Abstract
Humans will naturally synchronize their posture to the motion of a visual surround, but it is unclear if this visuomotor entrainment can be attenuated with an increased sensitivity to somatosensory information. Sub-threshold vibratory noise applied to the Achilles tendons has proven to enhance ankle proprioception through the phenomenon of stochastic resonance. Our purpose was to compare visuomotor entrainment during walking and standing, and to understand how this entrainment might be attenuated by applying sub-threshold vibratory noise over the Achilles tendons. We induced visuomotor entrainment during standing and treadmill walking for ten subjects (24.5 ± 2.9 years) using a speed-matched virtual hallway with continuous mediolateral perturbations at three different frequencies. Vibrotactile motors over the Achilles tendons provided noise (0-400 Hz) with an amplitude set to 90% of each participant’s sensory threshold. Mediolateral pelvic and head motion was greatly amplified (4-8x on average) at the perturbation frequencies during walking, but was much less pronounced during standing. During walking, individuals with greater mediolateral head motion at the fastest perturbation frequency saw the greatest attenuation of that motion with applied noise. Similarly, during standing, individuals who exhibited greater postural sway (as measured by the center of pressure) also saw the greatest reductions in sway with subthreshold noise applied in three of our summary metrics. Our results suggest that, at least for healthy young adults, sub-threshold vibratory noise over the Achilles tendons can slightly improve postural control during disruptive mediolateral visual perturbations, but the applied noise does not substantially attenuate visuomotor entrainment during walking or standing.
Keywords: Gait, Balance, Stochastic resonance, Optical Flow, Virtual Reality, Sub-Threshold Vibration
Introduction
Visuomotor entrainment is the process where motor responses instinctively synchronize to visual stimuli. For example, sacrum and trunk kinematics during walking will entrain to the mediolateral (ML) perturbations of a virtual reality environment (Franz, Francis, Allen, O’Connor, & Thelen, 2015). This entrainment is readily seen for a broad range and combination of perturbation frequencies that would be difficult to synchronize with consciously (Franz, Francis, Allen, & Thelen, 2017). Visuomotor entrainment also occurs when standing (Dijkstra, Schoner, Giese, & Gielen, 1994; van Asten, Gielen, & van der Gon, 1988), and we may expect that entrainment of head kinematics to a perturbed virtual reality environment when walking would be similar to entrainment when standing. It is hypothesized that this entrainment arises from postural regulation of the head and trunk to unify the motion sensed using visual feedback (i.e. optical flow) with the motion sensed using somatosensory and vestibular feedback (Stokes, Thompson, & Franz, 2017). Accordingly, visuomotor entrainment is more pronounced when individuals place a premium on visual feedback to maintain balance when walking. For example, older adults exhibit increased reliance on vision to maintain balance, likely compensating for diminished somatosensory feedback from the periphery (Francis, Franz, O ‘connor, & Thelen, 2015). As a result, older adults exhibit both increased gait variability and increased entrainment to visual perturbations (Franz et al., 2015). Thus, it may be feasible to affect entrainment by manipulating the relative weighting of visual and somatosensory information during walking.
Sub-threshold vibratory noise has shown promise to increase sensitivity to somatosensory information. This utilizes a phenomenon called “stochastic resonance”, whereby low-amplitude noise increases the likelihood that weak signals will exceed a sensory threshold and thus improves detection of small sensory fluctuations (J J Collins, Imhoff, & Grigg, 1996; Priplata, Niemi, Harry, Lipsitz, & Collins, 2003). For example, white noise tendon vibration can increase the sensitivity of afferent firing rates in the Golgi tendon organs and the adjoining muscle spindles (Cordo et al., 1996; Fallon, Carr, & Morgan, 2004). Further, ankle proprioception can be significantly improved by applying sub-threshold vibratory noise over the Achilles tendon (Ribot-Ciscar, Hospod, & Aimonetti, 2013). Thus, investigators have considered the possible influence of noise applied to the Achilles tendon to improve standing balance, as has been shown for noise applied to the bottom of the feet (James J. Collins et al., 2003). For example, Borel & Ribot-Ciscar found that the spectral power density of postural sway significantly decreased with an optimum level of noise at the Achilles tendons, but found no reduction in the sway area (Borel & Ribot-Ciscar, 2016). Sacco et al also found that no level of noise could consistently reduce the velocity of sway during quiet standing, but could improve the accuracy of postural positioning (Sacco, Gaffney, & Dean, 2017). However, we may better detect improvements due to applied noise at the Achilles tendons under conditions where visual information is conflicting, such as during ML visual perturbations, which may increase the reliance on somatosensory information to maintain balance. We may also see improvements during gait, as sub-threshold vibratory noise at the bottom of the feet has also shown to decrease gait variability (Aboutorabi, Arazpour, Bahramizadeh, Farahmand, & Fadayevatan, 2017; Galica et al., 2009; Stephen et al., 2012). These past improvements with applied noise likely arise from increased sensitivity to somatosensory information, which may also reduce the reliance on visual information to maintain balance (Dettmer, Pourmoghaddam, Lee, & Layne, 2015; Kabbaligere, Lee, & Layne, 2017).
The purpose of this study was to compare visuomotor entrainment during walking and standing, and to understand how this entrainment might be attenuated by applying sub-threshold vibratory noise over the Achilles tendons. As such, we measured kinematics for healthy young adults walking and standing in a virtual reality environment with ML visual perturbations. We first hypothesized that ML motion during walking and standing would be amplified at the driving frequencies of the ML visual perturbations. We also hypothesized that ML visual perturbations would increase gait variability and postural sway. Finally, we hypothesized that applying sub-threshold vibratory noise to the Achilles tendons would diminish the effect of ML visual perturbations during walking and standing.
Methods
Participants
Ten healthy young adults participated in the study (mean ± standard deviation, age: 24.5 ± 2.9 yrs, mass: 74.7 ± 14.1 kg, height: 1.73 ± 0.12 m, 5 female). The self-reported inclusion criteria were: 1) 18-30 years old; 2) Body mass index (BMI) < 30; 3) no current or prior orthopedic disorders affecting the lower limbs (such as bone fracture, ligament/tendon injury, arthritis, prosthetic); 4) no neurological, visual, vestibular, or balance disorders; 5) able to walk comfortably and unassisted for at least twenty consecutive minutes; 6) participates in mild-to-moderate exercise at least three times a week; and 7) no medication that may cause dizziness or affect neurological function. Participants wore their own shoes during the experiment. The experimental protocol was approved by the University of Wisconsin-Madison Health Sciences Institutional Review Board, and each subject provided written informed consent before participating in the study.
Sub-Threshold Vibratory Noise
We positioned a pair of voice coil vibrotactile motors (Haptuator BM3C, Tactile Labs Inc., Montreal, QC) bilaterally over the Achilles tendons in line with the malleoli. These were mounted in 3D-printed housings and held in place using Coban wrap. To drive the motors, we generated white noise at 2 kHz in LabView (v16.0, National Instruments, Austin, TX), low-pass filtered the noise signals to 400 Hz (Borel & Ribot-Ciscar, 2016), and then input the noise signals to a pair of audio amplifiers (LP-2024A+, Lepy Audio).
We determined a participant’s vibratory perception threshold on each side while standing with eyes open in a neutral position with equal weight on each foot. We first set the vibration level to maximum and gradually decreased the amplitude until the participant could no longer detect the vibration. Then we set the vibration level to zero and gradually increased the amplitude until the participant could first detect the vibration. We repeated finding the ascending and descending thresholds, and then took the average of the four amplitudes as the threshold of vibratory perception. We confirmed that this amplitude was at the threshold by checking that the subject could feel vibration at 110% of this amplitude and could not feel vibration at 90% of this amplitude, but was uncertain at the threshold amplitude (Miranda et al., 2016). We then set the amplitude of each motor to 90% of the determined threshold amplitude to ensure subsensory stimulation (Fig. 1D) (Stephen et al., 2012). At this amplitude, participants were blind to whether the vibration (i.e. vibratory noise) was on or off, and no participants reported being able to feel any vibration during the experimental protocol.
