Abstract
Objective:
To understand the association of frequent opioid use with disease phenotype and pain pattern and burden in children and adolescents with acute recurrent (ARP) or chronic pancreatitis (CP).
Methods:
Cross-sectional study of children <19 years with ARP or CP, at enrollment into the INSPPIRE cohort. We categorized patients as opioid “frequent use” (daily/weekly) or “non-frequent use” (monthly or less, or no opioids), based on patient and parent self-report.
Results:
Of 427 children with ARP or CP, 17% reported frequent opioid use. More children with CP (65%) reported frequent opioid use than with ARP (41%, p=0.0002). In multivariate analysis, frequent opioid use was associated with older age at diagnosis (OR 1.67 per 5 years, 95%CI 1.13-2.47, p=0.01), exocrine insufficiency (OR 2.44, 95%CI 1.13-5.24, p=0.02), constant/severe pain (OR 4.14, 95%CI 2.06-8.34, p<0.0001), and higher average pain impact score across all six functional domains (OR 1.62 per 1-point increase, 95%CI 1.28-2.06, p<0.0001). Children with frequent opioid use also reported more missed school days, hospitalizations, and emergency room visits in the past year than children with no frequent use (p<0.0002 for each). Participants in the U.S. West and Midwest accounted for 83% of frequent opioid users but only 56% of the total cohort.
Conclusions:
In children with CP or ARP, frequent opioid use is associated with constant pain, more healthcare use, and higher levels of pain interference with functioning. Longitudinal and prospective research is needed to identify risk factors for frequent opioid use and to evaluate non-opioid interventions for reducing pain and disability in these children.
Keywords: pancreatitis, chronic pain, pediatric, opioids, pain medication
Introduction:
Abdominal pain is the most common symptom in children and adolescents with acute recurrent pancreatitis (ARP) or chronic pancreatitis (CP), and it is often chronic and debilitating (1, 2). ARP is defined as more than one episode of acute pancreatitis, with relief from symptoms between episodes. CP denotes irreversible pancreatic damage, often with fibrosis, duct obstruction and eventual loss of pancreatic function (including exocrine and/or endocrine insufficiency) (3).
Similar to other chronic conditions of childhood, as pain from ARP or CP becomes more frequent and severe, it reduces health-related quality of life (HRQOL) across multiple domains of physical, psychological, and social functioning (4-7). Children with ARP or CP have a high disease burden, including frequent emergency room (ER) visits, missed school days and recurrent hospitalizations (8, 9). Many undergo multiple medical investigations, surgical interventions, and require opioids for pain management—both in the acute and chronic setting. A prior study by our group found that the use of pain medications, including opioids, was associated with higher healthcare costs in children with ARP or CP (8). However, little is known about the prevalence and patterns of opioid use, and the relationship to pain patterns and life impact, in these children.
Patterns of opioid use are important to consider in this population for a number of reasons. Opioid use has been associated with negative physical and psychological health in adults and children, and there is significant public health concern related to the substantial increase in opioid misuse, abuse, and overdose (3). Identifying whether chronic severe pain is associated with opioid use may also suggest avenues for developing more effective pain management options for children with ARP and CP.
The purpose of this current study was to determine patterns of self-reported opioid use in pediatric ARP and CP, the risk factors associated with opioid use, as well as the association of frequent opioid use with pain severity and pain impact using the INSPPIRE (INternational Study group of Pediatric Pancreatitis: In search for a cuRE) cohort. We hypothesized that opioid use would be more common in older children and those who report more constant and severe abdominal pain.
Methods:
Patients with ARP or CP with onset at or before 19 years of age were eligible for enrollment in the INSPPIRE registry. The INSPPIRE consortium and registry, which includes 19 centers in four countries, have been described in detail elsewhere (10). Briefly, INSPPIRE is a multicenter consortium that gathers data on the clinical presentation, risk factors, diagnosis (laboratory, radiologic, etc.), and management of children with ARP or CP. All centers obtained local Institutional Review Board approval or the equivalent for their country prior to enrolling subjects. All centers met the criteria of the Declaration of Helsinki (https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/). Consent was obtained from the parents of participants less than 18 years and directly from participants 18 years or older. Children gave assent at the age specified by the local institutional review board.
This is a cross-sectional study of baseline enrollment data of the cohort. Participants were enrolled September 1, 2012 through August 31, 2017. ARP was defined as at least two episodes of AP with resolution of pain (≥ 1 month between episodes) or normalization of pancreatic enzyme levels and resolution of abdominal pain between AP episodes. CP diagnosis required pancreatic histopathology consistent with CP or imaging findings suggestive of chronic pancreatic damage with at least one of the following: (1) abdominal pain consistent with pancreatic origin, (2) exocrine pancreatic insufficiency, or (3) endocrine pancreatic insufficiency.
