Abstract
Many herbs and spices have been recommended traditionally as galactagogues and several commercial formulations prepared using herbs. Due to the presence of various compounds such as polyphenols, flavonoids, isoflavones, and terpenes, bitter and stringent taste is elicited that make the consumption of these herbal preparations unpleasant. Moreover, these compounds are unstable when exposed to environmental conditions. In this regard, different approaches are used for taste masking such as microencapsulation. In the present study, microcapsules containing herbal galactagogue extract were developed through emulsification/external gelation and Box-Behnken design was used to investigate the effects of independent variables (sodium alginate: 1-1.5%, calcium chloride: 0.2-1% and extract concentrations: 1-5%) on encapsulation efficiency (EE%). Following evaluation of the model, the optimum condition of encapsulation process was selected as 1.49% sodium alginate, 0.84 CaCl2, and 1.58% extract with EE% of 77.97%. Microcapsules had an acceptable spherical morphology and the results of Fourier transform-infrared spectroscopy (FTIR) revealed the presence of the extract within the microcapsules. The mean diameters of the uncoated and chitosan-coated microcapsules were 52 and 123 μm and encapsulation yield was 50.21 and 69.7%, respectively. The polydispersity index of 0.45 and 0.48 were an indicative of polydisperse nature of the microcapsules. The loss of flavonoids in microcapsules stored at two different temperatures was insignificant. The in-vitro release in simulated gastric fluid (SGF; pH 1.2) and simulated intestinal fluid (SIF; pH 7.4) were 48.1% and 80.11%, respectively during 24 h. The prepared extract-loaded microcapsules have potential to be used in matrices with neutral pH.
Key Words: Galactagogues, Herbal extract, Microencapsulation, Sodium alginate, Chitosan, External gelation
Introduction
Galactagogues are pharmaceutical agents, foods, or herbal supplements used to support the initiation, continuation, or augmentation of breast milk production (1). Herbs and natural substances have been used traditionally since ancient times in order to stimulate milk production. The most frequently used herbs in commercial preparations and formulations are shatavari, torbangun, milk thistle, fenugreek, fennel, caraway, dill, cumin, and anise (2, 3). The main active components in these plants that contribute to galactapoetic effect are polyphenols, flavonoids, isoflavones, and terpenes (4) which elicit bitter and astringent taste in the derived extracts and therefore, consumption of the herbal preparations by breastfeeding women is aversive. In general, bitter-tasting products are unpleasant for consumers leading to noncompliance and thus decreased therapeutic efficacy. It has been proved that consumers prefer the products presenting health benefits without compromising flavor, taste, and color (5, 6). In this respect, different approaches for taste masking of pharmaceutical formulations have been proposed such as utilization of sweeteners and flavoring agents, granulation, ion exchange resins, solid dispersion system, mass extrusion method and microencapsulation (7, 8). Furthermore, polyphenols have poor stability and are usually affected by pH variation, light, temperature, oxygen, and presence of metal ions (9).
In this regard, microencapsulation is a technique that entraps active ingredients in polymeric materials and creates a physical barrier against environmental conditions as well as sustained release and masking of unpleasant taste (10, 11). Encapsulation process can be carried out through different approaches and polymeric substances as well as materials (12, 13). Ionotropic gelation of alginate with calcium chloride and formation of calcium alginate gel beads have been extensively used in encapsulation of different bioactive ingredients (14-16). Alginate is a water-soluble polyanionic polymer composed of 1, 4-linked-α-L-guluronic acid and β-D-mannuronic acid residues derived from brown see-weeds and marine algae (17, 18). Although the biocompatibility, biodegradability, immunogenecity, and non-toxicity of alginate have made it an excellent carrier, the macroporous structure of calcium alginate beads leads to rapid dissolution of microcapsules, sudden release of core substances, and low encapsulation efficiency (19). In order to increase the stability and improve the permeability, chitosan has been extensively employed as a coating for calcium alginate beads which forms a strong complex membrane via electrostatic interaction between its amino residues and the carboxyl residues present in alginate (20, 21). To the best of our knowledge, no study has been conducted to encapsulate herbal galactagogue extract in alginate microcapsules, thus this study was designed to develop and characterize microcapsules containing mixture of herbal galactagogue extract using W/O emulsification followed by external gelation which can be used in matrices with neutral pH and formulation of food products with galactapoetic property. RSM in combination with Box-Behnken was applied to explore the effects of three independent variables (sodium alginate, calcium chloride and extract concentrations) on encapsulation efficiency as well as to develop a mathematical model for prediction and determination of optimum conditions for the maximum encapsulation efficiency.
Experimental
Materials
Sodium alginate (molecular mass of 1.97×105 Da, mannuronate/guluronate ratio = 0.6) was from BDH (Poole, UK). Medium-molecular weight chitosan (deacetylation degree 75-85%), quercetin (≥ 95%), calcium chloride and Span 80 were purchased from Sigma-Aldrich (St. Louis, MO, USA). Herbal galactagogue extract (containing Foeniculum vulgare, Cuminum cyminum, Trigonella foenum and Anethum graveolens extracts) was purchased from Goldaru Co. (Isfahan, Iran). Sunflower oil with no added antioxidant was supplied by Ladan (Tehran, Iran).
Aluminum chloride and potassium acetate were purchased from Merck (Darmstadt, Germany).
Preparation of chitosan-coated alginate microcapsules containing herbal galactagogue extract
Microcapsules were prepared by W/O emulsion technique followed by external gelation. Alginate solution at different concentration was prepared in distilled water stirred by magnetic stirrer overnight. Herbal galactagogue extract was added to alginate solution and mixed completely. Afterwards, the W/O emulsion was prepared by blending alginate solution with sunflower oil (containing 1% v/v span 80) using an ultra-Turrax homogenizer (IKA T25- Digital Ultra Turrax, Staufen, Germany) at a speed of 8000 rpm for 10 min. Then, microcapsules were formed by adding CaCl2 dropwise to emulsion under stirring at 400 rpm. Stirring was continued for 45 min. Then, the microcapsules were recovered by centrifugation at 1800 rpm for 5 min followed by vacuum filtration. Microcapsules were washed severally with deionized water containing Tween 80. The obtained microcapsules were immersed in chitosan solution with pH of 4.5 (1% w/v in 1% v/v acetic acid) under stirring for 15 min followed by centrifugation at 2000 rpm for 3 min and washing with distilled water. Finally, the beads were frozen at -80°C for 2 h and lyophilized with a freeze drier for 14 h (ALPHA 2-4; Christ, Harz, Germany).
Formulation optimization of chitosan-coated alginate microcapsules loaded with herbal galactagogue extract
In order to optimize the conditions for the preparation of chitosan-coated alginate microcapsules, Design-Expert software (V.7.0.0, Stat-Ease, Inc., Minneapolis, USA) with Box-Behnken response surface methodology was used to determine relationships among the variables and responses. Three independent variables were chosen as sodium alginate concentration (X1), herbal galactagogue extract concentration (X2), and CaCl2 (X3) while the dependent variable was encapsulation efficiency (EE %). The coded levels and actual values of the independent variables are presented in Table 1. A set of 17 experiments were employed with five replicates (used to estimate experimental error) of the center point (Table 2). For the prediction of optimal point, a quadratic model was fitted to correlate relationship between independent variables and the response, which accounts for variations caused by linear and quadratic order effects as well as by interactions. For the factors, the equation is:
Table 1.
