Abstract
Background
A key component of schistosomiasis control is mass drug administration with praziquantel. While control interventions have been successful in several endemic regions, mass drug administration has been less effective in others. Here we focus on the impact of repeated praziquantel treatment on the population structure and genetic diversity of Schistosoma mansoni.
Methods
We examined S. mansoni epidemiology, population genetics, and variation in praziquantel susceptibility in parasites isolated from children across three primary schools in a high endemicity region at the onset of the Ugandan National Control Programme. Children were sampled at 11 timepoints over two years, including one week and four weeks post-praziquantel treatment to evaluate short-term impacts on clearance and evidence of natural variation in susceptibility to praziquantel.
Results
Prevalence of S. mansoni was 85% at baseline. A total of 3576 miracidia larval parasites, isolated from 203 individual children, were genotyped at seven loci. Overall, genetic diversity was high and there was low genetic differentiation, indicating high rates of parasite gene flow. Schistosome siblings were found both pre-treatment and four weeks post-treatment, demonstrating adult worms surviving treatment and natural praziquantel susceptibility variation in these populations at the beginning of mass drug administration. However, we did not find evidence for selection on these parasites. While genetic diversity decreased in the short-term (four weeks post-treatment), diversity did not decrease over the entire period despite four rounds of mass treatment. Furthermore, within-host genetic diversity was affected by host age, host sex, infection intensity and recent praziquantel treatment.
Conclusions
Our findings suggest that praziquantel treatments have short-term impacts on these parasite populations but impacts were transient and no long-term reduction in genetic diversity was observed. High gene flow reduces the likelihood of local adaptation, so even though parasites surviving treatment were observed, these were likely to be diluted at the beginning of the Ugandan National Control Programme. Together, these results suggest that MDA in isolation may be insufficient to reduce schistosome populations in regions with high genetic diversity and gene flow.
Keywords: Schistosomiasis, Mass drug administration, Praziquantel, Trematodes, Population genetics, Interventions, Natural variation
Background
Schistosomiasis is a neglected tropical disease that infects over 240 million people across 78 countries, predominantly within the developing world [1]. Adult Schistosoma mansoni sexually reproduces (predominantly) in humans and eggs are excreted in faeces. In high endemicity areas, worm burdens can be very heavy, producing as many as 9600 eggs per gram (epg) of stool [2]. In areas with inadequate containment of stool due to poor sanitation, eggs contact freshwater and hatch into free-swimming miracidia. Miracidia then infect suitable snail intermediate hosts and undergo asexual reproduction, releasing thousands of free-swimming clonal cercariae daily [3]. Cercariae burrow through the skin to infect humans when they contact infectious water, through activities such as bathing, gathering water or fishing. Despite the integral role of insufficient water, sanitation and hygiene (WASH) in maintaining transmission, preventative chemotherapy through mass drug administration (MDA) with praziquantel is currently the main strategy for controlling morbidity, and ultimately transmission, of schistosomiasis in endemic areas [4]. While MDA has been successful in reducing morbidity and prevalence or intensity of schistosomiasis across many parts of sub-Saharan Africa [5–7], persistent transmission hotspots of Schistosoma species remain [8, 9].
Studies investigating the genetic structure of Schistosoma populations and their response to MDAs have the ability to quantify the impact and potential limitations of MDAs [10, 11]. These findings could help identify parasite-specific characteristics contributing to persistent transmission. The impact of treatment on parasite populations depends on many factors including, but not exclusive to, population coverage, frequency of drug pressure, baseline levels of parasite genetic diversity [12, 13], and rates of parasite gene flow [14]. Drug selection has been linked to lower effective population sizes [15] and can reduce genetic diversity of parasites in the laboratory [16]. In the field, treatment generally reduces prevalence and intensity of parasites in targeted populations and individuals [17, 18], but can also measurably reduce transmission rates across the population, influencing infections in individuals beyond the treated group [19–21]. However, treatments can also select for reduced drug efficacy and/or increase in resistance in populations [22–25]. Therefore, it is important to understand how parasites are structured across the landscape, and within individuals, in order to monitor treatment impacts and manage for the potential emergence and spread of drug resistance.
Several studies in Africa support a lack of genetic population structure in Schistosoma species at relatively small scales, from within villages to between sites up to 60 km apart [26–30]. High rates of gene flow suggest there are minimal barriers for transmission, at least at these geographical scales. The only large-scale study to date, to the authors’ knowledge, which encompassed five African countries, also found little support for structure between geographically close sites but distinct parasite clusters at the country level [31]. However, in some parts of Brazil, gene flow has been observed to be limited, even between sites 6 km apart [32]. Human movement patterns and water flow have also been shown to facilitate parasite population structure between different boroughs within a single town [33]. Higher overall levels of genetic diversity and longer history of transmission of S. mansoni in East Africa relative to South America may contribute to these differences in population structure, but studies explicitly evaluating these hypotheses are lacking.
At least under laboratory conditions, praziquantel reduces the diversity of S. mansoni and drug resistance can be selected for in as few as six generations [34, 35]. Reduced drug efficacy has also been recorded in several endemic areas, including Uganda [24, 36], though the geographic spread of resistance has not yet been documented. There are no genetic markers for resistance or reduced susceptibility to praziquantel in any schistosomes and the mechanism of action for the drug is unknown, complicating understanding treatment failures. The effect of praziquantel treatment on S. mansoni genetic diversity in the field also offers conflicting results. Reduction of genetic diversity has been observed six months after a single praziquantel treatment in two schools in Tanzania [21, 37]. In contrast, studies in Kenya showed school-based praziquantel MDA did not reduce genetic diversity over a five-year period [2] and another study in Senegal showed no reduction in genetic diversity over two years [30]. Similarly, a study in Brazil demonstrated little differentiation between parasites isolated pre-treatment and four to six weeks post-treatment [38].
Here we focus on the structure and genetic diversity of S. mansoni at the beginning of the MDA in Uganda, the first schistosomiasis MDA programme in sub-Saharan Africa [39]. Few field studies to date have examined Schistosoma genetic diversity over short (less than one month) and medium-term (six months or more) follow-ups after praziquantel treatment. In this study, we use a unique longitudinal dataset to examine how repeated praziquantel treatments can affect schistosome populations. We examine evidence for adult worms surviving treatment, suggesting natural variation in tolerance or resistance to praziquantel treatment. We hypothesize that mean genetic diversity would decline immediately following praziquantel treatment but expect diversity to recover at longer time-scales because of high gene flow and high genetic diversity at the population level. We expect clearance of parasites to be high, as host populations were praziquantel-naïve and parasites had not undergone repeated rounds of praziquantel selection.
