Abstract
Background:
A link between selenium deficiency and inflammatory skin diseases have been noted by many, but this link is still not well understood. We have previously studied the efficacy of ceramide analogs, based on the fire ant venom Solenopsin A, against our psoriasis animal model. Treatment of animals with solenopsin analogs resulted in significantly improved skin as well as in a coordinate downregulation of selenoproteins, namely Glutathione Peroxidase 4 (GPX4). We thus hypothesize that ferroptosis may be a physiologic process that may protect the skin from both inflammatory and neoplastic processes.
Methods:
We analyze and compare gene expression profiles in the GEO database from clinical skin samples taken from healthy patients and psoriasis patients (both involved and noninvolved skin lesions). We validated the gene expression results against a second, independent, cohort from the GEO database.
Results:
Significant reduction in gene expression of GPX4, elevated expression of Nrf2 downstream targets, and expression profiles mirroring erastin-inhibition of Cystine/Glutamate Antiporter-System XC activity in psoriatic skin lesions, compared to both noninvolved skin and healthy patient samples, suggest an innately inducible mechanism of ferroptosis.
Conclusions:
We present data that may indicate selenoproteins, particularly GPX4, in resolving inflammation and skin cancer, including the novel hypothesis that the human organism may downregulate GPX4 and reactive oxygen (REDOX) regulating proteins in the skin as a way of resolving psoriasis and nonmelanoma skin cancer through increased reactive oxygen species. Further studies are needed to investigate ferroptosis as a possible physiologic mechanism for eliminating inflammatory and malignant tissues.
General significance:
This study provides a fresh framework for understanding the seemingly contradictory effects of selenium supplementation. In addition, it offers a novel explanation of how physiologic upregulation of ferroptosis and downregulation of selenoprotein synthesis may mediate resolution of inflammation and carcinogenesis. This is of therapeutic significance.
1. Introduction
Psoriasis is a common inflammatory disorder that is specific to humans, and is associated with a number of systemic disorders, including coronary artery disease, obesity, nonalcoholic steatohepatitis, arthritis, and inflammatory bowel disease. Multiple cytokines have been implicated in the pathogenesis of psoriasis, including tumor necrosis factor alpha (TNFα), interleukins 17, 22, and 23, and vascular endothelial growth factor (VEGF). It is on the basis of these cytokines that cytokine-based therapies have been employed to treat psoriasis [1]. These novel drugs are often dramatically effective, but may lose efficacy over time, either due to formation of antibodies against these reagents, or adaptation to different signaling pathways and cytokines [2,3]. Selenoproteins may play a role in resolving inflammation and skin cancer.
1.1. History of selenium in dermatology
Topical selenium sulfide was shown to have activity in psoriasis that was resistant to corticosteroids [4,5]. The form that was used, Selsun, was initially developed as a shampoo for Pityrosporum folliculitis, but appeared to be effective in selected cases of psoriasis. It is not known why this treatment is not widely used today, nor the metabolism of topical selenium sulfide. Unresolved questions include how much selenium penetrates the stratum corneum, and the metabolic fate of the topical selenium.
In an early study, levels of erythrocyte glutathione peroxidase, a selenoprotein, were found to be low in patients with psoriasis and other inflammatory conditions. The authors of the study administered tablets containing sodium selenite and tocopherol succinate, and noted a slow increase in glutathione peroxidase after 6 weeks. Some clinical benefit was noted. [6] Balneotherapy and oral ingestion of selenium-rich spa water (selenate 70/ig/l, and selenite 1/ig/l), resulted in a decreased PASI score of 47%. Plasma selenium levels were elevated as a result of selenium exposure, but levels of soluble CD25 did not change [7]. Defects in the absorption of selenomethionine from the gut of psoriatic and atopic dermatitis patients did not differ significantly from controls’ [8]. Other studies showed no difference in selenium levels in patients with psoriasis compared with normal controls [9]. In short, the connection between selenium and psoriasis is unclear, with poor understanding of the absorption, handling of inorganic vs organic selenium, and transport of active selenium moieties to the skin. Perhaps additional methods of introducing selenium to the skin are warranted, and selenium has been conjugated to nucleosides, which may facilitate uptake [10].
Many known inflammatory and pre-neoplastic disorders are associated with impaired barrier function. [11–18] These include psoriasis, atopic dermatitis and actinic keratosis, the most common precursor to cutaneous squamous cell carcinoma [19,20]. Emollients are employed in the treatment of inflammatory disorders, and application of sunscreen has been shown to cause regression of actinic keratosis [21]. This likely involves barrier restoration in part, as protection of the elderly from sunlight is unlikely by itself to revert lesions that have taken decades to develop. It also proves that actinic keratosis formation is a reversible event, unlike typical cutaneous squamous cell carcinoma, which does not regress in the presence of emollients.
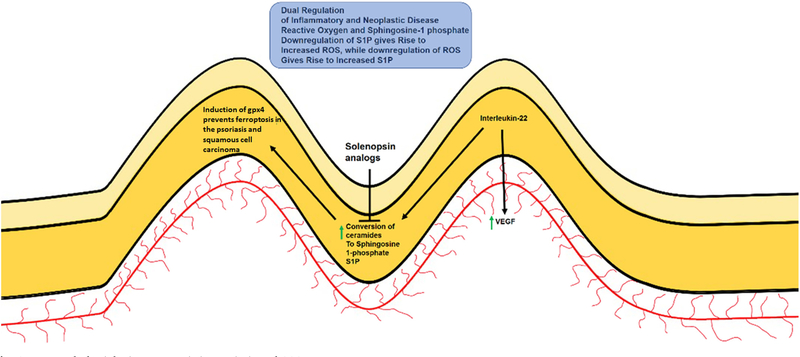
Ceramides are a family of lipids that are integral to cutaneous barrier function [22]. Once thought as inert fats, they have more recently been discovered to be biologically active molecules that have differing activities based upon the length of the fatty acid chains [4,23–25]. Ceramides are regulated both at the point of synthesis and degradation, and one of the major pathways of ceramide regulation is conversion to sphingosine-1 phosphate (S1P), a ligand that mediates pro-inflammatory and pro-neoplastic activities through a family of G protein based receptors [26]. Ceramide supplementation may correct ceramide deficiency, but can run the risk of being converted to S1P by enzymatic action. While impaired expression of barrier proteins such as filaggrin and claudins also play a major role in skin disorders, we would like to focus on deficiencies in ceramides [27–29], which have been found in human skin diseases [30] and murine knockouts of ceramide synthesis display cutaneous inflammation and alopecia [31,32]. We circumvented the problem of conversion of ceramide to S1P by the fortuitous discovery that solenopsin, the alkaloidal component of ant venom, acts biochemically similar to ceramide without being able to be converted to S1P [33]. Treatment of animals with solenopsin analogs resulted in a coordinate downregulation of selenoproteins and of the glutathione peroxidase family, and manganese superoxide dismutase, and especially downregulation of interleukin-22 (IL-22), a cytokine which is upregulated in both psoriasis and cutaneous squamous cell carcinoma [34–36]. The downregulation of these proteins are associated with ferroptosis (Fig. 1), with glutathione peroxidase 4 (GPX4) as an established master regulator. [37–40] Ceramides and solenopsin may act physiologically to coordinately reduce selenoproteins such as glutathione peroxidases and thus increase reactive oxygen (Table 1). We thus hypothesize that ferroptosis may be a physiologic process that may protect the skin from both inflammatory and neoplastic processes and looked toward clinical observations for clues.
Fig. 1.
Proposed Physiologic Ferroptosis in Psoriasis and SCC.
We hypothesize the concept of physiologic ferroptosis as a mechanistic target that may be induced by ceramide analogs, such as solenopsin derivatives, which suppress GPX4 expression is psoriatic lesions in mouse models. We believe that further suppression of GPX4 expression seen in clinical settings may play a role to resolving skin disorders.
Table 1.
