Abstract
Biventricular assist device (BiVAD) support is considered a risk factor for worse outcomes compared with left ventricular assist device (LVAD) alone for children with end-stage heart failure. It remains unclear whether this is because of the morbidity associated with a second device or the underlying disease severity. We aimed to show that early BiVAD support can result in good survival by analyzing our prospectively collected database for all pediatric patients who underwent BiVAD implantation. From 2005 to 2009, BiVADs were used exclusively. From 2010 to 2014, LVAD alone was considered, maintaining a low threshold for BiVAD support. All BiVADs were pulsatile devices. Thirty-one patients with median age of 3.5 years received BiVAD support. Diagnoses included dilated cardiomyopathy in 17 (55%), myocarditis in 6 (19%), and congenital heart disease in 3 (10%). Survival to transplant was achieved in 27 (87%) with a median duration of 41 days (interquartile range, 15–69). Adverse event rates (per 100 days of support) were bleeding at 0.52, infection at 1.17, and central nervous system dysfunction at 0.78. Of those who survived to transplant, 26 (96%) remain alive with a median follow-up of 55 months. These results show that BiVAD support can bridge patients to transplant with excellent long-term survival.
Keywords: biventricular assist device, VAD, pediatric, heart failure, survival, complications
The ventricular assist device (VAD) has provided a significant increase in survival to heart transplant (HT) over extracorporeal membrane oxygenation (ECMO) for children with end-stage heart failure.1–6 As the improved survival has become recognized, the utilization of pediatric VADs has dramatically increased, although not without significant morbidity.2,3 With increased VAD utilization, multiple risk factors for morbidity and mortality have been elucidated, including lower body surface area (BSA), younger patients, congenital heart disease (CHD), the presence of renal or hepatic dysfunction, and the use of biventricular assist device (BiVAD) support.2–4,6,7 Although some of these risk factors are clear, some are complicated by confounding factors. To make optimal decisions regarding morbidity for the child, expectations for the parent, and overall resource utilization, an accurate assessment of risk factors is essential.
Previously, many studies have shown a correlation between BiVAD implantation and a decreased survival.2,6,8,9 A recent study by Zafar et al.10 further illustrates an association of poor outcomes with BiVAD support in some patients. What remains unclear is how much of the reduced survival associated with BiVAD support should be attributed to the morbidity incurred with a second device and how much is because of the severity of the patient’s heart failure necessitating BiVAD support.7 In addition, morbidity may also be attributable to delays in right ventricular assist device (RVAD) implantation, as has been demonstrated in adults.11 As such, it has been our institutional bias for early RVAD implantation for BiVAD support.12 Herein, we describe our single-institution experience with BiVAD support using an extracorporeal pulsatile device.
Methods
Patients and Devices
Data for all pediatric patients, aged 0–18 years, admitted to St. Louis Children’s Hospital from April 2005 to June 2014 who underwent VAD implantation with a durable device were analyzed. Data were maintained in a prospectively collected database and subsequently confirmed by chart review. This study protocol was approved by the Washington University in St. Louis School of Medicine Internal Review Board with patient consent waived.
Statistical analysis was performed on all patients who underwent BiVAD support. Patients with single-ventricle anatomy were excluded. Patients who underwent support with a nondurable VAD were also excluded. All BiVADs implanted were the Berlin Heart EXCOR (Berlin Heart GmbH, Berlin, Germany), a pneumatically driven pulsatile device. Left ventricular assist device (LVAD)-only patients received one of the following: Berlin Heart EXCOR, HVAD (HeartWare, Inc., Framingham, MA), or HeartMate II (Thoratec Corp., Pleasanton, CA).
Preimplantation end-organ dysfunction was evaluated based on the kidney and hepatic function, as has been described previously.2,10 Renal impairment was determined by calculating the glomerular filtration rate (GFR) as determined by Schwartz et al.13 This was compared with their age and sex-adjusted predicted GFR by the national Kidney Foundation.14 Moderate impairment included all patients with a GFR from 30% to 99% of predicted, with severe impairment categorized as GFR < 30% of predicted. Hepatic impairment was based on the total serum bilirubin concentration and considered moderately increased from 0.7 to 1.1 mg/dl and severely increased if ≥1.2 mg/dl.
