G protein-coupled receptors (GPCRs) are transmembrane proteins that play a fundamental role in signalling, which can be described as transducing extracellular information into intracellular functional responses. GPCRs are ubiquitous throughout the human body and the extracellular signals vary from photons, ions, and small molecules to peptides and large proteins. To accommodate this vast functional diversity, the superfamily of GPCRs is encoded by approximately 1000 genes and has been divided into classes based on sequence homology and structural features [1]. Since GPCRs are a key transducer to mobilize cellular responses, these proteins have been successful pharmacological targets for decades and are currently targeted by over 30% of the pharmacotherapies approved by the FDA [2].
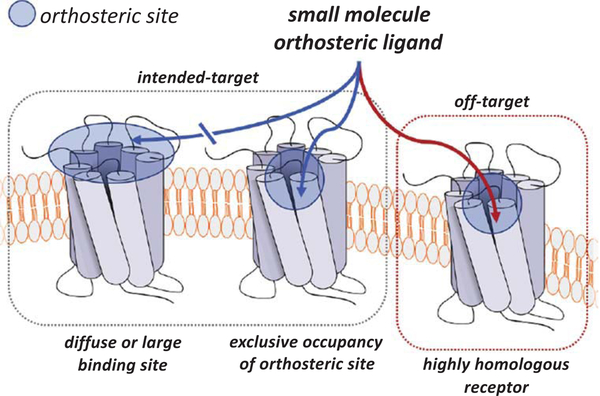
The majority of GPCR-targeted drugs are functionally active by binding to the orthosteric site of the receptor, which is responsible for the binding of endogenous GCPR ligands. As such, drugs targeting the orthosteric site can activate the receptor (e.g., agonists, partial agonists), inhibit receptor activation (e.g., antagonists), or produce an inversion of the functional response (e.g., inverse agonists). Despite the large number of orthosteric drugs, there are marked difficulties in designing orthosteric ligands that are safe and effective in certain biological circumstances. These circumstances include inducible off-target effects and poor selectivity due to highly homologous receptor orthosteric sites, the inability to target large, diffuse orthosteric sites that are activated by peptides or proteins, and the abrogation of spatial and/or temporal endogenous signalling patterns that are encoded by physiological systems (Fig. 1). As an alternative strategy towards GPCR drug design, allosteric modulation is an emerging approach where ligands are designed that can modulate the function of the receptor but, importantly, do not engage the receptor orthosteric site [3]. Allosteric modulation has been adopted as an innovative tool that can provide precision targeting of GPCRs and increasingly utilized throughout the past decade [4].
Fig. 1.
GPCR-targeted orthosteric small molecules may exhibit disadvantages such as difficulty binding diffuse orthosteric sites with high affinity, unintended kinetic or physiological effects from exclusively occupying the orthosteric site, and off-target effects through poor selectivity between highly homologous receptor subtypes.
Pure allosteric modulators, by definition, can either function as a positive allosteric modulator (PAM) by increasing the receptor’s functional response and/or intrinsic affinity for an orthosteric ligand, as a negative allosteric modulator (NAM) by fully or partially suppressing the receptor’s functional response to an orthosteric ligand, or as a neutral allosteric ligand (NAL) by binding to a receptor allosteric site and asserting no measurable changes to the receptor or orthosteric ligand behavior [5]. The NAL category of allosteric ligands is not widely pursued and the number of characterized NALs remains small. However, it may be postulated that with the increasing discovery of endogenous allosteric ligands, significant modulatory roles could be revealed for these endogenous ligands and strategically blocking the allosteric site with an otherwise benign NAL may yield therapeutic targets in the future. The remaining Current Frontiers will predominately highlight novel and innovative applications for PAMs and NAMs in drug discovery targeting GPCRs.
