Abstract
Despite graft-versus-host disease (GVHD) prophylactic agents, the success and wider utilization of allogeneic hematopoietic stem cell transplantation (allo-HSCT) is limited by GVHD. Although increasing donor graft regulatory (Treg): effector (Teff) T-cell ratios can substantially reduce GVHD in cancer patients, pre-HSCT conditioning regimens and GVHD create a challenging inflammatory environment for Treg stability, persistence and function. Metabolism plays a critical role in T-cell and Treg differentiation, and development of effector function. While glycolysis is a main driver of allogeneic T-cell-driven GVHD, oxidative phosphorylation is one main driver of Treg suppressor function. This review focuses on recent advances in our understanding of Treg metabolism in the context of GVHD, and discusses potential therapeutic applications of Tregs for the prevention or treatment of GVHD in cancer patients.
Keywords: Treg, Foxp3, GVHD, Microbiota, glycolysis, OXPHOS
Metabolic regulation of human donor T-cell mediated graft-versus-host disease
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a curative therapy for many cancer types [1]. Genetic disparities between donor and host can result in immune-mediated attack of host tissues, known as graft-versus-host disease (GVHD), a major cause of morbidity and mortality following HSCT [2]. Prophylactic GVHD regimens typically utilize non-specific immunosuppression, leaving cancer patients susceptible to relapse, infections, and potential secondary malignancies. Corticosteroids, the first-line of GVHD therapy, is effective in only ~50% of recipients, the remainder of which have refractory GVHD and a dismal prognosis [3]. Regulatory CD4+ T-cells (Tregs) are critical for immune system homeostasis, limiting activation and effector differentiation of T-cells that are self-reactive or stimulated by foreign antigenic exposure [4]. Foxp3+ Tregs can develop in the thymus (tTreg) in response to self-antigen or induced in the periphery (pTreg), in response to self-antigen or pathogen-specific signals [4]. Enhancing Treg:Teffector ratios, through therapeutic intervention -- such as via administration of ultra-low dose IL-2 (1 to 2 ×105 IU/m2/dose subcutaneously three times per week for 12 weeks) [5] or via adoptive transfer of tTregs or pTregs -- can ameliorate autoimmunity, organ graft rejection and GVHD in preclinical animal models [6–10]. These types of interventions were evaluated in completed single-arm, open label, phase 1 clinical trials for GVHD (; n=41; primary outcome, maximum tolerated dose of CD4+CD25+ Treg)I; Type 1 diabetes mellitus (; n=16; primary outcomes: adverse events and severe or life threatening laboratory abnormalities; CD4+CD127lo/−CD25+ polyclonal Tregs)II; and kidney transplant rejection (; end-stage renal disease; n= 10; primary outcome: safety; expanded Treg)III [11–13].
Metabolism plays a unique and intricate role in T-cell responses. Glycolysis and mitochondrial oxidative phosphorylation (OXPHOS, see Glossary) are the primary mechanisms used to generate ATP (Box 1). Naïve CD4+ and CD8+ T-cells use glycolysis for ATP generation [14]. Early studies indicated that CD28-dependent up-regulation of glycolysis was critical for in vitro human T-cell activation, independent of IL-2 [15]. While naive CD8+ T-cells differentiating into T effectors (Teffs) maintain a glycolytic phenotype, memory CD8+ (and CD4+) T-cells convert to OXPHOS, primarily generated through cellular fatty acid (FA) catabolism [14]. Following activation, naïve CD4+ T-cells differentiate into Teffs such as Th1, Th2 and Th17, regulated through metabolic reprogramming [14]. While the predominant metabolic program for murine and human Th1, Th2 and Th17 effector differentiation remains aerobic glycolysis, other key T-cell subset-specific metabolic regulators are required for ATP generation [16]. The importance of glycolysis for murine CD4+ Teff differentiation has been demonstrated by conditions that inhibit glycolysis (e.g. TGFß, rapamycin), and skew differentiation towards pTreg, which more readily use OXPHOS for energy than other effector subsets [17, 18].
Box 1. T cells, including Treg, engage multiple fuel sources to meet their needs during distinct cellular processes.
Glycolysis, the process by which glucose is catabolized to pyruvate, is inferior to OXPHOS in generating ATP (2 vs 36 molecules per glucose molecule) [100]. Despite being less efficient, glycolysis is frequently used by rapidly dividing cells, even in the presence of oxygen (a process known as the Warburg effect [100]), generating intermediates for other pathways without producing reactive oxygen species (ROS), a genotoxic OXPHOS byproduct. In addition to glycolysis-driven pyruvate, T-cells import extracellular amino acids. For example, glutamine transporters (ASCT1, ASCT2 or LAT1/CD98) provide intracellular glutamine that is converted to alpha (α-KG) that enters the tricarboxylic acid (TCA) cycle to fuel OXPHOS. In addition to providing an energy source, glutaminolysis supports amino acid production and provides reducing equivalents, NADH and NADPH, required for ATP generation and lowering ROS concentrations, respectively. T-cells can engage in OXPHOS through fatty acid oxidation (FAO), a process whereby fatty acids (FAs) enter the mitochondria, via carnitine palmitoyl transferase 1a (CPT1a), and 2-carbon units are cleaved to form acetyl-CoA that enters the TCA cycle. The high energy generation capacity of FAO is illustrated by the complete oxidation of the long-chain FA, palmitate, that produces 129 ATP molecules.
Pre-HSCT chemo-radiotherapy conditioning regimens and GVHD-mediated tissue injury cooperate to create a highly inflammatory environment that, along with lymphopenia, have a profound effect on the metabolites and metabolic pathways engaged in supporting donor T-cell proliferation, differentiation, and survival and Treg stability, persistence and function. This review highlights recent advances in our understanding of Treg metabolism in the context of GVHD, and discusses how this knowledge will direct potential therapeutic applications of Tregs for the prevention or treatment of GVHD in patients.
tTreg and FAO
Naive human and murine tTreg are highly glycolytic with only a minor percentage of OXPHOS generated through FAO [19, 20]. Activated tTreg further increase glycolysis, but also greatly increase OXPHOS relative to naïve cells [20]. Several past studies attributed increased OXPHOS in tTregs and in vitro inducible Tregs (iTregs) to FAO, by employing the CPT1a inhibitor etomoxir to inhibit FA mitochondrial entry (Box 1). However, recent studies have shown that the concentration of etoxomir used (>40 μM) also inhibits adenine nucleotide translocase, leading to inhibition of the electron transport chain and ROS production, and resulting in impaired Treg function relative to controls [21–23]. Furthermore, CPT1a deletion has not been found to affect Treg differentiation or homeostasis [24], pointing to ATP generation pathways other than FAs and FAO -- such as TCA cycle and aa oxidation – as potential contributors to OXPHOS. The importance of mitochondrial respiration in Treg function has been reinforced by the recent finding that Treg cell-specific ablation of mitochondrial respiratory electron transport chain complex III in mice (Uqcrqflox;Foxp3GFP-creERT2;ROSA26SorCAG-tdTomato deficient in complex III coenzyme Q: cytochrome c – oxidoreductase), markedly reduces Treg mediated suppression capacity in vivo, relative to wild type (WT) mice [25]. However, additional studies are required to better support the exact role of FAO in Treg metabolism.
Role of mTOR and glycolysis in Tregs and Teffs during GVHD
The mechanistic/mammalian target of rapamycin, mTOR, is a central metabolic regulator of immunity that functions, in part, by integrating signals from the TCR with environmental signals, such as growth factors, stress and certain metabolic intermediates, and by sensing amino acids, cholesterol and other metabolites signals to promote cell growth and function (Figure 1) [26]. mTOR is the catalytic subunit of two distinct multi-subunit kinases, mTORC1 (with the scaffold protein Raptor) and mTORC2 (with Rictor) [27]. Activated CD4+ and CD8+ T-cells and Tregs increase their glycolytic pathways by metabolic reprogramming controlled by mTOR; relative to controls, mTOR inhibition through rapamycin or by genetic knockout of mTOR (Frap1 knockout mice) prevents increased glycolysis following TCR stimulation and induces Foxp3 expression and suppressor function in both murine and human Tregs [28, 29]. tTreg cells are highly reliant on mTOR-driven glycolysis, lipogenesis and glutaminolysis for proliferation and function [17, 30, 31]. Relative to conventional T cells and tTreg, mTOR inhibition with rapamycin can support murine and human iTreg and pTreg generation, stabilize FoxP3 expression, increase suppressor function, and skew expansion cultures toward Tregs at the expense of Teff in a process concordant with de novo Foxp3 expression [32–34]. Consistent with their higher glycolytic activity compared to naïve murine T-cells, naïve tTregs have elevated steady-state mTORC1 activity [30]. Effector T cell differentiation under conditions that promote OXPHOS (pyruvate dehydrogenase kinase (PDHK) knockdown) leads to increased iTreg differentiation; and under conditions that restrict OXPHOS (etomoxir treatment) decreases iTreg differentiation relative to controls. In contrast, murine and human iTregs are more dependent on OXPHOS than tTRegs [35, 36]. Dichloroacetate, an inhibitor of pyruvate dehydrogenase kinase-1 and hence aerobic glycolysis, increases alloreactive T-cell Foxp3 expression [37], decreases Th17 differentiation, enhances iTreg generation and protects mice from experimental autoimmune encephalomyelitis (EAE) relative to controls [36]; this suggests that dichloroacetate may inhibit GVHD.