Criteria for BiVAD Implantation
From April 2005 to December 2009, BiVAD support was used exclusively. Since January 2010, a selective approach has been adopted, with a low threshold for RVAD implantation. Because no established criteria for RVAD implantation exist in pediatric patients, this was based on the clinical judgment and extrapolation from adult data. Preoperatively all patients had an echocardiogram as well as a cardiac catheterization if stable enough to tolerate the procedure. Right ventricular stroke work index was calculated, using a conservative value, ≤400 mmHg ml/m2, as a predictor of RV failure.15,16 Other preoperative factors used to assess the need for RVAD included patients on ECMO support, clinical symptoms of overt right heart failure (ascites, pleural effusions, renal, or hepatic dysfunction), severe tricuspid regurgitation, pulmonary vascular resistance index > 4 Woods units, or refractory ventricular arrhythmias. Intraoperatively, the need for RVAD was assessed with transesophageal echocardiogram and visual inspection after LVAD implantation, weaning from cardiopulmonary bypass and the initiation of vasopressors and pulmonary dilators.17 In addition, the assessment of LVAD function was used as a surrogate for RV dysfunction. By using these factors, either BiVAD or LVAD support was instituted based on the judgment of the heart failure team without any criteria considered an absolute indication for RVAD implantation.
Adverse Events
All adverse events (AEs) were recorded, focusing on infections, bleeding, and neurological events. Adverse events were documented according to their standard definitions described by the PediMACS criteria.18 In brief, their definitions were as follows: major bleeding, necessitating a reoperation or significant transfusion; major infection, clinical signs of an infection with a positive culture; neurological complication, new neurological deficit on physical examination or any findings on neurological imaging; renal dysfunction, any dialysis requirement or a serum creatinine >3 times normal; and hepatic dysfunction, an elevation >3 times normal of at least two of total bilirubin, aspartate transaminase and alanine transaminase.
Statistical Analysis
Continuous variables were expressed as medians with interquartile ranges (IQRs). A competing outcomes analysis was performed for all BiVAD patients, carried out at 8 months to capture all patients who underwent transplant, using three mutually exclusive events: HT, alive on support, and death on support.
Results
Study Cohort
During the study period, a total of 50 patients underwent VAD support with a durable device at our institution. Two patients with single-ventricle anatomy received a single VAD and were excluded. Of the remaining 48 patients who underwent VAD implantation, 17 (35%) received an LVAD only, all occurring after January 1, 2010 (Table 1). The remaining 31 (65%) patients received BiVAD support, all with a pulsatile device. Of the 31 patients who underwent BiVAD support, 21 were stable enough to undergo cardiac catheterization before VAD implantation, none of which had any catheter-associated AEs. After 2010, when a selective approach was implemented, there were a total of 29 patients who underwent VAD implantation: 12 (41%) BiVAD and 17 (59%) LVAD. The factors for BiVAD consideration are listed in Table 2 for patients who received BiVAD support. Although less frequent, some LVAD-only patients also had factors leading to the consideration of BiVAD support, although they were ultimately deemed likely to succeed with LVAD alone (Table 3). The 31 BiVAD patients were included in the statistical analysis.
Table 1.
Overall VAD Support
| Devices | |
|---|---|
| Total VAD support | 48 |
| BiVAD | 31 (65%) |
| LVAD | 17 (35%) |
| EXCOR* | 9 (53%) |
| HeartMate II† | 2 (12%) |
| HVAD‡ | 6 (35%) |
Berlin Heart EXCOR.
HeartMate II.
HeartWare HVAD.
BiVAD, biventricular assist device; LVAD, left ventricular assist device.
Table 2.
Indicators of RV Failure for Patients Who Received BiVAD Support During the Selective Era
| Factor | Patient | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9* | 10* | 11 | 12 | |
| ECMO support | X | X | X | X | ||||||||
| Clinical RV failure† | X | X | ||||||||||
| Gross RV dysfunction‡ | X | X | X | |||||||||
| Severe TR | X | X | ||||||||||
| PVRI > 4 | X | X | X | |||||||||
| RVSWI < 400 | X | X | ||||||||||
| Ventricular arrhythmias | X | X | X | X | ||||||||
| Inadequate LVAD filling | X | |||||||||||
Underwent initial LVAD and then later converted to BiVAD.
Determined by the presence of ascites, pleural effusions, renal failure, or hepatic failure.
Echo or gross inspection in the operating room after LVAD placement.
LVAD, left ventricular assist device; PVRI, pulmonary vascular resistance index: units, mmHg ml/m2; RV, right ventricular; RVSWI, right ventricular stroke work index: units, woods units; TR, tricuspid regurgitation.
Table 3.
