Abstract
Crocin, a component of saffron, showed hypotensive activity which is perhaps due to vascular smooth muscle relaxant effect. The relaxant effects of saffron on tracheal smooth muscle also could be due to its constituent, crocin. In the present study, the relaxant effects of crocin and its possible mechanisms on rat tracheal smooth muscle were investigated. The relaxant effects of three cumulative concentrations of crocin (30, 60, and 120 μM) or theophylline (0.2, 0.4, 0.6 mM) as positive control were examined on pre-contracted tracheal smooth muscle by methacholine or KCl in non-incubated or incubated conditions with different agents including atropine, chlorpheniramine, indomethacin, diltiazem, glibenclamide, and propranolol. In non-incubated tracheal smooth muscle, crocin showed significant relaxant effects on KCl induced muscle contraction (p < 0.001 for two higher concentrations). However, crocin did not show relaxant effect on methacholine induced tissue contraction. In incubated tissues with chlorpheniramine, indomethacin, diltiazem and propranolol, there were no significant differences in the relaxant effects of crocin between non-incubated and incubated tissues. However, the relaxant effects of crocin obtained in incubated tissues with atropine and glibenclamide were significant lower than non-incubated tracheal smooth muscle (p < 0.05 to p < 0.001). The EC50 value obtained in incubated tissue with propranolol was significantly increased. Theophylline showed significant relaxant effect on both KCl and methacholine induced tissue contraction (p < 0.01 to p < 0.001). A relatively potent relaxant effect of crocin on tracheal smooth muscle, lower than that of theophylline was shown. Muscarinic receptor blocking, potassium channels opening and ß2-adrenoreceptors stimulation were also suggested as possible mechanisms of this effect.
Key Words: Crocin, Smooth muscle, Trachea, Receptors, Adrenergic, Muscarinic
Introduction
The genus Crocus includes roughly 88 species among which Crocus sativus L. (C. sativus) or saffron, is the most important species which studied widely for its various pharmacological properties (1). Saffron, the dried stigma of the plant C. sativus, has three major characteristic components a) crocin, the principle coloring pigment (mono and diglycosyl esters of a polyene dicarboxylic acid, named crocetin), b) the glycoside picrocrocin which is a precursor of safranal and responsible for its distinctly bitter flavor and c) safranal, a monoterpen aldehyde which is the deglycosylated form of picrocrocin and the major organoleptic principle of the stigmas (1).
The crocins (C44H64O24) are a group of hydrophilic carotenoides that are either mono- or di-glycosyl polyene esters of crocetin in which D-glucose and/or D-gentiobiose occur as carbohydrate residues.
At present, saffron is almost exclusively used as a natural flavoring in the food industry. Recent studies have boosted interest in its medicinal properties as antioxidants (2-4), antitumorigenic (5-9), memory enhancers (10-13), antidepressants and anxiolytics (14-17), aphrodisiac (18-20), genoprotectives (21, 22), antitussives (23), cardioprotectives (24-27), and neuroprotectives (28, 29). The relaxant effect of the plant extract and safranal on tracheal smooth muscle (30, 31) and their effects on lung inflammation (32-35), pathological changes (32) and immunoregulation (34) in animal model of asthma were also shown.
The effects of saffron petals extracts on blood pressure, hypotensive effect of aqueous extract of C. sativus and its constituents, safranal and crocin, were shown (36). Administration of 50 mg/100 g of aqueous extract of saffron changed the blood pressure from 133.5 ± 3.9 to 117 ± 2.1 mmHg (36) and the effect of chronic administration of saffron stigma aqueous extract on systolic blood pressure was shown (37). In addition, the effect of saffron on uterine contraction was also investigated (38). For C. sativus and its constituent safranal, a stimulatory effect on ß2- adrenoceptors (31), an inhibitory effect on histamine H1 receptors (39) and a functional antagonistic effect on muscarinic receptors were demonstrated (40).
Abundant research has been conducted concerning the biological and pharmacological properties of two saffron ingredients, safranal (41) and crocin. It has been shown that crocin exhibits pharmacological effects on many organs including the nervous system, gastrointestinal, cardiovascular, genital, endocrine, immune systems, etc (42). Some of these pharmacological effects including learning and memory, depression and anxiety, cerebral ischemia via inhibition of reperfusion-induced oxidative/nitrative injury, atherosclerosis, hyperlipidemia and hypertension, myocardial injury, sexual function, carcinogenesis via delayed the formation of papillomas and strong cytotoxic effect, antioxidant activity, inflammation and genotoxicity (42). Hosseinzadeh and coworkers showed that crocin at pharmacological doses did not exhibit marked damages to all the major organs of the body (43). Crocin was shown Low toxicity in rats even in high experimental dosage (44).
The effects of chronic and subchronic crocin treatment in hypertension (45, 46) were demonstrated. Five weeks administration of three doses of crocin (50, 100 and 200 mg/kg/day) could reduce the mean systolic blood pressure (MSBP) in DOCA salt treated rats in a dose dependent manner. Crocin did not decrease the MSBP in normotensive rats (46). Although the last study did not show antihypertensive effect, this effect of crocin is perhaps due to its relaxant effect on vascular smooth muscle. The protective effect of crocin on reperfusion induced cardiac arrhythmias (47) as well as the lowering effect on heart rate and contractility was also documented (48). The results suggested that crocin is partially capable of suppressing reperfusion-induced arrhythmias (47).
Therefore, in the present study, the relaxant effect of crocin on rat tracheal smooth muscle and the possible underlying mechanism(s) responsible for this effect were examined.
Experimental
Animals
Sixty-four male Wistar rats weighing approximately 200–250 g (Animal house, School of Medicine, Mashhad University of Medical Sciences, Iran) were housed in Plexiglas cages in a temperature-controlled environment (20 ± 2 °C) on a 12-h light-dark schedule with standard diet and tap drinking water available ad libitum. The study was approved by the ethics committee of Mashhad University of Medical Sciences (No. 931653) for Animal Experiments.
Tissue preparation
For obtaining the trachea, the rats were sacrificed by blow on the neck and the chest was opened and excess of connective tissue and fat were dissected out. The trachea was cut into rings of 3 to 4 mm in width; each ring contains about four cartilages for the formation of tracheal ring. Each tracheal ring was hung between two Nichrome hooks inserted into the lumen, and placed in a 10 mL organ bath. The bath containing Krebs-Henseliet solution (KHS) composed of (mM): NaCl 120, KCl 4.72, KH2PO4 1.2, MgSO4·7H2O 0.5, CaCl2·2H2O 2.5, NaHCO3 25 and Dextrose 11 which was maintained at 37 ± 0.5 °C and bubbled constantly with 5% CO2-95% O2. Tissue was suspended under isotonic tension of 1 g and allowed to equilibrate for at least 1 h while it was washed with KHS solution every 15 min. In all experiments contraction responses were measured using an isotonic transducer (MLT0202, AD Instruments, Australia) which was connected to a power lab system (Power Lab 8/30, ML870, AD Instruments, Australia). The study duration was 12 months.
