Abstract
Despite the availability of global and regional guidelines to curtail the adverse clinical outcomes associated with chronic kidney disease–mineral and bone disorder (CKD-MBD), most CKD patients are still affected by the consequences of abnormalities of CKD-MBD. This important clinical complication of CKD continues to be studied, in order to improve the understanding and management of CKD-MBD. Some notable discoveries include the role of fibroblast growth factor 23 (FGF23) in the pathogenesis of CKD-MBD, leading to a shift from the previous well-established classic trade-off hypothesis to the updated trade-off hypothesis. More recently, there has been a shift from the treatment of CKD-MBD based on a single level of biomarkers to serial measurements of calcium, phosphate and parathyroid hormone (PTH). Furthermore, some clinical trials have emerged after the 2009 Kidney Disease-Improving Global Outcomes (KDIGO) Guidelines, leading to the 2017 KDIGO updated recommendations. Hence, this review gives an overview of the rapidly evolving trends in CKD-MBD, linking the past and current concepts of CKD-MBD.
Keywords: chronic kidney disease–mineral and bone disorder, updated pathogenesis, emerging trends, management
Introduction
Chronic kidney disease (CKD) is a worldwide health problem affecting 5–10% of the world’s population1,2 and the majority of these patients are at an increased risk of developing disturbances of bone and mineral metabolism. These disturbances lead to a constellation of bone lesions which was previously referred to as renal osteodystrophy (ROD), with affected patients manifesting with symptoms such as bone pain, muscle-tendon rupture, pruritus and high incidence of fractures.3,4 Subsequently, evidence has shown that patients with ROD are also predisposed to cardiovascular calcification with associated high morbidity and mortality rates.5,6 Unfortunately, the term ROD does not encompass this important extraskeletal manifestation. Therefore, to address these drawbacks and accommodate the extraskeletal manifestations, the Kidney Disease-Improving Global Outcomes (KDIGO) Foundation initiated a controversies conference with the aim of providing a globally acceptable definition and classification system for renal osteodystrophy. The KDIGO workgroup recommended a broader term, CKD–mineral and bone disorder (CKD-MBD) for the systemic disorder of mineral and bone metabolism due to CKD and that the term renal osteodystrophy should exclusively be used to describe disorders in bone morphology associated with CKD.6 However, in clinical settings, a bone biopsy is less frequently utilized because it is an invasive and cumbersome procedure and requires highly skilled personnel to interpret the tissue samples. For these reasons, clinicians largely depend on trends in the levels of parathyroid hormone in conjunction with levels of serum phosphate, calcium and alkaline phosphatase as markers of bone turnover to guide in the treatment of mineral bone disorder.4
Historical Perspectives
The association between kidney diseases and bone abnormalities dates back to 1883, when Lucas suggested the term “renal rickets” in patients with albuminuria and bone deformities.7 In 1930, Bauer et al8 established an association between bone lesions (osteitis fibrosa cystica) and the parathyroid gland following a review of 88 patients with endocrine bone disorders. Seven years later, Albright et al postulated that CKD patients with phosphate retention and low levels of calcium are prone to parathyroid gland hyperplasia and renal osteitis fibrosa. Subsequently, in the 1940s, the term renal osteodystrophy was coined and used interchangeably with renal rickets.9
The emergence of the “trade-off hypothesis” by Bricker and Slatopolsky10,11 provided an insight into the pathogenesis of renal osteodystrophy. The theory states that progressive nephron loss in CKD patients leads to several compensatory mechanisms such as elevated PTH in response to retained phosphate.
In the 1960s and 1970s, the two predominant forms of renal osteodystrophy in patients with end-stage kidney disease (ESKD) were osteitis fibrosa and mixed uraemic osteodystrophy with a minority of patients presenting with osteomalacia prior to dialysis.12 However, osteomalacia became a major problem following initiation of dialysis secondary to aluminum intoxication in some centers; the two most affected dialysis centers (Ottawa and Newcastle) had high concentrations of aluminum and fluoride in their tap water. This entity of renal osteodystrophy (osteomalacia) was characterized by microcytic anemia and encephalopathy.13 However, adynamic bone disease was not only peculiar to aluminum contamination of tap water used for dialysis but also associated with the use of large amounts of aluminum containing phosphate binders and active vitamin D therapy.14 Subsequently, there was a rapid decline in the occurrence of this disease entity with improvement in water purification systems and reduced prescription of aluminum-containing phosphate binders.
Definitions and Guidelines
Definitions
In 2003, the National Kidney Foundation proposed that renal osteodystrophy should be defined as a constellation of bone disorders present or exacerbated by CKD that lead to bone fragility and fractures, abnormal mineral metabolism, and extraskeletal manifestations.15 Despite incorporating a triad of abnormal mineral metabolism, skeletal and extraskeletal manifestations this definition failed to be accepted globally. Therefore, to ensure a widely acceptable definition, the second KDIGO controversies conference in 2005 came up with a broader term CKD-MBD. The conference participants agreed that CKD-MBD should be defined as:
A systemic disorder of mineral and bone metabolism due to CKD manifested by either one or a combination of the following: (i) abnormalities of calcium, phosphorus, PTH, or vitamin D metabolism; (ii) abnormalities in bone turnover, mineralization, volume, linear growth, or strength; or (iii) vascular or other soft tissue calcification.6
This internationally acceptable definition has facilitated valid comparison of studies in the field of CKD-MBD.
Guidelines
In an ongoing effort to reduce the adverse clinical events associated with CKD-MBD, several global and regional guidelines were proposed to assist clinicians in the management of patients with CKD-MBD. These guidelines provided recommended target reference values for intact PTH, phosphate and serum calcium. However, comparison of these guidelines has shown a lack of harmonization with the existence of relevant clinical differences in the target values16 (Table 1).
Table 1.
Recommended Guidelines by Different Professional Groups
| Group | Year | Recommended Levels | ||
|---|---|---|---|---|
| Corrected Calcium (mg/dL) | Phosphorous (mg/dL) | PTH (pg/mL) | ||
| KDIGO17 | 2017 | Near normal range | Near normal range | 2–9× upper limit of normal |
| KDIGO18 | 2009 | Within normal range | Within normal range | 2–9× upper limit of normal |
| K/DOQI15 | 2003 | 8.4–9.5 | 3.5–5.5 | 150–300 |
| Canadian Society of Nephrology19 | 2006 | Within normal range | Within normal range | 100–500 |
| Japanese Society for Dialysis Therapy20 | 2008 | 8.4–10.0 | 3.5–6.0 | 60–240 |
| UK Renal Association21 | 2002 | 8.8–10.4 | <5.6 | <4× upper normal range |
Pathogenesis of CKD-MBD
Classically, prior to the discovery of fibroblast growth factor 23 (FGF23), phosphate retention due to a decline in renal function had been considered as the main trigger of secondary hyperparathyroidism.22 The retained phosphate leads to a triad of hyperphosphatemia, low 1,25(OH)2D3 and hypocalcemia which are well-known stimuli for PTH secretion that in turn enhances phosphate excretion and development of secondary hyperparathyroidism in advanced CKD. However, what mitigates this process in the early stages of CKD continued to be a point of discussion. Some authors have observed that calcitriol deficiency occurred earlier than hyperphosphatemia and hypocalcemia suggesting that it may be the main initiator of secondary hyperparathyroidism. Therefore, the pathophysiology of secondary hyperparathyroidism is a complex process that involves an interaction between several factors. In the classic hypothesis, the trade-off of PTH for normalization of calcium and phosphate levels is the development of secondary hyperparathyroidism.10,23 The role of phosphate in the pathogenesis of secondary hyperparathyroidism was further supported by studies that demonstrated an association between high phosphate diets and parathyroid hyperplasia.24,25
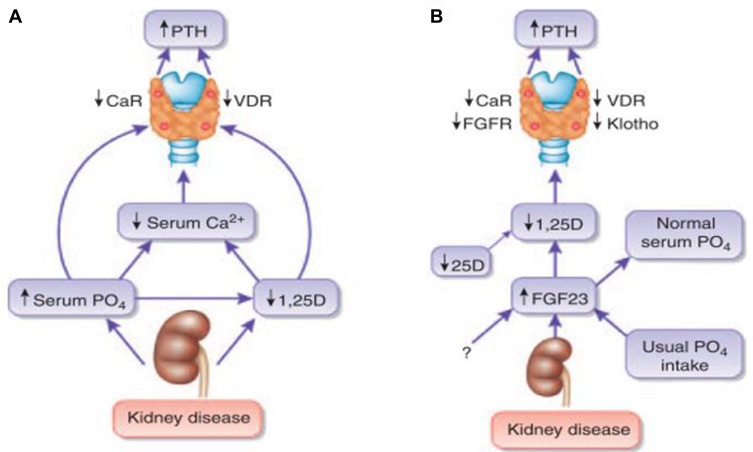
However, the pathophysiology of secondary hyperparathyroidism has evolved with new discoveries.4 For example, the emergence of FGF23 has revolutionized the understanding of the mechanisms underlying the development of secondary hyperparathyroidism, leading to an updated trade-off hypothesis. Plasma FGF23 levels become elevated with progressively worsening renal function, likely to occur before observed changes in the levels of phosphate and PTH.26 The updated trade-off hypothesis versus classic hypothesis is summarized in Figure 1.27
Figure 1.
