Abstract
Background:
South Asians are the second fastest growing ethnic group in the United States, and they have a high risk for cardiovascular disease (CVD). Moderate alcohol consumption has been associated with lower CVD risk in some race/ethnic groups, but the association of alcohol consumption and atherosclerosis in South Asians has not been investigated.
Methods:
We used data from 906 South Asian participants who participated in the Mediators of Atherosclerosis in South Asians Living in America (MASALA) cohort (2010-2012). Alcohol consumption was ascertained via questionnaire, coronary artery calcium (CAC) was measured with computed tomography, and common carotid artery intima-media thickness (cIMT) was measured using B-mode ultrasonography. We used multivariable regression models to examine cross-sectional associations of alcohol consumption with the presence and amount of CAC and cIMT.
Results:
Compared with never drinkers, participants consuming 4-7 drinks/week had a 63% decreased odds of any CAC after adjusting for potential confounders and mediators. Participants consuming 4-7 drinks/week had significantly lower odds of CAC score between 1-300 [OR (95% CI): 0.34 (0.16-0.72)]. A similar inverse association was seen for the odds of CAC>300 [OR (95% CI): 0.28 (0.07-0.97)]. Alcohol consumption of >7 drinks/week was associated with a 0.096 mm increase in common-cIMT.
Conclusion:
There was an inverse association between the amount of alcohol intake and CAC among South Asians while a positive association was found between alcohol consumption and common-cIMT. Long-term follow-up of the MASALA cohort will examine prospective associations of alcohol intake with the progression of subclinical atherosclerosis, incident CVD events, and mortality.
Keywords: South Asian, Alcohol, Subclinical atherosclerosis, Coronary artery calcium
Introduction
South Asians – individuals with ancestry from Bangladesh, Bhutan, India, the Maldives, Nepal, Pakistan, and Sri Lanka - are one of the fastest-growing ethnic groups in the United States[1] and make up one-quarter of the world’s population.[2] South Asians are more likely to develop coronary heart disease (CHD) at a younger age[3] and have higher mortality from ischemic heart disease compared to other populations[4] that is not explained by widely known risk factors. Ethnicity is known to account in part for inter-individual variability in the prevalence and incidence of cardiovascular disease (CVD). [5]
An important question yet to be answered definitively is whether alcohol influences the risk of cardiovascular disease (CVD). Alcohol has both protective and detrimental effects on CVD.[6] Epidemiological studies have consistently found that alcohol intake within recommended limits is associated with a lower risk of CVD.[7-10] One potential mechanism is the effect of alcohol consumption on atherogenesis.[11] The relationship between alcohol and subclinical atherosclerosis could provide clues about the mechanisms underlying the relationship between alcohol and CVD.[12] To understand this relationship; we can examine its association with subclinical atherosclerosis measured by surrogate markers, including coronary artery calcium (CAC) and carotid intima-media thickness (cIMT). However, the studies examining the relationship between alcohol consumption and subclinical atherosclerosis have shown inconsistent results, either no association[13-16], a dose-response relation[17, 18] or a U-shaped relationship.[19] Similarly, the studies examining the association of alcohol consumption and cIMT have yielded conflicting results. [20-22]
Among all the ethnicities and nations that have been studied previously, the cardioprotective effect of light to moderate drinking has not been consistently replicated. [23-25] The INTERHEART study that included 27,000 subjects from 52 different countries showed that moderate (but not high levels of) alcohol use was associated with a reduced risk of MI. Although moderate or low alcohol consumption was protective in most countries, such an effect was not seen in South Asia[26]. Our study may provide insights into the understanding of the association between alcohol intake and subclinical atherosclerosis in South Asians. We examined cross-sectional association between alcohol consumption and subclinical atherosclerosis using data from the Mediators of Atherosclerosis in South Asians Living in America (MASALA) study, a community-based sample of asymptomatic South Asians in the United States. We hypothesized that light to moderate alcohol consumption is associated with decreased prevalence of CAC and lower CIMT among asymptomatic South Asians aged 40-84 years in the U.S
Methods
Study population
A detailed description of the MASALA study design and methodology have been previously published. [27] MASALA is a prospective community-based cohort study of South Asians to examine the etiology and prognostic significance of subclinical atherosclerosis. This project is similar in measures and methods to a large ongoing Multi-Ethnic Study of Atherosclerosis (MESA).[28] The sampling frames were created by clinical site and included all 9 counties of the San Francisco Bay Area for the UCSF field site, and the 7 census tracts closest to the NWU medical center and secondary suburban locations around Chicago where census data revealed high proportions of South Asian residents. Between October 2010 and March 2013, 906 South Asians with a mean age of 55 ± 9 years were enrolled. All participants signed informed consent forms before undergoing study procedures. The study protocol was approved by the institutional review boards of the University of California, San Francisco, and Northwestern University.
Assessment of alcohol consumption
Based on the personal history questionnaire, alcohol consumption was assessed. The participants were asked, “Have you ever consumed alcoholic beverages?” If yes, they were then asked, “Do you presently drink alcoholic beverages?” Based on the answers given to these 2 questions, the participants were categorized into never, former, and current drinkers. Both current and former drinkers were asked, “For how many years did you drink alcoholic beverages?” They were also asked about the usual number of drinks consumed per week (before stopping drinking in the case of former drinkers). Current drinkers were asked about the number of drinks consumed during the past 24 hours, and the largest number of drinks consumed in 1 day in the past month. If the participant consumed ≥5 drinks on one occasion in the past month, it was defined as binge drinking. [27]
Measurement of coronary artery calcium
Non-contrast cardiac CT scans were performed using cardiac gated computed tomography scanners (UCSF: Phillips 16D scanner or a Toshiba MSD Aquilion 64; and NWU: Siemens Sensation Cardiac 64 Scanner). A four-sample calibration phantom was placed under the thorax for attenuation correction. All scans were interpreted at the CT reading center at Harbor-UCLA using the Rephot Imaging Software. Coronary Artery Calcium (CAC) Agatston scores were reported for each of the four major coronary arteries, and the summed score was used.
