Abstract
Background:
Beta-blockade administration after lung contusion, hemorrhagic shock and chronic stress has been shown to improve bone marrow function, decrease hypercatecholaminemia and reduce inflammation. microRNAs (miR) are critical biologic regulators, that can downregulate gene expression by causing messenger RNA degradation or inhibition of translation. This study sought to expand our understanding of the molecular mechanisms underlying the reduced inflammatory response following the administration of beta blockade in our rodent trauma model.
Study Design:
Male Sprague-Dawley rats aged 8–9 weeks were randomized to lung contusion, hemorrhagic shock with daily restraint stress (LCHS/CS) ± propranolol (LCHS/CS+BB). Restraint stress occurred two hours daily following LCHS. Propranolol (10 mg/kg) was given daily until day seven. Total RNA & miR were isolated from bone marrow and genome-wide miR expression patterns were assayed. Bone marrow cytokine expression was determined with qPCR.
Results:
LCHS/CS led to significantly increased bone marrow expression of IL-1β, TNF-α, IL-6, nitric oxide (NO) and plasma C-reactive protein. There were marked differences in expression of 45 miRs in the LCHS/CS+BB group when compared to the LCHS/CS, when using a p<0.001. Rno-miR-27a and miR-25 were upregulated 7–8 fold in the rodents who underwent LCHS/CS+BB when compared LCHS/CS alone and this correlated with reduced bone marrow expression of IL-1β, TNF-α, IL-6, NO and reduced plasma C-reactive protein in the LCHS/CS+BB group.
Conclusions:
The genomic and miR expression patterns in bone marrow following LCHS/CS differed significantly when compared to rodents that received propranolol following LCHS/CS. The use of beta blockade following severe trauma may help mitigate persistent inflammation by upregulating Rno-miR-27a and miR-25 and reducing inflammatory cytokines in those who remain critically ill.
Graphical Abstract
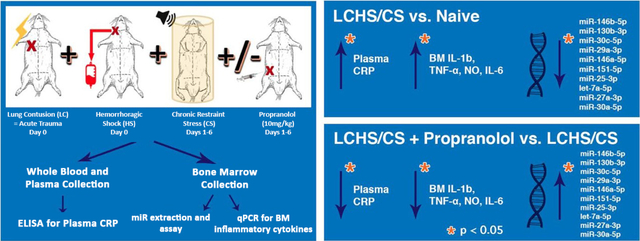
CONCLUSION: The use of beta blockade following severe trauma may help mitigate persistent inflammation by upregulating inflammation-related miRs and reducing inflammatory cytokines in those who remain critically ill.
Precis
Severe trauma and stress in a rodent model led to significantly altered bone marrow (BM) expression of 45 microRNAs (miR). The use of propranolol modified these expression patterns. BM miR-25–3p and miR-27a appear to play a major role in chronic inflammation since their reduced downregulation correlated with reduced BM expression of IL-1β, TNF-α, IL-6, nitric oxide.
Introduction
Severe traumatic injury and hemorrhage often lead to chronic anemia. Critically ill trauma patients who remain in the intensive care unit are universally anemic and this anemia persists for months after initial injury.(1, 2) Previous studies have demonstrated that this persistent injury-associated anemia is accompanied by a prolonged inflammatory response and persistent hypercatecholaminemia.(3–6) This clinical condition can be ‘reverse translated’ to a rodent model of lung contusion, hemorrhagic shock and chronic stress, which replicates this persistent injury-associated anemia and has elucidated a multifactorial etiology.(7, 8) Following trauma, bone marrow dysfunction manifests as myelo-erythroid reprioritization, decreased growth of bone marrow erythroid progenitor cells, loss of bone marrow progenitor cells from the bone marrow to peripheral blood and sites of injury, along with iron dysregulation that is linked to chronic inflammation.(9–12) In addition, both human and rodent studies have demonstrated that a reduction in hypercatecholaminemia and inflammation with propranolol or clonidine can mitigate this persistent injury-associated anemia and improve bone marrow erythropoietic function.(3, 13–15) Therefore, continuing to focus on potential targets of inflammatory processes would provide novel treatment strategies for the treatment of erythropoietic dysfunction following trauma.
Trauma and shock initiate a complex inflammatory response where many cytokines, including interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), C-reactive protein (CRP) and nitric oxide (NO), play a pivotal role. IL-6 and TNF-α are both pro-inflammatory cytokines that influence hematopoiesis and have altered expression following severe trauma.(16–19) IL-6 upregulates hepcidin production, leading to the sequestration of iron in macrophages, and also activates secretion of CRP.(20, 21) Norepinephrine, a key modulator of hematopoietic progenitor mobilization and an inhibitor of bone marrow erythroid cell growth at high levels, also directly stimulates IL-6 release.(22) Interleukin-1β (IL-1β) and IL-6 levels have also been found to be increased early after traumatic brain injury and there are marked alterations in the NO pathway following hemorrhagic shock, along with enhanced formation of NO following systemic inflammation.(23)
microRNA (miR) are 18–24 nucleotide non-coding RNAs, which act as epigenetic regulators in biologic processes and have been identified as major regulators of hematopoiesis.(24–27) These miRs are secreted by a variety of cells through exosomes or protein-mediated pathways. Extracellular miRs are highly stable and not only serve as biomarkers for various diseases, they also act as signaling molecules. As biologic regulators, circulating miRs can function as endogenous miRs regulating target genes. miRs can downregulate gene expression by sequence specific base pairing at the untranslated regions of the target gene messenger RNA, resulting in messenger RNA degradation or inhibition of translation.(25) In addition to translational repression, miRs can also act directly as physiologic ligands for certain RNA receptors but the functional consequences are yet to be defined. (28, 29)
The importance of miRs in the pathogenesis of erythropoietic dysfunction and the role of inflammation is unknown. Therefore, an understanding of the role of miRs in regulating inflammation presents a novel approach to improve bone marrow dysfunction following trauma. This study sought to determine if the expression of miRs is altered in rodents who undergo lung contusion, hemorrhagic shock and chronic stress (LCHS/CS) when compared to rodents who undergo LCHS/CS followed by daily propranolol injection. Since miR mainly result in mRNA degradation or inhibition of translation, we hypothesize that the use of propranolol following LCHS/CS may increase inflammation-related miRs, subsequently leading to a suppression of inflammatory cytokines.