Pathogenesis of disordered mineral metabolism in CKD.
Notes: (A) Traditional view of the mechanisms that maintain secondary hyperparathyroidism in advanced chronic kidney disease. (B) Updated view of the mechanisms that initiate secondary hyperparathyroidism in chronic kidney disease, emphasizing the central role of FGF23. Reprinted from Kidney International, 78(10). Isakova T, Wolf MS. FGF23 or PTH: which comes first in CKD? Kidney Int. 2010;78(10):947–9, Copyright 2010, with permission from Elsevier.
Abbrevaitions: CaR, calcium-sensing receptor; FGFR, fibroblast growth factor receptor; PTH, parathyroid hormone; VDR, vitamin D receptor.
Role of FGF23 in the Pathogenesis of Secondary Hyperparathyroidism
Fibroblast growth factor 23 (FGF23) is derived from osteocytes and plays a vital role in vitamin D and phosphate metabolism. It requires klotho (a transmembrane protein) to enable it to bind to the FGF receptor (FGFR) in classic target organs such as kidneys and parathyroid glands.28 Plasma FGF23 enhances phosphate excretion in the proximal renal tubule by decreasing the expression of luminal sodium-dependent phosphate transporters and may also decrease intestinal phosphate absorption by inhibiting NaPi cotransporter activity.29 In addition, it reduces the synthesis of 1,25-dihydroxyvitamin D [1,25(OH)2D3] by down-regulating the activity of 1α-hydroxylase and accentuating the activity of 24-hydroxylase.30,31 In the early stages of CKD, high levels of FGF23 attenuate hyperphosphatemia at the expense of 1,25(OH)2 vitamin D suppression, thus initiating the development of secondary hyperparathyroidism.31 The decrease in serum 1,25(OH)2D3 leads to decreased intestinal calcium absorption. The triad of low levels of calcium, calcitriol and hyperphosphatemia further enhances excessive PTH secretion. This excess PTH leads to mobilization of calcium from the bone and osteitis fibrosa. Other consequences of progressive worsening of kidney function include hypo-responsiveness of the vitamin D receptor (VDR) on the parathyroid gland with further enhancement of production of PTH and reduced expression of the calcium-sensing receptor on the parathyroid gland leading to parathyroid gland hyperplasia. In some subsets of patients, the parathyroid gland undergoes hypertrophy and becomes autonomous.32
Role of Klotho in CKD-MBD
Klotho is a transmembrane protein that confers tissue specificity to FGF23. The importance of this co-receptor was demonstrated in klotho null mice showing a phenotype similar to that of FGF23 null mice, with features of premature aging, vascular calcification, altered calcium/phosphate metabolism with hyperphosphatemia, and shortened lifespan.33,34 Disordered FGF23-Klotho axis, which is characterized by low level of serum klotho and high FGF23, has been shown to be an early feature of CKD. Klotho plays a fundamental role in mineral homeostasis through an interplay with other markers of CKD-MBD (parathyroid hormone, phosphate, fibroblast growth factor-23, and 1,25-[OH]2 vitamin D3).35 The loss of ability of FGF23 to normalize phosphate levels through its phosphaturic effect and regulate PTH secretion was evidenced in dialysis patients where higher levels of FGF23 corresponded to the highest PTH levels. This dysregulated compensatory mechanism by FGF23 was largely attributed to klotho deficiency in CKD, which is characterized by low expression of klotho and FGF23 receptor 1 in the parathyroid gland. Klotho is responsible for converting FGFR1(IIIc) into a specific receptor for FGF23. In addition, klotho is also termed a calciophosphoregulatory protein as demonstrated in its ability to enhance phosphaturia and prevent urinary calcium loss.36 Thus, klotho deficiency will lead to a constellation of disordered mineral metabolism, secondary hyperparathyroidism, vascular calcification, and cardiac hypertrophy,36 while exogenous administration of klotho may ameliorate or prevent the development of CKD-MBD.
Emerging Trends in the Pathogenesis of CKD-MBD
Recent studies have also reported the contribution of Wnt inhibitors (portmanteau of wingless and int) in the pathogenesis of CKD-MBD. These Wnt inhibitors which include Dickkopf-1 (Dkk1) and sclerotin are usually secreted during kidney injury. For instance, in CKD mouse models, Fang et al reported that in comparison with non-CKD diabetic controls, increased levels of Dkk1, sclerotin and Klotho were found in chronic kidney disease-2 mice and the use of a monoclonal antibody to reduce levels of Dkk1-enhanced bone formation rates, reversed the induced osteodystrophy and vascular calcification.37 In their treatment protocol, combination therapy of the monoclonal antibody with phosphate binders was found to completely ameliorate the induced CKD-MBD. Hence, these observations may serve as a therapeutic option for the management of CKD-MBD.
To further unravel the complex interplay between the implicated biomarkers of kidney injury in their pathogenic role in CKD-MBD, Activin A was also discovered to play a crucial role in the vascular and skeletal components of CKD-MBD. Activin A originates from peritubular myofibroblast of injured kidneys and exerts its effects via type 2 activin A receptor (ActRIIA). In another mouse model, the effects of activation of activin receptor type IIA (ActRIIA) through signal analysis and inhibition of ActRIIA by utilising a ligand trap for the receptor, RAP-011 (a soluble extracellular domain of ActRIIA fused to a murine IgG-Fc fragment) was evaluated.38 The researchers reported the development of CKD-MBD which was characterized by osteodystrophy, vascular calcification, cardiac hypertrophy, elevated levels of FGF23, PTH, hyperphosphatemia and reduced klotho with stimulation of ActRIIA. Reversal and attenuation of these features of CKD-MBD was observed with inhibition of ActRIIA signalling.38–40
Diagnosis of CKD-MBD
In 1983, Sherald et al41 proposed a classification for renal osteodystrophy based on bone histomorphometry findings, namely high turnover disease, low turnover and mixed uremic osteodystrophy. The emphasis on this classification was on bone turnover; however, since a bone biopsy is not routinely used for monitoring patients there is a need for reliable biomarkers for assessing and monitoring patients with CKD-MBD. Therefore, the KDIGO guidelines recommended the use of serum PTH in conjunction with total or bone-specific alkaline phosphatase (b-ALP) since high or low levels of these markers correlate with underlying bone turnover. To further support the diagnostic utility of these various biochemical markers of mineral bone disorders, the KDIGO group conducted one of the largest bone biopsy studies involving 492 dialysis patients. In their multivariate analysis, both intact PTH and whole PTH were found to remain significantly predictive in differentiating high from non-high bone turnover. In addition to PTH, they also assessed the additive value of bone-specific alkaline phosphatase and the amino-terminal propeptide of type 1 procollagen (PINP) in providing diagnostic accuracy. Surprisingly, the inclusion of specific b-ALP level added only non-statistically significant value to PTH while PINP did not.42 However, due to limited serum samples, they could not assess the diagnostic utility of FGF23, 25-OH vitamin D and other newer biomarkers of CKD-MBD.
