Abstract
Patients with ESKD who would benefit from a kidney transplant face a critical and continuing shortage of kidneys from deceased human donors. As a result, such patients wait a median of 3.9 years to receive a donor kidney, by which time approximately 35% of transplant candidates have died while waiting or have been removed from the waiting list. Those of blood group B or O may experience a significantly longer waiting period. This problem could be resolved if kidneys from genetically engineered pigs offered an alternative with an acceptable clinical outcome. Attempts to accomplish this have followed two major paths: deletion of pig xenoantigens, as well as insertion of “protective” human transgenes to counter the human immune response. Pigs with up to nine genetic manipulations are now available. In nonhuman primates, administering novel agents that block the CD40/CD154 costimulation pathway, such as an anti-CD40 mAb, suppresses the adaptive immune response, leading to pig kidney graft survival of many months without features of rejection (experiments were terminated for infectious complications). In the absence of innate and adaptive immune responses, the transplanted pig kidneys have generally displayed excellent function. A clinical trial is anticipated within 2 years. We suggest that it would be ethical to offer a pig kidney transplant to selected patients who have a life expectancy shorter than the time it would take for them to obtain a kidney from a deceased human donor. In the future, the pigs will also be genetically engineered to control the adaptive immune response, thus enabling exogenous immunosuppressive therapy to be significantly reduced or eliminated.
Keywords: xenotransplantation, clinical trial, kidney, nonhuman primates, pigs, genetically-engineered, patients, selection
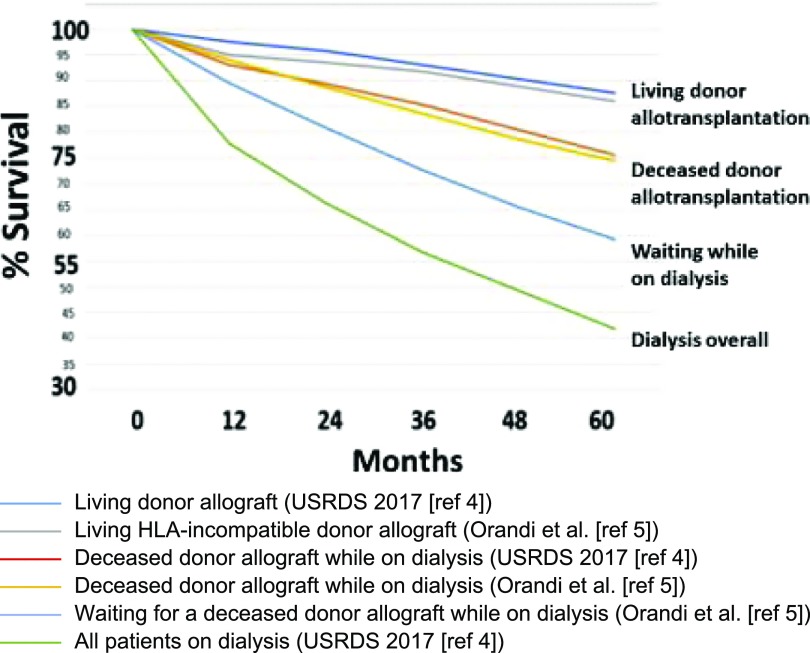
There is a continuing shortage of deceased human donor kidneys for transplantation in patients with ESKD.1–3 The overall survival of patients on chronic hemodialysis is 78% at 1 year, 57% at 3 years, and only 42% at 5 years (Figure 1).4 Forty percent of waitlisted patients are likely to die within 5 years.4,5 Importantly, in one analysis, most of those who died on the waitlist had been excellent candidates at the time of listing.6
Figure 1.
Percentage survival of patients with ESKD by treatment modality in 2010 (modified from reference 1, with permission, and on the basis of data from two sources: US Renal Data System [USRDS]4 and Orandi et al.5).
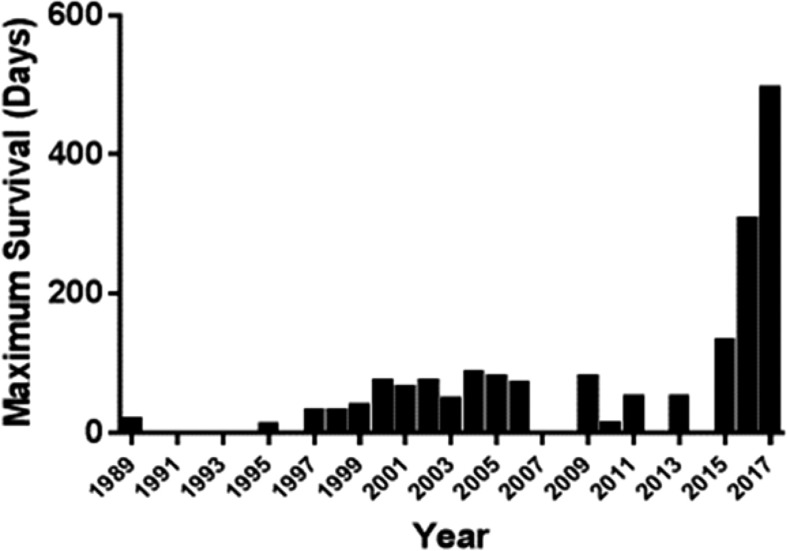
The lack of donor kidneys could be resolved if kidneys from genetically engineered pigs offered an alternative with an acceptable clinical outcome. The potential advantages of xenotransplantation are several (Table 1). The results of genetically engineered pig kidney transplantation in nonhuman primates (NHPs) have improved significantly in recent years (Figure 2), sufficiently to encourage consideration for initial clinical trials.
Table 1.
Advantages of xenotransplantation over allotransplantation
| Unlimited supply of “donor” organs. |
| Organs available electively, even when required urgently. |
| Avoids the detrimental effects of brain death on the donor organs. |
| The “donors” will be free of all known infectious microorganisms. |
| “Borderline” transplant candidates, i.e., those with health problems that may be detrimental to prolonged patient survival, e.g., poorly controlled diabetes, peripheral or cerebrovascular disease, will be more likely to be acceptable (as they will no longer be competing for scarce organs with other potential transplant candidates). |
| Avoids the “cultural” barriers to deceased human organ donation, e.g., in countries such as Japan. |
Figure 2.
Reported maximum survivals of nonhuman primates with life-supporting pig kidney grafts, 1989–2017. Details can be found in Lambrigts et al.,19 and Cooper et al.73 In some years, no results were reported.
Here we review advances in the experimental laboratory and consider which patients might ethically be offered the opportunity of receiving a pig kidney, either to reduce the length of time that they need be supported by chronic dialysis before undergoing allotransplantation or as a means of permanent support. The state of the science is sufficiently advanced to indicate that clinical trials of pig kidney transplantation are likely to be initiated within the next 2 years.