Methods
Parasite sampling
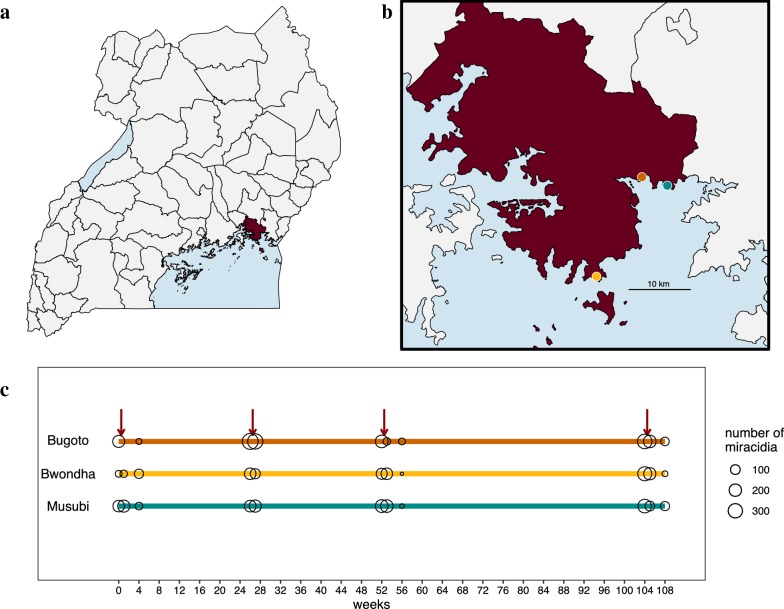
Children aged 6–12 years were initially recruited for this study in 2004 with an equal sex ratio from three primary schools on the shores of Lake Victoria in eastern Uganda (Fig. 1a, b). The primary schools are located in three separate villages within Mayuge District and between 4.35 km and 18.75 km apart (road and shoreline distances; as a proxy for actual travel distances are greater; Additional file 1: Table S1). Sample recruitment has been described previously [40, 41] and full details of new recruits and follow-ups are given in Additional file 1: Text S1 and Table S2. After the initial recruitment, an additional 30 praziquantel-naïve 6-year olds were recruited each year and included in follow-up surveys. Across a two-year period, there were a total of 11 sampling timepoints (Fig. 1c). Sample timepoints were designed to capture the effect of praziquantel treatment on parasite epidemiology and genetics in the short-term (one week and four weeks post-treatment) and medium-term (six months or more post-treatment). We acknowledge that medium-term is not reflective of an absolute definition but is used within this manuscript for convenience to describe discrete sampling windows.
Fig. 1.
Sampling locations and timeline of genetic samples. a Map of Uganda with b Mayuge district (dark red) and the three primary schools [Bugoto Lake View (Bugoto-orange), Bwondha (Bwondha-yellow) and Musubi Church of God (Musubi-teal)] indicated and the number of miracidia sampled at each timepoint (c). The red arrows indicate praziquantel given to the entire cohort after medium-term schistosomiasis surveys
At each timepoint, stool samples were collected for three consecutive days to measure infection intensity by duplicate Kato-Katz thick smears [42]. The number of S. mansoni eggs observed in a slide was multiplied by 24 to obtain eggs per gram (epg) (a standard 41.2 mg template was used to prepare). After Kato-Katz slides were prepared, the remainder of each stool sample was filtered through a Pitchford funnel to collect and hatch miracidia from eggs [43]. Filtered samples were exposed to sunlight and individual miracidia were picked up in 2.5–5.0 μl bottled spring water under a stereomicroscope. In 2004, single miracidia were placed in individual PCR tubes and kept cool until flash freezing each evening in a − 80 °C freezer and then shipped on dry ice to Imperial College London. From 2005 onwards, individual miracidia were placed on Whatman Indicating FTATM cards for cell lysis and DNA storage [44]. Cards were kept at room temperature in sealed plastic bags with desiccants in the field and during transport. As many miracidia as possible were collected for each child onto a single FTA card per timepoint resulting in a final range of 0–132 miracidia collected per child at any given timepoint. We use previous nomenclature and define all parasites isolated from a single child as an infrapopulation [37]. The cumulative number of miracidia at each timepoint is given in Fig. 1c.
The entire cohort was treated with praziquantel after each medium-term timepoint (no praziquantel treatment in the previous four weeks), indicated by red arrows in Figs. 1c and 2. At week 1, children with infection intensities greater than 100 epg were retreated with praziquantel. At all other timepoints, children were retreated with praziquantel if they had any S. mansoni eggs detected in any Kato-Katz slides. Children were treated with 40 mg/kg praziquantel, determined by weight. At all timepoints, observed treatment was recorded for each child.
Fig. 2.
Epidemiology and genetic diversity of S. mansoni in Mayuge District from 2004–2006. Prevalence of S. mansoni infection (a) and mean infection intensity, as eggs per gram of stool (epg) (b), estimated with three days of replicate Kato-Katz in each of the three schools sampled. Red arrows indicate timing of mass praziquantel treatment. c The mean allelic richness for all infrapopulations sampled at each timepoint for each school. No miracidia were isolated from infrapopulations in Bwondha at week 56
A randomly selected subset of miracidia from 11 children at 26 and 27 weeks were used for an in vitro assay that measured phenotypic praziquantel susceptibility of miracidia. This in vitro assay exposes miracidia to praziquantel and uses changes in shape, behaviour and activity levels as a proxy for susceptibility and has been validated in prior laboratory and field studies [40, 45]. Here we have linked key summary measures for each infrapopulation to genetic data from these corresponding hosts. Two measures that showed the most variation between individuals (but not among) were used as a proxy for praziquantel susceptibility of miracidia: the proportion of miracidia that had abnormal movement and the proportion of miracidia that were immobile/dead after seven minutes in vitro exposure to 2 × 10−6 M praziquantel. Resistant genotypes have fewer changes in shape and behavioural responses to praziquantel exposure and thus a higher proportion of these miracidia are still behaving normally at seven minutes [40, 45]. Because individual miracidia were not simultaneously phenotyped and genotyped, average metrics for infrapopulations at each timepoint were linked.
Laboratory analyses
DNA extraction and microsatellite analysis followed established protocols [44]. Briefly, individual miracidia were sized at seven microsatellite loci (Additional file 1: Table S3) in a single multiplex reaction that have low error rates in S. mansoni from Lake Albert, Uganda. Allele sizes were determined using ABI PRISM Genescan v2.7 and Genotyper v2.7 software (Applied Biosystems, Foster City, CA, USA).
While we aimed to amplify all microsatellites from 30 miracidia per infrapopulation per timepoint, one quarter of timepoints were represented by fewer than ten miracidia. This disparity in terms of sample size could affect the statistical power of the models and the accuracy of genetic diversity measures. However, a simulation study using similar microsatellite markers reported that more robust measures of genetic diversity are obtained when increasing the number of hosts rather than the number of miracidia per host [37]. Additional limitations of this study include genotypic errors inherent in these microsatellite markers [44] that may affect the conclusions. However, we were very stringent with allele calls and inclusion criteria to minimize these biases.
Data analyses
All analyses were carried out in R v3.5.1 [46]. Specific packages are cited alongside functions used and summary code for these analyses can be found on github (see section “Availability of data and materials” below).
Epidemiological summary statistics
Individual schistosome infection intensities were calculated as an arithmetic mean of epg estimates from daily Kato-Katz slides examined at that timepoint. Paired rank sum Wilcoxon tests were used to test differences between pre- and post-treatment infection intensities. Population prevalence was calculated at each timepoint for each school and 95% confidence intervals (CI) intervals were calculated with Agresti-Coull approximations [47].
Genetic diversity measures
Departure from Hardy–Weinberg equilibrium (HWE) was quantified in pegas v0.11 [48], implementing the Monte Carlo procedure present in the function hw.test with 1000 permutations. At each sampling point, infrapopulation schistosome diversity observed heterozygosity (Ho) and expected heterozygosity (He) were calculated in poppr v2.8.1 [49]. Allelic richness (AR), which corrects the number of alleles per locus for uneven sample size, was calculated for each infrapopulation and timepoint using the hierfstat package v0.04-22 [50].
