Abstract
CD8+ T cell immunological memory of past antigen exposure can confer long-lived protection against infections or tumors. The fact that CD8+ memory T cells can possess features of both naïve and effector cells has forced the field to struggle with several conceptual questions about the cell’s developmental origin, and consequently, the mechanism(s) that contribute to memory development. Here, we discuss recent conceptual advances in our understanding of memory T cell development that incorporate data describing a hybrid stem/effector state of differentiation. We theorize that the mechanisms involved in developing these cells could be, in part, mediated through epigenetic programs. Finally, we consider the potential therapeutic implications of inducing and/or utilizing such hybrid cells clinically.
Keywords: CD8+ T cells, naïve, effector, memory, exhaustion, TCF1, cancer immunotherapy
“I learned to recognise the thorough and primitive duality of man; I saw that, of the two natures that contended in the field of my consciousness, even if I could rightly be said to be either, it was only because I was radically both.”
Robert L. Stevenson, Strange Case of Dr. Jekyll and Mr. Hyde, 1886
Stem and effector properties of mammalian memory T cells
Upon encountering their cognate antigen, CD8+ naïve T cells (TN) clonally expand to generate effector cells that migrate to peripheral tissues and kill virally-infected and malignant cells. During this effector response, a bifurcation in the developmental capacity of the T cells occurs. The majority of the effector cells become terminally differentiated, termed terminal/short-lived effector cells (TE/SLEC), while a minor subset of effector cells, termed memory precursor effector cells (MP/MPECs) acquire the ability to survive the contraction stage of the immune response [1] and further differentiate into a heterogeneous pool of memory cells under optimal developmental conditions (i.e., in acute infections in which there is resolution of antigenic sources) [1, 2]. The discovery of developmentally permissive MPEC cells underscored the fact that the generation of T cell memory is controlled by an adaptive and dynamic set of cellular, transcriptional, and metabolic processes [3–9]. Ultimately, these signals can be integrated to establish a set of long-lived gene expression programs tailored to a multitude of factors including the source of antigen, anatomical location of antigenic encounter, and duration of antigen exposure [3–9].
Memory T cells can persist for long periods of time and maintain the ability to rapidly re-elicit an effector response upon secondary encounter with their cognate antigen. Generally, these memory T cells are subdivided into two major subpopulations: central memory (TCM) and effector memory (TEM) T cells [10]. Initially classified based on the expression of the lymphoid homing molecules CCR7 and CD62L (L-selectin), TEM cells have reduced expression of these receptors relative to naïve and TCM cells, and are mainly found recirculating between the blood and peripheral tissues, whereas, TCM express both CCR7 and CD62L, and are capable of circulating between the blood and lymphoid tissues under homeostatic conditions in mice [11], nonhuman primates [12, 13] and humans [14, 15]. Additionally, TEM from all these species have been shown to retain lower expression of molecules involved in long-term persistence while upregulating transcription factors (TFs) mediating terminal differentiation, and are thus considered a committed progeny with decreased self-renewal and multipotent capacity compared to TCM cells [2]. Over time, this model has become more nuanced based on observations that TCM cells can migrate into inflamed, peripheral tissues [16, 17] while CCR7− T cells can enter lymphoid tissues at steady state [12, 18] and during inflammation [19] (reviewed in [20]). Within the past decade, evidence for at least two novel memory subsets has emerged, and further highlighted the inherent diversity among these subtypes. Specifically, a non-circulating lineage of T cells termed tissue-resident memory (TRM) T cells express tissue-residency markers, CD69 and CD103, and reside in peripheral tissues [21–23]. TRM cells are localized at the port of entry of invading pathogens to provide a rapid first line of defense (as excellent reviews exist on the relationship between TRM and circulating memory cells, this topic will not be addressed here) [21–23]. Additionally, stem cell memory (TSCM) T cells, defined in humans by surface expression of CD122, CD95, and CXCR3 within the CD45RA+CD45RO−CCR7+CD62L+ naïve-like compartment, are characterized by enhanced homeostatic self-renewal, increased proliferative capacity, and a multipotent developmental potential compared to TCM and TEM; this enables them to generate differentiated progeny, including TCM and TEM cells in vitro and upon adoptive transfer in xenogeneic mouse models, as well as in humans [12, 24–28]. Despite being initially identified in the mouse in allogeneic bone marrow transplantation [29] or induced from activated naïve T cells by glycogen synthase kinase 3 β (GSK-3β) inhibition [30], antigen-specific TSCM remain poorly defined in murine infections; therefore, their role remains unclear. However, these features are also observed in TCM, and there may be overlap in the classification of these subtypes, thus explaining the observation that murine antigen-specific TCM have been shown to retain self-renewal and multipotency upon serial adoptive transfers in mice [31].
Although TSCM and TCM cells have the capacity to retain expression of naïve-associated genes while simultaneously displaying rapid cytokine production upon TCR stimulation, memory T cell subsets as a whole are often depicted as bearing mutually exclusive properties, mainly stem-like potential (TSCM and TCM) versus immediacy of effector functions/cytotoxicity (TEM) [2, 14, 24, 32, 33]. However, recent advances in single cell technologies at the proteomic and transcriptomic levels, as well as genome-wide epigenetic profiling studies have provided new insights that refine our current understanding of the gene expression programs and developmental origin of memory T cells. Notably, single cell transcriptomics have identified human and murine T cell subsets simultaneously co-expressing stem and effector genes, which we refer to as a ‘hybrid state’. Additionally, epigenetic profiling of stem-like T cells have identified several effector loci in these cells that remain epigenetically poised, while having limited transcriptional activity, thereby explaining their potential to rapidly recall an effector response [34, 35]. In this Opinion article, we discuss recent articles that provide evidence of a stem and effector hybrid state in long-lived memory CD8+ T cells in the context of T cell activation upon acute and chronic viral infections, or in response to tumors. We further theorize the therapeutic implications of generating stem/effector hybrid T cells that are intrinsically resistant to CD8+ T cell ‘exhaustion’ to improve responses to immune checkpoint blockade (ICB) or adoptive cell transfer (ACT) when treating cancers. We finally highlight fundamental questions that remain to be answered, and which in our view, have the potential of significantly advancing our understanding of memory CD8+ T cell differentiation.
Persistent antigen stimulation promotes adaptation of effector potential
Prolonged, high TCR stimulation that occurs during cancer and chronic viral infections has been shown to limit the effector response of T cells, a hallmark feature of what is now commonly referred to as T cell exhaustion (TEX) [36]. Collectively, this dysfunctional state is maintained through acquired gene expression programs that encompass decreased expression of effector proteins, a reduction in proliferative potential, and increased co-expression of multiple inhibitory receptors [37]. Attempts to therapeutically reverse this programming by disruption of inhibitory receptor-ligand interactions with monoclonal antibody blockade (such as blockade of programmed cell death protein 1 [PD-1/CD279], ICB) were found to modulate T cell function and lead to enhanced control of chronic viral infection or anti-tumor responses in various animal models and in humans [38–45]. Indeed, initial work with the mouse model of chronic lymphocytic choriomeningitis virus (LCMV) infection (Clone 13 strain), demonstrated that the PD-1 intermediate (PD-1int), but not the PD-1 high (PD-1high) cells expanded during blockade of PD-L1 (programmed death- ligand 1), and thus, could be ‘reinvigorated’ during therapeutic administration [46]. The same group later suggested in the same model that PD-1int cells act as precursors of the terminally differentiated PD-1high subset, and that deletion of either subset resulted in uncontrolled viral replication [47]; this indicated that terminally-differentiated TEX cells were not just bystander cells, but played an active role in limiting chronic LCMV disease progression [47]. Paradoxically, several studies have since demonstrated that aspects of this exhaustion programming were stable and heritable during T cell propagation and antigen-free maintenance [48–50], and may represent a distinct ‘differentiation state’ from functional memory cells [51], this also suggested that, once acquired, these cells might be committed to this exhaustive fate (see below).