Measurement of carotid artery intima-media thickness
High-resolution B-mode ultrasonography was conducted for measurement of right and left internal and common carotid artery intima-media thickness (cIMT). The vascular technician located the bifurcation of the carotid artery distinguished the internal from an external carotid artery, and identified the maximal wall thickening in the near or far wall, in the carotid bulb or internal carotid artery. Each of these images was collected in a specified order and recorded. The digitized data were batched and mailed on magneto-optical disks to the Reading Center at Wake Forest University for wall-thickness measurements. For quality control, a single reader completed 25 repeat CIMT measures. The intra-class correlation coefficient for the internal carotid IMT was 0.96 and 0.78 for the common carotid IMT.
Measurement of covariates
Other covariates include demographic characteristics and potential confounders and mediators of alcohol-subclinical atherosclerosis association. Information on participant’s demographic data, income, tobacco use, family history, medical history, and medication use was obtained using standard questionnaires and structured interview conducted by trained bilingual study staff. Education was categorized as having >=bachelor’s degree or <bachelor’s degree. Physical activity was assessed using the Typical Week’s Physical Activity Questionnaire[29]. Resting blood pressure was measured three times in the seated position, using an automated blood pressure monitor (V100 Vital sign monitor, GE Medical Systems, Fairfield, CT) and the average of the last two readings was used for analysis. Participant height was measured using a stadiometer, and weight was measured using a standard balance beam scale or digital weighing scale. Waist circumference was measured using a flexible tape measure tape at the site of maximum circumference midway between the lower ribs and the anterior superior iliac spine. Fasting plasma glucose was measured by the glucose oxidase method; total cholesterol, triglycerides, high-density lipoprotein (HDL) cholesterol and creatinine were measured by enzymatic methods (Quest, San Jose, CA) and low-density lipoprotein (LDL) cholesterol was calculated. Fasting serum samples were batched for insulin measured by the sandwich immunoassay method (Roche Elecsys 2010, Roche Diagnostics, Indianapolis, IN). Diabetes mellitus was defined as fasting glucose ≥126 mg/dL, or a post-challenge glucose ≥200 mg/dl, or by use of glucose-lowering medication. Hypertension was defined as systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg or medication use for hypertension. Use of an HMG-CoA reductase inhibitor, fibrate, or niacin was categorized as cholesterol medication use.
Statistical Analysis
Baseline characteristics of the total population were compared across alcohol consumption categories (never drinker, former drinker, 1-3 drinks/week, 4-7 drinks/week, and >7 drinks/week). Continuous variables were summarized using the mean (standard deviation) or median (interquartile range) depending on the normality of the data. Categorical variables were summarized using frequency (percentages). Analysis of variance (ANOVA) was used to compare the continuous variables while the chi-squared test was used to compare categorical variables. CAC scores were analyzed as a dichotomous outcome for the presence of any CAC (CAC>0), and as a categorical outcome for the degree of prevalent CAC (CAC = 0, 1–300, and >300). We used multivariable logistic regression models to compute odds ratios and 95% confidence intervals (CI) for the cross-sectional association between each alcohol consumption categories (never drinker (reference), former drinker, 1-3 drinks/week, 4–7 drinks/week, and >7 drinks/week) with the presence of any CAC, while multinomial logistic regression models were used to examine the association between alcohol consumption and degree of prevalent CAC (CAC = 0, 1–300, and >300). Linear regression models were used to examine the associations between categories of alcohol consumption and common CIMT modeled on a continuous scale. Model 1 was adjusted for age, sex, and education level. Model 2 was adjusted for model 1 plus smoking (never, former, current, and pack-years), BMI, self-reported physical activity and family history of heart attack, systolic and diastolic blood pressures, HDL-C, LDL-C, triglyceride, use of cholesterol medications, diabetes, C-reactive protein and eGFR (estimated glomerular filtration rate).
Additionally, we examined the interaction of alcohol consumption with sex, and age using the likelihood ratio χ2 test, by including interaction terms in model 2. A two-sided p-value of <0.05 was considered statistically significant. All statistical analyses were performed using with SAS version 9.4 (SAS Institute Inc., Cary, North Carolina).
Results
Baseline Characteristics
Sociodemographic characteristics by alcohol consumption categories are shown in Table 1. The average age of the 906 MASALA participants was 55.3 (±9.4) years, and 46% of participants were women. Among these, 304 (34.6%) were never drinkers, 303 (33.4%) were former drinkers, and 299 (33%) were current drinkers. Of the current drinkers, 182 (60.9%) reported consuming 1-3 drinks/week, 75 (25.1%) reported consuming 4-7 drinks/week, and 42 (18%) reported consuming >7 drinks/week. Participants consuming >7 drinks/week were more likely to be older, male, and current smokers. Higher categories of drinks per week were associated with higher blood pressure and waist circumference. In univariate analysis, higher alcohol consumption was associated with a higher cIMT score. The prevalence of any CAC was 37%, 43.2%, 44.4%, 43.8% and 63.4% in never drinkers, former drinkers, those who consume alcohol 1-3 drinks/week, 4–7 drinks/week, and >7 drinks/week, respectively.
Table 1.
