06-0514. [DOI] [PubMed] [Google Scholar]
- 4.Schistosomes, liver flukes and Helicobacter pylori IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7–14 June 1994. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1–241. [PMC free article] [PubMed] [Google Scholar]
- 5.Bases de datos de mortalidad, 1985-2002. Ministerio de Salud de Chile. Departamento de Estadísticas. Santiago, Chile. http://www.deis.cl/bases-de-datos-defunciones (2002).
- 6.Indicadores básicos de salud en Chile. Ministerio de Salud de Chile. Departamento de Estadísticas e Información de la Salud. Santiago, Chile. http://www.deis.cl/wpcontent/uploads/2013/12/IBS (2013).
- 7.Malfertheiner P, et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. 2017;66:6–30. doi: 10.1136/gutjnl-2016-312288. [DOI] [PubMed] [Google Scholar]
- 8.Guía Clínica AUGE. Tratamiento de erradicación de Helicobacter pylori en el paciente con úlcera péptica. Ministerio de Salud de Chile. https://diprece.minsal.cl/wrdprss_minsal/wpcontent/uploads/2014/09/Helicobacter-Pylori-en-paciente-con-%C3%BAlcera-p%C3%A9ptica.pdf (2013).
- 9.Hooi JKY, et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology. 2017;153(2):420–429. doi: 10.1053/j.gastro.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 10.Vester B, Douthwaite S. Macrolide resistance conferred by base substitutions in 23S rRNA. AntimicrobAgentsChemother. 2001;45(1):1–12. doi: 10.1128/AAC.45.1.1-12.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xuan SH, Wu LP, Zhou YG, Xiao MB. Detection of clarithromycin-resistant Helicobacter pylori in clinical specimens by molecular methods: A review. Journal of Global AntimicrobialResistance. Taibah University. 2016;4:35–41. doi: 10.1016/j.jgar.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Abadi AT, Taghvaei T, Ghasemzadeh A, Mobarez AM. High frequency of A2143G mutation in clarithromycin-resistant Helicobacter pyloriisolates recovered from dyspeptic patients in Iran. Saudi J Gastroenterol. 2011;17:396–399. doi: 10.4103/1319-3767.87181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen, J. et al. Application of next-generation sequencing to characterize novel mutations in clarithromycin-susceptible Helicobacter pylori strains with A2143G of 23S rRNA gene. Ann Clin Microbiol Antimicrob. 22;17(1):10 (2018). [DOI] [PMC free article] [PubMed]
- 14.Camargo MC, et al. The problem of Helicobacter pylori resistance to antibiotics: a systematic review in Latin America. Am J Gastroenterol. 2014;109(4):485–95. doi: 10.1038/ajg.2014.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Savoldi A, Carrara E, Graham DY, Conti M, Tacconelli E. Prevalence of Antibiotic Resistance in Helicobacter pylori: A Systematic Review and Meta-analysis in World Health Organization Regions. Gastroenterology. 2018;155(5):1372–1382. doi: 10.1053/j.gastro.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garrido L, Toledo H. Novel genotypes in Helicobacter pylori involving domain V of the 23S rRNA gene. Helicobacter. 2007;12(5):505–9. doi: 10.1111/j.1523-5378.2007.00506.x. [DOI] [PubMed] [Google Scholar]
- 17.Salinas A, et al. Detección de la resistencia del Helicobacter pylori a la claritromicina mediante nueva técnica de biología molecular (Abstract) Gastroenterol latinoam. 2015;26(Supl 2):S95–S96. [Google Scholar]
- 18.González-Hormazabal P, et al. Prevalence of clarithromycin resistance in Helicobacter pylori in Santiago, Chile, estimated by real-time PCR directly from gastric mucosa. BMC Gastroenterology. 2018;18:9. doi: 10.1186/s12876-018-0820-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Serrano CA, et al. Helicobacter pylori-Clarithromycin Resistance in Symptomatic Pediatric Patients in a High Prevalence Country. J Pediatr Gastroenterol Nutr. 2017;64(3):e56–e60. doi: 10.1097/MPG.0000000000001257. [DOI] [PubMed] [Google Scholar]
