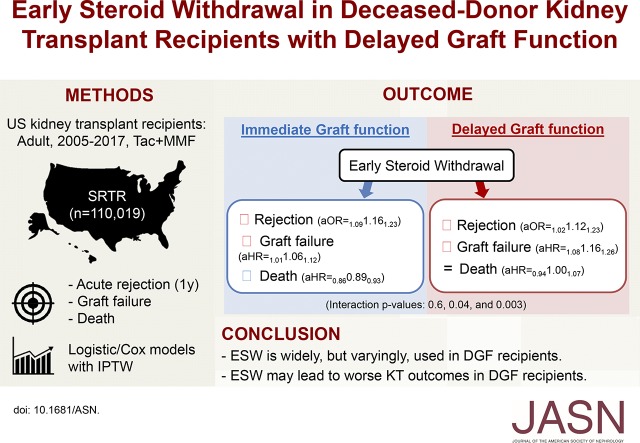
Significance Statement
Early steroid withdrawal (ESW) is a maintenance immunosuppression strategy to avoid the sequelae of long-term steroid use in kidney transplant (KT) recipients. Recipients with delayed graft function (DGF) may have a suboptimal allograft milieu, which may alter the risk/benefit equation of ESW. In this nationwide study, the authors found use of ESW in recipients with DGF varied at United States transplant centers. The authors also identified differences in outcomes after ESW in patients with and without DGF. Among recipients with immediate graft function, ESW was associated with possible harms such as increased rejection and benefits such as decreased mortality. However, among recipients with DGF, ESW was associated only with possible harms, including increased acute rejection and graft failure. Recipients with DGF also saw no change mortality with ESW. Our findings suggest ESW is harmful in KT recipients with DGF.
Keywords: immunosuppression, delayed graft function, transplantation
Visual Abstract
Abstract
Background
Early steroid withdrawal (ESW) is associated with acceptable outcomes in kidney transplant (KT) recipients. Recipients with delayed graft function (DGF), however, often have a suboptimal allograft milieu, which may alter the risk/benefit equation for ESW. This may contribute to varying practices across transplant centers.
Methods
Using the Scientific Registry of Transplant Recipients, we studied 110,019 adult deceased-donor KT recipients between 2005 and 2017. We characterized the association of DGF with the use of ESW versus continued steroid maintenance across KT centers, and quantified the association of ESW with acute rejection, graft failure, and mortality using multivariable logistic and Cox regression with DGF-ESW interaction terms.
Results
Overall 29.2% of KT recipients underwent ESW. Recipients with DGF had lower odds of ESW (aOR=0.600.670.75). The strength of this association varied across 261 KT centers, with center-specific aOR of <0.5 at 31 (11.9%) and >1.0 at 22 (8.4%) centers. ESW was associated with benefits and harms among recipients with immediate graft function (IGF), but only with harms among recipients with DGF. ESW was associated with increased acute rejection (aOR=1.091.161.23), slightly increased graft failure (aHR=1.011.061.12), but decreased mortality (aHR=0.860.890.93) among recipients with IGF. Among recipients with DGF, ESW was associated with a similar increase in rejection (aOR=1.12; 95% CI, 1.02 to 1.23), a more pronounced increase in graft failure (aHR=1.16; 95% CI, 1.08 to 1.26), and no improvement in mortality (aHR=1.00; 95% CI, 0.94 to 1.07). DGF-ESW interaction was statistically significant for graft failure (P=0.04) and mortality (P=0.003), but not for rejection (P=0.6).
Conclusions
KT centers in the United States use ESW inconsistently in recipients with DGF. Our findings suggest ESW may lead to worse KT outcomes in recipients with DGF.
Approximately 30% of deceased-donor kidney transplant (KT) recipients in the United States undergo early steroid withdrawal (ESW), a maintenance immunosuppression protocol that aims to reduce the adverse effects from long-term steroid use.1 Previous studies have suggested ESW leads to no or minimal increases in acute rejection and graft failure,2–10 while reducing steroid-related side effects such as new-onset diabetes, dyslipidemia, and fracture.11–14 However, this risk/benefit equation of ESW can be altered by the clinical characteristics of individual KT recipients. In particular, ESW may not achieve sufficient immunosuppression in certain subgroups, especially those with higher immunologic risk.15
One subgroup that might be unsuitable for ESW is recipients who develop delayed graft function (DGF). DGF is often a consequence of a suboptimal allograft milieu, which subsequently increases the risk of acute rejection and graft failure.16–19 This milieu may necessitate potent immunosuppression regimens that could also counterbalance the increased risks of acute rejection and graft failure,20 lending support to the use of continued steroid maintenance (CSM) over ESW in recipients with DGF. Alternatively, given that DGF is often attributable to ischemia-reperfusion injury rather than immune response,16,20,21 the pathophysiology of DGF may have little interaction with the pathway through which immunosuppression influences KT outcomes. In other words, DGF may not indicate high “immunologic” risk in the context of individualizing steroid use, despite being a risk factor for rejection and graft failure. There is no clinical evidence to support either ESW or CSM in the context of DGF. This knowledge gap may have negative implications for post-KT management, given that DGF is a common condition with an incidence of 25%–36% among recipients of deceased-donor KTs.17,22
In this nationwide study, we sought to understand whether DGF manifests a suboptimal allograft milieu in which ESW is not desirable for maintenance immunosuppression after KT. We first described the current practice by quantifying the association between DGF and the use of ESW (versus CSM) at each KT center across the country. We then investigated whether the association between ESW and post-KT outcomes varies by DGF status using interaction term analyses.