Protocol
Cumulative concentrations of crocin (30, 60, and 120 μM), purchased from Sigma Chemical Co (crocin-1, alpha-crocin, Crocetin di-β-D-gentiobiose ester, C44H64O24, molecular weight 976.972 g/mol, St Louis, MO, USA), or theophylline (0.2, 0.4, and 0.6 mM) as positive control (Sigma Chemical Co, St Louis, MO, USA) were added on pre-contracted tracheal smooth muscle in 5 min intervals to produce concentration response curves. As negative control, the effect of 1 mL saline on pre-contracted tracheal smooth muscle was also evaluated in each experiment. With regard to molecular weight of crocin (976.96 g/mol), the used studied concentrations of crocin are nearly 30, 60, and 120 μM.
The percentage of relaxation due to each concentration of crocin or theophylline in proportion to the maximum contraction due to contractile agent (methacholine or KCl) was plotted against crocin or theophylline concentration to produce concentration response curve in each experiment. The effective concentration of crocin causing 50% of maximum response (EC50) was also calculated as previously described (41).
Study groups
To examine the relaxant effect of crocin, tracheal smooth muscle was contracted by contractile agent for 5 min on non-incubated or incubated tissues with different substance for 10 min as follows:
A- Tracheal smooth muscle was contracted by 60 mM KCl (Merk, Germeny) in the following groups;
I) Non-incubated tissues (n = 8)
II) Incubated tissues with:
a. One μM atropine (Sigma Chemical Ltd UK), (n = 7)
b. One μM chlorpheniramine (Sigma Chemical Ltd UK), (n = 7)
c. One μM indomethacin (Sigma Chemical Ltd UK), (n = 6)
d. Five μM diltiazem (Sigma Chemical Ltd UK), (n = 5)
e. One μM glibenclamide (Sigma Chemical Ltd UK), (n = 5)
f. One μM propranolol (Sigma Chemical Ltd UK), (n = 6)
B- Non-incubated tracheal smooth muscle contracted by 10 μM methacholine (Sigma Chemical Ltd. UK), (n = 8).
Each experimental group was done in random order in one tissues. Occasionally, few experimental groups were performed in one tissue with one hour resting period between two experiments while washing tissues with KHS solution every 15 min. The effect of theophylline as positive control was only examined on non-incubated tissues (n = 6 for each group).
Data analysis
Data are presented as mean ± SEM. The results were analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s Multiple comparison test. Statistically significant was considered as p < 0.05.
Results
The relaxant effect of crocin in non-incubated tracheal smooth muscle contracted with KCl
The two higher concentrations of crocin showed significant and concentration-dependent relaxant effects in non-incubated tracheal smooth muscle contracted by KCl (p < 0.001 for both cases), (Figure 1).
Figure 1.
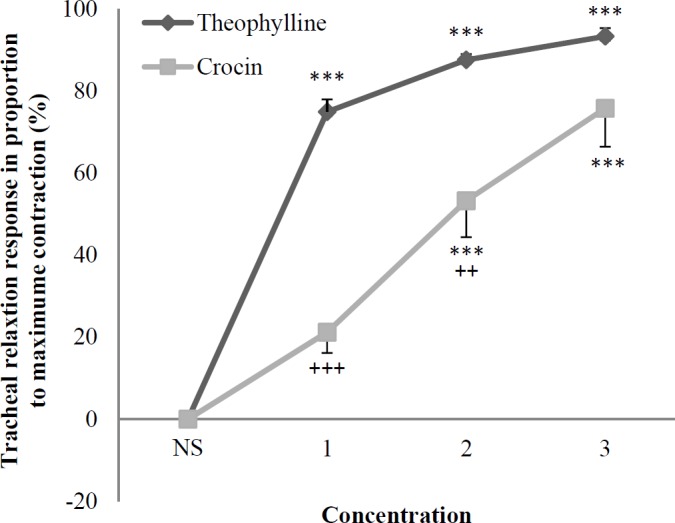
Concentration-response relaxant effect (mean ± SEM) of crocin (n = 8) and theophylline (n = 6) on KCl (60 mM) induced contraction of tracheal smooth muscle in non-incubated tissues. 1, 2 and 3 in X axis represent three concentration of crocin (30, 60, and 120 μM) and theophylline (0.2, 0.4, and 0.6 mM). ***p < 0.001 compared to saline (NS). ++p < 0.01, +++p < 0.001 compared to the effect of theophylline. Statistical comparisons were performed using ANOVA with Tukey Kramer post-test
The relaxant effect of crocin in incubated tracheal smooth muscle contracted with KCl
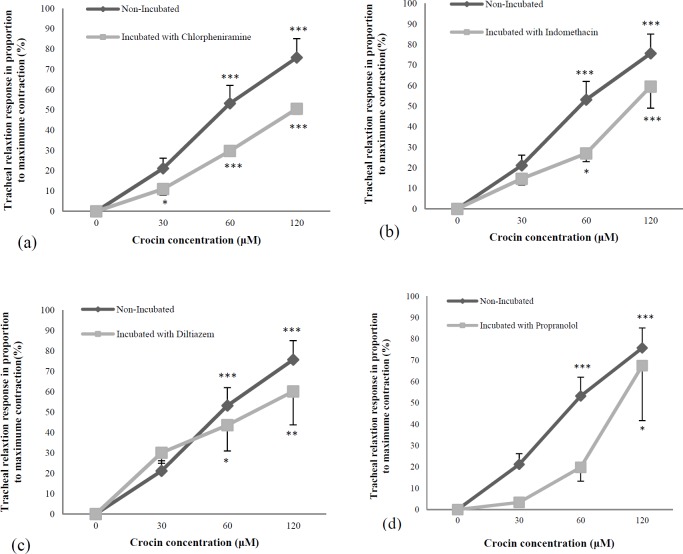
Crocin showed significant and concentration-dependent relaxant effect in incubated tracheal smooth muscle with chlorpheniramine, indomethacin (p < 0.05 to p < 0.001 for both cases), diltiazem (p < 0.05 to p < 0.01), and propranolol (p < 0.05), (Figure 2). There was no significant difference in the relaxant effects of crocin between non-incubated and incubated tissue with chlorpheniramine, indomethacin, diltiazem, and propranolol (Figure 2).
Figure 2.
