Abstract
Apnea of prematurity (AOP) defined as cessation of breathing for 15- 20 seconds, is commonly seen in preterm infants. Caffeine is widely used to treat AOP due to its safety and effectiveness. Caffeine releases respiratory arrest by competing with adenosine for binding to adenosine A1 and A2A receptors (A1R and A2AR). Long before its use in treating AOP, caffeine has been used as a psychostimulant in adult brains. However, the effect of caffeine on developing brains remains unclear. We found that A1R proteins for caffeine binding were expressed in the brains of neonatal rodents and preterm infants (26–27 weeks). Neonatal A1R proteins colocalized with PSD-95, suggesting its synaptic localization. In contrast, our finding on A2R expression in neonatal neurons was restricted to the mRNA level as detected by single cell RT/PCR due to the lack of specific A2AR antibody. Furthermore, caffeine (200 μM) at a dose twice higher than the clinically relevant dose (36–130 μM) had minor or no effects on several basic neuronal functions, such as neurite outgrowth, synapse formation, expression of A1R and transcription of CREB-1 and c-Fos, further supporting the safety of caffeine for clinical use. We found that treatment with CoCl2 (125 μM), a hypoxia mimetic agent, for 24 hours triggered neuronal death and nuclear accumulation of HIF-1α in primary neuronal cultures. Subsequent treatment with caffeine at a concentration of 100 μM alleviated CoCl2-induced cell death and prevented nuclear accumulation of HIF-1α. Consistently, caffeine treatment in early postnatal life of neonatal mice (P4-P7) also prevented subsequent hypoxia-induced nuclear increase of HIF-1α. Together, our data support the utility of caffeine in alleviating hypoxia-induced damages in developing neurons.
1. Introduction
Apnea of prematurity (AOP), defined as cessation of breathing for more than 20 seconds, is commonly seen in preterm infants (≤30 weeks of gestation). Preterm infants have a higher incidence of apnea due to exaggerated inhibition of the respiratory rhythm by adenosine signaling (Mathew, 2011). Methylxanthines, including caffeine, have been used to treat AOP for 40 years. Caffeine effectively releases this respiratory arrest by competing with adenosine for binding to adenosine receptors (Bairam et al., 1987). Clinically, relatively high concentrations of caffeine are used to treat AOP. The plasma concentrations of caffeine in preterm infants during AOP treatment were shown to be 10–100 times higher than that in infants receiving breast milk from mothers who drank moderate doses of coffee (Aden, 2011). The efficacy of caffeine was not evaluated until recently by a multicenter trial led by Erenberg and colleagues and by the international trial on caffeine for apnea (CAP trial) (Erenberg et al., 2000; Schmidt, 2005). The CAP trial suggested that caffeine is beneficial in lowering the incidence of several short-term morbidities, including bronchopulmonary dysplasia, and improving neurodevelopmental outcomes of premature infants at 18 to 21 months of age (Schmidt et al., 2007). These clinical trials not only validated the use of caffeine in alleviating AOP but also suggested that neonatal caffeine treatment may be beneficial to neurodevelopment in preterm infants.
Caffeine has been used to stimulate adult brains as a psychostimulant long before its use in treating AOP (Ferre, 2016; Frary et al., 2005). It is thought to modulate adult brain activity and plasticity by directly perturbing the purinergic system and indirectly affecting the transmission of other neurotransmitters (Fredholm et al., 1999). However, the effects of caffeine on developing brains remain unclear, especially when preterm infants receive such high concentrations of caffeine during AOP treatment.
Hypoxic ischemia is one of the major causes of early brain injury, which affects around 0.1–0.3% of term infants and the incidence in preterm infants is 0.1–0.8% (Davidson et al., 2018). Therapeutic strategies to treat early brain injury include neuroprotective agents and plasticity enhancing agents for functional recovery. Caffeine has been suggested to have both effects: 1) caffeine has been suggested to be neuroprotective in both rodents and preterm infants (Back et al., 2006; Cunha, 2001; Ferreira and Paes-de-Carvalho, 2001; Fredholm et al., 2011; Rivkees and Wendler, 2011; Winerdal et al., 2017) (Schmidt et al., 2007) and 2) caffeine is known to modulate plasticity in adult brains (Fredholm et al., 1999). We therefore propose that caffeine is a candidate agent for alleviating the damages caused by hypoxia ischemia in neonatal brains.
Adenosine A1 and A2A receptors (A1R and A2AR) are the primary targets of caffeine (Fredholm et al., 1999). Thus, characterizing the expression of these receptors in neonatal brains is essential before testing the effects of caffeine for treating early brain injury. A1R and A2AR can be detected in rat brains at perinatal stages by in situ hybridization and radioactive ligand binding assays, albeit at lower levels compared to adult brains (Aden et al., 2000; Aden et al., 2001; Rivkees, 1995). However, the protein expression and subcellular localization of these receptors in the brain have not been identified in neonatal rodents and preterm infants. Using an antibody specifically against A1R in wild type mice, but not in A1R deficient mice, we found that A1R displayed a punctate staining pattern and were synaptically localized in the brains of both neonatal mouse pups (postnatal day 3) and preterm infants (corrected age of 26–27 weeks). In contrast to A1R, the localization of A2AR in neonatal brains remains unclear due to the poor specificity of tested antibodies against A2AR. Furthermore, we also demonstrated the safety and therapeutic effects of caffeine on neonatal neurons. We found caffeine at a concentration of 200 μM did not affect basic neuronal functions, such as 1) expression and distribution of adenosine receptor, A1R, 2) mRNA levels of transcription factors, CREB-1, c-Fos and HIF-1α, and 3) neurite outgrowth and synapse formation. Importantly, we found that at a clinically relevant dose, caffeine promoted survival of primary neurons after hypoxic insults and prevented hypoxia-induced accumulation of HIF-1α both in primary culture (100 μM of caffeine) and in neonatal mouse pups (estimated serum concentration of caffeine is 26–77 μM).
2. Materials and Methods
The protocols for animal experiments were approved by IACUC at SUNY Downstate Medical Center, Rutgers University and Feinstein Medical Institute. The use of autopsy specimens of preterm brain tissue from the neuropathology laboratory was approved by IRB at Cohen Children’s Medical Center.
2.1. Primary neuronal cultures
Primary neuronal cultures were prepared from E18 or P8 rat pups as described. Briefly, the hippocampus, cerebellum or cortex was dissected followed by trypsinization and centrifugation. The pellet was then resuspended in neuronal culture medium (Neurobasal medium supplemented with 2% B27 and 25 mM KCl) and seeded at a density of 5×105 cells/ml (for RT/PCR and immunostaining) or 1×105 cells/ml (for MTT assays).
2.2. Induction of hypoxia in vitro
Cobalt chloride (CoCl2.6H2O) (Sigma) was used to induce hypoxia in primary neuronal cultures as previously described (Wu and Yotnda, 2011). Five-day old neuronal cultures were treated with various concentrations of CoCl2 (50–125 μM) for 24 hours. After washing off the CoCl2, cultures were replenished with conditioned medium which was supplemented with 100 μM caffeine or vehicle control for an additional 24 hours, followed by fixation and analysis.
2.3. Neonatal hypoxia and caffeine treatment in vivo
Mouse pups were given caffeine at a loading dose of 20 mg/kg/day on postnatal day 4 (P4), then 15 mg/kg/day for the next 3 days. Caffeine was dissolved in sterile water and given to mouse pups via orogastric tube 1.9 french to maintain a serum concentration of caffeine at 5–15 mg/l (approximately 26–77 μM) (Gaytan et al., 2006; Guillet and Kellogg, 1991). Sham groups were given equal volumes of sterile water via the orogastric route. In both groups, half of the pups were exposed to hypoxic environment (8% oxygen) for 20 min on P7, whereas the other half remained in normoxia. Pups were then deeply anesthetized with a lethal dose of xylazine (30mg/kg)/ketamine (300mg/kg) and perfused transcardially with 4% PFA.
2.4. Immunostaining
Immunostaining was performed using antibodies against A1R or A2AR (Antibody Online, GA and Genetex, CA and AbCam, MA), PSD-95 (Santa Cruz, CA), beta-III tubulin (Sigma), AMPA, CREB-1 and syntaxin (all from Genscript, NJ), HIF-1α (Novus, CO, Genetex, CA and AbCam, MA ) and c-Fos (Gentex, CA and Biorbyt, CA). The immune complexes were detected by FITC-conjugated anti-mouse antibodies or Cy3-conjugated goat anti-rabbit antibodies (Jackson Immunoresearch, PA). The resulting images were acquired using Axiovert fluorescent microscopy (primary neuronal cultures), Zeiss LSM710 (mouse brains) or Olympus Fluoroview 300 (preterm infant brains) confocal microscopy. The brains of mice deficient in A1R or A2AR were kindly provided by Dr. William Welch (Georgetown University) and Dr. Bruce Cronstein (NYU), respectively.