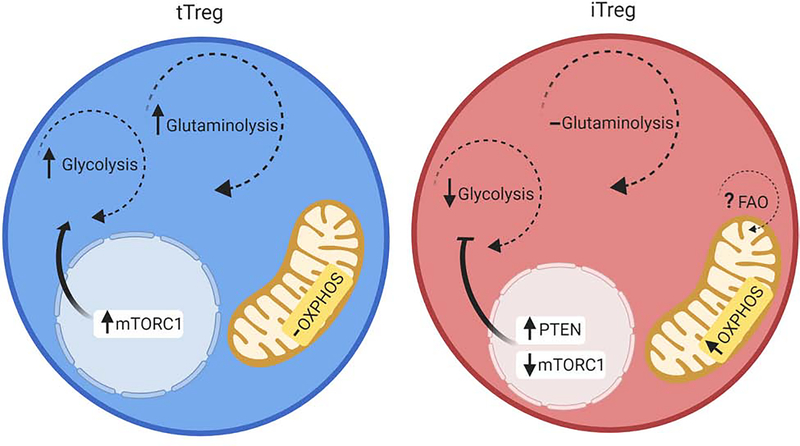
Figure 1: Dominant metabolic programs in tTreg vs iTreg.
Murine and human tTreg have been shown to be reliant on glycolysis and glutaminolysis for their energy needs. These processes are largely driven by increased mTORC1 activity. iTreg in contrast, have been shown to be highly reliant on OXPHOS, and less so on glycolysis. This is largely driven by increased PTEN activity, as well as decreased mTORC1 activity [17]. In addition, it has been suggested that FAO may be the primary driver of the elevated OXPHOS found in iTreg [35].
In preclinical murine studies, tTregs were seen to be more susceptible to calcineurin inhibitors such as cyclosporine A, than Teff [38]. In addition, in an acute GVHD model (FVB/N into BALB/c recipients), mice given tTregs and cyclosporine A exhibited significantly worse survival than those receiving concurrent rapamycin or mycophenolate [39]. In a completed phase 3 randomized multicenter clinical trial examining overall survival of 139 randomized lymphoma patients receiving HSCT with reduced intensity conditioning, rapamycin treatment reduced the risk of acute GVHD, especially in unrelated donor graft recipients (; non-Hodgkin and Hodgkin lymphoma)IV [40]. As a result of increased Treg Foxp3 expression and suppressor function, rapamycin is typically favored over calcineurin inhibitors for clinical trials designed to assess the efficacy of adoptively transferred Tregs added to standard-of-care GVHD prophylaxis. In a completed phase 1 trial assessing the safety and dose escalation of umbilical cord blood-derived Treg administration after HSCT in patients with advanced hematologic malignancies, rapamycin and mycophenolate given with high Treg doses virtually eliminated acute GVHD ()I [12, 41]. In other studies, human CD4+ iTregs generated in vitro with CD3/CD28 stimulation in the presence of rapamycin, TGFß, and IL2 supplementation led to high Foxp3 expression in iTregs, as well as ameliorated disease outcome in a xenogeneic humanized mouse model of GVHD, relative to controls [28]. Moreover, CD4+ iTregs are currently being evaluated in a completed phase 1 dose escalation clinical trial of leukemia, multiple myeloma, or myelodyplastic syndrome patients receiving matched sibling donor peripheral blood stem cell transplants under the cover of mycophenolate and cyclosporine A or rapamycin (; single group, open label, non-randomized; primary outcome: grade 3–5 infusional toxicity)V. Since rapamycin is anti-proliferative and associated with bone marrow transplantation complications (e.g. thrombocytopenia, transplant-associated thrombotic microangiopathy, venoocclusive disease) [42], more clinical studies are needed to understand how the administration of rapamycin with Treg adoptive transfer can be improved in the setting of HSCT.
Treg homeostasis is dependent on the balance of signals delivered by PTEN (phosphatase and tensin homolog) and the IL-2 signaling downstream pathway, lipid kinase PI(3)K (phosphatidylinositol-3-OH kinase), serine-threonine kinase Akt, and the metabolic checkpoint kinase complex mTORC1 [17, 43]. Via metabolic reprogramming, Toll-like receptor signaling, can impair the suppressive function of both iTreg and tTreg but via opposing mechanisms. Specifically, TLR1 and TLR2 engagement on murine iTreg was found to promote mTORC1 signaling and increased glycolysis, whereas TLR8 signaling in human tTreg inhibited glucose uptake and glycolysis, highlighting the notion that different Treg populations can have distinct metabolic needs despite similar Foxp3 expression [44, 45]. Conversely, mTORC1 activity can promote cholesterol and lipid synthesis required for proliferation and suppressive function by supporting endogenous FA stores, especially via cholesterol biosynthetic pathway gene expression [30]. Indeed, Tregs from mice deficient in the mTORC1 complex component Raptor (Foxp3creRptorfl/fl), have cholesterol and lipid metabolic defects that compromise Treg proliferation and maintenance, although the relative contributions of FA uptake and FA synthesis on providing ATP to Tregs via FAO requires further study [30].
The Forkhead transcription factor family member, Foxo1, supports Foxp3 expression [30]. Constitutive activation of Foxo1 in CD4+ T cells in mice (through transgenic expression of a Foxo1 mutant lacking serine residues critical for nuclear export, CD4creFoxo1AAA/+) limits cellular metabolism and provides these cells a Treg cell-intrinsic proliferative advantage, despite their inability to accumulate biomass [46]. However, under growth factor and cell competitive settings, survival and cell division of Treg from these animals are compromised, due to diminished Stat5 activation and heightened IL-2 dependency relative to WT mice [46]. Mechanistically, constitutively active Foxo1 has been reported to diminish mTORC1 signaling and free cholesterol accumulation, the latter deemed to be in a c-Myc independent fashion[46]. Thus, proper Foxo1 regulation is required for Treg competitive fitness (ATP production, OXPHOS, glycolysis, mitochondrial mass, mitochondrial membrane potential and mitochondrial DNA synthesis) and to couple Treg proliferation to cell growth, by controlling both Treg c-Myc expression and metabolism, as well as cholesterol accumulation [46]. PTEN, a primary negative regulator of PI(3)K-Akt signaling and glycolysis, can promote Treg stability by modulating the balance between glycolysis and mitochondrial fitness. Indeed, PTEN deficiency causes a loss of Treg functional stability that is dependent on increased mTORC2 activity[19]. Therefore, Treg depend on a number of transcription factors to maintain their metabolic fitness.
From another angle, Leptin, an adipose tissue-derived hormone, can also regulate energy metabolism through mTORC1 activation in murine and human tTregs which express the Leptin receptor (LepR) [47]. Specifically, Leptin-LepR signaling, which activates mTORC1, suppresses in vitro human Treg proliferation [47]. Consistent with these findings, Tregs are present in higher numbers in lean versus obese adipose tissues [48]. In vitro leptin neutralization has been found to decrease tTreg mTORC1 activity and glycolysis, and increase Treg proliferation relative to controls[49]. Moreover, in vivo antibody-mediated leptin neutralization has also been demonstrated to increase Treg frequency and decrease inflammation in mouse models relative to controls[49]. Of note, patients with hematological malignancies receiving allo-HSCT have shown higher serum leptin concentrations than controls or patients receiving autologous HSCT, and this may be relevant as it suggests that leptin-neutralization might be effective as a candidate GVHD therapy, although robust testing is clearly warranted [50].
Although it is well accepted that mTOR inhibition leads to iTreg or pTreg generation and acquisition of suppressor function, other studies in mice showed that Raptor deletion in Treg (Foxp3creRptorfl/fl) led to a fatal early onset inflammatory disorder, indicative of mTORC1 and a loss of Treg function [30]. These seemingly discordant findings are characteristic of effector and central memory Treg subsets, respectively, which have distinct metabolic profiles based on mTORC1 sensitivity [18]. The effector subset (eTreg), characterized as CD62LloCD44hi, requires mTOR function and maintains increased mTORC1 activity and glycolytic metabolism; conversely, mTOR inhibition by pharmacological agents or gene deletion (CD4creRptorfl/fl) drives naïve tTregs into eTregs that have decreased ICOS, CTLA-4, CD39 and PD-1 expression and reduced Treg suppressor function relative to eTreg with intact mTOR signaling [18, 51]. Consistent with these data, TGFß-induced iTreg have robust mTORC1 activation and maintain an eTreg phenotype [18]. In contrast, mTOR inhibition or mTORC1 deletion in tTregs promotes long-lived central Tregs (cTregs; CD62LhiCD44lo) with a memory-like phenotype and enhanced spare respiratory capacity, similar to memory CD8+ T-cells [18, 51]. Tregs induced with TGFß in the presence of rapamycin display a cTreg phenotype with decreased basal glycolytic rate and increased oxygen consumption rate relative to iTreg induced in the absence of rapamycin that display more of an eTreg phenotype [18]. In further support of an important role for mTOR driven glycolysis in Tregs, recent studies indicate that PI(3)K/ mTOR-C2 activation induces glucokinase (GCK), a key enzyme involved in the first step of glycolysis (i.e. phosphorylation of glucose to glucose-6-phosphate) [52]. PI(3)K/mTORC2 activation also downregulates the GCK regulatory subunit (GCKR) [52]. GCKR inhibits the activity of bound GCK and transports GCK from the cytoplasm (site of glycogen production for glucose storage) to the nucleus, when glucose concentrations are lowered [53]. Relevant to Tregs, GCK activation appears to be required for Treg migration into sites of inflammation, as GCK-dependent ATP is postulated to be consumed by ATPase activities critical for actin polymerization and myosin motors that facilitate Treg squeezing through small confinements and mechanical barriers [54]. Lastly, Tregs isolated from humans with a loss-of-function mutation in GCKR have demonstrated increased motility relative to WT Treg, though with intact suppressor function [52]. In addition to experiments assessing glycolysis, more work is needed to better understand the contributions of other metabolic pathways to T and Treg cell motility in the context of inflammation.