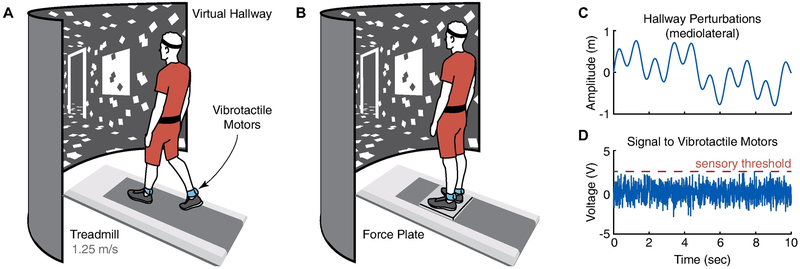
Figure 1.
During the walking trials, subjects faced a virtual reality hallway that progressed forward at the same speed as the treadmill (A). During the standing trials, subjects stood on a force plate and the hallway did not progress forward (B). To elicit mediolateral visuomotor entrainment, we experimentally perturbed the foreground of the virtual hallway. Here, the foreground swayed mediolaterally as the sum of three sinusoidal driving frequencies (0.10, 0.31, and 0.96 Hz) with prescribed amplitudes of 0.326 m (C). To increase sensitivity to ankle somatosensory information, we applied sub-threshold vibratory noise using a pair of vibrotactile motors placed over the Achilles tendons (D).
Experimental Protocol
We first determined each subject’s preferred overground walking speed as the average of two times taken to traverse the middle 4 m of a 6 m walkway. Participants then completed all walking trials on a treadmill at a fixed speed of 1.25 m/s, which was near the average preferred overground speed (1.34 ± 0.22 m/s). During these trials, a speed-matched virtual reality hallway was rear-projected onto a semicircular screen surrounding the front of the treadmill (height = 2.7 m, radius = 1 m)(Fig. 1 A), as described in previous publications (Acuña, Francis, Franz, & Thelen, 2019; Francis et al., 2015). We instructed subjects to simply look forward as if walking down an actual hallway, and to not fix their gaze on any point peripheral to the screen. Mediolateral perturbations to the virtual hallway were designed to elicit visuomotor entrainment in the mediolateral direction and increase gait variability (Franz et al., 2017; Thompson & Franz, 2017). When perturbed, the foreground of the virtual hallway would sway mediolaterally, while the projected end of the hallway remained stationary (i.e. provoking corrections to mediolateral position rather than corrections to heading). The perturbations swayed according to
where A prescribed an amplitude of 0.326 m and f described a base frequency of 0.10 Hz (Fig. 1C). We chose 3.1 as the scalar multiplier B to promote signal complexity that would be difficult to anticipate. Thus, the perturbed virtual hallway had driving frequencies of 0.10, 0.31, and 0.96 Hz with a maximum amplitude of 0.978 m. In order to minimize any adaptation effects immediately after being presented with a new walking condition, we provided time (at least 30 sec) for each subject to familiarize themselves with each condition before collecting data.
Each walking trial lasted three minutes providing ~300 steps per trial. In random order, subjects walked while viewing the virtual hallway with and without continuous ML perturbations. For half of these trials, we also applied sub-threshold vibratory noise. Each condition was repeated twice, for a total of eight trials. Upon completion of the walking trials, we positioned a low-profile balance plate (Legacy Balance Plate, Bertec Corp., Columbus, OH) over the center of the treadmill approximately where the subjects were walking. We instructed subjects to stand quietly on the force plate while facing the screen, feet placed shoulder-width apart, with their hands held in a neutral position along the vertical body axis (Fig. 1B).
In random order, subjects stood while viewing the virtual hallway with and without continuous ML perturbations. For half of these trials, we also applied sub-threshold vibratory noise. Each standing trial lasted 32 seconds, and each condition was repeated three times, for a total of eighteen trials. The perturbations to the virtual hallway were the same as during the walking trials, except the virtual hallway did not progress forward. To rule out the effect of fatigue, we required participants to step off the platform after each trial for ~20 seconds. Note that due to practical constraints involved with setting up the experiment, we did not randomize between the standing and walking trials, but we did provide subjects an opportunity to rest between these testing segments.
Measurement and Analysis
A motion capture system (Optotrak Certus, Northern Digital Inc., Waterloo, ON) recorded the 3D positions of markers placed over the back of the head, the C7 vertebra, the sacrum, and both heels at 100 Hz. Marker trajectories were first low-pass filtered at 12 Hz using a fourth-order, zero-lag Butterworth filter. We then used spectral analysis to quantify entrainment to the perturbation frequencies in the mediolateral trajectories of the head, C7, and sacrum markers (Hypotheses 1 and 3). Specifically, we computed the fast Fourier transform (FFT) on these data and extracted the magnitude of ML motion at the three perturbation frequencies (Franz et al., 2017). To improve our spectral resolution, we concatenated the marker trajectory time series for repeated trial conditions. This provided six minutes of concatenated kinematic data for each walking condition and ninety-six seconds of concatenated kinematic data for each standing condition.
We quantified gait variability both temporally and spatially (Hypotheses 2 and 3). To do this, we first identified heel contact from minimums in the vertical heel displacements (Desailly, Daniel, Sardain, & Lacouture, 2009) and divided each walking trial into a series of gait cycles. We then constructed time series of stride times and step widths. Stride time was measured for both legs. Step widths were constructed from the distance between the left and right heel markers over consecutive steps. Here, we defined step width as the mediolateral distance between heel marker positions averaged during midstance. Variability was described by the standard deviations of stride time and step width for each condition.
We quantified postural sway by measuring the motion of the net center of pressure (COP). For each standing trial, we acquired (1000 Hz) the COP from the balance plate, and then we low-pass filtered the COP data at 10 Hz using a fourth-order, zero-lag Butterworth filter (Baig, Dansereau, Chan, Remaud, & Bilodeau, 2012). We centered the mean COP at zero in both the mediolateral (ML) and anteroposterior (AP) directions. We then measured the overall amount of postural sway using five conventional measures: 1) the area covered by the COP displacement (defined as an ellipse containing 95% of the COP trajectory), 2) the mean velocity of the COP trajectory, 3) the mean radial displacement of the COP from center, 4) the standard deviation of the COP in the AP direction, and 5) the standard deviation of the COP in the ML direction (Baig et al., 2012; Duarte, Freitas, & Zatsiorsky, 2011; Schubert, Kirchner, Schmidtbleicher, & Haas, 2012). For each condition, we used the average measure of the three repetitions in our analyses. We also quantified entrainment of the COP displacement to the perturbation frequencies in the same manner as the marker trajectories (i.e. by extracting amplitudes of the FFT from concatenated COP data). We analyzed all kinematic and balance plate data using MATLAB (R2017a, MathWorks Inc., Natick, MA).
Statistical Analysis
We used a two-way factorial repeated measures analysis of variance (rmANOVA) to test for main effects of and interactions between sub-threshold vibratory noise (noise off, on) and virtual hallway conditions (ML perturbations off, on) on our measures of entrainment, gait variability, and postural sway. To further explore the effects of visual perturbations when walking, we performed an additional two-way rmANOVA to identify significant differences in ML motion by marker location (head, C7, and sacrum) and by frequency (0.10, 0.31, 0.96 Hz). When we found significant main effects during the rmANOVAs, we performed post-hoc pairwise comparisons using paired-samples t-tests. To provide context, we report effect sizes from the rmANOVAs as partial eta squared ()
Prior studies have argued that comparisons of mean values may not sufficiently assess changes due to sub-threshold vibratory noise because these changes may depend on an individual’s baseline performance without noise (Borel & Ribot-Ciscar, 2016; Kelty-Stephen & Dixon, 2013; Stephen et al., 2012). Thus, we explored this possibility as a post-hoc analysis of our data. Using Pearson’s correlations, we examined individual differences only for the outcome measures that were significantly affected by the ML hallway perturbations. Here, we evaluated the relationship between performance without noise to the corresponding changes in performance with noise. In this analysis, we did not include motion at the C7 or sacrum markers.
We performed all the statistical analyses using SPSS (v.25, IBM Corp., Armonk, NY), and defined significance as p < 0.05.