Unless specified, all mouse brains were immunostained using vibratome sections (50 μm thick). For A1R and PSD-95 co-localization experiments, cryosectioned slices were used. For study of the expression of A1R in postmortem preterm human brain, tissues were collected from the autopsy specimens of preterm infants (gestational age of 23–24 weeks) who were given caffeine on the first day of life and continued until death at three weeks of life. These tissues were fixed in 10% formalin, paraffin embedded and sectioned coronally at 6 μm thick. Following antigen retrieval, sections were incubated with A1R antibody.
The densitometries of single cell RT-PCR bands and the neurite length in the acquired images of beta-III tubulin immunostaining were quantified using NIH ImageJ. The number of A1R, syntaxin and AMPAR puncta was quantified using the Analyze Particle function of Fiji ImageJ (size=30–250, circularity=0.3–1.0) as previously described (Muller et al., 2018; Taylor et al., 2010). The pixel intensity of HIF-1α in the nucleus and cytoplasm was measured using ImageJ.
2.5. MTT viability assay
Primary neurons were seeded at a concentration of 1×105 cells/ml and cultured for 7 days prior to the addition of 125 μM of CoCl2 for an additional 24 hours. CoCl2 containing medium was then replaced with conditioned medium in the presence or absence of 100 μM caffeine for an additional 24 hours. Neuronal viability was tested using the MTT assay as follows.
MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] was added to medium at a final concentration of 0.5 mg/ml and incubated with the cells at 37°C for 2 hours. The resulting purple crystals (due to the action of mitochondrial reductase) were solubilized with DMSO and the optical density at 570 nm was subsequently measured.
2.6. Single cell RT-PCR
Glass cover slips coated with poly-L-lysine (Sigma) were cut at dimensions of approximately 2×3 mm. These cover slips were placed in 24 well tissue culture plates. Primary neurons were then seeded at a density of 5×105 cells/ml. After 48 hours, caffeine was added to the medium for an additional 5 days. Based on the morphology, cover glasses containing single neurons were harvested for RT-PCR as previously described (Dulac and Axel, 1995; Li et al., 2009). Reactions that were positive for the expression of GFAP, a glial cell marker, were discarded. All PCR products were subjected to sequencing analysis. The primers used to amplify the corresponding fragment of the genes are listed in the following table.
| Gene (Gene Bank No.) | Primers |
|---|---|
| Adenosine A1 Receptor (NM64299) | F: ACC TGC CTC ATG GTG GCC TG R: GTA GTA CTT CTG GGG GTC ACC G |
| Adenosine A2A Receptor (AF2228684) | F: GGA GCT GGC CAT CGC TGT GC R: TCG CCG CAG GTC TTC GTG GA |
| Actin (NM031144) | F: CCC TGT GCT GCT CAC CGA GG R: GCG GCA GTG GCC ATC TCT TG |
| CREB-1 (NM_031017.1) | F: GGG CAG ACA GTC CAG GTC CAT R: GCC AGC TGT ATT GCT CCT CCC |
| C-Fos (NM_022197) | F: ACG AGG CGT CAT CCT CCC GCT R: CCC TCC TCC GAT TCC GGC AC |
| HIF-1α (NM_24359.1) | F: GCA GCA CGA TCT CGG CGA AGC R: CAT CGT CCT CCC CCG GCT TG |
2.7. Statistics
Data were analyzed using GraphPad Prism 7 software (GraphPad Software Inc., CA.). Student’s t test was used to compare the data of two experimental groups, whereas the data of more than two groups were analyzed by ANOVA. The values of p < 0.001 were considered statistically significant. All data are shown as mean ± SEM.
3. Results
3.1. Adenosine A1 receptors are synaptically localized in neonatal brains
We first asked whether the brains of both neonatal mice and preterm infants express the primary receptors for caffeine, adenosine receptors A1R and A2AR by immunostaining. We chose immunostaining instead of Western blotting because Western blotting does not show the subcellular localization of these receptors and the tissue extracts often include non-neuronal cell types.
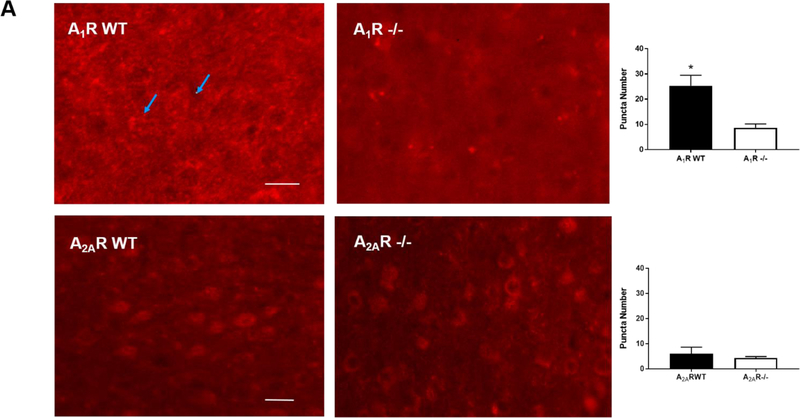
We screened the specificity of several commercially available antibodies against A1R and A2AR. We chose antibodies that showed high immunoreactivity in wild type mouse brains but not in the brains of the corresponding knockout mice. Using two antibodies against different epitopes of A1R, we consistently observed A1R distributed in a punctuate pattern in adult wild type brains (25.33±2.40 puncta), but not in A1R-deficient brains (8.67±0.88 puncta, p<0.001) (Figure 1A, top panel; enlarged images are in Supplementary Figure S1A).
Figure 1.
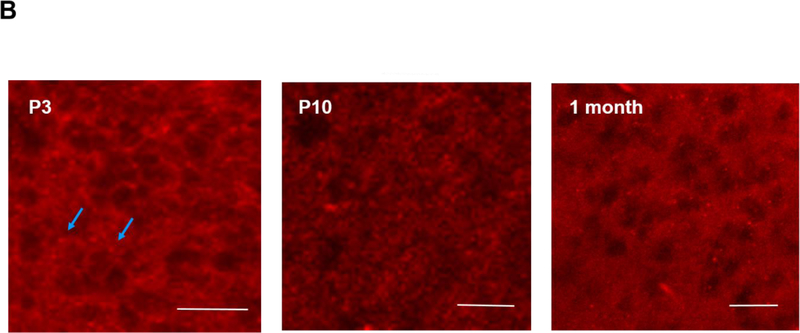
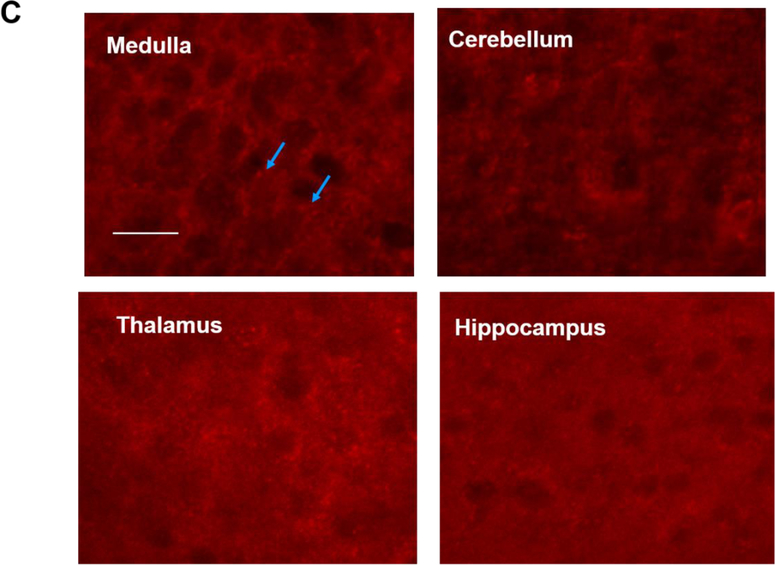
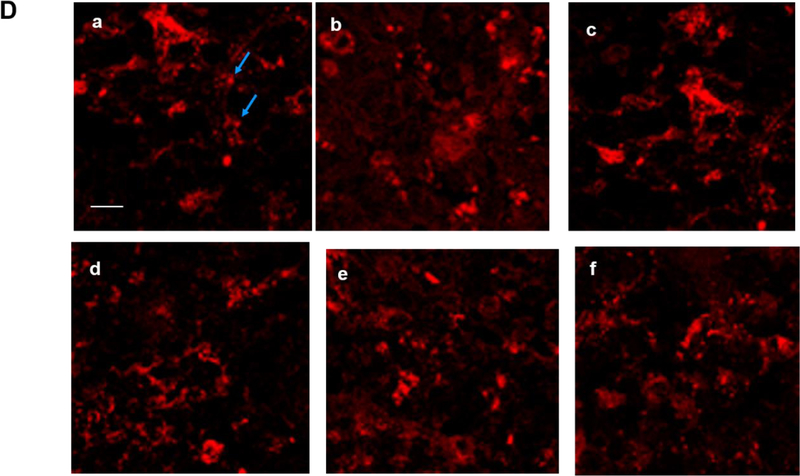
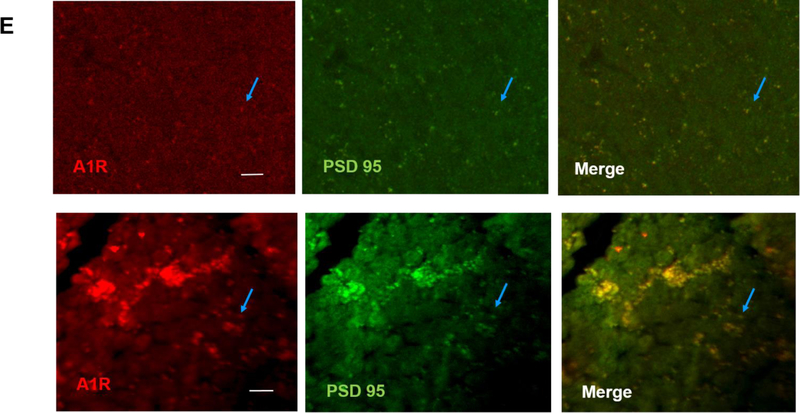