During murine GVHD, donor Teff rapidly proliferate, undergo metabolic stress, and rely upon multiple ATP sources including glutaminolysis, glycolysis, oxidative phosphorylation and FA metabolism for energy and cell survival [55–58]. Although there is some controversy as to which substrate(s) and pathway(s) are predominant and essential to meet the high energy demands of GVHD Teff, there is less debate regarding the metabolic need in Teff, for multiple substrates and pathways including those involved in aerobic glycolysis and OXPHOS [56]. During established GVHD, Teff acylcarnitine accumulation --indicative of FAO and mitochondrial ATPase inhibition-- has been reported, as well as that of increased superoxide production, relative to controls; this renders Teff vulnerable to therapeutic agents that can further increase oxidative stress[56]. This was confirmed in two murine GVHD models that displayed decreased disease burden following treatment with the superoxide inducing agent Bz-423 [56]. Furthermore, Teff in GVHD mouse models display increased FA uptake, elevated FAO enzyme concentrations and FAO rates relative to healthy mice. Indeed, a single intraperitoneal injection of etomoxir is sufficient to reduce GVHD in these mice [55].
Extracellular glutamine, a conditionally essential amino acid, can also be used as a fuel source by activated Tregs. Indeed, glutamine can be imported, incorporated directly into the TCA cycle, and catabolized through OXPHOS in a process known as glutaminolysis. Under in vitro conditions of glutamine deprivation, or in the presence of a glutaminase inhibitor (L-DON), Foxp3 expression is induced and Th1 differentiation is decreased relative to normal Th1 differentiation conditions [59]. Moreover, genetic ablation of one of the subunits required for glutamine transport (Slc1a5 knockout mice) resulted in inhibition of mTOR activation and Th1/Th17 differentiation, but did not affect Treg induction relative to WT mice [60]. Paradoxically, oral glutamine supplementation increased circulating Treg numbers and ameliorated murine GVHD compared with mice not receiving oral glutamine supplementation [61]. Moreover, oral glutamine supplementation has been reported to improve the clinical outcomes of patients with hematological malignancies undergoing HSCT, suggesting that its immunosuppressive effect might be potentially linked to increased Treg numbers, presumably in circulation [62]. Although an intriguing possibility, the exact role of glutamine in regulating Treg frequency and function, as well as T eff proliferation and differentiation in GVHD remains to be fully determined.
In further support of the needs for multiple energy requirements for GVHD Teffs, PD-1 activation in murine CD4+ T cells has been shown to lead to the activation of PTEN, the major inhibitor of the PI(3)K-Akt-mTOR pathway, and to metabolic reprogramming away from glycolysis to FAO by increasing CPT1A expression [43, 63]. Since PD-1/PD-L1 blockade has also been found to markedly accelerate lethality in multiple murine acute GVHD models [57, 58, 64],these studies suggest that alloreactive T-cells can use aerobic glycolysis and OXPHOS for ATP synthesis, dependent upon the magnitude of the T-cell response and the precise environment in which T-cells reside [57]. Alloreactive murine CD4+ and CD8+ T-cells can also express PD-L1, and donor T-cells lacking PD-L1 have been found to exhibit decreased aerobic glycolysis, OXPHOS, and FA metabolism, thus resulting in less severe GVHD compared with controls [58]. Others have observed that alloreactive CD4+ and CD8+ T-cells can switch from FAO and pyruvate oxidation via the TCA cycle, to aerobic glycolysis with increased glutaminolysis and pentose phosphate pathway dependence [65]; and furthermore, that inhibition of glycolysis in vivo via 2-deoxy-glucose, a small-molecule inhibitor of glucose hexokinase, or by targeting mTORC1 activation with rapamycin has been found to ameliorate GVHD morbidity and mortality following human HSCT[65]. Reasons for these discordant findings are unknown. Thus, strategies that target Teff metabolic pathways to reduce GVHD will need to take into account the particular GVHD setting, as well as the role Tregs might play, as these may have energy needs that overlap with Teff, thus potentially offsetting directed metabolism-based putative therapies for GVHD.
AMPK and OXPHOS in Treg metabolism and GVHD
The delicate balance between glycolysis and OXPHOS in Tregs is controlled by mTOR and AMP-activated protein kinase (AMPK) signaling, respectively, linking immunological and environmental inputs to Treg metabolism and function [16]. AMPK is a sensor of a low energy state activated by a high AMP/ATP ratio [29]. AMPK activation, by increasing cellular NAD+ concentrations[66], upregulates the activity of SIRT1, an NAD-dependent histone deacetylase that inhibits Foxo1 and deacetylates Foxp3 protein [67]. Pharmacological or genetic approaches that preclude effective sirtuin-1 function promote glucogenetic gene expression, increase iTreg stability and reduce GVHD lethality capacity, as well as decrease Teff proliferation, CD4+ IFNγ expression and iTreg conversion to pathogenic Teff, relative to controls in mice [67]. Moreover, AMPK activation can reprogram Treg metabolism to inhibit mTOR-dependent glycolysis and promote FAO via increased expression of Acetyl-CoA carboxylase 1 (ACC1), acetyl-CoA carboxylase 2 (ACC2), and CPT1a [29]. Indeed, ACC1, localized in the cytoplasm, and ACC2 localized in the outer mitochondrial membrane, produces malonyl-CoA that inhibits FA import into mitochondria and consequently long-chain FA beta-oxidation [68, 69]. The latter is important since FA synthesis appears to be more critical for Teff such as Th17 cells than for Tregs; and decreased ACCs lower malonyl-CoA resulting in increased FA import, shifting T-cells away from the Th17 and toward the Treg lineage [70, 71]. Moreover, ACC1 deficiency or treatment with soraphen A, an ACC inhibitor, limit alloreactive CD4+ T-cell expansion in vitro; accordingly, donor ACC1-deficient T-cells from CD4CreACC1fl/fl mice caused less severe GVHD than WT mice, and permitted higher Treg frequencies than WT CD4+ T-cells [71]. In addition, increased acetyl-CoA concentrations also support uridine diphosphate N-acetylglucosamine (UDP-GlcNAc) synthesis, which contributes to the suppressive capacity of Treg through a N-GlcNAcylation-mediated increase in CD25 stability [72], and via an O-GlcNAcylation-mediated increase in Foxp3 stability [73]. Metformin, an AMPK activator that functions by inhibiting mitochondrial complex I in the electron transport chain, can reduce murine GVHD severity compared to control groups in an AMPK-dependent manner [74]. Furthermore, AMPK can activate autophagy to initiate metabolic reprogramming towards catabolic reactions [75] during nutrient-limited states [76]. If autophagy fails to occur, Tregs upregulate mTOR signaling leading to increased glycolysis and impaired Treg suppressive function, while Th2 cells also experience increased glycolysis and undergo selective expansion [77]. Accordingly, murine recipients of grafts deficient in autophagy-related genes have displayed reduced Treg reconstitution, survival, and overall exacerbated GVHD relative to recipients that express autophagy-related genes [76]. Moreover, donor T-cells (CD4+ plus CD8+) from AMPK−/− mice have been documented to cause less severe GVHD through impaired survival, reduced GVHD target organ homing, and Treg preservation compared with WT mice [78]. Lastly, AMPK can promote FAO via ACC2 and inhibit de novo FA synthesis via ACC1, supporting Treg over Teff metabolic preference [79]. Thus, AMPK might represent a clinically relevant candidate target to promote Treg frequency and function, while inhibiting donor T cell responses, which may be relevant for GVHD therapeutic approaches.
Along these lines, Liver kinase B1 (LKB1; encoded by Stk11), an AMPK upstream activator, is an important metabolic sensor. In a T cell transfer colitis model, Treg isolated from Lkbfl/flFoxp3YFP−Cre mice showed decreased Treg frequency and suppressive function, and overall decreased metabolic function leading to an inability to control disease progression compared to mice treated with Treg from Lkb1+/+Foxp3YFPCre mice [80]. To further support the role of LKB1 in Treg function and mitochondrial fitness, one study reported that Foxp3CreStk11fl/fl mice developed a fatal inflammatory disease [81]. Of note, LKB1 also functions independently from the AMPK or mTORC1 pathways to suppress the expression of molecules associated with apoptosis and functional exhaustion [81]. A recent study uncovered another important role for OXPHOS in maintaining Treg suppressive function [25]; Treg disruption of mitochondrial complex III was shown to cause accumulation of α-KG metabolites which inhibit α-KG-dependent DNA demethylases, resulting in hyper-methylation and decreased expression of genes associated with Treg suppressive function (encoding PD-1, CD73, neuropilin-1, and TIGIT) relative to Treg that did express mitochondrial complex III [25]. Of note, in this study, methylation of the Foxp3 gene was unaltered and Foxp3 expression remained stable [25]. Finally, even with unaltered Foxp3 methylation and expression, as well as overall Treg numbers, mitochondrial complex III-deficient Tregs have not been found to be suppressive, and in this model, mice developed an early fatal inflammatory disease [25], reminiscent of scurfy mice [82]; this indicated that metabolic checks and balances can even override Foxp3 expression, and determine whether a CD4+ T cell engages in suppressive function or not. Overall, these studies demonstrate Treg survival, function, and stability are delicately regulated by multiple metabolic sensors and pathways that generally favor FAO over FAS; these observations may lead towards new investigations aiming to generate potential therapeutics that selectively support and expand Tregs while inhibiting Teff in GVHD.
