Abstract
Background
The precise role of cytomegalovirus (CMV) in ulcerative colitis (UC) remains disputed. We evaluated the association of CMV-specific host immune responses and systemic or local viral replication with responses to systemic steroids in patients with moderate to severe UC.
Methods
Patients who were hospitalized for moderate to severe UC between April 2015 and June 2016 were enrolled. At baseline, all enrolled patients underwent CMV-specific enzyme-linked immunospot assays, quantitative polymerase chain reaction (qPCR) analysis of blood and colonic tissue for CMV viral load, histopathological testing for CMV in colonic tissue by hematoxylin and eosin staining, and immunohistochemical (IHC) analysis. Clinical responses to steroid therapy based on the Oxford index were assessed on day 3.
Results
Of the 80 patients evaluated, 28 (35.0%) had poor responses to steroid therapy on day 3 of intensive treatment. The presence of inclusion bodies (32.1%) and high-grade (≥3) positivity on IHC (50.0%), as well as colonic (mean 1440.4 copies/mg) and blood (mean, 3692.6 copies/mL) CMV viral load, were higher in steroid-refractory UC patients than the control group (13.5%, 1.9%, mean 429.2 copies/mg, and mean 231.2 copies/mL, respectively; P = .046, .009, .017, and .002, respectively). However, CMV-specific T-cell responses were not associated with steroid-refractory UC. Multivariate analysis revealed that a higher Mayo score (odds ratio [OR], 2.00; P = .002) and higher blood CMV viral load via qPCR analysis (OR, 3.58; P = .044) were independent risk factors for steroid-refractory UC.
Conclusions
In patients with moderate to severe UC, higher Mayo score and blood CMV expression determined by qPCR are independently associated with steroid refractoriness.
ClinicalTrials.gov registration number
Keywords: cytomegalovirus, enzyme-linked immunospot, T-cell response, ulcerative colitis
Corticosteroids are the firstline of therapy for moderate to severe ulcerative colitis (UC); however, up to 30%–40% of patients fail to respond to treatment [1, 2]. In steroid-refractory colitis, the prevalence of cytomegalovirus (CMV) colitis appears to be particularly high [2]. Previous studies suggested that CMV infection should be suspected in patients with inflammatory bowel disease (IBD) who are refractory to steroid treatment. Current guidelines state that CMV infection should be excluded in patients with acute steroid-refractory colitis [2, 3].
However, the clinical importance of CMV colitis and its pathological contribution to the inflammatory process are still matters of debate. Whether CMV is an innocent bystander in inflamed intestinal tissue or increases the risk of colectomy is still unclear [3, 4]. Previous studies have reported that CMV infection might contribute to steroid refractoriness, megacolon, or exacerbation in UC patients [2]. However, other studies have shown that CMV infection is not associated with exacerbation of IBD [5, 6], because it resolved without antiviral therapy [7]. The causes of these conflicting results are not well understood.
Recently developed assays for investigating the CMV-specific T-cell response allow direct quantification of host immunity to CMV [8, 9]. Therefore, combined analysis of host immunity to CMV and quantitation of CMV replication in tissues, or at the systemic level in steroid-responsive or -unresponsive patients with UC, would provide further insight into this complex issue. We therefore evaluated the association of the CMV-specific host immune response and systemic or local viral replication with the response to systemic steroids in patients with moderate to severe UC.
METHODS
Patient Selection
Patients aged >16 years with moderate to severe UC who were admitted to Asan Medical Center, Seoul, South Korea, a tertiary care teaching hospital, were prospectively enrolled between April 2015 and June 2016. The diagnosis of UC was based on clinical, endoscopic, and histological parameters [10, 11]. Disease activity was assessed using the Mayo score [11]. Moderate to severe UC was defined as a Mayo score of 6 to 12 with an endoscopic subscore of 2 or more. Only patients with left-sided or extensive colitis were enrolled. There were no exclusion criteria for disease duration or medication such as anti–tumor necrosis factor (TNF) agents. The study protocol was approved by the institutional review board of the Asan Medical Center, which confirmed that it adhered to the ethical principles of the Declaration of Helsinki (IRB number 2015-0129). This study was also registered at ClinicalTrials.gov (NCT 02439372), and informed consent was obtained from each patient.
