Abstract
Neutrophils are phagocytic innate immune cells essential for killing bacteria via activation of a wide variety of effector responses and generation of large amounts of reactive oxygen species (ROS). Majority of the ROS in neutrophils is generated by activation of the superoxide-generating enzyme nicotinamide adenine dinucleotide phosphate (NADPH) oxidase. Independent of their anti-microbial function, NADPH oxidase-derived ROS have emerged as key regulators of host immune responses and neutrophilic inflammation. Data from patients with inherited defects in the NADPH oxidase subunit alleles that ablate its enzyme function as well as mouse models demonstrate profound dysregulation of host inflammatory responses, neutrophil hyperactivation and tissue damage in response to microbial ligands or tissue trauma. A large body of literature now demonstrates how oxidants function as essential signaling molecules that are essential for the regulation of neutrophil responses during priming, degranulation, neutrophil extracellular trap formation, and apoptosis, independent of their role in microbial killing. In this review we summarize how NADPH oxidase-derived oxidants modulate neutrophil function in a cell intrinsic manner and regulate host inflammatory responses. In addition, we summarize studies that have elucidated possible roles of oxidants in neutrophilic responses within the oral mucosa and periodontal disease.
Keywords: chronic granulomatous disease, gp91phox, NADPH oxidase, neutrophils, oxidative stress, periodontal disease, reactive oxygen species
1 |. INTRODUCTION
Reactive oxygen species (ROS) collectively refer to a large group of highly reactive derivatives of oxygen generated as a consequence of metabolic processes or during host stress response. Although associated with oxidative damage, when produced at low regulated levels, oxidants are essential for redox modulation of cellular pathways, immune effector function, cell signaling and anti-microbial responses. The majority of ROS generated in a cell is by the activation of the nicotinamide adenine dinucleotide phosphate (NADPH) oxidases or NOX enzyme complexes. NOX enzyme family members NOX1, NOX2, NOX3, NOX4, NOX5 and the dual oxidase (DUOX) enzymes DUOX1, and DUOX2 are membrane associated hetero-oligomeric complexes that are dedicated to generation of ROS by using oxygen as its substrate. NOX enzymes are expressed in a cell specific or tissue specific manner and are rapidly activated in response to external stressors to generate large amounts of ROS. NOX2 or gp91phox is the main catalytic subunit of the leukocyte NADPH oxidase complex. It is highly expressed in phagocytes (neutrophils, monocytes, macrophages, dendritic cells) and at much lower levels in lymphocytes, endothelial cells and colonic epithelial cells.1,2 NOX2 derived oxidants modulate multiple cellular processes independent of their anti-microbial function. For instance, NADPH oxidase activation is essential for antigen presentation in B cells3, T cell receptor stimulation4 and subset polarization.5,6 In addition, Nox2 has also been detected in colonic epithelial cells where its capacity to produce superoxide (O2−) in response to microbial stimulations may facilitate the attachment of commensals such as Escherichia coli to the colonic mucus layer.7 Epithelial cell-derived O2− within the gut has been implicated to modulate the composition of the gut microbiome, by altering gene transcription and crosstalk between bacteria. It should be noted that a homolog of Nox2, Noxl is expressed in the epithelial cells in the colon and may functionally overlap with Nox2 in the responses of these cells to the gut microbiome.8,9 These findings indicate that oxidants play diverse roles in the regulation of host responses. NOX2 has been extensively studied over the last 40–50 years for its role in the activation of the phagocyte oxidative burst (Phox), also known as the respiratory burst during phagocytosis; however, recent data from our lab and others now demonstrate a critical role for NOX2-derived ROS in the modulation of neutrophil responses during inflammation. In this review, we will specifically focus on the newly appreciated roles of NOX2 or the leukocyte NADPH oxidase in the regulation of neutrophil effector functions in the context of inflammation and inflammatory disorders.
Neutrophils are the most abundant leukocytes in human blood that are crucial for host innate immune responses during infection and inflammation. In response to soluble or particulate stimuli, neutrophils activate a battery of effector responses including the activation of NADPH oxidase to produce large quantities of ROS. Oxidants generated are crucial for pathogen elimination, and can further activate or modulate other effector responses in neutrophils such as priming, degranulation, apoptosis and formation of neutrophil extracellular traps (NET). Neutrophils are functionally and phenotypically heterogeneous cells that influence the outcome in multiple chronic inflammatory diseases such as arthritis, dermatitis, and periodontitis, thus playing a key role in immune regulation.10,11 Here we present mechanistic insights derived from published literate on how NADPH oxidase derived oxidants regulate neutrophil effector responses outside of their role in microbial killing. Excessive ROS generation by neutrophils is often associated with oxidative stress and chronic inflammation, however this view is now being challenged from observations in patients and mouse models where complete loss of NADPH oxidase activity was associated with hyperinflammation. We have summarized recent published data from our laboratory and others that supports the somehow counter-intuitive role of NADPH oxidase in dampening host inflammation and redox regulation of host inflammatory pathways.
2 |. COMPOSITION, ASSEMBLY AND ACTIVATION OF THE NADPH OXIDASE COMPLEX
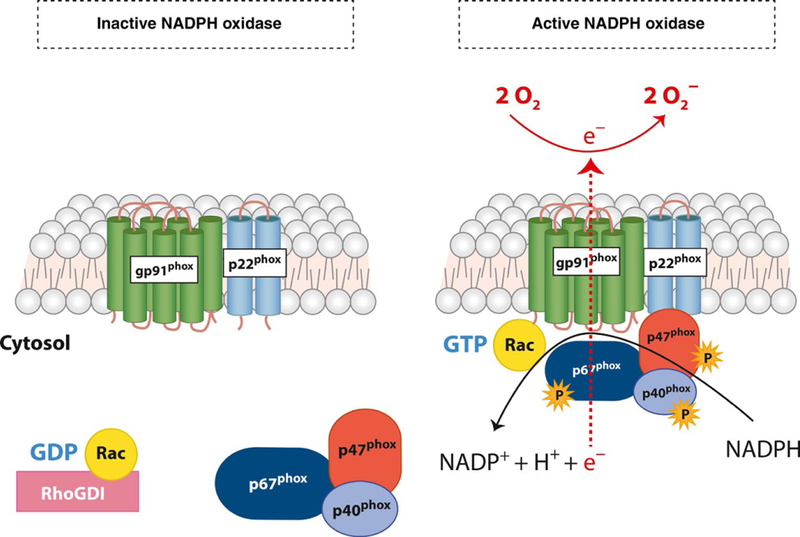
The NADPH oxidase complex is comprised of 2 membrane bound subunits and three cytosolic regulatory subunits, all encoded by distinct genes. The phagocyte oxidase or phox subunits are referred to by their molecular mass or gene names and assemble together to form an active complex that transfers NADPH-derived electrons to its substrate O2. The membrane-restricted flavocytochromeb558 is a homodimer formed by two integral membrane restricted proteins, the glycosylated gp91phox (NOX2 or CYBB), and the non-glycosylated p22phox (CYBA) subunits. The gp91phox subunit is the main redox center of the complex, and is responsible for the electron transferase activity of the complex. The cytosolic flavin adenine dinucleotide (FAD) domain of gp91phox receives two electrons from NADPH that are sequentially transferred along two internal heme moieties within the membrane-spanning gp91phox core. The p22phox subunit is an obligate binding partner of gp91phox and is essential for the stability of the heterodimer. It also provides docking sites critical for the assembly of the regulatory subunits of the oxidase that translocate from the cytosol.12 In resting neutrophils, the majority of the flavocytochromeb558 stores are localized within the specific (secondary) granules and secretory vesicles. Neutrophil activation or priming causes granule exocytosis delivering the flavocytochromeb558 to the plasma and phagosomal membranes.13
Activation of the enzyme complex is tightly regulated by a series of protein-protein and lipid binding interactions that mediate the translocation of the cytosolic subunits of NADPH oxidase to the plasma or phagosomal membrane. Concurrent events lead to Rac activation. Rac belongs to the Rho family of GTPases and is an obligate binding partner of the NADPH oxidase enzyme complex. The cytosolic subunits p47phox(Neutrophil Cytosolic Factor 1 [NCF1]), p67phox (NCF2), and p40phox (NCF4), form a tripartite complex in the cytosol of quiescent neutrophils (Figure 1). In resting cells, p47phox is present in an inactive or auto-inhibitory conformation. Upon phagocytosis or receptor ligation, downstream kinases such as protein kinase C (PKC) isoforms, protein kinase A (PKA), phosphoinositide 3-kinase (PI3K) and mitogen activated protein kinases (MAPKs) ERK1/2 and p38 mediate p47phox phosphorylation.14,15 Phosphorylation induced conformational changes in p47phox exposes its Src-homology (SH) domain that interacts with Proline residues on p22phox.16 p47phox also contains a PX (Phox homology) domains that bind to the transiently generated phosphoinositides within the membrane. This interaction allows for the retention of the subunit on the membrane.15
FIGURE 1.