Methods
Data Source
This study used data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes data on all donors, wait-listed candidates, and transplant recipients in the United States, which are submitted by the members of the Organ Procurement and Transplantation Network (OPTN). The Health Resources and Services Administration, US Department of Health and Human Services, provides oversight to the activities of the OPTN and SRTR contractors.
Study Population
Using the SRTR data, we studied adult (>18 years) recipients of deceased-donor kidney-alone transplants from January 1, 2005 to December 31, 2017 who received post-transplant maintenance immunosuppression using tacrolimus and mycophenolate. Recipients whose immunosuppression records (n=2285, 2.0%) or DGF status (n=13, 0.01%) could not be retrieved, and those who received maintenance agents other than tacrolimus and mycophenolate (n=16,188, 12.8%) were excluded. DGF was defined as the need for dialysis within the first week after transplant, as per the United Network for Organ Sharing/OPTN Kidney Transplant Recipient Registration form.23 For this reason, we excluded recipients who died or developed graft failure within 7 days post-KT or discharge (n=1154, 1.0%). Absence of DGF was termed immediate graft function (IGF). ESW was defined as withdrawal of maintenance steroid by the time of discharge from the KT admission; all other recipients were considered CSM. As the exact date of steroid withdrawal was not included in our data, we excluded the recipients who were not discharged within 30 days post-KT (n=1221, 1.1%) to exclude “late” steroid withdrawal cases (i.e., beyond the first few weeks post-KT) and their CSM counterparts. Our final study population included 110,019 recipients.
Use of ESW
To study nationwide temporal trends in ESW use, we quantified the proportion of recipients who underwent ESW by calendar year of transplant. To study center-level variation in ESW use, we quantified the proportion of recipients who underwent ESW at each center. To determine if DGF was associated with center-level decisions to use ESW, adjusting for recipient and donor characteristics, we used a multilevel logistic model that included a random slope term that estimates the association between DGF and ESW use at each transplant center. A large variance in this term indicates a large center-level variation in practice. We fitted this model using a Gibbs sampler with uninformative diffuse priors under the Bayesian framework.24
ESW and Inverse Probability of Treatment Weights
For KT outcomes analyses (acute rejection, graft survival, and patient survival), our primary exposure was ESW, as the initial maintenance immunosuppression regimen, which was treated as a time-fixed exposure. The time-fixed-exposure approach aims to estimate the effect of ESW, i.e., that of withdrawing steroid from the initial maintenance regimen, and does not require additional assumptions. This design is analogous to the intention-to-treat approach in clinical trials.25 In addition, reinitiations or late steroid withdrawals are often related to rejection or steroid-associated side effects, which can be regarded as mediators between the initial treatment assignment and the outcomes of interest under our conceptual framework.
When studying KT outcomes, we used the inverse probability of treatment weights (IPTW) to control for confounders, and confounding by indication (differences between the recipients that might have influenced the decision for ESW). The propensity score for undergoing ESW (psESW) was estimated using a generalized estimating equation with logistic link to account for clustering at transplant centers (Supplemental Table 1). Based on the SRTR Risk Adjustment Model, we adjusted for recipient factors (age, race, sex, panel-reactive antigen [PRA], previous transplants, body mass index, diabetes, hypertension, cause of ESKD, pre-emptive transplant, time on dialysis, serum albumin, symptomatic peripheral vascular diseases, previous malignancy, hepatitis B and C infection, education level, and primary payer), donor factors (age, race, sex, body mass index, hepatitis C infection, donation after cardiac death, diabetes, hypertension, stroke as the cause of death, donor pulsatile perfusion, and terminal serum creatinine), and transplant factors (HLA-A/-B/-DR mismatches, cold ischemic time, and induction immunosuppression agent). IPTW was derived as 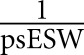 for the ESW group and
for the ESW group and 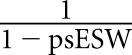 for the CSM group. We examined the distribution of the IPTW values to detect any observations with extremely large weights and found none.
for the CSM group. We examined the distribution of the IPTW values to detect any observations with extremely large weights and found none.
Acute Rejection
The OPTN queries centers for information on acute rejection according to periods covered by specific reporting forms (0–6 and 7–12 months, then annual periods), but exact dates of acute rejection within reporting periods are not collected. We defined acute rejection from SRTR records according to center reports of an acute rejection event occurring in a reporting period, as per prior methods for identifying acute rejection from United States registry data.26,27 For this reason, we studied acute rejection during the first year post-KT as a binary outcome. We conducted logistic regression to compare the odds of acute rejection between the ESW and CSM groups. We weighted the recipients using the IPTW described above to account for differences in recipient, donor, and transplant factors. We included an interaction term for DGF and ESW to test whether the association of ESW (versus CSM) with acute rejection varied in recipients with DGF versus IGF.