Study Protocol
At baseline, samples from all enrolled patients were subjected to CMV-specific enzyme-linked immunospot (ELISPOT) assays, and quantitative blood CMV polymerase chain reaction (PCR), hematoxylin and eosin (H&E) staining of colonic tissue, immunohistochemistry (IHC) analysis, and CMV PCR were also performed to assess CMV expression. Endoscopic activity was assessed by expert endoscopists using the Mayo endoscopic subscore and the Ulcerative Colitis Endoscopic Index of Severity (UCEIS) [12]. All enrolled patients with moderate to severe UC received intensive steroid treatment until clinical improvement was observed. The response to steroid therapy was assessed based on the Oxford index, which is the combination of C-reactive protein (CRP) >45 mg/L and stool frequency of 3–8 per day, or stool frequency >8 per day on day 3 [13]. Patients were classified as “poor responders” or “responders” to steroid treatment. We analyzed whether CMV infection was related to steroid responsiveness.
Laboratory Tests for CMV Infection
Real-time PCR was performed to assess CMV expression in the patients’ blood. DNA was isolated from the blood using a NucliSens easyMAG Nucleic Acid Extraction System (bioMérieux, Lyon, France). CMV DNA was then quantified using the Qiagen Artus CMV RGQ MDx kit (Qiagen, Doncaster, Australia) on a Rotor-Gene Q platform (Qiagen,Germantown, MD, USA). A positive result for CMV was defined as >250 copies/mL [14].
We also performed CMV-specific ELISPOT assays (IFN-γ; T-track CMV, Lophius Bioscience, Regensburg, Germany) to evaluate the host response to CMV infection, as previously described [8, 9].
Tissues from macroscopically active mucosal lesions (rectum, sigmoid, or descending colon) were obtained during sigmoidoscopy. Using the colonic biopsy tissues, we first checked for histologic signs of CMV infection, which include cell enlargement, basophilic intranuclear inclusion bodies (surrounded by clear halos described as “owl eyes”) [15, 16], thickened nuclear membranes, and granular intracytoplasmic inclusions. Next, we performed CMV IHC. Briefly, tissue sections were incubated with anti-CMV monoclonal antibody (clone CCH2+ DDG9, 1:50, Glostrup, Denmark). All staining procedures were performed using a BenchMark XT autostainer and the OptiView DAB IHC Detection Kit (Ventana Medical Systems, Tucson, AZ, USA). We graded CMV immunostaining using a 4-tier grading system based on the number of cells showing positive nuclear CMV staining per 2-mm tissue. The criteria are as follows: (i) grade 0: negative; (ii) grade 1: 1–2; (iii) grade 2: 3–5; and (iv) grade 3: >5. All biopsy specimens were reviewed by an experienced gastrointestinal pathologist (Dr. Jihun Kim). To detect CMV in the colonoscopic biopsy tissue, DNA was extracted using a QIAamp DNA FFPE Tissue Kit. CMV DNA was amplified by PCR, as described previously [17], with slight modifications. A positive result for tissue CMV PCR was defined as >10 copies/mg [18].
Assessment of Outcomes and Definition of Terms
The primary outcomes were risk factors for poor response to steroid treatment in patients with moderate to severe UC. The response to steroid treatment was assessed on day 3 based on the Oxford index, as described above [13]. We categorized CMV gastrointestinal (GI) disease in the patients using the following criteria, which are based on the recent Infectious Diseases Society of America guidelines [19], with some modifications. Patients were classified as having “proven CMV GI disease” if they had lower GI symptoms, macroscopic mucosal lesions, and CMV expression in tissues, as determined by histopathology or immunohistochemistry analysis. Patients were classified as having “probable CMV disease” if they had lower GI symptoms and CMV expression in tissues, as determined by histopathology or immunohistochemistry analysis, without macroscopic mucosal lesions. Lastly, patients were classified as having “possible CMV colitis” if they only had CMV expression in tissues, as determined by PCR analysis.
Statistical Analysis
Data are expressed as median and interquartile range (IQR) or mean ± standard deviation. Continuous data were compared using the Student t test or Mann-Whitney test, if the distribution was variable. Categorical data were described using contingency tables and a chi-square test or Fisher exact test. A univariate analysis was performed using a logistic regression to determine the independent risk factors associated with a poor response to steroid treatment. Subsequently, multiple logistic regression analysis was performed for variables with a P value <.2 in the univariate analysis, based on the backward Wald selection method. The results are reported as an odds ratio (OR) with 95% confidence interval (CI). A P value <.05 was considered significant. Calculations were performed using the SPSS for Windows software package, version 21.0 (IBM Co., Armonk, NY, USA).
