Abstract
Objectives:
To assess neurodevelopmental outcomes among children with biliary atresia (BA) surviving with their native liver at age 3-12 years and evaluate variables that associate with neurodevelopment.
Methods:
Participants (age 3-12 years) in a prospective, longitudinal, multicenter study underwent neurodevelopmental testing with Weschler Preschool and Primary Scale of Intelligence, 3rd edition (WPPSI-III, age 3-5 yrs.) and Weschler Intelligence Scale for Children, 4th edition (WISC-IV, age 6-12 yrs.). Continuous scores were analyzed using Kolmogorov-Smironov tests compared to a normal distribution (mean = 100±15). Effect of covariates on Full-Scale Intelligence Quotient (FSIQ) was analyzed using linear regression.
Results:
Ninety-three participants completed 164 WPPSI-III (mean age 3.9) and 51 WISC-IV (mean age 6.9) tests. WPPSI-III FSIQ (104±14, P<0.02), Verbal IQ (106±14, P<0.001), and General Language Composite (107±16, P<0.001) distributions were shifted higher compared to test norms. WISC-IV FSIQ (105±12, P<0.01), Perceptual Reasoning Index (107±12, P<0.01), and Processing Speed Index (105±10, P<0.02) also shifted upwards. In univariate and multivariable analysis, parent education (P<0.01) was a significant predictor of FSIQ on WPPSI-III and positively associated with WISC-IV FSIQ. Male sex and higher total bilirubin and gamma glutamyl transferase (GGT) predicted lower WPPSI-III FSIQ. Portal hypertension was predictive of lower WISC-IV FSIQ.
Conclusion:
This cohort of children with BA and native liver did not demonstrate higher prevalence of neurodevelopmental delays. Markers of advanced liver disease (higher total bilirubin and GGT for age ≤5 yrs; portal hypertension for age ≥6) correlate with lower FSIQ and may identify a vulnerable subset of patients who would benefit from intervention.
Keywords: Cognitive, pediatric, chronic liver disease
Introduction:
Biliary atresia (BA) is a fibroinflammatory and obliterative cholangiopathy affecting children. Uncertainty remains regarding whether BA arises during fetal life or postnatally, however, recent efforts have demonstrated evidence of BA in the first days of life, suggesting its presence at the time of birth.(1) Still, BA remains the most common cause of pediatric end-stage liver disease and the leading indication for pediatric liver transplant.(2–4) Prior studies have demonstrated that developmental deficits are present in young children with BA and other chronic liver diseases.(5–9) Rapid neurodevelopment occurs over the first years of life and insults that accrue during this stage may be irreversible.(10) Very young children with BA display characteristic profiles of neurodevelopmental delays (11) and early transplant may not alleviate the degree of impairment.(12) Timely Kasai hepatoportoenterostomy (HPE) has enabled improved survival of children with their native livers; however, the majority of affected children require liver transplantation before 20 years of life.(13) Thus, the progression of biliary cirrhosis in older children puts them at significant risk for the development of metabolic derangements, portal hypertension, minimal or overt hepatic encephalopathy, infection, nutritional deficiencies, and growth retardation, all of which may influence neurodevelopment across multiple domains. While overall health status and quality of life have been examined in older BA patients with their native livers (14, 15), neurodevelopment has been less-well characterized. Validated, age-based instruments to measure neurodevelopment are valuable tools to assess health outcomes in children. These cognitive-specific clinical outcome assessments (COAs) enable the determination of how a disease affects a patient’s neurocognitive function and are used to define efficacy endpoints when studying the response to interventions.(16) We have previously reported that neurodevelopmental delays were present in BA patients with native liver ≤ 2 years of age (17), thus we hypothesized that delays would also be seen at later ages. Understanding the continuum of neurodevelopment in older children with BA, alive with their native liver, will be highly valuable in determining appropriate interventions, as well as potential benefits of such therapies, provided to these children. The current study assesses neurodevelopmental outcomes using individually administered age-appropriate intelligence tests to reflect a child’s abilities in discrete cognitive domains in pre-school and school aged children with BA, alive with their native liver.
Methods:
Participants with BA and their native liver ages 3-12 years enrolled in the NIDDK-supported Childhood Liver Disease Research Network (ChiLDReN) study, A Prospective Database of Infants with Cholestasis (PROBE, ), underwent neurodevelopmental testing with the Weschler Preschool and Primary Scale of Intelligence, 3rd edition (WPPSI-III) and the Weschler Intelligence Scale for Children, 4th edition (WISC-IV) between June 2007 and April 2018. Informed consent was obtained and the protocol was carried out under institutional review board approval. Successful HPE was defined as total bilirubin serum < 1.5 mg/dl (<26 umol/L) within 6 months after HPE.(18) Biliary atresia with splenic malformation (BASM) was defined as BA with laterality features, including but not limited to polysplenia, asplenia, or right-sided spleen. Definite clinically evident portal hypertension (dCEPH) was defined as a history of specific complications of portal hypertension (clinically evident ascites or endoscopic evidence of varices) or clinical findings consistent with portal hypertension (splenomegaly [spleen > 2 cm below the costal margin] and thrombocytopenia [platelet count < 150,000/mm3]). (19–21) Exclusion criteria of the parent PROBE study included a birth weight of ≤2000 g, acute liver failure, previous hepatobiliary surgery (non-HPE), sepsis, hypoxia, shock, malignancy, primary hemolytic disease, parenteral nutritional associated cholestasis, or extracorporeal membrane oxygenation-associated cholestasis.
Data, including biomarkers associated with hepatobiliary disease and physical exam findings, were collected per protocol and entered into a centralized database as previously described.(17)
Neurodevelopmental Assessment Measures:
Protocol-established developmental testing was completed annually from age 3 through 5 years (WPPSI-III), and every other year from age 6-12 (WISC-IV). Composite scores include overall Full-Scale Intelligence Quotient (FSIQ), as well as Verbal IQ (VIQ)/ Verbal Comprehension Index (VCI) and Performance IQ (PIQ) / Perceptual Reasoning Index (PRI) (WPPSI-III/WISC-IV). At age 4, Processing Speed Quotient (PSQ) / Processing Speed Index (PSI) was added and Working Memory Index (WMI) was included from age 6 (WISC-IV only). General Language Composite (GLC) was also calculated from age 3-5 on the WPPSI-III. Although newer versions of both measures were published during the study period, we continued to use the older versions for consistency. Both newer versions (WPPSI-IV, 2012 and WISC-V, 2014) are considerably restructured compared to the previous versions, which would make interpretation of results in this longitudinal study challenging if the newer tests were adopted midway in this study.
