Abstract
Polycystic ovary syndrome (PCOS) is a common infertility disorder affecting a significant proportion of the global population. It is the main cause of anovulatory infertility in women and is the most common endocrinopathy affecting reproductive-aged women, with a prevalence of 8–13% depending on the criteria used and population studied. The disease is multifactorial and complex and, therefore, often difficult to diagnose due to overlapping symptoms. Multiple etiological factors have been implicated in PCOS. Due to the complex pathophysiology involving multiple pathways and proteins, single genetic diagnostic tests cannot be determined. Progress has been achieved in the management and diagnosis of PCOS; however, not much is known about the molecular players and signaling pathways underlying it. Conclusively PCOS is a polygenic and multifactorial syndromic disorder. Many genes have been associated with PCOS, which affect fertility either directly or indirectly. However, studies conducted on PCOS patients from multiple families failed to find a fully penetrant variant(s). The present study was designed to review the current genetic understanding of the disease. In the present review, we have discussed the clinical spectrum, the genetics, and the variants identified as being associated with PCOS. The mechanisms by which variants in the genes confer risk to PCOS and the nature of the physical and genetic interaction between the genetic elements underlying PCOS remain to be determined. Elucidation of genetic players and cellular pathways underlying PCOS will certainly increase our understanding of the pathophysiology of this syndrome. The study also discusses the current status of the treatment modalities for PCOS, which is important to find new ways of treatment.
Keywords: PCOS, infertility, genetics, treatment
Introduction
Polycystic ovary syndrome (PCOS) is a common endocrine disorder in females, especially in women of reproductive age. The worldwide prevalence of PCOS is estimated to be 5–10%.1 PCOS could be diagnosed by infertility, acne, amenorrhea or oligomenorrhea, hirsutism, insulin resistance, obesity, hyperandrogenism, and polycystic ovaries by ultrasonography.2,3 Association of PCOS with infertility is well studied and is thought to be responsible for 40% of female infertility.4 Moreover, it is a leading cause of endometrial carcinoma.2 Besides reproductive abnormalities, PCOS is also strongly associated with a wide range of metabolic disorders, such as hepatic steatosis, glucose intolerance, dyslipidemia, diabetes mellitus type II (T2DM), and hypertension.5
Polycystic ovary syndrome (PCOS) typically manifests with a combination of menstrual dysfunction and hyperandrogenism in the adolescent population. It is associated with derangements in insulin secretion and action, androgen synthesis and action, relative gonadotropin ratios, ovulatory function, and balance of pro- and antioxidant systems. PCOS is a lifelong disorder and the metabolic and reproductive ramifications of this syndrome are well documented in all stages of life. Women with PCOS are frequently obese with distinct abdominal or central obesity, which in turn aggravates insulin resistance. These women are at a higher risk of developing impaired glucose tolerance (IGT), T2DM, dyslipidemia, cardiovascular diseases, hypertension, and ultimately metabolic syndrome in their later lives.6 Apart from physiological maladies, studies have suggested that women with PCOS often show symptoms of negative body image perception, low self-esteem, depression, and decreased quality of life.7 PCOS is a multifactorial disorder where individual genes, gene–gene interaction, or gene–environment interactions have been reported to influence predisposition to PCOS development. Previously, the literature has reported the importance of genetic predisposition to PCOS development; however, no consensus has been reached on an established genetic marker for PCOS. Identifying causal variants in genes that may alter its expression or subsequent protein function helps to delineate the genetic architecture of this multifactorial disorder.8 Tissue-specific epigenetic alterations that do not affect the genetic code have been reported to be responsible for phenotypic plasticity and are mainly achieved by mechanisms involving the addition or removal of chemical groups in chromatin.9 Further, proteome profiling of pathophysiologically relevant tissues helps to elucidate the dynamic changes in the cellular proteome.10 Exploring genetic and epigenetic patterns with their consequent impact on protein profiles has contributed toward biomarker discovery and unraveling the molecular pathophysiology of PCOS. Thus, employing a multipronged approach and harnessing cutting-edge technologies has yielded copious and comprehensive data, which in turn have expanded and shaped the current understanding of PCOS etiology. Insight into this condition as well its far-reaching consequences will provide both researchers and clinicians new avenues into understanding the association between the various facets of this syndrome and treating this hormonal imbalance successfully and restoring fertility.
The presence of chronic anovulation and hyperandrogenism is required to establish a PCOS diagnosis.11 Recently, the diagnostic criteria have been extended and four clinical characteristics have been added to establish PCOS diagnosis.12 This expanded the definition of PCOS.
Inheritance of PCOS
Polycystic ovarian syndrome is a multifactorial disease affecting a significant number of people around the world. Individuals with PCOS face multiple social and stress-induced issues; therefore, various aspects of PCOS were studied to reach a definite conclusion. Historically, in 1972, infertile women with tiny, shiny ovaries were identified.13 Later, in 1844 another study on the degenerative ovaries was reported.14 Studies continued and different aspects such as cellular mechanism, hormonal involvement, environmental risk factors, and genetic determinants of PCOS were studied. The genetic basis of PCOS was first reported by Cooper and colleagues in 1968.15 Studies on PCOS reported multiple relatives and siblings in families with autosomal dominant inheritance. The prevalence of PCOS in the first-degree relative of the proband that was found in nearly 55–60% in several small families supported the hypothesis of autosomal dominant inheritance of PCOS. Later on, monogenic causes of hirsutism and oligomenorrhea in PCOS women and male-pattern baldness were identified.16
Twin studies in small cohorts of mono- and dizygotic twin pairs suggested that PCOS is neither an autosomal dominant nor a monogenic disease; rather, it is an X-linked polygenic disorder.17,18 Moreover, twin studies estimated 72% variance in risk of PCOS to be genetic in basis, highlighting the genetic involvement.19
Incidence and Prevalence
PCOS is diagnosed by criteria set by the National Institutes of Health (NIH) in 1990 and the estimation of the prevalence of PCOS depends on how many of the criteria were followed. The prevalence of PCOS was estimated in different populations, primarily in Caucasian and black races, and was estimated to be nearly 4.0% in both races.20 In a subsequent study involving 400 women (age range 18–45 years) it was estimated to be 6.6% in both black and white populations, while a significant difference was noted in white and black females, i.e., 8% and 4%, respectively.21 In Greek women, the prevalence estimated in females seeking help in free medical camps was 6.8%. A study conducted in Spanish Caucasians estimated the prevalence to be 6.8%.22 In a study conducted at Oxford University and a private medical center, a 6.8% prevalence of PCOS was estimated.23 The prevalence of PCOS in Chinese women of reproductive age is 5.6%.24 This prevalence is nearly similar to the other populations. PCOS is also quite prevalent in Indian females of reproductive age, at nearly 9.13%.25 The prevalence of PCOS is significantly higher in the South Asian population, especially in Pakistan, as compared to Caucasians. Akram and Roohiet al. (2015) reported a higher prevalence of PCOS (~50%) according to Rotterdam criteria 2003.26 Similarly, Zahida et al. (2010) reported a 40% prevalence of PCOS among infertile women seeking medical advice in Karachi, Pakistan.27
Clinical Description of PCOS
As the name indicates, the disease involves ovaries with many cysts. It is caused by a hormonal imbalance, which is further indicated by an irregular menstrual cycle, many cysts in the ovaries, amenorrhea, and hirsutism in adult females. PCOS is a multifactorial disease that primarily causes infertility and thus creates social imbalance.
Subcellular aberrations in theca cells are caused by elevated levels of androgen in the patient with PCOS. In theca cells of PCOS patients, a high level of androgen is secreted due to the intrinsic activation of theca cell steroidogenesis despite the absence of trophic factors.28 The granulosa cells are also affected by this intrinsic activation, which leads to elevated levels of serum anti-mullerian hormone in PCOS patients as compared to healthy women.29–31
Elevated pre-antral and small antral numbers of follicles in PCOS have also been reported in multiple studies.32 In maturing follicles, a defective apoptotic process further increases the number of follicles in PCOS women.33
The defect in the insulin signaling pathway independent of obesity is also due to intrinsic aberration in PCOS.34 Similarly, alteration in the insulin gene expression pathway has also been attributed to PCOS.35 Another pathway of glyco-oxidative stress has been reported as an underlying pathology of PCOS.36 Insulin resistance can also be elicited by oxidative stress, which further causes hypergonadism.37
PCOS is a complex disease and syndromic in pathology as the name indicates. The disease is multifactorial and often of variable symptoms. The disease is grouped into four phenotypes as discussed below.