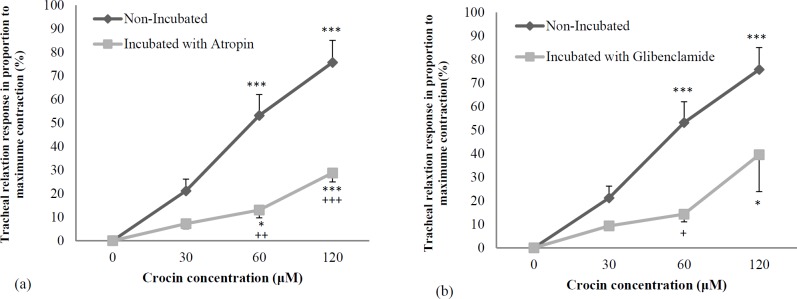
Concentration-response relaxant effect (mean ± SEM) of crocin on KCl (60 mM) induced contraction of tracheal smooth muscle in non-incubated (n = 8) and incubated tissues with (a) atropine (1 μM, n = 7), (b) glibenclamide (1 μM, n = 5). *p < 0.05, ***p < 0.001, compared to saline (as indicated by zero in X axis of the figure). +p < 0.05, ++p < 0.01 +++p < 0.001, compared to non-incubated tissues. Statistical comparisons were performed using ANOVA with Tukey Kramer post-test
In incubated tissues with atropine and glibenclamide, crocin showed significant and concentration-dependent relaxant effects (p < 0.05 to p < 0.001 and p < 0.05 to p < 0.01, respectively), (Figure 3). The relaxant effects of two higher concentrations of crocin were significantly lower in incubated tissue with atropine compared to non-incubated tracheal smooth muscle (p < 0.01 to p < 0.001), (Figure 3a). The relaxant effects of medium concentrations of crocin were significantly lower in incubated tissue with glibenclamide compared to non-incubated tracheal smooth muscle (p < 0.05), (Figure 3b).
Figure 3.
Concentration-response relaxant effect (mean ± SEM) of crocin on KCl (60 mM) induced contraction of tracheal smooth muscle in non-incubated (n = 8) and incubated tissues with (a) chlorpheniramine (1 μM, n = 7), (b) indomethacin (1 μM, n = 6), (c) diltiazem (5 μM, n = 5), and (d) propranolol (1 μM, n = 6). *p < 0.05, **p < 0.01, ***p < 0.001 compared to saline (as indicated by zero in X axis of the figure). Statistical comparisons were performed using ANOVA with Tukey Kramer post-test
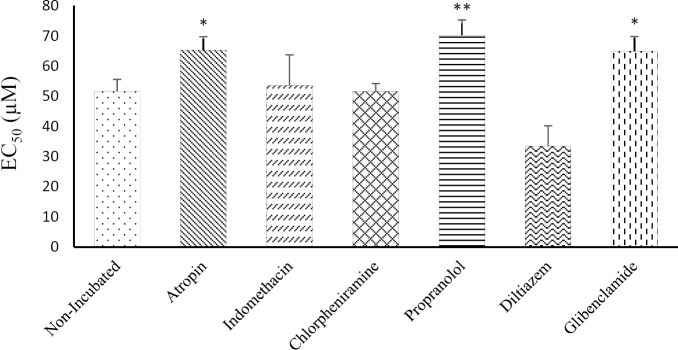
There was no significant difference in EC50 values of crocin between non-incubated (51.62 ± 4.01) and incubated tissue with chlorpheniramine (51.57 ± 2.67), indomethacin (53.5 ± 10.24), and diltiazem (33.4 ± 6.8). However, the results showed significant difference in EC50 values of crocin, between non-incubated and incubated tissue with atropine (65.28 ± 4.5), glibenclamide (65 ± 4.85), and propranolol (70.33 ± 5.01), (Figure 4).
Figure 4.
EC50 values of crocin induce relaxation obtained on contracted tracheal smooth muscles of rat with 60 mM KCl in non-incubated (n = 8) and incubated tissues with atropine (n = 7), chlorpheniramine (n = 7), indomethacin (n = 6), diltiazem (n = 5), glibenclamide (n = 5) and propranolol (n = 6). *p < 0.05, **p < 0.01 compared to non-incubated tissues. Statistical comparisons were performed using ANOVA with Tukey Kramer post-test
The relaxant effect of low concentrations of crocin in diltiazem incubated tracheal smooth muscle was significantly higher than those of incubated tissues with atropine (p < 0.001), chlorpheniramine (p < 0.001), indomethacin (p < 0.05), glibenclamide (p < 0.001) and propranolol (p < 0.001) in KCl induced muscle contraction. Additionally, the relaxant effect of medium concentrations of crocin in diltiazem incubated tissues was also significantly higher than those of incubated tracheal smooth muscle with atropine (p < 0.01) and glibenclamide (p < 0.05), (Table 1). The relaxant effects of medium and high concentrations of crocin in incubated tissues with atropine were significantly higher than those of incubated smooth muscles with chlorpheniramine and indomethacin (p < 0.05 to p < 0.01), (Table 1).
Table 1.
Comparison of the relaxant effects of three concentrations of crocin (percentage change in proportion to the maximum contraction) in different incubated tracheal smooth muscles contracted by 60 mM KCl
| Incubating substance |
Concentration (μM)
|
||
|---|---|---|---|
| 30 | 60 | 120 | |
| Atropine | 7.24 ± 2.21*** | 13.03 ± 3.28** | 28.8 ± 3.79 |
| Chlorpheniramine | 11.05 ± 3.1*** | 29.78 ± 2.2## | 50.55 ± 2.68# |
| Indomethacin | 14.69 ± 3.07 * + | 27.01 ± 4.04# | 59.61 ± 10.55## |
| Diltiazem | 30.08 ± 5.16 | 43.65 ± 12.67 | 60.21 ± 16.42 |
| Glibenclamide | 9.32 ± 1.76*** | 14.3 ± 3.26* | 39.56 ± 15.71 |
| Propranolol | 3.37 ± 0.82*** | 19.81 ± 6.51 | 67.44 ± 25.71 |
Data were presented as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001 compared to incubated tissues with diltiazem. +p < 0.05 compared to incubated tissues propranolol. #p < 0.05, ##p < 0.01 compared to incubated tissues with atropine.
The relaxant effect of crocin in non-incubated tracheal smooth muscle contracted by methacholine
In non-incubated tracheal smooth muscle contracted by methacholine, crocin did not show any relaxant effect (Figure 5).
Figure 5.
