Abstract
Background
Patients undergoing treatment for head and neck cancer commonly experience significant changes in quality of life (QoL) and levels of symptom distress. It is not known if a telehealth intervention would mitigate these changes.
Objective
To evaluate the impact of a telehealth intervention on QoL and symptom burden in patients undergoing initial treatment for head and neck cancers.
Methods
A randomized clinical trial comparing the impact on QoL and symptom distress of telehealth intervention and standard care was conducted with 80 patients (45 treatment, 35 control) who had been diagnosed with head or neck cancer and were receiving 1 or more treatment modalities. Treatment group participants responded daily to symptom management algorithms using a simple telehealth messaging device, QoL was evaluated by the Functional Assessment of Cancer Therapy-Head&Neck Scale (FACT-HN) and symptom burden by the Memorial Symptom Assessment Scale (MSAS). Control group participants completed assessments while they received routine care.
Results
In the posttreatment phase, the telehealth participants had significantly better scores than the controls for physical well-being (20.6 vs 17.0, P = .02) and trial outcome index (59.9 vs. 50.2, P = .04) on the FACT-HN, and total scores on the MSAS (0.9 vs. 1.2, P = .04).
Limitations
The moderate sample size of 80 patients limits the power to measure more subtle impacts of the intervention.
Conclusions
Using telehealth to provide support to patients with head and neck cancer during the acute phase of treatment improved some aspects of posttreatment QoL and symptom burden.
Recent technological advancements have allowed for innovative telehealth interventions of various levels of sophistication. In cancer care, telephone-based computer systems,1–3 handheld computers,4 and mobile phone technology5 have been used to monitor patients during chemotherapy treatment. Touchscreen computers have been successfully used by patients to report pain,6,7 symptoms, and QoL9 during clinic and office visits. Similar interventions have been used in head and neck cancer. In the Netherlands, touchscreen computers were used to gather data on QoL and distress for use by clinicians in assessing patients during treatment visits,10 and laptop computers were used to report symptoms and issues, communicate with physicians and other patients, and access information.11,12 The latter study found that patients who received the intervention had significantly improved QoL in 5 of the 22 studied parameters at the end of the intervention, but only 1 of those parameters remained significantly different at 12 weeks.
QoL considerations play a prominent role in head and neck cancer care. Previous QoL research in this population has consistently demonstrated a deterioration in QoL during the first 3 months after start of treatment followed by a slow recovery.14 Most recently, QoL scores have been found to predict survival in head and neck cancer patients,13,14 which further demonstrated the importance of addressing QoL in this population. However, there have been few hypothesis-driven studies of interventions designed to have an impact on QoL in these patients.15,16 A systematic review found emerging support for psycho-education interventions in head and neck cancer populations,16 but many of the studies had notable limitations, which precluded firm conclusions regarding about benefit. These studies included small samples or used pilot study methodologies that did not incorporate the random assignment or control group comparisons16,17 necessary to evaluate efficacy.
Knowing that treatment for head and neck cancer has a significant and adverse impact on QoL18–21 as well as physical22–24 and psychological25–29 symptom distress, we developed a multidisciplinary telehealth intervention to monitor, support, educate, and empower patients to become actively engaged in their care during active treatment. We hypothesized this intervention would have a positive impact on QoL and reduce the symptom burden in contrast to a standard-of-care/assessment-only condition.
Methods
Design
After receiving institutional review board approval, including an informed consent process, we conducted a parallel two-group randomized clinical trial comparing the telehealth intervention to standard-of-care/assessment-only in patients who had been recently diagnosed with head and neck cancer.
Site
Study participants were recruited over a two-year period from patients receiving care from the multidisciplinary head and neck cancer team in a metropolitan university teaching clinic. This team was composed of head and neck surgeons, medical oncologists, radiologists, nurses, speech therapists, registered dieticians, psychologists, and social workers. The team developed a comprehensive assessment and treatment plan during the patient’s initial visit to the cancer clinic.
Sample
Eligible patients met the following inclusion criteria: initial diagnosis of head or neck cancer, including cancers of the oral cavity, salivary glands, paranasal sinuses, nasal cavity, pharynx, and larynx; involvement in a treatment plan including 1 or more modalities (surgery, chemotherapy, radiation, or any combination); capacity to give independent informed consent; and ability to speak, read, and comprehend English on a 6th grade level or above. Patients were excluded from participation if they had a known active substance abuse problem, no access to a telephone landline, a thought disorder, compromised cognitive functioning, or were incarcerated.