RESULTS
Baseline Clinical Characteristics
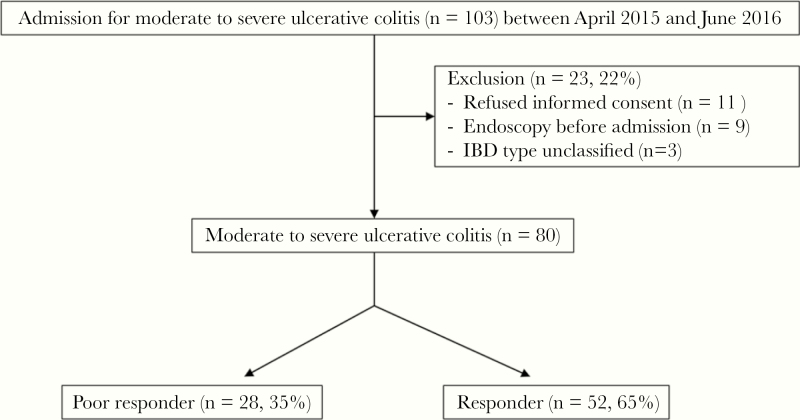
During the study period, 103 patients were admitted. However, 23 patients (22%) were excluded for the following reasons: refusal to provide informed consent (n = 11), endoscopy performed before admission (n = 9), and an unclassified IBD type (n = 3). Finally, 80 patients were officially enrolled (Figure 1). The baseline demographic characteristics at admission are shown in Table 1.
Figure 1.
Flowchart of the study.
Table 1.
Demographic and Clinical Characteristics of the Study Participants
| Patient Characteristic | Total (n = 80) | Poor Responder (n = 28) | Responder (n = 52) | P |
|---|---|---|---|---|
| Male, No. (%) | 47 (58.8) | 17 (60.7) | 30 (57.7) | .793 |
| Age, median (IQR), y | 41 (26–54) | 46 (34–57) | 39 (25–52) | .098 |
| Disease duration before admission, median (IQR), mo | 50.3 (7.4–74.6) | 25.9 (0.1–213.5) | 36.7 (0.1–227.5) | .824 |
| Disease extent at admission, No. (%) | ||||
| Left-sided colitis | 17 (21.3) | 2 (7.1) | 15 (28.8) | .024 |
| Extensive colitis | 63 (78.8) | 26 (92.9) | 37 (71.2) | .024 |
| Mayo score, mean ± SD | 9.9 ± 1.4 | 10.6 ± 1.1 | 9.4 ± 1.4 | <.001 |
| UCEIS score, mean ± SD | 6.2 ± 1.1 | 6.6 ± 0.8 | 5.9 ± 1.2 | .007 |
| Hemoglobin, mean ± SD, g/dL | 11.7 ± 2.4 | 11.1 ± 2.6 | 12.1 ± 2.3 | .103 |
| Albumin, mean ± SD, g/dL | 3.0 ± 0.66 | 2.7 ± 0.69 | 3.2 ± 0.58 | .002 |
| C-reactive protein, mean ± SD, mg/L | 48.0 ± 52.1 | 67.2 ± 52.4 | 37.7 ± 49.4 | .002 |
| Recent exposure to medication, No. (%) | ||||
| Anti-TNF agentsa | 12 (15.0) | 5 (17.9) | 7 (13.5) | .744 |
| Thiopurinesb | 24 (30.0) | 13 (46.4) | 11 (21.2) | .019 |
| Steroidsa | 42 (52.5) | 20 (71.4) | 22 (42.3) | .013 |
| Clostridium difficile infection, No. (%) | ||||
| Negative | 67 (83.8) | 23 (82.1) | 44 (84.6) | .778 |
| Positivec | 9 (11.3) | 4 (14.3) | 5 (9.6) | .534 |
| Not checked | 4 (5.0) | 1 (3.6) | 3 (5.8) | .672 |
| CMV colitis,d No. (%) | 33 (41.3) | 15 (53.6) | 18 (34.6) | .185 |
| Proven | 29 (36.3) | 14 (50) | 15 (28.8) | .062 |
| Possible | 4 (5.0) | 1 (4) | 3 (6) | .800 |
| Colonic tissue CMV PCR, mean ± SD, copies/mg | 783.1 ± 2736.7 | 1440.4 ± 3637.8 | 429.2 ± 2056.9 | .017 |
| Colonic tissue CMV PCR (>10 copies/mg), No. (%) | 29 (36.3) | 14 (50.0) | 15 (28.8) | .060 |
| IB on H&E staining, No. (%) | 16 (20.0) | 9 (32.1) | 7 (13.5) | .046 |
| Immunohistochemistry, No. (%) | 29 (36.2) | 14 (50.0) | 15 (28.8) | .062 |
| Grade 1 | 15/29 (51.7) | 6/14 (42.9) | 9/15 (60.0) | .355 |
| Grade 2 | 6/29 (20.7) | 1/14 (7.1) | 5/15 (33.3) | .081 |
| Grade 3 | 8/29 (27.6) | 7/14 (50.0) | 1/15 (6.7) | .009 |
