Abstract
Werner syndrome (WS) is an autosomal recessive progeroid disease characterized by patients’ early onset of aging, increased risk of cancer and other age-related pathologies. WS is caused by mutations in WRN, a RecQ helicase that has essential roles responding to DNA damage and preventing genomic instability. While human WRN has both an exonuclease and helicase domain, Drosophila WRNexo has high genetic and functional homology to only the exonuclease domain of WRN. Like WRN-deficient human cells, Drosophila WRNexo null mutants (WRNexoΔ) are sensitive to replication stress, demonstrating mechanistic similarities between these two models. Compared to age-matched wild-type controls, WRNexoΔ flies exhibit increased physiological signs of aging, such as shorter lifespans, higher tumor incidence, muscle degeneration, reduced climbing ability, altered behavior, and reduced locomotor activity. Interestingly, these effects are more pronounced in females suggesting sex-specific differences in the role of WRNexo in aging. This and future mechanistic studies will contribute to our knowledge in linking faulty DNA repair mechanisms with the process of aging.
Keywords: DNA repair, Werner syndrome, aging, tumor, locomotor function
1. Introduction
Werner Syndrome (WS) is an autosomal recessive progeroid disease that affects 1 in 1,000,000 to 1 in 10,000,000 individuals (reviewed in Shamanna et al., 2017). WS is characterized by accelerated aging that usually becomes apparent when patients lack a growth spurt during puberty. WS patients’ expected lifespan is 55 due to increased incidence of heart disease and cancer. Other aging-associated pathologies typical of WS patients include type II diabetes, cataracts, subcutaneous fat loss, osteoporosis, gonadal atrophy and atherosclerosis. Additionally, WS patients may have physical characteristics such as “pinched” face, truncal obesity, and thin limbs, which are indicative of muscle degeneration (reviewed in Yokote et al., 2017).
WS is caused by mutations in WRN, an essential gene for maintaining genomic stability. WRN is a member of the RecQ family of helicases and participates in essential cellular functions such as DNA replication, transcription, recombination, and repair, as well as telomere maintenance (reviewed in Shamanna et al., 2017). Like all RecQ helicases, WRN possesses 3’→5’ ATP-dependent helicase activity. Additionally, WRN is unique in that it has 3’→5’ exonuclease activity (Croteau et al., 2014). To date there are 83 unique WRN mutation variants described, most of which result in a truncation of the 1432-amino acid protein sequence and a loss of the nuclear localization signal (Chun, 2011; Yokote et al., 2017). Fibroblasts from WS patients have increased chromosomal translocations (Au et al., 2008) and an accelerated rate of senescence that is rescued by telomere elongation (Crabbe et al., 2007; Wyllie et al., 2000).
Various mouse models have been generated to study WS. However, unlike WS patients, WRN-−/− mice are phenotypically normal (Chang et al., 2004; Lebel and Leder, 1998; Lombard et al., 2000). Like WRN-−/− mice, WRN helicase-ablated mice (WRNΔhel/Δhel) are phenotypically normal for the first year, but have a shorter median life span overall (Lebel and Leder, 1998). WRNΔhel/Δhel mice also have increased autophagy, inflammatory cytokines, triglycerides, and reactive oxygen species (Lebel and Leder, 1998; Massip et al., 2006), possibly due to mislocalization of the WRN protein to the endoplasmic reticulum (Lombard et al., 2000). To predispose WRN null mice to increased tumor incidence similar to WS patients, researchers combined WRN deficiencies with a deletion of the tumor suppressor gene, p53. WRNΔhel/Δhel p53−/− mice showed rapid tumor growth and high variety of tumor types (Lebel et al., 2001). While WRN−/− p53−/− mice were shorter lived than WRN−/−, they had normal cell proliferation and lacked abnormal lesions or tumors (Lombard et al., 2000). Because of WRN’s role in telomere maintenance, WRN−/− mice were created in combination with a null allele of the telomerase RNA component Terc. WRN−/− Terc−/− mice showed premature aging symptoms such as heightened replicative senescence, DNA damage accumulation in cell culture, and increased tumor incidence, making this the most accurate mouse model of WS to date (Chang et al., 2004).
Drosophila are rapidly becoming a standard metazoan model organism with which to study mechanisms of human disease. Drosophila share approximately 75% similarity with human disease-causing genes (Rubin et al., 2000), making them a tractable genetic model for this purpose. Additionally, Drosophila have short generation time and lifespans, allowing for quicker manifestation of age-related pathologies. Furthermore, Drosophila are genetically malleable, allowing for creation of multiple alleles and conditional mutants that can be used to study physiological processes at different stages of development. To this effect, Drosophila models have been generated for neurodegenerative diseases associated with aging such as Parkinson’s, Amyotrophic Lateral Sclerosis (ALS), and Huntington’s (reviewed in Yamaguchi, 2018). Although faulty DNA repair mechanisms often lead to age related diseases, there are relatively few fly models directly linking faulty DNA repair and replication with aging and human disease genes (Garcia et al., 2011; Mounkes et al., 1992; Rimkus et al., 2008; Wu et al., 2008).
The Drosophila WRNexo gene shares 35% identity and 59% similarity to exonuclease domain of human WRN (Saunders et al., 2008). Because WRNexo does not contain a helicase domain, we can clearly investigate exonuclease-only roles of the protein, which are less explored in WS human cell culture and mouse models. Like human WRN, purified WRNexo displays ATP-dependent 3’→5’ exonuclease activity on a variety of DNA substrates, but does not digest blunt ends or abasic sites (Boubriak et al., 2008; Mason et al., 2013). Several hypomorphic WRNexo mutants have been characterized and include phenotypes such as hyperrecombination, female sterility, and sensitivity to the topoisomerase I inhibitor, camptothecin (Boubriak et al., 2008; Mason et al., 2013; Saunders et al., 2008). These phenotypes may be in part attributed to the p-element transposon insertion methodology by which the hypomorphs were created. Second site mutations are commonly generated in these, which may give rise to phenotypes that could be erroneously attributed to the gene of interest (Thomas et al., 2013; Venken and Bellen, 2014). True null WRNexo mutants, WRNexoΔ, are viable and fertile (Bolterstein et al., 2014). Additionally, WRNexoΔ mutants show enhanced embryonic DNA damage and lethality and are sensitive to the DNA replication fork stalling drug, hydroxyurea, suggesting a role for WRNexo in responding to replication stress, especially during early embryogenesis (Bolterstein et al., 2014). However, the physiology of this mutant and the presence of WS-like phenotypes have not yet been evaluated.
Here, we characterize the physiological phenotypes of the WRNexoΔ mutant Drosophila. In doing so, we have evaluated WS-like signs of accelerated aging by assessing adult mortality, tumor incidence, body composition, muscular degeneration, and locomotor activity. Together our results present the Drosophila WRNexoΔ mutant as a tractable WS model that we can use to better understand mechanisms of aging.
2. Material and Methods
2.1. Fly stocks
All fly stocks were maintained on solid cornmeal agar and kept at 25C under a 12h:12h light-dark cycle. WRNexoΔ null mutants were created as described in Bolterstein et al. (2014). For life span and tumor incidence analyses, we used an additional WRNexoΔ stock that was outcrossed four times to w1118 to remove the presence of second site mutations.
For all adult experiments, flies were allowed to mate for 24-48 hours following eclosion and then separated by sex under CO2 anesthetization. For aging experiments, flies were maintained in vials of approximately 20 individuals and transferred to new food every 2-3 days for the duration of aging. For our experimental purposes, “young” flies are 1-4 days old and “old” flies are 28 or 35 days old as noted. For lifespan analysis, 150-200 flies of the same sex and genotype were placed in a population cage that contained a vial of cornmeal agar. Population cages were maintained at 25C and every 2-3 days dead flies were counted and removed, and food was replaced. A total of 3 biological replicates were performed.
2.2. Histology
Flies selected for pathological analysis were placed in Telly’s fixative (20 parts 70% ethanol, 2 parts 37% formalin, 1 part glacial acetic acid) for at least 48 hours prior to sequential immersion in neutral buffered formalin, ethanol and xylene according to standard protocols. Flies were subsequently perfused with wax and embedded in paraffin blocks. They were then sliced into 6 micron thick sections and placed on positively charged glass slides. Slides were baked overnight at 65C to increase tissue adherence prior to staining with hematoxylin and eosin.
2.3. Larval Buoyancy Assay
Methods were modified from (Reis et al., 2010). Flies were allowed to lay eggs for 24 hours. After 5-6 days, third instar wandering larvae were removed from vials by adding 20% sucrose. Larvae were rinsed in PBS and sets of 30 wandering larvae were transferred to vials containing 4 mL of 8.5% sucrose in PBS. Larvae were agitated and allowed to settle before scoring floating as defined as larvae at the surface of the liquid. A solution of 50% sucrose in PBS was incrementally added and floating larvae scored until all larvae were floating.
