Significance Statement
Although mesenchymal stem cells might have potential for treating SLE, their immunoregulatory plasticity renders their therapeutic effects unpredictable. The authors genetically modified mesenchymal stem cells to overexpress IL-37—a protein with immunosuppressive activity—and assessed the modified cells’ effects on immune suppression in vitro, as well as the effects of transplanting such cells into a mouse model of SLE. Mice transplanted with IL-37–overexpressing cells displayed improved survival and reduced signs of SLE compared with controls. Expression of IL-37 by mesenchymal stem cells can maintain higher serum levels of IL-37, and these cells had prolonged survival after transplantation, perhaps through IL-37 suppressing the inflammatory microenvironment. The additive therapeutic effects of this approach might offer a way to enhance the stability and effectiveness of mesenchymal stem cells in treating SLE.
Keywords: mesenchymal stem cells, MSCs, systemic lupus erythematosus, SLE, interleukin-37, IL-37
Visual Abstract
Abstract
Background
Although mesenchymal stem cells (MSCs) might offer a promising strategy for treating SLE, their immunoregulatory plasticity makes their therapeutic effects unpredictable. Whether overexpressing IL-37, an IL-1 family member with immunosuppressive activity, might enhance the therapeutic effects of these cells for SLE is unknown.
Methods
We genetically modified MSCs to overexpress IL-37 and assessed their effects on immune suppression in vitro. We also evaluated the effects of such cells versus effects of various controls after transplanting them into MRL/lpr mice (model of SLE).
Results
Stem cell characteristics did not appear altered in MSCs overexpressing IL-37. These cells had enhanced immunosuppression in vitro in terms of inhibiting splenocyte proliferation, reducing proinflammatory factors (IL-1β, TNF-α, IL-17, and IL-6), and suppressing autoantibodies (anti-dsDNA and anti-ANA). Compared with animals receiving control MSCs or IL-37 treatment alone, MRL/lpr mice transplanted with IL-37–overexpressing cells displayed improved survival and reduced signs of SLE (indicated by urine protein levels, spleen weight, and renal pathologic scores); they also had significantly lower expression of proinflammatory factors, lower total antibody levels in serum and urine, lower autoantibody production, and showed reduced T cell numbers in the serum and kidney. Expression of IL-37 by MSCs can maintain higher serum levels of IL-37, and MSCs had prolonged survival after transplantation, perhaps through IL-37 suppressing the inflammatory microenvironment.
Conclusions
Mutually reinforcing interaction between MSCs and IL-37 appears to underlie their additive therapeutic effects. Genetic modification to overexpress IL-37 might offer a way to enhance the stability and effectiveness of MSCs in treating SLE.
SLE is a chronic autoimmune disease resulting in loss of self-tolerance and multiorgan destruction by an overactivated immune system.1–5 Renal involvement is one of the major characteristics of the SLE which normally results in renal failure.1–5 Current therapies have significantly reduced the morbidity and mortality of patients with SLE.6,7 However, they often experience some deleterious side effects, such as infection. Furthermore, some patients are refractory to these therapies.6,7 Therefore, more efforts should be made to develop novel alternative therapies.1
Both immune tolerance reconstruction and tissue regeneration are important for treating SLE. Thus mesenchymal stem cells (MSCs) might be a promising candidate for SLE treatment8 because of their regenerative capabilities9,10 and immunomodulatory functions.11,12 MSCs are multipotent stem cells that can be derived from various tissues.9,10,13 They can be safely harvested with no major ethical concerns and have low immunogenicity, and thus represent an attractive cell type for allogenic transplantation.10
Both preclinical and clinical studies have shown that transplantation of MSCs could alleviate the pathogenic symptoms of SLE.8,14–17 The main underlying mechanism behind this technique is believed to be the immune-suppression abilities of MSCs, which include reducing proinflammatory factors and upregulating anti-inflammatory factors.8 However, the immune-suppressive activities of MSCs could only be activated by strong inflammation signals or bacteria.18 In contrast, a low level of inflammation signals would reduce their immune-suppressive functions or even activate the immune system.19,20 Unfortunately, it is very difficult to define the exact levels of inflammation required in vivo, which might make the therapeutic effects of MSCs unpredictable. Thus it is very important to ensure that MSCs used for SLE treatments result in immune-suppression rather than immune-activation activities.
IL-37, a newly discovered member of the IL-1 family, has been demonstrated to have immune-suppression functions.21,22 The underlying mechanisms behind its activity include binding the IL-18 recepter α (IL-18Rα) and IL-1R8 as an extracellular cytokine,23,24 or reducing the expression of IL-6 in the nucleus.25 A previous study also showed IL-37 could suppress the expression of the inflammatory factors in the PBMCs of patients with SLE.26
Therefore, we conducted this study to investigate whether overexpressing IL-37 could enhance the therapeutic effects of MSCs for SLE treatment. Our results showed they had additive therapeutic effects in the mice model of SLE. The successful overexpression of IL-37 by the MSCs could extend the t1/2 time of IL-37, while IL-37 could prolong the survival of MSCs by suppressing the inflammatory microenvironment.
Methods
Isolation, Expansion, and Modification of MSCs
The MSCs were isolated from the bone marrow (BM) of healthy MRL/MpJ mice (female, 4 weeks old, stock number 000486; The Jackson Laboratory) as described before with modifications.27 The BM cells were flushed out of femurs and tibias, cultured with DMEM/high glucose (catalog number 10566032) plus 10% FBS (catalog number 26400044; both Thermo Fisher Scientific), 10 ng/ml basic fibroblast growth factor (bFGF, catalog number 450-33; PeproTech), 50 μg/ml ascorbic acid (catalog number S4245; Selleckchem), and antibiotics (catalog number 15240062; Thermo Fisher Scientific) in cell culture dishes. The nonadherent cells were removed 3 days later. MSCs were passaged with TrypLE (catalog number 12605010; Thermo Fisher Scientific), and passage-3 cells were used for IL-37 overexpression.