Classification Based on Phenotype
Four phenotypes can be observed in PCOS: phenotype A, phenotype B, phenotype C, and phenotype D. In general, it appears that the presence of hyperandrogenism (HA), body mass index (BMI),38 and degree of menstrual irregularity,39 but not ovarian morphology, is independent predictors of metabolic dysfunction. PCOS is classified into four phenotypes as:
By ultrasound, numerous polycystic ovaries, oligoanovulation, and hyperandrogenism.
By ultrasound, normal appearance of ovaries, oligoanovulation, and hyperandrogenism.
By ultrasound, polycystic ovaries with normal routine menses and hyperandrogenism.
By ultrasound, polycystic ovaries, oligoanovulation, without hyperandrogenism.
PCOS Phenotypes A and B
PCOS phenotypes A and B are termed classic PCOS. Women with the classic PCOS phenotypes A and B present with more significant menstrual dysfunction, increased secretion of insulin, increased insulin resistance, and increased risk for metabolic syndrome. A high incidence of obesity and atherogenic dyslipidemia (AD) is prevalent in classic PCOS as compared to other PCOS phenotypes.40 An increased risk of hepatic steatosis is also more common in PCOS phenotypes A and B in comparison with normal healthy controls and other phenotypes of PCOS with normal androgen.41 The classic PCOS is also characterized by a significantly elevated level of anti-mullerian hormone.42
Phenotype C – Ovulatory PCOS
Mildly elevated serum insulin, atherogenic lipids, and androgen levels, and high hirsutism scores are common in phenotype C (ovulatory PCOS) patients in comparison to classic and non-hyperandrogenic PCOS. Metabolic syndromes are also common ovulatory PCOS as compared to other types.43 In an Italian cohort of PCOS patients, the ovulatory phenotype was reported among individuals with higher socioeconomic status.44 In high socioeconomic groups, tissue fat distribution and insulin level due to eating habits could partially explain the difference in ovulation patterns.
Phenotype D – Nonhyperandrogenic PCOS
The classical characteristics of phenotype D include normal androgen with slightly elevated other endocrine levels and the lowest metabolic dysfunction45,46 in comparison to healthy controls.47,48 The endocrine finding includes an elevated level of sex hormone-binding globulin, a low level of T3 and T4 and a lower LH/FSH ratio in comparison to individuals with classic PCOS.49 Normally, individuals with PCOS phenotype D have regular menstrual cycles with intermittent irregularities.50
In fact, PCOS is a complex and multifactorial disease and not all investigators agree with the classifications. In German PCOS patients presented with different PCOS phenotypes, no significant differences in insulin resistance, dyslipidemia, or BMI were observed.51 Similar nonsignificant differences among different PCOS phenotypes were also reported in Greek women with PCOS.52 In the Greek study, insulin resistance was observed in individuals with a BMI of more than 25 kg/m2.52 Similarly, no significant variation in metabolic syndrome among women with PCOS from Sri Lanka and Brazil was reported.53,54 However, an elevated level of low-density lipoprotein (LDL) in ovulatory PCOS individuals from a nonhyperandrogenic Turkish PCOS cohort was reported,55 although the difference in androgen level could be attributed to the substandard estimation method of androgen, which leads to misclassification.56
Different diagnostic criteria have been employed in categorizing PCOS. The NIH criteria accept only phenotypes A and B,11 the Rotterdam consensus recognized all four categories of phenotypes,57 whereas the AES-PCOS declared the phenotypic categories A, B, and C.58
The most frequently identified phenotype is A, with 44–65%, followed by B with 8–33%, phenotype C with 3–29%, and D with 0–23%.43,48
Genetics of PCOS
PCOS is an extremely heterogenetic and complex disease. The genetic basis of PCOS is different between families and within families but is related to a common pathway. Due to complexity and heterogeneity single gene or related genes in a single family have not been reported.
The genetic susceptibility of different genes is different in patients from the same family.59 Recently, intrauterine programming as a susceptibly factor has been hypothesized for PCOS.60 Genome screening to search for a candidate gene in a complex disease like PCOS is unrealistic. Linkage analysis in such families always comes up with negative results. In such a disease, case-control studies of larger population size and genome-wide association studies (GWAS) are helpful to find possible associations. Parental analysis in such diseases is often impractical; however, known risk of disease can be estimated.61
Mutations
Many studies have been conducted in multiple families to find the causative gene/mutation, but no true penetrance of a single gene mutation has been reported until now. All genes/mutation s reported in familial aggregation show low penetrance associated with other covariant, hormonal or environmental factors to cause disease. Conclusively, PCOS is a polygenic and multifactorial syndromic disorder.
Variations Associated with PCOS
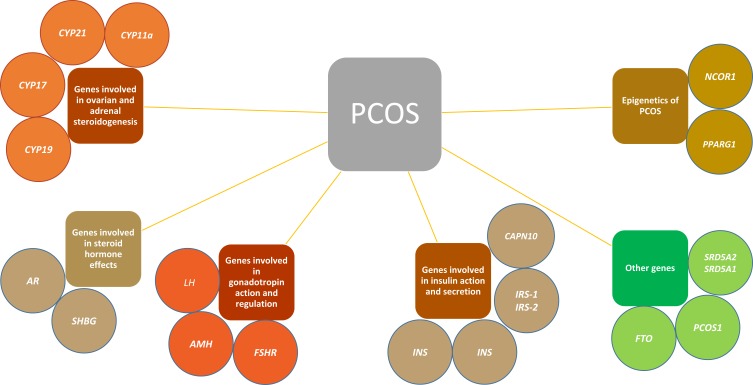
PCOS is a multifactorial disease and is caused by a number of abnormalities. All genes/mutations that affect ovaries directly or indirectly are associated with PCOS. An overview of the genetic picture of PCOS is depicted in Figure 1. These groups of related genes and their roles in PCOS are discussed below in detail.
Figure 1.
Summary of the genes involved in PCOS highlights the complexity of the disease.
Genes Involved in Ovarian and Adrenal Steroidogenesis
The most common endocrine disorder associated with PCOS is an elevated androgen level. Hence, in uncovering the reason for the elevated level of androgen, several genes have been reported to be associated with PCOS, as follows.
CYP11a
The gene CYP11a encodes an enzyme that is required in an intermediary step of cholesterol conversion to progesterone. This is a rate-limiting step in the conversion of cholesterol.62 Further more, Gharani et al. reported polymorphs and variation as associated factors in a study of 97 infertile women.63 Two other studies from China and Greece replicated the finding and reported CYP11a to be an association factor with PCOS.64,65 Later on, a large study conducted in the UK did not replicate the results.66
CYP21
In the synthesis of steroid hormones, the conversion of 17-hydroxyprogesterone to 11-deoxycortisol is catalyzed by an enzyme that is encoded by CYP21. A less-active enzyme due to variation leads to ineffective anabolism of steroidogenesis, which further causes PCOS.67 Witchel et al. reported heterozygous CYP21 associated with PCOS-like disease with hyperandrogenemia.67 Direct association of CYP21 variation with PCOS failed in 114 women with PCOS.68
CYP17
The conversion of pregnenolone and progesterone into 17-hydroxypregnenolone and 17-hydroxyprogesterone is catalyzed by an enzyme (P450c17α) that is encoded by CYP17.69 Rosenfield et al. reported elevated androgen levels in PCOS patients.70 Wickenheisser et al. reported increased expression of CYP17 in theca cells.71 Carey et al. reported a polymorph in the promoter region that is associated with PCOS.72
CYP19
The CYP19 gene responsible for aromatase p450, necessary for the formation of estrogen, lies on chromosome number 15q21.2.73 Lower activity of aromatase activity is reported in both obese and lean women with PCOS.
