Summary
To navigate complex environments, animals must generate highly robust, yet flexible, locomotor behaviors. For example, walking speed must be tailored to the needs of a particular environment: Not only must animals choose the correct speed and gait, they must also adapt to changing conditions and quickly respond to sudden and surprising new stimuli. Neuromodulators, particularly the small biogenic amine neurotransmitters, have the ability to rapidly alter the functional outputs of motor circuits. Here we show that the serotonergic system in the vinegar fly, Drosophila melanogaster, can modulate walking speed in a variety of contexts and also change how flies respond to sudden changes in the environment. These multifaceted roles of serotonin in locomotion are differentially mediated by a family of serotonergic receptors with distinct activities and expression patterns.
eTOC
Howard et al. describe the role of the neuromodulator serotonin in modifying Drosophila walking behavior. Serotonin release in the ventral nerve cord serves to slow walking speed, regardless of the context. Serotonin-mediated slowing of walking speed is required for a normal response to being startled by a novel stimulus.
Graphical Abstract
Introduction
Insects have a remarkable capacity to adapt their locomotor behaviors across a wide range of environmental contexts and to confront numerous challenges. They can walk forwards, backwards, and upside down, navigate complex terrains, and rapidly recover after injury [1–7]. To achieve this wide range of behaviors, insects regulate their walking speed and kinematic parameters, allowing them to modify stereotyped gaits as needed [3, 7–10]. Because overlapping sets of motor neurons and muscles are recruited for all of these behaviors, animals must be able to rapidly modulate the circuit dynamics that control locomotor parameters [11, 12].
As with limbed vertebrates, most insects use multi-jointed legs to walk [11, 13]. Neural circuits that orchestrate complex walking gaits are located in the ventral nerve cord (VNC), a functional analogue of the vertebrate spinal cord that includes three pairs of thoracic neuromeres (T1, T2, and T3) that coordinate the movements of three corresponding pairs of legs [14–17]. The insect VNC receives descending commands from the brain and sends motor output instructions via motor neurons to peripheral musculature [17–19]. Sensory neurons, which convey proprioceptive and tactile information, project axons from the appendages to the VNC by these same fiber tracts, where they arborize in the leg neuropils [20–24] (Figure 1A). Notably, the VNC is capable of executing coordinated leg motor behaviors, such as walking and grooming, even in decapitated animals [25]. Thus, as has been described in other insects, the VNC likely harbors neural networks that can drive the coordinated flexion and extension of each leg joint and also coordinated walking gaits [11, 26].
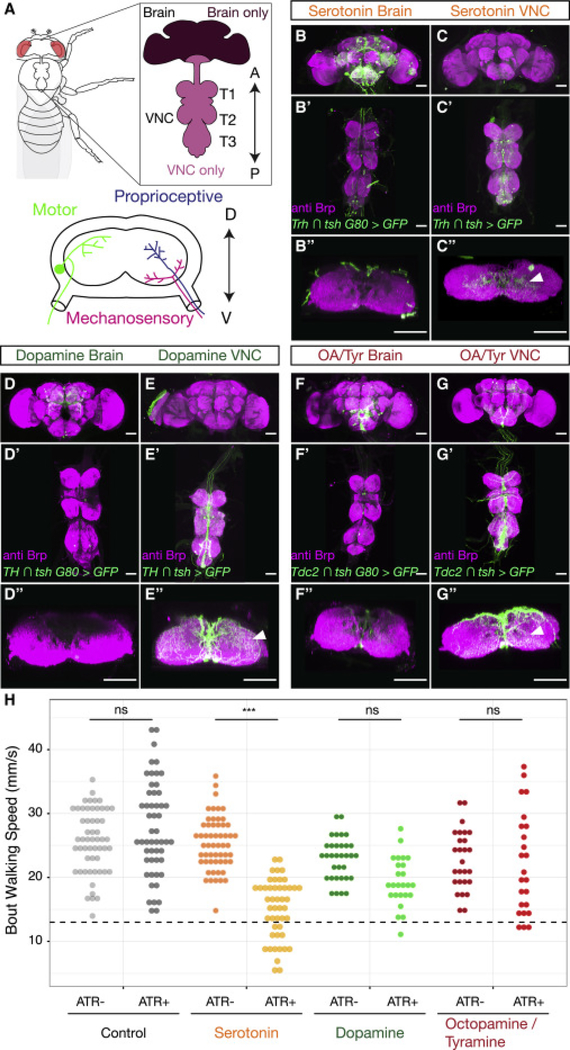
Figure 1. Neuromodulators in the Drosophila CNS.
A. The adult Drosophila CNS is composed of the brain and the VNC, which consists of three pairs of thoracic neuropils (T1, T2 and T3), each of which corresponds to a pair of adult legs, and an abdominal ganglion. The anterior (A)-posterior (P) axis is specified. Lower panel: Cross section of a thoracic neuropil illustrating projections of locomotor circuit components, including motor neurons, and sensory neurons that convey mechanosensory and proprioceptive information from the legs. The dorsal (D)– ventral (V) axis is specified.
B-G. Maximum intensity projections show the expression patterns driven by Gal4 lines labeling either brain-derived (B, D, F, Gal4 intersected with tsh Gal80) or VNC-derived (C, E, G, Gal4 intersected with tsh) serotonergic (B-C, Trh Gal4), dopaminergic (D-E, TH Gal4), or octopaminergic/tyraminergic (OA/Tyr) (F-G, Tdc2 Gal4) neurons. (B”-G”) Projection of a subset of cross sections of the VNC shows innervation of the T1 neuropil. Arrowheads point to innervation in the leg neuropils. All scale bars are 50 μm.
H. Optogenetic activation of serotonergic (Trh ∩ tsh > csChrimson) neurons in the Drosophila VNC, but not dopaminergic (TH ∩ tsh > csChrimson) or octopaminergic/tyraminergic (Tdc2 ∩ tsh > csChrimson) neurons, slows walking speed compared to all-trans-retinal (ATR) negative and non-Gal4 (w1118 ∩ tsh > csChrimson) controls. These experiments were carried out using the Flywalker assay (Mendes et al., 2013; see Figure S3 A for a schematic). * p<.05 **p<.01 ***p<.001 by Kruskal-wallis test with Dunn-Sidak correction for multiple comparisons. N walking bouts (animals) w1118 ATR− 55 (14–31); w1118 ATR+ 52 (14–36); Trh ATR− 56 (12–30); Trh ATR+ 47 (10–23); TH ATR− 33 (10–24); TH ATR+ 25 (10–26); Tdc2 ATR− 27 (10–27); Tdc2 ATR+ 24 (10–25).
See also Figure S1.
Numerous studies have established that sensory input from the legs is required for robust and stereotyped locomotor patterns, regulating the timing, magnitude, and coordination of locomotor activity [5, 10, 13, 23, 27–30]. However, sensory feedback cannot be the only means for tuning locomotion: mutation of proprioceptive receptors or even deafferenting limbs does not block coordinated walking [27, 31–35]. Beyond sensory feedback-driven tuning of gait patterns, larger behavioral changes must be accomplished by other circuits. These likely include neuromodulatory systems, including the monoamines dopamine, norepinephrine, and serotonin, which are highly conserved throughout the animal kingdom [36–38].
Monoamines have been shown to modulate, and even induce, the activities of central pattern generating (CPG) motor circuits. In crustaceans, neuromodulation causes the gastric CPG to generate distinct rhythmic activity patterns from the same neural network to address distinct behavioral demands [36, 39]. Remarkably, the same neuromodulatory systems appear to play similar roles across species. Serotonin has been shown to slow locomotor rhythms in animals as diverse as the lamprey, cat, and locust [40–42]. In the vinegar fly, Drosophila melanogaster, monoamine neurotransmitters have also been shown to modulate a wide range of behaviors. In addition to slowing walking speed, serotonin modulates sleep, aggression, and anxiety-related motor behaviors [43–49]. Dopamine, in contrast, has been linked to hyperactivity [25, 50–52]. Octopamine has been shown to mediate starvation induced hyperactivity, and in its absence animals walk more slowly [10, 53, 54]. As each of these neuromodulatory systems plays a variety of roles in regulating complex behaviors, it has thus far been challenging to tease apart which of the effects on walking behavior are due to direct modulation of motor circuitry or are a secondary consequence of modulating higher order circuits in the brain.
In this work, we show that the serotonergic neurons within the VNC have the ability to modulate walking speed across a diverse array of environmental contexts as well as in response to startling stimuli. Additionally, we demonstrate that these modulatory effects are enacted through serotonin’s action on specific receptors that are expressed in different parts of the locomotor circuit. Together, these findings reveal that neuromodulatory systems regulate multiple aspects of walking behavior, helping animals to effectively respond to rapidly changing environments.
Results
VNC serotonergic neurons arborize within the leg neuropils
To identify neuromodulatory neurons that might play a role in modulating walking behavior, we drove expression of a fluorescent reporter with Gal4 under the control of promoters encoding key synthetic enzymes for each neuromodulatory system - Tryptophan hydroxylase (Trh for serotonin (5-HT) [48]); tyrosine hydroxylase (TH or ple (pale) for dopamine [55]); and Tyrosine decarboxylase 2 (Tdc2 for octopamine and tyramine [56]). All of these drivers show extensive expression in cells both within the VNC and the brain, with processes that densely innervate VNC leg neuromeres (Figure 1).
To determine whether local neuromodulatory VNC neurons or descending neurons originating in the brain innervate the leg neuropils, we used genetic intersectional tools to limit the expression of these Gal4 lines to either the brain or VNC (Figure 1A) (see STAR Methods). These experiments show that neuromodulatory innervation of the leg neuropils arises almost entirely from VNC interneurons and not from descending neurons in the brain (Figure 1B–G). Moreover, these VNC neurons extensively innervate the leg neuropils. Thus, VNC neuromodulatory neurons are well-positioned to directly modulate VNC locomotor circuits.
Activation of VNC serotonergic neurons slows walking speed
Previous studies showed that neuromodulatory systems can regulate walking but have not addressed the role of VNC neuromodulatory subpopulations. Using the intersectional genetic tools described above, we addressed whether neuromodulatory neurons in the VNC alone are sufficient to modulate walking behavior. We optogenetically activated these neurons and measured walking speed using the Flywalker behavioral assay [27]. We found that activation of serotonergic VNC populations, but not dopaminergic or octopaminergic/tyraminergic VNC subpopulations, significantly reduced the average speed at which animals walk (Figure 1H).
Based on these results, we focused the remainder of our analysis on VNC serotonergic neurons (5-HTVNC). To validate the fidelity of our serotonergic Gal4 driver line, and to rule out co-secretion of other neurotransmitters, we performed immunostaining for markers of serotoninergic (5-HT), dopaminergic (TH), octopaminergic/tyraminergic (Tdc2), glutamatergic (VGlut), cholinergic (ChAT), and GABAergic (GABA) neurons (Figure S1). These experiments demonstrate that the Trh-Gal4 line drives expression in 5-HT-expressing neurons, and that these neurons do not express any of the other neurotransmitters we surveyed, suggesting that they are primarily serotonergic.
We next characterized the effects on locomotor behavior of activating 5-HTVNC neurons by studying animals freely walking within an arena [57, 58]. This approach allowed us to measure not only an animal’s speed, but also its walking frequency, angular velocity, and preferred position within the arena (Figure S2A–C). As with our initial experiments, activation of 5-HTvnc neurons is sufficient to dramatically slow average walking speed in this paradigm (Figure 2A). Interestingly, activation of 5-HTVNC neurons does not change the overall amount of time animals spend walking, suggesting that speed changes are not simply due to a decrease in overall activity, but instead reveal a bias towards slower walking speeds (Figure S2D and F). Unlike a previous study showing that overexpressing the serotonin transporter in all neurons caused flies to move away from the edge of the arena [45], we see no effect on the distribution of animals within the arena when we limit activation to 5-HTVNC neurons (Figure S2D). We also find that activation of 5-HTVNC neurons decreases the absolute angular velocity of walking flies (Figure S2D). Thus, although these flies walk slower, they also walk straighter than control flies. This latter observation is unexpected, because straighter trajectories are usually correlated with faster walking speeds (Figure S2F).
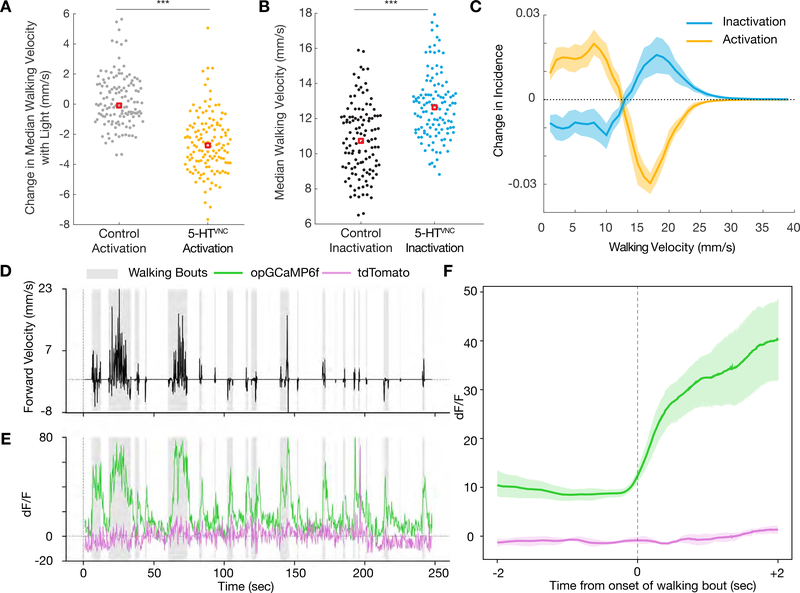
Figure 2. 5-HTVNC neurons modulate walking speed.