At face value, these observations were at odds – how can dysfunctional, terminally exhausted cells maintain these exhaustion programs over time, yet remain responsive to ICB intervention? Subsequently, these findings led to a nuanced view of T cell exhaustion in which different subsets of TEX cells might exist, some endowed with a higher degree of proliferative capacity and effector functions in response to TCR stimulation or ICB, and thus referred to as progenitors of TEX cells, as recently proposed [52]. These PD-1int Tim-3− cells retained expression of the TF Tcf1 (but not the PD-1high Tim-3+ Tcf1−), proliferated in response to ICB and further reduced viral load in mice chronically infected with LCMV [53–57]. These murine progenitor cells also expressed Bcl6 and Id3 TFs [54, 56], displaying a gene expression signature that was shared with T follicular helper CD4+ (TFH) T cells, including the TFH signature marker, chemokine receptor Cxcr5 (therefore, in some instances, these have been referred to as follicular CD8+ in both mice and humans) [54, 57]. In addition, the murine PD-1+ TEX cell subset exhibited reduced expression of Id2 and Prdm1 (Blimp-1) TFs relative to other subsets [53–57]. Mechanistically, the generation and maintenance of these PD-1int TEX progenitors appeared to be regulated by Tcf1 and Bcl6, while it was counteracted by Id2-E2A or Blimp-1 (Prdm1); indeed, CD8+ T cells lacking Tcf1 or Bcl6 generated a reduced number of of TEX progenitors, while mice lacking Id, E2A, or Blimp1, exhibited an increased number of progenitors in response to chronic LCMV infection, relative to controls [53–56] (Key Figure, Figure 1). Moreover, adoptive CD8+ T cell transfer of both Tcf1+ (Cxcr5+) Tim3− and Tcf1− (Cxcr5−) Tim3+ populations isolated from the spleens of mice chronically infected with LCMV and transferred into infection-matched recipients showed that the Tcf1+ Tim3− population consisted of cells that proliferated vigorously and resulted in a reduction in LCMV viral load following PD-1 blockade (ICB), suggesting that preferentially targeting this population might result in enhanced therapeutic benefit. These cells (Tcf1+Tim3−) were also capable of generating more differentiated Tim3+ progeny, given that purified Tim-3+ cells could not lead to the generation of Tcf1+ cells, thereby highlighting a unidirectional precursor-progeny relationship [53–55, 57]. Similar to these observations made in murine model systems, Hepatitis C virus (HCV)-specific CD8+ T cells in humans exhibit a TCF1+CD127+PD-1int phenotype. Thus, similar to progenitors of TEX described in mice, HCV-specific CD8+ T cell persistence may be supported following resolution of the infection by this subset, and suggests that these cells might harbor long-term memory potential [58]. Thus, PD-1int TCF1+ cells might be only partially exhausted, behaving as progenitors of the CD8+ TEX compartment. However, despite retaining memory-like gene expression and preferentially localizing to lymphoid tissues [53, 54], these TEX progenitors simultaneously display traits generally ascribed to effector-like CD8+ T cells, including a predominant CD62L−CD127− phenotype [53, 55], and copious IFNγ production upon cognate antigen stimulation (Figure 1) [55].
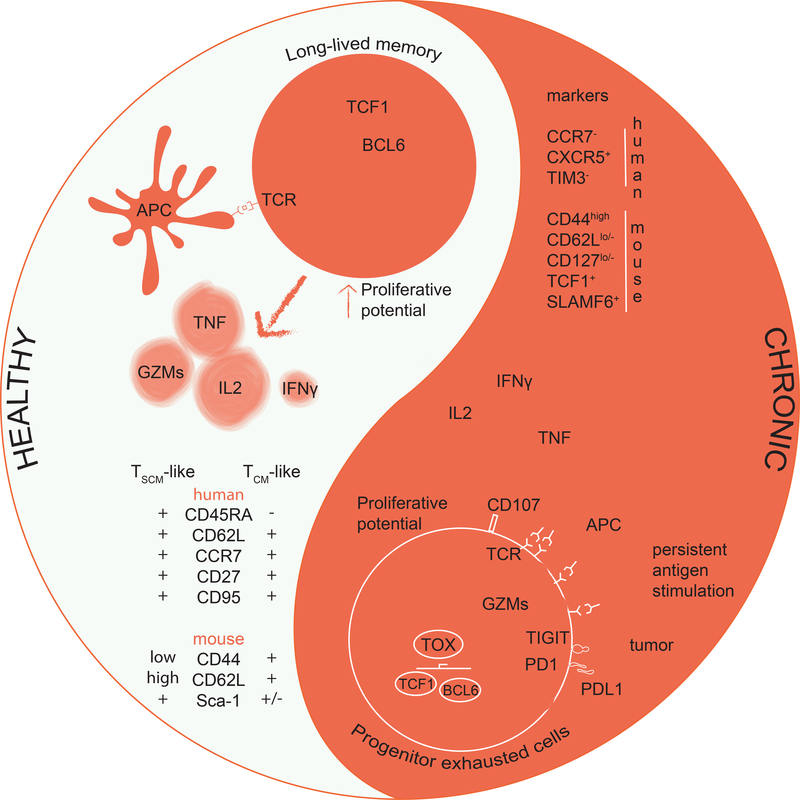
Figure 1. Long-lived T cell compartments in physiology and chronic stimulation.
Under healthy homeostatic conditions long-lived memory CD8+ T cells are endowed with a strong proliferation potential. Upon secondary encounter with cognate antigen these cells can rapidly re-elicit effector functions that include granzyme (GZM) and cytokine production. Multipotent memory CD8+ T cells express molecules that are also expressed in naïve T cells such as CD62L and CCR7. Additionally, they can express TCF1 and LEF1 that serve with BCL6 to enable self-renewal and long-term persistence.
Under chronic stimulation (i.e. cancer, chronic infections) progenitor exhausted T (TEX) cells are generated. These cells have a strong proliferative potential that is fully unleashed upon ICB. TCF1+ progenitor TEX are poised for rapid cytokine production and express a mixed phenotype of effector cells (lack of CCR7 and expression of GZMs) and exhausted cells (mainly expressing PD-1), along with the transcription factor TOX governing their development and survival among the pool of TEX cells. Progenitor TEX have also been identified as CXCR5+, a homing receptor for the lymph nodes’ B cell zone.
