Abstract
Background:
Commonly-used chemotherapies can be toxic to the ovaries. Most studies evaluating receipt of fertility counseling for women in their reproductive years were performed in specific settings, limiting generalizability.
Methods:
A nationwide sample of US women diagnosed with breast cancer before age 45 completed a survey assessing the prevalence of fertility counseling. Age-adjusted log-binomial regression was used to estimate prevalence ratios (PRs) and 95% confidence intervals (CIs) for fertility counseling.
Results:
Among 432 survivors diagnosed during 2004–2011, 288 (67%) had not discussed the effects of treatment on fertility with a healthcare provider before or during treatment. Fertility discussion was associated with younger age (for <35 vs 40+ years, PR: 3.49, CI: 2.66–4.58) and lower parity (for parity 1 vs. 2, PR: 1.81, CI: 1.29, 2.53). Twenty percent of respondents reported that they were interested in future fertility (87/432) at the time of their diagnosis, but not all (66/87) received counseling on the impact of treatment on their fertility, and few (8/87) utilized fertility preservation strategies. Among women with a fertility interest who provided reasons for not taking steps to preserve fertility (N=68), reasons cited included concern for adverse impact on cancer treatment (56%), lack of knowledge (26%), decision to not have a child (24%), and cost (18%).
Conclusions:
Across multiple treatment settings, most reproductive-age women diagnosed with breast cancer did not discuss fertility with a healthcare provider or use fertility preservation strategies. Discussing the potential impact of cancer treatment on future fertility is an important aspect of patient education.
Keywords: fertility, fertility counseling, fertility preservation, breast cancer
Precis:
In this nationwide sample, a higher proportion of reproductive-age women diagnosed with breast cancer did not receive counseling or utilize fertility preservation compared to published reports in fertility-focused studies in a cancer population. Decisions about fertility preservation were not based on informed discussion and suggest opportunity for improved patient education and outreach.
Introduction
Every year, over 23,000 American women aged <45 years are diagnosed with breast cancer.1 The majority of these women receive chemotherapy that can impair fertility and/or endocrine therapy that can delay pregnancy attempts until older ages when fertility declines.2 The overall 5-year relative survival rate for female breast cancer diagnosed before age 45 years is 87.6%.3, 4 Early detection and therapeutic advances permit most younger women to survive their initial diagnosis but also to realize the potential adverse impact of gonadotoxic therapies on fertility.5
In female cancer survivors of reproductive age, pretreatment counseling on the potential fertility-related complications of cancer treatment and options for fertility preservation has been associated with higher quality of life.6–8 However, many young women diagnosed with breast cancer report that they were not informed about infertility risks before initiating cancer treatment.9–11 Since 2006, national guidelines from the American Society of Clinical Oncology (ASCO)12–14, the National Comprehensive Cancer Network,15 and the American Society of Reproductive Medicine16 have recommended fertility counseling for reproductive-age patients with a cancer diagnosis.
Receipt of fertility counseling ranges from 34–81% across prior studies.17–28 Fertility counseling is more often received by women who are younger18, have lower parity,17, 18, 22, 29, 30 higher education,17, 18 higher income,22, 31 non-Hispanic White ethnicity,21, 32 and insurance coverage.22 Among women who do receive counseling, lower use of fertility preservation has been associated with concerns about cost,9, 33 treatment delay,11, 33 and/or an adverse impact on their cancer treatment.19, 33 In prior studies of reproductive-age women diagnosed with cancer who wanted to retain their fertility, 40–50% of women did not utilize fertility preservation.18, 34, 35
Most literature on fertility counseling, interest, and preservation is derived from academic centers or studies specifically designed to address fertility.22, 36, 37 It is unclear whether findings accurately reflect the experience of women seen outside of an academic setting. To address this gap, participants across the U.S. from the Sister Study38 and Two Sister Study39 were surveyed on their fertility-related experiences following a breast cancer diagnosis.
The aim of this analysis is to examine the prevalence of fertility interest, discussion, and use of preservation options among premenopausal, reproductive-age women with a breast cancer diagnosis in the Sister Study and Two Sister Study.
Methods
The Sister Study recruited a cohort of U.S. women whose sister had been diagnosed with breast cancer but who themselves had never had breast cancer at enrollment (2003–2009). Sister Study participants were recruited in the United States and Puerto Rico using brochures, flyers, mail, email, as well as free and paid media campaigns in English and Spanish as previously described.38 Between 2008 and 2010, the Two Sister Study enrolled the sisters (of Sister Study participants) who had been diagnosed with young-onset (<50 years) breast cancer.35 Data for the current analysis come from a survivor survey sent to Sister Study and Two Sister Study participants diagnosed with breast cancer prior to October 2012. Participants who had undergone tubal ligation, hysterectomy, or bilateral oophorectomy (permanent sterilization), or reported experiencing menopause prior to breast cancer diagnosis were excluded. The sample included 52 women from the Sister Study with breast cancer diagnosed at ages 35–44 and 474 women from the Two Sister Study with breast cancer diagnosed at ages 28–44 years. Study participants received treatment in both academic and community settings across the United States. 38, 39 Parity was defined as number of children birthed prior to cancer diagnosis and the referent group was defined by the most prevalent parity.