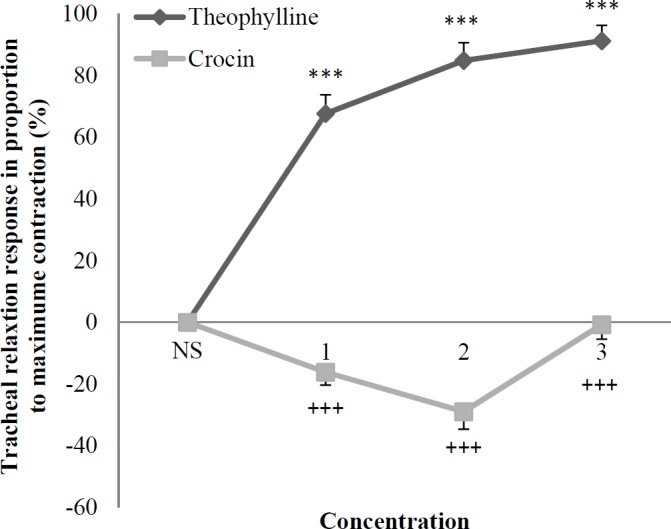
Concentration-response relaxant effect (mean ± SEM) of crocin (n = 8) and theophylline (n = 6) on methacholine (10 μM) induced contraction of tracheal smooth muscle in non-incubated tissues. 1, 2 and 3 in X axis represent three concentration of crocin (30, 60, and 120 μM) and theophylline (0.2, 0.4, and 0.6 mM). ***p < 0.001 compared to saline (NS). +++p < 0.01 compared to the effect of theophylline. Statistical comparisons were performed using ANOVA with Tukey Kramer post-test
The relaxant effects of various concentrations of crocin on tracheal smooth muscle contracted by methacholine were significantly lower than those obtained in KCl induced contraction (p < 0.01 to p < 0.001), (Figure 6).
Figure 6.
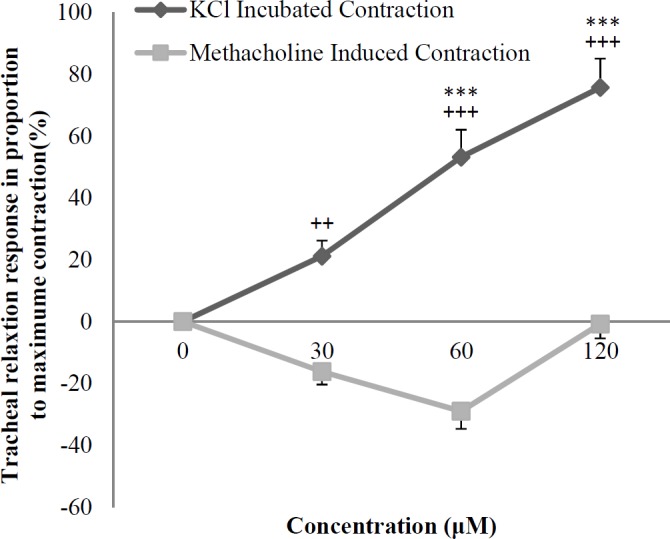
Concentration-response relaxant effect (mean ± SEM) of crocin on methacholine (10 μM) and KCl (60 mM) induced contraction of non-incubated tracheal smooth muscle (n = 8). ***p < 0.001 compared to saline (as indicated by zero in X axis of the figure). ++p < 0.01, +++p < 0.001, compared to the relaxant effect on methacholine induced muscle contraction. Statistical comparison of the effect of each concentration between two groups was performed using unpaired t-test
Comparison of the relaxant effect of crocin and theophylline
The relaxant effect of two lower concentrations of crocin was significantly lower than that of theophylline in KCl induced contraction of non-incubated tracheal smooth muscle (p < 0.01 to p < 0.001), (Figure 1).
The relaxant effects of all concentrations of theophylline were significantly higher than those of crocin in tracheal smooth muscle contracted by methacholine (p < 0.001 for all concentrations), (Figure 5).
Discussion
In this study, the relaxant effect of crocin in pre-contracted tracheal smooth muscle by KCl and methacholine was examined. In non-incubated tracheal smooth muscle contracted by KCl, crocin showed significant and concentration dependent relaxant effect. The relaxant effect of two higher concentrations of crocin was significantly lower than that of theophylline. These results indicated a relatively potent relaxant effect of crocin.
For C. sativus and its constituent safranal, a stimulatory effect on ß2- adrenoceptors (31), an inhibitory effect on histamine H1 receptors (47) and a functional antagonistic effect on muscarinic receptors were demonstrated (48). To evaluate these possible mechanisms, as well as other possible mechanisms for the relaxant effect of crocin, its effect was examined on tracheal smooth muscle incubated with atropine, chlorpheniramine, indomethacin, diltiazem, glibenclamide, and propranolol.
In incubated tracheal smooth muscle with atropine and contracted with KCl, the relaxant effect of crocin examined to assess the contribution of muscarinic receptor inhibitory effect on the relaxant property of crocin. The results showed significant lower relaxant effects of two higher concentrations of crocin in incubated tissues with atropine compared to non-incubated tracheal smooth muscle. These results indicated the inhibitory effect of crocin on muscarinic receptors which could be contributed in its relaxant effect on tracheal smooth muscle. In fact, the relaxant effect of muscarinic receptor blocking drugs on tracheal smooth muscle was previously documented (49). The study of Neamati et al. (2010) demonstrated the functional antagonistic effect of C. sativus and safranal on muscarinic receptors on tracheal muscle of guinea pigs. Both the extract and safranal shifted methacholine concentration-response curve to the right. However, the shift was not parallel and the maximum response to methacholine in the presence of extract and safranal was not obtained. These results indicated a functional antagonistic effect of the plant and safranal on muscarinic receptors (48). These two studies nearly supported the results of the present study regarding the inhibitory effect of crocin on muscarinic receptors.
To examine the effect of crocin on histamine (H1) receptor and the role of this mechanism on the relaxant effect of crocin on tracheal smooth muscle, the relaxant effect of crocin was examined on tracheal smooth muscle incubated with chlorpheniramine and contracted with KCl. The findings of this group showed non-significant difference in the relaxant effect of crocin in incubated tracheal smooth muscle with the results of non-incubated tissue. These results indicate the absence of the inhibitory effect of crocin on histamine (H1) receptor and therefore, this mechanism is not responsible for the relaxant effect of crocin on tracheal smooth muscle. However, the relaxant effect of histamine (H1) receptor blocking drugs on tracheal smooth muscle was shown previously (50). The inhibitory effect of extracts of C. sativus on histamine (H1) was also shown previously. The effect of three concentrations of aqueous-ethanolic extracts of C. sativus (0.025%, 0.05% and 0.1%) on histamine (H1) receptors was evaluated in guinea pig tracheal smooth muscles previously. Concentration-response curve of histamine was obtained in the presence of saline, saffron extract, and chlorpheniramine. The extract caused parallel rightward shift in histamine concentration-response curve similar to the effect of chlorpheniramine and the maximum response to histamine was obtained in the presence of the extract. These results showed an inhibitory effect of C. sativus (competitive antagonistic effect) on histamine H1 receptors which could be related to the relaxant effect of the plant on tracheal smooth muscle (47). The reason of the differences between the effect of the extract of saffron, safranal and corcin on histamine (H1) receptors is uncertain to us and should be examined in further studies.