| Inclusion body on H&E staining or immunohistochemistry (%) | 29 (36.3) | 14 (50.0) | 15 (28.8) | .062 |
| IB/IHC/colonic tissue PCR, No. (%) | ||||
| All positive | 16 (20.0) | 9 (32.1) | 7 (13.5) | .046 |
| Quantitative blood CMV PCR, mean ± SD, copies/mL | 1497.7 ± 4994.8 | 3692.6 ± 7823.2 | 231.2 ± 1036.1 | .002 |
| IE1-specific ELISPOT, mean ± SD, sfu/250 000 cells | 20.8 ± 53.7 | 7.9 ± 15.7 | 27.7 ± 64.7 | .200 |
| pp65-specific ELISPOT, mean ± SD, sfu/250 000 cells | 97.6 ± 126.3 | 84.9 ± 116.6 | 104.4 ± 131.8 | .642 |
Abbreviations: anti-TNF, anti–tumor necrosis factor; CMV, cytomegalovirus colitis; ELISPOT, enzyme-linked immune absorbent spot; H&E, hematoxylin and eosin; IB, inclusion body; IHC, immunohistochemistry; IQR, interquartile range; PCR, polymerase chain reaction; sfu, spot-forming unit; UCEIS, Ulcerative Colitis Endoscopic Index Of Severity.
aWithin the last 2 months.
bWithin the last month.
cPositive C. difficile infection was defined as C. difficile toxin-positive in enzyme-linked fluorescent assay or PCR or culture positive.
dCMV colitis was defined as proven CMV colitis and/or possible CMV colitis based on the criteria defined in the “Methods.”
Among these 80 patients, 28 (35.0%) were classified as poor responders to steroid therapy on day 3 based on the Oxford index. The remaining 52 patients (65%) were determined to be responders to steroid therapy (Figure 1). The median age of the poor responders (IQR) was 46 (34–57) years, and that of the responders was 39 (25–52) years. There was no statistical difference in disease duration at admission between the 2 groups. The median disease duration of the poor responders was 25.9 months, and that of the responders was 36.7 months (P = .824). Disease severity at admission was higher in the poor responders than the responders, with a mean Mayo score of 10.6 for the poor responders and 9.4 for the responders (P < .001).
The Link Between Various Laboratory Parameters and Steroid Refractoriness
Of the 80 patients with moderate to severe UC evaluated in this study, 33 (41.3%) were shown to have CMV colitis, including 29 with proven CMV colitis and 4 with possible CMV colitis, based on the criteria described above. CMV colitis was more frequent in the poor responders to steroid therapy than in the responders (53.6% vs 34.6%); however, the difference was not statistically significant (P = .185). Proven CMV colitis was diagnosed in 14 of 28 (50%) poor steroid responders and in 15 of 52 (28.9%) steroid responders (P = .062). Possible CMV colitis was diagnosed in 1 of 28 (4%) poor steroid responders and in 3 of 52 (6%) steroid responders (P = .800).