Results
Evidence for Visuomotor Entrainment during Walking and Standing
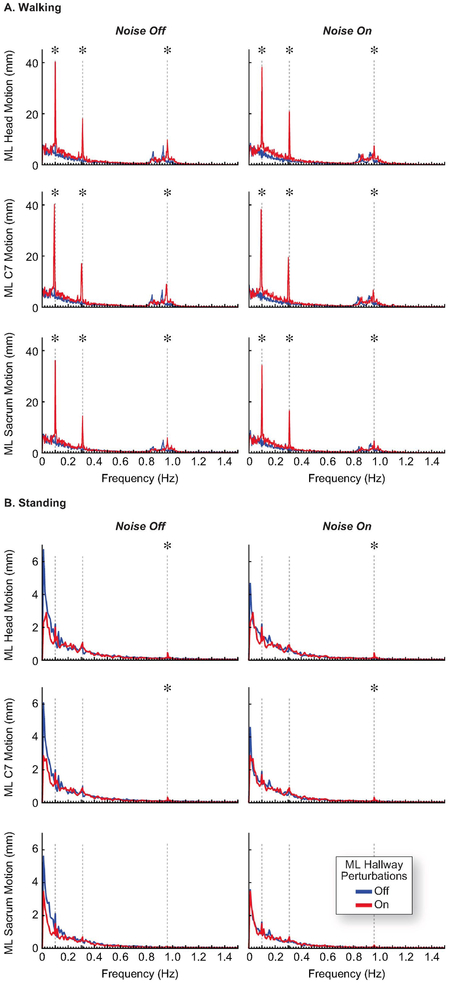
We found evidence of ML entrainment to each of the three perturbation frequencies for subjects walking in the perturbed virtual reality environment (Fig. 2A). The two-way rmANOVA revealed significant main effects of the virtual hallway perturbations on the magnitude of ML motion at hallway perturbation frequencies (Table 1). The increase in ML motion of the head, C7, and sacrum was substantial, averaging 4-8 times greater motion than when walking in the virtual hallway without perturbations. When walking with ML hallway perturbations, the magnitude of ML motion varied by anatomical location (p < 0.001, ) and perturbation frequency (p < 0.001, ). Independent of perturbation frequency, ML motion at the head was +5% greater than ML motion at the C7 (p= 0.001). ML motion at the C7 was +15% greater than ML motion at the sacrum (p = 0.009). Independent of marker location, ML motion at 0.10 Hz was +110% greater than ML motion at 0.31 Hz (p = 0.004). ML motion at 0.31 Hz was +143% greater than ML motion at 0.96 Hz (p = 0.004).
Figure 2.
Visuomotor entrainment to the driving frequencies of the perturbed virtual hallway was more pronounced during walking (A) than during standing (B). The entrainment we observed in both walking and standing was not significantly affected by sub-threshold vibratory noise. Data are plotted as the group average spectrum of mediolateral motion, and vertical dashed lines indicate the driving frequencies of the perturbed virtual hallway (0.10, 0.31, and 0.96 Hz). At these frequencies, asterisks indicate a significant difference between hallway conditions.
Table 1.
Group mean (± standard deviation) values of the magnitude of mediolateral motion at the perturbation frequencies of the virtual hallway. All units are in mm.
| Hallway Perturbations Off |
Hallway Perturbations On |
Main Effect of Perturbations |
Main Effect of Noise |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Task | Marker Location |
Perturbation Frequency |
Noise Off |
Noise On |
Noise Off | Noise On | p | p | ||
| Walking | Head | 0.10 | 7.6 (2.5) | 5.6 (2.0) | 40.2 (15.0) | 38.2 (14.2) | <0.001 | 0.874 | 0.168 | 0.224 |
| 0.31 | 2.3 (0.7) | 2.7 (1.1) | 18.1 (7.3) | 20.7 (7.5) | < 0.001 | 0.903 | 0.370 | 0.102 | ||
| 0.96 | 1.6 (1.3) | 1.9 (1.3) | 9.7 (6.8) | 7.4 (4.2) | 0.003 | 0.682 | 0.329 | 0.119 | ||
| C7 | 0.1 | 7.5 (2.5) | 5.5 (1.8) | 39.0 (14.4) | 36.9 (13.8) | < 0.001 | 0.871 | 0.145 | 0.245 | |
| 0.31 | 2.2 (0.7) | 2.4 (0.9) | 17.0 (7.0) | 19.5 (7.1) | < 0.001 | 0.901 | 0.390 | 0.094 | ||
| 0.96 | 1.5 (1.5) | 1.9 (1.3) | 9.0 (6.4) | 6.8 (4.6) | 0.006 | 0.627 | 0.333 | 0.117 | ||
| Sacrum | 0.10 | 6.6 (2.2) | 4.9 (1.6) | 36.1 (14.0) | 34.2 (13.6) | < 0.001 | 0.849 | 0.130 | 0.262 | |
| 0.31 | 2.0 (0.7) | 2.1 (0.7) | 14.5 (6.5) | 16.4 (6.1) | < 0.001 | 0.867 | 0.415 | 0.085 | ||
| 0.96 | 1.1 (0.9) | 1.6 (1.3) | 6.0 (5.4) | 4.7 (4.0) | 0.031 | 0.462 | 0.353 | 0.108 | ||
| Standing | Head | 0.10 | 2.2 (1.5) | 2.2 (0.8) | 2.0 (1.0) | 2.0 (0.8) | 0.613 | 0.030 | 0.983 | 0.000 |
| 0.31 | 1.1 (0.6) | 0.9 (0.6) | 1.2 (0.6) | 1.0 (0.3) | 0.316 | 0.111 | 0.130 | 0.424 | ||
| 0.96 | 0.1 (0.1) | 0.1 (0.1) | 0.3 (0.2) | 0.3 (0.2) | 0.016 | 0.496 | 0.201 | 0.175 | ||
| C7 | 0.10 | 2.0 (1.5) | 1.9 (0.8) | 1.8 (0.9) | 1.7 (0.8) | 0.578 | 0.036 | 0.341 | 0.101 | |
| 0.31 | 1.0 (0.5) | 0.8 (0.5) | 1.0 (0.6) | 0.9 (0.4) | 0.397 | 0.081 | 0.151 | 0.360 | ||
| 0.96 | 0.1 (0.0) | 0.1 (0.1) | 0.2 (0.2) | 0.2 (0.2) | 0.031 | 0.421 | 0.766 | 0.010 | ||
| Sacrum | 0.10 | 2.1 (1.4) | 1.6 (0.5) | 1.5 (0.7) | 1.5 (0.7) | 0.379 | 0.098 | 0.238 | 0.169 | |
| 0.31 | 0.7 (0.3) | 0.7 (0.5) | 0.7 (0.3) | 0.7 (0.2) | 0.877 | 0.003 | 0.702 | 0.019 | ||
| 0.96 | 0.1 (0.1) | 0.1 (0.0) | 0.1 (0.1) | 0.2 (0.1) | 0.282 | 0.143 | 0.878 | 0.003 | ||
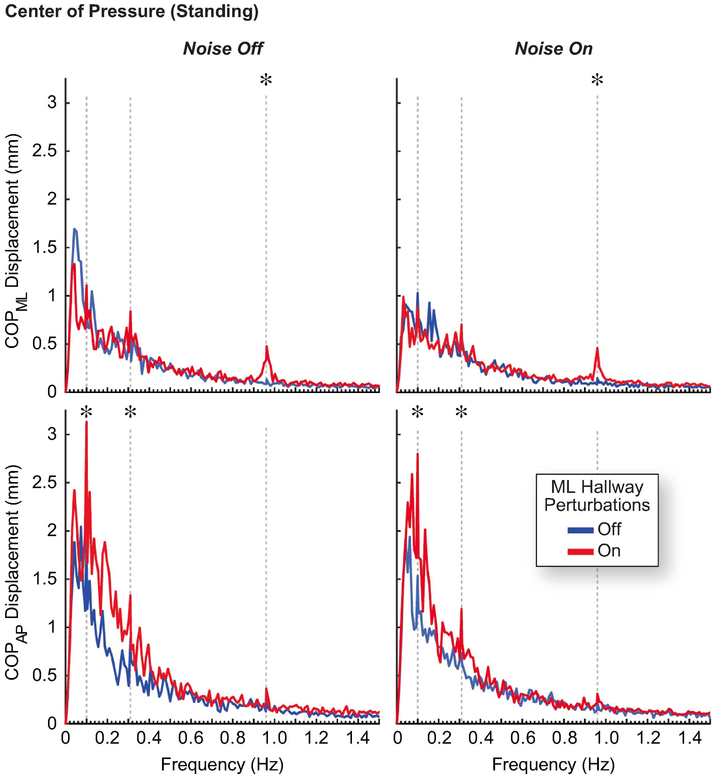
The effect of hallway perturbations on ML kinematics was not as pronounced during standing as compared to walking (Table 1, Fig. 2B). We saw a significant but small (i.e. < 1 mm) increase in ML head (+98% on average, p = 0.016) and C7 (+113% on average, p = 0.031) motion for only the 0.96 Hz perturbation frequency. We also saw an increase in the ML COP displacement at the 0.96 Hz perturbation frequency (+239% on average, p = 0.008)(Table 2, Fig. 3). We found evidence of visuomotor entrainment in the AP COP displacement to the 0.10 Hz and 0.31 Hz perturbation frequencies. However, there was a general increase in displacement for many frequencies below 0.5 Hz, including the 0.10 and 0.31 Hz perturbation frequencies, such that the amplified displacement at these perturbation frequencies did not appear as distinct peaks in the spectral plots.