The brains of neonatal mice and preterm infants expressed A1R receptors. (A) Immunostaining results showed the specificity of A1R antibody but not A2AR antibody. Using the tested A1R antibody, A1R immunostaining showed a punctate distribution of this receptor in the cortex of wild type adult mice, but not in that of A1R deficient mice (25.33±2.4 and 8.67±0.88 puncta, respectively) (top). In contrast, several antibodies against A2AR showed non-specific immunoreactivity in the nucleus and the cytoplasm in both wild type and A2AR deficient mice (bottom). No punctate pattern was observed (6±1.53 puncta for WT and 4.33±0.3 puncta for A2AR knockout). Representative images are shown here. The quantitative results are shown on the right. Blue arrows indicate puncta. Scale bar, 10 μm. (B) The punctate pattern of A1R could be detected in the cortex of P3 pups. The A1R puncta appeared more discrete at a lower magnification as the animals mature (10 day-old and 1 month-old pups). (C) A1R distributed in a punctate pattern in medulla, cerebellum, thalamus and hippocampus. The puncta were more prominent in medulla and cerebellum. Scale bar, 20 μm. (D) Immunostaining showed a punctate pattern of A1R in the cortex of preterm infants at the corrected age of 26–27 weeks. Representative images of the cortex from 6 preterm brains are shown. Scale bar, 10 μm. (E) A1R colocalized with a synaptic protein, PSD-95, in frozen sections of neonatal mouse brain (upper panel) and paraffin sections of preterm infant brains (lower panel). Scale bar, 20 μm.
In contrast to A1R, several commercially available antibodies against A2AR displayed low specificity, showing intense nuclear staining in both wild type and A2AR knockout brains (Figure 1A, bottom panel; enlarged images are in Supplementary Figure S1A). Thus, this study focused mainly on A1R, but not A2AR, in neonatal brains.
Similar to adult brain tissues, we found A1R also distributed in a punctate pattern in neonatal cortex (Figure 1B). This punctate pattern can be detected in neonatal mouse cortex as early as postnatal day 3 (P3), albeit the puncta are smaller in size compared to those in older pups (P10 and 1 month old) (Figure 1B; an enlarged image of P3 brain is in Supplementary Figure S1B). In addition to cortex, A1R was also expressed in a similar punctate pattern in various brain regions, such as medulla, cerebellum, thalamus and hippocampus (Figure 1C; an enlarged image of medulla is in Supplementary Figure S1C). A1R puncta were most prominent in medulla and cerebellum (Figure 1C), but weak in basal ganglia (data not shown). The punctuate pattern of A1R is reminiscent of that of synaptic markers. Using double immunostaining, we found that A1R co-localized with PSD-95, a synaptic protein, suggesting A1R is synaptically localized in neonatal mouse brain (the earliest time point we studied is P3) (Figure 1E, top panel; an enlarged image is in Supplementary Figure S1E).
We next sought to extend our findings to postmortem preterm brains. Most deceased preterm infants in our clinics were born at approximately gestational age of 23–24 weeks and died at the corrected age of 26–27 weeks. Among these patients, we selected infants with no discernible brain abnormalities on head ultrasound. It is worth noting that all selected infants received caffeine since it is our policy that all preterm infants with gestational age younger than 29 weeks be given caffeine within hours after birth. We therefore did not have age-matched preterm infants who did not receive caffeine among this age group. However, we still found detectable levels of A1R puncta in these preterm brains, as demonstrated in the cortex of 6 different preterm infants (a representative section of each patient is shown in Figure 1D; an enlarged image is in Supplementary Figure S1D). These A1R puncta co-localized with PSD-95 (Figure 1E, bottom panel). Together, these data supported the hypothesis that the preterm brain may be influenced by caffeine during therapy to treat AOP.
3.2. Caffeine promotes cell survival and suppresses hypoxia-induced nuclear accumulation of HIF-1α in vitro
Caffeine has been suggested to be neuroprotective, but its safety and effectiveness in neonatal neurons remained unclear (Aden, 2011; Natarajan et al., 2007; Rivkees and Wendler, 2011; Schmidt et al., 2007; Winerdal et al., 2017). Here, we sought to address these issues both in vitro and in vivo. When investigating whether caffeine perturbs the general neuronal functions, we used a higher concentration of caffeine (200–400 μM). However, we returned to the clinically relevant doses of caffeine (50–100 μM) to evaluate its neuroprotective effects on primary neurons after ischemic insults.