Historical Aspects of Kidney Xenotransplantation
Several attempts were made to transplant NHP kidneys into patients with ESKD, with little or no success.7–9 When Reemtsma et al. transplanted chimpanzee kidneys into six patients in 1963, although most of them succumbed from either rejection or infection within 8 weeks, one patient led an active life for 9 months, returning to work as a schoolteacher, before dying relatively suddenly from what was believed to be an acute electrolyte disturbance.10 The transplantation of baboon or monkey kidneys was less successful.
It was not until the 1980s that it was fully appreciated that, despite their phylogenetic closeness to humans, NHPs were for a variety of reasons unlikely to provide a solution to the lack of organs for kidney transplantation, and that pigs might make preferable sources of organs.11–13 However, the transplantation of an organ from a wildtype pig (i.e., a genetically unmodified pig) was followed by almost immediate graft rejection (hyperacute rejection) (Figure 3), as observed in some patients after kidney allotransplantation across the ABO-blood group barrier or when sensitized to donor HLAs. It was soon clear that the 80 million years during which humans (and NHPs) and pigs had been evolving differently had resulted in a complex primate immune/inflammatory response to a pig xenograft.14 However, importantly, xenotransplantation offers us the first real opportunity to modify the “donor” rather than just treat the recipient.
Figure 3.
Macroscopic appearances of a wildtype (i.e., genetically unmodified) pig kidney transplanted into a baboon (A) immediately after reperfusion, and (B) 10 minutes later, showing the typical features of hyperacute rejection. Modified from reference 14, with permission.
When the genetic engineering of pigs was suggested in the early 1990s,15,16 the initial approach was to introduce a human complement-regulatory protein, e.g., human decay-accelerating factor (hCD55) or membrane cofactor protein (CD46), that protected against the recipient NHP complement cascade. When the first hCD55 transgenic pigs were introduced,17,18 pig kidney graft survival was significantly increased, but graft failure still occurred from delayed antibody-mediated rejection.19 In 2004, Cozzi and colleagues reported graft survival for a maximum of 90 days (Figure 2), but the mean survival remained at 24 days.20
The identification of the major pig xenoantigen (Gal) against which humans and Old World NHPs (e.g., baboons) have natural (preformed) antibodies21 enabled pigs to be bred in which expression of this antigen had been deleted (GTKO pigs) (Table 2).22–24 The transplantation of kidneys from GTKO pigs into NHPs was associated with prolongation of graft function, similar to that achieved by the transplantation of CD55- or CD46-transgenic pig organs.25,26 The combination of GTKO and a human complement-regulatory protein reduced early graft failure further.27,28
Table 2.
Known carbohydrate xenoantigens expressed on pig cells
| Carbohydrate (Abbreviation) | Responsible Enzyme | Gene-Knockout Pig |
|---|---|---|
| Galactose-α1,3-galactose (Gal) | α1,3-galactosyltransferase | GTKO |
| N-glycolylneuraminic acid (Neu5Gc) | CMAHa | CMAHKO |
| Sda | β-1,4N-acetylgalactosaminyltransferase | β4GalNT2KO |
Cytidine monophosphate-N-acetylneuraminic acid hydroxylase.
In a GTKO/CD55 pig-to-rhesus monkey model of kidney transplantation, Higginbotham et al. reported that the selection of monkeys with low levels of anti-pig antibodies was associated with prolonged graft survival (>4 months),29 as had Yamada et al.,25 whereas a monkey with a high level of antibodies rejected the graft within a week. However, the immunosuppressive regimen had to target the CD40/CD154 T cell costimulation pathway, but not the CD28-B7 (CD80/CD86) pathway, confirming the observations of Iwase et al.30
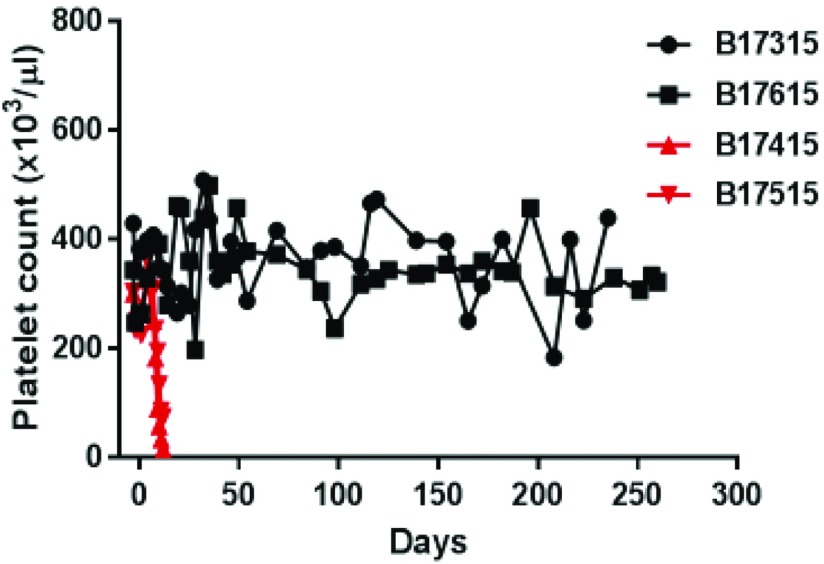
Furthermore, when post-transplant coagulation dysfunction became obvious, the importance of the additional expression of a human coagulation-regulatory protein was documented (Figure 4)31 (reviewed in Wang et al.32).
Figure 4.
Rapid development of thrombocytopenia (consumptive coagulopathy, a reliable indicator of graft rejection/failure) in two baboons with life-supporting GTKO/hCD46 pig kidney grafts (indicated in red), and maintenance of normal platelet counts in two baboons (treated identically) with life-supporting GKTO/hCD46/hTBM pig kidney grafts (indicated in black). Modified from reference 31, with permission.
Subsequently, genetic engineering has followed these two major paths: (1) deletion of pig xenoantigens, and (2) insertion of “protective” human transgenes.33 The introduction of more sophisticated techniques of genetic engineering has increased the speed and facility with which genetic manipulations can be made, and has reduced the costs (Table 3).
Table 3.
Timeline for application of evolving techniques for genetic engineering of pigs employed in xenotransplantation
| Year | Technique |
|---|---|
| 1992 | Microinjection of randomly integrating transgenes |
| 2000 | Somatic cell nuclear transfer (SCNT) |
| 2002 | Homologous recombination |
| 2011 | Zinc finger nucleases (ZFNs) |
| 2013 | Transcription activator-like effector nucleases (TALENs) |
| 2014 | CRISPR/Cas9a |
CRISPR/Cas9, clustered randomly interspaced short palindromic repeats and the associated protein 9.