Additional insights into the molecular mechanisms governing the development of this progenitor population have come from investigating the transcriptional regulator, Tox. Several reports recently demonstrated that Tox knockout (KO; Tox−/−) murine CD8+ T cells generate a reduced number of Tcf1+ progenitors compared to wild type (WT) CD8+ T cells in response to persistent TCR stimulation elicited by chronic LCMV infection, or by a tumor model in mice [59–61]. In these same studies, it was further demonstrated that Tox expression in mice promoted the expression of inhibitory receptors, thereby mitigating TCR-mediated activation of CD8+ T cells and ultimately preserving the development of TEX cells [59–61]. In summary, the TEX compartment appears to be organized in a hierarchy of developmental potential, analogous to that of conventional non-exhausted T cells featuring long-lived memory precursors (i.e., the TCF1+, similar to TSCM and TCM) and committed progeny (i.e., the TIM3+, similar to TEM). These findings highlight the importance of targeting the TCF1+ subset of TEX cells to ideally achieve enhanced therapeutic benefit following ICB.
Signals involved in the generation of a memory/effector hybrid state of gene expression
The data discussed above highlight a broader question: how does an activated T cell retain the stem-like capacity to self-renew while simultaneously developing into more differentiated progeny? TCF1 has been established as a critical factor in the TEX progenitor population, although other notable TFs, as well as additional molecular mechanisms are likely to play a pivotal role in sustaining stem/effector properties during homeostasis and following immune activation. While there is significant emphasis on TCF7/TCF1 and other notable TFs in regulating the persistence of TEX progenitors, TCF1 now has established roles in preserving this stem-like population [53, 55, 56]. However, it is less clear what the molecular mechanisms are that sustain these stem/effector properties during cellular division.
Epigenetic modifications are critical for establishing the commitment of various cell fates. Such modifications do so by regulating chromatin accessibility for TFs that then reinforce cell-type specific gene expression programs. This is highlighted during reproduction and development, where cell fate decisions are regulated by epigenetic programs that ultimately drive the formation of an entire organism [62, 63]. Similar to early developmental processes, recent studies aiming to understand the heritable nature of T cell memory have suggested that epigenetic modifications are a central mechanism governing the preservation of effector properties in memory T cells. Specifically, in recent work, our group examined whole-genome DNA methylation from cell-sorted memory CD8+ T cell subsets isolated from the blood of healthy human donors: the analysis revealed genes that were differentially targeted for methylation programming between TEM and TSCM cells, and which were enriched in pathways associated with cellular proliferation [34]. Equally important, however, TSCM cells contained demethylated loci coding for effector proteins, such as IFNγ, perforin, and granzymes [34]; this suggested that plastic stem-like memory cells might co-exist with poised effector programs, thereby establishing a molecular basis for the enhanced persistence and functionality of TSCM compared to more differentiated cell subsets.
Further mechanistic insights using murine KO models and adoptive transfer systems in mice have shed light on the molecular mechanisms leading to chromatin accessibility at effector loci in long-lived memory CD8+ T cells and further revealed chromatin dynamics during memory formation such as DNA methylation and histone modifications (Figure 2) [64–67]. To better understand the relationship between memory generation and epigenetic reprogramming, we used the LCMV murine model of acute virus infection (Armstrong strain) and tracked the changes in DNA methylation during the formation of terminal effector cells and effector cells with memory potential [67]. During the early stage of CD8+ T cell responses to LCMV infection, MPECs acquired DNA methylation at several naïve-associated genes (Sell [CD62L], Ccr7, Tcf7) consistent with the transcriptional repression of these genes. Additionally, MPECs exhibited demethylated loci of effector molecules (Prf1, Gzmb, IFNγ). The suppression of the examined naïve-associated genes (Sell, Ccr7, Tcf7) appeared to be dependent, in part, on de novo DNA methylation, as deletion of DNA methyltransferase 3a (Dnmt3a/Dnmt3a) prevented the methylation of these genes, and resulted in a heightened re-expression during the MPEC-to-memory T cell transition relative to WT mice [67]. These results prompted further exploration of the developmental origin of CD62L+ memory cells. In particular, do CD62L+ memory cells arise from CD62L− MPEC cells by epigenetic regulation of this promoter? To address this question, we isolated CD62L− MPEC and TE cells 8 days post-infection with Armstrong LCMV, labeled these cells with CFSE, and adoptively transferred these cells into naïve C57BL/6 mice [67]. 28 days later, we observed that, in an antigen-free environment, CD62L− MPECs re-expressed CD62L and underwent homeostatic proliferation which was not observed in the transferred CD62L− TE population [67]. Analysis of the undivided, rested MPEC population demonstrated that this was due to re-expression of CD62L, rather than the selective survival of an input CD62Lhi population. This re-expression of CD62L in undivided CD62Lhi MPECs was also associated with demethylation of the L-selectin promoter. Further, analysis of Ccr7, Bcl2, and Il7r (encoding CD127) also revealed similar re-expression in the adoptively transferred cells. These data suggest that after antigen clearance, MPECs undergo dynamic loci-specific epigenetic reprogramming during the antigen independent stage of memory generation in which they are able to re-express particular naïve-associated genes while maintaining demethylated (poised) effector loci [35, 67]. These findings further suggest that memory cells may undergo an obligatory transit through an effector phase during the primary immune response, which may explain the stable demethylation of effector genes in long-lived memory T cells [34, 35, 64, 67].
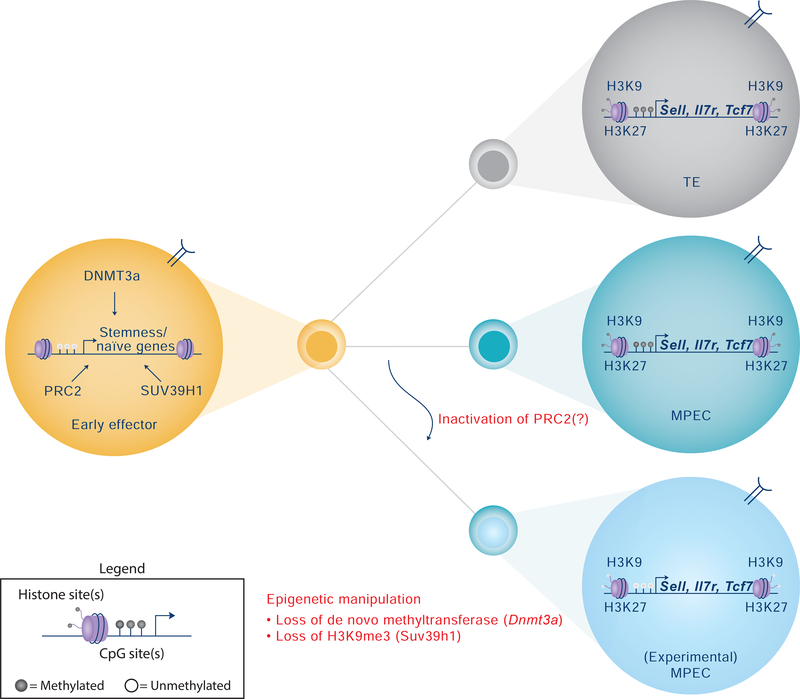
Figure 2. Epigenetic regulation of CD8+ terminal effector (TE) and memory-precursors effector cell (MPEC) differentiation.