Methods
Rodents
Male Sprague-Dawley rats (Charles River, Wilmington, MA) aged 8–9 weeks, weighing 275g to 325g were housed in pairs with ad lib access to food and water, during daily night/day cycles of 12 hours each. Female animals were excluded due to estrous cycle variability and its impact after hemorrhagic shock. The animal protocol was approved by the University of Florida Institutional Animal Care and Use Committee.
Experimental Rodent Model
Rodents were randomly assigned into one of three groups (n=6/group): 1) naïve control; 2) lung contusion followed by hemorrhagic shock and chronic stress in a restraint cylinder for 2h/day (LCHS/CS); 3) LCHS/CS with propranolol injection (LCHS/CS+BB). Naïve rodents underwent daily handling for seven days. Propranolol (10mg/kg) was administered by intraperitoneal injection ten minutes following LCHS and daily for six days following chronic stress. All rodents were weighed daily using a Kent Scientific animal weighing scale (Torrington, CT, USA). All rodents were sacrificed on day seven. Immediately after sacrifice, the left femur and peripheral blood were harvested. Peripheral blood was centrifuged for fifteen minutes at 2500g, and plasma was collected. Bone marrow and plasma were stored at −80°C.
Lung Contusion and Hemorrhagic Shock
As previously described, after intraperitoneal pentobarbital injection, a unilateral lung contusion (LC) was made by a percussive nail gun (Sears Brand, Chicago, IL) applied directly to a 12 mm metal plate that was placed in the right axilla of the rodent.(13) Ten minutes after LC, the right femoral artery and right internal jugular vein were cannulated using heparinized saline (10 units/ml). The femoral artery tubing was connected to a continuous BP-2 Digital Blood Pressure Monitor device (Columbus Instruments, Columbus, Ohio) for measurement of mean arterial pressure and heart rate. Blood was then withdrawn from the internal jugular vein to maintain a mean arterial pressure of 30–35 mmHg for 45 minutes. Shed blood was reinfused.
Chronic Restraint Stress
Chronic stress (CS) consisted of two hours of restraint in a nose cone rodent cylinder (Kent Scientific Corporation, Torrington, CT, USA) daily for six days. Rodents were repositioned in the cylinder every 30 minutes to prevent habituation and during which alarms sounded for 2 minutes. The rodents in LCH/CS models were restricted from food and water during chronic stress periods.
miRNA Isolation and Analysis
Following removal of the femur in the LCHS/CS and LCHS/CS+BB groups, the bone marrow was reamed using a 19g needle and 3mL syringe containing 1mL of Iscove’s Modified Dulvecco’s Medium (IMDM) with 2% FBS (Stem Cell, Vancouver, Canada). The bone marrow was centrifuged at 1200rpm in a microcentrifuge for 5 minutes. The supernatant was then decanted. 600μL of RNA lysis buffer (1mL of RLT from RNeasy Mini Kit, Qiagen and 10μL of 2-mercaptoethanol, Sigma®) was then added to the cell pellet and homogenized using Pro 200 Homogenizer (PRO Scientific, Oxford CT, USA). The samples were stored at −80°C.
RNA extraction was performed using RNeasy Mini Kit (Qiagen, Hilden, Germany) following the manufacturer’s instructions. RNA was quantified using Biotek Micro-volume plate in Biotek Instruments. Ratio of absorbance at 260/280nm was taken for RNA purity. Samples were diluted using RNA free water to a final concentration of 2μg RNA/ 10μL.
Genome wide gene and miR expression was assayed using a GeneChip miRNA 4.0 Assay using ThermoFisher standard protocols (Thermo Fisher Scientific, Waltham, Massachussetts). 1231 genes passed filtering criteria. Fold change expressions were calculated only for miRs with significant differences in expression (#p<0.01) between the LCHS/CS, LCHS/CS+BB and naïve groups and were expressed as fold expression changes over naïve. Statistical analysis was performed using BRB Array Tool (**p<0.01, t-test). For visualization, expression differences were clustered using a heat map.
Bone Marrow Analysis
Real-time polymerase chain reaction (PCR) was performed to analyze the expression of bone marrow inflammatory cytokines, including IL-1β, TNF-α, NO and IL-6. Following synthesis of bone marrow RNA, cDNA synthesis was performed using High-Capacity cDNA Reverse Transcription Kits (Applied Biosystems, Vilnius, Lithuania) as per manufacturer’s instructions. After the cDNA was made, a stock was made by diluting it five times with nuclease free water and this was stored frozen until use. Real-time polymerase chain reaction was performed using Brilliant II SYBR Green QPCR Master Mix (Agilent Technologies, Santa Clara, CA) and the Mx3005P qPCR System (Agilent Technologies, Santa Clara, CA), reported as mRNA fold change relative to controls. Primers were designed using Primer3 Web software (Table 1).
Table 1.
