Significance Statement
Kidney transplant recipients must take immunosuppressive medications to prevent rejection of their transplant kidney. Coverage of immunosuppressive drugs under Medicare’s ESKD program ends 36 months after transplantation, putting patients at risk for premature transplant failure. The authors analyzed the cost and benefits of extending Medicare immunosuppressive drug coverage for the entire duration of survival after transplantation using current generic immunosuppressive drug costs and estimates of increased transplant survival. From the Medicare payer perspective, extending immunosuppression drug coverage was cost-saving and led to better patient outcomes compared with the current policy. The findings may be useful in advancing legislative efforts to ensure kidney transplant recipients have access to essential life-saving immunosuppressive medications.
Keywords: kidney transplantation, Economic Analysis, transplant outcomes, Medicare
Abstract
Background
Kidney transplant recipients must take immunosuppressant drugs to prevent rejection and maintain transplant function. Medicare coverage of immunosuppressant drugs for kidney transplant recipients ceases 36 months after transplantation, potentially increasing the risk of transplant failure. A contemporary economic analysis of extending Medicare coverage for the duration of transplant survival using current costs of immunosuppressant medications in the era of generic equivalents may inform immunosuppressant drug policy.
Methods
A Markov model was used to determine the incremental cost and effectiveness of extending Medicare coverage for immunosuppressive drugs over the duration of transplant survival, compared with the current policy of 36-month coverage, from the perspective of the Medicare payer. The expected improvement in transplant survival by extending immunosuppressive drug coverage was estimated from a cohort of privately insured transplant recipients who receive lifelong immunosuppressant drug coverage compared with a cohort of Medicare-insured transplant recipients, using multivariable survival analysis.
Results
Extension of immunosuppression Medicare coverage for kidney transplant recipients led to lower costs of −$3077 and 0.37 additional quality-adjusted life years (QALYs) per patient. When the improvement in transplant survival associated with extending immunosuppressant coverage was reduced to 50% of that observed in privately insured patients, the strategy of extending drug coverage had an incremental cost–utility ratio of $51,694 per QALY gained. In a threshold analysis, the extension of immunosuppression coverage was cost-effective at a willingness-to-pay threshold of $100,000, $50,000, and $0 per QALY if it results in a decrease in risk of transplant failure of 5.5%, 7.8%, and 13.3%, respectively.
Conclusions
Extending immunosuppressive drug coverage under Medicare from the current 36 months to the duration of transplant survival will result in better patient outcomes and cost-savings, and remains cost-effective if only a fraction of anticipated benefit is realized.
Kidney transplantation is the preferred treatment for patients with ESKD as it is associated with longer survival and better quality of life compared with treatment with dialysis.1–4 Transplantation is also associated with lower health care costs than dialysis; the annual expenditure per patient by Medicare in 2016 for a patient treated with transplantation was $34,780 compared with $90,971 for a patient treated with hemodialysis.5
Maintenance of transplant function requires lifelong use of immunosuppressant medications. Since 1972, Medicare has provided coverage for the treatment of patients with kidney failure with dialysis, regardless of their age or disability status. In 2017, 58% of patients who underwent a kidney transplant relied on Medicare as a primary insurance provider.6 Medicare coverage for immunosuppressant medications is provided under Part B, and requires payment of an income-based monthly premium, annual deductible, and up to a 20% patient copayment. Although there is no time limit for Medicare coverage of patients on dialysis, kidney transplant recipients who are not otherwise eligible for Medicare because of age >65 years or medical disability lose their Medicare coverage 36 months after the date of transplantation. Transplant recipients who cannot afford to pay for their immunosuppressant medications after cessation of Medicare coverage are at significant risk for medication nonadherence, rejection, and premature failure of their kidney transplant.7–9
The current Medicare policy was implemented anticipating that most kidney transplant recipients would be able to obtain full-time employment and attendant health care coverage, as they would no longer be dialysis dependent. However, among persons of working age only 43%–56% of transplant recipients in the United States are employed.10,11 In part, this low rate of employment may be related to the fact that patients with other significant health burdens are treated with transplantation, and that transplantation alone may not restore their health status such that they can engage in work. However, fear of losing drug coverage and being unable to pay for immunosuppressive medications is commonly cited as a reason for patients to remain “disabled” and not return to work to enable continued coverage10,11; as such, the current policy may be a disincentive to those who could engage in the workforce. Although the costs of immunosuppressive medications have decreased over the past decade through market competition from generic immunosuppressive drugs, these costs remain relatively high; the mean annual cost for mycophenolate and tacrolimus in 2014 was $2064 and $2642, respectively.12 These costs comprise >20% of the annual income of patients in the lower 20th percentile of household income in the United States.13
The Comprehensive Immunosuppressive Drug Coverage for Kidney Patients Act (better known as “the immunosuppressive bill”) was proposed a decade ago to address this deficiency in ESKD Medicare coverage.14–17 This bill would allow Medicare-eligible kidney transplant recipients to receive life-saving immunosuppressant medications for as long as their transplant continues to function. Although the bill has received strong bipartisan support, it has not been enacted. A thorough understanding of health and budgetary consequences is a prerequisite for any proposed bill to be enacted into law. Existing published economic evaluations of extending immunosuppressive drug coverage for kidney transplant recipients are outdated and do not incorporate the lower contemporary cost of generic immunosuppressant drugs.18–20 We conducted an economic evaluation to provide contemporary evidence of the long-term costs and benefits of extending immunosuppression coverage from the current 36 months to the duration of transplant kidney survival.
