Abstract
Hippocampal injury and cognitive impairments are common after cardiac arrest and stroke and do not have an effective intervention despite much effort. Therefore we developed a new approach aimed at reversing synaptic dysfunction by targeting TRPM2 channels. Cardiac arrest/cardiopulmonary resuscitation (CA/CPR) in mice was used to investigate cognitive deficits and the role of the calcium-permeable ion channel transient receptor potential-M2 (TRPM2) in ischemia-induced synaptic dysfunction. Our data indicates that absence (TRPM2−/−) or acute inhibition of TRPM2 channels with tatM2NX reduced hippocampal cell death in males only, but prevented synaptic plasticity deficits in both sexes. Remarkably, administration of tatM2NX weeks after injury reversed hippocampal plasticity and memory deficits. Finally, TRPM2-dependent activation of calcineurin-GSK3β pathway contributes to synaptic plasticity impairments. These data suggest persistent TRPM2 activity following ischemia contributes to impairments of the surviving hippocampal network and that inhibition of TRPM2 channels at chronic time points may represent a novel strategy to improve functional recovery following cerebral ischemia that is independent of neuroprotection.
Keywords: neurorestoration, synaptic plasticity, LTP, cognitive impairment, stroke, cardiac arrest
Introduction
Global cerebral ischemia resulting from cardiac arrest (CA) occurs in approximately 560,000 people in the United States[1] leading to major cognitive disability in patients[2–6]. Metabolic failure and ionic imbalance following global ischemia results in cell death of vulnerable neuronal populations including hippocampal CA1 pyramidal cells[7, 8]. It is increasingly clear that in addition to neuronal injury following cerebral ischemia, impaired neuronal networks contribute to long-term functional deficits. In fact, despite numerous compounds reducing brain injury in experimental models of cerebral ischemia, translation to the clinic and improved outcomes in humans has been unsuccessful. Therefore, there is increased interest in developing therapeutic options that do not rely on neuroprotection to improve functional recovery[9–14]. What remains unclear is whether interventions administered beyond ischemic cell death time points can rescue neuronal networks, such as those in the hippocampus, to rescue synaptic plasticity and improve long-term cognitive recovery. In our laboratory, we have focused on synaptic plasticity in the surviving hippocampal network after ischemia, which is the leading model for the cellular changes that underlie learning and memory. Thus, we have utilized electrophysiological recordings of hippocampal long-term potentiation (LTP) as an indicator of network health and synaptic dysfunction following ischemia. Therefore, an optimal therapeutic intervention would have the capacity to reduce neuronal injury, maintain functional neuronal networks, and have the potential to recover cognitive function if administered in the subacute to late chronic ischemia phases.
Our CA/CPR mouse model displays impairment of long-term potentiation in CA1 hippocampal neurons and functional learning and memory for at least a month after cardiac arrest[15], however, the underlying molecular mechanisms remain elusive. The transient receptor potential melastatin 2 channel (TRPM2) is a Ca2+ permeable channel activated by oxidative stress. In the brain, TRPM2 expression is widespread throughout the hippocampus, cerebellum, and cortex[16] and has been implicated in pathologies such as Alzheimer’s disease, dementia, bipolar disorder and ischemia[17–22]. TRPM2 has sexually dimorphic roles in cell death and we have previously shown TRPM2 inhibition is neuroprotective only in males following focal and global cerebral ischemia[23, 22, 24–27]. In contrast, TRPM2 plays a minimal role in regulating hippocampal LTP[28]. However, the role of TRPM2 in hippocampal synaptic function following ischemia remains unknown and is the focus of the current study. Despite significant progress using transgenic mice, the lack of a specific TRPM2 channel antagonist has slowed efforts to unravel intrinsic neuronal TRPM2 function in cerebral ischemia and other neurological diseases. Therefore, we aimed to determine the role of TRPM2 in synaptic plasticity impairment following global cerebral ischemia using our recently described novel inhibitor of TRPM2, tatM2NX[23].
Here, we used our in vivo mouse model of CA/CPR[29, 15, 30, 31, 8] to investigate the role of TRPM2 activation in cognitive dysfunction observed in male and female mice after global ischemia. We hypothesize that TRPM2 is persistently activated at hippocampal CA3-CA1 synapses, leading to long-term LTP and memory impairments. We also provide evidence for TRPM2-dependent calcineurin-GSK3β signaling that contributes to LTP impairments. Therefore, this paper presents data that suggest a novel therapeutic strategy that provides benefit to hippocampal function when administered at acute or delayed times after injury, thus offering higher potential for clinical translation.
Results
Acute TRPM2 inhibition protects male neurons after CA/CPR but protects LTP in both sexes
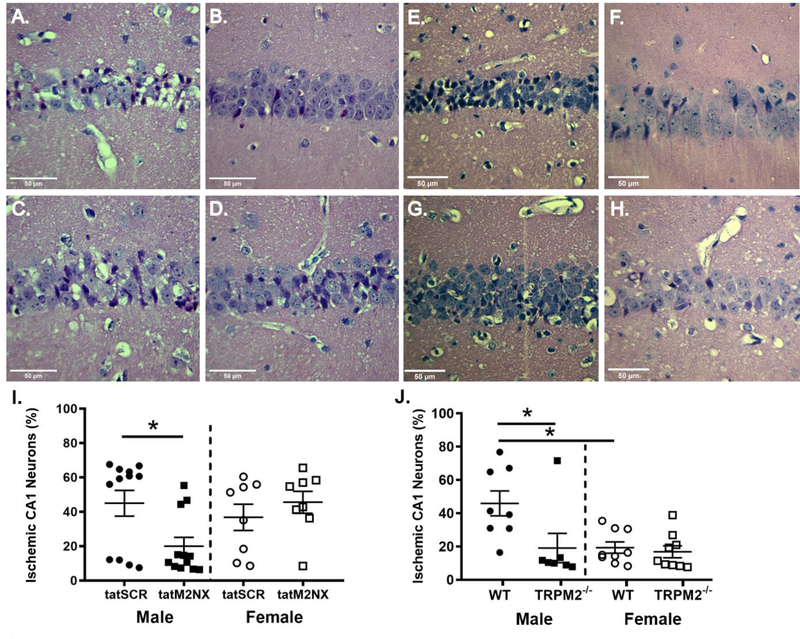
We recently developed a new peptide inhibitor of TRPM2 allowing us to perform more sophisticated pharmacology studies on the role of TRPM2 in acute and long-term functional outcomes. Global cerebral ischemia causes selective neuronal cell death in sensitive brain regions, such as hippocampus. We first sought to determine whether tatM2NX provides histological protection, therefore we tested the effect of acute administration of tatM2NX (20mg/kg, iv, dose chosen based upon our recent study[23]) or scrambled peptide (tat-SCR; 20 mg/kg) intravenously 30 minutes after reperfusion. Inhibition of TRPM2 by tatM2NX reduced neuronal injury in male mice compared to tatSCR (tatSCR: 44.9±7.5%, n=12 vs tatM2NX: 20.0±5.1%, n=12, p<0.05, Figure 1A,B, I). In females, inhibition of TRPM2 30 minutes after reperfusion did not decrease hippocampal injury (tatSCR: 36.8±7.7%, n=8 vs. tatM2NX: 45.6±6.4%, n=8, p=0.39, Figure 1C,D,I). This is consistent with our previous work demonstrating male-specific neuroprotection with TRPM2 inhibition or knock-down[22, 24, 25, 23]. We used mice lacking TRPM2 expression (TRPM2−/−) to confirm our pharmacological data. Histological assessment 3 days after global ischemia showed that TRPM2−/− male mice had reduced CA1 neuronal injury compared to wild type mice (19.2%, n=7 vs. 46.0%, n=8, respectively, p<0.05, Figure 1E,F,J). In contrast, TRPM2−/− female mice had no impact on cell death, showing equivalent CA1 neuronal injury compared to WT (16.9%, n=9 vs. 19.4%, n=9, respectively, Figure 1G,H,J). Female mice had less injury than male mice, independent of genotype, consistent with previous reports of reduced ischemic sensitivity in females [32].
Figure 1. Acute tatM2Nx reduces neuronal injury in males.