For human IL-37 overexpression in the mice BM MSCs, the full-length IL-37 (NM_014439.3) or the negative control Flag peptide (DYKDDDDK) was cloned into the lentiviral vector (pLVX-Puro, catalog number 632164; Clontech Laboratories). Virus production was performed in HEK293T cells (ATCC CRL-3216) by cotransfecting the lentiviral vector with the packaging lentiviral vectors pMD2.G (12259) and psPAX2 (12260; both Addgene). The lentivirus infection was performed with 8 μg/ml hexadimethrine bromide (catalog number H9268; Sigma) for 8 hours. The MSCs were then treated 2 days later with 2 μg/ml puromycin (catalog number A1113803; Thermo Fisher Scientific) for 3 days and then maintained in the cell culture medium plus 0.5 μg/ml puromycin. MSCs were passaged three times (passage 6) for subsequent experiments.
Animals
The MRL/lpr mice (female, 8 weeks old, 20.8±2.6 g), were purchased from The Jackson Laboratory (stock number 000485). The mice were maintained in specific pathogen-free conditions and randomly divided into different groups. The study was approved by the Animal Research Ethics Committee of the School of Medicine, Shenzhen University.
Transplantation of MSCs
Wild-type MSCs (1×106), MSCs overexpressing Flag (MSCs-Flag; 1×106), and MSCs overexpressing IL-37 (MSCs-IL37; 1×106) were resuspended in 200 μl PBS and injected into the mice via the tail vein at the age of 14 weeks, and every 2 weeks thereafter until the end of the experiment (at the age of 21 weeks). The IL-37 protein (1 mg/kg, diluted in 200 μl PBS, catalog number 9225-IL-025; R&D Systems) was injected via the tail vein every 2 days, starting at the age of 14 weeks until the age of 21 weeks. PBS was used as a negative control for the transplantation of MSCs and IL-37 protein injection. Each group contained eight mice.
Western Blot
The protein extraction and Western blot were performed as previously described.28 The primary antibody for IL-37 and glyceraldehyde-3-phosphate dehydrogenase were purchased from Thermo Fisher Scientific (catalog number PA5-30527 and AM4300).
Preparation of PBMCs and Mice Serum
For the culture of PBMCs, blood was collected from the hearts of MRL/lpr mice (female, 18 weeks old) which were treated with heparin (200 IU/mouse, intraperitoneal injection) for 15 minutes. The blood samples collected from ten mice were pooled together, diluted 1:1 in HBSS containing calcium and magnesium (catalog number 14025092; Thermo Fisher Scientific). The PBMCs were then isolated with the Ficoll-Paque PLUS density gradient media (catalog number 17144003; GE Healthcare) and cultured with RPMI1640 (catalog number 12633012; Thermo Fisher Scientific) plus 10% FBS and antibiotics. The mice serum was then centrifuged at 5000 rpm for 30 minutes at 4°C. The supernatant was stored at 4°C for subsequent applications.
Flow Cytometry
Antibody staining and flow cytometry were performed as previously described.28 Briefly, the MSCs were dissociated with TrypLE, centrifuged, and resuspended with PBS (catalog number 20012027; Thermo Fisher Scientific) plus 5% FBS. After 30 minutes of incubation, the cells were stained with FITC Rat Anti-Mouse CD44 Clone IM7 (for research use only [RUO], catalog number 561859), FITC Rat Anti-Mouse CD45 Clone 30-F11 (RUO, catalog number 553080), or FITC Rat IgG2b, κ Isotype Control Clone A95-1 (RUO, catalog number 556923; all BD Biosciences).
For immune cell population analysis, the PBMCs were prepared from the MRL/lpr mice after 4 weeks of MSC transplantation or IL-37 injection. Single-cell suspensions were obtained from the kidney by perfusing the kidney with PBS for 15 minutes, followed by 5 minutes with DMEM/high glucose, and then 1 mg/ml collagenase B (catalog number 11088815001) plus 1 mg/ml dispase (catalog number D4818; both Sigma) diluted in DMEM/high glucose for 30 minutes at 37°C. The single cells were then meshed and strained through a 70-μm cell strainer (catalog number CLS431751-50EA; Corning). Cells were stained with the indicated antibodies in dilution buffer (0.5% BSA in PBS) for 30 minutes on ice. The antibodies included FITC-labeled anti-B220 (catalog number 553087), allophycocyanin-labeled anti-CD3 (catalog number 565643), phycoerythrin (PE)-labeled anti-CD4 (catalog number 553049), FITC-labeled anti-CD8 (catalog number 561966), FITC-labeled anti-CD11c (catalog number 561045), PE-labeled anti-CD11b (catalog number 561689), allophycocyanin-labeled anti-CD138 (catalog number 561705), FITC-labeled anti-IgG1/2a/2b (catalog numbers 553399 and 553443), FITC-labeled anti-CD4 (catalog number 553055), PE-labeled anti-Foxp3 (catalog number 563101), and PE-CF594–labeled IL-17 (catalog number 562542; all BD Biosciences). Cells were analyzed with BD Accuri C6 Plus (BD Biosciences) and the data were analyzed with FlowJo software.
Differentiation of Adipocytes, Osteocytes, and Chondrocytes
The adipocytes, osteocytes and chondrocytes differentiation and characterization were performed as previously described.29
Isolation of Splenocytes
The MRL/lpr mice (female, 18 weeks old) were euthanized by cervical dislocation. Spleens were carefully isolated and incubated on ice in RPMI1640 plus 10% FBS and antibiotics. They were then mashed using frosted glass slides and the cells were filtered through 100 μm cell strainers (catalog number 08-771-19; Falcon). The splenocytes were cultured with RPMI1640 plus 10% FBS and antibiotics.
MSC Coculture Assay
The wild-type MSCs and lentivirus-modified MSCs including MSCs-Flag (the negative control) and MSCs-IL37 were plated onto 96-well plates at passage 6 with 1×104 cells per well. Cells were cultured for a further 3 days after they reached confluence.