Independent variables and their levels used in Box-Behnken design
| Independent variables | Symbol |
Coded levels
|
||
|---|---|---|---|---|
| -1 | 0 | 1 | ||
| Sodium alginate% | X1 | 1 | 1.25 | 1.5 |
| Herbal galactagogue extract% | X2 | 1 | 3 | 5 |
| CaCl2 | X3 | 0.2 | 0.6 | 1 |
Table 2.
Box-Behnken design and effect of independent variables on encapsulation efficiency as a response
| Runs | Sodium alginate (%) | Herbal galactagogue extract (%) | CaCl 2 (M) | Actual Encapsulation efficiency (%) | Predicted Encapsulation efficiency (%) |
|---|---|---|---|---|---|
| 1 | 1.25 | 5 | 0.2 | 37.61 | 35.62 |
| 2 | 1.25 | 3 | 0.6 | 62.29 | 63.54 |
| 3 | 1.25 | 3 | 0.6 | 64.76 | 63.54 |
| 4 | 1.25 | 1 | 1 | 68.15 | 70.14 |
| 5 | 1 | 5 | 0.6 | 35.5 | 34.83 |
| 6 | 1 | 3 | 0.2 | 50.09 | 52.75 |
| 7 | 1 | 1 | 0.6 | 53.2 | 51.79 |
| 8 | 1.25 | 3 | 0.6 | 65.1 | 63.54 |
| 9 | 1.5 | 3 | 0.2 | 56.54 | 57.12 |
| 10 | 1.25 | 1 | 0.2 | 61.12 | 59.87 |
| 11 | 1 | 3 | 1 | 52.15 | 51.57 |
| 12 | 1.5 | 5 | 0.6 | 32.18 | 33.59 |
| 13 | 1.25 | 3 | 0.6 | 61.11 | 63.54 |
| 14 | 1.5 | 1 | 0.6 | 74.22 | 74.89 |
| 15 | 1.25 | 3 | 0.6 | 64.42 | 63.54 |
| 16 | 1.5 | 3 | 1 | 71.73 | 69.07 |
| 17 | 1.25 | 5 | 1 | 34.89 | 36.14 |
| Equ. 1 |
Where Y is the dependent variable (EE%); Xi and Xj are levels of independent variables; β0 is a constant; βi, βii, and βij are interaction coefficients of linear, quadratic, and interaction coefficients, respectively; k is the number of independent parameters (k = 3 in this study); and ε is the error (22). Following determination and validation of the model, optimum formulation regarding to encapsulation efficiency was selected for preparation of chitosan-coated alginate microcapsules containing herbal galactagogue extract and the microcapsules were assessed in respect of physicochemical characteristics and release properties.
Encapsulation efficiency (EE%) of herbal galactogogue extract
In order to determine the EE%, accurately weighed amounts (200 mg) of microcapsules were suspended in 30 mL phosphate buffer (pH 7.4) and stirred at 150 rpm at room temperature until the complete dissolution of microcapsules. Then, the solution was centrifuged at 9000 rpm for 5 min and the content of total flavonoid was determined in the supernatant. EE% was calculated using the following equation:
| Equ. 2 |
Quantifications of the flavonoids content
Total flavonoid content was determined using the aluminum chloride colorimetric method (23). In brief, 0.5 mL of sample was mixed with 2.8 mL of distilled water, 1.5 mL of ethanol, 0.1 mL of 10% aluminum chloride solution and 0.1 mL of 1 mol/L potassium acetate.
The mixture was kept for 30 min and the absorbance of the samples was measured in a spectrophotometer (OPTIMA SP-3000 plus, Tokyo, Japan), at 415 nm. Quercetin was used as the standard in the range of 1-300 μg/mL.
Encapsulation yield (EY%)
The encapsulation yield was calculated as the ratio of the mass of microcapsules obtained at the end of the process to the mass of raw materials used in preparation of chitosan-coated and uncoated according to the following equation:
| Equ. 3 |
Morphological characterization of microcapsules
The morphology of chitosan-coated microcapsules prepared at optimum condition, was examined using a scanning electron microscope (SEM) (Philips XL30).
The samples were mounted to the specimen holder with a double-sided adhesive tape and vacuum coated with gold. SEM photographs were taken at the required magnification at room temperature and examined using an acceleration voltage of 25 kV.
Particle size analysis
The particle size distribution and the mean particle size of the samples were determined by dynamic light scattering using Malvern Nano ZS (red badge) ZEN 3600. Analyses were carried out using aqueous dispersions of loaded microcapsules in triplicate.
Fourier transform infrared (FTIR) spectroscopy
FTIR spectra of pure herbal galactagogue extract, sodium alginate, chitosan, chitosan-coated alginate beads without extract and chitosan-coated alginate beads with extract were obtained using an FTIR spectrophotometer (PerkinElmer Spectrum RX I, Waltham, MA, USA).
The samples were mixed with KBr and compressed to form discs. Each sample was scanned 16 times in the range of 4000-450 cm -1 at a resolution of 4 cm-1. Data were analyzed by Spectrum V 5.0.1 software (PerkinElmer, Waltham, MA, USA).
Stability studies
To evaluate the stability of flavonoids in microcapsules, 5 gr of freeze-dried chitosan-coated (prepared at optimum condition) microcapsule was placed in 40-mL bottles, tightly capped and stored away from light either at refrigerator temperature (4 °C) or room temperature (25 °C) for 120 days. The content of total flavonoids in the samples was determined after 0, 30, 60, 90, and 120 days of storage according to the method described in the total flavonoid quantification section. The results were reported as the percent loss of total flavonoids content.
In-vitro release studies
In-vitro release of extract from chitosan-coated microcapsules was investigated in simulated gastric fluid (SGF) and simulated intestinal fluid (SIF) during 24 h as follows: approximately 300 mg of microcapsules were suspended in 100 mL solution and maintained at 37 °C at 100 rpm.
The solutions were 0.1 mol/L HCL (pH 1.5) and phosphate buffer saline (PBS, pH 7). At predetermined time intervals, the samples (5 mL) were collected from release medium and replaced with fresh solutions. Aliquots (5 mL) were centrifuged at 9000 rpm for 10 min and the supernatant was analyzed for flavonoid concentration. All experiments were carried out in triplicate and percentage of cumulative amount of released extract was plotted against time.
Statistical analysis
Data analysis was performed using Design Expert software (V. 7, Stat-Ease, Inc., Minneapolis, USA) with appropriate ANOVA studies, used for mathematical modeling in response surface methodology.
Results and Discussion
Model evaluation
The encapsulation efficiencies obtained in different experiments are presented in Table 2. The quadratic model was selected as the best-fitting model having an insignificant lack of fit (0.1358) and maximum value of the adjusted R-square and the predicted R-square.
