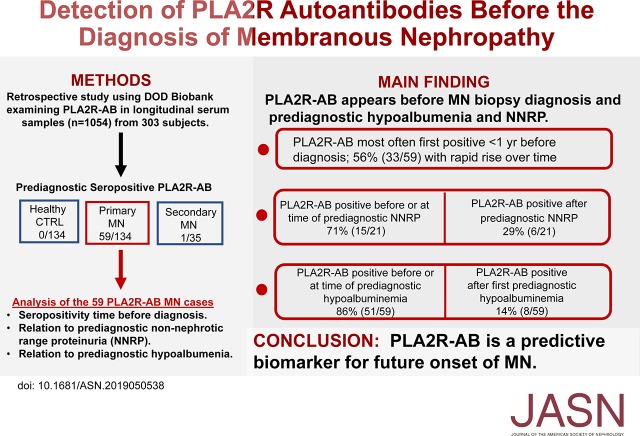
Significance Statement
Primary membranous nephropathy (MN) is an autoimmune glomerular disease associated with nephrotic syndrome and poor kidney prognosis. Autoantibodies against the M-type phospholipase A2 receptor (PLA2R-AB) are present at diagnosis in about 70% of cases. However, when PLA2R-AB first appear and their trajectory are unknown. The authors used the Department of Defense Serum Repository to describe the prediagnostic evolution of PLA2R-AB over time. In most patients who tested positive for the antibodies, PLA2R-AB appeared and rose rapidly before biopsy-proven MN and before the earliest preclinical evidence of disease, including non-nephrotic range proteinuria and hypoalbuminemia. Our data provides the strongest evidence to date of direct PLA2R-AB pathogenicity in humans and suggests that earlier screening of patients with unexplained NNRP may be warranted.
Keywords: membranous nephropathy, nephrotic syndrome, proteinuria, glomerular disease, glomerulopathy
Visual Abstract
Abstract
Background
Circulating serum autoantibodies against the M-type phospholipase A2 receptor (PLA2R-AB) are a key biomarker in the diagnosis and monitoring of primary membranous nephropathy (MN). However, little is known about the appearance and trajectory of PLA2R-AB before the clinical diagnosis of MN.
Methods
Using the Department of Defense Serum Repository, we analyzed PLA2R-AB in multiple, 1054 longitudinal serum samples collected before diagnosis of MN from 134 individuals with primary MN, 35 individuals with secondary MN, and 134 healthy volunteers. We evaluated the presence and timing of non-nephrotic range proteinuria (NNRP) and serum albumin measurements in relation to PLA2R-AB status.
Results
Analysis of PLA2R-AB in longitudinal serum samples revealed seropositivity in 44% (59 out of 134) of primary MN cases, 3% (one out of 35) of secondary MN cases, and in 0% of healthy controls. Among patients with MN, PLA2R-AB were detectable at a median of 274 days before renal biopsy diagnosis (interquartile range, 71–821 days). Approximately one third of the participants became seropositive within 3 months of MN diagnosis. Of the 21 individuals with documented prediagnostic NNRP, 43% (nine out of 21) were seropositive before NNRP was first documented and 28.5% (six out of 21) were seropositive at the same time as NNRP; 66% (39 out of 59) of those seropositive for PLA2R-AB had hypoalbuminemia present at the time antibody was initially detected. Twelve participants (20%) were seropositive before hypoalbuminemia became apparent, and eight participants (14%) were seropositive after hypoalbuminemia became apparent.
Conclusions
Circulating PLA2R-AB are detectable months to years before documented NNRP and biopsy-proven diagnosis in patients with MN.
Primary membranous nephropathy (MN) is a common autoimmune cause of nephrotic syndrome in adults and is associated with significant morbidity and mortality.1,2 The pathogenic mechanism involves circulating autoantibodies that bind to cell surface antigens on glomerular podocytes, forming immune complex deposits within the kidneys. Approximately 70% of patients with primary MN are seropositive for autoantibodies to the podocyte extracellular region of M-type phospholipase A2 receptor (PLA2R-AB) at the time of diagnosis.1–5 Significant clinical evidence suggests that PLA2R-AB are involved in disease pathogenesis. PLA2R-AB are elevated at diagnosis and downtrend preceding resolution of proteinuria. In addition, depletion of PLA2R-AB is associated with complete remission and reemergence of circulating antibodies predicts relapse.6–11 High PLA2R-AB titers at presentation are also associated with greater disease severity and subsequent decline in eGFR.
The Department of Defense Serum Repository (DoDSR) is a uniquely suitable resource for studying the natural history of a variety of autoimmune diseases because it provides access to blood samples collected well before a clinical diagnosis is made.12–17 To gain greater insight into the dynamics of autoantibody production in the preclinical phase of MN, we evaluated for the presence of circulating PLA2R-AB in serum samples from the DoDSR that were collected longitudinally before the diagnosis of MN.
Methods
Study Population
The protocol (#0042) was approved by the Institutional Review Board of the Walter Reed National Military Medical Center, Bethesda, Maryland. We performed a retrospective, matched case-control DoDSR to investigate the association between prediagnostic PLA2R-AB and future development of MN. Because of the innocuous nature of the study and the potential to acquire important medical information, the Walter Reed National Military Medical Center waived informed consent for the testing of deidentified serum samples from the DoDSR and for the review of clinical records. To protect the privacy of the participants, their names and unique personal information were not recorded or released.
Prospective MN cases of current or previous active duty personnel were identified by query of the military electronic medical record (EMR) for International Classification of Diseases, Ninth Revision codes 581.9 and 583.1. The EMR for each potential case was reviewed for evidence of biopsy-confirmed MN. In cases where multiple renal biopsies were performed, only the time of the first biopsy was considered for analysis. Each biopsy confirmed case was subcategorized into either primary MN or secondary MN. The time of biopsy-proven MN was used as the time of diagnosis. Primary MN was defined by the absence of identifiable secondary causes of MN, including evidence of a malignancy, autoimmune disease, infection, or culprit medication. Patients were designated as secondary MN cases if they had evidence of autoimmune disease, medications, malignancy, or infection that had a known association with MN that, on review of the EMR, were temporally related to diagnosis. There were 230 cases of biopsy-confirmed MN, comprising 169 cases of primary MN and 61 cases of secondary MN. After the DoDSR excluded cases without banked serum, there were 134 remaining cases of primary MN and 35 cases of secondary MN (Supplemental Figure 1).