Amino acid depletion and oxidative stress balance in Teffs and Tregs in GVHD
Oxidative stress has been implicated in both GVHD risk and severity. Pre-HSCT conditioning regimens can increase cellular ROS and free radicals, as well as deplete antioxidants in both human and mouse subjects [83, 84]. In a clinical study examining the metabolic profiles of allogeneic stem cell transplant recipients to identify metabolites altered in patients with chronic GVHD, the GVHD effector phase was also found to be associated with a dysregulation of redox balance, with increased measures of oxidative stress, including elevated γ-glutamyl amino acid and α-tocopherol, as well as decreased antioxidant enzyme activity in patients with disease relative to controls [85]. Moreover, in multiple murine models of GVHD, alloreactive T-cells demonstrate higher mitochondrial ROS production following increased metabolic activity, while exhibiting lower concentrations of the antioxidant glutathione [56, 86, 87]. Furthermore, recent work examining the bioenergetic demands of murine CD4+ T cells in the context of T cell-mediated immune responses has demonstrated that mitochondrial-derived ROS are needed for human and murine T-cell activation and antigen-specific responses [88, 89], and these findings are concordant with the general premise that GVHD may represent a state of high oxidative stress [83–85]. In studies using donor CD4+ and CD8+ T-cells from Sirtuin-3−/− mice, which exhibit decreased ROS production, murine GVHD development in allogeneic bone marrow transplant recipients was markedly reduced compared to animals receiving WT T cells [87]. Tregs have also been found to exhibit enhanced survival in conditions of oxidative stress, in part due to increased production of thioredoxin-1, an ROS scavenging molecule [90, 91]. CD4+ and CD8+ donor T-cells overexpressing transgenic thioredoxin-1 overexpression alleviated oxidative stress and significantly reduced GVHD severity when compared to WT T cells [92]. Recombinant thioredoxin-1 administration had similar effects in mice, leading to reduced CD4+ and CD8+ T-cell frequencies in the gut and lung target organs and increased Foxp3-expressing T-cells, particularly in the liver [92]. Lastly, the free radical scavenger NecroX-7 increased Treg frequency and reduced GVHD in mice[93]. Taken together, these studies suggest that modifying the global metabolic landscape by reducing oxidative stress may be beneficial for GVHD amelioration, although further studies are warranted.
From another perspective, Indoleamine 2,3-dioxygenase (IDO), the rate-limiting enzyme in catabolism of the essential amino acid tryptophan, is highly expressed in immunosuppressive dendritic cells and in multiple tumor types in both human and mouse, and is upregulated by IFNγ [94]. At the time of TCR stimulation, tryptophan depletion activates GCN2, an inhibitor of mTORC2, and prevents kinase activation [95]. Tryptophan metabolites, including kynurenines, serve as ligands of the aryl hydrocarbon receptor (AhR), and AhR activation has been shown to support pTreg generation in humans [96]. CD4+ T-cells activated in the presence of sub-optimal tryptophan concentrations plus kynurenines can induce Foxp3 expression and Treg suppressive function in mice and humans [97, 98]. Such iTregs have high glutaminolysis and express the LAT1 antiporter, capable of exchanging glutamine for tryptophan to maximize tryptophan and leucine uptake under conditions of limited availability [98]. Moreover, colonic antigen presenting cells express IDO that is induced by donor T-cell derived IFNγ in inflamed murine gut during GVHD; and host IDO expression can help mitigate GVHD by subduing CD4+ and CD8+ T-cell proliferation and cause T-cell apoptosis [8]. In another study, dendritic cells in IDO+/+ mice were also able to increase tTreg suppressive function, but the effect was lost in IDO−/− mice [99], thus raising the possibility that in situ activation of IDO expression in patients with GVHD might increase suppressive function of endogenous Treg. However, this possibility remains to be robustly tested, Alternatively, in vitro expansion of tTregs under conditions of low tryptophan and kynurenines might also generate tTregs with increased efficacy for preventing GVHD. Of note, several other metabolic pathways exist – such as those modulated by the microbiome -- that can influence Treg induction and function; these in turn, may be relevant when considering targeting approaches in the treatment of GVHD (Box 2).
Box 2. Gut microbiome produced short-chain fatty acids can support pTreg induction.
Changes to microbiota and loss of microbiome diversity due to chemotherapy and radiation, GVHD, and prophylactic antibiotic use, have been well-documented in both murine and human allo-HSCT studies [101–104]. Gut microbiota can both cause GVHD, or protect mice and patients from GVHD [101]. Alterations in gut microbiome in human patients include the loss of anaerobic commensal organisms such as Clostridia sp, known to support Treg induction, and expansion of Escherichia, Enterobacter, and Enterococcus species [103, 104]. During homeostasis, symbiotic gut microbiota break down resistant starch and dietary fiber into metabolites that can induce pTregs (Figure I) [105]. Clostridia sp., in the gut, can promote murine and human pTreg induction by secreting short-chain FAs (SCFAs), especially propionate and butyrate [106–108]. SCFAs directly affect murine colonic Treg Foxp3 expression via activation of a G-protein coupled receptor (GPR43) [108] and, by their inherent ability to inhibit HDAC, augment Foxp3 promoter histone acetylation, supporting greater gene transcription at conserved non-coding DNA sequence (CNS) elements of the Foxp3 locus: CNS1 (required for induction) and CNS3 (required for both thymic and pTreg) [109]. SCFA can also elicit murine and human intra-epithelial cell production of TGFß, inducing high, but unstable, Foxp3 expression in human CD4+ T cells, increasing DC aldehyde dehydrogenase 1A2 expression [110], retinoic acid (RA) production, and TGFß-induced pTreg stability [111]. However, RA production has also been implicated in driving GVHD lethality [112–114].
Not all commensal species of bacteria are symbionts that induce Tregs; colonization of murine small intestine with segmented filamentous bacteria induced MHC class II-restricted CD4+ T-cell activation and Th17 differentiation by activating CD11c+ lamina propria DC to produce Th17-polarizing cytokines (IL-6, IL-23) [115, 116]. However, Th17 cells can also have positive effects; the gut microbiome of B6 IL-17RA−/− mice can exacerbate GVHD when transferred into WT mice undergoing HSCT [117], and Th17 cells release IL-17A, inducing epithelial cells to secrete IL-8, thus recruiting neutrophils and more Th17 cells to sites of inflammation [118].
Consequently, therapeutically targeting microbiome dysbiosis might help in the prevention/treatment of GVHD. Indeed, Lactobacilli sp. produce tryptophan metabolites that activate the AhR, and oral Lactobacilli administration can reduce murine GVHD relative to controls; and safety has been demonstrated in allo-HSCT pediatric patients, although the impact on GVHD has yet to be reported (; completed Phase 1; single group, interventional; n=31 primary outcome, bacteremia)VI [119, 120]. Alternatively, fecal microbiota transplants (FMT) might be used to treat steroid-refractory or -dependent GVHD; in one study, 11 of 15 such patients with intestinal GVHD were FMT-treated in a single-arm interventional completed pilot study with nasoduodenal infusion of microbiota from a healthy donor; the patients presented with complete resolution of GVHD symptoms 4 weeks after FMT; the fecal microbial composition of those responders resembled their respective healthy donors (ISRCTN14530574; primary endpoint, GVHD resolution)VII [121]. Bacteroides fragilis, another prominent gut symbiont, induces regulatory function through secretion of Polysaccharide A, a TLR2 agonist: Colonization of germ-free mice with B. fragilis, or intra-gastric administration of polysaccharide A increased mesenteric lymph node Foxp3 expressing T-cell numbers with a concomitant increase in Treg IL-10 and TGFß relative to untreated germ-free mice [122]. Lastly, since direct SCFA administration has ameliorated GVHD severity in preclinical mouse models [123], purified microbial metabolites or drugs mimicking their action, might also represent promising candidate GVHD therapeutics. Indeed, Vorinostat, a histone deacetylase inhibitor mimicking butyrate/propionate-mediated histone deacetylation inhibition, was reported as both safe and effective in decreasing disease in murine models of GVHD, and in patients receiving HSCT for hematological malignancies (; single group, open label, interventional Phases 1 and 2; n= 61, primary endpoint, cumulative incidence of grade 2–4 GVHD)VIII [124, 125].
Concluding remarks
Although there are many unanswered questions regarding Treg metabolism and its role in GVHD, it is clear that Tregs utilize multiple metabolic pathways, and the balance between these pathways is critical in regulating Treg suppressive function and homeostasis (see Outstanding Questions). Thus, GVHD, as a highly inflammatory environment, together with lymphopenia, significantly impact metabolite availability, as well as the metabolic needs of Treg. While early clinical results have shown that adoptive transfer or in situ induction of Treg can ameliorate GVHD, robust investigation is needed to further elucidate the metabolic requirements for Treg survival under these conditions, as well as their specific influence on Treg function and stability to optimize their therapeutic efficacy.
Outstanding Questions.