2.4. Negative geotaxis assay
Flies were anesthetized using CO2 and separated by sex at approximately 20 flies per vial. Young flies were allowed 24-48 hours to recover from anesthetization prior to testing; aged flies were tested without anesthetization. Flies were then transferred to an empty vial with markings in 2 cm intervals. Flies were tapped to the bottom of the vial 5-6 times with 1 minute of rest in between trials. Experiments were videotaped and later scored for the number of flies to cross the 6 cm mark in 10 seconds.
2.5. Locomotor activity
Continuous monitoring of locomotor activity was assessed using Drosophila Activity Monitors (DAM2, TriKinetics). Each monitor contained 32 channels and each channel recorded the movements (breaks of an infrared beam in the center of the vial) of a single fly. Flies were anesthetized using CO2 and individual flies were placed into 5 mm plastic vials (TriKinetics) containing solid media (5% sucrose, 2% agar) and sealed with a small piece of cotton (Pike et al., 2010). Single flies were continually monitored in 1-minute intervals over a 6 day period (experimental days 2-7) using the TriKinetics software. 2-4 biological replicates were performed for each condition tested. Overall activity was calculated in R (R Core Team, 2017) by first calculating the average movements (beam breaks) per minute for each individual fly for each hour of the day (0-23 hr). These data were then averaged by genotype, sex, and age. Hourly activity was calculated in a similar manner: first, the average activity per minute for each individual fly for each hour of the day was calculated and then these data were averaged by hour, genotype, sex, and age.
2.6. Statistical analysis
Large data sets were assessed for Gaussian distribution using the D’Agostino & Pearson omnibus normality test; data that did not follow a Gaussian distribution was analyzed using a non-parametric test. Survival curves were compared by the Log-rank Mantel-Cox test and average lifespan, median lifespan, and 90% mortality were analyzed by 2-way ANOVA and multiple comparisons assessed using Tukey’s post-test. Total tumor frequency was assessed by Fisher’s exact test. Larval buoyancy at matched sucrose concentrations was assessed using the Kruskal-Wallis test with Dunn’s post-test for multiple comparisons and by comparisons of area under the curve (AUC) by the Kolmogorov-Smirnov test. Negative geotaxis response was analyzed by 2-way ANOVA and multiple comparisons assessed using Tukey’s post-test. Locomotor activity was analyzed using the Kruskal-Wallis test (indicated by χ2 score) with Dunn’s post-test for multiple comparisons and the Kolmogorov-Smirnov test for comparisons between the shape of continuous distributions. Statistical analysis was conducted using GraphPad Prism 6.
3. Results
3.1. WRNexo is required for normal life span
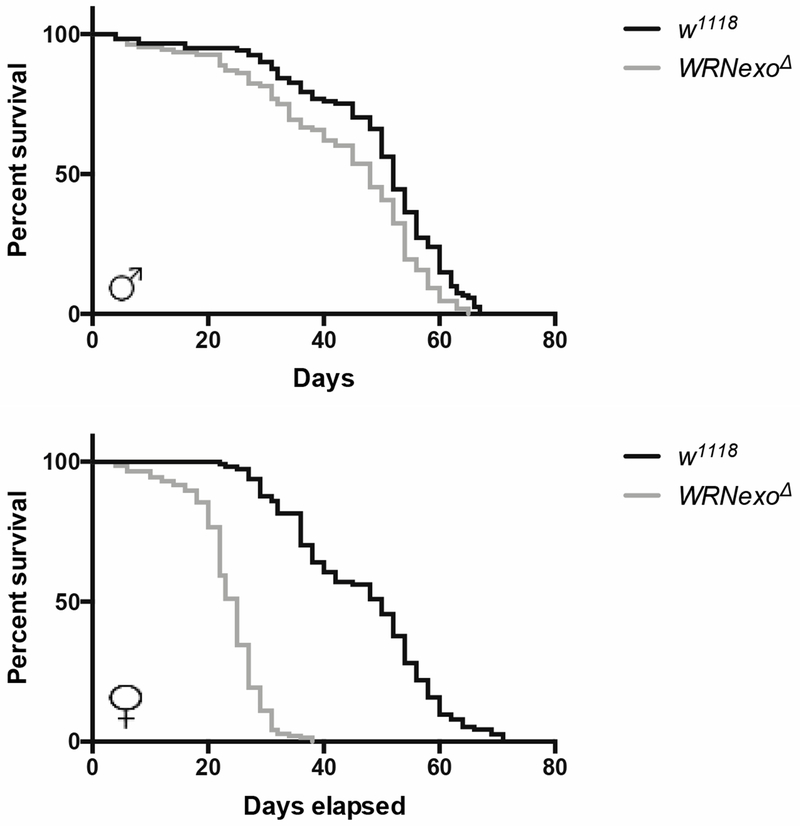
Because early onset of aging is a hallmark symptom of WS, we first measured lifespan of our WRNexoΔ flies. There was a significant reduction in median lifespan in WRNexoΔ mutants in comparison with same sex w1118 controls (Table 1) and a significant difference in survivorship curves for both sexes (Figure 1, Mantel-Cox log-rank p<0.0001). Median lifespan for WRNexoΔ was reduced to approximately 56% of w1118 lifespan in females and 81% in males (Table 1). For both sexes, WRNexoΔ survival began to diverge from w1118 at 10 days at which point mutant death rate increased. While w1118 showed no differences in survival between sexes (Table 1), WRNexoΔ females had a shorter median lifespan compared to WRNexoΔ males (p<0.01 by multiple t-test corrected by the Holm-Sidak method). WRNexoΔ females also exhibited significantly lower average lifespan and 90% mortality compared to w1118 females (Table 1).
Table 1:
Lifespan measurements for WRNexoΔ and w1118 mutants in days.
| Genotype | Sex | Average lifespan +/− SEM | Median lifespan +/− SEM | 90% mortality +/− SEM |
|---|---|---|---|---|
| w1118 | Male | 45.2 ± 1.1 | 48.0 ± 1.2 | 62.3 ± 1.5 |
| WRNexoΔ | Male | 36.6 ± 2.0 | 37.7 ± 2.3* | 52.7 ± 3.5 |
| w1118 | Female | 47.9 ± 1.1 | 50.0 ± 1.2 | 64.3 ± 1.8 |
| WRNexoΔ | Female | 28.4 ± 3.1** | 28.3 ± 2.0** | 38.3 ± 5.0** |
Data represent 4 independent experiments each containing 72-191 flies.
p < 0.05,
p < 0.001 compared to same sex w1118 control by two-way ANOVA and Tukey’s post-test.
Figure 1: WRNexoΔ mutants have a shortened lifespan.
Kaplan-Meyer survival curves for a representative experiment comparing homozygous WRNexoΔ and w1118 flies. Male: w1118 n = 121, WRNexoΔ n = 108; Female: w1118 n = 114, WRNexoΔ n = 145. There is a significant difference in survival curves between WRNexoΔ and w1118 flies of both sexes (Mantel-Cox log-rank p < 0.0001).
3.2. WRNexoΔ mutants exhibit age-related pathologies
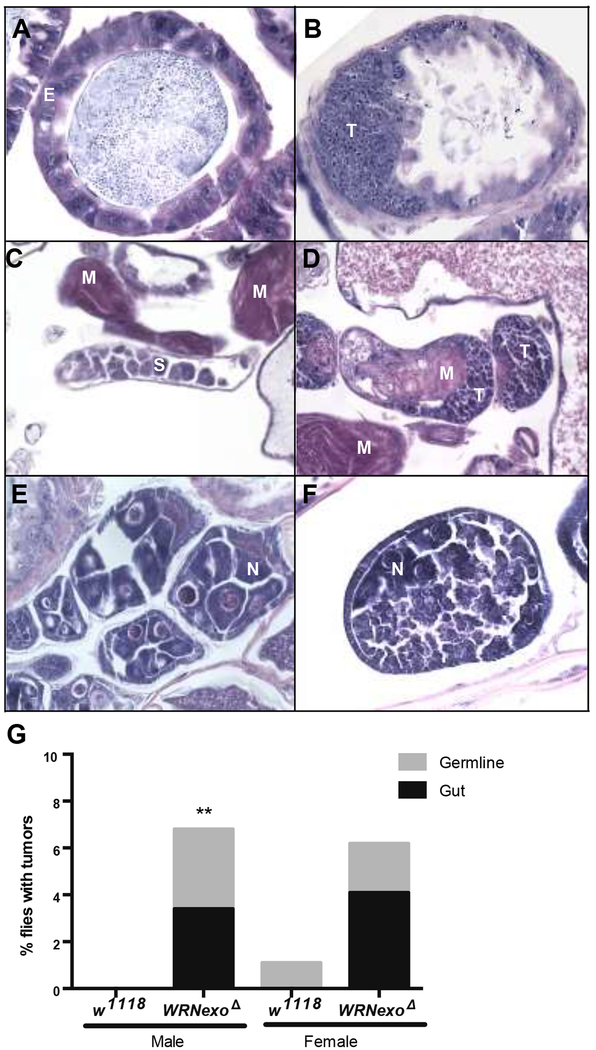
Another well-documented pathology of WS patients is high cancer incidence. Therefore, we evaluated aged WRNexoΔ (35 days old) for presence of masses of undifferentiated cells signifying tumors. By this definition, tumors were found in regions associated with highly proliferating cells: the gut and gonads (Figure 2A, F). Transverse midgut sections of age-matched w1118 controls showed epithelial cells with mild to moderate variation in nuclear size and shape, which is a common feature of gut epithelial cells in aging flies. In contrast, WRNexoΔ flies showed small pleomorphic tumor cells that infiltrate the gut wall and form a mass that protrudes into the lumen (Figure 2A, B). Similarly, the testes of aged WRNexoΔ males contained numerous tumor cells and fewer immature spermatagonia (Figure 2C, D). We also observed abnormal ovarian follicles in aged WRNexoΔ females while the ovaries were less affected in age-matched controls. The abnormal follicles contained fewer nurse cells and were filled with small, pleomorphic tumor cells whose morphology is reminiscent of germline stem cells (Figure 2E, F). WRNexoΔ males had a higher overall tumor incidence (p = 0.0029 by Fisher’s exact test), with no tumors detected in the age-matched w1118 controls. While we observed a six-fold increase in percentage of WRNexoΔ females that contained tumors compared to the age-matched w1118 controls, this difference was not statistically significant (p = 0.067) (Figure 2).