Representative photomicrographs of hippocampal CA1 neurons from male mice injected with 2o mg/kg scrambled control peptide; tat-SCR male (A) and female (C) or 2o mg/kg tatM2NX male (B) and female (D) 3o minutes after resuscitation. (I) Quantification of ischemic CA1 neurons 3 days after recovery from CA/CPR in mice treated with tatSCR vs tatM2NX. Representative photomicrographs of hippocampal CA1 neurons from WT male (E) and female (G) or TRPM2−/− male (F) and female (H) mice 3 days after recovery from CA/CPR. J) Quantification of ischemic CA1 neurons 3 days after recovery from CA/CPR. *p < 0.05. Data presented as mean±SEM.
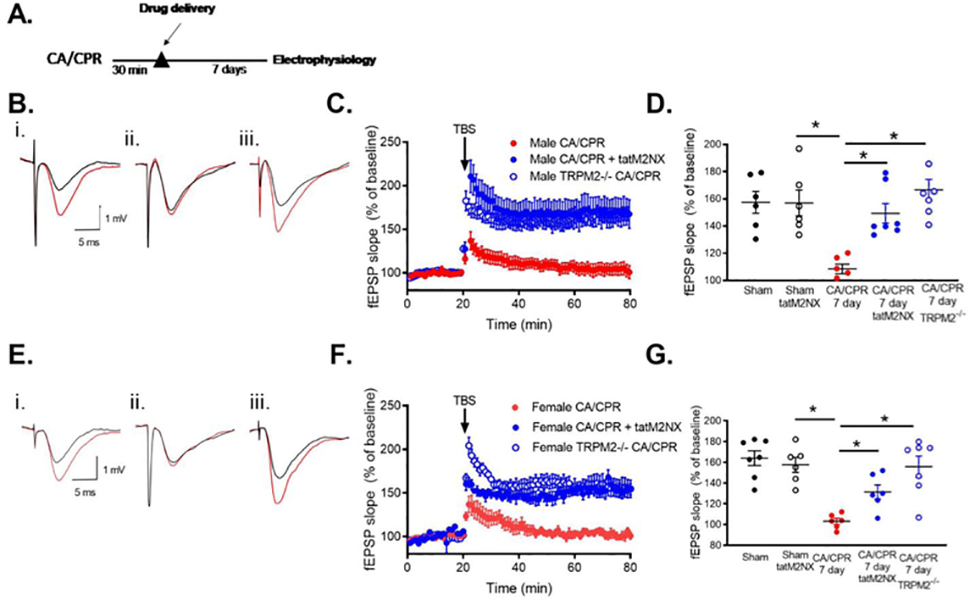
We next tested whether administration of tatM2NX 30 minutes after CA/CPR provides long-term benefit on hippocampal function in a sexually dimorphic manner as observed for neuroProtection (Figure 2A). We previously reported that CA/CPR in male mice impairs synaptically evoked NMDA receptor-dependent LTP when examining extracellular field recordings in acute hippocampal slices prepared 7 days after ischemia[33, 34]. This represents the sub-acute phase of recovery when cell death processes are complete and we can assess the function of surviving CA1 neurons. Here we confirm LTP impairment after CA/CPR in male mice by comparing the percent increase in synaptic response (in relation to baseline, set at 100%) in ischemic animals with sham controls at 7 days. In slices from sham mice, a brief theta burst stimulus (TBS: 40 pulses, 100Hz) at CA3 (Schaffer collateral)-CA1 synapses resulted in LTP that increased the slope of the field excitatory postsynaptic potential (fEPSP) to 161±9.2% of baseline (n=6, Figure 2B,C,D) after 60 minutes. In contrast, recordings obtained in slices from CA/CPR mice exhibited impairment of LTP with a fEPSP slope of 109±3.5% of baseline (n=6; p<0.05, compared to sham, p<0.05 compared to sham, Figure 2B,C,D). Administration of tatM2NX 30 min after reperfusion prevented the impairment of LTP 7 days after CA/CPR (179±20.2%, n=5), recovering to sham control levels (p<0.05 compared to CA/CPR,Figure 2B,C,D). There was no effect of tatM2NX on LTP in sham mice 7 days after surgery (160±7.0, n=8, p>0.05 compared to sham). Similar to males, we observed an LTP deficit in female mice 7 days after recovery from CA/CPR compared to shams (Sham: 160±7.4%, n=6; CA/CPR: 105±4.8%, n=6, p<0.05, Figure 2E,F,G). Despite the lack of histological protection with tatM2NX in females, we observed protection of LTP 7 days after CA/CPR, with an increase to 153.4±5.7% of baseline when tatM2NX was administered 30 min after reperfusion (n=6, p<0.05 compared to CA/CPR, Figure 2E, F,G). There was no effect on input-output curves or paired-pulse ratios by ischemia or tatM2NX in male or female mice (Supplemental Table 1). Consistent with acute pharmacologic inhibition, knockout of trpm2 gene preserved LTP 7 days after CA/CPR in both sexes (male TRPM2−/− after CA/CPR: 166.6±7.7%, n=7, p>0.05 compared to WT, Figure 2C,D); female TRPM2−/− after CA/CPR: 155.9±10.1%, n=7, p>0.05 compared to WT, Figure 2F,G). These data suggest that ischemia-induced activation of synaptic TRPM2 channels contributes to synaptic plasticity deficits in both sexes. This remarkable observation of synaptic protection in females without neuroprotection suggests disparate pathways involved in cell death and synaptic dysfunction.
Figure 2. Acute inhibition of TRPM2 with tatM2NX prevents synaptic plasticity deficits.
A) Experimental timeline B) Representative trace of recordings obtained in MALE (i) sham, (ii) 7 days after CA/CPR and (iii) 7 days after CA/CPR mice treated at 30 min with 20 mg/kg tatM2NX (iii). C) Time course of fEPSP slope from MALE mice 7 days after CA/CPR (red), 7 days after CA/CPR mice treated at 30 min with 20 mg/kg tatM2NX (blue) and male TRPM2−/− mice 7 days after CA/CPR (open blue). D) Quantification of change in fEPSP slope 60 minutes after TBS stimulation normalized to 20 minutes of baseline recording. E) Representative trace of recordings obtained in FEMALE (i) sham, (ii) 7 days after CA/CPR and (iii) 7 days after CA/CPR mice treated at 30 min with 20 mg/kg tatM2NX. F) Time course of fEPSP slope from FEMALE mice 7 days after CA/CPR (red), 7 days after CA/cPr mice treated at 30 min with 20 mg/kg tatM2NX (blue) and male TRPM2−/− mice 7 days after CA/CPR (open blue). G) Quantification of change in fEPSP slope 60 minutes after TBS stimulation normalized to 20 minutes of baseline recording.