Ceramide/Solenopsin analog S14 significantly affects gene expression in psoriasis mouse model.
| Gene Symbol | gene_assignment | p-Value (S14 vs Control) | Fold-change (S1 4 vs Control) | Fold-change (S14 vs Control) (Description) |
|---|---|---|---|---|
| Gpx4 | AK006441 // Gpx4 // glutathione peroxidase 4 // 10 C1|10 39.72 cM // 625249 /// AK14577 | 0.0390953 | −1.32133 | S14 down vs Control |
| Txn2 | AK002358 // Txn2 // thioredoxin 2 // 15 E1 |15 // 56551 /// AK010917 // Txn2 // thioredo | 0.0467511 | −1.23982 | S14 down vs Control |
| Txndc17 | AK004219 // Txndc17 // thioredoxin domain containing 17 // 11 B4|11 43.92 cM // 52700 / | 0.00314214 | −1.26646 | S14 down vs Control |
| Txndc15 | AK009878 // Txndc15 // thioredoxin domain containing 15 // 13 B1 13 // 69672 /// AK2137 | 0.00931008 | −1.31232 | S14 down vs Control |
| Sod2 | AK002428 // Sod2 // superoxide dismutase 2, mitochondrial // 17 A1|17 8.75 cM // 20656 | 0.0150224 | −1.33109 | S14 down vs Control |
| Txndc12 | AY548113 // Txndc12 // thioredoxin domain containing 12 (endoplasmic reticulum) // 4 C7 | 0.0307994 | −1.34924 | S14 down vs Control |
| Sod1 | BC057074 // Sod1 // superoxide dismutase 1, soluble // 16 B5-C3|16 51.56 cM // 20655 // | 0.000836736 | −1.42394 | S14 down vs Control |
| Blvrb | AK153153 // Blvrb // biliverdin reductase B (flavin reductase (NADPH)) // 7 A3|7 // 233 | 0.0179024 | −1.47698 | S14 down vs Control |
| Gpx1 | AK002245 // Gpx1 // glutathione peroxidase 1 // 9 F1|9 59.24 cM // 14775 /// AK010999 / | 0.00951611 | −1.60769 | S14 down vs Control |
A list of genes significantly downregulated by Solenospin S14 in mouse psoriasis model, which includes selenoprotein Gpx4.
2. Materials and methods
2.1. Retroactive psoriatic patient gene expression study
Data from gene expression data sets were analyzed from the Gene Expression Omnibus (GEO) database. For studies related to psoriasis, raw values from data GDS4602 (Ref GSE13355). (https://www.ncbi.nlm.nih.gov/sites/GDSbrowser?acc=GDS4602) [41] (shown) was analyzed and findings then validated on another data set GDS3539 (Ref GSE14905). The methods for collection of patient samples for human microarray data was previously described. Briefly, 58 psoriasis patients and 64 healthy control subjects recruited from Detroit, Michigan and surrounding areas. Patients were without systemic anti-psoriatic treatments for 2 weeks prior to biopsy, and without topical treatments 1 week prior to biopsy. In control subjects, biopsies were taken from the buttocks or upper thighs. Affymetrix Human Genome U133 Plus 2.0 Array,
The above date was validated against a second cohort from the Gene Expression Omnibus (GEO) database (Ref GSE14905) (https://www.ncbi.nlm.nih.gov/sites/GDSbrowser?acc=GDS3539) [42] (not shown). These data provided samples from healthy patient controls, as well as non-involved healthy skin and psoriatic lesions from the same patients. Using Affymetrix HGU133 Plus arrays, 54 total subjects donating skin biopsy samples from 21 healthy donors, 56 skin biopsy samples from 28 psoriasis patients who had match lesional and uninvolved tissue with 5 samples from psoriasis patients only providing lesional skin biopsies.
2.2. Retroactive INFɤ effect on psoriatic patient gene expression study
Data from gene expression data set GDS4607 (Ref GSE32407) (https://www.ncbi.nlm.nih.gov/sites/GDSbrowser?acc=GDS4607) [43] were analyzed from the Gene Expression Omnibus (GEO) database. Briefly, RNA isolation was performed on skin punch biopsies from healthy or non-lesional psoraisis patients at baseline or 24 h after placebo or IFNɤ injection. For studies related to psoriasis, raw values from data. https://www.ncbi.nlm.nih.gov/geo/info/disclaimer.html
2.3. Statistical analysis
Statistical Analysis was performed using PRISM 6 GraphPad on raw values obtained from the Gene Expression Omnibus (GEO) database. Values were assessed for normal distribution. Normally distributed data were analyzed using one-way ANOVA, with graphs representing mean of individual patient gene expression values and error bars indicating ± SEM. Significance threshold was set to p < 0.05. Skewed data were analyzed using the non-parametric Kruskal-Wallis Test, with graphs representing the median individual patient gene expression values and error bars indicating ± interquartile range. Significance threshold was set to p < 0.05.
3. Results
3.1. Psoriatic skin lesions show reduced GPX4 gene expression
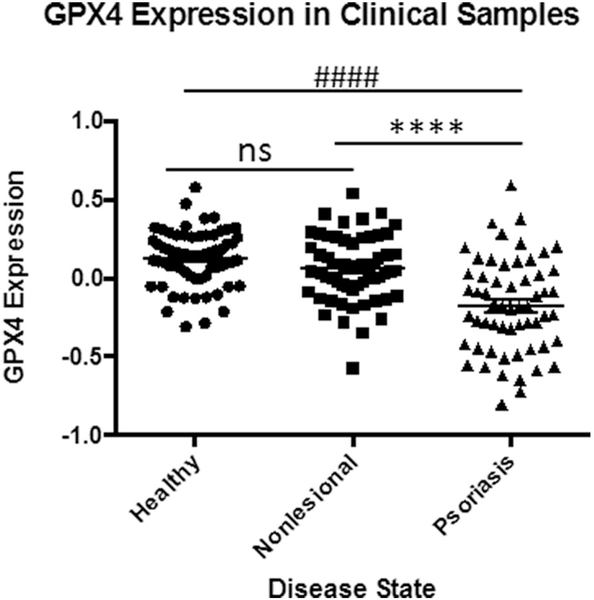
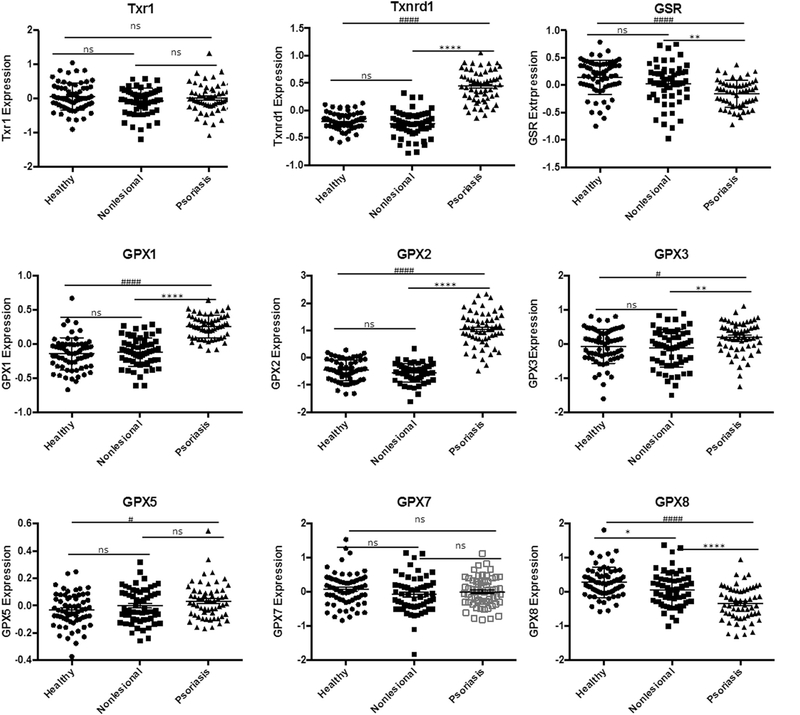
To assess for the possibility of ferroptosis, we first looked at GPX4 gene expression in the skin. A retroactive analysis of psoriasis patient samples indicated significant natural downregulation of GPX4 within psoriatic lesions when compared to non-involved skin or healthy patient controls (Fig. 2). These findings, which were validated in a second patient cohort, also showed what appears to be compensatory upregulation of other selenoproteins including, thioredoxin reductase1 (Txnrd1), and glutathione peroxidases 1, 2, 3, (GPX1, 2, 3), as well as, related proteins: thioredoxin (Trx1) and glutathione peroxidase 5 (GPX5) (Fig. 3). Interestingly, glutathione reductase (GSR) and glutathione peroxidase 8 (GPX8) were the only other proteins observed to be downregulated.