Recent Progress in Pig-to-NHP Kidney Transplantation Models
When conventional immunosuppressive therapy was administered, graft survival was short, unless very high blood levels of the agents were maintained, which resulted in infectious complications. When novel agents that blocked the CD40/CD154 costimulation pathway, e.g., with an anti-CD154 mAb,34 the adaptive immune response was suppressed, leading to prolonged graft survival.25,35 Although anti-CD154 mAbs have been associated with thrombogenic complications, anti-CD40 mAbs, which appear almost equally effective, are not,36 but are not yet approved by the US Food and Drug Administration (FDA) for use in organ transplantation. In contrast, blockade of the CD28-B7 (CD80/CD86) pathway, i.e., by CTLA4-Ig, is insufficient.30
A significant advance was made in 2015, when a baboon with a kidney graft from a GTKO pig that expressed human complement and coagulation-regulatory proteins functioned for 136 days before the recipient required euthanasia for a systemic infection (Myroides spp.), at which time the graft showed no histopathological features of rejection.37 Iwase et al.31 went on to document survival of up to 9 months, with the experiments again being terminated for infectious complications (that would probably have been prevented or treated successfully in human patients), although good graft function was maintained.
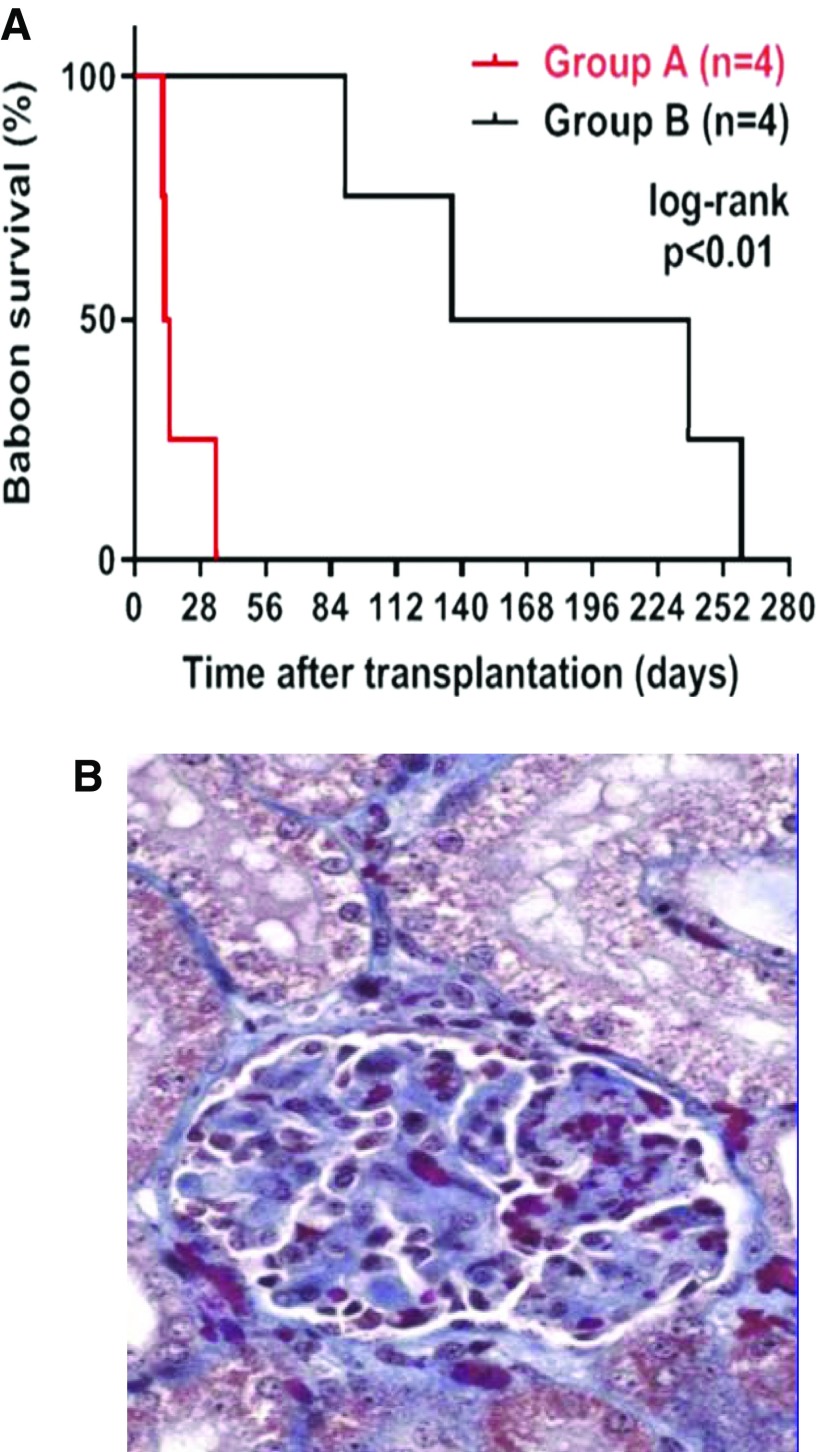
Confirmation of the inadequacy of conventional pharmacologic immunosuppressive therapy was provided by consistent pig kidney graft failure in contrast to consistent prolonged graft survival when the regimen used an anti-CD40 mAb38 (Figure 5A) with conventional immunosuppressive therapy, and graft failure was associated with histopathologic features of a thrombotic glomerulopathy (Figure 5B) despite expression of human coagulation-regulatory proteins, suggesting that blockade of the CD40/CD154 pathway may be critical.
Figure 5.
(A) Pig kidney graft survival in baboons receiving either conventional (tacrolimus-based; group A) or anti-CD40 mAb–based (group B) immunosuppressive therapy. Median pig kidney graft survival in group B (186 days) was significantly longer than in group A (13 days) (P<0.01). Reproduced from reference 38, with permission). (B) Histopathology of thrombotic glomerulopathy in a pig kidney of group A. Glomerular histopathology included thrombotic microangiopathic glomerulopathy, glomerular thrombi, mesangial thickening, and glomerular edema (expansion of Bowman’s space). Hematoxylin and eosin stain, original magnification ×400. Reproduced from reference 38, with permission.
Two other pig xenoantigens have been identified (Neu5Gc39,40 and Sda41) (Table 3), and have also been deleted from pigs, providing us with triple-knockout (TKO) pigs42 to which many humans have no antibody binding (Figure 6A),43 and no serum cytotoxicity (Figure 6B).
Figure 6.
(A) Human IgM (left) and IgG (right) antibody binding to wildtype (WT), GTKO, double knockout (DKO; i.e., knockout of Gal and Sda), and triple knockout (TKO) pig red blood cells (RBCs). Human serum antibody binding to pig RBCs (n=14) was measured by flow cytometry using the relative geometric mean (GM), which was calculated by dividing the GM value for each sample by the negative control (see Gao et al.,74 or Li et al.43). Negative controls were obtained by incubating the cells with secondary anti-human antibodies only (with no serum). Binding to TKO pig RBCs was not significantly different from human IgM and IgG binding to human RBCs of blood type O. **P<0.01. Modified and reproduced from reference 45, with permission. (B) Pooled human serum complement-dependent cytotoxicity (hemolysis) to WT, GTKO, and TKO (GTKO/β4GalKO/CMAHKO) pig RBCs was performed (Cooper et al.46). Briefly, RBCs were incubated with diluted serum for 30 minutes at 37°C. After washing, RBCs were incubated with rabbit complement (Sigma, St. Louis, MO) (final concentration 20%) for 150 minutes at 37°C. After centrifugation, supernatant was collected, and hemolysis was evaluated using a Multi-Label Microplate Reader (Victor3; Perkin Elmer, Waltham, MA). The absorbance of each sample at 541 nm was measured. Cytotoxicity of the same serum to autologous human blood type O RBCs was tested as a control. Serum cytotoxicity to TKO pig cells was not significantly different to human O blood type cells. Reproduced from reference 46, with permission. (C) Human (left) and baboon (right) IgM and IgG binding to TKO pig red blood cells. Baboon sera contain significantly more IgM directed to TKO pig cells than human sera. Modified from reference 43, with permission.