After securing informed consent, the study coordinator consulted a randomization grid which considered treatment modalities to ascertain if the participant would be assigned to treatment or control. Baseline data was also collected at the time of initial consent.
A power analysis considering the instruments to be used and using a conservative within group correlation of 0.50 for the measurements across time, had previously determined that with 40 participants per group, there would be at least 80% power.
Measures
The selected instruments were self-administered or completed by phone or in a face-to-face interview. The participants were instructed to respond independently (as opposed to allowing surrogate respondents). The Functional Assessment of Cancer Therapy-Head & Neck Scale (FACT-HN) and the Memorial Symptom Assessment Scale (MSAS) were used to assess primary outcomes of QoL and symptom burden.
FACT-HN.
The FACT-G (General) is a multidimensional QoL instrument designed for use with cancer patients. The instrument has 28 items divided into 4 subscales: Functional Well-Being, Physical Well-Being, Social Well-Being, Emotional Well-Being. The generic core questionnaire, validated over 2 decades, meets or exceeds requirements for use in oncology on the basis of ease of administration, brevity, reliability, validity, and responsiveness to clinical change.30 Added to the core questionnaire is the head and neck cancer-specific subscale consisting of 11 items specific to this cancer site. Together these questionnaires constitute the totality of the FACT-HN. The Trial Outcome Index (TOI), considered to be a measure of overall physical impact of the cancer, is obtained by adding together scores on the physical, functional, and cancer-specific subscales.
List and colleagues31 found the FACT-HN to be reliable and sensitive to differences in functioning for patients with head and neck cancers (Cronbach alpha for total FACT-G, 0.89; for head and neck subscale, 0.63). The patients found the FACT-HN was relevant to their problems, easy to understand, and preferable to other validated head and neck cancer QoL questionnaires.32 The FACT-HN was chosen for this study because it is nonspecific related to a treatment modality or subsite in head and neck cancers; allows comparison across cancer diagnoses while still probing issues specific to head and neck cancer; is short and can be completed quickly; includes the psychosocial domains of social/family and emotion subscales as well as physical and functional areas; and is self-administered.
MSAS.
This multidimensional scale measures the prevalence, severity, and distress of the most common symptoms experienced by cancer patients. Physical and emotional subscale scores as well as a Global Distress Index (GDI) are generated from patient responses. The MSAS has demonstrated validity and reliability in inpatient and outpatient cancer populations.33–35 Initial analysis used factor analysis to define 2 subscales: psychological and physical symptoms, with Cronbach coefficients of 0.88 and 0.83, respectively; convergent validity was also established.34 It was chosen for this study because of its proven ability to measure both the presence and the intensity of symptoms.33,35–37
All of the participants completed these 2 measures at baseline (pretreatment), at the midpoint of treatment (at least 3 weeks into treatment), and posttreatment (3 weeks after completion of treatment). In addition, basic demographic information, medical history, specific treatments, and planned timing of the treatments were documented.
Description of the intervention
Participants who had been randomly assigned to the treatment group had the telehealth device connected to a telephone landline in their home. Research study staff delivered, set up, and demonstrated equipment operation. The participants were instructed on how to reply to algorithm questions that appeared on the monitor screen by using 4 large keys below the possible answers (Figure 1). They began responding on the first day cf treatment and continued responding daily (unless hospitalized) throughout the study period. Daily response time took 5–10 minutes.
FIGURE 1.
Health Buddy© appliance used in the intervention
Symptom-control algorithms that had been developed with the use of participatory action research and evidence-based practice38 were programmed into the telehealth messaging system. Patients were asked questions related to the symptoms anticipated during their treatment scenario. Depending on their responses, they would receive specific information related to self-management of symptoms, including recommendations as to when to contact clinicians. Algorithms were constructed to encourage self-efficacy and independent action on the part of the participant when possible.
Responses to the previous day’s cuestions were automatically uploaded overnight and questions with related information for the next day were downloaded. Programming precluded interruption of phone service, completing the downloading and uploading of information during the middle of the night and could be repeated without data loss if service was somehow disrupted. After new algorithms had been transferred, a green light on the device would begin flashing to alert the participant that new questions were available for response.
Participant responses were stored in a secure server. Patient responses were risk-stratified according to the level of risk associated with the self-reported symptom or behavior and accessed electronically daily by study coordinators. Unrelieved symptoms or those targeted as requiring immediate intervention (eg, serious consideration of suicide) would result in the coordinator contacting the patient directly by phone and/or contacting clinicians to assure effective and immediate intervention. Direct intervention by study staff was infrequent because most of the symptoms were addressed independently by the participant in accordance with the study focus on self-management and self-efficacy. If a participant.did not respond for 3 consecutive days, then the coordinator would contact the patient to ascertain the reason for nonadherence.