Fig. 2.
Lower GPX4 expression in Psoriatic Lesions.
Significantly reduced GPX4 expression in clinical samples suggesting physiologic ferroptosis. Data from GEO data set GDS4602 [41] comparing healthy patients (n = 64) to Psoriasis Patients with samples from nonlesional healthy skin (n = 58) and psoriatic skin (n = 58). Statistical Analysis: Normal distribution, ANOVA test with bars indicating mean raw values, ± SEM. **** and #### indicating p < 0.0001; and ns as p > 0.05.
Fig. 3.
Expression of Selenoproteins and related family proteins in Psoriatic Lesions.
Expression levels of major selenoproteins thioredoxin reductase1 (Txnrd1), and glutathione peroxidases 1, 2, 3, (GPX1, 2, 3), as well as, related proteins: thioredoxin (Trx1) and glutathione peroxidase 5 (GPX5) in healthy and psoriatic skin. Data from GEO data set GDS4602 comparing healthy patients (n = 64) to Psoriasis Patients with samples from nonlesional healthy skin (n = 58) and psoriatic skin (n = 58). Statistical Analysis: Normal distribution, ANOVA test with bars indicating mean raw values, ± SEM. **** and #### indicating p < 0.0001; *** or ### p < 0.001, * or # p < 0.05 and ns as p > 0.05.
3.2. Upregulation of Nrf2 downstream targets observed in psoriatic skin lesions
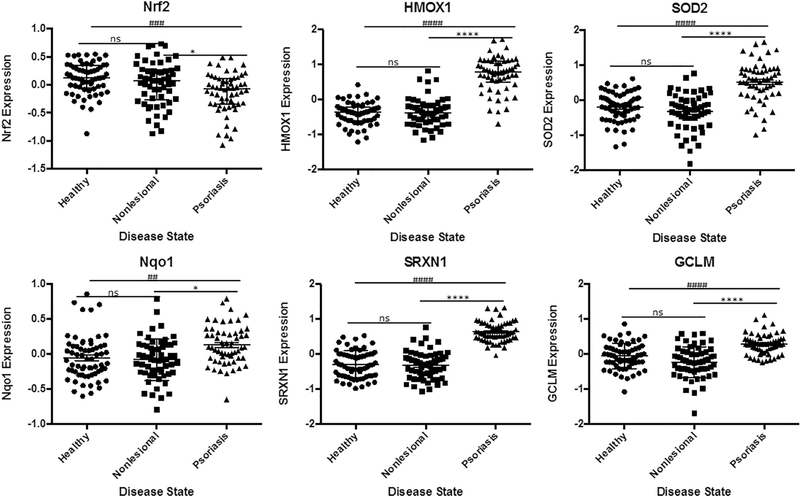
Given that ferroptosis is defined as non-apoptotic cell death by lipid peroxidation. We then looked for signs of increased oxidative stress in the absences of apoptopsis. We saw significantly elevated expression of downstream targets of nuclear factor (erythroid-derived 2)-like 2 (Nrf2) such as heme oxygenase (decycling) 1 (HMO1), superoxide dismutase 2 (SOD2), sulfiredoxin-1 (SRXN1), NAD(P)H dehydrogenase quinone 1 (NQ01) and glutamate-cysteine ligase regulatory subunit (GCLM). While not conclusive, the increased antioxidant response through Nrf2 activation suggested oxidative stress possibly linked to low GPX4 expression (Fig. 4).
Fig. 4.
Evidence of Nrf2 Activation and Elevated Expression of Downstream Targets in Psoriatic Lesions.
Significantly increased expression levels of downstream targets of Nrf2 suggesting oxidative stress in clinical samples from physiologic ferroptosis. Data from GEO data set GDS4602 comparing healthy patients (n = 64) to Psoriasis Patients with samples from nonlesional healthy skin (n = 58) and psoriatic skin (n = 58). Statistical Analysis: Normal distribution, ANOVA test with bars indicating mean raw values, ± SEM or nonparametric Kruskall-Wallis test with bars indicating median raw values, ± interquartile range. **** and #### indicating p < 0.0001; *** or ### p < 0.001, * or # p < 0.05 and ns as p > 0.05.
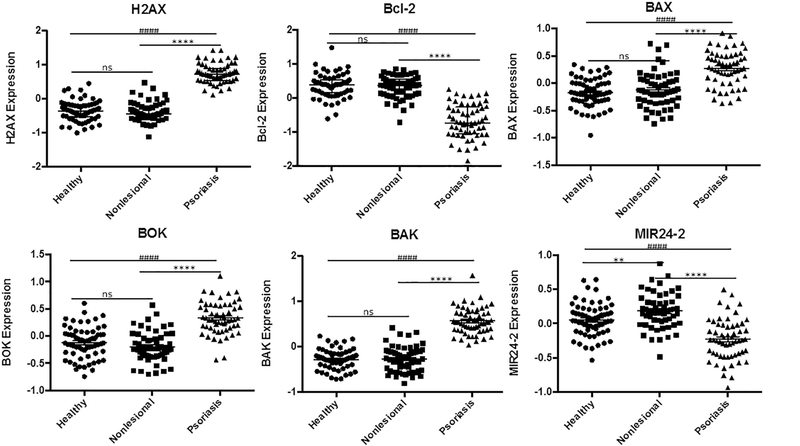
We also observed signs of double strained DNA repair through significant upregulation of H2A histone family member X (H2AX), [44] and looked further to see if relevant apoptotic pathways were activated (Fig. 5). Consequently, upregulation of proapoptotic B-cell lymphoma 2 (Bcl-2) family proteins were noticed, included Bcl-2-like protein 4 (BAX), Bcl-2 homologous antagonist/killer (BAK), Bcl-2 ovarian killer (BOK) suggesting apoptosis [45]. However, significant downregulation of MIR24–2, an essential mediator of H2AX/Bcl-2 apoptotic pathway, was also observed. [46,47]
Fig. 5.
Gene Expression Suggesting Halting of H2AX/Bcl-2 Apoptosis in Psoriatic Lesions.
Significant upregulation of H2A histone family member X (H2AX), proapoptotic B-cell lymphoma 2 (Bcl-2), Bcl-2-like protein 4 (BAX), Bcl-2 homologous antagonist/killer (BAK), and Bcl-2 ovarian killer (BOK) suggesting apoptosis likely halted by significant downregulation of MIR24–2, an essential mediator of H2AX/Bcl-2 apoptotic pathway. Data from GEO data set GDS4602 comparing healthy patients (n = 64) to Psoriasis Patients with samples from nonlesional healthy skin (n = 58) and psoriatic skin (n = 58). Statistical Analysis: nonparametric Kruskall-Wallis test with bars indicating median raw values, ± interquartile range. **** and #### indicating p < 0.0001; *** or ### p < 0.001, * or # p < 0.05 and ns as p > 0.05.
3.3. Gene expression in psoriatic skin lesions suggest an increased cellular import of iron
To investigate further if ferroptosis was plausible, we looked for indications linking the increased oxidative stress to intracellular iron levels. We looked at gene expressions of transferrins (TFRC or TFR1) and TFR2, which are known to import iron into the cell [48]. The data showed significant increases in expression of both genes, pointing to an innate effort to increase intracellular iron (Fig. 6).
Fig. 6.
Gene Expression Suggesting Increase in Intracellular Iron in Psoriatic Lesions.
Significant changes in gene expression of transferrins (TFRC or TFR1) and TFR2, metal regulatory transcription factor 1 (MTF1), specificity protein 1 (SP-1), glutaredoxin 3(GRX3), and ceruloplasmin (CP), involved cellular iron sensing suggest increases in intracellular iron in psoriatic skin. Data from GEO data set GDS4602 comparing healthy patients (n = 64) to Psoriasis Patients with samples from nonlesional healthy skin (n = 58) and psoriatic skin (n = 58). Statistical Analysis: Normal distribution, ANOVA test with bars indicating mean raw values, ± SEM. or nonparametric Kruskall-Wallis test with bars indicating median raw values, ± interquartile range. **** and #### indicating p < 0.0001; *** or ### p < 0.001, * or # p < 0.05 and ns as p > 0.05.