Methods
Study Design
In this cost-effectiveness analysis, we used a Markov model to simulate a fixed cohort of Medicare-insured adult kidney transplant recipients aged 18–62 years who survived with a functioning transplant for at least 36 months. The upper limit for age of 62 years was selected as patients over the age of 62 years at the time of transplantation will already be eligible for full Medicare coverage as they will be 65 years of age when their transplant survives to 36 months. The primary model compares the strategy of extending immunosuppression coverage for the lifetime of the kidney transplant to the current policy of discontinuing immunosuppression coverage at 36 months after transplantation from the perspective of Medicare as a health care payer. The primary study outcome is the incremental cost–utility ratio (ICUR) in dollars per quality-adjusted life year (QALY). Discounting of 3% was applied to both cost and utility as per current recommendations.21 A lifetime time horizon was set with a 3-month cycle length and half-cycle correction. The Markov model was created using TreeAge Pro 2018.22
Health States and Outcomes
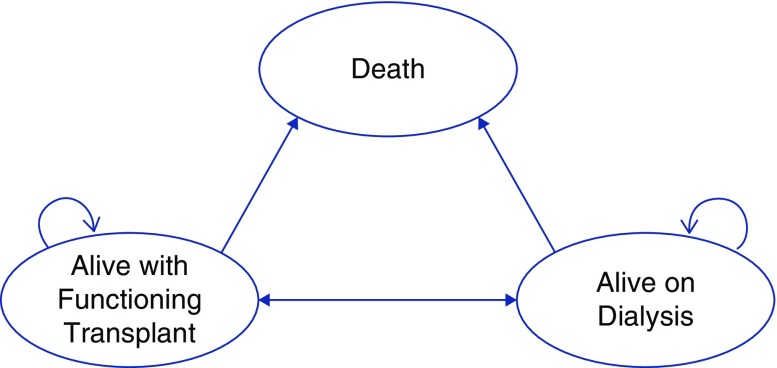
Possible patient health states in the model include alive with a functioning kidney transplant, alive with a failed transplant on dialysis, and death. The potential transitions between theses health states are depicted in Figure 1. Patients who have transplant failure may be retransplanted in the model. Patients in the model could transition to death from any health state.
Figure 1.
Health states and transitions in the Markov model.
Probability Estimates
To estimate the potential benefit of extended immunosuppressant drug coverage on transplant survival in Medicare-insured patients, we assumed that the extended immunosuppressant coverage would result in a risk of death-censored transplant failure similar to transplant recipients with private insurance coverage who receive immunosuppressant drug coverage for the entire duration of transplant survival.18,23
We conducted a survival analysis all patients aged 18–62 years who underwent a kidney only transplant between January 1, 2000 and October 1, 2016 recorded in the US Renal Data System (USRDS). The cohort was stratified by insurance payer status (private versus Medicare insurance). Patients were excluded if they received or were waitlisted for a nonkidney transplant, if they had no documented Medicare or private insurance, or if they did not survive with transplant function to 3 years post-transplant when their Medicare coverage on the basis of ESKD would expire. In addition to the outcomes of transplant failure (defined by the need to resume maintenance dialysis treatment or repeat transplantation), death with transplant function, repeat transplantation after transplant failure, and death after transplant failure, we included data on a priori selected confounders, including age, sex, cause of ESKD (diabetes, or nondiabetic kidney disease), donor type (deceased or living donor), history of prior kidney transplant (yes/no), and pre-emptive kidney transplant versus nonpre-emptive kidney transplant. The characteristics of the final cohort were described using means with SD and proportions as appropriate, stratified by payer status. Missing data were imputed using multiple imputation with chained equations. A multivariable survival analysis using a multistate survival model with Weibull distributions was used to generate adjusted survival curves of the primary outcomes of transplant failure and death, with function adjusting for age, sex, cause of ESKD, history of prior kidney transplantation, and pre-emptive transplantation. Similarly, a multistate survival model was used to generate adjusted survival curves for the outcomes of repeat transplantation after transplant failure, and death after transplant failure. These survival curves assumed a cohort with the mean characteristics of Medicare-insured patients. These adjusted survival curves directly informed the transition probabilities used in the final Markov model. This survival analysis was conducted using R software with the “mstate” package.24,25
Cost and Utility Estimates
It was assumed that all patients would receive standard immunosuppressant drugs, including mycophenolate mofetil and tacrolimus, and the annual costs were derived from a recent publication on the cost of immunosuppression to Medicare in the current era, accounting for market competition from generic immunosuppression.12 In the Medicare extension group, the costs of these medications were counted toward expenses which Medicare would pay for, whereas in the group with no extension of Medicare, these costs were not included. If a patient’s age increased to >65 years with a functioning transplant, or experienced transplant failure, we assumed these patients would be eligible for full Medicare Part A and Part B coverage. The costs to Medicare for full Part A and B coverage with a functioning transplant, death with transplant function, and transplant failure was derived from the Organ Procurement and Transplant Network Economic Report,26 and the costs associated with dialysis and death on dialysis were extracted from the USRDS Annual Data Report.5 The proportion of patients within the cohort that would reach an age >65 years is shown in Supplemental Figure 1. All cost estimates were adjusted to 2018 US dollars using the consumer price index. The utility estimates for patients on dialysis and with a functioning transplant in QALYs were extracted from the literature.27,28
Model Assumptions
A key assumption is that extension of Medicare coverage for immunosuppressive drugs beyond 3 years post-transplant will result in an improvement in death-censored transplant survival for the Medicare-insured cohort. The magnitude of improvement in death-censored transplant survival that would occur with extension of Medicare coverage is unknown. This model assumes transplant survival would be similar to that of patients with private insurance. We also assumed that Medicare coverage for immunosuppressant drugs would not improve rates of death with transplant function or death after transplant failure on dialysis.23 This model assumes that Medicare would continue with an 80% copayment system, and that Medicare would not be responsible for other health care costs after kidney transplantation. It is assumed that the cost of dialysis therapy after transplant failure is the same as it is for patients who have never had a kidney transplant. Furthermore, the model assumes the ratio of treatment with hemodialysis and peritoneal after transplant failure is the same as in patients who have never had a kidney transplant in the United States.5
Sensitivity Analyses
To ensure the robustness of this model we performed several sensitivity analyses. To explore the uncertainty in the improvement in death-censored transplant survival with extension of immunosuppressant drug coverage alternative scenarios where extension of immunosuppressive drug coverage resulted in only 75% and 50% of the observed benefit. Subsequently, we performed a threshold analysis to establish how much improvement in death-censored transplant survival would be required for extended immunosuppression coverage to be cost-effective at a willingness-to-pay threshold of $100,000, $50,000, and $0 per QALY gained. In this analysis, we varied the improvement in death-censored transplant survival until the ICUR from the model was equal to these willingness-to-pay thresholds. Additionally, we performed univariate sensitivity analyses on all our cost and utility inputs to explore the effect of uncertainty within individual parameters on the ICUR estimate.Given that the fear of losing drug coverage is a common reason for some patients to remain unemployed owing to medical disability, we performed a sensitivity analysis where extension of Medicare immunosuppression reduced the number of medically disabled patients that would require full Part A and B coverage. In this analysis, we assumed that 45% of Medicare-insured individuals are designated as medically disabled after kidney transplant.10 We then evaluated scenarios where up to 10% of these individuals no longer depended on disability status for insurance coverage.