When available, prespecified background clinical data were collected for each MN case. Deidentification requirements of the DoDSR prevented linking of individual clinical data to corresponding serum samples, but did allow for creation of study bins with more than five cases. Subgroup bins were established for MN cases complicated by ESKD, with serum creatinine >2 mg/dl, serum albumin <2.0 mg/dl, thromboembolic complications, and for evidence of spontaneous remission. Complete remission was defined as a reduction in proteinuria to <300 mg/d with a stable eGFR reflected by consecutive measurement within 15% value. Partial remission was defined by a 50% reduction in proteinuria to <3.5 gm/d with stable eGFR.18 The DoDSR provided up to four prediagnostic serum samples for each of the primary and secondary MN cases, in addition to one healthy control matched for age, race, sex, and the age of the serum sample. If there were four or less samples banked, all were selected. If there were more than four samples banked, the oldest serum sample and last sample before biopsy diagnosis were prioritized. The remaining two samples were chosen to be closest in time to the earliest documented proteinuria and to the most recent documented negative proteinuria in the patient record.
Among the 134 primary MN cases, there were 97 (72%) with four serum samples, 12 (9%) with three samples, 16 (12%) with two samples, and nine (7%) with one sample. Because of the stochastic nature of available serum samples from the primary MN cases, 93 out of 134 cases had serum samples within 2 years of diagnosis, whereas 30% (41 out of 134) only had samples 2 years or more before diagnosis. Moreover, 55% (74 out of 134) primary MN cases had at least one serum sample 1 year before diagnosis. Although all of the cases from the MN cohort had proteinuria documented in the EMR at diagnosis, no corresponding banked serum sample was available exactly at diagnosis. Additionally, an important limitation of our study is that urine samples are not collected by the Department of Defense and therefore we were unable to analyze proteinuria at the same time points as the collected serum. In total, 1054 serum samples were analyzed in blinded fashion for PLA2R-AB.
Quantitative Serum PLA2R-AB Measurements
We used fluid-phase luciferase immunoprecipitation systems (LIPS) assay utilizing light-emitting luciferase fusion proteins in this study.19 LIPS often has higher sensitivity, specificity, and dynamic range of detection than solid-phase immunoassays such as ELISA, because of the enhanced presentation of conformational epitopes in solution. A previously described extracellular region of PLA2R (amino acids 1–858) fused to nanoluciferase was used to measure autoantibodies in blinded samples.13 Internal positive controls from previously described seropositive PLA2R-AB serum samples were utilized for standardization and determination of cut-off values. Seropositive and seronegative status was assigned for each sample before unblinding. The PLA2R-AB levels obtained from luminometer readings represent raw light units (LU) and were not adjusted.
Non-Nephrotic Range Proteinuria and Serum Albumin Measurements
Non-nephrotic range proteinuria (NNRP) was used as surrogate marker for subclinical evidence of MN before biopsy confirmation and was on the basis of available medical record documentation. NNRP was determined by a urinalysis with 1–3+ proteinuria or 30–200 mg/dl on urinalysis, or between 300 and 3000 mg/dl proteinuria on spot urine protein-to-creatinine ratio for 24-hour urine collection. From the 134 total MN cases, 50 had a NNRP documented for the 24-hour urine collection.
Serum albumin levels were measured in all available serum samples from the seropositive MN cases as an indicator of clinically significant proteinuria. Serum albumin measurements were performed in samples at the same time points as the autoantibody testing using an established commercial test (Sigma-Millipore) with Bromocresol green, and a standard curve as previously described.20 The normal serum albumin range is between 3.5 and 5.0 g/dl and hypoalbuminemia was defined as serum albumin <3.0 g/dl.
Data and Statistical Analyses
Descriptive statistics include only values for the patients with primary MN because of deidentification requirement by the DoDSR. Data are summarized using counts and percentages for categorical variables and mean±SD, medians, and, at times, interquartile ranges (IQRs). The percentage of cases with elevated PLA2R-AB were compared among the patients with primary MN, secondary MN, and healthy controls.
Results
Clinical Samples and Characteristics of the Primary MN Cases
The cohort consisted of 134 patients with primary MN, 35 patients with secondary MN, and 134 healthy volunteers. As shown in Table 1, the majority of those with primary MN were men (87%) and white (40%), and the median age of diagnosis was 44 (IQR, 32–50) years. At diagnosis, most had nephrotic range proteinuria with median proteinuria of 6.6 g/d, and more than one quarter of patients had serum albumin <2 g/dl. Most of the patients with primary MN in our cohort were diagnosed before the discovery of PLA2R-AB and available clinical testing. As shown in Table 1, seven patients with MN had PLA2R-AB testing performed at the time of diagnosis, with four being seropositive (median titer, 353 u/ml; range, 52–1003) Similarly, biopsies from 16 patients with MN were evaluated for glomerular PLA2R staining, resulting in 12 positives.
Table 1.
Clinical characteristics of the primary MN cohort at time of biopsy diagnosis
| Characteristic | Primary Membranous Nephropathy Cases (n=134) |
|---|---|
| Median age, yr | 44 (IQR, 32–50) |
| Sex, % male | 87 |
| Race, % | |
| White | 40 |
| Black | 37 |
| Hispanic | 7 |
| Asian | 1 |
| Other | 7 |
| Unknown | 8 |
| Characteristics at diagnosis | |
| Median proteinuria, g/d | 6.6 (IQR, 4.2–9.1) |
| Median serum creatinine, mg/dl | 1.1 (IQR, 0.9–1.3) |
| Median serum albumin, g/dl | 2.4 (IQR, 1.7–2.8) |
| Median total cholesterol, mg/dl | 255 (IQR, 219–333) |
| Median LDL, mg/dl | 176 (IQR, 138–238) |
| Hypertension | 33% (44/134) |
| Diabetes mellitus | 6% (8/134) |
| Serum albumin, <2.0 g/d | 28% (37/134) |
| Venous thrombosis | 10% (13/134) |
| PLA2R-AB seropositive | 4 out of 7 tested |
| PLA2R tissue antigen positive | 12 out of 16 tested |
| Spontaneous remission after diagnosis | 33% (44/134) |
PLA2R-AB Seropositivity in the Total Cohort
A total of 1054 prospectively collected serum samples were assessed for the presence of circulating PLA2R-AB by LIPS assay. On the basis of the preassigned cut-off value (25,000 LU) from the standardized PLA2R-AB immunoassay,20 seropositive and seronegative status for each serum sample was determined before unblinding. After decoding, 44% (59 out of 134) of the cases classified as primary MN were PLA2R-AB seropositive. The median value of the PLA2R-AB levels in MN cases was 510,200 LU (IQR, 376,300–627,100 LU), which was approximately 20 times the cut-off value. Of the 35 cases classified as secondary MN cases, only one (3%) sample was seropositive for PLA2R-AB. Multiple longitudinal serum samples from the 134 matching healthy controls were all seronegative. It is important to note that 41 (30%) of the primary MN cases did not have a prediagnostic serum sample available for testing within 2 years of diagnosis and none of the cases had banked serum available at diagnosis.