The analysis included 427 of the 477 children and adolescents enrolled in INSPPIRE. Subjects missing sufficient patient-reported data on pain medication and opioid use (n=45 with no data on pain medication; n=5 taking pain medication but did not specify type) were excluded from the analysis. This included 11 for whom providers reported daily/weekly opioid use but were missing self-reported use of opioids to maintain internal consistency on the prioritization of patient/parent self-report in this analysis. Opioid use and frequency were classified based on patient-reported current medication lists and dose frequency ranging from once a month to daily. Subjects that reported a specific opioid medication and self-reported use as “daily” or “a few times a week” were classified as “frequent” opioid use, even if the corresponding provider survey reported less frequent use (n=29) or was missing (n=13). Patient/parent surveys that listed a specific opioid pain medication and for whom provider surveys noted daily or weekly use also were classified as “frequent” opioid use. Subjects that self-reported no opioid pain medication use or that listed specific opioid use as a “few times a month,” “once a month,” or “less than once a month” were classified as “non-frequent” opioid use. There is not consensus in the literature on what categorizes “frequent opioid use”, particularly in children with chronic health conditions. We use a definition of frequent opioid use as patient/parent report of using opioids at least weekly. This is consistent with categorization of chronic pain symptoms where symptoms occurring weekly or greater are considered frequent or recurrent.
Data collected from patient and physician questionnaires were standardized and included demographics, family history, phenotypic features of pancreatitis, risk factors, diagnostic evaluations, medications, emergency room (ER) visits, hospitalizations, treatments and therapeutic interventions, and pain variables, as previously described. (10) Patient and physician questionnaires were completed independently to reduce reporting bias. Physician reports were based on their interactions with subjects and review of available medical records at their institution. Results were recorded in the REDCap™ (Research Electronic Data Capture, Vanderbilt University, Nashville, Tennessee) system database to allow secure electronic capture of the data. Regions were determined by United States census region (Northeast, Midwest, South, West) of the INSPPIRE center at which the patient was enrolled into the registry. INSPPIRE sites “Outside the U.S.” are in Canada, Israel, and Australia.
Questions answered by the patient and/or parent included whether the patient had experienced abdominal pain associated with pancreatitis within the past year, severity and pattern of pain, visits to the ER and hospitalizations for pain, pain interference in several life domains, and medications specifically used for pain. Subjects rated pain severity using a 0-10 numeric scale with accompanying Wong-Baker Faces scale (11, 12). Pancreatitis-associated pain severity was categorized as none (0), mild (1-3), moderate (4-7), or severe (8-10). The survey asked separately about severity of constant pain (if any), severity of acute pain episodes, and usual length of pain episodes. Patients rated pain interference in 6 domains on a 5-point Likert scale (1 = “not at all” or “never”, 5=”very much” or “always”) (13). We then calculated a pain impact average score, as the average of all six pain impact questions in those who answered at least 4 items. Patient use of anti-depressant medication and adjunct medications was obtained from patient/parent self-report. We categorized medications into major classes for analysis.
Statistical Analysis:
Descriptive statistics were used to summarize the sample demographics and to report prevalence of frequent opioid use by risk factor. Between-group differences were compared using Pearson chi-square or Fisher’s exact tests for categorical variables, t-tests for age and disease duration variables, and Wilcoxon rank-sum tests for ordinal data and nonparametric continuous variables. Multivariate logistic regression was used to identify factors associated with membership in the frequent opioid use group. Given our limited sample size and distribution of the primary outcome (“frequent opioid use”), in our multivariate analysis we considered only a limited set of the variables that were significant in univariate analysis. For variables that correlated highly with each other, one variable was chosen to best represent that construct. To maximize the sample included in multivariate analysis, missing data in each of the included variables except “region” was imputed using multiple imputations with 10 imputed data sets. Multiple imputations is a statistical method in which missing values of key predictors are imputed, or estimated, based on other variables in the dataset and non-missing values of those key predictors. The missing values are imputed 10 times to create 10 datasets, each with plausible but slightly different values for those missing values. The multivariate model is derived from each of those 10 datasets. The reported multivariate model averages the 10 imputed versions, thereby reducing the variability introduced by the estimated missing data values. Only missing values of independent variables included in the multivariate model are imputed. In this analysis, data were missing for 11% of participants on exocrine insufficiency, 8% on past year ER visits, and <5% on other independent variables.” The Region variable had no missing data. All statistical analyses were performed using SAS/STAT 13.1 (Cary, North Carolina). A p-value < 0.05 was considered statistically significant in univariate analyses; all variables were retained in the multivariate model.
Results:
Cohort description:
This analysis included 427 participants, of which 233 (55%) met criteria for ARP and 194 (45%) for CP. The INSPPIRE cohort is diverse geographically and demographically; it includes children with a broad range of ages and disease durations (TABLE 1).