NADPH oxidase structure and assembly. The membrane-restricted heterodimer of NADPH oxidase is comprised of gp91phox and p22phox subunits. This heterodimer is known as flavocytochromeb558. In an inactive state, the cytosolic subunits p67phox, p47phox, and p40phox remain in the cytosol in a self-inhibitory confirmation. On activation, cellular kinases induce the phosphorylation of cytosolic subunits, releasing the inhibitory confirmation. p67phox, p47phox, and p40phox translocate to the membrane along with Rac-GTP and bind to the flavocytochromeb558 forming an active enzyme complex. NADPH-derived electrons are transferred to the substrate molecular oxygen (O2) generating superoxide (O2−) on the other side of the membrane.
Activation and membrane translocation of p47phox also brings p67phox and p40phox to the membrane. p67phox has an internal activation domain that regulates the transfer of NADPH-derived electrons to the flavin domain of gp91phox.17 p67phox interacts with GTP-bound Rac, an essential component of the NADPH oxidase holoenzyme. Concurrent to the activation of NADPH oxidase cytosolic subunits, Rac-GDP is converted to its active Rac-GTP form by guanine nucleotide exchange factors (GEFs). Rac-GTP translocates to the membrane and binds to gp91phox and p67phox.18‘22 p47phox and p40phox act as adaptor proteins that positively regulate enzyme assembly on the plasma membrane. p40phox plays a specialized role in regulating NADPH oxidase activation. It binds to the phosphatidylinositol 3-phosphate (PI(3)P) generated in the membrane via its PX domain. Matute et al reported a patient with missense mutations in the PI3P binding domain of p40phox had selective defects in phagosomal ROS generation in neutrophils, while the plasma membrane levels were unaffected.23,24 Thus, p40phox positively regulates NADPH oxidase function and is essential for high-level O2− generation within phagosomes.
Upon complete assembly, the NADPH oxidase transfers NADPH derived-electrons to molecular O2 generating O2−(Figure 1). Large amounts of O2− are generated by human neutrophils. For instance, 7–10 nmol min−1 10−6 cells of O2− are produced on stimulation with phorbol 12-myristate 13-acetate (PMA) as measured by ferric cyto-chrome C assays. Other factors contributing to sustained O2− production include a quick turnover of the enzyme complex and high affinity for its substrate O2. The Km for oxygen (NADPH oxidase substrate) is ~10, equivalent to ~7 mm Hg pO2. Thus, the NADPH oxidase complex can continue to produce O2− in tissues with low oxygen tension such as the gingival cavity.20,25
Excessive ROS generation or prolonged activation of the enzyme complex can cause oxidative damage and prolong inflammation. Thus, the activity of NADPH is tightly regulated and triggered only in response to stimuli via complex signaling pathways. Apart from the kinases that mediate subunit phosphorylation and membrane phospholipid generation, activation of voltage gated proton channels, chloride channels and accompanying calcium fluxes also regulate NADPH oxidase activity (reviewed by Nunes et al18). The absolute amount of ROS generated is also dependent on the priming status and complexing of activating receptors on neutrophils. Further, neutrophils also contain “deactivation pathways” that lead to disassembly of the NADPH oxidase and terminate O2− generation (reviewed by Decoursey et al26).
3 |. NADPH OXIDASE IN OXIDATIVE KILLING OF MICROBES
Neutrophils are phagocytic cells whose primary function is to defend the host against bacterial and fungal infections. Inherited defects that adversely affect neutrophil numbers or their effector functions are associated with higher susceptibility to recurrent infections indicating that neutrophils are indeed key in the early responses to an infection. To facilitate the recognition of microbial ligands, and their phagocytosis, neutrophils express a large arsenal of receptors. Ligation or activation of Toll like receptors (TLRs), Fc gamma receptors (FcR), G-protein coupled receptors (GPCRs), C-type lectin receptors and integrins on neutrophils potently activates NADPH oxidase, generating large amounts of oxidants.27,28 Separately, antimicrobial granule proteins (lactoferrin, myeloperoxidase, defensins, proteases, lysozyme, calprotectin, etc) are rapidly mobilized into phagosomes or into the extracellular environment that aid in microbial killing. Conventionally this has been viewed as a non-oxidative mechanism of killing. However, now it is increasingly apparent that the oxidative (ROS generation) and non-oxidative mechanisms synergize and work in concert to kill bacteria. We refer the readers to excellent reviews by Nauseef et al29 and Uriarte et al,30 that discuss the synergy and interdependence of these processes.
Although large amounts of O2− are produced during phagocytosis, O2− anions have low antibacterial activity and are short lived (microseconds). They either dismutate spontaneously or are enzymatically converted to hydrogen peroxide (H2O2) by superoxide dismutase. H2O2 is further converted to other forms of ROS with potent anti-microbial activity by the myeloperoxidase-hydrogen peroxide-halide system that converts H2O2 to hypochlorous acid on reaction with chloride ions (Cl−). Myeloperoxidase (MPO) released from neutrophil primary/azurophilic granules into the phagosomes is critical for this process. Neutrophils isolated from patients with MPO deficiency are less effective in killing bacteria, demonstrating a synergy between oxidative and non-oxidative killing pathways.31 Separately, H2O2 can also be oxidized by iron to produce hydroxyl radicals (OH−) via Fenton chemistry that is highly toxic to bacteria.27,32 The antimicrobial potential for ROS stems from its ability to cause irreversible damage to bacterial DNA, proteins, and lipids that eventually kills the bacteria. To prolong their survival, several pathogenic bacteria actively produce toxins or effector proteins to prevent the translocation of NADPH oxidase subunits to phagosomes and restrict NADPH oxidase assembly on the phagosome. Pathogens such as Francisella tularensis, Anaplasma phagocytophilum, and Helicobacter pylori have developed strategies to subvert killing by neutrophils. Virulence mechanisms employed by these pathogens are listed in Table 1.
TABLE 1.
Bacterial suppression of neutrophil respiratory burst
| Pathogen | Mechanism(s) |
|---|---|
| Anaplasma phagocytophilum | On internalization, the bacterium resides within a protective vacuole that excludes flavocytochromeb558103,104; down-regulates CYBB (gp91phox) gene expression.105 |
| Coxiella burnetii | Membrane translocation of p47phox and p67phox is inhibited, in part by the production of a diffusible acid phosphatase.106,107 |
| Francisella | Francisella tularensis (live vaccine strain) globally inhibits activation of respiratory burst by preventing flavocytochromeb558 as well as cytosolic p47phox and p67phox acquisition on phagosomes.108 Francisella novicida U112 produces an acid phosphatase that prevents phosphorylation and subsequent nuclear translocation of p47phox.109 |
| Helicobacter pylori | Although bacteria are internalized rapidly, O2− anions are produced extracellularly, but not inside phagosomes. p47phox, p67phox and gp91phox are excluded from the phagosome by unopsonized H. pylori strains.110 |
| Neisseria gonorrhoeae | Strains lacking Opacity (Opa)-Associated Protein on cell surface prevent the prevent p47phox recruitment to phagosomes.111 |
| Yersinia pestis | Produces YopE, a Type 3 secretion system (T3SS) effector protein that has GTPase like activity and inactivates Rac in neutrophils suppressing oxidative bust.112,113 |
| Pseudomonas aeruginosa | Produces T3SS effectors ExoS and ExoT that inhibit PI3K signaling essential for activation of NADPH oxidase.114 |
| Group A Streptococcus | Produces pore-forming toxin Streptolysin that inhibits NADPH oxidase activity via incompletely characterized mechanisms.115 |
| Bordetella pertussis | Produces adenylate cyclase toxin-hemolysin (CyaA) that breaks down ATP to cyclic-AMP to activate Src homology region 2 domain-containing phosphatase-1 (SHP-1). SHP-1 inactivates MAPKs (ERK and p38). This reduces p47phox phosphorylation and activation.116 |
| Bacillus anthracis | Produces lethal toxin (LTx) and edema toxin (EDx) that interfere with upstream MAPK signaling events preventing optimal activation of NADPH oxidase subunit and subsequent assembly of active enzyme complex. EDx can also upregulate conversion of ATP to cAMP and inhibit ROS via mechanism similar to CyaA.117 |
The critical role of NADPH oxidase in host antimicrobial responses was evident from chronic granulomatous disease (CGD), an immunodeficiency affecting ~1 out of 200 000 individuals in the US. X-linked (CYBB) or autosomal recessive mutations in NADPH oxidase genes (CYBA, NCF1, NCF2, and NCF4) that ablate enzyme activity cause CGD.23,24,33–35 CGD patients suffer from life-threatening bacterial and fungal infections resulting in high mortality and morbidity. Over-representation of certain bacterial infections caused by catalase positive microorganisms such as Staphylococcus aureus, Pseudomonas, Burkholderia cepacia as well as fungal species such as Aspergillus are common. Despite prophylactic use of antibiotics and antifungals, the average life span of CGD patients is short (four decades) and associated with significant morbidity. Thus, the oxidants generated by NADPH oxidase are critical mediators of host anti-microbial defense.