Biochemical Parameters of CKD-MBD
Parathyroid Hormone
The first-generation PTH assays were the radioimmunoassay (RIAs) that utilized an antibody to locate an epitope in the c-terminal or mid portions of the PTH molecule. The first-generation assays were later found to be associated with some drawbacks such as cross-reactivity with other fragments of PTH (mid and carboxyl terminal). These fragments are produced by the liver and excreted by the kidneys. Therefore, as a result of cross-reactivity, levels of PTH measured in CKD patients by these assays will be markedly elevated.43 Subsequently, in the 1980s, the two-site immunoradiometric assays were launched to address the inadequacies associated with the first-generation assays.44 The second-generation assay specifically measures the full-length PTH (intact PTH). Although, more recently, it was believed that the second-generation assays may also recognize other fragments such as PTH (7–84),45 it still remains the most widely used assay. The third-generation assays which are now believed to be specific for PTH (1–84) are also available.46 However, the improved diagnostic value of the third generation as compared to second-generation assays has not been established.47 In 2001, Monier et al48 assessed whether the use of the plasma PTH (1–84)/C-PTH fragment ratio could predict bone turnover better than individual PTH levels measured with second- or third-generation assays. Their results showed that the PTH-(1-84)/C-PTH fragment ratio was the best predictor of bone turnover, with a ratio >1 predicting high or normal bone turnover (sensitivity 100%), while a ratio <1 indicated a high probability (sensitivity 87.5%) of low bone turnover. However, subsequent studies did not find any advantage in the assessment of bone turnover with this ratio compared to a single value of PTH. Therefore, the KDIGO group is of the opinion that both second- and third-generation assays are comparable, with not enough evidence to recommend switching to the third-generation assay. Similarly, the use of both assays to arrive at a ratio will lead to a considerable increase in costs of evaluating CKD-MBD. Despite the limitations associated with PTH measurement, it remains the recommended marker for monitoring CKD-MBD. The choice of PTH as a marker for monitoring secondary hyperparathyroidism has been supported by studies that correlated elevated levels of these hormones with poor clinical outcomes. The US Renal Data System revealed reduction in fracture risk by 32% post parathyroidectomy after adjusting for confounding variables.49 In another study, independent of age and diabetes status, elevated levels of PTH were associated with a history of heart failure and myocardial infarction.50 Additionally, in a large randomized trial, decreased levels of PTH with paricalcitol therapy were significantly associated with decreased cardiovascular hospitalization.51 Furthermore, bone remodeling is a dynamic process with an average remodeling cycle of 3–6 months for an area of bone. Therefore, the use of multiple bone biopsies as a gold standard for diagnosing and monitoring renal osteodystrophy is impracticable. In clinical settings, an ideal biomarker for monitoring the management of CKD-MBD should be noninvasive and can be repeatedly measured.
Serum total alkaline phosphatase (TAP) is a marker of osteoid formation and mineralization, and widely utilized as a marker of bone formation because it is readily available and inexpensive. Although bone-specific alkaline phosphatase (b-ALP) is more specific and a better surrogate marker of bone formation, studies have reported a good correlation between TAP and (b-ALP).52 While TAP is cheaper than b-ALP, it is less specific and found in other tissues such as kidneys, liver, spleen and intestine.52 Other markers of bone turnover are summarized in Table 2.
Table 2.
Markers of Bone Turnover
| Marker | Tissue of Origin | Specimen | Comments |
|---|---|---|---|
| Bone Formation | |||
| Specific bone alkaline phosphatase (b-ALP) | Bone | Serum | Glycoprotein derives from osteoblasts. Good surrogate marker of bone formation. |
| Osteocalcin (OC) | Bone, Platelets |
Serum | Originates from osteoblasts, odontoblasts and hypertrophic chondrocytes. Plays a vital role in osteoid mineralization. |
| C-terminal propeptide of type I procollagen (PICP) | Bone, soft tissue, skin | Serum | Arises from proliferating osteoblasts and fibroblasts. Not recommended for routine use as a marker of mineral bone disorders. |
| N-terminal propeptide of type I procollagen (PINP) | Bone, soft tissue, skin | Serum | Arises from proliferating osteoblast and fibroblasts. |
| Bone Resorption | |||
| Hydroxyproline | Bone, cartilage, soft tissue, skin | Urine | Present in both the newly synthesized and the mature collagen. |
| Hydroxylysine glycosides | Bone, soft tissue, skin, serum & complement | Urine, serum | Hydroxylysine in collagen is glycosylated to varying degrees, depending on tissue type |
| Carboxyterminal crosslinked telopeptide of type I collagen (CTX-I) | Tissues containing type I collagen | Urine (a-/s), serum (s only) | Collagen type I, predominantly from the bone. Isomerisation of aspartyl to s-aspartyl occurs with ageing of collagen molecule. |
| Aminoterminal crosslinked telopeptide of type I collagen (NTX-I) | Tissues containing type I collagen | Urine, serum | Collagen type I, predominantly from the bone. |
| Bone Sialoprotein (BSP) | Bone, dentin, hypertrophic cartilage | Serum | Synthesized by osteoblasts and osteoclastic cells. |
| Tartrate-resistant acid phosphatase (TRAcP) | Bone, blood | Plasma, serum | Six isoenzymes are present in the human tissues. Expressed by Osteoclasts, dendritic cells and macrophages. TRAcP 5b is considered as a marker of bone resorption, while TRAcP 5a is a known marker of inflammatory conditions. |
Note: Modified from Clinical Biochemist Reviews. 2005;26(4):97-122. Seibel MJ. Biochemical markers of bone turnover: part 1: biochemistry and variability, with permission from the author and publisher of Clinical Biochemist Reviews.52
Clinical Impact of CKD-MBD
Several observational studies have shown an association between deranged markers of CKD-MBD and poor clinical outcomes in both predialysis and dialysis patients.53–56 For example, elevated levels of phosphate, calcium and PTH have been shown to be associated with cardiovascular-specific mortality in patients with CKD. In a large, prospective, multicenter, cohort study (Netherlands Cooperative Study on the Adequacy of Dialysis) involving 1629 hemodialysis and peritoneal dialysis patients, a significant increase in hazard ratio (HR) of 1.57 (1.07–2.30) in patients with the highest quartile of phosphate using both baseline and time-dependent values was reported.53 Similarly, Block et al57 reported an increased risk of death with increasing levels of phosphate, RR 1.07, 1.25, 1.43, 1.67, and 2.02 for serum phosphorus levels of 5.0 to 6.0, 6.0 to 7.0, 7.0 to 8.0, 8.0 to 9.0, and >9.0 mg/dL, respectively, in 40,538 patients on maintenance hemodialysis. The consistent association of hyperphosphatemia with increased mortality has been linked to its direct calcifying effect on coronary vessels and cardiac valves.
Studies relating to PTH have shown a “U” shaped association with increased risk of death at extreme values of PTH. The highest value of PTH that is associated with increased risk of death varies across studies from >400 pg/mL55 to >500 pg/mL58 and >600 pg/mL.57
On the lower end of PTH, some studies have associated PTH below the K/DOQI recommended lower threshold (<150 pg/mL) with increased death risk.55,59
Despite the differences in the cut-off points utilized by various studies, hypercalcemia has been consistently associated with increased risk of mortality in maintenance hemodialysis (MHD) patients.60–64 In the three phases of the dialysis outcomes and practice patterns study (DOPPSI, II and III) with 25,588 HD patients, calcium levels greater than 10.0 mg/dL (>2.5 mmol/L) were significantly associated with greater risk of all-cause and cardiovascular mortality in both baseline and time-dependent models.61 The reasons for this consistent association could be linked to the acceleration of arterial calcification by hypercalcemia.65,66
A triad of high calcium, elevated phosphate levels and high or low PTH levels was associated with increased mortality in MHD patients.67 Alkaline phosphatase which is one of the markers of high bone turnover was recommended to be measured annually by the KDIGO. This relatively cheap diagnostic test has been consistently associated with increased mortality in both predialysis and dialysis populations.68–70 For example, a USA multicenter observational study of hemodialysis patients reported that higher levels of alkaline phosphatase were associated with increased risk of hospitalization and death.68 Beddhu et al70 reported a similar association with higher levels of alkaline phosphatase in patients with CKD stages 3 and 4. Their findings suggested that independent of confounding variables such as liver function, serum phosphate and calcium, serum alkaline phosphatase was associated with increased risk of death in predialysis CKD patients. The role of high levels of alkaline phosphatase in the pathogenesis of vascular calcification was supported by a longitudinal study involving 134 stages 4 and 5 CKD patients.71 This 2 year prospective study revealed that higher levels of serum alkaline phosphatase were significantly associated with progressive vascular calcification.71 This relationship was independent of levels of serum fetuin-A, calcium, C-reactive protein and PTH. Table 3 summarizes the relationship between traditional markers of CKD-MBD (PTH, phosphate and calcium) and mortality.
Table 3.