Outcomes assessed included the prevalence of fertility interest, discussion, and use of preservation prior to breast cancer treatment. Fertility interest was defined as answering “Yes” to the question “Before your breast cancer diagnosis, did you think you wanted to get pregnant at some point in the future?” Fertility discussion was defined as answering “Yes” to the question “Did you ever have a discussion with a health care provider about the effect your treatment could have on your future fertility or ability to have children?” The use of fertility preservation was defined as answering “Yes” to “Before you began treatment, or during your treatment, did you take any additional steps to lessen your chances of becoming infertile as a result of your cancer treatment?” Women who responded “Yes” then selected one or more of the following response options: cryopreservation of embryos, cryopreservation of unfertilized eggs, cryopreservation of ovarian tissue, and/or GnRH agonist. Information on breast cancer diagnosis, tumor characteristics, and treatment data were abstracted from medical records as previously described.39–41 Endocrine therapy non-adherence was defined as non-initiation or reporting taking endocrine therapy most of the time, sometimes, rarely or never (rather than always) among women with estrogen receptor positive breast cancer.
Age-adjusted (<40/40+ years) log-binomial regression was used to estimate prevalence ratios (PRs) and 95% confidence intervals (CIs) for fertility discussion. All tests were two-sided, and p-values <0.05 were considered statistically significant. Analyses were performed using Statistical Analysis Software (SAS) version 9.4 (SAS Institute, Inc, Cary, NC). This analysis uses information from Data Release 6.0.
Results
In total, 526 women aged <45 years at breast cancer diagnosis completed the survivor survey. Of these, 432 (82%) were premenopausal and had not undergone permanent sterilization and were included in analyses. Most women were non-Hispanic White with an annual household income ≥$50,000 (Table 1).41, 42 Breast cancer stage was distributed such that 15% of women were Stage 0, 39% Stage I, 32% Stage II, 14% Stage III, and 1% were Stage IV at diagnosis. A minority (25%) of patients were diagnosed with breast cancer prior to 2006, the year that the ASCO guidelines about Fertility and Cancer were first published (Table 1).12 The majority of women (89%) completed the survey 5–8 years after diagnosis; years since diagnosis was not significantly associated with reported receipt of fertility counseling (Table 1). The prevalence of fertility counseling did not differ between the Sister Study (31%) and Two Sister Study (33%) in age-adjusted models (PR: 1.08, CI: 0.68, 1.72) (Table 1).
Table 1.
Participant characteristics among 432 women enrolled in the NIEHS Sister Study and Two Sister Study.
| Characteristic | N | % |
|---|---|---|
| Age at diagnosis, median (IQR) | 41.9 | (39.2, 43.5) |
| 28.3–34.9 | 24 | 6% |
| 35–39.9 | 112 | 26% |
| 40–44.9 | 296 | 69% |
| Race | ||
| Non-Hispanic White | 383 | 89% |
| Non-Hispanic Black | 15 | 3% |
| Hispanic | 25 | 6% |
| Other | 9 | 2% |
| Marital status | ||
| Never married | 36 | 8% |
| Legally married/living as married | 362 | 84% |
| Widowed/divorced/separated | 34 | 8% |
| Household income | ||
| Less than $20,000 | 5 | 1% |
| $20,000-$49,999 | 44 | 10% |
| $50,000-$99,999 | 152 | 35% |
| $100,000-$200,000 | 165 | 38% |
| More than $200,000 | 56 | 13% |
| Don’t know/refused | 10 | 2% |
| Education | ||
| High school or less | 33 | 8% |
| Some college | 92 | 21% |
| Bachelor’s degree or higher | 307 | 71% |
|
Rural/urban
(>50% of census tract population resides in rural/urban area) |
||
| Rural | 82 | 20% |
| Urban | 337 | 80% |
| Region | ||
| Northeast | 86 | 20% |
| Midwest | 141 | 33% |
| South | 111 | 26% |
| West | 89 | 21% |
| Calendar year of breast cancer diagnosis | ||
| 2004–2005 | 108 | 25% |
| 2006 | 150 | 35% |
| 2007–2011 | 174 | 40% |
| Time since diagnosis (years), median (IQR) | 6.2 | (5.5, 7.0) |
| 1.3-<5 | 47 | 11% |
| 5-<6 | 133 | 31% |
| 6-<7 | 142 | 33% |
| 7–8.2 | 110 | 25% |
| Study | N | % |
| Sister Study | 39 | 9% |
| Two Sister Study | 393 | 91% |
| Parity at breast cancer diagnosis | ||
| 0 | 131 | 30% |
| 1 | 58 | 13% |
| 2 | 159 | 37% |
| 3+ | 83 | 19% |
| Stage | ||
| 0 | 64 | 15% |
| I | 167 | 39% |
| II | 137 | 32% |
| III/IV | 63 | 15% |
| Missing | 1 | 0% |
| ER status | ||
| Negative | 96 | 23% |
| Positive † | 326 | 77% |
| Treatment | ||
| No chemotherapy or endocrine therapy | 49 | 11% |
| Endocrine therapy without chemotherapy | 71 | 16% |
| Chemotherapy without endocrine therapy | 83 | 19% |
| Chemotherapy and endocrine therapy | 229 | 53% |
Includes 2 women with borderline ER+ status
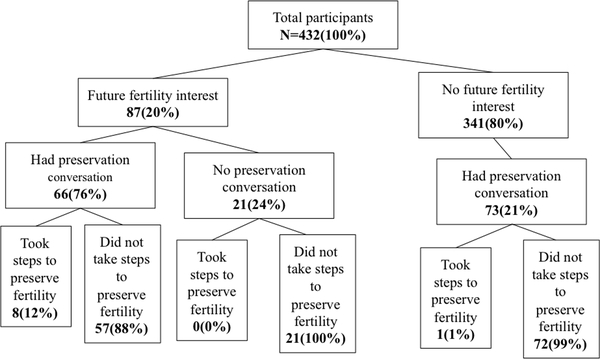
Overall, 67% of women (288/432) did not discuss the fertility impact of treatment with a healthcare provider before or during their cancer treatment (Figure 1). Across both the Sister Study and the Two Sister Study, 20% of women (87/432) reported having an interest in future fertility prior to their breast cancer diagnosis (Figure 1). A similar proportion (21%) reported that before treatment they were unaware that cancer treatment could affect a woman’s fertility (data not shown).