We believe that deletion of expression of the three known pig xenoantigens and strong expression of one or more human complement- and coagulation-regulatory proteins are essential to the success of clinical organ transplantation. These genetic manipulations do not appear to affect the health of the pigs.
A Remaining Immunologic Hurdle
One remaining experimental problem relates to the fact that most Old World NHPs, e.g., baboons, do have antibodies to TKO pig cells.43 This is because Old World NHPs, like wildtype pigs, express N-glycolylneuraminic acid. When this is deleted from the surface of the pig cell, it appears to expose another (a fourth) xenoantigen, against which Old World NHPs, but not humans, have natural antibodies (Figure 6C). In our limited experience to date, expression of the fourth xenoantigen in a pig kidney graft has resulted in rejection in four out of five cases. Proving the efficacy of both a pig genetic manipulation and/or a novel immunosuppressive regimen in the pig-to-Old-World-NHP model is therefore proving more challenging than it is likely to be when pig kidneys are transplanted clinically.
Deletion of both the Gal and Sda antigens in the pig (but not of Neu5Gc) provides a pig against which some Old World NHPs have low antibody binding, thus more closely mimicking the TKO pig-to-human model. Administering an anti-CD154 mAb–based regimen, Adams et al. carried out six kidney transplants in rhesus monkeys, and reported that three grafts underwent antibody-mediated rejection within a week, and a fourth at 35 days, although two grafts functioned for 100 and 435 days, respectively.44 As the pigs did not express any human protective proteins, these data strongly suggest that expression of human complement- and coagulation-regulatory proteins adds significantly to graft survival.
Optimal Genetically Engineered Pigs Today
TKO pigs that express no fewer than six human protective proteins (two complement-regulatory [CD46, CD55], two coagulation-regulatory [thrombomodulin, endothelial protein C receptor], the anti-inflammatory/antiapoptotic hemeoxygenase-1 [HO-1], and CD47 [with immunosuppressive effects]) have recently become available.45,46 Although most baboons still have antibodies against these pig cells, it is hoped that the expression of six protective human transgenes may prevent rejection from developing.
Nonimmunologic Observations in the Pig-to-NHP Model
Baboons with well functioning pig renal grafts remain alert and active, and are indistinguishable from healthy baboons without transplants. In the absence of innate and adaptive immune responses (which have only recently been overcome, and have affected renal graft function), function of the transplanted pig kidneys in NHPs (on the basis of measurements of serum creatinine, albumin, electrolytes, and specific gravity) has generally been excellent, although low phosphate levels have been observed.31,47 In particular, there is no or minimal proteinuria, and polyuria has not been observed. Because blood is drawn frequently from the (immunosuppressed) baboons, recombinant human erythropoietin is administered to maintain an adequate hematocrit. It is therefore uncertain whether pig erythropoietin functions in primates. However, two potential problems have been identified.
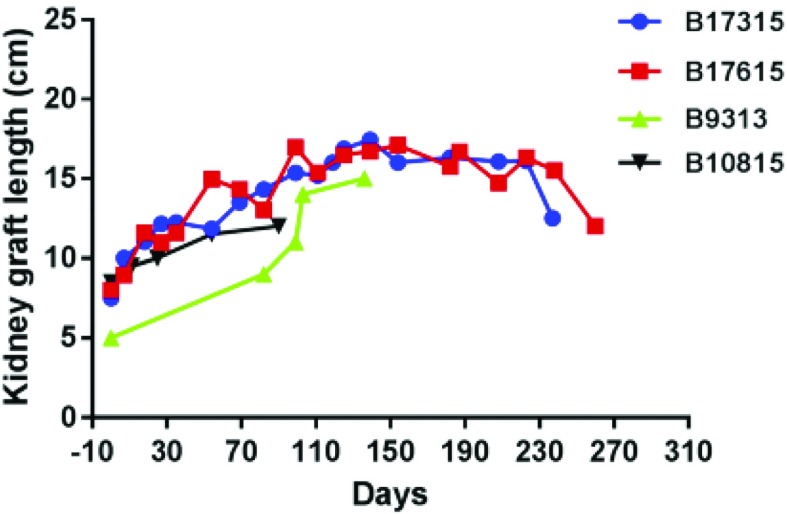
The first is rapid growth of the kidney for the first 2–3 months after transplantation, during which period it can double in length and/or mass31,37,47 (Figure 7). This can also occur after allotransplantation when a kidney from a rapidly growing pig is transplanted into a miniature swine that grows much more slowly.48 There appears to be an intrinsic factor that drives growth for the first few weeks. As there is space within the abdomen for the expanding kidney, we have not found this to be problematic (although it could be for a rapidly growing heart within the restricted confines of the chest). If it proves problematic, there are ways to genetically engineer the pigs to reduce the rate of growth.
Figure 7.
Increases in the lengths of the kidneys in four baboons with genetically engineered pig kidney grafts that functioned for 90, >136, >237, and >260 days, respectively. There were similar increases in the width and depth of the kidneys. A 2-month-old pig weighing 20 kg will grow rapidly to approximately 75–90 kg by 6 months of age, whereas a recipient 8 kg baboon may grow by only 1 kg within the following 6 months.
A second phenomenon that requires confirmation and investigation is the intermittent development of hypovolemia that we have observed in baboons with well functioning pig kidney grafts.47,49 Preliminary evidence suggests that this may be related to the inability of pig renin to cleave angiotensinogen in primates. This results in a reduced ability for vasoconstriction and retention of fluid. In the pig-to-primate experiments, we excise both baboon native kidneys to ensure that life is being supported only by the pig graft. In contrast, in humans, both native kidneys generally remain in situ, and so the problem should not arise. (If necessary, the organ-source pig could be genetically engineered to express human renin as well as pig renin.)
Progress in the experimental laboratory has been sufficiently encouraging to consider a clinical trial.
Selection of Patients for the First Clinical Trial
Patients with various conditions have been discussed (Table 4).1,50 An important point is that, because xenotransplantation will be offered to patients who are unable to receive a timely allograft, the results of the initial pig kidney xenotransplants should be compared with those for comparable patients maintained on chronic dialysis, but not with those for patients receiving kidney allografts. A second major point is that the patient’s general physical state should make him or her an acceptable candidate for allotransplantation. To select patients who are unlikely to survive after receiving an allograft (e.g., from general frailty, chronic infection, or previous or current neoplasia) would not prove to be an adequate trial of xenotransplantation, as the patient would be equally unlikely to survive.