Time Course of PLA2R-AB Development in MN
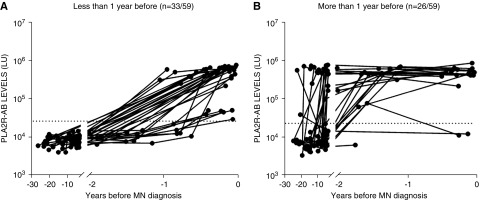
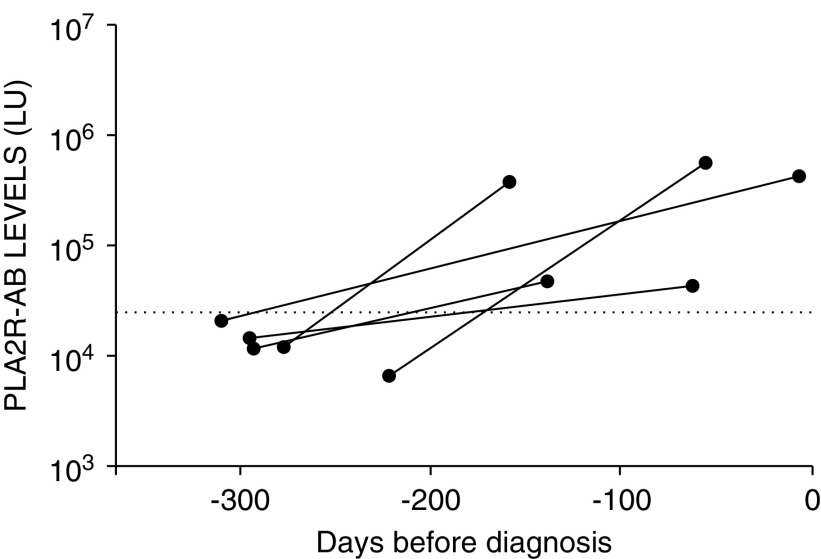
The median time of the earliest prediagnosis serum sample collected for the patients with MN was 12.7 years. Analysis of the trajectory of PLA2R-AB titers in the 59 seropositive MN cases revealed that the median onset of detectable autoantibodies occurred 274 days (IQR, 71–820 days) before biopsy-proven diagnosis. The 59 seropositive MN cases were broadly divided into two groups, in which 56% (33 out of 59) of the patients were first seropositive <1 year before MN diagnosis (Figure 1A) and 44% (26 out of 59) were first seropositive ≥1 year before MN diagnosis (Figure 1B). The majority of cases (83%, 49 out of 59) had a seropositive sample <1 year before diagnosis and a previous seronegative sample at some time point >1 year before diagnosis. A minority of cases (29%, 17 out of 59) had a seropositive sample both <1 year before diagnosis and >1 year before diagnosis. Additional analysis revealed no significant difference in the median age of diagnosis between these two groups. Of the patients with MN who became seropositive in <1 year, over half of these (58%, 19 out of 33) became seropositive <3 months before diagnosis. A subgroup of cases demonstrated a rise in PLA2R-AB from a seronegative status to seropositive in <1 year, and in as little as 118 days (Figure 2).
Figure 1.
PLA2R-AB is elevated months to years before MN diagnosis. Longitudinal profile of patients with seropositive PLA2R-AB before clinical diagnosis of MN. Autoantibody analysis identified 59 patients with MN with prediagnostic seropositive PLA2R-AB, in which (A) 33 patients developed seropositivity <1 year before MN diagnosis and (B) 26 patients demonstrated seropositivity >1 year before MN diagnosis. The PLA2R-AB levels in LU derived from serial samples of the seropositive cases are plotted on the y axis using a log10 scale. The cut-off value for determining PLA2R-AB seropositivity is shown by the dotted line. Time zero represents biopsy-proven diagnosis of MN.
Figure 2.
PLA2R-AB can significantly elevate over weeks to months prior to MN diagnosis. Rapid appearance of prediagnostic PLA2R-AB. Autoantibody analysis identified five patients with PLA2R-AB–positive MN that demonstrated a rapid rise from seronegative to significantly elevated in <1 year. The PLA2R-AB levels in LU derived from serial samples of the seropositive cases are plotted on the y axis using a log10 scale. The cut-off value for determining PLA2R-AB seropositivity is shown by the dotted line. Time zero represents biopsy-proven diagnosis of MN.