Which energy generating metabolic pathways in Tregs are dependent on the strength of TCR signaling and the inflammatory environment in which Tregs reside?
Are there detrimental effects associated with high concentrations of OXPHOS that subvert the beneficial effects on Treg differentiation and suppressive function?
What are other key ROS scavenging molecules and redox pathways that could be targeted to reduce the high oxidative stress in GVHD and increase Treg frequency?
How does the unique inflammatory environment in GVHD shape the metabolic programs that affect tTreg and pTreg differentiation and stability?
Since CPT1a deficient Treg develop normally and are present in equivalent numbers, what is the role of long-chain FAO (LC-FAO) in Treg, and can SCFA (2–6 carbons; e.g. butyrate) or medium chain fatty acids (6–12 carbons) substitute for long chain fatty acids 15 (LCFA)?
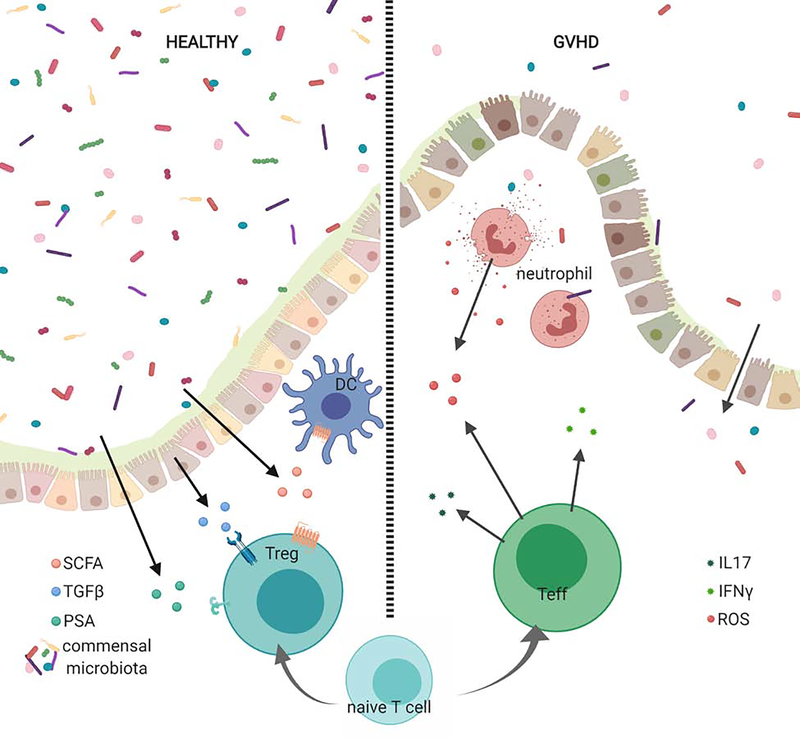
Figure I in Box 2: Gastrointestinal permeability in GVHD can alter the metabolic landscape.
In healthy gastrointestinal tissue, commensal organisms produce short chain fatty acids and polysaccharide A that promote Foxp3 expressing T cells. TGFβ production by intraepithelial cells also contributes to elevated Foxp3 expression. In GVHD, disruption of the epithelial barrier allows for the release of danger associated molecular pattern molecules (DAMPs) by damaged tissues and pathogen associated molecular pattern molecules (PAMPs) by invading microbiota [2]. Activated CD4+ T cells escalate utilization of glycolytic pathways and release pathogenic IL-17 and IFNγ [16]. Neutrophils contribute to redox imbalance by releasing ROS [2].
Table 1.
Treg Immunometabolic strategies for treating GVHD.
| Pathway | Target | Drug/Therapy | Model, Species | Treg frequency/function | Refs |
|---|---|---|---|---|---|
| ETC | Complex I | Metformin | GVHD, mice | Increased frequency | [74] |
| FAS | ACC1 | Soraphen A | EAE, mice | Increased frequency | [70] |
| Glycolysis | HK | 2-deoxyglucose | EAE, mice | Increased frequency | [126] |
| mTOR | Rapamycin | T1D, humans | Increased frequency | [127] | |
| GVHD, mice | Increased frequency | [128] | |||
| PDHK | Dichloroacetate | EAE, mice | Increased frequency | [129] | |
| FAO | CPT1a | Etomoxir | GVHD, mice | Decreased allogeneic T cell numbers without affecting Treg | [55] |
| AMPK | Metformin | GVHD, mice | Increased frequency | [74] | |
| AICAR | Model of IL-2 complex, mice | Increased frequency | [130] | ||
| Epigenetic modulation (HDACi) | Class I/II | Vorinostat | GVHD, humans | Increased frequency and suppression | [131] |
| Class I/II | Trichostatin A | Cardiac and islet transplant, mice | Increased frequency and suppression | [132] | |
| Class I | Entinostat | Renal and prostate cancer, mice | Decreased function, but not frequency | [133] | |
| Class III | Splitomicin | Cardiac allograft model, mice | Increased function | [87] | |
| Class III | Sirt3 knockout | GVHD | Decreased allogeneic T cell numbers without affecting Treg | [134] | |
| Other | Cytoskeleton | PKC-θ inhibitor | GVHD, mice | Increased function | [135] |
| ROS | Thioredoxin-1 | GVHD, mice | Increased frequency | [92] | |
| NecroX-7 | GVHD, mice | Increased frequency and function | [93] | ||
| Tryptophan depletion | IDO inhibition | GVHD | Increased allogeneic T cell numbers without affecting Treg | [136] | |
| Extracellular ATP | APYRASE | GVHD | Increased frequency | [137] |
Abbreviations: ACCI, acetyl-CoA carboxylase 1; AICAR, 5-Aminoimidazole-4-carboxamide ribonucleotide; AMPK, AMP- activated protein kinase; CPT1a, carnitine palmitoyltransferase 1a; DCA, dichloroacetate; EAE, experimental autoimmune encephalitis; ETC, electron transport chain; FAO, fatty acid oxidation; FAS, fatty acid synthesis; GVHD, graft-versus-host disease; HDACi, histone deacetylase inhibitors; HK, hexokinase; 2-DG, 2-deoxyglucose; IBD; inflammatory bowel disease; MS, multiple sclerosis; mTOR, mechanistic/mammalian target of rapamycin; PDHK, pyruvate dehydrogenase kinase; PKC-9, protein kinase C theta; ROS, reactive oxygen species; T1D, type 1 diabetes.
Highlights.
Graft-versus-host disease (GVHD) mediated tissue injury induces oxidative stress results in a high metabolic demand (aerobic glycolysis; mitochondrial oxidative phosphorylation and glutaminolysis) that can reprogram naïve T cells into effector cells.
Whereas naïve thymic-derived CD4+ Tregs are highly glycolytic with a minor proportion of energy derived from fatty acid oxidative phosphorylation, activated Tregs markedly increase fatty acid oxidative phosphorylation that is dependent upon mitochondrial complexes I-IV for energy and stability.
Naïve CD4+ T cells can be induced in vitro or in the periphery to become Tregs. While thymic-derived Tregs are reliant on mTOR, inhibition of mTOR enhances can induce Treg generation, Foxp3 expression and suppressive function.
Gut microbe dysbiosis after fecal matter transplant can interfere with the production of short chain fatty acids (SCFA) that support Treg generation in the gut, the major GVHD target organ.
Acknowledgments
This work was supported in part by research grants from the Children’s Cancer Research Fund (K.L.H.), Leukemia and Lymphoma Translational Research Grant R6029-07 (B.R.B.), R01 HL11879, P01 AI056299 (B.R.B.), and NCI P01 CA067493 (B.R.B.).
Glossary
- AMP-activated protein kinase (AMPK)
senses low-energy state (high AMP:ATP) and inhibits FAS through phosphorylation of ACC1 and inhibits mTORC1-mediated glycolysis through phosphorylation of TSK2.
- Autophagy
self-degrative cellular process in which macromolecules and organelles are recycled.
- Biomass
the amount of organic material inside the cell.
- Etomoxir
inhibits Cpt1a, the rate limiting step in fatty acid oxidation. At concentrations >40 μM, etomoxir also inhibits adenine nucleotide translocase, leading to inhibition of the electron transport chain.
- eTreg
regulatory T cell subset with an effector phenotype (mouse: CD44hi CD62Llo; human: FOXP3hiCD45RA−CD45RO+CD95+CTLA4+).
- Exhaustion
A state of T cell non-responsiveness that results from prolonged activation, for example during a chronic infection or in the presence of cancer.
- Fatty Acid (FA)
carboxylic acid with a long aliphatic chain, which is either saturated or unsaturated.
- Fatty acid oxidation (FAO)
Process whereby 2-carbon units are cleaved from fatty acids and enter the mitochondria via CPT1a, and are catabolized in the TCA cycle. The complete oxidation of palmitate produces 129 ATP molecules. ACC1, localized in the cytoplasm, and ACC2 localized in the outer mitochondrial membrane, produce malonyl-CoA that inhibits FA import into mitochondria for subsequent beta-oxidation
- Fecal microbiota transplant (FMT)
an approach to reconstitute microbiota, typically in colon, by transplanting with bacterial flora isolated from healthy individuals.
- Forkhead box protein O1 (FOXO1)
transcription factor with important roles in the regulation of gluconeogenesis and glycogenolysis
- Glutaminolysis
Catabolic process which, in T cells, results from the import of glutamine (ASCT1, ASCT2 or LAT1/CD98), which is shuttled to the mitochondria, where it is catabolized to alpha-KG and enters the TCA cycle. In addition to providing an energy source, glutaminolysis also supports amino acid production and provides reducing equivalents, NADH and NADPH, required for ATP generation and reducing ROS concentrations, respectively.