Indicators of RV Failure for Patients Who Received LVAD Support During the Selective Era
| Factor | Patient | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | |
| ECMO support | X | X | X | X | |||||||||||||
| Clinical RV failure* | X | X | |||||||||||||||
| Gross RV dysfunction† | X | X | |||||||||||||||
| Severe TR | |||||||||||||||||
| PVRI > 4 | X | X | X | ||||||||||||||
| RVSWI < 400 | X | X | |||||||||||||||
| Ventricular arrhythmias | X | ||||||||||||||||
| Inadequate LVAD filling | |||||||||||||||||
Determined by the presence of ascites, pleural effusions, renal failure, or hepatic failure.
Echo or gross inspection in the operating room after LVAD placement.
LVAD, left ventricular assist device; PVRI, pulmonary vascular resistance index: units, mmHg ml/m2; RV, right ventricular; RVSWI, right ventricular stroke work index: units, woods units; TR, tricuspid regurgitation.
BiVAD Patient Characteristics
The median age was 3.5 years with a BSA of 0.6 m2 (Table 4). Diagnoses included dilated cardiomyopathy in 17 (55%), myocarditis in 6 (19%), CHD in 3 (10%), and “other” in 5 (16%). The CHD group consisted of two patients with Tetralogy of Fallot and one patient with congenitally corrected transposition of the great arteries. Of the five patients classified as “other,” one suffered from allograft vasculopathy, two from noncompaction, one from Kawasaki disease, and one from restrictive cardiomyopathy. Thirteen (42%) patients were International Registry for Mechanically Assisted Circulatory Support (InTERMACS) profile 1 at the time of BiVAD implantation with 9 (29%) supported with ECMO. Moderate kidney dysfunction was present in nine (29%) patients, and severe hepatic impairment was present in eight (26%) patients and moderate in nine (29%) patients.
Table 4.
BiVAD Patient Preimplant Characteristics
| Preimplant Characteristics | |
|---|---|
| Age (years), median (IQR) | 3.5 (1.3–9.3) |
| Male | 18 (58%) |
| Weight (kg) | |
| <5 | 3 (10%) |
| 5–10 | 8 (26%) |
| >10 | 20 (65%) |
| BSA (m2), median (IQR) | 0.6 (0.4–0.9) |
| Diagnosis | |
| Dilated cardiomyopathy | 17 (55%) |
| Myocarditis | 6 (19%) |
| Congenital heart disease | 3 (10%) |
| Other | 5 (16%) |
| Previous surgeries | 5 (16%) |
| Status before VAD | |
| INTERMACS profile 1 | 13 (42%) |
| INTERMACS profile 2 | 18 (58%) |
| ECMO support | 9 (29%) |
| Ventilator support | 25 (81%)* |
| GFR† (ml/min/1.73 m2), median (IQR) | 150 (108–203) |
| Renal impairment | |
| Normal: GFR > 99% predicted | 22 (71%) |
| Moderate: GFR 30–99% predicted | 9 (29%) |
| Severe: GFR < 30% predicted | 0 |
| Total bilirubin (mg/dl), median (IQR) | 0.7 (0.4–1.3) |
| Hepatic impairment | |
| Normal (<0.6) | 14 (45%) |
| Moderate (0.7–1.1) | 9 (29%) |
| Severe (≥1.2) | 8 (26%) |
Includes patients who were on ECMO.
Calculated based on the formula described by Schwartz et al.13
BiVAD, biventricular assist device; BSA, body surface area; GFR, glomerular filtration rate; INTERMACS, International Registry for Mechanically Assisted Circulatory Support; IQR, interquartile range.
BiVAD Support
All patients who underwent BiVAD implantation received both VADs during a single operation, with the exception of two patients. One patient underwent LVAD implantation and a tricuspid annuloplasty. They progressed to RV failure with the inability to wean from the ventilator and continued vasopressor requirements. An RVAD was placed 6 days later without cardiopulmonary bypass. The second patient had reasonable RV function at the time of LVAD implantation but developed intractable RV failure with inability of the LVAD to deliver an adequate cardiac output. Right ventricular assist device implantation was performed 24 hours after LVAD implantation. Both patients were successfully bridged at 3 and 4 months, respectively, to HT. Both patients were included in the BiVAD statistical analysis. The 31 BiVAD patients were supported for a total of 1,538 days, a median of 41 days per patient.