Graft and Patient Survival
Graft survival was defined as the time from transplant to graft failure, censoring for death. Patient survival was defined as the time from transplant to death. The date and cause of death were ascertained from multiple sources, including follow-up reports from transplant centers, Centers for Medicare and Medicaid Services ESRD Death Notification Form (CMS 2746), and the Social Security Death Master File. For both outcomes, all recipients were censored at the end of study on December 31, 2017. Unlike acute rejection, we had the exact dates of graft failure and patient death for all recipients until the end of the study. The median length of follow-up was 4.2 years for graft failure and 4.7 years for death. We conducted Cox proportional hazard regression to compare the hazards of graft failure and death between the ESW and CSM groups. As with the rejection analysis, we weighted the recipients using the IPTW described above to account for differences in recipient, donor, and transplant factors, and we included an interaction term for DGF and ESW to test whether the association of ESW (versus CSM) with graft loss and death varied in recipients with DGF versus IGF.
Cause-Specific Mortality and Stratified Analyses
We conducted additional analyses to explore the potential mechanisms of the DGF-ESW interaction. First, we compared the cause-specific hazards of death between ESW and CSM groups to examine if the DGF-ESW interaction is primarily driven by any specific pathophysiology. We conducted Cox proportional hazard regression with IPTW weighting, treating death due to other causes as censoring events.28 We separately analyzed death due to cardiovascular diseases (including stroke), infection, malignancy, and all other causes. Second, we conducted stratified analysis by recipient age (19–64 versus ≥65 years), year of transplant (2005–2011 versus 2012–2017), induction immunosuppression agent (anti-thymocyte globulin, IL-2 receptor antagonists, alemtuzumab, none, and others),29 immune sensitivity as measured in peak PRA (0–9, 10–79, and 80–100), donation after cardiac death, and the number of HLA-A/-B/-DR mismatches.
Statistical Analysis
All analyses were performed using Stata 15.1/MP for Linux (College Station, TX) and R version 3.4. Confidence intervals are reported as per the method of Louis and Zeger.
Results
Study Population
Among the 110,019 recipients included in the study sample, 27,608 (25.1%) developed DGF, of whom 7083 underwent ESW and 20,525 underwent CSM. Among the 82,411 recipients with IGF, 25,032 underwent ESW and 57,379 underwent CSM. Compared with those with CSM, recipients who underwent ESW were more likely to be white (48.5% versus 42.3% in IGF; 37.2% versus 32.8% in DGF), have diabetes as the cause of ESKD (26.8% versus 24.3% in IGF; 35.7% versus 32.1% in DGF), have lower (less than ten) peak PRA (62.7% versus 52.9% in IGF; 62.9% versus 55.7% in DGF), and were less likely to have had a previous transplant (8.7% versus 15.0% in IGF; 9.0% versus 14.3% in DGF) (Table 1).
Table 1.
Population characteristics
| Characteristics | ESW; IGF (n=25,032) | CSM; IGF (n=57,379) | ESW; DGF (n=7083) | CSM; DGF (n=20,525) |
|---|---|---|---|---|
| Recipient factors | ||||
| Age (yr) | 55 (45, 63) | 53 (43, 62) | 56 (46, 63) | 55 (45, 63) |
| Female | 39.3% | 43.8% | 29.3% | 33.7% |
| Race | ||||
| White | 48.5% | 42.3% | 37.2% | 32.8% |
| Black | 28.6% | 32.3% | 35.9% | 41.2% |
| Hispanic/Latino | 14.4% | 16.6% | 17.0% | 17.6% |
| Other/multiracial | 8.5% | 8.8% | 9.9% | 8.4% |
| Preemptive transplant | 12.5% | 12.1% | 2.6% | 3.1% |
| Years on dialysis | 3.1 (1.3, 5.3) | 3.3 (1.4, 5.5) | 4.3 (2.6, 6.3) | 4.5 (2.7, 6.8) |
| Cause of ESKD | ||||
| GN | 20.7% | 24.1% | 16.7% | 19.6% |
| DM | 26.8% | 24.3% | 35.7% | 32.1% |
| HTN | 23.6% | 23.3% | 23.9% | 24.6% |
| Others | 29.0% | 28.3% | 23.7% | 23.7% |
| Peak PRA | ||||
| 0–9 | 62.7% | 52.9% | 62.9% | 55.7% |
| 10–79 | 24.7% | 26.2% | 25.7% | 25.5% |
| 80–100 | 12.6% | 20.9% | 11.4% | 18.8% |
| BMI (kg/m2) | 27.5 (24.1, 31.6) | 27.3 (23.8, 31.2) | 28.9 (25.2, 33.0) | 28.6 (25.0, 32.7) |
| Previous transplants | 8.7% | 15.0% | 9.0% | 14.3% |
| HLA mismatches | ||||
| 0 | 9.8% | 10.0% | 5.9% | 6.9% |
| 1 | 18.9% | 19.2% | 17.3% | 18.3% |
| 2+ | 71.4% | 70.8% | 76.8% | 74.8% |
| Cold ischemic time (hr) | 16.5 (11.2, 23.0) | 16.0 (11.0, 21.9) | 19.0 (13.0, 25.3) | 18.0 (13.0, 24.0) |
| Donor factors | ||||
| Age (yr) | 39 (25, 51) | 38 (24, 50) | 44 (31, 53) | 44 (30, 53) |
| Female | 40.9% | 40.3% | 36.6% | 37.2% |
| Race | ||||
| White | 70.8% | 66.9% | 70.5% | 69.1% |
| Black | 14.0% | 14.7% | 14.0% | 13.7% |
| Hispanic/Latino | 12.4% | 14.9% | 12.4% | 13.5% |
| Other/multiracial | 2.8% | 3.5% | 3.0% | 3.7% |
| Serum creatinine (mg/dl) | 0.9 (0.7, 1.3) | 0.9 (0.7, 1.2) | 1.1 (0.8, 1.6) | 1.0 (0.7, 1.5) |
| DCD | 13.0% | 12.6% | 25.8% | 25.6% |
| ECD | 16.4% | 14.3% | 20.5% | 18.3% |
Numbers are percentages for categorical variables and median (interquartile range) for continuous variables. DM, diabetes mellitus; HTN, hypertension; BMI, body mass index; DCD, donation after cardiac death; ECD, expanded criteria donor.