Finally, we conducted a probabilistic analysis using probability distributions to replace the estimates for the cost and utility parameters included in the model. In this analysis, the uncertainty of each model parameter is represented by a probability distribution (Table 1). We then analyzed the model repeatedly with various combinations of parameter estimates drawn randomly and independently from each of the probability distributions. For this probabilistic analysis, we conducted 10,000 individual analyses. The results of these analyses are presented in a cost-effectiveness acceptability curve, with the thresholds of willingness-to-pay varying to include a range of potentially acceptable willingness-to-pay thresholds.21,29
Table 1.
Cost and utility parameter inputs for the Markov model
| Input Variable | Estimate | Plausible Range | Distribution | Distribution Parameters | Reference |
|---|---|---|---|---|---|
| Cost | |||||
| Annual cost of tacrolimus | 2828 | (2750–2906) | Normal | Mean: 2828 SEM: 40 | 12 |
| Annual cost of mycophenolate | 2225 | (2141–2309) | Normal | Mean: 2225 SEM: 43 | 12 |
| Annual cost of dialysis therapy | 89,092 | (80,361–97,823) | Normal | Mean: 89,092 SEM: 4455 | 5 |
| Cost to Medicare in 1 yr for patient alive with transplant function after a yr with functiona | 23,253 | (20,974–25,532) | Normal | Mean: 23,253 SEM: 1163 | 26 |
| Cost to Medicare for the first yr after retransplantation | 76,094 | (68,637–83,551) | Normal | Mean: 76,094 SEM: 3805 | 26 |
| Cost to Medicare for organ acquisition for repeat transplantation | 64,713 | (45,113–84,813) | Normal | Mean: 64,713 SEM: 10,000 | 36 |
| Cost to Medicare in 1 yr for patient who dies with functiona | 127,048 | (123,525–150,367) | Normal | Mean: 136,946 SEM: 6847 | 26 |
| Cost to Medicare in 1 yr for patient who has transplant failurea | 90,560 | (81,685–99,435) | Normal | Mean: 90,560 SEM: 4528 | 26 |
| Cost of death while on dialysis | 30,531 | (27,539–33,523) | Normal | Mean: 30,531 SEM: 1527 | 5 |
| Utility | |||||
| Alive with a functioning transplant | 0.82 | (0.74–0.90) | β | α=74.8 β=16.4 | 27 |
| Alive with a failed transplant | 0.67 | (0.64–0.71) | β | α=369.7 β=182.1 | 28 |
| Death | 0 | — | — | — | — |
The estimate was used as the baseline in the deterministic model. The plausible range was used for the univariate sensitivity analyses and the distribution and distribution parameters were used for the probabilistic analyses.
These costs were only applied to the model for patients age >65 years. All costs are reported in 2018 US dollars.
Results
Survival Analysis
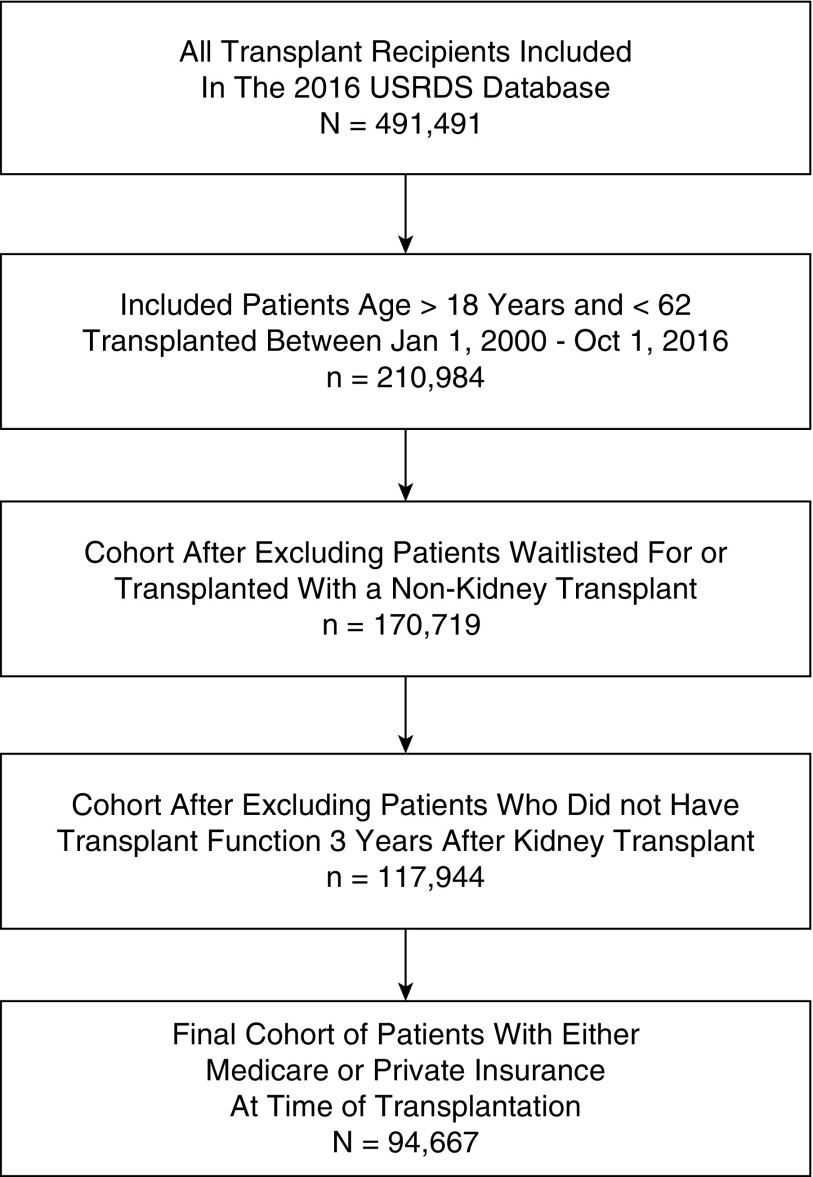
The survival analysis to inform the transition probabilities included n=94,667 patients reported in the 2016 USRDS database that met the study inclusion criteria (Figure 2).
Figure 2.
Cohort used in survival analysis to establish transition probabilities. The final analytic cohort included 94,667 patients.
In this cohort, n=65,811 patients had Medicare as a primary payer, whereas 28,856 patients had a private primary payer (Table 2). Patients with Medicare as a primary payer were more likely to be men, black, have diabetes or hypertension as the cause of kidney disease, or have a history of a previous kidney transplant. Patients with Medicare as the primary payer were less likely to have a living donor transplant or a pre-emptive kidney transplant.