Genes Involved in Steroid Hormone Effects
Androgen Receptor Gene
The “q” arm of chromosome X contains the AR gene, which is composed of 11 exons and encodes a 90-kb long tridomain protein.74 Mutations and structural disruption of the gene are reported to cause PCOS. “X” chromosome inactivation leads to disruption of the cellular pathway, causing elevated androgen hormone resulting in PCOS. As the AR gene is located on the X chromosome, a change in a single copy of the gene is sufficient to cause pathology. GWAS also reported a novel variation in the gene to be the cause of PCOS.75
Sex Hormone-Binding Globulin Gene
The SHBG gene is localized to chromosome 17p13-p12. SHBG synthesizes a protein of 373 amino acids. The protein product of SHBG controls the level of sex hormones in the body by binding to androgens, predominantly with estrogens and testosterone.76,77 Most of the SHBG is synthesized by hepatocytes in the liver. SHBG synthesis by hepatocytes is controlled by multiple metabolic factors, such as androgens and insulin.78–80 Concentrations of SHBG are lower in females with PCOS, which has been mainly attributed to an inhibitory consequence of hyperinsulinemia on SHBG synthesis.79 Single nucleotide polymorphism in the SHBG gene was described to be significantly associated with PCOS in numerous studies.81,82
Genes Involved in Gonadotropin Action and Regulation
Lutein Hormone (LH) and Its Receptor Gene
Both LH level and distorted function of LH are frequently reported as a cause of PCOS. These abnormalities cause annulations, thus producing PCOS.83
A high level of LH subsequently enhances the production of androgen.84 The negative feedback in response to elevated LH is reduced follicle-stimulating hormone (FSH), which may play an indirect role as a result of the decreased transfer of androgen to estrogen and exacerbating the excess androgen in the ovaries.85 The gene encoding for both LH and its receptor has been studied in PCOS to find the possible association. Initially, a point mutation (Trp8Arg and Ilg15Thr) in a gene encoding the B subunit was reported in patients with PCOS.86 The same mutation was reported to be nonpathogenic and is found in 15% of the normal population.87 Polymorphism in the LH β-subunit gene has also been reported to be associated with PCOS.88
AMH
The AMH gene is located on the long arm of chromosome 19 at cytogenetic location 13.3. The gene consists of five exons89 and encodes a protein that is involved in infertility. Variation in the AMH gene is associated with PCOS. Whole exome-sequencing and GWAS reported several variants of the AMH gene as strong predictors of PCOS.90
Follicular Stimulating Hormone Receptor (FSHR)
FSHR is located on the “p” arm of chromosome 2 and consists of 14 exons. The gene encodes a protein G-coupled receptor, which is required for gonad development.91 Mutation in the gene disrupts the structural protein, thus causing an imbalance of the hormone. Hormonal imbalance causes PCOS. A comparison of polymorphs in healthy and affected individuals in north Iraq showed a higher frequency of the gene among the affected individuals.92
Genes Involved in Insulin Action and Secretion
The Insulin Gene
Insulin also plays a significant role in the production of androgen by receptors present on theca cells.85 This act of insulin is provoked through the pathway (phosphoinositide 3-kinase/protein kinase B), which becomes active in PCOS theca cells.93 Similar to LH, a high level of insulin also further enhances the synthesis of androgens.94,95 The INS is a sandwich gene at 11p15.5 between tyrosine hydroxylase and IGF-II.96 The 5′ untranslated region is occupied by a tandem repeat of VNTR.97 The transcriptional rate of INS and IGF-II is regulated by VNTR polymorphism.98 The number of repeats of VNTR ranges from 26 to 200. This VNTR polymorphism is associated with PCOS.99
INSR
This gene encodes turmeric protein composed of two alphas and two beta chains.100 Several studies were conducted to find the association of infertility in obese women with PCOS, but they did not find any association.101 Conway et al. also studied the tyrosine kinase domain-encoding region of INSR in women with PCOS.102 Talbot et al. scanned the entire gene in women with PCOS.103 Neither of these studies found any association between PCOS and INSR. A larger part of chromosome 19p13.2 was searched and D19S884 was reported as the strongest association with PCOS.104 Thise region of the chromosome also contains the INSR gene.
Insulin Receptor Substrate Proteins
Insulin binds with its receptor. Activation of the receptor is autophosphorylated by the binding of insulin. Subsequently, the tyrosine kinase activity of INS receptor phosphorylates IRS-1 and IRS-2.34 These activated substrates are then further utilized in the downstream process. Several studies have been performed to find out the association in the genes of IRS-1 and IRS-2 and PCOS. Petermann et al. reported a higher frequency of Arg972 IRS-1 in women with PCOS,105 while El Mkadem et al. reported no significant difference between the mentioned mutation in PCOS patients and controls.106 Dilek et al. reported a much higher frequency of Gly972Arg in IRS-1 in Turkish women with PCOS.107 Reports of both association and no association have been reported. These differences highlight environmental and ethnic involvement.
Calpain10 Gene
CAPN10 is located on the long arm of “q” of chromosome 2 and consists of 12 exons. The gene encodes calcium-dependent cysteine protease, which is a heterodimeric protein. The gene is associated with diabetes mellitus type 1.108 The protein “calpain 10” interferes with insulin metabolism and secretion. The impaired insulin level causes PCOS; thus, a mutation in calpain 10 also causes PCOS. Hence, CAPN10 is a candidate gene causing PCOS.109
Other Genes
Fat Mass Obesity (FTO)
The FTO gene encodes an enzyme, alpha-ketoglutarate. The gene is located on the “q” arm of chromosome 16. The gene is reported as an association for obesity and T2DM.110
In a study conducted in Pakistani women with PCOS, single nucleotide polymorphism (SNP) rs9939609 was significantly associated with diseases. The SNP rs9939609 was significantly higher in affected women as compared to healthy participants of the study.111
PCOS1
The cytogenetic location of PCOS1 is 19p13.2. The gene has been associated with PCOS in several studies. The gene is also known as PCO and it is actually a susceptibility region on chromosome 19. Initially, it was identified in two sisters in 1971. Later on, the study was replicated in 2005 by Urbanek et al.112
SRD5A2 and SRD5A1
The cytogenetic location of SRD5A2 is 2p23.1. Increased activity of SRD5A among women with PCOS as compared to normal females was reported in 1999 by Jakimiuk et al.113 Later in 2006,SRD5A2 and SRD5A1 were tested as susceptibility to PCOS in hirsutism patients, and it was reported that a variant in SRD5A2 was associated with protection to PCOS while variants in SRD5A1 wereassociated with risk of hirsutism and thus PCOS.114
Epigenetics of PCOS
Heritable changes in gene expression that are not due to a sequence change of DNA but that are transgenerationally and mitotically heritable are known as epigenetic changes. Epigenetic involvement of many diseases has been reported, such as T2DM,115 PCOS,116 and prostate cancer.117 Higher secretion of androgens in fetal life has been reported to cause diseases in rats,118 monkeys,119 and sheep models. The symptoms of diseases are similar to PCOS. Although it is ethically restricted to use humans for experimentation, certain studies have shown that an increased level of androgen during fetal life predisposes the offspring to PCOS-like symptoms in later stages.120 Qu et al. reported a differential CPG island methylation in PPARG1, NCOR1 of granulosa cells that causes hyperandrogenism-induced epigenetic alteration, and thus the development of ovarian dysfunction.121 Ning Xu et al. reported an altered DNA methylation pattern in PCOS patients as compared to control groups.116
This epigenetic involvement of PCOS highlights the complexity of the disease.
Current State of Treatment
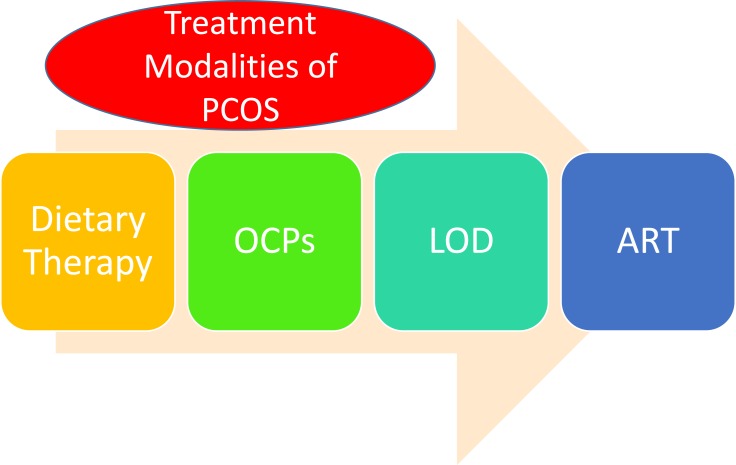
PCOS is not a curable disease; however, to assist females seeking conception, treatment modalities are available. The treatment options are highlighted in Figure 2 and are discussed in detail below.