Determining spatial, temporal and host effects on infrapopulation genetic diversity
To identify potential factors that affected the observed infrapopulation genetic diversity of parasites at a given timepoint, we constructed linear models using the function lm. Child ID (unique value identifying individual) was included as a random effect in a linear mixed effect model using lme4 [51] to account for repeated samples from the same infrapopulation over time, but was found to be insignificant. Explanatory variables included age, sex of child, cumulative number of observed praziquantel treatments, time since last observed treatment (in weeks), infection intensity at that sampling timepoint, and weeks from the beginning of MDA in that community. Sampling timepoints were also divided into three distinct categories: pre-treatment (weeks 0, 26, 52, 104); one week post-treatment (weeks 1, 27, 53, 58); and four weeks post-treatment (weeks 4, 56, 108). This was performed to increase statistical power, as post-treatment, especially at four weeks post-treatment, fewer miracidia were collected. The number of miracidia per infrapopulation per timepoint was included in the models as weights to reduce bias associated with estimates based on smaller sample sizes. Model comparison and selection was conducted using Akaike’s information criterion (AIC) [52].
Within-host dynamics
Some infrapopulations were sampled for miracidia at more than one timepoint. To further examine within-host dynamics, the genetic dataset was subset to include infrapopulations that were sampled at more than one timepoint, particularly pre-treatment and one week and four weeks post-treatment. Trees of infrapopulations over time were constructed using Nei’s distances in poppr v2.8.1 [49]. COLONY software was used to identify full-sibling pairs between miracidia within infrapopulations using the full likelihood method and long runs [53]. Because only seven microsatellite loci are used, the ability to detect half-siblings amongst this dataset was very limited and therefore the mating system was assumed to be monogamous. Miracidia with ≥ 0.75 probability of belonging to a family were included in the analysis (< 0.75 probability were assumed to be singletons). Our interest was to identify the occurrence of siblings between pre- and post-treatment sampling points, suggesting adult worms surviving treatment and reproducing viable offspring.
Quantifying population structure and gene flow
To determine the levels of gene flow, we used several methods to quantify structure of populations. Analysis of molecular variance (AMOVA), which detects population differentiation, was conducted using the function amova in poppr [49]. An AMOVA was carried out on the entire dataset to measure the genetic differentiation between schools, among children between schools and within children. AMOVAs were also carried out at each timepoint. P-values were calculated by 1000 random permutations. Population structure was also investigated using the discriminant analysis of principal components (DAPC) method [54] implemented in adegenet v2.1.1 [55], and by visualising Cavalli-Sforza & Edwards chord distances in hierfstat v0.04-22 with the neighbour-joining method implemented in ape v5.2 [56]. Phylogenetic trees were created using in vitro praziquantel data to elucidate whether infrapopulations with more drug-resistant phenotypes at that timepoint were genetically distinct from those infrapopulations which were more susceptible.
Results
A total of 468 unique children were sampled for S. mansoni over 11 timepoints during the two-year study (Additional file 1: Table S2). Miracidia were isolated and analysed from 207 of these children from at least one timepoint. Deviations from Hardy–Weinberg equilibrium (HWE) were tested on the whole dataset of 4743 miracidia. The majority of infrapopulations at each timepoint were found to strongly deviate from HWE. We then excluded miracidia that were not genotyped at all seven microsatellite loci, leaving a total dataset of 3576 from 203 children (Fig. 1c). Despite a smaller total sample size, this subset showed little deviation from HWE and only four children were removed from the genetic analyses. The number of miracidia successfully genotyped at seven loci within an infrapopulation ranged from 1 to 94 per timepoint (mean 25.3).
Baseline S. mansoni infections and genetic diversity
Schistosoma mansoni was found in 85.7% of individuals surveyed at the beginning of the study, indicating a high endemic transmission setting (Fig. 2a). The average infection intensity within an individual at the beginning of the study was 224.9 epg (moderate infection intensity; Fig. 2b). Genetic diversity of infrapopulations was also very high (Fig. 2c): average gene diversity among the loci (Hs) was 0.701 (range 0.280–0.888), while gene diversity among all populations (Ht) was 0.711 (range 0.282–0.901) (Additional file 1: Table S4). The number of alleles per locus ranged from 20 to 48. This supports the hypothesis that genetic diversity is high within these populations of S. mansoni.
Effect of praziquantel treatment on S. mansoni
One week post-treatment, genetic diversity was not significantly different than pre-treatment. This was also reflected in some of the epidemiological data; at a majority of time points and schools, the prevalence and infection intensity at one week post-treatment were not significantly different to pre-treatment (Additional file 1: Tables S5, S6).
However, prevalence and mean infection intensity significantly decreased at every four weeks post-treatment observation compared to pre-treatment (Fig. 2a, Additional file 1: Tables S5, S6). These data indicate a high level of success by praziquantel in reducing egg output four weeks after treatment and suggest there should be high levels of selection imposed on the parasites in treated infrapopulations. Concurrent to these epidemiological metrics, mean infrapopulation genetic diversity also significantly declined four weeks after each of the cohort treatments when accounting for age and sex of hosts (Fig. 3). This supports the hypothesis that praziquantel treatment reduces genetic diversity within treated individuals in the short-term following treatment.
Fig. 3.

Short-term declines in genetic diversity between pre- and 4 weeks post-treatment. Boxplots of infrapopulation mean allelic richness are shown for each primary school averaged pre-praziquantel timepoints (weeks 0, 26, 52, 104) and compared to 4 weeks after praziquantel treatment (weeks 4, 56, 108). Note the smaller sample size post treatment due to the lower number of individuals shedding miracidia
Although these short-term effects were significant, prevalence, intensity and genetic diversity recovered over timescales greater than four weeks. Genetic diversity declined from baseline (week 0) to subsequent pre-treatment samples (six months, one year and two years), but this decline was not significant (P > 0.05). This supports the hypothesis that genetic diversity of S. mansoni is resilient to praziquantel, at least within this observation period and setting.
Impact of treatment and host characteristics on S. mansoni infrapopulation genetic diversity
The best-fit model to explain infrapopulation genetic diversity, measured by AR at a given timepoint, contained infection intensity, short-term treatment, and an interaction between age and sex as significant predictor variables (Fig. 4). The mean AR of an infrapopulation for a female host pre-treatment, also the intercept in this model, was 1.65 (95% confidence interval (CI): 1.59 to 1.70). Each week post-treatment (up to four weeks), infrapopulation genetic diversity decreased (− 0.007, 95% CI: − 0.018 to − 0.001). Higher infection intensities slightly, but significantly, had higher genetic diversity; each additional 100 epg increased the mean allelic richness by 0.001 (95% CI: 0.0004 to 0.0020). Infrapopulations of males had a higher genetic diversity than those of females (0.07; 95% CI: 0.01 to 0.14). Schistosoma mansoni genetic diversity in female hosts increased with age (0.008; 95% CI: 0.002 to 0.015); however, in male hosts the interaction between age and sex reduced genetic diversity (− 0.010; 95% CI: − 0.018 to − 0.003).
Fig. 4.