A. Activation of 5-HTVNC neurons (Trh ∩ tsh > csChrimson fed with ATR) causes animals to walk slower than background matched non-Gal4 controls (w1118 ∩ tsh >csChrimson fed with ATR). ***p<.001 by Kruskal-wallis test. Red box indicates median. N = 130 animals for each genotype.
B. Inactivation of 5-HTVNC neurons (Trh ∩ tsh > Kir2.1) causes animals to walk faster than background matched non-Gal4 controls (w1118 ∩ tsh > Kir2.1). ***p<.001 by Kruskal-wallis test. Red box indicates median. N=119 animals per genotype.
C. The distribution of velocity shifts caused by activation and inhibition of 5-HTVNC neurons are symmetrical. Differences in population average histograms were calculated between control and experimental genotypes and were fit with 95% confidence intervals via bootstrapping. For activation experiments, behavior of w1118 ∩ tsh >csChrimson flies fed with ATR was compared to that of Trh ∩ tsh >csChrimson flies also fed with ATR for the light on period only.
D, E. Serotonergic processes passing through the cervical connective (labeled using Trh ∩ tsh) are active during walking. (D) A single animal’s forward velocity with overlaid boxes showing defined walking bouts. (E) While tdTomato baseline signal (purple line) is not affected by walking bouts, the calcium signal (green line) in these serotonergic processes rises during walking bouts (gray boxes).
F. Calcium signal but not tdTomato signal in these processes rises with the onset of walking bouts. For each animal, all walking bouts were synchronized around their onset, and an average was taken (between 80 and 130 walking bouts per animal). Plotted is the average of all animals (N=5) with a 95% confidence interval representing the spread between animals.
See also Figures S2 and S3.
Inhibition of 5-HTVNC neurons increases walking speed
Although activation of 5-HTVNC neurons causes flies to walk more slowly, gain-of-function experiments such as these cannot determine if and in what situations these neurons are normally used to modulate walking speed. To begin to address this question, we expressed the inward rectifying potassium channel Kir2.1 to constitutively inactivate 5-HTVNC neurons. Although neurons were inactivated throughout development and adulthood, we did not observe a change in the number or anatomy of 5-HTVNC neurons, suggesting that their development is not significantly affected (Figure S2H and I).
Consistent with the activation phenotype, inhibition of 5-HTVNC neurons causes animals to walk faster (Figure 2B). In fact, the shifts in velocity produced by either optogenetic activation or constitutive inhibition of 5-HTVNC neurons mirror each other (Figure 2C). In addition, 5-HTVNC inactivation causes animals to increase their angular velocity (Figure S2E and G) and increases the percentage of time that animals spend walking (Figure S2E). The reciprocal effects on speed from either activating or silencing 5-HTVNC neurons suggest that serotonin release in the VNC has the capacity to modulate baseline walking speed.
5-HTvnc neurons are active in walking flies
The opposing effects on speed when 5-HTVNC neurons are activated or inhibited suggest that the activity of these neurons will co-vary with walk-stop transitions and velocity changes during baseline walking. To test this prediction, we performed functional calcium imaging of 5-HTvnc processes while flies walked on a spherical treadmill (Figure S3A) [59]. To obtain the most robust signal we focused on fibers in the neck connective, which are likely derived from a subset of ascending 5-HTVNC neurons that target the brain (Figure S3B–D).
Activity in these fibers is highly correlated with walking (Figure 2D and E). Fluorescent calcium signals from these cells rise dramatically at the onset of each walking bout (Figure 2F). These signals are much weaker when animals perform other motor behaviors, like proboscis extension or grooming (Figure S3E). These results suggest that at least a subset of 5-HTVNC neurons are specifically active when flies walk, and are not generically active during all legged motor behaviors. We also find that the activity of these serotonergic processes positively correlates with the average speed of the walking bout, suggesting that these neurons may become more active when animals walk faster (Figure S3F).
The observation that the activity of some 5-HTVNC neurons is positively correlated with velocity is interesting in light of our behavioral data, which show that flies walk more slowly when 5-HTvnc neurons are optogenetically activated. There are several explanations for these apparently opposing observations. First, because the calcium imaging experiments only focus on a subset of 5-HTVNC processes, while the behavior experiments manipulate the entire set of 5-HTvnc neurons, it may be that the neurons being imaged have a unique function, and that their activity is not representative of the entire set. Alternatively, if their activity is representative of the broader population of 5-HTVNC neurons, it would suggest that as flies walk faster more serotonin release is needed to slow animals down. Regardless, the fact that activity in these neurons correlates with velocity supports a role for them in modulating walking speed and raises the possibility that some of them may function in a negative feedback loop.
5-HTvnc activation does not disrupt walking coordination
The slower walking speeds that result when 5-HTVNC neurons are activated could be the result of poor coordination or, alternatively, controlled adjustments of kinematic parameters, which naturally occur when flies walk more slowly [10, 27]. To distinguish between these two possibilities, we returned to the Flywalker assay, which measures kinematic parameters at high temporal and spatial resolution [27] (Figure S4A).
Using this assay, we find that optogenetic activation of 5-HTVNC neurons results in highly coordinated walking patterns. Representative traces of an individual’s footprints during a slow walking bout show that activation of these neurons does not perturb stereotyped foot placement or interfere with the straightness of the walking bout (Figure 3A and C). Step and stance traces show that these animals also use highly coordinated gaits, suggesting that interleg coordination is intact (Figure 3B and D). In fact, compared to control flies, 5-HTVNC neuron activation results in more precise foot placement at the onset and offset of each stance phase, suggesting that the walking behavior of these animals is more constrained compared to control animals (Figure S4B).
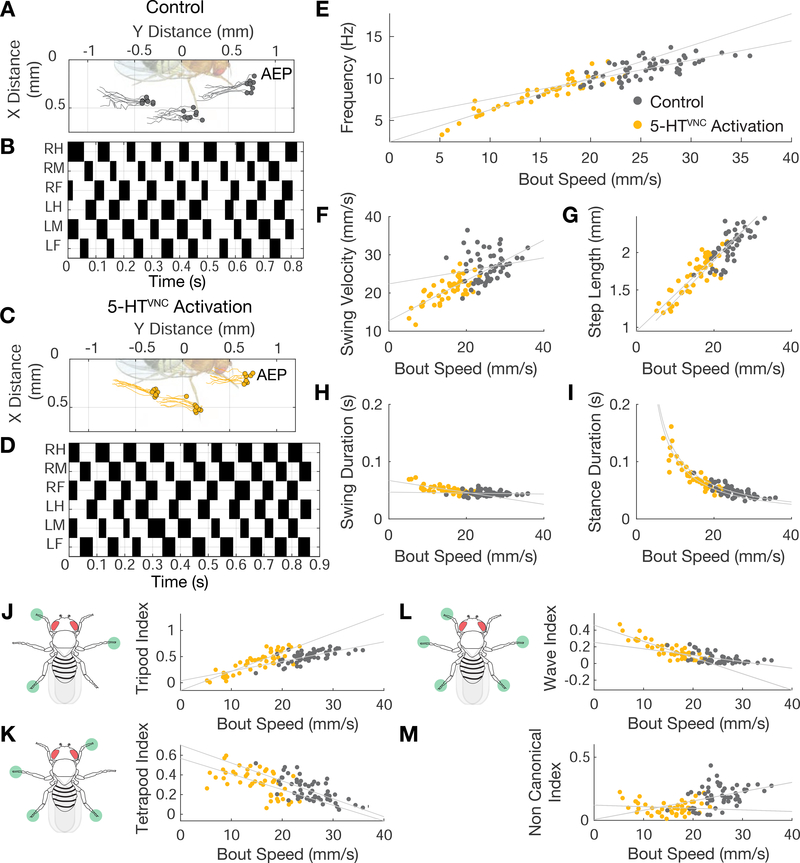
Figure 3. Walking is coordinated when 5-HTVNC neurons are activated.
A-D. Representative data from speed-matched slow (19 mm/s) walking bouts show that activation of 5-HTVNC neurons does not disrupt locomotor coordination. Footfalls (filled circles) and stance traces (lines) for all steps taken by the left front, middle, and hind legs show foot touchdown placement is consistent over time and stance traces are relatively straight in both control animals (Trh ∩ tsh >csChrimson grown on food lacking ATR) (A) and animals where 5-HTvnc neurons have been activated (Trh ∩ tsh >csChrimson fed with ATR) (C). Step trace for each leg during a walking bout for control (B) and experimental (D) animals. Stance phase is indicated in white and swing phase in black. The checkerboard pattern is consistent with a highly-coordinated walking gait.
E-I. Quantification of step parameters upon activation of 5-HTVNC neurons. The relationships between speed and frequency (E), swing velocity (F), step length (G), swing duration (H), and stance duration (I) extend the trends observed with control flies. N=47 bouts from 10–23 animals for Trh ∩ tsh >csChrimson ATR+ (yellow circles). N=56 bouts from 12–30 animals for Trh ∩ tsh >csChrimson ATR− (gray circles). Speed by ATR interaction effect in multivariable model: frequency (p<.001), swing velocity (p=.02), step length (p=.53), swing duration (p<.001), stance duration (p=.71).
J-M. Quantification of gait selection upon activation of 5-HTVNC neurons. Activation of 5-HTVNC neurons increases wave (L) and tetrapod (K) gait utilization while decreasing time spent using tripod (J) gait. There is a low frequency of non-canonical gait conformations upon activation (M). N=47 bouts from 10–23 animals for Trh ∩ tsh >csChrimson ATR+ (yellow circles). N=56 bouts from 12–30 animals for Trh ∩ tsh >csChrimson ATR− (gray circles). Speed by ATR interaction effect in multivariable model: tripod index (p<.001), tetrapod index (p=.60), wave index (p<.001), non canonical (p=.006).
See also Figure S4.
In wild type flies, most walking parameters are highly correlated with speed, shifting as animals walk faster or slower [10, 27]. For example, as animals walk slower, their step cycle frequency decreases, they take longer steps, and slow the velocity of their swinging legs. These shifts are accompanied by a shift in the step duty cycle, as stance duration increases while swing duration remains largely unchanged [10, 27]. When 5-HTVNC neurons are activated these relationships are maintained and extended into the slower speed range (Figure 3E–I). Similarly, as animals walk more slowly, their preferred gait shifts from the three-legged tripod gait to more stable tetrapod and wave gaits [10, 27]. Upon activation of 5-HTVNC neurons, animals continue the trend to preferentially use these slower walking gaits (Figure 3J–L). In some cases, such as step length (Figure 3G) and stance duration (Figure 3I), the parameter–speed relationship when 5-HTVNC neurons are activated is an extrapolation of the wild type relationship, while in other cases, such as the choice of tripod gait (Figure 3J), the relationship with speed is altered when these neurons are activated. Most of the other parameters fall in between these two extremes.
5-HTvnc neuron inactivation slows walking in multiple contexts
Results from our behavioral experiments suggest a model whereby the VNC serotonergic system is used to regulate walking speed: when the system is activated, flies walk more slowly and when the system is silenced, flies walk faster. Based on these observations, we next tested whether this system is required for flies to naturally adjust their baseline walking speeds. To test this hypothesis, we silenced 5-HTVNC neurons under conditions when flies normally walk at different speeds, including multiple temperatures, body orientations, nutritional states, and in response to mechanosensory stimulation [3, 53, 60–62]. Counter to our expectation, animals in which 5-HTVNC neurons were silenced are still able to adjust their speed in the same direction as wild type flies in all of these contexts (Figure 4A). For example, compared to 25°, flies walk slower at 18°C and fast er at 30°C even when 5-HT VNC neurons are silenced, arguing that this system is not required for flies to modulate their speed in response to this difference. In addition, these data reveal that, for all of the contexts tested here, animals in which 5-HTvnc neurons are silenced walk faster than their matched controls. For example, 5-HTVNC-silenced flies walk faster at 18°C compared to con trol flies at 18°C. Thus, we further conclude that serotonin release in the VNC slows walking speed across a wide spectrum of environmental contexts. These findings are consistent with a model where the VNC serotonergic system acts as a mild and constitutive break on walking speed, independently of how fast or slow this speed is set by other mechanisms. However, we note that because we are using constitutive inactivation tools, we cannot currently rule out that the observed phenotypes are in part the result of compensation within the network when 5-HTVNC neurons are silenced.
