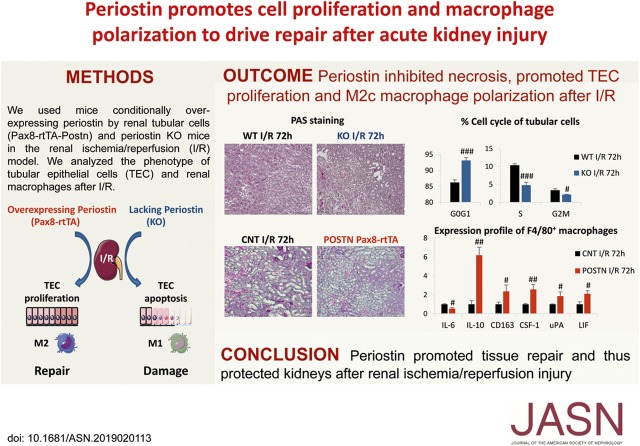
Significance Statement
Studies in animal models and human biopsy specimens have associated the matricellular protein periostin with CKD progression, but its role in AKI is unknown. To investigate periostin’s role in AKI in an ischemia-reperfusion injury model, they used mice with tubule-specific overexpression of periostin and mice lacking periostin expression. They also conducted in vitro studies in primary cultures of isolated tubular cells subjected to hypoxia reoxygenation. Periostin produced by damaged epithelial cells after acute ischemic injury protected epithelial cells from persistent cell cycle arrest and death and promoted a proregenerative macrophage phenotype, both of which contribute to more efficient repair of the injured epithelium. The study’s findings implicate periostin as a novel mediator of renal repair after AKI, and may provide insights into repair mechanisms after AKI.
Keywords: acute renal failure, apoptosis, cell survival, ischemia-reperfusion, macrophages, tubular epithelium
Visual Abstract
Abstract
Background
The matricellular protein periostin has been associated with CKD progression in animal models and human biopsy specimens. Periostin functions by interacting with extracellular matrix components to drive collagen fibrillogenesis and remodeling or by signaling through cell-surface integrin receptors to promote cell adhesion, migration, and proliferation. However, its role in AKI is unknown.
Methods
We used mice with conditional tubule-specific overexpression of periostin or knockout mice lacking periostin expression in the renal ischemia-reperfusion injury model, and primary cultures of isolated tubular cells in a hypoxia-reoxygenation model.
Results
Tubular epithelial cells showed strong production of periostin during the repair phase of ischemia reperfusion. Periostin overexpression protected mice from renal injury compared with controls, whereas knockout mice showed increased tubular injury and deteriorated renal function. Periostin interacted with its receptor, integrin-β1, to inhibit tubular cell cycle arrest and apoptosis in in vivo and in vitro models. After ischemia-reperfusion injury, periostin-overexpressing mice exhibited diminished expression of proinflammatory molecules and had more F4/80+ macrophages compared with knockout mice. Macrophages from periostin-overexpressing mice showed increased proliferation and expression of proregenerative factors after ischemia-reperfusion injury, whereas knockout mice exhibited the opposite. Coculturing a macrophage cell line with hypoxia-treated primary tubules overexpressing periostin, or treating such macrophages with recombinant periostin, directly induced macrophage proliferation and expression of proregenerative molecules.
Conclusions
In contrast to the detrimental role of periostin in CKD, we discovered a protective role of periostin in AKI. Our findings suggest periostin may be a novel and important mediator of mechanisms controlling renal repair after AKI.
AKI is a dynamic process involving hemodymamic changes, inflammation, and endothelial and epithelial cell damage, followed by repair and restoration of renal function. Ischemia/reperfusion (I/R) is among the primary causes of AKI.1 The nature of the recovery response is dependent on the degree to which the injured cells can regenerate and restore normal function. A maladaptive response to AKI has been associated to secondary CKD development.2 AKI was also linked with distal organ effects including pulmonary, cardiac, hepatic, and neurologic dysfunction.3 The mechanisms of injury and repair after AKI are not yet fully understood, but epithelial cells and macrophages are at the forefront of these processes.
Periostin is a 90-kDa secreted matricellular protein with high physiologic expression in bone and dental tissues.4 Although its expression in most adult tissues is low, periostin is de novo expressed during chronic disease of several organs, including the kidney, promoting inflammatory and fibrotic processes or proliferation.5–7 Periostin exerts its functions by interacting with extracellular matrix components to drive collagen fibrillogenesis and remodeling,8–10 or by signaling through cell-surface integrin receptors to promote cell adhesion, migration, and proliferation.11–13 Periostin is induced by different profibrotic factors like TGF-β1,4,10,14 angiotensin-II,14 and PDGF;15 the ILs IL-4 and IL-13;16,17 or proinflammatory transcription factors like NFκB.18 Although the association of periostin with CKD progression has been established in different experimental models and biopsy specimens,5,19 the role of periostin in AKI is still unknown.
Here, we investigated the role of periostin in the model of I/R-induced AKI. Periostin was strongly de novo expressed by renal tubular cells at the repair phase of I/R. Mice lacking periostin showed worsened kidney structure and function, whereas mice conditionally overexpressing periostin in the renal tubules displayed highly preserved renal phenotypes, both short term and long term after I/R. Subsequent studies revealed a direct effect of periostin in protecting epithelial cells from cell cycle arrest and death and promoting their proliferation. Moreover, periostin drove local macrophage proliferation after I/R and polarization to a proregenerative trophic phenotype. Our results describe for the first time a novel role for periostin as a protective factor in AKI, by regulating the fates of epithelial cells and macrophages to drive renal repair.
Methods
Animals
All procedures regarding animal experimentation were in accordance with the European Union Guidelines for the Care and Use of Laboratory Animals and approved by the local ethics committee of the National Institute for Health and Medical Research (Institut National de la Santé et de la Recherche Médicale, INSERM). Animals were housed at a constant temperature with free access to water and food.
The strain of periostin knockout (KO) mice was created at the laboratory of Dr. Simon Conway and has been previously described.20 The mice were used in the C57BL/6 background. Wild-type (WT) littermates were used as controls.
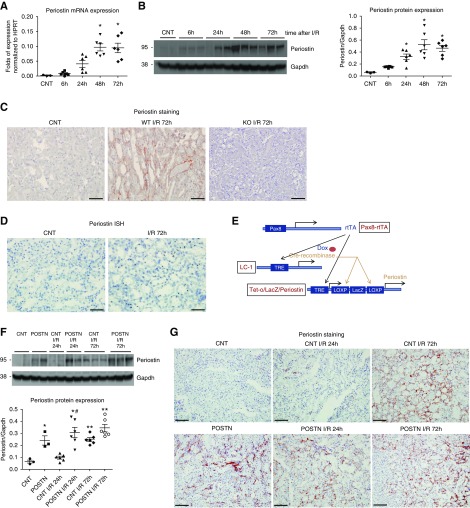
Transgenic mice conditionally expressing periostin (POSTN) in tubular epithelial cells (TECs) were created by crossing three different transgenic strains: (1) Pax8-rtTA (C57BL/6:DBA) mice expressing the reverse tetracycline-dependent transactivator (rtTA) under the control of the murine Pax8 promoter; (2) LC1 mice (C57BL/6:BALB/c) where the expression of Cre recombinase is under control of the bidirectional Ptet promoter—when combined, they drive the expression of Cre recombinase in the renal tubular compartment after doxycycline administration (these two strains were kindly provided by Dr. Robert Koesters and have been previously described21); and (3) Tet-o-Periostin (FVB/N) mice which carry the mouse periostin cDNA downstream of a loxP-LacZ-loxP cassette under control of the Ptet promoter and were created at the genomic facilities of the Pitié Salpêtrière Hospital in Paris. After extensive interbreeding, the genetic background of the transgenic mice was mixed, consisting of FVB/N:C57BL/6:DBA:BALB/c. The primers and conditions used for genotyping of the transgenic mice are presented in Supplemental Table 1. Periostin expression was induced by administration of 0.2 mg/ml doxycycline in drinking water containing 2.5% sucrose for a period of 5 weeks starting 1 month after birth, after which the mice were subjected to I/R. To examine the effect of periostin overexpression alone, doxycycline was administered to triple transgenic mice and double transgenic Pax8-rtTA/LC1-cre mice, serving as controls, for up to 4 months.