Temporal Relationship of Prediagnostic PLA2R-AB Seropositivity and Proteinuria
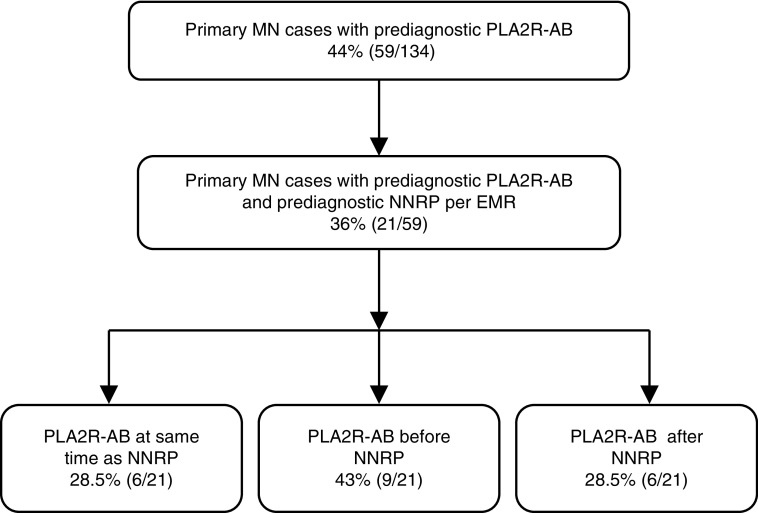
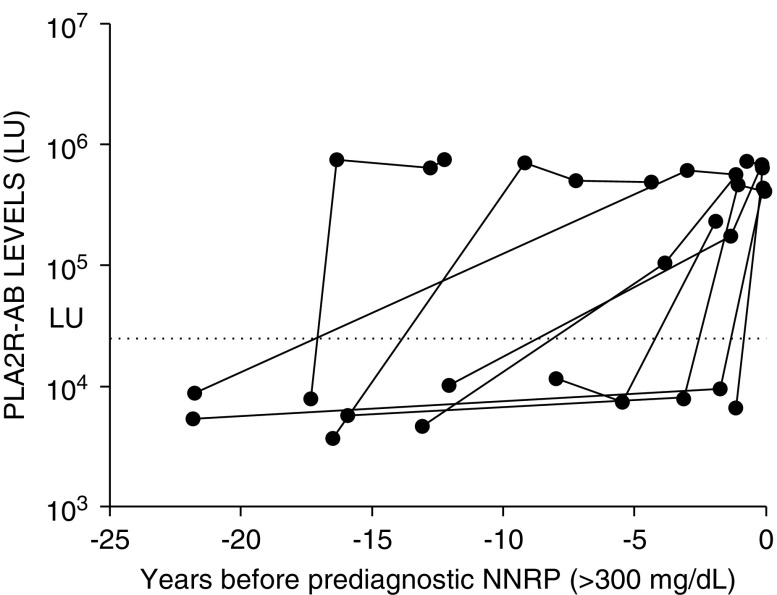
NNRP documented in the EMR was used as surrogate marker for potential evidence of MN before biopsy confirmation. Of the 59 PLA2R-AB–seropositive cases, 21 (36%) had available data regarding NNRP in their medical record (Figure 3). The median duration between NNRP and biopsy diagnosis was 213 days (IQR, 160–527 days). Documented NNRP was found at approximately the same time (within 1 month) that PLA2R-AB was initially detected in 28.5% (six out of 21) of the cases. In 43% (nine out of 21) of the cases, PLA2R-AB was seropositive before NNRP (median, 692 days; IQR, 385–1400 days) (Figure 3). Six other patients (28.5%) had documented NNRP before detectable PLA2R antibody (Figure 3). In these six cases (Figure 4), there was a median time gap of 244 days between the last PLA2R-AB negative sample and first documented, prediagnostic NNRP, during which time the PLA2R-AB may have become elevated. There were no cases where NNRP was followed initially by a negative and subsequently positive prediagnostic PLA2R-AB level. Twenty nine patients who were PLA2R-AB positive had a prediagnostic urinalysis negative for proteinuria in the EMR. In one unique case, the prediagnostic PLA2R-AB was elevated before a documented urinalysis negative for proteinuria to clearly signify that the antibody was present before the earliest clinical evidence of disease (Supplemental Figure 2). Nine patients who were PLA2R-AB seropositive had a urinalysis negative for proteinuria with subsequent NNRP documented in the EMR before biopsy-proven MN (Supplemental Figure 3).
Figure 3.
In the majority of MN cases, PLA2R-AB is elevated before or at the time of prediagnostic NNRP, a surrogate for the earliest evidence of clinical disease. A flow diagram of the temporal relationship between prediagnostic NNRP and PLA2R-AB. As described in the Methods, examination of the EMR from the 59 patients with MN who were positive for PLA2R-AB found evidence of prediagnostic NNRP in 21 patients. Further analysis was used to segregate the case according to whether NNRP occurred before, at the same time, or after detectable PLA2R seropositivity.
Figure 4.
PLA2R-AB is elevated months to years before NNRP as a surrogate marker for earliest clinical evidence of MN. Examples of MN cases showing seropositive PLA2R-AB before abnormal prediagnostic NNRP. The PLA2R-AB levels are plotted on the y axis and the horizontal dotted line is the cut-off value for determining seropositivity. The first clinical evidence of abnormal prediagnostic NNRP is indicated by time zero.
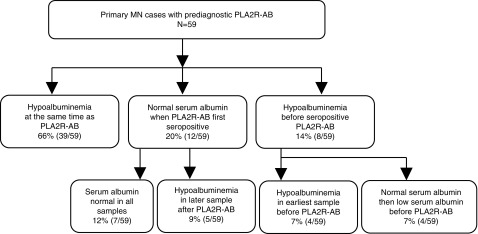
Temporal Relationship of PLA2R-AB Seropositivity to Hypoalbuminemia
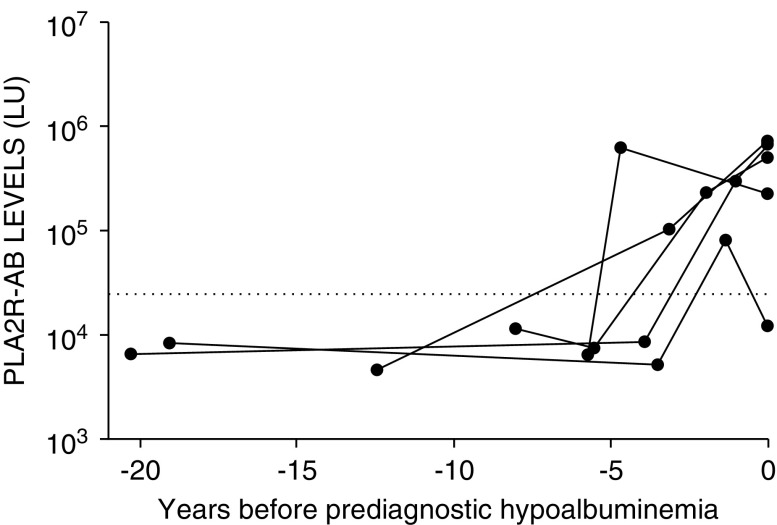
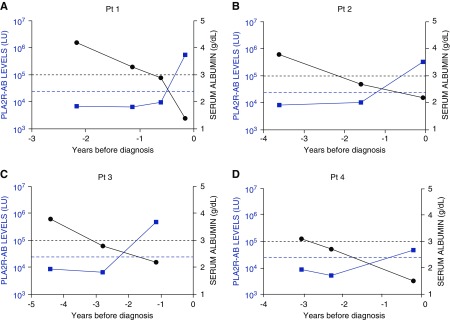
The majority of PLA2R-AB–positive MN cases (66%, 39 out of 59) had hypoalbuminemia present contemporaneously with the first PLA2R-positive serum sample (Figure 5). Serum albumin was preserved in 12 out of 59 (20%) of cases when PLA2R-AB was initially detected (see Supplemental Figure 4). Five of these cases demonstrated hypoalbuminemia in subsequent prediagnostic samples (Figures 5 and 6). In eight patients, hypoalbuminemia was evident before PLA2R-AB was detected (Figure 5). Four of these patients transitioned from a normal to low prediagnostic albumin level before PLA2R-AB seropositivity, and then became PLA2R-AB seropositive within 0.5–2 years from the first detection of hypoalbuminemia (Figure 7). The median duration between hypoalbuminemia and biopsy diagnosis was 310 days (IQR, 77–834 days).