Baseline Characteristics of MASALA Participants
| Alcohol consumption (number of drinks/week) | ||||||
|---|---|---|---|---|---|---|
|
Characteristics Mean± SD or N (%) |
Never N=304 |
Former N=303 |
1-3 N=182 |
4-7 N=75 |
>7 N=42 |
P value |
| Male | 93 (31) | 173 (57) | 116 (64) | 63 (84) | 41 (98) | <0.001 |
| Age (years) | 55.9 ± 9.4 | 54.6 ± 9.1 | 54.4 ± 9.6 | 56.1 ± 8.3 | 59 ± 11.5 | 0.021 |
| Systolic Blood Pressure (mm Hg) | 124.6 ± 16.5 | 123.4 ± 14.1 | 124.2 ± 16 | 128 ± 17.3 | 131.1 ± 14.5 | 0.014 |
| Diastolic Blood Pressure (mm Hg) | 71.7 ± 9.6 | 73.3 ± 9.9 | 74.4 ± 9.3 | 76.3 ± 9.4 | 78.4 ± 10.1 | <0.001 |
| LDL Cholesterol (mg/dL) | 111.7 ± 32.3 | 112.7 ± 32.4 | 108.2 ± 29.8 | 108.1 ± 33.6 | 116.5 ± 31.7 | 0.374 |
| HDL Cholesterol (mg/dL) | 50.9 ± 12.9 | 48.5 ± 12.9 | 50.5 ± 14 | 50.8 ± 15.3 | 51.7 ± 13.6 | 0.168 |
| Triglyceride (mg/dL) | 127.5 ± 55.9 | 135.3 ± 74.6 | 132.3 ± 90.8 | 136.1 ± 69.1 | 122.7 ± 49.3 | 0.594 |
| BMI (kg/m2) | 25.9 ± 4 | 26 ± 4 | 26.2 ± 4.5 | 25.8 ± 4 | 25.6 ± 3.8 | 0.879 |
| Physical activity (METs per week) * | 929 (689-1230) | 951 (744-1191) | 974 (805-1187) | 899 (739-1172 | 933 (746-1148) | 0.776 |
| Waist Circumference (cm) | 91.3 ± 10.4 | 93 ± 10.2 | 93.4 ± 10.2 | 94.4 ± 10.7 | 96.3 ± 9.6 | 0.009 |
| Family Income ≥ $75,000 per year | 121 (40) | 68 (23) | 152 (84) | 63 (84) | 32 (75) | 0.001 |
| Education < Bachelor’s Degree | 37 (12) | 36 (12) | 21(12) | 7 (9) | 9 (21) | <0.001 |
| Diabetes Mellitus † | 77 (26) | 80 (27) | 40 (22) | 24 (32) | 8 (19) | 0.47 |
| Smoker Status | <0.001 | |||||
| Never | 290 (95) | 265 (88) | 135 (74) | 45 (60) | 16 (38) | |
| Former | 11 (4) | 28 (9) | 42 (23) | 25 (33) | 18 (43) | |
| Current | 3 (1) | 10 (3) | 5 (3) | 5 (7) | 8 (19) | |
| Lipid-Lowering Medication use | 93 (31) | 80 (26) | 58 (32) | 28 (37) | 13 (31) | 0.39 |
| Family history of heart attack | 120 (40) | 157 (52) | 82 (45) | 36 (48) | 17 (40) | 0.04 |
| C-Reactive Protein (CRP) (ug/mL) * | 1.37 (0.7-3.2) | 1.3 (0.6-2.9) | 1.03 (0.7-2.5) | 0.96 (0.5-2.3) | 0.96 (0.5-2.6) | 0.376 |
| eGFR (ml/min/1.73 m2) $ | 95.3 ± 20.5 | 94.3 ± 20.6 | 92.9 ± 16.8 | 89.9 ± 16.1 | 90.3 ± 14.2 | 0.156 |
| Common Carotid IMT (mm) | 0.84 (0.73-0.95) | 0.84 (0.72-0.95) | 0.84 (0.72-1.00) | 0.89 (0.77-1.06) | 0.93 (0.82-1.17) | <0.001 |
| CAC Presence(CAC>0) | 112 (37) | 130 (43) | 80 (44) | 32 (44) | 26 (63) | 0.021 |
median (interquartile range) shown for skewed variables
diabetes defined by fasting plasma glucose ≥126 mg/dl, or a post-challenge glucose ≥200 mg/dl, or by use of a glucose-lowering medication
eGFR calculated from Modification of Diet in Renal Disease formula
LDL, low- density cholesterol; HDL, high-density cholesterol; BMI, body mass index; MET, metabolic equivalent; eGFR, estimated glomerular filtration rate; IMT, intima-media thickness; CAC, coronary artery calcium
Alcohol consumption and presence of CAC
Table 2 displays the association between levels of alcohol consumption and any prevalent CAC using multivariable logistic regression. In the model adjusted for sociodemographic factors, alcohol consumption of 4-7 drinks/week was associated with decreased odds of any CAC presence (odds ratio [OR] (95% CI):0.53 (0.28-1.00), P = 0.05). Compared with never drinkers, participants consuming 4-7 drinks/week had a 63% lower odds of any CAC after adjusting for potential confounders and mediators (OR (95% CI): 0.37 (0.18-0.75), P < 0.01). The interaction between alcohol consumption and sex or age was not statistically significant. After adjustment for all mediators and confounders, there was no association between binge drinking in the past month and any CAC presence.
Table 2.