Table 2.
Characteristics of cohort from the 2016 USRDS database used in final analysis stratified by insurance coverage at the time of kidney transplantation
| Variable | Private Insurance, n=28,856 | Medicare Insurance, n=65,811 | P Value |
|---|---|---|---|
| Age at the time of transplant, mean (SD) | 46.25 (10.7) | 45.18 (10.9) | <0.001 |
| Men, n (%) | 17,153 (59.4) | 39,539 (60.1) | 0.07 |
| Race, n (%) | <0.001 | ||
| Black | 4741 (16.4) | 21,799 (33.2) | |
| Other | 1650 (5.7) | 5496 (8.4) | |
| White | 22,441 (77.8) | 38,429 (58.5) | |
| Cause of ESKD, n (%) | <0.001 | ||
| Cystic kidney | 4785 (16.6) | 5109 (7.8) | |
| Diabetes | 5375 (18.6) | 13,965 (21.2) | |
| GN | 9754 (33.8) | 21,432 (32.6) | |
| Hypertension | 4180 (14.5) | 15,050 (22.9) | |
| Missing cause | 213 (0.7) | 275 (0.4) | |
| Other cause | 2893 (10.0) | 5994 (9.1) | |
| Other urologic | 672 (2.3) | 1444 (2.2) | |
| Unknown cause | 984 (3.4) | 2542 (3.9) | |
| Previous kidney transplant (%) | 4009 (13.9) | 11,227 (17.1) | <0.001 |
| Living donor kidney transplant (%) | 16,390 (56.8) | 15,624 (23.7) | <0.001 |
| Pre-emptive kidney transplant (%) | 6631 (23.0) | 2720 (4.1) | <0.001 |
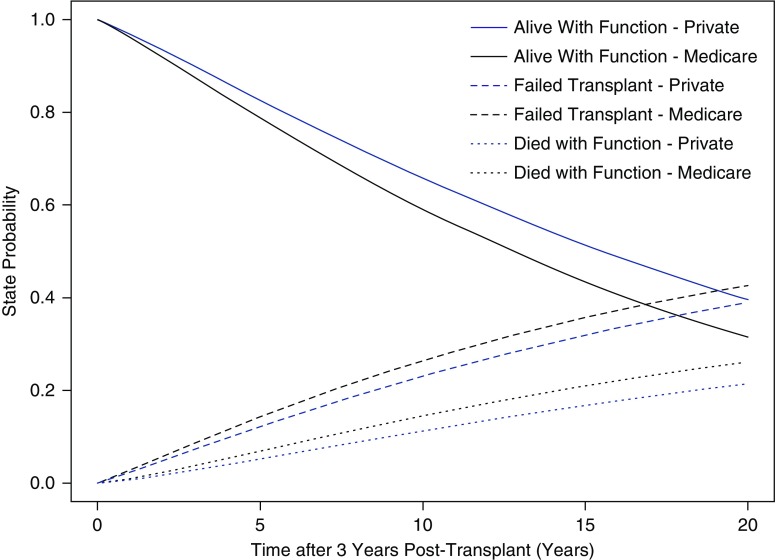
The results of the competing risk analysis with adjusted state probability curves stratified by insurance status are represented in Figure 3 for patients who were alive with a functioning transplant 36 months after transplantation. Unadjusted survival models of all-cause transplant failure and all-cause mortality using Kaplan–Meier curves are presented in Supplemental Figures 2 and 3, respectively. The proportion of patients alive with a functioning transplant was higher for patients with a private payer compared with patients with Medicare as primary payer during the study time horizon. After adjusting for age, sex, race, cause of ESKD, pre-emptive kidney transplantation, living donor kidney transplantation, and prior history of kidney transplantation, patients with Medicare insurance had a higher risk of death-censored transplant failure (hazard ratio, 1.18; 95% confidence interval, 1.14 to 1.23) compared with individuals with private insurance.
Figure 3.
State probabilities from multistate survival model adjusting for age, sex, race, cause of ESKD, history of prior kidney transplant, and type of kidney transplant stratified by insurance status. Health states presented in this figure include alive with function, failed transplant, and death with function. Individuals in the failed transplant group include those who are alive and those who have died after transplant failure.
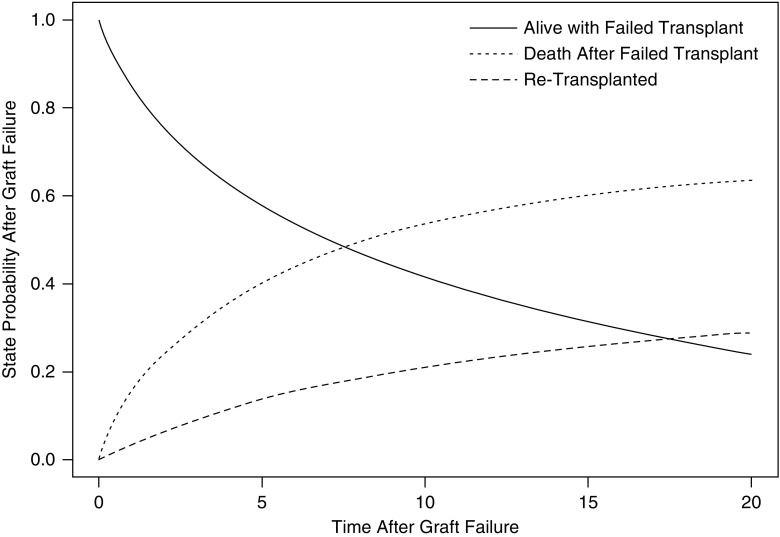
The adjusted state probability curves for patients after transplant failure are shown in Figure 4. Because we assumed no increase in patient survival after transplant failure with extended immunosuppressant drug coverage, the survival curves after transplant failure only represent outcomes for patients who are Medicare insured. The median expected survival of patients after transplant failure was 7.3 years.
Figure 4.
State probabilities of patients with a failed kidney transplant from multistate model. Only state probabilities for Medicare-insured individuals are reported in this diagram. Health states presented in this figure include alive with a failed transplant, retransplantation, and death after transplant failure.