Statistical Analyses:
Scores were compared to the population norms using the first test of each type for each participant. Continuous scores were analyzed using Kolmogorov-Smironov tests compared to a normal distribution (mean 100, standard deviation 15). Scores were also categorized into bins of ≥100, 85-99, and <85 and graphically compared to expected counts.
Covariates of interests included demographics, medical history, and physical exam and laboratory measures within +/− 6 months of testing. Height and weight z-scores were calculated using SAS macros provided by the Centers for Disease Control and Prevention (CDC) using the CDC growth charts.(22) Values flagged by the macro as being biologically implausible were excluded from analysis. Skewed laboratory variables were transformed to the log10 scale.
Univariate and multivariable linear regressions were performed on continuous FSIQ for the first test of each measure (WPPSI-III, WISC-IV) for each participant. Univariate p-values were adjusted for multiple testing using the adaptive Hochberg correction for family-wise error rate. For multivariable modeling, missing data for covariates was imputed using IVEware(23) version 2.0 with 21 imputations. Model selection was performed in R using MI-LASSO with all covariates available for selection regardless of significance on univariate analysis.(24) Variables selected in the model fit with the optimal lambda were used in the final model. Descriptive statistics and final analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Results:
Participant Characteristics:
A total of 597 PROBE participants with BA who underwent HPE were identified. Participants who underwent liver transplantation (n=253), died (n=23), had not reached the minimum age for appropriate WPPSI-III testing (n=57), or were lost to follow-up (n=32) did not undergo neurodevelopmental assessment. (Figure, Supplemental Digital Content 1 provides details of sample ascertainment) Eligible participants who completed neurodevelopmental testing (n=93) were more likely than those who were eligible but not tested (n=138) to be non-Hispanic and to have had a successful HPE (both P < 0.05, Table, Supplemental Digital Content 1). Demographic, medical, and clinical characteristics of all participants who underwent neurodevelopmental testing are provided in Table 1.
Table 1:
Demographic and Medical Characteristics for Neurocognitive Testing Participants by Age
| WPPSI-III (Age 3) (n=69) | WPPSI-III (Age 4) (n=50) | WPPSI-III (Age 5) (n=41) | WPPSI-III/WISC-IV (Age 6) (n=37) | WISC-IV (Age 8-12) (n=18)* | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | N | Stats or n (%) | N | Stats or n (%) | N | Stats or n (%) | N | Stats or n (%) | N | Stats or n (%) | |
| Demographics | |||||||||||
| Female sex | 69 | 28 (41%) | 50 | 26 (52%) | 41 | 23 (56%) | 37 | 20 (54%) | 18 | 9 (50%) | |
| Race | White | 69 | 42 (61%) | 49 | 33 (67%) | 41 | 29 (71%) | 37 | 26 (70%) | 18 | 10 (56%) |
| Black | 69 | 7 (10%) | 49 | 3 (6%) | 41 | 1 (2%) | 37 | 0 (0%) | 18 | 1 (6%) | |
| Other | 69 | 20 (29%) | 49 | 13 (27%) | 41 | 11 (27%) | 37 | 11 (30%) | 18 | 7 (39%) | |
| Hispanic ethnicity | 69 | 12 (17%) | 50 | 11 (22%) | 41 | 9 (22%) | 37 | 9 (24%) | 18 | 2 (11%) | |
| Highest Household Education | College degree or more | 65 | 37 (57%) | 49 | 32 (65%) | 39 | 25 (64%) | 35 | 23 (66%) | 18 | 13 (72%) |
| BASM Syndrome | 69 | 3 (4%) | 50 | 2 (4%) | 41 | 1 (2%) | 37 | 1 (3%) | 18 | 2 (11%) | |
| Medical History | |||||||||||
| Age at HPE (months), mean (SD) | 69 | 1.9 (0.7) | 50 | 1.9 (0.9) | 41 | 1.9 (0.6) | 37 | 2.0 (0.7) | 18 | 1.9 (0.5) | |
| Successful HPE | 69 | 63 (91%) | 50 | 44 (88%) | 41 | 39 (95%) | 37 | 35 (95%) | 18 | 17 (94%) | |
| Cholangitis | 69 | 34 (49%) | 50 | 26 (52%) | 41 | 17 (41%) | 37 | 19 (51%) | 18 | 9 (50%) | |
| GI bleed | 69 | 5 (7%) | 50 | 3 (6%) | 41 | 4 (10%) | 37 | 3 (8%) | 18 | 2 (11%) | |
| Ascites | 69 | 11 (16%) | 50 | 7 (14%) | 41 | 5 (12%) | 37 | 6 (16%) | 18 | 1 (6%) | |
| Splenomegaly | 69 | 46 (67%) | 50 | 35 (70%) | 41 | 27 (66%) | 37 | 24 (65%) | 18 | 14 (78%) | |
| Thrombocytopenia | 69 | 37 (54%) | 50 | 26 (52%) | 41 | 23 (56%) | 37 | 22 (59%) | 18 | 11 (61%) | |
| Esophageal or gastric varices | 69 | 2 (3%) | 50 | 2 (4%) | 41 | 4 (10%) | 37 | 5 (14%) | 18 | 1 (6%) | |
| Portal hypertension | 69 | 32 (46%) | 50 | 26 (52%) | 41 | 20 (49%) | 37 | 18 (49%) | 18 | 10 (56%) | |
| Measures taken at testing (+/− 6 months) | |||||||||||