Genetic diversity of infrapopulations by age and sex. Regression lines are based on individuals pre-treatment and with no detectable eggs. Female infrapopulation genetic diversity increases with age (yellow), whereas male infrapopulation genetic diversity begins higher and decreases slightly with age (navy)
Parasite structure and survival following treatment
Trees obtained from the Cavalli-Sforza & Edwards chord distances showed no clear clustering of infrapopulations between timepoints, suggesting no selection on these markers imposed by praziquantel treatment in the observed timeframe. Parasites excreted four weeks post-treatment were not more similar to one another compared with parasites collected pre-treatment within the same year and across all timepoints (Additional file 1: Figure S1). Additionally, infrapopulations with higher levels of in vitro drug-resistant phenotypes were not genetically distinct from infrapopulations with lower measures of drug-resistant phenotypes (Additional file 1: Table S7, Figure S2).
Miracidia that were collected from six infrapopulations before and after treatment showed evidence for clustering of pre-treatment and one week post-treatment (Fig. 5, Additional file 1: Figure S3). Parasites four weeks post-treatment were more distant, even compared to parasites sampled pre-treatment a year apart. We used COLONY to detect full siblings within these infrapopulations sampled both pre- and post-treatment. Analysis of miracidia from these infrapopulations identified siblings between pre- and post-treatment sampling points (Fig. 6), suggesting adult worm pairs survived treatment and produced viable miracidia, particularly when siblings were found four weeks post-treatment. There was a higher proportion of siblings detected at one week post-treatment compared to four weeks post-treatment, which was supported by phylogenies by timepoint (Fig. 5), but this is confounded by lower numbers of miracidia recovered four weeks post-treatment. Full siblings were found up to one year apart and following praziquantel treatment (Additional file 1: Tables S8, S9), but the number of miracidia recovered over longer timescales is very limited.
Fig. 5.
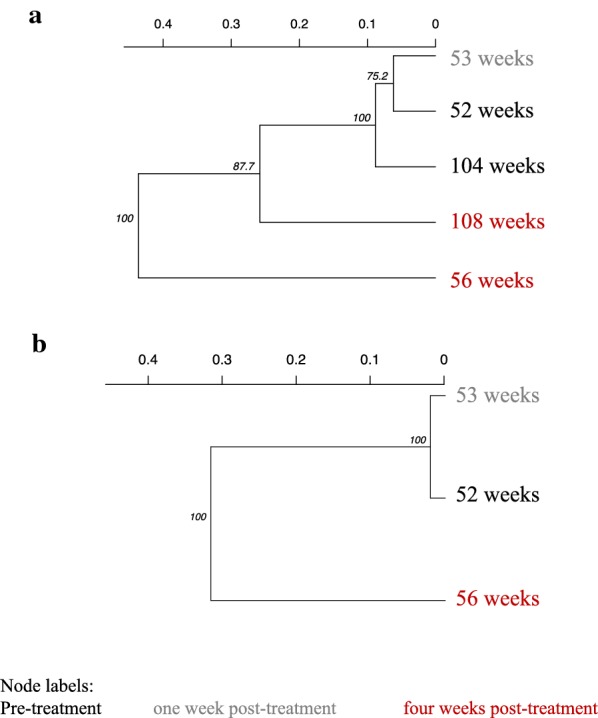
Phylogenies of infrapopulations from individual children sampled over time. The bootstrap support for each node is given and tips are labelled as the sampling time point. a A praziquantel naïve 6 year-old recruited in 2005 (52 weeks) in Musubi and followed-up at each time point afterwards. b A praziquantel naïve 6 year-old in 2005 (52 weeks) in Musubi that was followed at two post-treatment time point
Fig. 6.

Histograms of family structure of full-siblings from six infrapopulations sampled pre-treatment, one week post-treatment and four weeks post-treatment. The distribution of miracidia from reconstructed maximum likelihood families are shown in the histogram. The majority of miracidia are from single families or families from the same timepoint; however, some full siblings are found between pre- and post-treatment, with the highest frequency between pre-treatment and one week post-treatment
Gene flow between populations
The results of the AMOVA suggest a lack of structure between schools at different time points, showing that most of the variance in the dataset was explained by differences within hosts. Mean percentage of variation was 98.1% within hosts, 1.6% among hosts within schools and 0.3% between schools (Additional file 1: Table S10). FST was lower between schools, ranging from − 0.007 to 0.047, in the middle within schools, from 0.013 to 0.042 and higher within hosts, ranging from 0.015 to 0.088 (Additional file 1: Figure S4). The tree obtained from the Cavalli-Sforza & Edwards chord distances showed no clear clustering between villages (Additional file 1: Figure S5). Clustering algorithms implemented in DAPC also failed to identify an informative number of clusters in relation to school or timepoint (Additional file 1: Figure S6). These results support the hypothesis that gene flow is high between these populations.
Discussion
Using longitudinal epidemiological and genetic data from the start of MDA in Uganda, we show there are short-term effects of praziquantel treatment on S. mansoni but populations recover within six months. Although we identify parasites surviving treatment even at the beginning of MDA, there is no evidence that these parasites are selected for over this two-year period. High rates of gene flow between populations and refugia in snails and untreated individuals likely facilitate rapid recovery of parasite genetic diversity and prevent fixation of resistant/tolerant parasites.
Cure rates during this study were within praziquantel expectations at a population level for S. mansoni [57], suggesting that resistant/tolerant parasites are not overabundant in these study populations. However, we observed adult worms surviving treatment, as evidenced by full siblings found pre- and four weeks post-treatment in four of six infrapopulations with sufficient sampling frequency. We believe these are resistant or tolerant adult worms and are likely a subset of the natural variation (rather than a result of selection). Infrapopulation genetic diversity was significantly lower at four weeks post-treatment, supporting this idea. Juveniles at point of treatment could be contributing to some eggs observed at four weeks post-treatment, but the presence of siblings pre- and four weeks post-treatment suggest that at least some eggs are from adult worms that survive praziquantel. We also observed variation in phenotypic praziquantel susceptibility, but we did not directly sequence these parasites. The phenotypic and genetic data from this setting suggest that natural variation in this schistosome population has some praziquantel resistance or tolerance (we could not differentiate these with our data). This is consistent with evidence of natural variation in resistance within schistosomes predating drug use to a former anti-schistosomal drug, oxaminiquine, where resistance alleles are known [58].
Despite evidence of resistant/tolerant parasites in this population, there was no evidence for selection for these parasites in the timeframe observed. Parasites found four weeks after treatment did not cluster, nor did phenotypically resistant parasite populations. High rates of transmission and high rates of gene flow likely prevent population bottlenecking and could reduce the likelihood of resistance developing at a local level at the coverage levels and short- to medium- (under two years) time scales studied here [59]. Our genetic markers are likely not reflective of resistance; these microsatellite markers do not map to population (our study) or individual phenotypes [60]. It is not expected that microsatellites would be accurate markers for resistance, unless they were located physically close to praziquantel-resistant genes (which are not yet characterised in any Schistosoma species). Although these microsatellites do not seem to serve as resistance markers, they are useful for parentage analysis and identifying worms that survive treatment. Despite no evidence for selection in this study, concerted drug treatment across the area for several years may have selected for these resistant worms over longer timescales and resulted in the low cure rates currently observed in the region more recently [24].
Interestingly, there was very little difference in genetic diversity between pre- and one week post-treatment. We expect this is because eggs were still being excreted from adult worms that had produced the eggs before treatment, but which may have then died with treatment. Because genetic diversity and infection intensity were significantly lower at four weeks post-treatment, we expect most eggs of susceptible worms to be expelled by four weeks post-treatment. It is thought that eggs only survive up to three weeks after expulsion from the female [61]. This is supported by sibship analysis that finds a higher frequency of siblings between pre- and one week post-treatment compared to four weeks post-treatment. It is important to note that the majority of the host population are still shedding viable eggs one week post-treatment, meaning hosts contribute to transmission even a week after successful treatment. Infection intensities one week post-treatment in some schools and some timepoints were not significantly different from pre-treatment infection intensities, further emphasizing the potentially significant contribution to transmission in these communities.