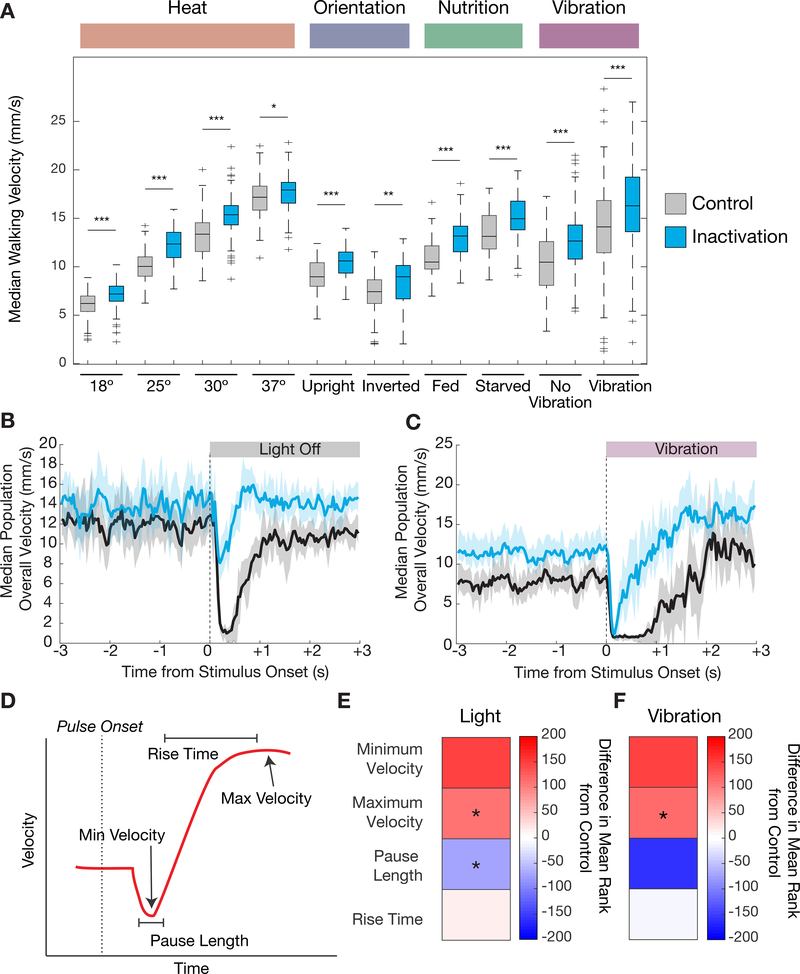
Figure 4. Changes in walking behavior when 5-HTVNC neurons are silenced.
A. Silencing 5-HTVNC neurons (Trh ∩ tsh > Kir2.1) causes an increase in walking speed compared to genetically background matched non-Gal4 controls (w1118 ∩ tsh > Kir2.1) across a diversity of behavioral contexts including different temperatures, orientations, nutritional states, and vibration stimuli. For each condition, genotypes were compared using a Kruskal Wallis test, ***p<.001, **p<.01, *p<.05 by Kruskal-wallis test. For all conditions, p<.001 for context effects for both control and experimental genotypes.18 °C, N=130 per genotype; 25 °C, N=120 per genotype; 30 °C, N=120 per genotype; 37 °C, N= w1118 (120) Trh (119); upright, N=90 per genotype; inverted w1118 (74) Trh (70); fed, N=86 per genotype; starved, N= w1118 (86) Trh (85); vibration, N= w1118 (165) Trh (164).
B-C. Silencing 5-HTVNC neurons changes the immediate behavioral responses to sudden contextual changes. When lights switch from on to off (B), control animals (w1118 ∩ tsh > Kir2.1, shown in black) show a brief behavioral pause and then resume activity. When 5-HTVNC neurons are silenced (Trh ∩ tsh > Kir2.1, shown in blue), animals still slow their speed but do not fully pause. In response to the onset of vibration (C) control animals stop, pause and then accelerate speed. When 5-HTVNC neurons are silenced (Trh ∩ tsh > Kir2.1, shown in blue), animals pause but re-accelerate more quickly than controls. Shaded areas show 95% confidence intervals. For light experiments, N= w1118 (150) Trh (140); for vibration experiments N=w1118 (167) Trh (166).
D. Schematic of behaviors in response to a sudden stimulus. This response is divided into four key parameters that describe different phases of the response, indicated with arrows and bounded lines.
E-F. Heatmaps quantifying the parameters schematized in (D) when 5-HTVNC neurons are inactivated in response to the blackout (E) and earthquake (f) scenarios. Plotted for every genotype is the difference in mean ranks (Kruskal-wallis test statistics) for each parameter compared to w1118 ∩ tsh > Kir2.1 control flies. Starred parameters are those where differences between control and experimental animals consistently reached significance (p<.05; see STAR Methods).
See also Figure S5.
Silencing 5-HTVNC neurons alters the response to sudden changes in the environment
Although the above results suggest the VNC serotonergic system is not needed for flies to adjust their baseline walking speed in many situations, another potentially valuable role for slowing walking speed might be as a response to when animals are startled. In mammals, stereotyped startle behaviors occur in response to a wide variety of sensory stimuli – acoustic, tactile, and vestibular. These responses take place on sub-second time scales, involve simultaneous contraction of muscles throughout the body, and are similar irrespective of the initiating stimulus [63, 64]. Like mammals, Drosophila display stereotyped responses to threatening looming stimuli, beginning with an initial freezing period lasting less than a second before escape behaviors are initiated [65–67]. Because these startle responses are contextually independent and have been shown to be mediated in part by serotonin in mammals [68], we asked whether 5-HTVNC neurons are required for these responses in Drosophila.
We tested this idea using two different startle-inducing paradigms: (i) one in which flies abruptly experience total darkness (‘blackout paradigm’) and (ii) one in which flies suddenly experience strong mechanical stimulation, such as an intense vibration (‘earthquake paradigm’) [61]. In both scenarios, control animals on average show a two-tiered response to these abrupt changes (Figure 4B and C): First, many animals rapidly come to a nearly complete stop and then they pause before resuming a behavior that is appropriate for the new context (schematized in Figure 4D). For both the blackout and earthquake paradigms, on average control animals stop within the first 0.25 seconds, pause for about a second, and then resume walking (Figure 4B and C). Animals lacking the ability to release serotonin in the VNC are deficient in these initial responses (Figure 4B, C, E, F), but still eventually achieve context-appropriate walking speeds (Figure 4A). Moreover, consistent with our earlier analyses, flies with silenced 5-HTVNC neurons walk faster compared to control flies, both before and after the startle-inducing stimulus (Figure 4B and C).
Thus, in addition to serving as a constitutive break on locomotor speed, these results suggest that the serotonergic system helps to facilitate an immediate and stimulus-independent pause response when flies are startled.
Different serotonin receptor mutants alter the startle response in different ways
All five serotonergic receptors in Drosophila – 5-HT1A, 5-HT1B, 5-HT2A, 5-HT2B, and 5-HT7 – are G-protein coupled receptors (GPCRs) [69, 70]. Like their mammalian orthologs, members of each serotonin receptor family (1, 2, and 7) have distinct effects upon activation. Receptors in the 1 family, 5-HT1A and 5-HT1B, act through the Gi pathway to inhibit the generation of cAMP, whereas 5-HT7, the only member of the 7 family in Drosophila, stimulates the production of cAMP [71, 72]. Receptors of the 2 family, 5-HT2A and 5-HT2B in Drosophila, act through the PLC-IP3 signaling pathway to increase intracellular calcium [70, 73, 74]. Together, this diversity of receptors is thought to allow serotonin to produce complex physiological responses that depend on both synaptic connectivity and receptor expression patterns.
Before characterizing the phenotypes of these receptor mutants, we analyzed a mutant of the Trh gene (Trh01), which is globally unable to produce serotonin [47]. Reassuringly, Trh01 animals show a similar phenotype to animals in which 5-HTVNC neurons were silenced: flies walk significantly faster and more frequently than controls and exhibit a similar startle response in the earthquake paradigm (Figure 5A, B, and G). In addition, Trh01 mutant animals walk closer to the edge of the arena compared to control animals, consistent with previous observations [45] (Figure S5A).
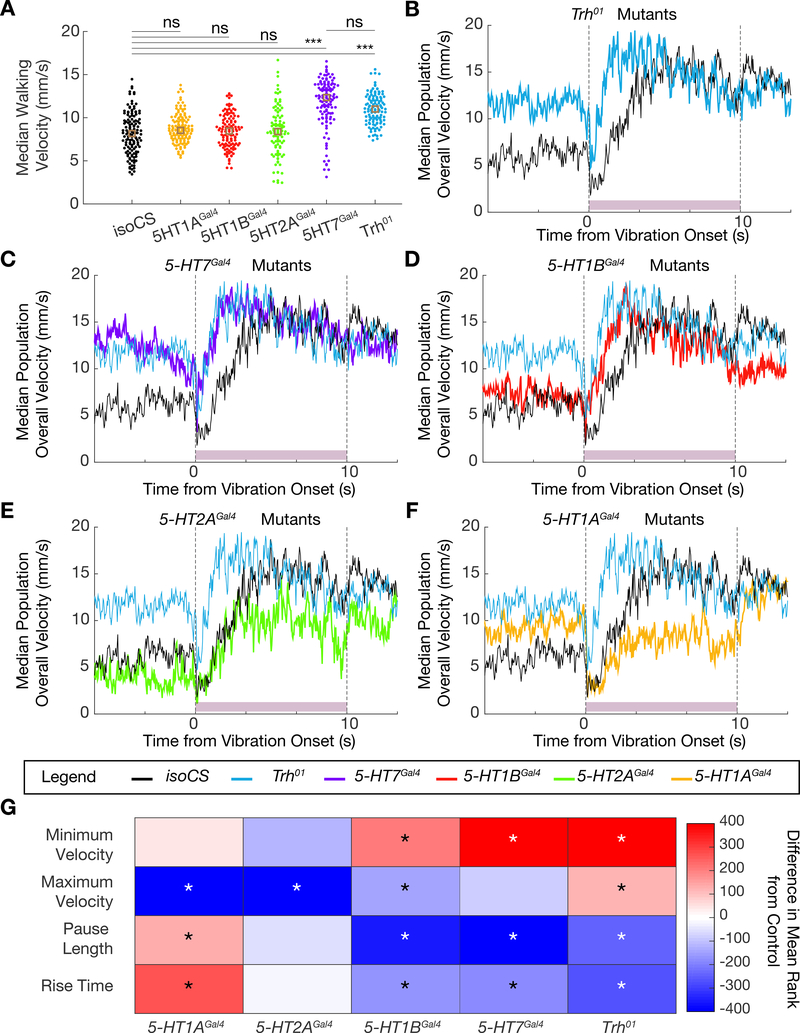
Figure 5. Phenotype of the startle response of Trh and serotonin receptor mutants.
A. Dotplot showing median population walking speed during a five-minute recording session for Trh01 mutants (blue), which walk faster than background matched isoCS controls (black), consistent with the 5-HTVNC inactivation experiments. 5-HT7Gal4 mutants (purple) also walk faster than controls, but the other receptor mutants do not. ***p<.001, **p<.01, *p<.05 ns p>.05 by Kruskal-wallis analysis with Dunn-sidak correction for multiple comparisons. Brown box indicates median. N= isoCS (130) 5-HT1AGal4 (130) 5-HT1BGal4 (120) 5-HT2AGal4 (100) 5-HT7Gal4 (120) Trh01 (120).
B-F. Median population walking speed sampled at 30 Hz in response to vibration stimulus. Trh01 mutants (B, blue line) show a blunted and shortened pause in response to the novel stimulus. 5-HT7Gal4 mutants (C, purple line) and 5-HT1BGal4 mutants (D, red line) show a similar phenotype to Trh01 mutants. 5-HT2AGal4 mutants (E, green line) and 5-HT1AGal4 mutants (F, yellow line) have a pause phase comparable to controls, but do not accelerate as much in response to the vibration stimulus. N= isoCS (140) 5-HT1AGal4 (115) 5-HT1BGal4 (124) 5-HT2AGal4 (88) 5-HT7Gal4 (120) Trh01 (139)
G. Heatmap showing how lack of Trh or serotonergic receptors affects the response to vibration stimulus. Plotted for every genotype is the difference in mean ranks (Kruskal-wallis test with Dunn-sidak correction for multiple comparisons statistics) for each parameter compared to isoCS control flies. Starred parameters are those where differences between control and experimentals consistently reached significance (p<.05, see STAR Methods).
See also Figure S5.
Null receptor mutants 5-HT1AGal4, 1BGal4, 2AGal4, and 7Gal4 all increase the percentage of time animals spend walking (Figure S5A) [47]. However, an increase in walking speed is only observed in 5-HT7Gal4 mutants (Figure 5A), suggesting that 5-HT7 is the primary receptor responsible for mediating the effects of serotonin on walking speed.
We next tested the receptor mutants in the earthquake paradigm. Interestingly, 5-HT7Gal4 and 5-HT1BGal4 mutants closely phenocopy the startle response seen in Trh01 mutants and also in the 5-HTvnc inactivation experiments (Figure 5C, D, and G, Figure S5C–E). By contrast, although 5-HT1AGal4 and 5-HT2AGal4 mutants do not show a dramatic change in pause length, they exhibit a sustained decrease in their final target speed in response to this stimulus (Figure 5E–G, Figure S5F and G).
These data are consistent with the idea that different receptors influence distinct aspects of the startle response. Notably, mutation of receptors that are predicted to have opposing effects on cAMP production, such as 5-HT1B and 5-HT7, can result in similar phenotypes. Further, some receptor mutants exhibit phenotypes that are not seen in Trh01 mutant animals. These complex changes in locomotor behavior might be explained by the differential expression of serotonin receptors in key components of the locomotor circuit.
