Abstract
Regulated synthesis and movement of proteins between cellular organelles are central to diverse forms of biological adaptation and plasticity. In neurons, the repertoire of channel, receptor, and adhesion proteins displayed on the cell surface directly impacts cellular development, morphology, excitability, and synapse function. The immensity of the neuronal surface membrane and its division into distinct functional domains present a challenging landscape over which proteins must navigate to reach their appropriate functional domains. This problem becomes more complex considering that neuronal protein synthesis is continuously refined in space and time by neural activity. Here we review our current understanding of how integral membrane and secreted proteins important for neuronal function travel from their sites of synthesis to their functional destinations. We discuss how unique adaptations to the function and distribution of neuronal secretory organelles may facilitate local protein trafficking at remote sites in neuronal dendrites to support diverse forms of synaptic plasticity.
Keywords: dendrite, synapse, intermediate compartment, Golgi apparatus, endoplasmic reticulum, recycling endosome
1. ORGANIZATION AND DYNAMICS OF NEURONAL SECRETORY ORGANELLES
1.1. Neuronal Morphology and the Constraints on the Secretory Machinery
Neurons are immense, polarized, and highly compartmentalized cells. The abundance and subcellular distribution of proteins displayed on neurons’ expansive surfaces determine their fundamental cellular properties, including excitability, synaptic strength, and remodeling during synaptic plasticity. How the surface proteome of neurons is established and regulated during development and neural plasticity has remained an enduring question in cellular neuroscience. The regulated synthesis of lipids, cell adhesion molecules, ion channels, voltage-gated channels, and neurotransmitter receptors and their trafficking to specific subcellular domains are central mechanisms in this context. However, where and when different classes of proteins are synthesized and how they travel from their birthplace to their functional sites remain central issues. Computational and experimental data suggest that decentralized protein synthesis in dendrites and axons plays a major role in protein targeting to remote sites (Cajigas et al. 2012, Shigeoka et al. 2016, Steward et al. 1998, Williams et al. 2016). However, this issue is complicated for integral membrane and secreted proteins since they must be processed through multiple networks of membrane-bound organelles, collectively termed the secretory pathway, where they are folded, assembled, and biochemically modified prior to their delivery to the cell surface. The distribution and dynamics of neuronal secretory organelle networks have raised fundamental questions concerning protein processing in morphologically complex cells: Can integral membrane and secreted factors be locally processed and trafficked in dendrites, and if so, are they biochemically distinct from proteins processed in the soma? Over what spatial scales and how specifically can new proteins target to specific compartments in neurons? Following synthesis, how long does it take for proteins to travel through the secretory pathway, and is this timescale compatible with current models of synaptic plasticity? Here we review literature describing the organization of the neuronal secretory network, current evidence for satellite protein processing and secretion in neuronal dendrites, the role of polarized secretion during cellular development, and the role of the secretory network in diverse forms of plasticity.
1.2. Organization of Cellular Secretory Organelles and Flow of Nascent Secretory Cargo
We begin with a basic introduction to how membrane and secreted proteins are synthesized, processed, and delivered to the cell surface through the complex and dynamic collection of organelles known as the secretory network. As classically defined, the secretory network consists of the endoplasmic reticulum (ER), the ER-Golgi intermediate compartment (IC), the Golgi apparatus (GA), the trans-Golgi network (TGN), and various classes of mobile carrier vesicles that emerge from the TGN to deliver processed cargo molecules to the appropriate subcellular address (for a review, see Lippincott-Schwartz et al. 2000). Each of the principal secretory organelles can be further subdivided into distinct functional compartments. For example, while the ER is a continuous membrane compartment, it exhibits diverse morphology as it extends from the nuclear envelope to the cell periphery, alternating zones enriched with or free of membrane-bound ribosomes that define the rough ER (RER) and the smooth ER (SER) (Baumann & Walz 2001). Likewise, the GA can be further subdivided into cis, medial, and trans compartments, each thought to host a distinct cast of enzymes and adaptor molecules responsible for protein modifications (e.g., proteases, glycosyltransferases, and other carbohydrate modifying enzymes) and sorting. Perhaps the most understudied and enigmatic compartment of the secretory network is the IC. Similar to the TGN, which constitutes a pleiomorphic membrane network at the interface between the GA and recycling endosomes (REs), the IC constitutes a pleiomorphic membrane network between the ER and the GA (Saraste & Kuismanen 1984, Saraste & Marie 2018, Saraste et al. 1987, Tooze et al. 1984).
During translation, most integral membrane and secreted proteins direct ribosomes to associate with the RER. During and following translation into the ER membrane, they are folded, posttranslationally modified, and in some cases assembled into multisubunit complexes. In the lumen of the ER, consensus amino acid sequences on 75% of membrane and secreted proteins are covalently modified with sugars, a process known as N-glycosylation (Moremen et al. 2012). Properly assembled and processed cargo molecules exit the ER at specialized ER exit sites (ERES) defined by the components of the coat protein complex II (COPII) (Zanetti et al. 2011). Following ER exit, nascent proteins are transported by COPII vesicles to the IC and from there to the GA in mobile Rab1- and COPI-dependent carriers (Saraste & Marie 2018). In the GA, proteins are further processed by proteolytic cleavage and glycosylation, and as mature proteins reach the TGN, they are sorted and packaged into mobile carrier vesicles destined for the plasma membrane (PM) or intracellular organelles. These mobile vesicles are classically defined by the small GTPase Rab8 (Huber et al. 1993), although considerable evidence exists for trafficking of secretory cargo through REs defined by the small GTPase Rab11 (Ang et al. 2004, Bowen et al. 2017). While rare exceptions have been reported (which we discuss in detail below), progression through the GA is generally thought to be a requirement for subsequent trafficking to the PM. Indeed, disrupting GA function with the fungal toxin brefeldin A (BFA), which inhibits clathrin-dependent and COPI-dependent transport at the TGN and the IC-GA interface, strongly impairs forward secretory trafficking in fibroblasts (Helms & Rothman 1992, Lippincott-Schwartz et al. 1989, Misumi et al. 1986, Orci et al. 1993).
1.3. Organization and Dynamics of Secretory Organelles in Neurons
While all the organelles that define the classical secretory network in fibroblasts are also present in neurons, their spatial distribution may be adapted for local protein processing and delivery at remote sites within the dendritic arbor (Figure 1).
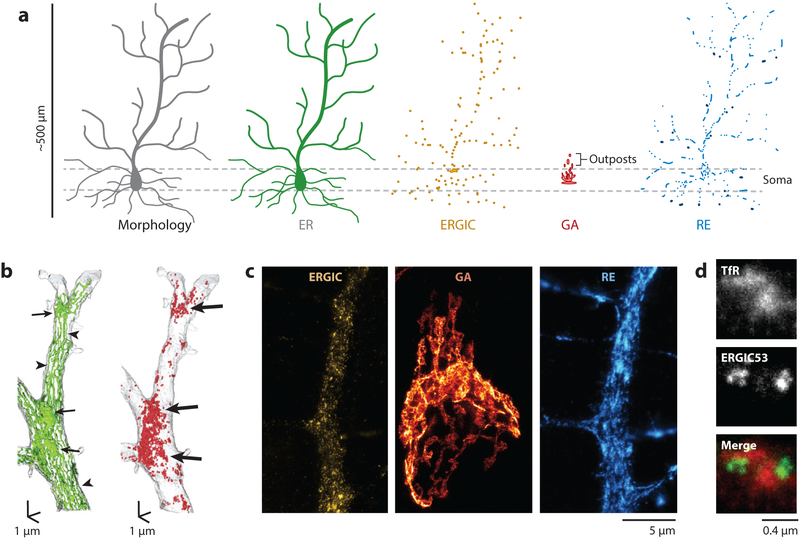
Figure 1.
Distribution of neuronal secretory organelles. (a) Schematic of the organelles that make up the secretory network in a neuron. (b) Reconstructions of serial electron micrographs showing the dendritic distribution of the ER (green, left) and ER-bound ribosomes (red, right) from hippocampal CA1 pyramidal neurons. (Left) Elongated ER tubules are found in straight dendritic segments (arrowheads), whereas more complex ER cisternal sheets are found at dendritic branch points (arrows). (Right) ER-bound ribosomes are concentrated at branch points (arrows). Adapted from Cui-Wang et al. (2012) with permission from Elsevier, copyright 2012. (c) Stimulated emission depletion microscopy images of a section of dendrite from a cultured cortical neuron expressing ERGIC53-GFP (left panel) and TfR-mCh (right panel) to visualize dendritic IC and REs, respectively. The middle panel shows the soma of a cultured cortical neuron stained with GM130 to label the GA. Adapted from Bourke et al. (2018) with permission from Elsevier. (d) Juxtaposition of IC and RE membranes. Superresolution image of REs (labeled with TfR-mCh, top) and IC (labeled with ERGIC53-GFP, middle). The bottom panel shows a merge of the two channels with TfR in red and ERGIC53-GFP in green. Adapted with permission from Bowen et al. (2017). Abbreviations: ER, endoplasmic reticulum; GA, Golgi apparatus; GFP, green fluorescent protein; IC, ER-Golgi intermediate compartment; mCh, mCherry; RE, recycling endosome; TfR, transferrin receptor.
1.3.1. The neuronal endoplasmic reticulum.
The neuronal ER is a continuous membrane network that distributes throughout the entire somatodendritic compartment and axons, including presynaptic terminals and a fraction of dendritic spines (Figure 1a,b) (Cooney et al. 2002, Cui-Wang et al. 2012, Spacek & Harris 1997, Wu et al. 2017). Occasional fragmentation is rapidly counteracted by homotypic membrane fusion so that the ER constitutes a single membrane forming a single, continuous, internal lumen throughout the neuron (Spacek & Harris 1997). As seen in 3D electron microscopy (3D-EM) reconstructions, the somatic ER consists of the nuclear envelope, flattened cisternae, and connecting reticulated tubules (Wu et al. 2017). In thin and larger axons, the ER consists of a single elongated tubule or multiple tubules with occasional anastomosis (Wu et al. 2017). In presynaptic terminals, ER tubules branch and expand into small cisternae, casting a web around mitochondria, clusters of synaptic vesicles, larger vacuoles, and occasional multivesicular bodies (Wu et al. 2017). In the dendritic shaft, the ER consists of long elongated tubules running along dendrites, which connect hot spots of increased membrane branching and bunching located at dendritic branch points and at the base of spines, from which it occasionally protrudes into the spine head (Figure 1b) (Cooney et al. 2002, Cui-Wang et al. 2012, Spacek & Harris 1997, Wu et al. 2017). Components of the translocon complex and ER-bound ribosomes (RER) are overall more abundant in the soma and proximal dendrites but are also found in distal dendrites (Cui-Wang et al. 2012, Spacek & Harris 1997, Torre & Steward 1996), indicating ongoing membrane protein synthesis at these locations (Figure 1b). Similarly, ERES are more abundant in the soma but are distributed throughout dendrites, where they are enriched at specialized locations (Aridor et al. 2004, Cui-Wang et al. 2012). They are largely excluded from axons but are occasionally observed in presynaptic terminals, suggesting local trafficking of nascent presynaptic membrane proteins (Luarte et al. 2018). While evidence suggests that membrane proteins can indeed be locally synthesized and trafficked to the surface in some presynaptic terminals (see below), to date, no ER-bound ribosomes (RER) have been described in axons or presynaptic terminals (Luarte et al. 2018).