TABLE 1:
Patient characteristics, pain patterns, and pain severity, by frequency of opioid use*
| On Opioids Daily/Weekly (Frequent, n=74) |
Not on Opioids Daily/Weekly (Non-Frequent, n=353) |
p | |
|---|---|---|---|
| Sex (Female) | 44 (59%) | 198 (56%) | 0.59 |
| Ethnicity (Hispanic) | 17 (23%) | 84 (24%) | 0.83 |
| Race | (n=66) | (n=330) | |
| White | 57 (86%) | 268 (81%) | 0.67 |
| African American | 2 (3%) | 10 (3%) | |
| Asian | 1 (2%) | 19 (6%) | |
| Multi-racial | 5 (8%) | 24 (7%) | |
| Other | 1 (2%) | 9 (3%) | |
| Age at enrollment (Mean±SD) | (n=73) 13.6±3.8 |
(n=346) 11.4±4.5 |
0.0001 |
| Chronic pancreatitis (vs. Acute recurrent pancreatitis) | 48 (65%) | 146 (41%) | 0.0002 |
| Age at 1st diagnosis of acute pancreatitis (Mean±SD) | (n=63) 10.4±4.6 |
(n=303) 8.6±4.6 |
0.004 |
| Duration of disease, years | (n=64) 1.76 (0.65-4.68) |
(n=307) 1.81 (0.81-4.40) |
0.86 |
| Exocrine insufficiency† | 22/63 (35%) | 49/315 (16%) | 0.0003 |
| Endocrine insufficiency‡ | 8/68 (12%) | 22/331 (7%) | 0.14 |
| Region | |||
| West | 30 (41%) | 81 (23%) | <0.0001 |
| Midwest | 31 (42%) | 95 (27%) | |
| Northeast | 4 (5%) | 38 (11%) | |
| South | 5 (7%) | 64 (18%) | |
| Outside US | 4 (5%) | 75 (21%) | |
| Genetic Risk Factors: | |||
| PRSS1 | 21/54 (39%) | 60/242 (25%) | 0.036 |
| SPINK1 | 12/52 (23%) | 45/222 (20%) | 0.65 |
| CFTR | 21/52 (40%) | 71/242 (29%) | 0.12 |
| Pancreatic enzymes (self-report) | 39/73 (53%) | 104/346 (30%) | 0.0001 |
| Vitamins/anti-oxidants (self-report) | 41/73 (56%) | 135/348 (39%) | 0.006 |
| On anti-depressant | 14% | 3% | 0.0002 |
| Other procedures reported as treatments for pancreatitis pain | |||
| Cholecystectomy | 22/72 (31%) | 12/341 (4%) | <0.0001 |
| Celiac nerve block | 5/71 (7%) | 1/346 (0.3%) | 0.0007 |
| Pancreatectomy/TP-IAT | 18/72 (25%) | 13/340 (4%) | <0.0001 |
| Octreotide (provider report) | 2/65 (3%) | 2/343 (1%) | 0.121 |
| Pain medication is helpful for controlling/treating pancreatitis (patient report) | (n=56) 50 (89%) |
(n=84) 66 (79%) |
0.099 |
| Pancreatitis pain pattern and severity (Patient-reported) | |||
| Pattern of abdominal pain from pancreatitis | (n=73) | (n=343) | |
| 0) No abdominal pain | 4 (5%) | 46 (13%) | <0.0001 |
| 1) Episodic mild-moderate pain, but usually pain free | 2 (3%) | 59 (17%) | |
| 2) Constant mild-moderate pain | 3 (4%) | 17 (5%) | |
| 3) Episodic severe pain, but usually pain free | 14 (19%) | 129 (38%) | |
| 4) Constant mild-mod pain and episodic severe pain | 39 (53%) | 75 (22%) | |
| 5) Constant severe pain | 11 (15%) | 17 (5%) | |
| Constant Pain score | (n=67) 2.1 (0-5.7) |
(n=329) 0 (0-0) |
<0.0001 |
| Episodic Pain score | (n=64) 7.1 (3.1-8.9) |
(n=315) 6.0 (4.0-8.2) |
0.129 |
| Episodes per month with moderate/severe pain | (n=58) 4.1 (0.33-24.0) |
(n=292) 0.33 (0.08-2.0) |
<0.0001 |
| Severe pain episode duration (of those with pain) | (n=63) | (n=221) | 0.059 |
| <24 hours | 19 (30%) | 78 (35%) | |
| 1-3 days | 16 (25%) | 72 (33%) | |
| More than 3 days | 19 (30%) | 65 (29%) | |
| Constant pain | 9 (14%) | 6 (3%) | |
| Moderate pain episode duration (of those with pain) | (n=52) | (n=187) | <0.0001 |
| <24 hours | 19 (31%) | 93 (50%) | |
| 1-3 days | 8 (15%) | 56 (30%) | |
| More than 3 days | 14 (27%) | 26 (14%) | |
| Constant pain | 14 (27%) | 12 (6%) | |
| ER visits – average per year, lifelong | (n=34) 1.1 (0.3-3.8) |
(n=199) 1.4 (0.4-2.5) |
0.854 |
| ER visits – past year | (n=67) 2 (1-5) |
(n=325) 2 (0-3) |
0.0002 |
| Hospitalizations – average per year, lifelong | (n=37) 1.5 (0.4-3.8) |
(n=202) 1.1 (0.4-2.1) |
0.382 |
| Hospitalizations – past year | (n=68) 2 (1-4) |
(n=326) 1 (0-2) |
0.0001 |
| Days missed school past month (school-aged children) | (n=57) 10 (3-20) |
(n=278) 0 (0-5) |
<0.0001 |
All continuous variables reported as median, IQR unless otherwise specified. All p-values from Wilcoxon rank-sum test.
Exocrine insufficiency reported by center, defined by fecal elastase, fecal chymotrypsin, stool fat excretion in 24-72 hours, or secretin/CCK stimulation test for duodenal aspirate.
Endocrine insufficiency as reported by center, defined as elevated fasting blood glucose, elevated HbA1c, abnormal glucose tolerance test, or diagnosed diabetes.
Prevalence of opioid use and predictors of opioid use:
Any opioid use was reported by 109 (26%) of the sample including 42 (18%) children with ARP and 67 (35%) children with CP. Frequent opioid use was reported by 74 children (17%), with significantly more children with CP (65%) reporting frequent opioid use compared to 41% with ARP (p=0.0002, TABLE 1).