Figure 2.
Summary of treatment modalities of PCOS.
Dietary Therapy
Obesity has been reported in 30% of PCOS patients. The symptoms of PCOS are also considered to be recovered by dietary therapies including resistance to insulin, annulations, and irregular menstrual cycle.122 However, dieting habit and exercise does not show long0term results; hence, bariatric surgery has been introduced to get more promising results.123
Oral Contraceptive Pills (OCPs)
Combined oral contraceptive pills (OCPs) are considered the choice of treatment in treating PCOS. These drugs regulate multiple endocrine abnormalities including hirsutism and acne.124 OCPs are safer compared to other therapies because of the low risk of endometrial cancer.125 The OCPs include an amalgamation of progestogen and estrogen, which increases SHBG which decreases LH and FSH, which in turn decreases free T and ovarian androgen production.126 Hence, a low dose of progestogen is recommended in OCPs. Some adverse effects have also been reported with OCPs, i.e., hyperglycemia, impaired glucose metabolism, insulin resistance, and diabetes mellitus127.
Laparoscopic Ovarian Drilling (LOD)
When clomiphene citrate therapy fails to produce ovulation, other methods of ovulation are used. LOD was used for ovulation in 1984 when ovarian wedge resection surgery failed. LOD is successful in 84% of patients and improves ovarian androgen production insulin resistance128 and increases the SHBG levels.129 Lower rates of miscarriages have been reported with LOD.130 Serum anti-mullerian hormone (AMH) level is used as an assessment tool for women treated with LOD.131 However, more studies are required to assess further the efficacy of LOD.
Assisted Reproductive Technology (ART)
Several methods are used for the treatment of fertility in PCOS patients. ART is the most frequently used. In this method, the ovaries are stimulated with exogenous gonadotropin which produces multiple follicles. However, exogenous gonadotropin causes ovarian hyperstimulation syndrome (OHSS) in these patients.132 Due to the use of this treatment modality, in vitro maturation (IVM) is used. Many studies have been conducted to assess the efficacy of ART compared to traditional IVF techniques. Results are comparable in both traditional IVF and IVM-IVF.133
Future Perspectives in the Treatment
Currently, COPs are used to treat PCOS as the first0line treatment. These include multiple medicines that increase ovulation. Combinations of medicines are used to treat the underlying associated pathology, which increases the chances of conception.134 However, the adverse effects of these medicines initiate multiple pathologies, such as cardiac pathology, diabetes mellitus, and depression.135
To reduce the risk of associated pathologies, interventional procedures were adopted. These procedures include IVF, IVM fertilization, and laparoscopic drilling. These procedures are also accompanied by a number of secondary diseases.136
Keeping in view the pathophysiology of PCOS as a multifactorial disease and the problems encountered in treating the disease, it is now suggested that a team composed of an endocrinologist, a physician, a gynecologist, and a reproductive medicine specialist will help these patients.
Author Contributions
Sulman Basit conceptualized the study idea and reviewed the manuscript; Anwar Ullah and Muhammad Jaseem Khan drafted the manuscript. All authors read and approved the final manuscript. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests in this work.
References
- 1.Kovanci E, Buster JE. Polycystic ovary syndrome. Clin Gynecol. 2015. Second Edition. [Google Scholar]
- 2.Hardiman P, Pillay OS, Atiomo W. Polycystic ovary syndrome and endometrial carcinoma. Lancet. 2003;361:1810–1812. doi: 10.1016/S0140-6736(03)13409-5 [DOI] [PubMed] [Google Scholar]
- 3.Diamanti-Kandarakis E, Piperi C, Spina J, et al. Polycystic ovary syndrome: the influence of environmental and genetic factors. Hormones (Athens). 2006;5:17–34. doi: 10.14310/horm.2002.1149 [DOI] [PubMed] [Google Scholar]
- 4.Krysiak R, Okopie B, Gdula-Dymek A, Herman ZS. Update on the management of polycystic ovary syndrome. Pharmacol Rep. 2006;58:614. [PubMed] [Google Scholar]
- 5.Liu AL, Xie HJ, Xie HY, et al. Association between fat mass and obesity associated (FTO) gene rs9939609 A/T polymorphism and polycystic ovary syndrome: a systematic review and meta-analysis. BMC Med Genet. 2017;18. doi: 10.1186/s12881-017-0452-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teede H, Deeks A, Moran L. Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010;8. doi: 10.1186/1741-7015-8-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deeks AA, Gibson-Helm ME, Paul E, Teede HJ. Is having polycystic ovary syndrome a predictor of poor psychological function including anxiety and depression? Hum Reprod. 2011;26:1399–1407. doi: 10.1093/humrep/der071 [DOI] [PubMed] [Google Scholar]
- 8.Lowe WL, Reddy TE. Genomic approaches for understanding the genetics of complex disease. Genome Res. 2015;25:1432–1441. doi: 10.1101/gr.190603.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feinberg AP. Phenotypic plasticity and the epigenetics of human disease. Nature. 2007;447:433–440. doi: 10.1038/nature05919 [DOI] [PubMed] [Google Scholar]
- 10.Hanash S. Disease proteomics. Nature. 2003;422: 226. [DOI] [PubMed] [Google Scholar]
- 11.Zawadzki JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rationale approach. Polycystic Ovary Syndrome. 1992. [Google Scholar]
- 12.Crespo RP, Bachega TASS, Mendonça BB, Gomes LG. An update of genetic basis of PCOS pathogenesis. Arch Endocrinol Metab. 2018;62:352–361. doi: 10.20945/2359-3997000000049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Insler V, Lunenfeld B. Polycystic ovarian disease: a challenge and controversy. Gynecol Endocrinol. 1990;4:51–70. doi: 10.3109/09513599009030691 [DOI] [PubMed] [Google Scholar]
- 14.Chereau. Mémoires Pour Servir À L’étude Des Maladies Des Ovaires. Paris: Fortin, Mason Et Cie, Libraires-Editeurs.1844. [Google Scholar]
- 15.Cooper HE, Spellacy WN, Prem KA, Cohen WD. Hereditary factors in the Stein-Leventhal syndrome. Am J Obstet Gynecol. 1968;100:371–387. doi: 10.1016/S0002-9378(15)33704-2 [DOI] [PubMed] [Google Scholar]
- 16.Carey AH, Chan KL, Short F, White D, Williamson R, Franks S. Evidence for a single gene effect causing polycystic ovaries and male pattern baldness. Clin Endocrinol (Oxf). 1993;38:653–658. doi: 10.1111/j.1365-2265.1993.tb02150.x [DOI] [PubMed] [Google Scholar]
- 17.Jahanfar S, Eden JA, Warren P, Seppala M, Nguyen TV. A twin study of polycystic ovary syndrome. Fertil Steril. 1995;63:478–486. [PubMed] [Google Scholar]
- 18.Jahanfar S, Eden JA, Nguyen T, Wang XL, Wilcken DEL. A twin study of polycystic ovary syndrome and lipids. Gynecol Endocrinol. 1997;11:111–117. doi: 10.3109/09513599709152521 [DOI] [PubMed] [Google Scholar]
- 19.Vink JM, Sadrzadeh S, Lambalk CB, Boomsma DI. Heritability of polycystic ovary syndrome in a Dutch twin-family study. J Clin Endocrinol Metab. 2006;91(6):2100–2104. doi: 10.1210/jc.2005-1494 [DOI] [PubMed] [Google Scholar]