4 |. NADPH OXIDASE IN NEUTROPHIL PRIMING AND DEGRANULATION
Neutrophil priming or pre-activation refers to the broad range of phenotypic and molecular changes that poise neutrophils in a state of heightened sensitivity or “readiness” to respond to subsequent secondary stimulation. Circulating neutrophils exist in a relatively quiescent state. Their activation is dynamically regulated and pro-gresses from a quiescent state to an intermediate “primed” state as they transmigrate to the site of infection or injury and encounter low levels of inflammatory cytokines (TNF-α, GM-CSF, IL-1β), chemoattractants (C5a, fMLF PAF, LTB4) or TLR agonists (LPS, flagellin, lipopeptides). A comprehensive list of neutrophil priming agents can be found in excellent reviews by Miralda et al36 and El-Benna et al.37
Priming augments the microbicidal capacity of neutrophils by enhancing O2− production, degranulation responses, inflammatory mediator production, and phagocytic capacity.36–38 However, priming alone does not cause preassembly of the NADPH oxidase complex. Priming agents can induce partial phosphorylation of NADPH oxidase cytosolic subunits with partial exocytosis of secretory vesicles and secondary granules that contain ~60%−70% flavocytochromeb558 to the plasma membrane. GM-CSF and TNF-α induce partial phosphorylation of p47phox at the Ser345 residue via activation of ERK1/2 and p38 MAPKs. Site-directed mutagenesis experiments demonstrated that phosphorylation of Ser345 on p38 is critical for neutrophil priming.39,40 The TNF-α-induced increase of gp91phox at the plasma membrane is dependent on granule exocytosis.41 Endotoxin induced priming responses elicited by low doses of lipopolysaccharides (LPS) induce the redistribution of flavocytochromeb558 from secondary granules to the plasma membrane42 and also into the recycling endosomal compartments. Thus changes in the location and/or phosphorylation of NADPH oxidase subunits upon priming leads to the generation of tonic or low basal levels of intracellular ROS critical in regulating the magnitude of neutrophil degranulation responses to secondary stimulation.43,44
Neutrophils contain four different types of granules. The primary (azurophilic) granules contain histotoxic enzymes, including elastase, MPO, and antimicrobial enzymes, cathepsins and defensins. The secondary and tertiary granules contain lactoferrin and matrix metalloprotease 9 (also known as gelatinase B), respec-tively. The secretory vesicles in human neutrophils contain human serum albumin, adhesion molecules and receptors such as CD11b and formyl peptide receptor (FPR1). Degranulation is a hierarchical process that is mediated by tightly regulated pathways.45 While exocytosis of tertiary granules and secretory vesicles can occur readily, exocytosis of azurophilic granules requires neutrophil priming, MAPK activation and low-level ROS generation.46,47 Potera et al suggested that NADPH oxidase-derived ROS are essential for preventing excessive degranulation responses. Further, neutrophils isolated from CGD patients that lack NADPH oxidase activity had significantly elevated elastase release from primary granules under both, unstimulated and TNF-α primed conditions.46 CGD neutrophils also have enhanced basal levels of activation markers like CDiib.47 These data indicate that NADPH oxidase-derived ROS are necessary to provide a negative feedback loop and prevent excessive degranulation that might lead to tissue damage. Alternatively, hyperactivation of other activation markers may reflect an attempt to compensate for the defect in ROS-mediated microbial killing due to the lack of NADPH oxidase activity.
While priming can facilitate the microbicidal potential of neutrophils, it can sustain or amplify neutrophil responses, thereby exacerbating chronic inflammatory disorders. Primed neutrophils have been observed in blood isolated from patients with systemic insults such as acute lung injury (ALI), trauma and hemorrhagic shock36 as well as from patients with chronic diseases such as inflammatory bowel disease,48 arthritis49 and periodontitis.50,51 Thus, understanding the molecular basis of how oxidants govern neutrophil priming responses is essential.
5 |. NADPH OXIDASE IN NET FORMATION
NETosis refers to the extrusion of decondensed chromatin that is in-terlaced with neutrophil granular proteins and mitochondrial mate-rial from neutrophils. NETs contain nuclear DNA, are proteolytically active, and form a mesh like structure to trap bacteria in vitro and in vivo. NETs have emerged as an important arm of innate immune anti-microbial responses since their first description by Brinkmann et al.52 However, the molecular pathways leading to NET formation are diverse and NET formation can occur via a NADPH oxidase dependent or independent manner.
A diverse range of stimuli such as bacterial and fungal pathogens, calcium ionophores, inflammatory cytokines, PMA and immune complexes can all induce NETosis in vitro. In the NADPH oxidase dependent pathway of NET generation, O2− is converted to H2O2 which is the substrate for MPO. Thus, ROS mediated MPO activation and azurophilic granule mobilization causes the release of neutrophil elastase into the cytosol. Once in the cytosol, neutrophil elastase cleaves F-actin to break down plasma membrane integrity and also translocates to the nucleus to cleave histones promoting chromatin decondensation and subsequent extrusion.53 Chromatin decondensation is also promoted by the activation of peptidylarginine deiminase 4, an enzyme that citrullinates histones H3.54 CGD neutrophils fail to form NETs in response to various stimuli.55,56 The role of ROS in NET formation has also been controversial due to discrepancies in comparing healthy neutrophils treated with ROS scavengers to neutrophils isolated from CGD patients. Separately, the requirement for NADPH oxidase-derived ROS can vary depending on the nature of the activating stimuli. While the NADPH oxidase was dispensable for NET formation in response to Candida albicans, CGD neutrophils produced significantly less NETs in response to Aspergillus fumigatus, another fungal pathogen associated with life-threaten-ing infections and mortality in CGD patients.
6 |. NADPH OXIDASE IN NEUTROPHIL LIFESPAN
Neutrophils are the most abundant immune cell in the blood and are recruited in large numbers to inflammatory sites where their collective histotoxic potential can prolong inflammation. Thus, timely regulation of neutrophil apoptosis is essential for resolution of inflammation and prevention of the release of its cytotoxic cargo into the tissue environment. Neutrophil apoptosis occurs via highly conserved programmed cell death pathways. The extrinsic pathway is triggered via ligation and oligomerization of death receptors (Fas, TRAIL, TNF-α receptors) that lead to the formation of a death inducing signaling complex and subsequent caspase activation. The intrinsic pathway is induced by incompletely characterized factors that precipitate in mitochondrial outer membrane permeabilization and the release of pro-apoptotic proteins (Bcl2 family members) into the cytosol. This leads to activation of executioner caspases.57–59
In neutrophils apoptosis is “potentiated” via the activation of the NADPH oxidase and ROS generation. Phagocytosis and consequent NADPH oxidase activation by bacteria trigger a third apoptosis pathway, known as the phagocytosis induced cell death (PICD) pathway.60–66 Activation of the NADPH oxidase is positively regulated by the numbers of bacteria phagocytosed (multiplicity of infection),63 as well the complexing of NADPH oxidase activating receptors.66 Certain pathogens actively subvert NADPH oxidase activity sub-verting PICD to survive in neutrophils (Table 1). CGD neutrophils lacking NADPH oxidase function are unable to undergo PICD.67 When injected with heat-killed S. aureus, oxidase null mice exhibited defective apoptosis in vivo leading to impaired recognition and subsequent clearance.68 Delayed neutrophil apoptosis can dysregulate resolution as neutrophils in inflammed tissues can actively sustain inflammation via prolonged production and release of inflammatory mediators.