Polymerase Chain Reaction Primers
| Variable | Forward primer | Reverse primer |
|---|---|---|
| TNF alpha | 5-GAAACACACGAGACGCTGAA-3 | 5-GAAAGCCCATTGGAATCCTT-3 |
| IL-1β | 5-CAGGAAGGCAGTGTCACTCA-3 | 5-AAAGAAGGTGCTTGGGTCCT-3 |
| IL-6 | 5-TGATGGATGCTTCCAAACTG-3 | 5-GAGCATTGGAAGTTGGGGTA-3 |
| Nitric oxide | 5-GGATCTAGACACCCGGACAA-3 | 5-CTGTACAGCACAGCCACGTT-3 |
| Beta actin | 5-AGCCATGTACGTAGCCATCC-3 | 5-CTCTCAGCTGTGGTGGTGAA-3 |
Plasma Analysis
Plasma CRP (ThermoFisher Scientific, Frederick, MD) was measured by sandwich enzyme linked immunosorbent assay. All samples were run in duplicate following the manufacturer’s protocol.
Statistical Analysis
Statistical analysis was performed using BRB Array Tool for the miRNA expression and statistical significance was set at #p<0.001. GraphPad Prism version 7.05 (GraphPad Software, La Jolla, CA) was used to determine differences in bone marrow PCR values and plasma ELISA values. Significance was set at *p<0.05 and data were reported as mean ± standard deviation (SD).
Results
Propranolol Use Altered miR Expression following Severe Trauma and Chronic Stress
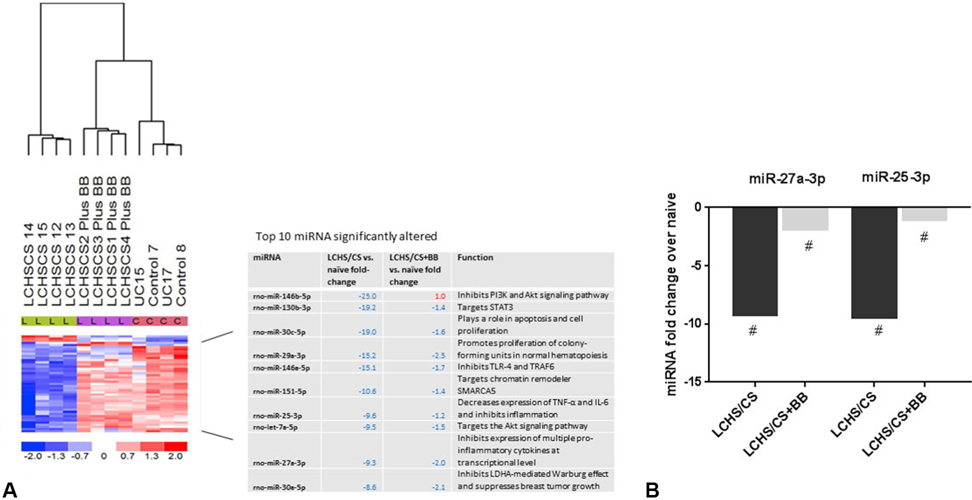
1232 genes passed filtering criteria in our comparison. Supervised hierarchical clustering heatmap found 45 miRs that had a significant difference in expression across LCHS/CS, LCHS/CS+BB and naïve groups (#p<0.001) (Figure 1A). This heat map shows a marked difference in the expression of 45 miRs, and the differences cluster by treatment group. The LCHS/CS group has a markedly different miR expression profile when compared to the naïve rodents. However, the use of propranolol following LCHS/CS altered the miR expression profile on the heat map to be similar to that of the naïve animals (Figure 1A).
Figure 1.
Altered bone marrow miRNA expression. (A) Supervised hierarchical clustering heatmap showing 45 miRNAs that had a significant difference in expression between the LCHS/CS, LCHS/CS+BB and naïve rodents. The use of propranolol following LCHS/CS resulted in similar miRNA expression to that of naïve rodents. Table shows top 10 miRNA most significantly altered in their expression when compared to naïve rodents. (B) miR-27a-3p and miR-25–3p expression is suppressed 9-fold in LCHS/CS when compared to naïve rodents and only 1–2 fold in LCHS/CS+BB when compared to naïve rodents. #p<0.001.
The 45 miRs that were significantly altered were queried for any relevance to inflammation or hematopoiesis. The top ten miRs suppressed 10–25-fold by LCHS/CS were focused on to narrow our search (Figure 1A). LCHS/CS led to significant 15–25 fold downregulation of miR-146b, miR-130b, miR-30c, miR-29a, and miR-146a. In particular, RnomiR-27a and miR-25 have been linked to the suppression of inflammatory cytokines (IL-1β, TNF-α, NO and IL-6) and were found to be downregulated 9.3 and 9.5 fold in the LCHS/CS group respectively when compared to the naïve (Figure 1B). Rno-miR-27a and miR-25 were found to be downregulated 2 and 1.2 fold respectively in the LCHS/CS+BB group when compared to the naïve rodent group (Figure 1B). Therefore, the use of propranolol following LCHS/CS upregulated Rno-miR-27a and miR-25 expression 7–8 fold when compared to the LCHS/CS alone.