The high value of R2 (0.9851) and R2 adjusted (0.9659) is an indicative of agreement between experimental results and the data predicted by the model. In order to find the significant parameters affecting the encapsulation efficiency, analysis of variance (ANOVA) was performed. The results are presented in Table 3.
Table 3.
Analysis of variance for response quadratic model
| Encapsulation efficiency (%) | |||||
|---|---|---|---|---|---|
| Source | Sum of Square | df* | Mean Square | F-value | Prob > F |
| Model | 2833.00 | 9 | 314.78 | 51.40 | < 0.0001** |
| X1 | 239.04 | 1 | 239.04 | 39.03 | 0.0004** |
| X2 | 1696.82 | 1 | 1696.82 | 277.06 | < 0.0001** |
| X3 | 58.10 | 1 | 58.10 | 9.49 | 0.0178** |
| X1X2 | 148.11 | 1 | 148.11 | 24.18 | 0.0017** |
| X1X3 | 43.10 | 1 | 43.10 | 7.04 | 0.0328** |
| X2X3 | 23.77 | 1 | 23.77 | 3.88 | 0.0895 |
| X1 2 |
60.42 | 1 | 60.42 | 9.86 | 0.0164** |
| X2 2 |
506.98 | 1 | 506.98 | 82.78 | < 0.0001** |
| X3 2 |
18.93 | 1 | 18.93 | 3.09 | 0.1221 |
| Residual | 42.87 | 7 | 6.12 | ||
| Lack of Fit | 30.71 | 3 | 10.24 | 3.37 | 0.1358 |
| Pure Error | 12.16 | 4 | 3.04 | ||
* Degree of freedom.
** Significant at 0.05 level.
The significance of each coefficient was specified by p-value. The values less than 0.05 indicate significant variables. Among the tested variables in this study, X1 (sodium alginate concentration), X2 (extract concentration), X3 (CaCl2 concentration), (X1)2 (sodium alginate concentration × sodium alginate concentration), (X2)2 (extract concentration × extract concentration), X1X2 (sodium alginate concentration × extract concentration) and X1X3 (sodium alginate × CaCl2 concentration) were significant terms of the model.
The coefficients of independent variables are given in equation as below:
Y = -96.6556 + 190.2000 X1 + 26.2182 X2 – 9.2493 X3- 12.1700 X1X2 + 32.8250 X1X3 – 60.6080 X12- 2.7432 X22
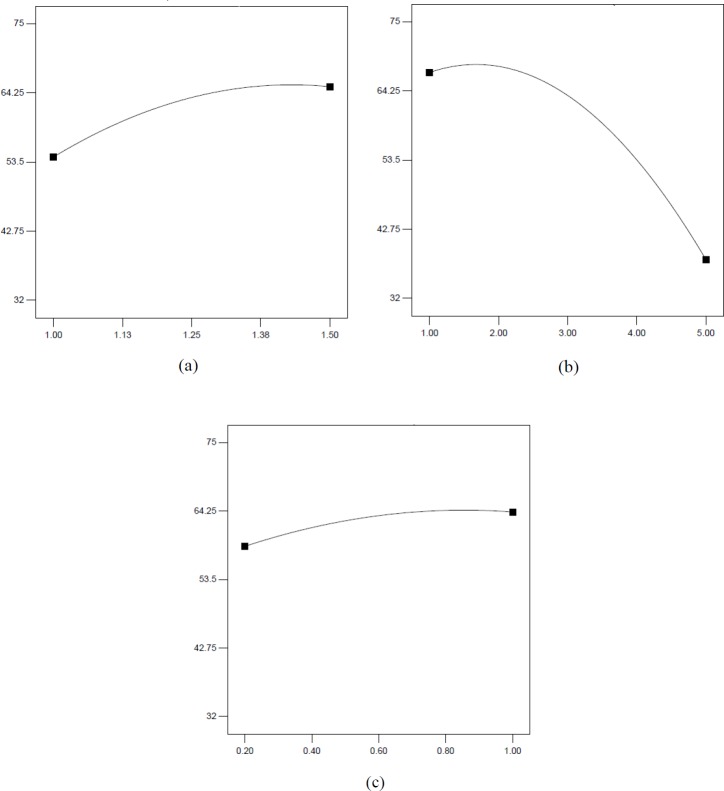
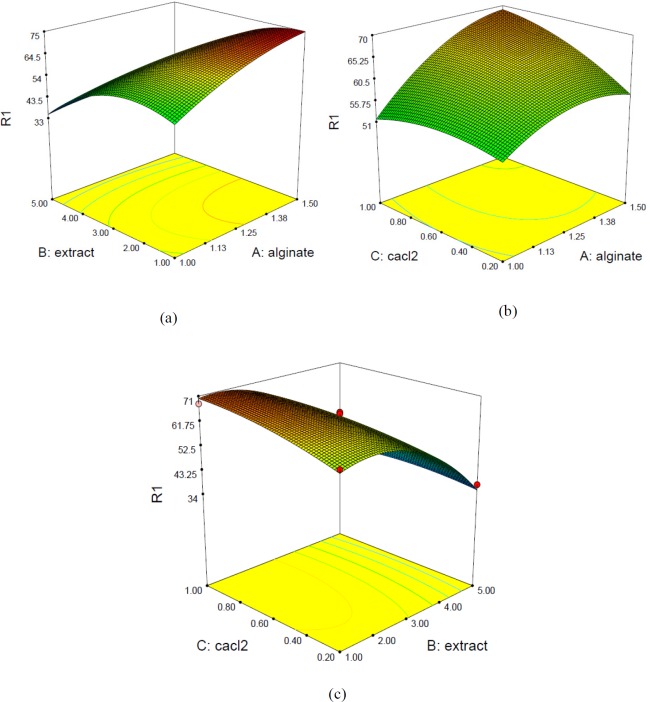
The equation shows that the sodium alginate and extract influenced EE% in a quadratic and linear model while CaCl2 had linear effect. Figure 1 illustrates linear effects of sodium alginate, extract and CaCl2 on EE%. Figure 2 illustrates the response surface plots for EE% with respect to sodium alginate and extract (a) and CaCl2 and sodium alginate (b).
Figure 1.
Effect of sodium alginate (a), extract (b) and CaCl2 (c) concentration on encapsulation efficiency
Figure 2.
3D surface plots for EE% with respect to sodium alginate and extract (a), CaCl2 and sodium alginate (b) and extract and CaCl2 (c)
Formulation optimization
The optimum point with a maximum predicated value of EE% (78.028 %) was obtained at alginate concentration of 1.49 %, extract concentration of 1.58 % and CaCl2 level of 0.84 %. The accuracy of predicated condition was examined by performing independent experiments at the optimal point. The results revealed that the predicated value from the model was reasonably close to observed value (77.97%).
