Abstract
The presence of quantifiable HIV RNA in cerebrospinal fluid (CSF) during antiretroviral therapy (ART) can associate with central nervous system (CNS) pathology, but the significance of RNA detected below the limit of quantification (LOQ) on a standard assay during ART remains unknown. We compared CNS parameters between individuals with CSF RNA detected below the LOQ (20 copies/mL) with those with HIV RNA not detected. Detection of CSF HIV RNA associated with decreased blood–brain barrier integrity and with decreased executive function, but not with CNS immune activation or poorer performance in overall neuropsychological testing.
Keywords: antiretroviral therapy, blood–brain barrier, HIV, viral persistence
Some people with HIV on suppressive ART have HIV detected but not quantifiable in cerebrospinal fluid (CSF). We evaluated markers of blood brain barrier breakdown, inflammation, and neurocognitive impairment in people with HIV detected but not quantifiable in CSF.
Long-term infection with HIV, even in the presence of antiretroviral therapy (ART), is associated with central nervous system (CNS) injury in a subset of individuals [1, 2]. One possible driver of CNS injury during suppressive ART is viral replication and associated immune activation within the brain. HIV enters the CNS during early infection, where it is associated with elevated cerebrospinal fluid (CSF) markers of myeloid cell activation and loss of blood–brain barrier (BBB) integrity [3]. In adults on suppressive ART, these markers remain elevated, along with measures of axonal injury. Taken together, this suggests that viral replication in the CNS ultimately may associate with neurological injury during plasma viral suppression [4, 5].
Some clues have emerged from the study of the subset of adults on ART that have HIV RNA detected in the CSF through sensitive nonclinical assays. For example, individuals with HIV detected in CSF by sensitive single-copy HIV-1 RNA assays have elevated CSF neopterin, a marker of myeloid cell activation, and worse global neurocognitive performance [6]. This suggests that, whether due to active viral replication via a compartmentalized reservoir or to a continued entry of virus from the systemic circulation, the presence of HIV RNA in the CNS may drive neuronal injury by promoting chronic immune activation. However, single-copy viral detection assays are research-only tools, and it remains unknown whether viral RNA detected in CSF below the limit of quantification (LOQ) on a standard clinical assay associates with measures of CNS injury.
Here we examine paired, cross-sectional blood and CSF samples from adults with HIV on chronic suppressive ART. We categorized our participants into 3 groups retrospectively based on their CSF HIV RNA, as determined by a widely used commercial assay: above the test’s limit of quantitation (LOQ, 20 copies/mL), detectable but below the LOQ, and not detected. Our objective was to determine whether HIV RNA detected in the CSF below the LOQ was associated with laboratory or clinical markers of CNS injury.
METHODS
Study Design and Participants
Research participants living with HIV were enrolled at Yale University in the context of a biorepository study. Participants were enrolled if they had plasma HIV viral load <20 copies/mL for >1 year, were on ART for >1 year, and had no contraindication to lumbar puncture. All participants underwent lumbar puncture, phlebotomy, and neuropsychological testing. Forty-one participants were enrolled, and 33 participants who had HIV RNA detected in CSF below the test limit of quantification (see below) or not detected in CSF were included in the analysis. The Institutional Review Board at Yale approved the protocol, and informed consent was obtained from all participants.
Laboratory Testing
HIV-1 RNA was measured in both plasma and CSF on fresh samples using the Roche Ampliprep Taqman assay (LOQ, 20 copies/mL). Blood T-cell subsets (CD4+ and CD8+) and albumin were measured by standard clinical laboratory assays, as was CSF cell count, albumin, and protein. The albumin ratio was calculated as [CSF albumin, mg/dL] / [serum albumin, g/dL].
Plasma and CSF neopterin were measured with the BRAHMS Neopterin enzyme immunoassay (BRAHMS, Hennigsdorf, Germany). Quantification of soluble plasma and CSF biomarkers sCD14, sCD163, CCL2/MCP-1, and CXCL10/IP-10 were measured using the human magnetic Luminex screening assay kit (R&D Systems, Minneapolis, MN, USA).