Statistical methods
Data were analyzed using SPSS software.39 Scores on the MSAS, FACT-HN subscales, FACT-HN TOI, and total FACT-HN were calculated for each patient at 3 time points (pretreatment, during treatment, posttreatment). Differences in scores on these scales were tested between the 2 groups over time. A priori power calculations suggested power of 81% to find a moderate-to-small effect size (Cohen’s d = 0.25).
Descriptive statistics for the 2 groups were calculated. To test for differences in continuous variables t-tests were used, and chi-squared techniques were used to test for differences in categorical variables. Subsequently, to test for differences in trends in outcomes over time, between the 2 groups, generalized linear mixed effects modeling techniques were developed. Group (treatment or control) was incorporated as a fixed effect, and time since randomization was incorporated as a repeated measures effect. Because it was hypothesized that a group effect could be observed simply because 1 group (eg, control group) was associated with decreasing values and the other group (eg, treatment group) was associated with unchanged values, a secondary analysis was performed to explore whether a significant change over time existed within each group.
Results
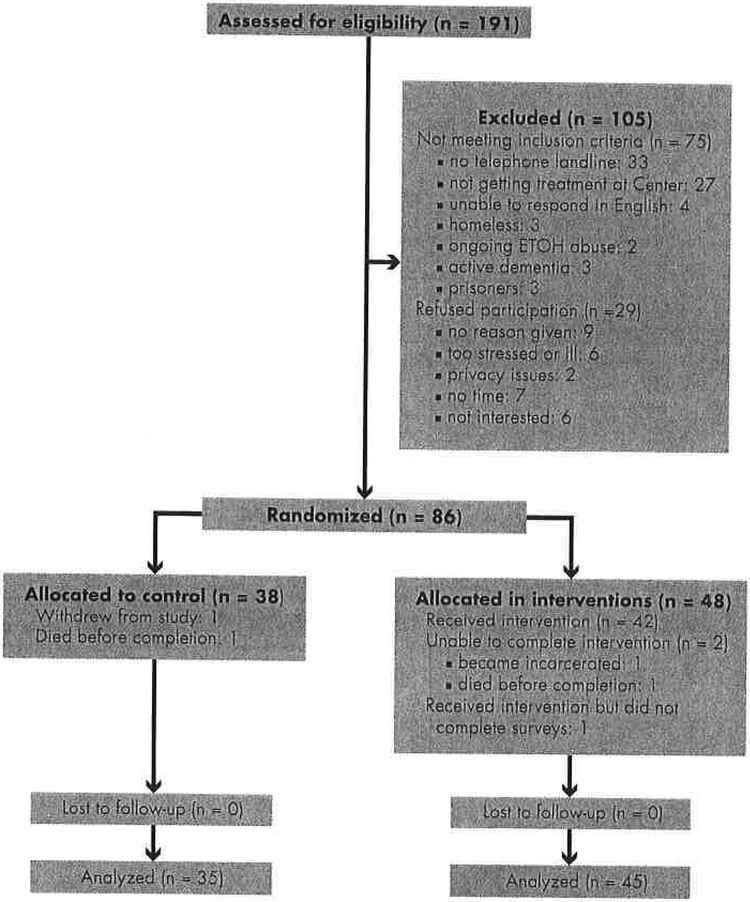
Of the 191 patients who were assessed for eligibility, 86 met the study criteria and were randomized to the treatment (n = 48) or control (n = 38) groups. Figure 2 depicts the CONSORT flow diagram of the screening and randomization process. In all, 80 patients completed the study protocol (45 treatment, 35 control).
FIGURE 2.
CONSORT patient flow diagram
There were no significant differences in demographic characteristics between the treatment and control groups at baseline (Table 1). In addition, there were no differences in any outcome measures between the 2 groups pretreatment. These results suggest the randomization scheme was successful. Although there were no significant differences in outcomes during treatment, differences were observed at follow-up. Because 69 of the 80 patients were men, we were not able to evaluate for gender-based outcome differences.
TABLE 1.