Serotonin receptors are expressed in distinct cell types
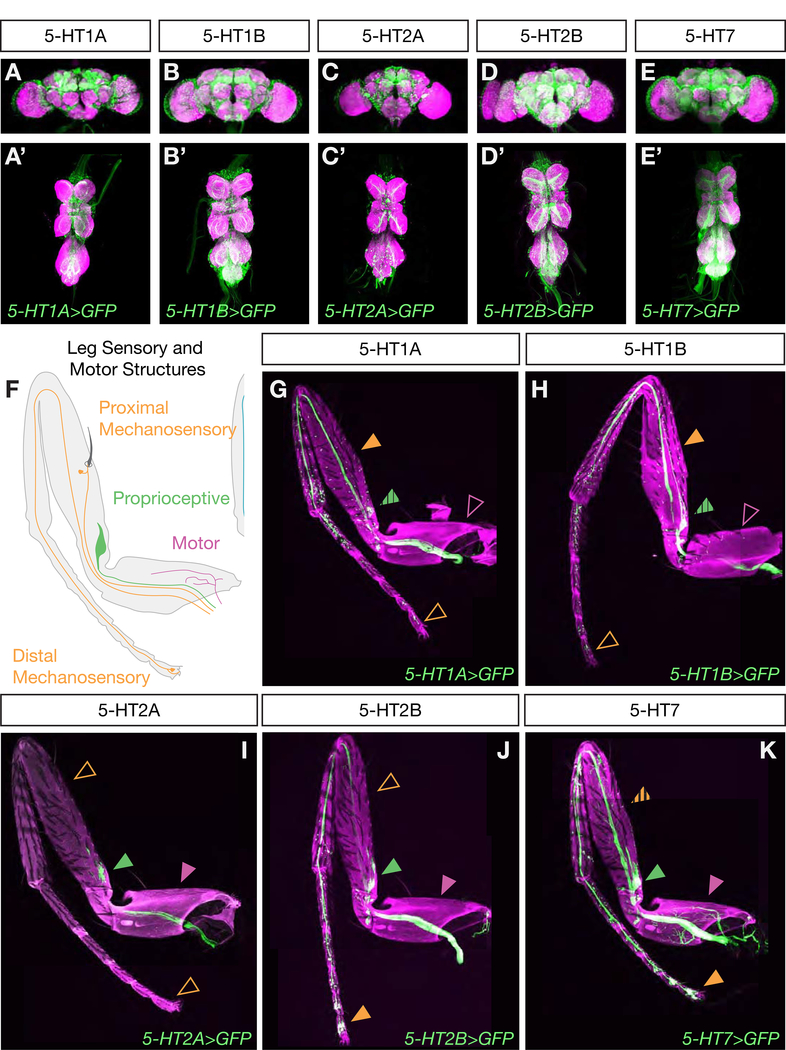
The different effects on walking observed in flies mutant for different serotonin receptors suggest that, in addition to distinct biochemical properties, they may also have different expression patterns within the locomotor circuit. To identify neurons that express these receptors, we used gene and protein trap Gal4 lines from the MiMIC library to drive expression of a GFP reporter in the pattern of each receptor subtype [75] (Figure S6G). Each receptor line drives expression in many neurons both within the brain and the VNC (Figure 6A–E). Many of these are uncharacterized interneurons that cannot yet be functionally studied. However, each serotonin receptor is also expressed in distinct subsets of leg motor and sensory neurons. In particular, while members of the 5-HT1 family are predominantly expressed in mechanosensory neurons throughout the legs, 5-HT2 and 5-HT7 receptors are expressed in proximal-targeting flexor and extensor motor neurons (Figure S6A–F), proprioceptive, and distal sensory neuron populations (Figure 6F–K). Thus, serotonin release in the VNC is likely to differentially affect these components of the locomotor circuit, which we hypothesize ultimately contributes to the observed changes in behavior.
Figure 6. Differential expression of serotonin receptors in locomotor circuit components.
A-E. Maximum intensity projections show Gal4-driven expression of serotonin receptors in both the brain (A-E) and VNC (A’-E’). All scale bars are 50 μm.
F. Schematic of sensory and motor neuron populations in an adult leg.
G-K. Maximum intensity projections show Gal4-driven expression of serotonin receptors in neuronal processes in the adult leg. Each receptor is expressed in a distinct pattern in sensory-motor components. While some are expressed in motor neurons (purple arrows, hatched arrows indicate limited or weak expression) and proprioceptive neurons (green arrows), others are not. All receptors are expressed in a subset of mechanosensory neurons (orange arrows), but some are preferentially expressed in proximal or distal leg segments. All scale bars are 50 μm.
See also Figure S6.
Discussion
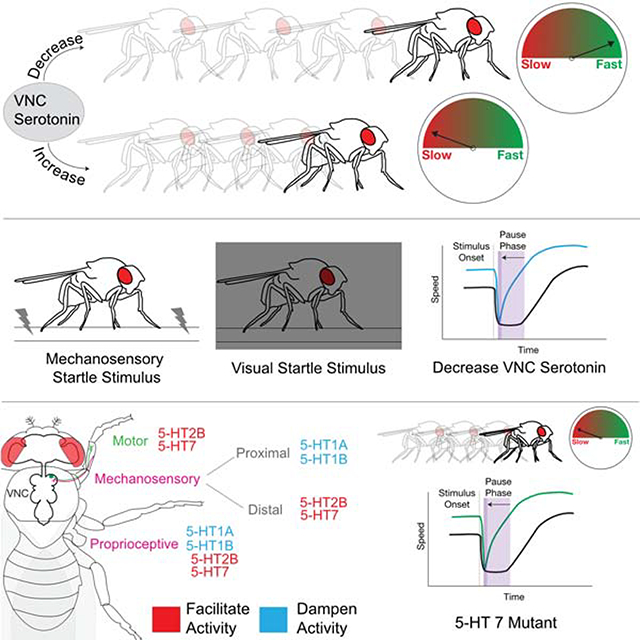
Walking is a highly stereotyped behavior, consisting of a small number of well-defined gaits, each with its own set of characteristic kinematic parameters. However, walking must also be flexible, to adapt to a wide variety of environments, complex terrains, and novel situations. How nervous systems manage to orchestrate behaviors that are simultaneously stereotyped and flexible is not well understood. Here we show in the fly that (i) serotonergic VNC neural activity can modulate walking speed in a wide variety of contexts and (ii) these neurons play a role in a fly’s response to the sudden onset of a startling stimulus. Below, we discuss these findings and present a preliminary model that summarizes serotonin’s role in modulating walking behavior.
A common role for serotonin in modulating walking speed across species
A central finding of our study is that serotonergic neuron activity in the VNC modulates baseline walking speed in Drosophila. This finding parallels previous observations in the motor systems of other organisms. For example, consistent with our calcium imaging experiments, activity of serotonergic neurons in the cat brainstem is correlated with motor behavior and walking speed [76, 77]. Additionally, in vertebrates as diverse as the lamprey and cat, serotonin induces an increase in oscillatory and step cycle period, respectively, slowing locomotion [40, 41]. One recent study in mice showed that activation of the dorsal raphe nucleus – a key serotonergic brain region – produces rapid suppression of spontaneous locomotion and locomotor speed, while showing minimal effect on kinematic parameters, such as gait, or on non-locomotor behaviors such as grooming [78]. Although this study focused on a subset of serotoninergic neurons targeting the forebrain, the parallels to our results suggest that the modulatory role of the serotonergic system in regulating locomotor speed is remarkably conserved across the animal kingdom.
Serotonergic modulation of the startle response
In our experiments, we observed that the onset of a startling stimulus (be it visual or mechanosensory), induces a brief period of pausing behavior in wild type flies. We hypothesize that these behavioral pauses are similar to startle responses seen in both mammalian systems and some species of insects, which also have a pause phase before animals embark on an appropriate behavioral action [63, 64, 66–68, 79, 80]. In Drosophila and the locust, these pauses are thought to optimally prepare animals for maximally effective escape behaviors. For example, prior to initiating flight in response to a looming threat, Drosophila pause for about 200 ms to execute a series of postural adjustments that help orient the direction of takeoff [67]. It may also be that pauses allow animals to collect additional sensory information before they select an appropriate response to the startling stimulus. Interestingly, however, not all insects appear to exhibit a pause phase; for example, studies of the American cockroach, Periplaneta americana, suggest that the escape behavior in response to a predator is immediate [81].
Although our 5HTVNC silencing experiments were done using constitutive inactivation tools, which makes it difficult to rule out a developmental defect, the similar phenotype exhibited by the Trh mutant argues that the inability to release serotonin from these neurons is the cause of the startle response defect. In mammals, the absence of serotonin, due to the lesion of key serotonergic brain regions or pharmacological blockade, is generally associated with an increase in the intensity of startle responses [82, 83]. Although this may seem counter to our results, other studies have shown that serotonin increases startle responses when injected directly into the lumbar spinal cord [84, 85]. Thus, serotonin may play distinct roles in the forebrain and in the spinal cord. Together with our results, we suggest that the role of spinal cord/VNC serotonin release is to extend the duration of and/or amplify the startle response.
A model for serotonin-mediated modulation of walking in flies
Putting the various lines of evidence presented here together, we can formulate a preliminary model for serotonin’s role in modulating Drosophila walking behavior. First, we suggest that one function of serotonin release in the VNC is to act as a mild constitutive brake. In non-startle contexts, the system would oppose forward acceleration that is driven by other inputs, such as descending commands from the brain [4, 17, 86]. Consistent with this idea, when the brake is removed (5-HTVNC neurons are silenced) flies walk slightly faster and when the brake is stronger (5-HTVNC neurons are activated), flies slow down. The calcium imaging results showing that the activity of a subset of 5-HTVNC neurons increases as flies walk faster suggest that the brake may be stronger at higher speeds. A caveat to this latter idea is that the calcium imaging experiments only monitored a subset of the 5-HTVNC neurons, raising the possibility that the activity of other 5-HTVNC neurons have a different relationship with speed.
When flies are startled, our data are consistent with the idea that serotonin release in the VNC serves to put a strong brake on walking speed, presumably to allow flies to prepare for an appropriate behavioral response. When the 5-HTVNC neurons are silenced, flies are unable to apply this brake and consequently have a compromised response. Notably, however, even when these neurons are silenced flies are still able to respond, albeit more weakly, to being started, suggesting that part of the response is still intact. One possibility is that, in addition to triggering a serotonin-mediated brake, the response to being startled may also independently dampen the accelerator, reducing walking drive.
The observation that serotonin release in the VNC similarly affects the response to both the earthquake and blackout paradigms, which are perceived by two very different sensory systems, suggests that this neuromodulator is affecting locomotor components that are shared by both systems. Although the expression of 5-HT receptors in sensory and motor neurons is consistent with this notion, we note that this model is likely incomplete as we cannot as of now incorporate the role of local interneurons that also express 5-HT receptors.
Nevertheless, because the primary receptors expressed in motor neurons are 5-HT7 and 5-HT2B, which have been shown to upregulate the production of cAMP and facilitate calcium entry [70], we hypothesize that serotonin amplifies activity in these motor neurons. There is ample evidence in the literature to support this role for serotonin both in rodent models as well as in human studies [87, 88]. Further, the motor neurons expressing these serotonergic receptors target both flexor and extensor muscles in the coxa and femur, two proximal leg segments. These observations suggest that serotonin acting on these motor neurons may facilitate co-contraction, a mechanism that would partially stiffen these leg joints, potentially resulting in slower walking speeds. Consistent with this notion, co-contraction has been shown to enable joint stability in the face of a complex environment and also during the preparatory phase for certain escape behaviors [67, 80, 89–91]. Co-contraction of tibia flexor and extensor muscles is also part of the initial pause phase of the startle response in the locust, suggesting that this mechanism may be shared [80].
In addition to motor neurons, serotonin receptors are expressed in distinct classes of leg sensory neurons that target the leg neuropils of the VNC. Considering the broad expression of serotonergic receptors in sensory organs, it is interesting that one of the behavioral roles of serotonin we identified is its ability to mediate the response to vibration. Vibration is sensed by the chordotonal organ, and our expression analysis reveals that serotonin receptors are expressed to different extents in chordotonal neurons [24, 92, 93]. Together, these observations suggest that modulation of sensory information as it is entering the VNC could play a key role in how serotonin modulates the response to a vibration stimulus.
Finally, the observed distribution of serotonergic receptor subtypes in sensory processes may serve to shift the balance of sensory information as a consequence of serotonergic input. Based on the known downstream signaling properties of these receptors, we predict that increased levels of serotonin in the VNC would amplify proprioceptive and distal sensory inputs at the expense of more proximal sensory information. These shifts in sensory processing may also contribute to increased stability and might be useful in other contexts where slow walking is preferred, such as navigating complex terrains where improved sensory information might be beneficial. It may also be that these shifts in sensory input are important during the pause phase of the startle response, as they may allow animals to gather valuable information in order to compute the next phase of escape behaviors.
STAR Methods
LEAD CONTACT AND MATERIAL AVAILABILITY
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Richard Mann (rsm10@columbia.edu).
Fly lines generated by this study are available upon request.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Fly husbandry
Experimental model for this study was the vinegar fly Drosophila melanogaster. A full list of strains used in the paper is included in the Key Resource Table. Unless otherwise described, flies were maintained at 25°C on dextrose cornmeal food using standard laboratory techniques.