Following activation by a professional APC, naïve CD8+ T cells undergo epigenetic reprogramming of genes related to stemness such as Tcf7 (TCF1) and Lef1, and tissue homing such as Sell (CD62L) and Il7R. These epigenetic changes include DNA methylation and demethylation events and an array of histone modifications. Inhibition of de novo DNA methylation or histone methylation restricts the progression of early effector cells into terminally differentiated T cells and/or increases the multipotency of MPECs. Deletion of Dnmt3a, a de novo methyltransferase, prior to effector differentiation has resulted in heightened re-expression of naïve-associated genes during the antigen-independent stage of memory CD8+ T cell differentiation in mice [67]. Genetic manipulation of H3K9me3 by Suv39h1 knockout (KO) in mice has resulted in increased expression of stem-like genes relative to wild type (WT) murine CD8+ T cells [66]. Likewise, manipulation of the polycomb repressive complex 2 (PRC2) has been demonstrated to promote increased skewing of cells towards an MPEC phenotype in mice following acute lymphocytic choriomeningitis virus (LCMV) infection [64]. However, conflicting reports of EZH2 on memory formation do exist [65]. Taken together, these studies highlight the role epigenetic programming plays in regulating the plasticity of CD8+ T cells during effector and memory differentiation.
Moreover, the data support the concept that epigenetic mechanisms, such as de novo DNA methylation, are, in part, responsible for regulating the progression of MPECs into developmentally plastic memory CD8+ T cells [67]. Similarly, others have determined that the epigenetic regulator Suv39h1 can promote H3K9me3 trimethylation deposition in ‘stemness’ genes such as Il7r and Sell in in vitro differentiated effector CD8+ T cells [66]. Specifically, using the Listeria monocytogenes expressing ovalbumin (LM-OVA) mouse infection model, erasure of H3K9me3 in Suv39h1−/− mice resulted in increased expression of several genes related to stem-like memory T cells in effector CD8+ T cells, upon challenge (e.g. Ccr7, Il7r and Satb); this was accompanied by increased animal survival and accelerated generation of long-lived memory following resolution of infection relative to WT mice [66]. By using a single-cell RNAseq (scRNAseq) approach, the study identified a stem/effector bipotent cycling intermediate at the peak of the expansion phase following acute infection with LM-OVA, with a putative role in generating both effector and memory cells [66]. Another independent series of experiments examined the role of the polycomb repressive complex (PRC2)—and more specifically, the histone methyltransferase, Ezh2: in Ezh2 KO mice (and by extension, PRC2 KO) – via tamoxifen-induced Cre under control of the Gzmb promoter -- the formation of TE cells during the effector phase of the primary response to acute LCMV was compromised relative to WT mice [64]. The study reported an enrichment of H3K27me3 at MPEC-signature genes in TE cells in Ezh2−/− compared to WT cells; this appeared to occur during late development of TE cells. By contrast, enrichment of methylation at TE-signature genes in MPEC cells was not found, possibly due to FOXO1-dependent shielding of these sites from H3K27me3 deposition, as determined by bioinformatic analysis of FOXO1 binding to DNA in naïve T cells [64]. These findings highlighted the link between Ezh2 programming of MPEC cells and differentiation potential [64]. However, contrasting results exist on the role of Ezh2, as a different study found that melanoma gp100 TCR-transgenic (Pmel-1) Ezh2−/− naïve CD8+ T cells restrained memory differentiation while promoting terminal differentiation following transfer in mice bearing B16 melanoma [65]; this indicated that epigenetic regulators might have opposite, important roles during different phases of the immune response. Overall, these studies suggest that the multipotency of CD8+ T cells can be dynamically regulated by several epigenetic mechanisms during the course of an immune response, as shown through their effects on MPECs (Figure 2). It is therefore plausible to postulate that targeted manipulation of these programs might result in the generation of stem/effector hybrid cells to potentially improve clinical therapeutics in a number of diseases, including cancer.
Stem-like cells with effector properties can provide benefit in cancer immunotherapy
Given that the presence of Tcf1+ TEX precursor is coupled to the clinical responses observed during PD-1 ICB blockade in viral infection model systems, these observations have recently been extended to analyses in tumor immunology. Early work from our group used high-dimensional single-cell profiling by flow cytometry and computational analysis to investigate the tumor-infiltrating CD8+ T cell compartment in non-small cell lung cancer (NSCLC); a small population of T cells was defined (∼2–3% of total CD8+ T cells in tumors) which shared phenotypic and transcriptional features with murine Tcf1+ memory-like CD8+ T cells found in mice chronically-infected with LCMV (Clone 13 strain) [68]. This investigation in human NSCLC identified a hierarchy in the exhausted T cell compartment according to which CXCR5+TIM3− progenitor TEX cells gave rise to both CXCR5−TIM3− effectors and CXCR5−TIM3+ terminally differentiated TEX cells upon TCR stimulation [68]. Likewise, adoptive cell transfer (ACT) of phenotypically similar tumor-infiltrating murine CD8+ T cell populations, identified by expression of SLAMF6 [69] or TCF1 [70] in the B16 melanoma-expressing OVA, or LCMV gp33 mouse models, respectively, defined the same precursor-progeny relationship. All studies agreed on the fact that both human and mouse TEX progenitors had a transcriptional signature and some surface immunophenotypic characteristics (e.g., expression of CD27, CD28 and CXCR3) of long-lived TSCM and TCM cells, albeit with some differences. Analysis of scRNAseq of human melanoma-infiltrating CD8+ T cells revealed cytolytic transcripts(with the exception of GZMB), whose expression was similar between human tumor-infiltrating CXCR5+TIM3− and CXCR5−TIM3+ CD8+ T cells [68]; this indicated the simultaneous presence of stem and cytotoxic CD8+ T cells traits in TEX progenitor cells. These results were corroborated by a subsequent study examining single-cell transcriptional profiles of immune cells from an extended cohort of melanoma patients [71]. The study demonstrated that the presence of TCF7-expressing memory-like cells correlated with a positive clinical outcome in response to ICB in melanoma patients, thereby suggesting a role for these cells in the regression of established melanoma tumors in humans [71]. Moreover, TCF1+TIM3− cells have been readily found at sites of metastasis [71], but their frequency has been shown to decrease with disease progression in primary lesions from treatment-naïve NSCLC patients [68]; this in turn, might suggest a less efficient anti-tumor immune response corresponding with erosion of this population with increasing antigenic stimulation (tumor); however, further studies are warranted to better elucidate this possibility. Nevertheless, collectively, these data support a model in which tumor control is mediated through the expansion and subsequent differentiation of a progenitor population of cells with effector traits, into terminally-differentiated TEX populations that can exert antitumor responses [53, 55, 72, 73].