There was no strong evidence for effects of praziquantel on genetic diversity over the medium-term. This is supported by other studies across sub-Saharan Africa [2, 26, 30, 60, 62]. Most studies focus on periods well after national control programmes begin. Only one other study, in addition to this one, examines parasite diversity and structure at the start of MDA. Norton et al. [21] found an initial decline six months after treatment at the start of MDA in Tanzania; however, a follow-up five years later demonstrated that parasite genetic diversity had recovered and even increased in these same schools [60]. One explanation for an initial decline in genetic diversity observed in Tanzania is a higher degree of population structure among the parasites compared to our sites. We did not observe genetic diversity declines after six months (only four weeks post-treatment), but recovery of genetic diversity of parasites at these Ugandan schools may be facilitated by higher rates of gene flow. Post-treatment parasite populations are small in comparison to the refugia in untreated humans within the community and other contributing communities as well as parasites in snails [63, 64]. Combined, these studies emphasize the resilience of schistosome parasite populations to repeated praziquantel treatments.
Many studies, including ours, find that the majority of the genetic diversity in S. mansoni occurs at the human host level, rather than village or district level [21, 28, 29, 31, 33]. This can be explained by a limited number of shared water contact sites and/or cercariae dispersing far enough to cover these sites. Genetic diversity was not significantly different between villages, suggesting similar exposure environments (all are along Lake Victoria) and further supporting a panmictic parasite population across the area surveyed. We also observed high levels of genetic diversity, similar to other studies that examined S. mansoni populations in Uganda [31, 65], and higher than that reported from other localities in East Africa. For example, a study focusing on four villages in Ethiopia [66] reported a total of six and 15 alleles for the SMD28 and SMDA28 loci, while in this study we recovered 26 and 54 alleles for the two loci, respectively. Lake Victoria is likely the origin of S. mansoni and larger scale surveys have reported the area to have the highest levels of genetic diversity across several markers [24, 31, 65, 67]. This high genetic diversity can increase chances of drug resistance developing and also help these populations recover from bottlenecking selection [12, 13]. However, high genetic diversity can also reduce the likelihood of an allele fixing in a population and can prevent resistance from spreading.
We found that infrapopulation genetic diversity is also significantly related to host age and sex. We interpret infrapopulation genetic diversity as the combined outcome of the genetic diversity of parasites circulating in the environment, variation in behaviour (particularly those related to water contact, i.e. location, duration and time of day), and establishment probability (dependent on host susceptibility and immune history and parasite infectiousness). We found that males had higher parasite genetic diversity than females. We expect this higher genetic diversity, particularly at younger ages, to reflect a difference in behaviour as young males have reported to play in the water more often than females of a similar age [68]. This effect of sex was dependent on age; males had similar genetic diversity across all ages surveyed here whereas each additional year significantly increased the genetic diversity of parasites observed in females. Older females (i.e. 10 years-old and above) help more in household chores like clothes washing and fetching water [68], which would increase their exposure to schistosomes and likely increase the genetic diversity observed in an infrapopulation. A study of S. haematobium in Mali also found significant impacts of host demographics; males had more unique genotypes and these private alleles increased with age [69]. Neither study, however, observed any decline in genetic diversity with age, as may be expected if immunity was developing. It may be that the genetic diversity is very high in these settings and new genotypes are constantly being encountered which hosts have not yet acquired immunity against. An alternative, non-exclusive, explanation is that the ages sampled here (6–14 years) are insufficient to detect this effect of an immune response on genetic diversity.
Conclusions
This study highlights the resilience of schistosome populations to repeated drug treatments in a high endemicity setting in Uganda. We found evidence for adult worms surviving treatment at the beginning of the national control programme, suggesting natural variation in resistance/tolerance. These may have been selected on with subsequent round of MDA and led to low cure rates observed a decade later. In settings with similar epidemiology and genetic diversity as observed here, MDA alone is unlikely to be sufficient for elimination and could even lead to long-term issues if drug resistance is selected.
Supplementary information
Additional file 1: Text S1. Additional methods. Table S1. Travel distances between schools. Table S2. Summary of infrapopulations (individuals) sampled at each timepoint (listed as weeks since start of study). Table S3. Microsatellite loci utilized in this study. Text S2. Additional results. Table S4. Comparison of genetic diversity at the seven microsatellite loci used in the study. Table S5. Proportion infected at pre- and post-treatment. Table S6. Infection intensity between pre- and post-treatment timepoints. Table S7. Summary of infrapopulation phenotyped. Table S8. Frequency of miracidia isolated per individual from relative timepoints. Table S9. Frequency of full-sibling miracidia belonging to a family structure (including singletons) per individual. Table S10. Analysis of molecular variance (AMOVA) for Schistosoma mansoni. Figure S1. Parasite structure through time. Figure S2. Trees of phenotype data. Figure S3. Phylogenies of infrapopulations from individual children sampled over time (continued from Fig. 5). Figure S4. Pairwise FST between each school and timepoint. Figure S5. Population differentiation by village. Figure S6. Clustering by discriminant analysis of principal components (DAPC).
Acknowledgements
We would like to thank all participants, community members and leaders for their involvement and support of the study. We would also like to thank the support provided by the Schistosomiasis Control Initiative to conduct this research.
Abbreviations
- AIC
akaike’s information criterion
- AMOVA
analysis of molecular variance
- CI
confidence interval
- DAPC
discriminant analysis of principal components
- epg
eggs per gram
- HWE
Hardy–Weinberg equilibrium
- MDA
mass drug administration
- WASH
water, sanitation and hygiene
Authors’ contributions
Study design: PHLL, NK, EMT and JPW. Study implementation and data collection: AM, DO, AW, MA and PHLL. Laboratory analysis: AJN, CMG and PHLL. Data analysis: CLF, MC and EKA. Interpretation: CLF, JPW and PHLL. Drafting and revising the manuscript: CLF, PHLL and JPW. All authors read and approved the final manuscript.
Funding
Field work was funded by the Schistosomiasis Control Initiative. During the field study, PHLL was funded by a Medical Research Council PhD studentship (under the supervision of JPW), CMG was funded by a Wellcome Trust Biodiversity Fellowship and JPW was funded by a Royal Society University Research Fellowship. PHLL and CLF are currently funded by a European Research Council Starting Grant (SCHISTO_PERSIST_680088) to PHLL. PHLL is also funded by a GCRF MRC Foundation award (MR/P025447/1) and an EPSRC grant (EP/R01437X/1). JPW currently funded by Biotechnology and Biological Sciences Research Council (BB/S013822/1), Global Challenges Research Fund (MR/S01313X/1), Research England (CCF-17-7779) and European and Developing Countries Clinical Trials Partnership grants.
Availability of data and materials
Archived raw data are deposited at researchdata.gla.ac.uk (10.5525/gla.researchdata.931). Code is available at https://github.com/cfaustus/ug_pop_gen_2004_2006.