Figure 1.
Fertility discussion and utilization of preservation among 432 NIEHS Sister Study and Two Sister Study participants who completed the breast cancer survivor survey.
Fertility discussion was positively associated with younger age, Hispanic ethnicity (compared to non-Hispanic whites), residing in the Western United States, lower parity, and more intensive cancer therapy (Table 2). Compared to women over 40 years old at the time of diagnosis, fertility discussion was more than three times as likely to occur in women aged 28–35 years (PR: 3.49, CI: 2.66–4.58) and nearly twice as likely in women aged 35–39 years (PR: 1.89, CI: 1.41–2.52). Fertility discussion was more prevalent in Hispanic women (PR: 2.01, CI: 1.25–3.24) compared to non-Hispanic White women. Receipt of fertility counseling varied by geographical location, determined by U.S. Census Bureau regional definitions.43 Compared to the 39% prevalence of fertility counseling among women residing in the Western U.S., fewer women (23% ) in the Midwest region received counseling (PR: 0.59, CI: 0.41–0.86). Hispanic ethnicity was related to geographic region whereby most Hispanic women lived in the South or West regions (South: N=10, 42%, West: N=10, 42%, Northeast: N=2, 8%, Midwest: N=2, 8%). Compared to women with two children, those with one child were more likely to receive fertility counseling (PR: 1.81, CI: 1.29–2.53). Compared to those who received chemotherapy or endocrine therapy, women who did not receive chemotherapy or endocrine therapy were 44% less likely to discuss fertility (PR: 0.56, CI: 0.32–0.99). Estrogen receptor status was not associated with receipt of fertility discussion whereby women with ER+ tumors were similarly likely to receive fertility counseling compared to their ER- counterparts (OR: 1.20, CI: 0.86–1.11). Rural or urban setting was associated with lower prevalence of receipt of fertility counseling in sensitivity analyses excluding women with Stage 0 disease (PR: 0.64, CI: 0.41–0.98, Supplementary Table 1) but was not significant when considering the entire cohort (Table 2).
Table 2.
Age-adjusted prevalence ratios (PR) and 95% confidence intervals (CI) for receipt of fertility counseling among 432 women enrolled in the NIEHS Sister Study and Two Sister Study.
| Characteristic | Received fertility counseling† N=140 (100%) |
No fertility counseling† N=288 (100%) |
Age-adjusted PR (95% CI) |
|---|---|---|---|
| Age at diagnosis, median (IQR) | |||
| 28.3–34.9 | 20 (14) | 4 (1) | 3.49 (2.66, 4.58) |
| 35–39.9 | 50 (36) | 61 (21) | 1.89 (1.41, 2.52) |
| 40–44.9 | 70 (50) | 223 (77) | 1 |
| Race | |||
| Non-Hispanic White | 116 (83) | 264 (92) | 1 |
| Non-Hispanic Black | 6 (4) | 8 (3) | 1.31 (0.76, 2.24) |
| Hispanic | 12 (9) | 13 (5) | 2.01 (1.25, 3.24) |
| Other | 6 (4) | 3 (1) | 1.65 (1.10, 2.48) |
| Marital status | |||
| Never married | 14 (10) | 22 (8) | 1 |
| Legally married/living as married | 115 (82) | 244 (85) | 0.85 (0.57, 1.26) |
| Widowed/divorced/separated | 11 (8) | 22 (8) | 0.88 (0.49, 1.57) |
| Household income | |||
| Less than $20,000 | 1 (71) | 4 (1) | 0.51 (0.09, 2.86) |
| $20,000-$49,999 | 14 (10) | 29 (10) | 0.94 (0.60, 1.49) |
| $50,000-$99,999 | 50 (36) | 101 (35) | 1 |
| $100,000-$200,000 | 52 (38) | 111 (39) | 0.98 (0.73, 1.32) |
| More than $200,000 | 22 (16) | 34 (12) | 1.21 (0.85, 1.74) |
| Education | |||
| High school or less | 6 (4) | 26 (9) | 0.65 (0.32, 1.35) |
| Some college | 31 (22) | 59 (20) | 1.00 (0.74, 1.36) |
| Bachelor’s degree or higher | 103 (74) | 203 (70) | 1 |
|
Rural/urban (>50% of census tract population resides in a rural/urban area) |
|||
| Rural | 19 (14) | 63 (22) | 0.69 (0.46, 1.04) |
| Urban | 114 (86) | 219 (78) | 1 |
| Region | |||
| Northeast | 26 (19) | 30 (35) | 0.84 (0.59, 1.20) |
| Midwest | 77 (55) | 32 (23) | 0.59 (0.41, 0.86) |
| South | 27 (19) | 42 (38) | 0.93 (0.67, 1.28) |
| West | 19 (14) | 35 (39) | 1 |
| Calendar year of breast cancer diagnosis |
Received fertility counseling† N=140 (100%) |
No fertility counseling† N=288 (100%) |
Age-adjusted PR (95% CI) |
| 2004–2005 | 34 (24) | 73 (25) | 0.97 (0.68, 1.37) |
| 2006 | 45 (32) | 102 (35) | 1 |
| 2007–2011 | 61 (44) | 113 (39) | 1.15 (0.85, 1.54) |
| Time between diagnosis and survey (years) | |||
| 1.3-<5 | 18 (13) | 29 (10) | 1.23 (0.82, 1.82) |
| 5-<6 | 43 (31) | 89 (31) | 1 |