Primary Model Results
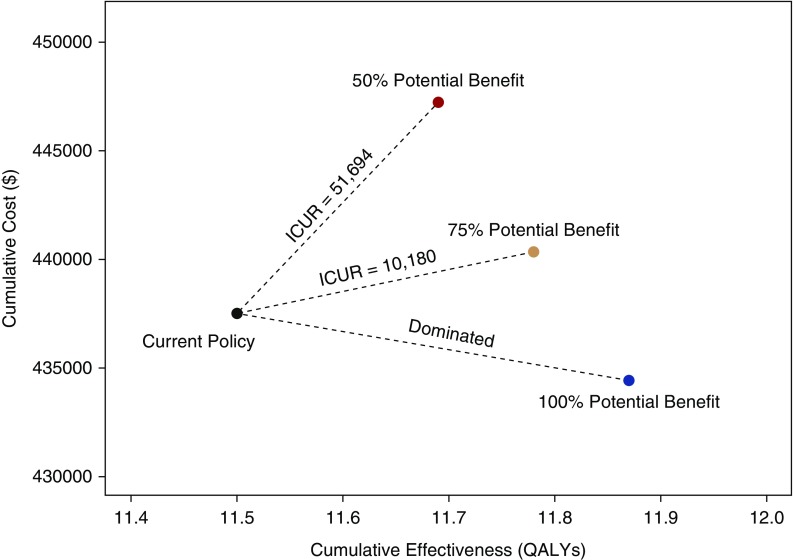
Extension of Medicare coverage for immunosuppressant drugs for the duration of transplant survival was less costly and led to better health compared with the current system of Medicare coverage. The reduction in cumulative cost with extended immunosuppressant drug coverage was $3077 per patient over the lifetime time horizon of the model (Figure 5), with an increase in projected QALYs of 0.37, assuming a risk reduction of 15.2% in death-censored transplant loss. Over the lifetime of a patient, there was an increase in the transplant associated costs of $35,039 but a reduction in dialysis associated costs of $38,117 (Table 3). If the improvement in transplant survival was reduced to 75% of observed (risk reduction of 11.4% in death-censored transplant loss), extension of Medicare coverage had a cumulative cost of $2838 and an incremental cost–utility ratio (ICUR) of $10,180 per QALY gained; a scenario with only 50% of observed benefit (risk reduction of 7.6% in death-censored transplant loss) had an incremental increase in cost of $9728 and an ICUR of $51,694/ QALY gained.
Figure 5.
The cumulative cost and cost-effectiveness of extending immunosuppressive drug coverage for the duration of transplant survival compared with the current Medicare policy in which drug coverage ceases at 36 months. In this plot, the results of the primary model and the sensitivity analyses where only 75% and 50% of this potential reduction in graft loss with extension of Medicare immunosuppression coverage were realized. The ICUR for each scenario is plotted. The scenario where the estimated benefit was realized dominated the current policy, meaning it was less costly with more effectiveness over the lifetime time horizon of the model.
Table 3.
Incremental costs per patient of extended Medicare coverage of immunosuppression compared with no extension of Medicare coverage separated into transplant associated, dialysis associated, and total costs at various times after 36 months post-transplant
| Time beyond 36 mo | Transplant-Associated Costs ($) | Dialysis-Associated Costs ($) | Total Costs ($) |
|---|---|---|---|
| 5 yr | 9953 | −5585 | 4368 |
| 10 yr | 17,127 | −14,308 | 2819 |
| 15 yr | 22,433 | −22,124 | 309 |
| 20 yr | 26,370 | −28,044 | −1674 |
| Lifetime | 35,039 | −38,117 | −3077 |
Sensitivity Analyses
In the threshold analysis, we found that the extension of immunosuppression coverage was cost-effective at a willingness-to-pay threshold of $100,000 per QALY gained if transplant survival was only 36% of the observed (risk reduction in death-censored transplant loss of 5.5%). If the willingness-to-pay threshold was lower (i.e., $50,000 per QALY gained), extending immunosuppressive coverage would be cost-effective if transplant survival was 51% of that observed (risk reduction in death-censored transplant loss of 7.8%). If the willingness-to-pay threshold was $0 per QALY gained, extending coverage would be cost-effective if transplant survival was 88% of that observed (risk reduction in death-censored transplant loss of 13.3%).
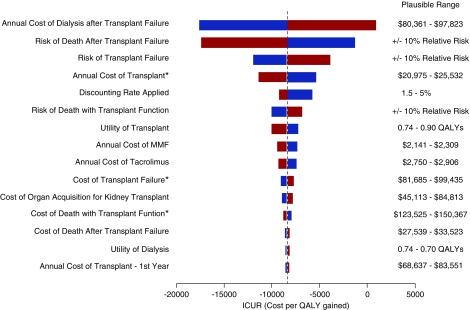
In other univariable sensitivity analysis, variation in the inputs informing the annual cost after dialysis therapy after transplant failure, the proportion of patients still with Medicare coverage after 36 months after transplant, the risk of death after transplant failure and the overall risk of transplant failure had the largest effect on the ICUR estimate (Figure 6). Extending coverage remained dominant in all analyses except for scenarios varying the annual cost of dialysis: if the annual cost of dialysis decreased below $81,235, then extension of Medicare coverage for immunosuppression medications was no longer cost-saving.
Figure 6.
Tornado diagram of univariate sensitivity analysis of extension of Medicare coverage compared with no extension of Medicare coverage. The base case is represented by the horizonal dashed line with incremental costs of −$3077 and incremental QALYs of 0.37, with an expected value (EV) of −$8387 per QALY gained. The plausible range for each variable is displayed on the left. The red bar represents the change in ICUR with the upper limit of the plausible range for each variable, and the blue bar represents the change in ICUR with the lower limit of the plausible range for each variable. Costs identified by an asterisk were only included in the model for patients who had full Part A and B Medicare coverage, either through disability or given their age was >65 years. Variation in the inputs informing the annual cost after dialysis therapy after transplant failure, the proportion of patients still with Medicare coverage after 36 months after transplant, the risk of death after transplant failure and the overall risk of transplant failure had the largest effect on the ICUR estimate.
In the scenario where extension of Medicare immunosuppressant drug coverage removed a disincentive to seek employment and therefore reduced the number individuals who retained Medicare insurance owing to medical disability by 10%, the cost-savings increased from $3077 per patient to $14,215 (Table 4). Furthermore, there were cost-savings of $8222 and $1188 per patient even when the improvement of transplant survival was only 75% and 50% of the observed, respectively.
Table 4.