The splenocytes or PBMCs isolated from the 18-week-old MRL/lpr mice were cocultured with MSCs with 100×104 cells per well. The cells were then stimulated with PMA at a concentration of 5 ng/ml for 48 hours in coculture medium (RPMI1640 plus 10% mice serum isolated from the 18-week-old MRL/lpr mice and antibiotics). The IL-37 protein (1 μg/ml) was also used to treat the splenocytes or PBMCs, and PBS was used as control. Cell proliferation was determined using the Cell Proliferation Kit I (catalog number 11465007001; Roche) according to the instructions. The light absorbance was measured at 570 nm by the automated microplate reader (model 550; Bio-Rad). The experiments were performed in triplicate.
RNA Extraction and Real-Time PCR
The RNA extraction, cDNA synthesis, and real-time PCR were performed as previously described.28 The primer sequences used were as follows:
IL-1β, forward 5′-TGGACCTTCCAGGATGAGGACA-3′ and reverse 5′-GTTCATCTCGGAGCCTGTAGTG-3′;
TNF-α, forward 5′-GGTGCCTATGTCTCAGCCTCTT-3′ and reverse 5′-GCCATAGAACTGATGAGAGGGAG-3′;
IL-17, forward 5′-CAGACTACCTCAACCGTTCCAC-3′ and reverse 5′-TCCAGCTTTCCCTCCGCATTGA-3′;
IL-6, forward 5′-TACCACTTCACAAGTCGGAGGC-3′ and reverse 5′-CTGCAAGTGCATCATCGTTGTTC-3′;
β-actin, forward 5′-CATTGCTGACAGGATGCAGAAGG-3′ and reverse 5′-TGCTGGAAGGTGGACAGTGAGG-3′.
ELISA
After 2 days of coculture, the cell culture supernatant was collected. The cytokines (IL-1β, TNF-α, IL-17, and IL-6; catalog numbers 432601, 430901, 432504, and 431304, respectively; BioLegend) and autoantibodies (anti–double stranded DNA [anti-dsDNA] and anti–antinuclear antibody [anti-ANA]; catalog numbers DEIA4488 and DEIA-BJ2332, respectively; Creative Diagnostics) were measured using an ELISA kit according to the instructions.
At 4 weeks after MSC transplantation or IL-37 injection, peripheral blood was collected from the eyes of the mice and urine was collected for 24 hours by using metabolism cages. Serum levels of the anti-dsDNA and anti-ANA antibodies were measured using an ELISA kit according to the instructions. The levels of total IgG1, IgG2a, IgG2b, and IgM (catalog numbers 3025, 3026, 3027, and 3024, respectively; Chondrex) in the serum and urine were measured using an ELISA kit according to the instructions. The cytokines (IL-1β, TNF-α, IL-17, and IL-6) were also measured using an ELISA kit according to the instructions.
The serum level of IL-37 was measured at the indicated time after one round of IL-37 protein injection or MSCs-IL37 transplantation. Blood was collected from the retro-orbital plexus. The ELISA (catalog number ab213798; Abcam) was performed according to the instructions.
Urine Protein Measurement
Four weeks after MSC transplantation or IL-37 injection, urine was collected for 24 hours using metabolic cages. The urine proteins were measured using a Creatinine Assay Kit (catalog number ab65340; Abcam) and a Mouse Albumin ELISA Kit (catalog number ab108792; Abcam) according to the instructions.
Spleen Index Measurement
The MRL/lpr mice (18 weeks old) were fasted for 12 hours, and then total body weight was measured. Spleens were dissected and weighed. The spleen index was presented as spleen/body weight.
Hematoxylin and Eosin Staining and Renal Scoring
At 4 weeks after MSC transplantation or IL-37 injection, mice were treated with heparin (200 IU/mouse, intraperitoneal injection) for 15 minutes and euthanized by cervical dislocation. Kidneys were perfused with PBS for 30 minutes and then fixed with 10% neutral-buffered formalin fixative at 4°C for 48 hours and then 70% ethanol until tissue processing and embedding in paraffin. Kidneys were sectioned (4-μm thick) and stained with a hematoxylin and eosin staining kit (catalog number ab245880; Abcam). The histopathologic examination was performed by three different investigators blindly. The interobserver agreement was assessed by the intraclass correlation coefficient.30 The final renal pathology score was then the average of these three scores for each picture (intraclass correlation coefficient, 0.96; 95% CI, 0.94 to 0.98). The renal pathology scoring was calculated as previously described.31,32 Briefly, each feature was scored from zero to three according to the levels of the glomerular proliferation, inflammation, necrosis, interstitial changes, vasculitis, and crescent formation. The final renal score was the sum of these scores.31,32
Bioluminescence Imaging
At 3 days before MSC transplantation, cells were infected with the Lentiviral Dual Reporter (green fluorescent protein and luciferase, catalog number BLIV101PA-1; System Biosciences) which was produced in 293T cells cotransfected with the packaging lentiviral vectors pMD2.G and psPAX2. The successful infection was confirmed by analyzing the expression of green fluorescent protein by flow cytometry. At 15 minutes before imaging, the D-luciferin (catalog number P1042; Promega) substrate solution (150 mg/kg in around 100 μl PBS) was injected intraperitoneally. Signals were detected using the IVIS Spectrum Preclinical In Vivo Imaging System (PerkinElmer) and analyzed using the IVIS Spectrum software.
Mimicking the Inflammatory Microenvironment In Vitro
The MSCs, MSCs-Flag, and MSCs-IL37 were stimulated with TNF-α (20 ng/ml, catalog number 315-01A) plus IFN-γ (50 ng/ml, catalog number 315-05; both PeproTech) for 24 hours. The recombinant IL-37 (1 μg/ml) was also used to treat the MSCs and MSCs-Flag. Cell apoptosis was measured by Annexin V-FITC Apoptosis Staining/Detection Kit (catalog number ab14085; Abcam).
Statistical Analyses
Data were analyzed using SPSS software for Windows (IBM) and are shown as mean±SEM. The t test was used for two group comparisons and one-way ANOVA was used for multiple group comparisons with normal data distribution, parametric test, and Tukey post hoc tests. For comparing the mice survival rates, we used both the Kruskal–Wallis test33 and Kaplan–Meier analysis.34 A level of P<0.05 was considered statistically significant.