- 20.Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R. Prevalence of the polycystic ovary syndrome in unselected black and white women of the Southeastern United States: a prospective study. J Clin Endocrinol Metab. 1998;83:3078–3082. [DOI] [PubMed] [Google Scholar]
- 21.Chan JL, Kar S, Vanky E, et al. Racial and ethnic differences in the prevalence of metabolic syndrome and its components of metabolic syndrome in women with polycystic ovary syndrome: a regional cross-sectional study. Am J Obstet Gynecol. 2017;217:189.e1–189.e8. doi: 10.1016/j.ajog.2017.04.007 [DOI] [PubMed] [Google Scholar]
- 22.Asunción M, Calvo RM, N JL SM, Sancho J, Avila S, Escobar-Morreale HF. A prospective study of the prevalence of the polycystic ovary syndrome in unselected Caucasian women from Spain. J Clin Endocrinol Metab. 2000;85:2434–2438. [DOI] [PubMed] [Google Scholar]
- 23.Michelmore KF, Balen AH, Dunger DB, Vessey MP. Polycystic ovaries and associated clinical and biochemical features in young women. Clin Endocrinol (Oxf). 1999;51:779–786. doi: 10.1046/j.1365-2265.1999.00886.x [DOI] [PubMed] [Google Scholar]
- 24.Li R, Zhang Q, Yang D, et al. Prevalence of polycystic ovary syndrome in women in China: a large community-based study. Hum Reprod. 2013;28:2562–2569. [DOI] [PubMed] [Google Scholar]
- 25.Nidhi R, Padmalatha V, Nagarathna R, Amritanshu R. Prevalence of polycystic ovarian syndrome in Indian adolescents. J Pediatr Adolesc Gynecol. 2011;24:223–227. doi: 10.1016/j.jpag.2011.03.002 [DOI] [PubMed] [Google Scholar]
- 26.Akram M, Roohi N. Endocrine correlates of polycystic ovary syndrome in Pakistani women. J Coll Physicians Surg Pakistan. 2015;25:22–26. [PubMed] [Google Scholar]
- 27.Baqai Z, Khanam M, Parveen S. Prevalence of PCOS in infertile patients. Med Chanel. 2010;16:255–260. [Google Scholar]
- 28.Nelson VL, Legro RS, Strauss JF, McAllister JM. Augmented androgen production is a stable steroidogenic phenotype of propagated theca cells from polycystic ovaries. Mol Endocrinol. 2014;13:946–957. [DOI] [PubMed] [Google Scholar]
- 29.Pellatt L, Hanna L, Brincat M, et al. Granulosa cell production of anti-Müllerian hormone is increased in polycystic ovaries. J Clin Endocrinol Metab. 2007;92:240–245. doi: 10.1210/jc.2006-1582 [DOI] [PubMed] [Google Scholar]
- 30.Azziz R, Carmina E, Dewailly D, et al. The androgen excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91:456–488. doi: 10.1016/j.fertnstert.2008.06.035 [DOI] [PubMed] [Google Scholar]
- 31.Villarroel C, Merino PM, López P, et al. Polycystic ovarian morphology in adolescents with regular menstrual cycles is associated with elevated anti-Mllerian hormone. Hum Reprod. 2011;26:2861–2868. doi: 10.1093/humrep/der223 [DOI] [PubMed] [Google Scholar]
- 32.Webber LJ, Stubbs S, Stark J, et al. Formation and early development of follicles in the polycystic ovary. Lancet. 2003;362:1017–1021. doi: 10.1016/S0140-6736(03)14410-8 [DOI] [PubMed] [Google Scholar]
- 33.Das M, Djahanbakhch O, Hacihanefioglu B, et al. Granulosa cell survival and proliferation are altered in polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93:881–887. doi: 10.1210/jc.2007-1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev. 1997;7:345–349. [DOI] [PubMed] [Google Scholar]
- 35.Cortón M, Botella-Carretero JI, Benguría A, et al. Differential gene expression profile in omental adipose tissue in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92:328–337. doi: 10.1210/jc.2006-1665 [DOI] [PubMed] [Google Scholar]
- 36.González F, Rote NS, Minium J, Kirwan JP. Reactive oxygen species-induced oxidative stress in the development of insulin resistance and hyperandrogenism in polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:336–340. doi: 10.1210/jc.2005-1696 [DOI] [PubMed] [Google Scholar]
- 37.Victor VM, Rocha M, Bañuls C, et al. Mitochondrial complex I impairment in leukocytes from polycystic ovary syndrome patients with insulin resistance. J Clin Endocrinol Metab. 2009;94:3505–3512. doi: 10.1210/jc.2009-0466 [DOI] [PubMed] [Google Scholar]
- 38.Ehrmann DA, Liljenquist DR, Kasza K, et al. Prevalence and predictors of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:48–53. doi: 10.1210/jc.2005-1329 [DOI] [PubMed] [Google Scholar]
- 39.Brower M, Brennan K, Pall M, Azziz R. The severity of menstrual dysfunction as a predictor of insulin resistance in pcos. J Clin Endocrinol Metab. 2013;98:E1967–E1971. doi: 10.1210/jc.2013-2815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lizneva D, Suturina L, Walker W, Brakta S, Gavrilova-Jordan L, Azziz R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil Steril. 2016;106:6–15. doi: 10.1016/j.fertnstert.2016.05.003 [DOI] [PubMed] [Google Scholar]
- 41.Goverde AJ, Van Koert AJB, Eijkemans MJ, et al. Indicators for metabolic disturbances in anovulatory women with polycystic ovary syndrome diagnosed according to the Rotterdam consensus criteria. Hum Reprod. 2009;24:710–717. [DOI] [PubMed] [Google Scholar]
- 42.Sahmay S, Atakul N, Oncul M, Tuten A, Aydogan B, Seyisoglu H. Serum anti-mullerian hormone levels in the main phenotypes of polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2013;170:157–161. doi: 10.1016/j.ejogrb.2013.05.019 [DOI] [PubMed] [Google Scholar]
- 43.Guastella E, Longo RA, Carmina E. Clinical and endocrine characteristics of the main polycystic ovary syndrome phenotypes. Fertil Steril. 2010;94:2197–2201. doi: 10.1016/j.fertnstert.2010.02.014 [DOI] [PubMed] [Google Scholar]
- 44.Di Fede G, Mansueto P, Longo RA, Rini G, Carmina E. Influence of sociocultural factors on the ovulatory status of polycystic ovary syndrome. Fertil Steril. 2009;91:1853–1856. doi: 10.1016/j.fertnstert.2008.02.161 [DOI] [PubMed] [Google Scholar]
- 45.Zhang HY, Zhu FF, Xiong J, Shi XB, Fu SX. Characteristics of different phenotypes of polycystic ovary syndrome based on the Rotterdam criteria in a large-scale Chinese population. BJOG an Int J Obstet Gynaecol. 2009;116:1633–1639. doi: 10.1111/j.1471-0528.2009.02347.x [DOI] [PubMed] [Google Scholar]
- 46.Dewailly D, Catteau-Jonard S, Reyss A-C, Leroy M, Pigny P. Oligoanovulation with polycystic ovaries but not overt hyperandrogenism. J Clin Endocrinol Metab. 2006;91:3922–3927. doi: 10.1210/jc.2006-1054 [DOI] [PubMed] [Google Scholar]
- 47.Zhuang J, Liu Y, Xu L, et al. Prevalence of the polycystic ovary syndrome in female residents of chengdu, china. Gynecol Obstet Invest. 2014;77:217–223. doi: 10.1159/000358485 [DOI] [PubMed] [Google Scholar]
- 48.Yilmaz M, Isaoglu U, Delibas IB, Kadanali S. Anthropometric, clinical and laboratory comparison of four phenotypes of polycystic ovary syndrome based on Rotterdam criteria. J Obstet Gynaecol Res. 2011;37:1020–1026. doi: 10.1111/jog.2011.37.issue-8 [DOI] [PubMed] [Google Scholar]
- 49.Jamil AS, Alalaf SK, Al-Tawil NG, Al-Shawaf T. Comparison of clinical and hormonal characteristics among four phenotypes of polycystic ovary syndrome based on the Rotterdam criteria. Arch Gynecol Obstet. 2016;293:447–456. doi: 10.1007/s00404-015-3889-5 [DOI] [PubMed] [Google Scholar]