Implications for immunotherapy
Long-lived memory T cells, such as human TSCM and TCM cells, have the capacity to retain expression of naïve-associated genes while simultaneously displaying immediacy of effector cytokine production upon TCR stimulation. As the latter increases with progressive peripheral differentiation, it originally served as a rationale for initial selection of these cells for ACT approaches to treating cancers [74, 75]. These cell products indeed induced objective responses in a subset of patients with metastatic melanoma [76]; yet, later studies reported the paradoxical evidence that the durability of anti-tumor responses was largely dependent not on the killing capacity of the CD8+ T cells at the time of transfer, but rather, on the persistence of the transferred cells [77]. A plethora of preclinical and clinical data now support the concept that anti-tumor potential is preferentially exerted by T cells with stem-like properties [24, 30, 78–81]. Indeed, a number of ACT protocols now favor the generation of these less differentiated cells over more differentiated subsets [78, 79, 82]. Recent efforts to obtain TSCM have been accomplished using modified human T cell expansion protocols in vitro, e.g., by replacing IL-2 with IL-7, IL-15 or IL-21 [83, 84], by diminishing TCR stimulation [84], by promoting Notch signaling [85], or by limiting ROS metabolism upon antioxidant treatment [81]. Additional strategies have involved the pharmacological inhibition of GSK-3β [30], AKT [86], or bromodomain and extra-terminal motif (BET) proteins [87], resulting in the suppression of genes associated with terminal effector differentiation such as T-bet, BATF and EOMES, and in the maintenance of stem-like genes such as those encoding TCF1 and LEF1 [85]. These strategies have led to obtaining stem-like CD8+ T cells with improved persistence and anti-tumor activity in vivo by using mouse lymphoma tumor models [85] or xenogeneic mouse models of human solid tumors [30, 87] or leukemias [81, 82, 87]. Alternatively, combining epigenetic modifiers with effector cytokines, such as IL-2 and IL-12, might be hypothesized as an additional strategy to simultaneously block silencing and retain expression of stem genes (increase persistence) while promoting effector and killing properties. In this way, the potential usage of a clonal population of stem-like/effector hybrid cells or a heterogenous population of cells with stem (progenitor-like) and effector-like properties with enhanced persistence may glean improved therapeutic potential (Figure 3). We posit that this certainly represents an important area of future investigation.
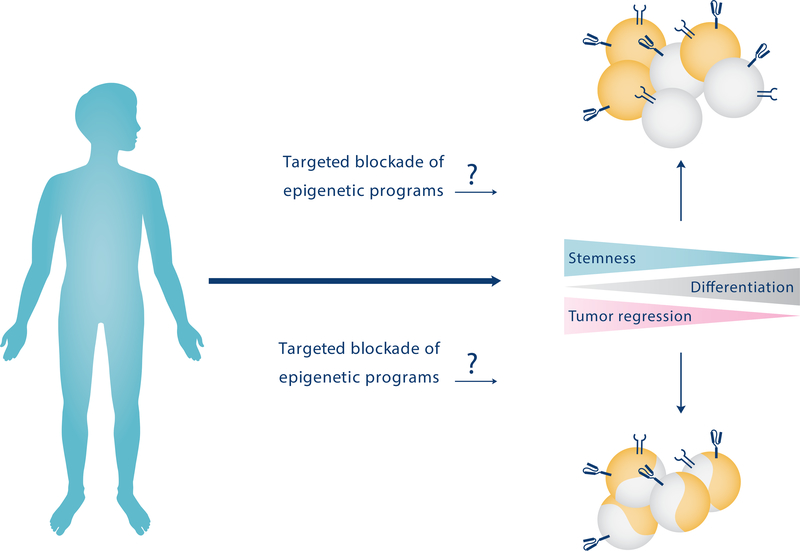
Figure 3. Potential therapeutic approaches for stem-effector T cell generation in the treatment of chronic diseases such as cancer.
A hypothetical therapeutic approach to treating certain cancers is to utilize DNA demethylating agents to target epigenetic programs that induce a de-differentiated pool of tumor-specific endogenous or adoptively-transferred T cells. These agents could be used to either create a uniform pool of stem-effector hybrid cells or a heterogeneous population of mixed progenitor and terminal effector cells capable of exerting anti-tumor functions after reinfusion into cancer patients.
Recent data in the chronic LCMV murine infection model has proposed that PD-1 blockade can promote effector functions without altering the epigenetic landscape of CD8+ TEX cells, as revealed by assessing transposase-accessible chromatin using sequencing (ATAC-seq) and DNA methylation profiling, thereby suggesting a potential limit of immunotherapy in actively reverting the dysfunctional state [50, 88]. Moreover, Tcf-1+ progenitors and terminally-differentiated TEX cells may exhibit a different chromatin landscape in chronic LCMV infection [89] and cancer [71], although it is still unknown whether such distinctions are maintained in the progeny of TEX following ICB. Defining this aspect of heterogeneity, particularly with regard to DNA methylation programming and ICB responsiveness, will be informative on the potential of TEX progenitors to exert prolonged anti-tumor immunity. Of note, differentiation of TEX cells is a dynamic process that involves progressive changes at the epigenetic level [51, 90] which impact the subsequent capacity for reprogramming [90]. Thus, interventions to the epigenome that inhibit the exhaustion program in cells that are not yet exhausted, or revert it in cells that are fully exhausted, may be a potential strategy to enhance existing therapeutic modalities.
Pursuant to this concept, recent data generated by our group has demonstrated that manipulation of the epigenome by deletion or inhibition of de novo DNA methylation programming prior to intervention with blockade of PD-1 can favor the maintenance of proliferating TCF1+ CD8+ T cells with long-lived effector potential [88]. Noteworthy, Dnmt3a-deficient CD8+ T cells (from conditional Dnmt3a KO mice) treated with ICB or WT T cells in mice, treated with a DNA demethylating agent (5-aza-2’-deoxycytidine; decitabine, DAC), underwent a striking proliferative burst in response to ICB treatment (Figure 4) [88]. These data served as proof-of-principle that epigenetic programs are not just a correlate of T cell exhaustion, but in fact, are causal in establishing T cell exhaustion. Subsequently, it may be possible and beneficial, then, to deliberately modify the differentiation status of T cells for utilization in therapeutic settings such as ACT.
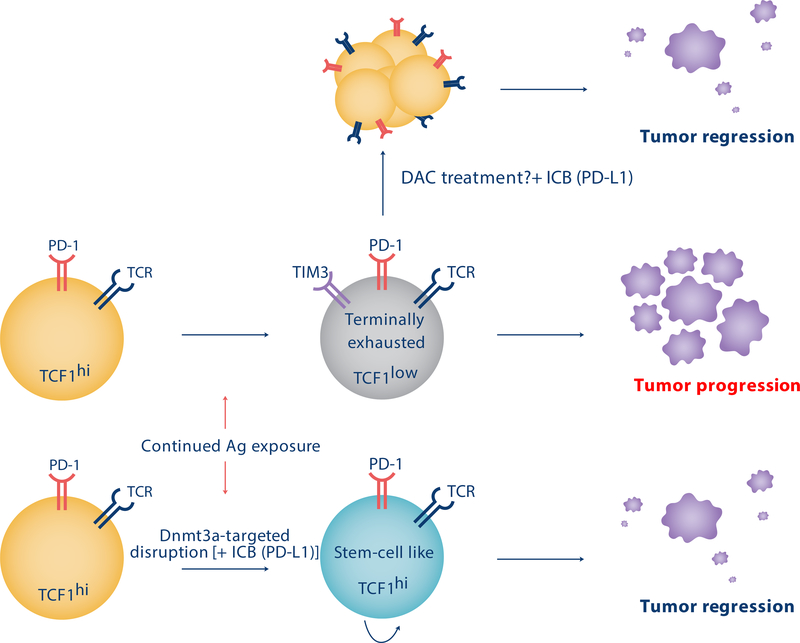
Figure 4. Manipulation of epigenetic programming could result in improved T cell therapeutic potential against tumors.