Use of ESW
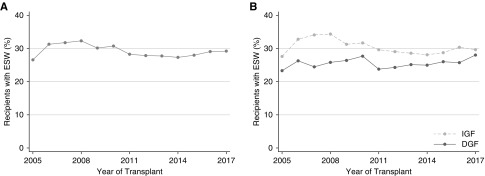
Overall 29.2% of the recipients underwent ESW. When stratified by DGF status, 25.7% of the recipients with DGF and 30.4% of the recipients with IGF underwent ESW. These proportions, overall (Figure 1A) as well as stratified by DGF status (Figure 1B), remained relatively stable over the study period. After adjusting for clinical factors and the variation among transplant centers, DGF was associated with significantly lower odds of ESW use (adjusted odds ratio [aOR]=0.600.670.75).
Figure 1.
Stable temporal trends in the use of ESW. (A) Entire population. (B) IGF versus DGF. After adjusting for recipient, donor, and transplant factors, DGF was associated with significantly lower odds of using ESW (aOR=0.600.670.75).
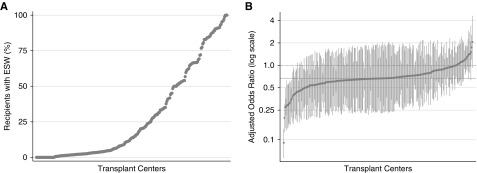
We found a substantial center-level variation in ESW use. First, the overall tendency to use ESW varied among centers (Figure 2A). For example, among 261 centers included in this analysis, 102 (39.1%) centers used ESW in <5% of their recipients, whereas 36 (13.8%) used ESW in >75%. Second, the association between DGF and ESW use also varied among centers (Figure 2B). After adjusting for clinical characteristics, recipients with DGF had half the odds of ESW compared with recipients with IGF (aOR<0.5) at 31 (11.9%) centers, but had higher odds of ESW compared with recipients with IGF (aOR>1.0) at 22 (8.4%) centers. Finally, the overall tendency to use ESW (the random intercept term) was not correlated (ρ=0.07) with the association between DGF and ESW use (the random slope term): in other words, how often a center uses ESW did not seem to be correlated with how strongly DGF was associated with ESW use at the center.
Figure 2.
Large variation in the use of ESW across transplant centers. (A) Center-specific prevalence of ESW use. (B) Center-specific aORs for ESW use in recipients with DGF, compared with those with IGF. Each marker indicates a single transplant center. Markers are sorted along the x axis. (A) The y axis indicates the proportion of the recipients with ESW at the corresponding center. (B) The y axis indicates the adjusted odds ratio for ESW use in recipients with DGF compared with those with IGF at the corresponding center. The solid horizontal line indicates the adjusted odds ratio over the entire population (aOR=0.67). The dashed horizontal line indicates no association (aOR=1.00).
Acute Rejection, Graft Failure, and Patient Death
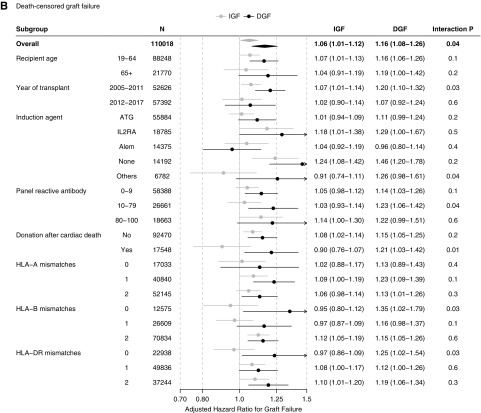
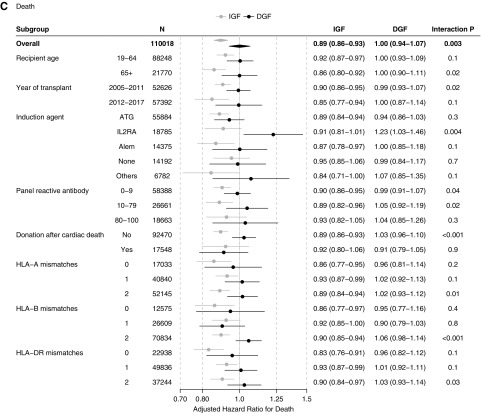
The association between ESW and transplant outcomes was modified by DGF status. Among recipients with IGF, ESW was significantly associated with increased odds of acute rejection (aOR=1.091.161.23), a slightly increased hazard of graft failure (adjusted hazard ratio [aHR]=1.011.061.12), and a decreased hazard of death (aHR=0.860.890.93). In contrast, there was no beneficial association between ESW and KT outcomes among recipients with DGF. ESW was associated with increased odds of acute rejection (aOR=1.021.121.23), an increased hazard of graft failure (aHR=1.081.161.26), and no difference in the hazard of death (aHR=0.941.001.07) in this subgroup (Table 2) (Supplemental Figure 1).