Figure 2: Aged WRNexoΔ mutants have increased tumor incidence.
A) Transverse midgut sections of 35 day-old w1118 controls show epithelial cells (E) with mild to moderate variation in nuclear size and shape, which is a common feature of gut epithelial cells in aging flies. B) In contrast, WRNexoΔ flies show small pleomorphic tumor cells (T) that infiltrate the gut wall and form a mass that protrudes into the lumen. Residual normal gut epithelial cells are present on the right. C) Normal testis in a 35-day old w1118 male sparsely populated with immature spermatocytes and spermatogonia (S) as well as mature spermatozoa (M). D) A 35-day old WRNexoΔ male showing tumor cells (T) in the testes. E) Example of normal follicles from the ovary of a 35 day-old wild-type fly. Normal nurse cells (N) are present within each follicle. F) Section through an abnormal follicle from a 35 day-old WRNexoΔ mutant fly. There is a reduction in nurse cells and the follicle is filled with small, pleomorphic tumor cells (T) whose morphology is reminiscent of germline stem cells. G) Higher total tumor incidence was observed in 35 day-old WRNexoΔ males (p = 0.0029 (males) and 0.067 (females) by Fisher’s exact test. Male: w1118 n = 123, WRNexoΔ n = 118; Female: w1118 n = 94, WRNexoΔ n = 195.
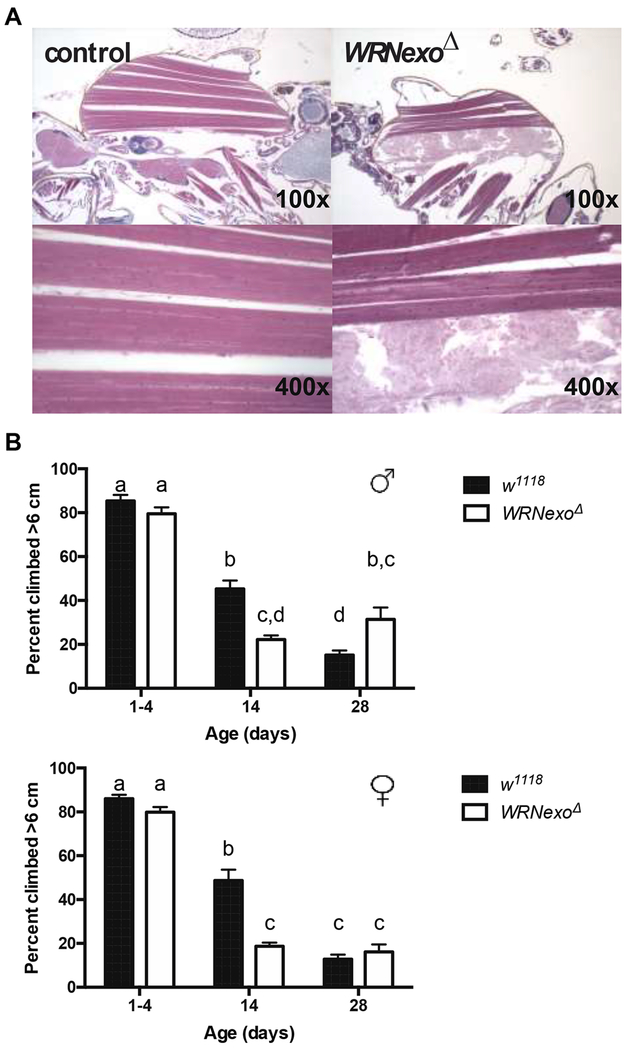
Another symptom of accelerated aging in both WS patients and the WRN−/− Terc−/− mouse model is sarcopenia (Chang et al., 2004). Aged WRNexoΔ (35 days old) demonstrate necrosis of indirect flight muscles as shown by complete loss of muscle cell nuclei and muscle fibers exhibiting a frayed appearance compared to well-defined striations in fully functioning muscles (Figure 3A). This degenerative muscle phenotype occurred in 5.1% and 3.8% of WRNexoΔ males and females respectively compared to 0% of age-matched w1118 controls (Male: w1118 n = 158, WRNexoΔ n = 171; Female: w1118 n = 142, WRNexoΔ n = 239). The degeneration of the indirect flight muscles may indicate lower motor function in the WRNexoΔ files, which is a common phenotype in aging.
Figure 3: Aged WRNexoΔ mutants exhibit muscle degeneration.
A) Example of normal muscle tissue in an aged male (left) compared to an aged WRNexoΔ male exhibiting indirect flight muscle necrosis. B) The loss of climbing capacity is exacerbated at 14 days but attenuated at 28 days in WRNexoΔ. Results were analyzed via two-way ANOVA and means compared by the Tukey post-hoc test. Letters denote statistical categories: p < 0.001 between subsequent letters. Error bars represent SEM of the averages of 5 climbing tests for each of 9-36 vials containing approximately 20 flies.
3.3. WRNexoΔ mutants show altered locomotor activity
To address the link between the observed degraded muscle structure in mutants and gross muscle function, we performed negative geotaxis assays. This simple, yet comprehensive assessment measures several behaviors including escape reflex, orientation response, locomotor activity, and climbing ability (reviewed in Iliadi et al., 2012). Both WRNexoΔ males and females showed a decline in climbing ability in comparison to age-matched w1118 controls in flies that were 14 days old (Figure 3B). There was no further climbing decline observed in 28-day old adults in either sex.
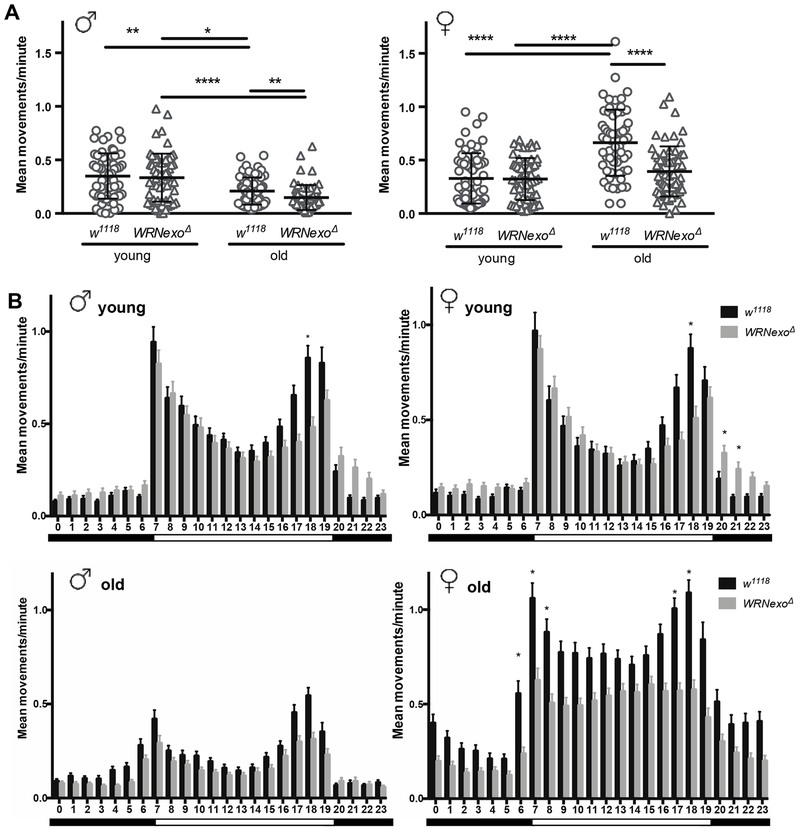
We further assessed locomotor activity though continuous monitoring using Drosophila activity monitors (DAMs). Overall locomotor activity levels of young (1-4 day old) and old (28 day old) male and female w1118 and WRNexoΔ were monitored over a six-day period (Figure. 4A and B). Within males, mean movements per minute varied significantly across groups (χ2 = 89.17, p ≤ 2.2e−16) with both w1118 and WRNexoΔ flies showing significant reductions in overall activity with age (Figure 4A; p ≤ 0.003). Comparisons between young male w1118 and WRNexoΔ revealed no significant difference in overall activity, while aged male WRNexoΔ mutants were significantly less active when compared to the w1118 controls (p ≤ 0.0001). Female mean movements per minute also varied significantly across groups (χ2 = 23.01, p ≤ 4.03e−5). However, unlike the males, overall activity in the w1118 and WRNexoΔ females did not significantly decrease with age (Figure 4A). Comparisons between young female w1118 and WRNexoΔ also revealed no significant difference in overall activity; however, aged WRNexoΔ mutant females were found to be significantly less active than the w1118 controls (Figure 4A; p ≤ 0.003).