Of children in the non-frequent use group (n=353), 35 (10%) listed an opioid in their self-reported medication list; 14 of these reported using an opioid “a few times a month,” 15 reported “less than once a month,” and 6 reported “once a month”. There were some inconsistencies between self-report and provider report surveys on opioid use. Of the 74 patients that self-reported frequent opioid use, 47 reported daily use, 16 “a few times per week,” and 11 had provider report of daily or weekly use but did not self-report frequency. Among these 74, 61% also had provider-report of frequent opioid use while 39% had provider-report of infrequent opioid use. Of the 353 patients that self-reported non-frequent opioid use, 94% also had provider-report of non-frequent use while 6% had provider-report of frequent use.
Children with frequent opioid use were more likely to have CP than ARP (p=0.0002), but ARP still accounted for 35% of those with frequent opioid use and CP for 41% of those without frequent opioid use (TABLE 1).
Opioid use:
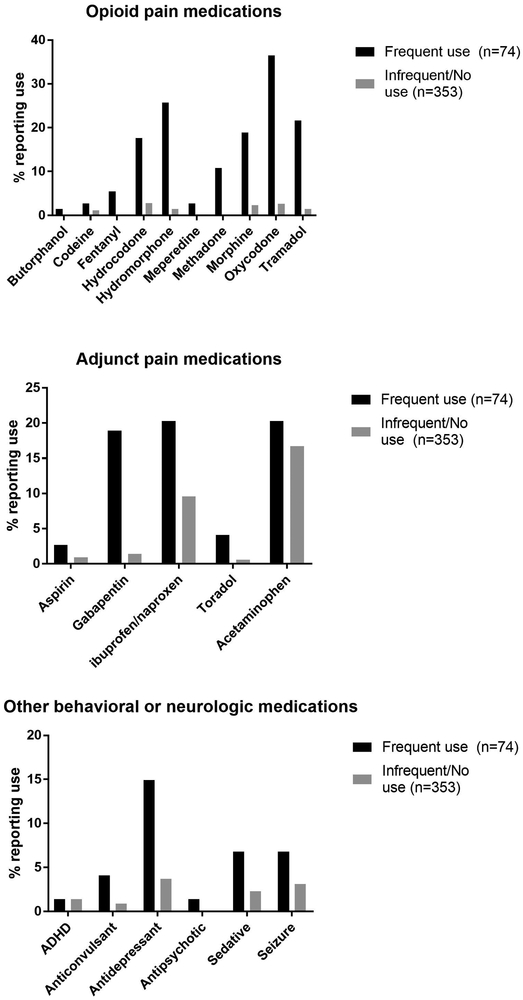
The most common opioid medication reported in the INSPPIRE cohort was oxycodone, followed by hydromorphone, tramadol, morphine, and hydrocodone. Acetaminophen, non-steroidal anti-inflammatories (ibuprofen, naproxen), and gabapentin were commonly reported non-opioid pain medications in both groups (FIGURE 1). Children who used opioids frequently were more likely to be on concomitant anti-depressant medications (14% vs .3%, p=0.0002). The majority of patients in both groups reported that their pain medications—both opioids and non-opioids—were helpful in controlling their pancreatitis symptoms (89%, 79%, p=0.099, TABLE 1).
FIGURE 1:
Percent of INSPPIRE cohort reporting any use of specific opioid, adjunct pain, or behavioral medications, by frequency of opioid use. Frequent opioid use categorized by self-reported daily or weekly use; non-frequent opioid user categorized by self-reported use monthly or less, or no opioid use.
Demographic predictors:
Prevalence of frequent opioid use did not differ by sex, race, or ethnicity. Although duration of pancreatitis symptoms was not different between groups, children reporting frequent opioid use were significantly older at age of initial pancreatitis diagnosis and at date of INSPPIRE enrollment. Frequent opioid use differed in prevalence by region (p<0.0001, TABLE 1); the U.S. West and Midwest accounted for 56% of the study cohort but 83% of these children reporting frequent opioid use (TABLE 1).
Clinical predictors:
Children using frequent opioids were more likely to have associated exocrine pancreatic insufficiency (35% vs. 16%, p=0.0003) but not diabetes (12% vs. 7%, p=0.14). The genetic mutation PRSS1, which encodes for cationic trypsinogen, occurred significantly more frequently in patients using frequent opioids (39% vs. 25%, p=0.036, TABLE 1) No other genetic mutation, pancreatobiliary anatomic abnormality (pancreas divisum, duct obstruction, gallstones, pancreatic duct malunion, sphincter of Oddi disorder), or toxic/metabolic risk factor (hypertriglyceridemia, medications, autoimmune pancreatitis, other autoimmune disease) was associated with frequent opioid use (data not shown).
Pain patterns:
Patients using frequent opioids were significantly more likely to report constant abdominal pain compared to the group using non-frequent opioids (71% vs. 32%) and more likely to report constant severe pain (vs. mild-moderate pain) (p<0.0001, TABLE 1). Children who did not use frequent opioids were more likely to report being usually pain-free but with episodes of severe (38%) or mild-moderate (17%) pain. For those with episodic pain, children who frequently used opioids reported longer duration of moderate severity pain episodes (p<0.0001) but not for severe episodes (p=0.059), TABLE 1).
Disease burden:
Children reporting frequent opioid use reported significantly more healthcare utilization, including more ER visits and hospitalizations in the preceding year (p=0.0002, p=0.0001 for respective comparison). They also reported more days of school missed in the preceding year compared to children infrequently using opioids (p<0.0001). However, the total number of lifetime ER visits and hospitalizations did not differ significantly between the groups (TABLE 1).