Results
IL-37 Overexpression Did Not Affect the Stem Cell Characteristics of MSCs
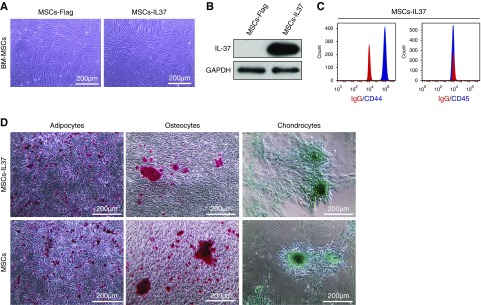
Overexpressing the IL-37 in the MSCs derived from the mice BM resulted in a fibroblast-like phenotype (Figure 1A). Successful overexpression was confirmed by Western blot (Figure 1B). Cell surface marker analysis indicated these MSCs-IL37 were positive for the mice MSC marker CD44 and negative for the hematopoietic marker CD45 (Figure 1C).27 Furthermore, the MSCs-IL37 could differentiate into adipocytes, osteocytes, and chondrocytes (Figure 1D), indicating the overexpression of IL37 in MSCs would not affect the major characteristics of the MSCs.
Figure 1.
IL-37 overexpression did not affect the stem cell characteristics of MSCs. MSCs were isolated from healthy MRL/MpJ mice. They were infected with the lentivirus expressing IL-37 (MSCs-IL37) or negative control (MSCs-Flag) at passage 3. (A) Cell morphology of the mice MSCs-IL37 or MSCs-Flag at passage 6. (B) Western blot showed the expression of IL-37 in the MSCs-IL37 at passage 6. (C) Flow cytometry analysis of the mice MSCs-IL37 at passage 6. (D) Differentiation of adipocytes, osteocytes, and chondrocytes of MSCs-IL37 and MSCs at passage 6. Scale bar, 200 μm. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
MSCs-IL37 Had an Enhanced Immune-Suppression Function In Vitro
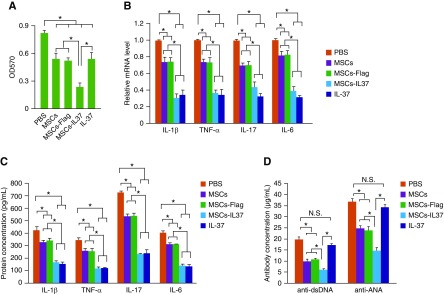
To study the immune-suppressive function of the MSCs-IL37, splenocytes were isolated from the MRL/lpr mice and stimulated by PMA. After 2 days of coculture, the proliferation of the splenocytes was analyzed by MTT assay. Data showed that the unmodified MSCs (MSCs), MSCs infected with lentivirus expressing Flag peptide (MSCs-Flag, negative control), MSCs overexpressing the IL-37 (MSCs-IL37) and the recombinant human IL-37 protein could significantly suppress the splenocytes proliferation (Figure 2A). Furthermore, the MSCs-IL37 had more suppressive effect on the proliferation of the splenocytes when comparing with the MSCs, MSCs-Flag or the IL-37 protein (Figure 2A). To eliminate the possibility that the proliferation of the MSCs also had been affected by the splenocytes or the PMA, the MSCs were cultured for three more days after they reached confluence and then subjected to the coculture assay, which should stop the MSCs proliferation through contact inhibition. In addition, the effects of splenocytes and/or PMA on these over-confluent MSCs were also analyzed by a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) assay. Both the PMA and the splenocytes had no obvious effects on the proliferation of MSCs after they reached confluence.
Figure 2.
MSCs-IL37 had enhanced immune suppression function in vitro. (A) The proliferation assay of splenocytes derived from the MRL/lpr mice by MTT after coculture with the MSCs, MSCs-Flag, MSCs-IL37, or the protein IL-37 (n=3). (B) The mRNA levels of proinflammatory factor IL-1β, TNF-α, IL-17, and IL-6 in the PBMCs of the MRL/lpr mice were determined by quantitative PCR after coculture with the MSCs, MSCs-Flag, MSCs-IL37, or the protein IL-37 (n=3). (C) The protein levels of proinflammatory factor IL-1β, TNF-α, IL-17, and IL-6 secreted by the PBMCs of MRL/lpr mice were determined by ELISA after coculture with the MSCs, MSCs-Flag, MSCs-IL37, or the protein IL-37 (n=3). (D) The levels of the autoantibodies, anti-dsDNA and anti-ANA, produced by the PBMCs of the MRL/lpr mice were determined by ELISA after coculture with the MSCs, MSCs-Flag, MSCs-IL37, or the protein IL-37 (n=3). *P<0.05.
The PBMCs were then also isolated from the MRL/lpr mice and cocultured with MSCs, MSCs-Flag, MSCs-IL37, and IL-37 protein. Both the mRNA and protein levels of the proinflammatory factors IL-1β, TNF-α, IL-17, and IL-6 had been significantly suppressed (Figure 2, B and C). The MSCs-IL37 and IL-37 protein showed similar anti-inflammatory effects, which were stronger than those of the MSCs or MSCs-Flag (Figure 2, B and C). The levels of the autoantibodies, anti-dsDNA and anti-ANA, were also decreased by the MSCs, MSCs-Flag, and MSCs-IL37 (Figure 2D). Although the IL-37 protein could not significantly suppress the autoantibody production, overexpressing IL-37 in the MSCs conferred enhanced inhibitory effects on the production of autoantibodies (Figure 2D). Thus MSCs-IL37 had enhanced immune suppressive activities in vitro.