To evaluate the involvement of prostacyclin mechanism in crocin induced tracheal smooth muscle relaxation, its effect was also examined in incubated tissues with indomethacin. Prostacyclin (PGI2) is an epithelium releasing factor produced by epithelial cyclooxygenase. Prostacyclin leads to elevation of cyclic AMP and finally reduces the availability of calcium and induces smooth muscle cell relaxation (51). The anti-inflammatory effect of crocin was also seen previously (52). Non-significant difference in the relaxant effect of crocin between non-incubated and incubated tissues with indomethacin showed that COX inhibitory effect of crocin does not affect its tracheal smooth muscle relaxation.
In incubated tracheal smooth muscle with diltiazem also the relaxant effect of crocin was studied to assess the role of calcium channel-blocking property in the relaxant effect of crocin. No significant difference was seen between the relaxant effects of crocin in non-incubated and incubated tissue with diltiazem. Therefore, calcium channel-blocking is not contributed in the relaxant effect of crocin on tracheal smooth muscle. Crocin could inhibit Ca2+ influx and release of intracellular Ca2+ stores in the endoplasmic reticulum in bovine aortic smooth muscle cells (53). It was also shown that reduction of intracellular Ca2+ release may contribute to relaxation of the corpus cavernosum, leading to erection (54). The effect of C. sativus on Ca2+ influx in isolated rat aortas was investigated using 45Ca as a radioactive tracer, and their calcium antagonistic effects were evaluated. Ca2+ uptake in isolated rat aorta rings in normal physiological status was not markedly altered by these drugs, whereas Ca2+ influxe induced by norepinephrine 1.2 µmol/L and KCl 100 mmol/L were significantly inhibited by C. sativus in a concentration-dependent manner. The results showed that Ca2+ influx through receptor-operated Ca2+ channels and potential-dependent Ca2+ channels can be blocked by the plant (55). It has been reported that crocetin decreased protein kinase C (PKC) activity in the membrane fraction, which led to reduced blood pressure by inhibition of proliferation in vascular smooth muscle cells (56). However, the results of the present study did not reveale a calcium channel inhibitory effect of crocin. The possible reason of the differences between the results of the and previously studies should be examined in further studies.
In incubated tracheal smooth muscle with glibenclamide as well as the relaxant effect of crocin was studied to assess the role of potassium channel-blocking property in its relaxant effect. The relaxant effects of medium concentrations of crocin were significantly lower in incubated tissue with glibenclamide compared to non-incubated tracheal smooth muscle. These results indicated the inhibitory effect of crocin on potassium channels which could be contributed in its relaxant effect on tracheal smooth muscle.
The most possible mechanism for the relaxant effect of agents on tracheal smooth muscle is their stimulatory effect on ß2-adrenergic receptors. To evaluate the effect of crocin on ß2- adrenoceptors and the role of this mechanism, the relaxant effect of crocin was examined on tracheal smooth muscle incubated with propranolol and contracted with KCl. The relaxant effect of ß2- adrenoceptors stimulatory drugs on tracheal smooth muscle was shown previously (50). There was no significant difference in the relaxant effects of crocin between non-incubated and incubated tissue with propranolol. These results indicate the absence of the stimulatory effect of crocin on ß2- adrenoceptors and therefore, this mechanism is not responsible for the relaxant effect of crocin on tracheal smooth muscle. However, the ß2-adrenergic stimulatory effect of the plant and safranal was tested by performing cumulative concentration-response curves of isoprenaline-induced relaxation of pre-contracted isolated guinea pig tracheal smooth muscle. The results showed leftward shifts in isoprenaline curves obtained in the presence of saffron extract and safranal compared to that of saline while propranolol caused rightward shift in isoprenaline response curve. The results indicated a relatively potent stimulatory effect of C. sativus extract and its constituent safranal on ß2-adrenoreceptors (31). Therefore, the results suggested that the major mechanism responsible for the relaxant effect of the plant and safranal is their stimulatory effect on ß2-adrenoreceptors. This discrepancy also should needs further investigations.
Higher EC50 values of crocin induced relaxant effect and lower relaxant effect of crocin obtained in incubated tissues with atropine and glibenclamide compared to non-incubated tissues also support the contribution of muscarinic receptor inhibitory and potassium channel-blocking properties of crocin in its relaxant effect on tracheal smooth muscle. The results also showed significant difference in EC50 values of crocin between non-incubated and incubated tissue with propranolol. Although, there was no significant difference in the relaxant effects of crocin between non-incubated and incubated tissue with propranolol, the higher EC50 value obtained in incubated tissues with propranolol may indicate a component of stimulatory effect of crocine on ß2-adrenoreceptors.
On methacholine induced contraction of tracheal smooth muscle, crocin did not show any significant relaxant effect. The absence of the relaxant effect of crocin in the methacholine induced contraction of smooth muscle almost excluded the role of the muscarinic receptor inhibitory effect of crocin on its smooth muscle relaxant property. The discrepancy between the results of crocin on methacholine induced muscle contraction and incubated tissue with atropine and contracted with KCl is unclear to us and should be evaluated in further studies.
The absence of the relaxant effect of crocin on methacholine induced muscle contraction and relatively potent relaxant on KCl induced muscle contraction may indicate that the main mechanism of the relaxant effect of crocin is its inhibitory effect on calcium channels and/or opening effect on potassium channels. In fact, the relaxant effect of potassium channels opening drugs (57-59) and calcium channel blocking drugs (60) on tracheal smooth muscle were shown previously.
Previous studies showed the relaxant effect of crocin in various types of smooth muscle which support the findings of the present study. Imenshahidi et al. compared the hypotensive effect of saffron aqueous extract and its two active ingredients in rats. Based on their results, aqueous extract of saffron stigma, safranal and crocin decreased mean arterial blood pressure in a dose-dependent manner. The hypotensive effect of the extract is perhaps due to its relaxant effect on vascular smooth muscle. The results also suggested that safranal, the major constituent of the plant, contributes to the hypotensive activity (25). The effect of crocin (50 mg/kg) on the reduction of systolic blood pressure (SBP) and the increased heart rate (HR) induced by diazinon (DZN) in rats, was shown which could be due to the relaxant effect of crocin on vascular muscle cells (38). It was also shown that crocetin (15, 30 mg/kg) dose-dependently improved endothelium-dependent relaxation (EDR) in response to acetylcholine (Ach) in high cholesterol diet (HCD)-fed rabbits. In addition, in bovine aortic endothelial cells (BAECs), oxidized LDL (oxLDL) treatment decreased nitric oxide production and down-regulated the activity and mRNA expression of endothelial nitric oxide synthase and these effects were inhibited by crocetin (0.1, 1, 10 mM) in a dose-dependent manner (61). The effect of C. sativus petals extracts on isolated guinea-pig ileum induced by electrical field stimulation (EFS) was studied. In rat isolated ileum, contractile responses to EFS were decreased by the petals extracts. Contractions of ileum to EFS are mediated by both noradrenaline and ATP released as co-transmitters from sympathetic nerves (36). The discrepancy regarding the possible mechanisms of the relaxant effect of crocin on smooth muscle observe in the above study and the current study may be due to the types of smooth muscles (caprine detrusor muscle vs tracheal) and the distribution of various receptor and channels in different types of smooth muscle.