Other interventions:
Children who reported frequent opioid use were more likely to be taking pancreatic enzymes (53% vs. 30%, p=0.0001) and vitamins/anti-oxidants (56% vs. 39%, p=0.006). They also were more likely to have undergone pancreatic or biliary surgical procedures to treat pain, compared to children infrequently using opioids, which included total pancreatectomy-islet auto transplantation (TP-IAT), cholecystectomy, and celiac nerve block (p<0.005 for all comparisons, TABLE 1).
Pain impact:
Although pain interfered with daily functioning in both groups, those using frequent opioids were more likely to report pain interfering “quite a bit” or “very much” across all six life domains queried. (FIGURE 2) Children reporting frequent opioid use had significantly higher overall pain impact scores compared to children with non-frequent opioid use (median 3.3, IQR 2.8-3.8 vs. median 2.0, IQR 0.2-3.5, p<0.0001).
FIGURE 2:
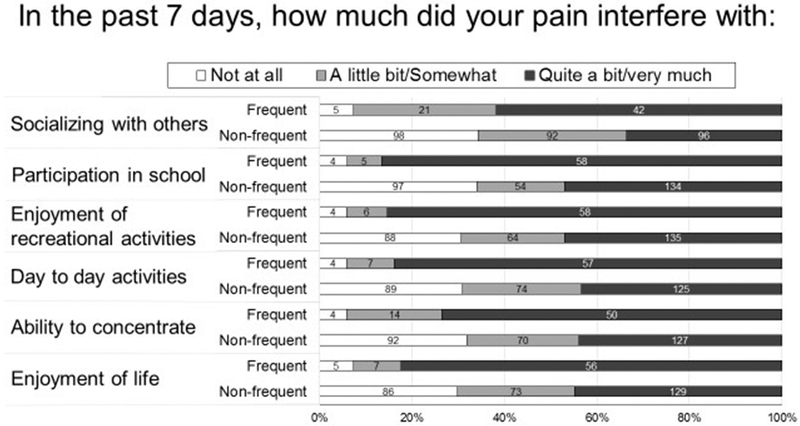
Association of pancreatitis-associated pain impact in children with acute recurrent or chronic pancreatitis, by frequency of opioid use. In all domains, children using opioids frequently (daily or weekly) were more likely to report that pain impacted their activity “quite a bit/very much” (p<0.0001 for all 6 comparisons)
Multivariate analysis of factors associated with frequent opioid use:
Multivariate analysis subsequently was performed using a limited set of variables that were significantly associated with frequent opioid use in univariate comparisons. Included variables are listed in TABLE 2, and excluded variables included missed school days, pancreatic enzyme use, and PRSS1 mutation. In multivariate analysis using multiple imputations to account for missing data, older age at INSPPIRE enrollment, patient report of constant pain +/− severe pain episodes, and exocrine insufficiency were associated with frequent opioid use. The association between frequent opioid use and pain impact was significant with 1.62 increased odds of frequent opioid use for every 1 point increase in the average pain impact score across all 6 domains (TABLE 2). In a sensitivity analysis excluding subjects with missing data (n=99), pain severity, average pain impact score, and exocrine insufficiency were still significantly associated with frequent opioid use. Additionally, anti-depressant use was more strongly associated with frequent opioid use (OR 3.89, 95% CI 1.02-14.86, p=0.05; other data not shown).
TABLE 2:
Factors associated with frequent opioid use in children with acute recurrent or chronic pancreatitis, multivariate analysis*
| Independent Variable | Odds Ratio |
(95% CI) | p-value |
|---|---|---|---|
| Age at enrollment (per 5 years) | 1.67 | (1.13, 2.47) | 0.010 |
| Region | |||
| West | 1.29 | (0.64, 2.60) | 0.470 |
| Northeast/South | 0.47 | (0.19, 1.16) | 0.101 |
| Outside the U.S. | 0.29 | (0.09, 0.95) | 0.041 |
| Midwest | REF | -- | -- |
| Anti-depressant use | 2.42 | (0.82, 7.10) | 0.108 |
| Exocrine insufficiency | 2.44 | (1.13, 5.24) | 0.023 |
| ER visits past year (per 1 additional visit) | 1.08 | (0.98, 1.17) | 0.127 |
| Constant and severe pain† | 4.14 | (2.06 - 8.34) | <0.0001 |
| Pain impact average score, across all 6 domains reported (per +1) ‡ | 1.62 | (1.28, 2.06) | <0.0001 |
Reported analysis includes n=427, using multiple imputations (10 imputed data sets) to account for missing data in each of the variables included in the model except Region, which had no missing data.
Includes children who reported “constant severe pain” or “constant mild/moderate pain with episodes of severe pain,” as detailed in Table 1.
Pain impact average score was the average of all 6 pain impact questions, in those who answered at least 4 items. An average score of zero was assigned to those without pain.
Discussion:
Almost 1 in 5 of the children and adolescents in INSPPIRE, the largest pediatric cohort of acute recurrent and chronic pancreatitis patients, reported using opioids on a daily or weekly basis. For the majority of patients who self-reported daily or weekly opioid use, the concurrent questionnaire from their physician did not correspond with their self-reported use. Children reporting frequent opioid use were slightly older at diagnosis and at cohort entry but did not differ by sex, ethnicity, or race. Frequent opioid use also was more common among children residing in the U.S. West and Midwest, which is consistent with previous findings of regional variance in opioid prescriptions as well as opioid misuse and poisonings. This may also reflect a referral bias, if children using frequent opioids were referred nationally to specialty centers in these U.S. regions for TP-IAT. These associations suggest practice variations as well as disease chronicity and severity as risk factors (13-15).