I/R Model
Male KO mice and their WT littermates (8–10 weeks old; n=7–8 per group) were anesthetized with intraperitoneal injection of a combination of ketamine (100 mg/kg) and xylazine (10 mg/kg) and subjected to right kidney nephrectomy. Right kidneys were used as controls. The left renal artery was clamped for 30 minutes of warm ischemia at 37°C followed by 24 hours, 72 hours, or 6 weeks of reperfusion. After reperfusion, the mice were euthanized and blood and renal tissues were collected for subsequent analyses.
I/R on POSTN male mice, 9–10 weeks old (n=5–7), was performed as described above, after administration of doxycycline for 5 weeks. Double transgenic Pax8-rtTA/LC1-cre male mice of the same age treated with doxycycline for the same period were used as controls. After right nephrectomy, the left renal artery was clamped for 35 minutes followed by 24 hours, 72 hours, or 6 weeks of reperfusion. The mice were euthanized and blood and renal tissues were collected for subsequent analyses. This strain (FVB/N:C57BL/6:DBA:BALB/c mixed background) required increased clamping time compared with the KO strain (C57BL/6 background) because it is less sensitive to I/R injury.
Cell Cycle Analysis of TECs after I/R
Mice that were reperfused for 72 hours were euthanized and the kidneys were collected, decapsulated, and manually minced using a scalpel followed by dissociation in PBS, 0.5% BSA, and 2 mM EDTA in a GentleMACS Dissociator. The homogenate was filtered through a 30-μm filter and centrifuged. The pellet was resuspended in PBS/BSA/EDTA. The cells were fixed and permeabilized with the Foxp3/Transcription Factor Staining Buffer Set (catalog number 00-5523-00; ThermoFisher) and incubated with an anti–Pax8-FITC antibody (orb16164; Biorbyt), followed by nuclear DNA staining with 4′,6-diamidino-2-phenylindole. An IgG-FITC isotype was used as control. The Pax8+ tubular cell population was selected, and the cell cycle phases were analyzed and quantified using MACSQuant Analyzer 10 and Flowlogic software.
Primary Culture of TECs
Primary tubular cells were isolated from periostin WT and KO mice or double transgenic Pax8-LC1 (CNT) and triple transgenic (POSTN) mice without prior doxycycline administration. The kidneys were manually minced and incubated with 1 mg/ml collagenase I in PBS with 0.5% BSA for 3 minutes at 37°C. A 10% FBS-containing RPMI medium was added to deactivate the collagenase, and the mixture was passed first through a 70-μm and then through a 40-μm filter (BD Falcon). The TECs were collected at the bottom of the 40-μm filter, seeded directly on culture plates in RPMI with 10% FBS medium, and cultured to confluence. For the immunofluorescence experiments, the cells were seeded on poly-l-lysine–coated glass coverslips in culture plates.
Hypoxia Treatment of Cultured Primary TECs
To induce periostin overexpression before hypoxia, 80%–90% confluent cultures of Pax8-LC1 (CNT) and triple transgenic (POSTN) primary tubular cells were treated with 1 μg/ml doxycycline in full medium for 2 days. To induce hypoxia, confluent cultures were incubated in four volumes of mineral oil on top of one volume of low-serum medium (RPMI with 1% FBS) for 3 hours at 37°C, followed by extensive washing and reperfusion in full medium for 1–2 hours or 24 hours. Nonhypoxia-treated cells were used as controls. After reperfusion, the cells were processed for protein extraction or immunofluorescence staining as described above. For the lactate dehydrogenase (LDH) assay, the cells were incubated with 75 μM or 125 μM of an integrin-β1 (Itgβ1) inhibitor (AS-62049; Anaspec) or a control arginylglycylaspartic acid peptide (AS-62527; Anaspec) in RPMI with 1% FBS medium for 2 hours before hypoxia.
Hydrogen Peroxide Treatment of Cultured Primary TECs
Confluent cultures were incubated with 400 µM hydrogen peroxide (H2O2) in full medium to induce oxidative stress and apoptosis for 2–24 hours. The cells were processed for protein extraction or immunofluorescence staining, LDH assays, or crystal violet assays as described below or in the Supplemental Methods (Supplemental Table 2).
Renal Macrophage Isolation
Mice that were reperfused for 72 hours were euthanized and the kidneys were collected, decapsulated, and manually minced using a scalpel followed by dissociation in PBS, 0.5% BSA, and 2 mM EDTA in a magnetic-cell-sorting dissociator. The homogenate was filtered through a 30-μm filter and centrifuged. The pellet was resuspended in PBS/BSA/EDTA and incubated with anti-F4/80 magnetic microbeads (130-110-443; Miltenyi Biotec) according to manufacturer’s instructions. F4/80+ cells were magnetically separated with LS columns and a magnetic-cell-sorting separator, and then processed for RNA and protein extraction as described above. All equipment and antibodies were purchased from Miltenyi Biotec.
Coculture of Primary TECs with Macrophages
The murine macrophage RAW 264.7 cell line was purchased from Sigma and maintained according to manufacturer’s instructions in 10% FBS-DMEM medium. Primary cultures of TECs, pretreated with doxycycline, were subjected to mineral oil–induced hypoxia as described above. RAW 264.7 macrophages were placed on top of TECs at reoxygenation, or normoxic TECs and cocultures were maintained in low-serum, 1% FBS-DMEM medium for 48 hours. Cocultures were then processed for RNA extraction, protein extraction, or immunofluorescence as described previously.
Itgβ1 Silencing of Cultured Primary TECs
Transfection of cultured primary TECs with Itgβ1 small interfering RNA (siRNA) or a negative control siRNA (TriFECTa DsiRNA Kit, mm.Ri.Itgb1.13; Integrated DNA Technologies) using HiPerFect transfection reagent (Qiagen) was performed according to manufacturer’s instructions. Following transfection, the cells were treated with doxycycline for 48 hours to induce expression of periostin and were harvested for Western blotting analysis or subjected to mineral oil–induced hypoxia followed by an LDH assay.
LDH Assay
The Cytotoxicity Detection KitPLUS (LDH) from Roche (catalog number 04744926001) was used according to manufacturer’s instructions. Confluent primary cultures transfected with Itgβ1 or a negative control siRNA and pretreated with doxycycline were subjected to mineral oil–induced hypoxia followed by 2 hours reperfusion, as described above. LDH release as an indicator of cell death was measured in culture medium using a spectrophotometer at 492 nm with a noise removal at 650 nm and normalized to the total LDH cell content. The results were expressed as percentage LDH release.
Crystal Violet Viability Assay
After washing with PBS, cells were fixed in ethanol for 15 minutes and colored with crystal violet solution (C.I.42555; Merck Millipore) for 20 minutes. Solubilization of the stain with 33% acetic acid was performed for an appropriate time (10–15 minutes), before measuring the absorbance at 590 nm in a spectrophotometer.
Treatment of RAW Macrophages with Recombinant Periostin
Subconfluent cultures of RAW 264.7 cells were incubated overnight with low-serum medium (1% FBS-DMEM) and treated with 1 μg/ml recombinant mouse periostin (catalog number 2955-F2-050; R&D Systems) in 1% FBS medium for 48 hours. Control cells were incubated with medium alone. The cells were collected for RNA extraction and quantitative real-time PCR or subjected to crystal violet assay for assessment of cell proliferation.