The Relationship Between Traditional Markers of CKD-MBD (PTH, Phosphate and Calcium) and Mortality
| Author, Year | Study Design | Dialysis Modality | Risk of Mortality with Markers of CKD-MBD | |||||
|---|---|---|---|---|---|---|---|---|
| Calcium | Phosphate | PTH | ||||||
| Low | High | Low | High | Low | High | |||
| Block et al 200457 | Retrospective | HD | Decreased | Increased | NS | Increased | NS | Increased |
| Noordzij et al 200553 | Prospective | HD, PD | NS | NS | NS | Increased | NS | NS |
| Kalantar-Zadeh et al 200655 | Prospective | HD | Increased | Increased | Increased | Increased | Increased | Increased |
| Melamed 200663 | Prospective | HD, PD | NS | Increased | NS | Increased | NS | Increased |
| Tentori et al 200861 | Retrospective | HD | Increased | Increased | Increased | Increased | Increased | Increased |
| Block et al 201064 | Prospective | HD | NS | Increased | NS | Increased | NS | Increased |
| Floege et al 201160 | Prospective | HD | Increased | Increased | Increased | Increased | Increased | Increased |
| Soohoo et al 201656 | Retrospective | HD | NS | Increased | Increased | Increased | Increased | Increased |
| Waziri et al 201954 | Prospective | HD, PD | NS | Increased | NS | Increased | NS | Increased |
Abbreviations: HD, hemodialysis; PD, peritoneal dialysis; NS, not significant.
FGF23 and CKD Progression
Studies have shown a strong correlation between serum FGF23 levels and eGFR. As renal function declines FGF23 levels increase. In ESKD, FGF23 levels can be up to 1000-fold above the normal range, likely due to retained phosphate or decreased renal clearance.72 In a prospective study involving 177 non-diabetic patients with CKD stages 1–5, with a median follow-up period of 53 months, both serum intact FGF23 (iFGF 23) and c-terminal FGF23 levels (cFGF) above optimal cut-off levels predicted a doubling of serum creatinine and/or the need for renal replacement therapy, independent of eGFR, proteinuria, and other indices of mineral metabolism, such as calcium, phosphate and parathyroid hormone.73
Similarly, in a Brazilian prospective study comprising type 2 diabetes mellitus patients with macroalbuminuric nephropathy, iFGF23 was an independent predictor of the composite primary outcome defined as death, doubling of baseline serum creatinine and/or need for dialysis, even after adjustment for creatinine clearance and intact parathyroid hormone.74 One of the limitations of the two aforementioned studies was the relatively small sample size. However, this finding was confirmed in a large multicenter prospective study CRIC (Chronic Renal Insufficiency Cohort) involving 3879 CKD stages 2–4 patients with a median follow-up of 3.5 years; high cFGF23 levels were independently associated with poor renal outcome.75 These studies were largely carried out in Caucasians or African Americans.
FGF23, Mortality and Cardiovascular Outcome
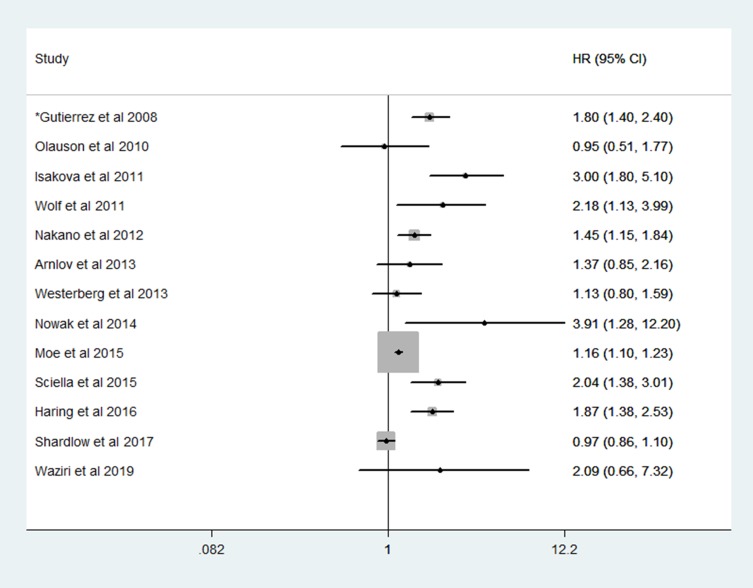
Studies evaluating the relationship between FGF23, all-cause and cardiovascular mortality in CKD, renal transplant and healthy population revealed mixed results. For example, while some studies have linked high levels of FGF23 with increased mortality,75–82 others revealed a lack of significant association.54,83–86 The findings from these studies are summarized in Figure 2.
Figure 2.
Hazard ratios of plasma fibroblast growth factor 23 with mortality by various studies.
Note: *Reported odd ratio.
In the homocysteine study (HOST), compared with patients with the lowest FGF23 levels, patients in the highest quartile of baseline FGF23 had a hazard ratio of 2.58 for future cardiovascular events in univariate analysis. High levels of FGF23 remained a significant predictor of cardiovascular outcome, while vitamin D, calcitriol and PTH did not. Regarding the composite cardiovascular endpoint, elevated FGF23 was an independent predictor of myocardial infarction and lower extremity amputation.87 These studies have also shown an association between elevated levels of FGF23 in CKD and left ventricular hypertrophy, vascular calcification and mortality.75,87
Management of CKD-MBD
The essence of CKD-MBD management is largely to prevent the adverse consequences associated with secondary hyperparathyroidism. Therefore, treatment of secondary hyperthyroidism is dependent on well-established measurable surrogate markers of disordered mineral bone metabolism.88 These markers are serum calcium, phosphate, intact parathyroid hormone and 25-hydroxyvitamin D. Thus, the current KDIGO guideline recommends treatment based on the serial trends of these biochemical markers.17
Although hyperphosphatemia has been linked to several adverse clinical outcomes, there is no evidence to show that normalization of phosphate levels improves patients’ outcomes. For instance, a recent randomized clinical trial revealed a significant decrease in serum phosphate and a non-significant decrease in the serum level of FGF23, and worsening of coronary calcification scores in patients that were given phosphate-lowering therapy.89 Hence, the updated KDIGO recommendation that prevention of hyperphosphatemia in patients with CKD stage G3a to G5D may be more important than treatment or normalization of phosphate levels.17
Prevention of hyperphosphatemia includes dietary restriction of phosphate, use of phosphate-lowering agents, and dialysis for patients with CKD stage G5D.
Phosphate Restriction
Efforts should be made to limit daily phosphate intake to less than 800 mg; this can be achieved by regulating the intake of high phosphate containing diets and carbonated beverages with phosphate additives.90 However, since most of the foods that are high in phosphate are also the major sources of protein, the nutritional status of these patients should be closely monitored to avoid malnutrition.
The dietary source of phosphate should be considered while making dietary recommendations. This is necessary since the intestinal absorptive capacity varied for different sources of the phosphate. The intestinal absorptive rate of inorganic phosphate as in additives and beverages is between 80% and 100%, while that of plant-based phosphate such as nuts is between 20% and 40%.90
Phosphate Binders
Phosphate binders are usually administered with meals to limit phosphate absorption from the gut by forming a non-absorbable complex with phosphate. The three main classes of phosphate binders are aluminium-based phosphate binders, Ca-based phosphate binders, and non-Ca-based phosphate binders.90 The long-term use of aluminum based phosphate binders has been limited by its associated side effects such as aluminium-induced osteomalacia and encephalopathy.91 The choice between the use of either calcium-containing or non-calcium-containing phosphate binders should be guided by serum levels of calcium and PTH of the patients. Overzealous use of calcium-based phosphate binders is linked to detrimental effects particularly in non-dialysis patients;89 for example, a previous study that compared calcium-containing phosphate binders with a non-calcium-based phosphate binder (sevelamer) in patients on maintenance hemodialysis showed that coronary artery calcification occurred more rapidly in patients on calcium-containing phosphate binders. In addition to reducing calcium overload, sevelamer was associated with lowering cholesterol and uric acid levels and has an anti-inflammatory effect.89 However, in another study, the mortality outcome between patients on calcium-based phosphate binders and sevelamer was comparable.92
Phosphate Removal Through Dialysis
Removal of phosphate through dialysis is dependent on the type of dialysis, session length and dialysate.