Prolonged exposure of T cells to their cognate antigen during chronic viral infection or cancer promotes the development of terminal exhaustion programming. These programs are reinforced by epigenetic modifications and result in a reduction in the T cell’s proliferative and cytotoxic potential and limit the T cell’s response to immune checkpoint blockade therapy (ICB). Inhibiting these epigenetic programs can preserve an ICB responsive population of T cells expressing TCF1, and has resulted in improved viral control in pre-clinical murine studies [88]. Treatment with the DNA demethylating agent, decitabine (DAC), can partially recapitulate the overall genetic deletion phenotype in mice and result in improved T cell responsiveness to ICB relative to controls [88]. Hypothetically, these strategies might be utilized to provide epigenetically permissible ICB-responsive CD8+ T cells that can lead to improved tumoral clearance.
Concluding remarks
The recent application of high-content single cell technologies and epigenetic profiling approaches have revealed that stem and effector/killing properties among CD8+ T cells are no longer considered mutually exclusive. The transcriptional programming which endows a T cell with a multipotent developmental capacity can co-exist with effector programs and establishes a long-lived population of memory T cells that are poised to mount a broad immunological response against a previously exposed pathogen. Recent studies examining these properties during T cell responses to chronic sources of antigen have illustrated an important role of these co-existing features in establishing therapeutic modalities that rely on select subsets of T cells. Outstanding questions surrounding these fundamental T cell properties are of great translational importance and we anticipate that they will be a matter of intense investigation in the near future (see Outstanding Questions box). Investigation of these processes will likely shed further insight into the cellular and molecular mechanisms of memory T cell differentiation and exhaustion during acute and chronic pathogenic challenges and ultimately enable the development of new therapeutic approaches that utilize the exquisite specificity and longevity of T cell-mediated immunity.
Outstanding Questions box.
How do stem/effector-hybrid CD8+ T cells relate to conventional memory CD8+ T cells in mouse and humans (Figure 1)? A thorough comparison of T cells at the transcriptional, epigenetic, and metabolic levels remains to be fully established; therefore, the developmental relationships between mouse and human memory T cells remain unresolved. Persistent antigenic/inflammatory stimulation, however, has been demonstrated to shape the long-lived memory compartment to acquire effector characteristics (enhanced inflammation and cytotoxicity), which may contribute to the question of effector function among stem-like memory cells.
What is the physiological requirement for establishing exhaustion in CD8+ TEX progenitors? Progressive suppression of effector functions (exhaustion) has, in part, likely evolved to avoid immunopathology. Notably, one of the major complications following ICB is autoimmune adverse events, especially in combination therapies. While exciting, strategies to induce stem/effector-hybrids in the absence of exhaustion would have to be approached with caution to avoid the generation of T cells that may harm the host.
What are the signals beyond epigenetic regulation leading to reshape the memory CD8+ T cell compartment in persistent infections and cancer? These chronic conditions profoundly alter tissue microenvironments, and memory CD8+ T cells are continuously exposed to excessive amounts of pro-inflammatory and/or immunosuppressive cytokines which might play a role in this regard. Analysis of transcriptional signatures (as described above) should provide more information in this context.
Highlights.
Murine models of infection indicate that exhausted CD8+ T (TEX) cells derive from effector cells that survived the contraction phase of the immune response.
CD8+ TEX cells from murine and human chronic infections and tumors are heterogeneous, featuring subsets of progenitor and terminally-differentiated cells.
Despite sharing similarities with memory cells, progenitor CD8+ TEX cells have unique features that we propose are shaped by persistent antigenic stimulation. The sustained signaling may induce the acquisition of effector properties that co-exist with the cell’s memory potential.
Mechanisms regulating the development of such stem/effector-hybrid CD8+ T cell state are unclear, although evidence suggests that epigenetics plays a non-redundant role.
T cell products containing stem/effector-hybrid CD8+ T cells may be exploited therapeutically in adoptive immunotherapy approaches for human cancer and chronic infections.
Acknowledgments
We wish to thank the members of the Lugli and Youngblood laboratories for fruitful discussions. Further, we would like to thank Emily VanGilder from St. Jude Biomedical Communications for her creative expertise in creating some of the figures. This work has been supported by grants from the Associazione Italiana per la Ricerca sul Cancro (AIRC; Investigator Grant No. 20607) to E.L., NIAID 5R01-AI114442, American Lebanese Syrian Associated Charities (ALSAC), ASSISI Foundation of Memphis, and Immune Tolerance Network (ITN) to B.Y. G.G. is supported by a AIRC fellowship for Italy.
Glossary
- Terminal/short-lived effector T cells
KLRG-1+ CD127− activated cells forming in the acute phase of an infection with potent effector functions but that are committed to die following the removal of the infected target
- Memory precursor effector T cells
KLRG-1− CD127+ cells forming in the acute phase of an infection that are capable to persist in the long-term, giving rise to long-lived memory cells
- Central memory
A subset of memory T cells expressing CD62L and CCR7, able to recirculate between the blood and secondary lymphoid organs
- Effector memory
A subset of memory T cells lacking CD62L and CCR7, generally excluded from secondary lymphoid organs, able to recirculate between the blood and peripheral tissues
- Tissue-resident memory cells
A specialized subset of non-circulating memory cells residing in peripheral tissues, important for protection at pathogen-entry sites
- Stem cell memory T cells
A long-lived memory population endowed with enhanced stem-like properties (self-renewal and multipotency) compared to central and effector memory T cells
- Hybrid state (T cell)
A memory T cell population simultaneously displaying stem and effector-like properties, as proposed in this manuscript
- Exhaustion
A differentiation state of T cells induced by persistent antigen stimulation as in chronic infections and cancer and characterized by the sustained expression of inhibitory receptors and diminished effector functions
- Immune checkpoint blockade
Therapeutic procedure for treating certain cancers involving the infusion of antibodies capable of blocking ligand-receptor interactions of checkpoint inhibitory receptors such as CTLA-4 and PD-1 on T cells
- Adoptive T cell transfer
Therapeutic procedure to treat chronic viral infections, certain malignancies, and transplantation; it involves the infusion of antigen-specific T cells or T cells genetically modified to express a chimeric antigen receptor or T cell receptor
- Progenitors of exhausted T cells
PD-1+ TCF-1+ subset of cells with features of partial exhaustion but retaining stem-like properties that enable an enhanced response to PD-1 blockade compared to terminally differentiated exhausted T cells
- T follicular helper CD4+ T cells
Found in B cell follicles and involved in the formation of germinal centers, specialized in stimulating antibody production by B cells
- Epigenetic modifications
Covalent modifications to the DNA and/or histones that can impact on chromatin accessibility and ultimately reinforce cell type-specific gene expression programs
- Homeostatic proliferation
A type of T cell proliferation that is driven by cytokines and which preserves lymphocyte numbers in the host
Footnotes
Competing interests.