Key Resources Table.
| Reagent or Resource | Source | Identifier | Additional Information |
|---|---|---|---|
| Antibodies | |||
| Rabbit anti Vglut | Aaron DiAntonio [94] | 1:10,000 | |
| Rabbit anti 5-HT | Millipore Sigma | RRID:AB_477522 | 1:1000 |
| Mouse anti TH | Immunostar | RRID:AB_572268 | 1:1000 |
| Mouse anti ChAT4B1 | Developmental Studies Hybridoma Bank (DSHB) | RRID:AB_528122 | 1:500 |
| Mouse anti nc82 c | DHSB | RRID:AB_2314866 | 1:20 – 1:100 |
| Rabbit anti GABA | Millipore Sigma | RRID:AB_477652 | 1:1000 |
| Rabbit anti Tdc2 | Cova Labs | pab0822-P | 1:200 |
| Rabbit anti dsRed | Takara Bio | RRID:AB_10013483 | 1:1000 |
| Experimental Models: Strains of D. melanogaster | |||
| ; ; Trh Gal4 | Bloomington Drosophila Stock Center (BDSC) [48] | RRID:BDSC_38389 | |
| ; ; TH Gal4 | BDSC [55] | RRID:BDSC_8848 | |
| ; Tdc2 Gal4 ; | BDSC [56] | RRID:BDSC_9313 | |
| w1118 ; IIiso ; IIIiso | BDSC [98] | RRID:BDSC_5905 | |
| w1118 ; IIiso ; Trh-Gal4 | This study | ||
| w1118 ; IIiso ; TH-Gal4 | This study | ||
| w1118 ; Tdc2 Gal4 ; IIIiso | This study | ||
| w1118 ; IIiso ; Trh iso Gal4 | This study | outcrossed 10 x to w1118 ; IIiso ; IIIiso | |
| ; UAS-mCD8::GFP; | [99] | ||
| tub>gal80> ; tsh-LexA, LexAop-Flp ; | [100] | Tsh LexA made by Julie Simpson. Combined tool provided by Wes Grueber | |
| ; tsh-Gal80 ; | Julie Simpson | ||
| ; ; UAS-csChrimson::mVenus | BDSC [101] | RRID:BDSC_55136 | |
| ; ; UAS-Kir2.1 | BDSC [102] | RRID:BDSC_6595 | |
| ; ; 20XUAS-hexameric-GFP | BDSC / Steve Stowers [103] | RRID:BDSC_52262 | |
| ; UAS-OpGCamp6f ; UAS-tdTomato | Pavan Ramdya [59] | ||
| IsoCS | Yi Rao [47] | ||
| w+ ; 5-HT1AGal4 ; | Yi Rao [47] | ||
| w+ ; 5-HT1BGal4 ; | Yi Rao [47] | ||
| w+; ; 5-HT2AGal4 | Yi Rao [47] | ||
| w+; ; 5-HT7Gal4 | Yi Rao [47] | ||
| w+; ; Trh01 | Yi Rao [47] | ||
| ; 5-HT1A-Gal4 (MI04464); | Herman A. Dierick [75] | ||
| ; 5-HT1B-Gal4 (MI05213); | Herman A. Dierick [75] | ||
| ; ; 5-HT2A-Gal4 (MI00459) | Herman A. Dierick [75] | ||
| ; ; 5-HT2A-Gal4 (MI03299) | Herman A. Dierick [75] | ||
| ; ; 5-HT2B-Gal4 (MI05208) | Herman A. Dierick [75] | ||
| ; ; 5-HT2B-Gal4 (MI06500) | Herman A. Dierick [75] | ||
| ; ; 5-HT2B-Gal4 (MI07403) | Herman A. Dierick [75] | ||
| ; ; 5-HT7-Gal4 (MI00215) | Herman A. Dierick [75] | ||
| Vglut>>LexAVP16, LexO-CD8GFP /FM7; Vglut>>LexAVP16, UASFlp, LexO-CD8GFP /CyO; LexO-CD8GFP, LexO-CD8GFP, Vglut>>LexAVP16 /TM2 | Myungin Baek | ||
| ; Mhc-RFP ; | BDSC | RRID:BDSC_38464 | |
| Software and Algorithms | |||
| Fiji | [95] | https://fiji.sc | |
| Caltech FlyTracker | [58] | http://www.vision.caltech.edu/Tools/FlyTracker/index.html | |
| Flywalker | [27] | ||
| Flycapture | https://www.flir.com/products/flycapture-sdk | ||
| Fview2 | Andrew Straw, modified from [97] | ||
| MATLAB 2018a | Mathworks | https://www.mathworks.com/products/matlab.html | |
| R Studio 3.3.2 | https://www.rstudio.com | ||
| Python 2.7 Anaconda | https://www.anaconda.com/distribution/ | ||
| NIS Elements AR | Nikon | https://www.microscope.healthcare.nikon.com/products/software/nis-elements | |
| PuTTY | https://www.chiark.greenend.org.uk/~sgtatham/putty/ | ||
| Arduino IDE 1.8.5 | Arduino | https://www.arduino.cc/en/Main/Software | |
Animal rearing for behavioral experiments
For Flywalker experiments, flies were maintained on dextrose cornmeal food at 25°C. For arena experiments, flies were maintained on Nutrifly German Sick food (Genessee Scientific 66–115) in an incubator humidified at 60% with a 12h:12h light:dark cycle. Crosses used for behavioral experiments were flipped every 2–3 days to prevent overcrowding. As animals eclosed, females of the appropriate genotype were collected under CO2 anesthesia every 2–3 days. Females were used for all experiments due to their larger size which facilitated accurate tracking. For non-optogenetic experiments, flies were collected onto Nutrifly Food without any additive. For optogenetic experiments, flies were collected onto Nutrifly food supplemented with .4 mM ATR dissolved in EtOH or an equal concentration of solvent alone (for arena experiments), or corn food supplemented with .4mM ATR dissolved in DMSO or an equal concentration of solvent alone for Flywalker experiments. Animals were aged in the dark (for optogenetic experiments) or on the same light:dark cycle for 2–3 more days at 25°C before being assayed. Gal4 driver lines used for arena experiments had been outcrossed ten times to an isogenized w1118 control population. Gal4 driver lines used for Flywalker experiments had been isogenized on two chromosomes, but not on the chromosome containing the Gal4 transgene. For a complete list of lines used in this paper please refer to the Key Resources Table.
Fly Genotypes with associated Figures
| Experimental Line | Main Figure | Supplementary |
|---|---|---|
| + /+; tsh Gal80 / UAS mCD8 GFP; Trh Gal4 / + | Figure 1B, B’, B” | |
| tub>gal80> / +; tsh LexA, lexAop Flp / +; Trh Gal4 / UAS mCD8 GFP | Figure 1C, C’, C” | |
| + /+; tsh Gal80 / +; UAS mCD8 GFP, TH Gal4 / + | Figure 1D, D’, D” | |
| tub>gal80> / +; tsh LexA, lexAop Flp / +; TH Gal4, UAS mCD8 GFP / + | Figure 1E, E’, E” | |
| + /+; tsh Gal80 / Tdc2 Gal4; UAS mCD8GFP / + | Figure 1F, F’, F” | |
| tub>gal80> / +; tsh LexA, lexAop Flp / Tdc2 Gal4; UAS mCD8 GFP / + | Figure 1G, G’, G” | |
| tub>gal80> / w1118; tsh LexA, lexAop Flp / isoII; UAS csChrimson::mVenus / isoIII | Figure 1H; 2A, C | S2 D, F |
| tub>gal80> / w1118; tsh LexA, lexAop Flp / isoII; UAS csChrimson::mVenus / Trh Gal4 | Figure 1H; 3A–M |
S3 D S4 B |
| tub>gal80> / w1118; tsh LexA, lexAop Flp / isoII; UAS csChrimson::mVenus / TH Gal4 | Figure 1H | |
| tub>gal80> / w1118; tsh LexA, lexAop Flp / Tdc2 Gal4; UAS csChrimson::mVenus / isoIII | Figure 1H | |
| tub>gal80> / w1118; tsh LexA, lexAop Flp / isoII; UAS csChrimson::mVenus / Trh iso Gal4 | Figure 2A, C |
S1 S2 D, F, H, I |
| tub>gal80> / w1118; tsh LexA, lexAop Flp / isoII; UAS Kir2.1::GFP / isoIII | Figure 2B and C; 4 | S2 E and G |
| tub>gal80> / w1118; tsh LexA, lexAop Flp / isoII; UAS Kir2.1::GFP / Trh iso Gal4 | Figure 2B and C; 4 |
S2 E, G, H, I S5 C |
| tub>gal80>/+; tsh-LexA, lexAop-FLP/UAS-opGCaMP6f; Trh-Gal4/UAS-tdTomato | Figure 2D–F | S4 B, C, E, F |
| isoCS | Figure 5A–G | S5 A,B |
| W+; 5-HT1AGal4; + | Figure 5A, F, G | S5 A,G |
| W+; 5-HT1BGal4; + | Figure 5A, D, G | S5 A,E |
| W+; +; 5-HT2AGal4 | Figure 5A, E, G | S5 A,F |
| W+; +; 5-HT7Gal4 | Figure 5A, C, G | S5 A,D |
| W+; +; Trh01 | Figure 5A–F, G | S5 A,B–G |
| 5-HT 1A-Gal4 (MI04464) / +; 20X-UAS hexameric GFP / + | Figure 6A,G | S6 G |
| 5-HT1B-Gal4 (MI05213) / +; 20X-UAS hexameric GFP / + | Figure 6B,H | S6 G |
| +/+; 5-HT2A-Gal4 (MI00459) / 20X-UAS hexameric GFP | Figure 6C,I | S6 G |
| +/+; 5-HT2B-Gal4 (MI05208) / 20X-UAS hexameric GFP | Figure 6D, J | S6 G |
| +/+; 5-HT7-Gal4 (MI0215) / 20X-UAS hexameric GFP | Figure 6E, K | S6 G |
| +/+; 5-HT2A-Gal4 (MI03299) / 20X-UAS hexameric GFP | S6 G | |
| +/+; 5-HT2B-Gal4 (MI06500) / 20X-UAS hexameric GFP | S6 G | |
| +/+; 5-HT2B-Gal4 (MI07403) / 20X-UAS hexameric GFP | S6 G | |
| Vglut>>LexA, LexO-CD8GFP / +; Vglut>>LexA, UASFlp, LexO-CD8GFP / +; LexO-CD8GFP, LexO-CD8GFP, Vglut>>LexAVP16 / 5-HT2B-Gal4 (MI05208) | S6 A | |
| Vglut>>LexA, LexO-CD8GFP / +; Vglut>>LexA, UASFlp, LexO-CD8GFP / +; LexO-CD8GFP, LexO-CD8GFP, Vglut>>LexAVP16 / 5-HT7-Gal4 (MI0215) | S6 B | |
| Vglut>>LexA, LexO-CD8GFP / +; Vglut>>LexA, UASFlp, LexO-CD8GFP / MHC-RFP; LexO-CD8GFP, LexO-CD8GFP, Vglut>>LexAVP16 / 5-HT2B-Gal4 (MI05208) | S6 C | |
| Vglut>>LexA, LexO-CD8GFP / +; Vglut>>LexA, UASFlp, LexO-CD8GFP / MHC-RFP; LexO-CD8GFP, LexO-CD8GFP, Vglut>>LexAVP16 / 5-HT7-Gal4 (MI0215) | S6 D |
METHOD DETAILS
Immunostaining brain and VNC
Brains and VNCs were dissected in phosphate buffered saline with 0.3% Triton (PBST) and fixed in 4% Paraformaldehyde (PFA) for 20 minutes. Samples were washed five times for 20 minutes in PBST with 0.1% Bovine serum albumin (BSA), and then blocked in PBST-BSA for one hour at room temperature, or overnight at 4° C. Samples were incubated with primary antibody diluted in PBST-BSA overnight at 4°C, and washed five times 20 minutes with PBST-BSA the next day. Primary antibodies used were: anti-VGlut (gift from Aaron DiAntonio, described in [94]); anti-5HT (1:1000 Sigma); anti-TH (1:1000 Immunostar); anti-ChAT (1:500 DSHB); anti-Brp (1:50–1:100 DSHB); anti-GABA (1:1000 Sigma); anti-Tdc2 (1:200 Cova Labs). Samples were then incubated in secondary antibody diluted in PBST-BSA overnight at 4° C. Secondary antibodies used Goat Anti-Rabbit Alexa 555 (1:500 Invitrogen); Donkey Anti-Mouse 647 (1:500 Jackson Immunolabs); Goat Anti-Mouse 555 (1:500 Life Technologies). The next day, samples were washed five times for 20 minutes in PBST, and then the liquid was replaced with Vectashield and samples were incubated overnight prior to mounting. Brains and VNCs from the same animals were mounted together, with the ventral surface of the VNC and the anterior surface of the brain facing up.
Validation of expression patterns during two photon experiments were performed as described in [59]. Briefly, brains and VNCs were dissected out of the animal, and fixed in 4% PFA for 20 min at room temperature. Samples were washed 2–3 × 10–15 min in 1% PBST and blocked for one hour at room temperature in 1% PBST with 5% NGS. Samples were stained overnight at room temperature with primary antibody diluted in blocking solution. Primary antibodies used included anti-Brp (1:20 DSHB anti-dsRed (1:1000 Takara Bio). The next day, samples were washed 2–3 × 10–15 min in 1% PBST. Samples were incubated with secondary antibody overnight at room temperature in the dark. Secondaries included Goat Anti-Rabbit Cy3 (1:400 Jackson); Goat Anti-Mouse 633 (Thermofisher). The next day, samples were washed 2–3 × 10–15 min 1% PBST and mounted dorsal side up in Slow Fade Gold mounting medium (ThermoFisher).
Confocal Imaging of Brains and VNCs
Mounted brains and VNCs were imaged on a Leica TCS SP5 confocal at 20X magnification with a resolution of 1024 × 512 pixels, and at a scanning rate of 200 Hz and 3x averaging. Sections were taken at 1 μm increments. Laser power and detector gain were maintained constant for the brain and VNC of the same animals, but were adjusted for optimal signal between animals.
Imaging of fixed samples following two photon live imaging experiments was performed as described in [59] on a Zeiss LSM 700 Laser Scanning Confocal Microscope at 20X magnification and 2X averaging, with a 0.52 × 0.52 μm pixel size. Z sections were taken at 1 μm intervals.
Cell Counting and Quantification
Images were analyzed in Fiji [95]. For quantification of the number of cells driven by Trh-Gal4 in the brain and VNC, mVenus or GfP positive and 5-HT positive cell bodies were counted from five or more individual animals.