For a dialysis session length of 4 hrs at a frequency of 3 times per week, an estimated 2.3–2.6 g of phosphate will be removed per week. If the session length is increased to 8 hrs 3 times per week (as in nocturnal dialysis), the phosphate removal increases to 3.0–3.6 g per week. For patients on peritoneal dialysis, an estimated 2.0–2.2 g of phosphate will be removed per week for 4 times per day, 2 L exchanges.90,93
Treatment of Secondary Hyperparathyroidism
Data suggesting an optimal level for PTH for patients with CKD stage G3a to G5 are lacking. The updated KDIGO guidelines recommend that treatment of elevated levels of PTH should be based on serial values rather than a single high value, bearing in mind that the elevated PTH may represent a compensatory response to hyperphosphatemia and increasing bone resistance to PTH.17 The routine use of calcitriol and vitamin D analogues to prevent secondary hyperparathyroidism is being discouraged in non-dialysis CKD patients. This recommendation was partly based on two randomized control trials (RCTs) that reported no benefit in clinical outcomes with the use of calcitriol in patients with CKD stage G3a to G5.51,94 Both RCTs: Paricalcitol Capsule Benefits in Renal Failure-Induced Cardiac Morbidity (PRIMO) and Oral Paricalcitol in Stages 3–5 Chronic Kidney Disease (OPERA) reported no improvement in LVH or diastolic dysfunction with the use of Paricalcitol in patients with CKD stage G3a to G5.51,94 Hypercalcemia was common in patients on Paricalcitol compared to placebo group. For CKD stage G5D requiring reduction of PTH level, the updated 2017 guidelines recommend the use of calcimimetics, calcitriol, or vitamin D analogues, or a combination of calcimimetics with calcitriol or vitamin D analogues.17
The emergence of cinacalcet as an alternative in the treatment of secondary hyperparathyroidism was as a result of concerted efforts to avert hypercalcemia and hyperphosphatemia induced by the use of large doses of calcium-based phosphate binders in combination with calcitriol/vitamin D sterols.
Cinacalcet is a calcimimetic that controls the release of parathyroid hormone by increasing the sensitivity of the calcium-sensing receptor to extracellular calcium ions, hence lowering PTH levels immediately post-administration. It is initially given at a dose of 30 mg and titrated upwards according to response;88 parenteral cinacalcet (Etelcalcetide) is administered thrice weekly post-dialysis.95 Hypocalcemia is one of the side effects associated with cinacalcet and thus regular measurement of serum calcium and phosphate is indicated.
The commonly available vitamin D agents for the treatment of secondary hyperparathyroidism are calcitriol, paricalcitol and doxercalciferol.
Calcitriol is a vitamin D receptor activator (VDRA) that is given daily or thrice weekly to suppress PTH. However, due to the widespread availability of vitamin D receptors beside the parathyroid tissues, the use of calcitriol also enhances the absorption of calcium and phosphorus from the gut.96 This may lead to hypercalcemia and elevated phosphate levels that are detrimental to vascular tissues.96,97 To mitigate the hypercalcemia and hyperphosphatemia associated with oral calcitriol, a more receptor-selective injectable paricalcitol became available in 1998.96 The recommended starting dose for parenteral paricalcitol is 2–5 μg per dialysis session and subsequently titrated downwards to maintain at 1–2 µg per dialysis session based on PTH levels.15 It is recommended to discontinue VDRs if the PTH level is less than 100 pg/mL.90
It is noteworthy that no special recommendation was made regarding the choice of agents in the management of secondary hyperparathyroidism. Thus, calcimimetic, calcitriol, or vitamin D analogues are all considered as first-line options for lowering PTH in CKD stage 5D and the choice of which agent to use should be guided by serum levels of calcium, phosphate and PTH.17
Finally, although parathyroidectomy is recommended for patients with secondary hyperparathyroidism who did not respond to medical therapy,17 the optimal choice between subtotal parathyroidectomy (subtotal PTX) or total parathyroidectomy with auto-transplantation (total PTX-AT) is yet to be established. Both surgical options have been found to be effective and have comparable outcomes.98
Emerging Novel Treatment Options for CKD-MBD
In recent years, iron-based, calcium-free phosphate binders have been introduced into clinical practice as a new class of phosphate binders.99 These include sucroferric oxyhydroxide (PA21) and ferric citrate (JTT-751). Both PA21 (Vifor Pharma) and Ferric citrate (Auryxia, Keryx Biopharmaceuticals Inc., New York, USA) have been shown to be as effective as sevelamer carbonate in lowering serum phosphate in CKD 5D.100,101 In addition to their phosphate-lowering effect, they are also effective in correcting anemia in CKD and attenuating vascular calcification.101
Correcting klotho deficiency states through supplementation with exogenous recombinant klotho may serve as a prophylactic or therapeutic therapy for preventing or reversing secondary hyperparathyroidism. Similarly, the use of anti-FGF23 monoclonal antibodies (FGF23-Ab) to neutralize the detrimental effects of high levels of FGF23 was evaluated in animal models.102 Although neutralization of FGF23 was characterized by improvement in secondary hyperparathyroidism, it was associated with increased levels of serum phosphate, aortic calcification and higher risk of mortality.102 Thus, the therapeutic applicability of FGF23-Ab in humans is yet to be established.
The use of a monoclonal antibody or a ligand trap for ActRIIA to downregulate circulating Dkk1 has been shown to be effective in improving and reversing the skeletal and vascular sequelae of CKD-MBD at preclinical phase.38
Conclusion
CKD-MBD has continued to evolve over time with regards to its pathophysiology, diagnosis, adverse clinical outcomes and management. However, even though CKD-MBD is widely studied, gaps in knowledge still exist, prompting the need for more RCTs to assist in the management of CKD-MBD.
Abbreviations
ActRIIA, activin A receptor; b-ALP, bone specific alkaline phosphatase; BSP, bone sialoprotein; CaSRs, calcium-sensing receptors; CKD, chronic kidney disease; CKD-MBD, chronic kidney disease–mineral and bone disorder; CTX, C-terminal telopeptide of type I collagen; DM, diabetes mellitus; DOPPS, Dialysis Outcomes and Practice Patterns; eGFR, estimated glomerular filtration rate; ESAs, erythropoiesis-stimulating agents; ESKD, end-stage kidney disease; FGF23, fibroblast growth factor 23; HR, hazard ratio; iPTH, intact parathyroid hormone; KDIGO, Kidney Disease – Improving Global Outcomes; K/DOQI, Kidney Disease Outcomes Quality Initiative; MHD, maintenance haemodialysis; MDRD, modified Diet Renal Disease; MESA, multi ethnic study of atherosclerosis; NHANES, National Health and Nutrition Examination Survey; NKF, National Kidney Foundation; 1,25(OH)2D3, 1,25-dihydroxyvitamin D; 25(OH) D, 25-hydroxyvitamin D; OR, odds ratio; OC, osteocalcin; PINP, procollagen type I N propeptide; PICP, carboxy (C-) terminal propeptide; RCTs, randomized controlled trials; ROD, renal osteodystrophy; TAP, total alkaline phosphatase; TRAP 5b, tartrate resistant acid phosphatase; UK, United Kingdom; USA, United States of America; VDR, vitamin D receptor.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Eknoyan G, Lameire N, Barsoum R, et al. The burden of kidney disease: improving global outcomes. Kidney Int. 2004;66(4):1310–1314. doi: 10.1111/j.1523-1755.2004.00894.x [DOI] [PubMed] [Google Scholar]
- 2.Coresh J, Eustace J. Epidemiology of kidney disease In: Brenner B, editor. Brenner and Rector’s the Kidney. 8th ed. Philadelphia: Saunders-Elsevier; 2008:615–632. [Google Scholar]