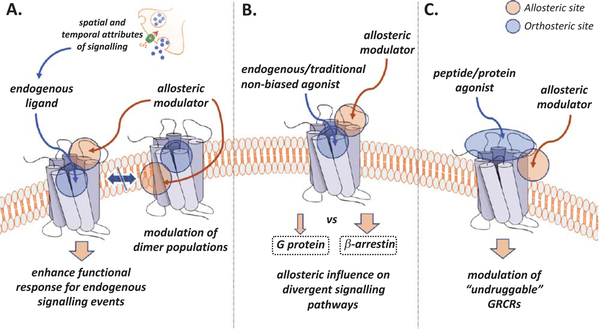
One of the first benefits theorized for PAM and NAM applications was the ability to tune a receptor’s functional response to endogenous orthosteric agonists while maintaining the system’s signalling profile (Fig. 2A) [6]. Physiological systems that depend on accurate and precise signalling profiles are exemplified by neurotransmission in the central nervous system (CNS) and by chemokine signalling that governs cell migration in immune and homeostatic processes. In both of these systems, gradients or “waves” of endogenous orthosteric ligands determine appropriate system responses, an effect that is difficult to recapitulate or maintain with synthetic agonists and antagonists. For example, the serotonin (5-HT) 5-HT2C receptor (5-HT2CR) is predominantly expressed in the CNS and therefore embedded into a dynamic framework of tightly controlled neurotransmission. The 5-HT2CR has been implicated in multiple CNS disorders and mediates behavioral traits that are implicated in drug use disorders and relapse [7]. Our group has recently employed the strategic enhancement of 5-HT-mediated signalling by 5-HT2CR PAMs to suppress these addiction-related behavioral traits in rodents, while achieving subtype selectivity over the highly homologous 5-HT2A receptor (5-HT2AR) [8]. The importance of spatial and temporal characteristics in signalling extends to many other GPCR-regulated systems in addition to neurotransmission and chemokine signalling and is receiving increased appreciation in the study of nutrient-sensing GPCRs (e.g. free fatty acid receptors; FFARs) that may present drug discovery targets for significant diseases such as obesity and diabetes [9].
Fig. 2.
Applications of GPCR allosteric modulators and theoretical functional outcomes. A. Spatial and temporal signalling control is exemplified by neuronal neurotransmitter release. Allosteric modulation of functional response is only achieved for receptors bound to the neurotransmitter. Additionally, allosteric modulators may be used to alter GPCR dimer populations and consequential signalling patterns. B. GPCR structural conformations are critical drivers for biased signalling. Allosteric modulators may stabilize, disrupt, or induce structural conformations that preferentially (biased) signal via one or multiple pathways, at magnitudes unlike the endogenous ligand. C. Many GPCRs are targeted by large endogenous ligands, such as peptides and proteins. Small molecules may not sufficiently interact with the orthosteric site to induce signalling. Allosteric sites may offer promising, novel druggable surfaces.
In some GPCR-mediated systems, single GPCRs can be responsible for numerous and diverse roles by accommodating multiple endogenous orthosteric ligands. For example, chemokine receptors can be differentially activated by multiple circulating chemokines that are implicated to various degrees in pathological states [10]. The application of a probe-dependent NAM to dampen pathological chemokine signalling, while retaining full signalling potentials for non-pathological chemokines or other receptor activities, could deliver novel therapeutics that limit adverse effects. An example of this phenomenon can be seen in work by Kenakin and colleagues, where the C-C chemokine receptor type 5 (CCR5) NAM 873140 blocked the binding of the endogenous orthosteric ligand CCL3 (also known as MIP-1α) but did not inhibit binding of the endogenous orthosteric ligand CCL5 (also known as RANTES) [11]. Signalling responses for CCL3 and CCL5 were both antagonized. However, ligand binding to a GPCR elicits numerous activities besides signalling that can be beneficially modulated, including receptor internalization and phosphorylation. Therefore, a NAM that blocks signalling of multiple ligands can probe-dependently regulate receptor phosphorylation or internalization via differentially allowing ligand binding. This allosteric modulation approach, which utilizes probe-dependence as a therapeutic advantage, should be considered for drug discovery campaigns targeting multi-agonist GPCRs.