Direct evidence on opioid use and its consequences in all children and adolescents remains limited. Although the number of opioid prescriptions written for children and adolescents over the past 15 years has not ballooned as it has for adults, the opioid crisis is a significant public health concern affecting youth in the United States (15, 16). One in ten U.S. deaths in 15-24 year olds, and one in five in 25-34 year olds, are opioid-related (17). Hospitalization of children and adolescents for opioid overdoses, often accidental, has increased more than 150% (18). Additionally, receiving prescription opioids before the 12th grade has an association with opioid misuse in adulthood (19). Recent guidelines on appropriate opioid use for painful conditions has exclusively focused on adults; however, little evidence-based guidance is available about the efficacy or appropriate prescribing of opioids in children and adolescents (20).
In our INSPPIRE cohort, children with frequent opioid use were older at INSPPIRE enrollment, reported more severe and frequent pain, a higher prevalence of exocrine insufficiency, and increased ER visits / hospitalizations over the past year. Additionally, pain interference with functioning in all categories was significantly worse in patients using frequent opioids. We have previously reported that children with CP and constant pain have higher rates of school absenteeism, ER visits, and hospitalizations than children with intermittent pain, but this had not previously been examined as it relates to their opioid use (21).
Children using frequent opioids also were prescribed adjunct medications including acetaminophen and gabapentin. They also reported greater use of antidepressant medications, and use of anti-depressant medication was strongly associated with frequent opioid use in our sensitivity analysis. Depression is a known co-morbidity in adult patients with CP undergoing frequent hospital re-admission (22, 23); however, rates of depressive symptoms in pediatric patients with ARP or CP have not yet been described. More detailed data is needed regarding the potential for adjunctive medications to effectively decrease pain, modify other health or mental health symptoms, and balance or reduce opioid use.
Given the high risk of constant pain, interference with life, and frequent opioid use in children with ARP or CP, better screening measures are needed to assess psychosocial health, to measure if impairment is exacerbating or abating, and to identify children in need of additional medical, mental health, or surgical interventions. For example, HRQOL and psychosocial risk instruments have been used for screening purposes in children with other chronic conditions to help determine treatment needs (24, 25).
Our findings also highlight the need for investigation of the etiology and mechanisms of chronic pain in children with ARP and CP. Pain in CP is theorized to involve multiple mechanisms, including peripheral sensitization, pancreatic neuropathy, and neuro-plastic changes in central pain pathways (26). Several studies have reported central sensitization in CP. This likely mirrors widespread sensitization of the central nervous system in a manner seen in other chronic pain disorders such as fibromyalgia and irritable bowel syndrome (27-29). Detailed investigations are needed to understand mechanisms of chronic pain in these children to guide effective interventions. There is little clinical evidence to support long-term opioid use for chronic abdominal pain; thus, there is an urgent need to consider the full medical, psychological, and surgical interventions that may improve pain management (30).
Mutation of the cationic trypsinogen (PRSS1) gene was the only etiologic risk factor associated with frequent opioid use in univariate analyses. The long-term risk of frequent opioid use in children with PRSS1 and other mutations may necessitate early intervention, including therapeutic endoscopic retrograde cholangiopancreatography (ERCP) and drainage-type surgery, to prevent continuation and acceleration of opioid use and dosing (31, 32).
We also found that children who used frequent opioids were more likely to have had a pancreatectomy or TP-IAT in univariate analysis, and indications for TP-IAT include opioid-dependent chronic pain. Continued postoperative opioid use is very common in adults after TP-IAT (33). However, a major goal after TP-IAT is to wean off of opioids, and the continued need for frequent opioids after TP-IAT suggests the importance of ongoing monitoring and treatment of chronic pain (29). It is unknown if our patients were on sustained dosing or were weaning from daily/weekly opioids.
Our findings should be interpreted in light of several limitations. One limitation is our reliance on self-reported opioid use. (34) This may have led to biases in reporting, (e.g., patients or parents may be reluctant to report frequent use of opioid medications or did not remember specific medication names). However, we felt that the survey may also have allowed patients/parents to report frequent opioid use that had not been discussed with their provider, and we have used this survey technique since the inception of the INSPPIRE study. (10) The discordance between patient/parent and provider report of opioid use warrants further consideration and may be clinically important. However, we were limited to understanding current medications and dose frequency only. To comprehensively assess opioid use, future studies could incorporate prescription records or pill counts to verify use frequency longitudinally and ensure appropriate patient classification. Additionally, we could not rule out that abdominal pain was due to other factors although inclusion criteria for patients included patients with known ARP or CP. Factors related to this discordant reporting, and more reliable methods including corroboration with pharmacy records or state opioid prescription registries, should be investigated in future research.
This INSPPIRE report only includes the baseline patient questionnaire; we have not yet addressed longitudinal patterns of opioid use in the cohort. Additionally, our data does not include opioid dosing or details of dose escalation or weaning. INSPPIRE does not include diagnostic screening for mental health comorbidities, including depression. Thus, we cannot verify the diagnosis for which anti-depressant medications were prescribed, and this medication class is not a known drug-induced cause of acute pancreatitis events (35, 36). We do not have data on adverse events associated with opioid use in this population; thus, additional research focused on a more comprehensive evaluation of chronic pain and mental health, and the impact of treatment strategies like cognitive behavioral therapy, will be critical (37). Prospective data collection is underway in INSPPIRE 2 which will provide important longitudinal information on these children as well as assessment of pain, HRQOL, depression and anxiety using validated questionnaires (38).