| 6-<7 | 45 (32) | 95 (33) | 0.97 (0.70, 1.34) |
| ≥7 | 34 (24) | 75 (26) | 0.89 (0.62, 1.26) |
| Study | |||
| Sister Study | 12 (9) | 27 (9) | 1.08 (0.68, 1.72) |
| Two Sister Study | 128 (91) | 261 (91) | 1 |
| Parity at breast cancer diagnosis | |||
| 0 | 47 (34) | 84 (29) | 1.35 (0.97, 1.88) |
| 1 | 29 (21) | 29 (10) | 1.81 (1.29, 2.53) |
| 2 | 41 (29) | 117 (41) | 1 |
| 3+ | 23 (16) | 58 (20) | 1.17 (0.77, 1.77) |
| Stage | |||
| 0 | 14 (10) | 50 (17) | 0.66 (0.40, 1.09) |
| I | 53 (38) | 112 (39) | 1 |
| II | 49 (35) | 86 (30) | 0.97 (0.72, 1.31) |
| III/IV | 23 (16) | 40 (14) | 1.05 (0.73, 1.51) |
| ER status | |||
| Negative | 28 (20) | 66 (23) | 1 |
| Positive | 110 (79) | 216 (75) | 1.20 (0.86, 1.66) |
| Treatment | |||
| No chemotherapy or endocrine therapy | 10 (7) | 39 (14) | 0.56 (0.32, 0.99) |
| Endocrine therapy without chemotherapy | 16 (11) | 55 (19) | 0.71 (0.45, 1.12) |
| Chemotherapy without endocrine therapy | 25 (18) | 56 (19) | 0.79 (0.56, 1.11) |
| Chemotherapy and endocrine therapy | 89 (64) | 138 (48) | 1 |
Column totals may not sum to 100% due to missing and/or rounding
Among women diagnosed with breast cancer before, in the same year, or after thef first publication of ASCO guidelines in 2006, the proportion who received fertility counseling was 32%, 31% and 35%, respectively. Calendar year of diagnosis was not associated with receipt of fertility counseling in age-adjusted models (Table 2). Sensitivity analysis excluding women with Stage 0 breast cancer (N=64) yielded similar findings (Supplementary Table 1).
Among women who discussed fertility with a provider, 73% (102/140) of conversations were initiated by a physician. In the subset of women interested in future fertility, 24% (21/87) reported that they did not receive fertility counseling and 9% (8/87) took steps to preserve fertility. Of the eight women who reported taking steps to preserve fertility, five received gonadotropin-releasing hormone (GnRH) agonists alone, one cryopreserved embryos only, one cryopreserved oocytes only, and one cryopreserved both oocytes and embryos.
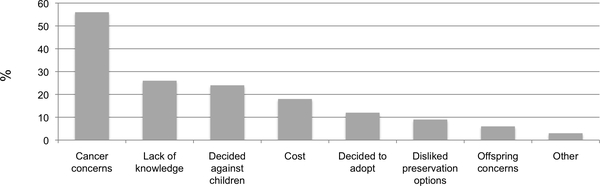
Women were allowed to select multiple reasons that they did not take steps to preserve fertility. Among women who reported an interest in future fertility prior to breast cancer diagnosis but did not take steps to preserve fertility (N=79), 68 women provided responses. Among these 68 women, the most common reasons cited included concern for adverse effect on cancer treatment (56%), lack of knowledge (26%), decision to not have children (24%), and cost (18%) (Figure 2).
Figure 2.
Distribution of reported reasons for not taking steps to preserve fertility among 68 NIEHS Sister Study and Two Sister Study participants who had been interested in a future pregnancy prior to their breast cancer diagnosis. Twelve possible responses were collapsed to eight categories defined as: Cancer concerns (56%: “I wanted to start cancer treatment right away,” or “Was afraid it would affect my breast cancer or the treatment,”); Lack of knowledge (26%: “Did not know there were any options”); Decided against children (24%: “Did not wish to have children after cancer treatment;); Cost (18% “It was too expensive,” or “Health insurance didn’t cover it”); Decided to adopt (12%: “Decided to adopt in the future”); Disliked preservation options (9%: “Fertility treatment options were overwhelming or invasive,” or “Did not like available options”); Offspring concerns (6%: “Concerned about passing on disease”); and Other (3%: “Decided to use egg or embryo donation in the future,” or “Decided to try to get pregnant at that time”
Among 340 women who did not report a fertility interest before their breast cancer diagnosis, eight (2%) changed their mind after cancer treatment about trying to conceive in the future. Among all 432 women, since cancer diagnosis sixteen women overall reported consulting with a fertility specialist, four women underwent infertility treatments, five adopted a child, and two women fostered a child. Of women prescribed endocrine therapy (N=305), 71% reported 100% adherence. Non-adherence appeared to be more common in women with an interest in future fertility (33 vs. 27%, respectively) but associations were not statistically significant (age-adjusted PR: 1.25, CI: 0.80–1.94, data not shown).