| Age at testing (years), mean (SD) | 69 | 3.0 (0.1) | 50 | 4.0 (0.1) | 41 | 5.1 (0.1) | 37 | 6.1 (0.2) | 18 | 8.3 (1.0) | |
| Weight Z-score, mean (SD) | 67 | 0.33 (1.06) | 49 | 0.52 (0.91) | 41 | 0.48 (0.92) | 36 | 0.38 (0.95) | 18 | 0.51 (0.90) | |
| Height Z-score, mean (SD) | 65 | −0.00 (1.04) | 50 | −0.00 (0.99) | 41 | 0.14 (0.95) | 37 | 0.18 (1.12) | 18 | 0.02 (0.74) | |
| Total bilirubin (mg/dL), median (IQR) | 65 | 0.6 (0.3-1.0) | 46 | 0.5 (0.3-0.7) | 39 | 0.6 (0.3-0.7) | 35 | 0.5 (0.3-0.9) | 16 | 0.8 (0.4-0.9) | |
| INR, median (IQR) | 52 | 1.0 (1.0-1.1) | 37 | 1.0 (1.0-1.1) | 32 | 1.1 (1.0-1.1) | 29 | 1.1 (1.0-1.1) | 13 | 1.1 (1.0-1.2) | |
| Hemoglobin (g/dl), median (IQR) | 60 | 12.6 (12.0-13.4) | 47 | 12.7 (12.2-13.2) | 37 | 13.2 (12.5-14.0) | 34 | 13.1 (12.1-13.7) | 16 | 13.8 (12.7-14.2) | |
| Platelet count, median (IQR) | 60 | 177 (118-307) | 46 | 183 (127-298) | 37 | 199 (110-270) | 34 | 175 (96-263) | 16 | 164 (68-260) | |
| GGTP, median (IQR) | 56 | 117 (22-287) | 40 | 72 (24-159) | 34 | 35 (19-81) | 30 | 81 (41-143) | 15 | 58 (19-155) | |
| ALT, median (IQR) | 65 | 79 (35-156) | 48 | 52 (32-126) | 39 | 40 (24-73) | 35 | 48 (32-80) | 16 | 38 (28-84) | |
| AST, median (IQR) | 64 | 76 (48-149) | 48 | 59 (43-108) | 39 | 54 (41-67) | 35 | 56 (45-86) | 16 | 49 (35-91) | |
| WBC count, median (IQR) | 61 | 6.4 (4.9-8.0) | 47 | 6.8 (4.5-8.5) | 37 | 5.7 (4.4-7.4) | 34 | 5.3 (4.4-7.1) | 16 | 5.6 (3.4-6.2) | |
Not all unique participants
Note: participants may contribute to more than one column.
Neurodevelopmental Test Scores and Distribution:
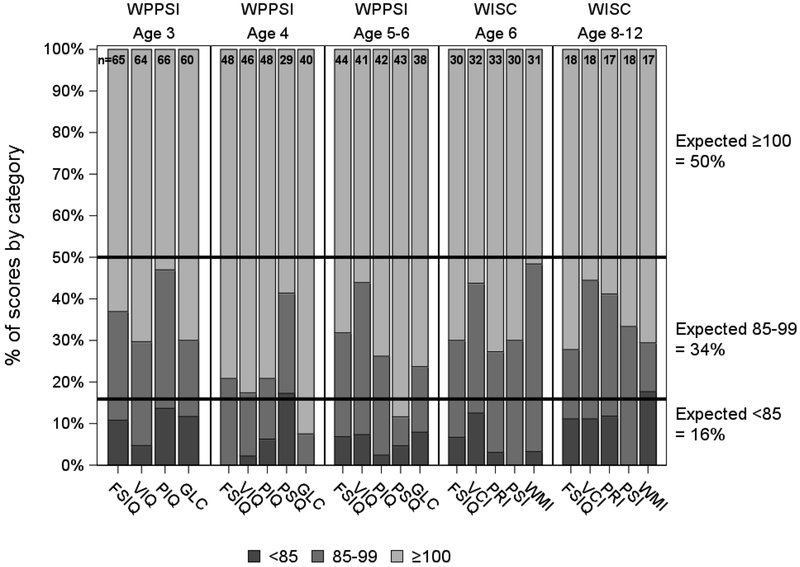
A total of 164 WPPSI-III and 51 WISC-IV tests from 93 participants were available for the final analysis. FSIQ distributions for both WPPSI-III (FSIQ 104 ± 14, P < 0.02) and WISC-IV (FSIQ 105 ± 12, P < 0.01) were shifted higher compared to test norms. Additional upward shifts were seen in WPPSI-III Verbal IQ (VIQ 106 ± 14, P < 0.001) and the general language composite score (GLC 107 ± 16, P < 0.001). WISC-IV Perceptual Reasoning Index (PRI 107 ± 12, P < 0.01) and Processing Speed Index (PSI 105 ± 10, P < 0.02) were also shifted upwards. (Table, Supplemental Digital Content 2) Categorical distribution of both WPPSI-III and WISC-IV scores demonstrated an overall upward shift, with higher proportion of average to above average scores than expected compared with the normal population. (Figure 1) Fewer than expected participants scored more than 1 standard deviation below the mean (FSIQ < 85) with only 8.4% (n=7) and 7.3% (n=3) of WPPSI-III and WISC-IV tested participants demonstrating severe deficits (2 or more standard deviations below the mean) at the time of first test.
Figure 1. Neurocognitive Score Distribution.
Distribution of WPPSI-III and WISC-IV scores compared to population norms. FSIQ: Full Scale Intelligent Quotient; GLC: General Language Composite; PIQ: Performance Intelligent Quotient; VIQ: Verbal Intelligent Quotient; PSQ: Processing Speed Quotient; PRI: Perceptual Reasoning Index; PSI: Processing Speed Index; VCI: Verbal Comprehension Index; WMI: Working Memory Index
Predictive Variables of FSIQ on WPPSI-III Testing:
Results of univariate and multivariable analyses identified demographic, medical history, and clinical variables predicted to associate with FSIQ scores. (Tables 2 and 3) Univariate analysis of WPPSI-III results (participants ≤ 5-years old, mean age 3.3 [SD 0.6], n=87) indicated that higher FSIQ associated with higher parent education (college degree or more vs less; P<0.01). Additionally, the models highlighted male sex and markers of advancing liver disease, including higher serum total bilirubin, GGT, ALT, and AST, as risk factors for lower FSIQ. Multivariable models using imputed data showed similar results with male sex and markers of advanced liver disease negatively influencing FSIQ on WPPSI-III tests. The pattern of highest household education (college degree or more) positively influencing FSIQ persisted. (Table 3) While models suggested non-white race was a factor associated with lower FSIQ, the overall demographic constitution of the cohort (Table 1) precludes a broader application of these findings. The median root mean squared error among the 21 imputations was 12.5 (range 12.3-12.7), a reduction from the general standard deviation for test scores of 15. The tight range additionally indicates stability of the imputation procedure for parameter estimation.