Figure 5.
PLA2R-AB most often occurs at or before the first documented prediagnostic hypoalbuminemia. A flow diagram of the temporal relationship between prediagnostic serum albumin and PLA2R-AB. As described in the Methods, the serial serum samples from the 59 PLA2R-AB–positive cases were also analyzed for serum albumin levels to determine whether albumin levels were normal or abnormally low before, at the same time, or after detectable PLA2R seropositivity.
Figure 6.
PLA2R-AB is elevated months to years before prediagnostic hypoalbuminemia in some cases. Five cases showing evidence of seropositive PLA2R-AB before prediagnostic hypoalbuminemia. The horizontal dotted line is the cut-off for determining PLA2R-AB seropositivity. The first clinical evidence of prediagnostic hypoalbuminemia is indicated by time zero.
Figure 7.
Prediagnostic hypoalbuminemia occurs before PLA2R-AB elevation in a minority of cases. Representative MN cases showing prediagnostic hypoalbuminemia before seropositive PLA2R-AB. The left y axis represents the scale of the PLA2R-AB levels and the values are plotted in blue. The blue, horizontal dotted line is the cut-off for determining PLA2R-AB seropositivity. The right y axis denotes the serum albumin levels, and the cut-off value for determining hypoalbuminemia is shown by the black dotted line. (A) In patient 1, prediagnostic hypoalbuminemia occurs before elevated PLA2R-AB. (B) In patient 2, hypoalbuminemia occurs before elevated PLA2R-AB. (C) In patient 3, hypoalbuminemia occurs before elevated PLA2R-AB. (D) In patient 4, hypoalbuminemia occurs before elevated PLA2R-AB.
Unusual PLA2R-AB–Seropositive MN Cases
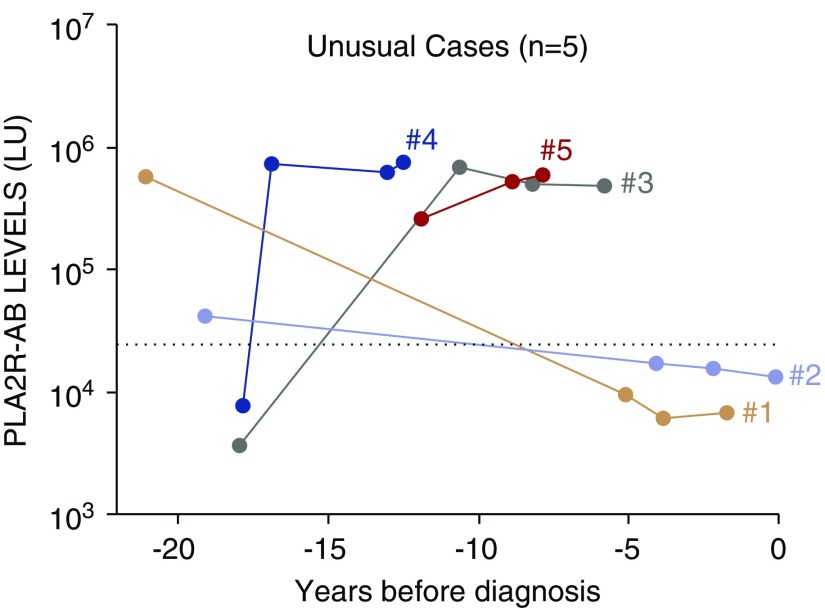
Of the 26 cases that were found to be seropositive more than 1 year before diagnosis, five cases had detectable autoantibody more than 10 years before diagnosis (Figure 8). Cases 1–3 all appeared to have at most mild disease during prediagnostic PLA2R-AB seropositivity, with serum albumin between 2.6 and 3.8 mg/dl. Cases 1 and 2 were only PLA2R-AB seropositive at 21 and 19 years before diagnosis and subsequently became seronegative in all serum samples closer to diagnosis, with a documented prediagnostic urinalysis negative for proteinuria to suggest spontaneous remission from this potential undiagnosed MN flare. Cases 1–3 all also experienced a spontaneous remission after the biopsy-proven case of MN. Unfortunately, for cases 1, 2, and 3, no additional serum samples were available for PLA2R and serum albumin analysis within 5 years before MN diagnosis that might have provided greater insight into trajectory of the PLA2R-AB closer to diagnosis. Cases 4 and 5, with elevated PLA2R-AB for three consecutive prediagnostic serum samples over 4.4 and 4.8 years, respectively (Figure 8), appeared to have a more severe flare of undocumented MN with serum albumin as low as 1.5 and 1.7 mg/dl, concurrent with prediagnostic PLA2R-AB seropositivity. Both appeared to have at least a spontaneous partial remission with NNRP recorded <1.5 years before biopsy-proven MN. Interestingly, neither of these cases had a spontaneous remission after official diagnosis and one progressed to ESKD.
Figure 8.