Reduction of Bone Marrow Inflammatory Cytokine Expression with Propranolol Use
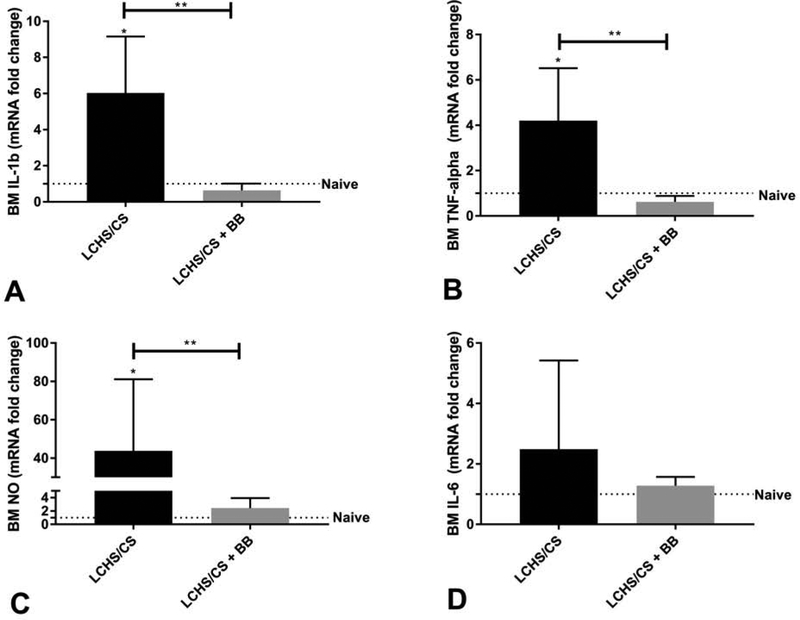
LCHS/CS led to a significant increase in the bone marrow expression of IL-1β when compared to naïve rodents (*p=0.0099). When propranolol was given following LCHS/CS, the bone marrow expression of IL-1β was significantly decreased when compared to LCHS/CS (**p=0.0092) (see Figure 2A). Following LCHS/CS+BB the expression of IL-1β was similar to that of naïve animals.
Figure 2.
Altered bone marrow inflammatory cytokine expression. (A) IL-1β expression was significantly increased in LCHS/CS, and significantly reduced after LCHS/CS+BB. (B) TNF-α expression was significantly increased in LCHS/CS, and significantly reduced after LCHS/CS+BB. (C) NO expression was significantly increased in LCHS/CS, and significantly reduced following LCHS/CS+BB. (D) Expression of IL-6 after similar patterns but was not statistically significant. *p<0.05 vs naïve rodents; **p<0.05 between groups.
Similarly, LCHS/CS led to significant increase in the bone marrow expression of TNF-α when compared to both naïve rodents and LCHS/CS+BB rodents (*p=0.0206). When propranolol was given following LCHS/CS, the bone marrow expression of TNF-α was significantly decreased when compared to LCHS/CS (**p=0.0113) (see Figure 2B). Following LCHS/CS+BB the expression of TNF-α was similar to that of naïve animals.
The bone marrow expression of NO following LCHS/CS rats was significantly increased when compared to naïve rodents (*p=0.0413). When propranolol was given following LCHS/CS, the bone marrow expression of NO was significantly decreased when compared to LCHS/CS (**p=0.0479) (see Figure 2C). Following LCHS/CS+BB the expression of NO was similar to that of naïve animals.
While LCHS/CS did lead to increased bone marrow expression of IL-6 there was no statistical significance (Figure 2D). In addition, the LCHS/CS+BB rodents had a decreased expression of IL-6 similar to naïve animals but again this was not statistically significant when compared to LCHS/CS alone.
Propranolol Use following Severe Trauma and Chronic Stress Reduced CRP Levels
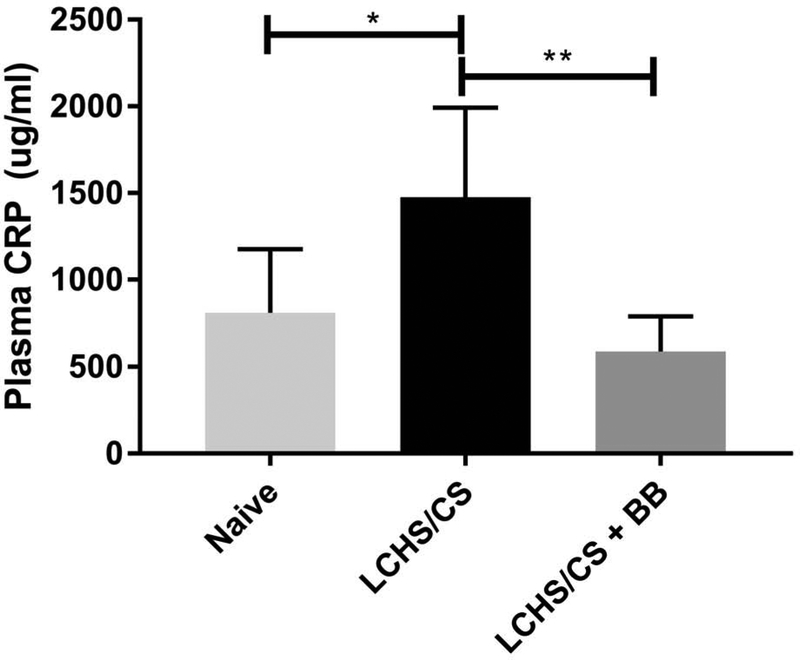
Plasma CRP concentration was significantly increased following LCHS/CS when compared to naïve rodents (*p=0.0063) (Figure 3). The use of propranolol following LCHS/CS led to a significant decrease in plasma CRP concentration when compared to LCHS/CS alone (**p=0.0004) (Figure 3).
Figure 3.
Plasma C-reactive protein (CRP) concentration after severe trauma. Plasma CRP concentration is significantly increased after LCHS/CS and significantly reduced after LCHS/CS+BB. *p<0.05 vs. naïve rodents; **p<0.05 between groups.