Demographics stratified by control and treatment group
| Group, n (%) | |||
|---|---|---|---|
| Variable | Control (n = 35) | Treatment (n = 45) | P |
| Categorical | |||
| Male | 30 (85.1) | 39 (87.1) | .48 |
| White | 31 (88.6) | 40 (87.9) | .20 |
| Urban setting | 27 (76.5) | 36 (79.1) | .10 |
| Tumor stage | .33 | ||
| I | 5 (14.2) | 7 (16.3) | |
| II | 12 (34.2) | 15 (33.8) | |
| III | 9 (26.9) | 13 (29.0) | |
| IV | 3 (9.5) | 4 (9.6) | |
| In situ | 1 (2.1) | 1 (1.2) | |
| Unknown | 5 (14.2) | 5 (10.1) | |
| Radiation | 31 (85.7) | 41 (91.1) | .79 |
| Chemotherapy | 20 (57) | 33 (73.3) | .13 |
| Surgery | 4 (11.4) | 4 (8.9) | .69 |
| Site | .15 | ||
| Oral cavity | 6 (17.1) | 8 (16,7) | |
| Pharynx | 10 (29.5) | 14 (31.1) | |
| Larynx | 11 (30.1) | 13 (29.0) | |
| Salivary glands | 3 (9.5) | 4 (9.6) | |
| Paranasal sinuses | 1 (2.1) | 1 (1.2) | |
| Angiosarcoma | 1 (2.1) | 1 (1.2) | |
| Unknown | 1 (2.1) | 5 (10.1) | |
| Continuous | |||
| Mean age, y (SD) | 59.67 (11.8) | 60.73 (10.2) | .20 |
| Mean BMI, kg/m2 (SD) | 28.1 (8.4) | 26.9 (7.6) | .82 |
As shown in Table 2, the generalized linear mixed effects model suggests that the telehealth approach was associated with better total scores over time for the MSAS (P = .013), MSAS-PSYCH (P = .009), Physical Well-Being (P = .001), and TOI (P < .001). No differences were observed globally over time, although post hoc comparisons at each time point are described hereinafter and in Table 3. In addition, no interaction effects existed.
TABLE 2.
Repeated measures ANOVA of outcomes over time stratified by group
| Outcome | Predictor | P |
|---|---|---|
| PWB | Group | .001* |
| Time | .510 | |
| Group × Time | .470 | |
| SWB | Group | .478 |
| Time | .316 | |
| Group × Time | .171 | |
| EWB | Group | .781 |
| Time | .436 | |
| Group × Time | .912 | |
| FWB | Group | .542 |
| Time | .508 | |
| Group × Time | .705 | |
| H&NCS | Group | .647 |
| Time | .521 | |
| Group × time | .733 | |
| TOI | Group | < .001* |
| Time | .249 | |
| Group × Time | .676 | |
| FACT-G | Group | .499 |
| Time | .501 | |
| Group × Time | .997 | |
| FACT-HN | Group | .129 |
| Time | .871 | |
| Group × Time | .148 | |
| Total MSAS | Group | .013* |
| Time | .058 | |
| Group × Time | .182 | |
| MSAS-PHYS | Group | .090 |
| Time | ,061 | |
| Group × Time | .338 | |
| MSAS-PSYCH | Group | .009* |
| Time | .141 | |
| Group × Time | .602 | |
| MSAS-GDI | Group | .341 |
| Time | .077 | |
| Group × Time | .697 |
EWB, Emotional Well-Being; FACT-G, Functional Assessment of Cancer Therapy-General: FACT-HN, Functional Assessment of Cancer Therapy-Head & Neck; FWB, Functional Welt-Being; H&NCS, Heed & Neck Cor1cer Specific; MSAS-GDI. Memorial Symptom Assessment Scale-Global Distress Index; MSAS-PHYS, Memorial Symptom Assessment Scale-Physical Symptom Distress; MSAS-PSYCH, Memorial Symptom Assessment Scale-Psychological Symptom Distress; PWB, Physical Well-Being; SWB, Social/Family Well-Being; TOI, Total Outcome Index; Totol MSAS, Total Memorial Symptom Assessment Scale
Significant at .05.
TABLE 3.