- 20.Fallone Carlo A., Chiba Naoki, van Zanten Sander Veldhuyzen, Fischbach Lori, Gisbert Javier P., Hunt Richard H., Jones Nicola L., Render Craig, Leontiadis Grigorios I., Moayyedi Paul, Marshall John K. The Toronto Consensus for the Treatment of Helicobacter pylori Infection in Adults. Gastroenterology. 2016;151(1):51-69.e14. doi: 10.1053/j.gastro.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 21.Rollan Antonio. Management ofHelicobacter pyloriinfection in Latin America: A Delphi technique-based consensus. World Journal of Gastroenterology. 2014;20(31):10969. doi: 10.3748/wjg.v20.i31.10969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chey WD, Leontidias G, Howden C, Moss S. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am J Gastroenterol. 2017;112(2):212–239. doi: 10.1038/ajg.2016.563. [DOI] [PubMed] [Google Scholar]
- 23.Fischbach L, Evans EL. Meta-analysis: the effect resistance status on the efficacy of triple and quadruple firstline therapies for Helicobacter pylori. Aliment Pharmacol T. 2007;26:343–57. doi: 10.1111/j.1365-2036.2007.03386.x. [DOI] [PubMed] [Google Scholar]
- 24.Kita T, et al. CYP2C19 genotype related effect of omeprazole on intragastric pH and antimicrobial stability. Pharm Res. 2001;18(5):615–21. doi: 10.1023/A:1011025125163. [DOI] [PubMed] [Google Scholar]
- 25.Solomon, A. et al. Cytochrome P450 family 2 subfamily C member 19 https://www.pharmvar.org/gene/CYP2C19 (2018).
- 26.Tomalik-Scharte D, Lazar A, Fuhr U, Kirchheiner J. The clinical role of genetic polymorphisms in drug-metabolizing enzymes. Pharmacogenomics J. 2008;8(1):4–15. doi: 10.1038/sj.tpj.6500462. [DOI] [PubMed] [Google Scholar]
- 27.Kuo, C.H. et al. CYP2C19 polymorphism influences Helicobacter pylori eradication. World J Gastroenterol. 21;20(43), 16029–36 (2014). [DOI] [PMC free article] [PubMed]
- 28.Lee JY, et al. Factors affecting first-line triple therapy of Helicobacter pylori including CYP2C19 genotype and antibiotic resistance. DigDisSci. 2014;59(6):1235–43. doi: 10.1007/s10620-014-3093-7. [DOI] [PubMed] [Google Scholar]
- 29.Tang HL, Li Y, Hu YF, Xie HG, Zhai S-D. Effects of CYP2C19 loss-of-function variants on the eradication of H. pylori infection in patients treated with proton pump inhibitor-based triple therapy regimens: a meta-analysis of randomized clinical trials. PLoSOne. 2013;8(4):e62162. doi: 10.1371/journal.pone.0062162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peduzzi P, et al. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49:1373–9. doi: 10.1016/S0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- 31.Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol. 2007;165(6):710–8. doi: 10.1093/aje/kwk052. [DOI] [PubMed] [Google Scholar]
- 32.Liberati A, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nyaga, V.N., Arbyn, M. & Aerts, M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 10;72(1):39 (2014). [DOI] [PMC free article] [PubMed]
- 34.Picoli SU, et al. Resistance to amoxicillin, clarithromycin and ciprofloxacin of Helicobacter pylori isolated from Southern Brazil patients. Rev Inst Med Trop Sao Paulo. 2014;56(3):197–200. doi: 10.1590/S0036-46652014000300003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coelho LG, Coelho MC. Clinical management of Helicobacter pylori: the Latin American perspective. Dig Dis. 2014;32(3):302–9. doi: 10.1159/000360615. [DOI] [PubMed] [Google Scholar]
- 36.Otth L, et al. Isolation of Helicobacter pylori in gastric mucosa and susceptibility to five antimicrobial drugs in Southern Chile. Braz J Microbiol. 2011;42(2):442–7. doi: 10.1590/S1517-83822011000200005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vallejos C, et al. Prevalence of metronidazole, clarithromycin and tetracycline resistance in Helicobacter pylori isolated from Chilean patients. Rev Med Chil. 2007;135(3):287–93. doi: 10.4067/S0034-98872007000300002. [DOI] [PubMed] [Google Scholar]