Table 2.
Group mean (± standard deviation) values of the magnitude of center of pressure (COP) displacement at the perturbation frequencies of the virtual hallway. All units are in mm.
| Hallway Perturbations Off |
Hallway Perturbations On |
Main Effect of Perturbations |
Main Effect of Noise |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Direction | Perturbation Frequency |
Noise Off | Noise On | Noise Off | Noise On | p | p | ||
| ML | 0.10 | 1.1 (1.0) | 1.0 (0.4) | 1.1 (0.5) | 0.9 (0.6) | 0.751 | 0.012 | 0.212 | 0.167 |
| 0.31 | 0.7 (0.4) | 0.6 (0.4) | 0.8 (0.4) | 0.7 (0.3) | 0.155 | 0.211 | 0.277 | 0.130 | |
| 0.96 | 0.1 (0.1) | 0.1 (0.1) | 0.5 (0.3) | 0.5 (0.4) | 0.008 | 0.561 | 0.840 | 0.005 | |
| AP | 0.10 | 1.9 (1.3) | 1.5 (0.8) | 3.1 (2.5) | 2.8 (1.5) | 0.018 | 0.483 | 0.310 | 0.114 |
| 0.31 | 0.9 (0.4) | 0.8 (0.5) | 1.3 (0.8) | 1.2 (0.7) | 0.006 | 0.589 | 0.301 | 0.118 | |
| 0.96 | 0.3 (0.1) | 0.2 (0.1) | 0.4 (0.2) | 0.3 (0.1) | 0.098 | 0.275 | 0.088 | 0.289 | |
Abbreviations: ML = mediolateral; AP = anteroposterior.
Figure 3:
Subjects entrained their ML center of pressure to the 0.96 Hz driving frequency while watching the perturbed virtual hallway, and this entrainment was not significantly affected by sub-threshold vibratory noise. We found evidence of visuomotor entrainment in the AP center of pressure to the 0.10 Hz and 0.31 Hz driving frequencies, although there was a general increase in displacement for many frequencies below 0.5 Hz. Data are plotted as the group average spectrum of center of pressure (COP) trajectories, and vertical dashed lines indicate the driving frequencies of the perturbed virtual hallway (0.10, 0.31, and 0.96 Hz). At these frequencies, asterisks indicate a significant difference between hallway conditions.
Effect of Visual Perturbations on Gait Variability and Postural Sway
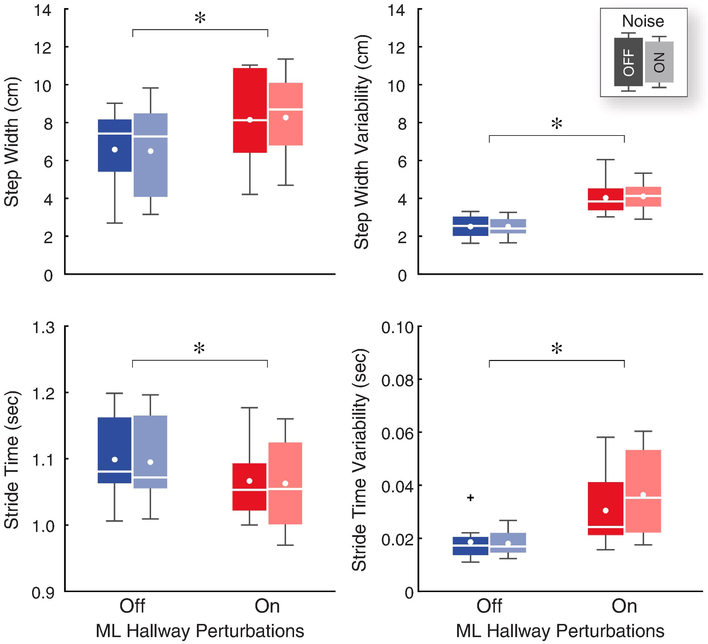
The two-way rmANOVA revealed significant main effects of the virtual hallway perturbations on step width, stride time, and their respective variabilities (Table 3). Here, walking while watching the perturbed virtual hallway significantly increased step width (+25% on average, p < 0.001) and decreased stride time (−3% on average, p = 0.005)(Fig. 4). We saw a significant increase in step width variability (+62% on average, p < 0.001) and stride time variability (+83% on average, p = 0.003).
Table 3.
Group mean (± standard deviation) values for our measures of gait variability and postural sway.
| Hallway Perturbations Off |
Hallway Perturbations On |
Main Effect of Perturbations |
Main Effect of Noise |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Measure | Units | Noise Off | Noise On | Noise Off | Noise On | p | p | ||
| Step Width | cm | 6.5 (2.0) | 6.5 (2.3) | 8.1 (2.4) | 8.3 (2.0) | <0.001 | 0.851 | 0.546 | 0.042 |
| Step Width Variability | cm | 2.6 (0.6) | 2.5 (0.5) | 4.0 (0.9) | 4.1 (0.7) | <0.001 | 0.862 | 0.505 | 0.051 |
| Stride Time | sec | 1.10 (0.07) | 1.09 (0.07) | 1.07 (0.06) | 1.06 (0.07) | 0.005 | 0.641 | 0.357 | 0.107 |
| Stride Time Variability | sec | 0.018 (0.007) | 0.018 (0.005) | 0.030 (0.014) | 0.036 (0.017) | 0.003 | 0.692 | 0.284 | 0.141 |
| Area of 95% Ellipse | cm2 | 3.7 (5.8) | 2.8 (2.5) | 4.9 (4.3) | 3.7 (4.0) | 0.120 | 0.084 | 0.082 | 0.104 |
| Mean Velocity | mm/s | 10.8 (4.3) | 10.6 (3.6) | 14.5 (6.4) | 12.5 (3.5) | <0.001 | 0.733 | 0.104 | 0.091 |
| Mean Radial Displacement | mm | 5.3 (3.9) | 4.7 (2.0) | 6.6 (3.4) | 5.7 (2.7) | 0.009 | 0.218 | 0.046 | 0.135 |
| Std. Dev. Of COPAP | mm | 4.8 (2.1) | 4.7 (2.2) | 7.0 (4.0) | 6.0 (2.9) | <0.001 | 0.519 | 0.082 | 0.104 |
| Std. Dev. Of COPML | mm | 3.5 (4.4) | 2.9 (1.3) | 3.3 (1.6) | 2.8 (1.5) | 0.775 | 0.003 | 0.195 | 0.059 |
Figure 4.
Gait variability significantly increased while watching the perturbed virtual hallway. We found no significant effects due to the sub-threshold vibratory noise. The middle lines of each box plot represent the medians, and the dots represent the group means. Single asterisks (*) indicate significant differences between hallway conditions. Plus signs (+) indicate outliers.