We followed up in determining if increased transferrin expression led to an increase in intracellular iron by looking at genes inducible by elevated iron presence or inducible by iron deficiency. We saw significant increases in metal regulatory transcription factor 1 (MTF1) in agreement with elevated intracellular metal concentration, perhaps iron. [49,50] We also saw significant reduction in expression of specificity protein 1 (SP-1), which is has been observed to increase in iron deficient conditions. [51] Interestingly glutaredoxin 3 (GRX3), known to be involved in intracellular iron sensing, [52] was also significantly upregulated. As expected, ceruloplasmin (CP), a ferroxidase and the human homologue to the yeast Fret3 downstream of GRX3, [53] was suppressed.
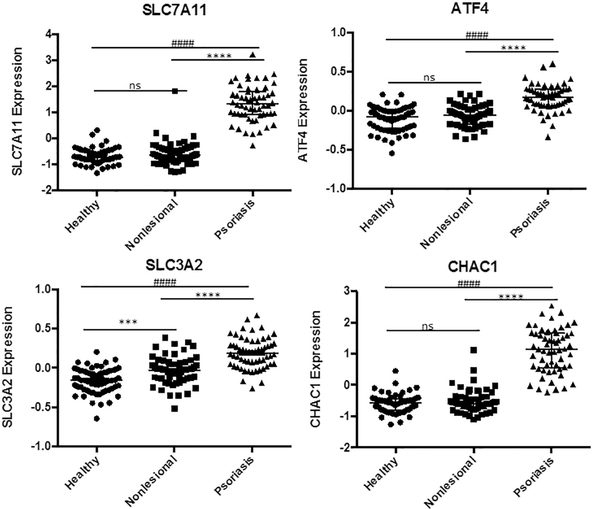
3.4. Psoriatic skin gene expression mirror erastm-induced ferroptosis, via Cystine/Glutamate Antiporter-System XC inhibition
Finally, we wanted to determine if events in psoriasis mirror known ferroptosis inducers with characterized mechanisms. We looked at previously described pharmacological mediators of ferroptosis, namely Erastin, RSL3, sorafenib, DPI, and FIN56 [54]. We found that psoratic skin lesions displayed a gene expression pattern that mimicked erastin-induced inhibition of Cystine/Glutamate Antiporter-System XC (System XC) (Fig. 7). Similar to erastin treatment, we observed upregulation of System XC subunits, namely SLC7A11 and SLC3A2, which has previously been attributed as a likely compensatory response to erastin. [55] Looking downstream of System XC, we observed significant upregulation of Activating transcription factor 4 (ATF4) and ChaC Glutathione Specific Gamma-Glutamylcyclotransferase 1 (CHAC1) in agreement with an erastin-liked induced inhibition of System XC. [55–57]
Fig. 7.
Gene Expression in Psoriatic Skin lesions mirror Erastin-like System XC inhibition.
Psoriatic lesions show gene expression pattern similar to erastin-like System XC inihibition. SLC7A11 and SLC3A2, were upregulated as compensatory response to similar erastin. Significant upregulation of Activating transcription factor 4 (ATF4), and ChaC Glutathione Specific Gamma-Glutamylcyclotransferase 1 (CHAC1) was also in agreement with an erastin-liked induced inhibition of System XC, the mechanism of erastin-mediated ferroptosis. Data from GEO data set GDS4602 comparing healthy patients (n = 64) to Psoriasis Patients with samples from nonlesional healthy skin (n = 58) and psoriatic skin (n = 58). Statistical Analysis: nonparametric Kruskall-Wallis test with bars indicating median raw values, ± interquartile range. ± SEM. **** and #### indicating p < 0.0001; *** or ### p < 0.001, * or # p < 0.05 and ns as p > 0.05.
4. Discussion
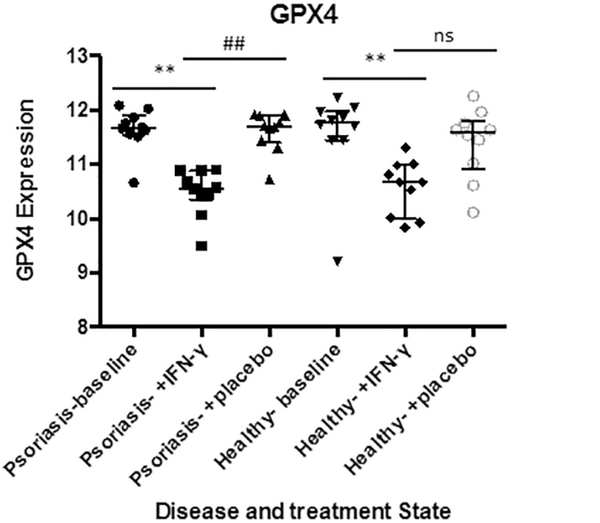
The possibility of physiologic ferroptosis, or a protective iron armor of the skin hints at a refined physiological ability to regulate specific antioxidant expression. We point to a nuanced understanding of skin biology, where ferroptosis may be a way of resolving cutaneous inflammation and malignancy with some effective treatments acting in part by further suppressing gpx4 expression in the skin, as suggested by interferon gamma (INFɤ) treatment (Fig. 8). We think that this hypothesis may explain several known phenomena in the skin, including: 1) Partial resolution of actinic keratosis and inflammatory conditions by emollients 2) Increased risk of cutaneous squamous cell carcinoma in transplant patients on cyclosporine A (CsA) 3) Presence of inactivating Notch mutations in cutaneous squamous cell carcinoma, and 4) Partial response of psoriasis to biologic therapies.
Fig. 8.
INFɣ suppresses GPX4 expression in Healthy skin and Psoriatic Lesions. Significantly reduced GPX4 expression in clinical samples from INFɣ treated patients suggesting effective therapies such cytokine-based therapies may act in part by physiologic ferroptosis. Data from GEO data set GDS4607 comparing healthy patients (n = 10) to Psoriasis Patients with samples from nonlesional healthy skin (n = 10) and psoriatic skin (n = 10). Statistical Analysis: non-parametric Kruskall-Wallis test with bars indicating median raw values, ± interquartile range. **** and #### indicating p < 0.0001; *** or ### p < 0.001, * or # p < 0.05 and ns as p > 0.05.
-
1)
Partial resolution of inflammatory and premalignant conditions by emollients
It is well established that both premalignancy and inflammatory processes are associated with impaired barrier function. We have previously shown that cutaneous barrier disruption results in upregulation of vascular endothelial growth factor (VEGF), a common factor in both psoriasis and squamous cell carcinoma [11,58]. Barrier disruption can be caused by chemical removal of ceramides, which may occur through water and detergent exposure, xerosis of aging, which may be due to loss of ceramide synthesis or increased degradation, and immunological disruption of cutaneous architecture, resulting in an impaired barrier function. Emollients are beneficial in the treatment of psoriasis, although they rarely induce complete remission [22,24,33,59]. A previous study also showed that use of sunscreen caused regression of actinic keratosis [21]. Sunscreens have emollient properties, and it is highly likely that partial barrier restoration results in decreased vascular endothelial growth factor and other cytokines [60]. The rapid response to sunscreens suggests an emollient effect over sun protection, as actinic keratosis may take decades to develop, and the protection from a few months of sun is probably insufficient to reverse decades of mutagenesis. However, abnormalities persist in both chronically sun damaged skin without clinically evident actinic keratosis, just as they do with uninvolved skin in psoriasis patients. These clinically unaffected areas are primed to undergo inflammatory or premalignant transformation, and restoration of ceramide signaling may aid in doing so.