Neuropsychological Testing
Neuropsychological testing was conducted across the following domains: language/fluency, executive function, speed of information processing, attention/working memory, verbal and visual learning, verbal and visual memory, and motor skills [7]. All measures, except timed gait, were normalized according to age, education, gender, and ethnicity and were evaluated as domain Z scores (average of test Z scores for each domain) and summarized as the total Z score for all tests.
Statistics
For laboratory biomarker data, the Mann-Whitney and Fisher exact tests were used to determine statistical significance. For demographic data, the t test and the chi-square or Fisher exact test were used. For neuropsychological tests, pairwise t tests were conducted for individual domain Z scores and for the composite Z score. All statistical analyses were conducted using GraphPad Prism 8.0 (GraphPad Software, Inc.). Differences were considered significant if P < .05.
RESULTS
Study Participant Characteristics
Forty-one participants had a mean age of 53 years, a mean CD4+ T-cell count of 607 cells/µL, and a median of 20 years on ART. Eight participants had CSF HIV RNA detected above the LOQ (median, 47 copies/mL), and these participants were excluded from all further analyses. Thirteen participants had CSF HIV RNA detected below the LOQ (LOQ ≤ 20 copies/mL); 20 had no detectable CSF HIV RNA. Participants with CSF HIV RNA <20 copies/mL did not differ from those with CSF HIV RNA not detected for age, sex, years on ART, nadir CD4+ T cells, current CD4+ T-cell count, CD4:CD8 ratio, race, or substance use history (Table 1). ART regimens differed between groups: Those with HIV RNA <20 copies/mL were more likely to be on a protease inhibitor at the time of enrollment, compared with those with HIV RNA not detected in CSF (46% vs 15%; P = .05).
Table 1.
Summary Characteristics of the Study Participants
| Characteristics | CSF HIV RNA Not Detected (n = 20) | CSF HIV RNA Detected <20 Copies/mL (n = 13) | P |
|---|---|---|---|
| Male sex, No. (%) | 16 (80) | 11 (85) | 1 |
| Age, No. (%) | 1 | ||
| 18–49 y | 5 (25) | 4 (31) | |
| 50+ y | 15 (75) | 9 (69) | |
| Race, No. (%) | .9 | ||
| African American/black | 13 (65) | 8 (62) | |
| White | 5 (25) | 3 (23) | |
| Other/unknown | 2 (10) | 2 (15) | |
| Reason for lumbar puncture | 1 | ||
| CNS symptoms | 5 (25) | 4 (31) | |
| Participation in biorepository study | 15 (75) | 9 (69) | |
| Years on antiretroviral therapy, median (IQR) | 20 (16–25) | 21 (12–26) | .47 |
| Current integrase inhibitor, No. (%) | 12 (60) | 7 (54) | .78 |
| Current protease inhibitor, No. (%) | 3 (15) | 6 (46) | .049 |
| Smoking, No. (%) | .07 | ||
| Current | 10 (50) | 6 (46) | |
| Former | 7 (35) | 1 (8) | |
| Never | 3 (15) | 6 (46) | |
| History of alcohol use disorder, No. (%) | 10 (50) | 4 (31) | .31 |
| History of substance use disorder, No. (%) | 13 (65) | 7 (54) | .81 |
| History of hepatitis C, No. (%) | 7 (35) | 4 (31) | .6 |
| Blood nadir CD4+ count, median (IQR), cells/µL | 280 (126–484) | 285 (138–396) | .47 |
| Blood CD4+ absolute count, median (IQR), cells/µL | 537 (418–881) | 616 (397–756) | .49 |
| Ratio blood CD4:CD8 cells, median (IQR) | 0.82 (0.66–1) | 0.8 (0.5–1) | .22 |
| Plasma HIV RNA | 1 | ||
| Not detected | 15 (75) | 9 (69) | |
| Detected, <20 copies/mL | 5 (25) | 4 (31) |
Metric variables were tested with t tests, whereas categorial variables were compared by chi-square and Fisher exact tests.
Abbreviations: CNS, central nervous system; CSF, cerebrospinal fluid; IQR, interquartile range.