Ethics approval and consent to participate
This study was undertaken as part of research conducted by the Schistosomiasis Control Initiative, Imperial College London and the Vector Control Division of the Ministry of Health, Uganda. Methods were approved by the Uganda National Council for Science and Technology (Memorandum of Understanding: sections 1.4, 1.5, 1.6) and the Imperial College Research Ethics Committee (EC NO: 03.36. R&D No: 03/SB/033E). Verbal assent was given by every child before inclusion in the study and at school committee meetings with parents, teachers and community leaders before the onset of the study. Written consent for the children to participate in the study was attained from each head teacher. Participation was voluntary and children could withdraw or be withdrawn from the study at any time. Access to treatment was not dependent on consenting to take part in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Joanne P. Webster and Poppy H. L. Lamberton are joint senior authors
Contributor Information
Christina L. Faust, Email: christina.faust@glasgow.ac.uk
Marco Crotti, Email: m.crotti.1@research.gla.ac.uk.
Arinaitwe Moses, Email: moses0772359814@gmail.com.
David Oguttu, Email: doguttu@gmail.com.
Aidah Wamboko, Email: wambokoaidah@gmail.com.
Moses Adriko, Email: adrikomoses@gmail.com.
Elizabeth K. Adekanle, Email: kennyadeks@gmail.com
Narcis Kabatereine, Email: vcdmoh@gmail.com.
Edridah M. Tukahebwa, Email: edmuheki@gmail.com
Alice J. Norton, Email: Alice.Norton@btinternet.com
Charlotte M. Gower, Email: charlottegowerox@gmail.com
Joanne P. Webster, Email: jowebster@rvc.ac.uk
Poppy H. L. Lamberton, Email: poppy.lamberton@glasgow.ac.uk
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13071-019-3860-6.
References
- 1.World Health Organization Schistosomiasis and soil-transmitted helminthiases: number of people treated in 2015. Wkly Epidemiol Rec. 2016;91:585–600. [PubMed] [Google Scholar]
- 2.Lelo AE, Mburu DN, Magoma GN, Mungai BN, Kihara JH, Mwangi IN, et al. No apparent reduction in schistosome burden or genetic diversity following four years of school-based mass drug administration in Mwea, central Kenya, a heavy transmission area. PLoS Negl Trop Dis. 2014;8:e3221. doi: 10.1371/journal.pntd.0003221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loker ES. A comparative study of the life-histories of mammalian schistosomes. Parasitology. 1983;87:343–369. doi: 10.1017/S0031182000052689. [DOI] [PubMed] [Google Scholar]
- 4.WHO . Preventive chemotherapy in human helminthiasis: coordinated use of anthelminthic drugs in control interventions: a manual for health professionals and programme managers. Geneva: World Health Organization; 2006. [Google Scholar]
- 5.Kabatereine NB, Tukahebwa E, Kazibwe F, Namwangye H, Zaramba S, Brooker S, et al. Progress towards countrywide control of schistosomiasis and soil-transmitted helminthiasis in Uganda. Trans R Soc Trop Med Hyg. 2006;100:208–215. doi: 10.1016/j.trstmh.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 6.Andrade G, Bertsch DJ, Gazzinelli A, King CH. Decline in infection-related morbidities following drug-mediated reductions in the intensity of Schistosoma infection: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2017;11:e0005372. doi: 10.1371/journal.pntd.0005372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Webster JP, Molyneux D, Hotez P, Fenwick A. The contribution of mass drug administration to global health: past, present and future. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130434. doi: 10.1098/rstb.2013.0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kittur N, Binder S, Campbell CH, King CH, Kinung’hi S, Olsen A, et al. Defining persistent hotspots: areas that fail to decrease meaningfully in prevalence after multiple years of mass drug administration with praziquantel for control of schistosomiasis. Am J Trop Med Hyg. 2017;97:1810–1817. doi: 10.4269/ajtmh.17-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pennance T, Person B, Muhsin MA, Khamis AN, Muhsin J, Khamis IS, et al. Urogenital schistosomiasis transmission on Unguja Island, Zanzibar: characterisation of persistent hot-spots. Parasite Vectors. 2016;9:646. doi: 10.1186/s13071-016-1847-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steinauer ML, Blouin MS, Criscione CD. Applying evolutionary genetics to schistosome epidemiology. Infect Genet Evol. 2010;10:433–443. doi: 10.1016/j.meegid.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Webster JP, Gower CM, Norton AJ. Evolutionary concepts in predicting and evaluating the impact of mass chemotherapy schistosomiasis control programmes on parasites and their hosts. Evol Appl. 2008;1:66–83. doi: 10.1111/j.1752-4571.2007.00012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDonald BA, Linde C. Pathogen population genetics, evolutionary potential, and durable resistance. Annu Rev Phytopathol. 2002;40:349–379. doi: 10.1146/annurev.phyto.40.120501.101443. [DOI] [PubMed] [Google Scholar]
- 13.Spielman D, Brook BW, Briscoe DA, Frankham R. Does inbreeding and loss of genetic diversity decrease disease resistance? Conserv Genet. 2004;5:439–448. doi: 10.1023/B:COGE.0000041030.76598.cd. [DOI] [Google Scholar]
- 14.Ariey F, Duchemin JB, Robert V. Metapopulation concepts applied to falciparum malaria and their impacts on the emergence and spread of chloroquine resistance. Infect Genet Evol. 2003;2:185–192. doi: 10.1016/S1567-1348(02)00099-0. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Mittler JE. Selection dramatically reduces effective population size in HIV-1 infection. BMC Evol Biol. 2008;8:133. doi: 10.1186/1471-2148-8-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coeli R, Baba EH, Araujo N, Coelho PM, Oliveira G. Praziquantel treatment decreases Schistosoma mansoni genetic diversity in experimental infections. PLoS Negl Trop Dis. 2013;7:e2596. doi: 10.1371/journal.pntd.0002596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mackenzie CD, Homeida MM, Hopkins AD, Lawrence JC. Elimination of onchocerciasis from Africa: possible? Trends Parasitol. 2012;28:16–22. doi: 10.1016/j.pt.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Deol A, Webster JP, Walker M, Basáñez MG, Hollingsworth TD, Fleming FM, et al. Development and evaluation of a Markov model to predict changes in schistosomiasis prevalence in response to praziquantel treatment: a case study of Schistosoma mansoni in Uganda and Mali. Parasit Vectors. 2016;9:543. doi: 10.1186/s13071-016-1824-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.French MD, Churcher TS, Gambhir M, Fenwick A, Webster JP, Kabatereine NB, et al. Observed reductions in Schistosoma mansoni transmission from large-scale administration of praziquantel in Uganda: a mathematical modelling study. PLoS Negl Trop Dis. 2010;4:e897. doi: 10.1371/journal.pntd.0000897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.French MD, Churcher TS, Webster JP, Fleming FM, Fenwick A, Kabatereine NB, et al. Estimation of changes in the force of infection for intestinal and urogenital schistosomiasis in countries with schistosomiasis control initiative-assisted programmes. Parasit Vectors. 