- Glycolysis
pathway in which glucose is catabolized to pyruvate + CO2, yielding 2 ATP molecules.
- iTreg
CD4+ T cell in which regulatory/suppressive function is induced in vitro.
- LAT1 antiporter (large neutral amino acid transporter)
heterodimer composed of SLC3A2 and SLC7A5 (CD98) that preferentially transports branched-chain (valine, leucine, isoleucine) and aromatic (tryptophan, tyrosine) amino acids.
- Scurfy mice
spontaneously arising mouse strain exhibiting extreme autoimmune pathology. The genetic defect causing the scurfy phenotype is a missense mutation in Foxp3, the master regulator of Treg differentiation.
- Th1, Th2 and Th17
CD4+ T cell differentiation states defined primarily on pattern of cytokine secretion (Th1: IFNgamma/TNFalpha; Th2: IL-4; and Th17: IL-17).
- Toll-like receptor
proteins that recognize structurally conserved molecules derived from microbes and/or viruses.
- Tricarboxylic acid (TCA) cycle
catabolic process whereby metabolites from glycolysis (pyruvate), glutaminolysis (glutamate) and FAO (acetyl-CoA) are catabolized to drive ATP production
- α-KG
Intermediate metabolite in the TCA cycle; especially important because it serves as a co-factor for demethylases (e.g. TET) that de-repress Treg associated genes, including Foxp3, CD73, TIGIT, and NRP1.
- mitochondrial complex I-Va
components of the electron transport chain (ETC) that oxidizes NADH produced by the TCA cycle to generate a proton gradient across the mitochondrial inner membrane and produce ATP.
- OXPHOS (oxidative phosphorylation)
process whereby metabolites enter the TCA cycle and generate ATP through mitochondrial complex 1–5.
- mTOR (mechanistic/mammalian target of rapamycin)
Serine/threonine kinase subunit of mTORC1 (with raptor) and mTORC2 (with rictor).
- NAD/NADH (nicotine adenine dinucleotide, oxidized and reduced form)
Co-factor involved in both REDOX reactions to generate ATP, and SIRT-dependent deacetylation reactions.
- Oxidative stress/ROS
damage to cellular structures resulting from over-production of reactive oxygen species, including: peroxides, superoxide and hydroxyl radicals.
- Pentose phosphate pathway
Side branch of glycolysis in which oxidation of glucose-6-P generates NADPH, a critical component for regenerating GSH, and de novo nucleotide synthesis.
- pTreg
CD4+ T cell that develops regulatory/suppressive functions after antigen encounter in the periphery.
- Short chain fatty acids (SCFA)
harbor less than six carbon atoms that result from bacterial fermentation of dietary fiber, and are highly enriched in the colon. Acetate, propionate, and butyrate are the three most common SCFAs
- Sirtuins (SIRTs)
histone/protein deacetylases. SIRT1 is nuclear and affects transcription via deacetylation of histones and transcription factors (e.g. FOXO, NFkB), while SIRT3 is in the mitochondrial matrix and its substrates include Complex 1 of the ETC.
- tTreg
Foxp3+ Tregs that develop in the thymus in response to self-antigen.
Footnotes
Resources:
This trial is registered at clinicaltrials.gov: https://www.clinicaltrials.gov/ct2/show/NCT00602693
This trial is registered at clinicaltrials.gov: https://clinicaltrials.gov/ct2/show/NCT01210664
This trial is registered at clinicaltrials.gov: https://clinicaltrials.gov/ct2/show/study/NCT02145325
This trial is registered at clinicaltrials.gov: https://clinicaltrials.gov/ct2/show/NCT00928018
This trial is registered at clinicaltrials.gov: https://clinicaltrials.gov/ct2/show/NCT01634217
This trial is registered at clinicaltrials.gov: https://clinicaltrials.gov/ct2/show/NCT01010867
This trial is registered at the ISRCTN Registry: http://www.isrctn.com/ISRCTN14530574
This trial is registered at clinicaltrials.gov: https://clinicaltrials.gov/ct2/show/NCT00810602
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Li HW and Sykes M (2012) Emerging concepts in haematopoietic cell transplantation. Nat Rev Immunol 12 (6), 403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeiser R and Blazar BR (2017) Acute Graft-versus-Host Disease - Biologic Process, Prevention, and Therapy. N Engl J Med 377 (22), 2167–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacMillan ML et al. (2002) Early antithymocyte globulin therapy improves survival in patients with steroid-resistant acute graft-versus-host disease. Biol Blood Marrow Transplant 8 (1), 40–6. [DOI] [PubMed] [Google Scholar]
- 4.Shevach EM and Thornton AM (2014) tTregs, pTregs, and iTregs: similarities and differences. Immunol Rev 259 (1), 88–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kennedy-Nasser AA et al. (2014) Ultra low-dose IL-2 for GVHD prophylaxis after allogeneic hematopoietic stem cell transplantation mediates expansion of regulatory T cells without diminishing antiviral and antileukemic activity. Clin Cancer Res 20 (8), 2215–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hippen KL et al. (2011) Massive ex vivo expansion of human natural regulatory T cells (Tregs) with minimal loss of in vivo functional activity. Science Translational Medicine 3 (78), 78ra33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Issa F et al. (2010) Ex vivo-expanded human regulatory T cells prevent the rejection of skin allografts in a humanized mouse model. Transplantation 90 (12), 1321–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jasperson LK et al. (2009) Inducing the tryptophan catabolic pathway, indoleamine 2,3-dioxygenase (IDO), for suppression of graft-versus-host disease (GVHD) lethality. Blood 114 (24), 5062–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang Q et al. (2004) In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. Journal of Experimental Medicine 199 (11), 1455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xia G et al. (2006) Targeting acute allograft rejection by immunotherapy with ex vivo-expanded natural CD4+ CD25+ regulatory T cells. Transplantation 82 (12), 1749–55. [DOI] [PubMed] [Google Scholar]
- 11.Bluestone JA et al. (2015) Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci Transl Med 7 (315), 315ra189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brunstein CG et al. (2016) Umbilical cord blood-derived T regulatory cells to prevent GVHD: kinetics, toxicity profile, and clinical effect. Blood 127 (8), 1044–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mathew JM et al. (2018) A Phase I Clinical Trial with Ex Vivo Expanded Recipient Regulatory T cells in Living Donor Kidney Transplants. Sci Rep 8 (1), 7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geltink RIK et al. (2018) Unraveling the Complex Interplay Between T Cell Metabolism and Function. Annu Rev Immunol 36, 461–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frauwirth KA et al. (2002) The CD28 signaling pathway regulates glucose metabolism Immunity 16 (6), 769–77. [DOI] [PubMed] [Google Scholar]
- 16.MacIver NJ et al. (2013) Metabolic regulation of T lymphocytes. Annu Rev Immunol 31, 259–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Priyadharshini B et al. (2018) Cutting Edge: TGF-β and Phosphatidylinositol 3-Kinase Signals Modulate Distinct Metabolism of Regulatory T Cell Subsets. J Immunol 201 (8), 2215–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun IH et al. (2018) mTOR Complex 1 Signaling Regulates the Generation and Function of Central and Effector Foxp3. J Immunol 201 (2), 481–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huynh A et al. (2015) Control of PI(3) kinase in Treg cells maintains homeostasis and lineage stability. Nat Immunol 16 (2), 188–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Procaccini C et al. (2016) The Proteomic Landscape of Human Ex Vivo Regulatory and Conventional T Cells Reveals Specific Metabolic Requirements. Immunity 44 (2), 406–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raud B et al. (2018) Etomoxir Actions on Regulatory and Memory T Cells Are Independent of Cpt1a-Mediated Fatty Acid Oxidation. Cell Metab 28 (3), 504–515.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao CH et al. (2018) Identifying off-target effects of etomoxir reveals that carnitine palmitoyltransferase I is essential for cancer cell proliferation independent of β-oxidation. PLoS Biol 16 (3), e2003782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Connor RS et al. (2018) The CPT1a inhibitor, etomoxir induces severe oxidative stress at commonly used concentrations. Sci Rep 8 (1), 6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raud B et al. (2018) Etomoxir Actions on Regulatory and Memory T Cells Are Independent of Cpt1a-Mediated Fatty Acid Oxidation. Cell Metab 28 (3), 504–515.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weinberg SE et al. (2019) Mitochondrial complex III is essential for suppressive function of regulatory T cells. Nature 565 (7740), 495–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chi H (2012) Regulation and function of mTOR signalling in T cell fate decisions. Nat Rev Immunol 12 (5), 325–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y et al. (2015) mTOR signaling in T cell immunity and autoimmunity. Int Rev Immunol 34 (1), 50–66. [DOI] [PubMed] [Google Scholar]
- 28.Hippen KL et al. (2011) Generation and large-scale expansion of human inducible regulatory T cells that suppress graft-versus-host disease. Am J Transplant 11 (6), 1148–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pollizzi KN and Powell JD (2014) Integrating canonical and metabolic signalling programmes in the regulation of T cell responses. Nat Rev Immunol 14 (7), 435–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeng H et al. (2013) mTORC1 couples immune signals and metabolic programming to establish T(reg)-cell function. Nature 499 (7459), 485–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee CF et al. (2015) Preventing Allograft Rejection by Targeting Immune Metabolism. Cell Rep 13 (4), 760–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Battaglia M et al. (2005) Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood 105 (12), 4743–8. [DOI] [PubMed] [Google Scholar]
- 33.Bocian K et al. (2010) Rapamycin, unlike cyclosporine A, enhances suppressive functions of in vitro-induced CD4+CD25+ Tregs. Nephrol Dial Transplant 25 (3), 710–7. [DOI] [PubMed] [Google Scholar]