Effect of microencapsulation conditions on encapsulation efficiency
Figure 1 illustrates linear effect of independent variables on EE%. As shown, encapsulation efficiency was increased by increasing sodium alginate concentration from 1 to 1.5% and extract concentration up to 2%. Increasing CaCl2 concentration enhanced EE% and was constant in the range of 0.8-1%. The simultaneous effects of two factors on response while keeping the other variable constant at its middle level (center value of testing ranges) are presented in forms of 3D surface plots in Figure 2. The tortuous surface shows a strong interaction between two factors. Figure 2a shows the 3D surface plot for combined effects of sodium alginate and galactagogue extract concentration (X1X2) on EE%. A higher encapsulation efficiency was obtained with extract concentration of 1-2% and sodium alginate concentration of 1.5%, but when extract level was enhanced, EE% decreased even at high concentration of sodium alginate. EE% increase by enhancing alginate level might be due to the formation of a more compact membrane which inhibits leakage of extract to external solution (24). Similarly, Chan (15) stated that EE% of a model oil in Ca-alginate beads increased from 60% for the 5 g/L alginate solution to 90% for the 25 g/L solution. The increase of EE% by enhancement of alginate concentration was confirmed in the study of Lotfipour et al. (25) who pointed that alginate concentration was the most affective factor on EE% of Lactobacillus acidophilus and also an increase in EE% of propranolol incorporated into calcium alginate beads (26). In a study conducted by Soliman et al. (27), EE% was decreased with increasing the alginate concentration over 2% due to formation of pores with smaller size and consequently a lower amount of ingredient can be entrapped within the polymer matrix. Calcium ions interact preferentially with guluronic sequences of sodium alginate and hydrogel networks are formed. Therefore, it can be assumed that sodium alginate with high guluronic acid denoted as high G (the alginate used in this study), are more susceptible to CaCl2 level.
By increasing extract level, the EE% was reduced. Similarly, Zam et al. (28) stated that the highest loading efficiency of pomegranate polyphenols in alginate beads was obtained by 1% extract. Extract is water soluble and the amount of encapsulated extract is dependent on the water amount of hydrogel. Therefore, applying high concentrations of lemon balm extract did not increase the encapsulation efficiency (24). Moreover, it can be explained by the saturation of extract loading into alginate microcapsules (29).
Figure 2b presents the 3D surface plot for combined effects of sodium alginate and CaCl2 concentration (X1X3) on EE%. Increasing the level of CaCl2 particularly in the range of 0.6-0.8% and simultaneously increase of sodium alginate concentration led to the highest EE%. Increasing the level of Ca+2 causes rapid cold setting and a densely cross-linked gel with lower porous structure; therefore, lower amount of herbal galactagogue extract can be entrapped in gel matrix and subsequently EE% would decrease (27). These results are in agreement with the study conducted by Najafi-Soulari et al. (24) who pointed that encapsulation efficiency of lemon balm extract decreased by increasing CaCl2 concentration up to 1% and became constant at higher concentrations. Consistently, it was reported that by increasing the level of CaCl2, more Ca+2 ions diffuse into the drug-loaded alginate beads and more drug would displace from alginate matrix by Ca+2 ions leading to a decrease in EE% (26).
Figure 2c shows combined effects of extract and CaCl2 on EE%. By increasing extract concentration in the range of 1-2% and CaCl2 in the range of 0.8-1%, EE% increased. Although, this increasing trend was higher at higher concentration of CaCl2, the difference was not remarkable. Therefore, the interaction of these two factors was not statistically significant (p ˃ 0.05).
Encapsulation yield
The microencapsulation yield (EY%) is important from the economic point of view in any encapsulation process, considering the cost of polymers and active principles used (30). EY% calculated in uncoated and chitosan-coated microcapsules was 50.21 ± 0.8 % and 69.7 ± 0.5 %, respectively. In a study by Samakradhamrongthai et al. (31), the encapsulation yield of freeze-dried Michelia alba D.C. extract was reported 50.01%. In another study, EY% of freeze-dried alginate microcapsules containing lactobacilli was 50.5 (30). Sabitha et al. (32) obtained EY% in the range of 55-68% for different antitubercular drugs encapsulated in chitosan-alginate matrix. In chitosan-coated microcapsules, due to an extra coating and increase in the thickness of the microcapsule wall, EY% was higher. It was reported that the higher amount of wall material available for formation of a thicker microcapsule wall resulted in increasing product recovery (33). Furthermore, using chitosan as a coating would increase the total solid content of feed solution in freeze drying process (34).
Morphology of microcapsules
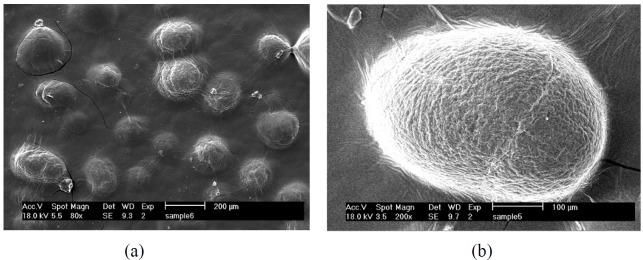
The morphology of chitosan-coated microcapsules prepared at optimum condition was studied by SEM as shown in Figure 3 As can be seen, an acceptable spherical morphology was obtained. The absence of ideal spherical morphology and rough structure can be probably attributed to the drying process that causes certain invaginations in the particles (35).
Figure 3.
SEM images of chitosan-coated microcapsules loaded with extract; 80× (a) and b) 200× (b)
Similarly, Saikia et al. (36) pointed out that freeze-dried encapsulates of polyphenol powder were irregular in the shape compared to spray-dried microcapsules. Dai et al. (37) reported a rough surface in alginate-chitosan hydrogel beads with cracks and wrinkles due to partial collapsing of the polymer network during dehydration. Insufficiency of ideal spherical structure and smoothness was also reported in other studies (21, 38).
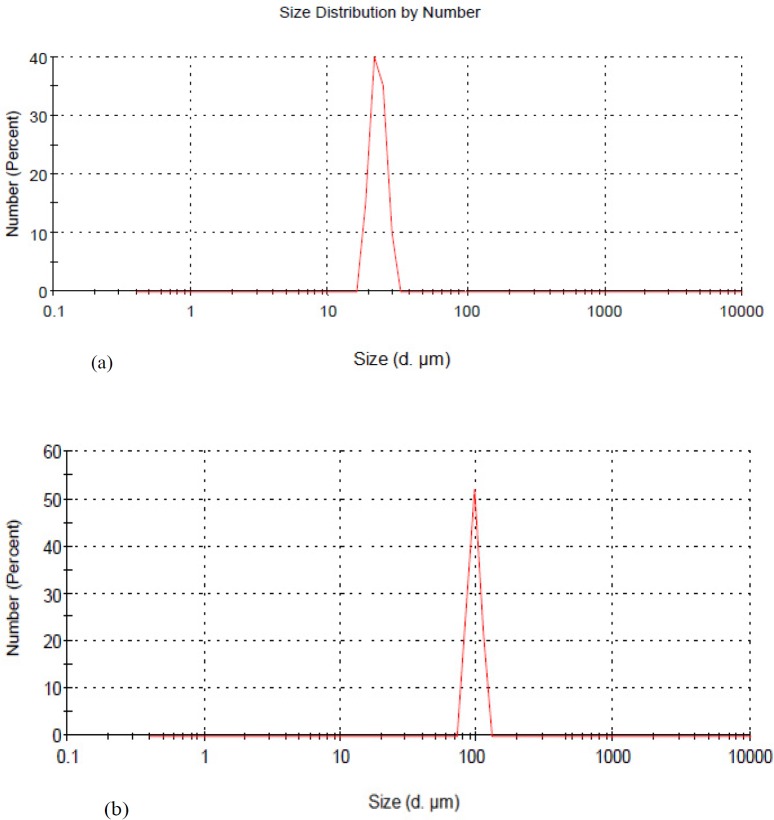
Mean particle size and particle size distribution of microcapsules
Figure 4 presents particle size distribution of chitosan-coated and uncoated microcapsules. Diameter of uncoated and chitosan-coated microcapsules were in the range of 46-75 μm and 90-150 μm and mean diameter of 52 ± 0.76 μm and 123 ± 2.3 μm, respectively. It has been reported that microcapsules smaller than 200 μm show a more prolonged passage time and the possibility of controlled release of the encapsulated drug substance (38, 39).