Certain classes of GPCRs (e.g. class C GPCRs) are known to form obligate dimers to achieve functional response; however, less is known about the roles of dimer populations in other classes of GPCRs (e.g. class A GPCRs) that are highly relevant to drug discovery [12]. It is gravely important to elucidate the functional relevance to health and disease of GPCR dimerization throughout all GPCR classes and strategically designed allosteric modulators can provide illumination into this question with novel chemical probes and innovative pharmacotherapies (Fig. 2A). A key example of utilizing an allosteric modulator to direct dimer populations can be seen in the FDA-approved CCR5 NAM maraviroc [13]. Maraviroc was discovered as an anti-HIV agent that inhibits viral cell entry and functions by targeting the HIV-1 co-receptor CCR5, subsequently disallowing the co-receptor formation that is requisite for viral-host cell membrane fusion [14]. Interestingly, it was discovered later that maraviroc’s mechanism of action is attributable to its ability to modulate CCR5 dimer populations and subsequent subcellular trafficking and localization to the cell membrane [13]. In the study by Jin, et al., two CCR5 dimer conformations that are imperative to membrane localization were present in the absence of maraviroc; however, after the addition of maraviroc, an additional CCR5 dimer conformation was discovered and all homodimer conformations were further stabilized. This finding is consistent with the observation that CCR5 dimers and oligomers inhibit HIV host-cell entry, likely by preventing the HIV-1 co-receptor formation. It is conceivable that dimer stabilization (via ligand chaperone activity) or dimer prevention with rationally designed allosteric modulators could be an attractive drug discovery strategy in certain circumstances.
GPCRs regulate cellular responses to extracellular stimuli by mobilizing multiple GPCR-activated signalling cascades. The binding of an endogenous or synthetic orthosteric agonist famously activates a heterotrimeric guanine nucleotide-binding protein (G protein) from which the receptor super-family derives its name. In recent years it has been increasingly recognized that additional GPCR-interacting proteins (e.g. β-arrestins) induce signalling cascades that can direct specific subsets of cellular responses that are independent of G protein activation [15]. Significantly, studies are now providing evidence that activation of specific signalling cascade pathways (e.g. G protein or β-arrestin) are responsible for either therapeutic or adverse effects and that pathway-selective modulation may hold potential for designing novel, precise pharmacotherapies. Brust and colleagues have demonstrated this phenomenon by designing G protein-biased agonists for the kappa opioid receptor (KOR), which maintain the desirable antinociceptive and antipruritic effects and eliminate the sedative and dissociative effects in rodent models [16]. The concept of a stimulated GPCR selectively activating certain pathways is known as biased signalling, and akin to all GPCR activation events, it is mechanistically a controlled conformational change. Although the majority of “biased ligands” being pursued for drug discovery purposes are synthetic agonists, PAMs and NAMs have the potential to further fine tune this response and yield therapeutics with a strikingly precise mechanism of action (Fig. 2B). Allosteric modulators can induce diverse conformational changes in GPCR structures and thus could be rationally designed to produce a pronounced biased signalling response from a GPCR activated by an otherwise non-biased orthosteric ligand. Recent advances in GPCR structural biology have provided insight into residues and domains that function as molecular switches for divergent pathway activation and further work in this area will enable the realization of rationally designed PAMs and NAMs that accomplish therapeutically beneficial biased signalling [17, 18].
It has been mentioned that GPCRs are activated by highly diverse stimuli that span photons and small molecules to peptides and proteins. Thus, across the GPCR superfamily orthosteric sites are highly divergent in both sequence and structure depending on the associated orthosteric ligand. GPCR-targeted peptides and proteins are responsible for binding to an orthosteric site and inducing subsequent conformational changes that lead to receptor activation. In simplest terms, these complexes are formed by protein-protein interactions (PPIs) and, broadly, PPIs are difficult to target with small molecules due to the intrinsically low-affinity interactions between the proteins that are diffused over a relatively large topological surface area [19]. In such cases, allosteric sites that are amenable to small molecule binding can be exploited to provide modulation of receptor function (Fig. 2C). This has been shown in the case of the neuropeptide galanin type 2 receptor (GalR2), which is considered as a promising target for seizures and epilepsy [20]. In this case, Lu and colleagues identify the small molecule PAM CYM2503 that potentiated the endogenous peptide agonism and resulted in seizure suppression in a rodent model. Numerous advantages may be realized through this approach, such as achieving the pharmacokinetics and pharmacodynamics of typical small molecules in comparison to peptide and antibody-based therapeutics.