Statistical Analysis
Data are expressed as mean values±SEM. Analyses were performed using GraphPad Prism software. Data were analyzed using one-way ANOVA followed by a Fisher test. Values of P<0.05 were considered significant.
Results
Periostin Is Strongly Expressed at the Repair Phase of I/R by Renal Epithelial Cells
Periostin expression was gradually augmented from 24 hours (injury phase) to 72 hours (repair phase) after I/R in WT mice (Figure 1, A and B). Periostin was secreted and localized at the tubulointerstitial area after I/R, showing no expression in KO mice (Figure 1C). In situ hybridization showed that it was predominantly produced by renal tubular cells (Figure 1D). Colocalization of periostin with tubule-specific markers after I/R revealed that periostin was more abundant around injured proximal tubules and loop of Henle, and less abundant around collecting ducts and distal tubular cells (Supplemental Figure 1).
Figure 1.
Periostin is strongly expressed at the repair phase of I/R by renal epithelial cells. (A) Periostin mRNA and (B) protein expression is gradually upregulated toward the repair phase of I/R. (C) Periostin is localized in the tubulointerstitial parenchyma around injured and dilated tubules after I/R in WT mice, showing no expression in KO mice. (D) Periostin is produced by renal tubular cells after I/R, as revealed by in situ hybridization staining. (E) Scheme of triple transgenic construct used to overexpress periostin in renal epithelial cells. The rtTA drives the expression of Cre recombinase specifically in the renal tubular compartment (controlled by Pax8 promoter) after doxycycline administration. Cre recombinase excises lacZ and moves periostin to the tetracycline response element (TRE) promoter which is expressed in response to doxycycline. (F) Periostin is significantly increased 72 hours after I/R in control mice, while it is stably upregulated in periostin-overexpressing mice. (G) Periostin is secreted around injured tubules at the repair phase of I/R (72 hours) in control mice, showing a similar localization in overexpressing mice at all time points. Representative images are shown. Scale bars, 50 μm in (C) and (D); 100 μm in (G). *P<0.05, **P<0.01 versus CNT; #P<0.05 versus CNT I/R 24 hours. n=3 for CNT and 6 per I/R group. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
To investigate the role of periostin in AKI, we performed I/R for 24 hours, 72 hours, and 6 weeks in two genetically modified strains: one conditionally overexpressing periostin by renal tubular cells (Pax8-rtTA system) (Figure 1E), and one lacking expression of periostin (periostin KO mice). As controls for these strains, we used double transgenic Pax8-rtTA/LC1-cre (named CNT) or WT littermates, respectively. Periostin mRNA and protein expression was significantly augmented at the repair phase of I/R in control mice (CNT), whereas it was increased in periostin-overexpressing mice (POSTN) at all time points (Figure 1F). In both control and overexpressing mice, periostin was secreted at the tubulointerstitial area, whereas its expression pattern in control mice at 72 hours was similar to that of the overexpressing mice (Figure 1G).
Periostin Protects from Deterioration of Renal Structure and Function after I/R
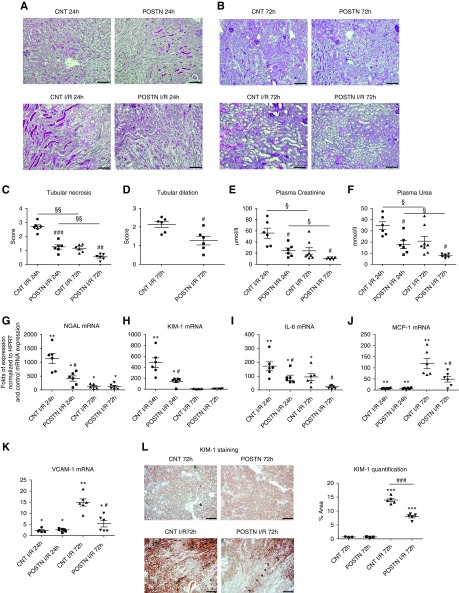
Periostin overexpression protected from severe tubular necrosis at 24 and 72 hours after I/R (Figure 2, A–C), while tubular dilation was also reduced (Figure 2D). Plasma creatinine and urea values were decreased in periostin-overexpressing mice at both time points, showing preserved renal function (Figure 2, E and F). The injury markers neutrophil gelatinase-associated lipocalin (NGAL) and kidney injury molecule-1 (KIM-1) and the expression of proinflammatory cytokines were attenuated in the overexpressing mice either at the injury phase (NGAL), the repair phase (monocyte chemoattractant protein-1, vascular cell adhesion molecule-1), or at both time points after I/R (KIM-1, IL-6) (Figure 2, G–L).
Figure 2.
Periostin overexpression protects from renal structural and functional damage after I/R. (A and B) Periodic acid–Schiff staining showing decreased epithelial damage in periostin-overexpressing mice at both (A) 24 hours and (B) 72 hours after I/R. (C) Tubular necrosis and (D) tubular dilation, depicted in quantification graphs, are decreased in periostin-overexpressing mice after I/R. (E) Plasma creatinine and (F) plasma urea are attenuated in periostin-overexpressing mice at both I/R time points. (G–K) Kidney injury markers (G) NGAL and (H) KIM-1, as well as proinflammatory mediators (I) IL-6, (J) monocyte chemoattractant protein-1 (MCP-1), and (K) vascular cell adhesion molecule-1 (VCAM-1) show reduced expression levels in periostin-overexpressing mice after I/R. The mRNA expression levels are presented relative to basal control levels. (L) Tubular KIM-1 staining is significantly lower in periostin-overexpressing mice 72 hours after I/R. Representative images are shown. Scale bars, 100 μm in (A) and (B); 200 μm in (L). *P<0.05, **P<0.01, ***P<0.001 versus CNT or POSTN; #P<0.05, ##P<0.01, ###P<0.001 versus CNT I/R 24 hours or CNT I/R 72 hours; §P<0.05, §§P<0.01 versus I/R 24 hours. n=3 for CNT and 5–6 per I/R group. HPRT, hypoxanthine-guanine phosphoribosyltransferase.
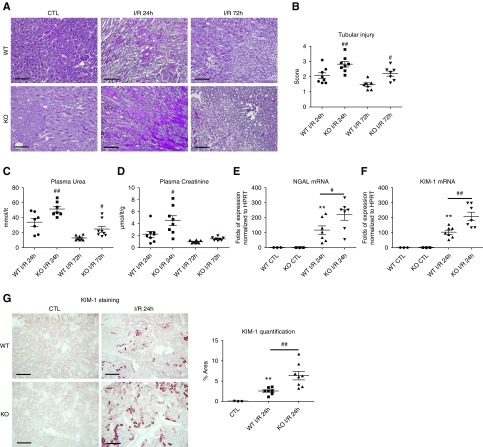
In contrast, periostin KO mice showed increased tubular injury, plasma creatinine, and urea at both 24 and 72 hours (Figure 3, A–D). The expression of KIM-1 and NGAL was augmented at 24 hours in periostin KO mice, indicating increased tubular damage (Figure 3, E–G). The kidney/body weight ratio was increased in both mouse strains after I/R, independently of periostin expression (Supplemental Figure 2).
Figure 3.
Periostin deletion exacerbates renal structural and functional damage after I/R. (A) Periodic acid–Schiff staining and (B) tubular injury score depicting increased epithelial damage in periostin KO mice at both 24 and 72 hours after I/R. (C) Plasma urea and (D) plasma creatinine are increased in periostin KO mice after I/R. (E and F) Injury markers (E) NGAL and (F) KIM-1 show increased expression levels in periostin KO mice at the injury phase of I/R. (G) Tubular KIM-1 staining is significantly higher in periostin KO mice after I/R. Representative images are shown. Scale bars, 100 μm. *P<0.05, **P<0.01 versus CTL; #P<0.05, ##P<0.01 versus WT I/R 24 hours or WT I/R 72 hours. n=3 for CTL and 7–8 per I/R group. CTL, control contralateral kidney.