-
2)
Increased risk of cutaneous squamous cell carcinoma in transplant patients on cyclosporine A (CsA)
Cyclosporine immunosuppression is associated with a high rate of cutaneous squamous cell carcinoma, at an incidence higher than immunosuppression with other agents such as sirolimus and tacrolimus [61,62]. Squamous cell carcinoma of the skin is a leading cause of death in transplant recipients. The increased rate of skin cancer has been attributed to decreased immunosurveillance, but other highly immunosuppressive agents do not have he same increased rate of squamous cell carcinoma. Thus, other factors must account for the high rate of squamous cell carcinogenesis in patients with cyclosporine. CsA is a molecule with a dual function [36]. It inhibits NFAT mediated transcription, which is the basis of its anti-inflammatory properties. In addition, it binds and inhibits the function of mitochondrial cyclophilin D. CsA protects cells from mitochondrial stability and reactive oxygen stress, preventing apoptosis [63]. Therefore, physiologic ferroptosis, which involves destruction of aberrant mutated keratinocytes with physiologic reactive oxygen, is compromised by CsA, and thus directly antagonizes the beneficial effects of ceramides.
-
3)
Presence of inactivating Notch mutations in cutaneous squamous cell carcinoma
We have previously discovered that Notch signaling is a major signaling pathway induced by reactive oxygen signaling. In fact, the gene that is most highly downregulated in our studies of NADPH oxidase inhibitors is Notch related Ankyrin related protein (Nrarp) [64]. Tumors that use reactive oxygen signaling invariably upregulate notch signaling as well. However, the most common mutation in cutaneous squamous cell carcinoma after p53 are missense mutations in Notch, which inactivate its function. Melanoma, which is predominantly wild type p53, and uses Notch and reactive oxygen signaling to avoid apoptosis, is far more resistant to oxidative stress [65]. These findings further confirm the role of physiologic ferroptosis in maintaining skin homeostasis.
-
4)
Partial response of psoriasis to biologic therapies
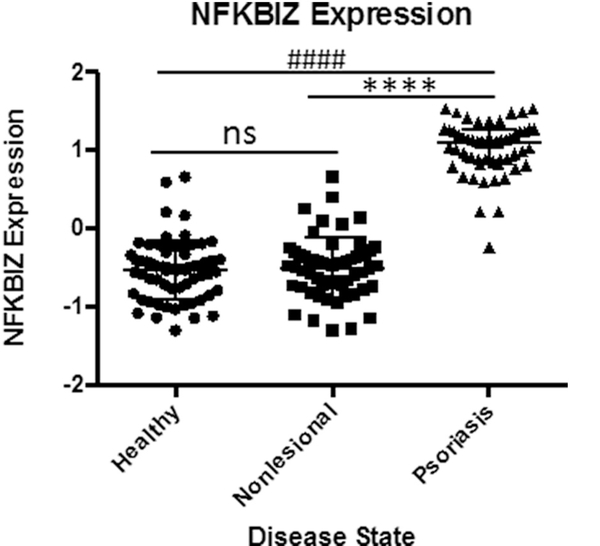
Use of biologic therapies has revolutionized the treatment of severe psoriasis. Initially beginning with targeting TNFα, more recent therapies have targeted IL-17 and IL-23. However, some patients do not fully respond or relapse to these therapies. Aside from immunity to these exogenous proteins, it is likely that psoriasis changes signaling pathways in response to therapies. It is instructive to look at signaling in psoriasis and cutaneous neoplasia as a combination of two distinct signaling pathways with crosstalk. One is a NADPH-superoxide-canonical NFkB signaling pathway, which can be activated by TNFα and other cytokines. The second is a ceramide-sphingosine-1 phosphate pathway resulting in activation of S1P receptors and stimulation of pro-inflammatory factors such as cyclooxygenase 2 (COX2). It is likely that the majority of pro-inflammatory factors credited to ceramides may result from conversion to pro-inflammatory S1P [66]. A potential candidate mediator of this crosstalk is IkBζ (NFKBIZ). The reasons for this is that first, IkBζ is overexpressed in psoriasis, and modulates NFkB signaling [67,68]. Second, IkBζ regulates secretion of IL-22 and is required for IL-17 mediated psoriasis [69]. Finally, psoriasis exhibits some characteristics of the senescence associated secretory phenomenon (SASP) as result, in part, of prolonged TNFα presence [70], in which senescent cells are not eliminated, but are present and contribute to the inflammatory milieu. A recent study has shown that NFKBIZ is required for the maintenance of the SASP phenotype, which has significantly elevated levels in psoriatic lesions (Fig. 9), and small molecules which downregulate NFKBIZ inhibit SASP [71,72].
There is likely feedback between these mechanisms, so when one is inhibited, the other is activated. Mechanistically, one could predict that selenium supplementation would lead to an increase in the selenoproteins involved selenoprotein-mediated reductive activity, followed by an increase in glutathione levels [73], which would inhibit the reactive species produced as a result of TNFα stimulation. This would lead to decreased signaling through NFkB, as reactive oxygen oxidizes IkB [74], and blockade of IkB oxidation would be predicted to result in decreased NFkB signaling. Reactive oxygen also oxidizes addition tyrosine phosphatases which have free sulfhydryl groups that are susceptible to oxidation [75]. In our model, we predict that ceramides, normal components of the skin, may increase reactive oxygen without activating NFkB, resulting in ferroptosis and therapeutic benefit. Psoriasis contains a balance of these two factors, with the proportion of signaling dependent on the genetic basis of the psoriasis as well as the current treatment. Since cutaneous squamous cell carcinoma is inhibited by reactive oxygen, it is likely to be ceramide-S1P predominant.
Fig. 9.
Elevated NFKBIZ expression in Psoriatic Lesions.
Significantly elevated expression NFKBIZ, a modulator of NFKB, in clinical samples linking psoriasis with senescence associated secretory phenomenon (SASP). Data from GEO data set GDS4602 comparing healthy patients (n = 64) to Psoriasis Patients with samples from nonlesional healthy skin (n = 58) and psoriatic skin (n = 58). Statistical Analysis: Normal distribution, ANOVA test with bars indicating mean raw values, ± SEM. **** and #### indicating p < 0.0001; *** or ### p < 0.001, * or # p < 0.05 and ns as p > 0.05.
5. Conclusion
We provide data from the field of dermatology that selenium plays an important role on skin homeostasis, spurred by findings from our studies on stable ceramide analogs that propose a more nuanced view of selenium and anti-oxidants in the skin. Having found that these stable ceramide analogs cause a downregulation of selenoproteins in affected skin, and downregulation of GPX4 promotes a novel form of iron dependent death, called ferroptosis. We thus proposed that ferroptosis is a physiological mechanism that can be used to resolve both inflammatory and neoplastic disorders of the skin. We show that gene expression patterns in psoariatic skin lesions are in alignment with erastin mediated ferroptosis, via Systemic XC inhibition. Further studies are needed to validate these findings and improve our understanding on selenoprotein regulation in the skin. We now hypothesis that improving effective treatments for resolving cutaneous inflammation and malignancy maybe achieved by promoting the skin’s innate response toward ferroptosis.
Acknowledgments
We would like to acknowledge the providers of the gene expression data used in this study, Gudjonsson JE, Ding J, Nair R, Stuart P, Voorhees JJ, Elder JT, Abecasis G, Higgs BW, Suárez-Fariñas M, Johnson-Huang LM, and Lowes MA, for their efforts which continue to contribute to our understanding of the skin.