Delayed TRPM2 inhibition reverses CA/CPR-induced synaptic impairments
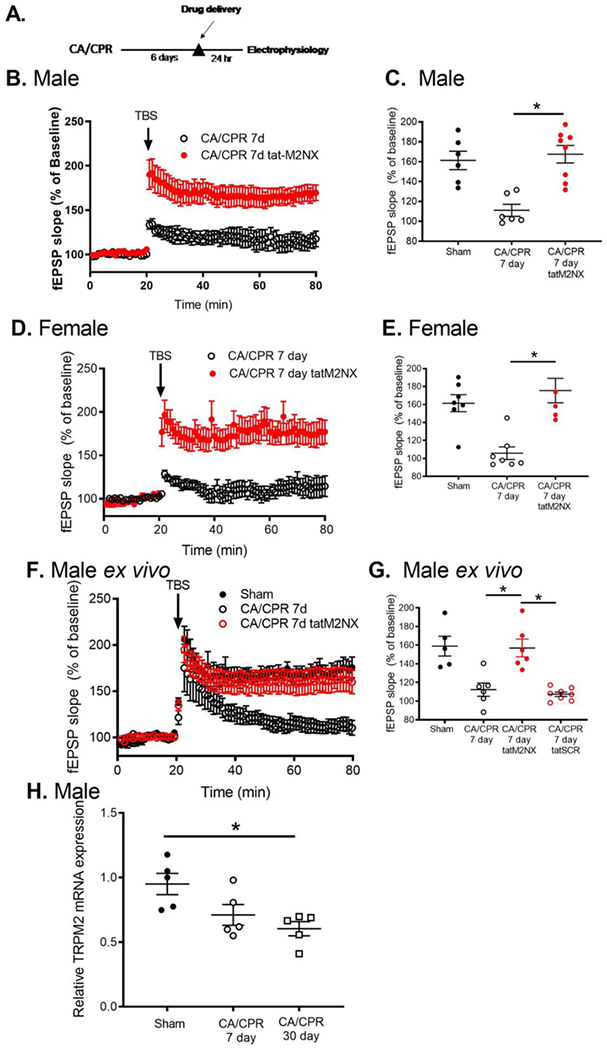
In light of our novel observation that TRPM2-mediated signaling contributes differently to cell death and synaptic dysfunction, we next aimed to determine whether TRPM2 inhibition is beneficial at delayed time points when cell death processes are complete (>3 days [35]) and synaptic dysfunction is present in the surviving network. Male and female mice were injected with tatM2NX (20mg/kg iv[23]) on day 6 after CA/CPR and hippocampal slices were prepared for LTP experiments the next day, on day 7 after CA/CPR (Figure 3A). In vivo inhibition of TRPM2 channels resulted in recovery of synaptic plasticity to sham levels in male (167.6±8.8%, n=8, p<0.05 compared to sham, Figure 3B,C) and female (175.5±13.6%, n=6, p<0.05 compared to sham, Figure 3D,E) CA/CPR mice. We extended our in vivo observation to ex vivo brain slice experiments in order to elucidate rapid signaling mechanisms. Brain slices prepared for field recording 7 days after CA/CPR were incubated in tatM2NX or tatSCR (1 μM in ACSF) for 2 hours prior to LTP experiments (ex vivo). Inhibition of TRPM2 at this delayed time point resulted in reversal of LTP impairment (154.0±6.7%, n=9, p<0.05 compared to 7d CA/CPR Figure 3F,G). Ischemia and delayed administration of tat-M2NX had no effect on presynaptic release (paired pulse stimulation) or axonal excitability as shown by input-output response (Supplemental Table 2). These data indicate that ischemia causes persistent synaptic TRPM2 channel activity which actively impairs plasticity. To assess whether this ischemia-induced increase in TRPM2 activity is related to TRPM2 expression, we performed quantitative RT-PCR. We did not observe increased TRPM2 channel mRNA expression consistent with increased activity (Figure 3H). We were unable to directly measure TRPM2 protein levels as Western blots using commercially available anti-TRPM2 antibodies showed positive bands in TRPM2−/− mice (Supplemental Figure 1).
Figure 3. Delayed inhibition of TRPM2 with tat-M2NX REVERSES synaptic plasticity deficits.
A) Experimental timeline. B) Time course of fEPSP slope from MALE mice 7 days after CA/CPR (red) and in mice administered 20 mg/kg tatM2NX 6 days after CA/CPR and recordings obtained 7 days after CA/CPR (black). C) Quantification of change in fEPSP slope 60 minutes after TBS stimulation normalized to 20 minutes of baseline recording. D) Time course of fEPSP slope from slices obtained 7 days after CA/CPR in FEMALE mice (black) and in mice administered 20 mig/kg tatM2NX 6 days after CA/cPr and recordings obtained from slices 7 days after CA/CPR (red). E) Quantification of change in fEPSP slope 60 minutes after TBS stimulation normalized to 20 minutes of baseline recording. F) Time course of fEPSP slope from male mice after sham surgery (black), 7 days after CA/CPR (red) and 7 days after CA/CPR slices treated 1 μM tatM2NX for 2 hours prior to recording (open black). G) Quantification of change in fEPSP slope 60 minutes after TBS stimulation normalized to 20 minutes of baseline recording. H) Quantitative real time RT-PCR analysis of TRPM2 mRNA expression from hippocampus obtained from sham mice and mice 7 or 30 days after CA/CPR.
Delayed inhibition of calcineurin and GSK3β rescues synaptic function
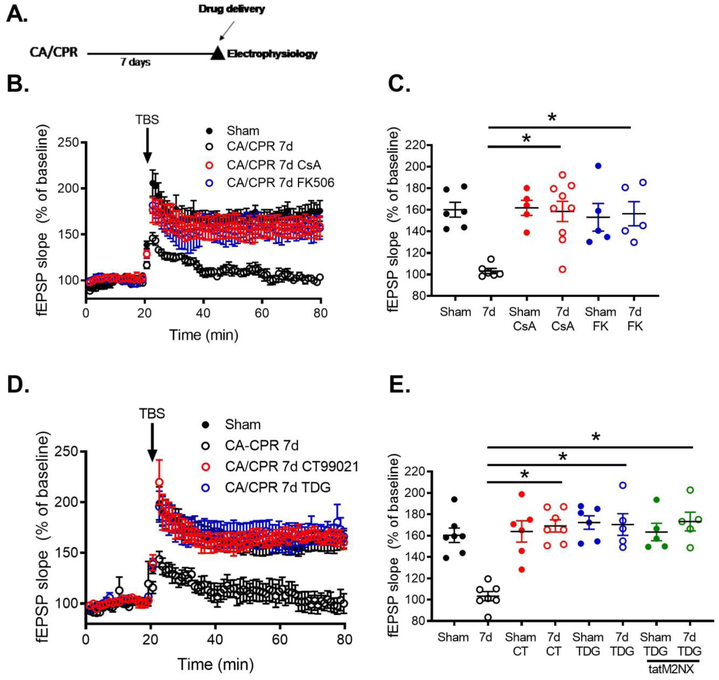
We next tested possible downstream targets that contribute to TRPM2-induced LTP impairments following ischemia. In light of recent studies showing TRPM2 alterations of long-term depression via increased GSK3β signaling [28], we hypothesize that TRPM2-induced calcium influx activates calcineurin (CaN) and consequently increased GSK3β. To assess the role of calcineurin (CaN) on post-ischemic LTP deficits, we used cyclosporine A (CsA) or FK-506 to inhibit CaN in acute slices taken from mice 7 days after recovery from CA/CPR in male mice. Figure 4 shows that bath application of CsA (1 μM) for 1 hour reverses the CA/CPR-induced loss of LTP in acute slices, recovering to 158±9.4% (n=9, p<0.05 compared to sham CsA treated slices, Figure 4B,C). There was no effect of CsA on sham slices (Figure 4B,C). Importantly, CsA had no effect on presynaptic release (paired pulse stimulation) or axonal excitability as shown by input-output response (Supplemental Table 3). We used a second CaN inhibitor to confirm our data showing a role for CaN activity in ischemia-induced LTP deficits. Consistent with our CsA data, FK-506 also reversed CA/CPR induced synaptic impairment after 1 hour incubation (10 μM), recovering to 156±11.2% (n=5, p<0.05 compared to CA/CPR, Figure 4B,C). FK-506 did not effect LTP in sham slices, nor did it change paired pulse stimulation or input-output response (Supplemental Table 2).
Figure 4. Inhibition of CaN or GSK3β reverses TRPM2-mediated LTP deficits.
A) Experimental timeline. B) Time course of fEPSP slope from MALE sham control mice (black), 7 days after CA/CPR (open black circles), 7 days after CA/CPR slices treated 1 μM cyclosporine (blue) or 10 μM FK-506 (red) for 1 hour prior to recording (blue) and sham control mice 7 days after 20 mg/kg tatM2NX (green). C) Quantification of change in fEPSP slope 60 minutes after TBS stimulation normalized to 20 minutes of baseline recording. D) Time course of fEPSP slope from male sham control mice (black), 7 days after CA/CPR (open black circles), 7 days after CA/CPR slices treated 1 μM CT99021 (blue) or 1 μM Tidegulib (red) for 1 hour prior to recording (blue) and sham control mice 7 days after 20 mg/kg tatM2NX (green). E) Quantification of change in fEPSP slope 60 minutes after TBS stimulation normalized to 20 minutes of baseline recording.