Outcomes
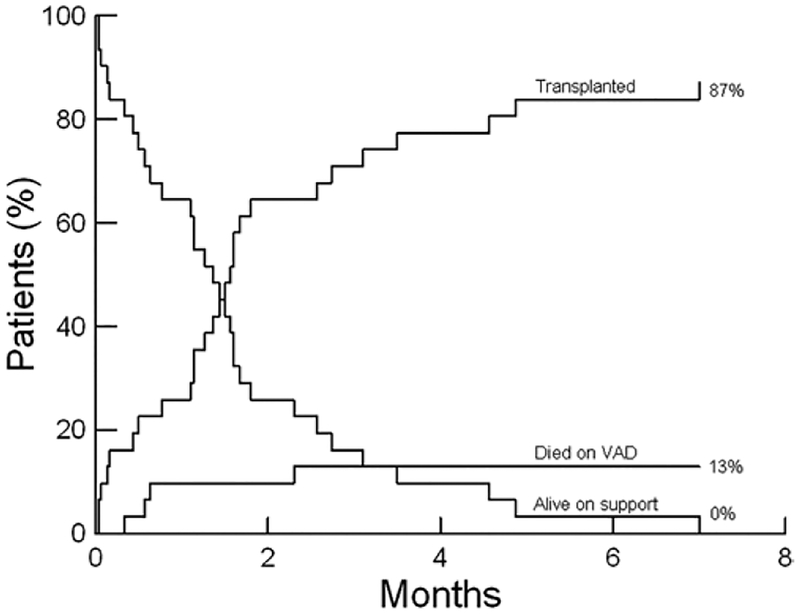
Forty-eight biventricular patients underwent VAD implantation during the study period with 41 patients (85%) surviving to transplant or explant. Forty (83%) patients survived to transplant, and one patient (3%) was weaned to explant. Of the 31 BiVAD patients, 27 (87%) patients survived to transplant, and none were weaned to explant (Table 5). The competing outcomes analysis is shown in Figure 1. Time to initial extubation was a median of 3.5 days, and initial intensive care unit (ICU) duration was a median of 11.5 days. For the four patients who died on BiVAD support, the causes of death were central nervous system (CnS) dysfunction in three and one with multisystem organ failure (Table 6).
Table 5.
Outcomes with BiVAD Support
| Outcomes | |
|---|---|
| BiVAD support | |
| Total duration of BiVAD support (days) | 1,538 |
| Patient duration of BiVAD support (days), median (IQR) | 41 (15–69) |
| Time to initial extubation (days), median (IQR) | 3.5 (1–7)* |
| Initial ICU duration (days, median (IQR) | 11.5 (7–27) |
| Survival | |
| Survived to transplant | 27 (87%) |
| Alive on support | 0 |
| Expired while on device | 4 (13%) |
| Current status | |
| Alive | 26 (84%) |
| Follow-up from BiVAD (months), median (IQR) | 50 (17–77) |
Two patients did not survive to extubation, and one patient underwent HT on day 1 of VAD support and was not extubated previously.
BiVAD, biventricular assist device; ICU, intensive care unit; IQR, interquartile range.
Figure 1.
Competing outcomes analysis over 8 months for 31 patients.
Table 6.
Patients Who Died on BiVAD Support
| Age (months) | Weight (kg) | Diagnosis | Complications | Duration (days) | Cause of Death | |
|---|---|---|---|---|---|---|
| 1 | 0.4 | 3 | Noncompaction | MB, CNS | 10 | MSOF |
| 2 | 78 | 22 | Myocarditis | CNS, Inf | 19 | CNS |
| 3 | 13 | 7 | Noncompaction | CNS | 17 | CNS |
| 4 | 8 | 8 | DCM | MB, Inf, CNS | 69 | CNS |
CNS, central nervous system; DCM, dilated cardiomyopathy; Inf, infection; MB, major bleeding; MSOF, multisystem organ failure.
Intention-to-Treat Analysis
Two patients who underwent LVAD implantation at their initial operation later required RVAD implantation and were included in the BiVAD statistical analysis. When using an intent-to-treat analysis based on the time of the initial operation, the BiVAD patient cohort included 29 patients, of which 25 (86%) patients survived to transplant. The LVAD cohort would include 19 patients, with 16 (84%) surviving to transplant (Table 7).
Table 7.
Intention-to-Treat Analysis
| Device | Patients | Survival to Transplant |
|---|---|---|
| BiVAD | 29 | 25 (86%) |
| LVAD | 19 | 16 (84%) |
BiVAD, biventricular assist device; LVAD, left ventricular assist device.
Survival by Era
During the initial era, when BiVADs were placed exclusively, 19 BiVADs were implanted with 17 (89%) patients surviving to transplant. During the latter era, when LVAD was considered, 29 VADs were implanted. Twelve of these were BiVADs, of which 10 (83%) survived to transplant. The remaining 17 had LVAD alone implanted and 14 (82%) survived to transplant (Table 8).