Figure 4: WRNexoΔ mutants exhibit altered activity.
Drosophila activity monitor data was collected for young (1-4 day old) and old (28 day old) w1118 and WRNexoΔ adults separated by sex over a 6-day period. A) Overall activity is dependent on age and genotype for both male and female flies (Kruskal-Wallis test, p < 0.0001). Dunn’s post showed significant differences between old w1118 and WRNexoΔ in both sexes, decreased activity in old males, and increased activity in old females (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001). B) Average hourly activity was calculated over a 24-hour day. Activity peaks are evident at light transition periods represented by the black and white bars. The Kolmogorov-Smirnov test showed a significant difference in activity distribution between w1118 and WRNexoΔ in old females (p < 0.01). Significant differences in activity at specific hourly intervals were determined using the Kruskal-Wallis test. (*p < 0.01). Data are represented by mean and SEM of single adults (n = 64/sex/genotype).
Hourly activity of young and old WRNexoΔ and w1118 flies of both sexes was also quantified across the 24-hour day (Figure 4B). All groups displayed significant oscillations in daily activity (200.86 ≤ χ2 ≤ 582.03, p ≤ 2.2e−16; Figure 4B) with the peaks of activity occurring at approximately the same time as the transitions of the light dark cycle (hours 7 and 19). Aged WRNexoΔ females show a significantly different distribution in activity, with plateaued activity lacking peaks during the day (Figure 4B, Kolmogorov-Smirnov Test, p ≤ 0.005). Young male and female WRNexoΔ flies show lower activity levels during the evening activity peak (hour 18) (Figure 4B).
3.4. WRNexo is required for normal body weight and body composition
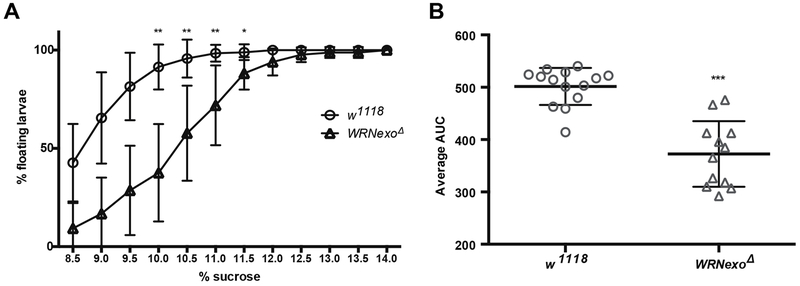
WS patients experience physiological abnormalities such as smaller size and subcutaneous fat loss. To measure potential fat loss in WRNexoΔ, we used the larval buoyancy assay, which has been shown to accurately correlate with body fat percentage (Reis et al., 2010). A smaller percentage of WRNexoΔ larvae float in 8.5-12.5% sucrose compared to w1118 controls, indicating lower fat composition (Figure 5A). This conclusion was confirmed by assessing the area under the curve (AUC) for percentage floating larvae across all sucrose concentrations tested (Figure 5B). While there was no significant difference in the dry weights of WRNexoΔ larvae, young female WRNexoΔ flies are significantly smaller than w1118 controls (Table 2). There was no difference in body size in WRNexoΔ males.
Figure 5: WRNexo deletion results in low larval body fat.
WRNexoΔ 3rd instar larvae have lower densi compared to w1118 controls as shown by A) a higher percentage of larvae that float in sucrose solutions ranging from 8.5-12% (*p < 0.001 by the Kruskal-Wallis test) and B) lower average area under the curve (AUC) calculated for each biological replicate of 30 larvae across all sucrose concentrations tested (***p = 0.0003 by the Kolmogorov-Smirnov test). Data are represented by mean and standard deviation of biological replicates (w1118: n = 14; WRNexoΔ: n = 12).
Table 2:
Larval and adult dry mass / 10 individuals (mg)
| Genotype | Larvae | Adult Male | Adult Female |
|---|---|---|---|
| w1118 | 4.2 ± 0.5 | 1.7 ± 0.3 | 2.6 ± 0.4 |
| WRNexoΔ | 3.5 ± 0.8 | 1.5 ± 0.3 | 2.0 ± 0.5* |
n = 10 groups of 10 individuals per sex/genotype.
p < 0.05 compared to same sex w1118 control by Student’s t-test.
4. Discussion
Because of flies’ genetic similarity to humans, short generation time, and our ability to control their environment, researchers have capitalized on the fly model system to better understand the process of aging. In Drosophila, lifespan has been shown to be heavily influenced by multiple factors including genetic background, environment, diet, and sex (reviewed in Iliadi et al., 2012). Drosophila have also been used to model progeroid and degenerative diseases that result in shortened life span including Parkinson’s disease, Type II diabetes, ALS, and cancer (Yamaguchi, 2018). Perhaps most relevant to this study, Drosophila have also been shown to be effective in studying DNA replication and repair-associated diseases such as Bloom syndrome, Rothman-Thomson syndrome, ataxia telangiectasia, and Xeroderma pigmentosum (Garcia et al., 2011; Mounkes et al., 1992; Rimkus et al., 2008; Wu et al., 2008). Though Drosophila WRNexo lacks the RecQ helicase domain found in human WRN, its mechanistic functions in DNA replication and repair are similar (Bolterstein et al., 2014; Boubriak et al., 2008; Saunders et al., 2008). This similarity in functionality may be possible due to WRNexo’s genetic association with Blm, another RecQ helicase that is critical in DNA replication and repair (Bolterstein, 2014), While WRNexo’s biochemical and genetic functions have been studied in flies (Bolterstein et al., 2014; Boubriak et al., 2008; Saunders et al., 2008), this is the first characterization of WS-like phenotypes in WRNexo mutant populations.
Unlike WS patient etiology, the accelerated aging phenotypes observed in WRNexoΔ mutants are manifested only in females. WRNΔhel/Δhel mice also display sex-specific phenotypes, where researchers have observed greater levels of blood glucose, triglycerides, and insulin resistance in mutant females (Massip et al., 2006). Likewise, constiutive overexpression of WRNexo in Drosophila causes increased lifespan in females, but decreased lifespan in males (Shaposhnikov et al., 2015). It is common to see sex-specific differences in Drosophila lifespan, which may in part be attributed to differential reproductive costs between males and females (reviewed in Iliadi et al., 2012). Mating has been shown to have a differential effect on the sexes: while females show lower mean longevity than males, males show increased early mortality (Pletcher, 1996). However, because we did not observe a difference in average lifespan between w1118 males and females, female-specific costs of mating are unlikely to be a large causative factor in lifespan decrease in WRNexoΔ mutants. Differential gene expression may also influence our sex-specific observations. WRNexo is more highly expressed in female adult flies, with the highest expression shown in the ovaries (Chintapalli, 2007). Because of WRNexo’s function in DNA replication during early embryogenesis, the high ovarian expression is likely due to maternal loading of the WRNexo protein into eggs (Bolterstein, 2014). However, an alternate explanation could be that WRNexo is required for normal ovarian function and protection against accelerated aging.