Scores over time stratified by treatment or control
| Variable | T1 | P | T2 | P | T3 | P |
|---|---|---|---|---|---|---|
| PWB | .983 | .312 | .021* | |||
| Treatment | 20.84 | 18.15 | 20.61 | |||
| Control | 22.63 | 15.54 | 17.04 | |||
| SWB | .953 | .432 | .477 | |||
| Treatment | 20.47 | 22.22 | 23.21 | |||
| Control | 21.13 | 21.38 | 21.91 | |||
| EWB | .892 | .534 | .250 | |||
| Treatment | 18.71 | 18.21 | 18.75 | |||
| Control | 18.33 | 16.79 | 18.04 | |||
| FWB | .795 | .925 | .191 | |||
| Treatment | 16.92 | 13.67 | 18.13 | |||
| Control | 15.75 | 13.21 | 16.21 | |||
| H&NCS | .142 | .560 | .052 | |||
| Treatment | 19.45 | 17.64 | 20.30 | |||
| Control | 16.37 | 16.41 | 17.83 | |||
| TOI | .914 | .782 | .042* | |||
| Treatment | 61.83 | 48.02 | 59.94 | |||
| Control | 58.00 | 44.92 | 50.16 | |||
| FACT-G | .825 | .667 | .180 | |||
| Treatment | 74.06 | 67.55 | 78.29 | |||
| Control | 78.04 | 67.71 | 73.84 | |||
| FACT-HN | .803 | .865 | .103 | |||
| Treatment | 100.25 | 85.63 | 101.53 | |||
| Control | 98.81 | 84.60 | 89.86 | |||
| Total MSAS | .902 | .140 | 0.87 | .044* | ||
| Treatment | 0.84 | 1.23 | 1.22 | |||
| Control | 0.85 | 1.47 | ||||
| MSAS-PHYS | .373 | .294 | .460 | |||
| Treatment | 0.70 | 1.46 | 1.13 | |||
| Control | 0.79 | 1.66 | 1.30 | |||
| MSAS-PSYCH | .531 | .595 | .095 | |||
| Treatment | 1.09 | 1.25 | 0.85 | |||
| Control | 0.95 | 1.39 | 1.37 | |||
| MSAS-GDI | .833 | .943 | .113 | |||
| Treatment | 1.14 | 1.77 | 1.27 | |||
| Control | 1.10 | 1.78 | 1.69 |
EWB, Emotional Well-Being; FACT-G, functional Assessment of Cancer Therapy-General; FACT-HN, Functional Assessment of Cancer Therapy-Head & Neck; FWB, Functional Well-Being; H&NCS, Head & Neck cancer Specific; MSAS-GDI, Memorial Symptom Assessment Scale-Global Distress Index; MSAS-PHYS, Memorial Symptom Assessment Scale-Physical Symptom Distress; MSAS-PSYCH, Memorial Symptom Assessment Scale-Psychological Symptom Distress; PWB Physical Well-Being; SWB, Social/Family Well-Being; T1, Time 1; T2, Time 2; T3, Time 3; TOI, Total Outcome Index; Total MSAS, Memorial Symptom Assessment Scale
Significant at 0.05.
Although differences in outcomes were observed during treatment, none reached statistical significance. At posttreatment, however, the telehealth participants had significantly better scores than the controls for Physical Well-Being (20.6 [SD, 12.1] vs 17.0 [11.7], respectively; P = .021), TOI (59.9 [26.3] vs 50.2 [28.4]; P= .042) and total MSAS (0.9 [0.5] vs 1.2 [0.6]; P = .0440). That is, although improvements in outcomes were observed during treatment, significant differences were not observed until after treatment. The telehealth approach, however, did not significantly improve the scores for Emotional Well-Being (18.04 control vs 18.75 treatment, P = .250) or Social Well-Being (21.91 control vs 23.21 treatment, P = .477). QoL measured by Total FACT-HN scores, which include the Social and Emotional Well-Being subcomponents, where also not significantly different between the 2 groups. Because these were post hoc comparisons at each time point, and each was viewed as important in isolation, no alpha-splicing for multiple testing (eg, Bonferroni) was performed.
Discussion
In this randomized, controlled trial, data analyses revealed that patients who were treated for head and neck cancer and were exposed to a simple telehealth intervention reported significantly better QoL and a lower symptom burden posttreatment compared with patients who received routine cancer care. As far as we know, this is the first published clinical trial in the United States to use a telehealth device for symptom management during head and neck cancer treatment. The devices were well accepted by patients and clinicians, had few technical issues, and none were lost or damaged.40
Physical symptoms, represented by the FACT-HN QoL subscale scores and the MSAS symptom burden measures, demonstrated the greatest improvement with the intervention. Patients’ social and emotional well-being were not improved, possibly because they were stable traits of individual patients during the treatment period. QoL and symptom burden were improved by the intervention in the weeks after treatment but not during the treatment phase. We hypothesize that the physical burdens during active multimodality cancer treatment produced strong symptoms that were resistant to an intervention. During the immediate recovery phase however, the telehealth intervention did return quality life and symptom burden to near pretreatment levels, findings from previous studies have shown a more gradual return to baseline QoL,41 whereas our findings demonstrated a more rapid improvement. Thus, telehealth interventions may shorten the recovery time and enhance survivorship in patients undergoing treatment for head and neck cancer.