7 |. NADPH OXIDASE IN EFFEROCYTOSIS OF APOPTOTIC NEUTROPHILS
Efferocytosis refers to the ingestion of apoptotic cells by professional phagocytes and other non-phagocytic cells. Prompt removal of apoptotic cells is essential to prevent the loss of cellular integrity and the leakage of cellular contents. Dying cells advertise their presence by the production of several secreted “find me” signals and simultaneously undergo molecular changes that generate “eat me” signals essential for uptake. Up-regulation of phosphatidylserine (PS) in the outer leaflet of the plasma membrane of apoptotic cells provides a key apoptosis associated ligand. PS receptors (TIM4, BAI1, TAM) then regulate the uptake of PS expressing cells and their subsequent clearance.69,70 Recent studies demonstrated that oxidation of PS at the fatty acyl chains transforms it to a more potent agonist of PS receptors such as CD36.71,72 The NADPH oxidase is involved in both PS oxidation and externalization that promote the recognition of apoptotic neutrophil and eventual clearance by macrophages.72,73 NADPH oxidase inhibitors that delayed lyso-PS generation delayed the uptake of apoptotic neutrophils in vitro. In vivo studies using gp91phox−/− mice demonstrated that lower levels of lyso-PS correlated with dysregulated clearance and prolonged inflammation in gp91phox−/− mice during zymosan-induced peritonitis.73
We recently demonstrated that NADPH oxidase was also involved in the efferocytic clearance of ingested apoptotic neutrophils by mouse peritoneal exudate macrophages (PEMs). ROS generation positively regulated efferosomal acidification and proteolysis of ingested apoptotic neutrophils. PEMs from gp91phox−/− mice that lack the capacity to generate ROS, exhibited significant delays in the clearance of ingested apoptotic cells. Cross presentation of apoptotic cells associated antigens to CD8 T cells was enhanced leading to CD8 T cell clonal expansion.74 Ingestion of apoptotic neutrophils is also an important resolution signal and reprograms macrophages from a pro-inflammatory (M1) phenotype to an anti-inflammatory or pro-resolving (M2) and augments the generation of pro-resolving mediators. Impaired efferocytosis in gp91phox−/− mice correlated with skewing of macrophages to the M1 phenotype and reprogramming defects that enhanced inflammation.75,76 Monocyte derived-macrophages from CGD patients produced significantly lower levels of anti-inflammatory mediators, prostaglandin D2 (PGD2) and TGF-B on efferocytosis.77 Thus, the NADPH oxidase is essential not only for the proper recognition and digestion of apoptotic neutrophils, but also plays an essential role in reprogramming efferocytosing macrophages to promote resolution of inflammation. CGD patients are markedly compromised in their ability to produce the anti-in-flammatory mediators PGD2 and TGF-β during the phagocytosis of apoptotic cells.
8 |. NADPH OXIDASE MODULATES ACUTE AND CHRONIC INFLAMMATORY RESPONSES IN VIVO
Excessive ROS generation has been associated with oxidative stress in multiple chronic inflammatory disorders such as periodontitis, cardiovascular disease, neurodegenerative disorders and inflammatory diseases.1 However these associations are based on correlative data linking oxidative stress biomarkers with disease and it is unclear whether the presence of oxidative stress is a cause or consequence of low-grade inflammation associated with these disorders. More di-rect approaches using deletion of NADPH oxidase genes in mouse models that entirely ablates NADPH oxidase derived ROS as well as data from CGD patients all paradoxically showed worse, often profound inflammation in the case of oxidase deficiency (Table 2). For instance, CGD patients are highly susceptible to sterile inflammatory complications, such as granulomatous inflammation in the genitourinary and gastrointestinal tract, and discoid mucocutaneous skin lesions. Further, mice lacking NADPH oxidase activity (due to deletion of Cybb [encoding gp91phox]) exhibited hyper-inflammatory responses characterized by elevated levels of pro-inflammatory cytokines and excessive neutrophilic recruitment in multiple models of sterile or microbe-elicited challenge.74,78‘80 Interestingly, hyperinflammation in these mice was observed even in the absence of active infection. In vivo challenge with heat killed A. fumigatus hyphae,81 LPS,82 Dectin-1 agonists,83 zymosan75,84 and even necrotic cells,79 all resulted in exuberant neutrophilic inflammation characterized by enhanced levels of pro-inflammatory cytokines, neutrophil and macrophage infiltration and delayed resolution (summarized in Table 2). We recently demonstrated excessive mobilization of neutrophils from the bone marrow reserves in Cybb−/− mice in a model of sterile peritoneal injury. Excessive neutrophil mobilization augmented tissue accrual of neutrophils and significantly delayed resolution of inflammation in Cybb−/− mice compared to wildtype mice.79 ROS deficiency also predisposes to autoimmune disorders. Oxidase null mice developed worse disease in models of collagen-induced arthritis,85,86 mannan-induced psoriasis85 and developed lupus-like disease with glomerular-nephritis.87–89 These data indicate that oxidants in fact are essential for suppressing excessive activation of host inflammatory responses triggered by endogenous and microbial stimulation.
TABLE 2.
Inhibitory role of oxidants during host inflammation
| Agonist/model | Inflammatory response |
|---|---|
| LPS intra-tracheal instillation | 1. Neutrophilic alveolitis; enhanced Nf-κB activation associate with aggravated lung injury in p47phox−/− mice.82,118 2. Decreased IL-10 production through attenuation of Akt-GSK3-β pathways in p47phox−/− mice.82 |
| Zymosan intra-tracheal | Neutrophilic inflammation and augmented inflammatory cytokine production in p47phox−/− mice.119 |
| Aspergillus fumigatus | 1. Invasive Aspergillosis, neutrophilic inflammation and granulomas in gp91phox−/− mice.120
2. Defective autophagy resulting in augmented IL-1β production that worsens inflammation in p47phox−/− mice.121 |
| Tobacco smoke exposure | Exacerbated inflammation and lung emphysema in oxidase null (gp91phox−/− and p47phox−/−) mice.122 |
| Gram negative sepsis | Enhanced PMN alveolar transmigration and acute lung injury in oxidase null mice (gp91phox−/− and p47phox−/−).123 |
| Systemic inflammatory response syndrome (SIRS) | Early mortality due to hyper-inflammation and advanced lung pathology in oxidase null mice (gp91phox−/−).84 |
| Necrotic cells/DAMPs | Leukocytosis, neutrophilia and elevated tissue accrual of neutrophils that prolongs inflammation gp91phox−/− mice.79,80 |
| Branched β-glucans | Skin granulomas.81,83 |
| Collagen induced arthritis | Enhanced T cell autoreactivity, and development of severe arthritis.85,124 |
| Influenza model | Increased inflammatory cell infiltration, Th1 driving cytokines in gp91phox−/− mice.125 |
Periodontal diseases collectively refer to a broad range of inflammatory conditions that affect the supporting structures of the teeth (gingiva, alveolar bone, and periodontal ligament). Neutrophil dysfunction, hyperactivation, and hyper-recruitment have all been associated with worse periodontal disease, pointing to a key role of neutrophils in gingival homeostasis.11 Interestingly, contrary to the protective role of NADPH oxidase in limiting host inflammation, some studies ascribe excessive ROS generation by neutrophils in response to periodontal bacteria as a significant contributing factor to the pathophysiology of periodontitis.50,90,91 Whether excessive ROS generation by neutrophils in this case is a cause or consequence of active inflammation associated with periodontitis is unclear. Proinflammatory cytokines such as TNF-α are often elevated in the gingival crevicular fluid and serum of patients with localized aggressive or chronic forms of periodontitis.92–94 TNF-α induces phosphorylation and membrane translocation of cytosolic regulatory subunits of the oxidase, resulting in the preassembly of active NADPH oxidase enzyme complex on endosomal membranes. Thus, neutrophils isolated from peritonitis patients with elevated circulating levels of cytokines are likely to have been “primed” in vivo, which accounts for an augmented oxidative burst on secondary in vitro challenge with other agonists as compared to neutrophils isolated from healthy individuals.18,43
Others report that inhibiting ROS by the use of ROS inhibitors such as N-acetylcysteine (NAC) ameliorates ROS mediated cytotoxicity, and inflammatory pathways relevant to gingival inflammation in vitro.95 However, it should be noted that ROS inhibitors, such as NAC, can independently and non-specifically inhibit inflammatory pathways, complicating interpretation.96 Thus, systematic studies using gene-targeted approaches to specifically delineate the role of NADPH oxidase-derived ROS in periodontal diseases as well as other chronic inflammatory disorders are needed to determine how ROS modulates host inflammatory response.