Multivariable Odds Ratio and 95% CI of association between alcohol consumption and CAC presence (CAC>0)
| Alcohol Consumption | Model 1 | Model 2 | ||
|---|---|---|---|---|
| Odds Ratio (95% CI) | p-value | Odds Ratio (95% CI) | p-value | |
| Never Drinker | reference | reference | ||
| Former Drinker | 1.03(0.68, 1.56) | 0.89 | 1.02(0.65, 1.59) | 0.92 |
| 1-3 drinks/week | 1.01 (0.62, 1.63) | 0.98 | 0.82(0.48, 1.40) | 0.47 |
| 4-7 drinks/week | 0.53 (0.28, 1.00) | 0.05 | 0.37 (0.18, 0.75) | 0.007 |
| >7 drinks/week | 0.92 (0.40, 2.16) | 0.85 | 0.61 (0.24, 1.53) | 0.29 |
Model 1 adjusted for age, sex, education, and income
Model 2 adjusted for Model 1 plus smoking (never, former, current, and pack-years), BMI, self-reported physical activity, family history of heart attack, systolic and diastolic blood pressures, HDL-C, LDL-C, triglyceride, use of cholesterol medications, diabetes, C-reactive protein and eGFR.
CAC, coronary artery calcium; BMI, body mass; HDL, high-density cholesterol; LDL, low- density cholesterol
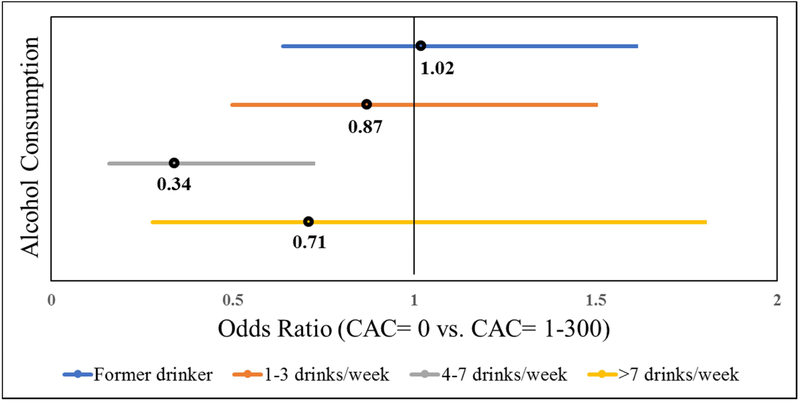
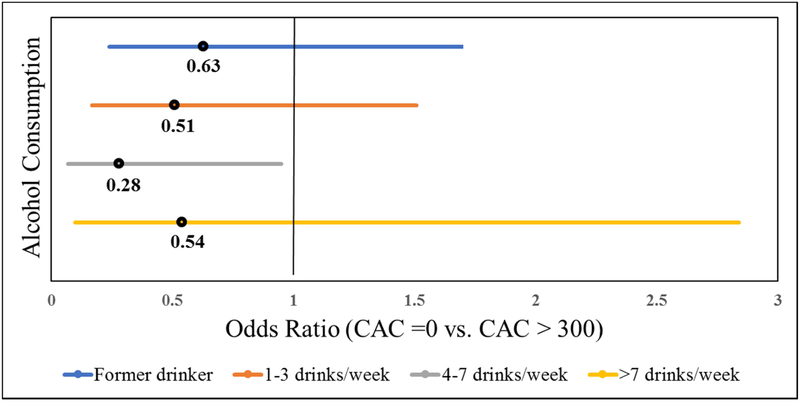
Figure 1 and 2 shows the association between alcohol consumption and the burden of CAC. For the categorical model, participants consuming 4-7 drinks/week had significantly lower odds of CAC = 1-300 compared with never drinkers (OR (95% CI): 0.34 (0.16-0.72), P = 0.005) (Figure 1). A similar inverse association was observed for the odds of CAC>300 (OR (95% CI): 0.28 (0.07-0.97), P = 0.04) (Figure 2).
Figure 1.
Multivariable Odds Ratio and 95% CI of association between alcohol consumption and degree of CAC (CAC = 1-300)
Reference group = Never drinker
Figure 2.
Multivariable Odds Ratio and 95% CI of association between alcohol consumption and degree of CAC (CAC > 300)
Reference group = Never drinker
Alcohol consumption and common cIMT
Table 3 shows the multivariable model result for the association between alcohol consumption categories and common cIMT. Alcohol consumption of >7 drinks/week was associated with a 0.096 mm increase in common cIMT compared to no alcohol intake in a fully adjusted model. (β (95% CI): 0.096 (0.024-0.167), P = 0.009). Using multiple linear regression analysis, we also calculated the least mean square and standard error (SE) of the association of alcohol consumption categories and cIMT (Table 4). There was an incremental increase in mean cIMT across alcohol consumption categories with the higher score in >7 drinks/week followed by 4-7 drinks/week followed by 1-3 drinks/week followed by former drinker and then never drinker (trend P-value of 0.01 for Model 1 and 0.07 for Model 2).
Table 3.
Multivariable Beta-coefficient and 95% CI of association between alcohol consumption Categories and common cIMT
| Alcohol Consumption | Model 1 | Model 2 | ||
|---|---|---|---|---|
| Beta-Coefficient (95% CI) | p-value | Beta-Coefficient (95% CI) | p-value | |
| Never Drinker | reference | reference | ||
| Former Drinker | 0.016 (−0.018, 0.050) | 0.37 | 0.012 (−0.022, 0.047) | 0.48 |
| 1-3 drinks/week | 0.039 (−0.00, 0.079) | 0.06 | 0.033 (−0.007, 0.074) | 0.10 |
| 4-7 drinks/week | 0.049 (−0.006, 0.103) | 0.08 | 0.045 (−0.010, 0.100) | 0.11 |
| >7 drinks/week | 0.114 (0.045, 0.183) | 0.001 | 0.096 (0.024, 0.167) | 0.009 |
Model 1 adjusted for age, sex, education, and income
Model 2 adjusted for Model 1 plus smoking (never, former, current, and pack-years), BMI, self-reported physical activity, family history of heart attack, systolic and diastolic blood pressures, HDL-C, LDL-C, triglyceride, use of cholesterol medications, diabetes, C-reactive protein and eGFR.
cIMT, carotid intima-media thickness; BMI, body mass; HDL, high-density cholesterol; LDL, low- density cholesterol
Table 4.