Table 2:
Variables included in univariate linear regression results for impacting FSIQ testing
| WPPSI-III |
WISC-IV |
||||||
|---|---|---|---|---|---|---|---|
| Variable | Estimate (95% CI) | Unadj P-value | Adj P-value* | Estimate (95% CI) | Unadj P-value | Adj P-value* | |
| Demographics | |||||||
| Male vs. Female | −6.72 (−12.85, −0.58) | 0.032 | 0.226 | 2.20 (−5.67, 10.07) | 0.575 | 0.907 | |
| Race | Black vs. White | −14.76 (−24.72, −4.81) | 0.004 | 0.029 | −15.77 (−40.99, 9.45) | 0.213 | 0.907 |
| Other vs. White | −4.41 (−11.33, 2.52) | 0.209 | 0.877 | −3.77 (−11.97, 4.43) | 0.358 | 0.907 | |
| Hispanic ethnicity | −6.17 (−14.03, 1.70) | 0.123 | 0.858 | −9.29 (−19.97, 1.39) | 0.086 | 0.907 | |
| Highest Household Education: College degree or more | 10.31 (4.21, 16.40) | 0.001 | 0.008 | 8.73 (0.33, 17.13) | 0.042 | 0.674 | |
| BASM | −3.68 (−20.53, 13.18) | 0.666 | 0.877 | −23.76 (−40.26, −7.26) | 0.006 | 0.095 | |
| Medical History | |||||||
| Age at HPE (months) | −1.36 (−5.15, 2.43) | 0.477 | 0.877 | −4.39 (−10.29, 1.51) | 0.140 | 0.907 | |
| Unsuccessful HPE | −3.92 (−14.56, 6.72) | 0.465 | 0.877 | −5.88 (−23.99, 12.22) | 0.515 | 0.907 | |
| Cholangitis | −2.62 (−8.90, 3.66) | 0.409 | 0.877 | −2.14 (−9.98, 5.69) | 0.583 | 0.907 | |
| GI bleed | −6.54 (−19.69, 6.62) | 0.326 | 0.877 | −5.65 (−18.74, 7.44) | 0.388 | 0.907 | |
| Portal hypertension | −0.49 (−6.80, 5.82) | 0.877 | 0.877 | −7.83 (−15.28, −0.39) | 0.040 | 0.635 | |
| Measures taken at testing (+/− 6 months) | |||||||
| Weight z-score | 1.81 (−1.20, 4.82) | 0.235 | 0.877 | 1.84 (−2.49, 6.16) | 0.395 | 0.907 | |
| Height z-score | 1.16 (−1.94, 4.26) | 0.459 | 0.877 | 3.08 (−0.68, 6.85) | 0.106 | 0.907 | |
| Total bilirubin, log10 scale | −9.62 (−18.59, −0.65) | 0.036 | 0.251 | 1.06 (−14.20, 16.32)** | 0.889 | 0.907 | |
| INR (per 0.1) | 0.76 (−3.12, 4.64) | 0.697 | 0.877 | 0.29 (−4.72, 5.30) | 0.907 | 0.907 | |
| Hemoglobin (g/dl) | 2.46 (−0.72, 5.65) | 0.128 | 0.877 | −1.56 (−5.22, 2.09) | 0.391 | 0.907 | |
| Platelet count (per 50) | 0.82 (−0.82, 2.45) | 0.322 | 0.877 | −0.33 (−2.30, 1.64) | 0.736 | 0.907 | |
| GGT, log10 scale | −7.11 (−13.41, −0.81) | 0.028 | 0.193 | −4.30 (−13.08, 4.48) | 0.326 | 0.907 | |
| ALT, log10 scale | −8.20 (−15.93, −0.47) | 0.038 | 0.265 | −1.28 (−13.01, 10.44) | 0.825 | 0.907 | |
| AST, log10 scale | −7.84 (−18.38, 2.70) | 0.143 | 0.877 | −1.77 (−18.86, 15.31) | 0.835 | 0.907 | |
| WBC count | 0.95 (−0.44, 2.34) | 0.176 | 0.877 | −0.75 (−2.64, 1.14) | 0.424 | 0.907 | |
Adaptive Hochberg correction for family-wise error rate
Excluding n=1 outlier with high influence
ALT = Alanine transaminase; AST = Aspartate transaminase; BASM = Biliary atresia with splenic malformation; FSIQ = Full-Scale Intelligence Quotient; GGT = Gamma glutamyl transferase; HPE = Hepatoportoenterostomy; INR = International normalized ratio; WBC = White blood cell; WISC-IV = Weschler Intelligence Scale for Children, 4th edition; WPPSI-III = Weschler Preschool and Primary Scale of Intelligence, 3rd edition
Table 3:
Variables included in multivariable linear regression results for impacting FSIQ testing
| Variable | Estimate (95% CI) | P-value | |
|---|---|---|---|
| WPPSI-III (21 imputed datasets of N=87 each, df=[p=6,d=66114])* | |||
| Intercept | 106.02 (91.78, 120.25) | <.001 | |
| Male vs. Female | −6.76 (−12.38, −1.15) | 0.018 | |
| Race | Black vs. White | −9.95 (−19.32, −0.59) | 0.037 |
| Other vs. White | −6.82 (−13.22, −0.43) | 0.037 | |
| Highest Household Education: College degree or more | 8.58 (2.65, 14.52) | 0.005 | |
| Total bilirubin, log10 scale | −8.50 (−17.14, 0.14) | 0.054 | |
| GGT, log10 scale | −1.43 (−7.38, 4.53) | 0.638 | |
| WISC-IV (21 imputed datasets of N=44 each, df=[p=3,d=314656])* | |||
| Intercept | 104.37 (95.90, 112.84) | <.001 | |
| Hispanic Ethnicity | −4.54 (−15.48, 6.39) | 0.416 | |
| Highest Household Education: College degree or more | 5.65 (−2.75, 14.05) | 0.187 | |
| Portal hypertension | −5.45 (−13.06, 2.15) | 0.160 | |
for an overall test of the model, which references an F distribution with p,d degrees of freedom with p corresponding to the number of parameters in the model and d being the estimated degrees of freedom for the variability
FSIQ = Full-Scale Intelligence Quotient; GGT = Gamma glutamyl transferase
Predictive Variables of FSIQ on WISC-IV Testing:
For participants ≥ 6-years old who completed WISC-IV testing (mean age 6.7 [SD 0.9], n=44), univariate analyses did not uncover any variables that were significantly predictive of lower FSIQ scores after adjusting for multiple testing. (Table 2) However, multivariable modeling using imputed data suggested participants with clinical evidence of portal hypertension were expected to have lower FSIQ than those without such evidence. Higher household education was again associated with higher FSIQ scores in participants who came from households where members had achieved a college degree or more. (Table 3) The median root mean squared error among the 21 imputations was 11.5 (range 11.5-11.7), a reduction from the general standard deviation for test scores of 15. The tight range additionally indicates stability of the imputation procedure for parameter estimation.