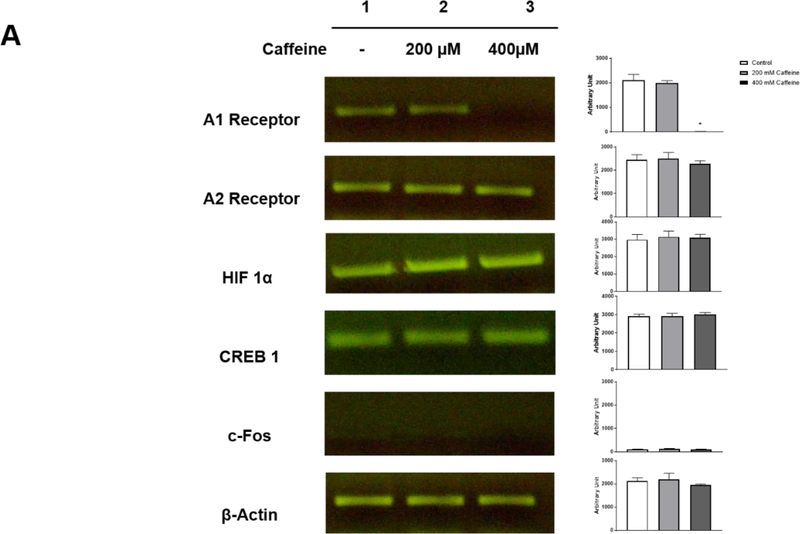
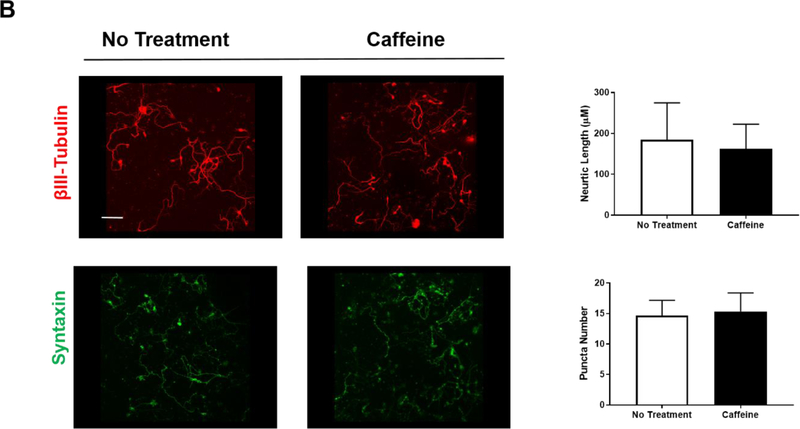
We examined three neuronal aspects to gauge the safety of caffeine: 1) expression of A1R and A2AR, 2) levels of several transcription factors, CREB-1, c-Fos and HIF-1α, and 3) synapse formation and neurite outgrowth. First, we analyzed the effects of caffeine on the transcription of A1R and A2AR by single cell RT/PCR as well as their protein levels by immunostaining. To ensure specificity, all RT/PCR products were sequenced to verify the identity of adenosine A1R and A2AR. The intensities of the PCR products were quantified using the densitometry function of ImageJ in arbitrary units. We found that neonatal neuronal cultures expressed mRNA for both adenosine A1R and A2AR in untreated controls (2104±138 and 2446±130). With 200 μM caffeine treatment, the transcription of A1R and A2AR was comparable to that of untreated neurons (2000±136 and 2500±150). However, when the concentration of caffeine was raised to 400 μM, caffeine selectively impaired the transcription of A1R, but not A2R (360±0.3 and 2280±77) (Figure 2A). Second, caffeine treatment was suggested to regulate the expression of several transcription factors, such as c-Fos, CREB-1, and HIF-1α (Connolly and Kingsbury, 2010; Gaytan and Pasaro, 2012) (Maugeri et al., 2018; Merighi et al., 2007). CREB-1 and c-Fos are immediate early genes induced by neural activity, whereas HIF-1α is often induced by hypoxic conditions. We examined the effects of caffeine on these transcription factors in neuronal cultures under normoxic conditions using single cell RT/PCR. In the absence of caffeine, there was low baseline expression of c-Fos but higher levels in HIF-1α and CREB-1 mRNA (HIF-1α, 2978±17.5; CREB-1, 2928±64; c-Fos, 105±7.5) (Figure 2A). With treatment of either 200 μM or 400 μM caffeine, we did not observe any significant changes in the transcription of HIF-1α, CREB-1 and c-Fos (HIF-1α, 3141±193 and 3095±111; CREB-1, 2933±88 and 3007±64; c-Fos, 132±7.6 and114±4) (Figure 2A). Third, beta-III tubulin and syntaxin are commonly used markers for the measurement of neurite length and synaptogenesis (Katsetos et al., 2003; Shin, 2014). We found that the average neurite length (162.1±17.45 μm) and the number of syntaxin puncta (15.33±1.76) in neurons that were treated with 200 μM caffeine for 5 days is comparable to that of the untreated neurons (184.9±27.6 μm and 14.67±1.45, respectively) (Figure 2B). Together, our data defined the safety threshold for caffeine at 200 μM since this dose did not perturb basic neuronal functions and characteristics in vitro.
Figure 2.
(A) Treatment with 200 μM caffeine has minimal/no effect on the transcription of A1R and A2AR, HIF-1α, CREB-1 or c-Fos in vitro. Single cell RT/PCR was performed on neuronal culture that were treated with or without caffeine for 5 days (n=10). The intensities of the RT-PCR bands were quantified by densitometry in arbitrary units. The results of control and caffeine groups are as follows: A1R (2104±138, 2000±136), A2AR (2446±130, 2500±150), HIF-1α (2978±17.5, 3141±19.3), CREB-1 (2928±64, 2933±88) and c-Fos (105±7.5, 132±7.6). The levels of β-actin were used as a loading control. The transcription levels of CREB-1 and HIF-1α were discernible, but the level of c-Fos was barely detectable. A high dose of caffeine (400 μM) selectively decreased the transcription of A1R (36±0.3, p<0.001) but not other tested factors. (B) Treatment with 200 μM caffeine did not affect neurite length (beta-III tubulin, red) (184.9±27.16 μm for control and 162.1±17.45 μm for caffeine treatment group) and synapse formation (syntaxin, green) (14.67±1.45 for control and 15.33±1.76 for caffeine treatment group). The quantitative results are shown on the right. Scale bar, 100 μm (n=5).
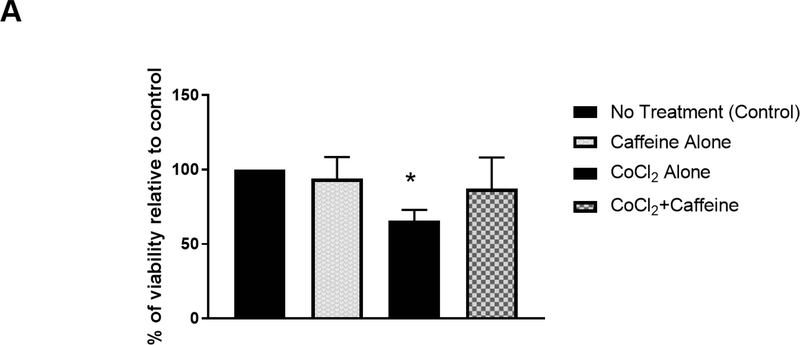
We next sought to determine whether caffeine treatment promotes neuronal survival after hypoxic ischemia. Many of the cellular responses induced by hypoxia are mediated by changes in gene expression, which are often regulated by HIF-1α (Ke and Costa, 2006; Semenza, 2007; Shi, 2009). Under normal conditions, HIF-1α is constitutively degraded by prolyl-hydroxylase enzymes. However, hypoxia stabilizes HIF-1α by inhibiting these enzymes. Cobalt chloride (CoCl2) is a hypoxia mimetic agent in vitro since it also inhibits prolyl-hydroxylase enzymes, resulting in accumulation and nuclear translocation of HIF-1α (Li et al., 2015; Piret et al., 2002). Here, we used CoCl2 to mimic hypoxia in vitro. Primary neurons (5–7 days in culture) were treated with 50–125 μM CoCl2 for 24 hours as previously described (Wu and Yotnda, 2011). The culture medium was discarded and replenished with conditioned medium in the presence or absence of 100 μM caffeine for an additional 24 hours. Subsequently, cells were subjected to MTT assay to measure cell survival rate (Denizot and Lang, 1986). In this experiment, the MTT reading of control group (no treatment) was defined as 100% viability. The MTT readings of other groups were compared with that of the control group, and the percentage of viability was derived accordingly. We found that the viability of neuronal cultures was comparable in the presence or absence of caffeine (Figure 3A; control, 100%; caffeine alone, 94±7.1%), suggesting caffeine does not affect baseline cell survival. As expected, CoCl2 decreased the viability of neuronal cultures compared to that of untreated cultures (Figure 3A; CoCl2 treatment, 65.6±3.6%, p<0.001). Interestingly, the low viability caused by CoCl2 was alleviated significantly by subsequent treatment with 100 μM caffeine (Figure 3A; CoCl2+caffeine, 87.2±10.5%, n=4. p<0.001).
Figure 3.
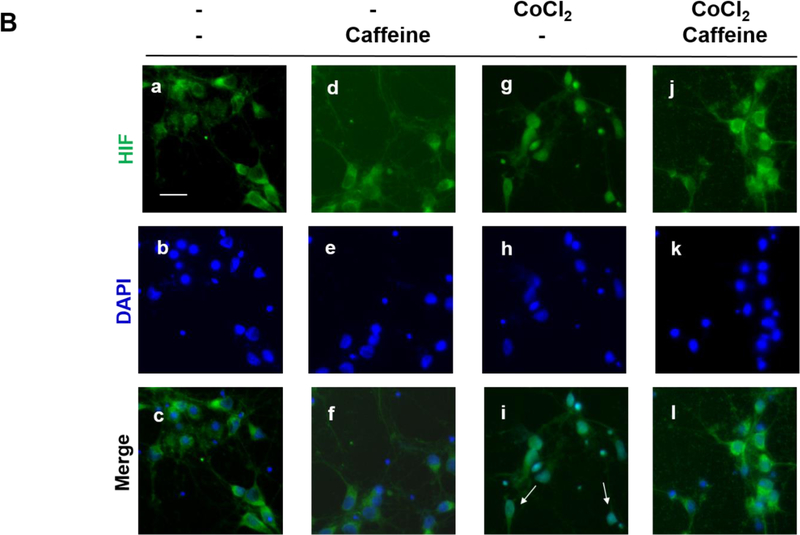
Caffeine promoted cell survival after hypoxia and decreased CoCl2-induced nuclear accumulation of HIF-1α in vitro. (A) Primary cortical neuronal cultures were treated with CoCl2 or vehicle (control) for 24 h. Each group was divided into two subgroups that were either treated with 100 μM caffeine or vehicle for an additional 24 h. The viability of each treatment group was subsequently measured by MTT assays. Data are presented as percentage of viability in each group relative to that of the control group (control, 100%; caffeine alone, 94±7.1%; CoCl2 treatment, 65.6±3.6%; CoCl2+caffeine, 87.2±10.5% n=4. p<0.001).