Periostin Protects from Cell Cycle Arrest and Apoptosis and Promotes Proliferation after I/R
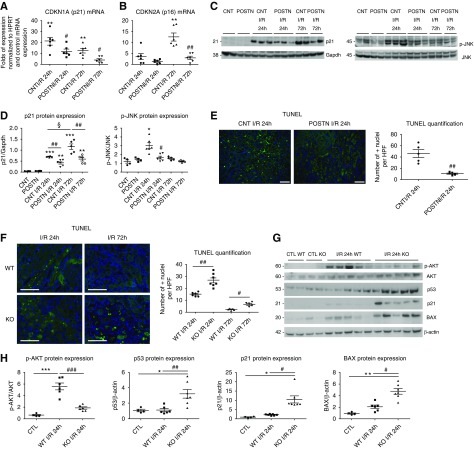
Because epithelial cell death and subsequent proliferation are major mechanisms of injury and repair in AKI, respectively, we examined the interaction of periostin with pathways of apoptosis and proliferation. Indeed, periostin-overexpressing mice displayed reduced expression of the cell cycle arrest genes p21 and p16 at both 24 and 72 hours after I/R (Figure 4, A–D). Activation of c-Jun N-terminal kinase (JNK), a kinase associated with cell stress and death, was prevented in periostin-overexpressing mice (Figure 4, C and D). As confirmation, terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling (TUNEL; Figure 4E) and cleaved caspase-3 staining (Supplemental Figure 3A) were highly reduced in the overexpressing mice, indicating protection against apoptosis/cell death pathways. Cell cycle analysis of Pax8+ tubular cells with flow cytometry 72 hours after I/R demonstrated a decreased proportion of periostin-overexpressing tubular cells in G0G1 and an increased proportion in S phase (Supplemental Figure 4, A and B).
Figure 4.
Periostin protects from cell cycle arrest and apoptosis after I/R. (A and B) The expression levels of the cell cycle arrest mediators (A) p21 and (B) p16 is highly attenuated in periostin-overexpressing mice after I/R. mRNA expression is presented relative to basal control levels. (C and D) Western blots of p21 and p-JNK and their quantifications showing decreased protein levels in periostin-overexpressing mice after I/R. (E) Cell death, assessed by TUNEL staining, is reduced in periostin-overexpressing mice after I/R. (F) TUNEL staining showing increased cell death in periostin KO mice after I/R. (G) Western blots and (H) quantifications of p-AKT, p53, p21, and BAX, showing decreased protein levels of p-AKT and increased levels of p53, p21, and BAX in periostin KO compared with WT mice after I/R. Representative images are shown. Scale bars, 100 μm in (E); 50 μm in (F). *P<0.05, **P<0.01, ***P<0.001 versus CNT or CTL; #P<0.05, ##P<0.01, ###P<0.001 versus CNT I/R or WT I/R; §P<0.05 versus I/R 24 hours. n=4 for CNT or CTL and 5–7 per I/R group. CTL, control contralateral kidney; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HPF, high-powered field; HPRT, hypoxanthine-guanine phosphoribosyltransferase.
TUNEL (Figure 4F) and cleaved caspase-3 staining (Supplemental Figure 3B) were increased in periostin KO mice after I/R, indicating increased apoptosis. Activation of protein kinase B (AKT), a major prosurvival pathway, was higher in WT mice; whereas proteins associated with cell cycle arrest and death, like p53, p21, and BAX, were more highly induced in KO mice after I/R (Figure 4, G and H). A higher proportion of Pax8+ tubular cells from KO mice was in the G0G1 phase after I/R compared with WT littermates, whereas a lower proportion was in the S and G2M cell cycle phases (Supplemental Figure 4C), indicating that periostin attenuates cell cycle arrest and promotes proliferation after I/R.
Periostin Inhibits Hypoxia-Induced Epithelial Cell Death Partially through Interacting with Itgβ1
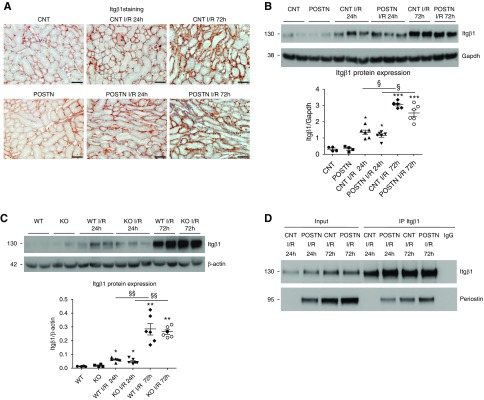
To investigate the mechanisms of periostin-mediated protection after AKI, we examined the expression of potential periostin receptors. Itgβ1 expression was gradually augmented on the basal side of TECs from 24 to 72 hours after I/R (Figure 5A), with no major expression differences between groups (Figure 5, B and C). Coimmunoprecipitation with an Itgβ1 antibody revealed that periostin specifically interacted with Itgβ1 after I/R (Figure 5D). The Itgβ1 downstream targets focal adhesion kinase (FAK) and extracellular signal–regulated kinase (ERK) were more highly activated in KO mice, and phosphorylated ERK (p-ERK) was less increased in periostin-overexpressing mice at 24 hours (Supplemental Figure 5). This suggests the periostin-Itgβ1 interaction leads to lower Itgβ1-associated signaling after I/R, which may be linked to periostin-mediated protection.
Figure 5.
Periostin interacts with Itgβ1 on epithelial cells after I/R. (A) Itgβ1 is gradually upregulated in TECs after I/R, (B) whereas its levels are similarly induced in control and periostin-overexpressing mice, or (C) WT and periostin KO mice. (D) Coimmunoprecipitation with an Itgβ1 antibody showed that Itgβ1 interacts with periostin after I/R. Representative images are shown. Scale bars, 50 μm. *P<0.05, **P<0.01, ***P<0.001 versus CNT, POSTN, WT, or KO; §P<0.05, §§P<0.01 versus I/R 24 hours. n=4 for CNT or CTL and 6 per I/R group. CTL, control contralateral kidney; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
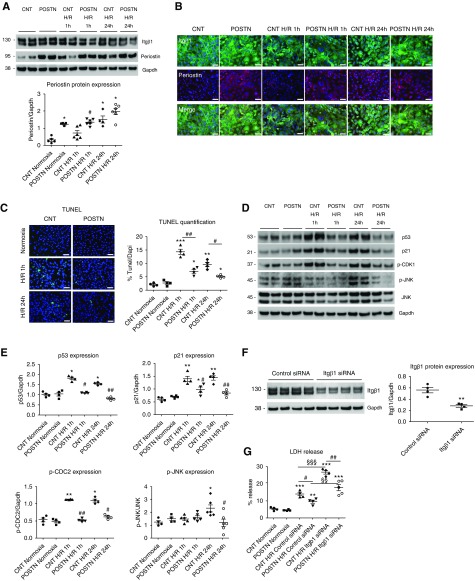
We studied the direct effects of periostin in primary cultures of isolated tubular cells subjected to mineral oil–induced hypoxia. Although Itgβ1 expression did not change after reoxygenation, periostin gradually increased from 1 to 24 hours of reoxygenation after hypoxia, mimicking its expression pattern in the mouse model (Figure 6A). Immunofluorescence studies showed the epithelial layer lost its integrity and the interaction pattern mediated by Itgβ1 after hypoxia; this phenotype was markedly ameliorated in cells overexpressing periostin (Figure 6B). Periostin-overexpressing cells showed decreased apoptosis (Figure 6C) and a blunted induction of cell cycle arrest and proapoptotic proteins after hypoxia, including p53, p21, phosphorylated cyclin-dependent kinase (p-CDK1), and p-JNK (Figure 6, D and E). Periostin-overexpressing cells displayed lower LDH release and death compared with control cells after hypoxia, whereas Itgβ1 silencing increased the LDH release of both strains, indicating that both Itgβ1 and periostin are implicated in cell survival after hypoxia (Figure 6, F and G).
