Abstract
Objective:
Exaggerated neutrophil activation and formation of neutrophil extracellular traps (NETs) are linked to inflammation and autoimmunity, including rheumatoid arthritis (RA). However, whether NETs are present in the circulation of RA patients and contribute to inflammation and disease progression has not been carefully addressed. Here we assess markers of neutrophil activation and NET formation in plasma samples, asking whether they add clinical value in improving on determination of prognosis and monitoring in RA patients.
Methods:
Markers of neutrophil activation (calprotectin) and cell death (NET) were analyzed in plasma from three cross-sectional RA cohorts and healthy individuals using ELISA. A longitudinal inception cohort (n=247), seen for follow-up a median of 8 years later was used for predictive analyses.
Results:
Markers of neutrophil activation and cell death were increased in RA patients compared to healthy individuals (p<0.0001). Calprotectin levels correlated with CDAI (r=0.53, p<0.0001) and distinguished between patients in remission and active disease, an observation not seen with CRP. A biomarker panel consisting of ACPA and calprotectin could predict erosive disease (OR=7.5, p<0.0001) and joint space narrowing (OR=4.9, p=0.001). Levels of NETs were associated with heightened levels of cell-free citrulline (p=0.02) and inflammation (p=0.0002). Furthermore, NETs, and a ‘neutrophil activation signature’ biomarker panel, had good predictive value in identifying patients developing extra-articular nodules (OR=5.6, p=0.006).
Conclusion:
Neutrophils undergo marked activation and cell death in RA. Neutrophil biomarkers provide added clinical value in monitoring and prognosis of RA patients, and may allow for early preventive treatment intervention.
Introduction
Neutrophils are the most abundant immune cells in the circulation, participating in host defense mechanisms through production of reactive oxygen species (ROS), phagocytosis and formation of neutrophil extracellular traps (NETs). NET formation, also called NETosis, is a neutrophil cell death process in which DNA is extruded together with cytoplasmic and granular contents in a web-like structure to eliminate extracellular pathogens (1–6). Although beneficial from a host-pathogen perspective, exaggerated neutrophil activation has been linked to inflammation and autoimmunity including rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) (2,3,7–9). In RA, neutrophils are well-known contributors of local inflammation and participate in tissue destruction and erosions (10). Further, neutrophils express high levels of peptidyl-arginine deiminase (PAD)2 and PAD4, key proteins in the enzymatic conversion of arginine into citrulline, the autoantigen towards which anti-citrullinated protein antibodies (ACPA) are targeted. Two main processes through which citrullination may occur have emerged; i) pore-formation leading to ‘hypercitrullination’ of cytosolic molecules (11,12), and ii) NETosis, exposing citrullinated histones and vimentin, key targets for RA autoantibodies (9,13–15). The latter process may be of particular importance in the synovium where local B cells have been found to produce anti-NET antibodies (13).
RA neutrophils are prone to undergo spontaneous NETosis when examined ex vivo, as well as upon stimulation with inflammatory cytokines and autoantibodies (9,16). The released NETs induce local inflammation as well as present citrullinated peptides to antigen-specific T cells by fibroblast-like synoviocytes (9,17). Illustrating the important role of NETs in the disease setting of RA, Cl-amidine, a PAD4 inhibitor, ameliorated disease development in the CIA model (18). However, PAD4, inhibition did not affect arthritis phenotype in the K/BxN serum-transfer model (19). In RA patients, neutrophil activation markers, including calprotectin, are elevated and associated with active disease, and may predict radiographic progression (20–22). Serum levels of NETs and NET-derived products, including cell-free DNA, are also elevated in RA patients and associated with disease activity (16,23,24). However, given that the fragile neutrophils undergo spontaneous NET formation upon serum processing, e.g. coagulation (16), serum levels may not reflect the true physiological levels of NETs experienced by patients with RA. Thus, whether RA patients have NETs in the circulation, as well as their relation to disease activity and inflammation is still not known.
In the current study we investigate true levels of neutrophil activation markers, including NETs, in large cross-sectional and longitudinal RA cohorts and ask whether these markers could add value with regard to diagnosis, prognosis, and monitoring of RA patients. In brief, we found that neutrophil activation markers were superior to CRP in identifying patients with active disease. Finally, neutrophil biomarkers improved the prognostic capacity of ACPA in predicting radiographic changes, and development of extra-articular nodules (EAN). Thus, neutrophils are clearly implicated in the RA pathogenesis, contributing to inflammation, organ damage and exposure of autoantigens. As such, we propose that neutrophil activation biomarkers may help guide the clinician in diagnosis and prognosis. Further, neutrophil activation biomarkers may help identify patients with active disease and allow for early preventive treatment strategies, thus reducing disabling morbidities and improving the quality of life in these patients.