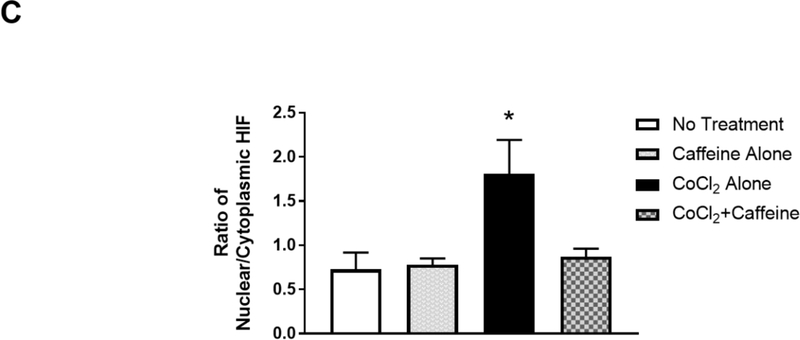
(B) Treated neurons were double-immunostained to label HIF-1α (green, top row) and neuron-specific beta-III tubulin (red, not shown) and counter-stained with DAPI for nuclei (blue, middle row). The merged images of HIF-1α and DAPI are shown in the bottom row. Beta-III tubulin immunostaining (not shown) was used as a neuronal marker. In the absence of CoCl2, HIF-1α was primarily localized in the cytoplasm regardless of whether neurons received caffeine or not (Lane 1, a, b, c and Lane 2, d, e, f). In contrast, CoCl2 triggered nuclear co-localization of HIF-1α with DAPI (Lane 3, g.h.i), suggesting nuclear accumulation of HIF-1α. White arrows indicate co-localization of HIF-1α and DAPI. This nuclear accumulation of HIF-1α was alleviated by subsequent treatment with caffeine (Lane 4, j, k,l). Scale bar, 20 μm. (C) Quantification of the ratio of nuclear vs cytoplasmic HIF-1α in neurons of each treatment group (control, 0.73±0.07; caffeine alone, 0.78±0.03; CoCl2 treatment, 1.81±0.17, CoCl2 and caffeine, 0.82±0.04, p<0.001, n=5).
HIF-1α appears to have complex effects in hypoxic brains in a time-dependent manner (Supplementary Table S1). Accumulation of HIF-1α after ischemia has been shown to facilitate cell death at the very early phase (<24 h, pro-death effect) (Barteczek et al., 2017; Cheng et al., 2014; Yang et al., 2017), but HIF-1α has also been shown to limit infarct size at a later stage (>4 d, pro-survival effect) (Baranova et al., 2007; Bergeron et al., 2000; Sheldon et al., 2014; Sheldon et al., 2009). Since caffeine treatment increased neuronal viability immediately after hypoxia, we hypothesized that caffeine could decrease the accumulation of HIF-1α, therefore inhibiting its pro-death effect. To test this hypothesis, neuronal cultures were treated as in Figure 3A. We then co-immunostained the neuronal culture with 1) antibody against beta-III tubulin for neuronal labeling, 2) antibody against HIF-1α transcription factor and 3) DAPI for nuclear staining. The ratio of the pixel intensity of HIF-1α immunostaining in the nucleus vs cytoplasm of the neurons was computed using Image J. Prior to the induction of hypoxia, the ratio of nuclear vs cytoplasmic HIF-1α is comparable in cultures treated with 100 μM caffeine or vehicle (0.78±0.03 and 0.73±0.07, respectively) (Figure 3B and 3C, Lane 1 and 2), suggesting that caffeine alone did not affect nuclear levels of HIF-1α. However, this ratio increased significantly in CoCl2-treated cultures (1.81±0.17, p<0.001) compared to that of untreated cultures, suggesting nuclear accumulation of HIF-1α in CoCl2-induced hypoxia (Figure 3B and 3C). For most of the study, we used 125 μM CoCl2 since this concentration triggered a significant nuclear accumulation of HIF-1α and cell death (Figure 3B and 3C). Interestingly, subsequent treatment with 100 μM of caffeine alleviated this hypoxia-induced nuclear accumulation of HIF-1α (0.82±0.04, p<0.001) (Figure 3B and 3C).
3.3. Caffeine suppresses hypoxia-induced nuclear accumulation of HIF-1α factor in vivo
We next sought to examine whether caffeine treatment in newborn mice is neuroprotective for subsequent hypoxia exposure. Here, caffeine was given to neonatal pups before induction of hypoxia in order to 1) avoid the high mortality rate when carotid ligation is performed in newborn mice at the age of P0-P4, and 2) avoid the injury-induced plasticity obscuring the potential effects of caffeine on neuroprotection and 3) mimic the timing of caffeine therapy in preterm infants.
Neonatal pups were gavage fed with caffeine as previously described (Gaytan et al., 2006). After caffeine treatment, the animals were exposed to a hypoxic or normoxic environment for 20 min before euthanization (Zaghloul et al., 2014). Their brains were removed immediately after the procedure and the brain sections were immunostained with HIF-1α antibody and counter stained with DAPI.
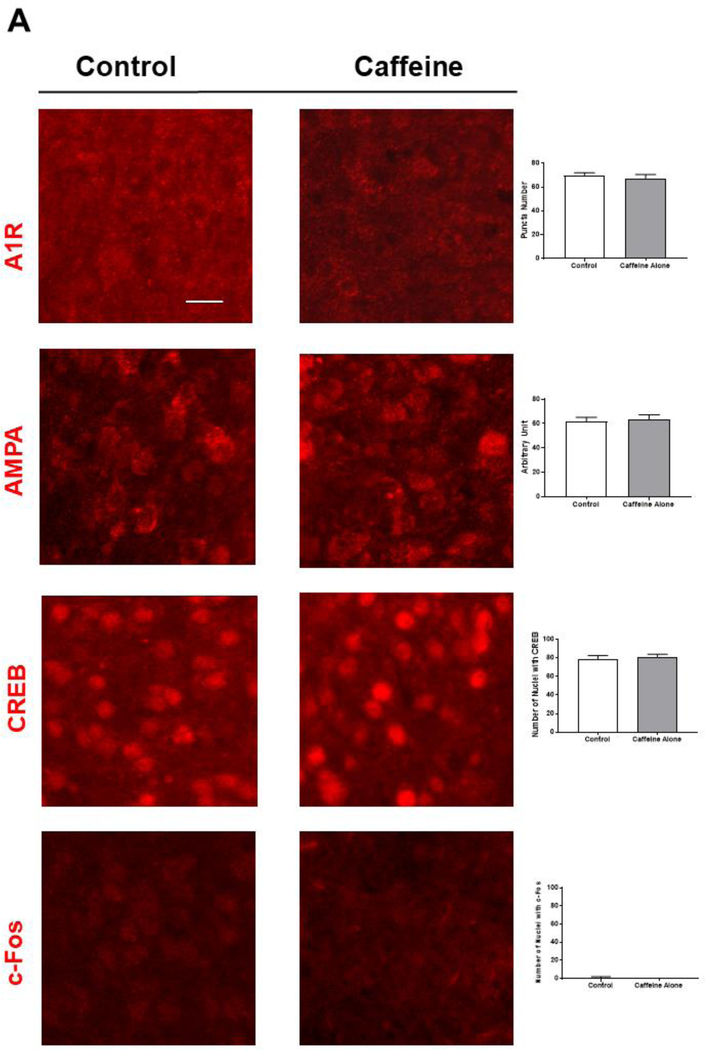
We first ensured caffeine treatment did not perturb basic neuronal functions. We found that the expression and subcellular localization of A1R in various brain areas were similar whether neonatal pups received caffeine or not (Figure 4A, the puncta number in cortex is 70±1.15 in control group and 67.3±1.86 in caffeine group). Caffeine treatment also did not affect the punctate pattern of A1R in cerebellum and brain stem (data not shown). In addition, we did not observe any significant changes in immunostaining for AMPA receptors, CREB-1 and c-Fos in the brains of neonatal rodents that were treated with vehicle or caffeine (AMPAR puncta, 67.6±2 and 63.6±2; CREB-1, 79±2.1, 81.3±1.5; c-Fos, 1±0.58, 1±0.1, respectively) (Figure 4A; an enlarged image of AMPA receptor puncta is in Supplementary Figure S3). Together, these data suggested that this clinically relevant dose of caffeine did not have a discernible impact on A1R expression and the protein levels of AMPAR, CREB-1, and c-Fos.