MSCs-IL37 Alleviated SLE Symptoms in the MRL/lpr Mice
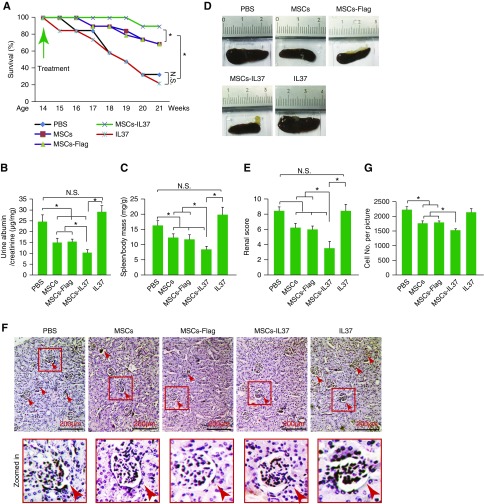
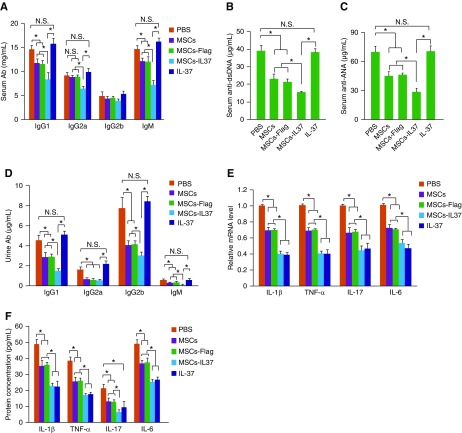
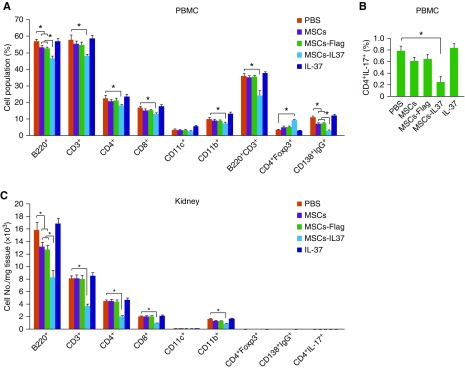
The therapeutic effects of the MSCs-IL37 were then evaluated in the MRL/lpr mice, the mice model of SLE. Transplantation of MSCs improved the overall survival of the MRL/lpr mice and overexpression of IL-37 (MSCs-IL37) further boosted this therapeutic effect (Figure 3A). However, the IL-37 protein did not show any obvious therapeutic effects (Figure 3A). Urine protein concentration, spleen weight, and renal pathology scoring analysis also indicated the MSCs-IL37 had enhanced therapeutic effects compared with the MSCs, whereas the IL-37 protein showed limited effects (Figure 3, B–G). This was further confirmed by the total antibody and autoantibody (anti-dsDNA and anti-ANA) measurements in the serum and urine (Figure 4, A–D). However, the mRNA and protein levels of the proinflammatory factors (IL-1β, TNF-α, IL-17, and IL-6) were significantly suppressed by the IL-37 protein, indicating the IL-37 protein was functional in vivo (Figure 4, E and F). Cell population analysis showed MSCs-IL37 could significantly suppress B220+, CD3+, CD4+, CD8+, CD11b+, B220+CD3+, CD138+IgG+, and CD4+IL17+ cells while increasing the CD4+Foxp3+ cells in the PBMCs of the MRL/lpr mice (Figure 5, A and B). Furthermore, MSCs-IL37 could reduce the number of B220+, CD3+, CD4+, CD8+, and CD11b+ cells in the kidney (Figure 5C). Therefore, the IL-37 protein had the anti-inflammation effects in vivo but could not reverse the symptoms of the MRL/lpr mice. MSCs-IL37 could alleviate the lupus symptoms and also suppress the proinflammatory factors. This therapeutic effect might also include rebalancing of immune cell populations.
Figure 3.
MSCs-IL37 alleviated the SLE symptoms of the MRL/lpr mice. (A) The survival kinetics of the MRL/lpr mice transplanted with MSCs, MSCs-Flag, MSCs-IL37, or injected with the IL-37 protein. PBS was used as negative control (n=20). The treatments were performed at the age of 14 weeks of the MRL/lpr mice. The MSCs were then transplanted every 2 weeks and the IL-37 protein was injected every 2 days. *P<0.05 in both the Kruskal–Wallis test33 and Kaplan–Meier analysis.34 (B) The urine proteins were measured by ELISA kits at the age of 18 weeks, 4 weeks after the first treatment (n=8). (C) The ratio of the spleen/body mass was measured at the age of 18 weeks, 4 weeks after the first treatment (n=8). (D) Representative figures of the spleens isolated from different groups. (E) Renal pathology scoring was conducted at the age of 18 weeks, 4 weeks after the first treatment (n=8). The renal pathology scoring was calculated according to the levels of the glomerular proliferation, inflammation, necrosis, interstitial changes, vasculitis, and crescent formation, each feature was scored from zero to three. The final renal score was the sum of these scores. (F) Representative figures of the affected glomeruli from different groups. The bottom row shows a larger magnification of the affected glomeruli framed in red in the top row. Arrows indicate the affected glomeruli. (G) Counting number of cells in hematoxylin and eosin figures from different groups. *P<0.05. Scale bar, 200 μm.
Figure 4.
MSCs-IL37 reduced the expression of proinflammatory factors and antibody production in MRL/lpr mice. (A) Serum levels of total antibodies (Ab) were measured by ELISA at the age of 18 weeks, 4 weeks after the first treatment (n=8). (B) Serum levels of the anti-DNA antibody were measured by ELISA at the age of 18 weeks, 4 weeks after the first treatment (n=8). (C) Serum levels of the anti-ANA antibody were measured by ELISA at the age of 18 weeks, 4 weeks after the first treatment (n=8). (D) Urine levels of the total antibodies were measured by ELISA at the age of 18 weeks, 4 weeks after the first treatment (n=8). (E) The mRNA levels of proinflammatory factors IL-1β, TNF-α, IL-17, and IL-6 in PBMCs of the MRL/lpr mice were determined by quantitative PCR at the age of 18 weeks, 4 weeks after the first treatment (n=8). (F) Serum levels of the proinflammatory factor IL-1β, TNF-α, IL-17, and IL-6 were determined by ELISA at the age of 18 weeks, 4 weeks after the first treatment (n=8). *P<0.05.
Figure 5.