Finally, in light of the prescription of opioid abuse crisis seen in the United States and to varying degrees worldwide, allosteric modulator pharmacotherapies of the mu opioid receptor (MOR) may provide a mechanistic barrier to drug overdose and the reduction of adverse effects or abuse potential [21]. By definition, the obligate occupation of the receptor orthosteric site must be satisfied for an allosteric modulator to modulate a receptor’s functional response to said orthosteric site-occupying ligand. Thus, there exists a “ceiling effect” or saturation of PAM/NAM effect that is dependent on orthosteric ligand function. This ceiling of allosteric modulator effect could be a feature for GPCR-targeted pharmacotherapies that traditionally carry dangerous overdose liabilities, such as opioids for analgesia.
GPCRs are a highly valuable class of proteins for drug discovery and have been actively targeted for multiple decades. Due to the functional role of GPCRs in vast physiological systems, they remain efficient targets for discovering disease interventions and the available targeting approaches must increase to meet the need of drug discovery campaigns. Allosteric modulation is an attractive approach that can provide innovative mechanisms of action at GPCRs, broaden the chemical space for a given target, and increase the tools available to medicinal chemists and chemical biologists. In GPCR-mediated physiological systems of interest that are highly nuanced, applying allosteric modulators is projected to be a viable and novel approach for future pharmacotherapy discovery.
ACKNOWLEDGEMENTS
This work was supported by grants R01 DA038446, T32 DA07287, and F31 DA045511 from the National Institute on Drug Abuse and the Ruth L. Kirschstein National Research Service Award of the National Institutes of Health.
LIST OF ABBREVIATIONS
- GPCRs
G protein-coupled Receptors
- PAM
Positive Allosteric Modulator
- NAM
Negative Allosteric Modulator
- NAL
Neutral Allosteric Ligand
- CNS
Central Nervous System
- 5-HT
Serotonin
- 5-HT2CR
5-HT2C Receptor
- 5-HT2AR
5-HT2A Receptor
- FFARs
Free Fatty Acid Receptors
- CCR5
C-C Chemokine Receptor Type 5
- KOR
Kappa Opioid Receptor
- PPIs
Protein-protein Interactions
- GalR2
Galanin Type 2 Receptor
- MOR
Mu Opioid Receptor
Footnotes
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- [1].Fredriksson R; Lagerström MC; Lundin L-G; Schiöth HB The G protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol. Pharmacol, 2003, 63(6), 1256–1272. [DOI] [PubMed] [Google Scholar]
- [2].Hauser AS; Attwood MM; Rask-Andersen M; Schiöth HB; Gloriam DE Trends in GPCR drug discovery: new agents, targets and indications. Nat. Rev. Drug Discov, 2017, 16, 829–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Christopoulos A Allosteric binding sites on cell-surface receptors: novel targets for drug discovery. Nat. Rev. Drug Discov, 2002, 1, 198. [DOI] [PubMed] [Google Scholar]
- [4].Wold EA; Chen J; Cunningham KA; Zhou J Allosteric modulation of class A GPCRs: targets, agents, and emerging concepts. J. Med. Chem, 2018, DOI: 10.1021/acs.jmedchem.8b00875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lindsley CW; Emmitte KA; Hopkins CR; Bridges TM; Gregory KJ; Niswender CM; Conn PJ Practical strategies and concepts in GPCR allosteric modulator discovery: recent advances with metabotropic glutamate receptors. Chem. Rev, 2016, 116(11), 6707–6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Foster DJ; Conn PJ Allosteric modulation of GPCRs: new insights and potential utility for treatment of schizophrenia and other CNS disorders. Neuron, 2017, 94(3), 431–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zhou J; Cunningham KA Positive-allosteric modulation of the 5-HT2C receptor: implications for