While the ER is a continuous membrane, it does exhibit some degree of compartmentalization. Prior to ER exit, membrane and luminal proteins can explore large fractions of the neuronal ER by lateral diffusion (Cui-Wang et al. 2012, Goyal & Blackstone 2013). This free diffusion is, however, counteracted by protein binding to the cytoskeleton or motor proteins (Valenzuela et al. 2014) and by confinement in zones of increased morphological complexity, which act as diffusion traps to increase the dwell time of proteins, particularly at dendritic branch points and near synapses (Cui-Wang et al. 2012). This constrained diffusion is particularly important for the local forward trafficking of nascent dendritic proteins, as it opposes ER-cargo exchange between the soma and dendrites and favors local ER exit by local enrichment of ERES at complex ER hot spots (Bowen et al. 2017, Cui-Wang et al. 2012). Interestingly, ER-bound ribosomes are also frequent at zones of increased ER complexity, suggesting coordination between protein synthesis and ER exit (Cui-Wang et al. 2012). Moreover, RER and ERES hot spots are spatially coordinated with IC clusters, where membrane cargo accumulates after exiting the ER (Cooney et al. 2002, Cui-Wang et al. 2012, Spacek & Harris 1997, Wu et al. 2017). As seen for the SNARE protein VAMP2, insertion into the PM occurs preferentially at branch points, suggesting that the entire secretory network can be locally organized (Cui-Wang et al. 2012).
The structure of the ER in all cell types is dynamic and is regulated by multiple proteins that control ER membrane curvature (REEP, reticulons, CLIMP63), fission, and branching (atlastin) (Goyal & Blackstone 2013). As shown in fibroblasts, the binding of ribosomes favors the transition from tubules to sheet morphology (Puhka et al. 2007). Local demand for membrane proteins (and resulting ER-bound ribosomes) could thus potentially modify the morphology of the dendritic ER to facilitate compartmentalized processing of nascent proteins to satisfy local demands. The morphology and dynamics of the neuronal ER are regulated by the cytoskeleton through microtubule and actin motors (kinesin, dynein, Myosin Ic, and Myosin Va) and binding proteins (REEP1–4, p600, p180, CLIMP63, 4.1N, kinectin) (Goyal & Blackstone 2013, Ramírez & Couve 2011). For example, tight ER binding to microtubules through CLIMP63 favors an elongated, low-complexity ER morphology (Cui-Wang et al. 2012). Release from microtubules causes the ER to adopt a more convoluted morphology that can impair lateral diffusion, potentially trapping nascent proteins for ER exit and downstream trafficking. ER interactions with the microtubule network are dynamic and regulated by group I metabotropic glutamate receptors (mGluRs) and PKC-dependent phosphorylation of CLIMP63, which abrogates microtubule binding (Cui-Wang et al. 2012). Thus, synaptic activity can tune nascent cargo confinement, and presumably influence where a cargo leaves the ER, by altering the degree of ER complexity. As documented for GABAB receptors, the mobility and distribution of nascent membrane proteins in the dendritic ER (or post-ER compartments) may also be actively regulated through cargo binding to motor proteins such as dynein and kinesin 1 (Valenzuela et al. 2014, Vidal et al. 2007). Interestingly, recent evidence in fibroblasts suggests that ER tubules actively contract in a pulsatile manner, enabling directional flow of luminal proteins that, in neurons, could bias how nascent cargoes or associated chaperones redistribute from ER tubules to defined hot spots within the dendritic or axonal arbor (Holcman et al. 2018).
1.3.2. The ER-Golgi intermediate compartment.
The IC is a dynamic system of pleiomorphic tubulovesicular elements that sorts and bidirectionally traffics lipids and proteins between the ER and the GA (see Saraste & Marie 2018 for an excellent review). At the interface with the ER, the IC receives nascent proteins and lipids exiting the ER from COPII-coated vesicles budding off at ERES. These vesicles then coalesce to form relatively stable elements from which proteins are sorted and incorporated into budding tubular elements and vesicles. At the interface with the GA, the IC receives COPI-coated vesicles, enabling retrograde trafficking and recycling of lipids and proteins back to the ER. While the IC has long been thought of as merely a recycling platform, accumulating evidence indicates that it may provide membranes for autophagy and participate in the folding and quality control of nascent proteins, in O- and N-glycosylation, and in the synthesis of glycosaminoglycans and lipids (Ge et al. 2013, Jönsson et al. 2003, Krijnse-Locker et al. 1995, Sannerud et al. 2006, Saraste & Marie 2018, Sirkis et al. 2017).
As shown by the distribution of Rab1, Rab2, COPI, and ERGIC53, the neuronal IC is distributed throughout dendrites and presynaptic terminals (Figure 1c,d) (Bowen et al. 2017, Hanus et al. 2014, Krijnse-Locker et al. 1995). In dendrites, the IC seems to be excluded from dendritic spines and is nearly exclusively found in the dendritic shaft. As seen in 3D-EM, the neuronal IC closely resembles endosomes, making it difficult to unambiguously distinguish the two types of organelles (Wu et al. 2017). The issue is further complicated by the fact that the two types of membranes are closely apposed with one another, potentially participating in direct transfer of lipids and proteins between the compartments (Figure 1d) (Bowen et al. 2017).
As reported with multiple thermosensitive, pharmacologically or optogenetically controlled trafficking cargoes, dendritic proteins exiting the ER accumulate in clusters of IC membranes, which are coordinated with hot spots of ER complexity (Bowen et al. 2017, Chen et al. 2013, Cui-Wang et al. 2012, Hanus et al. 2014). From there, nascent cargoes may be processed locally or distribute over large segments of the somatodendritic compartment through microtubule-dependent transport (Hanus et al. 2014, Horton & Ehlers 2003). This key step in early secretory trafficking is directly regulated by synaptic activity through control of microtubule motors. In hippocampal neurons, for example, increasing neuronal activity and cytoplasmic calcium decreases Kif17 binding to IC through CaMKII-dependent phosphorylation of the kinesin, hence impairing long-range transport of nascent cargo (Hanus et al. 2014). Importantly, increased synaptic activity prevents long-range transport without blocking cargo access to the cell surface, suggesting that dendritic regions with increased synaptic activity may drive local surface trafficking of nascent proteins from stable IC clusters (Hanus et al. 2014).
1.3.3. Golgi apparatus.
As in fibroblast cells, in pyramidal neurons the GA is predominantly localized in proximity to the nucleus and is usually polarized toward the large, apical dendrite. Smaller, independent Golgi outposts (GOs) are present in some dendrites. However, numerous studies agree that the majority of dendrites lack a detectable GA, at least defined by classical GA markers, including GM130 (cis-Golgi), mannosidase II, GRASP55 (medial Golgi and trans-Golgi), and TGN38 (trans-Golgi), all of which are present in the somatic GA. Studies both in vivo and in vitro indicate that GOs are found in only 5% of dendrites (~20% of hippocampal neurons contain GOs in one of their approximately four to five central dendritic processes), typically within the first 30 μm of the apical dendrite (Figure 1a,c) (Bowen et al. 2017, Hanus et al. 2014, Horton et al. 2005). In contrast, expressed, fluorescent protein–tagged versions of various GA-resident proteins have been observed throughout the dendritic arbor. These include galactosyl transferase, α-mannosidase II, TGN38, and a hybrid GA reporter containing elements of calneuron-2, which localizes to the TGN (Horton & Ehlers 2003, Mikhaylova et al. 2016, Ori-McKenney et al. 2012, Zhou et al. 2014). However, a major caveat to these observations is that exogenous proteins may localize to dendritic organelles due to fluorescent protein tags interfering with normal trafficking or high expression levels exceeding the capacity of the target organelle (Mikhaylova et al. 2016). These results have been difficult to reconcile with the absence of staining with antibodies against many of the endogenous GA organizing proteins (Bowen et al. 2017, Hanus et al. 2014, Horton et al. 2005). Dendritic Golgi organelles may lack the defining features of the somatic GA, including cis and trans stacked-membrane structures, and may therefore not require many of the structural proteins that have long been used as markers of the GA. Alternatively, a distinct organelle may fulfill the role of the GA at dendritic sites. Recent studies demonstrated that the IC is present in dendrites and that cargo released from the ER accumulates in ICs with similar kinetics as the somatic GA (Bowen et al. 2017, Hanus et al. 2014). Because the GA houses key enzymes involved in refining the glycosylation patterns of membrane proteins, it will be important to understand whether these refinements can occur in dendrites lacking GA.
1.3.4. The spine apparatus.
The spine apparatus (SA) is a stacked-membrane organelle that is found within a subset of dendritic spines as an extension of the SER (Gray & Guillery 1963, Spacek & Harris 1997). One of the few molecules known to localize to the SA is synaptopodin, which links the ER to the spine actin cytoskeleton (Deller et al. 2000, Mundel et al. 1997). In mice lacking synaptopodin, SER can still invade dendritic spines, but the ER fails to elaborate into the cisternal membrane structures emblematic of the SA (Deller et al. 2003). These mice display deficits in long-term potentiation and behavioral phenotypes in learning and memory tasks (Deller et al. 2003, Jedlicka et al. 2009, Vlachos et al. 2009). Whether these deficits are a sole consequence of SA disruption and whether synaptopodin plays unappreciated roles in synaptic function remain to be seen. SA-containing spines appear to potentiate more robustly relative to neighboring SA-negative spines following strong synaptic activation due to amplification of spine calcium signaling mediated by calcium release from the SA (Korkotian et al. 2014, Vlachos et al. 2009).
In addition to calcium regulation, the SA has been hypothesized to play a role in local secretory trafficking based on its membrane-stacked, GA-like appearance. This idea was initially supported by studies using postembedding immunogold labeling for a panel of proteins that localize to the ER, the IC, and the GA. These proteins include ERGIC53 and Rab1b (the IC); α-mannosidase II, Giantin, and Rab6 (GA); and TGN38 (TGN). Antibodies against these proteins labeled membrane stacks of the SA by using a postembedding immunogold labeling protocol (Pierce et al. 2001). However, subsequent studies using fluorescence-based immunolabeling or expression of tagged versions of some of these proteins have generally failed to observe localization within dendritic spines. For example, in neuronal cultures, TGN38 staining is sparse within dendrites, with the majority of the signal confined to the cell body or GOs located within proximal regions of the apical dendrite (Bowen et al. 2017, Hanus et al. 2014, Horton et al. 2005). Likewise, little if any spine-localized signal was observed for ERGIC53, either by overexpressing GFP-tagged ERGIC53 or by immunolabeling endogenous ERGIC53 (Bowen et al. 2017, Hanus et al. 2014). However, these studies were carried out in cultured neurons at a relatively young age, while the immunogold studies were carried out in tissue from adult rat. Thus, it remains controversial which, if any, of the defining factors for the IC or GA localize to dendritic spines.