Results of sensitivity analysis where extension of Medicare immunosuppression coverage results in a reduction in Medicare-insured patients who are dependent on disability as an indication for Part A and B Medicare insurance
| Percent Reduction in Disability-Dependent Patients | Anticipated Benefit in Reduction of Transplant Loss with Extended Immunosuppression Coverage | |||||
|---|---|---|---|---|---|---|
| 100% | 75% | 50% | ||||
| Incremental Cost ($) | ICUR | Incremental Cost ($) | ICUR | Incremental Cost ($) | ICUR | |
| 0% (Reference case) | −3077 | Dominates | 2838 | 10,180 | 9728 | 51,694 |
| 2.5% | −5862 | Dominates | 73 | 261 | 6999 | 37,192 |
| 5% | −8646 | Dominates | −2692 | Dominates | 4270 | 22,689 |
| 7.5% | −11,431 | Dominates | −5457 | Dominates | 1541 | 8187 |
| 10% | −14,215 | Dominates | −8222 | Dominates | −1188 | Dominates |
Dominates refers to scenarios that are less costly but also result in an increase in expected utility.
The incremental effectiveness in QALYs is not presented in this table as this did not vary in this sensitivity analysis.
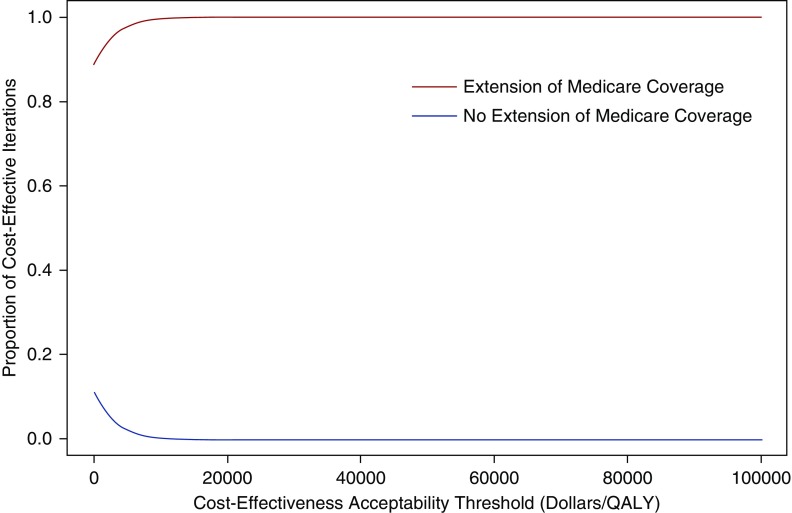
In probabilistic analysis, extension of Medicare immunosuppressive drug coverage for the duration of transplant survival was preferred when payers were not willing to pay anything ($0 per QALY) (i.e., resulted in cost-savings and improved health) in 89% of the iterations (Supplemental Figure 4). The extension of Medicare immunosuppression coverage was below a willingness-to-pay threshold of $20,000 per QALY in 100% of the iterations (Figure 7).
Figure 7.
Cost-effectiveness acceptability curves of the results of the probabilistic analysis for the incremental cost-effectiveness of extension of Medicare immunosuppression coverage compared with no extension of Medicare immunosuppression coverage. This graph shows the proportion of iterations where each option was cost-effective according to various cost-effectiveness acceptability (also known as willingness-to-pay) thresholds ($/QALY). In this analysis, extension of Medicare immunosuppression coverage was cost-effective in the majority of iterations regardless of the cost-effectiveness acceptability threshold.
Discussion
The inferior long-term transplant survival of transplant recipients in the United States compared with other developed countries is well recognized and has been attributed in part to cessation of Medicare coverage for immunosuppressant drugs required to prevent rejection.9,30 Despite long-standing efforts to extend Medicare coverage, legislative change has not been enacted in part because of the high cost of immunosuppressant medications. The availability of generic immunosuppressant medications has dramatically reduced the cost of the most commonly used drugs (i.e., tacrolimus and mycophenolate) from $10,000 per patient year in 2008 to $5000 per patient year in 2013,12 prompting the need for re-evaluation of the cost and benefits of extending Medicare coverage of immunosuppressant drugs.
In this cost-effectiveness analysis, extension of Medicare coverage of immunosuppressant drugs for the lifetime of kidney transplant function was cost-saving and led to better patient outcomes compared with the current policy of stopping Medicare coverage after 36 months of transplantation. The analysis assumed transplant survival would be similar to that observed in a cohort of patients with private drug coverage who receive immunosuppressant drugs throughout transplantation. This assumption is consistent with previous literature describing the effect of Medicare extension from 1 year to 3 years in the 1990s; with Medicare coverage extension from 1 to 3 years post-transplant, the risk of transplant failure in patients with lower income became similar to that among patients with a higher income.18,31 Additionally, in the setting of a universal health care system, it has been shown that a low income does not affect the risk of transplant failure.23 However, there may be unique and unmeasured confounders in the United States health care system, which may limit the improvement in transplant failure that can occur with extension of immunosuppression to the lifetime of a kidney transplant patient. An analysis by Hart et al. found that individuals who lost Medicare coverage at 36–38 months after transplant did not have an increased risk of transplant failure compared with those who continued to have Medicare coverage, whereas those who lost coverage either before 36 months or after 38 months did have a significantly increased risk of transplant failure.32 This analysis differs from ours because it compares outcomes of patients who discontinue Medicare coverage with patients who continue Medicare coverage. Patients who continue Medicare coverage in their analysis likely include patients who are disabled owing to medical comorbidities and therefore may have inferior outcomes. The estimates of the improvement in transplant survival with extension of Medicare coverage of immunosuppression may therefore be underestimated in that analysis. Overall, there is uncertainty in the benefit that may be observed with extension of Medicare immunosuppression coverage. We modeled conservative scenarios where the benefit of extension of Medicare was less than anticipated; extension of Medicare coverage remained a cost-effective strategy even it if it only reduced the difference in transplant survival between Medicare and privately insured patients by 36%, representing good value for health care dollars spent in the United States, on the basis of based on commonly cited thresholds.29,33 Stated alternatively, extension of immunosuppression coverage was cost-effective at a willingness-to-pay threshold of $100,000, $50,000, and $0 per QALY gained if it results in a decrease in the risk of transplant failure of 5.5%, 7.8%, and 13.3%, respectively.