PLA2R-AB is elevated years to decades before MN diagnosis in a minority of cases. Five unusual cases with detectable seropositive PLA2R-AB over 10 years before diagnosis of MN. The longitudinal PLA2R-AB profile is shown for the unusual MN cases. The cut-off value for determining PLA2R-AB seropositivity is shown by the dotted line and time zero represents biopsy-proven diagnosis of MN. Cases 1–5 are shown by the orange, magenta, green, blue, and red lines, respectively.
Relationship between PLA2R-AB, Hypoalbuminemia, and Spontaneous Remission
Out of the 59 cases with prediagnostic PLA2R-AB seropositivity, 21 out of 59 (36%) achieved spontaneous remission, which was not significantly different from the 23 out of 75 (31%) spontaneous remission rate for cases without PLA2R-AB. However, prediagnostic albumin <2.0 mg/dl with PLA2R-AB seropositivity was associated with not achieving spontaneous remission (29 out of 38 [76%] versus four out of 21 [19%]; P<0.001; odds ratio, 13.7 [95% confidence interval, 3.7–51.3]).
Discussion
This is the first description of the natural history of the PLA2R-AB profile before biopsy diagnosis of primary MN. Using a quantitative PLA2R LIPS assay, our study documents the temporal relationship between prediagnostic PLA2R-AB and surrogates for earliest subclinical evidence of MN, including NNRP and hypoalbuminemia. In the DODSR cohort, 44% of patients with primary MN were seropositive for PLA2R-AB. This may be an underestimation of the percentage of true PLA2R seropositivity in this cohort as 30% of the cases classified as primary MN did not have a prediagnostic sample available within 2 years of diagnosis. Excluding these patients, 63% of the primary MN cases were positive for PLA2R-AB, a percentage consistent with the reported seroprevalence.4 We did not test for antibodies to thrombospondin type 1 domain-containing 7A, a second antigen responsible for up to 5% of PLA2R-negative MN cases.21,22
The majority of the patients with primary MN had PLA2R-AB detectable within 1 year of biopsy-proven MN. In cases with short intervals between prediagnostic serum samples, PLA2R-AB rose rapidly from normal to significantly elevated in <1 year. This observation contrasts with other autoimmune diseases, such as SLE,12,23 Sjogren syndrome,24 rheumatoid arthritis,25 and systemic sclerosis,26 where patients harbor prediagnostic autoantibodies against intracellular autoantigens 5–25 years before diagnosis. PLA2R-AB seropositivity occurred close to or before prediagnostic NNRP, when documented in the EMR, in the majority of cases. This is consistent with the concept that PLA2R-AB is directly pathogenic and that there is a lag period between PLA2R-AB production and clinical evidence of MN.27
A minority of patients did manifest NNRP and hypoalbuminemia before the first recorded prediagnostic PLA2R-AB. There are five potential explanations. First, this subclinical evidence of MN before PLA2R-AB may reflect the known interval delay in normalization of proteinuria and hypoalbuminemia after the spontaneous remission of a previous undocumented flare of PLA2R-AB–positive MN, during which there were no prediagnostic samples. The subsequent PLA2R seropositivity may represent the earliest evidence of MN relapse that led to the first formal diagnosis. Second, the proteinuria and hypoalbuminemia are related to a separate process such as diabetic nephropathy. However, none of the eight diabetic cases required insulin, had histopathology consistent with diabetic nephropathy on kidney biopsy, or had other evidence of end-organ damage. Third, although increased PLA2R-AB production may have occurred before proteinuria and/or hypoalbuminemia, the majority of the antibodies may have been deposited in the glomerulus leaving the serum autoantibody levels in the seronegative range. Fourth, LIPS may have failed to detect low-level seropositivity in a prediagnostic sample before NNRP that may have been detectable by Western blot assay.5 Lastly, it is possible that there was a second mechanism of podocyte injury, with associated proteinuria causing exposure of the pathogenic PLA2R epitope, and subsequent PLA2R-AB production and MN.
One intriguing finding was that in five provocative cases, the first detectable seropositive PLA2R-AB was present 10–20 years before diagnosis. The subclinical trajectory of PLA2R-AB, serum albumin, and proteinuria in these cases suggest that some patients diagnosed with MN have previous undocumented MN flares with spontaneous remission, and that sustained PLA2R-AB seropositivity with hypoalbuminemia may portend a lower rate of spontaneous remission. This conclusion was bolstered by our discovery that hypoalbuminemia in combination with PLA2R-AB seropositivity before MN diagnosis was strongly associated with a low spontaneous remission rate after formal biopsy diagnosis.
This study had inherent limitations. The DoDSR cohort skews male and younger because it consists of current and prior active duty military personnel. Misclassification of MN cases as primary versus secondary may have occurred as it relies on historical charting. Similarly, the timing of the diagnosis of MN relies on proper medical record documentation. The time intervals between serum samples and diagnosis were variable for each case, and some cases (19%) had only one or two serum samples available. Many cases (n=41) did not have a serum sample available within 2 years of diagnosis, which may have led to underestimation of the seropositive cases. It is also possible that there were false-negative prediagnostic serum samples that would have been detected by gold-standard Western blot analysis. The DoDSR only allows up to four serum samples per case, which limited more detailed analysis of PLA2R-AB trajectory before diagnosis. Each case likely developed PLA2R-AB seropositivity, NNRP, and hypoalbuminemia before the first time it was appreciated before diagnosis. This is an inherent limitation of evaluating prediagnostic serum samples that were not specifically collected at intervals to support this specific study. Also, urine collection is not part of the research repository, which prevented simultaneous quantification of proteinuria. Because of DoDSR deidentification requirements, we were unable to link background clinical information and outcomes to the prediagnostic serum sample results for each case. Although cases required biopsy confirmation of MN for inclusion, not all cases had comprehensive background clinical data to include proteinuria quantification or serum albumin prior time of biopsy, limiting our ability to draw conclusions regarding onset of antibody detection, titers and clinical outcomes. Although some patients did have NNRP and/or hypoalbuminemia before detectable autoantibody, deidentification requirements meant that we could not further determine if they had preexisting diabetes, hypertension, or other comorbid conditions. Finally, because the luciferase assay was not compared directly with the commercially available Euroimmun assay, the results may be more difficult to interpret in the clinical setting.