In support of a trafficking role for the SA, the majority of spines that harbor SA also contain distinct endosomal membranes, as observed by 3D-EM (Spacek & Harris 1997, Wu et al. 2017). Using fluorescent imaging, Bowen et al. (2017) observed that SA-containing spines identified by synaptopodin staining nearly always contain REs identified by transferrin receptor. Whether these endosomes represent newly formed vesicular structures that arose from the SA or whether they formed independently, through either spine PM endocytosis or translocation of mobile dendritic shaft REs into spines, remains to be determined. Because REs can act as trafficking intermediaries between the GA and the PM, spine REs, regardless of their origin, may receive secretory cargo from the SA for sorting and delivery to the spine PM. However, a definitive role for the SA in forward biosynthetic trafficking remains to be demonstrated.
2. POLARIZATION OF SECRETORY TRAFFICKING DURING NEURONAL MORPHOGENESIS
The acquisition of neuronal polarity and the growth of long and ramified dendrites and axons put a colossal demand on the secretory system to generate and traffic new lipids and proteins. Accordingly, neurite outgrowth is accompanied by a profound spatial reorganization of secretory organelles where membrane components are synthesized and sorted for trafficking. While most studies have focused on reorganization of the GA during neuronal morphogenesis, recent evidence indicates that other organelle networks may experience even more dramatic reorganization during cellular development.
2.1. Polarization of the Somatic Golgi Apparatus
As is typical in animal cells, the neuronal GA is localized in close proximity to the centriole near the nucleus. Both the centriole and the GA are important microtubule nucleating centers from which microtubules radiate toward the cell periphery (Efimov et al. 2007; but see Stiess et al. 2010). This organization enables optimal membrane collection (e.g., pre-Golgi trafficking, which is centripetal) and redistribution throughout the cell (e.g., post-Golgi trafficking, which is centrifugal). The centrosome-Golgi vector (CGV) thus constitutes a core cellular polarization axis whose orientation varies with and sustains asymmetric cell growth. As shown in multiple neuron types across vertebrates and invertebrates, such variations in CGV orientation are particularly important when neurons acquire their axon-dendrite polarity, with first the specification and elongation of a single axon and then the growth of multiple dendrites (Barnes & Polleux 2009, Sakakibara & Hatanaka 2015). For example, in cultured hippocampal neurons, the orientation of the CGV aligns with the site of axon outgrowth (Calderon de Anda et al. 2005, 2008). In the cortex in vivo, the CGV reorients as developing pyramidal neurons migrate into the cortical plate (Calderon de Anda et al. 2005). Initially oriented toward the basal pole (rear) of the cell, the CGV subsequently reorients toward the opposite pole (front), presumably to favor asymmetric growth of the leading process used for radial migration that subsequently becomes the apical dendrite (Barnes & Polleux 2009, Horton et al. 2005, Sakakibara & Hatanaka 2015).
As seen both in vitro and in vivo, the polarization of the somatic GA precedes the formation of the apical dendrite but does not occur in neurons with symmetrical dendritic trees, including GABAergic interneurons (Horton et al. 2005). This polarization is associated with a bias in post-Golgi trafficking to satisfy the demands of the apical dendrite and may thus be important for asymmetric growth (Horton et al. 2005). Indeed, fragmentation of the GA by overexpression of the Golgi stacking protein GRASP65 prevents the preferential growth of the apical dendrite without diminishing the overall rate and extent of dendritic outgrowth (Horton et al. 2005). However, the central roles that the GA plays in membrane trafficking and cytoskeleton dynamics are so intricately interdependent that it is often challenging to decipher the underlying cause of cellular phenotypes resulting from GA manipulations. For example, the GA is a major hub for microtubule nucleation, actin remodeling, and integration of signaling pathways that may not be directly linked to secretory trafficking (Makhoul et al. 2018). The polarization of the GA toward growing axons and dendrites may thus reflect local remodeling of the cytoskeleton rather than a need for vectoral delivery of new lipids and proteins. Indeed, in young neurons, local stabilization of microtubules is both necessary and sufficient for the specification of the axon (Witte et al. 2008). Also, in older neurons in which the GA is no longer polarized toward the axon, membrane cargo is still efficiently delivered to this compartment. In hippocampal neurons, for instance, dendritic proteins that exit the GA are equally likely to enter dendrites and axons. Dendritic proteins are then excluded from the latter compartment by motor proteins at the axon initial segment, while axonal proteins are allowed to enter (Al-Bassam et al. 2012, Kuijpers et al. 2016). Nonetheless, the polarization of the somatic GA that occurs in these multiple examples is accompanied by important changes in how and to which extent axons and dendrites rely on post-Golgi trafficking.
2.2. Developmental and Compartment-Specific Reliance on Secretory Trafficking
Dendrites and axons surprisingly differ in their immediate reliance on the secretory pathway. For example, in rat neurons after axonal polarization, disrupting Golgi function with BFA or inhibitory mutants of Arf1 (a component of the COPI machinery) and protein kinase D (PKD) (a protein regulating membrane fission at the TGN) prevents dendritic growth and maintenance (Horton et al. 2005). In contrast, these manipulations have no measurable effect on axonal outgrowth (Horton et al. 2005). Similarly, mutagenesis screens in Drosophila mechanosensory neurons identified mutations in the genes encoding Sar1 and Sec23 (ERES/IC) and Rab1 (IC), three proteins of the ER-Golgi interface that are involved in forward secretory trafficking and that selectively disrupt growth or maintenance of dendrites but not axons in a cell-autonomous manner (Ye et al. 2007). Intriguingly, if secretory trafficking is disrupted early in cellular development (prior to axon specification), axonal polarization is prevented. Furthermore, if secretory trafficking is blocked at an early time following axon specification, axon growth and maintenance are disrupted (Jareb & Banker 1997). Consistent with an early role of local secretory trafficking supporting axon generation and growth, Sar1 and active ERES are enriched in newly specified axons (Aridor & Fish 2009). Indeed, inhibiting Sar1 prevents axonal growth in cultured neurons in their first day in vitro but has no measurable effect on the length of axons in neurons cultured for 2 or 3 days (Aridor & Fish 2009). As discussed above, BFA prevents the growth of developing dendrites (Horton et al. 2005). Yet surprisingly, in hippocampal neurons the drug has only a moderate effect on the maintenance of mature dendrites (with a 20% decrease in total length over the course of a day) (Horton et al. 2005). This result thus indicates that neuronal reliance on conventional, Golgi-dependent, secretory trafficking may strongly vary during development and maturation (see also Section 3.4).
PKD has also been implicated in development-dependent variations in the sensitivity of axons and dendrites to disrupted secretory trafficking. As discussed above, inhibition or loss of PKD1 in hippocampal neurons prevents dendritic growth and maintenance but does not affect the axon (Horton et al. 2005). In contrast, similar manipulations in cultured neurons at an earlier stage resulted in the formation of multiple axons (Yin et al. 2008). At this stage, PKD1 inhibition prevents the asymmetric growth of a single neurite while preserving total neurite outgrowth, suggesting that PKD is involved in determining polarization rather than in controlling the bulk level of secretory trafficking to support growth. This effect may be due to a neuron-specific regulation of post-GA trafficking of PKD, as evidence suggests that PKD1 regulates the appropriate packaging of polarized (dendritic versus axonal) cargo in the TGN rather than bulk membrane fission for cargo destined for the cell surface, as also seen in fibroblasts and cancer cells (Bisbal et al. 2008, Malhotra & Campelo 2011). In contrast to PKD1, knockdown of LIM kinase 1 (LIMK1), yet another kinase controlling post-Golgi trafficking, inhibits total neurite growth (both axons and dendrites), thus likely controlling growth through the overall membrane output of the GA but not the polarity of membrane trafficking (Rosso et al. 2004).
2.3. Genesis of Golgi Outposts and Role in Dendritic Development
As discussed in Section 1.3, dendritic GOs are rare in mammalian hippocampal and cortical neurons. They are, however, occasionally observed in the most proximal segment of major dendrites such as the apical dendrites of pyramidal neurons, where they form by fission of the somatic GA (Quassollo et al. 2015, Ye et al. 2007). This process is controlled by RhoA through Rock, PKD1, LIMK1, cofilin, and dynamin (Quassollo et al. 2015). An opposite mechanism has been described for Citron-N, a brain-specific Rho binding protein, which promotes integrity of the GA through recruitment of RockII and profilin (Camera et al. 2003). Both mechanisms share multiple features with mechanisms controlling GA fragmentation and reformation during and after mitosis (Colanzi et al. 2003). While it is not clear whether such GA fragmentation impacts dendritic morphology and function, these mechanisms indicate that multiple actin regulatory proteins activated by polarity proteins directly impact GA morphology.
In contrast to vertebrate neurons, dendritic GOs appear to be abundant in mechanosensory neurons of the fly larva body wall (class IV da neurons), whose planar and stereotyped dendritic patterning (albeit devoid of synapses) has been extensively used as a genetic model for understanding neuronal morphogenesis. By using heterologously expressed Golgi reporters, dendritic GOs have been reported to distribute throughout the dendritic arbors of these neurons (Lin et al. 2015, Ori-McKenney et al. 2012, Ye et al. 2007, Zhou et al. 2014). To our knowledge, the ultrastructure of these structures has not been reported, and thus whether they display the canonical dictyosomal morphology of GOs observed in proximal hippocampal dendrites remains unknown (Horton et al. 2005). As observed for other endomembranes (Satoh et al. 2008), Drosophila GOs are preferentially positioned at branch points and are thus ideally positioned to regulate cargo trafficking between multiple dendrites (Ye et al. 2007). Altering GO localization by disrupting the interaction of Golgi membranes with microtubule motors resulted in reduced and aberrant dendrite branching, supporting a role for GOs in driving dendritic growth and branching (Lin et al. 2015, Ye et al. 2007).
Owing to the peculiar orientation of dendritic microtubules in class IV da neurons (Stone et al. 2008), localization of GOs requires dynein and the Golgi dynein adaptor protein Lava lamp (Lin et al. 2015, Ye et al. 2007). As is generally the case for most organelles, GO positioning is determined by a tug-of-war between dynein and kinesins. Mutations in Drosophila that disrupt dynein (Zheng et al. 2008) or prevent the autoinhibition of kinesin 1 result in redistributed GOs in proximal dendrites and axons (Kelliher et al. 2018, Lin et al. 2015, Ye et al. 2007). GO interaction with dynein is dynamic and is inhibited by phosphorylation of Lava lamp by Lrkk, which promotes GO stability and dendritic branching (Lin et al. 2015).
Consistent with the notion that GOs exert local control over dendritic geometry, their stability and escape from specific branch points are correlated with the extension and retraction of nearby terminal branches (Ye et al. 2007). While remodeling is equally frequent at branch points with or without GOs, local disruption by laser irradiation selectively diminishes both extension and retraction of terminal dendritic branches when GOs are present, suggesting a causal relationship between GO and branching dynamics (Ye et al. 2007). The correlation between GO stability and branch elongation supports the view that local membrane biogenesis favors neurite extension. In contrast, the correlation between GO and branch retraction points toward nontrafficking functions of Golgi membranes. Indeed, GOs are important sites of microtubule nucleation in class IV neurons, a function that requires γ-tubulin and CP309 (Ori-McKenney et al. 2012; but see Nguyen et al. 2014). As shown by disruption of these two proteins, microtubule nucleation at the GA is required for proper extension of terminal dendritic branches but does not impact branch retraction or GO distribution throughout the dendritic tree (Ori-McKenney et al. 2012).