In univariable sensitivity analysis, the results were sensitive to the annual cost to Medicare of providing dialysis therapy. Recently, President Trump issued an executive order that 80% of individuals with ESKD should be treated with either a home dialysis modality or transplantation.34 If we assume that the uptake of peritoneal dialysis increases enough to achieve this goal, the mean annual cost of dialysis per patient per year would be $84,399 (see Supplemental Appendix 1 for calculation). At this value, extension of immunosuppression in the primary model would still be cost-saving. It should also be noted that this analysis does not evaluate the effect of newer immunosuppressant drugs, such as belatacept, which may increase both the cost and effectiveness of transplantation.To our knowledge, this study provides the first estimate of the cost-effectiveness of extending Medicare immunosuppressive drug coverage in the era of generic immunosuppressant drugs. A recent government-commissioned estimate of the financial effect of extending immunosuppressive drug coverage found this strategy would be cost-saving. However, this analysis was not a cost-effectiveness analysis in that it did not account for patient quality of life with transplantation and on dialysis.35 Although prior economic evaluations have estimated the cost-effectiveness of extended immunosuppression coverage, these studies are over 10 years old, and are also limited by not considering the percentage of patients who continue to have full Part A and B Medicare insurance owing to medical disability.18–20 We uniquely evaluated scenarios where the extension of Medicare immunosuppression coverage of immunosuppression resulted in a decrease in the number of individuals dependent on medical disability by up to 10%. In these scenarios, the cost-savings realized from extension of Medicare immunosuppression coverage was even more pronounced. These estimates may be conservative, as one study reported up to 18% of individuals on disability cite the fear of losing insurance coverage as a reason to not seek employment, whereas another study reported that the fear of losing insurance coverage was a consideration for the majority of individuals who were unemployed after transplantation.10,11 Additionally, this analysis used updated cost estimates for immunosuppressants, reflecting the decrease in costs over time given market competition with generic tacrolimus and mycophenolate.12
The model was conducted only from the perspective of Medicare as a health care payer. The choice of this perspective is intentional, as the goal of the study was to inform Medicare health insurance policy; however, the potential addition benefits from a societal perspective are not observed. For example, patients with a functioning kidney transplant are more likely to be employed and there are less out-of-pocket costs associated with a functioning kidney transplant compared with dialysis; therefore, a strategy that prolongs transplant kidney survival may lead to positive effects in these areas. Additionally, a reduction in transplant failure may lead to a reduction in the number of individuals waitlisted for a kidney transplant and therefore may reduce the overall organ shortage in the United States. Of note, the model assumes there will be no benefit in reduction in death with function; however, it is plausible that extending immunosuppression could reduce complications associated with rejection, which may also reduce the outcome of death with function. Finally, the model does not consider the societal value of a deceased donor kidney as a limited resource, and that reducing premature transplant failure would further extend the societal benefit of kidney transplantation.
In summary, extension of Medicare immunosuppression coverage is a cost-saving strategy from the perspective of Medicare as a health care payer, assuming it results in an improvement in transplant survival. This finding may support current efforts to enact legislation to extend immunosuppressive drug coverage under Medicare.
Disclosures
None.
Funding
Dr. Kadatz is supported by the University of British Columbia through the Clinician Investigator Program and the American Society of Transplantation Research Network Clinical Fellowship grant. Dr. John S. Gill is funded by a Foundation Award from the Canadian Institutes of Health Research. Dr. Jagbir Gill is funded by a Scholar Award from the Michael Smith Foundation for Health Research. Dr. Klarenbach is supported by the Kidney Health Research Chair and the Division of Nephrology at the University of Alberta, Edmonton, Canada.
Supplementary Material
Acknowledgments
Dr. Kadatz, Dr. Klarenbach, and Dr. John S. Gill designed this analysis. Dr. Kadatz completed the analysis and created the figures and tables. Dr. Kadatz, Dr. Klarenbach, Dr. Jagbir Gill, Dr. John S. Gill, and Dr. Formica drafted the manuscript. All authors have approved the final version of this manuscript.
The data reported in this study has been supplied by the US Renal Data System. The interpretation and reporting of this data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US Government.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019070646/-/DCSupplemental.
Supplemental Figure 1. Age distribution of Medicare-insured patients included in the survival analysis.
Supplemental Figure 2. Kaplan–Meier curves of all-cause transplant failure stratified by insurance status. Privately insured patients are represented by the solid line and Medicare-insured patients are represented by the dashed line.
Supplemental Figure 3. Kaplan–Meier curves of all-cause mortality stratified by insurance status. Privately insured patients are represented by the solid line and Medicare-insured patients are represented by the dashed line.
Supplemental Figure 4. Plot of results of 10,000 probabilistic analyses iterations. Each point on the graph represents the results of one iteration of the probabilistic analysis. The black circle represents the 95% confidence ellipse.
Supplemental Appendix 1. Calculation to determine the mean annual cost per patient per year of dialysis, assuming 80% of individuals with ESKD are treated with home therapies or transplantation.