- 50.Panidis D, Tziomalos K, Papadakis E, et al. Associations of menstrual cycle irregularities with age, obesity and phenotype in patients with polycystic ovary syndrome. Hormones. 2015. doi: 10.14310/horm.2002 [DOI] [PubMed] [Google Scholar]
- 51.Cupisti S, Haeberle L, Schell C, et al. The different phenotypes of polycystic ovary syndrome: no advantages for identifying women with aggravated insulin resistance or impaired lipids. Exp Clin Endocrinol Diabetes. 2011;119:502–508. doi: 10.1055/s-0031-1277136 [DOI] [PubMed] [Google Scholar]
- 52.Panidis D, Tziomalos K, Misichronis G, et al. Insulin resistance and endocrine characteristics of the different phenotypes of polycystic ovary syndrome: a prospective study. Hum Reprod. 2012;27:541–549. doi: 10.1093/humrep/der418 [DOI] [PubMed] [Google Scholar]
- 53.Wijeyaratne CN, Seneviratne RDA, Dahanayake S, et al. Phenotype and metabolic profile of South Asian women with polycystic ovary syndrome (PCOS): results of a large database from a specialist endocrine clinic. Hum Reprod. 2011;26:202–213. doi: 10.1093/humrep/deq310 [DOI] [PubMed] [Google Scholar]
- 54.Melo AS, Vieira CS, Romano LGM, Ferriani RA, Navarro PA. The frequency of metabolic syndrome is higher among PCOS Brazilian women with menstrual irregularity plus hyperandrogenism. Reprod Sci. 2011;18:1230–1236. doi: 10.1177/1933719111414205 [DOI] [PubMed] [Google Scholar]
- 55.Ates S, Sevket O, Sudolmus S, et al. Different phenotypes of polycystic ovary syndrome in Turkish women: clinical and endocrine characteristics. Gynecol Endocrinol. 2013;29:931–935. doi: 10.3109/09513590.2013.819082 [DOI] [PubMed] [Google Scholar]
- 56.Rosner W, Auchus RJ, Azziz R, Sluss PM, Raff H. Position statement: utility, limitations, and pitfalls in measuring testosterone: an endocrine society position statement. J Clin Endocrinol Metab. 2007;92:405–413. doi: 10.1210/jc.2006-1864 [DOI] [PubMed] [Google Scholar]
- 57.PCOS Consensus Workshop Group TRE-SPCWG. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81(1):19–25. doi: 10.1016/j.fertnstert.2003.10.004 [DOI] [PubMed] [Google Scholar]
- 58.Azziz R, Carmina E, Dewailly D, et al. Position statement: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an androgen excess society guideline. J Clin Endocrinol Metab. 2006;91:4237–4245. doi: 10.1210/jc.2006-0178 [DOI] [PubMed] [Google Scholar]
- 59.Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nature Rev Endocrinol. 2011;7:219–231. doi: 10.1038/nrendo.2010.217 [DOI] [PubMed] [Google Scholar]
- 60.Abbott DH, Dumesic DA, Franks S. Developmental origin of polycystic ovary syndrome - A hypothesis. J Endocrinol. 2002;174:1–5. doi: 10.1677/joe.0.1740001 [DOI] [PubMed] [Google Scholar]
- 61.Urbanek M, Legro RS, Driscoll DA, et al. Thirty-seven candidate genes for polycystic ovary syndrome: strongest evidence for linkage is with follistatin. Proc Natl Acad Sci U S A. 1999;96:8573–8578. doi: 10.1073/pnas.96.15.8573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Franks S, Gilling-Smith C, Gharani N, McCarthy M. Pathogenesis of polycystic ovary syndrome: evidence for a genetically determined disorder of ovarian androgen production. Hum Fertil. 2000;3:77–79. doi: 10.1080/1464727002000198731 [DOI] [PubMed] [Google Scholar]
- 63.Gharani N, Waterworth DM, Batty S, et al. Association of the steroid synthesis gene CYP11a with polycystic ovary syndrome and hyperandrogenism. Hum Mol Genet. 1997;6:397–402. doi: 10.1093/hmg/6.3.397 [DOI] [PubMed] [Google Scholar]
- 64.Diamanti-Kandarakis E, Bartzis MI, Bergiele AT, Tsianateli TC, Kouli CR. Microsatellite polymorphism (tttta)(n) at −528 base pairs of gene CYP11α influences hyperandrogenemia in patients with polycystic ovary syndrome. Fertil Steril. 2000;73:735–741. doi: 10.1016/S0015-0282(99)00628-7 [DOI] [PubMed] [Google Scholar]
- 65.Wang Y, Wu XK, Cao Y, Yi L, Chen J. A microsatellite polymorphism (tttta)n in the promoter of the CYP11a gene in Chinese women with polycystic ovary syndrome. Fertil Steril. 2006;86:223–226. [DOI] [PubMed] [Google Scholar]
- 66.Gaasenbeek M, Powell BL, Sovio U, et al. Large-scale analysis of the relationship between CYP11A promoter variation, polycystic ovarian syndrome, and serum testosterone. J Clin Endocrinol Metab. 2004;89:2408–2413. doi: 10.1210/jc.2003-031640 [DOI] [PubMed] [Google Scholar]
- 67.Witchel SF, Aston CE. The role of heterozygosity for CYP21 in the polycystic ovary syndrome. J Pediatr Endocrinol Metab. 2000;13:1315–1317. [PubMed] [Google Scholar]
- 68.Witchel SF, Kahsar-Miller M, Aston CE, White C, Azziz R. Prevalence of CYP21 mutations and IRS1 variant among women with polycystic ovary syndrome and adrenal androgen excess. Fertil Steril. 2005;83:371–375. doi: 10.1016/j.fertnstert.2004.10.027 [DOI] [PubMed] [Google Scholar]
- 69.Picado-Leonard J, Miller WL. Cloning and sequence of the human gene for P450cl7 (Steroid 17α-hydroxylase/17,20 Lyase): similarity with the gene for P450c21. DNA. 1987;6:439–448. doi: 10.1089/dna.1987.6.439 [DOI] [PubMed] [Google Scholar]
- 70.Rosenfield RL, Barnes RB, Cara JF, Lucky AW. Dysregulation of cytochrome P450c17α as the cause of polycystic ovarian syndrome. Fertil Steril. 1990;53:785–791. doi: 10.1016/S0015-0282(16)53510-9 [DOI] [PubMed] [Google Scholar]
- 71.Wickenheisser JK, Quinn PG, Nelson VL, Legro RS, Strauss JF, Mcallister JM. Differential activity of the cytochrome P450 17α-hydroxylase and steroidogenic acute regulatory protein gene promoters in normal and polycystic ovary syndrome theca cells. J Clin Endocrinol Metab. 2000;85:2304–2311. [DOI] [PubMed] [Google Scholar]
- 72.Carey AH, Waterworth D, Patel K, et al. Polycystic ovaries and premature male pattern baldness are associated with one allele of the steroid metabolism gene CYP17. Hum Mol Genet. 1994;3:1873–1876. doi: 10.1093/hmg/3.10.1873 [DOI] [PubMed] [Google Scholar]
- 73.Bulun SE, Takayama K, Suzuki T, Sasano H, Yilmaz B, Sebastian S Organization of the human aromatase p450 (CYP19) gene. Seminars in Reproductive Medicine; 2004;22(1):5–9. doi: 10.1055/s-2004-823022 [DOI] [PubMed] [Google Scholar]
- 74.Gottlieb B, Beitel LK, Nadarajah A, Paliouras M, Trifiro M. The androgen receptor gene mutations database: 2012 update. Hum Mutat. 2012;33:887–894. doi: 10.1002/humu.22046 [DOI] [PubMed] [Google Scholar]
- 75.Urbanek M. The genetics of the polycystic ovary syndrome. Nature Clin Pract Endocrinol Metab. 2007;3:103–111. doi: 10.1038/ncpendmet0400 [DOI] [PubMed] [Google Scholar]
- 76.Bérubé D, Séralini GE, Gagné R, Hammond GL. Localization of the human sex hormone-binding globulin gene (SHBG) to the short arm of chromosome 17 (17p12→p13). Cytogenet Genome Res. 1990;54:65–67. [DOI] [PubMed] [Google Scholar]
- 77.Hammond GL. Molecular properties of corticosteroid binding globulin and the sex-steroid binding proteins. Endocr Rev. 1990;11:65–79. doi: 10.1210/edrv-11-1-65 [DOI] [PubMed] [Google Scholar]