- 34.Tresoldi E et al. (2011) Stability of human rapamycin-expanded CD4+CD25+ T regulatory cells. Haematologica 96 (9), 1357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Michalek RD et al. (2011) Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol 186 (6), 3299–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gerriets VA et al. (2015) Metabolic programming and PDHK1 control CD4+ T cell subsets and inflammation. J Clin Invest 125 (1), 194–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eleftheriadis T et al. (2016) In human alloreactive CD4(+) T-cells, dichloroacetate inhibits aerobic glycolysis, induces apoptosis and favors differentiation towards the regulatory T-cell subset instead of effector T-cell subsets. Mol Med Rep 13 (4), 3370–6. [DOI] [PubMed] [Google Scholar]
- 38.Zeiser R et al. (2006) Inhibition of CD4+CD25+ regulatory T-cell function by calcineurin-dependent interleukin-2 production. Blood 108 (1), 390–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeiser R et al. (2008) Differential impact of mammalian target of rapamycin inhibition on CD4+CD25+Foxp3+ regulatory T cells compared with conventional CD4+ T cells. Blood 111 (1), 453–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Armand P et al. (2016) The addition of sirolimus to the graft-versus-host disease prophylaxis regimen in reduced intensity allogeneic stem cell transplantation for lymphoma: a multicentre randomized trial. Br J Haematol 173 (1), 96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brunstein CG et al. (2011) Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood 117 (3), 1061–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lutz M and Mielke S (2016) New perspectives on the use of mTOR inhibitors in allogeneic haematopoietic stem cell transplantation and graft-versus-host disease. Br J Clin Pharmacol 82 (5), 1171–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fan MY and Turka LA (2018) Immunometabolism and PI(3)K Signaling As a Link between IL-2, Foxp3 Expression, and Suppressor Function in Regulatory T Cells. Front Immunol 9, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gerriets VA et al. (2016) Foxp3 and Toll-like receptor signaling balance T. Nat Immunol 17 (12), 1459–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li L et al. (2019) TLR8-Mediated Metabolic Control of Human Treg Function: A Mechanistic Target for Cancer Immunotherapy. Cell Metab 29 (1), 103–123 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Newton RH et al. (2018) Maintenance of CD4 T cell fitness through regulation of Foxo1. Nat Immunol 19 (8), 838–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Galgani M et al. (2016) Role of Metabolism in the Immunobiology of Regulatory T Cells. J Immunol 197 (7), 2567–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feuerer M et al. (2009) Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med 15 (8), 930–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Rosa V et al. (2007) A key role of leptin in the control of regulatory T cell proliferation. Immunity 26 (2), 241–55. [DOI] [PubMed] [Google Scholar]
- 50.Tauchmanova L et al. (2004) High serum leptin in patients with chronic graft-versus-host disease after hematopoietic stem cell transplantation. Transplantation 78 (9), 1376–83. [DOI] [PubMed] [Google Scholar]
- 51.Chapman NM et al. (2018) mTOR coordinates transcriptional programs and mitochondrial metabolism of activated Treg subsets to protect tissue homeostasis. Nat Commun 9 (1), 2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kishore M et al. (2017) Regulatory T Cell Migration Is Dependent on Glucokinase-Mediated Glycolysis. Immunity 47 (5), 875–889.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matschinsky FM and Wilson DF (2019) The Central Role of Glucokinase in Glucose Homeostasis: A Perspective 50 Years After Demonstrating the Presence of the Enzyme in Islets of Langerhans. Front Physiol 10, 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alon R (2017) A Sweet Solution: Glycolysis-Dependent Treg Cell Migration. Immunity 47 (5), 805–807. [DOI] [PubMed] [Google Scholar]
- 55.Byersdorfer CA et al. (2013) Effector T cells require fatty acid metabolism during murine graft-versus-host disease. Blood 122 (18), 3230–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gatza E et al. (2011) Manipulating the bioenergetics of alloreactive T cells causes their selective apoptosis and arrests graft-versus-host disease. Sci Transl Med 3 (67), 67ra8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saha A et al. (2013) Host programmed death ligand 1 is dominant over programmed death ligand 2 expression in regulating graft-versus-host disease lethality. Blood 122 (17), 3062–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saha A et al. (2016) Programmed death ligand-1 expression on donor T cells drives graft-versus-host disease lethality. J Clin Invest 126 (7), 2642–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Klysz D et al. (2015) Glutamine-dependent alpha-ketoglutarate production regulates the balance between T helper 1 cell and regulatory T cell generation. Sci Signal 8 (396), ra97. [DOI] [PubMed] [Google Scholar]
- 60.Nakaya M et al. (2014) Inflammatory T cell responses rely on amino acid transporter ASCT2 facilitation of glutamine uptake and mTORC1 kinase activation. Immunity 40 (5), 692–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Song EK et al. (2013) Glutamine protects mice from acute graft-versus-host disease (aGVHD). Biochem Biophys Res Commun 435 (1), 94–9. [DOI] [PubMed] [Google Scholar]
- 62.da Gama Torres HO et al. (2008) Efficacy of glutamine-supplemented parenteral nutrition on short-term survival following allo-SCT: a randomized study. Bone Marrow Transplant 41(12), 1021–7. [DOI] [PubMed] [Google Scholar]
- 63.Patsoukis N et al. (2015) PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nat Commun 6, 6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blazar BR et al. (2003) Blockade of programmed death-1 engagement accelerates graft versus-host disease lethality by an IFN-gamma-dependent mechanism. J Immunol 171 (3), 1272–7. [DOI] [PubMed] [Google Scholar]
- 65.Nguyen HD et al. (2016) Metabolic reprogramming of alloantigen-activated T cells after hematopoietic cell transplantation. J Clin Invest 126 (4), 1337–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chalkiadaki A and Guarente L (2012) Sirtuins mediate mammalian metabolic responses to nutrient availability. Nat Rev Endocrinol 8 (5), 287–96. [DOI] [PubMed] [Google Scholar]
- 67.Daenthanasanmak A et al. (2019) Targeting Sirt-1 controls GVHD by inhibiting T-cell allo-response and promoting Treg stability in mice. Blood 133 (3), 266–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McGarry JD and Brown NF (1997) The mitochondrial carnitine palmitoyltransferase system. From concept to molecular analysis. Eur J Biochem 244 (1), 1–14. [DOI] [PubMed] [Google Scholar]
- 69.Abu-Elheiga L et al. (2000) The subcellular localization of acetyl-CoA carboxylase 2. Proc Natl Acad Sci U S A 97 (4), 1444–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Berod L et al. (2014) De novo fatty acid synthesis controls the fate between regulatory T and T helper 17 cells. Nat Med 20 (11), 1327–33. [DOI] [PubMed] [Google Scholar]
- 71.Raha S et al. (2016) Disruption of de novo fatty acid synthesis via acetyl-CoA carboxylase 1 inhibition prevents acute graft-versus-host disease. Eur J Immunol 46 (9), 2233–8. [DOI] [PubMed] [Google Scholar]
- 72.Araujo L et al. (2017) Glycolysis and glutaminolysis cooperatively control T cell function by limiting metabolite supply to N-glycosylation. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu B et al. (2019) The lineage stability and suppressive program of regulatory T cells require protein O-GlcNAcylation. Nat Commun 10 (1), 354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Park MJ et al. (2016) Metformin attenuates graft-versus-host disease via restricting mammalian target of rapamycin/signal transducer and activator of transcription 3 and promoting adenosine monophosphate-activated protein kinase-autophagy for the balance between T helper 17 and Tregs. Transl Res 173, 115–130. [DOI] [PubMed] [Google Scholar]
- 75.Mihaylova MM and Shaw RJ (2011) The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol 13 (9), 1016–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Le Texier L et al. (2016) Autophagy-dependent regulatory T cells are critical for the control of graft-versus-host disease. JCI Insight 1 (15), e86850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kabat AM et al. (2016) The autophagy gene Atg16l1 differentially regulates Treg and TH2 cells to control intestinal inflammation. Elife 5, e12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Byersdorfer CA et al. , Deletion of AMP-activated protein kinase (AMPK) in donor T cells mitigates graft-versus-host disease without impacting graft-versus-leukemia effects, Am Assoc Immnol, 2017. [Google Scholar]
- 79.Herzig S and Shaw RJ (2018) AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol 19 (2), 121–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.He N et al. (2017) Metabolic control of regulatory T cell (Treg) survival and function by Lkb1. Proc Natl Acad Sci U S A 114 (47), 12542–12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang K et al. (2017) Homeostatic control of metabolic and functional fitness of Treg cells by LKB1 signalling. Nature 548 (7669), 602–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Das M et al. (2019) Regulatory T Cells under the Mercy of Mitochondria. Cell Metab 29 (2), 243–245. [DOI] [PubMed] [Google Scholar]
- 83.Jonas CR et al. (2000) Plasma antioxidant status after high-dose chemotherapy: a randomized trial of parenteral nutrition in bone marrow transplantation patients. Am J Clin Nutr 72 (1), 181–9. [DOI] [PubMed] [Google Scholar]
- 84.Suh JH et al. (2014) Thiol/redox metabolomic profiling implicates GSH dysregulation in early experimental graft versus host disease (GVHD). PLoS One 9 (2), e88868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Reikvam H et al. (2017) Patients with Treatment-Requiring Chronic Graft versus Host Disease after Allogeneic Stem Cell Transplantation Have Altered Metabolic Profiles due to the Disease and Immunosuppressive Therapy: Potential Implication for Biomarkers. Front Immunol 8, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Maeda Y et al. (2005) Both perforin and Fas ligand are required for the regulation of alloreactive CD8+ T cells during acute graft-versus-host disease. Blood 105 (5), 2023–7. [DOI] [PubMed] [Google Scholar]