Table 4.
Potential conditions for which initial clinical trials of pig kidney xenotransplantation may be justified
| Elderly patients without significant concomitant disease |
| Patients of blood group B or O often wait for >5 yr for a suitable donor. The mortality of waitlist patients is 40% at 5 yr, and so many of these patients, particularly in the age range 55–65 yr, will not survive until a deceased human kidney becomes available. |
| Recurrent kidney disease |
| Recurrent FSGS |
| Recurrence can be very rapid in some patients. If recurrence occurs rapidly in the pig kidney, this may not be a valid test of xenotransplantation. |
| Other potentially recurrent diseases |
| Recurrence is slower in several other disease states, e.g., Ig A nephropathy, membranoproliferative GN type 2, and so these patients might possibly be candidates for a trial of pig kidney transplantation. |
| High sensitization to HLA |
| There is evidence for some cross-reactivity between anti-HLA antibodies and swine leukocyte antigens, suggesting that patients sensitized to HLA should be excluded from the first clinical trials. |
| Loss of vascular access for dialysis |
| These patients have often been on dialysis for some time (years rather than months) and may have diseased blood vessels making kidney transplantation technically difficult. They are frequently less than ideal candidates even for allotransplantation. |
Modified from reference 50, with permission.
We suggest it would be ethical to offer a pig kidney transplant to selected patients whose life expectancy is less than the time it will take for them to obtain a deceased human donor organ. The median waiting period for a patient with ESKD to obtain a human donor kidney is 3.9 years,4 by which time approximately 35% of transplant candidates may have died or removed from the waitlist (Figure 1). Those of blood group B or O may experience a wait of ≥7 years,4 even when the patient has no antibodies directed to HLA. Many patients might choose to receive a life-supporting pig kidney as long as a reasonable period of graft function could be anticipated without the need for excessive immunosuppressive therapy (compared with that required to maintain an allograft). Other parameters that may be important are listed in Table 5.
Table 5.
Factors considered in selection of patients for first clinical trials of pig kidney xenotransplantation
| Age 55–65 yr. As the anticipated period of pig graft survival remains uncertain, younger patients, who are more likely to survive until a suitable allograft becomes available, should perhaps be excluded from the initial trials. |
| No significant health problems except ESKD. A patient with a pig xenograft may possibly (1) require more immunosuppressive therapy than one with an allograft, and (2) may need to return to chronic dialysis, which may be associated with a higher morbidity than initially. It is therefore important that the patient should have no other health problems except ESKD. A patient with isolated polycystic kidney disease might be a preferred candidate. |
| Blood type B or O, as these patients spend longer on the waiting list for a deceased human donor kidney. |
| No anti-HLA antibodies (to avoid any risk of cross-reactivity of anti-HLA antibodies with swine leukocyte antigens). |
| Supported by dialysis, but for <12 mo. Initiation of dialysis will confirm to the patient and his/her family that ESKD has advanced sufficiently to warrant kidney transplantation, but the period of dialysis has not been so long to be associated with complications or lead to general debility. |
| Fulfill all other criteria for allotransplantation, e.g., absence of potentially life-threatening infections or malignant disease. |
| Vascular access may be problematic, or is likely to become limited. |
At present, the FDA recommends that a patient should only be considered for a pig organ transplant if his or her life expectancy is anticipated to be <2 years. It may be difficult to predict the exact survival of a patient, and this guideline, if followed, might rule out many of the patients we have suggested above. Furthermore, some patients who are unlikely to survive for 2 years have already been supported by dialysis for several years and have developed comorbidities and are no longer ideal transplant candidates. In this case, patients in whom vascular access for dialysis is becoming difficult could be considered, but again many of these will have been undergoing dialysis for a prolonged period of time and, for this and other reasons, may not be suitable candidates for inclusion in a clinical trial. Selection of the initial patients, therefore, will require very careful consideration.
A method of predicting the potential benefit of a pig kidney transplant would be valuable. This could possibly be indicated by a similar method to that suggested by Bae et al.51 On the basis of the Kidney Donor Profile Index and the Estimated Post-Transplant Survival, this group calculated the predicted survival of a patient on the waitlist and compared it with the predicted survival after kidney allotransplantation, A similar approach is worth exploration in relation to xenotransplantation.
A second group of patients who could be considered as possible candidates for xenotransplantation is those with an underlying disease that has recurred in a second or even third allograft (Table 4).50 However, some of these patients will likely have developed antibodies to HLA, and therefore, if we follow our current criteria (see below), would be excluded. It is unknown whether any of these diseases will recur in a pig kidney but, even if they do, the xenograft will have allowed for the allocation of an allograft to another recipient where it may have been better utilized. For the first clinical trial, however, we suggest that these patients, particularly those in whom the disease might recur rapidly, e.g., FSGS, may not be ideal candidates.
At present, we would exclude patients with any sensitization to HLA. Although our own studies have repeatedly indicated that sensitization to HLA would not be detrimental to survival of a pig kidney graft,52,53 several other studies have indicated that there can be crossreactivity between anti-HLA antibodies and some swine leukocyte antigens (SLA).53,54 Methods are being developed to delete or replace specific SLA against which there might be crossreactivity.55,56 Although HLA-sensitized patients may be those who ultimately benefit most from pig kidney xenotransplantation, we submit that no risks in this respect should be taken in the first clinical trial.
In addition, the kidney allocation system introduced in the United States in December 2014 prioritizes allocation of donor kidneys to HLA highly sensitized recipients if there are no preformed antibodies against that specific donor. This has resulted in increased rates of transplantation for highly sensitized patients, some of whom may now have a shorter waitlist time than less-sensitized patients.50,57
Although a pre-emptive transplant might be most beneficial, for the first clinical trial we suggest that recipient selection should be limited to those already on dialysis. This removes the additional variable of native renal function from the interpretation of the study results. Furthermore, if the patient is already requiring dialysis, there will be no doubt that ESKD had advanced enough to warrant a kidney transplant.
Importantly, the current (limited) evidence is that a failed pig xenograft, even if anti-pig antibodies are developed, would not be detrimental to a subsequent allograft.54
The Potential Risk of Xenozoonosis
Breeding and housing of the organ-source pigs in a biosecure, designated pathogen-free environment should rid them of all potentially pathogenic microorganisms. Nevertheless, expert opinion is that the immunosuppressed recipients will be susceptible to the same infectious complications as recipients of allografts, but that these will be manageable.58
There has, however, been special concern about the transfer of porcine endogenous retroviruses (PERVs, that are present in every pig cell). Although not pathogenic in pigs, whether these will be pathogenic in humans is unknown, but considered unlikely. Furthermore, there are drugs that can be administered to prevent or treat PERV activation. Among the numerous genetic manipulations in pigs that have been explored is inactivation or deletion of PERVs,59–61 although the present opinion is that this will not be necessary.