2.4. Polarization of Pre-Golgi Compartments and Endosomes
As discussed above, the polarization of the somatic GA varies during neuronal morphogenesis, reflecting important changes in the organization of the cytoskeleton and the directionality of membrane trafficking. Yet these changes are relatively minor compared to the massive reorganization of pre-Golgi membranes and endosomes. For example, the ER rapidly extends in growing neurites through microtubule binding proteins and motors. For example, knocking down p600 demonstrated that ER binding to microtubules is required for proper neuronal migration and neuritogenesis (Shim et al. 2008). In growing dendrites, the appearance of local zones of ER complexity is spatially and temporally correlated with dendritic branching. Increasing or decreasing ER complexity augments or diminishes dendritic growth and complexity, respectively, thus indicating that ER complexity, and consequently local ER cargo processing, is limiting for dendritogenesis (Cui-Wang et al. 2012). Consistently, active ERES first are abundant throughout the axon during early cellular development but then become progressively more abundant in dendrites as dendrites grow and mature (Aridor 2004, Aridor & Fish 2009). Multiple regulators of ER morphology, notably REEP, reticulons, and atlastin, have been linked to normal or pathological dendritic and axonal outgrowth, which is consistent with the idea that morphology and dynamics of the neuronal ER strongly impact neuronal growth and maturation (Goyal & Blackstone 2013, Liu et al. 2019). In growing neuronal processes, most notably at growth cones, the ER maintains multiple membrane contact sites with the PM and endosomes (Wu et al. 2017). As shown for the SNARE Sec22b, these ER-PM contact sites are structured by nonfusogenic SNAREs. Such membrane contact sites are required for proper neurite outgrowth and are thought to contribute to membrane expansion by nonvesicular lipid transfer (Gallo et al. 2016, Petkovic et al. 2014).
The IC and endosomes are also abundant in neuronal processes (Lasiecka & Winckler 2011). In mature neurons, endosomes play a major role in trafficking membranes in the growth and plasticity of dendritic spines (Chambers et al. 1986, da Silva et al. 2015, Kennedy et al. 2010). Endosomes play a comparable role in developing dendrites. For example, in Drosophila class IV neurons, endosomes are distributed throughout the dendritic tree through interactions with dynein and kinesin 1, and their dynamics closely match those of growing terminal branches (Satoh et al. 2008). As seen for GOs, dendritic endosomes redistribute from distal to proximal dendritic segments upon dynein disruption (Satoh et al. 2008). This redistribution may thus also explain the increased branching that occurs in proximal dendrites in the dynein mutants described above. Indeed, disrupting endosome function with dominant-negative mutants of Rab5 prevented this proximal branching phenotype (Satoh et al. 2008). Endosomes likely play a major role in vectorial trafficking of new membranes in dendritic branching and local growth. For example, protrudin localizes to ER–endosomal membrane contact sites and is important for neurite extension (Shirane & Nakayama 2006). Evidence suggests that ER–late endosome (LE) contacts enable the transfer of kinesin 1 from protrudin to LEs, hence favoring LE translocation and exocytosis in growing neurites (Raiborg et al. 2015).
3. COMPARTMENTALIZED SYNTHESIS AND PROCESSING OF NEURONAL MEMBRANE PROTEINS
Neurons continuously integrate synaptic activity and firing patterns to regulate protein abundance and subcellular positioning over spatial scales ranging from individual synapses to large sections of the dendritic arbor or even the entire cell. While abundant protein synthesis occurs within the soma, modeling studies suggest that synthesis in the neuronal cell body followed by long-range trafficking to distal regions in dendrites would be too slow and nonspecific to selectively target cargo proteins to small and potentially dispersed subcellular domains undergoing plasticity (Williams et al. 2016). To overcome issues of central somatic production and trafficking, models for cellular and synaptic plasticity rely on local translation of relevant plasticity proteins to satisfy nearby demand. For example, mRNAs encoding neurotransmitter receptors, ion channels, adhesion molecules, and secreted neurotrophins thought to play key roles in plasticity have been reported to localize to dendrites, where the mRNAs are presumably translated, processed, and trafficked (Cajigas et al. 2012). mRNA translation can be triggered by plasticity stimuli, but the postsynthesis trafficking itinerary of new membrane proteins is still emerging. Here we review current evidence for compartmentalized dendritic secretory processing and trafficking at remote dendritic sites.
3.1. Synaptic Plasticity, Local Protein Synthesis, and Secretory Trafficking
Diverse forms of synaptic plasticity rely on the synthesis and delivery of new proteins to synaptic sites. Protein synthesis is a key requirement for long-term potentiation (LTP), some forms of long-term depression (LTD), and homeostatic synaptic strengthening during prolonged periods of neural inactivity (Frey & Morris 1997, Huber et al. 2000, Kang & Schuman 1996, Kauderer & Kandel 2000, Linden 1996, Martin et al. 1997, Sutton et al. 2006, Wong et al. 2017). While the requirement for protein synthesis in most cases is well documented, the complete repertoire of proteins generated in response to diverse plasticity stimuli and precisely where these proteins are synthesized have only recently begun emerging (Schanzenbächer et al. 2016). However, experiments from multiple groups support plasticity-induced synthesis of neurotransmitter receptors (Ju et al. 2004, Nayak et al. 1998, Smith et al. 2005, Sutton et al. 2006). Homeostatic synaptic strengthening during chronic blockade of neural firing can be mediated by synaptic incorporation of GluA2-lacking AMPA-type glutamate receptors both in cultured neurons and in vivo (Goel et al. 2006, Kim & Ziff 2014, Sutton et al. 2006). These receptors have higher single-channel conductance and calcium permeability than do their GluA2-containing counterparts, resulting in larger postsynaptic excitatory currents. When both action potentials and NMDA receptors (NMDARs) were blocked for several hours, new proteins, including the AMPA receptor subunit GluA1, were generated (Sutton et al. 2006). Surprisingly, when action potentials and NMDARs were locally blocked over a subregion of the dendritic arbor, newly synthesized GluA1 appeared on the surface selectively within the manipulated region (Sutton et al. 2006). This result indicates compartmentalized trafficking of new GluA1 receptors independent of the soma, consistent with previous studies demonstrating AMPA receptor synthesis in mechanically isolated dendrites (Ju et al. 2004). Importantly, the timescale over which synapses strengthen following NMDAR block (~3 h) is consistent with the reported timescale of GluA1 trafficking through the secretory network to the cell surface. Multiple groups have used inducible ER retention and release systems to observe homomeric GluA1 receptors trafficking from the ER to the cell surface within approximately 30 min to 1 h (Bowen et al. 2017, Hangen et al. 2018). However, this measurement is likely an underestimate since this approach bypasses the time it takes for synthesis, folding, subunit assembly, and posttranslational modifications, which could add tens of minutes to hours (Greger et al. 2002).
Rapid synaptic depression has been attributed to synthesis and trafficking of GluA2-containing AMPA receptors. Following cocaine exposure, high-conducting GluA2-lacking AMPA receptors are incorporated into synapses of dopamine neurons in the ventral tegmental area (Wolf & Tseng 2012). Subsequent depression of these synapses can be driven by activating group 1 mGluRs (mGluR LTD) (Bellone & Lüscher 2005). This form of LTD requires protein synthesis and is thought to occur through replacement of GluA2-lacking receptors with GluA2-containing receptors (Mameli et al. 2007). Acute introduction of interfering RNAs or antisense oligonucleotides against GluA2 mRNA into cells through a patch pipette blocks this form of plasticity, suggesting that depression is mediated by newly synthesized GluA2-containing AMPA receptors. However, it is hard to reconcile the kinetics of mGluR LTD in this preparation (synaptic depression maximally expresses within ~5–10 min following activation of mGluRs) with the relatively slow kinetics of AMPA receptor assembly, ER release, and subsequent trafficking through the secretory network, which can take tens of minutes to hours (Greger et al. 2002). However, new proteins generated in response to plasticity stimuli may access an accelerated secretory route for rapid transit to the cell surface. For example, elevated synaptic activity increases the rate of surface delivery of AMPA receptors released from the ER by using an inducible ER retention approach (Hangen et al. 2018).
Finally, the requirement for protein synthesis is a hallmark of LTP. If protein synthesis is transiently blocked during and shortly following LTP induction, synaptic responses potentiate but gradually return to baseline levels ~2 h following induction (Frey & Morris 1997, Stanton & Sarvey 1984). While many soluble proteins that do not depend on the secretory network are generated in response to LTP stimuli, some integral membrane and/or secreted proteins may also be synthesized that would not contribute to synapse function at short timescales (seconds to minutes) following LTP induction but that could contribute to LTP maintenance at later time points. Indeed, LTP is sensitive to BFA, which blocks forward secretory trafficking through the GA (Broutman & Baudry 2001, Kramar et al. 2012). The identity and trafficking itineraries of the newly generated plasticity proteins required for LTP remain unknown, but some evidence exists for synthesis of AMPA receptor subunits following LTP (Nayak et al. 1998). The time it takes for AMPA receptors to progress through the secretory pathway to the cell surface is consistent with the delay between the time of LTP-triggered protein synthesis (minutes following induction) and when new proteins actually influence synapse function (1–2 h following induction). Involvement of newly synthesized secretory cargo for late-phase potentiation is also consistent with experiments demonstrating that potentiated synapses gradually decline to baseline values if BFA is transiently applied 10–45 min following LTP induction (Kramar et al. 2012), roughly the time window when newly synthesized secretory proteins would be assembled, modified, and exiting the ER.
3.2. Evidence for Compartmentalized Secretory Trafficking in Dendrites
Evidence for compartmentalized forward trafficking first came from studies describing the organization and morphology of the dendritic ER (Cui-Wang et al. 2012, Spacek & Harris 1997). As discussed above, the dendritic ER is a continuous meshwork of membranes with convoluted highly branched or bunched structures associated with dendritic branch points and spines. The juxtaposition of ER-associated ribosomes, ERES, and IC at regions of high membrane complexity suggests that protein synthesis occurs at regions where new molecules may be poised for rapid exit and subsequent processing through downstream organelles. These sites appear to be enriched at locations of dendritic branching, possibly allowing a single secretory hub to accommodate the demands of multiple dendritic branches. Thus, some degree of compartmentalization of protein trafficking appears to occur in the earliest steps following protein synthesis through regulated morphology of the ER.