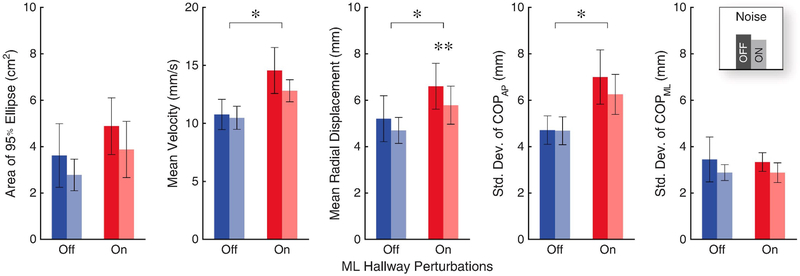
Standing while watching the perturbed virtual hallway significantly increased subjects’ overall postural sway, as measured by the center of pressure (Fig. 5). The two-way rmANOVA revealed significant main effects of the virtual hallway perturbations on mean velocity, mean radial displacement, and the standard deviation of the COPAP, but not for the area or the standard deviation of the COPML (Table 3). Specifically, we observed an average increase of +34% in sway velocity (p < 0.001), and an average increase of +25% in the mean radial displacement of the COP (p = 0.050). We also saw a significant increase in the standard deviation of COPAP (+47% on average, p < 0.001).
Figure 5.
Postural sway significantly increased while watching the perturbed virtual hallway. When we applied sub-threshold vibratory noise during these perturbations, we found a significant decrease in the mean center of pressure (COP) radial displacement. Data are plotted as group averages with errors bars representing the standard error. Asterisks (*) indicate significant differences between hallway conditions. Double asterisks (**) indicate significant differences with applied noise found during post-hoc comparisons.
Effect of Sub-Threshold Vibratory Noise
Our two-way rmANOVA did not reveal any significant main effects of sub-threshold vibratory noise on the magnitude of ML motion at the perturbation frequencies (Table 1). We also saw no significant main effects of noise on the magnitude of COP displacement at the perturbation frequencies (Table 2, Fig. 3). We found no significant main effects of noise on step width, stride time, or their respective variabilities (Table 3, Fig. 4). We did not find significant interaction effects between noise and the virtual hallway conditions on the magnitude of ML motion or our measures of gait variability.
On average, our metrics of postural sway decreased when we applied sub-threshold vibratory noise (Fig. 5). We found a significant main effect of noise on the mean radial displacement of the COP (p = 0.046) (Table 3), and main effects approaching significance for the area of the 95% ellipse (p = 0.082) and the standard deviation of the center of pressure in the AP direction (p = 0.082). In post-hoc tests, we found that when subjects watched the perturbed virtual hallway, applying noise significantly decreased the mean radial displacement (−14% on average, p = 0.026). We did not find significant main effects of noise for mean velocity or standard deviation in the ML direction. We also did not observe any significant interaction effects between noise and the virtual hallway conditions on our measures of postural sway.
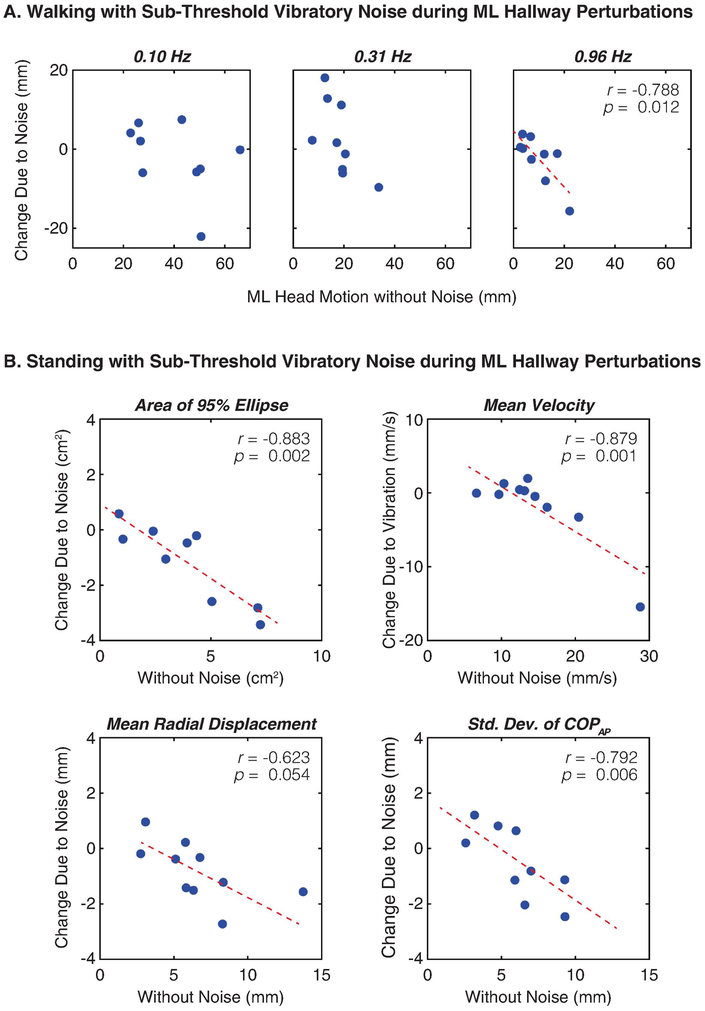
As part of our post-hoc exploration of our data, we found the effect of sub-threshold vibratory noise while watching the perturbed virtual hallway was related to an individual’s baseline performance without noise (Fig. 6). For example, subjects who exhibited greater ML head motion at the 0.96 Hz perturbation frequency during the walking trials generally saw the greatest attenuation in motion at this frequency when we applied noise (p = 0.012). However, we found no significant correlations between baseline performance and change in ML head motion due to noise at the 0.10 or 0.31 Hz perturbation frequencies. We also found no correlation between baseline gait variability and change in gait variability due to noise.
Figure 6.
In a post-hoc exploration of our data, we found the effect of sub-threshold vibratory noise was related to an individual’s performance without noise. (A) During the walking trials, subjects who exhibited greater ML head motion at the 0.96 Hz perturbation frequency generally saw the greatest attenuation when we applied noise. However, we found no significant relationships between baseline performance and change in ML head motion due to noise at the 0.10 or 0.31 Hz perturbation frequencies. (B) During the standing trials, subjects who exhibited greater postural sway generally saw the largest improvements when we applied noise.
During the standing trials, subjects who exhibited greater postural sway generally saw the largest reductions when we applied noise. Specifically, we found significant correlations between baseline performance and change due to noise in sway area (p = 0.002), mean velocity (p = 0.001), and standard deviation of the COP in the AP direction (p = 0.006). The mean radial displacement also exhibited a baseline-dependent relationship, approaching significance (p = 0.054). One subject displayed a remarkable reduction in mean velocity with noise (−15 mm/s), but even without this outlier, the baseline-dependent relationship for mean velocity was still significant (p = 0.029). Despite these relationships for postural sway, we found no significant baseline-dependent correlations for ML head motion or ML COP displacement at the 0.96 Hz perturbation frequency during the standing trials.
Discussion
In this study, we investigated visuomotor entrainment to ML visual perturbations during walking and standing, and explored how this entrainment might be attenuated by applying sub-threshold vibratory noise over the Achilles tendon. As we first hypothesized, we found that ML motion during walking was greatly amplified at the driving frequencies of the ML visual perturbations. We also detected ML entrainment during standing, but this was much less pronounced than it was in walking. Consistent with our second hypothesis, we found ML visual perturbations increased gait variability and postural sway. Our last hypothesis that sub-threshold vibratory noise would diminish the effect of ML visual perturbations was partially supported. We found sub-threshold noise significantly decreased only one measure of postural sway. However, when we examined individual responses to the applied noise, we found that some improvements due to noise were significantly related to an individual’s baseline performance without noise. We conclude that, at least for healthy young adults, sub-threshold vibratory noise over the Achilles tendons can slightly improve postural control during disruptive ML visual perturbations via stochastic resonance, but the increased sensitivity to somatosensory information does not substantially attenuate visuomotor entrainment to these perturbations.