MSCs-IL37 altered the composition of the major immune cell types in MRL/lpr mice. (A and B) The composition of the major immune cell types in PBMCs of the MRL/lpr mice were analyzed by flow cytometry at the age of 18 weeks, 4 weeks after the first treatment (n=8). (C) The composition of the major immune cell types in the kidney of the MRL/lpr mice were analyzed by flow cytometry at the age of 18 weeks, 4 weeks after the first treatment (n=8). *P<0.05.
Mutual Reinforcement between the IL-37 and MSCs Conferred the Additive Therapeutic Effects
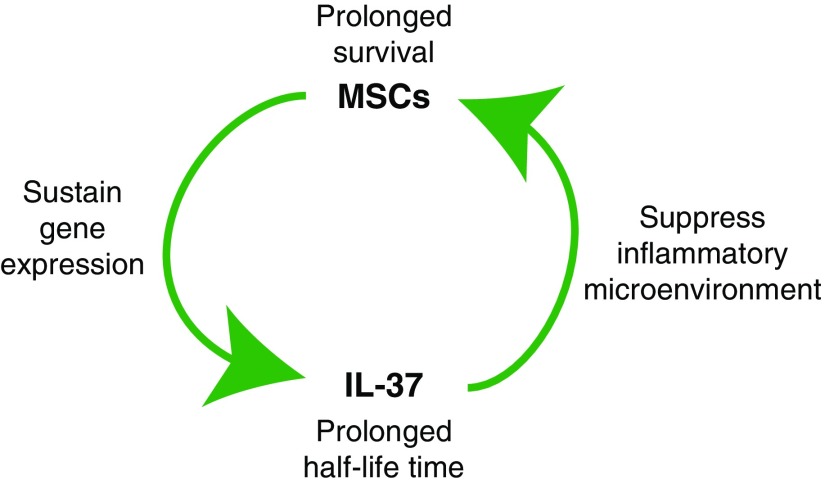
The in vitro and in vivo data here demonstrated that IL-37 had suppressive effects on the expression of proinflammatory cytokines but minimal effects on the autoantibody production and alleviation of lupus symptoms, whereas MSCs had both anti-inflammation and autoantibody-reduction functions, resulting in lupus recovery. It is reasonable that MSCs-IL37 had better anti-inflammation function, but why it also had improved therapeutic effects—including reduction of autoantibodies—remains unclear. We hypothesized that the anti-inflammatory effects of IL-37 might prolong the survival of transplanted MSCs by improving the microenvironment, whereas MSCs-IL37 could successively supply the IL-37 protein. They both benefit each other and the long-lived MSCs-IL37 showed improved therapeutic effects.
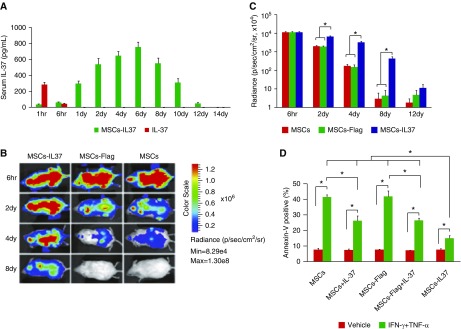
To verify the validity of our hypothesis, the serum level of IL-37 protein was measured after IL-37 protein injection or transplantation of MSCs-IL37. The treatment was performed only once on the 14-week-old MRL/lpr mice. The data showed that IL-37 protein decreased quickly and became undetectable 1 day later (Figure 6A). However, MSCs-IL37 had elevated production of IL-37 after transplantation, reaching a maximum level after 6 days and decreasing slowly until levels were undetectable 14 days later (Figure 6A). On the other hand, luciferase-reporter tracing showed that the MSCs-IL37 had improved survival after transplantation (Figure 6, B and C). To further confirm that the prolonged survival of the transplanted MSCs-IL37 resulted from the anti-inflammatory effects of IL-37, the inflammatory microenvironment was mimicked in vitro by adding the IFN-γ plus TNF-⍺ to the culture medium.35 Cell apoptosis analysis showed that adding the recombinant IL-37 protein or the MSCs-IL37 significantly reduced the levels of apoptosis (Figure 6D). Thus, the IL-37 and MSCs could mutually reinforce each other, resulting in the additive therapeutic effects (Figure 7).
Figure 6.
Mutual reinforcement between the IL-37 and MSCs. (A) The serum level of IL-37 protein was measured by ELISA after IL-37 protein injection or MSCs-IL37 transplantation (n=8). The MRL/lpr mice were transplanted with MSCs-37 or injected with IL-37 protein at the age of 14 weeks; treatments were performed only once. (B) Representative figures of MSCs survival after transplantation, analyzed by luciferase-reporter tracing (n=8). The MRL/lpr mice were transplanted with MSCs-IL37, MSCs-Flag, or MSCs at the age of 14 weeks; treatments were performed only once. (C) Average radiance of the luciferase-labeled MSCs (n=8). (D) Cell apoptosis analysis showed that the MSCs-IL37 had significantly reduced levels of apoptosis (n=3). *P<0.05.
Figure 7.
Proposed underlying mechanism of the mutual reinforcement between IL-37 and MSCs. IL-37 improved the graft survival of the MSCs, possibly through inhibiting the inflammatory microenvironment. The continuous expression of IL-37 by MSCs conferred the successive immune suppression.