Head and neck cancer patients may have unique features that make telehealth interventions more effective. The patient’s ability to communicate is often impaired, disfigurement can lead to social isolation, and initial surgical treatment can have a long recovery period. In addition, surgeons, medical oncologists, radiation oncologists, and allied therapists are all typically involved, and telehealth devices may provide some continuity among multiple disciplines’ treatment plans. Daily messaging related to self-management of symptoms may establish belief in one’s self-efficacy, which may extend into the recovery period and result in improved disease management and return to baseline functioning.
We purposefully chose to use a simple device for this trial, one using a landline and a simple four-button keypad, with daily prompting from a green flashing light. Previous studies of telehealth modalities in cancer patients have shown mixed use of websites and smart phone technologies1–5 that may not be as feasible in this specific patient population. Our methodology prompted the patient to respond, as opposed to patient-initiated interaction with specific websites or applications. This immediacy and prompting may have improved patient engagement, which was strong, and confirms the feasibility and acceptance of this study’s particular approach.40
Our study has several limitations that should be considered when interpreting these results. First, the sample size of 80 patients is moderate and limits the power to measure more subtle influences of the telehealth intervention. This could be particularly true of several favorably trending, but statistically nonsignificant findings. In addition, our study was conducted at a single institution and before there was more widespread use of surgical robotics in head and neck cancer care. Our patients differed demographically from national norms in head and neck cancer by gender (86% men in our study vs 73% men nationally42). Finally, our trial was in one type of cancer and cannot be extrapolated to other types of cancer patients.
Future studies are needed to replicate our findings in other settings and other types of cancers. Our focus was on the initial treatment phase; the value of telehealth support in other phases of the clinical cancer trajectory is unknown. Finally, new technologies are constantly emerging that will also require evaluation.
Head and neck cancer is a common and devastating condition that commonly induces a high level of physical and psychosocial symptom burden. Based on these results, basic telehealth technology offers hope and support for patients to improve QoL and reduce symptom burden. Modalities for such interventions are becoming more affordable and accessible to the general population. If focused on self-management and patient education, interventions have the potential to reduce the costs related to professional oversight, clinic visits, emergency calls and inpatient stays. While more evidence is needed to justify routine use of telehealth in cancer care, a growing body of evidence supports the efficacy of such approaches.
Acknowledgments
Funding Supported in part by NIH R21CA115345 from the National Cancer Institute.
Footnotes
Disclosures: The authors have no disclosures.
Ms Keeney is now with Big White Wall.
ClinicalTrials.gov identifier: NIH R21CA115345.JCSO 2015;13:14–21.
References
- 1.Mooney KH, Beck SL, Friedman RH, et al. Telephone-linked care for cancer symptom monitoring: a pilot study. Cancer Pract. 2002;1:147–154. [DOI] [PubMed] [Google Scholar]
- 2.Davis K, Yount S, Cello KD, et al. An innovative symptom monitoring tool for people with advanced lung cancer: a pilot demonstration. J Support Oncol. 2007;5:381–387. [PubMed] [Google Scholar]
- 3.Kroenke K, Theobald D, Wu J, et al. Effect of telecare management on pain and depression in patients with cancer: a randomized trial. JAMA. 2010;304:163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kearney N, Kidd L, Miller M, et al. Utilising handheld computers to monitor and support patients receiving chemotherapy: results of a UK-based feasibility study. Support Care Cancer. 2006;14:742–752. [DOI] [PubMed] [Google Scholar]