Figure 4.
Size distribution by number for uncoated alginate microcapsules loaded with extract (a) and chitosan-coated alginate microcapsules loaded with extract (b)
As evident in Figures 4, the microcapsules had a narrow size distribution. The polydispersity index (PDI) values of 0.48 and 0.45 for uncoated and chitosan-coated alginate microcapsules confirm acceptable polydisperse nature of microcapsules in aqueous. Coating of alginate microcapsules with chitosan increased the particle size. This is in agreement with the results obtained by Nualkaekul et al. (40) that reported an increase in particle size of alginate beads loaded with Lactobacillus plantarum by chitosan coating. Furthermore, loading of extract in microcapsules was affective on particle size increment. Similarly, Khaksar et al. (41) stated an increase of particle size by entrapment of nisin in alginate-high methoxy pectin microparticles. Hui et al. (42) also reported an increase in microcapsules’ size loaded with PentaHerbs extract compared to unloaded microcapsules.
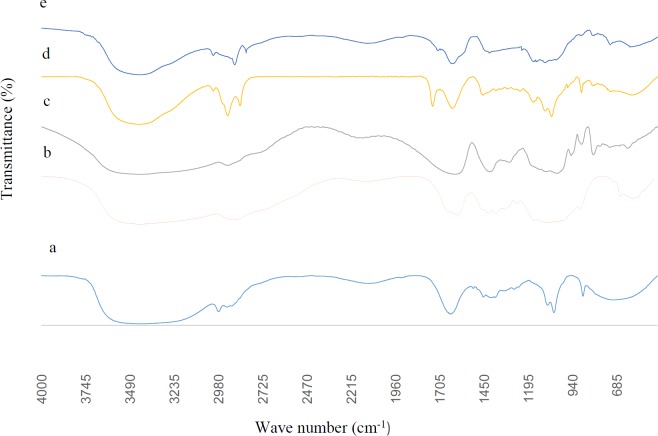
FTIR analysis
The functional groups and intermolecular interactions within the microcapsules were investigated by FTIR. Figure 5 represents FTIR spectrum of herbal galactagogue extract, chitosan, sodium alginate, and chitosan-coated alginate microcapsules containing extract. Figure. 5a shows the spectrum of galactagogue extract band at 3419, 2979, 1645, 1411, 1047, and 713 cm-1 that can be assigned to OH stretching, sp3 C-H stretching, C=O stretching, α-CH2 bending, C–O stretching, and C–H bending and ring puckering, respectively (42, 43). As shown in Figure. 5b, the main bands in chitosan powder were 3433 cm-1 (O-H stretching), 2875 cm-1 (C-H stretching), 1655 cm-1 (C=O stretching, amide I representing the structure of N-acetylglucosamine), 1580 cm-1 (bending vibrations of the N-H representing N-acetylated residues), 1424 cm-1 (N-H stretching, amide II representing glucosamine functional groups), 1385 cm-1 (NH stretching, amide III), 1159 cm-1 (C-O-C stretching) and 896 cm-1 (pyranose ring) (37, 44).
Figure 5.
FTIR spectra of herbal galactagogue extract (a), chitosan (b), sodium alginate (c), chitosan-coated alginate microcapsules containing extract (d) and blank chitosan-coated alginate microcapsules (e)
Sodium alginate spectrum (Figure. 5c) showed characteristic bands at 3433 cm-1 (OH), 2924 cm-1 (CH), 1630 cm-1 (COO- asymmetric), 1416 cm-1 (COO- symmetric), and 1031 cm-1 (C-O-C). Similar absorption bands were reported by other authors (37, 45). By comparing the spectrum of blank chitosan-coated alginate microcapsules (Figure 5e) with chitosan (b) and sodium alginate (c), it was found that most specific peaks of chitosan and alginate were present in microcapsules spectrum with some shifts. Stretching vibration of O-H at 3433 cm-1 shifted to 3429 cm-1.
Appearance of new peak at 2855 cm-1 and increase of the sharpness of the peak at 1631 cm−1 as a result of COO- groups in alginate and the disappearance of the chitosan amino band at 1580 cm-1, suggest the formation of a polyelectrolyte complex between sodium alginate and chitosan (46, 47). A new peal at 1746 cm-1 belonging to COOH is an indicative of acidic condition in which the microcapsules were prepared (37).
Moreover, some peaks in chitosan spectrum disappeared due to the presence of multi-interactions of polymers like electrostatic and hydrogen bonding (48, 49). Compared to the blank chitosan-coated alginate microcapsules and herbal galactagogue extract spectra, the spectrum of extract-loaded microcapsules (Figure. 5d) is a combination of the two aforementioned spectra and also the appearance of some peaks at 1099, 1061 and 887 cm-1 is an indicative of herbal galactagogue extract encapsulation in chitosan-sodium alginate matrix.
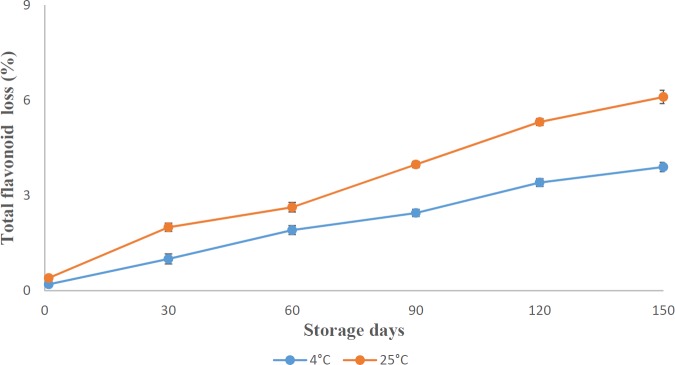
Stability of entrapped flavonoids in microcapsules
Figure 6 shows the percent loss of flavonoids in microcapsules stored at two different temperatures. As can be seen in Figure 6, although flavonoid loss increased during storage from day 1 to day 120, the loss of flavonoids in the microcapsules was insignificant during the storage. Similarly, in a study by Sansone et al. (50), the spray-dried microcapsules containing quercetin were stored at 25 °C for 12 months and after this period, no degradation products or decrease of concentration was observed. Accordingly, Nori et al. (51) also pointed out that storage temperatures of 10 and 25 °C had no effect on flavonoid content of microencapsulated propolis extract. The microcapsules stored at 4 °C showed lower percent loss (3.9 ± 0.14%) compared to the samples (6.11 ± 0.21%) stored at 25 °C during 120 days storage. This is in agreement with the results of Abedi et al. (52) who reported a higher loss of thymoquinone in microcapsules stored at 20 °C compared to 4 °C. In general, it can be expressed that alginate and chitosan as wall materials are suitable in protection of flavonoids from environmental conditions.