Despite the recognized limitations, this study is provocative and highlights the need for further studies to understand the properties of the autoantibodies in MN and how epitope spreading, changes in antibody conformation, intermolecular spreading, genetics, and other factors might lead to the observed variability in disease presentation, progression, remission, and outcomes.28–34 Overall, the subclinical presence of seropositive PLA2R-AB with NNRP in our study introduces the potential to screen current unexplained cases of NNRP for PLA2R-AB. Taken a step further, people determined to have genetic risk for MN in the future could be followed with serial urinalysis with analysis of PLA2R-AB at the onset of NNRP. Because our data suggest that persistent MN is associated with lower spontaneous remission, the observation period for spontaneous remission could potentially be accelerated to reduce the interval of sustained nephrotic range proteinuria with associated risk of thrombosis and decline in renal function. High PLA2R-AB titers are associated with a reduced rate of spontaneous remission and passive monitoring of high-titer PLA2R-AB while awaiting potential spontaneous remission may lead to epitope spreading, which is also associated with worse renal outcomes.2,10,35–40 It is also possible that PLA2R-AB IgG subclass 1,3 predominate early in the disease, activating the deleterious classic complement pathway. Therefore, it is even possible that the benefit of preemptive therapeutic intervention may outweigh the risk of observation. Thus, our findings align with recently published proposals for closer monitoring and potential earlier treatment in patients with high-titer PLA2R-AB by De Vriese et al.41
Disclosures
Dr. Burbelo has a patent “Methods of Using Neodymium Magnet for Rapid Diagnosis of Infectious and Autoimmune Diseases” pending. Dr. Little reports employment as a clinical research physician at AstraZeneca, and as a credentialed nephrologist at Walter Reed National Military Medical Center (WRNMMC). AstraZeneca has no products for membranous nephropathy, and is not developing any products for membranous nephropathy. Involvement in this project stems solely from his role at WRNMMC, and is completely independent of and not influenced by AstraZeneca. All of the remaining authors have nothing to disclose.
The views expressed in this manuscript are those of the authors and do not reflect the official policy of the Department of the Army, Department of Defense, or the US Government.
Funding
This research was supported by the Division of Intramural Research of the National Institute of Dental and Craniofacial Research and National Institute of Diabetes and Digestive and Kidney Diseases. This research was also supported by the National Institute of Dental and Craniofacial Research Combined Technical Research Core (ZIC DE000729-09).
Supplementary Material
Acknowledgments
We thank Dr. Angelia Cost from the Department of Defense Serum Repository for her invaluable contributions.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Refining Our Understanding of the PLA2R-Antibody Response in Primary Membranous Nephropathy: Looking Forward, Looking Back,” and article, “Clinical Relevance of Domain-Specific Phospholipase A2 Receptor 1 Antibody Levels in Patients with Membranous Nephropathy” on pages 8–11 and 197–207, respectively.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019050538/-/DCSupplemental.
Supplemental Figure 1. Flow chart for selection of the primary and secondary MN cases.
Supplemental Figure 2. One MN case showing seropositive PLA2R-AB before NNRP.
Supplemental Figure 3. Representative MN cases showing seropositive PLA2R-AB after negative NNRP, and then showing positive NNRP.
Supplemental Figure 4. Representative MN cases showing seropositive PLA2R-AB before hypoalbuminemia.
References
- 1.Couser WG: Primary membranous nephropathy. Clin J Am Soc Nephrol 12: 983–997, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ronco P, Debiec H: Pathophysiological advances in membranous nephropathy: Time for a shift in patient’s care. Lancet 385: 1983–1992, 2015 [DOI] [PubMed] [Google Scholar]
- 3.Ancian P, Lambeau G, Mattéi MG, Lazdunski M: The human 180-kDa receptor for secretory phospholipases A2. Molecular cloning, identification of a secreted soluble form, expression, and chromosomal localization. J Biol Chem 270: 8963–8970, 1995 [DOI] [PubMed] [Google Scholar]
- 4.Beck LH Jr., Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, et al.: M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 361: 11–21, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qin W, Beck LH Jr., Zeng C, Chen Z, Li S, Zuo K, et al.: Anti-phospholipase A2 receptor antibody in membranous nephropathy. J Am Soc Nephrol 22: 1137–1143, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beck LH Jr., Fervenza FC, Beck DM, Bonegio RG, Malik FA, Erickson SB, et al.: Rituximab-induced depletion of anti-PLA2R autoantibodies predicts response in membranous nephropathy. J Am Soc Nephrol 22: 1543–1550, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blosser CD, Ayalon R, Nair R, Thomas C, Beck LH Jr.: Very early recurrence of anti-Phospholipase A2 receptor-positive membranous nephropathy after transplantation. Am J Transplant 12: 1637–1642, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Debiec H, Martin L, Jouanneau C, Dautin G, Mesnard L, Rondeau E, et al.: Autoantibodies specific for the phospholipase A2 receptor in recurrent and De Novo membranous nephropathy. Am J Transplant 11: 2144–2152, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Debiec H, Hanoy M, Francois A, Guerrot D, Ferlicot S, Johanet C, et al.: Recurrent membranous nephropathy in an allograft caused by IgG3κ targeting the PLA2 receptor. J Am Soc Nephrol 23: 1949–1954, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hofstra JM, Beck LH Jr., Beck DM, Wetzels JF, Salant DJ: Anti-phospholipase A2 receptor antibodies correlate with clinical status in idiopathic membranous nephropathy. Clin J Am Soc Nephrol 6: 1286–1291, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stahl R, Hoxha E, Fechner K: PLA2R autoantibodies and recurrent membranous nephropathy after transplantation. N Engl J Med 363: 496–498, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, et al.: Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med 349: 1526–1533, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Burbelo PD, Beck LH Jr., Waldman M: Detection and monitoring PLA2R autoantibodies by LIPS in membranous nephropathy. J Immunol Methods 444: 