neuropsychopharmacology and neurotherapeutics. Neuropsychopharmacology, 2019, 44(1), 230–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wild CT; Miszkiel JM; Wold EA; Soto CA; Ding C; Hartley RM; White MA; Anastasio NC; Cunningham KA; Zhou J Design, synthesis, and characterization of 4-undecylpiperidine-2-carboxamides as positive allosteric modulators of the serotonin (5-HT) 5-HT2C receptor. J. Med. Chem, 2018, DOI: 10.1021/acs.jmedchem.8b00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Holliday N; Watson S-J; Brown A Drug discovery opportunities and challenges at G protein coupled receptors for long chain free fatty acids. Front. Endocrinol, 2012, 2(112). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kohout TA; Nicholas SL; Perry SJ; Reinhart G; Junger S; Struthers RS Differential desensitization, receptor phosphorylation, β-arrestin recruitment, and ERK1/2 activation by the two endogenous ligands for the CC chemokine receptor 7. J. Biol. Chem, 2004, 279(22), 23214–23222. [DOI] [PubMed] [Google Scholar]
- [11].Watson C; Jenkinson S; Kazmierski W; Kenakin T The CCR5 receptor-based mechanism of action of 873140, a potent allosteric noncompetitive HIV entry inhibitor. Mol. Pharmacol, 2005, 67(4), 1268. [DOI] [PubMed] [Google Scholar]
- [12].Gurevich VV; Gurevich EV How and why do GPCRs dimerize? Trends Pharmacol. Sci, 2008, 29(5), 234–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Jin J; Momboisse F; Boncompain G; Koensgen F; Zhou Z; Cordeiro N; Arenzana-Seisdedos F; Perez F; Lagane B; Kellenberger E; Brelot A CCR5 adopts three homodimeric conformations that control cell surface delivery. Sci. Signal, 2018, 11(529), 2869. [DOI] [PubMed] [Google Scholar]
- [14].Tan Q; Zhu Y; Li J; Chen Z; Han GW; Kufareva I; Li T; Ma L; Fenalti G; Li J; Zhang W; Xie X; Yang H; Jiang H; Cherezov V; Liu H; Stevens RC; Zhao Q; Wu B Structure of the CCR5 chemokine receptor-HIV entry inhibitor maraviroc complex. Science, 2013, 341(6152), 1387–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Eichel K; Jullié D; Barsi-Rhyne B; Latorraca NR; Masureel M; Sibarita J-B; Dror RO; von Zastrow M Catalytic activation of β-arrestin by GPCRs. Nature, 2018, 557(7705), 381–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Brust TF; Morgenweck J; Kim SA; Rose JH; Locke JL; Schmid CL; Zhou L; Stahl EL; Cameron MD; Scarry SM; Aubé J; Jones SR; Martin TJ; Bohn LM Biased agonists of the kappa opioid receptor suppress pain and itch without causing sedation or dysphoria. Sci. Signal, 2016, 9(456), ra117–ra117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Latorraca NR; Wang JK; Bauer B; Townshend RJL; Hollingsworth SA; Olivieri JE; Xu HE; Sommer ME; Dror RO Molecular mechanism of GPCR-mediated arrestin activation. Nature, 2018, 557(7705), 452–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].McCorvy JD; Butler KV; Kelly B; Rechsteiner K; Karpiak J; Betz RM; Kormos BL; Shoichet BK; Dror RO; Jin J; Roth BL Structure-inspired design of β-arrestin-biased ligands for aminergic GPCRs. Nat. Chem. Biol, 2017, 14, 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Liu Z; Chen H; Wold EA; Zhou J In Comprehensive Medicinal Chemistry III. Chackalamannil S; Rotella D; Ward SE Eds.; Elsevier: Oxford, 2017, pp 329–353. [Google Scholar]
- [20].Lu X; Roberts E; Xia F; Sanchez-Alavez M; Liu T; Baldwin R; Wu S; Chang J; Wasterlain CG; Bartfai T GalR2-positive allosteric modulator exhibits anticonvulsant effects in animal models. Proc. Natl. Acad. Sci. U. S. A, 2010, 107(34), 15229–15234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Burford NT; Traynor JR; Alt A Positive allosteric modulators of the μ-opioid receptor: a novel approach for future pain medications. Br. J. Pharmacol, 2015, 172(2), 277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]