Table 8.
Survival by Era
| Device | Patients | Survival to Transplant |
|---|---|---|
| BiVAD exclusive | 19 | 17 (89%) |
| Selective | 29 | 24 (83%) |
| BiVAD | 12 | 10 (83%) |
| LVAD | 17 | 14 (82%) |
BiVAD, biventricular assist device; LVAD, left ventricular assist device.
Adverse Events on BiVAD Support
The most common adverse event (AE) was major infection, 18 occurred in 15 (48%) patients for a rate of 1.17 per 100 days of support (Table 9). A total of 12 episodes of CnS dysfunction occurred in 11 (35%) patients, a rate of 0.78 per 100 days of support. All four patients who died experienced a significant cerebrovascular accident (CVA), three of which were direct causes of the patient’s death. Of the remaining seven patients who developed CnS dysfunction, four have no residual deficits. Major bleeding events occurred at a rate of 0.52 per 100 days of support, eight events in six (19%) patients. Renal dysfunction occurred in five (16%) and hepatic dysfunction in three (10%) while on device support. The total number of pump changes was 74, or 4.8 per 100 days of support.
Table 9.
Complications Occurring on VAD Support
| Event | |
|---|---|
| Patients with cerebrovascular accident | 11 (35%) |
| Total CNS dysfunction (rate*) | 12 (0.78) |
| Patients with major infection | 15 (48%) |
| Total infections (rate*) | 18 (1.17) |
| Patients with major bleeding | 6 (19%) |
| Total major bleeding events (rate†) | 8 (0.52) |
| Patients with renal dysfunction | 5 (16%) |
| Patients with hepatic dysfunction | 3 (10%) |
| Patients requiring pump change† | 21 (68%) |
| Total pump changes (rate*) | 74 (4.8) |
Rate is events per 100 days of BiVAD support.
Changing of both pumps at one time was considered two pump changes.
BiVAD, biventricular assist device; CNS, central nervous system dysfunction.
Post-HT Survival
For the 27 BiVAD patients who survived to transplant, 26 (96%) remain alive with an overall median follow-up of 55 (IQR, 37–76) months from HT (Table 10). One patient died 3 years after HT because of allograft rejection, secondary to patient noncompliance.
Table 10.
Postheart Transplant outcomes
| Outcome | |
|---|---|
| Time to initial extubation (days), median (IQR) | 1.2 (0.5–3.0) |
| Initial ICU duration (days), median (IQR) | 5 (4–7) |
| Survival (N = 27) | |
| 1 month survival | 27 (100%) |
| 6 month survival | 25 (100%) |
| At last follow-up* | 26 (96%) |
| Last follow-up from transplant (months), median (IQR) | 55 (37–76) |
One patient alive at less than 6 months of follow-up. ICU, intensive care unit; IQR, interquartile range.
Comment
Herein, we report a large single-institution experience using early BiVAD support in children with end-stage heart failure with a survival to transplant of 87%. These results compare favorably with the multiinstitutional experience with the same device reported by Almond et al., who described a 75% 1 year survival. In this study, 36% of patients received BiVAD support with a survival of 71%, compared with 78% survival for patients who received LVAD alone.2
Previous studies have shown that BiVAD support is a significant risk factor for poor outcomes.2,6,8 This study is the first to illustrate a high survival rate for pediatric patients receiving BiVAD support. The reasons for this are likely multifactorial. Our strategy of early RVAD implantation allows for immediate total cardiac support that is especially beneficial in patients with marginal RV function after LVAD implantation. The avoidance of post-LVAD RV failure is critical, because it has been shown to lead to improved survival in adult studies.11,19 In addition, our institutional experience may have contributed to our outcomes. It has been shown that institutions that have implanted greater than 10 VADs have an 89% survival, attributed largely to improved patient selection.2,20
Another concern with BiVAD support is the perceived increase in risk of AEs associated with a second device. In this study, AEs continue to be significant with this pulsatile device, however, not appreciably worse than previously reported.1,2,6,10 In this study, the risk of major infection and bleeding were less than published data.2,21 Contrary to this, our risk of CnS dysfunction was slightly higher. It is unlikely that a right-sided device resulted in significantly higher CnS complications. Rather, this may be attributed to a more aggressive anticoagulation strategy, as 55% of the CVAs that occurred in our patients were hemorrhagic rather than embolic.