Leg dissection, imaging, and image processing
To prepare legs for imaging, fly heads and abdomens were removed, and thoraces with legs attached were fixed overnight in 4% PFA at 4°C. Ca rcasses were washed 5x with 0.03% PBST, and then placed in Vectashield overnight before legs were mounted. Imaging was performed on a Leica SP5 confocal at 20X magnification and 1024 × 1024 pixel resolution with 3x averaging, and Z sections were taken at 1 μm. Two PMT detectors were set to capture green fluorescent signal and the green autofluorescence of the cuticle. Laser power was adjusted independently for each line to achieve optimal visualization of structures. Images were processed in Fiji [95]. Autofluorescence was subtracted from the green channel to allow for clearer visualization of leg structures.
Arena Experiments
Hardware
The skeleton of the system was built of 80–20 bars and acrylic plates and the arena itself was machined out of polycarbonate to the specifications published in [96]. The polycarbonate plastic arena was embedded in an aluminum plate to maintain a level surface. During experiments, the arena was covered with an acrylic disc with a small hole for mouth pipetting in flies. The inside of the lid was coated in a thin layer of Fluon (Amazon, B00UJLH12A) to prevent flies from walking on the ceiling.
A Point Grey Blackfly Mono USB3 camera fitted with a Tamron 1/2” F/1.2 IR C-mount lens (B&H photo) was mounted above the arena and connected by USB 3 cable to a System 76 Leopard WS computer running Ubuntu 14.04 LTS. A Kodax 3×3” 89B Opaque IR filter (B&H photo) was placed in front of the camera detector to allow for detection of IR but not visible light.
Backlighting and optogenetic stimulation was provided by a plate of LEDs sitting under the arena. An acrylic diffuser was placed between the lighting plate and the arena. Each plate was designed with two sets of LEDs – one for IR backlighting (ledlightsworld.com SMD3528–300) and one for optogenetic or white light stimulation (superbrightLEDS.com NFLS-x-LC2 in Red or Natural White). These plates were swapped out when experiments required different color LEDs. To allow for detection of the on state of non-IR lights, an additional IR light was wired in series with each visible light array, and placed within the field of view of the camera.
Each set of lights was powered separately by an Arduino Uno driver, allowing for modulation of light intensity via Pulse Width Modulation (PWM). Commands to set LED brightness and start and end experiments were sent to this driver using a PuTTY terminal and USB serial interface. For all the experiments described here, both IR and visible spectrum LEDs were set at 100% brightness. At the center of the arena this corresponded roughly to intensities of:
| Light On | Percent | Intensity | Wavelength Measured |
|---|---|---|---|
| Infrared | 100 | .13 mW | 1050 nm |
| .08 mW | 635 nm | ||
| .08 mW | 535 nm | ||
| Infrared + Red | 100 | .6 mW | 635 nm |
| Infrared + White | 100 | .62 mW | 635 nm |
| .68 mW | 535 nm |
Data Acquisition
All behavioral recordings were done during the three-hour morning activity peak. Prior to the experiment, the arena was leveled, the lid cleaned, and a new layer of Fluon applied. For each experiment, videos were recorded of cohorts of 10 flies. For each recording session, flies were mouthpipetted into the arena through a small hole and then the arena lid was slid to move the hole out of the field of view. A blackout curtain cover (Thor Labs, BK5) was used to surround the arena, protecting it from any contaminating light.
Experimental protocols were programmed into the Arduino through serial communication via a PuTTY terminal. Videos were recorded at a rate of 30 frames per second and stored in a compressed “fly movie format” using custom software written by Andrew Straw at the University of Freiberg based on work previously described [97].
Orientation experiments: For inverted experiments, animals were introduced into the arena setup when it was upright, and the lid of the arena was taped in place. The entire arena was manually inverted and propped up on two overturned ice buckets. Flies were either recorded upright and then inverted, for five minutes each, or in the opposite order.
Starvation: 24 hours prior to behavioral assay, half of the flies were transferred to an empty tube with a wet Kim Wipe. Behavioral recordings were collected as described above and lasted for five minutes.
Heat: Heated experiments were carried out inside a walk-in temperature-controlled incubator, which was set at either 18, 25, 30, or 37° C and 40% humidity. Flies were introduced to the arena immediately after entering the temperature-controlled room, recording began immediately thereafter and lasted for five minutes.
Light: For experiments examining responses to light stimuli, flies were first exposed to five minutes of white light, and then a one minute period of darkness.
Vibration: To provide a vibration stimulus, four 3V haptic motors (1670–1023-ND, Digikey) were attached to the aluminum plate in which the arena sat using 3D printed holders. The motors were wired in series and driven by the same Arduino system driving the arena’s LED lighting array. For all experiments described, vibration was set at 10% power. The protocol for vibration experiments consisted of a brief habituation period (five minutes for inactivation experiments, 30 seconds for mutant experiments) followed by a 10 second vibration pulse and a 110 sec recovery period.
Flywalker Experiments
Hardware
The Flywalker was constructed as described in [27] with modifications. The rig consisted of a frame of 80/20 supporting a sheet of 6 mm Borofloat optical glass with polished edges placed over an Andor Zyla 4.2 Magapixel sCMOS camera with an AF Nikkor 24–85mm 1:2.8–4 D lens (Nikon). On each edge of the glass were placed four Luxeon Neutral White (4100K) Rebel LED on a SinkPAD-II 10mm Square Base (230 lm @ 700mA) wired in series. Each set of lights was driven by a dedicated 700mA, Externally Dimmable, BuckPuck DC Driver (Luxeon), and all four of these drivers were connected to a single power supply. Each driver was independently adjustable.
Chambers were 3D printed by Protolabs (schematics available upon request). The ceiling and walls of the chamber were painted with Fluon mixed with india ink, to prevent flies from walking on the ceiling while maintaining an effective dark background. Small far-red LEDS were embedded in the walls of the chamber for Chrimson optogenetic experiments (LXM3-PD01 LUXEON). These lights were controlled by an Arduino driver that used pulsewitdth modulation to adjust light brightness. Commands were sent to this driver using a PuTTY terminal and USB serial interface. For all the experiments described here, LEDs were set at 20% brightness.
Data Acquisition
The Flywalker was calibrated using a calibration reticle prior to use on each day. On the day of the experiment, animals were mouthpipetted into a clean glass tube and allowed to equilibrate for five minutes to get rid of as much dirt and food as possible, to prevent contamination of the glass surface. 2–3 flies were added to the chamber by mouth pipette.
Videos were recorded using the NIS Elements AR software. A constant region of interest was defined such that the rate of recording was 226 fps. Each group of animals was recorded for one minute. Videos were cut to select traces where flies walked straight without touching the wall for >6 steps without other flies in the frame.
Functional Imaging Experiments
Functional imaging experiments on Trh ∩ tsh > opGCaMP6f, tdTomato animals were performed as described in [59].
QUANTIFICATION AND STATISTICAL ANALYSIS
Arena Experiments
Tracking
Videos were tracked using the FlyTracker software from the Caltech vision lab [58]. Prior to tracking, pixel to mm conversion was calibrated using an inbuilt GUI. One calibration file was generated for all videos taken on the same day. Background model and thresholds were adjusted to provide optimal recognition of animals and were not standardized between recording sessions. If present, the state of an indicator light was annotated by custom-written MATLAB software.
Behavioral classifiers
Each frame of an individual’s walking bout was assigned a behavioral classifier based on the definitions below:
Jump: Jumps were classified as frames where the velocity of the animal exceeded 50 mm/s.
Walk: Walking frames were defined using a dual threshold Schmitt trigger filter. Speed thresholds were set at 1 and 2.5 mm/s, and time thresholds were 0.1 s. Walking frames were also specified to be those in which the fly was not already engaged in a jump.
Stop: Stop frames were classified as any frames where animals were not performing walking or jumping behaviors.
Parameters
Baseline walking parameters:
Walk Frequency: the percent of frames classified as walking during the recording period.
Overall Velocity: the median of all velocities over the recording period.
Walking Velocity: the median of velocities during all frames when the animal is classified as walking.
Maximum Walking Velocity: the maximum velocity an animal reaches during walking.
Angular velocity: the median value of angular velocity. This parameter takes into account directionality of turning.
Absolute Angular Velocity: the median of the absolute value of angular velocities. This parameter does not take into account directionality of turning.
Distance from Wall: the median distance from the closest point on the arena wall during the recording period.
Walking bout number, bouts were defined as contiguous frames of walking (longer than .1 s as specified in the walking classifier). The number of bouts was calculated for the entire recording period.
Walking bout duration: the length of each bout was calculated, and the median of all bout lengths was taken for each animal.
Stop bout number and duration: calculated as for walking bouts.
Jump Frequency: the percent of time that an animal spends in the jump state as defined above.
Startle parameters:
Startle responses were highly variable between individuals. To generate smoother average traces and model fitting, the data was serially resampled, with the median velocity of 20 animals selected for each frame, generating an average trace of random animals each run. The number of resampling runs for each genotype matched the actual number of animals studied.
Parameters calculated for each averaged trace included:
Minimum Velocity: defined as the minimum velocity reached within the first three seconds of the walking bout. This time threshold was selected based on aggregate traces that show the initial pause phase is well below this threshold.
Maximum Velocity: defined as the maximum velocity reached during the vibration stimulus period.
Pause Length: a Schmitt trigger classifier was used to define pause bouts. For inactivation light data and mutant vibration data, speed thresholds were defined as 2 mm/s faster than the minimum speed, and 5 mm/s faster than the minimum speed. For inactivation vibration data thresholds were 2 mm/s faster than the minimum speed and 3 mm/s faster than the minimum speed. Time thresholds were defined as .17 seconds for the “not paused” classification. No constraints were set on the length of the pause phase. The length of the first pause bout after the onset of the stimulus was recorded as “pause length”. The thresholds of the classifier were tested with ten average traces per genotype and classifier fit was validated by eye.
Rise Time: defined as the time from the end of the pause phase to the maximum speed during the vibration pulse.
Statistics
For optogenetic arena experiments, behavior parameters described above were calculated for the five-minute light on period and compared to the same metric calculated during the light off period for each individual. For each activation experiment, we recorded behavior from both experimental (Trh ∩ tsh > csChrimson) and control (w1118 ∩ tsh > csChrimson) flies. For each genotype, we analyzed data from flies that had been fed with ATR, the required co-factor for optogenetic activation, and flies from the same cross that had been fed on food depleted of ATR. Figures show comparisons between ATR+ control and experimental animal behavior, as we found these populations had the most similar light off behavior pattern. However, significant differences in parameters are consistent even when all controls are included in the analysis.
For constitutive inhibition arena experiments, behavior during the five-minute light off period was analyzed for experimental (Trh ∩ tsh > Kir2.1) and control (w1118 ∩ tsh > Kir2.1) flies fed on the same ATR negative food we used for optogenetic experiments.
All analysis on data from arena experiments was performed in MATLAB using custom-written scripts. For each individual and parameter, a Z score was calculated comparing that individual’s behavior to the mean and standard deviation of the control group. Z scores of different genotypes were compared using Kruskal-wallis analysis with Dunn-sidak multiple correction testing when multiple groups were being compared. To compare changes in velocity distribution, bootstrapping (1000 replicates) was used to estimate the median difference between two genotypes and fit a 95% confidence interval around this difference.
For experiments under different conditions (heat, orientation, starvation, vibration), median walking speed was compared between control and experimental animals under each condition using Kruskal-wallis analysis. For all conditions except vibration, animals who spent less than 30 seconds of the five minute recording period walking were excluded from the analysis. The response of each genotype to contextual shifts was independently assessed by Kruskal-wallis analysis with Dunn-sidak correction for multiple comparions.
As described above, for vibration and light experiments, parameters were defined for each average trace, with the number of traces per genotype corresponding to the actual number of animals in the experimental group. For a particular parameter, genotypes were compared using Kruskal Wallis test with subsequent Dunn-sidak correction for multiple comparisons. The model and statistical analysis was run ten times for each data set to identify parameters that consistently were found to be significant.
The statistical tests performed for specific experiments are described in the figure legend for each experiment.
Flywalker Behavior Experiments
Tracking
Flywalker videos were automatically tracked using custom software written by Imre Bartos as described in [27]. Tracking was then validated by eye and incorrect footprint calls were corrected. Summary plots were then screened by eye for gross errors and for linear traces. If traces were short (<3 traces per foot) or excessively turning, they were excluded.
Parameters
Behavioral parameters were calculated as described in [27]. Gait parameters were defined as follows. Leg order in combination: LF RF LM RM LH RH. 1 indicates the leg is in stance phase, 0 indicates the leg is in swing phase.
| Tripod | Tetrapod | Wave | Non-Canonical |
|---|---|---|---|
| 100110 | 011011 | 011111 | All Other |
| 011001 | 011110 | 101111 | |
| 100111 | 110111 | ||
| 110110 | 111011 | ||
| 101101 | 111101 | ||
| 111001 | 111110 |
Statistics
For Flywalker experiments, behavior was recorded for a one minute walking bout with red light illumination. Light off conditions were not possible as the white light LEDs required to generate fTIR signal contained the red wavelength used to activate our optogenetic tool. For each activation experiment, we recorded behavior from both experimental (Trh – or other neuromodulatory Gal4 driver – ∩ tsh > csChrimson) and control (w1118 ∩ tsh > csChrimson) flies. For each genotype, we analyzed data from flies that had been fed with ATR, the required co-factor for optogenetic activation, and flies from the same cross that had been fed on food depleted of ATR. Figures show comparisons between ATR+ and ATR− controls, as these populations provide the best genetic control and had the most similar behavioral pattern. However, significant differences in parameters are consistent even when all controls are included in our multivariate model (described below).