Figure 6.
Periostin inhibits hypoxia-induced cell death of primary tubular cells through interaction with Itgβ1. (A) Cultured primary tubular cells from control mice gradually overexpress periostin after mineral oil–induced hypoxia to a similar level with cells from periostin-overexpressing mice, whereas Itgβ1 expression remains stable. (B) Coimmunofluorescence staining of Itgβ1 and periostin in normoxic conditions and after 1 or 24 hours of mineral oil–induced hypoxia. The interaction pattern of control epithelial cells mediated by Itgβ1 is lost after hypoxia, whereas it is markedly preserved in periostin-overexpressing cells. (C) Cell death, assessed by TUNEL, is reduced in tubular cells overexpressing periostin at both early and late reoxygenation intervals after hypoxia. (D and E) Western blots and quantifications of cell cycle arrest and apoptosis mediators, showing blunted expression in periostin-overexpressing cells after hypoxia. (F) Western blot and quantification of Itgβ1 expression in primary tubular cells treated with control or Itgβ1 siRNA, showing efficient inhibition of Itgβ1 protein synthesis in Itgβ1 siRNA–treated cells. (G) Itgβ1 silencing increases the LDH release, indicative of cell death, of both control and periostin-overexpressing tubular cells after hypoxia; whereas periostin-overexpressing cells demonstrate highly decreased LDH release both before and after Itgβ1 silencing compared with control cells. Representative images are shown. Scale bars, 100 μm. *P<0.05, **P<0.01, ***P<0.001 versus normoxia; #P<0.05, ##P<0.01 versus CNT H/R 1 hour, CNT H/R 24 hours, CNT H/R control siRNA, or CNT H/R Itgβ1 siRNA; §§P<0.01, §§§P<0.001 versus H/R control siRNA. Quantifications of two to three independent experiments performed in duplicates or triplicates are shown. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; H/R, hypoxia and reoxygenation.
Moreover, H2O2-induced oxidative stress increased the death of primary periostin in KO tubular cells compared with WT, reflected by the increased caspase-3 activation and LDH release, and the decreased cell survival of KO cells (Supplemental Figure 6, A–C). Periostin was produced by WT tubular cells and it was absent in KO cells, whereas Itgβ1 was similarly expressed in both cell types. As in the mouse model, AKT activation was higher in periostin-expressing cells, whereas p53 was more increased in KO cells after H2O2 (Supplemental Figure 6D).
Periostin Induces Local Proliferation of Macrophages in the Kidney and Polarization to a Proregenerative Phenotype
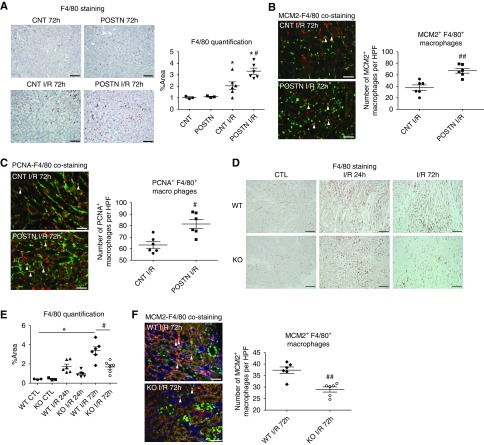
F4/80+ macrophages were more abundant in periostin-overexpressing mice at the repair phase of I/R (Figure 7A). This increase was associated with higher proliferation of F4/80+ macrophages in the overexpressing mice, assessed by costaining with the proliferation markers MCM2 and proliferating cell nuclear antigen (Figure 7, B and C). In periostin KO mice, F4/80+ macrophages were less abundant and showed lower proliferation compared with WT mice (Figure 7, D and F).
Figure 7.
Periostin promotes proliferation of macrophages in the kidney. (A) F4/80 staining and quantification show a higher abundance of macrophages in the kidneys of periostin-overexpressing mice after I/R. (B) MCM2 (green)-F4/80 (red) and (C) proliferating cell nuclear antigen (PCNA) (red)-F4/80 (green) costaining 72 hours after I/R and quantification of the number of the MCM2+ or PCNA+ macrophages reveal increased proliferation of macrophages in periostin-overexpressing mice. (D) F4/80 staining and (E) quantification show a lower abundance of kidney macrophages in periostin KO mice after I/R. (F) MCM2 (green)-F4/80 (red) costaining 72 hours after I/R and quantification of the number of the MCM2+ macrophages reveal decreased proliferation of macrophages in periostin KO mice. Arrowheads indicate MCM2+ or PCNA+ macrophages. Representative images are shown. Scale bars, 100 μm in (A) and (D); 50 μm in (B), (C), and (F). *P<0.05 versus CNT, POSTN, or CTL; #P<0.05, ##P<0.01 versus CNT I/R or WT I/R. n=3 for CNT or CTL and 6 per I/R group. CTL, control contralateral kidney; HPF, high-powered field.
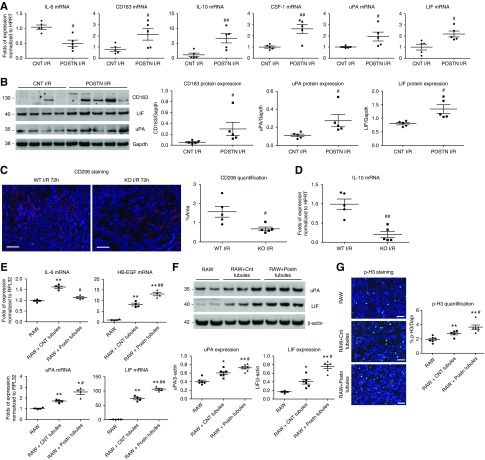
F4/80+ macrophages isolated from periostin-overexpressing mice showed decreased expression of IL-6, a proinflammatory cytokine linked to damage caused by I/R, and increased expression of macrophage genes/markers associated to M2c polarization and cell regeneration, like CD163, IL-10, colony stimulating factor-1 (CSF-1), urokinase-type plasminogen activator (uPA), and leukemia inhibitory factor (LIF) (Figure 8A). The increased expression of CD163, uPA, and LIF was further confirmed at the protein level (Figure 8B). Consistently, periostin KO mice showed a decreased abundance of CD206+ macrophages and lower macrophage expression of IL-10 (Figure 8, C and D), indicating decreased M2 polarization.
Figure 8.
Periostin promotes polarization of kidney macrophages to a proregenerative phenotype. (A) mRNA expression of different markers in F4/80+ macrophages isolated from control and periostin-overexpressing mice 72 hours after I/R. Macrophages from periostin-overexpressing mice show decreased levels of IL-6 and higher levels of CD163, IL-10, CSF-1, uPA, and LIF; indicating a polarization to a M2c regenerative phenotype. (B) Western blots and quantifications of different proteins in F4/80+ macrophages 72 hours after I/R. Macrophages from periostin-overexpressing mice produce higher levels of CD163, a M2c marker, and the trophic proregenerative factors LIF and uPA. (C) CD206+ macrophages in the kidney and (D) IL-10 expression levels in isolated renal macrophages are lower in periostin KO mice after I/R, showing a decreased M2 polarization. (E) mRNA expression of different factors in RAW macrophages cocultured with primary tubular cells for 48 hours at normal conditions (normoxia) or after subjection of cells to mineral oil–induced hypoxia. RAW macrophages cocultured with periostin-overexpressing tubular cells express lower levels of IL-6 and higher levels of heparin-binding EGF-like growth factor (HB-EGF), uPA, and LIF, only after coculture with cells subjected to hypoxia. (F) Macrophages show increased protein production of uPA and LIF after coculture with periostin-overexpressing tubular cells subjected to hypoxia, compared with coculture with control cells. (G) p-H3 staining and quantification, showing a higher induction in the proliferation of macrophages after coculture with hypoxia-treated tubular cells overexpressing periostin. Representative images are shown. Scale bars, 100 μm. *P<0.05, **P<0.01 versus RAW; #P<0.05, ##P<0.01 versus CNT I/R, WT I/R, or RAW + Cnt tubules. n=5 mice per group. For cell cultures, quantifications of two to three independent experiments performed in duplicate or triplicate are shown. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HPRT, hypoxanthine-guanine phosphoribosyltransferase.