The authors declare no competing financial interests.
Bibliography
- 1.Kaech SM et al. (2003) Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol 4 (12), 1191–8. [DOI] [PubMed] [Google Scholar]
- 2.Mahnke YD et al. (2013) The who’s who of T-cell differentiation: Human memory T-cell subsets. Eur J Immunol 43 (11), 2797–809. [DOI] [PubMed] [Google Scholar]
- 3.Kaech SM et al. (2002) Molecular and functional profiling of memory CD8 T cell differentiation. Cell 111 (6), 837–51. [DOI] [PubMed] [Google Scholar]
- 4.Intlekofer AM et al. (2005) Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol 6 (12), 1236–44. [DOI] [PubMed] [Google Scholar]
- 5.Joshi NS et al. (2007) Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity 27 (2), 281–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Windt GJ et al. (2012) Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity 36 (1), 68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buck MD et al. (2016) Mitochondrial Dynamics Controls T Cell Fate through Metabolic Programming. Cell 166 (1), 63–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaech SM and Cui W (2012) Transcriptional control of effector and memory CD8+ T cell differentiation. Nat Rev Immunol 12 (11), 749–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pearce EL et al. (2013) Fueling immunity: insights into metabolism and lymphocyte function. Science 342 (6155), 1242454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.allusto F et al. (2004) Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol 22, 745–63. [DOI] [PubMed] [Google Scholar]
- 11.Masopust D et al. (2001) Preferential localization of effector memory cells in nonlymphoid tissue. Science 291 (5512), 2413–7. [DOI] [PubMed] [Google Scholar]
- 12.Lugli E et al. (2013) Superior T memory stem cell persistence supports long-lived T cell memory. J Clin Invest 123 (2), 594–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pitcher CJ et al. (2002) Development and homeostasis of T cell memory in rhesus macaque. J Immunol 168 (1), 29–43. [DOI] [PubMed] [Google Scholar]
- 14.Sallusto F et al. (1999) Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401 (6754), 708–12. [DOI] [PubMed] [Google Scholar]
- 15.Thome JJ et al. (2014) Spatial map of human T cell compartmentalization and maintenance over decades of life. Cell 159 (4), 814–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nolz JC and Harty JT (2014) IL-15 regulates memory CD8+ T cell O-glycan synthesis and affects trafficking. J Clin Invest 124 (3), 1013–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osborn JF et al. (2017) Enzymatic synthesis of core 2 O-glycans governs the tissue-trafficking potential of memory CD8(+) T cells. Sci Immunol 2 (16). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sathaliyawala T et al. (2013) Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity 38 (1), 187–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guarda G et al. (2007) L-selectin-negative CCR7- effector and memory CD8+ T cells enter reactive lymph nodes and kill dendritic cells. Nat Immunol 8 (7), 743–52. [DOI] [PubMed] [Google Scholar]
- 20.Jameson SC and Masopust D (2018) Understanding Subset Diversity in T Cell Memory. Immunity 48 (2), 214–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schenkel JM and Masopust D (2014) Tissue-resident memory T cells. Immunity 41 (6), 886–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mueller SN and Mackay LK (2016) Tissue-resident memory T cells: local specialists in immune defence. Nat Rev Immunol 16 (2), 79–89. [DOI] [PubMed] [Google Scholar]
- 23.Milner JJ and Goldrath AW (2018) Transcriptional programming of tissue-resident memory CD8(+) T cells. Curr Opin Immunol 51, 162–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gattinoni L et al. (2011) A human memory T cell subset with stem cell-like properties. Nat Med 17 (10), 1290–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cieri N et al. (2015) Generation of human memory stem T cells after haploidentical T-replete hematopoietic stem cell transplantation. Blood 125 (18), 2865–74. [DOI] [PubMed] [Google Scholar]
- 26.Roberto A et al. (2015) Role of naive-derived T memory stem cells in T-cell reconstitution following allogeneic transplantation. Blood 125 (18), 2855–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biasco L et al. (2015) In vivo tracking of T cells in humans unveils decade-long survival and activity of genetically modified T memory stem cells. Sci Transl Med 7 (273), 273ra13. [DOI] [PubMed] [Google Scholar]
- 28.Oliveira G et al. (2015) Tracking genetically engineered lymphocytes long-term reveals the dynamics of T cell immunological memory. Sci Transl Med 7 (317), 317ra198. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y et al. (2005) Host-reactive CD8+ memory stem cells in graft-versus-host disease. Nat Med 11 (12), 1299–305. [DOI] [PubMed] [Google Scholar]
- 30.Gattinoni L et al. (2009) Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med 15 (7), 808–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Graef P et al. (2014) Serial transfer of single-cell-derived immunocompetence reveals stemness of CD8(+) central memory T cells. Immunity 41 (1), 116–26. [DOI] [PubMed] [Google Scholar]
- 32.Hamann D et al. (1997) Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med 186 (9), 1407–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Restifo NP et al. (2012) Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol 12 (4), 269–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abdelsamed HA et al. (2017) Human memory CD8 T cell effector potential is epigenetically preserved during in vivo homeostasis. J Exp Med 214 (6), 1593–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akondy RS et al. (2017) Origin and differentiation of human memory CD8 T cells after vaccination. Nature 552 (7685), 362–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blank CU et al. (2019) Defining ‘T cell exhaustion’. Nat Rev Immunol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wherry EJ (2011) T cell exhaustion. Nat Immunol 12 (6), 492–9. [DOI] [PubMed] [Google Scholar]
- 38.Barber DL et al. (2006) Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439 (7077), 682–7. [DOI] [PubMed] [Google Scholar]
- 39.Day CL et al. (2006) PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443 (7109), 350–4. [DOI] [PubMed] [Google Scholar]
- 40.Petrovas C et al. (2006) PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med 203 (10), 2281–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trautmann L et al. (2006) Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med 12 (10), 1198–202. [DOI] [PubMed] [Google Scholar]
- 42.Hodi FS et al. (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363 (8), 711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Larkin J et al. (2015) Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med 373 (1), 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Topalian SL et al. (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366 (26), 2443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Velu V et al. (2009) Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature 458 (7235), 206–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blackburn SD et al. (2008) Selective expansion of a subset of exhausted CD8 T cells by alphaPD-L1 blockade. Proc Natl Acad Sci U S A 105 (39), 15016–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paley MA et al. (2012) Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science 338 (6111), 1220–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Utzschneider DT et al. (2013) T cells maintain an exhausted phenotype after antigen withdrawal and population reexpansion. Nat Immunol 14 (6), 603–10. [DOI] [PubMed] [Google Scholar]