Discussion
In the present study, we found significant differences in 45 bone marrow miRs and several bone marrow cytokine profiles following LCHS/CS and LCHS/CS+BB when compared to naïve animals. Our rodent trauma model of lung contusion and hemorrhagic shock followed by chronic stress led to significantly increased bone marrow expression of IL-1β, TNF-α, IL-6, NO and plasma levels of CRP seven days after initial injury. The addition of chronic stress following lung contusion and hemorrhagic shock maintained the elevation of these inflammatory markers. Previous studies have shown that circulating IL-1β, TNF-α, and CRP are significantly elevated early after hemorrhagic shock, hypoxia and ischemia-reperfusion injury.(30–32) Elevations of circulating IL-6 occur early and have been shown to correlate with severe trauma and degree of tissue injury.(33) In addition, our analysis revealed 45 bone marrow miRs with significantly altered expression following LCHS/CS when compared to those rodents that received propranolol following LCHS/CS and naïve rodents. This study provides evidence that bone marrow miR-25–3p and Rno-miR-27a play a major role in chronic inflammation following lung injury, hemorrhagic shock and chronic stress, which is consistent with previously published studies. The reduced downregulation of Rno-miR-25–3p and Rno-miR-27a correlated with reduced bone marrow expression of IL-1β, TNF-α, IL-6, NO and reduced plasma levels of CRP following the use of propranolol after LCHS/CS.
Several studies have studied miRs in relation to important inflammatory cytokines, such as cyclooxygenase-2, IL-2, IL-4, IL-5, IL-9 and prostaglandin E2.(16–19) Specific miRs have been shown to have differential expression in asthma, COPD, and idiopathic pulmonary fibrosis where these inflammatory cytokines are elevated.(34–37) In addition to inflammation, miRs are essential to stem cell renewal, lineage selection, cell expansion, differentiation with myeloid or erythroid commitment and apoptosis. In our study, Rno-miR-146b-5p was the most suppressed miRNA in the LCHS/CS group when compared to those rodents that received propranolol following LCHS/CS and naïve rodents, with a 25-fold suppression in expression. Li et al.(38) showed that miR-146b-5p was downregulated in hepatocellular carcinoma and correlated with poor prognosis. miR-146b-5p inhibited tumor growth by a suppression of proliferation, migration and invasion through inhibiting the phosphoinositide 3-kinase (PI3K) and Akt (protein kinase B) signaling pathway.(38) miR Let-7a-5p expression was also suppressed 9.5-fold following LCHS/CS when compared to LCHS/CS+BB and naïve animals and its expression has been studied in thyroid carcinoma and cellular proliferation in relation to the Akt pathway.(39) The PI3K-Akt signaling pathway plays an important role in erythropoiesis and can downregulate apoptosis of erythroblasts.(40) miR-146a was also suppressed 15-fold in the LCHS/CS group when compared to LCHS/CS+BB and naïve rodents. He et al.(41) found a decreased expression of miR-146a in patients with small intestine ischemia and ischemia reperfusion injury and determined that improved intestine epithelial cell survival occurs with inhibition of toll like receptor 4 and tumor necrosis factor receptor-associated factor-6 (TRAF6) which subsequently led to decreased nuclear factor kappa light chain enhancer of activated B cells (NF-κB). NF-κB has been shown to play a role in early erythroid proliferation and suppression of erythroid specific genes.(42) These miRNA findings correlate with our previous studies demonstrating significant erythroid growth suppression following LCHS/CS and improvement in inflammation and erythropoiesis with the use of propranolol following LCHS/CS.(3, 13, 14, 43)
Also, bone marrow miR-130b expression was suppressed in the LCHS/CS group when compared to LCHS+BB and naïve rodents. Previous studies have demonstrated that miR-130b downregulation played a role in prostate and pancreatic cancer likely through its targeting of STAT3.(34, 35) STAT3 can act as negative regulator of erythroid differentiation.(44) Bone marrow miR-30c-5p expression was decreased 19-fold and miR-29a expression was decreased 15-fold in the LCHS/CS group when compared to LCHS/CS+BB and naïve rodents. Both miR-30c-5p and miR-29a play a role in apoptosis and cellular proliferation (36–38). Bone marrow miR-29a has also been shown to promote proliferation of colony-forming unit (CFU)-GM, CFU-G and CFU-M in normal hematopoiesis and its expression has been found to be significantly decreased in acute myeloid leukemia patients, suggesting that it might be a biomarker of abnormal myeloid differentiation.(45) This correlates with several previous studies demonstrating dysfunctional myelopoiesis after severe trauma and burns.(46, 47)
Several studies, including our rodent model of lung contusion, hemorrhagic shock and chronic stress have demonstrated that LCHS/CS is associated with a hypercatecholaminemia which leads to a prolonged inflammatory state and dysfunctional erythropoiesis.(5, 6, 9, 12, 48, 49) Treatment with propranolol has been shown to improve erythroid progenitor growth, decrease plasma granulocyte-colony stimulating factor, decrease mobilization of hematopoietic progenitor cells from the bone marrow, reduce inflammation and improve erythropoiesis after severe trauma.(3, 13, 14, 43) In the current study, both miR-25–3p and Rno-miR-27a were downregulated 9-fold following LCHS/CS when compared to LCHS/CS and naïve animals. Both miR-25–3p and Rno-miR-27a have been shown to play a role in inflammation. Yao et al. (50) demonstrated in Sprague Dawley rats that improved expression of miR-25 decreased expression of TNF-α and IL-6 following lipopolysaccharide (LPS) stimulation and inhibited inflammation.(51, 52) These findings correlate with our study, demonstrating that use of propranolol following LCHS/CS was associated with both the improved expression of miR-25 and reduced bone marrow expression of TNF-α and IL-6. Min et al (53) revealed that miR-27a influenced the production of pro-inflammatory cytokines by targeting mitogen-activated protein kinases (MAPK) in the dendritic cell-mediated differentiation of T cells. Human monocyte-derived dendritic cells transfected with miR-27a inhibited the expression of multiple cytokines, including TNF- α, IL-1β, IL-6 and IL-12, at both the transcriptional and protein levels after exposure to LPS. Cheng et al. (54) further studied the downstream targets of miR-27a and determined that upregulation of miR-27a targeted monocyte chemotactic protein-1 induced protein-1, which when overexpressed led to 50% suppression of IL-6 and IL-1β.(55) In addition, Lv et al. (56) investigated the effect of miR-27a on LPS-induced microglial inflammation, and found that over-expression of miR-27a caused suppression of IL-1β, TNF-α, NO and IL-6 microglia expression and downregulation of miR-27a expression significantly increased the expression of these pro-inflammatory cytokines. Both miR-25–3p and Rno-miR-27a may play key roles in the sustained inflammation seen after LCHS/CS and the reduced inflammation seen following the use of propranolol after LCHS/CS.