9 |. CONCLUSIONS
Our understanding of the function of the NADPH oxidase has greatly evolved from it being simply an antimicrobial effector to a master regulator of host inflammatory pathways. Besides its role in the regulation of neutrophil effector responses (Figure 2), NADPH oxidase activation is also essential for antigen presenta-tion, autophagy, chemotaxis, and redox signaling in other immune cells. The global role played by the NADPH oxidase in the modulation of host inflammatory responses is also supported by genome wide association studies that link single nucleotide polymorphisms and hypomorphic NADPH oxidase subunit alleles with various chronic inflammatory and autoimmune disorders such as arthritis,97 lupus,98–100 and inflammatory bowel disease.101,102 We along with others demonstrated that oxidants were in fact essential for regulating both the duration and magnitude of host immune responses in multiple murine models. Thus although excessive ROS production might indeed cause oxidative stress, low or tonic levels of ROS are essential to regulate host inflammatory pathways and prevent chronic inflammation. These functions of ROS are independent of its anti-microbial role and we are only beginning to un-derstand the complexities through the use of total or conditional oxidase null mice.
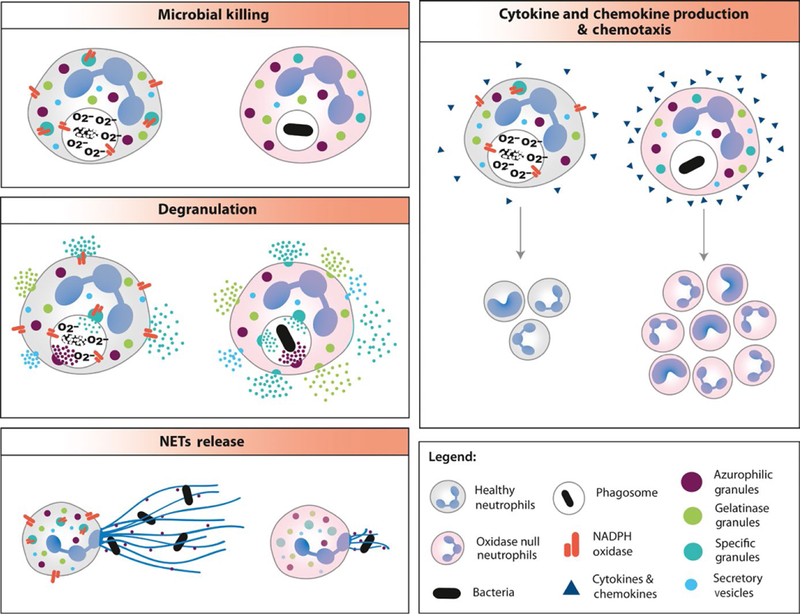
FIGURE 2.
NADPH oxidase derived ROS differentially modulate neutrophil effector responses. NADPH oxidase deficient (oxidase null) neutrophils are deficient in killing of catalase positive microorganisms and Aspergillus species. NET production is also compromised in response to select stimuli. ROS deficiency enhances degranulation, cytokine and chemokine generation that might lead to persistent inflammation by recruitment of other immune cells and/or their differential polarization. NADPH, nicotinamide adenine dinucleotide phosphate; NET, neutrophil extracellular traps; ROS, reactive oxygen species
ACKNOWLEDGEMENTS
This work was supported by DE024509 to SMU; DE28031 and GM125504 to JB.
Funding information
National Institute of General Medical Sciences, Grant/Award Number: GM125504; National Institute of Dental and Craniofacial Research, Grant/Award Number: DE024509 and DE028031
Footnotes
CONFLICT OF INTEREST
None declared.
REFERENCES
- 1.Egea J, Fabregat I, Frapart YM, et al. European contribution to the study of ROS: a summary of the findings and prospects for the future from the COST action BM1203 (EU-ROS). Redox Biol. 2017;13:94–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lambeth JD, Neish AS. Nox enzymes and new thinking on reactive oxygen: a double-edged sword revisited. Annu Rev Pathol. 2014;9:119–145. [DOI] [PubMed] [Google Scholar]
- 3.Crotzer VL, Matute JD, Arias AA, et al. Cutting edge: NADPH oxidase modulates MHC class II antigen presentation by B cells. J Immunol. 2012;189:3800–3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jackson SH, Devadas S, Kwon J, Pinto LA, Williams MS. T cells express a phagocyte-type NADPH oxidase that is activated after T cell receptor stimulation. Nat Immunol. 2004;5:818–827. [DOI] [PubMed] [Google Scholar]
- 5.Padgett LE, Tse HM. NADPH oxidase-derived superoxide provides a third signal for CD4 T cell effector responses. J Immunol. 2016;197:1733–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yarosz EL, Chang CH. The role of reactive oxygen species in regulating T cell-mediated immunity and disease. Immune Netw. 2018;18:e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banskota S, Regmi SC, Gautam J, et al. Serotonin disturbs colon epithelial tolerance of commensal E. coli by increasing NOX2-derived superoxide. Free Radic Biol Med. 2017;106:196–207. [DOI] [PubMed] [Google Scholar]
- 8.Aviello G, Knaus UG. NADPH oxidases and ROS signaling in the gastrointestinal tract. Mucosal Immunol. 2018;11:1011–1023. [DOI] [PubMed] [Google Scholar]
- 9.Szanto I, Rubbia-Brandt L, Kiss P, et al. Expression of NOX1, a superoxide-generating NADPH oxidase, in colon cancer and inflammatory bowel disease. J Pathol. 2005;207:164–176. [DOI] [PubMed] [Google Scholar]
- 10.Hellebrekers P, Vrisekoop N, Koenderman L. Neutrophil phenotypes in health and disease. Eur J Clin Invest. 2018;48(suppl 2):e12943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hajishengallis G, Korostoff JM. Revisiting the Page & Schroeder model: the good, the bad and the unknowns in the periodontal host response 40 years later. Periodontol 2000. 2017;75: 116–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biberstine-Kinkade KJ, DeLeo FR, Epstein RI, LeRoy BA, Nauseef WM, Dinauer MC. Heme-ligating histidines in flavocytochrome b(558): identification of specific histidines in gp91(phox). J Biol Chem. 2001;276:31105–31112. [DOI] [PubMed] [Google Scholar]
- 13.DeLeoFR Allen et al. NADPH oxidase activation and assembly during phagocytosis. J Immunol. 1999;163:6732–6740. [PubMed] [Google Scholar]
- 14.Lam GY, Huang J, Brumell JH. The many roles of NOX2 NADPH oxidase-derived ROS in immunity. Semin Immunopathol. 2010;32:415–430. [DOI] [PubMed] [Google Scholar]
- 15.Hawkins PT, Stephens LR, Suire S, Wilson M. PI3K signaling in neutrophils. Curr Top Microbiol Immunol. 2010;346:183–202. [DOI] [PubMed] [Google Scholar]
- 16.Dusi S, Della Bianca V, Grzeskowiak M, Rossi F. Relationship between phosphorylation and translocation to the plasma membrane of p47phox and p67phox and activation of the NADPH oxidase in normal and Ca(2+)−depleted human neutrophils. Biochem J. 1993;290:173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nisimoto Y, Motalebi S, Han CH, Lambeth JD. The p67(phox) activation domain regulates electron flow from NADPH to flavin in flavocytochrome b(558). J Biol Chem. 1999;274:22999–23005. [DOI] [PubMed] [Google Scholar]
- 18.Nunes P, Demaurex N, Dinauer MC. Regulation of the NADPH oxidase and associated ion fluxes during phagocytosis. Traffic. 2013;14:1118–1131. [DOI] [PubMed] [Google Scholar]
- 19.Winterbourn CC, Kettle AJ, Hampton MB. Reactive oxygen species and neutrophil function. Annu Rev Biochem. 2016;85:765–792. [DOI] [PubMed] [Google Scholar]
- 20.Nauseef WM. Nox enzymes in immune cells. Semin Immunopathol. 2008;30:195–208. [DOI] [PubMed] [Google Scholar]
- 21.Groemping Y, Rittinger K. Activation and assembly of the NADPH oxidase: a structural perspective. Biochem J. 2005;386:401–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Groemping Y, Lapouge K, Smerdon SJ, Rittinger K. Molecular basis of phosphorylation-induced activation of the NADPH oxidase. Cell. 2003;113:343–355. [DOI] [PubMed] [Google Scholar]
- 23.Matute JD, Arias AA, Wright NA, et al. A new genetic subgroup of chronic granulomatous disease with autosomal recessive mutations in p40 phox and selective defects in neutrophil NADPH oxidase activity. Blood. 2009;114:3309–3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bagaitkar J, Matute JD, Austin A, Arias AA, Dinauer MC. Activation of neutrophil respiratory burst by fungal particles requires phosphatidylinositol 3-phosphate binding to p40(phox) in humans but not in mice. Blood. 2012;120:3385–3387. [DOI] [PubMed] [Google Scholar]
- 25.Gabig TG, Bearman SI, Babior BM. Effects of oxygen tension and pH on the respiratory burst of human neutrophils. Blood. 1979;53:1133–1139. [PubMed] [Google Scholar]