The post-HT survival for pediatric patients successfully bridged with a VAD approaches 90%.22 A recent study even suggests an early survival advantage for some HT recipients bridged with a VAD compared with those who did not receive mechanical circulatory support.23 A strategy of early BiVAD support, theoretically, may provide analogous advantages over LVAD in patients with marginal RV function. To that point, our BiVAD patients had a short time to extubation and ICU length of stay. Overall, 72% of the duration of BiVAD support was spent outside of the ICU. This allowed for extensive rehabilitation before HT and likely contributed substantially to our post-HT survival of 96%.
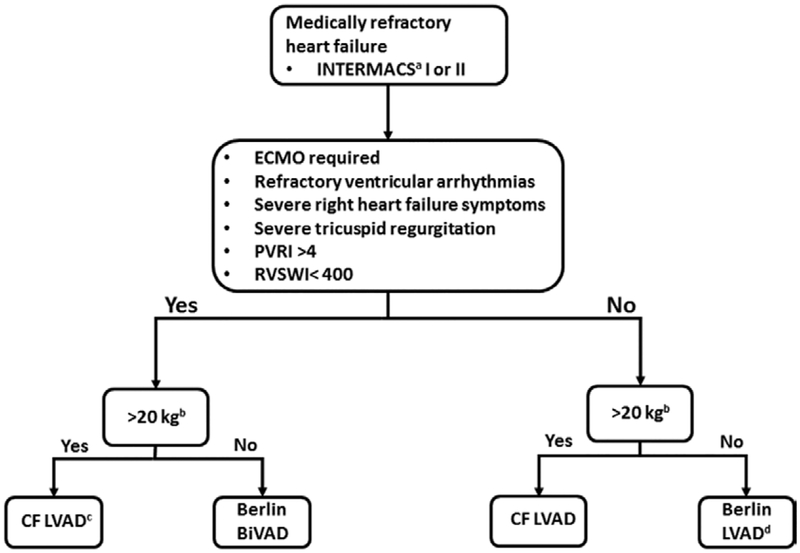
Our management of patients with a VAD has evolved into a selective approach allowing for LVAD only, but maintaining a low threshold for BiVAD support. Unfortunately, predicting RV failure after LVAD implantation can be problematic with limited data available for pediatric patients.8 Although metrics have been devised in the adult population, this is far from ideal because of the differing patient population and unique pathology.15,24 Furthermore, it has been suggested that at least some degree of RV dysfunction occurs nearly universally with some pediatric pathologies.25 Indeed most pediatric studies have at least a 30% RVAD implantation rate.2–4 This high rate of RV failure has influenced our low threshold for RVAD implantation. Using our selective approach, 89% of patients who underwent LVAD implantation alone did not require subsequent RVAD implantation. Our strategy for VAD support has evolved based on our extensive experiences, modes of evaluating RV function, and devices presently available. An algorithm that reflects our current strategy for determining the type of VAD support is illustrated in Figure 2.
Figure 2.
Current strategy for durable mechanical circulatory support in pediatric patients. aINTERMACS, International Registry for Mechanically Assisted Circulatory Support. b20 kg cutoff chosen to have sufficient size for HVAD placement. cAdditional intraoperative assessment for potential CentriMag placement as RVAD. dAdditional intraoperative assessment for the need for BiVAD support with an additional Berlin Heart EXCOR. BiVAD, biventricular assist device; Berlin, Berlin Heart EXCOR; CF, continuous flow; PVRI, pulmonary vascular resistance index, units: woods units; RVAD, right ventricular assist device RVSWI, right ventricular stroke work index, units: mmHg ml/m2.
The current generation of continuous-flow devices has shown superior outcomes compared with pulsatile devices in the adult population.26 In some studies, they have shown a decrease in the incidence of RVAD implantation or RV failure.27,28 This is attributed to their ability to more effectively unload the LV leading to decreased pulmonary artery pressures and improved RV function.18,29 Their use in pediatric patients has been increasing because of the potential advantages of improved morbidity profile, survival, and the potential for patient discharge.30,31 Increased utilization of the current continuous-flow devices, as well as continuous-flow devices under the development for smaller pediatric patients,32 may lead to less need for RVAD implantation.
Limitations
This represents a single-institution study from an institution with a significant experience with BiVAD implantation and management. Therefore, these results may not be fully generalizable. As well, these data may not compare with data from other institutions easily for many reasons including institutional differences in patient selection and management strategies. This study also has a small sample size that limits overall data interpretation and any subgroup analysis.