Figure 6.
Total flavonoid loss (%) in chitosan-coated microcapsules stored at 4 °C and 25 °C during storage
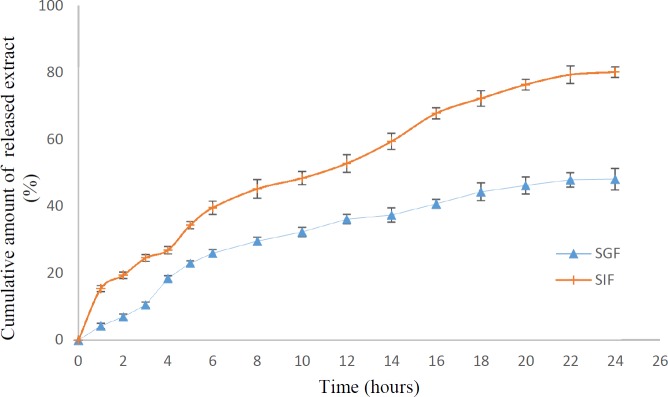
Release studies within simulated gastrointestinal conditions
Figure 7 shows the release of extract from microcapsules in SGF and SIF. Under SGF condition, a fast initial release (18%) during 4 h occurred followed by a slow and continuous release (48.1%) till 24 h. In a study by Dai et al. (37), the release of nifedipine from chitosan-coated alginate beads at pH 1.5 was 18% while at pH 6.8, the release increased significantly up to 99%. Similarly, Finotelli et al. (53) reported a very fast release of insulin (18%) from alginate/chitosan nanoparticles corresponding to insulin physically entrapped to bead’s external layer. The release of extract from alginate matrix was relatively low due to applying a chitosan coating. The barrier property of chitosan was confirmed in other studies (53-55). At low pH, the carboxyl groups of alginate were protonated and electrostatic repulsion among these groups led to formation of insoluble alginic acid and a reversible shrinkage took place that hindered the release of the core substance (21, 56). Similarly, it was reported that in acidic environment, calcium ions in alginate beads were totally discharged and the carboxyl groups shifted to an un-ionized form (57). In SIF with pH 7, a rapid increase in the release rate was observed up to 80% during 24 h. According to Lacerda et al. (21), increase of the pH led to deprotonation of chitosan that attenuated the extent of the interactions inside the microparticle and the ionization of the sodium alginate carboxyl groups. Anal et al. (58) reported a negligible release of bovine serum albumin from chitosan-alginate beads in SGF medium (pH 1.2), but in SIF medium (pH 7.5), the release was faster. It was expressed that high affinity of phosphate ions present in intestinal fluid for Ca+2 induced disruption of calcium-alginate gel matrix.
Figure 7.
In-vitro cumulative release of herbal galactagogue extract from microcapsules in SGF and SIF
Conclusion
Encapsulation of herbal galactagogue extract in chitosan-coated alginate microcapsules using emulsification/external gelation method in optimum condition (sodium alginate concentration of 1.49%, extract concentration of 1.58% and CaCl2 concentration of 0.84%) led to preparation of spherical microcapsules with mean diameter of 123 μm and EE% of 77.97%. FTIR analysis revealed successful extract loading in alginate microcapsules. The in-vitro release studies demonstrated a controlled release of extract in SGF and SIF. Based on the results obtained and physicochemical properties of chitosan-coated microcapsules, it can be concluded that these microcapsules can be incorporated into food matrices with neutral pH.
Acknowledgment
We gratefully acknowledge the National Nutrition and Food Technology Research Institute of Shahid Beheshti University of Medical Sciences for their supports. This article is a part of Ph.D dissertation at Shahid Beheshti University of Medical Sciences, Tehran, Iran.
References
- 1.Zapantis A, Steinberg JG, Schilit L. Use of herbals as galactagogues. J. Pharm. Pract. 2012;25:222–31. doi: 10.1177/0897190011431636. [DOI] [PubMed] [Google Scholar]
- 2.Forinash AB, Yancey AM, Barnes KN, Myles TD. The use of galactogogues in the breastfeeding mother. Ann. Pharmacother. 2012;46:1392–404. doi: 10.1345/aph.1R167. [DOI] [PubMed] [Google Scholar]
- 3.Shinde P, Patil P, Bairagi V. Herbs in pregnancy and lactation: a review appraisal. Int. J. Pharm. Sci. Res. 2012;3:3001–6. [Google Scholar]
- 4.Mohanty I, Senapati M, Jena D, Behera P. Ethnoveterinary importance of herbal galactogogues-α review. Vet. World. 2014;7:325–30. [Google Scholar]
- 5.Nakamura T, Tanigake A, Miyanaga Y, Ogawa T, Akiyoshi T, Matsuyama K, Uchida T. The effect of various substances on the suppression of the bitterness of quinine–human gustatory sensation, binding, and taste sensor studies. Chem. Pharm. Bull. 2002;50:1589–93. doi: 10.1248/cpb.50.1589. [DOI] [PubMed] [Google Scholar]
- 6.Sun-Waterhouse D, Wadhwa SS. Industry-relevant approaches for minimising the bitterness of bioactive compounds in functional foods: a review. Food. Bioprocess. Tech. 2013;6:607–27. [Google Scholar]
- 7.Kulkarni L, Metkari V, Bamane G, Jadhav P, Raut G. Recent trends on achieving taste masking of bitter drug. JCPR . 2014;4:1246–57. [Google Scholar]
- 8.Tripathi A, Parmar D, Patel U, Patel G, Daslaniya D, Bhimani B. Taste masking: a novel approach for bitter and obnoxious drugs. JPSBR . 2011;1:36–142. [Google Scholar]
- 9.Aizpurua-Olaizola O, Navarro P, Vallejo A, Olivares M, Etxebarria N, Usobiaga A. Microencapsulation and storage stability of polyphenols from Vitis vinifera grape wastes. Food. Chem. 2016;190:614–621. doi: 10.1016/j.foodchem.2015.05.117. [DOI] [PubMed] [Google Scholar]
- 10.Nazzaro F, Orlando P, Fratianni F, Coppola R. Microencapsulation in food science and biotechnology. Curr. Opin. Biotech. 2012;23:182–6. doi: 10.1016/j.copbio.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Paulo F, Santos L. Design of experiments for microencapsulation applications: A review. Mater. Sci. Eng. C. 2017;77:1327–40. doi: 10.1016/j.msec.2017.03.219. [DOI] [PubMed] [Google Scholar]
- 12.Hoyos-Leyva JD, Bello-Pérez LA, Alvarez-Ramirez J, Garcia HS. Microencapsulation using starch as wall material: A review. Food Rev. Int. 2018;34:148–61. [Google Scholar]
- 13.Singh M, Hemant K, Ram M, Shivakumar H. Microencapsulation: A promising technique for controlled drug delivery. Res. Pharm. Sci. 2010;5:65. [PMC free article] [PubMed] [Google Scholar]