- 3.Jadoul M, Albert JM, Akiba T, et al. Incidence and risk factors for hip or other bone fractures among hemodialysis patients in the dialysis outcomes and practice patterns study. Kidney Int. 2006;70(7):1358–1366. doi: 10.1038/sj.ki.5001754 [DOI] [PubMed] [Google Scholar]
- 4.Delanaye P, Souberbielle JC, Lafage-Proust MH, Jean G, Cavalier E. Can we use circulating biomarkers to monitor bone turnover in CKD haemodialysis patients? Hypotheses and facts. Nephrol Dial Transplant. 2014;29(5):997–1004. doi: 10.1093/ndt/gft275 [DOI] [PubMed] [Google Scholar]
- 5.Gal-Moscovici A, Sprague SM. Bone health in chronic kidney disease-mineral and bone disease. Adv Chronic Kidney Dis. 2007;14(1):27–36. doi: 10.1053/j.ackd.2006.10.010 [DOI] [PubMed] [Google Scholar]
- 6.Moe S, Drueke T, Cunningham J, et al. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: improving Global Outcomes (KDIGO). Kidney Int. 2006;69(11):1945–1953. doi: 10.1038/sj.ki.5000414 [DOI] [PubMed] [Google Scholar]
- 7.Lucas RC. On a form of late rickets associated with albuminuria. Lancet. 1883;1(3119):993–994. doi: 10.1016/S0140-6736(02)37965-0 [DOI] [Google Scholar]
- 8.Bauer W, Albright F, Aub JC. A case of osteitis fibrosa cystica (osteomalacia?) With evidence of hyperactivity of the para-thyroid bodies. Metabolic study II. J Clin Invest. 1930;8(2):229–248. doi: 10.1172/JCI100262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu SH, Chu HI. Treatment of renal osteodystrophy with dihydrotachysterol (A.T.10) and Iron. Science. 1942;95(2467):388–389. doi: 10.1126/science.95.2467.388 [DOI] [PubMed] [Google Scholar]
- 10.Deykin D, Balko C, Bricker NS. On the pathogenesis of the uremic state. An exposition of the “trade-off hypothesis”. N Engl J Med. 1972;286(20):1093–1099. doi: 10.1056/NEJM197205182862009 [DOI] [PubMed] [Google Scholar]
- 11.Slatopolsky E, Caglar S, Pennell JP, et al. On the pathogenesis of hyperparathyroidism in chronic experimental renal insufficiency in the dog. J Clin Invest. 1971;50(3):492–499. doi: 10.1172/JCI106517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drueke TB, Massy ZA. Changing bone patterns with progression of chronic kidney disease. Kidney Int. 2016;89(2):289–302. doi: 10.1016/j.kint.2015.12.004 [DOI] [PubMed] [Google Scholar]
- 13.Ward MK, Feest TG, Ellis HA, Parkinson IS, Kerr DN. Osteomalacic dialysis osteodystrophy: evidence for a water-borne aetiological agent, probably aluminium. Lancet. 1978;1(8069):841–845. doi: 10.1016/S0140-6736(78)90191-5 [DOI] [PubMed] [Google Scholar]
- 14.Andreoli SP, Bergstein JM, Sherrard DJ. Aluminum intoxication from aluminum-containing phosphate binders in children with azotemia not undergoing dialysis. N Engl J Med. 1984;310(17):1079–1084. doi: 10.1056/NEJM198404263101704 [DOI] [PubMed] [Google Scholar]
- 15.Eknoyan G, Levin A, Levin NW. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42(Suppl 4):S1–S201. doi: 10.1016/S0272-6386(03)00905-3 [DOI] [PubMed] [Google Scholar]
- 16.Vanbelleghem H, Vanholder R, Levin NW, et al. The kidney disease: improving global outcomes website: comparison of guidelines as a tool for harmonization. Kidney Int. 2007;71(10):1054–1061. doi: 10.1038/sj.ki.5002177 [DOI] [PubMed] [Google Scholar]
- 17.Ketteler M, Block GA, Evenepoel P, et al. Executive summary of the 2017 KDIGO Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) guideline update: what’s changed and why it matters. Kidney Int. 2017;92(1):26–36. doi: 10.1016/j.kint.2017.04.006 [DOI] [PubMed] [Google Scholar]
- 18.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int. 2009;113:S1–S130. [DOI] [PubMed] [Google Scholar]
- 19.Jindal K, Chan CT, Deziel C, et al. Hemodialysis clinical practice guidelines for the Canadian society of nephrology. J Am Soc Nephrol. 2006;17(3 Suppl 1):S1–S27. doi: 10.1681/ASN.2005121372 [DOI] [PubMed] [Google Scholar]
- 20.Guideline Working Group, Japanese Society for Dialysis Therapy. Clinical practice guideline for the management of secondary hyperparathyroidism in chronic dialysis patients. Ther Apher Dial. 2008;12(6):514–525. doi: 10.1111/j.1744-9987.2008.00648.x [DOI] [PubMed] [Google Scholar]
- 21.Burden R, Tomson C. Identification, management and referral of adults with chronic kidney disease: concise guidelines. Clin Med. 2005;5(6):635–642. doi: 10.7861/clinmedicine.5-6-635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moe SM, Drueke T, Lameire N, Eknoyan G. Chronic kidney disease-mineral-bone disorder: a new paradigm. Adv Chronic Kidney Dis. 2007;14(1):3–12. doi: 10.1053/j.ackd.2006.10.005 [DOI] [PubMed] [Google Scholar]
- 23.Gutierrez OM. Fibroblast growth factor 23 and disordered vitamin D metabolism in chronic kidney disease: updating the “trade-off” hypothesis. Clin J Am Soc Nephrol. 2010;5(9):1710–1716. doi: 10.2215/CJN.02640310 [DOI] [PubMed] [Google Scholar]
- 24.Laflamme GH, Jowsey J. Bone and soft tissue changes with oral phosphate supplements. J Clin Invest. 1972;51(11):2834–2840. doi: 10.1172/JCI107106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jowsey J, Reiss E, Canterbury JM. Long-term effects of high phosphate intake on parathyroid hormone levels and bone metabolism. Acta Orthop Scand. 1974;45(6):801–808. doi: 10.3109/17453677408989691 [DOI] [PubMed] [Google Scholar]
- 26.Isakova T, Wahl P, Vargas GS, et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011;79(12):1370–1378. doi: 10.1038/ki.2011.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Isakova T, Wolf MS. FGF23 or PTH: which comes first in CKD? Kidney Int. 2010;78(10):947–949. doi: 10.1038/ki.2010.281 [DOI] [PubMed] [Google Scholar]
- 28.Kuro-o M. Overview of the FGF23-klotho axis. Pediatr Nephrol. 2010;25(4):583–590. doi: 10.1007/s00467-009-1260-4 [DOI] [PubMed] [Google Scholar]
- 29.Miyamoto K, Ito M, Kuwahata M, Kato S, Segawa H. Inhibition of intestinal sodium-dependent inorganic phosphate transport by fibroblast growth factor 23. Ther Apher Dial. 2005;9(4):331–335. doi: 10.1111/tap.2005.9.issue-4 [DOI] [PubMed] [Google Scholar]
- 30.Pateinakis P, Papagiannni A. Fibroblast growth factor-23 and adverse clinical outcomes in chronic kidney disease patients. OA Nephrology. 2013;1(1):4. doi: 10.13172/2053-0293 [DOI] [Google Scholar]
- 31.Shimada T, Hasegawa H, Yamazaki Y, et al. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res. 2004;19(3):429–435. doi: 10.1359/JBMR.0301264 [DOI] [PubMed] [Google Scholar]
- 32.Lewis R. Mineral and bone disorders in chronic kidney disease: new insights into mechanism and management. Ann Clin Biochem. 2012;49(Pt 5):432–440. doi: 10.1258/acb.2012.012004 [DOI] [PubMed] [Google Scholar]
- 33.Kuro-o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390(6655):45–51. doi: 10.1038/36285 [DOI] [PubMed] [Google Scholar]
- 34.Sitara D, Razzaque MS, Hesse M, et al. Homozygous ablation of fibroblast growth factor-23 results in hyperphosphatemia and impaired skeletogenesis, and reverses hypophosphatemia in phex-deficient mice. Matrix Biol. 2004;23(7):421–432. doi: 10.1016/j.matbio.2004.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Canalejo R, Canalejo A, Martinez-Moreno JM, et al. FGF23 fails to inhibit uremic parathyroid glands. J Am Soc Nephrol. 2010;21(7):1125–1135. doi: 10.1681/ASN.2009040427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu MC, Kuro-o M, Moe OW. Renal and extrarenal actions of klotho. Semin Nephrol. 2013;33(2):118–129. doi: 10.1016/j.semnephrol.2012.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fang Y, Ginsberg C, Seifert M, et al. CKD-induced wingless/integration1 inhibitors and phosphorus cause the CKD-mineral and bone disorder. J Am Soc Nephrol. 2014;25(8):1760–1773. doi: 10.1681/ASN.2013080818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Agapova OA, Fang Y, Sugatani T, Seifert ME, Hruska KA. Ligand trap for the activin type IIA receptor protects against vascular disease and renal fibrosis in mice with chronic kidney disease. Kidney Int. 2016;89(6):1231–1243. doi: 10.1016/j.kint.2016.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams MJ, Sugatani T, Agapova OA, et al. The activin receptor is stimulated in the skeleton, vasculature, heart, and kidney during chronic kidney disease. Kidney Int. 2018;93(1):147–158. doi: 10.1016/j.kint.2017.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sugatani T. Systemic activation of activin a signaling causes chronic kidney disease-mineral bone disorder. Int J Mol Sci. 2018;19(9):2490. doi: 10.3390/ijms19092490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sherrard DJ, Hercz G, Pei Y, et al. The spectrum of bone disease in end-stage renal failure – an evolving disorder. Kidney Int. 1993;43(2):436–442. doi: 10.1038/ki.1993.64 [DOI] [PubMed] [Google Scholar]