Discussion
SLE is characterized by aberrant immune responses to the body’s own tissues, resulting in immune tolerance collapse, local tissue destruction, and chronic inflammation.1–5 MSCs have been proposed as a promising candidate for SLE treatment because of their immune-modulation functions and regenerative capabilities.8 Immune suppression is one of the underlining mechanisms for alleviating the SLE symptoms by MSCs.8 However, the immune-suppressive function is not an innate characteristic of MSCs. MSCs can either promote or suppress the immune system, and their function is determined by their microenvironment.36 For example, the immune-suppressive activities of MSCs could be stimulated by proinflammatory factors, such as IFN-γ, TNF-⍺, and IL-1β.9,11,12,37 However, low levels of proinflammatory signals could cause the MSCs to promote immune responses instead due to an insufficient production of immune suppressors, such as inducible nitric-oxide synthase or indoleamine-pyrrole 2,3-dioxygenase.38 Thus the level of the proinflammatory signals and the host microenvironments determine the therapeutic effects of MSCs.12,39,40
To overcome the uncertainty of MSC therapy in vivo, genetically modified or chemically primed MSCs might be more suitable for SLE treatment.8 Indeed, MSCs combined with immunosuppressive drugs ameliorate lupus symptoms with reduced doses of immunosuppressive drugs, potentially also alleviating their adverse side effects.41–44 Here we demonstrated that genetically modified MSCs overexpressing IL-37, a strong immune suppressor, could boost the therapeutic effects of MSCs. IL-37 improved the graft survival of the MSCs, possibly through inhibiting the inflammatory microenvironment. The continuous expression of IL-37 by MSCs conferred the successive immune suppression. Therefore, IL-37 and MSCs showed additive therapeutic effects for the treatment of SLE in MRL/lpr mice. One of the major limitations of this study is that the mice do not have the gene IL-37,21 thus whether IL-37 has the same function in human as in mice requires further investigation.
However, continuous immune suppression might not be the way to cure SLE, which has been proven by immune-suppression therapies.1–5 Immune-tolerance reconstruction should be the best way to a cure but, unfortunately, it is not feasible thus far. Therefore, the genetically modified MSCs could alleviate SLE symptoms by suppressing the immune system as a short-term therapy. For the long term, the transplanted MSCs might improve the BM microenvironment, which is impaired in the SLE.16,45 The immune tolerance might then be reconstructed by the newly differentiated hematopoietic stem cells in the refined microenvironment or by transplanting extra hematopoietic stem cells. Furthermore, genetic modification using genome-editing tools might provide a better way to overexpress exogenous genes.29
Disclosures
None.
Funding
This work was supported by the Medical Science and Technology Foundation of Guangdong Province (A2018308), National Natural Science Foundation of China for Shenzhen University (2017083), and National Natural Science Foundation of China for Shenzhen (KQJSCX20180328093434771, JCYJ20170302152735071, JCYJ20180305163407913, and JCYJ20170818093720089).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Murphy G, Lisnevskaia L, Isenberg D: Systemic lupus erythematosus and other autoimmune rheumatic diseases: Challenges to treatment. Lancet 382: 809–818, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Tsokos GC, Lo MS, Costa Reis P, Sullivan KE: New insights into the immunopathogenesis of systemic lupus erythematosus. Nat Rev Rheumatol 12: 716–730, 2016 [DOI] [PubMed] [Google Scholar]
- 3.Lisnevskaia L, Murphy G, Isenberg D: Systemic lupus erythematosus. Lancet 384: 1878–1888, 2014 [DOI] [PubMed] [Google Scholar]
- 4.van Kempen TS, Wenink MH, Leijten EF, Radstake TR, Boes M: Perception of self: Distinguishing autoimmunity from autoinflammation. Nat Rev Rheumatol 11: 483–492, 2015 [DOI] [PubMed] [Google Scholar]
- 5.Banchereau R, Cepika AM, Banchereau J, Pascual V: Understanding human autoimmunity and autoinflammation through transcriptomics. Annu Rev Immunol 35: 337–370, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiong W, Lahita RG: Pragmatic approaches to therapy for systemic lupus erythematosus. Nat Rev Rheumatol 10: 97–107, 2014 [DOI] [PubMed] [Google Scholar]
- 7.Hahn BH: Belimumab for systemic lupus erythematosus. N Engl J Med 368: 1528–1535, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Xu J: Therapeutic applications of mesenchymal stem cells for systemic lupus erythematosus. Adv Exp Med Biol 1089: 73–85, 2018 [DOI] [PubMed] [Google Scholar]
- 9.Uccelli A, Moretta L, Pistoia V: Mesenchymal stem cells in health and disease. Nat Rev Immunol 8: 726–736, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Chen X, Cao W, Shi Y: Plasticity of mesenchymal stem cells in immunomodulation: Pathological and therapeutic implications. Nat Immunol 15: 1009–1016, 2014 [DOI] [PubMed] [Google Scholar]
- 11.Keating A: Mesenchymal stromal cells: New directions. Cell Stem Cell 10: 709–716, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Bernardo ME, Fibbe WE: Mesenchymal stromal cells: Sensors and switchers of inflammation. Cell Stem Cell 13: 392–402, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al.: Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8: 315–317, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Liao J, Chang C, Wu H, Lu Q: Cell-based therapies for systemic lupus erythematosus. Autoimmun Rev 14: 43–48, 2015 [DOI] [PubMed] [Google Scholar]