- 5.Weaver A, Young AM, Rowntree J, et al. Application of mobile phone technology for managing chemotherapy-associated side-ef-fects. Ann Oncol. 2007;18:1887–1892. [DOI] [PubMed] [Google Scholar]
- 6.Wilkie DJ, Judge MK, Berry DL, Dell J, Zong S, Gilcspie R. Usability of a computerized PAlNReportlt in the general public with pain and people with cancer pain.J Pain Symptom Manage. 2003;25:213–224. [DOI] [PubMed] [Google Scholar]
- 7.Huang H, Wilkie DJ, Zong S, et al. Developing a computerized data collection and decision support system for cancer pain management. Compute Inform Nurs. 2003;21:206–217. [DOI] [PubMed] [Google Scholar]
- 8.Berry D, Trigg LJ, Lober WB, et al. Computerized symptom and quality-of-life assessment for patients with cancer part I: development and pilot testing. Oncol Nurs Forum. 2004;31:E75–83. [DOI] [PubMed] [Google Scholar]
- 9.Mullen K, Berry D, Zierler B. Computerized symptom and quality-of-life assessment for patients with cancer part II: acceptability and usability. Oncol Nurs Forum. 2004;31:E84–89. [DOI] [PubMed] [Google Scholar]
- 10.de Bree R, Verdonck-de Leeuw IM, Keizer AL, Houffelaar A, Lee-mans CR. Touch screen computer-assisted health-related QoL and distress data collection in head and neck cancer patients. Clin Otolaryngol. 2008;33:138–142. [DOI] [PubMed] [Google Scholar]
- 11.van den Brink JL, Moorman PW, de Boer MF, et al. Impact on QoL of a telemedicine system supporting head and neck cancer patients: a controlled trial during the postoperative period at home. J Am Med Inform Assoc. 2007;14:198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van den Brink JL, Moorman PW, de Boer MF, Pruyn JFA, Verwoerd CDA, van Bemmel JH. Involving the patient: a prospective study on use, appreciation and effectiveness of an information system in head and neck cancer care. Int J Med Inform. 2005;74:839–849. [DOI] [PubMed] [Google Scholar]
- 13.Meyer F, Fortin A, Gelinas M, et al. Health-related QoL as a survival predictor for patients with localized head and neck cancer treated with radiation therapy. J Clin Oncol. 2009;27:2970–2976. [DOI] [PubMed] [Google Scholar]
- 14.Karvonen-Gutierrez CA, Ronis DL, Fowler KE, Terrell JE, Gruber SB, Duffy SA. QoL scores predict survival among patients with head and neck cancer. J Clin Oncol. 2008;26:2754–2760. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz S, Donald P, Yueh B. Quality-of-life outcomes in the evaluation of head and neck cancer treatments. Arch Otolaryngol Head Neck Surg. 2001;127:673–678. [DOI] [PubMed] [Google Scholar]
- 16.Luckett T, Britton B, Clover K, Rankin NM. Evidence for interventions to improve psychological outcomes in people with head and neck cancer: a systematic review of the literature. Support Care Cancer. 2011;19:871–881. [DOI] [PubMed] [Google Scholar]
- 17.Semple CJ, Sullivan K, Dunwoody L, Kernohan WG. Psychosocial interventions for patients with head and neck cancer: past, present, and future. Cancer Nurs. 2004;27:434–441. [DOI] [PubMed] [Google Scholar]
- 18.Dropkin MJ. Literatrue review on QoL following head and neck cancer: 1996–1997. Head Neck Nurs, 1998;16:22–23. [PubMed] [Google Scholar]
- 19.Chang VT, Hwang SS, Feuerman M, Kasimis BS. Symptom and QoL survey of medical oncology patients at a veterans affairs medical center: a role for symptom assessment. Cancer. 2000;88:1175–1183. [DOI] [PubMed] [Google Scholar]
- 20.Terrell JE, Ronis DL, Fowler KE, et al. Clinical predictors of QoL in patients with head and neck cancer. Arch Otolaryngol Head Neck Surg. 2004;130:401–408. [DOI] [PubMed] [Google Scholar]
- 21.Ferlito A, Rogers SN, Shaha AR, Bradley PJ. Quality of life in head and neck cancer. Acta Otolaryngol. 2003;123:5–7. [DOI] [PubMed] [Google Scholar]
- 22.Chua K, Reddy S, Lee M, Patt R. Pain and loss of function in head and neck cancer survivors. J Pain Symptom Manage. 1999;18:193–202. [DOI] [PubMed] [Google Scholar]
- 23.Connor NP, Cohen SB, Kammer RE, et al. Impact of conventional radiotherapy on health-related QoL and critical functions of the head and neck. Int J Radiat Oncol Biol Phys. 2006;65:1051–1062. [DOI] [PubMed] [Google Scholar]