Regulatory, Psychosocial, and Ethical Aspects of Clinical Xenotransplantation
Clinical trials will clearly be overseen by national regulatory authorities. Archiving of specimens (blood, tissues) from the organ-source pig will be essential so that, in the event that an unusual complication develops in the human recipient (e.g., infection, neoplasia), suitable testing can be carried out to determine whether this can be attributed to the presence of the pig xenograft.
Several studies of the attitudes to clinical xenotransplantation of patients awaiting organ transplantation, their family members, medical and nursing staff, or of other groups within the community (e.g., religious groups62) have been undertaken, with those personally affected by terminal disease (e.g., ESKD) being more positive in accepting this potential new form of therapy than those with no immediate threat to life (W.D. Paris, unpublished data).
Ethical aspects of xenotransplantation have been discussed for many years,63 with particular consideration on whether a patient with a xenograft (who, in the event of developing a porcine infection, might present a risk to society) can withdraw from a clinical trial.64
The Future
The transplantation of organs, tissues, and cells from genetically engineered pigs has immense clinical therapeutic potential. The genetic manipulations that have been introduced in the pigs to date have largely been directed to overcome the innate immune response, for which effective drug therapy is limited. In future, however, the pigs will also be manipulated to control the adaptive immune response, thus enabling exogenous immunosuppressive therapy to be significantly reduced or, indeed, ultimately unnecessary. For example, pigs have already been produced in which expression of SLA class 1 has been deleted65 or SLA class 2 has been downregulated,66 or in which PD-L1,67–69 the immunosuppressive agent CTLA4-Ig,70,71 or HLA-E or G (providing protection from NK cell activity)72 have been expressed.
The ultimate goal of both allotransplantation and xenotransplantation is the induction of immunologic tolerance, in which the recipient no longer attempts to reject the graft. Although efforts in this respect in xenotransplantation have to date been unsuccessful, in view of the potential offered by genetic engineering of the donor, it would seem it is more likely to be achieved in xenotransplantation than in allotransplantation.
Disclosures
Prof. Cooper reports grants from National Institutes of Health National Institute of Allergy and Infectious Diseases during the conduct of the study. Prof. Eckhoff reports grants from United Therapeutics, during the conduct of the study. Prof. Kumar reports receiving honoraria from Mallinckrodt, outside the submitted work. All remaining authors have nothing to disclose.
Funding
Work on xenotransplantation at the University of Alabama at Birmingham is supported in part by National Institutes of Health National Institute of Allergy and Infectious Diseases U19 grant AI090959, and in part by a grant from United Therapeutics, Silver Spring (the parent company of Revivicor, Blacksburg, VA, that provides University of Alabama at Birmingham with genetically engineered pigs).
Acknowledgments
Prof. Hara, Prof. Iwase, Dr. Yamamoto, Dr. Jagdale, Prof. Anderson, Prof. Eckhoff, and Prof. Cooper carried out the preclinical studies outlined in the manuscript. Prof. Kumar, Prof. Mannon, Prof. Hanaway, Prof. Eckhoff, and Prof. Cooper contributed to the section on selection of potential patients. Prof. Cooper wrote the original draft of the manuscript. All authors reviewed the manuscript, after which revisions were made and approved by all authors.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Jagdale A, Cooper DKC, Iwase H, Gaston RS: Chronic dialysis in patients with end-stage renal disease: Relevance to kidney xenotransplantation. Xenotransplantation 26: e12471, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scientific Registry of Transplant Recipients: Annual Data Report, 2017. Available at: https://srtr.transplant.hrsa.gov/annual_reports/2017_ADR_Preview.aspx. Accessed August 4, 2019
- 3.United States Renal Data System: Annual Data Report, 2018. Available at: https://www.usrds.org/adr.aspx. Accessed August 4, 2019
- 4.United States Renal Data System: Annual Data Report, 2017. Available at: https://www.usrds.org/2017/view/Default.aspx. Accessed August 4, 2019
- 5.Orandi BJ, Luo X, Massie AB, Garonzik-Wang JM, Lonze BE, Ahmed R, et al. : Survival benefit with kidney transplants from HLA-incompatible live donors. N Engl J Med 374: 940–950, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casingal V, Glumac E, Tan M, Sturdevant M, Nguyen T, Matas AJ: Death on the kidney waiting list--good candidates or not? Am J Transplant 6: 1953–1956, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Carrel A: The surgery of blood vessels. Johns Hopkins Hospital Bulletin. 190: 18–28, 1907 [Google Scholar]
- 8.Taniguchi S, Cooper DKC: Clinical xenotransplantation: Past, present and future. Ann R Coll Surg Engl 79: 13–19, 1997 [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper DKC: A brief history of cross-species organ transplantation. Proc Bayl Univ Med Cent 25: 49–57, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reemtsma K, McCracken BH, Schlegel JU, Pearl MA, Pearce CW, Dewitt CW, et al. : Renal heterotransplantation in man. Ann Surg 160: 384–410, 1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lexer G, Cooper DKC, Rose AG, Wicomb WN, Rees J, Keraan M, et al. : Hyperacute rejection in a discordant (pig to baboon) cardiac xenograft model. J Heart Transplant 5: 411–418, 1986 [PubMed] [Google Scholar]
- 12.Cooper DKC, Human PA, Lexer G, Rose AG, Rees J, Keraan M, et al. : Effects of cyclosporine and antibody adsorption on pig cardiac xenograft survival in the baboon. J Heart Transplant 7: 238–246, 1988 [PubMed] [Google Scholar]
- 13.Cooper DKC, Gollackner B, Sachs DH: Will the pig solve the transplantation backlog? Annu Rev Med 53: 133–147, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Cooper DKC, Ezzelarab MB, Hara H, Iwase H, Lee W, Wijkstrom M, et al. : The pathobiology of pig-to-primate xenotransplantation: A historical review. Xenotransplantation 23: 83–105, 2016 [DOI] [PubMed] [Google Scholar]
- 15.Dalmasso AP, Vercellotti GM, Platt JL, Bach FH: Inhibition of complement-mediated endothelial cell cytotoxicity by decay-accelerating factor. Potential for prevention of xenograft hyperacute rejection. Transplantation 52: 530–533, 1991 [DOI] [PubMed] [Google Scholar]
- 16.White DJ, Oglesby T, Liszewski MK, Tedja I, Hourcade D, Wang MW, et al. : Expression of human decay accelerating factor or membrane cofactor protein genes on mouse cells inhibits lysis by human complement. Transpl Int 5[Suppl 1]: S648–S650, 1992 [DOI] [PubMed] [Google Scholar]