Abbreviations:
- GPX4
Glutathione Peroxidase 4
- System XC
Cystine/Glutamate Antiporter-System XC
- TNFα
Tumor necrosis factor alpha
- VEGF
Vascular endothelial growth factor
- S1P
Sphingosine-1 phosphate
- IL-22
Interleukin-22
- Trx1
Thioredoxin
- Txnrd1
Thioredoxin reductase1
- GPX1, 2, 3, 5
Glutathione peroxidases 1, 2, 3, 5
- GSR
Glutathione reductase
- GPX8
Glutathione peroxidase 8
- Nrf2
Nuclear factor (erythroid-derived 2-like 2
- HMO1
Heme oxygenase (decycling 1)
- SOD2
Superoxide dismutase 2
- SRXN1
Sulfiredoxin-1
- NQ01
NAD(PH dehydrogenase quinone 1
- GCLM
Glutamate-cysteine ligase regulatory subunit
- H2AX
H2A histone family member X
- Bcl-2
B-cell lymphoma 2
- BAX
Bcl-2-like protein 4
- BAK
Bcl-2 homologous antagonist/killer
- BOK
Bcl-2 ovarian killer
- TFRC or TFR1
Transferrin receptor 1
- TFR2
Transferrin receptor 2
- MTF1
Metal regulatory transcription factor 1
- SP-1
Specificity protein 1
- GRX3
Glutaredoxin 3
- CP
Ceruloplasmin
- ATF4
Activating transcription factor 4
- CHAC1
ChaC Glutathione Specific Gamma-Glutamylcyclotransferase 1
- INFɤ
Interferon gamma
- CsA
Cyclosporine A
- Nrarp
Notch related Ankyrin related protein
- COX2
Cyclooxygenase 2
- NFKBIZ
IkBζ
- SASP
Senescence associated secretory phenomenon
References
- [1].Wu Y, Chen J, Li YH, Ma GZ, Chen JZ, Gao XH, Chen HD, Treatment of psoriasis with interleukin-12/23 monoclonal antibody: a systematic review, Eur. J. Dermatol. 22 (1) (2012) 72–82. [DOI] [PubMed] [Google Scholar]
- [2].van Schouwenburg PA, Krieckaert CL, Nurmohamed M, Hart M, Rispens T, Aarden L, Wouters D, Wolbink GJ, IgG4 production against adalimumab during long term treatment of RA patients, J. Clin. Immunol. 32 (5) (2012) 1000–1006. [DOI] [PubMed] [Google Scholar]
- [3].Chiu HY, Chu TW, Cheng YP, Tsai TF, The association between clinical response to ustekinumab and immunogenicity to ustekinumab and prior adalimumab, PLoS One 10 (11) (2015) e0142930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Arbiser JL, Bips M, Seidler A, Bonner MY, Kovach C, Combination therapy of imiquimod and gentian violet for cutaneous melanoma metastases, J. Am. Acad. Dermatol. 67 (2) (2012) e81–e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Borglund E, Enhamre A, Treatment of psoriasis with topical selenium sulphide, Br. J. Dermatol. 117 (5) (1987) 665–666. [DOI] [PubMed] [Google Scholar]
- [6].Juhlin L, Edqvist LE, Ekman LG, Ljunghall K, Olsson M, Blood glutathione-peroxidase levels in skin diseases: effect of selenium and vitamin E treatment, Acta Derm. Venereol. 62 (3) (1982) 211–214. [PubMed] [Google Scholar]
- [7].Pinton J, Friden H, Kettaneh-Wold N, Wold S, Dreno B, Richard A, Bieber T, Clinical and biological effects of balneotherapy with selenium-rich spa water in patients with psoriasis vulgaris, Br. J. Dermatol. 133 (2) (1995) 344–347. [DOI] [PubMed] [Google Scholar]
- [8].Fairris GM, Perkins PJ, Lawson AD, Blake GM, The pharmacokinetics of selenium in psoriasis and atopic dermatitis, Acta Derm. Venereol. 68 (5) (1988) 434–436. [PubMed] [Google Scholar]
- [9].Donadini A, Fiora C, Regazzini R, Perini D, Minoia C, Selenium plasma levels in psoriasis, Clin. Exp. Dermatol. 17 (3) (1992) 214–216. [DOI] [PubMed] [Google Scholar]
- [10].Schinazi RF, Arbiser J, Lee JJ, Kalman TI, Prusoff WH, Synthesis and biological activity of 5-phenylselenenyl-substituted pyrimidine nucleosides, J. Med. Chem. 29 (7) (1986) 1293–1295. [DOI] [PubMed] [Google Scholar]
- [11].Elias PM, Arbiser J, Brown BE, Rossiter H, Man MQ, Cerimele F, Crumrine D, Gunathilake R, Choi EH, Uchida Y, Tschachler E, Feingold KR, Epidermal vascular endothelial growth factor production is required for permeability barrier homeostasis, dermal angiogenesis, and the development of epidermal hyperplasia: implications for the pathogenesis of psoriasis, Am. J. Pathol. 173 (3) (2008) 689–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Jiang M, Li B, Zhang J, Hu L, Dang E, Wang G, Vascular endothelial growth factor driving aberrant keratin expression pattern contributes to the pathogenesis of psoriasis, Exp. Cell Res. 360 (2) (2017) 310–319. [DOI] [PubMed] [Google Scholar]
- [13].Armstrong AW, Voyles SV, Armstrong EJ, Fuller EN, Rutledge JC, Angiogenesis and oxidative stress: common mechanisms linking psoriasis with atherosclerosis, J. Dermatol. Sci. 63 (1) (2011) 1–9. [DOI] [PubMed] [Google Scholar]
- [14].Davis BP, Stucke EM, Khorki ME, Litosh VA, Rymer JK, Rochman M, Travers J, Kottyan LC, Rothenberg ME, Eosinophilic esophagitis-linked calpain 14 is an IL-13-induced protease that mediates esophageal epithelial barrier impairment, JCI Insight 1 (4) (2016) e86355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Boehncke WH, Schon MP, Psoriasis, Lancet 386 (9997) (2015) 983–994. [DOI] [PubMed] [Google Scholar]
- [16].Ye L, Lv C, Man G, Song S, Elias PM, Man MQ, Abnormal epidermal barrier recovery in uninvolved skin supports the notion of an epidermal pathogenesis of psoriasis, J. Invest Dermatol. 134 (11) (2014) 2843–2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Guttman-Yassky E, Nograles KE, Krueger JG, Contrasting pathogenesis of atopic dermatitis and psoriasis—part I: clinical and pathologic concepts, J. Allergy Clin. Immunol. 127 (5) (2011) 1110–1118. [DOI] [PubMed] [Google Scholar]
- [18].Zibert JR, Wallbrecht K, Schon M, Mir LM, Jacobsen GK, Trochon-Joseph V, Bouquet C, Villadsen LS, Cadossi R, Skov L, Schon MP, Halting angiogenesis by non-viral somatic gene therapy alleviates psoriasis and murine psoriasiform skin lesions, J. Clin. Invest. 121 (1) (2011) 410–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cho JM, Davis DMR, Wetter DA, Bartley AC, Brewer JD, Association between atopic dermatitis and squamous cell carcinoma: a case-control study, Int. J. Dermatol. 57 (3) (2018) 313–316. [DOI] [PubMed] [Google Scholar]
- [20].Mittelbronn MA, Mullins DL, Ramos-Caro FA, Flowers FP, Frequency of pre-existing actinic keratosis in cutaneous squamous cell carcinoma, Int. J. Dermatol. 37 (9) (1998) 677–681. [DOI] [PubMed] [Google Scholar]
- [21].Thompson SC, Jolley D, Marks R, Reduction of solar keratoses by regular sunscreen use, N. Engl. J. Med. 329 (16) (1993) 1147–1151. [DOI] [PubMed] [Google Scholar]
- [22].Motta S, Monti M, Sesana S, Mellesi L, Ghidoni R, Caputo R, Abnormality of water barrier function in psoriasis. Role of ceramide fractions, Arch. Dermatol. 130 (4) (1994) 452–456. [PubMed] [Google Scholar]
- [23].Cho Y, Lew BL, Seong K, Kim NI, An inverse relationship between ceramide synthesis and clinical severity in patients with psoriasis, J. Korean Med. Sci. 19 (6) (2004) 859–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Tawada C, Kanoh H, Nakamura M, Mizutani Y, Fujisawa T, Banno Y, Seishima M, Interferon-gamma decreases ceramides with long-chain fatty acids: possible involvement in atopic dermatitis and psoriasis, J. Invest Dermatol. 134 (3) (2014) 712–718. [DOI] [PubMed] [Google Scholar]
- [25].Nakajima K, Terao M, Takaishi M, Kataoka S, Goto-Inoue N, Setou M, Horie K, Sakamoto F, Ito M, Azukizawa H, Kitaba S, Murota H, Itami S, Katayama I, Takeda J, Sano S, Barrier abnormality due to ceramide deficiency leads to psoriasiform inflammation in a mouse model, J. Invest Dermatol. 133 (11) (2013) 2555–2565. [DOI] [PubMed] [Google Scholar]