Discussion
Results from this nationwide sample demonstrate that most reproductive-age women diagnosed with breast cancer did not receive counseling on the potential impact of cancer treatment on fertility and did not utilize fertility preservation strategies. Fertility-related discussions were reported by a modest proportion (33%) of women overall, suggesting an area where improvements are needed in breast cancer care delivery.44 Approximately 1 in 5 women in our sample reported that they had hoped to become pregnant in the future before receiving their cancer diagnosis.
In the present study, an overwhelming 90% of women with an interest in future fertility did not utilize fertility preservation and 24% never discussed fertility with a provider. The low incidence of receipt of fertility counseling in our nationwide cohort is similar to the previously reported 9% prevalence of fertility counseling in the community setting, and suggests that counseling rates outside of academic or major cancer centers may be suboptimal.45 Among women who indicated they wanted a future pregnancy prior to their breast cancer diagnosis, few used fertility preservation in contrast to published reports of 40–50% utilization of fertility preservation.32, 34, 35 Our findings are more consistent with Canadian reports in which 446-9%28 of young female cancer patients utilized preservation strategies. The proportion of women diagnosed with cancer before (2004–2005), in the same year (2006), and in the years after (2007–2011) publication of the ASCO guidelines in 2006 was similar. During these years, embryo cryopreservation was the only accepted, non-experimental fertility preservation option recommended.47 However, only two women used this strategy, one of whom also preserved oocytes. Two women underwent oocyte cryopreservation which had an experimental designation until 2013.47 The most common strategy reported (by five women) was use of a GnRH agonist, which may reduce susceptibility to gonadotoxic effects of chemotherapy but is likely to provide only a modest benefit 48 and was not a recommended fertility preservation strategy by ASCO at the time this survey was conducted.14 The most recent ASCO recommendations support the use of a GnRH agonist but not in lieu of a proven fertility method. 14
Receipt of fertility discussion was associated with younger age in our cohort. Letourneau and colleagues reported that women over 35 years old at the time of cancer diagnosis during 1997 to 2007 were less likely to receive fertility counseling than younger women, but this difference did not achieve statistical significance.18 Our finding that women with higher parity are less likely to receive fertility counseling is consistent with prior reports in reproductive-age women with any cancer diagnosis between 1990 and 2009. 17 In our study, both nulliparous and uniparous women were more likely to receive fertility counseling than women with 2 children. Previous investigations have often evaluated parity as a dichotomous variable (“having at least one child/children at diagnosis”).6, 17, 18, 25 Chin and colleagues demonstrated that desired family size is associated with fertility counseling whereby women with fewer children than desired were more likely to receive counseling.17 Though the comparison of nulliparous and uniparous women was limited by sample size, our results could indicate that primiparous women have contemplated ideal family size and may be more likely to advocate for future fertility to complete their desired family size. Lower receipt of fertility discussion was associated with rural setting in sensitivity analyses excluding women with Stage 0 disease. This finding suggests that the lower prevalence of fertility counseling in the present work may be related to differences in counseling by population density, or more plausibly, proximity to major cancer or academic centers..
Frequently cited reasons for not using fertility preservation in our study and others are cancer treatment concerns, including concern for treatment delay and concern for an adverse impact of fertility preservation on cancer33, 49–51, as well as lack of knowledge of options.11 Assumptions that fertility preservation will delay and adversely affect cancer treatment are not supported by existing, albeit limited, evidence. Letourneau and colleagues demonstrated no difference in time to neoadjuvant chemotherapy in women with breast cancer who underwent fertility preservation compared to those who did not (38 vs. 39 days, respectively, P=0.7)52 and Chien et al. similarly found no difference (42 vs. 36 days, respectively, P=0.5).53 Research also counters concerns that fertility preservation may impair long-term survival but current evidence is limited to one investigation.54 Among 262 reproductive-age women diagnosed with breast cancer, Moravek et al. demonstrated no significant difference in mortality between those who underwent oocyte or embryo cryopreservation before treatment compared to those who did not (1.8 vs. 3.4%, respectively).54 However, these findings were published only recently and were not available to address potential concerns at the time of breast cancer diagnosis among women in our analysis.
Previous studies of young women with breast cancer show that fertility-related concerns impact treatment decisions.23, 34, 37 In an academic center, women who endorsed concern about the gonadotoxic impact of cancer treatment or who desired future childbearing were one-fifth as likely to initiate endocrine therapy as their counterparts.55 This association was not apparent in our sample. Similarly, our data support the lower end of the range of previous reports of how often reproductive-age women diagnosed with cancer receive fertility counseling (34–81%).17–26 The low prevalence (33%) of fertility counseling in our sample may reflect practice differences in our geographically diverse sample.15 Uptake of in vitro fertilization services may also vary based on state-mandated insurance coverage.56, 57
Our findings may be limited by recall, whereby some women who discussed fertility do not recall this discussion36 due to the overwhelming gravity, complexity, or sheer volume of medical information shared with patients around the time of cancer diagnosis.16 The majority of participants (68%) were over age 40 at the time of breast cancer diagnosis such that practitioners may have been less likely to discuss fertility preservation due to lower success rates with oocyte cryopreservation in older women.58 Selection bias in this volunteer cohort is possible; however a low prevalence of receipt of fertility counseling among a relatively well-educated and high-resourced sample is additionally concerning for what prevalence may be among women with fewer resources. Our study population was largely non-Hispanic White and estimates were adjusted for age only, leaving the potential for residual confounding or incomplete assessment of the experience of minority women. A higher prevalence of fertility counseling in the West and South regions of the U.S., which contain higher concentrations of Hispanic women relative to the Midwest may confound assessment of the Hispanic woman’s experience. Whether women received their fertility counseling, cancer diagnosis and/or treatment at an academic or community setting was not assessed. The low proportion that recalled discussing fertility with their doctor in our cohort may partially reflect the inclusion of diagnosis years that pre-dated the ASCO guideline recommending fertility discussion for all reproductive-age cancer patients.12, 59 However, the proportion of women who reported fertility counseling varied only slightly before and after 2006 (32–35%). Strengths of this investigation include using a sample that was not recruited to the study based on fertility interests and was treated in diverse settings across the country. Tumor hormone receptor status, chemotherapy and endocrine therapy were abstracted directly from medical records.