Our finding that ML motion during walking was amplified at the frequencies of the ML visual perturbations is consistent with previous studies on visuomotor entrainment (Franz et al., 2015, 2017; Thompson & Franz, 2017). The synchronization to these frequencies was quite pronounced, with the spectral analysis revealing distinct peaks in ML motion (Fig. 2). We found that the lower perturbation frequencies (0.10 and 0.31 Hz) elicited the greatest increases in ML motion. However, the relationship between the increased motion and perturbation frequency is certainly more complex: depending on frequency, the amplitude of that frequency, and even the age of the subject (Franz et al., 2015, 2017; Thompson, Plummer, & Franz, 2018). Further, we found that amplitude of ML motion at the perturbation frequencies was greater at the head, which was greater than at the C7, which was greater than the sacrum. This supports the notion that visuomotor entrainment arises from postural regulation of the head to unify visual feedback with somatosensory and vestibular feedback. Further, as walking is accompanied by inherently more ML body motion than during standing (Fig. 2), this may explain, in part, greater entrainment during walking than during standing.
ML visuomotor entrainment was much less pronounced when standing. We saw a small amplification in ML head motion, and then only for the highest perturbation frequency (0.96 Hz, Fig. 2B). This entrainment was reflected more clearly in the ML center of pressure (Fig. 3), although the increases in postural sway were driven primarily by changes in AP center of pressure (Fig. 5). We note that we cannot exclude the possibility that the experimental order may be a confounding factor to these observations, as all the standing trials were performed after the walking trials. Indeed, the less pronounced entrainment seen during standing may be influenced by fatigue or habituation to the visual perturbations. However, our findings align with observations by O’Connor and Kuo, who found that postural sway increased more for visual perturbations in the AP direction than the ML direction (O’Connor & Kuo, 2009). Standing has an inherently wider base of support than walking, which may require less active control of ML balance than AP balance during visual perturbations. Accordingly, most studies investigating entrainment to visual perturbations during standing exclusively use perturbations in the AP direction (Baumberger, Isableu, & FlUckiger, 2004; Dijkstra et al., 1994; Dokka, Kenyon, & Keshner, 2009; Greffou, Bertone, Hanssens, & Faubert, 2008; Loughlin & Redfern, 2001). Our findings also suggest that entrainment to ML visual perturbations during standing may be more prominent at a different range of frequencies than entrainment to AP visual perturbations. For example, van Asten et al. found AP visuomotor entrainment was greatest for AP visual perturbations at frequencies between 0.10 and 0.40 Hz (van Asten et al., 1988), but we found no evidence that ML postural sway synchronized with the ML visual perturbations in this perturbation frequency range. However, we would likely see more evidence of ML visuomotor entrainment at lower perturbation frequencies with decreased stance width, such as during tandem standing, which can increase the sensitivity of postural sway to ML visual perturbations (O’Connor & Kuo, 2009).
In partial support of our last hypothesis, we found sub-threshold vibratory noise improved postural sway during disruptive ML visual perturbations. As a group, the improvements we observed were admittedly modest. Although all five average posturography measures decreased when we applied noise, this reduction was only significant for the mean COP radial displacement. We also saw a reduction in the area covered by the center of pressure trajectories, approaching significance. Consistent with two recent studies (Borel & Ribot-Ciscar, 2016; Sacco et al., 2017), and in contrast to a third (Ross, Linens, Wright, & Arnold, 2013), we found no evidence that the applied noise reduced the mean velocity of the COP, which is considered to be the most informative summary metric of COP (Raymakers, Samson, & Verhaar, 2005). Our results follow a common theme found throughout previous studies: sub-threshold vibratory noise at the ankles can improve postural control, but these improvements are generally small, inconsistent, and dependent on the choice of outcome measure (Aboutorabi et al., 2017; Borel & Ribot-Ciscar, 2016; Dettmer et al., 2015; Magalhaes & Kohn, 2012; Ross et al., 2013; Sacco et al., 2017). When we explored individual responses to the applied noise as part of a post-hoc analysis, we found that the subjects who exhibited greater postural sway without noise saw the greatest improvements in their sway when we applied noise (Fig. 6B). Prior studies of postural sway have also observed this baseline-dependent effect of noise (Borel & Ribot-Ciscar, 2016; Kelty-Stephen & Dixon, 2013; Priplata et al., 2006), although caution must be taken to not falsely attribute these changes as a noise effect as opposed to regression to the mean. However, by repeating experimental conditions three times for each subject and presenting conditions in random order, we hope our averaged data better represents true individual differences with noise, although we cannot exclude the possibility that regression to the mean may explain part of this observation. Taken together, our findings suggest we may have achieved a beneficial stochastic resonance effect, whereby the improvements in postural sway arose from increased sensitivity to somatosensory information due to the sub-threshold vibratory noise. This interpretation is consistent with a recent study by Borel and Ribot-Ciscar, who suggest that with increased postural sway there are a greater number of muscle spindles near the sensory threshold, and thus the addition of sub-threshold vibratory noise leads to greater improvement in movement coding and postural performance (Borel & Ribot-Ciscar, 2016).
Our subjects took faster, wider, and more variable steps when walking with ML visual perturbations, as others have shown (Francis et al., 2015; Martelli, Xia, Prado, & Agrawal, 2019; O’Connor & Kuo, 2009; O’Connor, Xu, & Kuo, 2012; Stokes et al., 2017; Thompson & Franz, 2017). However, we did not see an effect of sub-threshold vibratory noise on our measures of gait variability (Fig. 4). Although this study is the first to investigate changes in gait with sub-threshold vibratory noise applied over the Achilles tendons, these results are generally consistent with a prior study applying noise to the bottom of the feet using shoe insoles (Lipsitz et al., 2015). In this study of healthy elderly adults, sub-threshold vibratory noise significantly reduced the area covered by the center of pressure trajectories when standing, but had no effect on step width or step width variability. However, inconsistent with our results, the authors found a small reduction in stride time and stride time variability, which might be explained by the advanced age of their study participants. Advanced age may also explain the results of a similar insole study by Stephen et al., who found that sub-threshold vibratory noise decreased gait variability for elderly subjects who already had more variable gait (Stephen et al., 2012). However, we found no baseline-dependent relationships for our measures of gait variability. Although, we might have seen differences in gait variability for our healthy young adults if we had chosen a more vigorous, fatiguing activity (Miranda et al., 2016).
We were surprised to find that sub-threshold vibratory noise over the Achilles tendons had such a small influence on visuomotor entrainment to the perturbation frequencies of the virtual hallway. ML visual perturbations induce a visual perception of lateral motion, thus creating conflict between visual feedback and somatosensory and vestibular feedback (Franz et al., 2017). We hypothesized that this would create an environment in which the body would rely more on somatosensory information for motor planning and execution—primed for sensory reweighting (Horak, 2006; Peterka, 2002). Utilizing sub-threshold vibratory noise to create a stochastic resonance effect, we expected the increased sensitivity to ankle somatosensory information would manifest as a significant attenuation in ML motion at the perturbation frequencies. On average, visuomotor entrainment was unaffected by the applied noise during walking and standing. This finding is similar to a prior observation that noise at the Achilles tendons did not affect stabilization of the head while standing on an oscillating platform (Borel & Ribot-Ciscar, 2016). However, our post-hoc analysis suggested there may be a baseline-dependent attenuation in ML head motion during walking, but only for the fastest perturbation frequency (0.96 Hz, Fig. 6A). Future studies with larger sample sizes and various perturbation frequencies will be required to fully investigate how the beneficial effects of noise may be related to specific perturbation frequencies. For now, we interpret our current findings to suggest that, at least for healthy young adults, increased sensitivity to ankle somatosensory information is not effective at attenuating visuomotor entrainment to ML visual perturbations. This may be explained, in part, by the highly reflexive nature of visuomotor entrainment during gait. We may expect to find more noticeable noise effects during more voluntary postural tasks (Sacco et al., 2017).