- 24.Shuman AG, Terrell JE, Light E, et al. Predictors of pain among patients with head and neck cancer. Arch Otolaryngol Head Neck Surg. 2012;138:1147–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katz M, Irish J, Devins G, Rodin G, Gullane P. Psychosocial adjustment in head and neck cancer: the impact of disfigurement, gender, and social support. Head Neck. 2003;25:103–112. [DOI] [PubMed] [Google Scholar]
- 26.D’Antonio L, Long S, Zimmerman G, Peterman A, Petti G, Chonkich G. Relationship between QoL and depression in patients with head and neck cancer, Laryngoscope. 1998;108:806–811. [DOI] [PubMed] [Google Scholar]
- 27.Kugaya A, Akechi T, Okamura H, Mikami I, Uchitomi Y. Correlates of depressed mood in ambulatory head and neck cancer patients. Psychooncology. 1999;8:494–499. [DOI] [PubMed] [Google Scholar]
- 28.Pandey M, Devi N, Thomas BC, Kumar SV, Krishnan R. Distress overlaps with anxiety and depression in patients with head and neck cancer. Psychooncology. 2007;16:582–586. [DOI] [PubMed] [Google Scholar]
- 29.Aarstad HJ, Aarstad AKH, Heimdal J-H, Olofsson J. Mood, anxiety and sense of humor in head and neck cancer patients in relation to disease stage, prognosis and quality of life. Acta Otolaryngol. 2005;125:557–565. [DOI] [PubMed] [Google Scholar]
- 30.Celia DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy (FACT) Scale: development and validation of the general measure. J Clin Oncol. 1993;11:570–579. [DOI] [PubMed] [Google Scholar]
- 31.List MA, D'Antonio LL, Celia DF, et al. The Performance Status Scale for head and neck cancer patients and the Functional Assessment of Cancer Therapy-Head and Neck Scale, a study of utility and validity. Cancer. 1996;77:2294–2301. [DOI] [PubMed] [Google Scholar]
- 32.Mehanna HM, Morton RP, Patients’views on the utility of QoL questionnaires in head and neck cancer: a randomised trial, Clin Otolaryngol. 2006;31:310–316. [DOI] [PubMed] [Google Scholar]
- 33.Chang V, Hwang S, Feuerman M, Kasimis B,Thaler H.The memorial symptom assessment scale short form (MSAS-SF). Cancer, 2000;89:1162–1171. [DOI] [PubMed] [Google Scholar]
- 34.Portenoy R, Thaler H, Kornblith A. The Memorial Symptom Assessment Scale: an instrument for the evaluation of symptom prevalence, characteristics and distress, Eur J Cancer. 1994;30A: 1326–1336. [DOI] [PubMed] [Google Scholar]
- 35.Tranmer JE, Heyland D, Dudgeon D, Groll D, Squires-Graham M,Coulson K. Measuring the symptom experience of seriously ill cancer and noncanccr hospitalized patients near the end of life with the Memorial Symptom Assessment Scale. J Pain Symptom Manage. 2003;25:420–429. [DOI] [PubMed] [Google Scholar]
- 36.Nelson JF, Meier DE, Litke A, Natale DA, Siegel RE, Morrison RS. The symptom burden of chronic critical illness. Crit Care Med. 2004;32:1527–1534. [DOI] [PubMed] [Google Scholar]
- 37.Harrison LB, Zelefsky MJ, Pfister DG, et al. Detailed QoL assessment in patients treated with primary radiotherapy for squamous cell cancer of the base of the tongue. Head Neck. 1997;19:169–175. [DOI] [PubMed] [Google Scholar]
- 38.Head BA, Studts JL, Bumpous JM, Gregg JL, Wilson L, Keeney C, Scharfenberger JA, Pfeifer MP. Development of a telehealth intervention for head and neck cancer patients. TelemedJ E Health. 2009;15:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.IBM SPSS Statistics for Windows, Version 21.0. [computer pro-glam]. Armonk, NY: 2012. [Google Scholar]
- 40.Head BA, Keeney C, Studts JL, Khayat M, Bumpous J, Pfeifer M. Feasibility and acceptance of a telehealth intervention to promote symptom management during treatment for head and neck cancer.J Supp Oncol. 2011;9:el–ell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ledeboer QC, Velden LA, Boer MF, Feenstra L, Pruyn JF. Physical and psychosocial correlates of head and neck cancer: an update of the literature and challenges for the future (1996–2003). Clin Otolaryngol. 2005;30:303–319. [DOI] [PubMed] [Google Scholar]
- 42.American Cancer Society. Cancer Facts & Figures 2014.2014: http://www.cancer.0rg/acs/gr0ups/c0ntent/@research/d0cuments/webcontent/acspc-042151.pdf. Accessed March 15,2014.