Table 2.
Association between ESW and KT outcomes in recipients with IGF and with DGF
| Outcomes (ESW versus CSM) | IGF (n=82,411) | DGF (n=27,608) | Interaction P Value |
|---|---|---|---|
| Acute rejection (1-yr) | 1.091.161.23 | 1.021.121.23 | 0.6 |
| Death-censored graft failure | 1.011.061.12 | 1.081.161.26 | 0.04 |
| Death (all cause) | 0.860.890.93 | 0.941.001.07 | 0.003 |
| Death (cause specific) | |||
| Cardiovascular disease | 0.740.820.92 | 0.921.081.26 | 0.004 |
| Infection | 0.650.750.86 | 0.670.821.00 | 0.5 |
| Malignancy | 0.860.991.13 | 0.771.011.31 | 0.9 |
| Others | 0.921.011.11 | 0.911.061.23 | 0.5 |
Acute rejection was treated as a binary variable at 1 yr post-KT. Other outcomes were treated as time-to-event variables. The median length of follow-up was 4.2 yr for graft failure and 4.7 for death. Estimates are aORs for acute rejection and aHRs for other outcomes, with 95% CIs in subscripts. A low (<0.05) interaction P value indicates the association between ESW and the transplant outcome was significantly different between the IGF and DGF populations.
Although no interaction was found in the acute rejection analysis (P=0.6), there was statistically significant interactions between ESW and DGF in the graft failure (P=0.04) and death (P=0.003) analyses. In other words, the association of ESW with acute rejection did not differ by DGF status, whereas those with graft failure and with death were more adverse among recipients with DGF than they were among those with IGF.
Cause-Specific Mortality
In our analyses on cause-specific mortality, DGF modified the association of ESW with cardiovascular disease–related death, but not those with infection- or malignancy-related death. Among the 20,257 deaths included in our study, 3003 (14.8%) were related to cardiovascular disease, 1970 (9.7%) were related to infection, 1508 (7.4%) were related to malignancy, and 3647 (18.0%) were due to other causes. The remaining 10,129 (50.0%) did not have cause of death data in our records. DGF modified the association between ESW and cardiovascular disease–related death, with a decreased hazard among recipients with IGF (aHR=0.740.820.92) but not among those with DGF (aHR=0.921.081.26; interaction P=0.004). However, the association between ESW and death due to infection, malignancy, or other causes did not vary significantly between the IGF and DGF recipients (interaction P=0.5, 0.9, and 0.5, respectively) (Table 2).
Stratified Analyses
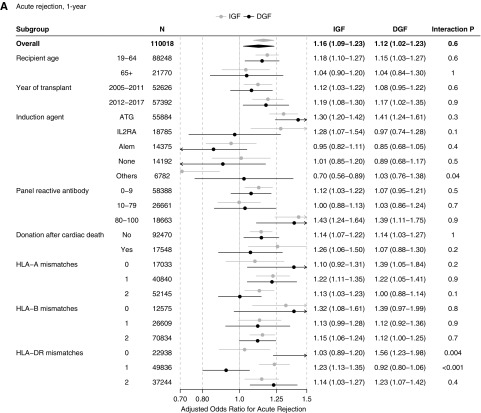
When stratified by recipient age, we observed the ESW-DGF interaction in the younger (19–64 years; n=88,248) as well as in the older (≥65 years; n=21,770) recipients. In both subgroups, the association of ESW with increased acute rejection did not vary by DGF status, that with increased graft failure was more pronounced among recipients with DGF, and that with decreased death was only observed among recipients with IGF (Figure 3). Likewise, the ESW-DGF interaction was similar across the calendar year strata (2005–2011 versus 2012–2017) (Figure 3).
Figure 3.
DGF-ESW interaction was generally homogeneous across subgroups and across KT outcomes. (A) Acute rejection, 1-year. (B) Death-censored graft failure. (C) Death. Estimates are aORs for acute rejection and aHRs for other outcomes, followed by 95% CIs. A low (<0.05) interaction P value indicates the association between ESW and the transplant outcome was significantly different between the IGF and DGF populations. Alem, alemtuzumab; ATG, anti-thymocyte globulin; IL2RA, IL-2 receptor antagonists.
Overall 55,884 (50.8%) recipients received anti-thymocyte globulin, 18,785 (17.1%) received IL-2 receptor antagonists, 14,375 (13.1%) received alemtuzumab, 14,192 (12.9%) recipients received no induction agent, and 6782 (6.2%) received others. Our findings on the DGF-ESW interaction were generally consistent over induction agent strata (Figure 3). However, the ESW-DGF interaction was slightly different among those who received IL-2 receptor antagonists for induction. In this subpopulation, the ESW-DGF interaction on death was more pronounced (aHR=0.810.911.01 in IGF versus 1.031.231.46 in DGF; interaction P=0.004) and the interaction on acute rejection, although not statistically significant, appeared to be in the opposite direction (aOR=1.071.281.54 in IGF versus 0.740.971.28 in DGF; interaction P=0.1).