To assess the role of GSK3β, we used two GSK3β inhibitors (CT99027, tideglusib) in our ex vivo LTP experiments. Figure 4 shows that exposure of post-ischemic slices to the GSK3β inhibitor CT99027 (1 μM) for 1 hour reversed LTP impairment, recovering to 169±5.6% (n=7, p<0.05 compared to CA/CPR, Figure 4D,E). Consistent with previous reports[36], bath application of CT99021 had no effect on the induction of LTP in sham control slices (Figure 4E). A different and more specific GSK3β inhibitor, tideglusib (TDG)[37], was also tested. Post-ischemic hippocampal slices exposed to TDG (1 μM) displayed reversed LTP impairment, recovering to 170±10.1%, (n=5, p<0.05 compared to CA/CPR, Figure 4D,E). TDG had no effect on sham slices (Figure 4E). Finally, to causally link TRPM2 activity and GSK3β activity, post-ischemic hippocampal slices were exposed to tatM2NX (1μM) for 2 hours in combination with TDG (1 μM). We observed similar recovery of LTP (173±8.7%, n=5, p>0.05, Figure 4E) compared to either drug alone. There was no effect of this combination of drugs on sham LTP (Figure 4E), paired pulse stimulation or input-output responses (Supplemental Table 3). Together, these data indicate that TRPM2 channels impair synaptic activity via CaN-GSK3β signaling following global cerebral ischemia.
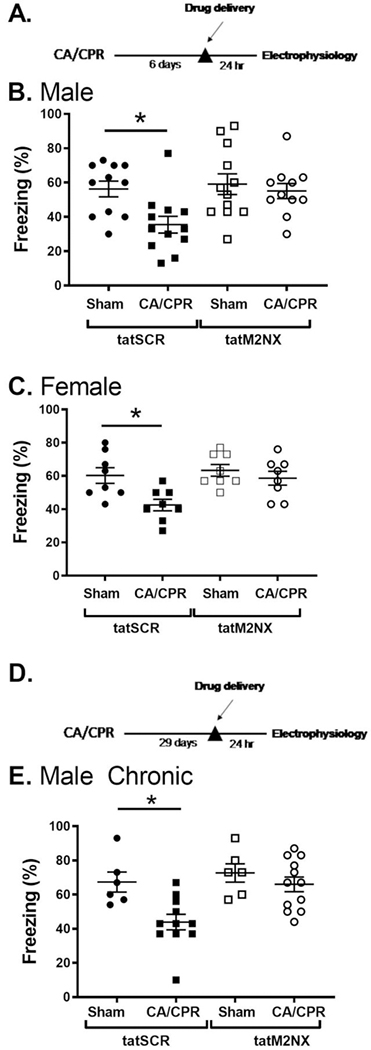
To determine the effect of persistent TRPM2 channel on hippocampal function, we performed contextual fear conditioning to analyze hippocampal-dependent learning and memory[38, 39]. Figure 5 shows that sham mice administered control peptide (tatSCR, 20mg/kg, ip) exhibited intact memory, as evidence by freezing behavior in 55.1±4.4% of time epochs (n=11) 24 hours after training. However, mice 7 days after CA/CPR administered tatSCR demonstrated decreased freezing (35.5±4.9%, n=12, p<0.05), indicating impairment in memory. Male mice tested 7 days after recovery from CA/CPR given tatM2NX (20mg/kg, ip) on day 6 after CA/CPR exhibited recovery of memory function (59.1±6.0%, n=12), similar to sham control levels. In the absence of ischemia, tatM2NX did not improve memory function (56.3±4.6%, n=11), indicating an ischemia-specific role for TRPM2 channels in memory function (Figure 5B). Female mice exhibited similar recovery of learning and memory after delayed inhibition of TRPM2 channels (Figure 5C). One week after sham surgery, female mice given tatSCR exhibited freezing behavior in 58.6.1±4.2% (n=8) of time epochs 24 hours after training. Females given tatSCR after CA/CPR had decreased freezing (42.5±3.5%, n=8, p<0.05). Administration of tatM2NX reversed memory impairments after CA/CPR (63.4±3.4%, n=8) and had no effect after sham surgeries (6o.3±4.7%, n=8). The benefit of delayed tatM2NX administration on memory behavior are consistent with our electrophysiology data showing that delayed administration of tatM2NX reverses ischemia-induced LTP deficits.
Figure 5. Delayed inhibition of TRPM2 with tatM2NX improves memory function.
A) Experimental timeline. B) Quantification of freezing behavior 24 hr after fear conditioning in a novel environment in MALE mice administered 20 mg/kg tatM2NX 6 days after recovery from CA/CPR and behavior testing initiated on day 7 after CA/CPR. C) Quantification of freezing behavior 24 hr after fear conditioning in a novel environment in FEMALE mice administered 20 mg/kg tatM2NX 6 days after recovery from CA/CPR and behavior testing initiated on day 7 after CA/CPR. D) Experimental timeline. E) Quantification of freezing behavior 24 hr after fear conditioning in a novel environment in male mice administered 20 mg/kg tatM2NX 29 days after recovery from CA/CPR and behavior testing initiated on day 30 after CA/CPR.
We went on to test whether administering tatM2NX at a chronic time point (four weeks) after CA/CPR had a similar effect (Figure 5D,E). Male mice were given a single injection of tatM2NX or tatSCR (20mg/kg, ip) on day 29 after CA/CPR or sham surgeries. There was no difference between tatM2NX and tatSCR in sham mice (72±5.4% and 67±5.9%, respectively, n=6 each, Figure 5). CA/CPR mice receiving tatSCR froze 44±4.5% (n=11), consistent with a persistent memory impairment at 30 days after CA/CPR. However, inhibition of TRPM2 with tatM2NX in mice having undergone CA/CPR rescued memory formation to sham levels with mice freezing 66±4.2% of the time (n=12, p<0.05).
Discussion
The results presented here is consistent with several recent studies showing that ischemia causes a persistent impairment of hippocampal LTP[15, 13, 14] and importantly that these LTP deficits correlate well with our behavioral data indicating impairments in memory and learning function. Our new data show that inhibition of TRPM2 channels (with tatM2NX) 7 days after recovery from cerebral ischemia (in vivo or ex vivo) reverses ischemia-induced deficits in synaptic plasticity and improves memory function in both males and females. This delayed administration of a pharmacological agent to enhance synaptic function in the hippocampus is a unique approach to improve recovery. While a unique approach to ischemia-induced memory complications, our data is consistent with several recent studies showing that delayed treatment can improve sensorimotor function following experimental stroke [9–12]. Further, we show that treatment with tatM2NX one month after recovery from cardiac arrest reverses the stable memory deficit observed. A major roadblock in the study of TRPM2 channels in brain ischemia and neurodegenerative disease has been the lack of specific inhibitors that are amenable to use in vivo. Our recent study was the first to report the safety and efficacy of our novel TRPM2 channel inhibitor to reduce brain injury following experimental stroke[23, 27]. The lack of additional effect of tatM2NX in TRPM2−/− mice provides strong evidence for specificity. Consequently, tatM2NX serves as an important new pharmacological tool in the current study to understand how TRPM2 is involved in hippocampal function in ischemic mice. Therefore, our novel strategy of delayed TRPM2 inhibition with a novel specific inhibitor unlocks potential new therapeutic avenues following cerebral ischemia, and potentially other neurologic diseases associated with memory deficits due to TRPM2.
A role for TRPM2 channels in mediating ischemia/reperfusion injury in the brain has been proposed for several years[40, 41, 21]. However, we were the first to directly demonstrate the role of TRPM2 channels following experimental stroke, making the remarkable observation that inhibitors, and knockdown, of TRPM2 reduce ischemic injury following experimental stroke (MCAO) in the male brain, while having no effect in the female brain[42, 25]. Other studies using male TRPM2−/− mice observe similar neuroprotection[43, 20, 44], although none of these studies used female mice to confirm our observation regarding male-specific role of TRPM2 channels in neuroprotection. We have gone on to confirm this initial observation and demonstrate the same sex-specific phenomena following global cerebral ischemia[29] and in vitro[45]. Our recent data has shed some light on the mechanism of sex-specific activation of TRPM2 during the acute phase, implicating male-specific activation of PARP-1 as the upstream mediator of TRPM2 channel engagement in the male brain following ischemia[25]. In contrast, data presented in the current study demonstrates that despite male-specific neuroprotection in TRPM2−/− mice and acute tatM2NX treated mice, both male and female mice show improved synaptic plasticity and memory function when TRPM2 is inhibited or genetically knocked out. These data indicate disparate signaling pathways engaging TRPM2 in cell death in males only and TRPM2-mediated synaptic dysfunction of surviving neurons observed in both male and females.