High cancer incidence has been widely reported in human WS patients (reviewed in Yokote et al., 2017). This etiology may be explained by the Somatic Mutation Theory of Aging, which posits that accumulation of mutations in somatic cells drive formation of tumors during the process of aging (reviewed in Kennedy et al., 2011). Interestingly, high tumor incidence has been inconsistently reported in WS mouse models. While embryonic stem cells from WRN−/− mice show higher mutation frequencies, a gross tumor phenotype was not observed in either WRN−/− mice or mice containing deletions in both WRN and the tumor suppressor gene, p53 (WRN−/− p53−/−) (Lebel and Leder, 1998; Lombard et al., 2000). The lack of a tumor phenotype in WS mouse models could be attributed to several differences in WRN function between humans and mice. While WRN protein is localized to the nucleolus in humans, its murine expression is diffuse across the nucleoplasm (Marciniak et al., 1998). A possible functional redundancy of WRN may also be present in the mouse (Lombard et al., 2000). Perhaps the most impactful difference between mice and humans is their differences in telomere maintenance: mice have long telomeres and constitutively active telomerase, which may negate the effect of a lack of WRN (Kipling and Cooke, 1990). Similarly, premature senescence and chromosomal abnormalities were prevented in WS cells expressing telomerase (Crabbe et al., 2007; Wyllie et al., 2000). Therefore, a telomerase-deficient WS mouse model was developed (WRN−/− Terc−/−), which was shown to have accumulation of DNA damage, chromosomal instability, and higher tumor incidence (Chang et al., 2004). Because telomeres are maintained by a transposon-based mechanism in Drosophila that is different from human and mouse models (Celniker et al., 2006), it is unlikely that WRNexo is involved in this capacity.
When we remove the possibility that the high tumor incidence in WRNexoΔ flies may indicate improper telomere maintenance, the more likely cause of our observed phenotypes is deficiencies in DNA replication and repair. We observed significantly higher tumor incidence in aged WRNexoΔ males, but not females, compared to age-matched controls. This result was surprising given shortened lifespans of WRNexoΔ females and it is possible that younger WRNexoΔ females have tumors, but die before our 28-day collection. Tumors were observed in gut and germline tissues where WRNexo expression is highest in the adult fly (Chintapalli et al., 2007). Cells in the gut, ovaries, and testes are highly proliferative, exacerbating the mutation frequency caused by replicative errors. Because WRNexo is required for normal replication (Bolterstein et al., 2014), replication defects may occur at a higher frequency in these cell populations, leading to tumor-forming mutations. Exacerbating this effect, aged Drosophila have reduced ability to repair double-strand breaks with age as was demonstrated through elevated γ-H2Av in 29 day-old spermatogonia (Delabaere et al., 2017). Importantly, we are the first to report a sex-dependent difference in tumor incidence in a WS model as sex was not a factor investigated in WRN-depleted mice (Chang et al., 2004; Lebel and Leder, 1998; Lombard et al., 2000).
Other DNA repair deficiencies have been shown to cause heightened tumorigenesis in Drosophila. For example, Garcia et al. (2011) reported higher tumor incidence in flies lacking Blm, another RecQ helicase (Garcia et al., 2011). At 35 days-old, blm males possessed predominantly gut tumors while blm females possessed tumors in both the gut and ovaries (Garcia, 2011), demonstrating that tumors derived in both WRNexoΔ and blm aged adults are derived from epithelial cells. This epithelial tumor origin is similar to Bloom syndrome patients who most commonly show carcinomas, but in contrast with WS patients display more mesenchymal cell-derived sarcomas (reviewed in Chu and Hickson, 2009). This difference in tumor tissue specificity between human patients and flies may be attributed to the different roles of the WRNexo and Blm proteins in each species. Drosophila WRNexo lacks the RecQ helicase domain, but because of its epistatic relationship with Blm in DNA replication (Bolterstein, 2014), these proteins may more closely associate with each other in Drosophila leading to similar tumor manifestations.
Sarcopenia, or loss of muscle mass is a classic sign of age-associated senescence that is present in both invertebrates and mammals (reviewed in Iliadi et al., 2012). Muscular deterioration has been observed in both WS patients and WRN−/− Terc−/− mice (Chang et al., 2004; Yamaga et al., 2017). In Drosophila, indirect flight muscle structure and function have been shown to degrade with age (Das et al., 2015; Ferguson et al., 2005; Grotewiel et al., 2005). Similarly, researchers have observed an age-associated decline in flight ability, where 56 day-old flies show no flight capacity (Miller et al., 2008). This decline of flight ability has been correlated with an age-related change in myofibril structure in which the myofibril heads move closer to the filaments resulting in greater muscle fiber stiffness, tension, and more power output. Further muscular deterioration in old flies (56 days) was linked to deterioration of mitochondria in the muscle fibers, suggesting that the age-related structural changes in muscle fiber structure may be compensatory reactions to low levels of available ATP (Miller et al., 2008).
A decline in locomotor activity is one consequence of muscular deterioration and is easily measured in Drosophila (Iliadi et al., 2012; Iliadi and Boulianne, 2010). To capture different aspects of behavioral changes, we measured fly locomotor activity using two complementary methods: negative geotaxis and continuous monitoring using Drosophila activity monitors (DAM). It has been found that aged and short-lived strains of Drosophila show lower climbing ability in a strain and sex-dependent manner (Fernández et al., 1999; Le Bourg, 1987; Niveditha et al., 2017; Rhodenizer et al., 2009), which was supported by our data showing decreased negative geotaxis responses at 14 days in both sexes of WRNexoΔ compared to w1118. It is unlikely that flight muscle deterioration influenced these results as flight does not contribute to negative geotaxis performance (Rhodenizer et al., 2009). Our result that 28 day-old WRNexoΔ males have higher climbing ability may suggest selective mortality in the negative geotaxis assay in which the weakest individuals are excluded from analysis. In contrast to the negative geotaxis data, DAM data showed an age-related decline in overall activity in males only. While this result was unexpected, it is not unusual as aging has been shown to influence locomotor activity in a strain- and sex-dependent manor and in some instances, female activity has been reported to increase with age (Fernández et al., 1999; Le Bourg, 1987; Rhodenizer et al., 2009). These sex-dependent differences may be attributed to a shift in behaviors that occur as flies age, favoring stationary activities that are not detectable by our methods, such as preening (Carey et al., 2006). It is also possible that because of the shortened median lifespan in WRNexoΔ females (27 days), at 28 days we may be capturing a less vulnerable population with increased activity. However, our measurements of locomotor activity using DAM also showed lower overall activity levels in both male and female aged WRNexoΔ compared to their w1118 counterparts, demonstrating that a WRNexo deletion has a negative impact on locomotor activity.
The circadian system synchronizes daily physiological functions so that organisms can optimally respond to predictable changes in environment. Characteristic diurnal patterns in Drosophila show peaks in locomotor activity around transitions of the light/dark cycle (reviewed in Allada and Chung, 2010). Using DAM to monitor hourly levels of locomotor activity, we found that w1118 exhibited the expected strong activity peaks at light transition times independent of sex or age. Young WRNexoΔ of both sexes also exhibited activity peaks associated with light transitions, however hourly analysis shows reduced activity in both WRNexoΔ males and females during the evening activity peak (hour 18).
Because many physiological functions are governed by circadian rhythms, it is unsurprising that disruptions in daily circadian patterns are associated with aging and disease. To this point, the strength of Drosophila diurnal activity peaks and consistency of their rhythms has been shown to weaken with age (Driver et al., 2004; Koh et al., 2006; Rakshit et al., 2012). Furthermore, researchers have described sex-specific effects on Drosophila aging, lifespan, and sleep patterns caused by mutations in circadian regulatory genes (reviewed in Iliadi and Boulianne, 2010). Our data show altered diurnal activity in WRNexoΔ females only: while hourly activity was lower in aged WRNexoΔ males compared to w1118, the transition period activity peaks were still evident. However, light transition-associated activity peaks were absent in aged WRNexoΔ females with relatively constant daytime activity levels. Expression of WRNexo in females remains comparatively high as flies age (Graveley et al., 2010) and therefore the requirement of WRNexo in maintaining normal activity levels and behavior may be greater in older flies.
The connection between aging, circadian rhythms, and tumorigenesis may be partially explained through interactions between clock regulatory genes and genes that regulate cell cycle and DNA repair (Dakup et al., 2018; Krishnan et al., 2008; Miki et al., 2013, 2012; Oklejewicz et al., 2008; Pregueiro et al., 2006). Specifically, the clock regulator, PER2, is directly regulated by p53 (Miki, 2013), a tumor suppressor that is commonly mutated in human cancers (Hollstein et al., 2016). Similarly, DNA damage induced by ionizing radiation has been shown to cause phase shifts in circadian rhythms through interactions through involvement of the ATM/ATR DNA damage signaling pathway (Oklejewicz et al., 2008). Studies have shown that mutations in circadian regulatory genes ablate circadian oscillations in expression of DNA damage response genes in the presence of cisplatin in mice (Dakup et al., 2018) and oxidative stress in Drosophila (Krishnan et al., 2008). On a global level, the interactions between the cell cycle and circadian oscillations have been suggested to explain the higher rates of cancer in shift workers (Feillet et al., 2015). WRN’s DNA repair and replication functions may interact with processes controlling circadian rhythms. However, additional experiments are required to thoroughly address the relationship between WS and altered circadian rhythms.