References
- 1.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, et al.: Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 341: 1725–1730, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Laupacis A, Keown P, Pus N, Krueger H, Ferguson B, Wong C, et al.: A study of the quality of life and cost-utility of renal transplantation. Kidney Int 50: 235–242, 1996 [DOI] [PubMed] [Google Scholar]
- 3.Axelrod DA, Schnitzler MA, Xiao H, Irish W, Tuttle-Newhall E, Chang SH, et al.: An economic assessment of contemporary kidney transplant practice. Am J Transplant 18: 1168–1176, 2018 [DOI] [PubMed] [Google Scholar]
- 4.Tonelli M, Wiebe N, Knoll G, Bello A, Browne S, Jadhav D, et al.: Systematic review: Kidney transplantation compared with dialysis in clinically relevant outcomes. Am J Transplant 11: 2093–2109, 2011 [DOI] [PubMed] [Google Scholar]
- 5.United States Renal Data System : 2018 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2018 [Google Scholar]
- 6.U.S. Department of Health & Human Services : U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: Transplant Data 1988-2018, Rockville, MD, Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation; Richmond, VA, United Network for Organ Sharing; Ann Arbor, MI, University Renal Research and Education Association, 2018 [Google Scholar]
- 7.Evans RW, Applegate WH, Briscoe DM, Cohen DJ, Rorick CC, Murphy BT, et al.: Cost-related immunosuppressive medication nonadherence among kidney transplant recipients. Clin J Am Soc Nephrol 5: 2323–2328, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gordon EJ, Prohaska TR, Sehgal AR: The financial impact of immunosuppressant expenses on new kidney transplant recipients. Clin Transplant 22: 738–748, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gill JS, Tonelli M: Penny wise, pound foolish? Coverage limits on immunosuppression after kidney transplantation. N Engl J Med 366: 586–589, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Markell MS, DiBenedetto A, Maursky V, Sumrani N, Hong JH, Distant DA, et al.: Unemployment in inner-city renal transplant recipients: Predictive and sociodemographic factors. Am J Kidney Dis 29: 881–887, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Slakey DP, Rosner M: Disability following kidney transplantation: The link to medication coverage. Clin Transplant 21: 224–228, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Helmuth ME, Liu Q, Turenne MN, Park JM, Oguntimein M, Dutcher SK, et al.: Secular trends in the cost of immunosuppressants after solid organ transplantation in the United States. Clin J Am Soc Nephrol 14: 421–430, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Denavas-Walt C, Proctor B; United States Census Bureau : Income and Poverty in the United States: 2014, Suitland, MD, United States Census Bureau, U.S. Department of Commerce, 2015 [Google Scholar]
- 14.United States Congress: Comprehensive immunosuppressive drug coverage for kidney transplant patients act of: 2011. HR 2969, 2011. Available at: https://www.congress.gov/bill/112th-congress/house-bill/2969. Accessed March 28, 2019
- 15.United States Congress: Comprehensive Immunosuppressive Drug Coverage for Kidney Transplant Patients Act of 2009. HR 1458, 2009. Available at: https://www.congress.gov/bill/111th-congress/house-bill/1458. Accessed March 28, 2019
- 16.United States Congress: Comprehensive Immunosuppressive Drug Coverage for Kidney Transplant Patients Act of 2013. HR 1325, 2013. Available at: https://www.congress.gov/bill/113th-congress/house-bill/1325. Accessed March 28, 2019
- 17.United States Congress: Comprehensive Immunosuppressive Drug Coverage for Kidney Transplant Patients Act of 2016. HR6139, 2016. Available at: https://www.congress.gov/bill/114th-congress/house-bill/6139. Accessed March 28, 2019
- 18.Woodward RS, Schnitzler MA, Lowell JA, Spitznagel EL, Brennan DC: Effect of extended coverage of immunosuppressive medications by medicare on the survival of cadaveric renal transplants. Am J Transplant 1: 69–73, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Yen EF, Hardinger K, Brennan DC, Woodward RS, Desai NM, Crippin JS, et al.: Cost-effectiveness of extending Medicare coverage of immunosuppressive medications to the life of a kidney transplant. Am J Transplant 4: 1703–1708, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Page TF, Woodward RS: Cost of lifetime immunosuppression coverage for kidney transplant recipients. Health Care Financ Rev 30: 95–104, 2008 [PMC free article] [PubMed] [Google Scholar]
- 21.Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, et al.: Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: Second panel on cost-effectiveness in health and medicine. JAMA 316: 1093–1103, 2016 [DOI] [PubMed] [Google Scholar]
- 22.TreeAge Pro : R2.1, Williamstown, MA, TreeAge Software, 2018 [Google Scholar]
- 23.Naylor KL, Knoll GA, Shariff SZ, McArthur E, Garg AX, Van Walraven C, et al.: Socioeconomic status and kidney transplant outcomes in a universal healthcare system: A Population-based Cohort Study. Transplantation 103: 1024–1035, 2019 [DOI] [PubMed] [Google Scholar]
- 24.R Core Development Team : R: A Language and Environment for Statistical Computing, Vienna, Austria, R Foundation for Statistical Computing, 2017 [Google Scholar]
- 25.Putter H, de Wreede L, Fiocco M: Data Preparation, Estimation and Prediction in Multi-State Models, Vienna, Austria, CRAN, 2018 [Google Scholar]
- 26.Schnitzler MA, Skeans MA, Axelrod DA, Lentine KL, Randall HB, Snyder JJ, et al.: OPTN/SRTR 2016 annual data report: Economics. Am J Transplant 18[Suppl 1]: 464–503, 2018 [DOI] [PubMed] [Google Scholar]
- 27.Wyld M, Morton RL, Hayen A, Howard K, Webster AC: A systematic review and meta-analysis of utility-based quality of life in chronic kidney disease treatments. PLoS Med 9: e1001307, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perl J, Zhang J, Gillespie B, Wikström B, Fort J, Hasegawa T, et al.: Reduced survival and quality of life following return to dialysis after transplant failure: The Dialysis Outcomes and Practice Patterns Study. Nephrol Dial Transplant 27: 4464–4472, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neumann PJ, Cohen JT, Weinstein MC: Updating cost-effectiveness--the curious resilience of the $50,000-per-QALY threshold. N Engl J Med 371: 796–797, 2014 [DOI] [PubMed] [Google Scholar]
- 30.Merion RM, Goodrich NP, Johnson RJ, McDonald SP, Russ GR, Gillespie BW, et al.: Kidney transplant graft outcomes in 379 257 recipients on 3 continents. Am J Transplant 18: 1914–1923, 2018 [DOI] [PubMed] [Google Scholar]
- 31.Woodward RS, Page TF, Soares R, Schnitzler MA, Lentine KL, Brennan DC: Income-related disparities in kidney transplant graft failures are eliminated by Medicare’s immunosuppression coverage. Am J Transplant 8: 2636–2646, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hart A, Gustafson SK, Wey A, Salkowski N, Snyder JJ, Kasiske BL, et al.: The association between loss of Medicare, immunosuppressive medication use, and kidney transplant outcomes. Am J Transplant 19: 1964–1971, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grosse SD: Assessing cost-effectiveness in healthcare: History of the $50,000 per QALY threshold. Expert Rev Pharmacoecon Outcomes Res 8: 165–178, 2008 [DOI] [PubMed] [Google Scholar]
- 34.White House : Executive order on advancing American Kidney Health, 2019. Available at: https://www.whitehouse.gov/presidential-actions/executive-order-advancing-american-kidney-health/. Accessed September 16, 2019
- 35.Assessing the costs and benefits of extending coverage of immunosuppressive drugs under Medicare. In: SERVICES, D. O. H. A. H. (Ed.), Office of the Assistant Secretary for Planning and Evaluation, 2019. Available at: https://aspe.hhs.gov/system/files/pdf/261746/Savings_From_Extending_Coverage_For_Immunosuppressive_Drugs_Final.pdf. Accessed September 16, 2019
- 36.Sullivan B: Maximizing Medicare cost report reimbursement. 2015. Available at: http://organdonationalliance.org/wp-content/uploads/2015/08/ATC_BSullivan_CostReport_062016_S5N0001.pdf. Accessed June 6, 2019
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.