- 26.Decoursey TE, Ligeti E. Regulation and termination of NADPH oxidase activity. Cell Mol Life Sci. 2005;62:2173–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen GT, Green ER, Mecsas J. Neutrophils to the ROScue: mechanisms of NADPH oxidase activation and bacterial resis-tance. Front Cell Infect Microbiol. 2017;7:373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Futosi K, Fodor S, Mocsai A. Neutrophil cell surface receptors and their intracellular signal transduction pathways. Int Immunopharmacol. 2013;17:638–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nauseef WM. How human neutrophils kill and degrade microbes: an integrated view. Immunol Rev. 2007;219:88–102. [DOI] [PubMed] [Google Scholar]
- 30.Uriarte SM, Edmisson JS, Jimenez-Flores E. Human neutrophils and oral microbiota: a constant tug-of-war between a harmonious and a discordant coexistence. Immunol Rev. 2016;273:282–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klebanoff SJ, Kettle AJ, Rosen H, Winterbourn CC, Nauseef WM. Myeloperoxidase: a front-line defender against phagocytosed microorganisms. J Leukoc Biol. 2013;93:185–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winterbourn CC, Hampton MB, Livesey JH, Kettle AJ. Modeling the reactions of superoxide and myeloperoxidase in the neutrophil phagosome: implications for microbial killing. J Biol Chem. 2006;281:39860–39869. [DOI] [PubMed] [Google Scholar]
- 33.Dinauer M, Newburger P, Borregaard N. The phagocyte system and disorders of granulopoiesis and granulocyte function In: Nathan DG, Orkin SH, eds. Nathan and Oski’s Hematology of Infancy and Childhood. Philadelphia, PA: Elsevier/Saunders; 2014:773–847. [Google Scholar]
- 34.Dinauer MC. Disorders of neutrophil function: an overview. Methods Mol Biol. 2014;1124:501–515. [DOI] [PubMed] [Google Scholar]
- 35.Dinauer MC. Primary immune deficiencies with defects in neutrophil function. Hematology Am Soc Hematol Educ Program. 2016;2016:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miralda I, Uriarte SM, McLeish KR. Multiple phenotypic changes define neutrophil priming. Front Cell Infect Microbiol. 2017;7:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.El-Benna J, Dang PM, Gougerot-Pocidalo MA. Priming of the neutrophil NADPH oxidase activation: role of p47phox phosphorylation and NOX2 mobilization to the plasma membrane. Semin Immunopathol. 2008;30:279–289. [DOI] [PubMed] [Google Scholar]
- 38.Vogt KL, Summers C, Chilvers ER, Condliffe AM. Priming and de-priming of neutrophil responses in vitro and in vivo. Eur J Clin Invest. 2018;48:e12967. [DOI] [PubMed] [Google Scholar]
- 39.Dang PM-C. A specific p47phox -serine phosphorylated by con-vergent MAPKs mediates neutrophil NADPH oxidase priming at inflammatory sites. J Clin Invest. 2006;116:2033–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown GE, Stewart MQ, Bissonnette SA, Elia AE, Wilker E, Yaffe MB. Distinct ligand-dependent roles for p38 MAPK in priming and activation of the neutrophil NADPH oxidase. J Biol Chem. 2004;279:27059–27068. [DOI] [PubMed] [Google Scholar]
- 41.Uriarte SM, Rane MJ, Luerman GC, et al. Granule exocytosis con-tributes to priming and activation of the human neutrophil respiratory burst. J Immunol. 2011;187:391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DeLeo FR, Renee J, McCormick S, et al. Neutrophils exposed to bacterial lipopolysaccharide upregulate NADPH oxidase assembly. J Clin Invest. 1998;101:455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lamb FS, Hook JS, Hilkin BM, Huber JN, Volk AP, Moreland JG. Endotoxin priming of neutrophils requires endocytosis and NADPH oxidase-dependent endosomal reactive oxygen species. J Biol Chem. 2012;287:12395–12404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moreland JG, Davis AP, Matsuda JJ, et al. Endotoxin priming of neutrophils requires NADPH oxidase-generated oxidants and is regulated by the anion transporter ClC-3. J Biol Chem. 2007;282:33958–33967. [DOI] [PubMed] [Google Scholar]
- 45.Lacy P. Mechanisms of degranulation in neutrophils. Allergy Asthma Clin Immunol. 2006;2:98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Potera RM, Jensen MJ, Hilkin BM, et al. Neutrophil azurophilic granule exocytosis is primed by TNF-alpha and partially regulated by NADPH oxidase. Innate Immun. 2016;22:635–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Volk AP, Barber BM, Goss KL, et al. Priming of neutrophils and differentiated PLB-985 cells by pathophysiological concentrations of TNF-alpha is partially oxygen dependent. J Innate Immun. 2011;3:298–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murch SH, Lamkin VA, Savage MO, Walker-Smith JA, MacDonald TT. Serum concentrations of tumour necrosis factor alpha in child-hood chronic inflammatory bowel disease. Gut. 1991;32:913–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cascao R, Rosário HS, Souto-Carneiro MM, Fonseca JE. Neutrophils in rheumatoid arthritis: more than simple final effectors. Autoimmun Rev. 2010;9:531–535. [DOI] [PubMed] [Google Scholar]
- 50.Matthews JB, Wright HJ, Roberts A, Cooper PR, Chapple IL. Hyperactivity and reactivity of peripheral blood neutrophils in chronic periodontitis. Clin Exp Immunol. 2007;147:255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guentsch A, Puklo M, Preshaw PM, et al. Neutrophils in chronic and aggressive periodontitis in interaction with Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans. J Periodontal Res. 2009;44:368–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. [DOI] [PubMed] [Google Scholar]
- 53.Metzler KD, Goosmann C, Lubojemska A, Zychlinsky A, Papayannopoulos V. A myeloperoxidase-containing complex regulates neutrophil elastase release and actin dynamics during NETosis. Cell Rep. 2014;8:883–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li P, Li M, Lindberg MR, Kennett MJ, Xiong N, Wang Y. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J Exp Med. 2010;207:1853–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fuchs TA, Abed U, Goosmann C, et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176:231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gazendam RP, van Hamme JL, Tool AT, et al. Human neutrophils use different mechanisms to kill Aspergillus fumigatus co-nidia and hyphae: evidence from phagocyte defects. J Immunol. 2016;196:1272–1283. [DOI] [PubMed] [Google Scholar]
- 57.Witko-Sarsat V, Pederzoli-Ribeil M, Hirsh E, Sozzani S, Cassatella MA. Regulating neutrophil apoptosis: new players enter the game. Trends Immunol. 2011;32:117–124. [DOI] [PubMed] [Google Scholar]
- 58.McCracken JM, Allen LA. Regulation of human neutrophil apoptosis and lifespan in health and disease. J Cell Death. 2014;7:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kobayashi SD, Malachowa N, DeLeo FR. Neutrophils and bacterial immune evasion. J Innate Immun. 2018;10:432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kobayashi SD, Voyich JM, Buhl CL, Stahl RM, DeLeo FR. Global changes in gene expression by human polymorphonuclear leukocytes during receptor-mediated phagocytosis: cell fate is regulated at the level of gene expression. Proc Natl Acad Sci USA. 2002;99:6901–6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamamoto A, Taniuchi S, Tsuji S, Hasui M, Kobayashi Y. Role of reactive oxygen species in neutrophil apoptosis following ingestion of heat-killed Staphylococcus aureus. Clin Exp Immunol. 2002;129:479–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang B, Hirahashi J, Cullere X, Mayadas TN. Elucidation of molecular events leading to neutrophil apoptosis following phagocytosis: crosstalk between caspase 8, reactive oxygen species, and MAPK/ERK activation. J Biol Chem. 2003;278:28443–28454. [DOI] [PubMed] [Google Scholar]