- 15.Sun L, Wang D, Liang J, Zhang H, Feng X, Wang H, et al.: Umbilical cord mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus. Arthritis Rheum 62: 2467–2475, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Sun L, Akiyama K, Zhang H, Yamaza T, Hou Y, Zhao S, et al. : Mesenchymal stem cell transplantation reverses multiorgan dysfunction in systemic lupus erythematosus mice and humans. Stem Cells 27: 1421–1432, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou K, Zhang H, Jin O, Feng X, Yao G, Hou Y, et al. : Transplantation of human bone marrow mesenchymal stem cell ameliorates the autoimmune pathogenesis in MRL/lpr mice. Cell Mol Immunol 5: 417–424, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan MC, Kuok DI, Leung CY, Hui KP, Valkenburg SA, Lau EH, et al.: Human mesenchymal stromal cells reduce influenza A H5N1-associated acute lung injury in vitro and in vivo. Proc Natl Acad Sci U S A 113: 3621–3626, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Che N, Li X, Zhang L, Liu R, Chen H, Gao X, et al.: Impaired B cell inhibition by lupus bone marrow mesenchymal stem cells is caused by reduced CCL2 expression. J Immunol 193: 5306–5314, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rasmusson I, Le Blanc K, Sundberg B, Ringdén O: Mesenchymal stem cells stimulate antibody secretion in human B cells. Scand J Immunol 65: 336–343, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Nold MF, Nold-Petry CA, Zepp JA, Palmer BE, Bufler P, Dinarello CA: IL-37 is a fundamental inhibitor of innate immunity. Nat Immunol 11: 1014–1022, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu WD, Zhao Y, Liu Y: Insights into IL-37, the role in autoimmune diseases. Autoimmun Rev 14: 1170–1175, 2015 [DOI] [PubMed] [Google Scholar]
- 23.Li S, Neff CP, Barber K, Hong J, Luo Y, Azam T, et al.: Extracellular forms of IL-37 inhibit innate inflammation in vitro and in vivo but require the IL-1 family decoy receptor IL-1R8. Proc Natl Acad Sci U S A 112: 2497–2502, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lunding L, Webering S, Vock C, Schröder A, Raedler D, Schaub B, et al.: IL-37 requires IL-18Rα and SIGIRR/IL-1R8 to diminish allergic airway inflammation in mice [published correction appears in Allergy 71: 910, 2016]. Allergy 70: 366–373, 2015 [DOI] [PubMed] [Google Scholar]
- 25.Bulau AM, Nold MF, Li S, Nold-Petry CA, Fink M, Mansell A, et al.: Role of caspase-1 in nuclear translocation of IL-37, release of the cytokine, and IL-37 inhibition of innate immune responses. Proc Natl Acad Sci U S A 111: 2650–2655, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ye L, Ji L, Wen Z, Zhou Y, Hu D, Li Y, et al.: IL-37 inhibits the production of inflammatory cytokines in peripheral blood mononuclear cells of patients with systemic lupus erythematosus: Its correlation with disease activity. J Transl Med 12: 69, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soleimani M, Nadri S: A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat Protoc 4: 102–106, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Xu JY, Lee YK, Ran X, Liao SY, Yang J, Au KW, et al.: Generation of induced cardiospheres via reprogramming of skin fibroblasts for myocardial regeneration. Stem Cells 34: 2693–2706, 2016 [DOI] [PubMed] [Google Scholar]
- 29.Jiang W, Lian W, Chen J, Li W, Huang J, Lai B, et al.: Rapid identification of genome-edited mesenchymal stem cell colonies via Cas9. Biotechniques 66: 231–234, 2019 [DOI] [PubMed] [Google Scholar]
- 30.Haifler M, Lask A, Gal J, Verhovsky G, Kord E, Zisman A: Interobserver agreement of the estimated contact surface area system for renal masses. Clin Genitourin Cancer 17: e802–e805, 2019 [DOI] [PubMed] [Google Scholar]
- 31.Mishra N, Reilly CM, Brown DR, Ruiz P, Gilkeson GS: Histone deacetylase inhibitors modulate renal disease in the MRL-lpr/lpr mouse. J Clin Invest 111: 539–552, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watson ML, Rao JK, Gilkeson GS, Ruiz P, Eicher EM, Pisetsky DS, et al.: Genetic analysis of MRL-lpr mice: Relationship of the Fas apoptosis gene to disease manifestations and renal disease-modifying loci. J Exp Med 176: 1645–1656, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwarting A, Paul K, Tschirner S, Menke J, Hansen T, Brenner W, et al.: Interferon-beta: A therapeutic for autoimmune lupus in MRL-Faslpr mice. J Am Soc Nephrol 16: 3264–3272, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Kulkarni O, Pawar RD, Purschke W, Eulberg D, Selve N, Buchner K, et al. : Spiegelmer inhibition of CCL2/MCP-1 ameliorates lupus nephritis in MRL-(Fas)lpr mice. J Am Soc Nephrol 18: 2350–2358, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Dang S, Yu ZM, Zhang CY, Zheng J, Li KL, Wu Y, et al.: Autophagy promotes apoptosis of mesenchymal stem cells under inflammatory microenvironment. Stem Cell Res Ther 6: 247, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, et al.: Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2: 141–150, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Krampera M: Mesenchymal stromal cell ‘licensing’: A multistep process. Leukemia 25: 1408–1414, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Li W, Ren G, Huang Y, Su J, Han Y, Li J, et al.: Mesenchymal stem cells: A double-edged sword in regulating immune responses. Cell Death Differ 19: 1505–1513, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sheng H, Wang Y, Jin Y, Zhang Q, Zhang Y, Wang L, et al.: A critical role of IFNgamma in priming MSC-mediated suppression of T cell proliferation through up-regulation of B7-H1. Cell Res 18: 846–857, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Mai S, Zou L, Tian X, Liao X, Luan Y, Han X, et al. : Double-edged effect of hydroxychloroquine on human umbilical cord-derived mesenchymal stem cells treating lupus nephritis in MRL/lpr mice. Mol Pharm 15: 1800–1813, 2018 [DOI] [PubMed] [Google Scholar]
- 41.Lee HK, Kim KH, Kim HS, Kim JS, Lee JH, Ji A, et al. : Effect of a combination of prednisone or mycophenolate mofetil and mesenchymal stem cells on lupus symptoms in MRL.Faslpr mice. Stem Cells Int 2018: 4273107, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hajkova M, Hermankova B, Javorkova E, Bohacova P, Zajicova A, Holan V, et al.: Mesenchymal stem cells attenuate the adverse effects of immunosuppressive drugs on distinct T cell subopulations. Stem Cell Rev Rep 13: 104–115, 2017 [DOI] [PubMed] [Google Scholar]
- 43.Eggenhofer E, Renner P, Soeder Y, Popp FC, Hoogduijn MJ, Geissler EK, et al.: Features of synergism between mesenchymal stem cells and immunosuppressive drugs in a murine heart transplantation model. Transpl Immunol 25: 141–147, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Wang H, Qi F, Dai X, Tian W, Liu T, Han H, et al.: Requirement of B7-H1 in mesenchymal stem cells for immune tolerance to cardiac allografts in combination therapy with rapamycin. Transpl Immunol 31: 65–74, 2014 [DOI] [PubMed] [Google Scholar]
- 45.Liu S, Liu D, Chen C, Hamamura K, Moshaverinia A, Yang R, et al. : MSC transplantation improves osteopenia via epigenetic regulation of notch signaling in lupus. Cell Metab 22: 606–618, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]