- 38.Vallejos C, Cerda O, Valenzuela M, Toledo H. Antimicrobial resistance of Helicobacter pylori: clinical and molecular aspects. Rev Med Chil. 2003;131(11):1313–20. doi: 10.4067/S0034-98872003001100014. [DOI] [PubMed] [Google Scholar]
- 39.González C, et al. In vitro antimicrobial susceptibility of Helicobacter pylori strains: isolation of strains resistant to clarithromycin] Rev Med Chil. 2001;129(6):643–6. doi: 10.4067/S0034-98872001000600007. [DOI] [PubMed] [Google Scholar]
- 40.Oporto Marcelo, Pavez Monica, Troncoso Claudia, Cerda Alvaro, Hofmann Edmundo, Sierralta Armando, Rios Eddy, Coppelli Luis, Barrientos Leticia. Prevalence of Infection and Antibiotic Susceptibility of Helicobacter pylori: An Evaluation in Public and Private Health Systems of Southern Chile. Pathogens. 2019;8(4):226. doi: 10.3390/pathogens8040226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Graham DY, Lu H, Yamaoka Y. A report card to grade Helicobacter pylori therapy. Helicobacter. 2007;12:275e8. doi: 10.1111/j.1523-5378.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- 42.Greenberg ER, et al. 14-day triple, 5-day concomitant, and 10-day sequential therapies for Helicobacter pylori infection in seven Latin American sites: A randomised trial. Lancet. 2011;378:507–14. doi: 10.1016/S0140-6736(11)60825-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alba C, Blanco A, Alarcón T. Antibiotic resistance in Helicobacter pylori. Curr Opin Infect Dis. 2017;30(5):489–497. doi: 10.1097/QCO.0000000000000396. [DOI] [PubMed] [Google Scholar]
- 44.Miyaki A, Yamaguchi K, Ida A, Miyauchi T. An assessment of the efficacy of first-line Helicobacter pylori-eradication therapy based on clarithromycin susceptibility. Minerva Gastroenterol Dietol. 2016;62:234–9. [PubMed] [Google Scholar]
- 45.Muñoz, N. et al. Systematic review, meta-analysis, and meta-regression: Successful second-line treatment for Helicobacter pylori. Helicobacter. e12488 (2018). [DOI] [PubMed]
- 46.Zagari RM, et al. The “three-in-one” formulation of bismuth quadruple therapy for Helicobacter pylori eradication with or without probiotics supplementation: Efficacy and safety in daily clinical practice. Helicobacter. 2018;23(4):e12502. doi: 10.1111/hel.12502. [DOI] [PubMed] [Google Scholar]
- 47.Federico A, et al. Efficacy of 5-day levofloxacin-containing concomitant therapy in eradication of Helicobacter pylori infection. Gastroenterology. 2012;143(1):55–6.e1. doi: 10.1053/j.gastro.2012.03.043. [DOI] [PubMed] [Google Scholar]
- 48.Molina-Infante J, et al. Optimized nonbismuth quadruple therapies cure most patients with Helicobacter pylori infection in populations with high rates of antibiotic resistance. Gastroenterology. 2013;145(1):121–128.e1. doi: 10.1053/j.gastro.2013.03.050. [DOI] [PubMed] [Google Scholar]
- 49.Tai WC, et al. A 14-day esomeprazole- and amoxicillin-containing high-dose dual therapy regimen achieves a high eradication rate as first-line anti-Helicobacter pylori treatment in Taiwan: a prospective randomized trial. J Antimicrob Chemother. 2019;74:1718–1724. doi: 10.1093/jac/dkz046. [DOI] [PubMed] [Google Scholar]
- 50.Yang X, Wang JX, Han SX, Gao CP. High dose dual therapy versus bismuth quadruple therapy for Helicobacter pylori eradication treat- ment: a systematic review and meta-analysis. Medicine (Baltimore). 2019;98(7):e14396. doi: 10.1097/MD.0000000000014396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roco A, et al. Frequencies of 23 functionally significant variant alleles related with metabolism of antineoplastic drugs in the Chilean population: comparison with Caucasian an Asian populations. Front Genet. 2012;2(3):229. doi: 10.3389/fgene.2012.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saldaña-Cruz AM, et al. CYP2C9 and CYP2C19 Allele and Haplotype Distributions in Four Mestizo Populations from Western Mexico: An Interethnic Comparative Study. Genet Test Mol Biomarkers. 2016;20(11):702–709. doi: 10.1089/gtmb.2016.0115. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.