2015;8:558. doi: 10.1186/s13071-015-1138-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Norton AJ, Gower CM, Lamberton PH, Webster BL, Lwambo NJ, Blair L, et al. Genetic consequences of mass human chemotherapy for Schistosoma mansoni: population structure pre- and post-praziquantel treatment in Tanzania. Am J Trop Med Hyg. 2010;83:951–957. doi: 10.4269/ajtmh.2010.10-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krücken J, Fraundorfer K, Mugisha JC, Ramünke S, Sifft KC, Geus D, et al. Reduced efficacy of albendazole against Ascaris lumbricoides in Rwandan schoolchildren. Int J Parasitol Drugs Drug Resist. 2017;7:262–271. doi: 10.1016/j.ijpddr.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soukhathammavong PA, Sayasone S, Phongluxa K, Xayaseng V, Utzinger J, Vounatsou P, et al. Low efficacy of single-dose albendazole and mebendazole against hookworm and effect on concomitant helminth infection in Lao PDR. PLoS Negl Trop Dis. 2012;6:e1417. doi: 10.1371/journal.pntd.0001417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crellen T, Walker M, Lamberton PH, Kabatereine NB, Tukahebwa EM, Cotton JA, et al. Reduced efficacy of praziquantel against Schistosoma mansoni is associated with multiple rounds of mass drug administration. Clin Infect Dis. 2016;63:1151–1159. doi: 10.1093/cid/ciw506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaplan RM, Vidyashankar AN. An inconvenient truth: global worming and anthelmintic resistance. Vet Parasitol. 2012;186:70–78. doi: 10.1016/j.vetpar.2011.11.048. [DOI] [PubMed] [Google Scholar]
- 26.Agola LE, Steinauer ML, Mburu DN, Mungai BN, Mwangi IN, Magoma GN, et al. Genetic diversity and population structure of Schistosoma mansoni within human infrapopulations in Mwea, central Kenya assessed by microsatellite markers. Acta Trop. 2009;111:219–225. doi: 10.1016/j.actatropica.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steinauer ML, Hanelt B, Agola LE, Mkoji GM, Loker ES. Genetic structure of Schistosoma mansoni in western Kenya: the effects of geography and host sharing. Int J Parasitol. 2009;39:1353–1362. doi: 10.1016/j.ijpara.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thiele EA, Sorensen RE, Gazzinelli A, Minchella DJ. Genetic diversity and population structuring of Schistosoma mansoni in a Brazilian village. Int J Parasitol. 2008;38:389–399. doi: 10.1016/j.ijpara.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van den Broeck F, Meurs L, Raeymaekers JA, Boon N, Dieye TN, Volckaert FA, et al. Inbreeding within human Schistosoma mansoni: do host-specific factors shape the genetic composition of parasite populations? Heredity (Edinb). 2014;113:32–41. doi: 10.1038/hdy.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huyse T, Van den Broeck F, Jombart T, Webster BL, Diaw O, Volckaert FA, et al. Regular treatments of praziquantel do not impact on the genetic make-up of Schistosoma mansoni in northern Senegal. Infect Genet Evol. 2013;18:100–105. doi: 10.1016/j.meegid.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 31.Gower CM, Gouvras AN, Lamberton PH, Deol A, Shrivastava J, Mutombo PN, et al. Population genetic structure of Schistosoma mansoni and Schistosoma haematobium from across six sub-Saharan African countries: implications for epidemiology, evolution and control. Acta Trop. 2013;128:261–274. doi: 10.1016/j.actatropica.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 32.Barbosa LM, Reis EA, Dos Santos CR, Costa JM, Carmo TM, Aminu PT, et al. Repeated praziquantel treatments remodel the genetic and spatial landscape of schistosomiasis risk and transmission. Int J Parasitol. 2016;46:343–350. doi: 10.1016/j.ijpara.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Curtis J, Sorensen RE, Minchella DJ. Schistosome genetic diversity: the implications of population structure as detected with microsatellite markers. Parasitology. 2002;125:S51–S59. doi: 10.1017/S0031182002002020. [DOI] [PubMed] [Google Scholar]
- 34.Cioli D, Botros SS, Wheatcroft-Francklow K, Mbaye A, Southgate V, Tchuenté LA, et al. Determination of ED50 values for praziquantel in praziquantel-resistant and -susceptible Schistosoma mansoni isolates. Int J Parasitol. 2004;34:979–987. doi: 10.1016/j.ijpara.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 35.Fallon PG, Doenhoff MJ. Drug-resistant schistosomiasis: resistance to praziquantel and oxamniquine induced in Schistosoma mansoni in mice is drug specific. Am J Trop Med Hyg. 1994;51:83–88. doi: 10.4269/ajtmh.1994.51.83. [DOI] [PubMed] [Google Scholar]
- 36.Melman SD, Steinauer ML, Cunningham C, Kubatko LS, Mwangi IN, Wynn NB, et al. Reduced susceptibility to praziquantel among naturally occurring Kenyan isolates of Schistosoma mansoni. PLoS Negl Trop Dis. 2009;3:e504. doi: 10.1371/journal.pntd.0000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.French MD, Churcher TS, Basáñez MG, Norton AJ, Lwambo NJ, Webster JP. Reductions in genetic diversity of Schistosoma mansoni populations under chemotherapeutic pressure: The effect of sampling approach and parasite population definition. Acta Trop. 2013;128:196–205. doi: 10.1016/j.actatropica.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 38.Blanton RE, Blank WA, Costa JM, Carmo TM, Reis EA, Silva LK, et al. Schistosoma mansoni population structure and persistence after praziquantel treatment in two villages of Bahia. Brazil. Int J Parasitol. 2011;41:1093–1099. doi: 10.1016/j.ijpara.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fenwick A, Webster JP, Bosque-Oliva E, Blair L, Fleming F, Zhang Y, et al. The Schistosomiasis Control Initiative (SCI): rationale, development and implementation from 2002–2008. Parasitology. 2009;136:1719–1730. doi: 10.1017/S0031182009990400. [DOI] [PubMed] [Google Scholar]
- 40.Lamberton PHL, Hogan SC, Kabatereine NB, Fenwick A, Webster JP. In vitro praziquantel test capable of detecting reduced in vivo efficacy in Schistosoma mansoni human infections. Am J Trop Med Hyg. 2010;83:1340–1347. doi: 10.4269/ajtmh.2010.10-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lamberton PH, Kabatereine NB, Oguttu DW, Fenwick A, Webster JP. Sensitivity and specificity of multiple Kato-Katz thick smears and a circulating cathodic antigen test for Schistosoma mansoni diagnosis pre- and post-repeated-praziquantel treatment. PLoS Negl Trop Dis. 2014;8:e3139. doi: 10.1371/journal.pntd.0003139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in Schistosoma mansoni. Rev Inst Med Trop Sao Paulo. 1972;14:397–400. [PubMed] [Google Scholar]
- 43.Pitchford RJ, Visser PS. A simple and rapid technique for quantitative estimation of helminth eggs in human and animal excreta with special reference to Schistosoma sp. Trans R Soc Trop Med Hyg. 1975;69:318–322. doi: 10.1016/0035-9203(75)90126-1. [DOI] [PubMed] [Google Scholar]
- 44.Gower CM, Shrivastava J, Lamberton PH, Rollinson D, Webster BL, Emery A, et al. Development and application of an ethically and epidemiologically advantageous assay for the multi-locus microsatellite analysis of Schistosoma mansoni. Parasitology. 2007;134:523–536. doi: 10.1017/S0031182006001685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liang YS, Coles GC, Doenhoff MJ, Southgate VR. In vitro responses of praziquantel-resistant and -susceptible Schistosoma mansoni to praziquantel. Int J Parasitol. 2001;31:1227–1235. doi: 10.1016/S0020-7519(01)00246-6. [DOI] [PubMed] [Google Scholar]
- 46.R Core Team: R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2018.