- 42.Sprague SM, Bellorin-Font E, Jorgetti V, et al. Diagnostic accuracy of bone turnover markers and bone histology in patients with CKD treated by dialysis. Am J Kidney Dis. 2016;67(4):559–566. doi: 10.1053/j.ajkd.2015.06.023 [DOI] [PubMed] [Google Scholar]
- 43.Souberbielle JC, Roth H, Fouque DP. Parathyroid hormone measurement in CKD. Kidney Int. 2010;77(2):93–100. doi: 10.1038/ki.2009.374 [DOI] [PubMed] [Google Scholar]
- 44.Nussbaum SR, Zahradnik RJ, Lavigne JR, et al. Highly sensitive two-site immunoradiometric assay of parathyrin, and its clinical utility in evaluating patients with hypercalcemia. Clin Chem. 1987;33(8):1364–1367. [PubMed] [Google Scholar]
- 45.Souberbielle JC, Boutten A, Carlier MC, et al. Inter-method variability in PTH measurement: implication for the care of CKD patients. Kidney Int. 2006;70(2):345–350. doi: 10.1038/sj.ki.5001606 [DOI] [PubMed] [Google Scholar]
- 46.Gao P, Scheibel S, D’Amour P, et al. Development of a novel immunoradiometric assay exclusively for biologically active whole parathyroid hormone 1–84: implications for improvement of accurate assessment of parathyroid function. J Bone Miner Res. 2001;16(4):605–614. doi: 10.1359/jbmr.2001.16.4.605 [DOI] [PubMed] [Google Scholar]
- 47.Sprague SM, Moe SM. The case for routine parathyroid hormone monitoring. Clin J Am Soc Nephrol. 2013;8(2):313–318. doi: 10.2215/CJN.04650512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Monier-Faugere MC, Geng Z, Mawad H, et al. Improved assessment of bone turnover by the PTH-(1–84)/large C-PTH fragments ratio in ESRD patients. Kidney Int. 2001;60(4):1460–1468. doi: 10.1046/j.1523-1755.2001.00949.x [DOI] [PubMed] [Google Scholar]
- 49.Rudser KD, de Boer IH, Dooley A, Young B, Kestenbaum B. Fracture risk after parathyroidectomy among chronic hemodialysis patients. J Am Soc Nephrol. 2007;18(8):2401–2407. doi: 10.1681/ASN.2007010022 [DOI] [PubMed] [Google Scholar]
- 50.Hagstrom E, Hellman P, Larsson TE, et al. Plasma parathyroid hormone and the risk of cardiovascular mortality in the community. Circulation. 2009;119(21):2765–2771. doi: 10.1161/CIRCULATIONAHA.108.808733 [DOI] [PubMed] [Google Scholar]
- 51.Thadhani R, Appelbaum E, Pritchett Y, et al. Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease: the PRIMO randomized controlled trial. JAMA. 2012;307(7):674–684. doi: 10.1001/jama.2012.120 [DOI] [PubMed] [Google Scholar]
- 52.Seibel MJ. Biochemical markers of bone turnover: part I: biochemistry and variability. Clin Biochem Rev. 2005;26(4):97–122. [PMC free article] [PubMed] [Google Scholar]
- 53.Noordzij M, Korevaar JC, Boeschoten EW, et al. The Kidney Disease Outcomes Quality Initiative (K/DOQI) guideline for bone metabolism and disease in CKD: association with mortality in dialysis patients. Am J Kidney Dis. 2005;46(5):925–932. doi: 10.1053/j.ajkd.2005.08.013 [DOI] [PubMed] [Google Scholar]
- 54.Waziri B, Musenge E, Duarte R, et al. Associations of plasma fibroblast growth factor 23 and other markers of chronic kidney disease-mineral and bone disorder with all-cause mortality in South African patients on maintenance dialysis: a 3-year prospective cohort study. PLoS One. 2019;14(5):e0216656–e. doi: 10.1371/journal.pone.0216656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kalantar-Zadeh K, Kuwae N, Regidor DL, et al. Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int. 2006;70(4):771–780. doi: 10.1038/sj.ki.5001514 [DOI] [PubMed] [Google Scholar]
- 56.Soohoo M, Feng M, Obi Y, et al. Changes in markers of mineral and bone disorders and mortality in incident hemodialysis patients. Am J Nephrol. 2016;43(2):85–96. doi: 10.1159/000444890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Block GA, Klassen PS, Lazarus JM, et al. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15(8):2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2 [DOI] [PubMed] [Google Scholar]
- 58.Lowrie EG, Lew NL. Death risk in hemodialysis patients: the predictive value of commonly measured variables and an evaluation of death rate differences between facilities. Am J Kidney Dis. 1990;15(5):458–482. doi: 10.1016/S0272-6386(12)70364-5 [DOI] [PubMed] [Google Scholar]
- 59.Avram MM, Mittman N, Myint MM, Fein P. Importance of low serum intact parathyroid hormone as a predictor of mortality in hemodialysis and peritoneal dialysis patients: 14 years of prospective observation. Am J Kidney Dis. 2001;38(6):1351–1357. doi: 10.1053/ajkd.2001.29254 [DOI] [PubMed] [Google Scholar]
- 60.Floege J, Kim J, Ireland E, et al. Serum iPTH, calcium and phosphate, and the risk of mortality in a European haemodialysis population. Nephrol Dial Transplant. 2011;26(6):1948–1955. doi: 10.1093/ndt/gfq219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tentori F, Blayney MJ, Albert JM, et al. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2008;52(3):519–530. doi: 10.1053/j.ajkd.2008.03.020 [DOI] [PubMed] [Google Scholar]
- 62.Waziri B, Duarte R, Naicker S. High serum alkaline phosphatase, hypercalcaemia, race, and mortality in South African maintenance haemodialysis patients. Int J Nephrol. 2017;2017:2795432. doi: 10.1155/2017/2795432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Melamed ML, Eustace JA, Plantinga L, et al. Changes in serum calcium, phosphate, and PTH and the risk of death in incident dialysis patients: a longitudinal study. Kidney Int. 2006;70(2):351–357. doi: 10.1038/sj.ki.5001542 [DOI] [PubMed] [Google Scholar]
- 64.Block GA, Zaun D, Smits G, et al. Cinacalcet hydrochloride treatment significantly improves all-cause and cardiovascular survival in a large cohort of hemodialysis patients. Kidney Int. 2010;78(6):578–589. doi: 10.1038/ki.2010.167 [DOI] [PubMed] [Google Scholar]
- 65.London GM, Marchais SJ, Guérin AP, et al. Association of bone activity, calcium load, aortic stiffness, and calcifications in ESRD. J Am Soc Nephrol. 2008;19(9):1827–1835. doi: 10.1681/ASN.2007050622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Noordzij M, Cranenburg EM, Engelsman LF, et al. Progression of aortic calcification is associated with disorders of mineral metabolism and mortality in chronic dialysis patients. Nephrol Dial Transplant. 2011;26(5):1662–1669. doi: 10.1093/ndt/gfq582 [DOI] [PubMed] [Google Scholar]
- 67.Block GA, Hulbert-Shearon TE, Levin NW, Port FK. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis. 1998;31(4):607–617. doi: 10.1053/ajkd.1998.v31.pm9531176 [DOI] [PubMed] [Google Scholar]
- 68.Blayney MJ, Pisoni RL, Bragg-Gresham JL, et al. High alkaline phosphatase levels in hemodialysis patients are associated with higher risk of hospitalization and death. Kidney Int. 2008;74(5):655–663. doi: 10.1038/ki.2008.248 [DOI] [PubMed] [Google Scholar]
- 69.Beddhu S, Baird B, Ma X, Cheung AK, Greene T. Serum alkaline phosphatase and mortality in hemodialysis patients. Clin Nephrol. 2010;74(08):91–96. doi: 10.5414/CNP74091 [DOI] [PubMed] [Google Scholar]
- 70.Beddhu S, Ma X, Baird B, Cheung AK, Greene T. Serum alkaline phosphatase and mortality in African Americans with chronic kidney disease. Clin J Am Soc Nephrol. 2009;4(11):1805–1810. doi: 10.2215/CJN.01560309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sigrist MK, Taal MW, Bungay P, McIntyre CW. Progressive vascular calcification over 2 years is associated with arterial stiffening and increased mortality in patients with stages 4 and 5 chronic kidney disease. Clin J Am Soc Nephrol. 2007;2(6):1241–1248. doi: 10.2215/CJN.02190507 [DOI] [PubMed] [Google Scholar]