- 78.Edmunds SEJ, Stubbs AP, Santos AA, Wilkinson ML. Estrogen and androgen regulation of sex hormone binding globulin secretion by a human liver cell line. J Steroid Biochem Mol Biol. 1990;37:733–739. doi: 10.1016/0960-0760(90)90358-R [DOI] [PubMed] [Google Scholar]
- 79.Nestler JE, Powers LP, Matt DW, et al. A direct effect of hyperinsulinemia on serum sex hormone-binding globulin levels in obese women with the polycystic ovary syndrome. J Clin Endocrinol Metab. 1991;72:83–89. doi: 10.1210/jcem-72-1-83 [DOI] [PubMed] [Google Scholar]
- 80.Plymate SR, Matej LA, Jones RE, Friedl KE. Inhibition of sex hormone-binding globulin production in the human hepatoma (Hep G2) cell line by insulin and prolactin. J Clin Endocrinol Metab. 1988;67:460–464. doi: 10.1210/jcem-67-3-460 [DOI] [PubMed] [Google Scholar]
- 81.Wickham EP, Ewens KG, Legro RS, Dunaif A, Nestler JE, Strauss JF. Polymorphisms in the SHBG gene influence serum SHBG levels in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2011;96:E719–E727. doi: 10.1210/jc.2010-1842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen C, Smothers J, Lange A, Nestler JE, Strauss Iii JF, Wickham Iii EP. Sex hormone-binding globulin genetic variation: associations with type 2 diabetes mellitus and polycystic ovary syndrome. Minerva Endocrinol. 2010;35(4):271–280. [PMC free article] [PubMed] [Google Scholar]
- 83.Franks S, Stark J, Hardy K. Follicle dynamics and anovulation in polycystic ovary syndrome. Hum Reprod Update. 2008;14:539. doi: 10.1093/humupd/dmn028 [DOI] [PubMed] [Google Scholar]
- 84.Pigny P, Merlen E, Robert Y, et al. Elevated Serum level of anti-mullerian hormone in patients with polycystic ovary syndrome: relationship to the ovarian follicle excess and to the follicular arrest. J Clin Endocrinol Metab. 2003;88:5957–5962. doi: 10.1210/jc.2003-030727 [DOI] [PubMed] [Google Scholar]
- 85.Nardo LG, Patchava S, Laing I. Polycystic ovary syndrome: pathophysiology, molecular aspects and clinical implications. Panminerva Med. 2008;50:267–278. [PubMed] [Google Scholar]
- 86.Furui K, Suganuma N, Tsukahara SI, et al. Identification of two point mutations in the gene coding luteinizing hormone (LH) β-subunit, associated with immunologically anomalous LH variants. J Clin Endocrinol Metab. 1994;78:107–113. [DOI] [PubMed] [Google Scholar]
- 87.Nilsson C, Matzuk MM, Pettersson K, et al. Worldwide frequency of a common genetic variant of luteinizing hormone: an international collaborative research. Fertil Steril. 1997;67:998–1004. doi: 10.1016/S0015-0282(97)81430-6 [DOI] [PubMed] [Google Scholar]
- 88.Roy AC, Liao W-X, Chen Y, Arulkumaran S, Ratnam SS. Identification of seven novel mutations in LH ?-Subunit gene by SSCP. Mol Cell Biochem. 1996;165(2):151–153. doi: 10.1007/BF00229477 [DOI] [PubMed] [Google Scholar]
- 89.Cate RL, Mattaliano RJ, Hession C, et al. Isolation of the bovine and human genes for müllerian inhibiting substance and expression of the human gene in animal cells. Cell. 1986;45:685–698. doi: 10.1016/0092-8674(86)90783-X [DOI] [PubMed] [Google Scholar]
- 90.Gorsic LK, Kosova G, Werstein B, et al. Pathogenic anti-Müllerian hormone variants in polycystic ovary syndrome. J Clin Endocrinol Metab. 2017;102:2862–2872. doi: 10.1210/jc.2017-00612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gromoll J, Simoni M. Genetic complexity of FSH receptor function. Trends Endocrinol Metab. 2005;16:368–373. doi: 10.1016/j.tem.2005.05.011 [DOI] [PubMed] [Google Scholar]
- 92.Baban ASS, Korsheed SH, Al Hayawi AY. The FSHR polymorphisms association with polycystic ovary syndrome in women of Erbil, Kurdistan in North of Iraq. Ibn AL- Haitham J Pure Appl Sci. 2018;262. doi: 10.30526/2017.IHSCICONF.1799 [DOI] [Google Scholar]
- 93.Munir I, Yen H-W, Geller DH, et al. Insulin augmentation of 17α-Hydroxylase activity is mediated by phosphatidyl inositol 3-kinase but not extracellular signal-regulated kinase-1/ 2 in human ovarian theca cells. Endocrinology. 2004;145:175–183. doi: 10.1210/en.2003-0329 [DOI] [PubMed] [Google Scholar]
- 94.Poretsky L, Cataldo NA, Rosenwaks Z, Giudice LC. The insulin-related ovarian regulatory system in health and disease. Endocr Rev. 1999;20:535–582. doi: 10.1210/edrv.20.4.0374 [DOI] [PubMed] [Google Scholar]
- 95.Baillargeon J-P, Carpentier A. Role of insulin in the hyperandrogenemia of lean women with polycystic ovary syndrome and normal insulin sensitivity. Fertil Steril. 2007;88:886–893. doi: 10.1016/j.fertnstert.2006.12.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Junien C, Van Heyningen V, Gillett GT. Report of the committee on the genetic constitution of chromosome 11. Cytogenet Cell Genet. 1991;459–554. doi: 10.1159/000133171 [DOI] [PubMed] [Google Scholar]
- 97.Kennedy GC, German MS, Rutter WJ. The minisatellite in the diabetes susceptibility locus IDDM2 regulates insulin transcription. Nat Genet. 1995;9:293–298. doi: 10.1038/ng0395-293 [DOI] [PubMed] [Google Scholar]
- 98.Paquette J, Giannoukakis N, Polychronakos C, Vafiadis P, Deal C. The INS 5′ variable number of tandem repeats is associated with IGF2 expression in humans. J Biol Chem. 1998;273:14158–14164. doi: 10.1074/jbc.273.23.14158 [DOI] [PubMed] [Google Scholar]
- 99.Waterworth DM, Bennett ST, Gharani N, et al. Linkage and association of insulin gene VNTR regulatory polymorphism with polycystic ovary syndrome. Lancet. 1997;349:986–990. doi: 10.1016/S0140-6736(96)08368-7 [DOI] [PubMed] [Google Scholar]
- 100.Signaling T, Goldfine IRAD. The insulin receptor: molecular biology and transmembrane signaling. Endocrine Rev. 2015;8:3. [DOI] [PubMed] [Google Scholar]
- 101.Sorbara LR, Tang Z, Cama A, et al. Absence of insulin receptor gene mutations in three insulin-resistant women with the polycystic ovary syndrome. Metabolism. 1994;43:1568–1574. doi: 10.1016/0026-0495(94)90018-3 [DOI] [PubMed] [Google Scholar]
- 102.Kashima K, Yahata T, Fujita K, Tanaka K. Polycystic ovary syndrome: association of a C/T Single nucleotide polymorphism at tyrosine kinase domain of insulin receptor gene with pathogenesis among lean Japanese women. J Reprod Med. 2013;58:491–496. [PubMed] [Google Scholar]
- 103.Diamanti-Kandarakis E, Papavassiliou AG. Molecular mechanisms of insulin resistance in polycystic ovary syndrome. Trends Mol Med. 2006;12:324–332. doi: 10.1016/j.molmed.2006.05.006 [DOI] [PubMed] [Google Scholar]
- 104.Urbanek M, Woodroffe A, Ewens KG, et al. Candidate gene region for polycystic ovary syndrome on chromosome 19p13.2. J Clin Endocrinol Metab. 2005;90(12):6623–6629. doi: 10.1210/jc.2005-0622 [DOI] [PubMed] [Google Scholar]
- 105.Sir-Petermann T, Pérez-Bravo F, Angel B, Maliqueo M, Calvillan M, Palomino A. G972R polymorphism of IRS-1 in women with polycystic ovary syndrome [3]. Diabetologia. 2001;44:1200–1201. [DOI] [PubMed] [Google Scholar]
- 106.El Mkadem SA, Lautier C, Macari F, et al. Role of allelic variants Gly972Arg of IRS-1 and Gly1057Asp of IRS-2 in moderate-to-severe insulin resistance of women with polycystic ovary syndrome. Diabetes. 2001;50:2164–2168. doi: 10.2337/diabetes.50.9.2164 [DOI] [PubMed] [Google Scholar]