Least Mean Square and SE of common cIMT across alcohol categories.
| Alcohol categories | Model 1 | Model 2 | ||
|---|---|---|---|---|
| Mean ± SE | Trend p-value | Mean ± SE | Trend p-value | |
| Never Drinker | 0.86±0.013 | 0.01 | 0.86±0.013 | 0.07 |
| Former Drinker | 0.87±0.012 | 0.87±0.012 | ||
| 1-3 drinks/week | 0.89±0.015 | 0.89±0.015 | ||
| 4-7 drinks/week | 0.90±0.024 | 0.90±0.024 | ||
| >7 drinks/week | 0.97±0.032 | 0.95±0.033 |
Least mean square and standard error calculated from multiple linear regression analysis
Model 1 adjusted for age, sex, race, and education
Model 2 adjusted for Model 1 plus smoking (never, former, current, and pack-years), BMI, self-reported physical activity, family history of heart attack, systolic and diastolic blood pressures, HDL-C, LDL-C, triglyceride, use of cholesterol medications, diabetes, C-reactive protein and eGFR.
SE, standard error; cIMT, carotid intima-media thickness; BMI, body mass; HDL, high-density cholesterol; LDL, low- density cholesterol
Discussion
Main Findings
Several important insights were obtained in this study about alcohol consumption and subclinical atherosclerosis among middle-aged US South Asian adults free of CVD at baseline. We found an inverse association of light alcohol consumption of 4-7 drinks/week with presence and amount of CAC. In contrast, moderate to high alcohol consumption of >7 drinks/week was associated with higher cIMT. Moreover, there was a graded association with the number of drinks/week and common cIMT, with higher alcohol consumption associated with the higher cIMT. Our study was designed to further the understanding of the relationship between alcohol consumption and subclinical atherosclerosis. To our knowledge, this is the first study assessing the association of alcohol consumption and measures of subclinical atherosclerosis in South Asians in the U.S.
Comparison with previous studies
The studies examining the relationship between alcohol consumption and CAC have shown inconsistent results. Tofferi et al. [14] used a cohort of 731 consecutive, consenting, active-duty US Army personnel (39 to 45 years of age) without known CVD. This cross-sectional study found no relationship between the CAC score and the alcohol intake. It was one of the first studies focusing on the relation between alcohol consumption and CAC in a group at low risk for cardiovascular disease and with early atherosclerosis. Using CAC and atherosclerotic plaque in the aorta as surrogate markers of subclinical atherosclerosis, Ellison et al. [15] investigated this relationship using data from a prospective cohort study including 3166 white and African American subjects with a mean age around 55 years. They found no evidence of an association between total alcohol intake and CAC, although there was a tendency for the increased prevalence of CAC in the highest category of alcohol intake. Analyses of aortic calcification showed similar nonsignificant associations. Yang et al. [16] tried to determine if alcohol intake was associated with reduced coronary risk in a high-risk asymptomatic population and whether this effect was independent of coronary risk factors and coronary calcium. The study showed no relation between alcohol use and CAC. The definition of moderate alcohol consumption was different at that time. Also, the study lacked detail data on alcohol consumption. The first large study to evaluate the association of alcohol with CAC in 4 racial-ethnic groups and to evaluate the progression of calcification, was done by McClelland et al. [13] The researchers studied the association between alcohol consumption and CAC prevalence, incidence and progression in The Multi-Ethnic Study of Atherosclerosis (MESA) which is a prospective community-based cohort study including 6814 participants aged 45–84 y who identified themselves as white, African American, Hispanic, or Chinese. They showed that overall alcohol consumption was not associated with the prevalence of any CAC > 0 at baseline regardless of alcohol type.
In contrast to these studies, Vliegenthart et al. [19] found a U-shaped association between alcohol consumption and CAC performing a cross-sectional analysis using data from the population-based Rotterdam Coronary Calcification Study. By using prospective study design. Interestingly, Pletcher et al. [17] found a dose-response relation between alcohol and CAC. Our study did have some evidence of J-shaped association of alcohol with the presence and degree of CAC.
Carotid IMT is another non-invasive measure that can be used as a surrogate marker of subclinical atherosclerosis. To address the relationship between alcohol consumption and carotid atherosclerosis in older adults, Mukamal et al. [20] used data from the Cardiovascular Health Study (CHS), including 5888 adults aged 65 years and older. The result of this cross-sectional study found that relative to older adults who abstain from alcohol, consumption of 1 to 6 drinks per week had an inverse association with carotid atherosclerosis whereas consumption of 14 or more drinks had a positive association. The CHS participants represent a relatively healthy group of older adults, so the results can only be generalized to older adults in similar health. Kim at al[21] tried to find relationships between alcohol consumption and subclinical atherosclerosis in 5539 Korean subjects (2121 men and 3418 women) who were participants in the Multi-Rural Communities cohort (MRcohort) study. The result showed that carotid artery intima-media wall thickness (CCA-IMT) was lower with alcohol consumption in men only. Zyriax at al[22] conducted a cross-sectional study with the aim of investigating the relationship between alcohol intake and c-IMT in a selectively healthy population of the Stress Atherosclerosis and ECG Study (STRATEGY study). The results revealed a significant positive correlation between daily alcohol consumption and IMT in men, whereas in women the positive correlation was not significant. Our study found a positive association between alcohol consumption and cIMT. In our study, we could not do a stratified analysis by gender given a small sample size of women in 4-7 drinks/week and >7 drinks/week categories.