Together, our findings may suggest that increased DNA damage and/or replication stress during early development may negatively impact larval growth and subsequent development into an adult. WS patients generally have repressed growth during puberty, which leads to short stature and subcutaneous fat loss as adults, as well as thin limbs as a sign of muscular degeneration (reviewed in Yokote et al., 2017). Likewise, WRN−/− Terc−/− mice show lower body weight and a reduction in adipose tissue (Chang et al., 2004). We observed changes in body composition in WRNexoΔ, as marked by lower larval body fat and smaller size of young adult females. While larvae trended toward lower weight, the difference was not statistically significant. Reduced body size alone is unlikely to influence lifespan as body size has been shown to inversely correlate with lifespan (Khazaeli, 2005). Because larval growth and development is a determining factor of adult body size (reviewed in Yongmei Xi, 2015), reduced body fat in WRNexoΔ larvae may lead to smaller adult flies.
Low larval body fat may also indicate underdevelopment of the larval fat body, which is an important organ for storage and utilization of nutrients, endocrine regulation, immune response, and detoxification (reviewed in Estela and L. Soulages. Jose, 2010). The fat body is responsible for the synthesis and secretion of proteins that aid in organ development (e.g. growth factors for wing discs, insulin-like peptide for brain development) (Yongmei Xi, 2015) and in the oxidation of fatty acids used as fuel (Arrese and Soulages, 2010). Cells from the larval fat body persist through morphogenesis (Butterworth et al., 1965) and function in the young adult as a food source (Aguila et al., 2007). Therefore, an underdeveloped fat body is likely to contribute to suboptimal metabolic function in the adult fly, which may contribute to a physiology unable to adequately handle the stresses of aging. The fat body is also responsible for inducing autophagy during metamorphosis (reviewed in Yongmei Xi, 2015). WRN has been shown to transcriptionally regulate proteins involved in autophagy (Maity et al., 2018, 2014), further demonstrating the importance of Drosophila WRNexo during this developmental period.
Lower metabolic function may lead to an environment high in oxidative stress, where the production of mitochondrial free radicals overpowers the cell’s ability to scavenge them leading to damage of DNA, proteins, lipids, and other cellular structures. To this point, WRN has been linked with protecting against damage caused by oxidative stress (Aumailley et al., 2015a, 2015b; Massip et al., 2010; Pagano et al., 2005; Seco-Cervera et al., 2014; Talaei et al., 2013). Analysis of WS cells has shown differential expression of antioxidant genes such as glutathione peroxidase, catalase, and superoxide dismutase (Seco-Cervera et al., 2014). Similarly, treatment with vitamin C has been shown to rescue aging-related pathologies in WRN-deficient mice (Aumailley et al., 2015a; Massip et al., 2010). On a molecular level, WRN protein has been associated with the base excision repair pathway, which is the mechanism of removing 8oxo-G DNA lesions caused by oxidative stress (Das et al., 2007; Harrigan et al., 2007, 2006). Specifically, WRN helicase has been shown to unwind substrates for long-patch base excision repair (BER) (Harrigan et al., 2003). While long-patch BER is likely the preferential BER mechanism in flies (Sekelsky, 2017), WRNexo alone is likely insufficient to aid in this pathway as it does not contain a helicase domain. Instead, WRNexo may recruit a “partner” helicase (e.g. Blm) to participate in the long-patch BER mechanism. Therefore, it is possible that in our model, WRNexo protects against damage caused by oxidative stress that contributes to our observed accelerated aging phenotypes.
In conclusion, we have shown that Drosophila can be used to model accelerated aging phenotypes similar to those seen in WS patients. Because the causative factors of aging are so numerous, more research needs to be done to determine how WRNexo promotes longevity. By using model systems of degenerative diseases, we can learn more about the mechanisms behind normal aging.
Highlights.
Deficiencies in Drosophila WRNexo result in some Werner syndrome-like phenotypes
WRNexo mutants have shorter lifespans, higher tumors, and lower locomotor activity
Signs of premature aging are more pronounced in WRNexo mutant females
5. Acknowledgements
Research reported in this publication was supported, in part by the National Science Foundation (MCB0643253), the US Department of Education Title III Award (P031C110157), Northeastern Illinois University, the National Institutes of Health’s National Institute of General Medical Sciences IRACDA program (K12GM074869), and National Cancer Institute, Grant Numbers U54CA202995, U54CA202997, and U54CA203000. The authors would also like to thank Mary Kimble for her proofreading and all of the Bolterstein Lab undergraduate trainees for their help with experiments and in maintaining the fly stocks.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. References
- Aguila JR, Suszko J, Gibbs AG, Hoshizaki DK, 2007. The role of larval fat cells in adult Drosophila melanogaster. J. Exp. Biol 210, 956–963. 10.1242/jeb.001586 [DOI] [PubMed] [Google Scholar]
- Allada R, Chung BY, 2010. Circadian Organization of Behavior and Physiology in Drosophila. Annu. Rev. Physiol 72, 605–624. 10.1146/annurev-physiol-021909-135815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrese EL, Soulages JL, 2010. Insect Fat Body: Energy, Metabolism, and Regulation. Annu. Rev. Entomol 55, 207–225. 10.1146/annurev-ento-112408-085356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au K, Salk D, Martina GM, Stenchever MR, Hoehn H, 2008. Evidence of clonal attenuation, clonal succession, and clonal expansion in mass cultures of aging Werner’s syndrome skin fibroblasts. Cytogenet. Genome Res 30, 108–117. 10.1159/000131597 [DOI] [PubMed] [Google Scholar]
- Aumailley L, Dubois MJ, Garand C, Marette A, Lebel M, 2015a. Impact of vitamin C on the cardiometabolic and inflammatory profiles of mice lacking a functional Werner syndrome protein helicase. Exp. Gerontol 72, 192–203. 10.1016/j.exger.2015.10.012 [DOI] [PubMed] [Google Scholar]
- Aumailley L, Garand C, Dubois MJ, Johnson FB, Marette A, Lebel M, 2015b. Metabolic and Phenotypic Differences between Mice Producing a Werner Syndrome Helicase Mutant Protein and Wrn Null Mice. PLoS One 10, e0140292 10.1371/journal.pone.0140292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolterstein E, Rivero R, Marquez M, Mcvey M, 2014. The Drosophila Werner Exonuclease Participates in an Exonuclease-Independent Response to Replication Stress. 10.1534/genetics.114.164226 [DOI] [PMC free article] [PubMed]
- Boubriak I, Saunders RDC, Mason PA, Clancy DJ, Cox LS, Dockray J, 2008. DmWRNexo is a 3′–5′ exonuclease: phenotypic and biochemical characterization of mutants of the Drosophila orthologue of human WRN exonuclease. Biogerontology 10, 267–277. 10.1007/s10522-008-9181-3 [DOI] [PubMed] [Google Scholar]
- Butterworth FM, Bodenstein D, King RC, 1965. Adipose tissue of Drosophila melanogaster. I. An experimental study of larval fat body. J. Exp. Zool 10.1002/jez.1401580203 [DOI] [PubMed] [Google Scholar]
- Carey JR, Papadopoulos N, Kouloussis N, Katsoyannos B, MCiller H-G, Wang J-L, Tseng Y-K, 2006. Age-specific and lifetime behavior patterns in Drosophila melanogaster and the Mediterranean fruit fly, Ceratitis capitata. Exp. Gerontol 41, 93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celniker SE, Pardue M-L, DeBaryshe PG, Traverse KL, George JA, 2006. Genomic organization of the Drosophila telomere retrotransposable elements. Genome Res. 16, 1231–1240. 10.1101/gr.5348806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S, Multani AS, Cabrera NG, Naylor ML, Laud P, Lombard D, Pathak S, Guarente L, DePinho RA, 2004. Essential role of limiting telomeres in the pathogenesis of Werner syndrome. Nat. Genet 36, 877–882. 10.1038/ng1389 [DOI] [PubMed] [Google Scholar]