Discussion:
Our findings represent the largest prospective, multi-center study of neurodevelopmental outcomes in preschool and school age patients with BA and native liver to date. We found children with BA and native liver (age 3-12 years) did not demonstrate an increased prevalence of neurodevelopmental delays. In fact, our sample demonstrated higher IQ scores compared to test norms on the WPPSI-III and WISC-IV. This is contrary to our initial hypothesis, in part based on prior studies (17) that delays would be prevalent in this population. There are likely several factors that contributed to these unexpected findings which we describe below. In those who did demonstrate lower IQ scores, we looked to better understand risks, and potentially identify treatment targets, when delays were present. We found that male sex and markers of advanced liver disease were risk factors for lower IQ.
As the success of HPE for BA is most dependent on earliest age of surgical intervention, there has been an increased focus on detection and timely referral.(1, 2, 25) However, challenges persist and the prevention of progressive liver injury and fibrosis that often occurs, even after successful HPE, contributes to the heavy disease burden for affected children, their families, and society as a whole.(3) Children with chronic liver diseases are known to be at increased risk of deficits in multiple neurodevelopmental domains; however, there remains a paucity of data on neurodevelopmental outcomes in children with hepatobiliary disease and their native liver, compared with those who have undergone liver transplantation.(9) Our group’s recent report on the neurodevelopmental outcomes of 1- and 2 year old children with BA and their native liver after HPE demonstrated that substantial risk of delays are indeed present.(17) Collectively, and compounded by a renewed interest to incorporate neurodevelopmental outcomes as acceptable endpoints for clinical trial design (26), we examined IQ longitudinally in 3-12 year old children with BA and their native liver. Increased risk for neurodevelopmental delays in very young children with BA has been primarily associated with an unsuccessful HPE; however, even with evidence of an effective drainage procedure, vulnerability remains.(17) While the majority of our tested cohort was determined to have had a successful HPE, it was still surprising that we did not find persistent neurodevelopmental delays. It is possible that death (occurring in 23 participants ≤ 3 years of age; and 2 participants ages of 3-12 years) and transplantation (required in 253 subjects ≤ 3 years of age; and 22 participants ages of 3 to 12 years) over the course of the study may account for some of the testing results in our cohort as the patients with the most advanced liver disease either died or had their disease course interrupted by transplant. Then again, neurodevelopmental stabilization in the older cohort may reflect overall improvements in health status, a focus on nutritional rehabilitation, developmental interventions, and fewer disease related complications. This is supported by trajectory plots of participants who demonstrated neurodevelopmental deficits at younger age (≤ 2 years, Bayley Scales of Infant Development, 2nd edition) and higher functioning at older time points. (Figure Supplemental Digital Content 1) However, caution should be taken to not over-interpret these results as neurodevelopmental testing in very young children, including Bayley measurements, have been shown to be insufficient predictors of future IQ.(27)
Alternatively, our findings may also be framed by the growing understanding of the “recovery continuum” whereby the concepts of early plasticity and early vulnerability are thought to represent two extremes explaining the wide spectrum of clinical outcomes in children who recover from early brain insults.(28) Here, it is proposed that an individual child’s recovery will depend upon a number of influences including injury-related factors (e.g. nature, severity, timing), constitutional factors (e.g. developmental stage, cognitive capacity, genetic make-up, gender) and environment (e.g. family function, social status, access to rehabilitation/intervention). Nevertheless, strategies are needed to identify those participants who are most at risk for neurodevelopmental delays so that appropriate interventions on modifiable variables may be pursued that can act to optimize outcomes for patients with progressive, chronic disease.
The clinical course of older children with BA with their native liver is well-described, in which effective biliary drainage and markers of cholestasis essentially may normalize in the months following successful HPE yet progressive hepatobiliary disease continues, usually manifesting with minimal aminotransferase elevations, advancing fibrosis, and portal hypertension. In our study, elevations in clinical markers of cholestasis were predictive of lower FSIQ scores in the cohort of participants age 3 to 5 years. For the oldest cohort ≥ 6 years, the only clinical finding predictive of lower FSIQ in the best fit model was the presence of clinically evident portal hypertension. The effect of persistent cholestasis on WPPSI-III testing and portal hypertension on the WISC-IV would seem to extend findings reported in the very young (<2 years), where persistent or advancing liver disease, such as ascites and growth deficits, negatively influences neurodevelopment. (17)
Similar to our group’s prior findings (17), others have also reported on the neurodevelopmental deficits that are often seen in very young children with BA.(11, 29, 30) In older children with BA, the majority of the published literature describes those who have undergone liver transplant. In these reports, the intelligence scores were below the average of the norm population with mean FSIQ ranging from 86.6-98.0.(9, 30–39) We report for the first time on intelligence scores for older children with BA alive with their native liver who have avoided transplantation and demonstrate scores statistically shifted upwards and solidly within the average range vs test norms (WPPSI-III FSIQ 104±14, p=0.01; WISC-IV FSIQ 105±12, p<0.01). One explanation for these discrepant findings may be the under-appreciated effect of the transplant procedure itself. Major surgery, extended anesthesia, prolonged hospitalization, and immunosuppression are only a few of the many factors that may unduly influence the neurodevelopmental recovery potential for a given child. Future efforts would benefit from a better understanding of the peri-operative variables that can affect neurodevelopmental outcomes. Alternatively, our results may reflect the significant contributions of the higher socio-economic status of our population. In our cohort, 60% had a college degree or more, compared to 25-28% in the WPPSI-III/WISC-IV normative samples. Parent education is a well-known and important predictor of IQ for multiple reasons and it is possible the higher parent education in our sample may have resulted in an underestimate of neurocognitive deficits. In addition, assessments that include measures of executive function, attention, and academic performance may provide a more sensitive appraisal of neurodevelopmental outcomes and yield a fuller understanding of the cognitive profiles of older children with BA.