We examined whether qualitative or quantitative evidence of CMV replication in colonic tissue or blood and CMV-specific T-cell responses were associated with steroid refractoriness. When we evaluated CMV expression in colonic tissue by PCR, we observed that viral load was higher in the poor responders (mean ± SD, 1440 ± 3637.8 copies/mg) than in the responders (mean ± SD, 429.2 ± 2056.9 copies/mg; P = .017). Inclusion bodies in colonic tissue, as assessed by H&E staining, were more frequent in the poor responders (32.1%, 9 of 28) than in the responders (13.5%, 7 of 52; P = .046). The overall positive expression of CMV by IHC analysis was higher in the poor responders (50.0%, 14 of 28) than in the responders (28.8%, 15 of 52); however, this was not statistically significant (P = .062). Among the IHC-positive patients, histologic grade 3 IHC was significantly higher in the poor responders (25.0%, 7 of 14) than in the responders (28.8%, 1 of 15; P = .009).
We also measured CMV replication in the blood using qPCR. CMV was significantly higher in steroid-refractory UC (mean ± SD, 3692.6 ± 7823.2 copies/mL) than in the control samples (mean ± SD, 231.2 ± 1036.1 copies/mL; P = .002).
ELISPOT assays with stimulation by IE-1 and pp65 demonstrated that CMV-specific T-cell responses were slightly lower in the poor responders; however, this effect was not statistically significant. The IE-1-specific ELISPOT (mean ± SD) was 7.9 ± 15.7 spot-forming units (sfu)/250 000 cells in the poor responders and 27.7 ± 64.7 sfu/250 000 cells in the steroid responders (P = .200). The results of the pp65-specific ELISPOT (mean ± SD) were 84.9 ± 116.6 sfu/250 000 cells in the poor responders and 104.4 ± 131.8 sfu/250 000 cells in the responders (P = .642).
The multivariate analysis (Table 2) demonstrated that a higher Mayo score (OR, 2.00; 95% CI, 1.28–3.14; P = .002) and higher CMV expression in the blood, as assessed by qPCR (OR, 3.58; 95% CI, 1.03–12.34; P = .044), are independent risk factors for steroid-refractory UC. Neither proven nor possible CMV colitis, as determined using the above-mentioned criteria, was an independent risk factor for steroid-refractory UC. However, there was a strong trend for proven CMV colitis as a risk factor for steroid-refractory UC based on univariate analysis (OR, 2.47; 94% CI, 0.95–6.40; P = .063).
Table 2.
Univariate and Multivariate Analyses of the Risk Factors Linked to Poor Steroid Response in Patients with Moderate to Severe UC
| Univariate Analysis | Multivariate Analysisb | |||
|---|---|---|---|---|
| Characteristics | Unadjusted OR (95% CI) | P | Adjusted OR (95% CI) | P |
| Age | 1.03 (0.99–1.06) | .21 | ||
| Mayo score | 6.93 (2.38–20.16) | <.001 | 2.00 (1.28–3.14) | .002 |
| General laboratory test results | ||||
| Albumin, g/dL | 0.25 (0.11–0.61) | .02 | ||
| C-reactive protein, mg/L | 1.11 (1.02–1.22) | .02 | ||
| Recent use of steroids | 3.41 (1.27–9.15) | .015 | ||
| Laboratory tests for CMV infection | ||||
| Quantitative blood CMV PCR | 1.68 (1.21–2.32) | .002 | 3.58 (1.03–12.34) | .044 |
| Colonic tissue CMV PCR (>10 copies/mg) | 2.47 (0.95–6.40) | .063 | ||
| CMV colitisa | 1.89 (0.74–4.81) | .183 | ||
| Proven | 2.47 (0.95–6.40) | .063 | ||
| Possible | 0.73 (0.62–8.55) | .798 | ||
Abbreviations: CI, confidence interval; CMV, cytomegalovirus colitis; OR, odds ratio; PCR, polymerase chain reaction.
aCMV colitis was defined as proven CMV colitis and/or possible CMV colitis.s based on the criteria defined in the “Methods.”
bThe following variables were included in the multivariate analysis: Mayo score, albumin, C-reactive protein, recent use of steroids, quantitative blood CMV PCR, colonic tissue CMV PCR, and CMV colitis (proven and possible).
DISCUSSION
Several studies indicate that CMV infection is associated with, or causes, refractory colitis and/or steroid refractoriness [2, 20–24]. However, some studies have contradicted these findings [5, 6]. The varying results of these studies could be attributed to the small sample sizes and heterogeneity in the study designs. Another possible source of bias is likely the variety of techniques used to characterize CMV infection and disease. In addition, the broad spectrum of investigated CMV markers was not evaluated in the previous studies [22, 23]. Owing to these limitations, no definite conclusions can be drawn from the available data.