Subsequently, we cocultured primary tubular cells from control and overexpressing mice after being submitted to hypoxia with a macrophage cell line (RAW 264.7). Our results showed inhibition of IL-6 and a bigger induction of heparin-binding EGF-like growth factor, uPA, and LIF (Figure 8, E and F), as well as cell proliferation (Figure 8G) in macrophages cocultured with periostin-overexpressing cells. Moreover, treatment of RAW macrophages with recombinant periostin directly inhibited the expression of IL-6 and IL-1β, and induced the expression of IL-10, CD163, uPA, and LIF, as well as cell proliferation (Supplemental Figure 7).
Periostin Does Not Induce Chronic Fibrosis Development Either Alone or after I/R
To examine whether periostin overexpression could induce spontaneous fibrosis development, we administered doxycycline to the transgenic mice for up to 4 months. Collagen 1 and collagen 3 expression as well as interstitial Sirius-Red staining were similar in control and periostin-overexpressing mice after up to 4 months of doxycycline administration, despite high periostin levels in the overexpressing mice (Supplemental Figure 8, A–E). Moreover, although the expression of profibrotic genes was increased at the repair phase of I/R, it was less induced in periostin-overexpressing mice (Supplemental Figure 8, F–H). This effect was maintained for up to 6 weeks after I/R, because periostin-overexpressing mice displayed less Sirius-Red and collagen-1 staining, with KO mice showing more interstitial fibrosis and collagen-1 accumulation, compared with their respective controls (Supplemental Figure 9, A–D). Of note, periostin levels were lower 6 weeks after I/R (Supplemental Figure 9, E and F) compared with the increase observed at the repair phase in both strains (Figure 1).
Discussion
The major finding of this study is that periostin plays a protective role after I/R-induced AKI, thus contrasting its detrimental role during CKD. Using two different transgenic strains, mice conditionally overexpressing periostin in the renal epithelium and mice lacking periostin, we showed for the first time that periostin prevents renal epithelial damage after I/R by at least two mechanisms: by protecting epithelial cells from cell cycle arrest and death, and by promoting a proregenerative macrophage phenotype, thus favoring epithelial repair. The successful regeneration of the injured epithelium and the switching of macrophages to a proreparative phenotype are believed to play a major role in the response to AKI.
Periostin was de novo expressed and secreted by damaged epithelial cells after I/R. Periostin protected from early tubular damage and aggravation of renal function by inhibiting epithelial cell death 24 hours after I/R. This was demonstrated by the increased levels of proapoptotic proteins (p53, p21, BAX) and TUNEL staining in KO mice. Deletion of p53 or BAX in TECs inhibits renal damage and apoptosis after I/R.22,23 Activation of AKT, a prosurvival kinase, was increased in WT mice, potentially limiting proapoptotic signals. Periostin was shown to directly activate AKT in several diseases and contexts.24–26 p21 is strongly induced by p53 in epithelial cells after AKI.22,27 p21 and p16 contribute to growth arrest and stress-induced premature senescence, leading to JNK activation, proliferative arrest, and apoptosis.28,29 However, the consequences of tubular growth arrest or proliferation in AKI were shown to be time and context dependent. Early proliferation of heavily damaged cells may promote further damage, therefore initial cell cycle blockade, e.g., through ischemic preconditioning30 or CDK4/6 inhibition,31 before I/R led to ameliorated kidney injury. Later in the course of AKI or in the case of less-damaged cells, cell cycle activation is beneficial. Periostin diminished the induction of p21 and p-JNK after I/R and inhibited a late increase of both p21 and p16 in overexpressing mice, enabling cell cycle progression.
Renewal of the injured tubular epithelium is believed to be the predominant mechanism of repair after ischemic AKI.32 Derepression of cell cycling by periostin was associated with induction of reparative proliferation 72 hours after I/R. Lack of periostin resulted in a higher G0G1 proportion and a reduced S and G2M phase abundance of tubular cells, whereas periostin overexpression led to decreased tubular G0G1 and increased S phase abundance at the repair phase of I/R. Integrins allow cells to sense and respond to microenvironmental signals and are thus crucial for the adhesion, survival, proliferation, and differentiation of most cell types.33 Itgβ1 controls proliferation and cell cycle regulation and its conditional deletion leads to defective growth of different epithelial cell types.33 In a recent study, Itgβ1 was essential in maintaining skeletal muscle cell homeostasis, regeneration, and growth after experimentally induced muscle injury or in aged and dystrophic muscles through activation of ERK and AKT pathways.34 In I/R-induced AKI, Itgβ1 was increased in tubular cells toward the repair phase and directly interacted with periostin. Except for increased AKT activation in WT mice, other Itgβ1 signaling targets like FAK and ERK were more highly activated in KO mice after I/R. This may suggest that the periostin-Itgβ1 interaction differentially affects Itgβ1 signaling, and/or that increased FAK and ERK activation reflect higher tubular damage. In a model of mineral oil–induced hypoxia of cultured primary tubular cells, periostin was strongly induced at the late phase of reoxygenation after hypoxia, consistent with the I/R model. Cells overexpressing periostin maintained the epithelial layer integrity and showed lower activation of proapoptotic and cell cycle arrest pathways, evidenced by the reduced TUNEL and p-JNK levels, as well as the decreased expression of p53, p21, and p-CDK1.35–37 Consistently, H2O2-induced oxidative stress increased the expression of p53 in primary cultures of periostin KO tubular cells, which was associated with a lack of p-AKT signaling. Itgβ1 silencing in vitro before hypoxia increased further the LDH release and death of periostin-overexpressing cells, indicating that both Itgβ1 and periostin are implicated in cell survival after injury. On the other hand, recombinant periostin was shown to augment the death and expression of fibrotic proteins in cultured tubular cells after 24 hours of hypoxia.38 These results are not contradictory to those presented here because the authors performed a longer hypoxia treatment without reoxygenation, which reflects the long-term injury conditions associated with CKD.