Our study constitutes the largest examination of the neurodevelopmental outcomes in a prospective cohort of children with BA alive with their native livers; however, we acknowledge several potential biases and limitations which may limit the generalizability of our findings. Study ascertainment and completion of testing were challenging. Over the course of the study, 81 participants were lost to follow-up and only 40% of eligible participants completed their neurodevelopmental assessments likely resulting in ascertainment bias which is underscored by the disproportionate percentage of non-Hispanic white participants in our cohort. Secondly, there were few participants in PROBE with failed Kasai (a known risk for neurodevelopmental deficits) who survived without liver transplant in this age group. Furthermore, we recognize that the participants with the most advanced liver disease received a transplant or died prior to potential testing at age 3 years, and were thus not included in this analysis, biasing this analysis to children who survived to an older age with native liver. Another important limitation, especially considering above average IQ findings, is the use of older test versions throughout the study. The WPPSI-III and WISC-IV normative samples were based on 2000 census data, while the updated versions (WPPSI-IV 2012 and WISC-V 2014) were based on 2010 data. Described as the “Flynn Effect”, IQ scores have been observed to rise over time (40), meaning the IQ scores reported here are likely somewhat inflated. Our study group chose to continue testing with the older measures rather than shift to the newer versions during the course of data collection because interpretation of aggregated data would be very challenging. Lastly, we note the narrowness in which FSIQ is reflective of more comprehensive neurocognitive health and the potential disparity between statistical significance and clinical effect. Measurement of multidimensional constructs, such as the health-related quality of life (HRQOL), which depict a group’s perceived physical and psychosocial functioning, have demonstrated school functioning to be low in children with BA and their native livers. (41) While these patient-reported measurements are by definition subjective, they may more clearly signal clinically relevant issues.
In conclusion, we present for the first time the results of neurodevelopmental testing of older children with BA alive with their native liver from a large, multi-center prospective study. These findings did not demonstrate developmental delays but instead found a solidly average, if not an overall higher FSIQ in older participants (age 3-12 years) with BA and their native liver. However, elevations in clinical markers of cholestasis and the presence of portal hypertension did negatively affect neurodevelopmental measurement at varying timepoints. These data will be useful to future investigators determining surrogates of disease progression for interventional studies and clinical trials that will target subgroups of patients with initial drainage following HPE. Additionally, these data can be used currently to counsel patients and families about neurodevelopmental functions that can be expected in children with this devastating disease who are fortunate enough to avoid the need for early liver transplantation.
Supplementary Material
Figure, Supplemental Digital Content 1. Spaghetti plot of WPPSI and WISC scores by Bayley Performance. Trajectory plots of participants who demonstrated neurodevelopmental deficits at younger age (≤ 2 years, Bayley Scales of Infant Development, 2nd edition) and higher functioning at older time points. Each dot represents a test score for WPPSI-III or WISC-IV. Blue lines represent participants who had previous FSIQ scores of ≤ 85 on Bayley testing. Black lines represent participants who had previous FSIQ scores of > 85 on Bayley testing.
Table, Supplemental Digital Content 1: Select demographics and medical variables of participants (with testing) vs eligible non-participants (not tested but could have been)
*Wilcoxon for continuous variables, chi-square or Fisher for categorical.
Table, Supplemental Digital Content 2: Comparison of patient scores to the normal population, first test of each WPPSI-III and WISC-IV
*Kolmogorov-Smirnov
Normal (100,15)
Figure, Supplemental Digital Content 2. Study Participant Ascertainment. Flow diagram demonstrating study participation and ascertainment. Left vertical column shows total enrollment and participant eligibility at each time point, as well as percentage of those tested vs. eligible but not tested. Right vertical column shows ineligible participants and reasons for not being tested.
What is Known:
Biliary atresia (BA) is the most common cause of pediatric end-stage liver disease and the leading indication for pediatric liver transplant.
Young children with BA display characteristic profiles of neurodevelopmental delays and early transplant may not alleviate impairment.
Rapid neurodevelopment occurs over the first years of life and insults during this stage may be irreversible.
What is New:
Children with BA and native liver (age 3-12 years) did not demonstrate an increased prevalence of neurodevelopmental delays.
Intelligence scores increased for this cohort within the average range vs test norms.
Markers of advanced liver disease did negatively affect neurodevelopmental measurement at varying timepoints.
Acknowledgments
Conflicts of Interest and Source of Funding
This work was supported by U01 grants from the National Institute of Diabetes, Digestive and Kidney Diseases (DK 62445 [Mount Sinai School of Medicine], DK 62497 [Cincinnati Children’s Hospital Medical Center], DK 62470 [Children’s Healthcare of Atlanta], DK 62481 [The Children’s Hospital of Philadelphia], DK 62456 [The University of Michigan], DK 84536 [Riley Hospital for Children], DK 84575 [Seattle Children’s Hospital], DK 62500 [UCSF Children’s Hospital], DK 62503 [Johns Hopkins School of Medicine], DK 62466 [Children’s Hospital of Pittsburgh of UPMC], DK 62453 [University of Colorado Denver], DK 62452 [Washington University School of Medicine], DK 84538 [Children’s Hospital Los Angeles], DK 62436 [Ann & Robert H Lurie Children’s Hospital of Chicago], DK103149 [Texas Children’s Hospital], DK103135 [The Hospital for Sick Children], DK103140 [University of Utah]).
In addition, the project described was supported by National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award (NCATS CTSA) grants: University of Colorado UL1 TR002535, Cincinnati Children’s Hospital Medical Center UL1 TR000077, UCSF Children’s Hospital UL1 TR001872, Indiana University Hospital for Children UL1 TR001108, Children’s Hospital of Pittsburgh of UPMC UL1 TR001857, The Children’s Hospital of Philadelphia UL1 TR001878, Seattle Children’s Hospital UL1 TR000423 and UL1 RR025014, Children’s Healthcare of Atlanta UL1 TR000454, Children’s Hospital of Los Angeles UL1TR00130.