Although the concentrations of thephylline and crocin were not the same, their concentrations were chosen according the previous studies (30, 45). In addition, theophylline was used as a positive control in the present study as previous studies. Therefore, the effects of high, medium, and low concentrations of crocin were compared to the effect of corresponding concentrations of theophylline.
In conclusion, the present study has demonstrated a relatively potent relaxant effect of crocin on tracheal smooth muscle which was lower compared to the effect of theophylline at studied concentration. The findings also suggested that the possible mechanisms of the relaxant effect of the crocin on tracheal smooth muscle including muscarinic receptor blocking, potassium channels opening, and ß2-adrenoreceptors stimulation.
Acknowledgment
This study was financially supported by a grant from Research Council of Mashhad University of Medical Sciences. The results of this paper are a part of MSc thesis of Mahsa Yasavoli.
References
- 1.Alavizadeh SH, Hosseinzadeh H. Bioactivity assessment and toxicity of crocin: a comprehensive review. Food Chem. Toxicol. 2014;64:65–80. doi: 10.1016/j.fct.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 2.Hosseinzadeh H, Shamsaie F, Mehri S. Antioxidant activity of aqueous and ethanolic extracts of Crocus sativus L stigma and its bioactive constituents, crocin and safranal. Pharmacogn. Mag. 2009;5:419. [Google Scholar]
- 3.Ochiai T, Ohno S, Soeda S, Tanaka H, Shoyama Y, Shimeno H. Crocin prevents the death of rat pheochromyctoma (PC-12) cells by its antioxidant effects stronger than those of α-tocopherol. Neurosci. Lett. 2004;362:61–4. doi: 10.1016/j.neulet.2004.02.067. [DOI] [PubMed] [Google Scholar]
- 4.Soeda S, Ochiai T, Shimeno H, Saito H, Abe K, Tanaka H, Shoyama Y. Pharmacological activities of crocin in saffron. J. Nat. Med. 2007;61:102–11. [Google Scholar]
- 5.Abdullaev FI. Cancer chemopreventive and tumoricidal properties of saffron (Crocus sativus L) Exp. Biol. Med. 2002;227:20–5. doi: 10.1177/153537020222700104. [DOI] [PubMed] [Google Scholar]
- 6.Aung H, Wang C, Ni M, Fishbein A, Mehendale S, Xie J, Shoyama A, Yuan C. Crocin from Crocus sativus possesses significant anti-proliferation effects on human colorectal cancer cells. Exp. Oncol. 2007;29:175. [PMC free article] [PubMed] [Google Scholar]
- 7.Escribano J, Alonso GL, Coca-Prados M, Fernández JA. Crocin, safranal and picrocrocin from saffron (Crocus sativus L) inhibit the growth of human cancer cells in-vitro. Cancer Lett. 1996;100:23–30. doi: 10.1016/0304-3835(95)04067-6. [DOI] [PubMed] [Google Scholar]
- 8.Garc-Olmo DC, Riese HH, Escribano J, Ontan J, Fernandez JA, Atiénzar M, Garcí-Olmo D. Effects of long-term treatment of colon adenocarcinoma with crocin, a carotenoid from saffron (Crocus sativus L): an experimental study in the rat. Nutr. Cancer. 1999;35:120–6. doi: 10.1207/S15327914NC352_4. [DOI] [PubMed] [Google Scholar]
- 9.Mousavi SH, Moallem SA, Mehri S, Shahsavand S, Nassirli H, Malaekeh-Nikouei B. Improvement of cytotoxic and apoptogenic properties of crocin in cancer cell lines by its nanoliposomal form. Pharm. Biol. 2011;49:1039–45. doi: 10.3109/13880209.2011.563315. [DOI] [PubMed] [Google Scholar]
- 10.Abe K, Saito H. Effects of saffron extract and its constituent crocin on learning behaviour and long‐term potentiation. Phytother. Res. 2000;14:149–52. doi: 10.1002/(sici)1099-1573(200005)14:3<149::aid-ptr665>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 11.Ghadrdoost B, Vafaei AA, Rashidy-Pour A, Hajisoltani R, Bandegi AR, Motamedi F, Haghighi S, Sameni HR, Pahlvan S. Protective effects of saffron extract and its active constituent crocin against oxidative stress and spatial learning and memory deficits induced by chronic stress in rats. Eur. J. Pharmacol. 2011;667:222–9. doi: 10.1016/j.ejphar.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 12.Hosseinzadeh H, Ziaei T. Effects of Crocus sativus stigma extract and its constituents, crocin and safranal, on intact memory and scopolamine-induced learning deficits in rats performing the Morris water maze task. J. Med. Plants . 2006;3:40–50. [Google Scholar]
- 13.Pitsikas N, Zisopoulou S, Tarantilis PA, Kanakis CD, Polissiou MG, Sakellaridis N. Effects of the active constituents of Crocus sativus L crocins on recognition and spatial rats’ memory. Behav. Brain Res. . 2007;183:141–6. doi: 10.1016/j.bbr.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 14.Hosseinzadeh H, Karimi G, Niapoor M. Antidepressant effects of Crocus sativus stigma extracts and its constituents, crocin and safranal, in mice. J. Med. Plants . 2004;3:48–8. [Google Scholar]
- 15.Hosseinzadeh H, Motamedshariaty V, Hadizadeh F. Antidepressant effect of kaempferol, a constituent of saffron (Crocus sativus) petal, in mice and rats. Pharmacologyonline . 2007;2:367–70. [Google Scholar]
- 16.Hosseinzadeh H, Noraei NB. Anxiolytic and hypnotic effect of Crocus sativus aqueous extract and its constituents, crocin and safranal, in mice. Phytother. Res. . 2009;23:768–74. doi: 10.1002/ptr.2597. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Han T, Zhu Y, Zheng CJ, Ming QL, Rahman K, Qin LP. Antidepressant properties of bioactive fractions from the extract of Crocus sativus L. J. Nat. Med. . 2010;64:24–30. doi: 10.1007/s11418-009-0360-6. [DOI] [PubMed] [Google Scholar]