This study presents evidence for the first time that ischemia causes sustained TRPM2 activity that impairs synaptic plasticity and memory function after cardiac arrest-induced global cerebral ischemia. The rapid reversal of LTP deficits in acute brain slices provides strong evidence that ischemia causes aberrant synaptic TRPM2 channel activity. It was recently shown that TRPM2−/− mice have reduced hippocampal NMDA receptor function[20]. However, our data shows that acute inhibition of TRPM 2 channels does not impair NMDA receptor function, as tatM2NX administered either in vivo or ex vivo increases NMDA-receptor dependent LTP in post-ischemic mice. Further, our recent studies indicate intact NMDA receptor activity 7 and 30 days after global cerebral ischemia [15, 31]. Importantly, our data in sham control animals show no effect of tatM2NX on excitability or LTP, consistent with recent studies showing no effect of TRPM2 channels on hippocampal LTP [28]. This is an important finding as it increases the clinical impact of using TRPM2 channel inhibitors that restore LTP without impacting physiological signaling.
The mechanism of ischemia-induced increase in TRPM2 channel activity in the hippocampus remains to be determined. Our current data shows no increase of TRPM2 channel expression 7–30 days following ischemia, implicating upstream signaling pathways in TRPM2 channel activity following ischemia. To attempt to understand the mechanism of ischemia-induced activation of TRPM2 channels during these delayed time-points, we interrogated several proteins known to regulate TRPM2 channel activity. TRPM2 channels are activated by increased levels of intracellular ADPribose. In particular, it has been demonstrated that ADPribose generated following activation of PARP-1 stimulates TRPM2 channel activity[46–48] and we have observed this pathway as fundamental in the sex-specific activation of TRPM2 channels during the acute phase following cerebral ischemia[49, 25]. Quantification of PARP-1 mRNA at delayed time-points (7 and 30 days), showed a small decline in expression (Supplemental Figure 2). Therefore, PARP-1 is unlikely to explain the enhanced activity observed at these delayed time-points. In addition, PARP-1 is a nuclear protein and thus generates increased ADPribose within the soma of neurons, which is not consistent with the increased synaptic TRPM2 channel activity implicated in the current study. Similarly, the enzyme sirtuin 2 (SirT2), has been demonstrated to produce O-acetyl-ADPribose which can activate TRPM2 channels[50]. Supplemental Figure 2 illustrates that ischemia does not alter SirT2 expression. Therefore, we hypothesize alternative mechanisms of TRPM2 regulation following cerebral ischemia. Unfortunately, the lack of specific anti-TRPM2 antibodies (Supplemental Figure 1) limits our ability to assess post-translational modifications that likely impact TRPM2 channel activity. Clearly, further studies are needed to fully elucidate the mechanism of ischemia-induced TRPM2 channel activity. Nonetheless, the data presented in this study indicate that our novel TRPM2 inhibitor, tatM2NX, represents a new restorative therapeutic strategy, eg. pharmacological rehabilitation.
Lastly, our data implicates increased CaN-GSK3B signaling as the downstream mediator of TRPM2-induced synaptic plasticity dysfunction following ischemia. Glycogen synthase kinase 3β (GSK3β) is a neuron-specific serine/threonine kinase that is abundant within the central nervous system and is involved in several cellular process. GSK3β contributes to ischemic brain injury, as evidence by increased GSK3β activity following ischemia and the ability of GSK3β inhibitors to reduce ischemic brain injury[51, 52]. In addition to its well-established role in determining sensitivity to injury, GSK3β activation plays an integral role in synaptic plasticity, particularly the induction of long-term depression (LTD) (for review see[53–56]). Activation of LTD impairs the consequent induction of LTP. Therefore, we hypothesize that ischemia activates intracellular signaling pathways involved in LTD, thereby inhibiting/impairing LTP. Indeed, a recent study showed that that TRPM2−/− mice have impaired LTD, likely due to loss of GSK3β activity[28]. Relevant to our hypothesis, excessive activity of GSK3β impairs LTP[57]. Our data is consistent with this mechanism of impaired LTP, as inhibition of GSK3β reverses ischemia-induced LTP deficits. We do not observe an additive impact on LTP with the co-application of the TRPM2 channel inhibitor tatM2NX and GSK3β inhibitor, providing further evidence for TRPM2-mediated activation of GSK3β. The protein phosphatase calcineurin (CaN) is a well-established regulator of GSK3β activity, via dephosphorylation of the S9 residue of GSK3β. CaN is a calcium-activated phosphatase and therefore a logical mediator of TRPM2 channel activation of GSK3β. Consistent with this, it has recently been demonstrated that TRPM2 can activate (dephosphorylate at S9) GSK3β in hippocampal neurons[28, 58]. We show here that ischemia-induced hippocampal dysfunction is reversed by inhibition of calcineurin and GSK3β. Thus, our data leads us to favor a model of ischemia-induced TRPM2 channel activity resulting in increased Ca2+, which activates the calcium-dependent phosphatase CaN causing GSK3β activation and impaired LTP. Interestingly, hippocampal dysfunction observed in models of Alzheimer’s disease have also been linked to increased GSK3β activity (for review see[59]), and a recent study showed that aged APP/PS1 Alzheimer’s mice crossed with the TRPM2−/− mice exhibit improved hippocampal plasticity, memory and normalized GSK3β activity[17]. Therefore, further studies are needed to elucidate the shared mechanisms between acute cerebral ischemia and neurodegenerative diseases with regards to TRPM2 channel signaling, synaptic plasticity and cognitive decline.
Conclusions
We provide the first direct evidence that hippocampal synaptic dysfunction can be targeted at delayed timepoints to produce reversal of chronic memory deficits following cerebral ischemia. We show efficacy of our novel TRPM2 channel inhibitor, tatM2NX, administered in vivo at timepoints beyond those observed for neuroprotective strategies, thus describing the potential neuro-restorative function of tatM2NX. We further describe that ischemia-induced memory deficits are mediated at least in part by aberrant activity of the TRPM2-CaN-GSK3β signaling cascade that actively inhibits synaptic plasticity. Our strategy of targeting delayed TRPM2 channel inhibition weeks to months after cardiac arrest affords a unique new opportunity to reverse learning and memory impairments.
Materials and Methods
Experimental Models and Subject Details
All experimental protocols were approved by the University of Colorado-Denver Institutional Animal Care and Use Committee (IACUC) and conformed to the National Institutes of Health guidelines for care and use of animals. Adult C57Bl/6 (20–25g) mice (Charles River Laboratory) were used for this study. The mice were housed in a standard 12h light and 12h dark cycle and had free access to food and water. All experiments in the study adhered to the ARRIVE guidelines for animal experiments. Mice were randomly assigned to experimental groups and the investigator was blinded as stated below.
Cardiac Arrest and Cardiopulmonary Resuscitation (CA/CPR)
Cardiac arrest in 8–12 week old mice was performed as previously described[60]. Briefly, mice were anesthetized using 3% isoflurane and maintained with 1.5–2% isoflurane in 25% fraction of inspired oxygen (FiO2) via face-mask. Body temperature was maintained at 37°C using a heat lamp and heating pad while being monitored with temperature probes placed into the left ear canal and rectum. For drug administration, a PE-10 catheter was inserted into the right internal jugular vein and flushed with heparinized 0.9% normal saline solution. Animals were endotracheally intubated using a 24G intravenous catheter and connected to a mouse ventilator (Minivent, Hugo Sachs Elektronik, March-Hugstetten, Germany) set to a respiratory rate of 160 breaths per minute. Cardiac function was monitored throughout the experiment with electrocardiography (EKG). Cardiac arrest was induced by injection of 30μL of 0.5M KCl via the jugular catheter and confirmed by asystole on EKG and absence of spontaneous breathing. The endotracheal tube was disconnected from the ventilator during cardiac arrest and no spontaneous breathing was observed. During this time anesthesia was not being delivered. Body warming was ceased 1 min prior to cardiac arrest. During cardiac arrest the pericranial temperature was maintained at 37.5±o.5°C by using a water-filled coil. Body temperature was allowed to fall spontaneously to 35°C. Resuscitation was begun 8 minutes after the initiation of cardiac arrest by slow injection of 0.2–0.5mL of epinephrine (16μg epinephrine/mL 0.9% saline), administration of chest compressions at a rate of approximately 300 min−1 and resumption of ventilation with 100% FiO2 at a rate of 210 breaths/min. Chest compressions were stopped upon return of spontaneous circulation (ROSC), defined as electrical evidence of cardiac contractions. If ROSC was not achieved within 3 minutes of CPR initiation, resuscitation was stopped and the animal was excluded from the study. Five minutes following ROSC, FiO2 was decreased to 50%. When the spontaneous respiratory rate was 30 breaths/min, the ventilator was adjusted to 150 breaths/min and when the animals had at least 60 spontaneous breaths/min, the endotracheal tube was removed. Temperature probes and intravascular catheters were removed and the surgical wounds were closed.