Figure 4.
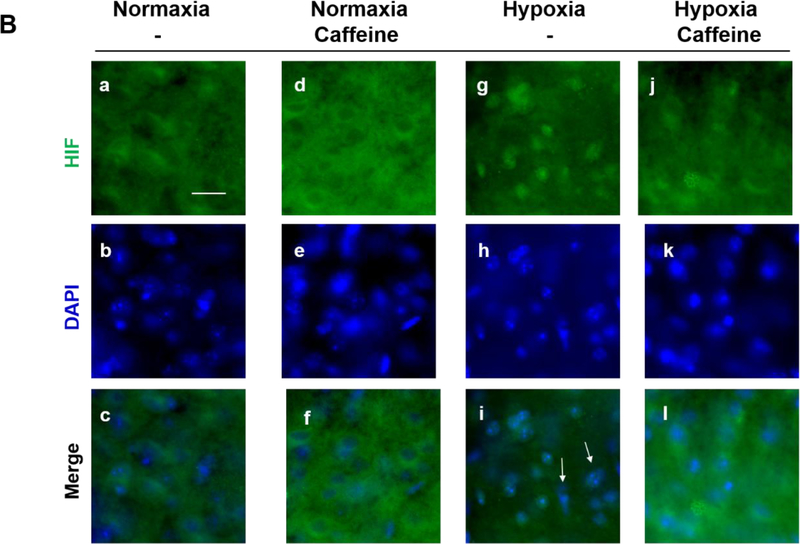
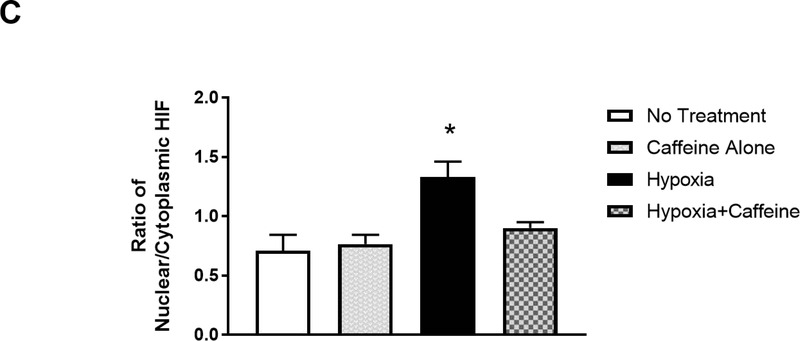
Caffeine blocked hypoxia-induced nuclear accumulation of HIF1-α in vivo. (A) Immunostaining for A1R, AMPA receptors, CREB-1 and c-Fos showed that caffeine treatment has minimal/no effect on the expression of these factors in neonatal cortex (n=5). Scale bar, 20 μm. The quantitative results in control and caffeine-treated groups are shown on the right; the number of A1R (70±1.15, 67.3±1.86) or AMPA receptor puncta (67.6±2, 63.6±2), the number of nuclear CREB-1 (79±2.1, 81.3±1.5) or c-Fos (1±0.58, 1±0.01). (B) Neonatal mouse pups were gavage fed with caffeine or vehicle from P4-P7. Subsequently, pups were placed under normoxic (room air) or hypoxic (8% O2) conditions for 20 min prior to cardiac perfusion and isolation of their brains. Brain sections were immunostained with anti-HIF-1α (green, top row) and counter-stained with DAPI to show nuclei (blue, middle row). The overlaid images of HIF-1α immunostaining and DAPI staining are shown in the bottom row. Representative cortical images are shown here. Under normoxic conditions, HIF-1α primarily distributed in the cytoplasm of cortical neurons whether mouse pups received caffeine therapy or not (Lane 1, a. b. c. and Lane 2, d, e, f). In pups without caffeine therapy, hypoxia induced nuclear accumulation of HIF-1α (Lane 3, g, h, i). White arrows indicate co-localization of HIF-1α and DAPI. Pre-treatment with caffeine prevented subsequent hypoxia-induced nuclear accumulation of HIF-1α in neonatal cortex (Lane 4, j, k, l). Scale bar, 20 μm. (C) Quantification of the ratio of nuclear vs cytoplasmic HIF-1α in neonatal cortex in each group (control, 0.71±0.05; caffeine alone, 0.77±0.03; hypoxia, 1.33±0.05; hypoxia and caffeine, 0.9±0.02, p<0.001, n=5)
Under normoxia, caffeine treatment did not affect the ratio of nuclear vs cytoplasmic HIF-1α in the brain of P7 pups whether the animals received caffeine or not (0.71±0.05 for control group and 0.77±0.03 for caffeine treatment group) (Figure 4B and 4C, Lane 1 and 2). Hypoxia increased the ratio of nuclear vs cytoplasmic HIF-1α in P7 pups that did not receive caffeine therapy (1.33±0.05, p<0.001) (Figure 4B and 4C, Lane 3). In contrast, the same hypoxic condition failed to trigger a significant nuclear accumulation of HIF-1α in neonatal pups that had received caffeine feeding previously (the ratio of nuclear vs cytoplasmic HIF-1α is 0.9±0.02, p<0.001) (Figure 4B and 4C, Lane 4), suggesting a prophylactic effect of caffeine in preventing upregulation in HIF-1α by subsequent hypoxia.
4. Discussion
Caffeine therapy is widely used to treat AOP because of its effectiveness and safety. Caffeine relieves AOP by competing with adenosine for binding to its receptors, A1R and A2AR, in brain stem. However, the effects of caffeine at this high dose on neonatal brains remained unclear. In this study, we first showed that the neonatal brain of both rodents and preterm infants expressed A1R and that this receptor was enriched in synapses. In addition, we also detected A2AR mRNA by single cell RT/PCR in primary neuronal cultures. These data suggested that neonatal brains could be responsive to caffeine via A1R and potentially A2AR. We then showed that caffeine was considered safe in neonatal neurons since it did not perturb several basic neuronal functions, including the expression and distribution of A1R, transcription of CREB-1, c-Fos and HIF-1α and neurite outgrowth and synapse formation. Lastly, we determined the beneficial effects of caffeine in alleviating hypoxia-induced neuronal death. We found that caffeine at clinically relevant doses suppressed hypoxia-induced nuclear accumulation of HIF-1α both in vitro and in vivo, and promoted viability in neuronal cultures exposed to hypoxia.
4.1. A1R is synaptically localized in neonatal brains
The major receptors for caffeine are adenosine A1R and A2AR (Aden et al., 2000; Fredholm, 1995). However, the protein expression and subcellular localization of these receptors in developing neurons remain unclear. We found that the brains of neonatal mice and deceased preterm infants expressed A1R in brain areas, such as cortex, basal ganglia, thalamus, and hippocampus. We also found A1R was enriched in synapses. The synaptic targeting of A1R began at neonatal stages, albeit to a lesser extent, since the A1R puncta became more prominent as the mouse pups matured into adulthood (Figure 1B). Interestingly, the synaptic targeting of A1R is functionally linked to its neuromodullatory role in the adult brain (Rebola et al., 2003; Sheth et al., 2014).
In contrast to A1R, we found A2AR mRNA by single cell RT/PCR was expressed in neonatal neuronal culture, but the protein distribution of A2AR in neonatal brains remained unclear. Previous reports showed soma enrichment of A2AR in the respiratory nuclei of neonatal brain stem (Gaytan and Pasaro, 2012). We also observed soma/nuclear enrichment of A2AR by immunostaining, but unfortunately, this strong immunostaining was present in both wild type brains and A2AR deficient brains, rendering inconclusive results (Figure 1A). As a control, we also tested these antibodies in adult brains since radio-ligand binding assays have clearly shown that the expression of A2AR is more enriched in striatum and olfactory bulb in adult brains (Fredholm et al., 2011), but none of these commercial antibodies showed this immunostaining pattern in adult brains.
4.2. Clinically relevant doses of caffeine had minor/no effects on synapse formation and expression of A1R, CREB-1 and c-Fos
Caffeine has been shown to regulate the synaptic functions in adult cortical regions in several in vitro experiments (Greene et al., 1985; Yoshimura, 2005). However, we did not observe any differences in neurite length, synaptic density or the expression of several transcription factors between caffeine-treated and untreated neonatal neuronal culture. Moreover, under normoxic condition, the morphology of neonatal brains from pups receiving caffeine treatment appeared similar to that from untreated group.