When stratified by PRA, donation after cardiac death, and HLA mismatches, the DGF-ESW interactions were overall consistent over the strata (Figure 3). Nonetheless, the DGF-ESW interaction on acute rejection was slightly more pronounced among recipients with zero HLA-DR mismatch (aOR=0.891.031.20 in IGF versus 1.231.561.98 in DGF; interaction P=0.004) (Figure 3A). Similarly, the DGF-ESW interaction on graft failure was also slightly more pronounced among those with zero HLA-B mismatch (aHR=0.800.951.12 in IGF versus 1.021.351.79 in DGF; interaction P=0.03), and among those with zero HLA-DR mismatch (aHR=0.860.971.09 in IGF versus 1.021.251.54; interaction P=0.03) (Figure 3B).
Discussion
In this nationwide registry analysis, we found that the associations between ESW and KT outcomes vary by DGF status. Among recipients with IGF, ESW was associated with a 6% increase in the hazard of graft failure, offset by an 11% decrease in the hazard of death. However, among recipients with DGF, ESW was associated with a 16% increase in the hazard of graft failure and no difference in the hazard of death. These interactions between ESW and DGF were statistically significant. Our findings suggest DGF alters the risk/benefit equation of ESW, such that ESW might possibly confer a mixture of beneficial and harmful effects among recipients of KT with IGF, but overall harmful effects among those with DGF.
Practice patterns regarding ESW among recipients with DGF were substantially different among transplant centers. Although DGF was associated with lower odds of ESW use (aOR=0.600.670.75) over the entire study population, the strength of this association varied among transplant centers. At some centers, recipients with DGF were at lower than half the odds of undergoing ESW compared with recipients with IGF, whereas recipients at other centers were treated similarly regardless of their DGF status. This variation underscores the lack of consensus in whether DGF should be regarded as a contraindication to ESW. Resembling the inconsistent practice pattern observed in our study, previous clinical trials of ESW have used mixed approaches on DGF. Some investigators viewed DGF as a contraindication for ESW. Woodle et al.2 excluded recipients with DGF at enrollment, stating it is a known risk factor for acute rejection. The FREEDOM trial3 also excluded those with DGF from their per-protocol analyses. In contrast, other trials did not restrict the study participants by their DGF status. The ATLAS trial4 and a trial by Laftavi et al.5 included those with DGF, who constituted about 30% of their study populations. Montagnino et al.6 included those with DGF, using a more flexible protocol in which the withdrawal of steroid could be delayed (beyond 7 days post-KT) or cancelled based on the rate of graft function recovery. These variations, found both in practice and research, demonstrates the knowledge gap in the interaction between DGF and ESW.
The effect modification observed in our study supports that DGF could be a reason to consider CSM over ESW for initial maintenance immunosuppression. ESW has been deemed viable because previous studies showed only marginal increases in the risk of graft failure, accompanied by decreases in adverse events.3,4,11–13,31–33 However, we found the increase in the hazard of graft failure associated with ESW was more pronounced (16% versus 6%) among recipients with DGF than among those with IGF, and that the decrease in the hazard of death associated with ESW was observed only among recipients with IGF. Although the choice of ESW versus CSM should be individualized according to the recipient’s overall risk profile rather than a single risk factor,15,34,35 DGF appears to be an element of the risk profile that suggests CSM over ESW. In addition, DGF is unique in that it is a post-KT outcome that can still inform the initial maintenance immunosuppression strategy. The withdrawal of steroid can be postponed or cancelled according to the rate of graft function recovery. A similar protocol was used by Montagnino et al.6
Although the exact mechanism through which DGF modifies the association between ESW and KT outcomes remains unclear, the findings from our cause-specific mortality analyses and stratified analyses provide some implications. First, the effect modification of DGF on the association between ESW and patient death seems to have been primarily driven by death due to cardiovascular disease. ESW was associated with a decreased hazard of cardiovascular disease–related death among those with IGF but not among those with DGF. In contrast, the association of ESW with death due to infection, malignancy, or other causes did not significantly vary by DGF status. However, this finding should be interpreted with caution given that cause of death was unavailable in our data set for 50% of the deaths. Second, our various stratified analyses mostly showed a consistent DGF-ESW interaction across the strata. For instance, despite being a possible risk factor for DGF36 and arguably an indication for ESW,37 older recipient age does not seem to influence the DGF-ESW interaction observed in our study. On the other hand, the DGF-ESW interaction appeared slightly heterogeneous when stratified by induction agent and by the number of HLA mismatches.
Our study has several limitations. First, the findings from this observational study do not directly signify causal effects. Our findings are hypothesis generating but not confirmatory because they are at risk of selection bias, confounding by indication, and unmeasured confounders. We attempted to minimize this limitation by using IPTW and controlling for an extensive set of potential confounders. Second, we treated acute rejection as a binary outcome and thus were not able to capture any differences in timing, severity, or recurrences of acute rejection. Additionally, we did not have data on other relevant clinical information, such as steroid dose or the duration or severity of DGF. Lastly, our study population was restricted to those who received tacrolimus and mycophenolate. Our finding might not be generalizable to those who receive other agents for maintenance immunosuppression.