17–23, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olson SW, Arbogast CB, Baker TP, Owshalimpur D, Oliver DK, Abbott KC, et al.: Asymptomatic autoantibodies associate with future anti-glomerular basement membrane disease. J Am Soc Nephrol 22: 1946–1952, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olson SW, Lee JJ, Prince LK, Baker TP, Papadopoulos P, Edison J, et al.: Elevated subclinical double-stranded DNA antibodies and future proliferative lupus nephritis. Clin J Am Soc Nephrol 8: 1702–1708, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olson SW, Owshalimpur D, Yuan CM, Arbogast C, Baker TP, Oliver D, et al.: Relation between asymptomatic proteinase 3 antibodies and future granulomatosis with polyangiitis. Clin J Am Soc Nephrol 8: 1312–1318, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olson SW, Lee JJ, Poirier M, Little DJ, Prince LK, Baker TP, et al.: Anti-myeloperoxidase antibodies associate with future proliferative lupus nephritis. Autoimmune Dis 2017: 1872846, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson A, Cattran DC, Blank M, Nachman PH: Complete and partial remission as surrogate end points in membranous nephropathy. J Am Soc Nephrol 26: 2930–2937, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burbelo PD, Lebovitz EE, Notkins AL: Luciferase immunoprecipitation systems for measuring antibodies in autoimmune and infectious diseases. Transl Res 165: 325–335, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doumas BT, Watson WA, Biggs HG: Albumin standards and the measurement of serum albumin with bromcresol green. Clin Chim Acta 31: 87–96, 1971 [DOI] [PubMed] [Google Scholar]
- 21.Tomas NM, Beck LH Jr., Meyer-Schwesinger C, Seitz-Polski B, Ma H, Zahner G, et al.: Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med 371: 2277–2287, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zaghrini C, Seitz-Polski B, Justino J, Dolla G, Payré C, Jourde-Chiche N, et al.: Novel ELISA for thrombospondin type 1 domain-containing 7A autoantibodies in membranous nephropathy. Kidney Int 95: 666–679, 2019 [DOI] [PubMed] [Google Scholar]
- 23.Eriksson C, Kokkonen H, Johansson M, Hallmans G, Wadell G, Rantapää-Dahlqvist S: Autoantibodies predate the onset of systemic lupus erythematosus in northern Sweden. Arthritis Res Ther 13: R30, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Theander E, Jonsson R, Sjöström B, Brokstad K, Olsson P, Henriksson G: Prediction of Sjögren’s syndrome years before diagnosis and identification of patients with early onset and severe disease course by autoantibody profiling. Arthritis Rheumatol 67: 2427–2436, 2015 [DOI] [PubMed] [Google Scholar]
- 25.Jørgensen KT, Wiik A, Pedersen M, Hedegaard CJ, Vestergaard BF, Gislefoss RE, et al.: Cytokines, autoantibodies and viral antibodies in premorbid and postdiagnostic sera from patients with rheumatoid arthritis: Case-control study nested in a cohort of Norwegian blood donors. Ann Rheum Dis 67: 860–866, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Burbelo PD, Gordon SM, Waldman M, Edison JD, Little DJ, Stitt RS, et al.: Autoantibodies are present before the clinical diagnosis of systemic sclerosis. PLoS One 14: e0214202, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beck LH Jr., Salant DJ: Membranous nephropathy: From models to man. J Clin Invest 124: 2307–2314, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta S, Köttgen A, Hoxha E, Brenchley P, Bockenhauer D, Stanescu HC, et al.: Genetics of membranous nephropathy. Nephrol Dial Transplant 33: 1493–1502, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le WB, Shi JS, Zhang T, Liu L, Qin HZ, Liang S, et al.: HLA-DRB1*15:01 and HLA-DRB3*02:02 in PLA2R-related membranous nephropathy. J Am Soc Nephrol 28: 1642–1650, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fresquet M, Jowitt TA, Gummadova J, Collins R, O’Cualain R, McKenzie EA, et al.: Identification of a major epitope recognized by PLA2R autoantibodies in primary membranous nephropathy. J Am Soc Nephrol 26: 302–313, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seitz-Polski B, Dolla G, Payré C, Girard CA, Polidori J, Zorzi K, et al.: Epitope spreading of autoantibody response to PLA2R associates with poor prognosis in membranous nephropathy. J Am Soc Nephrol 27: 1517–1533, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kao L, Lam V, Waldman M, Glassock RJ, Zhu Q: Identification of the immunodominant epitope region in phospholipase A2 receptor-mediating autoantibody binding in idiopathic membranous nephropathy. J Am Soc Nephrol 26: 291–301, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doi T, Mayumi M, Kanatsu K, Suehiro F, Hamashima Y: Distribution of IgG subclasses in membranous nephropathy. Clin Exp Immunol 58: 57–62, 1984 [PMC free article] [PubMed] [Google Scholar]
- 34.Huang CC, Lehman A, Albawardi A, Satoskar A, Brodsky S, Nadasdy G, et al.: IgG subclass staining in renal biopsies with membranous glomerulonephritis indicates subclass switch during disease progression. Mod Pathol 26: 799–805, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Beck LH, Jr.: PLA2R and THSD7A: Disparate paths to the same disease? J Am Soc Nephrol 28: 2579–2589, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Francis JM, Beck LH Jr., Salant DJ: Membranous nephropathy: A Journey from bench to bedside. Am J Kidney Dis 68: 138–147, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hofstra JM, Debiec H, Short CD, Pellé T, Kleta R, Mathieson PW, et al.: Antiphospholipase A2 receptor antibody titer and subclass in idiopathic membranous nephropathy. J Am Soc Nephrol 23: 1735–1743, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hofstra JM, Wetzels JF: Phospholipase A2 receptor antibodies in membranous nephropathy: Unresolved issues. J Am Soc Nephrol 25: 1137–1139, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoxha E, Harendza S, Pinnschmidt H, Panzer U, Stahl RA: M-type phospholipase A2 receptor autoantibodies and renal function in patients with primary membranous nephropathy. Clin J Am Soc Nephrol 9: 1883–1890, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kanigicherla D, Gummadova J, McKenzie EA, Roberts SA, Harris S, Nikam M, et al.: Anti-PLA2R antibodies measured by ELISA predict long-term outcome in a prevalent population of patients with idiopathic membranous nephropathy. Kidney Int 83: 940–948, 2013 [DOI] [PubMed] [Google Scholar]
- 41.De Vriese AS, Glassock RJ, Nath KA, Sethi S, Fervenza FC: A proposal for a serology-based approach to membranous nephropathy. J Am Soc Nephrol 28: 421–430, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.