We chose the Achilles tendons as the location to apply our sub-threshold vibratory noise. Given that the Achilles tendons are primary contributors to AP motion during walking and standing (Winter, 1995), this may not be an optimal location to attenuate entrainment to ML visual perturbations. We may have seen more pronounced ML attenuation had we chosen to apply the noise in a different location, such as the bottom of the feet (Lipsitz et al., 2015), or over more medially and laterally positioned ankle tendons. For context, we initially hoped that by stimulating a primary structure of the ankle we might introduce a remote stochastic resonance effect, which increases sensitivity to somatosensory information in structures more distal to the point of application (Enders, Hur, Johnson, & Seo, 2013; Lakshminarayanan, Lauer, Ramakrishnan, Webster, & Seo, 2015; Seo, Lakshminarayanan, Bonilha, Lauer, & Schmit, 2015). For example, Enders et al found that applying sub-threshold vibratory noise to the wrist improves tactile sensation at the fingertips (Enders et al., 2013). If a similar remote application of noise could be implemented at the ankle and increase sensitivity of the feet, this would allow for more practical designs of vibration devices that do not interfere with footwear (e.g. vibrating insoles) and would not require tuning an array of actuators to deliver sub-sensory vibration. As some prior work has shown that sub-threshold vibratory noise over the Achilles tendon may affect balance (Borel & Ribot-Ciscar, 2016), we considered the possibility of a remote stochastic resonance effect throughout the ankles and feet, which may contribute to balance in both ML and AP directions. However, as improvements from sub-sensory vibration are typically small for quiet standing, we chose to include ML visual perturbations in our study design because they are known to disrupt balance during gait for healthy young adults (O’Connor & Kuo, 2009; Stokes et al., 2017), which may increase their reliance on somatosensory information to maintain balance and thus might increase our potential to detect a beneficial effect of noise. Regardless, the present study did not directly test whether Achilles tendon vibration may induce a remote stochastic resonance effect, and future studies should explore this potential mechanism.
This study has several important limitations to consider when interpreting our results. First, the intensity of the sub-threshold vibratory noise was set to 90% of each subject’s sensory threshold, which may not be the optimal intensity for every subject. Optimal noise intensity can vary widely between participants (Haynes, Tenan, Passaro, & Tweedell, 2018; Lipsitz et al., 2015), and can also vary depending on the summary metric used to describe a task (Ross et al., 2013). We may have seen greater effects of noise when comparing group averages had we optimized the noise intensity for each subject. Second, this study involved healthy young adults who had good balance and may have already been near optimal control during the ML visual perturbations. The effects of noise might have been more pronounced had we investigated aging or pathological populations (Dettmer et al., 2015; Francis et al., 2015; Galica et al., 2009; Priplata et al., 2006; Zarkou, Lee, Prosser, Hwang, & Jeka, 2018). Third, there was no randomization between the walking and the standing tasks, and this may introduce a bias due to fatigue or habituation. Fourth, the exploratory post-hoc analysis is limited by a small sample size. The main study was not designed with this analysis in mind, but given that prior researchers have emphasized the importance of considering individual differences (Borel & Ribot-Ciscar, 2016; Kelty-Stephen & Dixon, 2013; Stephen et al., 2012), we thought it would be prudent to consider this possibility. Future research should examine the extent of a possible baseline-dependent attenuation with applied noise. Finally, we cannot exclude the possibility that the effects of noise might manifest only after a sufficient adaptation period, both to the noise and to the visual perturbations (Thompson & Franz, 2017).
In conclusion, our results provide evidence that sub-threshold vibratory noise over the Achilles tendons can improve postural control when walking and standing in visually perturbed environments. Future studies should investigate populations with impaired somatosensory function who may exhibit visuomotor entrainment that is more responsive to attenuation with noise.
Highlights.
Walking and standing entrained to the motion of a virtual reality hallway
Sub-threshold vibratory noise improved one measure of postural sway
Sub-threshold vibratory nose did not substantially attenuate visuomotor entrainment
Acknowledgments
Funding
Research reported in this publication was supported by the UW-Madison Faustin Prinz Undergraduate Research Fellowship and the National Institutes of Health under award number AG051748
Footnotes
Conflicts of interest: None to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aboutorabi A, Arazpour M, Bahramizadeh M, Farahmand F, & Fadayevatan R (2017). Effect of vibration on postural control and gait of elderly subjects : a systematic review. Aging Clinical and Experimental Research. 10.1007/S40520-017-0831-7 [DOI] [PubMed] [Google Scholar]
- Acuna SA, Francis CA, Franz JR, & Thelen DG (2019). The effects of cognitive load and optical flow on antagonist leg muscle coactivation during walking for young and older adults. Journal of Electromyography and Kinesiology, 44, 8–14. 10.1016/j.jelekin.2018.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baig S, Dansereau RM, Chan ADC, Remaud A, & Bilodeau M (2012). Cluster Analysis of Center-of-Pressure Measures. International Journal of Electrical and Computer Engineering, 1(1). 10.11159/ijecs.2012.002 [DOI] [Google Scholar]
- Baumberger B, Isableu B, & FlUckiger M (2004). The visual control of stability in children and adults: postural readjustments in a ground optical flow. Experimental Brain Research, 159(1), 33–46. 10.1007/S00221-004-1930-1 [DOI] [PubMed] [Google Scholar]
- Borel L, & Ribot-Ciscar E (2016). Improving postural control by applying mechanical noise to ankle muscle tendons. Experimental Brain Research, 234(8), 2305–2314. 10.1007/s00221-016-4636-2 [DOI] [PubMed] [Google Scholar]
- Collins JJ, Imhoff TT, & Grigg P (1996). Noise-enhanced tactile sensation. Nature. 10.1038/383770a0 [DOI] [PubMed] [Google Scholar]
- Collins JJ, Priplata AA, Gravelle DC, Niemi J, Harry J, & Lipsitz LA (2003). Noise-Enhanced Human Sensorimotor Function. IEEE Engineering in Medicine and Biology Magazine. 10.1109/MEMB.2003.1195700 [DOI] [PubMed] [Google Scholar]
- Cordo P, Inglis JT, Verschueren S, Collins JJ, Merfeld DM, Rosenblum S, ... Moss F (1996). Noise in human muscle spindles. Nature, 383, 769. [DOI] [PubMed] [Google Scholar]
- Desailly E, Daniel Y, Sardain P, & Lacouture P (2009). Foot contact event detection using kinematic data in cerebral palsy children and normal adults gait. Gait & Posture, 29(1), 76–80. 10.1016/j.gaitpost.2008.06.009 [DOI] [PubMed] [Google Scholar]
- Dettmer M, Pourmoghaddam A, Lee B-C, & Layne CS (2015). Effects of aging and tactile stochastic resonance on postural performance and postural control in a sensory conflict task. Somatosensory & Motor Research, 32(2), 128–135. 10.3109/08990220.2015.1004045 [DOI] [PubMed] [Google Scholar]
- Dijkstra TMH, Schoner G, Giese MA, & Gielen CCAM (1994). Frequency dependence of the action-perception cycle for postural control in a moving visual environment: relative phase dynamics. Biological Cybernetics, 71(6), 489–501. 10.1007/BF00198467 [DOI] [PubMed] [Google Scholar]
- Dokka K, Kenyon RV, & Keshner EA (2009). Influence of visual scene velocity on segmental kinematics during stance. Gait & Posture, 30(2), 211–216. 10.1016/j.gaitpost.2009.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte M, Freitas SMSF, & Zatsiorsky V (2011). Control of Equilibrium in Humans - Sway over sway. In Danion F & Latash ML (Eds.), Motor Control: Theories, Experiments, and Applications (pp. 219–242). New York, NY: Oxford University Press. 10.1093/acprof:oso/9780195395273.003.0010 [DOI] [Google Scholar]
- Enders LR, Hur P, Johnson MJ, & Seo NJ (2013). Remote vibrotactile noise improves light touch sensation in stroke survivors’ fingertips via stochastic resonance. Journal of Neuroengineering and Rehabilitation, 10(1), 105. 10.1186/1743-0003-10-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon JB, Carr RW, & Morgan DL (2004). Stochastic Resonance in Muscle Receptors. Journal of Neurophysiology, 91(6), 2429–2436. 10.1152/jn.00928.2003 [DOI] [PubMed] [Google Scholar]