- [26].Yamaguchi H, Kitayama J, Takuwa N, Arikawa K, Inoki I, Takehara K, Nagawa H, Takuwa Y, Sphingosine-1-phosphate receptor subtype-specific positive and negative regulation of Rac and haematogenous metastasis of melanoma cells, Biochem. J. 374 (Pt 3) (2003) 715–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Batista DI, Perez L, Orfali RL, Zaniboni MC, Samorano LP, Pereira NV, Sotto MN, Ishizaki AS, Oliveira LM, Sato MN, Aoki V, Profile of skin barrier proteins (filaggrin, claudins 1 and 4) and Th1/Th2/Th17 cytokines in adults with atopic dermatitis, J. Eur. Acad. Dermatol. Venereol. 29 (6) (2015) 1091–1095. [DOI] [PubMed] [Google Scholar]
- [28].Yuki T, Tobiishi M, Kusaka-Kikushima A, Ota Y, Tokura Y, Impaired tight junctions in atopic dermatitis skin and in a skin-equivalent model treated with interleukin-17, PLoS One 11 (9) (2016) e0161759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, Goudie DR, Sandilands A, Campbell LE, Smith FJ, O’Regan GM, Watson RM, Cecil JE, Bale SJ, Compton JG, DiGiovanna JJ, Fleckman P, Lewis-Jones S, Arseculeratne G, Sergeant A, Munro CS, El Houate B, McElreavey K, Halkjaer LB, Bisgaard H, Mukhopadhyay S, McLean WH, Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis, Nat. Genet. 38 (4) (2006) 441–446. [DOI] [PubMed] [Google Scholar]
- [30].Choi MJ, Maibach HI, Role of ceramides in barrier function of healthy and diseased skin, Am. J. Clin. Dermatol. 6 (4) (2005) 215–223. [DOI] [PubMed] [Google Scholar]
- [31].Ebel P, Imgrund S, Vom Dorp K, Hofmann K, Maier H, Drake H, Degen J, Dormann P, Eckhardt M, Franz T, Willecke K, Ceramide synthase 4 deficiency in mice causes lipid alterations in sebum and results in alopecia, Biochem. J. 461 (1) (2014) 147–158. [DOI] [PubMed] [Google Scholar]
- [32].Jennemann R, Rabionet M, Gorgas K, Epstein S, Dalpke A, Rothermel U, Bayerle A, van der Hoeven F, Imgrund S, Kirsch J, Nickel W, Willecke K,Riezman H, Grone HJ, Sandhoff R, Loss of ceramide synthase 3 causes lethal skin barrier disruption, Hum. Mol. Genet. 21 (3) (2012) 586–608. [DOI] [PubMed] [Google Scholar]
- [33].Arbiser JL, Nowak R, Michaels K, Skabytska Y, Biedermann T, Lewis MJ, Bonner MY, Rao S, Gilbert LC, Yusuf N, Karlsson I, Fritz Y, Ward NL, Evidence for biochemical barrier restoration: topical solenopsin analogs improve inflammation and acanthosis in the KC-Tie2 mouse model of psoriasis, Sci. Rep. 7 (1) (2017) 11198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Shimauchi T, Hirakawa S, Suzuki T, Yasuma A, Majima Y, Tatsuno K, Yagi H, Ito T, Tokura Y, Serum interleukin-22 and vascular endothelial growth factor serve as sensitive biomarkers but not as predictors of therapeutic response to biologics in patients with psoriasis, J. Dermatol. 40 (10) (2013) 805–812. [DOI] [PubMed] [Google Scholar]
- [35].Ma HL, Liang S, Li J, Napierata L, Brown T, Benoit S, Senices M, Gill D, Dunussi-Joannopoulos K, Collins M, Nickerson-Nutter C, Fouser LA, Young DA, IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation, J. Clin. Invest. 118 (2) (2008) 597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Abikhair M, Mitsui H, Yanofsky V, Roudiani N, Ovits C, Bryan T, Oberyszyn TM, Tober KL, Gonzalez J, Krueger JG, Felsen D, Carucci JA, Cyclosporine A immunosuppression drives catastrophic squamous cell carcinoma through IL-22, JCI Insight 1 (8) (2016) e86434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascon S, Hatzios SK, Kagan VE, Noel K, Jiang X, Linkermann A, Murphy ME, Overholtzer M, Oyagi A, Pagnussat GC, Park J, Ran Q, Rosenfeld CS, Salnikow K, Tang D, Torti FM, Torti SV, Toyokuni S, Woerpel KA, Zhang DD, Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease, Cell 171 (2) (2017) 273–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Viswanathan VS, Ryan MJ, Dhruv HD, Gill S, Eichhoff OM, Seashore-Ludlow B, Kaffenberger SD, Eaton JK, Shimada K, Aguirre AJ, Viswanathan SR, Chattopadhyay S, Tamayo P, Yang WS, Rees MG, Chen S, Boskovic ZV, Javaid S, Huang C, Wu X, Tseng YY, Roider EM, Gao D, Cleary JM, Wolpin BM, Mesirov JP, Haber DA, Engelman JA, Boehm JS, Kotz JD, Hon CS, Chen Y, Hahn WC, Levesque MP, Doench JG, Berens ME, Shamji AF, Clemons PA, Stockwell BR, Schreiber SL, Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway, Nature 547 (7664) (2017) 453–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Doll S, Proneth B, Tyurina YY, Panzilius E, Kobayashi S, Ingold I, Irmler M, Beckers J, Aichler M, Walch A, Prokisch H, Trumbach D, Mao G, Qu F, Bayir H, Fullekrug J, Scheel CH, Wurst W, Schick JA, Kagan VE, Angeli JP, Conrad M, ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition, Nat. Chem. Biol. 13 (1) (2017) 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji AF, Clish CB, Brown LM, Girotti AW, Cornish VW, Schreiber SL, Stockwell BR, Regulation of ferroptotic cancer cell death by GPX4, Cell 156 (1–2) (2014) 317–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Nair RP, Duffin KC, Helms C, Ding J, Stuart PE, Goldgar D, Gudjonsson JE, Li Y, Tejasvi T, Feng BJ, Ruether A, Schreiber S, Weichenthal M, Gladman D, Rahman P, Schrodi SJ, Prahalad S, Guthery SL, Fischer J, Liao W, Kwok PY, Menter A, Lathrop GM, Wise CA, Begovich AB, Voorhees JJ, Elder JT, Krueger GG, Bowcock AM, AbecasisP GR. Collaborative Association Study of, Genome-wide scan reveals association of psoriasis with IL-23 and NF-kappaB pathways, Nat. Genet. 41 (2) (2009) 199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Yao Y, Richman L, Morehouse C, de los Reyes M, Higgs BW, Boutrin A, White B, Coyle A, Krueger J, Kiener PA, Jallal B, Type I interferon: potential therapeutic target for psoriasis? PLoS One 3 (7) (2008) e2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Johnson-Huang LM, Suarez-Farinas M, Pierson KC, Fuentes-Duculan J, Cueto I, Lentini T, Sullivan-Whalen M, Gilleaudeau P, Krueger JG, Haider AS, Lowes MA, A single intradermal injection of IFN-gamma induces an inflammatory state in both non-lesional psoriatic and healthy skin, J. Invest Dermatol. 132 (4) (2012) 1177–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Sedelnikova OA, Pilch DR, Redon C, Bonner WM, Histone H2AX in DNA damage and repair, Cancer Biol. Ther. 2 (3) (2003) 233–235. [DOI] [PubMed] [Google Scholar]
- [45].Llambi F, Wang YM, Victor B, Yang M, Schneider DM, Gingras S, Parsons MJ, Zheng JH, Brown SA, Pelletier S, Moldoveanu T, Chen T, Green DR, BOK is a non-canonical BCL-2 family effector of apoptosis regulated by ER-associated degradation, Cell 165 (2) (2016) 421–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Srivastava N, Manvati S, Srivastava A, Pal R, Kalaiarasan P, Chattopadhyay S, Gochhait S, Dua R, Bamezai RN, miR-24–2 controls H2AFX expression regardless of gene copy number alteration and induces apoptosis by targeting antiapoptotic gene BCL-2: a potential for therapeutic intervention, Breast Cancer Res. 13 (2) (2011) R39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Wang S, Zhang R, Claret FX, Yang H, Involvement of microRNA-24 and DNA methylation in resistance of nasopharyngeal carcinoma to ionizing radiation, Mol. Cancer Ther. 13 (12) (2014) 3163–3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Hentze MW, Muckenthaler MU, Galy B, Camaschella C, Two to tango: regulation of Mammalian iron metabolism, Cell 142 (1) (2010) 24–38. [DOI] [PubMed] [Google Scholar]