The reparative capacity of the injured epithelium is influenced by local immune responses to tissue damage, with macrophages being a central coordinator of tissue injury and repair.39 Upon injury, resident macrophages are supplemented by actively recruited infiltrating monocytes which differentiate into a proinflammatory pool known as M1 or classically activated macrophages. Macrophages respond to a combination of factors which drives their transition to the M2a or alternatively activated phenotype to coordinate tissue repair.40,41 Several mechanisms contribute to their deactivation and priming toward an anti-inflammatory and proregenerative phenotype, namely M2c, displaying immunosuppressive effects.40,41 The switch from the M1 to the M2 phenotype manifests the transition from the injury to the repair phase in the ischemically injured kidney and is necessary for tubular cell proliferation and functional recovery.42
Our results indicate that epithelial cell–derived periostin promotes a M2 macrophage phenotype assisting in tubular repair. Renal macrophages from WT mice displayed increased levels of M2 markers like mannose receptor (CD206) and IL-10 at the repair phase of I/R compared with those of KO mice. This tendency toward an M2 phenotype was associated with a higher intrarenal macrophage proliferation in WT mice. In glioblastoma xenografts, periostin secreted by stem cells was shown to promote the tumor recruitment of macrophages and their polarization to an M2 phenotype.43
Accordingly, periostin-overexpressing mice displayed a more abundant macrophage population at the repair phase of I/R, which was attributed to increased local macrophage proliferation compared with the control mice. Recently, it was shown that resident macrophages can directly proliferate in tissue without the requirement for blood monocyte recruitment in the context of T helper type 2 cell–mediated inflammation.44,45 Macrophages isolated from periostin-overexpressing mice displayed a less proinflammatory and a more proregenerative phenotype, showing increased expression of M2c markers like CD163 and IL-10 and higher production of proregenerative factors like CSF-1, LIF, and uPA. Inhibition of CSF-1 was associated with decreased numbers of renal macrophages and impaired recovery after AKI,46 whereas LIF and uPA were linked to a M2c macrophage phenotype promoting regeneration after muscle injury.47 LIF was also associated with tubular regeneration after renal I/R in rats.48 M2a markers Arg1 and Ym1 were downregulated in isolated macrophages of periostin-overexpressing mice (Supplemental Figure 10). This could be attributed to the fact that periostin overexpression protected from early tubular injury, limiting the requirement for proreparative M2a macrophages. Coculture of macrophages with primary tubular cells from periostin-overexpressing mice subjected to hypoxia induced a higher expression of proregenerative factors and an increased proliferation of macrophages than coculture with control cells. Moreover, treatment of macrophages with exogenous periostin had a similar effect, indicating that periostin directly promotes macrophage proliferation and polarization. Periostin secreted by cancer stem cells was shown to recruit macrophages through direct interaction with its receptor avβ3, followed by receptor-associated AKT activation.43
In contrast to the previously established detrimental role of periostin in CKD, we unexpectedly discovered a protective role of periostin in AKI. Previous studies in various animal models of CKD5,6,15,18,20 and chronic diseases of other organs5–7 showed that periostin promotes maladaptive epithelial proliferation, inflammatory cell infiltration, and matrix deposition, which contributes to fibrosis and organ dysfunction. However, in acute injury conditions, periostin was protective and promoted kidney repair by inhibiting epithelial cell death, inducing a differential macrophage polarization, and promoting epithelial proliferation. The beneficial effect of periostin was maintained 6 weeks after AKI, with KO mice showing increased interstitial fibrosis and overexpressing mice displaying the opposite effect. Development of chronic fibrosis after AKI was dependent on the initial injury response in this model. Besides, periostin alone did not induce spontaneous fibrosis after 4 months of chronic overexpression, further indicating that the distinctive functions of periostin in acute versus chronic conditions may depend on the different contexts and mechanisms implicated in these diseases.
In summary, our results demonstrate a novel protective role of periostin in AKI. It has two roles: (1) periostin inhibits proapoptotic pathways and promotes proliferation of renal epithelial cells at least in part through direct interaction with Itgβ1, favoring preservation of renal epithelium and a more efficient repair; and (2) periostin promotes a regenerative trophic macrophage phenotype during the repair phase of AKI by driving proliferation of macrophages in the kidney and their secretion of proregenerative factors. These results position for the first time periostin as a repair mechanism after AKI and contribute to our better understanding and more efficient design of AKI-targeted treatments.
Disclosures
None.
Funding
This work was supported by the recurring annual funding of Inserm and an Inserm-Copoc grant of Inserm-Transfert.
Supplementary Material
Acknowledgments
The authors thank the biochemical platform of the Centre de Recherche des Cordeliers for the measurements of renal function and the animal facility of INSERM UMRS 1155 for their valuable help with animal breeding.
Dr. Kormann, Dr. Prakoura, Dr. Chadjichristos, and Dr. Chatziantoniou designed the study, analyzed data, and prepared the manuscript. Dr. Kormann, Dr. Prakoura, Dr. Kavvadas, Mrs. Placier, Mrs. Vandermeersch, and Dr. Dorison carried out experiments. Prof. Dussaule provided scientific input. All authors approved the final version of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019020113/-/DCSupplemental.
Supplemental Table 1. PCR reaction and products used for the genotyping of the triple transgenic Pax8-rtTA/LC1-Cre/Tet-o-Periostin mice.
Supplemental Table 2. Primer sequences used for quantitative Real-time PCR.
Supplemental Figure 1. Co-localization of periostin with different renal tubular markers in WT mice 72 hours after I/R.
Supplemental Figure 2. Kidney/body weight ratio of periostin-overexpressing and KO mice compared with their respective controls at basal conditions and 72 hours after I/R.
Supplemental Figure 3. Periostin protects from apoptosis induction after I/R.
Supplemental Figure 4. Periostin promotes proliferation of tubular cells after I/R.
Supplemental Figure 5. Periostin inhibits the activation of the Itgβ1 downstream targets FAK and ERK.
Supplemental Figure 6. Periostin protects primary tubular cells from oxidative stress-induced cell death.
Supplemental Figure 7. Recombinant periostin directly promotes the polarization and proliferation of cultured macrophages.
Supplemental Figure 8. Chronic periostin overexpression is not linked to a profibrotic phenotype.
Supplemental Figure 9. Periostin protects from fibrosis development 6w after I/R.
Supplemental Figure 10. M2a markers are not increased in renal macrophages derived from periostin-overexpressing mice.
References
- 1.Bonventre JV, Yang L: Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest 121: 4210–4221, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basile DP, Anderson MD, Sutton TA: Pathophysiology of acute kidney injury. Compr Physiol 2: 1303–1353, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grams ME, Rabb H: The distant organ effects of acute kidney injury. Kidney Int 81: 942–948, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Horiuchi K, Amizuka N, Takeshita S, Takamatsu H, Katsuura M, Ozawa H, et al.: Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J Bone Miner Res 14: 1239–1249, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Prakoura N, Chatziantoniou C: Periostin in kidney diseases. Cell Mol Life Sci 74: 4315–4320, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prakoura N, Chatziantoniou C: Matricellular proteins and organ fibrosis. Curr Pathobiol Rep 5: 111–121, 2017 [Google Scholar]
- 7.Malanchi I, Santamaria-Martínez A, Susanto E, Peng H, Lehr HA, Delaloye JF, et al.: Interactions between cancer stem cells and their niche govern metastatic colonization. Nature 481: 85–89, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Norris RA, Damon B, Mironov V, Kasyanov V, Ramamurthi A, Moreno-Rodriguez R, et al.: Periostin regulates collagen fibrillogenesis and the biomechanical properties of connective tissues. J Cell Biochem 101: 695–711, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kii I, Nishiyama T, Li M, Matsumoto K, Saito M, Amizuka N, et al.: Incorporation of tenascin-C into the extracellular matrix by periostin underlies an extracellular meshwork architecture. J Biol Chem 285: 2028–2039, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Snider P, Hinton RB, Moreno-Rodriguez RA, Wang J, Rogers R, Lindsley A, et al.: Periostin is required for maturation and extracellular matrix stabilization of noncardiomyocyte lineages of the heart. Circ Res 102: 752–760, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gillan L, Matei D, Fishman DA, Gerbin CS, Karlan BY, Chang DD: Periostin secreted by epithelial ovarian carcinoma is a ligand for alpha(V)beta(3) and alpha(V)beta(5) integrins and promotes cell motility. Cancer Res 62: 5358–5364, 2002 [PubMed] [Google Scholar]