Potential mechanisms
An explanation for these conflicting results is unclear. Moderate alcohol acts upon the liver and can, therefore, serve to directly increase the hepatic production and secretion of apolipoproteins and lipoprotein particles, increase triglyceride concentrations, and decrease the removal of circulating high-density lipoprotein cholesterol. [8] We did not find any association between alcohol consumption categories and HDL or triglyceride in MASALA participants (Table 1). Systemic inflammation is a known aggravating factor in the pathogenesis of CVD, and studies have shown that moderate alcohol intake is associated with lower levels of inflammatory markers such as IL-6, TNF-α, and CRP. [30-34] Some alcoholic beverages, especially red wine, contains polyphenols.[35] One of the polyphenols, resveratrol, is postulated to be an important contributor in explaining the cardio-protective effect of red wine through its anti-inflammatory effect. [36, 37] This anti-inflammatory effect may mediate the observed reduction of atherosclerotic burden. Alcohol also reduces hyperglycemia through the inhibition of hepatic gluconeogenesis, with a resulting reduction in plasma glucose levels.[38] Chronic kidney disease (CKD) has shown to be associated with atherosclerosis. [39, 40] Although few studies have suggested a harmful association between alcohol consumption and CKD, our study found no such association.[41, 42]
In contrast, moderate to heavy alcohol intake has been shown to increases blood pressure in a dose-dependent fashion. [43-45] Our study confirmed this finding showing that both SBP and DBP increased with higher levels of alcohol consumption. Alcohol is the second most energy-dense macronutrient consumed and also has an appetite enhancing the effect, which could lead to an increased energy-intake, thereby causing weight gain. [6] We found that the level of alcohol consumption was positively associated with waist circumference in MASALA participants. These findings, if causal, could partly explain the dose-response relationship of alcohol consumption and cIMT in MASALA cohort.
Lastly, genetic factors of South Asians may play a role in modifying the relationship between alcohol consumption and atherosclerosis. The study by Hines et al. [46] showed that moderate drinkers who were homozygous for the slow-oxidizing ADH3(alcohol dehydrogenase type 3) allele have higher HDL levels and a substantially decreased risk of myocardial infarction. A meta-analysis of 56 epidemiological studies containing individuals of European descent revealed that individuals with a genetic variant associated with non-drinking and lower alcohol consumption had a more favorable cardiovascular profile. [47] Although, we could not find any studies examining the role of genetic factors in the association of alcohol and heart disease among South Asians.
Implications
Due to the diversity of language, cultural and religious practices, it is difficult to stereotype degree and pattern of alcohol use among a group as diverse as South Asians. Moreover, differences between generations and increased alcohol consumption due to acculturation further complicate the picture.[48] The recommended limit of alcohol consumption varies substantially across different national guidelines. [49] Despite multiple studies revealing the benefits of moderate alcohol consumption to lower CVD risk, a recent study analyzing data from nearly 600,000 people concluded that in current drinkers of alcohol in high-income countries, the threshold for lowest risk of all-cause mortality was about 100 g/week (<7 drinks/week).[50] Even though our study showed the inverse association of consuming 4-7 drinks/week with respect to CAC, we also observed that >7 drinks/week of alcohol consumption was related to greater cIMT. Thus, it would be difficult to make any recommendations regarding a safe threshold in South Asian population.
Strengths and limitations
The major strength of our study is the understudied and unique population of South Asian. Also, we were able to adjust for many potential confounders and mediators, including lifestyle variables and CVD risk factors. There are certain limitations that need to be taken into consideration in the interpretation of our study. First, alcohol consumption was based on questionnaire responses, and participants may have underreported heavy or any consumption. These would most likely attenuate the association due to misclassification. Second, our study design was cross-sectional, and therefore, a causal relationship between alcohol and subclinical atherosclerosis cannot be established. Third, there were very few participants with heavy alcohol consumption defined as >14 drinks/week, which limited our ability to explore alcohol associations among this subset. Finally, we adjusted for several confounders, but residual confounding remains a possibility.
Conclusion
In conclusion, we found different associations of alcohol consumption with surrogate markers of subclinical atherosclerosis. We observed an inverse association of alcohol consumption and presence and burden of CAC while alcohol seems to have an unfavorable association with cIMT. Future research can examine the association between alcohol consumption and incidence and progression of CAC as well as incident coronary heart disease in MASALA cohort.
Highlights.
Moderate alcohol consumption has been associated with lower CVD risk in some race/ethnic groups, but the association of alcohol consumption and atherosclerosis in South Asians has not been investigated
We found an inverse association of light alcohol consumption of 4-7 drinks/week with presence and amount of CAC among South Asians
In contrast, moderate to high alcohol consumption of >7 drinks/week was associated with higher cIMT
Acknowledgment
The authors would like to thank the other investigators, staff, and participants of the MASALA Study for their valuable contribution. A full list of participating MASALA investigators and institutions can be found at http://www.masalastudy.org.