To resolve these issues, we used both blood-based and tissue-based tests to identify CMV. First, we performed qPCR to evaluate CMV in the blood to diagnose CMV viremia. Second, we measured CMV in colonic tissue using qPCR. To avoid observer bias, a single experienced pathologist graded the CMV IHC results on a scale from 0 to 3. We also used cutting edge technology to identify the host response to CMV infection, including evaluating CMV-specific T-cell responses. We then comprehensively analyzed all the variables to identify which viral and host risk factors for CMV infection were associated with steroid refractoriness. We found that only higher blood CMV expression, as determined by qPCR, was independently associated with poor steroid response in patients with moderate to severe UC. Therefore, our data suggest that increased CMV replication in colonic tissue and decreased CMV-specific T-cell responses may not be associated with steroid refractoriness in moderate to severe UC but may merely reflect disease severity.
We initially thought that if CMV-specific T-cell responses and systemic or local viral replication were evaluated together, it would be possible to predict the host immunomodulatory or immunopathological effects of CMV infection as well as the response to steroid treatment in UC patients with CMV colitis. However, we found that host immune responses were not independently related to a poor response to steroid treatment. However, systemic viral replication was associated with a poor response to steroid treatment. Recent studies have indicated that measuring CMV-specific T cells may provide important information about CMV infection and help predict the risk of developing CMV disease, especially in immunocompromised patients, such as those with hemato-oncologic malignancies or transplant recipients [8, 9]. To the best of our knowledge, few studies have used CMV-specific T-cell response assays to evaluate CMV infection in IBD patients. Nowacki et al. showed that CMV-specific granzyme B and perforin-producing CD8 T cells in blood were elevated in IBD patients. In addition, active long-lasting IBD exhibited increased CMV expression, although CMV DNA could not be detected in the blood by qPCR analysis during flares. However, their study compared IBD patients with healthy controls and failed to establish whether CMV reactivation was a cause or consequence of IBD or whether increased CMV-specific T-cell activity was directly associated with CMV colitis [25]. In the present study, we performed CMV-specific ELISPOT to determine whether the IE-1 protein and pp65 were associated with steroid-refractory UC; however, we did not obtain any evidence that the decreased T-cell response to CMV was associated with a poor response to steroid treatment in patients with moderate to severe UC. This discrepancy between our study and that of Nowacki et al. may be due to the difference in the level of immunosuppression between hemato-oncologic malignancy patients/transplant recipients and UC patients.
Previous studies have indicated that higher CMV load is associated with a poor clinical outcome in patients with IBD [20–22]. Recent studies also showed that positive CMV DNA expression in colonic tissue via PCR is associated with a longer time to steroid-free UC remission, with an increased rate and earlier need for proctocolectomy [23, 24]. In the current study, colonic tissue CMV viral load was not independently associated with a poor steroid response in moderate to severe UC. We assumed that expression of CMV DNA in colonic tissue could not distinguish active CMV colitis from CMV colonization or past CMV infection. Instead, high CMV expression in the blood via qPCR was independently associated with a poor response to steroid therapy, which is consistent with a previous study reporting that CMV antigenemia was associated with subsequent colectomy [14]. CMV infection or reactivation may induce persistent immunological dysfunction, which not only plays a key role in the aggravation of UC but is also related to steroid refractoriness [26]. We therefore speculate that evidence for systemic CMV infection or reactivation, such as CMV viremia, may reflect this pathophysiologic sequence. We suggest that, of the various laboratory parameters, simple and noninvasive quantitative CMV PCR using blood samples could represent the most useful strategy to investigate CMV infection in patients with moderate to severe UC.
There were, however, several limitations to this study. First, the seropositivity of CMV is high in Korea; thus, caution needs to be exercised while applying our findings to other populations. Second, although both pp65 and IE-1 are considered to play an important role in targeting T cells in CMV infections [9], the optimal target antigens for achieving a protective T-cell response in CMV infection are not known. Therefore, further studies are needed before applying our findings to other target antigens. Third, we measured CMV-specific cell-mediated immunity only at the time of steroid treatment. Thus, we could not assess whether CMV-specific immune responses were already affected before steroid therapy or in mild to moderate UC. Cell-mediated immunity is not static, and dynamic changes in CMV cell-mediated immunity may depend on the severity of UC or steroid treatment. Fourth, some host and viral risk factors for CMV were statistically significant in the univariate but not in the multivariate analysis. This may be attributed to the small sample size and heterogeneity of disease severity, which may have reduced the statistical power. In addition, although a trend was observed in the proven CMV colitis patients with steroid refractory moderate to severe UC, the small sample size is a barrier to identifying any association between CMV colitis and response to steroid treatment in these patients.