CSF and Plasma Biomarkers
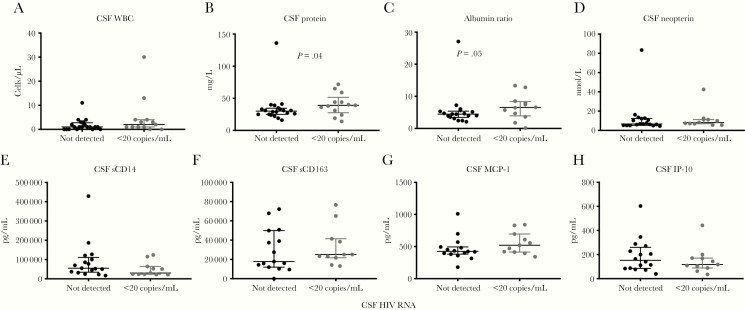
There were no significant differences between participants with CSF HIV RNA <20 copies/mL and CSF HIV RNA not detected when assessed for CSF white blood cell count (median, 2 vs 1 white blood cells; P = .09) (Figure 1A). However, we found that participants with CSF HIV RNA <20 copies/mL demonstrated significant differences in markers of BBB integrity when compared with participants with CSF HIV RNA not detected. The former group showed increased CSF total protein (median, 39 vs 30 mg/dL; P = .041) (Figure 1B) and increased albumin ratio (median, 6.5 vs 4.5; P = .047) (Figure 1C), indicating a decrease in blood–brain barrier integrity.
Figure 1.
A–H, Cerebrospinal fluid biomarkers in HIV+ participants with CSF HIV RNA not detected or detected below the limit of quantification (<20 copies/mL). Shown are medians and interquartile ranges. Albumin ratio refers to the value of CSF albumin divided by plasma albumin. P values were calculated using the Mann-Whitney test. Abbreviations: CSF, cerebrospinal fluid; WBC, white blood cell count.
CSF and plasma markers of immune cell activation were similar between the 2 groups. There was no difference between the 2 groups in the measured level of CSF neopterin, soluble CD163, soluble CD14, MCP-1, or IP-10 (Figure 1D–H). Likewise, plasma markers of immune activation were similar between the 2 groups (Supplementary Table 1).
Neuropsychological Outcomes
When compared for overall performance on neuropsychological testing of 11 cognitive and motor domains, participants with HIV detected <20 copies/mL in CSF did not differ from those with HIV not detected in CSF (combined Z score mean, –1.5 vs –1.3; P > 0.5). However, when we examined performance in the individual cognitive and motor domains, we found a difference in performance on tests of executive function (domain Z score mean, –1.7 vs –0.6; P < .009) (Supplementary Figure 1).
Discussion
This study examined whether study participants with HIV on ART with HIV RNA in CSF detected but below the LOQ on a widely used commercial assay demonstrated abnormalities in laboratory or clinical CNS parameters. We hypothesized that patients with CSF HIV RNA detected below the LOQ would demonstrate increased markers of CNS immune activation and BBB injury and poorer neuropsychological performance when compared with those HIV not detected in CSF. We found that, while adults with HIV detected in CSF <20 copies/mL had CSF biomarker changes that suggested a decrease in BBB integrity, the 2 groups had no significant differences in CSF markers of immune activation. We also found that the 2 groups performed similarly across a battery of tests of neuropsychological function, with a decrease noted only in tests of executive function. In contrast to studies that used a research-only single-copy viral RNA detection assay, this analysis focuses on distinguishing participants’ HIV burden using a commercial, widely available assay. Thus, these data may be applicable to clinical care [6, 8, 9].
During HIV infection, the BBB is compromised, with the production of pro-inflammatory cytokines causing loosening of BBB tight junctions [10]. However, the relationship between viral replication in the CNS and BBB integrity is incompletely understood [11]. To assess BBB integrity, we calculated the CSF:plasma albumin ratio, an index that is known to be elevated in other neuroinflammatory and neurodegenerative conditions. We found that despite being on longstanding ART, participants with CSF HIV RNA detected below the LOQ had increased albumin ratios and increased CSF protein levels compared with participants without detected HIV RNA in CSF. Prior studies have suggested that the albumin ratio may underestimate the level of BBB injury, as CSF albumin levels may be influenced by proteolytic cleavage, as well as by increased albumin uptake by inflammatory cells [12]. Our findings suggest that even minimally detectable levels of viral CNS infiltration may associate with mild loss of BBB integrity in individuals on suppressive therapy.