Drug treatment in CA/CPR mice
For acute neuroprotection experiments, male and female mice were injected with tatSCR (20mg/kg) or tatM2NX (20mg/kg) via intravenous (iv) route 30min after sham or CA/CPR surgery. For restoration of function experiments, male and female mice were injected via intraperitoneal route (ip) with tatSCR (20mg/kg) or tatM2NX (20mg/kg) 6 or 29 days after sham or CA/CPR surgery and studied on day 7 or 30.
Histology
At 3 days after CA/CPR mice were transcardially perfused with 4% paraformaldehyde (PFA) and post-fixed in PFA at 4 °C overnight. Brains were paraffin embedded and coronal sections containing hippocampus (6 μm at 100 μm intervals) were cut and stained with hematoxylin and eosin (H&E). Staining was visualized with a bright field Leica DM2000 microscope (Leica Microsystems, Buffalo Grove, IL, USA) and analysis of cell morphology was performed bilaterally on 3 sections containing anterior hippocampus. Injured CA1 neurons were identified by hypereosinophilic cytoplasm and pyknotic nuclei and were presented as a percent of total CA1 neurons (% ischemic neurons ± SEM) as previously described[27].
Acute Hippocampal Slice Preparation
Hippocampal slices were prepared at 7 or 30 days after recovery from CA/CPR or sham surgeries. Mice were anesthetized with 3% isoflurane in an O2-enriched chamber. Mice were transcardially perfused with ice-cold (2–5°C) oxygenated (95% O2/5% CO2) artificial cerebral spinal fluid (aCSF) for 2 minutes prior to decapitation. The brains were then extracted and placed in the same aCSF. The composition of aCSF was the following (in mmol/L): 126 NaCl, 2.5 KCl, 25 NaHC03, 1.3 NaH2PO4, 2.5 CaCl2, 1.2 MgCl2 and 12 glucose[34]. Horizontal hippocampal slices (300μm thick) were cut with a Vibratome 1200 (Leica) and transferred to a holding chamber containing aCSF for at least 1 hour before recording.
Electrophysiology
Synaptically evoked field potentials were recorded from hippocampal CA1 slices that were placed on a temperature controlled (31±0.5°C) interface chamber perfused with aCSF at a rate of 1.5ml/min. Excitatory post-synaptic potentials (fEPSP) were produced by stimulating the Schaffer collaterals (CA3 axons) and recording in the stratum radiatum of the CA1 region. The fEPSPs were adjusted to 50% of the maximum slope and test pulses were evoked every 20 seconds. Paired pulse responses were recorded using a 50ms interpulse interval (20Hz) and expressed as a ratio of the slopes of the second pulse over the first pulse. A 20 min stable baseline was established before delivering a theta burst stimulation (TBS) train of four pulses delivered at 100Hz in 30ms bursts repeated 10 times with 200ms interburst intervals[34]. Following TBS, the fEPSP was recorded for 60 min. The averaged 10 min slope from 50–60 min after TBS was divided by the average of the 10 min baseline (set to 100%) prior to TBS to determine the amount of potentiation. Analog fEPSPs were amplified (1000X) and filtered through a pre-amplifier (Model LP511 AC, Grass Instruments) at 1.0kHz, digitized at 10kHz and stored on computer for later off-line analysis (Clampfit 10.4, Axon Instruments). The derivative (dV/dT) of the initial initial fEPSP slope was measured. For time course graphs, normalized fEPSP slope values were averaged and plotted as the percent change from baseline. Two electrophysiologists (RD and JO) independently verified all LTP results in this report.
Quantitative real-time PRC
For quantitative PCR measurement of TRPM2, PARP2 and SIRT2 transcripts, hippocampi were harvested at various times after CA/CPR or sham surgeries. Total RNA was isolated using the RNAqueous-4 PCR kit (Ambion, Austin, TX, USA) per the manufacturer’s instructions. Briefly, approximately 1–3 mg of tissue was lysed in lysis buffer and total RNA was isolated and eluted from a column with 50μL RNase-free elution buffer, and further treated with Turbo DNase (Ambion, Austin, TX, USA,). First strand cDNA was reverse transcribed from 500ng total RNA with High Capacity cDNA archive Kit (Applied Biosystems, Foster City, CA, USA). Real-time PCR reactions using SsoFast PCR mastermix was performed on BioRad CFX connect detection system in duplicate using 50ng cDNA. Primers used to detect TRPM2, PARP2 and SIRT2 were synthesized by Invitrogen. The housekeeping gene 18s was also assayed for each sample using 5ng of cDNA. Cycle parameters used were 95°C for 10min followed by 40 cycles of 95°C for 15s and 60°C for 30s. Relative expression levels were calculated using the ΔΔCT as the ratio of the target gene to 18S.
Behavioral test
The contextual fear conditioning (CFC) paradigm was utilized as a hippocampal-dependent memory task[61]. The apparatus consisted of two fear-conditioning chambers with shock grid floors, consisting of 16 stainless steel rods connected to a shock generator (Colbourn Instruments, Model H13–15, Whitehall, PA, USA). Mice were transported in white buckets during the training and testing sessions. During training, mice were allowed to habituate to the conditioning chamber for two separate 2 minute pre-exposure sessions followed by a foot shock (2-sec/1.0 mA electric shock) immediately after the second exposure. Following shock, mice were returned to their home cages. Memory was tested 24 hours later by transporting mice in white buckets and placed back into the fear conditioning chambers. Memory was determined by percentage of freezing behavior, measured in 10 sec intervals across a 5-minute test by a blinded observer and was defined as the absence of movement except for heart beat or respiration.
Quantification and Statistical Analysis
All data is presented as mean±SEM. Sample size and power analyses were performed using previous data generated in our laboratory. To determine group size for LTP recordings, to observe a 40% change in LTP between two groups with a standard deviation of 20 and an alpha error of 5% and a beta error of 80%, a group of 6 slices per group are required, with no more than 2 slices per animal used in analysis per condition. For behavior studies, to observe a 25% change in object exploration between two groups with a standard deviation of 15%, with an alpha error of 5% and a beta error of 80% 8 animals per group are require for behavior experiments. Statistical analysis was performed using Student’s t-test for two-group comparisons and one-way ANOVA with Tukey post-hoc test for comparison of multiple groups.
Supplementary Material
Acknowledgments
We thank Joan Yonchek for her expert assistance with histology preparation and unwavering support.