- 87.Toubai T et al. (2018) Mitochondrial Deacetylase SIRT3 Plays an Important Role in Donor T Cell Responses after Experimental Allogeneic Hematopoietic Transplantation. J Immunol 201 (11), 3443–3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Franchina DG et al. (2018) Reactive Oxygen Species: Involvement in T Cell Signaling and Metabolism. Trends Immunol 39 (6), 489–502. [DOI] [PubMed] [Google Scholar]
- 89.Sena LA et al. (2013) Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity 38 (2), 225–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mougiakakos D et al. (2011) Increased thioredoxin-1 production in human naturally occurring regulatory T cells confers enhanced tolerance to oxidative stress. Blood 117 (3), 857–61. [DOI] [PubMed] [Google Scholar]
- 91.Mougiakakos D et al. (2009) Naturally occurring regulatory T cells show reduced sensitivity toward oxidative stress-induced cell death. Blood 113 (15), 3542–5. [DOI] [PubMed] [Google Scholar]
- 92.Sofi MH et al. (2019) Thioredoxin-1 confines T cell alloresponse and pathogenicity in graft-versus-host disease. J Clin Invest 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Im KI et al. (2015) The Free Radical Scavenger NecroX-7 Attenuates Acute Graft-versus-Host Disease via Reciprocal Regulation of Th1/Regulatory T Cells and Inhibition of HMGB1 Release. J Immunol 194 (11), 5223–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.O’Neill LA et al. (2016) A guide to immunometabolism for immunologists. Nat Rev Immunol 16 (9), 553–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Munn DH and Mellor AL (2016) IDO in the Tumor Microenvironment: Inflammation, Counter-Regulation, and Tolerance. Trends Immunol 37 (3), 193–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gandhi R et al. (2010) Activation of the aryl hydrocarbon receptor induces human type 1 regulatory T cell-like and Foxp3(+) regulatory T cells. Nat Immunol 11 (9), 846–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen W et al. (2008) The Indoleamine 2,3-Dioxygenase Pathway Is Essential for Human Plasmacytoid Dendritic Cell-Induced Adaptive T Regulatory Cell Generation. J Immunol 181 (8), 5396–5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hippen KL et al. (2017) In Vitro Induction of Human Regulatory T Cells Using Conditions of Low Tryptophan Plus Kynurenines. Am J Transplant 17 (12), 3098–3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sharma MD et al. (2007) Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. The Journal of clinical investigation 117 (9), 2570–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vander Heiden MG et al. (2009) Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324 (5930), 1029–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shono Y et al. (2016) Increased GVHD-related mortality with broad-spectrum antibiotic use after allogeneic hematopoietic stem cell transplantation in human patients and mice. Sci Transl Med 8 (339), 339ra71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Holler E et al. (2014) Metagenomic analysis of the stool microbiome in patients receiving allogeneic stem cell transplantation: loss of diversity is associated with use of systemic antibiotics and more pronounced in gastrointestinal graft-versus-host disease. Biol Blood Marrow Transplant 20 (5), 640–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Khoruts A et al. (2017) Toward revision of antimicrobial therapies in hematopoietic stem cell transplantation: target the pathogens but protect the indigenous microbiota. Transl Res 179, 116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Taur Y et al. (2014) The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood 124 (7), 1174–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kim CH et al. (2014) Gut microbiota-derived short-chain Fatty acids, T cells, and inflammation. Immune Netw 14 (6), 277–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Arpaia N et al. (2013) Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504 (7480), 451–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Atarashi K et al. (2013) Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 500 (7461), 232–6. [DOI] [PubMed] [Google Scholar]
- 108.Smith PM et al. (2013) The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341 (6145), 569–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zheng Y et al. (2010) Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature 463 (7282), 808–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Singh N et al. (2014) Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 40 (1), 128–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lu L et al. (2010) Characterization of protective human CD4CD25 FOXP3 regulatory T cells generated with IL-2, TGF-beta and retinoic acid. PloS one 5 (12), e15150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Aoyama K et al. (2013) Inhibiting retinoic acid signaling ameliorates graft-versus-host disease by modifying T-cell differentiation and intestinal migration. Blood 122 (12), 2125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Choi SW et al. (2015) Histone deacetylase inhibition regulates inflammation and enhances Tregs after allogeneic hematopoietic cell transplantation in humans. Blood 125 (5), 815–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Thangavelu G et al. (2019) Dendritic Cell Expression of Retinal Aldehyde Dehydrogenase-2 Controls Graft-versus-Host Disease Lethality. J Immunol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ivanov II et al. (2009) Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139 (3), 485–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Goto Y et al. (2014) Segmented filamentous bacteria antigens presented by intestinal dendritic cells drive mucosal Th17 cell differentiation. Immunity 40 (4), 594–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Varelias A et al. (2017) Acute graft-versus-host disease is regulated by an IL-17-sensitive microbiome. Blood 129 (15), 2172–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bettelli E et al. (2008) Induction and effector functions of T(H)17 cells. Nature 453 (7198), 1051–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ladas EJ et al. (2016) The safety and feasibility of probiotics in children and adolescents undergoing hematopoietic cell transplantation. Bone Marrow Transplant 51 (2), 262–6. [DOI] [PubMed] [Google Scholar]
- 120.Staffas A et al. (2017) The intestinal microbiota in allogeneic hematopoietic cell transplant and graft-versus-host disease. Blood 129 (8), 927–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.van Lier YF et al. (2019) Fecal Microbiota Transplantation Can Cure Steroid-Refractory Intestinal Graft-Versus-Host Disease. Biology of Blood and Marrow Transplantation 25 (3), S241. [Google Scholar]
- 122.Round JL and Mazmanian SK (2010) Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci U S A 107 (27), 12204–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mathewson ND et al. (2016) Gut microbiome-derived metabolites modulate intestinal epithelial cell damage and mitigate graft-versus-host disease. Nat Immunol 17 (5), 505–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Choi SW et al. (2014) Vorinostat plus tacrolimus and mycophenolate to prevent graft-versus-host disease after related-donor reduced-intensity conditioning allogeneic haemopoietic stem-cell transplantation: a phase 1/2 trial. Lancet Oncol 15 (1), 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Choi SW et al. (2017) Vorinostat plus tacrolimus/methotrexate to prevent GVHD after myeloablative conditioning, unrelated donor HCT. Blood 130 (15), 1760–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shi LZ et al. (2011) HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. The Journal of experimental medicine 208 (7), 1367–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Battaglia M et al. (2006) Rapamycin Promotes Expansion of Functional CD4<sup>+</sup>CD25<sup>+</sup>FOXP3<sup>+</sup> Regulatory T Cells of Both Healthy Subjects and Type 1 Diabetic Patients. The Journal of Immunology 177 (12), 8338. [DOI] [PubMed] [Google Scholar]
- 128.Shin H-J et al. (2011) Rapamycin and IL-2 reduce lethal acute graft-versus-host disease associated with increased expansion of donor type CD4<sup>+</sup>CD25<sup>+</sup>Foxp3<sup>+</sup> regulatory T cells. Blood 118 (8), 2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gerriets VA et al. (2015) Metabolic programming and PDHK1 control CD4+ T cell subsets and inflammation. The Journal of clinical investigation 125 (1), 194–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gualdoni GA et al. (2016) The AMP analog AICAR modulates the Treg/Th17 axis through enhancement of fatty acid oxidation. Faseb j 30 (11), 3800–3809. [DOI] [PubMed] [Google Scholar]
- 131.Choi SW et al. (2015) Histone deacetylase inhibition regulates inflammation and enhances Tregs after allogeneic hematopoietic cell transplantation in humans. 125 (5), 815–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shen L et al. (2012) Class I histone deacetylase inhibitor entinostat suppresses regulatory T cells and enhances immunotherapies in renal and prostate cancer models. PLoS One 7 (1), e30815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Beier UH et al. (2011) Sirtuin-1 Targeting Promotes Foxp3+ T-Regulatory Cell Function and Prolongs Allograft Survival. Mol.Cell.Biol 31 (5), 1022–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tao R et al. (2007) Deacetylase inhibition promotes the generation and function of regulatory T cells. Nature Medicine 13, 1299. [DOI] [PubMed] [Google Scholar]
- 135.McDonald-Hyman C et al. (2018) The vimentin intermediate filament network restrains regulatory T cell suppression of graft-versus-host disease. J Clin Invest 128 (10), 4604–4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jasperson LK et al. (2008) Indoleamine 2,3-dioxygenase is a critical regulator of acute graft-versus-host disease lethality. Blood 111 (6), 3257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wilhelm K et al. (2010) Graft-versus-host disease is enhanced by extracellular ATP activating P2X7R. Nat Med 16 (12), 1434–8. [DOI] [PubMed] [Google Scholar]