This study also examined a subset of markers of systemic and CNS immune activation in study participants. Previous work has noted a correlation between the albumin ratio and CSF neopterin levels in untreated HIV-infected individuals, though this finding has not been noted in patients on long-term ART [6, 13]. We identified no elevations in CSF neopterin or other CSF markers of CNS myeloid cell activation in participants with HIV RNA detected in CSF below the LOQ when compared with those with HIV not detected in CSF.
Motta and colleagues recently reported that HIV detected below the LOQ in CSF using the Roche Amplicor assay is associated with elevated CSF neopterin [14]. In contrast, we found no association between HIV detected in CSF below the LOQ and elevated CSF neopterin. This may be due to a difference in the sample populations between the studies: 54% of the participants in the Motta et al. study underwent lumbar puncture because of a neurological or neuroimaging abnormality, compared with 27% of the participants in our study, suggesting that there may have been higher rates of symptomatic neuroinflammation in that group compared with the present study group. The duration of plasma viral suppression also differed between the 2 groups: in the present study, all participants had a duration of plasma viral suppression of at least 12 months. In contrast, Motta et al. included participants with as little as 1 month of plasma viral suppression, a factor that may have also contributed to the overall rates of CSF neopterin in that study group. Motta et al. also found that the presence of HIV RNA detected below the LOQ in CSF was associated with HIV RNA detected below the LOQ in plasma. Likewise, we recently reported that PWH on ART with detectable cell-free HIV RNA in CSF had higher levels of plasma HIV RNA by integrase single-copy assay than those without detectable CSF HIV RNA [15]. However, in the current study, plasma HIV detected below the LOQ was not associated with CSF HIV detected below the LOQ on a commercial assay. This may be due to overall lower sensitivity of the commercial assay compared with research assays that can detect single copies of HIV RNA.
Our study is the first to investigate the relationship between CSF HIV detected but not quantifiable in CSF and neurocognitive outcomes. Although we found overall similar performance between the 2 groups across most measures of neuropsychological testing, we did note worsened performance in tests of executive function in those participants with CSF HIV detected below the LOQ. Impairments in executive function are a hallmark of HIV-associated neurocognitive impairment and occur alongside accelerated volume loss in several brain regions that are associated with maintaining executive function, including the prefrontal cortex and the thalamus [16]. Likewise, loss of executive function in PWH associates with hypometabolism of the thalamus [17]. Our results suggest that viral persistence in the CNS also associates with loss of executive function, though it remains unclear if this loss of function is mediated through brain volume loss or another neuropathogenic mechanism.
We found a trend toward an association between the current use of a protease inhibitor and the presence of HIV RNA detected in CSF. This finding is consistent with a study based on the CHARTER cohort of adults with HIV on ART, which found protease inhibitor–based regimens to be independent predictors of HIV RNA in CSF [18]. However, the mechanism for this correlation remains incompletely understood, though it is possibly related to reduced penetrance of protease inhibitors into the CNS compartment.
In summary, our study found that in individuals with HIV on ART with CSF HIV RNA detected below the LOQ on a commercial assay, there is reduced blood–brain barrier integrity and executive function relative to those with no HIV RNA detected. By using a commercially available assay, our findings have implications for the clinical evaluation of a “detected <20 copies/mL” HIV RNA result in CSF. Practitioners may be encouraged that the presence of HIV RNA below the LOQ does not appear to correlate with any of the markers of immune activation that we tested. However, the present study has important limitations that, at present, preclude it from forming a basis for clinical decision-making. In addition to being limited by small sample size, this study is limited in scope due to its focus on a nonexhaustive list of measures of BBB injury and immune activation. Future analyses should examine more extensive measures of myeloid and T-cell activation in participants with CSF HIV RNA detected below the LOQ of standard assays. Finally, as this was a cross-sectional study, our results are restricted to a single time point and can be expanded though longitudinal studies that may assess the stability of CSF biomarkers and neurological outcomes over time. Overall, our findings lay the groundwork for future analysis into the pathogenesis and clinical outcomes of persistent viral detection in the CNS.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Financial support. This work was supported by NIH K23MH118999 (SF), R21MH110260 (SS), R21MH118109 (SS), and by the American Federation for Aging Research and the Robert E. Leet and Clara Guthrie Patterson Trust. This work was presented in a platform presentation at the 2019 Conference on Retroviruses and Opportunistic Infections (Seattle, WA).