- 49.Youngblood B et al. (2011) Chronic virus infection enforces demethylation of the locus that encodes PD-1 in antigen-specific CD8(+) T cells. Immunity 35 (3), 400–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pauken KE et al. (2016) Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD-1 blockade. Science 354 (6316), 1160–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sen DR et al. (2016) The epigenetic landscape of T cell exhaustion. Science 354 (6316), 1165–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blank C et al. (2019) Defining T cell exhaustion. Nat Rev Immunol in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Im SJ et al. (2016) Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature 537 (7620), 417–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leong YA et al. (2016) CXCR5(+) follicular cytotoxic T cells control viral infection in B cell follicles. Nat Immunol 17 (10), 1187–96. [DOI] [PubMed] [Google Scholar]
- 55.Utzschneider DT et al. (2016) T Cell Factor 1-Expressing Memory-like CD8(+) T Cells Sustain the Immune Response to Chronic Viral Infections. Immunity 45 (2), 415–27. [DOI] [PubMed] [Google Scholar]
- 56.Wu T et al. (2016) The TCF1-Bcl6 axis counteracts type I interferon to repress exhaustion and maintain T cell stemness. Sci Immunol 1 (6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.He R et al. (2016) Follicular CXCR5- expressing CD8(+) T cells curtail chronic viral infection. Nature 537 (7620), 412–428. [DOI] [PubMed] [Google Scholar]
- 58.Wieland D et al. (2017) TCF1(+) hepatitis C virus-specific CD8(+) T cells are maintained after cessation of chronic antigen stimulation. Nat Commun 8, 15050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alfei F et al. (2019) TOX reinforces the phenotype and longevity of exhausted T cells in chronic viral infection. Nature 571 (7764), 265–269. [DOI] [PubMed] [Google Scholar]
- 60.Khan O et al. (2019) TOX transcriptionally and epigenetically programs CD8(+) T cell exhaustion. Nature 571 (7764), 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seo H et al. (2019) TOX and TOX2 transcription factors cooperate with NR4A transcription factors to impose CD8(+) T cell exhaustion. Proc Natl Acad Sci U S A 116 (25), 12410–12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leseva M et al. (2015) Erase-Maintain-Establish: Natural Reprogramming of the Mammalian Epigenome. Cold Spring Harb Symp Quant Biol 80, 155–63. [DOI] [PubMed] [Google Scholar]
- 63.Messerschmidt DM et al. (2014) DNA methylation dynamics during epigenetic reprogramming in the germline and preimplantation embryos. Genes Dev 28 (8), 812–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gray SM et al. (2017) Polycomb Repressive Complex 2-Mediated Chromatin Repression Guides Effector CD8(+) T Cell Terminal Differentiation and Loss of Multipotency. Immunity 46 (4), 596–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.He S et al. (2017) Ezh2 phosphorylation state determines its capacity to maintain CD8(+) T memory precursors for antitumor immunity. Nat Commun 8 (1), 2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pace L et al. (2018) The epigenetic control of stemness in CD8(+) T cell fate commitment. Science 359 (6372), 177–186. [DOI] [PubMed] [Google Scholar]
- 67.Youngblood B et al. (2017) Effector CD8 T cells dedifferentiate into long-lived memory cells. Nature 552 (7685), 404–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brummelman J et al. (2018) High-dimensional single cell analysis identifies stem-like cytotoxic CD8(+) T cells infiltrating human tumors. J Exp Med 215 (10), 2520–2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miller BC et al. (2019) Subsets of exhausted CD8(+) T cells differentially mediate tumor control and respond to checkpoint blockade. Nat Immunol 20 (3), 326–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Siddiqui I et al. (2019) Intratumoral Tcf1(+)PD-1(+)CD8(+) T Cells with Stem-like Properties Promote Tumor Control in Response to Vaccination and Checkpoint Blockade Immunotherapy. Immunity 50 (1), 195–211 e10. [DOI] [PubMed] [Google Scholar]
- 71.Sade-Feldman M et al. (2018) Defining T Cell States Associated with Response to Checkpoint Immunotherapy in Melanoma. Cell 175 (4), 998–1013 e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kratchmarov R et al. (2018) TCF1 expression marks self-renewing human CD8(+) T cells. Blood Adv 2 (14), 1685–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang Y et al. (2019) The Transcription Factor TCF1 Preserves the Effector Function of Exhausted CD8 T Cells During Chronic Viral Infection. Front Immunol 10, 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rosenberg SA et al. (1988) Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med 319 (25), 1676–80. [DOI] [PubMed] [Google Scholar]
- 75.Gattinoni L et al. (2006) Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol 6 (5), 383–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rosenberg SA et al. (1994) Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin 2. J Natl Cancer Inst 86 (15), 1159–66. [DOI] [PubMed] [Google Scholar]
- 77.Gattinoni L et al. (2005) Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest 115 (6), 1616–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chapuis AG et al. (2013) Transferred WT1-Reactive CD8+ T Cells Can Mediate Antileukemic Activity and Persist in Post-Transplant Patients. Sci Transl Med 5 (174), 174ra27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xu Y et al. (2014) Closely related T-memory stem cells correlate with in vivo expansion of CAR.CD19-T cells and are preserved by IL-7 and IL-15. Blood 123 (24), 3750–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fraietta JA et al. (2018) Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pilipow K et al. (2018) Antioxidant metabolism regulates CD8+ T memory stem cell formation and antitumor immunity. JCI Insight 3 (18). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sabatino M et al. (2016) Generation of clinical-grade CD19-specific CAR-modified CD8+ memory stem cells for the treatment of human B-cell malignancies. Blood. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cieri N et al. (2013) IL-7 and IL-15 instruct the generation of human memory stem T cells from naive precursors. Blood 121 (4), 573–84. [DOI] [PubMed] [Google Scholar]
- 84.Zanon V et al. (2017) Curtailed T-cell activation curbs effector differentiation and generates CD8(+) T cells with a naturally-occurring memory stem cell phenotype. Eur J Immunol 47 (9), 1468–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kondo T et al. (2017) Notch-mediated conversion of activated T cells into stem cell memory-like T cells for adoptive immunotherapy. Nat Commun 8, 15338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mousset CM et al. (2018) Ex vivo AKT-inhibition facilitates generation of polyfunctional stem cell memory-like CD8(+) T cells for adoptive immunotherapy. Oncoimmunology 7 (10), e1488565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kagoya Y et al. (2016) BET bromodomain inhibition enhances T cell persistence and function in adoptive immunotherapy models. J Clin Invest. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ghoneim HE et al. (2017) De Novo Epigenetic Programs Inhibit PD-1 Blockade-Mediated T Cell Rejuvenation. Cell 170 (1), 142–157 e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jadhav RR et al. (2019) Epigenetic signature of PD-1+ TCF1+ CD8 T cells that act as resource cells during chronic viral infection and respond to PD-1 blockade. Proc Natl Acad Sci U S A 116 (28), 14113–14118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Philip M et al. (2017) Chromatin states define tumour-specific T cell dysfunction and reprogramming. Nature 545 (7655), 452–456. [DOI] [PMC free article] [PubMed] [Google Scholar]