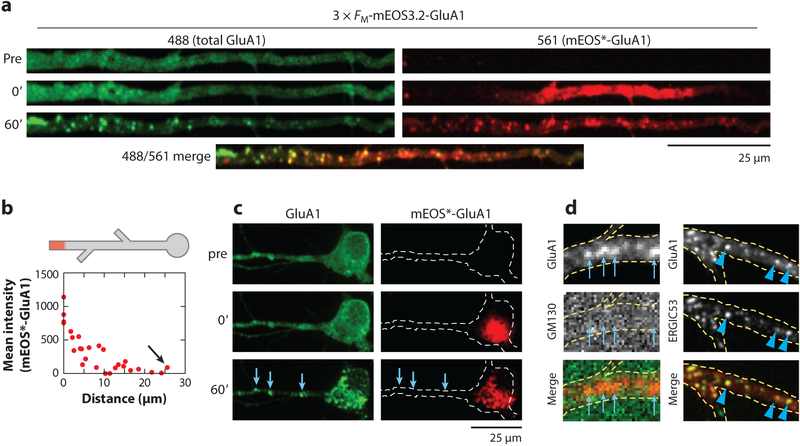
Experiments investigating subsequent trafficking following ER exit in dendrites have largely been carried out using various strategies for conditionally releasing cargo molecules from the ER with user-defined timing. Pioneering studies utilized a temperature-sensitive mutation in the vesicular stomatitis viral glycoprotein (VSV-Gts045) (Hirschberg et al. 1998, Presley et al. 1997, Toomre et al. 2000). At elevated temperature (39°C), VSV-Gts045 is retained in the ER, but when the temperature is lowered, the protein quickly exits the ER and traffics to the PM. Using fluorescent versions of VSV-Gts045 allows for real-time visualization of protein trafficking, revealing the rapid appearance of mobile punctate structures in dendrites within minutes following ER release. This approach has been generalized to diverse secretory proteins with the introduction of modular ER retention and release systems that can be adapted to nearly any protein (Boncompain et al. 2012, Chen et al. 2013, Rivera et al. 2000). These approaches allow ER exit to be triggered with small molecules or light rather than with a temperature shift. Evidence for distinct, compartmentalized somatic and dendritic secretory networks came from experiments coupling ER retention with locally photoswitching fluorescent tags (Figure 2a–c) (Bowen et al. 2017, Chen et al. 2013). This approach allows for specific labeling of ER-retained cargo molecules within targeted subcellular domains (e.g., soma versus dendrites), followed by ER release to track where the photoconverted protein ends up. For example, Bowen et al. (2017) demonstrated that AMPA receptors exiting the somatic ER are very efficiently trafficked to the somatic GA, while cargo exiting the ER in dendrites enters the dendritic IC, with little retrograde trafficking to the somatic GA (Figure 2a,d). This appears to be a general feature of the secretory network, as different studies demonstrated that a nonneuronal trafficking cargo (VSV-G) exhibits similar behavior (Chen et al. 2013, Hanus et al. 2014). While early secretory trafficking (the ER to the IC/GA) appears to be compartmentalized, few studies have investigated the spatial relationship between where cargo molecules exit the ER and where they end up on the cell surface. This is an important issue because, following accumulation in the IC, dendritic secretory cargo moves into highly mobile organelles, including REs and other post-GA vesicles, which may rapidly distribute nascent proteins away from their sites of synthesis (Bowen et al. 2017). The extent to which secretory cargoes spread from their sites of synthesis prior to PM delivery is uncharacterized but is likely influenced by the identity of the cargo, by the location of ER exit, and by ongoing synaptic activity (Valenzuela & Perez 2015).
Figure 2.
Compartmentalization of dendritic and somatic trafficking. (a) A section of dendrite from a neuron expressing ER-retained mEOS3.2-GluA1 before (top) and immediately following (middle) photoconversion of mEOS3.2 from green to red. The bottom panels show the same section of dendrite 60 min after GluA1 was released from the ER. (b) The schematic represents local dendritic photoconversion of mEOS3.2-GluA1 prior to ER release (top). The plot shows quantification of the spread of photoconverted mEOS3.2-GluA1 from the edge of the targeted region, 60 min following ER release. Nearly none of the photoconverted signal traffics to the somatic GA (arrow). (c) A cultured hippocampal neuron expressing ER-retained mEOS3.2-GluA1 before (top) and immediately following (middle) photoconversion of mEOS3.2 from green to red in the soma. The bottom panels show the same neuron 60 min after GluA1 was released from the ER. The blue arrows denote sites of dendritic accumulation of GluA1 following ER release. (d) GluA1 exiting the ER in dendrites localizes to the IC. Shown is mCh-GluA1 60 min following ER release (top). Cells were stained with anti-GM130 to identify the GA (middle left; the blue arrows denote sites of GluA1 accumulation) or coexpressed with ERGIC53-GFP to label the IC (middle right; the blue arrowheads denote sites of GluA1 accumulation). The bottom panels display a merge of GluA1 and GM130 (left) and a merge of GluA1 and ERGIC53 (right). Panels a–d adapted from Bowen et al. (2017). Abbreviations: ER, endoplasmic reticulum; GA, Golgi apparatus; IC, ER-Golgi intermediate compartment.
3.3. Unconventional Secretory Trafficking in Neurons: Bypassing the Golgi Apparatus?
The subcellular distribution of the ER, IC, and GA has raised the key issue of whether all or a subset of dendritically synthesized membrane and secreted proteins can traffic to the neuronal PM without first visiting the somatic GA (Figure 3). While processing in the GA is thought to be a requisite step for trafficking most integral membrane and secreted proteins in nonneuronal cells, there is an ever-expanding list of exceptions. For example, cystic fibrosis transmembrane conductance regulator (CFTR), CD45, Connexins 26 and 30, and the Drosophila integrin αPS1 can be delivered to the cell surface in the presence of BFA (Baldwin & Ostergaard 2002, Martin et al. 2001, Qu et al. 2009, Schotman et al. 2008). In some cases, surface proteins display immature glycosylation patterns, consistent with GA bypass, as discussed below. While BFA disrupts GA function, off-target effects of BFA may cause artificial surface expression of a subset of proteins. However, further evidence for GA bypass comes from genetic studies. Schotman et al. (2008) used a null allele of syntaxin 5 (Stx5) to generate mosaic Drosophila embryos in which the GA was disrupted in a subset of cells. Remarkably, the integral membrane protein αPS1 could still traffic to the cell surface of Stx5 knockout cells, even though trafficking of other protein cargoes was disrupted. In addition to a potential role for GA bypass in normal protein traffic, considerable evidence indicates that GA bypass can occur in response to cellular stress. For example, misfolded proteins in the ER can trigger translocation of GFP-tagged GRASP55 from the GA to the ER, where it is thought to act as an adaptor for the generation of COPII-independent carriers that could transport proteins, such as CFTR, to the cell surface without first transiting through the GA (Gee et al. 2011).
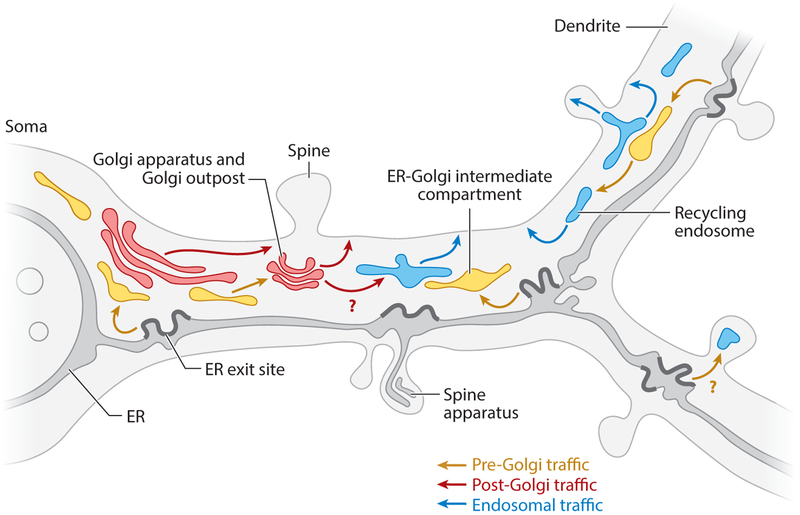
Figure 3.
Golgi-dependent and Golgi-independent secretory routes in dendrites. Nascent secretory proteins exit the endoplasmic reticulum (ER) at ER exit sites at hot spots of complex ER and accumulate in ER-Golgi intermediate compartments distributed throughout the somatodendritic compartment. From the ER-Golgi intermediate compartment, nascent proteins may either traffic to the somatic Golgi apparatus and to Golgi outposts in the most proximal segments of dendrites or directly traffic to recycling endosomes, hence using a Golgi-independent pathway to reach the plasma membrane.
As discussed in Section 2.2, GA disruption with BFA blocks dendritic growth in developing neurons but has only a moderate effect on mature dendrites (Horton et al. 2005). Thus, Golgi-independent trafficking becomes more prominent as neurons mature. Indeed, in mature hippocampal neurons, metabolic labeling of endogenous proteins combined with cell fractionation revealed that a surprisingly large fraction of newly synthesized proteins reach the PM despite GA disruption with BFA (Hanus et al. 2016). Further support for GA bypass comes from experiments utilizing BFA in conjunction with inducible ER retention/release systems. Bowen et al. (2017) observed impaired but significant AMPA receptor trafficking from the ER to REs and ultimately to the neuronal PM following GA disruption with BFA. This effect was specific for AMPA receptors since, under identical conditions, surface expression of two other cargoes (VSV-G and neuroligin1) was blocked.
If some cargoes can bypass the GA, how do they reach the surface? Bowen et al. (2017) demonstrated robust accumulation of AMPA receptors in REs prior to surface delivery, even in the presence of BFA. Dendritic ICs are closely associated with REs, raising the possibility that cargo may traffic directly through ICs to the RE network (Figure 3). Disrupting RE function with dominant-negative Rab11 but not Rab8 impaired early (within 2 h of ER release) surface accumulation (Bowen et al. 2017). Thus, nascent cargo may access the neuronal surface independently of the GA via a previously unrecognized dendritic IC-RE trafficking pathway. While a fraction of AMPA receptors appear to gain access to the surface in a GA-independent manner, other cargoes, like neuroligin 1, require GA function. Further support for GA bypass comes from biochemical analyses of integral membrane proteins purified from the neuronal cell surface, as discussed below.
3.4. Biochemical Consequences of Decentralized Protein Processing
Proteins are chemically modified by glycosylation and proteolytic cleavage as they progress through the GA. Thus, if proteins synthesized in dendrites do not progress through the GA, they will lack the specific biochemical modifications typically associated with this organelle. N-glycosylation is particularly intriguing in this regard, as this process is controlled sequentially in distinct secretory organelles. More than 75% of membrane and secreted proteins are N-glycosylated (Moremen et al. 2012). N-glycosylation is particularly prevalent in the brain (Zielinska et al. 2010) and is thought to have a strong impact on the structure and function of neuronal proteins (Scott & Panin 2014). Indeed, numerous mutagenesis studies have shown that loss of specific glycosylation sites impairs the proper expression of synaptic receptors and associated proteins (Lichnerova et al. 2015, Zheng et al. 2015).