Potential conflicts of interest. The authors declare no commercial or other associations that might pose a conflict of interest.
References
- 1. Calcagno A, Atzori C, Romito A, et al. . Blood brain barrier impairment is associated with cerebrospinal fluid markers of neuronal damage in HIV-positive patients. J Neurovirol 2016; 22:88–92. [DOI] [PubMed] [Google Scholar]
- 2. Heaton RK, Clifford DB, Franklin DR Jr, et al. ; CHARTER Group HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER study. Neurology 2010; 75:2087–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Spudich S, Gisslen M, Hagberg L, et al. . Central nervous system immune activation characterizes primary human immunodeficiency virus 1 infection even in participants with minimal cerebrospinal fluid viral burden. J Infect Dis 2011; 204:753–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jessen Krut J, Mellberg T, Price RW, et al. . Biomarker evidence of axonal injury in neuroasymptomatic HIV-1 patients. PLoS One 2014; 9:e88591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rahimy E, Li FY, Hagberg L, et al. . Blood-brain barrier disruption is initiated during primary HIV infection and not rapidly altered by antiretroviral therapy. J Infect Dis 2017; 215:1132–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dahl V, Peterson J, Fuchs D, et al. . Low levels of HIV-1 RNA detected in the cerebrospinal fluid after up to 10 years of suppressive therapy are associated with local immune activation. AIDS 2014; 28:2251–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Robertson K, Yosief S. Neurocognitive assessment in the diagnosis of HIV-associated neurocognitive disorders. Semin Neurol 2014; 34:21–6. [DOI] [PubMed] [Google Scholar]
- 8. Dahl V, Peterson J, Spudich S, et al. . Single-copy assay quantification of HIV-1 RNA in paired cerebrospinal fluid and plasma samples from elite controllers. AIDS 2013; 27:1145–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Anderson AM, Muñoz-Moreno JA, McClernon DR, et al. ; CHARTER Group Prevalence and correlates of persistent HIV-1 RNA in cerebrospinal fluid during antiretroviral therapy. J Infect Dis 2017; 215:105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ivey NS, MacLean AG, Lackner AA. Acquired immunodeficiency syndrome and the blood-brain barrier. J Neurovirol 2009; 15(2):111–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Atluri VSR, Hidalgo M, Samikkannu T, et al. . Effect of human immunodeficiency virus on blood-brain barrier integrity and function: an update. Front Cell Neurosci 2015; 9:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sweeney MD, Sagare AP, Zlokovic BV. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol 2018; 14:133–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Andersson LM, Hagberg L, Fuchs D, et al. . Increased blood-brain barrier permeability in neuro-asymptomatic HIV-1-infected individuals—correlation with cerebrospinal fluid HIV-1 RNA and neopterin levels. J Neurovirol 2001; 7:542–7. [DOI] [PubMed] [Google Scholar]
- 14. Motta I, Allice T, Romito A, et al. . Cerebrospinal fluid viral load and neopterin in HIV-positive patients with undetectable viraemia. Antivir Ther 2017; 22:539–43. [DOI] [PubMed] [Google Scholar]
- 15. Spudich S, Robertson KR, Bosch RJ, et al. . Persistent HIV-infected cells in cerebrospinal fluid are associated with poorer neurocognitive performance. J Clin Invest 2019; 129:3339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pfefferbaum A, Rogosa DA, Rosenbloom MJ, et al. . Accelerated aging of selective brain structures in human immunodeficiency virus infection: a controlled, longitudinal magnetic resonance imaging study. Neurobiol Aging 2014; 35:1755–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hammoud DA, Sinharay S, Steinbach S, et al. . Global and regional brain hypometabolism on FDG-PET in treated HIV-infected individuals. Neurology 2018; 91(17):e1591–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mukerji SS, Misra V, Lorenz DR, et al. . Impact of antiretroviral regimens on cerebrospinal fluid viral escape in a prospective multicohort study of antiretroviral therapy-experienced human immunodeficiency virus-1-infected adults in the United States. Clin Infect Dis 2018; 67:1182–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.