A previous investigation of young women with breast cancer determined that the preferred method of obtaining fertility-related information is consultation with a fertility specialist followed by a decision aid, such as an information booklet.34, 60 Supplanting counseling with provision of an information booklet or referral to a web-based information or telethealth platform may help patients interpret data on the fertility-related impact of cancer treatment in a more convenient, memorable, and usable way.34, 37, 60, 61 In this nation-wide sample, the majority of reproductive-age women did not receive fertility counseling, and among those interested in future pregnancy before diagnosis, few utilized fertility preservation. Our findings reinforce the continued need to implement guideline-concordant care by providing fertility counseling regarding the potential impact of breast cancer treatment on fertility and available fertility preservation options. 62 Additional investigation of disparities in provision of fertility counseling, as well as research to identify strategies that enhance receipt of recommended counseling, such as web-based or telehealth decision aids to augment in-office counseling sessions, is merited.
Supplementary Material
Acknowledgements:
We thank the former and current Sister Study research staff, our many volunteer recruiters and spokespersons for the Sister Study, and the dedicated Sister Study participants. The Sister Study was funded by the Intramural Research Program of the NIH, NIEHS (Z01ES044005). This study was also supported by the Centers for Disease Control and Prevention, and by the St. Baldrick’s Foundation.
Funding source(s) of support (organizations): the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences (Z01-ES044005, DPS), by the Centers for Disease Control and Prevention, Division of Cancer Prevention and Control (Survivorship Survey, DPS), and by the St. Baldrick’s Foundation (523803, HBN).
Footnotes
Conflict of interest disclosures:
LHB has no conflicts of interest to disclose.
JEM has no conflicts of interest to disclose.
CA has no conflicts of interest to disclose.
JLR has no conflicts of interest to disclose.
EH has no conflicts of interest to disclose.
CRW has no conflicts of interest to disclose.
DPS has no conflicts of interest to disclose.
HBN has no conflicts of interest to disclose.
References
- 1.CDC. USCS United States Cancer Statistics 2018. [updated July 13, 2018]. Available from: https://www.cdc.gov/cancer/npcr/uscs/index.htm
- 2.Trivers KF, Fink AK, Partridge AH, et al. Estimates of young breast cancer survivors at risk for infertility in the U.S. Oncologist. 2014;19(8):814–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noone AM, Cronin KA, Altekruse SF, et al. Cancer Incidence and Survival Trends by Subtype Using Data from the Surveillance Epidemiology and End Results Program, 1992–2013. Cancer Epidemiol Biomarkers Prev. 2017;26(4):632–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Group USCSW. U.S. Cancer Statistics Data Visualizations Tool based on November 2017 submission data (1999–2015). U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; [updated June 2018]. Available from: www.cdc.gov/cancer/dataviz,. [Google Scholar]
- 5.Larsen EC, Muller J, Schmiegelow K, et al. Reduced ovarian function in long-term survivors of radiation- and chemotherapy-treated childhood cancer. J Clin Endocrinol Metab. 2003;88(11):5307–14. [DOI] [PubMed] [Google Scholar]
- 6.Letourneau JM, Ebbel EE, Katz PP, et al. Pretreatment fertility counseling and fertility preservation improve quality of life in reproductive age women with cancer. Cancer. 2012;118(6):1710–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deshpande NA, Braun IM, Meyer FL. Impact of fertility preservation counseling and treatment on psychological outcomes among women with cancer: A systematic review. Cancer. 2015;121(22):3938–47. [DOI] [PubMed] [Google Scholar]
- 8.Howard-Anderson J, Ganz PA, Bower JE, Stanton AL. Quality of life, fertility concerns, and behavioral health outcomes in younger breast cancer survivors: a systematic review. Journal of the National Cancer Institute. 2012;104(5):386–405. [DOI] [PubMed] [Google Scholar]
- 9.Ruddy KJ, Greaney ML, Sprunck-Harrild K, et al. A qualitative exploration of supports and unmet needs of diverse young women with breast cancer. J Community Support Oncol. 2015;13(9):323–9. [DOI] [PubMed] [Google Scholar]
- 10.Ruddy KJ, Greaney ML, Sprunck-Harrild K, et al. Young Women with Breast Cancer: A Focus Group Study of Unmet Needs. J Adolesc Young Adult Oncol. 2013;2(4):153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bann CM, Treiman K, Squiers L, et al. Cancer Survivors’ Use of Fertility Preservation. J Womens Health (Larchmt). 2015;24(12):1030–7. [DOI] [PubMed] [Google Scholar]
- 12.Lee SJ, Schover LR, Partridge AH, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24(18):2917–31. [DOI] [PubMed] [Google Scholar]
- 13.Oktay K, Harvey BE, Partridge AH, et al. Fertility Preservation in Patients With Cancer: ASCO Clinical Practice Guideline Update. J Clin Oncol. 2018;36(19):1994–2001. [DOI] [PubMed] [Google Scholar]
- 14.Loren AW, Mangu PB, Beck LN, et al. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31(19):2500–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson RH, Kroon L. Optimizing fertility preservation practices for adolescent and young adult cancer patients. J Natl Compr Canc Netw. 2013;11(1):71–7. [DOI] [PubMed] [Google Scholar]