- Chintapalli VR, Wang J, Dow JAT, 2007. Using FlyAtlas to identify better Drosophila models of human disease. Nat. Genet 39, 715–720. 10.1038/ng2049 [DOI] [PubMed] [Google Scholar]
- Chu WK, Hickson ID, 2009. RecQ helicases: Multifunctional genome caretakers. Nat. Rev. Cancer 10.1038/nrc2682 [DOI] [PubMed] [Google Scholar]
- Chun S, 2011. The Werner’s Syndrome RecQ Helicase/Exonuclease at the Nexus of Cancer and Aging. Hawai’i Med. J 70, 52–55. [PMC free article] [PubMed] [Google Scholar]
- Crabbe L, Jauch A, Naeger CM, Karlseder J, Holtgreve-Grez H, 2007. Telomere dysfunction as a cause of genomic instability in Werner syndrome. Proc. Natl. Acad. Sci 104, 2205–2210. 10.1073/pnas.0609410104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croteau DL, Popuri V, Opresko PL, Bohr VA, 2014. Human RecQ Helicases in DNA Repair, Recombination, and Replication. Annu. Rev. Biochem 83, 519–552. 10.1146/annurev-biochem-060713-035428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakup PP, Porter KI, Little AA, Gajula RP, Gaddameedhi S, Kemp MG, Zhang H, Skornyakov E, Van Dongen HPA, 2018. The circadian clock regulates cisplatin-induced toxicity and tumor regression in melanoma mouse and human models. Oncotarget 9, 14524–14538. 10.18632/oncotarget.24539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Boldogh I, Jae WL, Harrigan JA, Hegde ML, Piotrowski J, Pinto NDS, Ramos W, Greenberg MM, Hazra TK, Mitra S, Bohr VA, 2007. The human Werner syndrome protein stimulates repair of oxidative DNA base damage by the DNA glycosylase NEIL1. J. Biol. Chem 282, 26591–26602. 10.1074/jbc.M703343200 [DOI] [PubMed] [Google Scholar]
- Das N, Levine RL, Orr WC, Sohal RS, 2015. Selectivity of protein oxidative damage during aging in Drosophila melanogaster. Biochem. J 360, 209–216. 10.1042/bj3600209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delabaere L, Ertl HA, Massey DJ, Hofley CM, Sohail F, Bienenstock EJ, Sebastian H, Chiolo I, LaRocque JR, 2017. Aging impairs double-strand break repair by homologous recombination in Drosophila germ cells. Aging Cell 16, 320–328. 10.1111/acel.12556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driver C, Georgiou A, Georgiou G, 2004. The contribution by mitochondrially induced oxidative damage to aging in Drosophila melanogaster. Biogerontology 5, 185–192. 10.1023/B:BGEN.0000031156.75376.e3 [DOI] [PubMed] [Google Scholar]
- Feillet C, van der Horst GTJ, Levi F, Rand DA, Delaunay F, 2015. Coupling between the circadian clock and cell cycle oscillators: Implication for healthy cells and malignant growth. Front. Neurol 6, 1–7. 10.3389/fneur.2015.00096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson M, Mockett RJ, Shen Y, Orr WC, Sohal RS, 2005. Age-associated decline in mitochondrial respiration and electron transport in Drosophila melanogaster. Biochem. J 390, 501–511. 10.1042/bj20042130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández JR, Grant MD, Tulli NM, Karkowski LM, McClearn GE, 1999. Differences in locomotor activity across the lifespan of Drosophila melanogaster. Exp. Gerontol 34, 621–631. 10.1016/S0531-5565(99)00040-6 [DOI] [PubMed] [Google Scholar]
- Garcia A, Salomon RN, Witsell A, Liepkalns J, Calder RB, Lee M, Lundell M, Vijg J, McVey M, 2011. Loss of the bloom syndrome helicase increases DNA ligase 4-independent genome rearrangements and tumorigenesis in aging Drosophila. Genome Biol. 12, R121 10.1186/gb-2011-12-12-r121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graveley BR, Brooks AN, Carlson JW, Duff MO, Landolin JM, Yang L, Artieri CG, van Baren MJ, Boley N, Booth BW, Brown JB, Cherbas L, Davis CA, Dobin A, Li R, Lin W, Malone JH, Mattiuzzo NR, Miller D, Sturgill D, Tuch BB, Zaleski C, Zhang D, Blanchette M, Dudoit S, Eads B, Green RE, Hammonds A, Jiang L, Kapranov P, Langton L, Perrimon N, Sandler JE, Wan KH, Willingham A, Zhang Y, Zou Y, Andrews J, Bickel PJ, Brenner SE, Brent MR, Cherbas P, Gingeras TR, Hoskins RA, Kaufman TC, Oliver B, Celniker SE, 2010. The developmental transcriptome of Drosophila melanogaster. Nature 471, 473–479. 10.1038/nature09715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotewiel MS, Martin I, Bhandari P, Cook-Wiens E, 2005. Functional senescence in Drosophila melanogaster. Ageing Res. Rev 10.1016/j.arr.2005.04.001 [DOI] [PubMed] [Google Scholar]
- Harrigan JA, Fan J, Momand J, Perrinc FW, Bohr VA, Wilson DM, 2007. WRN exonuclease activity is blocked by DNA termini harboring 3′ obstructive groups. Mech. Ageing Dev 128, 259–266. 10.1016/j.mad.2006.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrigan JA, Opresko PL, Von Kobbe C, Kedar PS, Prasad R, Wilson SH, Bohr VA, 2003. The Werner syndrome protein stimulates DNA polymerase β strand displacement synthesis via its helicase activity. J. Biol. Chem 278, 22686–22695. 10.1074/jbc.M213103200 [DOI] [PubMed] [Google Scholar]
- Harrigan JA, Wilson DM, Prasad R, Opresko PL, Beck G, May A, Wilson SH, Bohr VA, 2006. The Werner syndrome protein operates in base excision repair and cooperates with DNA polymerase β. Nucleic Acids Res. 34, 745–754. 10.1093/nar/gkj475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollstein M, Sidransky D, Vogelstein B, Harris CC, Hollstein M, Sidransky D, Vogelstein B, Harrist CC, 2016. p53 Mutations in Human Cancers. Science (80-. ). 253, 49–53. 10.1126/science.2157286 [DOI] [PubMed] [Google Scholar]
- Iliadi KG, Boulianne GL, 2010. Age-related behavioral changes in Drosophila, in: Annals of the New York Academy of Sciences, pp. 9–18. 10.1111/j.1749-6632.2009.05372.x [DOI] [PubMed] [Google Scholar]
- Iliadi KG, Knight D, Boulianne GL, 2012. Healthy aging -insights from Drosophila. Front. Physiol 3 APR, 1–11. 10.3389/fphys.2012.00106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy SR, Loeb LA, Herr AJ, 2012. Somatic mutations in aging, cancer and neurodegeneration. Mech. Ageing Dev 133, 118–126. 10.1016/j.mad.2011.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipling D, Cooke HJ, 1990. Hypervariable ultra-long telomeres in mice. Nature 347, 400–402. 10.1038/347400a0 [DOI] [PubMed] [Google Scholar]
- Koh K, Evans JM, Hendricks JC, Sehgal A, 2006. A Drosophila model for age-associated changes in sleep:wake cycles. Proc. Natl. Acad. Sci 103, 13843–13847. 10.1073/pnas.0605903103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan N, Davis AJ, Giebultowicz JM, 2008. Circadian regulation of response to oxidative stress in Drosophila melanogaster. Biochem. Biophys. Res. Commun 374, 299–303. 10.1016/j.bbrc.2008.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bourg E, 1987. The rate of living theory. Spontaneous locomotor activity, aging and longevity in Drosophila melanogaster. Exp. Gerontol 22, 359–369. 10.1016/0531-5565(87)90034-9 [DOI] [PubMed] [Google Scholar]
- Lebel M, Cardiff RD, Leder P, 2001. Tumorigenic effect of nonfunctional p53 or p21 in mice mutant in the Werner syndrome helicase. Cancer Res. 61, 1816–1819. [PubMed] [Google Scholar]
- Lebel M, Leder P, 1998. A deletion within the murine Werner syndrome helicase induces sensitivity to inhibitors of topoisomerase and loss of cellular proliferative capacity. Proc. Natl. Acad. Sci 95, 13097–13102. 10.1073/pnas.95.22.13097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard DB, Beard C, Johnson B, Marciniak RA, Dausman J, Bronson R, Buhlmann JE, Lipman R, Curry R, Sharpe A, Jaenisch R, Guarente L, 2000. Mutations in the WRN gene in mice accelerate mortality in a p53-null background. Mol. Cell. Biol 20, 3286–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maity J, Bohr VA, Laskar A, Karmakar P, 2014. Transient overexpression of Werner protein rescues starvation induced autophagy in Werner syndrome cells. Biochim. Biophys. Acta - Mol. Basis Dis 1842, 2387–2394. 10.1016/j.bbadis.2014.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maity J, Das B, Bohr VA, Karmakar P,2018. Acidic domain of WRNp is critical for autophagy and up-regulates age associated proteins. DNA Repair (Amst). 68, 1–11. 10.1016/j.dnarep.2018.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marciniak RA, Lombard DB, Bradley Johnson F, Guarente L, 1998. Nucleolar localization of the Werner syndrome protein in human cells. [DOI] [PMC free article] [PubMed]