Similarly, CRP is an acute phase reactants released in response to IL-6, and has been used as a marker for cardiac outcomes, acute tissue injury and inflammation.(57) Moreover, trends in CRP have been used to monitor ongoing inflammation in severely injured trauma patients.(58) Due to the transient nature of the pro-inflammatory cytokines in the plasma following injury, this study analyzed CRP as a surrogate for persistent systemic inflammation. Our study demonstrated a correlation between the bone marrow inflammatory cytokine expression and the plasma CRP concentration in the LCHS/CS rodents, as well as their reduction to naïve levels with the use of propranolol following LCHS/CS. Plasma CRP has been shown to influence miR profiles in damaged myocardium following ischemia-reperfusion injury.(59) Systemic infusion of CRP led to differential regulation of miR when comparing myocardial ischemia to non-ischemic myocardium.(59)
Epigenetics are a fascinating new avenue of study in trauma and inflammation because studies have already shown that the disruption of epigenetic processes is a major contributor in cancer development, and that we can target certain drivers of cancer on a DNA methylation or histone modification level to pharmacologically reverse these altered epigenetics.(59) We have shown in multiple studies that beta blockade is effective at reversing the bone marrow dysfunction and improving the inflammation seen in a trauma population. (3, 13, 15) However, because propranolol is a drug that may not be tolerated by all critically ill trauma patients, we can now study mechanisms whereby we could imitate the effects of propranolol that mitigates the inflammatory milieu that is seen in our severely injured trauma patients by directly targeting the inflammation-related miRs that are altered. These novel epigenetic therapeutics could help us to improve the persistent injury-associated anemia seen in our trauma patients long-term.
Limitations of this study are the small number of rodents which could impact the power of our study. We also excluded female rodents due to their variability in response to hemorrhagic shock dependent on their estrus state. Our study is further limited by the lack of knowledge regarding many miRs and their role following trauma and hemorrhagic shock as well their specific role in erythropoiesis and persistent inflammation. In order to link the modulation of pro-inflammatory cytokines in the bone marrow to the altered expression of these miRs, evaluation of their downstream targets needs to be performed with either a luciferase reporter assay or western blot of protein expression. miR-25 suppresses the activity of phosphatase and tensin homolog (PTEN) and miR27a impacts monocyte chemotactic protein-1 induced protein-1 (MCP1P1).(50, 54) Both PTEN and MCP1P1 are associated with decreased secretion of TNF-α, IL-6 IL-1β and IL-10.(50, 54) In addition, further studies are needed to determine whether the effects of these regulatory miRs can be replicated with selective beta blockade following severe trauma and chronic stress and determine if epigenetic modifiers can be targeted.
Conclusions
In summary, this study characterized the bone marrow inflammatory state that is present following severe trauma and prolonged stress. It also demonstrated that propranolol use was associated with both the mitigation of the inflammatory state with a reduction of bone marrow cytokine expression, as well as, an alteration of the expression of regulatory miRs. We identified two key regulators of systemic inflammation, miR-25–3p and Rno-miR-27a, that may play role in the improved inflammatory milieu in those rodents that received propranolol following severe trauma and prolonged stress. These data highlight how manipulation of miR expression may become important in clinical settings.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Information: Nothing to disclose.
Presented at the American College of Surgeons 105th Annual Clinical Congress, Scientific Forum, San Francisco, CA, October 2019.
References
- 1.Corwin HL, Gettinger A, Pearl RG, et al. The CRIT Study: Anemia and blood transfusion in the critically ill--current clinical practice in the United States. Crit Care Med. 2004;32(1):39–52. [DOI] [PubMed] [Google Scholar]
- 2.Rodriguez RM, Corwin HL, Gettinger A, et al. Nutritional deficiencies and blunted erythropoietin response as causes of the anemia of critical illness. J Crit Care. 2001;16(1):36–41. [DOI] [PubMed] [Google Scholar]
- 3.Mohr AM, ElHassan IO, Hannoush EJ, et al. Does beta blockade postinjury prevent bone marrow suppression? J Trauma. 2011;70(5):1043–9; discussion 9–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Millar JK, Kannan KB, Loftus TJ, et al. Persistent injury-associated anemia: the role of the bone marrow microenvironment. J Surg Res. 2017;214:240–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woolf PD, McDonald JV, Feliciano DV, et al. The catecholamine response to multisystem trauma. Arch Surg. 1992;127(8):899–903. [DOI] [PubMed] [Google Scholar]
- 6.Fonseca RB, Mohr AM, Wang L, et al. Adrenergic modulation of erythropoiesis following severe injury is mediated through bone marrow stroma. Surg Infect (Larchmt). 2004;5(4):385–93. [DOI] [PubMed] [Google Scholar]