Funding
The MASALA study was supported by the National Institutes of Health grant no. R01-HL093009 . Data collection at UCSF was also supported by NIH/NCRR UCSF-CTSI Grant Number UL1 RR024131 . Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors declared they do not have anything to disclose regarding conflict of interest with respect to this manuscript
References
- 1.SAALT (South Asians Learning Together). A demographic snapshot of South Asians in the United States. 2015. http://saalt.org/wp-content/uploads/2016/01/Demographic-Snapshot-updated_Dec-2015.pdf.
- 2.Central Intelligence Agency. The World Fact Book. 2017. https://www.cia.gov/library/publications/the-world-factbook/geos/in.html.
- 3.Enas EA, et al. , Coronary heart disease and its risk factors in first-generation immigrant Asian Indians to the United States of America. Indian Heart J, 1996. 48(4): p. 343–53. [PubMed] [Google Scholar]
- 4.Jose PO, et al. , Cardiovascular Disease Mortality in Asian Americans. Journal of the American College of Cardiology, 2014. 64(23): p. 2486–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Budoff MJ, et al. , Progression of Coronary Calcium and Incident Coronary Heart Disease Events MESA (Multi-Ethnic Study of Atherosclerosis). Journal of the American College of Cardiology, 2013. 61(12): p. 1231–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gronbaek M, The positive and negative health effects of alcohol- and the public health implications. Journal of Internal Medicine, 2009. 265(4): p. 407–420. [DOI] [PubMed] [Google Scholar]
- 7.O'Keefe JH, Bybee KA, and Lavie CJ, Alcohol and cardiovascular health - The razor-sharp double-edged sword. Journal of the American College of Cardiology, 2007. 50(11): p. 1009–1014. [DOI] [PubMed] [Google Scholar]
- 8.Rimm EB, et al. , Moderate alcohol intake and lower risk of coronary heart disease: metaanalysis of effects on lipids and haemostatic factors. British Medical Journal, 1999. 319(7224): p. 1523–1528D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao J, et al. , Alcohol Consumption and Mortality From Coronary Heart Disease: An Updated Meta-Analysis of Cohort Studies. Journal of Studies on Alcohol and Drugs, 2017. 78(3): p. 375–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chevli PA, et al. , Electrocardiographic subclinical myocardial injury and alcohol consumption: a cross-sectional analysis of data from the Third National Health and Nutrition Examination Survey. Am J Cardiovasc Dis, 2018. 8(5): p. 58–65. [PMC free article] [PubMed] [Google Scholar]
- 11.Gaziano JM, et al. , Light-to-moderate alcohol consumption and mortality in the physicians' health study enrollment cohort. Journal of the American College of Cardiology, 2000. 35(1): p. 96–105. [DOI] [PubMed] [Google Scholar]
- 12.Rehm JT, et al. , Alcohol consumption and coronary heart disease morbidity and mortality. American Journal of Epidemiology, 1997. 146(6): p. 495–501. [DOI] [PubMed] [Google Scholar]
- 13.McClelland RL, et al. , Alcohol and coronary artery calcium prevalence, incidence, and progression: results from the Multi-Ethnic Study of Atherosclerosis (MESA). American Journal of Clinical Nutrition, 2008. 88(6): p. 1593–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tofferi JK, et al. , Alcohol intake is not associated with subclinical coronary atherosclerosis. American Heart Journal, 2004. 148(5): p. 803–809. [DOI] [PubMed] [Google Scholar]
- 15.Ellison RC, et al. , Is alcohol consumption associated with calcified atherosclerotic plaque in the coronary arteries and aorta? American Heart Journal, 2006. 152(1): p. 177–182. [DOI] [PubMed] [Google Scholar]
- 16.Yang T, et al. , Alcohol consumption, coronary calcium, and coronary heart disease events. American Journal of Cardiology, 1999. 84(7): p. 802–806. [DOI] [PubMed] [Google Scholar]
- 17.Pletcher MJ, et al. , Alcohol consumption, binge drinking, and early coronary calcification: Findings from the coronary artery risk development in young adults (CARDIA) study. American Journal of Epidemiology, 2005. 161(5): p. 423–433. [DOI] [PubMed] [Google Scholar]
- 18.Yun KE, et al. , Alcohol and coronary artery calcification: an investigation using alcohol flushing as an instrumental variable. International Journal of Epidemiology, 2017. 46(3): p. 950–962. [DOI] [PubMed] [Google Scholar]
- 19.Vliegenthart R, et al. , Alcohol consumption and coronary calcification in a general population. Archives of Internal Medicine, 2004. 164(21): p. 2355–2360. [DOI] [PubMed] [Google Scholar]
- 20.Mukamal KJ, et al. , Alcohol consumption and carotid atherosclerosis in older adults - The cardiovascular health study. Arteriosclerosis Thrombosis and Vascular Biology, 2003. 23(12): p. 2252–2259. [DOI] [PubMed] [Google Scholar]
- 21.Kim MK, et al. , Harmful and beneficial relationships between alcohol consumption and subclinical atherosclerosis. Nutrition Metabolism and Cardiovascular Diseases, 2014. 24(7): p. 767–776. [DOI] [PubMed] [Google Scholar]
- 22.Zyriax BC, et al. , Association between alcohol consumption and carotid intima-media thickness in a healthy population: data of the STRATEGY study (Stress, Atherosclerosis and ECG Study). European Journal of Clinical Nutrition, 2010. 64(10): p. 1199–1206. [DOI] [PubMed] [Google Scholar]