- 14.Chan E-S. Preparation of Ca-alginate beads containing high oil content: Influence of process variables on encapsulation efficiency and bead properties. Carbohydr. Polym. 2011;84:1267–75. [Google Scholar]
- 15.Natrajan D, Srinivasan S, Sundar K, Ravindran A. Formulation of essential oil-loaded chitosan–alginate nanocapsules. J. Food Drug Anal. 2015;23:560–8. doi: 10.1016/j.jfda.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mortazavian AM, Ehsani MR, Azizi A, Mousavi SM, Sohrabvandi S, Reinheimer JA. Viability of calcium-alginate microencapsulated probiotic bacteria in Iranian yogurt drink (Doogh) during refrigerated storage and under simulated gastrointestinal conditions. Aust. J. Dairy. Technol. 2008;63:25–30. [Google Scholar]
- 17.Luo Y, Wang Q. Recent development of chitosan-based polyelectrolyte complexes with natural polysaccharides for drug delivery. Int. J. Biol. Macromol. 2014;64:353–67. doi: 10.1016/j.ijbiomac.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 18.Jaafari M, Tafaghodia M, Eskandari M, Khamesipour A. Immunization against cutaneous leishmaniasis by alginate microspheres loaded with autoclaved leishmania major (ALM) and quillaja saponins. Iran. J. Pharm. Res. 2016;15:578–81. [PMC free article] [PubMed] [Google Scholar]
- 19.Goh CH, Heng PWS, Chan LW. Alginates as a useful natural polymer for microencapsulation and therapeutic applications. Carbohydr Polym. 2012;88:1–12. [Google Scholar]
- 20.Kim W-T, Chung H, Shin I-S, Yam KL, Chung D. Characterization of calcium alginate and chitosan-treated calcium alginate gel beads entrapping allyl isothiocyanate. Carbohydr. Polym. 2008;71:566–73. [Google Scholar]
- 21.Lacerda L, Parize AL, Fávere V, Laranjeira MCM, Stulzer HK. Development and evaluation of pH-sensitive sodium alginate/chitosan microparticles containing the antituberculosis drug rifampicin. Mater. Sci. Eng. C. 2014;39:161–7. doi: 10.1016/j.msec.2014.01.054. [DOI] [PubMed] [Google Scholar]
- 22.Maran JP, Manikandan S, Priya B, Gurumoorthi P. Box-Behnken design based multi-response analysis and optimization of supercritical carbon dioxide extraction of bioactive flavonoid compounds from tea (Camellia sinensis L) leaves. J. Food Sci. Tech. 2015;52:92–104. [Google Scholar]
- 23.Chang C-C, Yang M-H, Wen H-M, Chern J-C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002;10:178–82. [Google Scholar]
- 24.Najafi-Soulari S, Shekarchizadeh H, Kadivar M. Encapsulation optimization of lemon balm antioxidants in calcium alginate hydrogels. J. Biomater. Sci. Polym. Ed. 2016;27:1631–44. doi: 10.1080/09205063.2016.1226042. [DOI] [PubMed] [Google Scholar]
- 25.Lotfipour F, Mirzaeei S, Maghsoodi M. Evaluation of the effect of CaCl2 and alginate concentrations and hardening time on the characteristics of Lactobacillus acidophilus loaded alginate beads using response surface analysis. Adv. PharM. Bull. 2012;2:71–8. doi: 10.5681/apb.2012.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halder A, Maiti S, Sa B. Entrapment efficiency and release characteristics of polyethyleneimine-treated or-untreated calcium alginate beads loaded with propranolol–resin complex. Int. J. Pharm. 2005;302:84–94. doi: 10.1016/j.ijpharm.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 27.Soliman EA, El-Moghazy AY, El-Din MM, Massoud MA. Microencapsulation of essential oils within alginate: formulation and in-vitro evaluation of antifungal activity. JEAS . 2013;3:48–55. [Google Scholar]
- 28.Zam W, Bashour G, Abdelwahed W, Khayata W. Alginate-pomegranate peels› polyphenols beads: effects of formulation parameters on loading efficiency. Braz. J. Pharm. Sci. 2014;50:741–8. [Google Scholar]
- 29.Hosseini SF, Zandi M, Rezaei M, Farahmanghavi F. Two-step method for encapsulation of oregano essential oil in chitosan nanoparticles: Preparation, characterization and in-vitro release study. Carbohydr. Polym. 2013;95:50–6. doi: 10.1016/j.carbpol.2013.02.031. [DOI] [PubMed] [Google Scholar]
- 30.Ale CE, Torres Luque A, Gonzalez Moreno C, Otero MC. Microencapsulation of bovine vaginal lactobacilli in alginate using emulsion-gelation: Freeze-drying, storage and antimicrobial activity. J. Bioprocess Biotech. 2015;5:1–8. [Google Scholar]
- 31.Samakradhamrongthai R, Thakeow P, Kopermsub P, Utama-ang N. Encapsulation of Michelia alba DC Extract Using spray drying and freeze drying and application on Thai dessert from rice flour. Int. J. Food Eng. 2015;1:77–85. [Google Scholar]
- 32.Sabitha P, Ratna J, Reddy KR. Design and evaluation of controlled release chitosan-calcium alginate microcapsules of antitubercular drugs for oral use. Int J. Chemtech. Res. 2010;2:88–98. [Google Scholar]
- 33.Quispe-Condori S, Saldaña MDA, Temell F. Microencapsulation of flax oil with zein using spray and freeze drying. LWT-Food Sci. Technol. 2011;44:1880–7. [Google Scholar]
- 34.Laokuldilok N, Thakeow P, Kopermsub P, Utama-ang N. Optimisation of microencapsulation of turmeric extract for masking flavor. Food Chem. 2016;194:695–704. doi: 10.1016/j.foodchem.2015.07.150. [DOI] [PubMed] [Google Scholar]
- 35.Mladenovska K, Cruaud O, Richomme P, Belamie E, Raicki R, Venier-Julienne M-C, Popovski E, Benoit J-P, Goracinova K. 5-ASA loaded chitosan–Ca–alginate microparticles: Preparation and physicochemical characterization. Int. J. Pharm. 2007;345:59–69. doi: 10.1016/j.ijpharm.2007.05.059. [DOI] [PubMed] [Google Scholar]
- 36.Saikia S, Mahnot NK, Mahanta CL. Optimisation of phenolic extraction from Averrhoa carambola pomace by response surface methodology and its microencapsulation by spray and freeze drying. Food Chem. 2015;171:144–52. doi: 10.1016/j.foodchem.2014.08.064. [DOI] [PubMed] [Google Scholar]