- 63.Watson RW, Redmond HP, Wang JH, Condron C, Bouchier-Hayes D. Neutrophils undergo apoptosis following ingestion of Escherichia coli. J Immunol. 1996;156:3986–3992. [PubMed] [Google Scholar]
- 64.Kennedy AD, DeLeo FR. Neutrophil apoptosis and the resolution of infection. Immunol Res. 2009;43:25–61. [DOI] [PubMed] [Google Scholar]
- 65.Kasahara Y, Iwai K, Yachie A, et al. Involvement of reactive oxygen intermediates in spontaneous and CD95 (Fas/APO-1)-mediated apoptosis of neutrophils. Blood. 1997;89:1748–1753. [PubMed] [Google Scholar]
- 66.Coxon A, Rieu P, Barkalow FJ, et al. A novel role for the beta 2 integrin CD11b/CD18 in neutrophil apoptosis: a homeostatic mechanism in inflammation. Immunity. 1996;5:653–666. [DOI] [PubMed] [Google Scholar]
- 67.Kobayashi SD, Voyich JM, Braughton KR, et al. Gene expression profiling provides insight into the pathophysiology of chronic granulomatous disease. J Immunol. 2004;172:636–643. [DOI] [PubMed] [Google Scholar]
- 68.Hampton MB, Vissers MC, Keenan JI, Winterbourn CC. Oxidant-mediated phosphatidylserine exposure and macrophage uptake of activated neutrophils: possible impairment in chronic granulomatous disease. J Leukoc Biol. 2002;71:775–781. [PubMed] [Google Scholar]
- 69.Hochreiter-Hufford A, Ravichandran KS. Clearing the dead: apoptotic cell sensing, recognition, engulfment, and digestion. Cold Spring Harb Perspect Biol. 2013;5:a00874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gordon S, Pluddemann A. Macrophage clearance of apoptotic cells: a critical assessment. Front Immunol. 2018;9:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Greenberg ME, Sun M, Zhang R, Febbraio M, Silverstein R, Hazen SL. Oxidized phosphatidylserine-CD36 interactions play an essential role in macrophage-dependent phagocytosis of apoptotic cells. J Exp Med. 2006;203:2613–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arroyo A, Modriansky M, Serinkan FB, et al. NADPH oxidase-dependent oxidation and externalization of phosphatidylserine during apoptosis in Me2SO-differentiated HL-60 cells. Role in phagocytic clearance. J Biol Chem. 2002;277:49965–49975. [DOI] [PubMed] [Google Scholar]
- 73.Frasch SC, Berry KZ, Fernandez-Boyanapalli R, et al. NADPH oxidase-dependent generation of lysophosphatidylserine enhances clearance of activated and dying neutrophils via G2A. J Biol Chem. 2008;283:33736–33749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bagaitkar J, Huang J, Zeng MY, et al. NADPH oxidase activation regulates apoptotic neutrophil clearance by murine macrophages. Blood. 2018;131:2367–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fernandez-Boyanapalli RF, Frasch SC, McPhillips K, et al. Impaired apoptotic cell clearance in CGD due to altered macrophage pro-gramming is reversed by phosphatidylserine-dependent production of IL-4. Blood. 2009;113:2047–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fernandez-Boyanapalli R, Frasch SC, Riches DW, Vandivier RW, Henson PM, Bratton DL. PPARgamma activation normalizes resolution of acute sterile inflammation in murine chronic granulomatous disease. Blood. 2010;116:4512–4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brown JR, Goldblatt D, Buddle J, Morton L, Thrasher AJ. Diminished production of anti-inflammatory mediators during neutrophil apoptosis and macrophage phagocytosis in chronic granulomatous disease (CGD). J Leukoc Biol. 2003;73: 591–599. [DOI] [PubMed] [Google Scholar]
- 78.Zeng MY, et al. An efferocytosis-induced, IL-4-dependent macrophage-iNKT cell circuit suppresses sterile inflammation and is defective in murine CGD. Blood. 2013;121:3473–3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zeng MY, Pham D, Bagaitkar J, et al. NADPH oxidase controls neutrophilic response to sterile inflammation in mice by regulating the IL-1alpha/G-CSF axis. Blood. 2015;126:2724–2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bagaitkar J, Barbu EA, Perez-Zapata LJ, et al. PI(3)P-p40phox binding regulates NADPH oxidase activation in mouse macrophages and magnitude of inflammatory responses in vivo. J Leukoc Biol. 2017;101:449–457. [DOI] [PubMed] [Google Scholar]
- 81.Morgenstern DE, Gifford MA, Li LL, Doerschuk CM, Dinauer MC. Absence of respiratory burst in X-linked chronic granulomatous disease mice leads to abnormalities in both host defense and inflammatory response to Aspergillus fumigatus. J Exp Med. 1997;185:207–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Deng J, Wang X, Qian F, et al. Protective role of reactive oxygen species in endotoxin-induced lung inflammation through modulation of IL-10 expression. J Immunol. 2012;188:5734–5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schäppi M, Deffert C, Fiette L, et al. Branched fungal beta-glucan causes hyperinflammation and necrosis in phagocyte NADPH oxidase-deficient mice. J Pathol. 2008;214:434–444. [DOI] [PubMed] [Google Scholar]
- 84.Whitmore LC, Hilkin BM, Goss KL, et al. NOX2 protects against prolonged inflammation, lung injury, and mortality following systemic insults. J Innate Immun. 2013;5:565–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hultqvist M, Backlund J, Bauer K, Gelderman KA, Holmdahl R. Lack of reactive oxygen species breaks T cell tolerance to colla-gen type II and allows development of arthritis in mice. J Immunol. 2007;179:1431–1437. [DOI] [PubMed] [Google Scholar]
- 86.Wing K, Klocke K, Samuelsson A, Holmdahl R. Germ-free mice deficient of reactive oxygen species have increased arthritis susceptibility. Eur J Immunol. 2015;45:1348–1353. [DOI] [PubMed] [Google Scholar]
- 87.Kelkka T, Kienhofer D, Hoffmann M, et al. Reactive oxygen species deficiency induces autoimmunity with type 1 interferon signature. Antioxid Redox Signal. 2014;21:2231–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kienhofer D, Boeltz S, Hoffmann MH. Reactive oxygen homeostasis - the balance for preventing autoimmunity. Lupus. 2016;25:943–954. [DOI] [PubMed] [Google Scholar]
- 89.Jacob CO, Yu N, Yoo D-G, et al. Haploinsufficiency of NADPH oxidase subunit neutrophil cytosolic factor 2 is sufficient to ac-celerate full-blown lupus in NZM 2328 mice. Arthritis Rheumatol. 2017;69:1647–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chapple IL, Matthews JB. The role of reactive oxygen and antiox-idant species in periodontal tissue destruction. Periodontol 2000. 2007;43:160–232. [DOI] [PubMed] [Google Scholar]
- 91.Chapple IL. Reactive oxygen species and antioxidants in inflammatory diseases. J Clin Periodontol. 1997;24:287–296. [DOI] [PubMed] [Google Scholar]
- 92.Gümüş P, Nizam N, Lappin DF, Buduneli N. Saliva and serum levels of B-cell activating factors and tumor necrosis factor-alpha in patients with periodontitis. J Periodontol. 2014;85:270–280. [DOI] [PubMed] [Google Scholar]
- 93.Duarte PM, da Rocha M, Sampaio E, et al. Serum levels of cytokines in subjects with generalized chronic and aggressive periodontitis before and after non-surgical periodontal therapy: a pilot study. J Periodontol. 2010;81:1056–1063. [DOI] [PubMed] [Google Scholar]