- 7.Efron PA, Mohr AM, Moore FA, Moldawer LL. The future of murine sepsis and trauma research models. J Leukoc Biol. 2015;98(6):945–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alamo IG, Kannan KB, Loftus TJ, et al. Severe trauma and chronic stress activates extramedullary erythropoiesis. J Trauma Acute Care Surg. 2017;83(1):144–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bible LE, Pasupuleti LV, Gore AV, et al. Chronic restraint stress after injury and shock is associated with persistent anemia despite prolonged elevation in erythropoietin levels. J Trauma Acute Care Surg. 2015;79(1):91–6; discussion 6–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biffl WL, Moore EE, Moore FA, Peterson VM. Interleukin-6 in the injured patient. Marker of injury or mediator of inflammation? Ann Surg. 1996;224(5):647–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunser MW, Hasibeder WR. Sympathetic overstimulation during critical illness: adverse effects of adrenergic stress. J Intensive Care Med. 2009;24(5):293–316. [DOI] [PubMed] [Google Scholar]
- 12.Epstein J, Breslow MJ. The stress response of critical illness. Crit Care Clin. 1999;15(1):17–33, v. [DOI] [PubMed] [Google Scholar]
- 13.Alamo IG, Kannan KB, Bible LE, et al. Daily propranolol administration reduces persistent injury-associated anemia after severe trauma and chronic stress. J Trauma Acute Care Surg. 2017;82(4):714–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beiermeister KA, Keck BM, Sifri ZC, et al. Hematopoietic progenitor cell mobilization is mediated through beta-2 and beta-3 receptors after injury. J Trauma. 2010;69(2):338–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bible LE, Pasupuleti LV, Alzate WD, et al. Early propranolol administration to severely injured patients can improve bone marrow dysfunction. J Trauma Acute Care Surg. 2014;77(1):54–60; discussion 59–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gentile LF, Cuenca AG, Efron PA, et al. Persistent inflammation and immunosuppression: a common syndrome and new horizon for surgical intensive care. J Trauma Acute Care Surg. 2012;72(6):1491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Volpin G, Cohen M, Assaf M, et al. Cytokine levels (IL-4, IL-6, IL-8 and TGFbeta) as potential biomarkers of systemic inflammatory response in trauma patients. Int Orthop. 2014;38(6):1303–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kishimoto T IL-6: from its discovery to clinical applications. Int Immunol. 2010;22(5):347–52. [DOI] [PubMed] [Google Scholar]
- 19.Uddin MN, Zhang Y, Harton JA, et al. TNF-alpha-dependent hematopoiesis following Bcl11b deletion in T cells restricts metastatic melanoma. J Immunol. 2014;192(4):1946–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nemeth E, Rivera S, Gabayan V, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113(9):1271–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Golabek-Dropiewska K, Pawlowska J, Witkowski J, et al. Analysis of selected pro- and anti-inflammatory cytokines in patients with multiple injuries in the early period after trauma. Cent Eur J Immunol. 2018;43(1):42–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takaki A, Huang QH, Somogyvari-Vigh A, Arimura A. Immobilization stress may increase plasma interleukin-6 via central and peripheral catecholamines. Neuroimmunomodulation. 1994;1(6):335–42. [DOI] [PubMed] [Google Scholar]
- 23.Bamberger T, Masson I, Mathieu J, et al. Nitric oxide mediates the depression of lymphoproliferative responses following burn injury in rats. Biomed Pharmacother. 1992;46(10):495–500. [DOI] [PubMed] [Google Scholar]
- 24.Mattick JS. A new paradigm for developmental biology. J Exp Biol. 2007;210(Pt 9):1526–47. [DOI] [PubMed] [Google Scholar]
- 25.Hong SH, Kim KS, Oh IH. Concise review: Exploring miRNAs--toward a better understanding of hematopoiesis. Stem Cells. 2015;33(1):1–7. [DOI] [PubMed] [Google Scholar]
- 26.Kim M, Civin CI, Kingsbury TJ. MicroRNAs as regulators and effectors of hematopoietic transcription factors. Wiley Interdiscip Rev RNA. 2019:e1537. [DOI] [PubMed] [Google Scholar]
- 27.Montagner S, Deho L, Monticelli S. MicroRNAs in hematopoietic development. BMC Immunol. 2014;15:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elbashir SM, Harborth J, Lendeckel W, et al. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411(6836):494–8. [DOI] [PubMed] [Google Scholar]
- 29.Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T. Identification of novel genes coding for small expressed RNAs. Science. 2001;294(5543):853–8. [DOI] [PubMed] [Google Scholar]
- 30.Sato H, Kasai K, Tanaka T, et al. Role of tumor necrosis factor-alpha and interleukin-1beta on lung dysfunction following hemorrhagic shock in rats. Med Sci Monit. 2008;14(5):BR79–87. [PubMed] [Google Scholar]
- 31.Sadatomo A, Inoue Y, Ito H, et al. Interaction of Neutrophils with Macrophages Promotes IL-1beta Maturation and Contributes to Hepatic Ischemia-Reperfusion Injury. J Immunol. 2017;199(9):3306–15. [DOI] [PubMed] [Google Scholar]
- 32.Bian A, Shi M, Flores B, et al. Downregulation of autophagy is associated with severe ischemia-reperfusion-induced acute kidney injury in overexpressing C-reactive protein mice. PLoS One. 2017;12(9):e0181848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qiao Z, Wang W, Yin L, et al. Using IL-6 concentrations in the first 24 h following trauma to predict immunological complications and mortality in trauma patients: a meta-analysis. Eur J Trauma Emerg Surg. 2018;44(5):679–87. [DOI] [PubMed] [Google Scholar]