KFM reports research support <$10K: Gilead, Shire/Mirum, Merck and consulting for Gilead. No additional support for this work; JPM reports funding from Abbvie, Gillead, Mirum for other studies not related to this work. VLN reports consulting fee or honorarium and support for travel to meetings for the study or other purposes. PR reports grant support from the NIH; VLN reports consulting fee or honorarium and support for travel to meetings for the study or other purposes.
References:
- 1.Harpavat S, Garcia-Prats JA, Shneider BL. Newborn Bilirubin Screening for Biliary Atresia. N Engl J Med. 2016;375(6):605–6. [DOI] [PubMed] [Google Scholar]
- 2.Wang KS, Section on S, Committee on F, Newborn, Childhood Liver Disease Research N. Newborn Screening for Biliary Atresia. Pediatrics. 2015;136(6):e1663–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bezerra JA, Wells RG, Mack CL, Karpen SJ, Hoofnagle JH, Doo E, et al. BILIARY ATRESIA: Clinical and Research Challenges for the 21(st) Century. Hepatology. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanchez-Valle A, Kassira N, Varela VC, Radu SC, Paidas C, Kirby RS. Biliary Atresia: Epidemiology, Genetics, Clinical Update, and Public Health Perspective. Adv Pediatr 2017;64(1):285–305. [DOI] [PubMed] [Google Scholar]
- 5.Chin SE, Shepherd RW, Cleghorn GJ, Patrick MK, Javorsky G, Frangoulis E, et al. Survival, growth and quality of life in children after orthotopic liver transplantation: a 5 year experience. Journal of paediatrics and child health. 1991;27(6):380–5. [DOI] [PubMed] [Google Scholar]
- 6.Moser JJ, Veale PM, McAllister DL, Archer DP. A systematic review and quantitative analysis of neurocognitive outcomes in children with four chronic illnesses. Paediatric anaesthesia. 2013;23(11):1084–96. [DOI] [PubMed] [Google Scholar]
- 7.Stewart SM, Hiltebeitel C, Nici J, Waller DA, Uauy R, Andrews WS. Neuropsychological outcome of pediatric liver transplantation. Pediatrics. 1991;87(3):367–76. [PubMed] [Google Scholar]
- 8.Stewart SM, Uauy R, Waller DA, Kennard BD, Benser M, Andrews WS. Mental and motor development, social competence, and growth one year after successful pediatric liver transplantation. The Journal of pediatrics. 1989;114(4 Pt 1):574–81. [DOI] [PubMed] [Google Scholar]
- 9.Rodijk LH, den Heijer AE, Hulscher JBF, Verkade HJ, de Kleine RHJ, Bruggink JLM. Neurodevelopmental Outcomes in Children With Liver Diseases: a Systematic Review. J Pediatr Gastroenterol Nutr 2018;67(2):157–68. [DOI] [PubMed] [Google Scholar]
- 10.Johnson RJ, Warady BA. Long-term neurocognitive outcomes of patients with end-stage renal disease during infancy. Pediatric nephrology. 2013;28(8):1283–91. [DOI] [PubMed] [Google Scholar]
- 11.Caudle SE, Katzenstein JM, Karpen SJ, McLin VA. Language and motor skills are impaired in infants with biliary atresia before transplantation. The Journal of pediatrics. 2010;156(6):936–40, 40, e1. [DOI] [PubMed] [Google Scholar]
- 12.Wayman KI, Cox KL, Esquivel CO. Neurodevelopmental outcome of young children with extrahepatic biliary atresia 1 year after liver transplantation. The Journal of pediatrics. 1997;131(6):894–8. [DOI] [PubMed] [Google Scholar]
- 13.Sundaram SS, Mack CL, Feldman AG, Sokol RJ. Biliary atresia: Indications and timing of liver transplantation and optimization of pretransplant care. Liver Transpl 2017;23(1):96–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lind RC, de Vries W, Keyzer CM, Peeters PM, Verkade HJ, Hoekstra-Weebers JE, et al. Health status and quality of life in adult biliary atresia patients surviving with their native livers. European journal of pediatric surgery : official journal of Austrian Association of Pediatric Surgery [et al] = Zeitschrift fur Kinderchirurgie. 2015;25(1):e2. [DOI] [PubMed] [Google Scholar]
- 15.Ng VL, Haber BH, Magee JC, Miethke A, Murray KF, Michail S, et al. Medical status of 219 children with biliary atresia surviving long-term with their native livers: results from a North American multicenter consortium. The Journal of pediatrics. 2014;165(3):539–46 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walton MK, Powers JH 3rd, Hobart J, Patrick D, Marquis P, Vamvakas S, et al. Clinical Outcome Assessments: Conceptual Foundation-Report of the ISPOR Clinical Outcomes Assessment - Emerging Good Practices for Outcomes Research Task Force. Value in health : the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2015;18(6):741–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ng VL, Sorensen LG, Alonso EM, Fredericks EM, Ye W, Moore J, et al. Neurodevelopmental Outcome of Young Children with Biliary Atresia and Native Liver: Results from the ChiLDReN Study. The Journal of pediatrics. 2018;196:139–47 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bezerra JA, Spino C, Magee JC, Shneider BL, Rosenthal P, Wang KS, et al. Use of corticosteroids after hepatoportoenterostomy for bile drainage in infants with biliary atresia: the START randomized clinical trial. Jama. 2014;311(17):1750–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shneider BL, Abel B, Haber B, Karpen SJ, Magee JC, Romero R, et al. Portal hypertension in children and young adults with biliary atresia. Journal of pediatric gastroenterology and nutrition. 2012;55(5):567–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bass LM YW, Hawthorne K, Leung DH, Murray KF, Molleston JP, Romero R, Sokol RJ, Karpen SJ, Rosenthal P, Loomes KM, Wang KS, Squires RH, Bezerra JA, Kamath BM, Guthery SL, Magee JC, Shneider BL, for the ChiLDReN Network. Natural History and Risk Factors for Variceal Hemorrhage in Biliary Atresia: Results of a Prospective Multi-Center Longitudinal Analysis. Digestive Diseases Week (DDW), Washington DC: June 2-5, 2018. 2018. [Google Scholar]