A previous study has shown that caffeine increased A1R transcription in the brain stem and the respiratory nuclei of the hypothalamus when caffeine was given to neonatal pups directly (Gaytan and Pasaro, 2012). The same study also demonstrated a caffeine-induced early shift of the postnatal increase in c-Fos in these regions. However, in our study, we did not observe any changes in 1) the protein levels of A1R in the neonatal cortex and other regions (hippocampus/medulla/cerebellum/thalamus), and 2) the transcription levels of A1R in neuronal cultures. We also found that the protein levels of c-Fos were low in both control and caffeine-treated groups. Since we also fed neonatal pups with caffeine directly or added caffeine into neuronal cultures directly, this discrepancy may arise from analyzing different brain regions and using different methodology. For example, we used single cell RT-PCR to analyze pure neuronal population, whereas Gaytan and colleagues used qPCR to analyze the whole extracts of hypothalamus and respiratory nuclei.
4.3. Caffeine blocks hypoxia-induced nuclear accumulation in HIF-1α and promotes neuronal survival
It has been suggested that caffeine has opposite effects on embryonic vs neonatal brains because caffeine is a potent inhibitor of A1R. During the embryonic stage, inhibition of A1R rendered cells more susceptible to hypoxia, whereas inhibition of A1R in neonatal brains after birth was neuroprotective in white matter injury and hypoxic ischemia in rodents (Rivkees and Wendler, 2011). Notably, caffeine therapy for AOP and most related research in neonatal rodents employed postnatal treatment. Thus, caffeine has been suggested to have neuroprotective effects during the neonatal period (Back et al., 2006; Cunha, 2001; Ferreira and Paes-de-Carvalho, 2001; Fredholm et al., 2011; Rivkees and Wendler, 2011; Winerdal et al., 2017) and are beneficial to neurodevelopment in preterm infants (Murner-Lavanchy et al., 2018; Schmidt et al., 2007).
Here, we showed a novel mechanism by which caffeine exerts its neuroprotective effects in neonatal brains. Hypoxia generally results in tissue damage immediately after ischemia. The “pro-death” effect of HIF-1 at this stage has been shown to facilitate death of severely injured neurons (Supplementary Table S1) (Barteczek et al., 2017; Cheng et al., 2014; Liu et al., 2015; Yang et al., 2017). We found that caffeine alleviated hypoxia-induced accumulation of HIF-1α both in vivo and in vitro and therefore increased the viability of primary neurons. This inhibitory effect is specific to nuclear accumulation of HIF-1α, not on its transcription (Supplementary Figure S2). In addition, caffeine had neuroprotective effects in vivo whether caffeine was administered before or after hypoxic insults. A recent study showed that a single dose of caffeine (5 mg/ml via intraperitoneal injection) immediately after neonatal hypoxic ischemia in neonatal pups decreased brain atrophy and improved behavioral outcomes at 2 weeks after hypoxic insults (Winerdal et al., 2017). However, in our study, caffeine was given to neonatal pups (P4-P7) before induction of mild hypoxia (8% O2 in 20 minutes). We found that early caffeine therapy could prevent subsequent hypoxia-induced accumulation of HIF-1α. Clinically, our data suggest that caffeine therapy in the NICU may protect preterm brains from hypoxic damages that may occur during their NICU stay.
In conclusion, our results further support the utility of caffeine in preventing hypoxia-induced nuclear accumulation of HIF-1α and promotes neuronal survival.
Supplementary Material
Highlights.
Neonatal brains express adenosine A1R in synapses for caffeine binding.
Caffeine treatment does not affect the basal neuronal function.
Caffeine promotes cell survival and inhibits hypoxia-induced accumulation of HIF-1α.
Acknowledgement
We thank Drs. Raddy Ramos and Benjamin Huang and Ms. Seto Chice for critical reading of early drafts of this paper.
Funding
This work was supported by grants from Little Giraffe Foundation (1111135 to HLL), Cerebral Palsy Foundation (R-804-12 to HLL) and NJCBIR (CBIR14IRG019 to BLF). A.O. is supported by the National Institutes of Health T32 GM008339–28 from the NIGMS.
Footnotes
Conflict of Interest
All authors have read the journal’s policy on disclosure of potential conflicts of interest and have none to declare. This study was supported by two non-profit foundations. The funders played no role in the design and conclusion of this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aden U, 2011. Methylxanthines during pregnancy and early postnatal life. Handb Exp Pharmacol, 373–389. [DOI] [PubMed] [Google Scholar]
- Aden U, Herlenius E, Tang LQ, Fredholm BB, 2000. Maternal caffeine intake has minor effects on adenosine receptor ontogeny in the rat brain. Pediatr Res 48, 177–183. [DOI] [PubMed] [Google Scholar]
- Aden U, Leverin AL, Hagberg H, Fredholm BB, 2001. Adenosine A(1) receptor agonism in the immature rat brain and heart. Eur J Pharmacol 426, 185–192. [DOI] [PubMed] [Google Scholar]
- Back SA, Craig A, Luo NL, Ren J, Akundi RS, Ribeiro I, Rivkees SA, 2006. Protective effects of caffeine on chronic hypoxia-induced perinatal white matter injury. Ann Neurol 60, 696–705. [DOI] [PubMed] [Google Scholar]
- Bairam A, Boutroy MJ, Badonnel Y, Vert P, 1987. Theophylline versus caffeine: comparative effects in treatment of idiopathic apnea in the preterm infant. J Pediatr 110, 636–639. [DOI] [PubMed] [Google Scholar]
- Baranova O, Miranda LF, Pichiule P, Dragatsis I, Johnson RS, Chavez JC, 2007. Neuron-specific inactivation of the hypoxia inducible factor 1 alpha increases brain injury in a mouse model of transient focal cerebral ischemia. J Neurosci 27, 6320–6332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barteczek P, Li L, Ernst AS, Bohler LI, Marti HH, Kunze R, 2017. Neuronal HIF-1alpha and HIF-2alpha deficiency improves neuronal survival and sensorimotor function in the early acute phase after ischemic stroke. J Cereb Blood Flow Metab 37, 291–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron M, Gidday JM, Yu AY, Semenza GL, Ferriero DM, Sharp FR, 2000. Role of hypoxia-inducible factor-1 in hypoxia-induced ischemic tolerance in neonatal rat brain. Ann Neurol 48, 285–296. [PubMed] [Google Scholar]
- Cheng YL, Park JS, Manzanero S, Choi Y, Baik SH, Okun E, Gelderblom M, Fann DY, Magnus T, Launikonis BS, Mattson MP, Sobey CG, Jo DG, Arumugam TV, 2014. Evidence that collaboration between HIF-1alpha and Notch-1 promotes neuronal cell death in ischemic stroke. Neurobiol Dis 62, 286–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly S, Kingsbury TJ, 2010. Caffeine modulates CREB-dependent gene expression in developing cortical neurons. Biochem Biophys Res Commun 397, 152–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha RA, 2001. Adenosine as a neuromodulator and as a homeostatic regulator in the nervous system: different roles, different sources and different receptors. Neurochem Int 38, 107–125. [DOI] [PubMed] [Google Scholar]
- Davidson JO, Dean JM, Fraser M, Wassink G, Andelius TC, Dhillon SK, Bennet L, Gunn AJ, 2018. Perinatal brain injury: mechanisms and therapeutic approaches. Front Biosci (Landmark Ed) 23, 2204–2226. [DOI] [PubMed] [Google Scholar]
- Denizot F, Lang R, 1986. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods 89, 271–277. [DOI] [PubMed] [Google Scholar]
- Dulac C, Axel R, 1995. A novel family of genes encoding putative pheromone receptors in mammals. Cell 83, 195–206. [DOI] [PubMed] [Google Scholar]
- Erenberg A, Leff RD, Haack DG, Mosdell KW, Hicks GM, Wynne BA, 2000. Caffeine citrate for the treatment of apnea of prematurity: a double-blind, placebo-controlled study. Pharmacotherapy 20, 644–652. [DOI] [PubMed] [Google Scholar]
- Ferre S, 2016. Mechanisms of the psychostimulant effects of caffeine: implications for substance use disorders. Psychopharmacology (Berl) 233, 1963–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira JM, Paes-de-Carvalho R, 2001. Long-term activation of adenosine A(2a) receptors blocks glutamate excitotoxicity in cultures of avian retinal neurons. Brain Res 900, 169–176. [DOI] [PubMed] [Google Scholar]