In conclusion, ESW was associated with a minimal increase in graft failure and a decrease in mortality among recipients of KT with IGF, but with a more pronounced increase in graft failure and no difference in mortality among those with DGF. KT centers appear to have inconsistent practices regarding the use of ESW in recipients with DGF. Our findings suggest that DGF is a reason to consider CSM over ESW for initial maintenance immunosuppression after KT.
Disclosures
Dr. McAdams-DeMarco reports grants from the National Institutes of Health (NIH), during the conduct of the study. Dr. Coresh reports grants from the NIH and grants from the National Kidney Foundation, during the conduct of the study. Dr. Segev reports honoraria from Sanofi, honoraria from Novartis, and honoraria from CSL Behring, outside the submitted work.
Funding
This work was supported by the American Society of Nephrology and Mogam Science Scholarship Foundation (Dr. Bae), and the National Institute of Diabetes and Digestive and Kidney Diseases (K23DK115908 to Dr. Garonzik Wang, F32DK113719 to Dr. Jackson, R01DK120518 to Dr. McAdams-DeMarco, R01DK102981 to Dr. Brennan, Dr. Lentine, and Dr. Segev, and K24DK101828 to Dr. Segev).
Supplementary Material
Acknowledgments
The analyses described here are the responsibility of the authors alone and do not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government. The data reported here have been supplied by the Minneapolis Medical Research Foundation as the contractor for the SRTR. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the US Government.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “When Less Becomes More: Life and Losses without the ‘Roids’?” on pages 6–8.
Supplemental material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019040416/-/DCSupplemental.
Supplemental Figure 1. Kaplan-Meier cumulative incidence plot for death-censored graft failure and death.
Supplemental Table 1. Coefficients from the inverse probability of treatment weights model.
References
- 1.Hart A, Smith JM, Skeans MA, Gustafson SK, Wilk AR, Robinson A, et al.: OPTN/SRTR 2016 annual data report: Kidney. Am J Transplant 18[Suppl 1]: 18–113, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woodle ES, First MR, Pirsch J, Shihab F, Gaber AO, Van Veldhuisen P; Astellas Corticosteroid Withdrawal Study Group : A prospective, randomized, double-blind, placebo-controlled multicenter trial comparing early (7 day) corticosteroid cessation versus long-term, low-dose corticosteroid therapy. Ann Surg 248: 564–577, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Vincenti F, Schena FP, Paraskevas S, Hauser IA, Walker RG, Grinyo J; FREEDOM Study Group : A randomized, multicenter study of steroid avoidance, early steroid withdrawal or standard steroid therapy in kidney transplant recipients. Am J Transplant 8: 307–316, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Vítko S, Klinger M, Salmela K, Wlodarczyk Z, Tydèn G, Senatorski G, et al.: Two corticosteroid-free regimens-tacrolimus monotherapy after basiliximab administration and tacrolimus/mycophenolate mofetil-in comparison with a standard triple regimen in renal transplantation: Results of the Atlas study. Transplantation 80: 1734–1741, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Laftavi MR, Stephan R, Stefanick B, Kohli R, Dagher F, Applegate M, et al.: Randomized prospective trial of early steroid withdrawal compared with low-dose steroids in renal transplant recipients using serial protocol biopsies to assess efficacy and safety. Surgery 137: 364–371, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Montagnino G, Sandrini S, Iorio B, Schena FP, Carmellini M, Rigotti P, et al.: A randomized exploratory trial of steroid avoidance in renal transplant patients treated with everolimus and low-dose cyclosporine. Nephrol Dial Transplant 23: 707–714, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Rostaing L, Cantarovich D, Mourad G, Budde K, Rigotti P, Mariat C, et al. CARMEN Study Group : Corticosteroid-free immunosuppression with tacrolimus, mycophenolate mofetil, and daclizumab induction in renal transplantation. Transplantation 79: 807–814, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Cantarovich D, Rostaing L, Kamar N, Saint-Hillier Y, Ducloux D, Mourad G, et al. FRANCIA Study Trial Investigators Group : Corticosteroid avoidance in adult kidney transplant recipients under rabbit anti-T-lymphocyte globulin, mycophenolate mofetil and delayed cyclosporine microemulsion introduction. Transpl Int 23: 313–324, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Matas AJ, Kandaswamy R, Gillingham KJ, McHugh L, Ibrahim H, Kasiske B, et al.: Prednisone-free maintenance immunosuppression-a 5-year experience. Am J Transplant 5: 2473–2478, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Rizzari MD, Suszynski TM, Gillingham KJ, Dunn TB, Ibrahim HN, Payne WD, et al.: Ten-year outcome after rapid discontinuation of prednisone in adult primary kidney transplantation. Clin J Am Soc Nephrol 7: 494–503, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knight SR, Morris PJ: Steroid avoidance or withdrawal after renal transplantation increases the risk of acute rejection but decreases cardiovascular risk. A meta-analysis. Transplantation 89: 1–14, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Rike AH, Mogilishetty G, Alloway RR, Succop P, Roy-Chaudhury P, Cardi M, et al.: Cardiovascular risk, cardiovascular events, and metabolic syndrome in renal transplantation: Comparison of early steroid withdrawal and chronic steroids. Clin Transplant 22: 229–235, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Nikkel