Footnotes
Compliance with Ethical Standards: The authors declare that they have no conflict of interest, with the exception of the study being funded by extramural grant support. This research was supported by National Institutes of Health grants T32GM007635 (Pharmacology training grant), K08NS097586 (R.M.D), R01NS092645 (to P.S.H). Ethical approval: All applicable national and institutional guidelines for the care and use of animals were followed. Ethical approval: This article does not contain any studies with human participants.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019:CIR0000000000000659. doi: 10.1161/CIR.ooooooooooooo659. [DOI] [PubMed] [Google Scholar]
- 2.Tiainen M, Poutiainen E, Kovala T, Takkunen O, Happola O, Roine RO. Cognitive and neurophysiological outcome of cardiac arrest survivors treated with therapeutic hypothermia. Stroke; a journal of cerebral circulation. 2007;38(8):2303–8. doi: 10.1161/STROKEAHA.107.483867. [DOI] [PubMed] [Google Scholar]
- 3.Moulaert VR, Verbunt JA, van Heugten CM, Wade DT. Cognitive impairments in survivors of out-of-hospital cardiac arrest: a systematic review. Resuscitation. 2009;8o(3):297–305. doi: 10.1016/j.resuscitation.2008.10.034. [DOI] [PubMed] [Google Scholar]
- 4.Cronberg T, Lilja G, Rundgren M, Friberg H, Widner H. Long-term neurological outcome after cardiac arrest and therapeutic hypothermia. Resuscitation. 2009;80(10):1119–23. doi: 10.1016/j.resuscitation.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 5.Mateen FJ, Josephs KA, Trenerry MR, Felmlee-Devine MD, Weaver AL, Carone M et al. Long-term cognitive outcomes following out-of-hospital cardiac arrest: a population-based study. Neurology. 2011;77(15):1438–45. doi: 10.1212/WNL.obo13e318232ab33. [DOI] [PubMed] [Google Scholar]
- 6.Beesems SG, Wittebrood KM, de Haan RJ, Koster RW. Cognitive function and quality of life after successful resuscitation from cardiac arrest. Resuscitation. 2014;85(9):1269–74. doi: 10.1016/j.resuscitation.2014.05.027. [DOI] [PubMed] [Google Scholar]
- 7.Horn M, Schlote W. Delayed neuronal death and delayed neuronal recovery in the human brain following global ischemia. Acta Neuropathol. 1992;85(1):79–87. [DOI] [PubMed] [Google Scholar]
- 8.Deng G, Yonchek JC, Quillinan N, Strnad FA, Exo J, Herson PS et al. A novel mouse model of pediatric cardiac arrest and cardiopulmonary resuscitation reveals age-dependent neuronal sensitivities to ischemic injury. Journal of neuroscience methods. 2014;222:34–41. doi: 10.1016/j.jneumeth.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clarkson AN, Huang BS, Macisaac SE, Mody I, Carmichael ST. Reducing excessive GABA-mediated tonic inhibition promotes functional recovery after stroke. Nature. 2010;468(7321):305–9. doi: 10.1038/nature09511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carmichael ST. Brain excitability in stroke: the yin and yang of stroke progression. Archives of neurology. 2012;69(2):161–7. doi: 10.1001/archneurol.2011.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joy MT, Ben Assayag E, Shabashov-Stone D, Liraz-Zaltsman S, Mazzitelli J, Arenas M et al. CCR5 Is a Therapeutic Target for Recovery after Stroke and Traumatic Brain Injury. Cell. 2019;176(5):1143–57 e13. doi: 10.1016/j.cell.2019.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin YH, Dong J, Tang Y, Ni HY, Zhang Y, Su P et al. Opening a New Time Window for Treatment of Stroke by Targeting HDAC2. J Neurosci. 2017;37(28):6712–28. doi: 10.1523/JNEUROSCI.0341-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orfila JE, Grewal H, Dietz RM, Strnad F, Shimizu T, Moreno M et al. Delayed inhibition of tonic inhibition enhances functional recovery following experimental ischemic stroke. J Cereb Blood Flow Metab. 2017:271678X17750761. doi: 10.1177/0271678X17750761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dietz RM, Orfila JE, Rodgers KM, Patsos OP, Deng G, Chalmers N et al. Juvenile cerebral ischemia reveals age-dependent BDNF-TrkB signaling changes: Novel mechanism of recovery and therapeutic intervention. J Cereb Blood Flow Metab. 2018:271678X18766421. doi: 10.1177/0271678X18766421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orfila JE, Shimizu K, Garske AK, Deng G, Maylie J, Traystman RJ et al. Increasing small conductance Ca2+-activated potassium channel activity reverses ischemia-induced impairment of long-term potentiation. The European journal of neuroscience. 2014;40(8):3179–88. doi: 10.1111/ejn.12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fonfria E, Murdock PR, Cusdin FS, Benham CD, Kelsell RE, McNulty S. Tissue distribution profiles of the human TRPM cation channel family. J Recept Signal Transduct Res. 2006;26(3):159–78. doi: 10.1080/10799890600637506. [DOI] [PubMed] [Google Scholar]
- 17.Ostapchenko VG, Chen M, Guzman MS, Xie YF, Lavine N, Fan J et al. The Transient Receptor Potential Melastatin 2 (TRPM2) Channel Contributes to beta-Amyloid Oligomer-Related Neurotoxicity and Memory Impairment. J Neurosci. 2015;35(45):15157–69. doi: 10.1523/JNEUROSCI.4081-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alawieyah Syed Mortadza S, Sim JA, Neubrand VE, Jiang LH. A critical role of TRPM2 channel in Abeta42 -induced microglial activation and generation of tumor necrosis factor-alpha. Glia. 2018;66(3):562–75. doi: 10.1002/glia.23265. [DOI] [PubMed] [Google Scholar]
- 19.Jang Y, Lee SH, Lee B, Jung S, Khalid A, Uchida K et al. TRPM2, a Susceptibility Gene for Bipolar Disorder, Regulates Glycogen Synthase Kinase-3 Activity in the Brain. J Neurosci. 2015;35(34):11811–23. doi: 10.1523/JNEUROSCI.5251-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alim I, Teves L, Li R, Mori Y, Tymianski M. Modulation of NMDAR subunit expression by TRPM2 channels regulates neuronal vulnerability to ischemic cell death. J Neurosci. 2013;33(44):17264–77. doi: 10.1523/JNEUROSCI.1729-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rempe DA, Takano T, Nedergaard M. TR(I)Pping towards treatment for ischemia. NatNeurosci. 2009;12(10):1215–6. [DOI] [PubMed] [Google Scholar]
- 22.Jia J, Verma S, Nakayama S, Quillinan N, Grafe MR, Hurn PD et al. Sex differences in neuroprotection provided by inhibition of TRPM2 channels following experimental stroke. JCerebBlood Flow Metab. 2011;31:2160–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shimizu T, Dietz RM, Cruz-Torres I, Strnad F, Garske AK, Moreno M et al. Extended therapeutic window of a novel peptide inhibitor of TRPM2 channels following focal cerebral ischemia. Experimental neurology. 2016;283(Pt A):151–6. doi: 10.1016/j.expneurol.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakayama S, Vest R, Traystman RJ, Herson PS. Sexually Dimorphic Response of TRPM2 Inhibition Following Cardiac Arrest-Induced Global Cerebral Ischemia in Mice. Journal of molecular neuroscience : MN. 2013. doi: 10.1007/s12031-013-0005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shimizu T, Macey TA, Quillinan N, Klawitter J, Perraud AL, Traystman RJ et al. Androgen and PARP-1 regulation of TRPM2 channels after ischemic injury. J Cereb Blood Flow Metab. 2013;33(10):1549–55. doi: 10.1038/jcbfm.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quillinan N, Grewal H, Klawitter J, Herson PS. Sex Steroids Do Not Modulate TRPM2-Mediated Injury in Females following Middle Cerebral Artery Occlusion. eNeuro. 2015;1:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimizu K, Quillinan N, Orfila JE, Herson PS. Sirtuin-2 mediates male specific neuronal injury following experimental cardiac arrest through activation of TRPM2 ion channels. Experimental neurology. 2016;275 Pt 1:78–83. doi: 10.1016/j.expneurol.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xie YF, Belrose JC, Lei G, Tymianski M, Mori Y, Macdonald JF et al. Dependence of NMDA/GSK-3beta mediated metaplasticity on TRPM2 channels at hippocampal CA3-CA1 synapses. Mol Brain. 2011;44. doi: 10.1186/1756-6606-4-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakayama S, Vest R, Traystman RJ, Herson PS. Sexually dimorphic response of TRPM2 inhibition following cardiac arrest-induced global cerebral ischemia in mice. J Mol Neurosci. 2013;51(1):92–8. doi: 10.1007/s12031-013-0005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deng G, Carter J, Traystman RJ, Wagner DH, Herson PS. Pro-inflammatory T-lymphocytes rapidly infiltrate into the brain and contribute to neuronal injury following cardiac arrest and cardiopulmonary resuscitation. Journal of neuroimmunology. 2014;274(1–2):132–40. doi: 10.1016/j.jneuroim.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dietz RM, Deng G, Orfila JE, Hui X, Traystman RJ, Herson PS. Therapeutic hypothermia protects against ischemia-induced impairment of synaptic plasticity following juvenile cardiac arrest in sex-dependent manner. Neuroscience. 2016;325:132–41. doi: 10.1016/j.neuroscience.2016.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herson PS, Hurn PD. Gender and the Injured Brain. ProgBrain Res. 2010;In Press. [DOI] [PubMed] [Google Scholar]