- 12.Li G, Jin R, Norris RA, Zhang L, Yu S, Wu F, et al.: Periostin mediates vascular smooth muscle cell migration through the integrins alphavbeta3 and alphavbeta5 and focal adhesion kinase (FAK) pathway. Atherosclerosis 208: 358–365, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lambert AW, Wong CK, Ozturk S, Papageorgis P, Raghunathan R, Alekseyev Y, et al.: Tumor cell-derived periostin regulates cytokines that maintain breast cancer stem cells. Mol Cancer Res 14: 103–113, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li L, Fan D, Wang C, Wang JY, Cui XB, Wu D, et al.: Angiotensin II increases periostin expression via Ras/p38 MAPK/CREB and ERK1/2/TGF-β1 pathways in cardiac fibroblasts. Cardiovasc Res 91: 80–89, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Zhao X, Hao J, Duan H, Rong Z, Li F: Phosphoinositide 3-kinase/protein kinase B/periostin mediated platelet-derived growth factor-induced cell proliferation and extracellular matrix production in lupus nephritis. Exp Biol Med (Maywood) 242: 160–168, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takayama G, Arima K, Kanaji T, Toda S, Tanaka H, Shoji S, et al.: Periostin: A novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J Allergy Clin Immunol 118: 98–104, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Masuoka M, Shiraishi H, Ohta S, Suzuki S, Arima K, Aoki S, et al.: Periostin promotes chronic allergic inflammation in response to Th2 cytokines. J Clin Invest 122: 2590–2600, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prakoura N, Kavvadas P, Kormann R, Dussaule JC, Chadjichristos CE, Chatziantoniou C: NFκB-Induced periostin activates Integrin-β3 signaling to promote renal injury in GN. J Am Soc Nephrol 28: 1475–1490, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prakoura N, Chatziantoniou C: Periostin and discoidin domain receptor 1: New biomarkers or targets for therapy of renal disease. Front Med (Lausanne) 4: 52, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mael-Ainin M, Abed A, Conway SJ, Dussaule JC, Chatziantoniou C: Inhibition of periostin expression protects against the development of renal inflammation and fibrosis. J Am Soc Nephrol 25: 1724–1736, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koesters R, Kaissling B, Lehir M, Picard N, Theilig F, Gebhardt R, et al.: Tubular overexpression of transforming growth factor-beta1 induces autophagy and fibrosis but not mesenchymal transition of renal epithelial cells. Am J Pathol 177: 632–643, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang D, Liu Y, Wei Q, Huo Y, Liu K, Liu F, et al.: Tubular p53 regulates multiple genes to mediate AKI. J Am Soc Nephrol 25: 2278–2289, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei Q, Dong G, Chen JK, Ramesh G, Dong Z: Bax and Bak have critical roles in ischemic acute kidney injury in global and proximal tubule-specific knockout mouse models. Kidney Int 84: 138–148, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hakuno D, Kimura N, Yoshioka M, Mukai M, Kimura T, Okada Y, et al.: Periostin advances atherosclerotic and rheumatic cardiac valve degeneration by inducing angiogenesis and MMP production in humans and rodents. J Clin Invest 120: 2292–2306, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghatak S, Misra S, Norris RA, Moreno-Rodriguez RA, Hoffman S, Levine RA, et al.: Periostin induces intracellular cross-talk between kinases and hyaluronan in atrioventricular valvulogenesis. J Biol Chem 289: 8545–8561, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsuzawa M, Arai C, Nomura Y, Murata T, Yamakoshi Y, Oida S, et al.: Periostin of human periodontal ligament fibroblasts promotes migration of human mesenchymal stem cell through the αvβ3 integrin/FAK/PI3K/Akt pathway. J Periodontal Res 50: 855–863, 2015 [DOI] [PubMed] [Google Scholar]
- 27.Ying Y, Kim J, Westphal SN, Long KE, Padanilam BJ: Targeted deletion of p53 in the proximal tubule prevents ischemic renal injury. J Am Soc Nephrol 25: 2707–2716, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferenbach DA, Bonventre JV: Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat Rev Nephrol 11: 264–276, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV: Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med 16: 535–543, 1p following 143, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishioka S, Nakano D, Kitada K, Sofue T, Ohsaki H, Moriwaki K, et al.: The cyclin-dependent kinase inhibitor p21 is essential for the beneficial effects of renal ischemic preconditioning on renal ischemia/reperfusion injury in mice. Kidney Int 85: 871–879, 2014 [DOI] [PubMed] [Google Scholar]
- 31.DiRocco DP, Bisi J, Roberts P, Strum J, Wong KK, Sharpless N, et al.: CDK4/6 inhibition induces epithelial cell cycle arrest and ameliorates acute kidney injury. Am J Physiol Renal Physiol 306: F379–F388, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Humphreys BD, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS, et al.: Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell 2: 284–291, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Moreno-Layseca P, Streuli CH: Signalling pathways linking integrins with cell cycle progression. Matrix Biol 34: 144–153, 2014 [DOI] [PubMed] [Google Scholar]
- 34.Rozo M, Li L, Fan CM: Targeting β1-integrin signaling enhances regeneration in aged and dystrophic muscle in mice. Nat Med 22: 889–896, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor WR, Stark GR: Regulation of the G2/M transition by p53. Oncogene 20: 1803–1815, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown JP, et al.: Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science 282: 1497–1501, 1998 [DOI] [PubMed] [Google Scholar]
- 37.Norbury C, Blow J, Nurse P: Regulatory phosphorylation of the p34cdc2 protein kinase in vertebrates. EMBO J 10: 3321–3329, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.An JN, Yang SH, Kim YC, Hwang JH, Park JY, Kim DK, et al.: Periostin induces kidney fibrosis after acute kidney injury via the p38 MAPK pathway. Am J Physiol Renal Physiol 316: F426–F437, 2019 [DOI] [PubMed] [Google Scholar]
- 39.Forbes SJ, Rosenthal N: Preparing the ground for tissue regeneration: From mechanism to therapy. Nat Med 20: 857–869, 2014 [DOI] [PubMed] [Google Scholar]
- 40.Lech M, Anders HJ: Macrophages and fibrosis: How resident and infiltrating mononuclear phagocytes orchestrate all phases of tissue injury and repair. Biochim Biophys Acta 1832: 989–997, 2013 [DOI] [PubMed] [Google Scholar]
- 41.Lech M, Gröbmayr R, Weidenbusch M, Anders HJ: Tissues use resident dendritic cells and macrophages to maintain homeostasis and to regain homeostasis upon tissue injury: The immunoregulatory role of changing tissue environments. Mediators Inflamm 2012: 951390, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee S, Huen S, Nishio H, Nishio S, Lee HK, Choi BS, et al.: Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol 22: 317–326, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou W, Ke SQ, Huang Z, Flavahan W, Fang X, Paul J, et al.: Periostin secreted by glioblastoma stem cells recruits M2 tumour-associated macrophages and promotes malignant growth. Nat Cell Biol 17: 170–182, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N, et al.: Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science 332: 1284–1288, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jenkins SJ, Ruckerl D, Thomas GD, Hewitson JP, Duncan S, Brombacher F, et al.: IL-4 directly signals tissue-resident macrophages to proliferate beyond homeostatic levels controlled by CSF-1. J Exp Med 210: 2477–2491, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Y, Chang J, Yao B, Niu A, Kelly E, Breeggemann MC, et al.: Proximal tubule-derived colony stimulating factor-1 mediates polarization of renal macrophages and dendritic cells, and recovery in acute kidney injury. Kidney Int 88: 1274–1282, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Capote J, Kramerova I, Martinez L, Vetrone S, Barton ER, Sweeney HL, et al.: Osteopontin ablation ameliorates muscular dystrophy by shifting macrophages to a pro-regenerative phenotype. J Cell Biol 213: 275–288, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoshino J, Monkawa T, Tsuji M, Hayashi M, Saruta T: Leukemia inhibitory factor is involved in tubular regeneration after experimental acute renal failure. J Am Soc Nephrol 14: 3090–3101, 2003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.