- 17.Fodor WL, Williams BL, Matis LA, Madri JA, Rollins SA, Knight JW, et al. : Expression of a functional human complement inhibitor in a transgenic pig as a model for the prevention of xenogeneic hyperacute organ rejection. Proc Natl Acad Sci U S A 91: 11153–11157, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cozzi E, White DJ: The generation of transgenic pigs as potential organ donors for humans. Nat Med 1: 964–966, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Lambrigts D, Sachs DH, Cooper DKC: Discordant organ xenotransplantation in primates: World experience and current status. Transplantation 66: 547–561, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Baldan N, Rigotti P, Calabrese F, Cadrobbi R, Dedja A, Iacopetti I, et al. : Ureteral stenosis in HDAF pig-to-primate renal xenotransplantation: A phenomenon related to immunological events? Am J Transplant 4: 475–481, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Good AH, Cooper DKC, Malcolm AJ, Ippolito RM, Koren E, Neethling FA, et al. : Identification of carbohydrate structures that bind human antiporcine antibodies: Implications for discordant xenografting in humans. Transplant Proc 24: 559–562, 1992 [PubMed] [Google Scholar]
- 22.Cooper DKC, Koren E, Oriol R: Genetically engineered pigs. Lancet 342: 682–683, 1993 [DOI] [PubMed] [Google Scholar]
- 23.Phelps CJ, Koike C, Vaught TD, Boone J, Wells KD, Chen SH, et al. : Production of alpha 1,3-galactosyltransferase-deficient pigs. Science 299: 411–414, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kolber-Simonds D, Lai L, Watt SR, Denaro M, Arn S, Augenstein ML, et al. : Production of alpha-1,3-galactosyltransferase null pigs by means of nuclear transfer with fibroblasts bearing loss of heterozygosity mutations. Proc Natl Acad Sci U S A 101: 7335–7340, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamada K, Yazawa K, Shimizu A, Iwanaga T, Hisashi Y, Nuhn M, et al. : Marked prolongation of porcine renal xenograft survival in baboons through the use of α1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med 11: 32–34, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Shimizu A, Yamada K, Robson SC, Sachs DH, Colvin RB: Pathologic characteristics of transplanted kidney xenografts. J Am Soc Nephrol 23: 225–235, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Azimzadeh AM, Kelishadi SS, Ezzelarab MB, Singh AK, Stoddard T, Iwase H, et al. : Early graft failure of GalTKO pig organs in baboons is reduced by expression of a human complement pathway-regulatory protein. Xenotransplantation 22: 310–316, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGregor CG, Ricci D, Miyagi N, Stalboerger PG, Du Z, Oehler EA, et al. : Human CD55 expression blocks hyperacute rejection and restricts complement activation in Gal knockout cardiac xenografts. Transplantation 93: 686–692, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Higginbotham L, Mathews D, Breeden CA, Song M, Farris AB 3rd, Larsen CP, et al. : Pre-transplant antibody screening and anti-CD154 costimulation blockade promote long-term xenograft survival in a pig-to-primate kidney transplant model. Xenotransplantation 22: 221–230, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iwase H, Ekser B, Satyananda V, Bhama J, Hara H, Ezzelarab M, et al. : Pig-to-baboon heterotopic heart transplantation--exploratory preliminary experience with pigs transgenic for human thrombomodulin and comparison of three costimulation blockade-based regimens. Xenotransplantation 22: 211–220, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iwase H, Hara H, Ezzelarab M, Li T, Zhang Z, Gao B, et al. : Immunological and physiological observations in baboons with life-supporting genetically engineered pig kidney grafts. Xenotransplantation 24: 1–13, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang L, Cooper DKC, Burdorf L, Wang Y, Iwase H: Overcoming coagulation dysregulation in pig solid organ transplantation in nonhuman primates: Recent progress. Transplantation 102: 1050–1058, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cooper DKC, Ekser B, Ramsoondar J, Phelps C, Ayares D: The role of genetically engineered pigs in xenotransplantation research. J Pathol 238: 288–299, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bühler L, Awwad M, Basker M, Gojo S, Watts A, Treter S, et al. : High-dose porcine hematopoietic cell transplantation combined with CD40 ligand blockade in baboons prevents an induced anti-pig humoral response. Transplantation 69: 2296–2304, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Kuwaki K, Tseng YL, Dor FJ, Shimizu A, Houser SL, Sanderson TM, et al. : Heart transplantation in baboons using α1,3-galactosyltransferase gene-knockout pigs as donors: Initial experience. Nat Med 11: 29–31, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Mohiuddin MM, Singh AK, Corcoran PC, Thomas Iii ML, Clark T, Lewis BG, et al. : Chimeric 2C10R4 anti-CD40 antibody therapy is critical for long-term survival of GTKO.hCD46.hTBM pig-to-primate cardiac xenograft. Nat Commun 7: 11138, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iwase H, Liu H, Wijkstrom M, Zhou H, Singh J, Hara H, et al. : Pig kidney graft survival in a baboon for 136 days: Longest life-supporting organ graft survival to date. Xenotransplantation 22: 302–309, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamamoto T, Hara H, Foote J, Wang L, Li Q, Klein EC, et al. : Life-supporting kidney xenotransplantation from genetically-engineered pigs in baboons: A comparison of two immunosuppressive regimens. Transplantation 103: 2090–2104, 2019 [DOI] [PubMed] [Google Scholar]
- 39.Bouhours D, Pourcel C, Bouhours JE: Simultaneous expression by porcine aorta endothelial cells of glycosphingolipids bearing the major epitope for human xenoreactive antibodies (Gal alpha 1-3Gal), blood group H determinant and N-glycolylneuraminic acid. Glycoconj J 13: 947–953, 1996 [DOI] [PubMed] [Google Scholar]
- 40.Zhu A, Hurst R: Anti-N-glycolylneuraminic acid antibodies identified in healthy human serum. Xenotransplantation 9: 376–381, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Byrne GW, Du Z, Stalboerger P, Kogelberg H, McGregor CG: Cloning and expression of porcine β1,4 N-acetylgalactosaminyl transferase encoding a new xenoreactive antigen. Xenotransplantation 21: 543–554, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Estrada JL, Martens G, Li P, Adams A, Newell KA, Ford ML, et al. : Evaluation of human and non-human primate antibody binding to pig cells lacking GGTA1/CMAH/β4GalNT2 genes. Xenotransplantation 22: 194–202, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Q, Shaikh S, Iwase H, Long C, Lee W, Zhang Z, et al. : Carbohydrate antigen expression and anti-pig antibodies in New World capuchin monkeys: Relevance to studies of xenotransplantation. Xenotransplantation 26: e12498, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adams AB, Kim SC, Martens GR, Ladowski JM, Estrada JL, Reyes LM, et al. : Xenoantigen deletion and chemical immunosuppression can prolong renal xenograft survival. Ann Surg 268: 564–573, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cooper