- 16.Practice Committee of American Society for Reproductive M. Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion. Fertility and sterility. 2013;100(5):1214–23. [DOI] [PubMed] [Google Scholar]
- 17.Chin HB, Howards PP, Kramer MR, et al. Which female cancer patients fail to receive fertility counseling before treatment in the state of Georgia? Fertility and sterility. 2016;106(7):1763–71 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Letourneau JM, Smith JF, Ebbel EE, et al. Racial, socioeconomic, and demographic disparities in access to fertility preservation in young women diagnosed with cancer. Cancer. 2012;118(18):4579–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niemasik EE, Letourneau J, Dohan D, et al. Patient perceptions of reproductive health counseling at the time of cancer diagnosis: a qualitative study of female California cancer survivors. Journal of cancer survivorship : research and practice. 2012;6(3):324–32. [DOI] [PubMed] [Google Scholar]
- 20.Chan JL, Letourneau J, Salem W, et al. Regret around fertility choices is decreased with pre-treatment counseling in gynecologic cancer patients. Journal of cancer survivorship : research and practice. 2017;11(1):58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salsman JM, Yanez B, Smith KN, et al. Documentation of Fertility Preservation Discussions for Young Adults With Cancer: Examining Compliance With Treatment Guidelines. J Natl Compr Canc Netw. 2016;14(3):301–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shnorhavorian M, Harlan LC, Smith AW, et al. Fertility preservation knowledge, counseling, and actions among adolescent and young adult patients with cancer: A population-based study. Cancer. 2015;121(19):3499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Partridge AH, Gelber S, Peppercorn J, et al. Web-based survey of fertility issues in young women with breast cancer. J Clin Oncol. 2004;22(20):4174–83. [DOI] [PubMed] [Google Scholar]
- 24.Knobf MT. The menopausal symptom experience in young mid-life women with breast cancer. Cancer Nurs. 2001;24(3):201–10; quiz 10–1. [PubMed] [Google Scholar]
- 25.Duffy CM, Allen SM, Clark MA. Discussions regarding reproductive health for young women with breast cancer undergoing chemotherapy. J Clin Oncol. 2005;23(4):766–73. [DOI] [PubMed] [Google Scholar]
- 26.Biglia N, Cozzarella M, Cacciari F, et al. Menopause after breast cancer: a survey on breast cancer survivors. Maturitas. 2003;45(1):29–38. [DOI] [PubMed] [Google Scholar]
- 27.Just Mayor S. 4% of young women treated for cancer take up fertility preservation services. BMJ. 2012;344:e2336. [DOI] [PubMed] [Google Scholar]
- 28.Yee S. Factors associated with the receipt of fertility preservation services along the decision-making pathway in young Canadian female cancer patients. J Assist Reprod Genet. 2016;33(2):265–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quinn GP, Block RG, Clayman ML, et al. If you did not document it, it did not happen: rates of documentation of discussion of infertility risk in adolescent and young adult oncology patients’ medical records. J Oncol Pract. 2015;11(2):137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewin J, Ma JMZ, Mitchell L, et al. The positive effect of a dedicated adolescent and young adult fertility program on the rates of documentation of therapy-associated infertility risk and fertility preservation options. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2017;25(6):1915–22. [DOI] [PubMed] [Google Scholar]
- 31.Mersereau JE, Goodman LR, Deal AM, et al. To preserve or not to preserve: how difficult is the decision about fertility preservation? Cancer. 2013;119(22):4044–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goodman LR, Balthazar U, Kim J, Mersereau JE. Trends of socioeconomic disparities in referral patterns for fertility preservation consultation. Human reproduction. 2012;27(7):2076–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones G, Hughes J, Mahmoodi N, et al. What factors hinder the decision-making process for women with cancer and contemplating fertility preservation treatment? Hum Reprod Update. 2017;23(4):433–57. [DOI] [PubMed] [Google Scholar]
- 34.Thewes B, Meiser B, Rickard J, Friedlander M. The fertility- and menopause-related information needs of younger women with a diagnosis of breast cancer: a qualitative study. Psychooncology. 2003;12(5):500–11. [DOI] [PubMed] [Google Scholar]
- 35.Meneses K, McNees P, Azuero A, Jukkala A. Development of the Fertility and Cancer Project: an Internet approach to help young cancer survivors. Oncol Nurs Forum. 2010;37(2):191–7. [DOI] [PubMed] [Google Scholar]
- 36.Burns KC, Boudreau C, Panepinto JA. Attitudes regarding fertility preservation in female adolescent cancer patients. J Pediatr Hematol Oncol. 2006;28(6):350–4. [DOI] [PubMed] [Google Scholar]
- 37.Peate M, Meiser B, Hickey M, Friedlander M. The fertility-related concerns, needs and preferences of younger women with breast cancer: a systematic review. Breast Cancer Res Treat. 2009;116(2):215–23. [DOI] [PubMed] [Google Scholar]