- 72.Gutierrez O, Isakova T, Rhee E, et al. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol. 2005;16(7):2205–2215. doi: 10.1681/ASN.2005010052 [DOI] [PubMed] [Google Scholar]
- 73.Fliser D, Kollerits B, Neyer U, et al. Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) study. J Am Soc Nephrol. 2007;18(9):2600–2608. doi: 10.1681/ASN.2006080936 [DOI] [PubMed] [Google Scholar]
- 74.Titan SM, Zatz R, Graciolli FG, et al. FGF-23 as a predictor of renal outcome in diabetic nephropathy. Clin J Am Soc Nephrol. 2011;6(2):241–247. doi: 10.2215/CJN.04250510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Isakova T, Xie H, Yang W, et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011;305(23):2432–2439. doi: 10.1001/jama.2011.826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gutierrez OM, Mannstadt M, Isakova T, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359(6):584–592. doi: 10.1056/NEJMoa0706130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nowak A, Friedrich B, Artunc F, et al. Prognostic value and link to atrial fibrillation of soluble Klotho and FGF23 in hemodialysis patients. PLoS One. 2014;9(7):e100688. doi: 10.1371/journal.pone.0100688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wolf M, Molnar MZ, Amaral AP, et al. Elevated fibroblast growth factor 23 is a risk factor for kidney transplant loss and mortality. J Am Soc Nephrol. 2011;22(5):956–966. doi: 10.1681/ASN.2010080894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nakano C, Hamano T, Fujii N, et al. Intact fibroblast growth factor 23 levels predict incident cardiovascular event before but not after the start of dialysis. Bone. 2012;50(6):1266–1274. doi: 10.1016/j.bone.2012.02.634 [DOI] [PubMed] [Google Scholar]
- 80.Haring R, Enserro D, Xanthakis V, et al. Plasma fibroblast growth factor 23: clinical correlates and association with cardiovascular disease and mortality in the framingham heart study. J Am Heart Assoc. 2016;5:7. doi: 10.1161/JAHA.116.003486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Scialla JJ, Parekh RS, Eustace JA, et al. Race, mineral homeostasis and mortality in patients with end-stage renal disease on dialysis. Am J Nephrol. 2015;42(1):25–34. doi: 10.1159/000438999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moe SM, Chertow GM, Parfrey PS, et al. Cinacalcet, fibroblast growth factor-23, and cardiovascular disease in hemodialysis: the evaluation of cinacalcet HCl therapy to lower cardiovascular events (EVOLVE) Trial. Circulation. 2015;132(1):27–39. doi: 10.1161/CIRCULATIONAHA.114.013876 [DOI] [PubMed] [Google Scholar]
- 83.Olauson H, Qureshi AR, Miyamoto T, et al. Relation between serum fibroblast growth factor-23 level and mortality in incident dialysis patients: are gender and cardiovascular disease confounding the relationship? Nephrol Dial Transplant. 2010;25(9):3033–3038. doi: 10.1093/ndt/gfq191 [DOI] [PubMed] [Google Scholar]
- 84.Westerberg PA, Tivesten A, Karlsson MK, et al. Fibroblast growth factor 23, mineral metabolism and mortality among elderly men (Swedish MrOs). BMC Nephrol. 2013;14:85. doi: 10.1186/1471-2369-14-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Arnlov J, Carlsson AC, Sundstrom J, et al. Higher fibroblast growth factor-23 increases the risk of all-cause and cardiovascular mortality in the community. Kidney Int. 2013;83(1):160–166. doi: 10.1038/ki.2012.327 [DOI] [PubMed] [Google Scholar]
- 86.Shardlow A, McIntyre NJ, Fluck RJ, McIntyre CW, Taal MW. Associations of fibroblast growth factor 23, vitamin D and parathyroid hormone with 5-year outcomes in a prospective primary care cohort of people with chronic kidney disease stage 3. BMJ Open. 2017;7(8):e016528. doi: 10.1136/bmjopen-2017-016528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kendrick J, Cheung AK, Kaufman JS, et al. FGF-23 associates with death, cardiovascular events, and initiation of chronic dialysis. J Am Soc Nephrol. 2011;22(10):1913–1922. doi: 10.1681/ASN.2010121224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tomasello S. Secondary hyperparathyroidism and chronic kidney disease. Diabetes Spectr. 2008;21(1):19–25. doi: 10.2337/diaspect.21.1.19 [DOI] [Google Scholar]
- 89.Block GA, Wheeler DC, Persky MS, et al. Effects of phosphate binders in moderate CKD. J Am Soc Nephrol. 2012;23(8):1407–1415. doi: 10.1681/ASN.2012030223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hwang E, Choi BS, Oh KH, Kwon YJ, Kim GH. Management of chronic kidney disease-mineral and bone disorder: Korean working group recommendations. Kidney Res Clin Pract. 2015;34(1):4–12. doi: 10.1016/j.krcp.2015.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Alfrey AC. Aluminum toxicity in patients with chronic renal failure. Ther Drug Monit. 1993;15(6):593–597. doi: 10.1097/00007691-199312000-00025 [DOI] [PubMed] [Google Scholar]
- 92.St Peter WL, Liu J, Weinhandl E, Fan Q. A comparison of sevelamer and calcium-based phosphate binders on mortality, hospitalization, and morbidity in hemodialysis: a secondary analysis of the Dialysis Clinical Outcomes Revisited (DCOR) randomized trial using claims data. Am J Kidney Dis. 2008;51(3):445–454. doi: 10.1053/j.ajkd.2007.12.002 [DOI] [PubMed] [Google Scholar]
- 93.Cupisti A, Gallieni M, Rizzo MA, et al. Phosphate control in dialysis. Int J Nephrol Renovasc Dis. 2013;6:193–205. doi: 10.2147/IJNRD.S35632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang AY, Fang F, Chan J, et al. Effect of paricalcitol on left ventricular mass and function in CKD–the OPERA trial. J Am Soc Nephrol. 2014;25(1):175–186. doi: 10.1681/ASN.2013010103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Alexander ST, Hunter T, Walter S, et al. Critical cysteine residues in both the calcium-sensing receptor and the allosteric activator AMG 416 underlie the mechanism of action. Mol Pharmacol. 2015;88(5):853–865. doi: 10.1124/mol.115.098392 [DOI] [PubMed] [Google Scholar]
- 96.Cheng S, Coyne D. Paricalcitol capsules for the control of secondary hyperparathyroidism in chronic kidney disease. Expert Opin Pharmacother. 2006;7(5):617–621. doi: 10.1517/14656566.7.5.617 [DOI] [PubMed] [Google Scholar]
- 97.Goldsmith D, Ritz E, Covic A. Vascular calcification: a stiff challenge for the nephrologist: does preventing bone disease cause arterial disease? Kidney Int. 2004;66(4):1315–1333. doi: 10.1111/j.1523-1755.2004.00895.x [DOI] [PubMed] [Google Scholar]
- 98.Anderson K Jr, Ruel E, Adam MA, et al. Subtotal vs. total parathyroidectomy with autotransplantation for patients with renal hyperparathyroidism have similar outcomes. Am J Surg. 2017;214(5):914–919. doi: 10.1016/j.amjsurg.2017.07.018 [DOI] [PubMed] [Google Scholar]
- 99.Locatelli F, Del Vecchio L. Iron-based phosphate binders: a paradigm shift in the treatment of hyperphosphatemic anemic CKD patients? J Nephrol. 2017;30(6):755–765. doi: 10.1007/s40620-017-0421-y [DOI] [PubMed] [Google Scholar]
- 100.Floege J, Covic AC, Ketteler M, et al. A phase III study of the efficacy and safety of a novel iron-based phosphate binder in dialysis patients. Kidney Int. 2014;86(3):638–647. doi: 10.1038/ki.2014.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yang WC, Yang CS, Hou CC, et al. An open-label, crossover study of a new phosphate-binding agent in haemodialysis patients: ferric citrate. Nephrol Dial Transplant. 2002;17(2):265–270. doi: 10.1093/ndt/17.2.265 [DOI] [PubMed] [Google Scholar]
- 102.Shalhoub V, Shatzen EM, Ward SC, et al. FGF23 neutralization improves chronic kidney disease-associated hyperparathyroidism yet increases mortality. J Clin Invest. 2012;122(7):2543–2553. doi: 10.1172/JCI61405 [DOI] [PMC free article] [PubMed] [Google Scholar]