DKC, Ezzelarab M, Iwase H, Hara H: Perspectives on the optimal genetically engineered pig in 2018 for initial clinical trials of kidney or heart xenotransplantation. Transplantation 102: 1974–1982, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cooper DKC, Hara H, Iwase H, Yamamoto T, Li Q, Ezzelarab M, et al. : Justification of specific genetic modifications in pigs for clinical organ xenotransplantation. Xenotransplantation 26: e12516, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iwase H, Klein EC, Cooper DK: Physiologic aspects of pig kidney transplantation in Nonhuman primates. Comp Med 68: 332–340, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tanabe T, Watanabe H, Shah JA, Sahara H, Shimizu A, Nomura S, et al. : Role of intrinsic (graft) versus extrinsic (host) factors in the growth of transplanted organs following allogeneic and xenogeneic transplantation. Am J Transplant 17: 1778–1790, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iwase H, Yamamoto T, Cooper DKC: Episodes of hypovolemia/dehydration in baboons with pig kidney transplants: A new syndrome of clinical importance? Xenotransplantation 26: e12472, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cooper DKC, Wijkstrom M, Hariharan S, Chan JL, Singh A, Horvath K, et al. : Selection of patients for initial clinical trials of solid organ xenotransplantation. Transplantation 101: 1551–1558, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bae S, Massie AB, Thomas AG, Bahn G, Luo X, Jackson KR, et al. : Who can tolerate a marginal kidney? Predicting survival after deceased donor kidney transplant by donor-recipient combination. Am J Transplant 19: 425–433, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cooper DK, Tseng YL, Saidman SL: Alloantibody and xenoantibody cross-reactivity in transplantation. Transplantation 77: 1–5, 2004 [DOI] [PubMed] [Google Scholar]
- 53.Zhang Z, Hara H, Long C, Iwase H, Qi H, Macedo C, et al. : Immune responses of HLA highly sensitized and nonsensitized patients to genetically engineered pig cells. Transplantation 102: e195–e204, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li Q, Hara H, Zhang Z, Breimer ME, Wang Y, Cooper DKC: Is sensitization to pig antigens detrimental to subsequent allotransplantation? Xenotransplantation 25: e12393, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martens GR, Reyes LM, Li P, Butler JR, Ladowski JM, Estrada JL, et al. : Humoral reactivity of renal transplant-waitlisted patients to cells from GGTA1/CMAH/B4GalNT2, and SLA class I knockout pigs. Transplantation 101: e86–e92, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ladowski JM, Reyes LM, Martens GR, Butler JR, Wang ZY, Eckhoff DE, et al. : Swine leukocyte antigen Class II Is a xenoantigen. Transplantation 102: 249–254, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jackson KR, Covarrubias K, Holscher CM, Luo X, Chen J, Massie AB, et al. : The national landscape of deceased donor kidney transplantation for the highly sensitized: Transplant rates, waitlist mortality, and posttransplant survival under KAS. Am J Transplant 19: 1129–1138, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fishman JA: Infectious disease risks in xenotransplantation. Am J Transplant 18: 1857–1864, 2018 [DOI] [PubMed] [Google Scholar]
- 59.Dieckhoff B, Petersen B, Kues WA, Kurth R, Niemann H, Denner J: Knockdown of porcine endogenous retrovirus (PERV) expression by PERV-specific shRNA in transgenic pigs. Xenotransplantation 15: 36–45, 2008 [DOI] [PubMed] [Google Scholar]
- 60.Ramsoondar J, Vaught T, Ball S, Mendicino M, Monahan J, Jobst P, et al. : Production of transgenic pigs that express porcine endogenous retrovirus small interfering RNAs. Xenotransplantation 16: 164–180, 2009 [DOI] [PubMed] [Google Scholar]
- 61.Niu D, Wei HJ, Lin L, George H, Wang T, Lee IH, et al. : Inactivation of porcine endogenous retrovirus in pigs using CRISPR-Cas9. Science 357: 1303–1307, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Paris W, Seidler RJH, FitzGerald K, Padela AI, Cozzi E, Cooper DKC: Jewish, Christian and Muslim theological perspectives about xenotransplantation. Xenotransplantation 25: e12400, 2018 [DOI] [PubMed] [Google Scholar]
- 63.Smetanka C, Cooper DKC: The ethics debate in relation to xenotransplantation. Rev Sci Tech 24: 335–342, 2005 [PubMed] [Google Scholar]
- 64.Hurst DJ, Padilla LA, Walters W, Hunter JM, Cooper DKC, Eckhoff DM, et al.: Pediatric xenotransplantation clinical trials and the right to withdraw. J Med Ethics 2019, doi: 10.1136/medethics-2019-105668 [DOI] [PubMed] [Google Scholar]
- 65.Reyes LM, Estrada JL, Wang ZY, Blosser RJ, Smith RF, Sidner RA, et al. : Creating class I MHC-null pigs using guide RNA and the Cas9 endonuclease. J Immunol 193: 5751–5757, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hara H, Witt W, Crossley T, Long C, Isse K, Fan L, et al. : Human dominant-negative class II transactivator transgenic pigs - effect on the human anti-pig T-cell immune response and immune status. Immunology 140: 39–46, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jeon DH, Oh K, Oh BC, Nam DH, Kim CH, Park HB, et al. : Porcine PD-L1: Cloning, characterization, and implications during xenotransplantation. Xenotransplantation 14: 236–242, 2007 [DOI] [PubMed] [Google Scholar]
- 68.Plege A, Borns K, Beer L, Baars W, Klempnauer J, Schwinzer R: Downregulation of cytolytic activity of human effector cells by transgenic expression of human PD-ligand-1 on porcine target cells. Transpl Int 23: 1293–1300, 2010 [DOI] [PubMed] [Google Scholar]
- 69.Buermann A, Petkov S, Petersen B, Hein R, Lucas-Hahn A, Baars W, et al. : Pigs expressing the human inhibitory ligand PD-L1 (CD 274) provide a new source of xenogeneic cells and tissues with low immunogenic properties. Xenotransplantation 25: e12387, 2018 [DOI] [PubMed] [Google Scholar]
- 70.Phelps CJ, Ball SF, Vaught TD, Vance AM, Mendicino M, Monahan JA, et al. : Production and characterization of transgenic pigs expressing porcine CTLA4-Ig. Xenotransplantation 16: 477–485, 2009 [DOI] [PubMed] [Google Scholar]
- 71.Klymiuk N, van Buerck L, Bähr A, Offers M, Kessler B, Wuensch A, et al. : Xenografted islet cell clusters from INSLEA29Y transgenic pigs rescue diabetes and prevent immune rejection in humanized mice. Diabetes 61: 1527–1532, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Crew MD: Play it in E or G: Utilization of HLA-E and -G in xenotransplantation. Xenotransplantation 14: 198–207, 2007 [DOI] [PubMed] [Google Scholar]
- 73.Cooper DKC, Satyananda V, Ekser B, van der Windt DJ, Hara H, Ezzelarab MB, et al. : Progress in pig-to-non-human primate transplantation models (1998-2013): A comprehensive review of the literature. Xenotransplantation 21: 397–419, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gao B, Long C, Lee W, Zhang Z, Gao X, Landsittel D, et al. : Anti-Neu5Gc and anti-non-Neu5Gc antibodies in healthy humans. PLoS One 12: e0180768, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]