N-glycosylation starts in the ER, where a common mannose-rich precursor is transferred en bloc to consensus sequences as proteins are synthesized (Moremen et al. 2012). This N-glycan precursor mediates protein interactions with ER chaperones such as calnexin and calreticulin and is progressively modified, enabling cells to time and control proper folding during protein quality control. Glycoproteins exiting the ER thus display specific N-glycans, which are termed high-mannose glycans or core glycans, which are then further trimmed and modified by branched carbohydrates and the addition of diverse monosaccharides in the GA (Moremen et al. 2012). This maturation of core glycans into so-called hybrid and complex glycans in the GA renders glycoproteins insensitive to certain glycosidases, which have classically been used to biochemically characterize the maturation state of a glycoprotein. However, accumulating evidence indicates that, for neurons, such immature proteins (i.e., those that are core glycosylated) are abundant at the PM. For example, studies in photoreceptor in the retina indicate that, while the initiation of N-glycosylation in the ER is necessary, the maturation of N-glycans in the GA is dispensable for proper rhodopsin expression (Chambers et al. 1986, Tian et al. 2014). As recently shown, this core glycosylation is not restricted to a few neuronal surface proteins but is emerging as a much more widespread phenomenon than anticipated. Indeed, in hippocampal neurons, hundreds of key surface-expressed neuronal proteins, including neurotransmitter and growth factor receptors, adhesion molecules, and voltage-gated channels, are core glycosylated (Hanus et al. 2016). Of particular interest are some AMPA and GABAA receptors and likely many other proteins, which are expressed on the neuronal surface in both their mature and core-glycosylated states. As shown for candidate proteins, this core glycosylation correlates with a Golgi-independent trafficking to the neuronal surface, probably explaining why a surprisingly large fraction of the nascent surface neuronal proteome is insensitive to GA disruption (Hanus et al. 2016). As shown in acute brain slices by total lectin binding and deglycosylation of candidate proteins, this atypical prevalence of core glycosylation at the neuronal surface also occurs in vivo (Hanus et al. 2016).
While glycosylation is associated with protein trafficking and expression at the cell surface, the maturation of these glycans in the GA appears largely dispensable for dendritic growth and synaptic transmission in cultured hippocampal neurons (Hanus et al. 2016). As mentioned in Section 2.2, GA disruption with BFA impairs dendritic morphology in young neurons but has little effect on mature neurons. In contrast, blocking N-glycan maturation in GA neurons seems not to have much impact on either young or older neurons. Similarly, in conditional MGAT1 and MGAT2 knockout mice in vivo, neuron-specific disruption of glycan maturation in the GA induced apoptosis in specific neuronal populations and resulted in severe locomotion deficits (Ye & Marth 2004). Surprisingly, however, the density of neurons and the overall anatomy of the brain appear largely unaffected by loss of mature N-glycans (Ye & Marth 2004). Similarly, in conditional GM130 knockout mice, neuron-specific disruption of the GA has severe deleterious effects on the cerebellum but leaves the cortex and the hippocampus largely unaffected (Liu et al. 2017). Altogether, these findings indicate that Golgi-independent trafficking, and consequently protein core glycosylation, is atypically prominent across multiple neuron types.
What could the functional outcome be for proteins bypassing the GA? As shown for some AMPA receptors, core-glycosylated proteins are much less stable than their mature glycoform counterparts (Hanus et al. 2016). Increasing core glycosylation accelerates the turnover of these receptors (D. Miremont & C. Hanus, unpublished results), suggesting a causal link between core glycosylation and protein lifetime. Glycans can also influence the biophysical properties of receptor proteins. For example, kainate and AMPA receptors are glycosylated at a site predicted to influence receptor gating and desensitization. Indeed, different glycans impacted receptor function in a manner that was dependent on the subunit composition of the receptors (Copits et al. 2014, Vernon et al. 2017, Weigand & Keller 1998). Thus, the subcellular location of protein synthesis and processing could dictate the longevity and biophysical properties of the same protein. For example, long-lived somatic GA-processed proteins with mature N-linked glycans could be tailored for time-consuming long-range transport to distant locations in the axonal or dendritic arbor. Alternatively, shorter-lived, locally processed core-glycosylated proteins could be better suited for local and transient synaptic modifications.
FUTURE ISSUES.
Is synthesis of the different subunits of multisubunit proteins (e.g., neurotransmitter receptors) spatially coordinated to facilitate assembly and trafficking in neuronal dendrites?
Are the sites of protein synthesis and ER release spatially and/or functionally coordinated?
Following plasticity, can newly synthesized integral membrane proteins be fast-tracked to local synaptic sites on the PM?
How is the molecular and functional heterogeneity of surface proteins established and maintained within neuronal dendrites?
What is the complete repertoire of proteins locally translated in dendrites following plasticity signals?
How far do new integral membrane proteins traffic from their sites of synthesis before insertion into the PM?
Do all integral membrane and secreted proteins locally synthesized in dendrites undergo unconventional trafficking?
Can the SA support protein trafficking to individual synapses?
ACKNOWLEDGMENTS
We apologize to our colleagues whose work we could not cite because of space limitations. We would like to thank Aaron Bowen and Ashley Bourke for critical discussions and help with the figures. Work in the lab of M.J.K. is supported by grants R01NS082271, R01NS10755, and UF1NS107710 from the National Institute of Neurological Disorders and Stroke. Work in the lab of C.H. is supported by Agence Nationale de la Recherche (ANR-6-CE16-0009-01), Fondation NRJ, INSERM, and Université de Paris.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- Al-Bassam S, Xu M, Wandless TJ, Arnold DB. 2012. Differential trafficking of transport vesicles contributes to the localization of dendritic proteins. Cell Rep. 2(1):89–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang AL, Taguchi T, Francis S, Fisch H, Murrells LJ, et al. 2004. Recycling endosomes can serve as intermediates during transport from the Golgi to the plasma membrane of MDCK cells. J. Cell Biol. 167(3):531–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aridor M, Fish KN. 2009. Selective targeting of ER exit sites supports axon development. Traffic 10(11):1669–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aridor M, Guzik AK, Bielli A, Fish KN. 2004. Endoplasmic reticulum export site formation and function in dendrites. J. Neurosci 24(15):3770–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin TA, Ostergaard HL. 2002. The protein-tyrosine phosphatase CD45 reaches the cell surface via Golgi-dependent and -independent pathways. J. Biol. Chem 277(52):50333–40 [DOI] [PubMed] [Google Scholar]
- Barnes AP, Polleux F. 2009. Establishment of axon-dendrite polarity in developing neurons. Annu. Rev. Neurosci 32:347–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann O, Walz B. 2001. Endoplasmic reticulum of animal cells and its organization into structural and functional domains. Int. Rev. Cytol 205:149–214 [DOI] [PubMed] [Google Scholar]
- Bellone C, Lüscher C. 2005. mGluRs induce a long-term depression in the ventral tegmental area that involves a switch of the subunit composition of AMPA receptors. Eur. J. Neurosci 21(5):1280–88 [DOI] [PubMed] [Google Scholar]
- Bisbal M, Conde C, Donoso M, Bollati F, Sesma J, et al. 2008. Protein kinase D regulates trafficking of dendritic membrane proteins in developing neurons. J. Neurosci 28(37):9297–9308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boncompain G, Divoux S, Gareil N, de Forges H, Lescure A, et al. 2012. Synchronization of secretory protein traffic in populations of cells. Nat. Methods 9(5):493–98 [DOI] [PubMed] [Google Scholar]
- Bourke AM, Bowen AB, Kennedy MJ. 2018. New approaches for solving old problems in neuronal protein trafficking. Mol. Cell. Neurosci 91:48–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen AB, Bourke AM, Hiester BG, Hanus C, Kennedy MJ. 2017. Golgi-independent secretory trafficking through recycling endosomes in neuronal dendrites and spines. eLife 6:e27362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broutman G, Baudry M. 2001. Involvement of the secretory pathway for AMPA receptors in NMDA-induced potentiation in hippocampus. J. Neurosci 21(1):27–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajigas IJ, Tushev G, Will TJ, tom Dieck S, Fuerst N, Schuman EM. 2012. The local transcriptome in the synaptic neuropil revealed by deep sequencing and high-resolution imaging. Neuron 74(3):453–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon de Anda F, Gartner A, Tsai LH, Dotti CG. 2008. Pyramidal neuron polarity axis is defined at the bipolar stage. J. Cell Sci 121(2):178–85 [DOI] [PubMed] [Google Scholar]
- Calderon de Anda F, Pollarolo G, Da Silva JS, Camoletto PG, Feiguin F, Dotti CG. 2005. Centrosome localization determines neuronal polarity. Nat. Cell Biol 436(7051):704–8 [DOI] [PubMed] [Google Scholar]
- Camera P, Da Silva JS, Griffiths G, Giuffrida MG, Ferrara L, et al. 2003. Citron-N is a neuronal Rho-associated protein involved in Golgi organization through actin cytoskeleton regulation. Nat. Cell Biol 5(12):1071–78 [DOI] [PubMed] [Google Scholar]
- Chambers JP, Tsin AT, Raymond NY, Aldape FG, Rodriguez KA. 1986. Effects of glycosylation inhibitors on the frog retina. Brain Res. Bull 17(2):259–63 [DOI] [PubMed] [Google Scholar]
- Chen D, Gibson ES, Kennedy MJ. 2013. A light-triggered protein secretion system. J. Cell Biol 201(4):631–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colanzi A, Suetterlin C, Malhotra V. 2003. Cell-cycle-specific Golgi fragmentation: how and why? Curr. Opin. Cell Biol 15(4):462–67 [DOI] [PubMed] [Google Scholar]
- Cooney JR, Hurlburt JL, Selig DK, Harris KM, Fiala JC. 2002. Endosomal compartments serve multiple hippocampal dendritic spines from a widespread rather than a local store of recycling membrane. J. Neurosci 22(6):2215–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copits BA, Vernon CG, Sakai R, Swanson GT. 2014. Modulation of ionotropic glutamate receptor function by vertebrate galectins. J. Physiol 592(10):2079–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui-Wang T, Hanus C, Cui T, Helton T, Bourne J, et al. 2012. Local zones of endoplasmic reticulum complexity confine cargo in neuronal dendrites. Cell 148(1–2):309–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva ME, Adrian M, Schätzle P, Lipka J, Watanabe T, et al. 2015. Positioning of AMPA receptor–containing endosomes regulates synapse architecture. Cell Rep 13(5):933–43 [DOI] [PubMed] [Google Scholar]