- Francis CA, Franz JR, O ‘connor SM, & Thelen DG (2015). Gait variability in healthy old adults is more affected by a visual perturbation than by a cognitive or narrow step placement demand. Gait & Posture, 42(3), 380–385. 10.1016/j.gaitpost.2015.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz JR, Francis CA, Allen MS, O’Connor SM, & Thelen DG (2015). Advanced age brings a greater reliance on visual feedback to maintain balance during walking. Human Movement Science, 40, 381–392. 10.1016/j.humov.2015.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz JR, Francis CA, Allen MS, & Thelen DG (2017). Visuomotor Entrainment and the Frequency-Dependent Response of Walking Balance to Perturbations. IEEE Transactions on Neural Systems and Rehabilitation Engineering, 25(8), 1135–1142. 10.1109/TNSRE.2016.2603340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galica AM, Kang HG, Priplata AA, D’Andrea SE, Starobinets OV, Sorond FA, ... Lipsitz LA (2009). Subsensory vibrations to the feet reduce gait variability in elderly fallers. Gait and Posture, 30(3), 383–387. 10.1016/j.gaitpost.2009.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greffou S, Bertone A, Hanssens J-M, & Faubert J (2008). Development of visually driven postural reactivity: A fully immersive virtual reality study. Journal of Vision, 8(11), 15. [DOI] [PubMed] [Google Scholar]
- Haynes CA, Tenan MS, Passaro AD, & Tweedell AJ (2018). Optimal Noise Level for Imperceptible Vibrotactile Stimulation during a Force Stability Task. Preprints, (November), 1–15. [Google Scholar]
- Horak FB (2006). Postural orientation and equilibrium: What do we need to know about neural control of balance to prevent falls? Age and Ageing, 35(SUPPL.2), 7–11. 10.1093/ageing/afl077 [DOI] [PubMed] [Google Scholar]
- Kabbaligere R, Lee B-C, & Layne CS (2017). Balancing sensory inputs: Sensory reweighting of ankle proprioception and vision during a bipedal posture task, 52, 244–250. 10.1016/j.gaitpost.2016.12.009 [DOI] [PubMed] [Google Scholar]
- Kelty-Stephen DG, & Dixon JA (2013). Temporal correlations in postural sway moderate effects of stochastic resonance on postural stability. Human Movement Science, 32(1), 91–105. 10.1016/j.humov.2012.08.006 [DOI] [PubMed] [Google Scholar]
- Lakshminarayanan K, Lauer AW, Ramakrishnan V, Webster JG, & Seo NJ (2015). Application of vibration to wrist and hand skin affects fingertip tactile sensation. Physiological Reports, 3(7), e12465. 10.14814/phy2.12465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsitz LA, Lough M, Niemi J, Travison T, Howlett H, & Manor B (2015). A shoe insole delivering subsensory vibratory noise improves balance and gait in healthy elderly people. Archives of Physical Medicine and Rehabilitation, 96(3), 432–9. 10.1016/j.apmr.2014.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughlin PJ, & Redfern MS (2001). Spectral characteristics of visually induced postural sway in healthy elderly and healthy young subjects. IEEE Transactions on Neural Systems and Rehabilitation Engineering, 9(1), 24–30. 10.1109/7333.918273 [DOI] [PubMed] [Google Scholar]
- Magalhaes FH, & Kohn AF (2012). Imperceptible electrical noise attenuates isometric plantar flexion force fluctuations with correlated reductions in postural sway. Experimental Brain Research, 217(2), 175–186. 10.1007/s00221-011-2983-6 [DOI] [PubMed] [Google Scholar]
- Martelli D, Xia B, Prado A, & Agrawal SK (2019). Gait adaptations during overground walking and multidirectional oscillations of the visual field in a virtual reality headset. Gait & Posture, 67, 251–256. 10.1016/j.gaitpost.2018.10.029 [DOI] [PubMed] [Google Scholar]
- Miranda DL, Hsu W-H, Petersen K, Fitzgibbons S, Niemi J, Lesniewski-Laas N, & Walsh CJ (2016). Sensory Enhancing Insoles Modify Gait during Inclined Treadmill Walking with Load. Medicine & Science in Sports & Exercise, 48(5), 860–868. 10.1249/MSS.0000000000000831 [DOI] [PubMed] [Google Scholar]
- O’Connor SM, & Kuo AD (2009). Direction-dependent control of balance during walking and standing. Journal of Neurophysiology, 102(3), 1411–1419. 10.1152/jn.00131.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor SM, Xu HZ, & Kuo AD (2012). Energetic cost of walking with increased step variability. Gait & Posture, 36(1), 102–107. 10.1016/j.gaitpost.2012.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterka RJ (2002). Sensorimotor Integration in Human Postural Control. Journal of Neurophysiology, 88(3), 1097–1118. 10.1152/jn.2002.88.3.1097 [DOI] [PubMed] [Google Scholar]
- Priplata AA, Niemi JB, Harry JD, Lipsitz LA, & Collins JJ (2003). Vibrating insoles and balance control in elderly people. Lancet, 362(9390), 1123–1124. 10.1016/S0140-6736(03)14470-4 [DOI] [PubMed] [Google Scholar]
- Priplata AA, Patritti BL, Niemi JB, Hughes R, Gravelle DC, Lipsitz LA, ... Collins JJ (2006). Noise-enhanced balance control in patients with diabetes and patients with stroke. Annals of Neurology, 59(1), 4–12. 10.1002/ana.20670 [DOI] [PubMed] [Google Scholar]
- Raymakers J. a., Samson MM, & Verhaar HJJ (2005). The assessment of body sway and the choice of the stability parameter(s). Gait and Posture, 21(1), 48–58. 10.1016/j.gaitpost.2003.11.006 [DOI] [PubMed] [Google Scholar]
- Ribot-Ciscar E, Hospod V, & Aimonetti J-M (2013). Noise-enhanced kinaesthesia: a psychophysical and microneurographic study. Experimental Brain Research, 228(4), 503–511. 10.1007/s00221-013-3581-6 [DOI] [PubMed] [Google Scholar]
- Ross SE, Linens SW, Wright CJ, & Arnold BL (2013). Customized noise-stimulation intensity for bipedal stability and unipedal balance deficits associated with functional ankle instability. Journal of Athletic Training, 48(4), 463–70. 10.4085/1062-6050-48.3.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco CC, Gaffney EM, & Dean JC (2017). Effects of White Noise Achilles Tendon Vibration on Quiet Standing and Active Postural Positioning. Journal of Applied Biomechanics, 1–27. 10.1123/jab.2016-0359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert P, Kirchner M, Schmidtbleicher D, & Haas CT (2012). About the structure of posturography: Sampling duration, parametrization, focus of attention (part I). Journal of Biomedical Science and Engineering, 05(09), 496–507. 10.4236/jbise.2012.59062 [DOI] [Google Scholar]
- Seo NJ, Lakshminarayanan K, Bonilha L, Lauer AW, & Schmit BD (2015). Effect of imperceptible vibratory noise applied to wrist skin on fingertip touch evoked potentials - an EEG study. Physiological Reports, 3(11), e12624. 10.14814/phy2.12624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephen DG, Wilcox BJ, Niemi JB, Franz J, Casey Kerrigan D, & D’Andrea SE (2012). Baseline-dependent effect of noise-enhanced insoles on gait variability in healthy elderly walkers. Gait and Posture, 36(3), 537–540. 10.1016/j.gaitpost.2012.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes HE, Thompson JD, & Franz JR (2017). The Neuromuscular Origins of Kinematic Variability during Perturbed Walking. Scientific Reports, 7(1), 808. 10.1038/s41598-017-00942-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, & Franz JR (2017). Do kinematic metrics of walking balance adapt to perturbed optical flow? Human Movement Science, 54(January), 34–40. 10.1016/j.humov.2017.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Plummer P, & Franz JR (2018). Age and falls history effects on antagonist leg muscle coactivation during walking with balance perturbations. Clinical Biomechanics, 59, 94–100. 10.1016/j.clinbiomech.2018.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Asten WNJC, Gielen CCAM, & van der Gon JJD (1988). Postural adjustments induced by simulated motion of differently structured environments. Experimental Brain Research, 73(2), 371–383. 10.1007/BF00248230 [DOI] [PubMed] [Google Scholar]
- Winter DA (1995). Human blance and posture control during standing and walking. Gait & Posture, 3(4), 193–214. 10.1016/0966-6362(96)82849-9 [DOI] [Google Scholar]
- Zarkou A, Lee SCK, Prosser LA, Hwang S, & Jeka J (2018). Stochastic resonance stimulation improves balance in children with cerebral palsy: a case control study. Journal of NeuroEngineering and Rehabilitation, 15(1), 115. 10.1186/s12984-018-0467-7 [DOI] [PMC free article] [PubMed] [Google Scholar]