- 94.Chauhan A, Yadav SS, Dwivedi P, Lal N, Usman K, Khattri S. Correlation of serum and salivary cytokines level with clinical pa-rameters in metabolic syndrome with periodontitis. J Clin Lab Anal. 2016;30:649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kanzaki H, Wada S, Narimiya T, et al. Pathways that regulate ROS scavenging enzymes, and their role in defense against tissue destruction in periodontitis. Front Physiol. 2017;8:351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zafarullah M, Li WQ, Sylvester J, Ahmad M. Molecular mechanisms of N-acetylcysteine actions. Cell Mol Life Sci. 2003;60:6–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Olsson LM, Lindqvist AK, Källberg H, et al. A case-control study of rheumatoid arthritis identifies an associated single nucleotide polymorphism in the NCF4 gene, supporting a role for the NADPH-oxidase complex in autoimmunity. Arthritis Res Ther. 2007;9:R98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Armstrong DL, Eisenstein M, Zidovetzki R, Jacob CO. Systemic lupus erythematosus-associated neutrophil cytosolic factor 2 mutation affects the structure of NADPH oxidase complex. J Biol Chem. 2015;290:12595–12602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jacob CO, Eisenstein M, Dinauer MC, et al. Lupus-associated causal mutation in neutrophil cytosolic factor 2 (NCF2) brings unique insights to the structure and function of NADPH oxidase. Proc Natl Acad Sci USA. 2012;109:E59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cunninghame Graham DS, Morris DL, Bhangale TR, et al. Association of NCF2, IKZF1, IRF8, IFIH1, and TYK2 with systemic lupus erythematosus. PLoS Genet. 2011;7:e1002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dhillon SS, Fattouh R, Elkadri A, et al. Variants in nicotinamide adenine dinucleotide phosphate oxidase complex components determine susceptibility to very early onset inflammatory bowel disease. Gastroenterology. 2014;147:680–689.e2. [DOI] [PubMed] [Google Scholar]
- 102.Schwerd T, Bryant RV, Pandey S, et al. NOX1 loss-of-function genetic variants in patients with inflammatory bowel disease. Mucosal Immunol. 2018;11:562–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Carlyon JA, Latif DA, Pypaert M, Lacy P, Fikrig E. Anaplasma phagocytophilum utilizes multiple host evasion mechanisms to thwart NADPH oxidase-mediated killing during neutrophil infection. Infect Immun. 2004;72:4772–4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jw IJ, Mueller AC. Neutrophil NADPH oxidase is reduced at the Anaplasma phagocytophilum phagosome. Infect Immun. 2004;72:5392–5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Thomas V, et al. Anaplasma phagocytophilum modulates gp91phox gene expression through altered interferon regulatory factor 1 and PU.1 levels and binding of CCAAT displacement protein. Infect Immun. 2005;73:208–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Siemsen DW, Kirpotina LN, Jutila MA, Quinn MT. Inhibition of the human neutrophil NADPH oxidase by Coxiella burnetii. Microbes Infect. 2009;11:671–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hill J, Samuel JE. Coxiella burnetii acid phosphatase inhibits the release of reactive oxygen intermediates in polymorphonuclear leukocytes. Infect Immun. 2011;79:414–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.McCaffrey RL, Allen LA. Francisella tularensis LVS evades killing by human neutrophils via inhibition of the respiratory burst and phagosome escape. J Leukoc Biol. 2006;80:1224–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mohapatra NP, Soni S, Rajaram MV, et al. Francisella acid phos-phatases inactivate the NADPH oxidase in human phagocytes. J Immunol. 2010;184:5141–5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Allen LA, Beecher BR, Lynch JT, et al. Helicobacter pylori disrupts NADPH oxidase targeting in human neutrophils to induce extracellular superoxide release. J Immunol. 2005;174:3658–3667. [DOI] [PubMed] [Google Scholar]
- 111.Smirnov A, Daily KP, Criss AK. Assembly of NADPH oxidase in human neutrophils is modulated by the opacity-associated protein expression State of Neisseria gonorrhoeae. Infect Immun. 2014;82:1036–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Songsungthong W, Higgins MC, Rolan HG, Murphy JL, Mecsas J. ROS-inhibitory activity of YopE is required for full virulence of Yersinia in mice. Cell Microbiol. 2010;12:988–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Spinner JL, Cundiff JA, Kobayashi SD. Yersinia pestis type III secretion system-dependent inhibition of human polymorphonuclear leukocyte function. Infect Immun. 2008;76:3754–3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vareechon C, Zmina SE, Karmakar M, Pearlman E, Rietsch A. Pseudomonas aeruginosa effector ExoS inhibits ROS production in human neutrophils. Cell Host Microbe. 2017;21:611–618.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Uchiyama S, Döhrmann S, Timmer AM, et al. Streptolysin O rapidly impairs neutrophil oxidative burst and antibacterial responses to group A Streptococcus. Front Immunol. 2015;6:581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cerny O, Anderson KE, Stephens LR, Hawkins PT, Sebo P. cAMP signaling of adenylate cyclase toxin blocks the oxidative burst of neutrophils through epac-mediated inhibition of phospholipase C activity. J Immunol. 2017;198:1285–1296. [DOI] [PubMed] [Google Scholar]
- 117.Crawford MA, Aylott CV, Bourdeau RW, Bokoch GM. Bacillus anthracis toxins inhibit human neutrophil NADPH oxidase activity. J Immunol. 2006;176:7557–7565. [DOI] [PubMed] [Google Scholar]
- 118.Han W, et al. NADPH oxidase limits lipopolysaccharide-induced lung inflammation and injury in mice through reduction-oxidation regulation of NF-kappaB activity. J Immunol. 2013;190:4786–4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Han W, Li H, Cai J, et al. NADPH oxidase limits innate immune responses in the lungs in mice. PLoS ONE. 2010;5:e9631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pollock JD, Williams DA, Gifford M, et al. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat Genet. 1995;9:202–209. [DOI] [PubMed] [Google Scholar]
- 121.de Luca A, Smeekens SP, Casagrande A, et al. IL-1 receptor block-ade restores autophagy and reduces inflammation in chronic granulomatous disease in mice and in humans. Proc Natl Acad Sci USA. 2014;111:3526–3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yao H, Edirisinghe I, Yang SR, et al. Genetic ablation of NADPH oxidase enhances susceptibility to cigarette smoke-induced lung inflammation and emphysema in mice. Am J Pathol. 2008;172:1222–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gao XP, Standiford TJ, Rahman A, et al. Role of NADPH oxidase in the mechanism of lung neutrophil sequestration and microvessel injury induced by Gram-negative sepsis: studies in p47phox−/− and gp91phox−/− mice. J Immunol. 2002;168:3974–3982. [DOI] [PubMed] [Google Scholar]
- 124.Hultqvist M, Olofsson P, Holmberg J, Backstrom BT, Tordsson J, Holmdahl R. Enhanced autoimmunity, arthritis, and encephalomyelitis in mice with a reduced oxidative burst due to a mutation in the Ncf1 gene. Proc Natl Acad Sci USA. 2004;101:12646–12651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Snelgrove RJ, Edwards L, Rae AJ, Hussell T. An absence of reactive oxygen species improves the resolution of lung influenza infection. Eur J Immunol. 2006;36:1364–1373. [DOI] [PubMed] [Google Scholar]