- 18.Hosseinzadeh H, Ziaee T, Sadeghi A. The effect of saffron, Crocus sativus stigma, extract and its constituents, safranal and crocin on sexual behaviors in normal male rats. Phytomedicine . 2008;15:491–5. doi: 10.1016/j.phymed.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 19.Shamsa A, Hosseinzadeh H, Molaei M, Shakeri MT, Rajabi O. Evaluation of Crocus sativus L(saffron) on male erectile dysfunction: a pilot study. Phytomedicine . 2009;16:690–3. doi: 10.1016/j.phymed.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 20.Sumalatha K, Kumar S, Lakshmi SM. Review on natural aphrodisiac potentials to treat sexual dysfunction. Int. J. Pharm. Ther. . 2010;1:10–8. [Google Scholar]
- 21.Hosseinzadeh H, Sadeghnia HR. Effect of safranal, a constituent of Crocus sativus (saffron), on methyl methanesulfonate (MMS)-induced DNA damage in mouse organs: an alkaline single-cell gel electrophoresis (comet) assay. DNA Cell Biol. 2007;26:841–6. doi: 10.1089/dna.2007.0631. [DOI] [PubMed] [Google Scholar]
- 22.Premkumar K, Abraham SK, Santhiya S, Gopinath P, Ramesh A. Inhibition of genotoxicity by saffron (Crocus sativus L) in mice. Drug Chem. Toxicol. 2001;24:421–8. doi: 10.1081/dct-100106266. [DOI] [PubMed] [Google Scholar]
- 23.Hosseinzadeh H, Ghenaati J. Evaluation of the antitussive effect of stigma and petals of saffron (Crocus sativus) and its components, safranal and crocin in guinea pigs. Fitoterapia . 2006;77:446–8. doi: 10.1016/j.fitote.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 24.Goyal S, Arora S, Sharma A, Joshi S, Ray R, Bhatia J, Kumari S, Arya D. Preventive effect of crocin of Crocus sativus on hemodynamic, biochemical, histopathological and ultrastuctural alterations in isoproterenol-induced cardiotoxicity in rats. Phytomedicine . 2010;17:227–32. doi: 10.1016/j.phymed.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 25.Imenshahidi M, Hosseinzadeh H, Javadpour Y. Hypotensive effect of aqueous saffron extract (Crocus sativus L) and its constituents, safranal and crocin, in normotensive and hypertensive rats. Phytother. Res. . 2010;24:990–4. doi: 10.1002/ptr.3044. [DOI] [PubMed] [Google Scholar]
- 26.Xu GL, Qian ZY, Yu SQ, Gong ZN, Shen XC. Evidence of crocin against endothelial injury induced by hydrogen peroxide in-vitro. J. Asian Nat. Prod. Res. . 2006;8:79–85. doi: 10.1080/10286020500044732. [DOI] [PubMed] [Google Scholar]
- 27.Zhang R, Zhi-Yu Q, Xiao-Yuan H, Zhen C, Jun-Ling Y, Hamid A. Comparison of the effects of crocetin and crocin on myocardial injury in rats. Chin. J. Nat. Med. 2009;7:223–7. [Google Scholar]
- 28.Essa MM, Vijayan RK, Castellano-Gonzalez G, Memon MA, Braidy N, Guillemin GJ. Neuroprotective effect of natural products against Alzheimer’s disease. Neurochem. Res. 2012;37:1829–42. doi: 10.1007/s11064-012-0799-9. [DOI] [PubMed] [Google Scholar]
- 29.Mehri S, Abnous K, Mousavi SH, Shariaty VM, Hosseinzadeh H. Neuroprotective effect of crocin on acrylamide-induced cytotoxicity in PC12 cells. Cell. Mol. Neurobiol. 2012;32:227–35. doi: 10.1007/s10571-011-9752-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boskabady Ma, Aslani M. Relaxant effect of Crocus sativus (saffron) on guinea‐pig tracheal chains and its possible mechanisms. J. Pharm. Pharmacol. 2006;58:1385–90. doi: 10.1211/jpp.58.10.0012. [DOI] [PubMed] [Google Scholar]
- 31.Nemati H, Boskabady M, Vostakolaei HA. Stimulatory effect of Crocus sativus (saffron) on β 2-adrenoceptors of guinea pig tracheal chains. Phytomedicine . 2008;15:1038–45. doi: 10.1016/j.phymed.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 32.Boskabady M, Tabatabaee A, Byrami G. The effect of the extract of Crocus sativus and its constituent safranal, on lung pathology and lung inflammation of ovalbumin sensitized guinea-pigs. Phytomedicine . 2012;19:904–11. doi: 10.1016/j.phymed.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 33.Byrami G, Boskabady MH, Jalali S, Farkhondeh T. The effect of the extract of Crocus sativus on tracheal responsiveness and plasma levels of IL-4, IFN-γ, total NO and nitrite in ovalbumin sensitized Guinea-pigs. J. Ethnopharmacol. . 2013;147:530–5. doi: 10.1016/j.jep.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 34.Boskabady MH, Farkhondeh T. Antiinflammatory, antioxidant, and immunomodulatory effects of Crocus sativus L and its main constituents. Phytother. Res. . 2016 doi: 10.1002/ptr.5622. [DOI] [PubMed] [Google Scholar]
- 35.Boskabady MH, Byrami G, Feizpour A. The effect of safranal, a constituent of Crocus sativus (saffron), on tracheal responsiveness, serum levels of cytokines, total NO and nitrite in sensitized guinea pigs. Pharmacol. Rep. . 2014;66:56–61. doi: 10.1016/j.pharep.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 36.Fatehi M, Rashidabady T, Fatehi-Hassanabad Z. Effects of Crocus sativus petals’ extract on rat blood pressure and on responses induced by electrical field stimulation in the rat isolated vas deferens and guinea-pig ileum. J. Ethnopharmacol. . 2003;84:199–203. doi: 10.1016/s0378-8741(02)00299-4. [DOI] [PubMed] [Google Scholar]
- 37.Imenshahidi M, Razavi BM, Faal A, Gholampoor A, Mousavi SM, Hosseinzadeh H. Effects of chronic crocin treatment on desoxycorticosterone acetate (doca)-salt hypertensive rats. Iran. J. Basic. Med. Sci. . 2014;17:9–13. [PMC free article] [PubMed] [Google Scholar]
- 38.Razavi M, Hosseinzadeh H, Abnous K, Motamedshariaty VS, Imenshahidi M. Crocin restores hypotensive effect of subchronic administration of diazinon in rats. Iran J. Basic. Med. Sci. 2013;16:64–72. [PMC free article] [PubMed] [Google Scholar]