- Deller T, Korte M, Chabanis S, Drakew A, Schwegler H, et al. 2003. Synaptopodin-deficient mice lack a spine apparatus and show deficits in synaptic plasticity. PNAS 100(18):10494–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deller T, Merten T, Roth SU, Mundel P, Frotscher M. 2000. Actin-associated protein synaptopodin in the rat hippocampal formation: localization in the spine neck and close association with the spine apparatus of principal neurons. J. Comp. Neurol 418(2):164–81 [DOI] [PubMed] [Google Scholar]
- Efimov A, Kharitonov A, Efimova N, Loncarek J, Miller PM, et al. 2007. Asymmetric CLASP-dependent nucleation of noncentrosomal microtubules at the trans-Golgi network. Dev. Cell 12(6):917–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey U, Morris RG. 1997. Synaptic tagging and long-term potentiation. Nature 385(6616):533–36 [DOI] [PubMed] [Google Scholar]
- Gallo A, Vannier C, Galli T. 2016. Endoplasmic reticulum–plasma membrane associations: structures and functions. Annu. Rev. Cell Dev. Biol 32:279–301 [DOI] [PubMed] [Google Scholar]
- Ge L, Melville D, Zhang M, Schekman R. 2013. The ER-Golgi intermediate compartment is a key membrane source for the LC3 lipidation step of autophagosome biogenesis. eLife 2:e00947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee HY, Noh SH, Tang BL, Kim KH, Lee MG. 2011. Rescue of ΔF508-CFTR trafficking via a GRASP-dependent unconventional secretion pathway. Cell 146(5):746–60 [DOI] [PubMed] [Google Scholar]
- Goel A, Jiang B, Xu LW, Song L, Kirkwood A, Lee HK. 2006. Cross-modal regulation of synaptic AMPA receptors in primary sensory cortices by visual experience. Nat. Neurosci 9(8):1001–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal U, Blackstone C. 2013. Understanding the web: mechanisms underlying ER network formation. Biochim. Biophys. Acta Mol. Cell Res 1833(11):2492–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray EG, Guillery RW. 1963. A note on the dendritic spine apparatus. J. Anat 97:389–92 [PMC free article] [PubMed] [Google Scholar]
- Greger IH, Khatri L, Ziff EB. 2002. RNA editing at Arg607 controls AMPA receptor exit from the endoplasmic reticulum. Neuron 34(5):759–72 [DOI] [PubMed] [Google Scholar]
- Hangen E, Cordelières FP, Petersen JD, Choquet D, Coussen F. 2018. Neuronal activity and intracellular calcium levels regulate intracellular transport of newly synthesized AMPAR. Cell Rep 24(4):1001–12.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanus C, Geptin H, Tushev G, Garg S, Alvarez-Castelao B, et al. 2016. Unconventional secretory processing diversifies neuronal ion channel properties. eLife 5:e20609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanus C, Kochen L, tom Dieck S, Racine V, Sibarita J-B, et al. 2014. Synaptic control of secretory trafficking in dendrites. Cell Rep 7(6):1771–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms JB, Rothman JE. 1992. Inhibition by brefeldin A of a Golgi membrane enzyme that catalyses exchange of guanine nucleotide bound to ARF. Nature 360(6402):352–54 [DOI] [PubMed] [Google Scholar]
- Hirschberg K, Miller CM, Ellenberg J, Presley JF, Siggia ED, et al. 1998. Kinetic analysis of secretory protein traffic and characterization of Golgi to plasma membrane transport intermediates in living cells. J. Cell Biol 143(6):1485–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holcman D, Parutto P, Chambers JE, Fantham M, Young LJ, et al. 2018. Single particle trajectories reveal active endoplasmic reticulum luminal flow. Nat. Cell Biol 20:1118–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton AC, Ehlers MD. 2003. Dual modes of endoplasmic reticulum–to–Golgi transport in dendrites revealed by live-cell imaging. J. Neurosci 23(15):6188–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton AC, Rácz B, Monson EE, Lin AL, Weinberg RJ, Ehlers MD. 2005. Polarized secretory trafficking directs cargo for asymmetric dendrite growth and morphogenesis. Neuron 48(5):757–71 [DOI] [PubMed] [Google Scholar]
- Huber KM, Kayser MS, Bear MF. 2000. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science 288(5469):1254–57 [DOI] [PubMed] [Google Scholar]
- Huber LA, Pimplikar S, Parton RG, Virta H, Zerial M, Simons K. 1993. Rab8, a small GTPase involved in vesicular traffic between the TGN and the basolateral plasma membrane. J. Cell Biol 123(1):35–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jareb M, Banker G. 1997. Inhibition of axonal growth by brefeldin A in hippocampal neurons in culture. J. Neurosci 17(23):8955–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedlicka P, Schwarzacher SW, Winkels R, Kienzler F, Frotscher M, et al. 2009. Impairment of in vivo theta-burst long-term potentiation and network excitability in the dentate gyrus of synaptopodin-deficient mice lacking the spine apparatus and the cisternal organelle. Hippocampus 19(2):130–40 [DOI] [PubMed] [Google Scholar]
- Jönsson M, Eklund E, Fransson L-Å, Oldberg Å. 2003. Initiation of the decorin glycosaminoglycan chain in the endoplasmic reticulum–Golgi intermediate compartment. J. Biol. Chem 278(24):21415–20 [DOI] [PubMed] [Google Scholar]
- Ju W, Morishita W, Tsui J, Gaietta G, Deerinck TJ, et al. 2004. Activity-dependent regulation of dendritic synthesis and trafficking of AMPA receptors. Nat. Neurosci 7(3):244–53 [DOI] [PubMed] [Google Scholar]
- Kang H, Schuman EM. 1996. A requirement for local protein synthesis in neurotrophin-induced hippocampal synaptic plasticity. Science 273(5280):1402–6 [DOI] [PubMed] [Google Scholar]
- Kauderer BS, Kandel ER. 2000. Capture of a protein synthesis–dependent component of long-term depression. PNAS 97(24):13342–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelliher MT, Yue Y, Ng A, Kamiyama D, Huang B, et al. 2018. Autoinhibition of kinesin-1 is essential to the dendrite-specific localization of Golgi outposts. J. Cell Biol 217(7):2531–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy MJ, Davison IG, Robinson CG, Ehlers MD. 2010. Syntaxin-4 defines a domain for activity-dependent exocytosis in dendritic spines. Cell 141(3):524–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Ziff EB. 2014. Calcineurin mediates synaptic scaling via synaptic trafficking of Ca2+-permeable AMPA receptors. PLOS Biol 12(7):e1001900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkotian E, Frotscher M, Segal M. 2014. Synaptopodin regulates spine plasticity: mediation by calcium stores. J. Neurosci 34(35):11641–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramar EA, Babayan AH, Gavin CF, Cox CD, Jafari M, et al. 2012. Synaptic evidence for the efficacy of spaced learning. PNAS 109(13):5121–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krijnse-Locker J, Parton RG, Fuller SD, Griffiths G, Dotti CG. 1995. The organization of the endoplasmic reticulum and the intermediate compartment in cultured rat hippocampal neurons. Mol. Biol. Cell 6(10):1315–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijpers M, van de Willige D, Freal A, Chazeau A, Franker MA, et al. 2016. Dynein regulator NDEL1 controls polarized cargo transport at the axon initial segment. Neuron 89(3):461–71 [DOI] [PubMed] [Google Scholar]
- Lasiecka ZM, Winckler B. 2011. Mechanisms of polarized membrane trafficking in neurons—focusing in on endosomes. Mol. Cell. Neurosci 48(4):278–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichnerova K, Kaniakova M, Park SP, Skrenkova K, Wang Y-X, et al. 2015. Two N-glycosylation sites in the GluN1 subunit are essential for releasing NMDA receptors from the endoplasmic reticulum. J. Biol. Chem 290(30):18379–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C-H, Li H, Lee Y-N, Cheng Y-J, Wu R-M, Chien C-T. 2015. Lrrk regulates the dynamic profile of dendritic Golgi outposts through the golgin Lava lamp. J. Cell Biol 210(3):471–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden DJ. 1996. A protein synthesis–dependent late phase of cerebellar long-term depression. Neuron 17(3):483–90 [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Roberts TH, Hirschberg K. 2000. Secretory protein trafficking and organelle dynamics in living cells. Annu. Rev. Cell Dev. Biol 16:557–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Yuan LC, Bonifacino JS, Klausner RD. 1989. Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell 56(5):801–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Mei M, Li Q, Roboti P, Pang Q, et al. 2017. Loss of the golgin GM130 causes Golgi disruption, Purkinje neuron loss, and ataxia in mice. PNAS 114(2):346–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Guo X, Niu L, Li X, Sun F, et al. 2019. Atlastin-1 regulates morphology and function of endoplasmic reticulum in dendrites. Nat. Commun 10:568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luarte A, Cornejo VH, Bertin F, Gallardo J, Couve A. 2018. The axonal endoplasmic reticulum: one organelle—many functions in development, maintenance, and plasticity. Dev. Neurobiol 78(3):181–208 [DOI] [PubMed] [Google Scholar]
- Makhoul C, Gosavi P, Gleeson PA. 2018. The Golgi architecture and cell sensing. Biochem. Soc. Trans 46(5):1063–72 [DOI] [PubMed] [Google Scholar]
- Malhotra V, Campelo F. 2011. PKD regulates membrane fission to generate TGN to cell surface transport carriers. Cold Spring Harb. Perspect. Biol 3(2):a005280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mameli M, Balland B, Luján R, Lüscher C. 2007. Rapid synthesis and synaptic insertion of GluR2 for mGluR-LTD in the ventral tegmental area. Science 317(5837):530–33 [DOI] [PubMed] [Google Scholar]
- Martin KC, Casadio A, Zhu H, Yaping E, Rose JC, et al. 1997. Synapse-specific, long-term facilitation of Aplysia sensory to motor synapses: a function for local protein synthesis in memory storage. Cell 91(7):927–38 [DOI] [PubMed] [Google Scholar]
- Martin PE, Blundell G, Ahmad S, Errington RJ, Evans WH. 2001. Multiple pathways in the trafficking and assembly of connexin 26, 32 and 43 into gap junction intercellular communication channels. J. Cell Sci 114(Pt 21):3845–55 [DOI] [PubMed] [Google Scholar]
- Mikhaylova M, Bera S, Kobler O, Frischknecht R, Kreutz MR. 2016. A dendritic Golgi satellite between ERGIC and retromer. Cell Rep 14(2):189–99 [DOI] [PubMed] [Google Scholar]
- Misumi Y, Misumi Y, Miki K, Takatsuki A, Tamura G, Ikehara Y. 1986. Novel blockade by brefeldin A of intracellular transport of secretory proteins in cultured rat hepatocytes. J. Biol. Chem 261(24):11398–403 [PubMed] [Google Scholar]
- Moremen KW, Tiemeyer M, Nairn AV. 2012. Vertebrate protein glycosylation: diversity, synthesis and function. Nat. Rev. Mol. Cell Biol 13(7):448–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundel P, Heid HW, Mundel TM, Krüger M, Reiser J, Kriz W. 1997. Synaptopodin: an actin-associated protein in telencephalic dendrites and renal podocytes. J. Cell Biol 139(1):193–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak A, Zastrow DJ, Lickteig R, Zahniser NR, Browning MD. 1998. Maintenance of late-phase LTP is accompanied by PKA-dependent increase in AMPA receptor synthesis. Nature 394(6694):680–83 [DOI] [PubMed] [Google Scholar]
- Nguyen MM, McCracken CJ, Milner ES, Goetschius DJ, Weiner AT, et al. 2014. γ-Tubulin controls neuronal microtubule polarity independently of Golgi outposts. Mol. Biol. Cell 25(13):2039–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L, Palmer DJ, Ravazzola M, Perrelet A, Amherdt M, Rothman JE. 1993. Budding from Golgi membranes requires the coatomer complex of non-clathrin coat proteins. Nature 362(6421):648–52 [DOI] [PubMed] [Google Scholar]