Conclusion
Early implantation of pulsatile BiVAD support can be used to bridge pediatric patients with end-stage heart failure to HT with good outcomes and with AE rates comparable with LVAD support alone. Furthermore, patients are able to participate in rehabilitation early after BiVAD implantation and have excellent post-HT survival. Future studies are needed to establish criteria for RVAD implantation to prevent the morbidity associated with RV failure after LVAD implantation. Until this is more completely understood, a strategy of maintaining a low threshold for RVAD implantation for early BiVAD support can allow for good survival.
Acknowledgment
The use of a continuous-flow ventricular assist device in pediatric patients is off-label; this pertains to both the HeartWare HVAD and the HeartMate II.
This study was supported by NIH grant T32 HL007776.
Footnotes
Disclosure:
Charles E. Canter received travel reimbursement from Berlin Heart, GmbH, Berlin, Germany. The remaining authors have no conflicts of interest to report.
References
- 1.Fraser CD Jr, Jaquiss RD, Rosenthal Dn, et al. ; Berlin Heart Study Investigators: Prospective trial of a pediatric ventricular assist device. N Engl J Med 367: 532–541, 2012. [DOI] [PubMed] [Google Scholar]
- 2.Almond CS, Morales DL, Blackstone EH, et al. : Berlin Heart EXCOR pediatric ventricular assist device for bridge to heart transplantation in US children. Circulation 127: 1702–1711, 2013. [DOI] [PubMed] [Google Scholar]
- 3.Cassidy J, Dominguez T, Haynes S, et al. : A longer waiting game: Bridging children to heart transplant with the Berlin Heart EXCOR device—The United Kingdom experience. J Heart Lung Transplant 32: 1101–1106, 2013. [DOI] [PubMed] [Google Scholar]
- 4.Morales DL, Almond CS, Jaquiss RD, et al. : Bridging children of all sizes to cardiac transplantation: The initial multicenter north American experience with the Berlin Heart EXCOR ventricular assist device. J Heart Lung Transplant 30: 1–8, 2011. [DOI] [PubMed] [Google Scholar]
- 5.Blume ED, Naftel DC, Bastardi HJ, Duncan BW, Kirklin JK, Webber SA; Pediatric Heart Transplant Study Investigators: Outcomes of children bridged to heart transplantation with ventricular assist devices: A multi-institutional study. Circulation 113: 2313–2319, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Stein ML, Robbins R, Sabati AA, et al. : Interagency Registry for Mechanically Assisted Circulatory Support (InTERMACS)- defined morbidity and mortality associated with pediatric ventricular assist device support at a single US center: The Stanford experience. Circ Heart Fail 3: 682–688, 2010. [DOI] [PubMed] [Google Scholar]
- 7.Aissaoui N, Morshuis M, Paluszkiewicz L, Lauenroth V, Börgermann J, Gummert J: Comparison of biventricular and left ventricular assist devices for the management of severe right ventricular dysfunction in patients with end-stage heart failure. ASAIO J 60: 400–406, XXX2014. [DOI] [PubMed] [Google Scholar]
- 8.Karimova A, Pockett CR, Lasuen N, et al. : Right ventricular dysfunction in children supported with pulsatile ventricular assist devices. J Thorac Cardiovasc Surg 147: 1691–1697, 2014. [DOI] [PubMed] [Google Scholar]
- 9.Rockett SR, Bryant JC, Morrow WR, et al. : Preliminary single center north American experience with the Berlin Heart pediatric EXCOR device. ASAIO J 54: 479–482, 2008. [DOI] [PubMed] [Google Scholar]
- 10.Zafar F, Jefferies JL, Tjossem CJ, et al. : Biventricular Berlin Heart EXCOR pediatric use across the United States. Ann Thorac Surg 99: 1328–1334, 2015. [DOI] [PubMed] [Google Scholar]
- 11.Fitzpatrick JR III, Frederick JR, Hiesinger W, et al. : Early planned institution of biventricular mechanical circulatory support results in improved outcomes compared with delayed conversion of a left ventricular assist device to a biventricular assist device. J Thorac Cardiovasc Surg 137: 971–977, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gandhi SK, Huddleston CB, Balzer DT, Epstein DJ, Boschert TA, Canter CE: Biventricular assist devices as a bridge to heart transplantation in small children. Circulation 118 (14 suppl): S89–S93, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz GJ, Haycock GB, Edelmann CM Jr, Spitzer A: A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics 58: 259–263, 1976. [PubMed] [Google Scholar]
- 14.National Kidney Foundation: Guidelines. http://www2.kidney.org/professionals/KDOQI/guidelines_ckd/p4_class_g1.htm. Accessed January 5, 2015.