- 39.Imenshahidi M, Razavi BM, Faal A, Gholampoor A, Mousavi SM, Hosseinzadeh H. The effect of chronic administration of saffron (Crocus sativus) stigma aqueous extract on systolic blood pressure in rats. Jundishapur J. Nat. Pharm. Prod. 2013;8:175–9. doi: 10.17795/jjnpp-12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rezaee R, Hosseinzadeh H. Safranal: from an aromatic natural product to a rewarding pharmacological agent. Iran. J. Basic. Med. Sci. 2013;16:12–26. [PMC free article] [PubMed] [Google Scholar]
- 41.Mokhtari-Zaer A, Khazdair MR, Boskabady MH. Smooth muscle relaxant activity of Crocus sativus (saffron) and its constituents: possible mechanisms. Avicenna J. Phytomed. 2015;5:365. [PMC free article] [PubMed] [Google Scholar]
- 42.Hosseinzadeh H, Shariaty M, Sameni A, Vahabzadeh M. Acute and sub-acute toxicity of crocin, a constituent of Crocus sativus L (saffron), in mice and rats. Pharmacologyonline . 2010;2:943–51. [Google Scholar]
- 43.Wang C, Hwang LS, Lin J. Reversible hepatic black pigmentation and enzyme alteration induced by prolonged feeding of high dose of crocin dyes in rats. Proc. Natl. Sci. Counc. Repub. China B . 1984;8:246–53. [PubMed] [Google Scholar]
- 44.Jahanbakhsh Z, Rasoulian B, Jafari M, Shekarforoush Sh EM, Mohammadi M, Aghai H, Salehi M. Protective effect of crocin against reperfusion-induced cardiac arrhythmias in anaesthetized rats. EXCLI J. . 2012;11:20–9. [PMC free article] [PubMed] [Google Scholar]
- 45.Boskabady M, Shafei M, Shakiba A, Sefidi HS. Effect of aqueous‐ethanol extract from Crocus sativus (saffron) on guinea‐pig isolated heart. Phytother. Res. . 2008;22:330–4. doi: 10.1002/ptr.2317. [DOI] [PubMed] [Google Scholar]
- 46.Chang P, Wang C, Liang J, Kuo W. Studies on the pharmacological action of Zang Hong Hua (Crocus sativus L) I Effects on uterus and estrus cycle. Yao Xue Xue Bao . 1964;11:94. [PubMed] [Google Scholar]
- 47.Boskabady MH, Rahbardar MG, Nemati H, Esmaeilzadeh M. Inhibitory effect of Crocus sativus (saffron) on histamine (H1) receptors of guinea pig tracheal chains. Pharmazie . 2010;65:300–5. [PubMed] [Google Scholar]
- 48.Neamati N, Boskabady MH. Effect of Crocus sativus (saffron) on muscarinic receptors of guinea pig tracheal chains. Funct. Plant Sci. Biotechnol. . 2010;4:128–31. [Google Scholar]
- 49.Loenders B, Rampart M, Herman AG. Selective M3 muscarinic receptor antagonists inhibit smooth muscle contraction in rabbit trachea without increasing the release of acetylcholine. J. Pharmacol. Exp. Ther. 1992;263:773–9. [PubMed] [Google Scholar]
- 50.Popa VT, Somani P, Simon V. The Effect of Inhaled Verapamil on Resting Bronchial Tone and Airway Contractions Induced by Histamine and Acetylcholine in Normal and Asthmatic Subjects 1–4. Am. Rev. Respir. Dis. 1984;130:1006–13. doi: 10.1164/arrd.1984.130.6.1006. [DOI] [PubMed] [Google Scholar]
- 51.Mombouli JV, Vanhoutte PM. Endothelial dysfunction: from physiology to therapy. J. Mol. Cell. Cardiol. 1999;31:61–74. doi: 10.1006/jmcc.1998.0844. [DOI] [PubMed] [Google Scholar]
- 52.Nam KN, Park YM, Jung HJ, Lee JY, Min BD, Park SU, Jung WS, Cho KH, Park JH, Kang I. Anti-inflammatory effects of crocin and crocetin in rat brain microglial cells. Eur. J. Pharmacol. 2010;648:110–6. doi: 10.1016/j.ejphar.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 53.He SY, Qian ZY, Tang FT. Effect of crocin on intracellular calcium concentration in cultured bovine aortic smooth muscle cells. Yao Xue Xue Bao . 2004;39:778–81. [PubMed] [Google Scholar]
- 54.Williams BA, Liu C, DeYoung L, Brock GB, Sims SM. Regulation of intracellular Ca2+ release in corpus cavernosum smooth muscle: synergism between nitric oxide and cGMP. Am. J. Physiol. Cell Physiol. . 2005;288:C650–C8. doi: 10.1152/ajpcell.00475.2004. [DOI] [PubMed] [Google Scholar]
- 55.Liu N, Yang Y, Mo S, Liao J, Jin J. Calcium antagonistic effects of Chinese crude drugs: preliminary investigation and evaluation by 45 Ca. Appl. Radiat. Isot. 2005;63:151–5. doi: 10.1016/j.apradiso.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 56.Zhou CH, Xiang M, He SY, Qian ZY. Protein kinase C pathway is involved in the inhibition by crocetin of vascular smooth muscle cells proliferation. Phytother. Res. 2010;24:1680–6. doi: 10.1002/ptr.3194. [DOI] [PubMed] [Google Scholar]
- 57.Buckle D, Arch J, Bowring N, Foster K, Taylor J, Taylor S, Shaw D. Relaxant effects of the potassium channel activators BRL 38227 and pinacidil on guinea-pig and human airway smooth muscle, and blockade of their effects by glibenclamide and BRL 31660. Pulm. Pharmacol. 1993;6:77–86. doi: 10.1006/pulp.1993.1011. [DOI] [PubMed] [Google Scholar]
- 58.Laurent F, Michel A, Bonnet P, Chapat J, Boucard M. Evaluation of the relaxant effects of SCA40, a novel charybdotoxin‐sensitive potassium channel opener, in guinea‐pig isolated trachealis. Br. J. Pharmacol. 1993;108:622–6. doi: 10.1111/j.1476-5381.1993.tb12851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miura M, Belvisi MG, Ward JK, Tadjkarimi S, Yacoub M, Barnes P. Bronchodilating effects of the novel potassium channel opener HOE 234 in human airways in-vitro. Br. J. Clin. Pharmacol. 1993;35:318–20. doi: 10.1111/j.1365-2125.1993.tb05701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sonna LA, Hirshman CA, Croxton TL. Role of calcium channel blockade in relaxation of tracheal smooth muscle by extracellular Mg2+ Am. J. Physiol. Lung Cell Mol. Physiol. 1996;271:L251–L7. doi: 10.1152/ajplung.1996.271.2.L251. [DOI] [PubMed] [Google Scholar]
- 61.Tang F, Qian Z, Liu P, Zheng S, He S, Bao L, Huang H. Crocetin improves endothelium-dependent relaxation of thoracic aorta in hypercholesterolemic rabbit by increasing eNOS activity. Biochem. Pharmacol. 2006;72:558–65. doi: 10.1016/j.bcp.2006.05.018. [DOI] [PubMed] [Google Scholar]