- 23.Schooling CM, et al. , Moderate Alcohol Use and Mortality from Ischaemic Heart Disease: A Prospective Study in Older Chinese People. Plos One, 2008. 3(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Halanych JH, et al. , Alcohol Consumption in Young Adults and Incident Hypertension: 20-Year Follow-up From the Coronary Artery Risk Development in Young Adults Study. American Journal of Epidemiology, 2010. 171(5): p. 532–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nunez-Cordoba JM, et al. , Alcohol Consumption and the Incidence of Hypertension in a Mediterranean Cohort: the SUN Study. Revista Espanola De Cardiologia, 2009. 62(6): p. 633–641. [DOI] [PubMed] [Google Scholar]
- 26.Leong DP, et al. , Patterns of Alcohol Consumption and Myocardial Infarction Risk Observations From 52 Countries in the INTERHEART Case-Control Study. Circulation, 2014. 130(5): p. 390–398. [DOI] [PubMed] [Google Scholar]
- 27.Kanaya AM, et al. , Mediators of Atherosclerosis in South Asians Living in America (MASALA) Study: Objectives, Methods, and Cohort Description. Clinical Cardiology, 2013. 36(12): p. 713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bild DE, et al. , Multi-ethnic study of atherosclerosis: Objectives and design. American Journal of Epidemiology, 2002. 156(9): p. 871–881. [DOI] [PubMed] [Google Scholar]
- 29.Ainsworth BE, et al. , Moderate physical activity patterns of minority women: the Cross-Cultural Activity Participation Study. J Womens Health Gend Based Med, 1999. 8(6): p. 805–13. [DOI] [PubMed] [Google Scholar]
- 30.Libby P, Inflammation in atherosclerosis. Nature, 2002. 420(6917): p. 868–874. [DOI] [PubMed] [Google Scholar]
- 31.Becker AE, de Boer OJ, and van der Wal AC, The role of inflammation and infection in coronary artery disease. Annual Review of Medicine, 2001. 52: p. 289–297. [DOI] [PubMed] [Google Scholar]
- 32.Wood D and Joint F European Soc Task, Established and emerging cardiovascular risk factors. American Heart Journal, 2001. 141(2): p. S49–S57. [DOI] [PubMed] [Google Scholar]
- 33.Albert MA, Glynn RJ, and Ridker PM, Alcohol consumption and plasma concentration of C-reactive protein. Circulation, 2003. 107(3): p. 443–447. [DOI] [PubMed] [Google Scholar]
- 34.Volpato S, et al. , Relationship of alcohol intake with inflammatory markers and plasminogen activator inhibitor-1 in well-functioning older adults: the Health, Aging, and Body Composition study. Circulation, 2004. 109(5): p. 607–12. [DOI] [PubMed] [Google Scholar]
- 35.Stockley C, et al. , Bioavailability of wine-derived phenolic compounds in humans: a review. Food Funct, 2012. 3(10): p. 995–1007. [DOI] [PubMed] [Google Scholar]
- 36.Bianconi V, et al. , Cholesterol-Lowering Nutraceuticals Affecting Vascular Function and Cardiovascular Disease Risk. Curr Cardiol Rep, 2018. 20(7): p. 53. [DOI] [PubMed] [Google Scholar]
- 37.de Sa Coutinho D, et al. , Anti-Inflammatory Effects of Resveratrol: Mechanistic Insights. Int J Mol Sci, 2018. 19(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siler SQ, et al. , The inhibition of gluconeogenesis following alcohol in humans. American Journal of Physiology-Endocrinology and Metabolism, 1998. 275(5): p. E897–E907. [DOI] [PubMed] [Google Scholar]
- 39.Olechnowicz-Tietz S, et al. , The risk of atherosclerosis in patients with chronic kidney disease. Int Urol Nephrol, 2013. 45(6): p. 1605–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cano-Megias M, et al. , Coronary calcification as a predictor of cardiovascular mortality in advanced chronic kidney disease: a prospective long-term follow-up study. Bmc Nephrology, 2019. 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pan CS, et al. , Alcohol use disorder tied to development of chronic kidney disease: A nationwide database analysis. Plos One, 2018. 13(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.White SL, et al. , Alcohol consumption and 5-year onset of chronic kidney disease: the AusDiab study. Nephrology Dialysis Transplantation, 2009. 24(8): p. 2464–2472. [DOI] [PubMed] [Google Scholar]
- 43.Puddey IB and Beilin LJ, Alcohol is bad for blood pressure. Clinical and Experimental Pharmacology and Physiology, 2006. 33(9): p. 847–852. [DOI] [PubMed] [Google Scholar]
- 44.Santana NMT, et al. , Consumption of alcohol and blood pressure: Results of the ELSA-Brasil study. Plos One, 2018. 13(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aladin A, et al. , Alcohol Consumption and Risk of Hypertension. Journal of the American College of Cardiology, 2019. 73(9): p. 12–12. [Google Scholar]
- 46.Hines LM, et al. , Genetic variation in alcohol dehydrogenase and the beneficial effect of moderate alcohol consumption on myocardial infarction. N Engl J Med, 2001. 344(8): p. 549–55. [DOI] [PubMed] [Google Scholar]
- 47.Holmes MV, et al. , Association between alcohol and cardiovascular disease: Mendelian randomisation analysis based on individual participant data. Bmj-British Medical Journal, 2014. 349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Orford J, Johnson M, and Purser B, Drinking in second generation black and Asian communities in the English Midlands. Addiction Research & Theory, 2004. 12(1): p. 11–30. [Google Scholar]
- 49.Kalinowski A and Humphreys K, Governmental standard drink definitions and low-risk alcohol consumption guidelines in 37 countries. Addiction, 2016. 111(7): p. 1293–8. [DOI] [PubMed] [Google Scholar]
- 50.Wood AM, et al. , Risk thresholds for alcohol consumption: combined analysis of individual-participant data for 599 912 current drinkers in 83 prospective studies. Lancet, 2018. 391(10129): p. 1513–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]