- Mason PA, Boubriak I, Robbins T, Lasala R, Saunders R, Cox LS, 2013. The Drosophila orthologue of progeroid human WRN exonuclease, DmWRNexo, cleaves replication substrates but is inhibited by uracil or abasic sites: Analysis of DmWRNexo activity in vitro. Age (Omaha). 35, 793–806. 10.1007/s11357-012-9411-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massip L, Garand C, Paquet ER, Cogger VC, O’Reilly JN, Tworek L, Hatherell A, Taylor CG, Thorin E, Zahradka P, Le Couteur DG, Lebel M, 2010. Vitamin C restores healthy aging in a mouse model for Werner syndrome. FASEB J. 24, 158–172. 10.1096/fj.09-137133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massip L, Garand C, Turaga RVN, Deschênes F, Thorin E, Lebel M, 2006. Increased insulin, triglycerides, reactive oxygen species, and cardiac fibrosis in mice with a mutation in the helicase domain of the Werner syndrome gene homologue. Exp. Gerontol 41, 157–168. 10.1016/j.exger.2005.10.011 [DOI] [PubMed] [Google Scholar]
- Miki T, Matsumoto T, Zhao Z, Lee CC, 2013. P53 regulates Period2 expression and the circadian clock. Nat. Commun 4 10.1038/ncomms3444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki T, Xu Z, Chen-Goodspeed M, Liu M, Van Oort-Jansen A, Rea MA, Zhao Z, Lee CC, Chang KS, 2012. PML regulates PER2 nuclear localization and circadian function. EMBO J. 31, 1427–1439. 10.1038/emboj.2012.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MS, Lekkas P, Braddock JM, Farman GP, Ballif BA, Irving TC, Maughan DW, Vigoreaux JO, 2008. Aging enhances indirect flight muscle fiber performance yet decreases flight ability in Drosophila. Biophys. J 95, 2391–2401. 10.1529/biophysj.108.130005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounkes LC, Jones RS, Liang BC, Gelbart W, Fuller MT, 1992. A Drosophila model for xeroderma pigmentosum and Cockayne’s syndrome. haywire encodes the fly homolog of ERCC3, a human excision repair gene. Cell 71, 925–937. 10.1016/0092-8674(92)90389-T [DOI] [PubMed] [Google Scholar]
- Niveditha S, Deepashree S, Ramesh SR, Shivanandappa T, 2017. Sex differences in oxidative stress resistance in relation to longevity in Drosophila melanogaster. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol 187, 899–909. 10.1007/s00360-017-1061-1 [DOI] [PubMed] [Google Scholar]
- Oklejewicz M, Destici E, Tamanini F, Hut RA, Janssens R, van der Horst GTJ, 2008. Phase Resetting of the Mammalian Circadian Clock by DNA Damage. Curr. Biol 18, 286–291. 10.1016/j.cub.2008.01.047 [DOI] [PubMed] [Google Scholar]
- Pagano G, Zatterale A, Degan P, D’Ischia M, Kelly FJ, Pallardó FV, Kodama S, 2005. Multiple involvement of oxidative stress in Werner syndrome phenotype. Biogerontology 6, 233–243. 10.1007/s10522-005-2624-1 [DOI] [PubMed] [Google Scholar]
- Pike DH, Yildirim E, Low KH, Chiu JC, Edery I, 2010. Assaying Locomotor Activity to Study Circadian Rhythms and Sleep Parameters in Drosophila. J. Vis. Exp 2157 10.3791/2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pregueiro AM, Liu Q, Baker CL, Dunlap JC, Loros JJ, 2006. The Neurospora checkpoint kinase 2: A regulatory link between the circadian and cell cycles. Science (80-. ). 313, 644–649. 10.1126/science.1121716 [DOI] [PubMed] [Google Scholar]
- R Core Team (2017), 2017 R: A language and environment for statistical computing. R Found. Stat. Comput. Vienna, Austria. [Google Scholar]
- Rakshit K, Krishnan N, Guzik EM, Pyza E, Giebultowicz JM, 2012. Effects of aging on the molecular circadian oscillations in Drosophila. Chronobiol. Int 29, 5–14. 10.3109/07420528.2011.635237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis T, van Gilst MR, Hariharan IK, 2010. A buoyancy-based screen of drosophila larvae for fat-storage mutants reveals a role for Sir2 in coupling fat Storage to Nutrient Availability. PLoS Genet. 6 10.1371/journal.pgen.1001206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodenizer D, Martin I, Bhandari P, Pletcher SD, 2009. Genetic and environmental factors impact age-related impairment of negative geotaxis in Drosophila by altering age-dependent climbing speed 43, 739–748. 10.1016/j.exger.2008.04.011.Genetic [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimkus SA, Katzenberger RJ, Trinh AT, Dodson GE, Tibbetts RS, Wassarman DA, 2008. Mutations in String/CDC25 inhibit cell cycle re-entry and neurodegeneration in a Drosophila model of Ataxia telangiectasia. Genes Dev. 22, 1205–1220. 10.1101/gad.1639608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin GM, Hong L, Brokstein P, Evans-Holm M, Frise E, Stapleton M, Harvey DA, 2000. A Drosophila complementary DNA resource. Science (80-. ). 10.1126/science.287.5461.2222 [DOI] [PubMed] [Google Scholar]
- Saunders RDC, Boubriak I, Clancy DJ, Cox LS, 2008. Identification and characterization of a Drosophila ortholog of WRN exonuclease that is required to maintain genome integrity. Aging Cell 7, 418–425. 10.1111/j.1474-9726.2008.00388.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seco-Cervera M, Spis M, García-Giménez JL, Ibañez-Cabellos JS, Velázquez-Ledesma A, Esmorís I, Bañuls S, Pérez-Machado G, Pallardó FV, 2014. Oxidative stress and antioxidant response in fibroblasts from Werner and Atypical Werner Syndromes. Aging (Albany. NY). 6, 231–245. 10.18632/aging.100649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekelsky J, 2017. DNA repair in Drosophila: Mutagens, models, and missing genes. Genetics 205, 471–490. 10.1534/genetics.116.186759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamanna RA, Croteau DL, Lee J-H, Bohr VA, 2017. Recent Advances in Understanding Werner Syndrome. F1000Research 6, 1779 10.12688/f1000research.12110.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaposhnikov M, Proshkina E, Shilova L, Zhavoronkov A, Moskalev A, 2015. Lifespan and Stress Resistance in Drosophila with Overexpressed DNA Repair Genes. Sci. Rep 5, 1–12. 10.1038/srep15299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talaei F, Van Praag VM, Henning RH, 2013. Hydrogen sulfide restores a normal morphological phenotype in Werner syndrome fibroblasts, attenuates oxidative damage and modulates mTOR pathway. Pharmacol. Res 74, 34–44. 10.1016/j.phrs.2013.04.011 [DOI] [PubMed] [Google Scholar]
- Thomas AM, Hui C, South A, McVey M, 2013. Common Variants of Drosophila melanogaster Cyp6d2 Cause Camptothecin Sensitivity and Synergize With Loss of Brca2 . G3: Genes|Genomes|Genetics 3, 91–99. 10.1534/g3.112.003996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venken KJT, Bellen HJ, 2014. Chemical mutagens, transposons, and transgenes to interrogate gene function in Drosophila melanogaster. Methods 68, 15–28. 10.1016/j.ymeth.2014.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Capp C, Feng L, Hsieh T. shih , 2008. Drosophila homologue of the Rothmund-Thomson syndrome gene: Essential function in DNA replication during development. Dev. Biol 323, 130–142. 10.1016/j.ydbio.2008.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie FS, Jones CJ, Skinner JW, Haughton MF, Wallis C, Wynford-Thomas D, Faragher RGA, Kip D, 2000. Telomerase prevents the accelerated cell ageing of Werner syndrome fibroblasts. Nat. Genet 24, 16–17. [DOI] [PubMed] [Google Scholar]
- Yamaga M, Takemoto M, Shoji M, Sakamoto K, Yamamoto M, Ishikawa T, Koshizaka M, Maezawa Y, Kobayashi K, Yokote K, 2017. Werner syndrome: A model for sarcopenia due to accelerated aging. Aging (Albany. NY). 9, 1738–1744. 10.18632/aging.101265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M, 2018. Drosophila Models for Human Diseases. 10.1007/978-981-13-0529-0 [DOI] [Google Scholar]
- Yokote K, Chanprasert S, Lee L, Eirich K, Takemoto M, Koizumi N, Lessel D, Mori T, Hisama FM, Paula D, Angle B, Baris H, Cefle K, Palanduz S, Ozturk S, 2017. WRN Mutation Update: Mutation Spectrum, Patient Registries, and Translational Prospects. Hum. Mutat 38, 7–15. 10.1002/humu.23128.WRN [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yongmei Xi YZ, 2015. Fat Body Development and its Function in Energy Storage and Nutrient Sensing in Drosophila melanogaster. J. Tissue Sci. Eng 06, 1–8. 10.4172/2157-7552.1000141 [DOI] [Google Scholar]