- 34.Kishore A, Borucka J, Petrkova J, Petrek M. Novel insights into miRNA in lung and heart inflammatory diseases. Mediators Inflamm. 2014;2014:259131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sato T, Liu X, Nelson A, et al. Reduced miR-146a increases prostaglandin E(2)in chronic obstructive pulmonary disease fibroblasts. Am J Respir Crit Care Med. 2010;182(8):1020–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pinkerton M, Chinchilli V, Banta E, et al. Differential expression of microRNAs in exhaled breath condensates of patients with asthma, patients with chronic obstructive pulmonary disease, and healthy adults. J Allergy Clin Immunol. 2013;132(1):217–9. [DOI] [PubMed] [Google Scholar]
- 37.Tomankova T, Petrek M, Kriegova E. Involvement of microRNAs in physiological and pathological processes in the lung. Respir Res. 2010;11:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li C, Miao R, Liu S, et al. Down-regulation of miR-146b-5p by long noncoding RNA MALAT1 in hepatocellular carcinoma promotes cancer growth and metastasis. Oncotarget. 2017;8(17):28683–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou B, Shan H, Su Y, et al. Let-7a inhibits migration, invasion and tumor growth by targeting AKT2 in papillary thyroid carcinoma. Oncotarget. 2017;8(41):69746–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao C, Li Z, Ji L, et al. PI3K-Akt Signal Transduction Molecules Maybe Involved in Downregulation of Erythroblasts Apoptosis and Perifosine Increased Its Apoptosis in Chronic Mountain Sickness. Med Sci Monit. 2017;23:5637–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He X, Zheng Y, Liu S, et al. MiR-146a protects small intestine against ischemia/reperfusion injury by down-regulating TLR4/TRAF6/NF-kappaB pathway. J Cell Physiol. 2018;233(3):2476–88. [DOI] [PubMed] [Google Scholar]
- 42.Liu JJ, Hou SC, Shen CK. Erythroid gene suppression by NF-kappa B. J Biol Chem. 2003;278(21):19534–40. [DOI] [PubMed] [Google Scholar]
- 43.Bible LE, Pasupuleti LV, Gore AV, et al. Daily propranolol prevents prolonged mobilization of hematopoietic progenitor cells in a rat model of lung contusion, hemorrhagic shock, and chronic stress. Surgery. 2015;158(3):595–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kirito K, Uchida M, Takatoku M, et al. A novel function of Stat1 and Stat3 proteins in erythropoietin-induced erythroid differentiation of a human leukemia cell line. Blood. 1998;92(2):462–71. [PubMed] [Google Scholar]
- 45.Wang XS, Gong JN, Yu J, et al. MicroRNA-29a and microRNA-142–3p are regulators of myeloid differentiation and acute myeloid leukemia. Blood. 2012;119(21):4992–5004. [DOI] [PubMed] [Google Scholar]
- 46.Nacionales DC, Szpila B, Ungaro R, et al. A Detailed Characterization of the Dysfunctional Immunity and Abnormal Myelopoiesis Induced by Severe Shock and Trauma in the Aged. J Immunol. 2015;195(5):2396–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gamelli RL, He LK, Liu H. Marrow granulocyte-macrophage progenitor cell response to burn injury as modified by endotoxin and indomethacin. J Trauma. 1994;37(3):339–46. [DOI] [PubMed] [Google Scholar]
- 48.Fonseca RB, Mohr AM, Wang L, et al. The impact of a hypercatecholamine state on erythropoiesis following severe injury and the role of IL-6. J Trauma. 2005;59(4):884–9; discussion 9–90. [DOI] [PubMed] [Google Scholar]
- 49.Weissman C The metabolic response to stress: an overview and update. Anesthesiology. 1990;73(2):308–27. [DOI] [PubMed] [Google Scholar]
- 50.Yao Y, Sun F, Lei M. miR-25 inhibits sepsis-induced cardiomyocyte apoptosis by targetting PTEN. Biosci Rep. 2018;38(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yao L, Liu Z, Zhu J, et al. Clinical evaluation of circulating microRNA-25 level change in sepsis and its potential relationship with oxidative stress. Int J Clin Exp Pathol. 2015;8(7):7675–84. [PMC free article] [PubMed] [Google Scholar]
- 52.Benz F, Roy S, Trautwein C, et al. Circulating MicroRNAs as Biomarkers for Sepsis. Int J Mol Sci. 2016;17(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Min S, Li L, Zhang M, et al. TGF-beta-associated miR-27a inhibits dendritic cell-mediated differentiation of Th1 and Th17 cells by TAB3, p38 MAPK, MAP2K4 and MAP2K7. Genes Immun. 2012;13(8):621–31. [DOI] [PubMed] [Google Scholar]
- 54.Cheng Y, Du L, Jiao H, et al. Mmu-miR-27a-5p-Dependent Upregulation of MCPIP1 Inhibits the Inflammatory Response in LPS-Induced RAW264.7 Macrophage Cells. Biomed Res Int. 2015;2015:607692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mizgalska D, Wegrzyn P, Murzyn K, et al. Interleukin-1-inducible MCPIP protein has structural and functional properties of RNase and participates in degradation of IL-1beta mRNA. FEBS J. 2009;276(24):7386–99. [DOI] [PubMed] [Google Scholar]
- 56.Lv YN, Ou-Yang AJ, Fu LS. MicroRNA-27a Negatively Modulates the Inflammatory Response in Lipopolysaccharide-Stimulated Microglia by Targeting TLR4 and IRAK4. Cell Mol Neurobiol. 2017;37(2):195–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gebhard F, Pfetsch H, Steinbach G, et al. Is interleukin 6 an early marker of injury severity following major trauma in humans? Arch Surg. 2000;135(3):291–5. [DOI] [PubMed] [Google Scholar]
- 58.Giannoudis PV, Hildebrand F, Pape HC. Inflammatory serum markers in patients with multiple trauma. Can they predict outcome? J Bone Joint Surg Br. 2004;86(3):313–23. [DOI] [PubMed] [Google Scholar]
- 59.Kelly AD, Issa JJ. The promise of epigenetic therapy: reprogramming the cancer epigenome. Curr Opin Genet Dev. 2017;42:68–77. [DOI] [PubMed] [Google Scholar]