Conclusion:
Pediatric patients with ARP or CP have a high prevalence of frequent opioid use. Factors including disease severity, chronicity, and pain burden are associated with an increased likelihood of frequent opioid use. The association of PRSS1 mutations with frequent opioid use is intriguing and warrants further study. Additional research is needed to determine risk factors that precede the onset of frequent opioid use and to develop medical, psychological, and surgical therapies that can more effectively treat chronic pain and its interference with the lives of these children.
What is Known:
Chronic pancreatitis and acute recurrent pancreatitis are increasingly recognized in children and adolescents, often driven by genetic risk factors.
Pain is a major symptom of chronic and acute recurrent pancreatitis, and is a substantial burden on young people with this incurable chronic illness.
Opioids are often used for acute pain management in these and other conditions, but opioid misuse/abuse has become epidemic in the U.S., raising concerns about frequent use.
What is New:
In the INSPPIRE cohort, almost 1 in 5 children with chronic or acute recurrent pancreatitis reported using opioids daily or weekly to manage their pancreatitis pain.
Physician reports of opioid use commonly under-estimated patient-reported frequency of opioid use.
Frequent opioid use was associated with increasing age at diagnosis, exocrine insufficiency, constant or severe pain, and functional impairment.
Acknowledgments
Conflicts of Interest and Sources of Funding: The authors have no relevant conflicts of interest. Research reported in this publication was supported by National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award numbers R21 DK096327, U01 DK108334. INSPPIRE registry was developed by CTSA (2UL1 TR000442) and REDCap. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- ARP
Acute recurrent pancreatitis
- CP
Chronic pancreatitis
- ER
Emergency room
- HRQOL
health-related quality of life
- INSPPIRE
INternational Study group of Pediatric Pancreatitis: In search for a cuRE
- TP-IAT
total pancreatectomy-islet auto transplantation
Footnotes
Author disclosures: Dr. Mark Lowe is on the Board of Directors of the National Pancreas Foundation; receives royalties from Millipore Inc and UpToDate. Dr. Tanja Gonska received a research grant from Vertex Pharmaceuticals and she is a consultant for Cystic Fibrosis Foundation. Dr. Husain has equity in Prevcon, LLC. Dr. John Pohl is on the speaker’s bureau for Medical Education Resources, Inc. Dr. Melena Bellin is a consultant for ARIEL Precision Medicine and receives research support from ViaCyte and Dexcom. Dr. Chee Y. Ooi is a consultant for Vertex Pharmaceuticals. Dr. Aliye Uc is a member of American Board of Pediatrics, Subboard of Pediatric Gastroenterology and a consultant for Cystic Fibrosis Foundation. The other authors declare no conflicts of interest.
Prior presentations: This work was presented in abstract form at the National Association of Pediatric Gastroenterology, Hepatology, and Nutrition Annual Meeting 2018 (poster) and at the American Pancreatic Association Annual Meeting 2018 (oral abstract).
References:
- 1.Frossard J, Steer M, Pastor C. Acute Pancreatitis. Lancet 2008;371:143–52. [DOI] [PubMed] [Google Scholar]
- 2.Kleef J, Whitcomb D, Shimosegawa T, et al. Chronic pancreatitis. Nat Rev Dis Primers 2017;3:17060. [DOI] [PubMed] [Google Scholar]
- 3.Morinville V, Husain S, Bai H, et al. Definitions of pediatric pancreatitis and survey of present clinical practices. J Pediatr Gastroenterol Nutr 2012;55:261–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pendharkar S, Salt K, Plank L, et al. Quality of life after acute pancreatitis: a systemic review and meta-analysis. Pancreas 2014;43:1194–200. [DOI] [PubMed] [Google Scholar]
- 5.Pezzilli R, Bini L, Fantini L, et al. Quality of life in chronic pancreatitis. World J Gastroenterol 2006;12:6249–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keller C, Wilcox C, Gudleski G, et al. Beyond abdominal pain: pain beliefs, pain affect, and distress as determinants of quality of life in patients with chronic pancreatitis. J Clin Gastroenterol 2018;52:563–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gachago C, Draganov P. Pain Management in chronic pancreatitis. World J Gastroenterol 2008;14:3137–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ting J, Wilson L, Schwarzenberg S, et al. Direct costs of acute recurrent and chronic pancreatitis in children in the INSPPIRE study. J Pediatr Gastroenterol Nutr 2016;62:443–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar S, Ooi C, Werlin S, et al. Risk factors associated with pediatric acute recurrent and chronic pancreatitis: lessons from INSPPIRE. JAMA Pediatr 2016;170:562–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morinville V, Lowe M, Ahuja M, et al. Design and implementation of INSPPIRE. J Pediatr Gastroenterol Nutr 2014;59:360–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong D,Baker C. Pain in children: comparison of assessment scales. Pediatr Nurs 1988;14:917. [PubMed] [Google Scholar]
- 12.Keck J, Gerkensmeyer J, Joyce B, et al. Reliability and validity of the FACES and Word Descriptor Scales to measure procedural pain. J Pediatr Nurs 1996;11:368–74. [DOI] [PubMed] [Google Scholar]