- 37.Dai YN, Li P, Zhang JP, Wang AQ, Wei Q. Swelling characteristics and drug delivery properties of nifedipine‐loaded pH sensitive alginate–chitosan hydrogel beads. Biomed. Mater. Res. Part B Appl. Biomater. 2008;86:493–500. doi: 10.1002/jbm.b.31046. [DOI] [PubMed] [Google Scholar]
- 38.Crcarevska MS, Dodov MG, Goracinova K. Chitosan coated Ca–alginate microparticles loaded with budesonide for delivery to the inflamed colonic mucosa. Eur. J. Pharm. Biopharm. 2008;68:565–78. doi: 10.1016/j.ejpb.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 39.McMaster L, Kokott S. Micro-encapsulation of Bifidobacterium lactis for incorporation into soft foods. World J. Microbiol. Biotechnol. 2005;21:723–8. [Google Scholar]
- 40.Nualkaekul S, Lenton D, Cook MT, Khutoryanskiy VV, Charalampopoulos D. Chitosan coated alginate beads for the survival of microencapsulated Lactobacillus plantarum in pomegranate juice. Carbohydr Polym. 2012;90:1281–7. doi: 10.1016/j.carbpol.2012.06.073. [DOI] [PubMed] [Google Scholar]
- 41.Khaksar R, Hosseini SM, Hosseini H, Shojaee‐Aliabadi S, Mohammadifar MA, Mortazavian AM, khosravi‐Darani K, Haji Seyed Javadi N, Komeily R. Nisin‐loaded alginate‐high methoxy pectin microparticles: preparation and physicochemical characterisation. Int. J. Food Sci. Tech. 2014;49:2076–82. [Google Scholar]
- 42.Hui PC-L, Wang W-Y, Kan C-W, Ng FS-F, Wat E, Zhang VX, Chan C-L, Bik-San Lau C, Leung P-C. Microencapsulation of traditional Chinese herbs—PentaHerbs extracts and potential application in healthcare textiles. Colloids. Surf. B Biointerfaces . 2013;111:156–61. doi: 10.1016/j.colsurfb.2013.05.036. [DOI] [PubMed] [Google Scholar]
- 43.Krishnaiah D, Sarbatly R, Nithyanandam R. Microencapsulation of Morinda citrifolia L extract by spray-drying. Chem. Eng. Res. Des. . 2012;90:622–32. [Google Scholar]
- 44.Mirzaei F, Mohammadpour Dounighi N, Avadi MR, Rezayat M. A New Approach to Antivenom Preparation Using Chitosan Nanoparticles Containing EchisCarinatus Venom as A Novel Antigen Delivery System. Iran. J. Pharm. Res. 2017;16:858–67. [PMC free article] [PubMed] [Google Scholar]
- 45.Angadi SC, Manjeshwar LS, Aminabhavi TM. Novel composite blend microbeads of sodium alginate coated with chitosan for controlled release of amoxicillin. Int. J. Biol. Macromol. 2012;51:45–55. doi: 10.1016/j.ijbiomac.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 46.Abreu FO, Bianchini C, Forte MM, Kist TB. Influence of the composition and preparation method on the morphology and swelling behavior of alginate–chitosan hydrogels. Carbohydr. Polym. 2008;74:283–9. [Google Scholar]
- 47.Li D, Li P, Zang J, Liu J. Enhanced hemostatic performance of tranexamic acid-loaded chitosan/alginate composite microparticles. Biomed. Res. Int. 2012;2012:1–9. doi: 10.1155/2012/981321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bulatao RM, Samin JPA, Salazar JR, Monserate JJ. Encapsulation of anthocyanins from black rice (Oryza Sativa L) bran extract using chitosan-alginate nanoparticles. J. Food Res. 2017;6:40–7. [Google Scholar]
- 49.Sankalia MG, Mashru RC, Sankalia JM, Sutariya VB. Reversed chitosan–alginate polyelectrolyte complex for stability improvement of alpha-amylase: optimization and physicochemical characterization. Eur. J. Pharm. Biopharm. 2007;65:215–32. doi: 10.1016/j.ejpb.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 50.Sansone F, Picerno P, Mencherini T, Villecco F, D’ursi AM, Aquino RP, Lauro MR. Flavonoid microparticles by spray-drying: Influence of enhancers of the dissolution rate on properties and stability. J Food Eng. 2011;103:188–96. [Google Scholar]
- 51.Nori MP, Favaro-Trindade CS, de Alencar SM, Thomazini M, de Camargo Balieiro JC, Castillo CJ. Microencapsulation of propolis extract by complex coacervation. LWT-Food Sci. Technol. 2011;44:429–35. [Google Scholar]
- 52.Abedi AS, Rismanchi M, Shahdoostkhany M, Mohammadi A, Hosseini H. Microencapsulation of Nigella sativa seeds oil containing thymoquinone by spray‐drying for functional yogurt production. Int. J. Food Sci. Technol. 2016;51:2280–9. [Google Scholar]
- 53.Finotelli PV, Da Silva D, Sola-Penna M, Rossi AM, Farina M, Andrade LR, Takeuchi AY, Rocha-Leão MH. Microcapsules of alginate/chitosan containing magnetic nanoparticles for controlled release of insulin. Colloids. Surf. B Biointerfaces . 2010;81:206–11. doi: 10.1016/j.colsurfb.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 54.Anbinder PS, Deladino L, Navarro AS, Amalvy JI, Martino MN. Yerba mate extract encapsulation with alginate and chitosan systems: interactions between active compound encapsulation polymers. JEAS . 2011;1:80–7. [Google Scholar]
- 55.Sezer A, Akbuga J. Release characteristics of chitosan treated alginate beads: II Sustained release of a low molecular drug from chitosan treated alginate beads. J. Microencapsul. 1999;16:687–96. doi: 10.1080/026520499288636. [DOI] [PubMed] [Google Scholar]
- 56.Takka S, Gürel A. Evaluation of chitosan/alginate beads using experimental design: formulation and in-vitro characterization. AAPS PharmSciTech. 2010;11:460–6. doi: 10.1208/s12249-010-9406-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mirghani A, Idkaidek N, Salem MS, Najib NM. Formulation and release behavior of diclofenac sodium in compritol 888 matrix beads encapsulated in alginate. Drug Dev. Ind. Pharm. 2000;26:791–5. doi: 10.1081/ddc-100101301. [DOI] [PubMed] [Google Scholar]
- 58.Anal AK, Bhopatkar D, Tokura S, Tamura H, Stevens WS. Chitosan-alginate multilayer beads for gastric passage and controlled intestinal release of protein. Drug Dev. Ind. Pharm. 2003;29:713–24. doi: 10.1081/ddc-120021320. [DOI] [PubMed] [Google Scholar]