Statistical analysis of Flywalker data was performed using custom scripts written in MATLAB and R. For each walking bout, an average was calculated for every parameter across three to five footprints per leg. For parameters that exponentially related to speed, the natural logarithm was taken of both the bout speed and parameter values. A multivariable regression model was then run on the data for every kinematic parameter. The formula for this model was as follows:
This model was designed to analyze the effects of activation while controlling for speed, which is the largest contributor to behavioral shifts.
We also ran a version of this model that contained all control data, to validate our results:
To prevent model overfitting, we selected our model based on Akaike information criterion using the R step() package.
Functional Imaging
Analysis
Initial image Processing: TIFF videos from two-photon microscopy were processed in Fiji to merge green (opGCaMP6f) and red (tdTomato) channels [95]. No brightness or contrast adjustments were performed, in order to standardize region-of-interest (ROI) selection.
ROI Selection: The tdTomato channel was used to select ROIs containing neuronal processes, using custom Python software relying on OpenCV and Numpy libraries. Images were converted into 8-bits, color ranges were extended, and contrast was augmented to better detect ROIs. Baseline signals were subtracted and then brightness was scaled to a maximum value of 255. A blur filter was applied to the image (blur value =10), and then an Otsu Threshold was applied to binarize the grayscale image. After the image was thus thresholded, an erosion function (kernel size 5) was used to avoid the detection of overly large or small ROIs. The contours of all ROIs were detected on the eroded image and a copy of the contrast-augmented image was returned with ROI contours drawn super-imposed. A minimum threshold of 150 pixels was set on the ROI size to avoid overly small detections.
Fluorescence extraction: Mean fluorescence values for the tdTomato, or opGCaMP6f channels were calculated over all ROIs combined. Baseline signals for dF/F calculations were defined as mean raw fluorescence binned over 2.5 s.
Synchronization: Fluorescence measurements, behavior videography, and optic flow of spherical treadmill rotations were all recorded at different frame rates. Thus, we used interpolation to upsample fluorescence signals and behavioral videography acquisition rates to that of optic flow. Optic flow and fluorescence data were then smoothed (window size 200 ms). Optic flow data was then translated into mm/s in the anterior-posterior and medial-lateral directions and into degrees/s for yaw.
Automatic Walking Classifier: An automatic walking classifier was used to define walking bouts. A velocity of 0.31 mm/s was empirically determined as a threshold for distinguishing between walking and standing. The minimum threshold for bout length was empirically set to 2 s.
Manual behavioral annotation: Videos showing a side view of the fly on the spherical treadmill were manually annotated to capture four behaviors: (1) walk, (2) stop, (3) proboscis extension reflex, and (4) groom. All frames that could not neatly be classified as one of these four behaviors were defined as (5) other.
Statistics
Manually Annotated Behaviors: For each animal, the average dF/F for frames labeled a particular behavior classification was calculated. Comparisons between behaviors were made using Kruskal-Wallis testing with Dunn’s correction for multiple comparisons.
Timecourses: For each behavioral classification, an average time course was determined for each animal by averaging dF/F for all behavioral bouts (between 80 and 130 bouts per animal), centering them on bout onset. Averages across all animals were then calculated, and 95% confidence intervals fit by bootstrapping.
Correlation Analysis: A Pearson R correlation coefficient was calculated between walking velocity and dF/F.
DATA AND CODE AVAILABILITY
Tracking of flies in Flywalker and arena experiments was done using published tracking code [27, 58]. Analysis performed on Flywalker data was completed in R. Analysis performed on arena data sets was completed in MATLAB. Analysis of functional imaging data was completed in Python. Custom scripts written for these analyses and associated data are available for download at Zenodo.org, DOI 10.5281/zenodo.3497586
Supplementary Material
Highlights.
Serotonergic neurons in the fly VNC extensively innervate the leg neuropils
Activating these neurons causes flies to walk slower but maintain coordination
Silencing these neurons causes flies to walk faster in many contexts
Silencing these neurons alters how flies respond to being startled
Acknowledgements
We thank Cesar Mendes for helping to optimize the Flywalker system, Imre Bartos for help in updating the Flywalker analysis code, Andrew Straw for editing software, Meredith Peterson, Floris van Bruegel, Irene Kim and Michael Dickinson for assistance with hardware and data analysis, Randy Bruno for assistance with analysis, and Laura Hermans for assistance with data analysis. We thank Daniel Wolpert and the anonymous referees for helpful comments on the manuscript. This work was supported by NIH grants to R.S.M (1U01NS090514 and 1U19NS104655) the Columbia MD/PhD Training program (GM007367) and the Columbia Neuroscience Program (5T32NS064928). P.R. acknowledges support from the Swiss National Science Foundation (31003A_175667)
Footnotes
Declaration of Interests
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ritzmann RE, and Büschges A (2007). Adaptive motor behavior in insects. Curr. Opin. Neurobiol 17, 629–636. [DOI] [PubMed] [Google Scholar]
- 2.Ritzmann RE, Quinn RD, and Fischer MS (2004). Convergent evolution and locomotion through complex terrain by insects, vertebrates and robots. Arthropod Struct Dev 33, 361–379. [DOI] [PubMed] [Google Scholar]
- 3.Mendes CS, Rajendren SV, Bartos I, Márka S, and Mann RS (2014). Kinematic responses to changes in walking orientation and gravitational load in Drosophila melanogaster. PLoS ONE 9, e109204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bidaye SS, Machacek C, Wu Y, and Dickson BJ (2014). Neuronal control of Drosophila walking direction. Science 344, 97–101. [DOI] [PubMed] [Google Scholar]
- 5.Isakov A, Buchanan SM, Sullivan B, Ramachandran A, Chapman JKS, Lu ES, Mahadevan L, and de Bivort B (2016). Recovery of locomotion after injury in Drosophila depends on proprioception. J. Exp. Biol [DOI] [PubMed] [Google Scholar]
- 6.Pick S, and Strauss R (2005). Goal-driven behavioral adaptations in gap-climbing Drosophila. Current Biology 15, 1473–1478. [DOI] [PubMed] [Google Scholar]
- 7.Blaesing B, and Cruse H (2004). Stick insect locomotion in a complex environment: climbing over large gaps. J. Exp. Biol 207, 1273–1286. [DOI] [PubMed] [Google Scholar]
- 8.Strauss R, and Heisenberg M (1990). Coordination of legs during straight walking and turning in Drosophila melanogaster. J. Comp. Physiol. A 167, 403–412. [DOI] [PubMed] [Google Scholar]
- 9.Couzin-Fuchs E, Kiemel T, Gal O, Ayali A, and Holmes P (2015). Intersegmental coupling and recovery from perturbations in freely running cockroaches. J. Exp. Biol 218, 285–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wosnitza A, Bockemühl T, Dübbert M, Scholz H, and Büschges A (2013). Interleg coordination in the control of walking speed in Drosophila. J. Exp. Biol 216, 480–491. [DOI] [PubMed] [Google Scholar]
- 11.Pearson KG (1993). Common principles of motor control in vertebrates and invertebrates. Annu. Rev. Neurosci 16, 265–297. [DOI] [PubMed] [Google Scholar]
- 12.Harris-Warrick RM, and Marder E (1991). Modulation of neural networks for behavior. Annu. Rev. Neurosci 14, 39–57. [DOI] [PubMed] [Google Scholar]
- 13.Graham D (1985). Pattern and Control of Walking in Insects In Advances in Insect Physiology Advances in Insect Physiology. Berridge MJ, Treherne JE, and Wigglesworth VB, eds. (Academic Press; ), pp. 31–140. [Google Scholar]
- 14.Court RC, Armstrong JD, Borner J, Card G, Costa M, Dickinson M, Duch C, Korff W, Mann R, Merritt D, et al. (2017). A Systematic Nomenclature for the Drosophila Ventral Nervous System. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Venkatasubramanian L, and Mann RS (2019). The development and assembly of the Drosophila adult ventral nerve cord. Curr. Opin. Neurobiol 56, 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Truman JW, Schuppe H, Shepherd D, and Williams DW (2004). Developmental architecture of adult-specific lineages in the ventral CNS of Drosophila. Development 131, 5167–5184. [DOI] [PubMed] [Google Scholar]
- 17.Namiki S, Dickinson MH, Wong AM, Korff W, and Card GM (2018). The functional organization of descending sensory-motor pathways in Drosophila. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baek M, and Mann RS (2009). Lineage and birth date specify motor neuron targeting and dendritic architecture in adult Drosophila. J. Neurosci 29, 6904–6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brierley DJ, Blanc E, Reddy OV, VijayRaghavan K, and Williams DW (2009). Dendritic targeting in the leg neuropil of Drosophila: the role of midline signalling molecules in generating a myotopic map. PLoS Biol. 7, e1000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tuthill JC, and Azim E (2018). Proprioception. Curr. Biol 28, R194–R203. [DOI] [PubMed] [Google Scholar]
- 21.Murphey RK, Possidente DR, Vandervorst P, and Ghysen A (1989). Compartments and the topography of leg afferent projections in Drosophila. J. Neurosci 9, 3209–3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tuthill JC, and Wilson RI (2016). Mechanosensation and Adaptive Motor Control in Insects. Current Biology 26, R1022–R1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Büschges A (2005). Sensory control and organization of neural networks mediating coordination of multisegmental organs for locomotion. J. Neurophysiol 93, 1127–1135. [DOI] [PubMed] [Google Scholar]
- 24.Field L, and Matheson T (1998). Chordotonal Organs of Insects. Advances in Insect Physiology 27. [Google Scholar]
- 25.Yellman C, Tao H, He B, and Hirsh J (1997). Conserved and sexually dimorphic behavioral responses to biogenic amines in decapitated Drosophila. Proc. Natl. Acad. Sci. U.S.A 94, 4131–4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burrows M (1992). Local circuits for the control of leg movements in an insect. Trends Neurosci. 15, 226–232. [DOI] [PubMed] [Google Scholar]
- 27.Mendes CS, Bartos I, Akay T, Márka S, and Mann RS (2013). Quantification of gait parameters in freely walking wild type and sensory deprived Drosophila melanogaster. elifesciences.org. [DOI] [PMC free article] [PubMed]
- 28.Stein W, Büschges A, and Bässler U (2006). Intersegmental transfer of sensory signals in the stick insect leg muscle control system. J. Neurobiol 66, 1253–1269. [DOI] [PubMed] [Google Scholar]
- 29.Büschges A, and Manira AE (1998). Sensory pathways and their modulation in the control of locomotion. Curr. Opin. Neurobiol 8, 733–739. [DOI] [PubMed] [Google Scholar]
- 30.Pearson KG (1995). Proprioceptive regulation of locomotion. Curr. Opin. Neurobiol 5, 786–791. [DOI] [PubMed] [Google Scholar]
- 31.Grillner S, and Zangger P (1979). On the central generation of locomotion in the low spinal cat. Exp Brain Res 34, 241–261. [DOI] [PubMed] [Google Scholar]
- 32.Bekoff A, Nusbaum MP, Sabichi AL, and Clifford M (1987). Neural control of limb coordination. I. Comparison of hatching and walking motor output patterns in normal and deafferented chicks. J. Neurosci 7, 2320–2330. [PMC free article] [PubMed] [Google Scholar]
- 33.Marder E, and Bucher D (2001). Central pattern generators and the control of rhythmic movements. Current Biology 11, R986–96. [DOI] [PubMed] [Google Scholar]
- 34.Rossignol S, Dubuc R, and Gossard J-P (2006). Dynamic sensorimotor interactions in locomotion. Physiol. Rev 86, 89–154. [DOI] [PubMed] [Google Scholar]
- 35.Pearson KG (2000). Neural adaptation in the generation of rhythmic behavior. Annu. Rev. Physiol 62, 723–753. [DOI] [PubMed] [Google Scholar]
- 36.Marder E (2012). Neuromodulation of neuronal circuits: back to the future. Neuron 76, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dickinson PS (2006). Neuromodulation of central pattern generators in invertebrates and vertebrates. Curr. Opin. Neurobiol 16, 604–614. [DOI] [PubMed] [Google Scholar]
- 38.Harris-Warrick RM (2011). Neuromodulation and flexibility in Central Pattern Generator networks. Curr. Opin. Neurobiol 21, 685–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morton DW, and Chiel HJ (1994). Neural architectures for adaptive behavior. Trends Neurosci. 17, 413–420. [DOI] [PubMed] [Google Scholar]
- 40.Barbeau H, and Rossignol S (1991). Initiation and modulation of the locomotor pattern in the adult chronic spinal cat by noradrenergic, serotonergic and dopaminergic drugs. Brain Res. 546, 250–260. [DOI] [PubMed] [Google Scholar]
- 41.Harris-Warrick RM, and Cohen AH (1985). Serotonin modulates the central pattern generator for locomotion in the isolated lamprey spinal cord. J. Exp. Biol 116, 27–46. [DOI] [PubMed] [Google Scholar]
- 42.Parker D (1995). Serotonergic modulation of locust motor neurons. J. Neurophysiol 73, 923–932. [DOI] [PubMed] [Google Scholar]
- 43.Albin SD, Kaun KR, Knapp J-M, Chung P, Heberlein U, and Simpson JH (2015). A Subset of Serotonergic Neurons Evokes Hunger in Adult Drosophila. Curr. Biol 25, 2435–2440. [DOI] [PubMed] [Google Scholar]