- 107.Dilek S, Ertunc D, Tok EC, Erdal EM, Aktas A. Association of Gly972Arg variant of insulin receptor substrate-1 with metabolic features in women with polycystic ovary syndrome. Fertil Steril. 2005;84:407–412. doi: 10.1016/j.fertnstert.2005.01.133 [DOI] [PubMed] [Google Scholar]
- 108.Sáez ME, González-Sánchez JL, Ramírez-Lorca R, et al. The CAPN10 gene is associated with insulin resistance phenotypes in the spanish population. PLoS One. 2008;3:e2953. doi: 10.1371/journal.pone.0002953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ajmal N, Khan SZ, Shaikh R. European journal of obstetrics & gynecology and reproductive biology: X polycystic ovary syndrome (PCOS) and genetic predisposition: a review article. Eur J Obstet Gynecol Reprod Biol X. 2019;3:100060. doi: 10.1016/j.eurox.2019.100060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wojciechowski P, Lipowska A, Rys P, et al. Impact of FTO genotypes on BMI and weight in polycystic ovary syndrome: a systematic review and meta-analysis. Diabetologia. 2012;55:2636–2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rizwan S, Ghazanvi S, Rasheed N, Mi U. Association of FTO common RS9939609 polymorphism with obesity and association of FTO common RS9939609 polymorphism with obesity and polycystic ovarian syndrome in Pakistani women. J Med Res Biol Stud 1. 2018. [Google Scholar]
- 112.Urbanek M, Woodroffe A, Ewens KG, et al. Candidate gene region for polycystic ovary syndrome on chromosome 19p13.2. J Clin Endocrinol Metab. 2005;90:6623–6629. doi: 10.1210/jc.2005-0622 [DOI] [PubMed] [Google Scholar]
- 113.Jakimiuk AJ, Weitsman SR, Magoffin DA. 5α-Reductase activity in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1999;84:2414–2418. [DOI] [PubMed] [Google Scholar]
- 114.Goodarzi MO, Shah NA, Antoine HJ, Pall M, Guo X, Azziz R. Variants in the 5α-reductase type 1 and type 2 genes are associated with polycystic ovary syndrome and the severity of hirsutism in affected women. J Clin Endocrinol Metab. 2006;91:4085–4091. doi: 10.1210/jc.2006-0227 [DOI] [PubMed] [Google Scholar]
- 115.Martin-Gronert MS, Ozanne SE. Programming of appetite and type 2 diabetes. Early Hum Dev. 2005;81:981–988. doi: 10.1016/j.earlhumdev.2005.10.006 [DOI] [PubMed] [Google Scholar]
- 116.Xu N, Azziz R, Goodarzi MO. Epigenetics in polycystic ovary syndrome: a pilot study of global DNA methylation. Fertil Steril. 2010;94:781–783.e1. doi: 10.1016/j.fertnstert.2009.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ho SM, Tang WY, Belmonte De Frausto J, Prins GS. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res. 2006;66:5624–5632. doi: 10.1158/0008-5472.CAN-06-0516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang F, Yu B, Yang W, Liu J, Lu J, Xia X. Polycystic ovary syndrome resembling histopathological alterations in ovaries from prenatal androgenized female rats. J Ovarian Res. 2012;5:15. doi: 10.1186/1757-2215-5-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Abbott DH, Tarantal AF, Dumesic DA. Fetal, infant, adolescent and adult phenotypes of polycystic ovary syndrome in prenatally androgenized female rhesus monkeys. Am J Primatol. 2009;71:776–784. doi: 10.1002/ajp.v71:9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hague WM, Adams J, Rodda C, et al. The prevalence of polycystic ovaries in patients with congenital adrenal hyperplasia and their close relatives. Clin Endocrinol (Oxf). 1990;33:501–510. [DOI] [PubMed] [Google Scholar]
- 121.Qu F, Wang FF, Yin R, et al. A molecular mechanism underlying ovarian dysfunction of polycystic ovary syndrome: hyperandrogenism induces epigenetic alterations in the granulosa cells. J Mol Med. 2012;90:911–923. doi: 10.1007/s00109-012-0881-4 [DOI] [PubMed] [Google Scholar]
- 122.Crave JC, Fimbel S, Lejeune H, Cugnardey N, Déchaud H, Pugeat M. Effects of diet and metformin administration on sex hormone-binding globulin, androgens, and insulin in hirsute and obese women. J Clin Endocrinol Metab. 1995;80:2057–2062. [DOI] [PubMed] [Google Scholar]
- 123.Sjöström L, Narbro K, Sjöström CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–752. doi: 10.1056/NEJMoa066254 [DOI] [PubMed] [Google Scholar]
- 124.Ehrmann DA, Kasza K, Azziz R, Legro RS, Ghazzi MN. Effects of race and family history of type 2 diabetes on metabolic status of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:66–71. doi: 10.1210/jc.2004-0229 [DOI] [PubMed] [Google Scholar]
- 125.Schlesselman JJ. Risk of endometrial cancer in relation to use of combined oral contraceptives. A practitioner’s guide to meta-analysis. Human Reprod. 1997;12:1851–1863. doi: 10.1093/humrep/12.9.1851 [DOI] [PubMed] [Google Scholar]
- 126.Moghetti P, Toscano V. Treatment of hirsutism and acne in hyperandrogenism. Best Pract Res. 2006;20:221–234. doi: 10.1016/j.beem.2006.03.003 [DOI] [PubMed] [Google Scholar]
- 127.Rimm EB, Manson JE, Stampfer MJ, et al. Oral contraceptive use and the risk of Type 2 (non-insulin-dependent) diabetes mellitus in a large prospective study of women. Diabetologia. 1992;35:967–972. doi: 10.1007/BF00401427 [DOI] [PubMed] [Google Scholar]
- 128.Seow KM, Juan CC, Hsu YP, Hwang JL, Huang LW, Ho LT. Amelioration of insulin resistance in women with PCOS via reduced insulin receptor substrate-1 Ser312 phosphorylation following laparoscopic ovarian electrocautery. Hum Reprod. 2007;22:1003–1010. doi: 10.1093/humrep/del466 [DOI] [PubMed] [Google Scholar]
- 129.Farquhar C, Brown J, Marjoribanks J. Laparoscopic drilling by diathermy or laser for ovulation induction in anovulatory polycystic ovary syndrome. Cochrane Database Syst Rev. 2012. doi: 10.1002/14651858.CD001122.pub4 [DOI] [PubMed] [Google Scholar]
- 130.Cocksedge KA, Li T-C, Saravelos SH, Metwally M. A reappraisal of the role of polycystic ovary syndrome in recurrent miscarriage. Reprod Biomed Online. 2008;17:151–160. doi: 10.1016/S1472-6483(10)60304-5 [DOI] [PubMed] [Google Scholar]
- 131.Amer SA, Li TC, Ledger WL. The value of measuring anti-Müllerian hormone in women with anovulatory polycystic ovary syndrome undergoing laparoscopic ovarian diathermy. Hum Reprod. 2009;24:2760–2766. doi: 10.1093/humrep/dep271 [DOI] [PubMed] [Google Scholar]
- 132.Tummon I, Gavrilova-Jordan L, Allemand MC, Session D. Polycystic ovaries and ovarian hyperstimulation syndrome: a systematic review. Acta Obstet Gynecol Scand. 2005;84:611–616. [DOI] [PubMed] [Google Scholar]
- 133.Shalom-Paz E, Holzer H, Young Son W, Levin I, Tan SL, Almog B. PCOS patients can benefit from in vitro maturation (IVM) of oocytes. Eur J Obstet Gynecol Reprod Biol. 2012;165:53–56. doi: 10.1016/j.ejogrb.2012.07.001 [DOI] [PubMed] [Google Scholar]
- 134.Legro RS. Evaluation and treatment of polycystic ovary syndrome. 2017. [Google Scholar]
- 135.Rédei GP. Polycystic ovarian disease (Stein-Leventhal syndrome). Encyclopedia Genetics Genomics Proteomics Inf. 2008:1528. [Google Scholar]
- 136.Melo AS, Ferriani RA, Navarro PA. Treatment of infertility in women with polycystic ovary syndrome: approach to clinical practice. Clinics. 2015;70:765–769. doi: 10.6061/clinics [DOI] [PMC free article] [PubMed] [Google Scholar]