- 33.Deng G, Orfila JE, Dietz RM, Moreno-Garcia M, Rodgers KM, Coultrap SJ et al. Autonomous CaMKII Activity as a Drug Target for Histological and Functional Neuroprotection after Resuscitation from Cardiac Arrest. Cell Rep. 2017;18(5):1109–17. doi: 10.1016/j.celrep.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Increasing small conductance Ca(2+) -activated potassium channel activity reverses ischemia-induced impairment of long-term potentiation, (2014). [DOI] [PMC free article] [PubMed]
- 35.Deng G, Yonchek JC, Quillinan N, Strnad FA, Exo J, Herson PS et al. A novel mouse model of pediatric cardiac arrest and cardiopulmonary resuscitation reveals age-dependent neuronal sensitivities to ischemic injury. Journal of neuroscience methods. 2013. doi: 10.1016/j.jneumeth.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jo J, Whitcomb DJ, Olsen KM, Kerrigan TL, Lo SC, Bru-Mercier G et al. Abeta(1–42) inhibition of LTP is mediated by a signaling pathway involving caspase-3, Akt1 and GSK-3beta. Nature neuroscience. 2011;14(5):545–7. doi: 10.1038/nn.2785. [DOI] [PubMed] [Google Scholar]
- 37.Hicks JW, VanBrocklin HF, Wilson AA, Houle S, Vasdev N. Radiolabeled small molecule protein kinase inhibitors for imaging with PET or SPECT. Molecules. 2010;15(11):8260–78. doi: 10.3390/molecules15118260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu W, Wang L, Zhang L, Palmateer JM, Libal NL, Hurn PD et al. Isoflurane preconditioning neuroprotection in experimental focal stroke is androgen-dependent in male mice. Neuroscience. 2010;169(2):758–69. doi: 10.1016/j.neuroscience.2010.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uchida M, Palmateer JM, Herson PS, DeVries AC, Cheng J, Hurn PD. Dose-dependent effects of androgens on outcome after focal cerebral ischemia in adult male mice. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2009;29(8):1454–62. doi: 10.1038/jcbfm.2009.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller BA, Zhang W. TRP channels as mediators of oxidative stress. Advances in experimental medicine and biology. 2011;704:531–44. doi: 10.1007/978-94-007-0265-3_29. [DOI] [PubMed] [Google Scholar]
- 41.Szydlowska K, Tymianski M. Calcium, ischemia and excitotoxicity. Cell Calcium. 2010;47(2):122–9. [DOI] [PubMed] [Google Scholar]
- 42.Jia J, Verma S, Nakayama S, Quillinan N, Grafe MR, Hurn PD et al. Sex differences in neuroprotection provided by inhibition of TRPM2 channels following experimental stroke. J Cereb Blood Flow Metab. 2011;31(11):2160–8. doi: 10.1038/jcbfm.2011.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toda T, Yamamoto S, Umehara N, Mori Y, Wakamori M, Shimizu S. Protective Effects of Duloxetine against Cerebral Ischemia-Reperfusion Injury via Transient Receptor Potential Melastatin 2 Inhibition. J Pharmacol Exp Ther. 2019;368(2):246–54. doi: 10.1124/jpet.118.253922. [DOI] [PubMed] [Google Scholar]
- 44.Gelderblom M, Melzer N, Schattling B, Gob E, Hicking G, Arunachalam P et al. Transient receptor potential melastatin subfamily member 2 cation channel regulates detrimental immune cell invasion in ischemic stroke. Stroke. 2014;45(11):3395–402. doi: 10.1161/STROKEAHA.114.005836. [DOI] [PubMed] [Google Scholar]
- 45.Verma S, Quillinan N, Yang YF, Nakayama S, Cheng J, Kelley MH et al. TRPM2 channel activation following in vitro ischemia contributes to male hippocampal cell death. Neuroscience letters. 2012;530(1):41–6. doi: 10.1016/j.neulet.2012.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fonfria E, Marshall IC, Benham CD, Boyfield I, Brown JD, Hill K et al. TRPM2 channel opening in response to oxidative stress is dependent on activation of poly(ADP-ribose) polymerase. BrJPharmacol. 2004;143(1):186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miller BA. Inhibition of TRPM2 function by PARP inhibitors protects cells from oxidative stress-induced death. British journal of pharmacology. 2004;143(5):515–6. doi: 10.1038/sj.bjp.0705923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang KT, Chang WL, Yang PC, Chien CL, Lai MS, Su MJ et al. Activation of the transient receptor potential M2 channel and poly(ADP-ribose) polymerase is involved in oxidative stress-induced cardiomyocyte death. Cell DeathDiffer. 2006;13(10):1815–26. [DOI] [PubMed] [Google Scholar]
- 49.Fairbanks SL, Vest R, Verma S, Traystman RJ, Herson PS. Sex stratified neuronal cultures to study ischemic cell death pathways. Journal of visualized experiments : JoVE. 2013(82):e50758. doi: 10.3791/50758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grubisha O, Rafty LA, Takanishi CL, Xu X, Tong L, Perraud AL et al. Metabolite of SIR2 reaction modulates TRPM2 ion channel. JBiolChem. 2006;281(20):14057–65. [DOI] [PubMed] [Google Scholar]
- 51.Kelly S, Zhao H, Hua Sun G, Cheng D, Qiao Y, Luo J et al. Glycogen synthase kinase 3beta inhibitor Chir025 reduces neuronal death resulting from oxygen-glucose deprivation, glutamate excitotoxicity, and cerebral ischemia. Experimental neurology. 2004;188(2):378–86. doi: 10.1016/j.expneurol.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 52.Endo H, Nito C, Kamada H, Nishi T, Chan PH. Activation of the Akt/GSK3beta signaling pathway mediates survival of vulnerable hippocampal neurons after transient global cerebral ischemia in rats. J Cereb Blood Flow Metab. 2006;26(12):1479–89. doi: 10.1038/sj.jcbfm.9600303. [DOI] [PubMed] [Google Scholar]
- 53.Bradley CA, Peineau S, Taghibiglou C, Nicolas CS, Whitcomb DJ, Bortolotto ZA et al. A pivotal role of GSK-3 in synaptic plasticity. Frontiers in molecular neuroscience. 2012;5:13. doi: 10.3389/fnmol.2012.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peineau S, Taghibiglou C, Bradley C, Wong TP, Liu L, Lu J et al. LTP inhibits LTD in the hippocampus via regulation of GSK3beta. Neuron. 2007;53(5):703–17. doi: 10.1016/j.neuron.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 55.Peineau S, Nicolas CS, Bortolotto ZA, Bhat RV, Ryves WJ, Harwood AJ et al. A systematic investigation of the protein kinases involved in NMDA receptor-dependent LTD: evidence for a role of GSK-3 but not other serine/threonine kinases. Molecular brain. 2009;2:22. doi: 10.1186/1756-6606-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Collingridge GL, Peineau S, Howland JG, Wang YT. Long-term depression in the CNS. Nat Rev Neurosci. 2010;11(7):459–73. doi: 10.1038/nrn2867. [DOI] [PubMed] [Google Scholar]
- 57.Hooper C, Markevich V, Plattner F, Killick R, Schofield E, Engel T et al. Glycogen synthase kinase-3 inhibition is integral to long-term potentiation. The European journal of neuroscience. 2007;25(1):81–6. doi: 10.1111/j.1460-9568.2006.05245.x. [DOI] [PubMed] [Google Scholar]
- 58.Fourgeaud L, Dvorak C, Faouzi M, Starkus J, Sahdeo S, Wang Q et al. Pharmacology of JNJ-28583113: A novel TRPM2 antagonist. European journal of pharmacology. 2019. doi: 10.1016/j.ejphar.2019.03.043. [DOI] [PubMed] [Google Scholar]
- 59.King MK, Pardo M, Cheng Y, Downey K, Jope RS, Beurel E. Glycogen synthase kinase-3 inhibitors: Rescuers of cognitive impairments. Pharmacol Ther. 2014;141(1):1–12. doi: 10.1016/j.pharmthera.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.A novel mouse model of pediatric cardiac arrest and cardiopulmonary resuscitation reveals age-dependent neuronal sensitivities to ischemic injury, (2014). [DOI] [PMC free article] [PubMed]
- 61.Rudy JW, O’Reilly RC. Conjunctive representations, the hippocampus, and contextual fear conditioning. Cognitive, affective & behavioral neuroscience. 2001;1(1):66–82. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.