- 38.Sandler DP, Hodgson ME, Deming-Halverson SL, et al. The Sister Study Cohort: Baseline Methods and Participant Characteristics. Environ Health Perspect. 2017;125(12):127003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Brien KM, Fei C, Sandler DP, et al. Hormone therapy and young-onset breast cancer. Am J Epidemiol. 2015;181(10):799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buchanan ND, Dasari S, Rodriguez JL, et al. Post-treatment Neurocognition and Psychosocial Care Among Breast Cancer Survivors. Am J Prev Med. 2015;49(6 Suppl 5):S498–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anderson C, Sandler DP, Weinberg CR, et al. Age- and treatment-related associations with health behavior change among breast cancer survivors. Breast. 2017;33:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lehrer S, Green S, Rosenzweig KE. Affluence and Breast Cancer. Breast J. 2016;22(5):564–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.United States Census Bureau GD. “Census Regions and Divisions of the United States” U.S. Census Bureau; 2013. [cited 2018 October 22]. Available from: http://webarchive.loc.gov/all/20130107113900/http://www.census.gov/geo/www/us_regdiv.pdf. [Google Scholar]
- 44.Pavone ME, Hirshfeld-Cytron J, Lawson AK, et al. Fertility preservation outcomes may differ by cancer diagnosis. J Hum Reprod Sci. 2014;7(2):111–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goldfarb SB, Kamer SA, Oppong BA, et al. Fertility Preservation for the Young Breast Cancer Patient. Ann Surg Oncol. 2016;23(5):1530–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yee S, Buckett W, Campbell S, et al. A national study of the provision of oncofertility services to female patients in Canada. J Obstet Gynaecol Can. 2012;34(9):849–58. [DOI] [PubMed] [Google Scholar]
- 47.Practice Committees of American Society for Reproductive M, Society for Assisted Reproductive T. Mature oocyte cryopreservation: a guideline. Fertility and sterility. 2013;99(1):37–43. [DOI] [PubMed] [Google Scholar]
- 48.Hasky N, Uri-Belapolsky S, Goldberg K, et al. Gonadotrophin-releasing hormone agonists for fertility preservation: unraveling the enigma? Human reproduction. 2015;30(5):1089–101. [DOI] [PubMed] [Google Scholar]
- 49.Balthazar U, Deal AM, Fritz MA, et al. The current fertility preservation consultation model: are we adequately informing cancer patients of their options? Human reproduction. 2012;27(8):2413–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Balthazar U, Fritz MA, Mersereau JE. Fertility preservation: a pilot study to assess previsit patient knowledge quantitatively. Fertility and sterility. 2011;95(6):1913–6. [DOI] [PubMed] [Google Scholar]
- 51.Kim J, Oktay K, Gracia C, et al. Which patients pursue fertility preservation treatments? A multicenter analysis of the predictors of fertility preservation in women with breast cancer. Fertility and sterility. 2012;97(3):671–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Letourneau JM, Sinha N, Wald K, et al. Random start ovarian stimulation for fertility preservation appears unlikely to delay initiation of neoadjuvant chemotherapy for breast cancer. Human reproduction. 2017;32(10):2123–9. [DOI] [PubMed] [Google Scholar]
- 53.Chien AJ, Chambers J, McAuley F, et al. Fertility preservation with ovarian stimulation and time to treatment in women with stage II-III breast cancer receiving neoadjuvant therapy. Breast Cancer Res Treat. 2017;165(1):151–9. [DOI] [PubMed] [Google Scholar]
- 54.Moravek MB, Confino R, Smith KN, et al. Long-term outcomes in cancer patients who did or did not pursue fertility preservation. Fertility and sterility. 2018;109(2):349–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Llarena NC, Estevez SL, Tucker SL, Jeruss JS. Impact of Fertility Concerns on Tamoxifen Initiation and Persistence. Journal of the National Cancer Institute. 2015;107(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jain T, Harlow BL, Hornstein MD. Insurance coverage and outcomes of in vitro fertilization. N Engl J Med. 2002;347(9):661–6. [DOI] [PubMed] [Google Scholar]
- 57.Inhorn MC, Birenbaum-Carmeli D, Westphal LM, et al. Medical egg freezing: How cost and lack of insurance cover impact women and their families. Reprod Biomed Soc Online. 2018;5:82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goldman RH, Racowsky C, Farland LV, et al. Predicting the likelihood of live birth for elective oocyte cryopreservation: a counseling tool for physicians and patients. Human reproduction. 2017;32(4):853–9. [DOI] [PubMed] [Google Scholar]
- 59.Lee JA, Barritt J, Moschini RM, et al. Optimizing human oocyte cryopreservation for fertility preservation patients: should we mature then freeze or freeze then mature? Fertility and sterility. 2013;99(5):1356–62. [DOI] [PubMed] [Google Scholar]
- 60.Thewes B, Meiser B, Taylor A, et al. Fertility- and menopause-related information needs of younger women with a diagnosis of early breast cancer. J Clin Oncol. 2005;23(22):5155–65. [DOI] [PubMed] [Google Scholar]
- 61.Bloom JR, Kessler L. Risk and timing of counseling and support interventions for younger women with breast cancer. J Natl Cancer Inst Monogr. 1994(16):199–206. [PubMed] [Google Scholar]
- 62.Oktay K, Harvey BE, Loren AW. Fertility Preservation in Patients With Cancer: ASCO Clinical Practice Guideline Update Summary. J Oncol Pract. 2018;14(6):381–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.