In conclusion, in patients with moderate to severe UC, higher disease severity and positive blood CMV expression by qPCR rather than high local CMV replication or low systemic CMV-specific T-cell response appear to be independently associated with steroid refractoriness.
Acknowledgments
Author contributions. Suk-Kyun Yang and Sung-Han Kim: study concept and design. Kyung Hwa Jung: drafting. Kyung Hwa Jung, Jene Choi, Ho-Su Lee, Sung-Han Kim: analysis and interpretation. Jihun Kim, Sung-Han Kim: critical revision of the manuscript. Jihun Kim, Se Jin Jang, Jiwon Jung, Min Jae Kim, Yong Pil Chong, Sang-Oh Lee, Sang-Ho Choi, Yang Soo Kim, Jun Hee Woo, Sang Hyoung Park, Dong-Hoon Yang, Byong Duk Ye, Suk-Kyun Yang, Sung-Han Kim: study supervision.
Financial support. This work was supported by a grant from the National Research Foundation of Korea, funded by the Ministry of Science, Information Communication Technology & Future Planning (MSIT) (grant NRF-2018R1D1A1A09082099) and the Asan Institute for Life Sciences (2015-0129). H.-S. Lee was supported by a National Research Foundation of Korea (NRF) Medical Research Council (MRC) grant funded by the Korean government (MSIT, 2018R1A5A2020732).
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Kumar S, Ghoshal UC, Aggarwal R, et al. Severe ulcerative colitis: prospective study of parameters determining outcome. J Gastroenterol Hepatol 2004; 19:1247–52. [DOI] [PubMed] [Google Scholar]
- 2. Lee HS, Park SH, Kim SH, et al. Risk factors and clinical outcomes associated with cytomegalovirus colitis in patients with acute severe ulcerative colitis. Inflamm Bowel Dis 2016; 22:912–8. [DOI] [PubMed] [Google Scholar]
- 3. Rubin DT, Ananthakrishnan AN, Siegel CA, et al. ACG clinical guideline: ulcerative colitis in adults. Am J Gastroenterol 2019; 114:384–413. [DOI] [PubMed] [Google Scholar]
- 4. Lawlor G, Moss AC. Cytomegalovirus in inflammatory bowel disease: pathogen or innocent bystander? Inflamm Bowel Dis 2010; 16:1620–7. [DOI] [PubMed] [Google Scholar]
- 5. do Carmo AM, Santos FM, Ortiz-Agostinho CL, et al. Cytomegalovirus infection in inflammatory bowel disease is not associated with worsening of intestinal inflammatory activity. PLoS One 2014; 9:e111574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Delvincourt M, Lopez A, Pillet S, et al. The impact of cytomegalovirus reactivation and its treatment on the course of inflammatory bowel disease. Aliment Pharmacol Ther 2014; 39:712–20. [DOI] [PubMed] [Google Scholar]
- 7. Matsuoka K, Iwao Y, Mori T, et al. Cytomegalovirus is frequently reactivated and disappears without antiviral agents in ulcerative colitis patients. Am J Gastroenterol 2007; 102:331–7. [DOI] [PubMed] [Google Scholar]
- 8. Bae S, Jung J, Kim SM, et al. The detailed kinetics of cytomegalovirus-specific T cell responses after hematopoietic stem cell transplantation: 1 year follow-up data. Immune Netw 2018; 18:e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim T, Lee HJ, Kim SM, et al. Diagnostic usefulness of the cytomegalovirus (CMV)-specific T cell-based assay for predicting CMV infection after kidney transplant. Korean J Intern Med. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yang SK, Hong WS, Min YI, et al. Incidence and prevalence of ulcerative colitis in the Songpa-Kangdong District, Seoul, Korea, 1986–1997. J Gastroenterol Hepatol 2000; 15:1037–42. [DOI] [PubMed] [Google Scholar]
- 11. Lennard-Jones JE. Classification of inflammatory bowel disease. Scand J Gastroenterol Suppl 1989; 170:2–6; discussion 16–9. [DOI] [PubMed] [Google Scholar]