LE, Mohan S, Zhang A, McMahon DJ, Boutroy S, Dube G, et al.: Reduced fracture risk with early corticosteroid withdrawal after kidney transplant. Am J Transplant 12: 649–659, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dharnidharka VR, Schnitzler MA, Chen J, Brennan DC, Axelrod D, Segev DL, et al.: Differential risks for adverse outcomes 3 years after kidney transplantation based on initial immunosuppression regimen: A national study. Transpl Int 29: 1226–1236, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sprangers B, Kuypers DR, Vanrenterghem Y: Immunosuppression: Does one regimen fit all? Transplantation 92: 251–261, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Siedlecki A, Irish W, Brennan DC: Delayed graft function in the kidney transplant. Am J Transplant 11: 2279–2296, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu WK, Famure O, Li Y, Kim SJ: Delayed graft function and the risk of acute rejection in the modern era of kidney transplantation. Kidney Int 88: 851–858, 2015 [DOI] [PubMed] [Google Scholar]
- 18.Yarlagadda SG, Coca SG, Formica RN Jr., Poggio ED, Parikh CR: Association between delayed graft function and allograft and patient survival: A systematic review and meta-analysis. Nephrol Dial Transplant 24: 1039–1047, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Tapiawala SN, Tinckam KJ, Cardella CJ, Schiff J, Cattran DC, Cole EH, et al.: Delayed graft function and the risk for death with a functioning graft. J Am Soc Nephrol 21: 153–161, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perico N, Cattaneo D, Sayegh MH, Remuzzi G: Delayed graft function in kidney transplantation. Lancet 364: 1814–1827, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Schröppel B, Legendre C: Delayed kidney graft function: From mechanism to translation. Kidney Int 86: 251–258, 2014 [DOI] [PubMed] [Google Scholar]
- 22.Irish WD, Ilsley JN, Schnitzler MA, Feng S, Brennan DC: A risk prediction model for delayed graft function in the current era of deceased donor renal transplantation. Am J Transplant 10: 2279–2286, 2010 [DOI] [PubMed] [Google Scholar]
- 23.United Network for Organ Sharing : Adult kidney transplant recipient registration worksheet. Available at: https://unos.org/wp-content/uploads/unos/Adult_TRR_Kidney.pdf. Accessed September 25, 2019
- 24.Gilks WR, Clayton DG, Spiegelhalter DJ, Best NG, McNeil AJ, Sharples LD, et al. : Modelling complexity: Applications of Gibbs sampling in medicine. J R Stat Soc B 55: 39–52, 1993 [Google Scholar]
- 25.Hernán MA, Alonso A, Logan R, Grodstein F, Michels KB, Willett WC, et al.: Observational studies analyzed like randomized experiments: An application to postmenopausal hormone therapy and coronary heart disease. Epidemiology 19: 766–779, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lentine KL, Gheorghian A, Axelrod D, Kalsekar A, L’italien G, Schnitzler MA: The implications of acute rejection for allograft survival in contemporary U.S. kidney transplantation. Transplantation 94: 369–376, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Massie AB, Kucirka LM, Segev DL: Big data in organ transplantation: Registries and administrative claims [published correction appears in Am J Transplant 14: 2673, 2014]. Am J Transplant 14: 1723–1730, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lau B, Cole SR, Gange SJ: Competing risk regression models for epidemiologic data. Am J Epidemiol 170: 244–256, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ravindra KV, Sanoff S, Vikraman D, Zaaroura A, Nanavati A, Sudan D, et al.: Lymphocyte depletion and risk of acute rejection in renal transplant recipients at increased risk for delayed graft function. Am J Transplant 19: 781–789, 2019 [DOI] [PubMed] [Google Scholar]
- 30.Louis TA, Zeger SL: Effective communication of standard errors and confidence intervals. Biostatistics 10: 1–2, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pascual J, Royuela A, Galeano C, Crespo M, Zamora J: Very early steroid withdrawal or complete avoidance for kidney transplant recipients: A systematic review. Nephrol Dial Transplant 27: 825–832, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Haller MC, Royuela A, Nagler EV, Pascual J, Webster AC: Steroid avoidance or withdrawal for kidney transplant recipients. Cochrane Database Syst Rev CD005632, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cantarovich D, Rostaing L, Kamar N, Ducloux D, Saint-Hillier Y, Mourad G, et al. FRANCIA Study Trial Investigators Group : Early corticosteroid avoidance in kidney transplant recipients receiving ATG-F induction: 5-year actual results of a prospective and randomized study. Am J Transplant 14: 2556–2564, 2014 [DOI] [PubMed] [Google Scholar]
- 34.Kidney disease: Improving Global Outcomes (KDIGO) transplant work Group : KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 9: S1–S155, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Wavamunno MD, Chapman JR: Individualization of immunosuppression: Concepts and rationale. Curr Opin Organ Transplant 13: 604–608, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Giral M, Bertola JP, Foucher Y, Villers D, Bironneau E, Blanloeil Y, et al.: Effect of brain-dead donor resuscitation on delayed graft function: Results of a monocentric analysis. Transplantation 83: 1174–1181, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Krenzien F, ElKhal A, Quante M, Rodriguez Cetina Biefer H, Hirofumi U, Gabardi S, et al.: A rationale for age-adapted immunosuppression in organ transplantation. Transplantation 99: 2258–2268, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.