- Ori-McKenney KM, Jan LY, Jan YN. 2012. Golgi outposts shape dendrite morphology by functioning as sites of acentrosomal microtubule nucleation in neurons. Neuron 76(5):921–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkovic M, Jemaiel A, Daste F, Specht CG, Izeddin I, et al. 2014. The SNARE Sec22b has a non-fusogenic function in plasma membrane expansion. Nat. Cell Biol 16(5):434–44 [DOI] [PubMed] [Google Scholar]
- Pierce JP, Mayer T, McCarthy JB. 2001. Evidence for a satellite secretory pathway in neuronal dendritic spines. Curr. Biol 11(5):351–55 [DOI] [PubMed] [Google Scholar]
- Presley JF, Cole NB, Schroer TA, Hirschberg K, Zaal KJ, Lippincott-Schwartz J. 1997. ER-to-Golgi transport visualized in living cells. Nature 389(6646):81–85 [DOI] [PubMed] [Google Scholar]
- Puhka M, Vihinen H, Joensuu M, Jokitalo E. 2007. Endoplasmic reticulum remains continuous and undergoes sheet-to-tubule transformation during cell division in mammalian cells. J. Cell Biol 179(5):895–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu C, Gardner P, Schrijver I. 2009. The role of the cytoskeleton in the formation of gap junctions by Connexin 30. Exp. Cell Res 315(10):1683–92 [DOI] [PubMed] [Google Scholar]
- Quassollo G, Wojnacki J, Salas DA, Gastaldi L, Marzolo MP, et al. 2015. A RhoA signaling pathway regulates dendritic Golgi outpost formation. Curr. Biol 25(8):971–82 [DOI] [PubMed] [Google Scholar]
- Raiborg C, Wenzel EM, Pedersen NM, Olsvik H, Schink KO, et al. 2015. Repeated ER-endosome contacts promote endosome translocation and neurite outgrowth. Nature 520(7546):234–38 [DOI] [PubMed] [Google Scholar]
- Ramírez OA, Couve A. 2011. The endoplasmic reticulum and protein trafficking in dendrites and axons. Trends Cell Biol 21(4):219–27 [DOI] [PubMed] [Google Scholar]
- Rivera VM, Wang X, Wardwell S, Courage NL, Volchuk A, et al. 2000. Regulation of protein secretion through controlled aggregation in the endoplasmic reticulum. Science 287(5454):826–30 [DOI] [PubMed] [Google Scholar]
- Rosso S, Bollati F, Bisbal M, Peretti D, Sumi T, et al. 2004. LIMK1 regulates Golgi dynamics, traffic of Golgi-derived vesicles, and process extension in primary cultured neurons. Mol. Biol. Cell 15(7):3433–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara A, Hatanaka Y. 2015. Neuronal polarization in the developing cerebral cortex. Front. Neurosci 9:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sannerud R, Marie M, Nizak C, Dale HA, Pernet-Gallay K, et al. 2006. Rab1 defines a novel pathway connecting the pre-Golgi intermediate compartment with the cell periphery. Mol. Biol. Cell 17(4):1514–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste J, Kuismanen E. 1984. Pre- and post-Golgi vacuoles operate in the transport of Semliki Forest virus membrane glycoproteins to the cell surface. Cell 38(2):535–49 [DOI] [PubMed] [Google Scholar]
- Saraste J, Marie M. 2018. Intermediate compartment (IC): from pre-Golgi vacuoles to a semi-autonomous membrane system. Histochem. Cell Biol 150(5):407–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste J, Palade GE, Farquhar MG. 1987. Antibodies to rat pancreas Golgi subfractions: identification of a 58-kD cis-Golgi protein. J. Cell Biol 105(5):2021–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh D, Sato D, Tsuyama T, Saito M, Ohkura H, et al. 2008. Spatial control of branching within dendritic arbors by dynein-dependent transport of Rab5-endosomes. Nat. Cell Biol 10(10):1164–71 [DOI] [PubMed] [Google Scholar]
- Schanzenbächer CT, Sambandan S, Langer JD, Schuman EM. 2016. Nascent proteome remodeling following homeostatic scaling at hippocampal synapses. Neuron 92(2):358–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schotman H, Karhinen L, Rabouille C. 2008. dGRASP-mediated noncanonical integrin secretion is required for Drosophila epithelial remodeling. Dev. Cell 14(2):171–82 [DOI] [PubMed] [Google Scholar]
- Scott H, Panin VM. 2014. The role of protein N-glycosylation in neural transmission. Glycobiology 24(5):407–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeoka T, Jung H, Jung J, Turner-Bridger B, Ohk J, et al. 2016. Dynamic axonal translation in developing and mature visual circuits. Cell 166(1):181–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim SY, Wang J, Asada N, Neumayer G, Tran HC, et al. 2008. Protein 600 is a microtubule/endoplasmic reticulum–associated protein in CNS neurons. J. Neurosci 28(14):3604–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirane M, Nakayama KI. 2006. Protrudin induces neurite formation by directional membrane trafficking. Science 314(5800):818–21 [DOI] [PubMed] [Google Scholar]
- Sirkis DW, Aparicio RE, Schekman R. 2017. Neurodegeneration-associated mutant TREM2 proteins abortively cycle between the ER and ER-Golgi intermediate compartment. Mol. Biol. Cell 28(20):2723–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith WB, Starck SR, Roberts RW, Schuman EM. 2005. Dopaminergic stimulation of local protein synthesis enhances surface expression of GluR1 and synaptic transmission in hippocampal neurons. Neuron 45(5):765–79 [DOI] [PubMed] [Google Scholar]
- Spacek J, Harris KM. 1997. Three-dimensional organization of smooth endoplasmic reticulum in hippocampal CA1 dendrites and dendritic spines of the immature and mature rat. J. Neurosci 17(1):190–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton PK, Sarvey JM. 1984. Blockade of long-term potentiation in rat hippocampal CA1 region by inhibitors of protein synthesis. J. Neurosci 4(12):3080–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Wallace CS, Lyford GL, Worley PF. 1998. Synaptic activation causes the mRNA for the IEG Arc to localize selectively near activated postsynaptic sites on dendrites. Neuron 21(4):741–51 [DOI] [PubMed] [Google Scholar]
- Stiess M, Maghelli N, Kapitein LC, Gomis-Rüth S, Wilsch-Bräuninger M, et al. 2010. Axon extension occurs independently of centrosomal microtubule nucleation. Science 327(5966):704–7 [DOI] [PubMed] [Google Scholar]
- Stone MC, Roegiers F, Rolls MM. 2008. Microtubules have opposite orientation in axons and dendrites of Drosophila neurons. Mol. Biol. Cell 19(10):4122–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton MA, Ito HT, Cressy P, Kempf C, Woo JC, Schuman EM. 2006. Miniature neurotransmission stabilizes synaptic function via tonic suppression of local dendritic protein synthesis. Cell 125(4):785–99 [DOI] [PubMed] [Google Scholar]
- Tian G, Ropelewski P, Nemet I, Lee R, Lodowski KH, Imanishi Y. 2014. An unconventional secretory pathway mediates the cilia targeting of peripherin/rds. J. Neurosci 34(3):992–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toomre D, Steyer JA, Keller P, Almers W, Simons K. 2000. Fusion of constitutive membrane traffic with the cell surface observed by evanescent wave microscopy. J. Cell Biol 149(1):33–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze J, Tooze S, Warren G. 1984. Replication of coronavirus MHV-A59 in sac− cells: determination of the first site of budding of progeny virions. Eur. J. Cell Biol 33(2):281–93 [PubMed] [Google Scholar]
- Torre ER, Steward O. 1996. Protein synthesis within dendrites: glycosylation of newly synthesized proteins in dendrites of hippocampal neurons in culture. J. Neurosci 16(19):5967–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela JI, Jaureguiberry-Bravo M, Salas DA, Ramirez OA, Cornejo VH, et al. 2014. Transport along the dendritic endoplasmic reticulum mediates the trafficking of GABAB receptors. J. Cell. Sci 127(15):3382–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela JI, Perez F. 2015. Diversifying the secretory routes in neurons. Front. Neurosci 9:358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon CG, Copits BA, Stolz JR, Guzmán YF, Swanson GT. 2017. N-glycan content modulates kainate receptor functional properties. J. Physiol 595(17):5913–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal RL, Ramírez OA, Sandoval L, Koenig-Robert R, Härtel S, Couve A. 2007. Marlin-1 and conventional kinesin link GABAB receptors to the cytoskeleton and regulate receptor transport. Mol. Cell. Neurosci 35(3):501–12 [DOI] [PubMed] [Google Scholar]
- Vlachos A, Korkotian E, Schonfeld E, Copanaki E, Deller T, Segal M. 2009. Synaptopodin regulates plasticity of dendritic spines in hippocampal neurons. J. Neurosci 29(4):1017–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigand E, Keller BU. 1998. Functional diversity of synaptic AMPA/KA receptors from rat as revealed by subtype-specific antagonists. Eur. J. Neurosci 10(1):64–70 [DOI] [PubMed] [Google Scholar]
- Williams AH, O’Donnell C, Sejnowski TJ, O’Leary T. 2016. Dendritic trafficking faces physiologically critical speed-precision tradeoffs. eLife 5:e20556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte H, Neukirchen D, Bradke F. 2008. Microtubule stabilization specifies initial neuronal polarization. J. Cell Biol 180(3):619–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME, Tseng KY. 2012. Calcium-permeable AMPA receptors in the VTA and nucleus accumbens after cocaine exposure: when, how, and why? Front. Mol. Neurosci 5:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong HH, Lin JQ, Ströhl F, Roque CG, Cioni JM, et al. 2017. RNA docking and local translation regulate site-specific axon remodeling in vivo. Neuron 95(4):852–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Whiteus C, Xu CS, Hayworth KJ, Weinberg RJ, et al. 2017. Contacts between the endoplasmic reticulum and other membranes in neurons. PNAS 114(24):E4859–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye B, Zhang Y, Song W, Younger SH, Jan LY, Jan YN. 2007. Growing dendrites and axons differ in their reliance on the secretory pathway. Cell 130(4):717–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z, Marth JD. 2004. N-glycan branching requirement in neuronal and postnatal viability. Glycobiology 14(6):547–58 [DOI] [PubMed] [Google Scholar]
- Yin DM, Huang YH, Zhu YB, Wang Y. 2008. Both the establishment and maintenance of neuronal polarity require the activity of protein kinase D in the Golgi apparatus. J. Neurosci 28(35):8832–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanetti G, Pahuja KB, Studer S, Shim S, Schekman R. 2011. COPII and the regulation of protein sorting in mammals. Nat. Cell Biol 14(1):20–28 [DOI] [PubMed] [Google Scholar]
- Zheng C-Y, Chang K, Suh YH, Roche KW. 2015. TARP γ−8 glycosylation regulates the surface expression of AMPA receptors. Biochem. J 465(3):471–77 [DOI] [PubMed] [Google Scholar]
- Zheng Y, Wildonger J, Ye B, Zhang Y, Kita A, et al. 2008. Dynein is required for polarized dendritic transport and uniform microtubule orientation in axons. Nat. Cell Biol 10(10):1172–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Chang J, Wang X, Savelieff MG, Zhao Y, et al. 2014. GM130 is required for compartmental organization of dendritic Golgi outposts. Curr. Biol 24(11):1227–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinska DF, Gnad F, Wiśniewski JR, Mann M. 2010. Precision mapping of an in vivo N-glycoproteome reveals rigid topological and sequence constraints. Cell 141(5):897–907 [DOI] [PubMed] [Google Scholar]