- 13.Groenewald C, Law E, Fisher E, et al. Associations between chronic pain and prescription opioid misuse in adulthood. J Pain 2019;20:28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fortuna R, Robbins B, Caiola E, et al. Prescribing of controlled medications to adolescents and young adults in the United States. Pediatrics 2010;126:1108–16. [DOI] [PubMed] [Google Scholar]
- 15.Groenewald C, Rabbitts J, Gebert T, et al. Trends in opioid prescriptions among children and adolescents in the U.S.: a nationally representative study 1996–2012. Pain. 2016;157:1021–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shipton E, Shipton E, Shipton A. A review of the opioid epidemic: what do we do about it? Pain Ther 2018;7:23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gomes T, Tadrous M, Mamdani M, et al. The burden of opioid-related mortality in the United States. JAMA Netw Open 2018;1:e180217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaither J, Leventhal J, Ryan S, et al. National trends in hospitalizations for opioid poisonings among children and adolescents, 1997 to 2012. JAMA Pediatr 2016;170:1195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miech R, Johnston L, O’Malley P, et al. Prescription opioids in adolescence and future opioid misuse. Pediatrics 2015;136:e1169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manchikanti L, Kaye A, Knezevic N, et al. Responsible, safe, and effective prescriptions of opioids for chronic non-cancer pain: American Society of Interventional Pain Physicians (ASIPP) guidelines. Pain Physician 2017;20:S3–92. [PubMed] [Google Scholar]
- 21.Schwarzenberg S, Bellin M, Husain S, et al. Pediatric chronic pancreatitis is associated with genetic risk factors and substantial disease burden. J Pediatr 2015;166:890–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cote G, Yadav D, Abberbock J, et al. Recurrent acute pancreatitis significantly reduces qualify of life in the absence of overt chronic pancreatitis. Am J Gastroenterol. 2018;113:906–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keller C, Wilcox C, Gudleski G, et al. Beyond abdominal pain: pain beliefs, pain affect, and distress as determinants of quality of life in patients with chronic pancreatitis. J Clin Gastroenterol 2019;52:563–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hill R, Lewindon P, Muir R, et al. Quality of life in children with Crohn disease. J Pediatr Gastroenterol Nutr 2010;51:35–40. [DOI] [PubMed] [Google Scholar]
- 25.Arvantis M, DeWalt D, Martin C, et al. Patient-reported outcomes measurement information system in children with Crohn’s disease. J Pediatr 2016;174:153–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drewes A, Bousense S, Campbell C, et al. Guidelines for the understanding and management of pain in chronic pancreatitis. Pancreatology 2017;17:720–31. [DOI] [PubMed] [Google Scholar]
- 27.Anaparthy R and Pasricha P. Pain and chronic pancreatitis: is it the plumbing or the wiring? Curr Gastroenterol Rep 2008;10:101–6. [DOI] [PubMed] [Google Scholar]
- 28.Poulsen J, Olesen S, Malver L, et al. Pain and chronic pancreatitis: a complex interplay of multiple mechanisms. World J Gastroenterol 2013;19:7282–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chinnakotla S, Radosevich D, Dunn T, et al. Long-term outcomes of total pancreatectomy and islet auto transplantation for hereditary/genetic pancreatitis. J Am Coll Surg 2014;218:530–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang D Opioid medications in the management of chronic abdominal pain. Curr Pain Headache Rep 2017;21:40. [DOI] [PubMed] [Google Scholar]
- 31.Sun X, Hu L, Xia T, et al. Clinical features and endoscopic treatment of Chinese patients with hereditary pancreatitis. Pancreas 2015;44:59–63. [DOI] [PubMed] [Google Scholar]
- 32.Bellin M, Forlenza G, Majumder K, et al. Total pancreatectomy with islet autotransplantation resolved pain in young children with severe chronic pancreatitis. J Pediatr Gastroenterol Nutr 2017;64:440–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moran R, Klapheke R, John G, et al. Prevalence and predictors of pain and opioid analgesic use following total pancreatectomy with islet autotransplantation for pancreatitis. Pancreatology 2017;17:732–7. [DOI] [PubMed] [Google Scholar]
- 34.Rosenman R, Tennekon V, Hill LG. Measuring bias in self-reported data. Int J Behav Healthc Res 2011;2:320–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Teitelbaum J, Arora R. Long-term efficacy of low-dose tricyclic antidepressants for children with functional gastrointestinal disorders. J Pediatr Gastroenterol Nutr 2011;53:260–4. [DOI] [PubMed] [Google Scholar]
- 36.Norgaard M, Jacobsen J, Gasse C, et al. Selective serotonin reuptake inhibitors and risk of acute pancreatitis: a population-based case-control study. J Clin Psychopharmacol 2007;27:259–62. [DOI] [PubMed] [Google Scholar]
- 37.Chung C, Callahan S, Cooper W, et al. Outpatient opioid prescriptions for children and opioid-related adverse events. Pediatrics 2018;142. pii: e20172156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uc A, Perito ER, Pohl JF, et al. INternational Study Group of Pediatric Pancreatitis: In Search for a CuRE Cohort Study: Design and Rationale for INSPPIRE 2 From the Consortium for the Study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer. Pancreas 2018;47:1222–8. [DOI] [PMC free article] [PubMed] [Google Scholar]