- 21.Bass LM, Shneider BL, Henn L, Goodrich NP, Magee JC, Childhood Liver Disease Research N. Clinically Evident Portal Hypertension: An Operational Research Definition for Future Investigations in the Pediatric Population. J Pediatr Gastroenterol Nutr 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.A SAS Program for the 2000 CDC Growth Charts (ages 0 to <20 years) Division of Nutrition, Physical Activity, and Obesity, National Center for Chronic Disease Prevention and Health Promotion; >2016. [Available from: https://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm. [Google Scholar]
- 23.Raghunathan TS, Hoewyk PW, JV. IVEware: Imputation and Variance Estimation Software User Guide In: Survey Research Center IfSR, University of Michigan, editor. Survey Methodology Program; 2002. p. 1–73. [Google Scholar]
- 24.Chen Q, Wang S. Variable selection for multiply-imputed data with application to dioxin exposure study. Stat Med. 2013;32(21):3646–59. [DOI] [PubMed] [Google Scholar]
- 25.Harpavat S, Lupo PJ, Liwanag L, Hollier J, Brandt ML, Finegold MJ, et al. Factors Influencing Time-to-diagnosis of Biliary Atresia. J Pediatr Gastroenterol Nutr. 2018;66(6):850–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyers CA, Brown PD. Role and relevance of neurocognitive assessment in clinical trials of patients with CNS tumors. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24(8):1305–9. [DOI] [PubMed] [Google Scholar]
- 27.Mannson J SK, Serenius F, Aden U, Kallen K. Agreement Between Bayley-III Measurements and WISC-IV Measurements in Typically Developing Children. Journal of Psychoeducational Assessment. 2018:1–14. [Google Scholar]
- 28.Anderson V, Spencer-Smith M, Wood A. Do children really recover better? Neurobehavioural plasticity after early brain insult. Brain. 2011;134(Pt 8):2197–221. [DOI] [PubMed] [Google Scholar]
- 29.Caudle SE, Katzenstein JM, Karpen S, McLin V. Developmental assessment of infants with biliary atresia: differences between boys and girls. J Pediatr Gastroenterol Nutr 2012;55(4):384–9. [DOI] [PubMed] [Google Scholar]
- 30.Gold A, Rogers A, Cruchley E, Rankin S, Parmar A, Kamath BM, et al. Assessment of School Readiness in Chronic Cholestatic Liver Disease: A Pilot Study Examining Children with and without Liver Transplantation. Can J Gastroenterol Hepatol 2017;2017:9873945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adeback P, Nemeth A, Fischler B. Cognitive and emotional outcome after pediatric liver transplantation. Pediatr Transplant. 2003;7(5):385–9. [DOI] [PubMed] [Google Scholar]
- 32.Alonso EM, Sorensen LG. Cognitive development following pediatric solid organ transplantation. Curr Opin Organ Transplant. 2009;14(5):522–5. [DOI] [PubMed] [Google Scholar]
- 33.Gilmour S, Adkins R, Liddell GA, Jhangri G, Robertson CM. Assessment of psychoeducational outcomes after pediatric liver transplant. Am J Transplant. 2009;9(2):294–300. [DOI] [PubMed] [Google Scholar]
- 34.Gritti A, Di Sarno AM, Comito M, De Vincenzo A, De Paola P, Vajro P. Psychological impact of liver transplantation on children’s inner worlds. Pediatr Transplant. 2001;5(1):37–43. [DOI] [PubMed] [Google Scholar]
- 35.Kaller T, Langguth N, Petermann F, Ganschow R, Nashan B, Schulz KH. Cognitive performance in pediatric liver transplant recipients. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013;13(11):2956–65. [DOI] [PubMed] [Google Scholar]
- 36.Krull K, Fuchs C, Yurk H, Boone P, Alonso E. Neurocognitive outcome in pediatric liver transplant recipients. Pediatr Transplant. 2003;7(2):111–8. [DOI] [PubMed] [Google Scholar]
- 37.Robertson CM, Dinu IA, Joffe AR, Alton GY, Yap JY, Asthana S, et al. Neurocognitive outcomes at kindergarten entry after liver transplantation at <3 yr of age. Pediatr Transplant. 2013;17(7):621–30. [DOI] [PubMed] [Google Scholar]
- 38.Sorensen LG, Neighbors K, Martz K, Zelko F, Bucuvalas JC, Alonso EM, et al. Cognitive and academic outcomes after pediatric liver transplantation: Functional Outcomes Group (FOG) results. Am J Transplant. 2011;11(2):303–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sorensen LG, Neighbors K, Martz K, Zelko F, Bucuvalas JC, Alonso EM, et al. Longitudinal study of cognitive and academic outcomes after pediatric liver transplantation. J Pediatr 2014;165(1):65–72 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trahan LH, Stuebing KK, Fletcher JM, Hiscock M. The Flynn effect: a meta-analysis. Psychol Bull 2014;140(5):1332–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sundaram SS, Alonso EM, Haber B, Magee JC, Fredericks E, Kamath B, et al. Health related quality of life in patients with biliary atresia surviving with their native liver. J Pediatr 2013;163(4):1052–7 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure, Supplemental Digital Content 1. Spaghetti plot of WPPSI and WISC scores by Bayley Performance. Trajectory plots of participants who demonstrated neurodevelopmental deficits at younger age (≤ 2 years, Bayley Scales of Infant Development, 2nd edition) and higher functioning at older time points. Each dot represents a test score for WPPSI-III or WISC-IV. Blue lines represent participants who had previous FSIQ scores of ≤ 85 on Bayley testing. Black lines represent participants who had previous FSIQ scores of > 85 on Bayley testing.
Table, Supplemental Digital Content 1: Select demographics and medical variables of participants (with testing) vs eligible non-participants (not tested but could have been)
*Wilcoxon for continuous variables, chi-square or Fisher for categorical.
Table, Supplemental Digital Content 2: Comparison of patient scores to the normal population, first test of each WPPSI-III and WISC-IV
*Kolmogorov-Smirnov
Normal (100,15)
Figure, Supplemental Digital Content 2. Study Participant Ascertainment. Flow diagram demonstrating study participation and ascertainment. Left vertical column shows total enrollment and participant eligibility at each time point, as well as percentage of those tested vs. eligible but not tested. Right vertical column shows ineligible participants and reasons for not being tested.