- [49].Rutherford JC, Bird AJ, Metal-responsive transcription factors that regulate iron, zinc, and copper homeostasis in eukaryotic cells, Eukaryot. Cell 3 (1) (2004) 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Troadec MB, Ward DM, Lo E, Kaplan J, De Domenico I, Induction of FPN1 transcription by MTF-1 reveals a role for ferroportin in transition metal efflux, Blood 116 (22) (2010) 4657–4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Ruiz IG, de la Torre P, Diaz T, Esteban E, Morillas JD, Munoz-Yague T, Solis-Herruzo JA, Sp family of transcription factors is involved in iron-induced collagen alpha1(I) gene expression, DNA Cell Biol. 19 (3) (2000) 167–178. [DOI] [PubMed] [Google Scholar]
- [52].Muhlenhoff U, Molik S, Godoy JR, Uzarska MA, Richter N, Seubert A, Zhang Y, Stubbe J, Pierrel F, Herrero E, Lillig CH, Lill R, Cytosolic monothiol glutaredoxins function in intracellular iron sensing and trafficking via their bound iron-sulfur cluster, Cell Metab. 12 (4) (2010) 373–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].de Silva D, Davis-Kaplan S, Fergestad J, Kaplan J, Purification and characterization of Fet3 protein, a yeast homologue of ceruloplasmin, J. Biol. Chem. 272 (22) (1997) 14208–14213. [DOI] [PubMed] [Google Scholar]
- [54].Yang WS, Stockwell BR, Ferroptosis: death by lipid peroxidation, Trends Cell Biol. 26 (3) (2016) 165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Shin CS, Mishra P, Watrous JD, Carelli V, D’Aurelio M, Jain M, Chan DC, The glutamate/cystine xCT antiporter antagonizes glutamine metabolism and reduces nutrient flexibility, Nat. Commun. 8 (2017) 15074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Dixon SJ, Patel DN, Welsch M, Skouta R, Lee ED, Hayano M, Thomas AG, Gleason CE, Tatonetti NP, Slusher BS, Stockwell BR, Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis, elife 3 (2014) e02523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B 3rd, Stockwell BR, Ferroptosis: an iron-dependent form of nonapoptotic cell death, Cell 149 (5) (2012) 1060–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Larcher F, Murillas R, Bolontrade M, Conti CJ, Jorcano JL, VEGF/VPF over-expression in skin of transgenic mice induces angiogenesis, vascular hyperpermeability and accelerated tumor development, Oncogene 17 (3) (1998) 303–311. [DOI] [PubMed] [Google Scholar]
- [59].Alessandrini F, Pfister S, Kremmer E, Gerber JK, Ring J, Behrendt H, Alterations of glucosylceramide-beta-glucosidase levels in the skin of patients with psoriasis vulgaris, J. Invest Dermatol. 123 (6) (2004) 1030–1036. [DOI] [PubMed] [Google Scholar]
- [60].Liu X, German GK, The effects of barrier disruption and moisturization on the dynamic drying mechanics of human stratum corneum, J. Mech. Behav. Biomed. Mater. 49 (2015) 80–89. [DOI] [PubMed] [Google Scholar]
- [61].Berg D, Otley CC, Skin cancer in organ transplant recipients: epidemiology, pathogenesis, and management, J. Am. Acad. Dermatol. 47 (1) (2002) 1–17; quiz 18–20. [DOI] [PubMed] [Google Scholar]
- [62].Marcil I, Stern RS, Squamous-cell cancer of the skin in patients given PUVA and ciclosporin: nested cohort crossover study, Lancet 358 (9287) (2001) 1042–1045. [DOI] [PubMed] [Google Scholar]
- [63].Norman KG, Canter JA, Shi M, Milne GL, Morrow JD, Sligh JE, Cyclosporine A suppresses keratinocyte cell death through MPTP inhibition in a model for skin cancer in organ transplant recipients, Mitochondrion 10 (2) (2010) 94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Bhandarkar SS, Jaconi M, Fried LE, Bonner MY, Lefkove B, Govindarajan B, Perry BN, Parhar R, Mackelfresh J, Sohn A, Stouffs M, Knaus U, Yancopoulos G, Reiss Y, Benest AV, Augustin HG, Arbiser JL, Fulvene-5 potently inhibits NADPH oxidase 4 and blocks the growth of endothelial tumors in mice, J. Clin. Invest. 119 (8) (2009) 2359–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Balint K, Xiao M, Pinnix CC, Soma A, Veres I, Juhasz I, Brown EJ, Capobianco AJ, Herlyn M, Liu ZJ, Activation of Notch1 signaling is required for beta-catenin-mediated human primary melanoma progression, J. Clin. Invest. 115 (11) (2005) 3166–3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Motta S, Monti M, Sesana S, Caputo R, Carelli S, Ghidoni R, Ceramide composition of the psoriatic scale, Biochim. Biophys. Acta 1182 (2) (1993) 147–151. [DOI] [PubMed] [Google Scholar]
- [67].Bertelsen T, Ljungberg C, Boye Kjellerup R, Iversen L, Johansen C, IL-17F regulates psoriasis-associated genes through IkappaBzeta, Exp. Dermatol. 26 (3) (2017) 234–241. [DOI] [PubMed] [Google Scholar]
- [68].Johansen C, Mose M, Ommen P, Bertelsen T, Vinter H, Hailfinger S, Lorscheid S, Schulze-Osthoff K, Iversen L, IkappaBzeta is a key driver in the development of psoriasis, Proc. Natl. Acad. Sci. U. S. A. 112 (43) (2015) E5825–E5833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Okamoto K, Iwai Y, Oh-Hora M, Yamamoto M, Morio T, Aoki K, Ohya K, Jetten AM, Akira S, Muta T, Takayanagi H, IkappaBzeta regulates T(H)17 development by cooperating with ROR nuclear receptors, Nature 464 (7293) (2010) 1381–1385. [DOI] [PubMed] [Google Scholar]
- [70].Khan SY, Awad EM, Oszwald A, Mayr M, Yin X, Waltenberger B, Stuppner H, Lipovac M, Uhrin P, Breuss JM, Premature senescence of endothelial cells upon chronic exposure to TNFalpha can be prevented by N-acetyl cysteine and plumericin, Sci. Rep. 7 (2017) 39501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Lim H, Park H, Kim HP, Effects of flavonoids on senescence-associated secretory phenotype formation from bleomycin-induced senescence in BJ fibroblasts, Biochem. Pharmacol. 96 (4) (2015) 337–348. [DOI] [PubMed] [Google Scholar]
- [72].Alexander E, Hildebrand DG, Kriebs A, Obermayer K, Manz M, Rothfuss O, Schulze-Osthoff K, Essmann F, IkappaBzeta is a regulator of the senescence-associated secretory phenotype in DNA damage- and oncogene-induced senescence, J. Cell Sci. 126 (Pt 16) (2013) 3738–3745. [DOI] [PubMed] [Google Scholar]
- [73].Panee J, Stoytcheva ZR, Liu W, Berry MJ, Selenoprotein H is a redox-sensing high mobility group family DNA-binding protein that up-regulates genes involved in glutathione synthesis and phase II detoxification, J. Biol. Chem. 282 (33) (2007) 23759–23765. [DOI] [PubMed] [Google Scholar]
- [74].Midwinter RG, Cheah FC, Moskovitz J, Vissers MC, Winterbourn CC, IkappaB is a sensitive target for oxidation by cell-permeable chloramines: inhibition of NF-kappaB activity by glycine chloramine through methionine oxidation, Biochem. J. 396 (1) (2006) 71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Boivin B, Zhang S, Arbiser JL, Zhang ZY, Tonks NK, A modified cysteinyl-labeling assay reveals reversible oxidation of protein tyrosine phosphatases in angiomyolipoma cells, Proc. Natl. Acad. Sci. U. S. A. 105 (29) (2008) 9959–9964. [DOI] [PMC free article] [PubMed] [Google Scholar]