- Frary CD, Johnson RK, Wang MQ, 2005. Food sources and intakes of caffeine in the diets of persons in the United States. J Am Diet Assoc 105, 110–113. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, 1995. Astra Award Lecture. Adenosine, adenosine receptors and the actions of caffeine. Pharmacol Toxicol 76, 93–101. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, AP IJ, Jacobson KA, Linden J, Muller CE, 2011. International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors--an update. Pharmacol Rev 63, 1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB, Battig K, Holmen J, Nehlig A, Zvartau EE, 1999. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev 51, 83–133. [PubMed] [Google Scholar]
- Gaytan SP, Pasaro R, 2012. Neonatal caffeine treatment up-regulates adenosine receptors in brainstem and hypothalamic cardio-respiratory related nuclei of rat pups. Exp Neurol 237, 247–259. [DOI] [PubMed] [Google Scholar]
- Gaytan SP, Saadani-Makki F, Bodineau L, Frugiere A, Larnicol N, Pasaro R, 2006. Effect of postnatal exposure to caffeine on the pattern of adenosine A1 receptor distribution in respiration-related nuclei of the rat brainstem. Auton Neurosci 126–127, 339–346. [DOI] [PubMed] [Google Scholar]
- Greene RW, Haas HL, Hermann A, 1985. Effects of caffeine on hippocampal pyramidal cells in vitro. Br J Pharmacol 85, 163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillet R, Kellogg C, 1991. Neonatal exposure to therapeutic caffeine alters the ontogeny of adenosine A1 receptors in brain of rats. Neuropharmacology 30, 489–496. [DOI] [PubMed] [Google Scholar]
- Katsetos CD, Legido A, Perentes E, Mork SJ, 2003. Class III beta-tubulin isotype: a key cytoskeletal protein at the crossroads of developmental neurobiology and tumor neuropathology. J Child Neurol 18, 851–866; discussion 867. [DOI] [PubMed] [Google Scholar]
- Ke Q, Costa M, 2006. Hypoxia-inducible factor-1 (HIF-1). Mol Pharmacol 70, 1469–1480. [DOI] [PubMed] [Google Scholar]
- Li HL, Huang BS, Vishwasrao H, Sutedja N, Chen W, Jin I, Hawkins RD, Bailey CH, Kandel ER, 2009. Dscam mediates remodeling of glutamate receptors in Aplysia during de novo and learning-related synapse formation. Neuron 61, 527–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Zhang J, Yang H, Wu C, Dang X, Liu Y, 2015. Copper depletion inhibits CoCl2-induced aggressive phenotype of MCF-7 cells via downregulation of HIF-1 and inhibition of Snail/Twist-mediated epithelial-mesenchymal transition. Sci Rep 5, 12410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu FJ, Kaur P, Karolina DS, Sepramaniam S, Armugam A, Wong PT, Jeyaseelan K, 2015. MiR-335 Regulates Hif-1alpha to Reduce Cell Death in Both Mouse Cell Line and Rat Ischemic Models. PLoS One 10, e0128432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew OP, 2011. Apnea of prematurity: pathogenesis and management strategies. J Perinatol 31, 302–310. [DOI] [PubMed] [Google Scholar]
- Maugeri G, D’Amico AG, Rasa DM, Saccone S, Federico C, Magro G, Cavallaro S, D’Agata V, 2018. Caffeine inhibits angiogenesis in human glioblastoma cells via HIFs modulation. Anticancer Agents Med Chem. [DOI] [PubMed] [Google Scholar]
- Merighi S, Benini A, Mirandola P, Gessi S, Varani K, Simioni C, Leung E, Maclennan S, Baraldi PG, Borea PA, 2007. Caffeine inhibits adenosine-induced accumulation of hypoxia-inducible factor-1alpha, vascular endothelial growth factor, and interleukin-8 expression in hypoxic human colon cancer cells. Mol Pharmacol 72, 395–406. [DOI] [PubMed] [Google Scholar]
- Muller T, Braud S, Juttner R, Voigt BC, Paulick K, Sheean ME, Klisch C, Gueneykaya D, Rathjen FG, Geiger JR, Poulet JF, Birchmeier C, 2018. Neuregulin 3 promotes excitatory synapse formation on hippocampal interneurons. EMBO J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murner-Lavanchy IM, Doyle LW, Schmidt B, Roberts RS, Asztalos EV, Costantini L, Davis PG, Dewey D, D’Ilario J, Grunau RE, Moddemann D, Nelson H, Ohlsson A, Solimano A, Tin W, Anderson PJ, Caffeine for Apnea of Prematurity Trial, G., 2018. Neurobehavioral Outcomes 11 Years After Neonatal Caffeine Therapy for Apnea of Prematurity. Pediatrics 141. [DOI] [PubMed] [Google Scholar]
- Natarajan G, Botica ML, Thomas R, Aranda JV, 2007. Therapeutic drug monitoring for caffeine in preterm neonates: an unnecessary exercise? Pediatrics 119, 936–940. [DOI] [PubMed] [Google Scholar]
- Piret JP, Mottet D, Raes M, Michiels C, 2002. CoCl2, a chemical inducer of hypoxia-inducible factor-1, and hypoxia reduce apoptotic cell death in hepatoma cell line HepG2. Ann N Y Acad Sci 973, 443–447. [DOI] [PubMed] [Google Scholar]
- Rebola N, Pinheiro PC, Oliveira CR, Malva JO, Cunha RA, 2003. Subcellular localization of adenosine A(1) receptors in nerve terminals and synapses of the rat hippocampus. Brain Res 987, 49–58. [DOI] [PubMed] [Google Scholar]
- Rivkees SA, 1995. The ontogeny of cardiac and neural A1 adenosine receptor expression in rats. Brain Res Dev Brain Res 89, 202–213. [DOI] [PubMed] [Google Scholar]
- Rivkees SA, Wendler CC, 2011. Adverse and protective influences of adenosine on the newborn and embryo: implications for preterm white matter injury and embryo protection. Pediatr Res 69, 271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt B, 2005. Methylxanthine therapy for apnea of prematurity: evaluation of treatment benefits and risks at age 5 years in the international Caffeine for Apnea of Prematurity (CAP) trial. Biol Neonate 88, 208–213. [DOI] [PubMed] [Google Scholar]
- Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, Solimano A, Tin W, Caffeine for Apnea of Prematurity Trial, G., 2007. Long-term effects of caffeine therapy for apnea of prematurity. N Engl J Med 357, 1893–1902. [DOI] [PubMed] [Google Scholar]
- Semenza GL, 2007. Hypoxia-inducible factor 1 (HIF-1) pathway. Sci STKE 2007, cm8. [DOI] [PubMed] [Google Scholar]
- Sheldon RA, Lee CL, Jiang X, Knox RN, Ferriero DM, 2014. Hypoxic preconditioning protection is eliminated in HIF-1alpha knockout mice subjected to neonatal hypoxia-ischemia. Pediatr Res 76, 46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon RA, Osredkar D, Lee CL, Jiang X, Mu D, Ferriero DM, 2009. HIF-1 alpha-deficient mice have increased brain injury after neonatal hypoxia-ischemia. Dev Neurosci 31, 452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth S, Brito R, Mukherjea D, Rybak LP, Ramkumar V, 2014. Adenosine receptors: expression, function and regulation. Int J Mol Sci 15, 2024–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, 2009. Hypoxia inducible factor 1 as a therapeutic target in ischemic stroke. Curr Med Chem 16, 4593–4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin OH, 2014. Exocytosis and synaptic vesicle function. Compr Physiol 4, 149–175. [DOI] [PubMed] [Google Scholar]
- Taylor AM, Dieterich DC, Ito HT, Kim SA, Schuman EM, 2010. Microfluidic local perfusion chambers for the visualization and manipulation of synapses. Neuron 66, 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winerdal M, Urmaliya V, Winerdal ME, Fredholm BB, Winqvist O, Aden U, 2017. Single Dose Caffeine Protects the Neonatal Mouse Brain against Hypoxia Ischemia. PLoS One 12, e0170545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Yotnda P, 2011. Induction and testing of hypoxia in cell culture. J Vis Exp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XS, Yi TL, Zhang S, Xu ZW, Yu ZQ, Sun HT, Yang C, Tu Y, Cheng SX, 2017. Hypoxia-inducible factor-1 alpha is involved in RIP-induced necroptosis caused by in vitro and in vivo ischemic brain injury. Sci Rep 7, 5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura H, 2005. The potential of caffeine for functional modification from cortical synapses to neuron networks in the brain. Curr Neuropharmacol 3, 309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaghloul N, Patel H, Codipilly C, Marambaud P, Dewey S, Frattini S, Huerta PT, Nasim M, Miller EJ, Ahmed M, 2014. Overexpression of extracellular superoxide dismutase protects against brain injury induced by chronic hypoxia. PLoS One 9, e108168. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.