- 12. Travis SP, Schnell D, Krzeski P, et al. Reliability and initial validation of the ulcerative colitis endoscopic index of severity. Gastroenterology 2013; 145:987–95. [DOI] [PubMed] [Google Scholar]
- 13. Travis SP. Predicting outcome in severe ulcerative colitis. Dig Liver Dis 2004; 36:448–9. [DOI] [PubMed] [Google Scholar]
- 14. Kim JW, Boo SJ, Ye BD, et al. Clinical utility of cytomegalovirus antigenemia assay and blood cytomegalovirus DNA PCR for cytomegaloviral colitis patients with moderate to severe ulcerative colitis. J Crohns Colitis 2014; 8:693–701. [DOI] [PubMed] [Google Scholar]
- 15. Beaugerie L, Cywiner-Golenzer C, Monfort L, et al. Definition and diagnosis of cytomegalovirus colitis in patients infected by human immunodeficiency virus. J Acquir Immune Defic Syndr Hum Retrovirol 1997; 14:423–9. [DOI] [PubMed] [Google Scholar]
- 16. Kandiel A, Lashner B. Cytomegalovirus colitis complicating inflammatory bowel disease. Am J Gastroenterol 2006; 101:2857–65. [DOI] [PubMed] [Google Scholar]
- 17. Kishore J, Ghoshal U, Ghoshal UC, et al. Infection with cytomegalovirus in patients with inflammatory bowel disease: prevalence, clinical significance and outcome. J Med Microbiol 2004; 53:1155–60. [DOI] [PubMed] [Google Scholar]
- 18. Pillet S, Williet N, Pouvaret A, et al. Distribution of cytomegalovirus DNA load in the inflamed colon of ulcerative colitis patients. Am J Gastroenterol 2016; 111:439–41. [DOI] [PubMed] [Google Scholar]
- 19. Ljungman P, Boeckh M, Hirsch HH, et al. ; Disease Definitions Working Group of the Cytomegalovirus Drug Development Forum Definitions of cytomegalovirus infection and disease in transplant patients for use in clinical trials. Clin Infect Dis 2017; 64:87–91. [DOI] [PubMed] [Google Scholar]
- 20. Long MD, Onyiah JC, Miller M, Herfarth HH. Cytomegalovirus viral load in the colon and risk of colectomy in hospitalized patients with inflammatory bowel diseases. Inflamm Bowel Dis 2016; 22:E21–2. [DOI] [PubMed] [Google Scholar]
- 21. Jones A, McCurdy JD, Loftus EV Jr, et al. Effects of antiviral therapy for patients with inflammatory bowel disease and a positive intestinal biopsy for cytomegalovirus. Clin Gastroenterol Hepatol 2015; 13:949–55. [DOI] [PubMed] [Google Scholar]
- 22. Zagórowicz E, Bugajski M, Wieszczy P, et al. Cytomegalovirus infection in ulcerative colitis is related to severe inflammation and a high count of cytomegalovirus-positive cells in biopsy is a risk factor for colectomy. J Crohns Colitis 2016; 10:1205–11. [DOI] [PubMed] [Google Scholar]
- 23. Schenk W, Klugmann T, Borkenhagen A, et al. The detection of the cytomegalovirus DNA in the colonic mucosa of patients with ulcerative colitis is associated with increased long-term risk of proctocolectomy: results from an outpatient IBD clinic. Int J Colorectal Dis 2019; 34:393–400. [DOI] [PubMed] [Google Scholar]
- 24. Tun GSZ, Raza M, Hale MF, Lobo AJ. Polymerase chain reaction for detection of mucosal cytomegalovirus infection in patients with acute ulcerative colitis. Ann Gastroenterol 2019; 32:81–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nowacki TM, Bettenworth D, Ross M, et al. Cytomegalovirus (CMV)-specific perforin and granzyme B ELISPOT assays detect reactivation of CMV infection in inflammatory bowel disease. Cells 2012; 1:35–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xue M, Chen SJ, Wang LJ, et al. Cytomegalovirus: a probable cause of steroid-refractory ulcerative colitis. J Dig Dis 2013; 14:160–5. [DOI] [PubMed] [Google Scholar]