- 15.Fitzpatrick JR III, Frederick JR, Hsu VM, et al. : Risk score derived from pre-operative data analysis predicts the need for biventricular mechanical circulatory support. J Heart Lung Transplant 27: 1286–1292, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ochiai Y, McCarthy PM, Smedira NG, et al. : Predictors of severe right ventricular failure after implantable left ventricular assist device insertion: Analysis of 245 patients. Circulation 106 (12 suppl 1): I198–I202, 2002. [PubMed] [Google Scholar]
- 17.Lovich MA, Pezone MJ, Wakim MG, et al. : Inhaled nitric oxide augments left ventricular assist device capacity by ameliorating secondary right ventricular failure. ASAIO J 61: 379–385, 2015. [DOI] [PubMed] [Google Scholar]
- 18.Atluri P, Fairman AS, MacArthur JW, et al. : Continuous flow left ventricular assist device implant significantly improves pulmonary hypertension, right ventricular contractility, and tricuspid valve competence. J Card Surg 28: 770–775, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morgan JA, John R, Lee BJ, Oz MC, Naka Y: Is severe right ventricular failure in left ventricular assist device recipients a risk factor for unsuccessful bridging to transplant and post-transplant mortality. Ann Thorac Surg 77: 859–863, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Morales DL, Zafar F, Rossano JW, et al. : Use of ventricular assist devices in children across the United States: Analysis of 7.5 million pediatric hospitalizations. Ann Thorac Surg 90: 1313–1318, 2010. [DOI] [PubMed] [Google Scholar]
- 21.Cabrera AG, Khan MS, Morales DL, et al. : Infectious complications and outcomes in children supported with left ventricular assist devices. J Heart Lung Transplant 32: 518–524, 2013. [DOI] [PubMed] [Google Scholar]
- 22.Eghtesady P, Almond CS, Tjossem C, et al. ; Berlin Heart Investigators: Post-transplant outcomes of children bridged to transplant with the Berlin Heart EXCOR Pediatric ventricular assist device. Circulation 128 (11 suppl 1): S24–S31, 2013. [DOI] [PubMed] [Google Scholar]
- 23.Davies RR, Haldeman S, McCulloch MA, Pizarro C: Ventricular assist devices as a bridge-to-transplant improve early post-transplant outcomes in children. J Heart Lung Transplant 33: 704–712, 2014. [DOI] [PubMed] [Google Scholar]
- 24.Atluri P, Goldstone AB, Fairman AS, et al. : Predicting right ventricular failure in the modern, continuous flow left ventricular assist device era. Ann Thorac Surg 96: 857–863, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Groner A, Yau J, Lytrivi ID, et al. : The role of right ventricular function in paediatric idiopathic dilated cardiomyopathy. Cardiol Young 23: 409–415, 2013. [DOI] [PubMed] [Google Scholar]
- 26.Slaughter MS, Rogers JG, Milano CA, et al. ; HeartMate II Investigators: Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med 361: 2241–2251, 2009. [DOI] [PubMed] [Google Scholar]
- 27.Patel ND Weiss ES, Schaffer J, et al. : Right heart dysfunction after left ventricular assist device implantation: A comparison of the pulsatile HeartMate I and axial-flow HeartMate II devices. Ann Thorac Surg 86: 832–840, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Kormos RL, Teuteberg JJ, Pagani FD, et al. ; HeartMate II Clinical Investigators: Right ventricular failure in patients with the HeartMate II continuous-flow left ventricular assist device: Incidence, risk factors, and effect on outcomes. J Thorac Cardiovasc Surg 139: 1316–1324, 2010. [DOI] [PubMed] [Google Scholar]
- 29.Morgan JA, Paone G, Nemeh HW, et al. : Impact of continuous-flow left ventricular assist device support on right ventricular function. J Heart Lung Transplant 32: 398–403, 2013. [DOI] [PubMed] [Google Scholar]
- 30.Sparks J, Epstein D,Baltagi S, et al. : Continuous flow device support in children using the HeartWare HVAD: 1000 days of lessons learned from a single center experience. ASAIO J 61: 569–573, 2015. [DOI] [PubMed] [Google Scholar]
- 31.Cabrera AG, Sundareswaran KS, Samayoa AX, et al. : Outcomes of pediatric patients supported by the HeartMate II left ventricular assist device in the United States. J Heart Lung Transplant 32: 1107–1113, 2013. [DOI] [PubMed] [Google Scholar]
- 32.Baldwin JT, Borovetz HS, Duncan BW, Gartner MJ, Jarvik RK, Weiss WJ: The national heart, lung, and blood institute pediatric circulatory support program: A summary of the 5-year experience. Circulation 123: 1233–1240, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]