- 44.Majeed ZR, Abdeljaber E, Soveland R, Cornwell K, Bankemper A, Koch F, and Cooper RL (2016). Modulatory Action by the Serotonergic System: Behavior and Neurophysiology in Drosophila melanogaster. Neural Plast. 2016, 7291438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mohammad F, Aryal S, Ho J, Stewart JC, Norman NA, Tan TL, Eisaka A, and Claridge-Chang A (2016). Ancient Anxiety Pathways Influence Drosophila Defense Behaviors. Curr. Biol 26, 981–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pooryasin A, and Fiala A (2015). Identified Serotonin-Releasing Neurons Induce Behavioral Quiescence and Suppress Mating in Drosophila. J. Neurosci 35, 12792–12812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qian Y, Cao Y, Deng B, Yang G, Li J, Xu R, Zhang D, Huang J, and Rao Y (2017). Sleep homeostasis regulated by 5HT2b receptor in a small subset of neurons in the dorsal fan-shaped body of drosophila. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alekseyenko OV, Lee C, and Kravitz EA (2010). Targeted manipulation of serotonergic neurotransmission affects the escalation of aggression in adult male Drosophila melanogaster. PLoS ONE 5, e10806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alekseyenko OV, Chan YB, Fernandez M. de L. P., Bülow T, Pankratz MJ, and Kravitz EA (2014). Single serotonergic neurons that modulate aggression in Drosophila. Curr. Biol. 24, 2700–2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Riemensperger T, Isabel G, Coulom H, Neuser K, Seugnet L, Kume K, Iché-Torres M, Cassar M, Strauss R, Preat T, et al. (2011). Behavioral consequences of dopamine deficiency in the Drosophila central nervous system. Proc. Natl. Acad. Sci. U.S.A 108, 834–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ueno T, Masuda N, Kume S, and Kume K (2012). Dopamine modulates the rest period length without perturbation of its power law distribution in Drosophila melanogaster. PLoS One 7, e32007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pendleton RG, Rasheed A, Sardina T, Tully T, and Hillman R (2002). Effects of tyrosine hydroxylase mutants on locomotor activity in Drosophila: a study in functional genomics. Behav. Genet 32, 89–94. [DOI] [PubMed] [Google Scholar]
- 53.Yang Z, Yu Y, Zhang V, Tian Y, Qi W, and Wang L (2015). Octopamine mediates starvation-induced hyperactivity in adult Drosophila. Proc. Natl. Acad. Sci. U.S.A. 112, 5219–5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brembs B, Christiansen F, Pflüger HJ, and Duch C (2007). Flight initiation and maintenance deficits in flies with genetically altered biogenic amine levels. J. Neurosci 27, 11122–11131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Friggi-Grelin F, Coulom H, Meller M, Gomez D, Hirsh J, and Birman S (2003). Targeted gene expression in Drosophila dopaminergic cells using regulatory sequences from tyrosine hydroxylase. J. Neurobiol 54, 618–627. [DOI] [PubMed] [Google Scholar]
- 56.Cole SH, Carney GE, McClung CA, Willard SS, Taylor BJ, and Hirsh J (2005). Two functional but noncomplementing Drosophila tyrosine decarboxylase genes: distinct roles for neural tyramine and octopamine in female fertility. J. Biol. Chem 280, 14948–14955. [DOI] [PubMed] [Google Scholar]
- 57.Branson K, Robie AA, Bender J, Perona P, and Dickinson MH (2009). High-throughput ethomics in large groups of Drosophila. Nat. Methods 6, 451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eyjolfsdottir E, Branson S, Burgos-Artizzu XP, Hoopfer ED, Schor J, Anderson DJ, and Perona P (2014). Detecting Social Actions of Fruit Flies In, Fleet D, Pajdla T, Schiele B, and Tuytelaars T, eds. (Cham: Springer International Publishing; ), pp. 772–787. [Google Scholar]
- 59.Chen C-L, Hermans L, Viswanathan MC, Fortun D, Aymanns F, Unser M, Cammarato A, Dickinson MH, and Ramdya P (2018). Imaging neural activity in the ventral nerve cord of behaving adult Drosophila. Nature Communications 9, 459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gibert P, Huey RB, and Gilchrist GW (2001). Locomotor performance of Drosophila melanogaster: interactions among developmental and adult temperatures, age, and geography. Evolution 55, 205–209. [DOI] [PubMed] [Google Scholar]
- 61.Cho W, Heberlein U, and Wolf FW (2004). Habituation of an odorant-induced startle response in Drosophila. Genes Brain Behav. 3, 127–137. [DOI] [PubMed] [Google Scholar]
- 62.Lee G, and Park JH (2004). Hemolymph sugar homeostasis and starvation-induced hyperactivity affected by genetic manipulations of the adipokinetic hormone-encoding gene in Drosophila melanogaster. Genetics 167, 311–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yeomans JS, Li L, Scott BW, and Frankland PW (2002). Tactile, acoustic and vestibular systems sum to elicit the startle reflex. Neurosci Biobehav Rev 26, 1–11. [DOI] [PubMed] [Google Scholar]
- 64.Yeomans JS, and Frankland PW (1995). The acoustic startle reflex: neurons and connections. Brain Res. Brain Res. Rev 21, 301–314. [DOI] [PubMed] [Google Scholar]
- 65.Card GM (2012). Escape behaviors in insects. Curr. Opin. Neurobiol 22, 180–186. [DOI] [PubMed] [Google Scholar]
- 66.Zacarias R, Namiki S, Card GM, Vasconcelos ML, and Moita MA (2018). Speed dependent descending control of freezing behavior in Drosophila melanogaster. Nature Communications 9, 3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Card G, and Dickinson MH (2008). Visually mediated motor planning in the escape response of Drosophila. Current Biology 18, 1300–1307. [DOI] [PubMed] [Google Scholar]
- 68.Davis M (1984). The Mammalian Startle Response In Neural Mechanisms of Startle Behavior, Eaton RC, ed. (Boston, MA: Springer US; ), pp. 287–351. [Google Scholar]
- 69.Saudou F, and Hen R (1994). 5-Hydroxytryptamine receptor subtypes in vertebrates and invertebrates. Neurochem. Int 25, 503–532. [DOI] [PubMed] [Google Scholar]
- 70.Tierney AJ (2018). Invertebrate serotonin receptors: a molecular perspective on classification and pharmacology. J. Exp. Biol 221, jeb184838. [DOI] [PubMed] [Google Scholar]
- 71.Saudou F, Boschert U, Amlaiky N, Plassat JL, and Hen R (1992). A family of Drosophila serotonin receptors with distinct intracellular signalling properties and expression patterns. EMBO J. 11, 7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Witz P, Amlaiky N, Plassat JL, Maroteaux L, Borrelli E, and Hen R (1990). Cloning and characterization of a Drosophila serotonin receptor that activates adenylate cyclase. Proc. Natl. Acad. Sci. U.S.A 87, 8940–8944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Blenau W, Daniel S, Balfanz S, Thamm M, and Baumann A (2017). Dm5-HT2B: Pharmacological Characterization of the Fifth Serotonin Receptor Subtype of Drosophila melanogaster. Front Syst Neurosci 11, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Colas JF, Launay JM, Kellermann O, Rosay P, and Maroteaux L (1995). Drosophila 5-HT2 serotonin receptor: coexpression with fushi-tarazu during segmentation. Proc. Natl. Acad. Sci. U.S.A 92, 5441–5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gnerer JP, Venken KJT, and Dierick HA (2015). Gene-specific cell labeling using MiMIC transposons. Nucleic Acids Res. 43, e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Veasey SC, Fornal CA, Metzler CW, and Jacobs BL (1995). Response of serotonergic caudal raphe neurons in relation to specific motor activities in freely moving cats. J. Neurosci 15, 5346–5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Veasey SC, Fornal CA, Metzler CW, and Jacobs BL (1997). Single-unit responses of serotonergic dorsal raphe neurons to specific motor challenges in freely moving cats. Neuroscience 79, 161–169. [DOI] [PubMed] [Google Scholar]
- 78.Correia PA, Lottem E, Banerjee D, Machado AS, Carey MR, and Mainen ZF (2017). Transient inhibition and long-term facilitation of locomotion by phasic optogenetic activation of serotonin neurons. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gibson WT, Gonzalez CR, Fernandez C, Ramasamy L, Tabachnik T, Du RR, Felsen PD, Maire MR, Perona P, and Anderson DJ (2015). Behavioral responses to a repetitive visual threat stimulus express a persistent state of defensive arousal in Drosophila. Curr. Biol. 25, 1401–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pearson KG, and O’Shea M (1984). Escape Behavior of the Locust In Neural Mechanisms of Startle Behavior Neural Mechanisms of Startle Behavior. Eaton RC, ed. (Boston, MA: Springer US; ), pp. 163–178. [Google Scholar]
- 81.Ritzmann RE (1984). The Cockroach Escape Response In Neural Mechanisms of Startle Behavior Neural Mechanisms of Startle Behavior. Eaton RC, ed. (Boston, MA: Springer US; ), pp. 93–131. [Google Scholar]
- 82.Davis M, and Sheard MH (1974). Habituation and sensitization of the rat startle response: effects of raphe lesions. Physiol. Behav 12, 425–431. [DOI] [PubMed] [Google Scholar]
- 83.Carlton PL, and Advokat C (1973). Attenuated habituation due to parachlorophenylalanine. Pharmacol. Biochem. Behav 1, 657–663. [DOI] [PubMed] [Google Scholar]
- 84.Davis M, Strachan DI, and Kass E (1980). Excitatory and inhibitory effects of serotonin on sensorimotor reactivity measured with acoustic startle. Science 209, 521–523. [DOI] [PubMed] [Google Scholar]
- 85.Astrachan DI, and Davis M (1981). Spinal modulation of the acoustic startle response: the role of norepinephrine, serotonin and dopamine. Brain Res. 206, 223–228. [DOI] [PubMed] [Google Scholar]
- 86.Cande J, Namiki S, Qiu J, Korff W, Card GM, Shaevitz JW, Stern DL, and Berman GJ (2018). Optogenetic dissection of descending behavioral control in Drosophila. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Perrier J-F, and Cotel F (2014). Serotonergic modulation of spinal motor control. Curr. Opin. Neurobiol 33C, 1–7. [DOI] [PubMed] [Google Scholar]
- 88.Johnson MD, and Heckman CJ (2014). Gain control mechanisms in spinal motoneurons. Front Neural Circuits 8, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cappellini G, Ivanenko YP, Dominici N, Poppele RE, and Lacquaniti F (2010). Motor patterns during walking on a slippery walkway. J. Neurophysiol 103, 746–760. [DOI] [PubMed] [Google Scholar]
- 90.Voloshina AS, and Ferris DP (2015). Biomechanics and energetics of running on uneven terrain. J. Exp. Biol 218, 711–719. [DOI] [PubMed] [Google Scholar]
- 91.Nakazawa K, Kawashima N, Akai M, and Yano H (2004). On the reflex coactivation of ankle flexor and extensor muscles induced by a sudden drop of support surface during walking in humans. J. Appl. Physiol 96, 604–611. [DOI] [PubMed] [Google Scholar]
- 92.Kavlie RG, and Albert JT (2013). Chordotonal organs. Curr. Biol. 23, R334–5. [DOI] [PubMed] [Google Scholar]
- 93.Mamiya A, Gurung P, and Tuthill JC (2018). Neural Coding of Leg Proprioception in Drosophila. Neuron 100, 636–650.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Daniels RW, Gelfand MV, Collins CA, and Diantonio A (2008). Visualizing glutamatergic cell bodies and synapses inDrosophila larval and adult CNS. J. Comp. Neurol 508, 131–152. [DOI] [PubMed] [Google Scholar]
- 95.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. (2012). Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Simon JC, and Dickinson MH (2010). A new chamber for studying the behavior of Drosophila. PLoS ONE 5, e8793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Straw AD, and Dickinson MH (2009). Motmot, an open-source toolkit for realtime video acquisition and analysis. Source Code Biol Med 4, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ryder E, Blows F, Ashburner M, Bautista-Llacer R, Coulson D, Drummond J, Webster J, Gubb D, Gunton N, Johnson G, et al. (2004). The DrosDel collection: a set of P-element insertions for generating custom chromosomal aberrations in Drosophila melanogaster. Genetics 167, 797–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Enriquez J, Venkatasubramanian L, Baek M, Peterson M, Aghayeva U, and Mann RS (2015). Specification of individual adult motor neuron morphologies by combinatorial transcription factor codes. Neuron 86, 955–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Burgos A, Honjo K, Ohyama T, Qian CS, Shin GJ-E, Gohl DM, Silies M, Tracey WD, Zlatic M, Cardona A, et al. (2018). Nociceptive interneurons control modular motor pathways to promote escape behavior in Drosophila. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Klapoetke NC, Murata Y, Kim SS, Pulver SR, Birdsey-Benson A, Cho YK, Morimoto TK, Chuong AS, Carpenter EJ, Tian Z, et al. (2014). Independent optical excitation of distinct neural populations. Nat. Methods 11, 338–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hardie RC, Raghu P, Moore S, Juusola M, Baines RA, and Sweeney ST (2001). Calcium influx via TRP channels is required to maintain PIP2 levels in Drosophila photoreceptors. Neuron 30, 149–159. [DOI] [PubMed] [Google Scholar]
- 103.Shearin HK, Macdonald IS, Spector LP, and Stowers RS (2014). Hexameric GFP and mCherry reporters for the Drosophila GAL4, Q, and LexA transcription systems. Genetics 196, 951–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Tracking of flies in Flywalker and arena experiments was done using published tracking code [27, 58]. Analysis performed on Flywalker data was completed in R. Analysis performed on arena data sets was completed in MATLAB. Analysis of functional imaging data was completed in Python. Custom scripts written for these analyses and associated data are available for download at Zenodo.org, DOI 10.5281/zenodo.3497586