- 47.Agresti A, Coull B. Approximate is better than ‘exact’ for interval estimation of binomial proportions. Amer Statist. 1998;52:119–126. [Google Scholar]
- 48.Paradis E. pegas: an R package for population genetics with an integrated-modular approach. Bioinformatics. 2010;26:419–420. doi: 10.1093/bioinformatics/btp696. [DOI] [PubMed] [Google Scholar]
- 49.Kamvar ZN, Tabima JF, Grünwald NJ. Poppr: an R package for genetic analysis of populations with clonal, partially clonal, and/or sexual reproduction. PeerJ. 2014;2:e281. doi: 10.7717/peerj.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goudet J. Hierfstat, a package for R to compute and test hierarchical F-statistics. Mol Ecol Notes. 2005;5:184–186. doi: 10.1111/j.1471-8286.2004.00828.x. [DOI] [Google Scholar]
- 51.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1–48. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- 52.Burnham KP, Anderson DR. Model selection and inference: a practical information-theoretical approach. New-York: Springer Science+Business Media; 1998. [Google Scholar]
- 53.Jones OR, Wang J. COLONY: a program for parentage and sibship inference from multilocus genotype data. Mol Ecol Notes. 2010;10:551–555. doi: 10.1111/j.1755-0998.2009.02787.x. [DOI] [PubMed] [Google Scholar]
- 54.Jombart T, Devillard S, Balloux F. Discriminant analysis of principal components: a new method for the analysis of genetically structured populations. BMC Genet. 2010;11:94. doi: 10.1186/1471-2156-11-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jombart T. adegenet: a R package for the multivariate analysis of genetic markers. Bioinformatics. 2008;24:1403–1405. doi: 10.1093/bioinformatics/btn129. [DOI] [PubMed] [Google Scholar]
- 56.Paradis E, Claude J, Strimmer K. APE: analyses of phylogenetics and evolution in R language. Bioinformatics. 2004;20:289–290. doi: 10.1093/bioinformatics/btg412. [DOI] [PubMed] [Google Scholar]
- 57.Zwang J, Olliaro PL. Clinical efficacy and tolerability of praziquantel for intestinal and urinary schistosomiasis - a meta-analysis of comparative and non-comparative clinical trials. PLoS Negl Trop Dis. 2014;8:e3286. doi: 10.1371/journal.pntd.0003286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chevalier FD, Le Clec’h W, McDew-White M, Menon V, Guzman MA, Holloway SP, et al. Oxamniquine resistance alleles are widespread in Old World Schistosoma mansoni and predate drug deployment. PLoS Pathog. 2019;15:e1007881. doi: 10.1371/journal.ppat.1007881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lenormand T. Gene flow and the limits to natural selection. Trends Ecol Evol. 2002;17:183–189. doi: 10.1016/S0169-5347(02)02497-7. [DOI] [Google Scholar]
- 60.Gower CM, Gehre F, Marques SR, Lamberton PHL, Lwambo NJ, Webster JP. Phenotypic and genotypic monitoring of Schistosoma mansoni in Tanzanian schoolchildren five years into a preventative chemotherapy national control programme. Parasite Vectors. 2017;10:593. doi: 10.1186/s13071-017-2533-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jourdane J, Theron A. Larval development: eggs to cercariae. In: Rollinson D, Simpson AJG, editors. The biology of schistosomes: from genes to latrines. New York: Academic Press; 1987. pp. 83–106. [Google Scholar]
- 62.Betson M, Sousa-Figueiredo JC, Kabatereine NB, Stothard JR. New insights into the molecular epidemiology and population genetics of Schistosoma mansoni in Ugandan pre-school children and mothers. PLoS Negl Trop Dis. 2013;7:e2561. doi: 10.1371/journal.pntd.0002561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Adriko M, Faust CL, Carruthers LV, Moses A, Tukahebwa EM, Lamberton PHL. Low praziquantel treatment coverage for Schistosoma mansoni in Mayuge District, Uganda, due to the absence of treatment opportunities, rather than systematic non-compliance. Trop Med Infect Dis. 2018;3:E111. doi: 10.3390/tropicalmed3040111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chami GF, Kontoleon AA, Bulte E, Fenwick A, Kabatereine NB, Tukahebwa EM, et al. Profiling nonrecipients of mass drug administration for schistosomiasis and hookworm infections: a comprehensive analysis of praziquantel and albendazole coverage in community-directed treatment in Uganda. Clin Infect Dis. 2016;62:200–207. doi: 10.1093/cid/civ829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stothard JR, Webster BL, Weber T, Nyakaana S, Webster JP, Kazibwe F, et al. Molecular epidemiology of Schistosoma mansoni in Uganda: DNA barcoding reveals substantial genetic diversity within Lake Albert and Lake Victoria populations. Parasitology. 2009;136:1813–1824. doi: 10.1017/S003118200999031X. [DOI] [PubMed] [Google Scholar]
- 66.Aemero M, Boissier J, Climent D, Moné H, Mouahid G, Berhe N, et al. Genetic diversity, multiplicity of infection and population structure of Schistosoma mansoni isolates from human hosts in Ethiopia. BMC Genet. 2015;16:137. doi: 10.1186/s12863-015-0297-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morgan JA, Dejong RJ, Adeoye GO, Ansa ED, Barbosa CS, Brémond P, et al. Origin and diversification of the human parasite Schistosoma mansoni. Mol Ecol. 2005;14:3889–3902. doi: 10.1111/j.1365-294X.2005.02709.x. [DOI] [PubMed] [Google Scholar]
- 68.Hedges S, Sear R, Todd J, Urassa M, Lawson DW. Trade-offs in children’s time allocation: mixed support for embodied capital models of the demographic transition in Tanzania. Curr Anthrop. 2018;59:644–654. doi: 10.1086/699880. [DOI] [Google Scholar]
- 69.Gower CM, Gabrielli AF, Sacko M, Dembelé R, Golan R, Emery AM, et al. Population genetics of Schistosoma haematobium: development of novel microsatellite markers and their application to schistosomiasis control in Mali. Parasitology. 2011;138:978–994. doi: 10.1017/S0031182011000722. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Text S1. Additional methods. Table S1. Travel distances between schools. Table S2. Summary of infrapopulations (individuals) sampled at each timepoint (listed as weeks since start of study). Table S3. Microsatellite loci utilized in this study. Text S2. Additional results. Table S4. Comparison of genetic diversity at the seven microsatellite loci used in the study. Table S5. Proportion infected at pre- and post-treatment. Table S6. Infection intensity between pre- and post-treatment timepoints. Table S7. Summary of infrapopulation phenotyped. Table S8. Frequency of miracidia isolated per individual from relative timepoints. Table S9. Frequency of full-sibling miracidia belonging to a family structure (including singletons) per individual. Table S10. Analysis of molecular variance (AMOVA) for Schistosoma mansoni. Figure S1. Parasite structure through time. Figure S2. Trees of phenotype data. Figure S3. Phylogenies of infrapopulations from individual children sampled over time (continued from Fig. 5). Figure S4. Pairwise FST between each school and timepoint. Figure S5. Population differentiation by village. Figure S6. Clustering by discriminant analysis of principal components (DAPC).
Data Availability Statement
Archived raw data are deposited at researchdata.gla.ac.uk (10.5525/gla.researchdata.931). Code is available at https://github.com/cfaustus/ug_pop_gen_2004_2006.