Materials and Methods
Patient cohorts
Patients with RA (n=101, Cohort I) and age- and gender-matched healthy individuals (n=20) were recruited to participate in research studies at the University of Washington Medical Center, Seattle. Disease activity was recorded using the Clinical Disease Activity Index (CDAI) for RA taking into account tender and swollen joints, patient global assessment, and provider global assessment. Median CDAI was 11 (moderate activity), ranging from 0–46 in Cohort I. One cross-sectional cohort with established RA (n=93, Cohort II), age- and gender-matched healthy individuals (n=100), as well as one RA inception cohort (n=247, Cohort III) seen for follow-up after a median of 8.3 years (range: 4.4–19.8 years) were recruited in Washington State. For additional patient characteristics, see Supplemental Table 1. The study was approved by regional ethics boards (#3100 and #810), and informed written consent was obtained from all participants in accordance with the Helsinki Declaration.
Neutrophil activation and cell death markers
Levels of calprotectin (S100A8/A9) were analyzed using a commercial ELISA kit according to the manufacturer’s instructions (R&D Systems). For the detection of NETs, a 96-well microtiter plate (Corning) was coated with anti-MPO antibody (4 μg/mL, Biorad) overnight at 4°C, followed by blocking with 1% bovine serum albumin (BSA) in PBS for 2 hours at room temperature. After blocking, plasma or serum samples (10% in PBS-BSA) were added and incubated overnight at 4°C. For detection, anti-dsDNA-HRP antibody (diluted 1/100, Roche Diagnostic) was added for 2 hours, room temperature. The reaction was developed with 3,3’,5,5’-tetramethylbenzidine (TMB, BD Biosciences), and ended by the addition of 2 N sulfuric acid. Absorbance was measured at 450 nm by a plate reader (Synergy, BioTek). For cohorts I-II, plasma samples were used, and for cohort III, serum samples were used. Isolated NETs were used as a standard curve with 1 U/mL equaling NETs released by 10,000 neutrophils.
NET and DNA degradation
NET degradation was assessed using our previously published protocol with some modifications (26). Briefly, neutrophils were isolated through density gradient (Polymorphprep, Axis-Shield) and seeded at 1×106 cells/mL in poly-L-lysine-coated black 96 well microtiter plate. Neutrophils were induced to undergo NETosis by addition of 20 nM PMA for 4 hours. Upon washing, attached NETs were stained with Sytox Green (1/5,000, Life Technologies), followed by subsequent wash steps. After recording baseline fluorescence, sera (5%, diluted in nuclease buffer; 10 mM Tris-HCl pH 7.5, 10 mM MgCl2, 2 mM CaCl2, 50 mM NaCl) were added and incubated for 90 minutes at 37°C. As a positive control, micrococcal nuclease was used. After the incubation, the wells were washed and residual NETs analyzed by plate reader. NET degradation was calculated as the relative loss of NETs (Sytox Green signal) in each well, using the standard curve as a reference value. For DNA degradation, Sytox Green-labeled DNA (5 μg/mL) was bound to poly-L-lysine-coated plates, and the capacity to degrade the DNA assessed in presence of 10% sera in nuclease buffer.
Serum-mediated neutrophil activation
Neutrophils, isolated as above, were incubated with 10% serum for 3 hours, and analyzed for cell surface expression of CD11b and CD66b (BioLegend) by flow cytometry. The CD11b antibody recognizes all forms of CD11b, including the non-activated. The results are presented as the relative MFI signal as compared to non-activated neutrophils.
IL-6 and CRP ELISA
IL-6 levels were measured by sandwich ELISA. Briefly, a 96-well microtiter plate (Corning™ Clear Polystyrene flat-bottomed, 96-Well, medium binding), was coated with 100 μL of capture antibody (4 μg/mL, anti-human IL-6, BioLegend) diluted in PBS and incubated overnight at 4°C. After blocking with 1% BSA in PBS for 2 hours at room temperature, samples were added and incubated overnight at 4°C. For detection, plates were incubated with biotinylated detection antibody (1 μg/mL in blocking buffer, Biotin anti-human IL-6) for 2 hours at room temperature, followed by addition of HRP-streptavidin (BioLegend) for an additional 2 hours at room temperature. The reaction was visualized by the addition of TMB. The reaction was stopped by addition of 2N sulfuric acid and the absorbance was measured at 450 nm by plate reader. Wells were washed thoroughly three times in PBS-Tween between every step. CRP was analyzed using ELISA according to the manufacturer’s protocol (Enzo Life Sciences Inc. Farmingdale, NY, USA).
Statistics
For sample sets with a non-Gaussian distribution, Mann-Whitney U test, and Spearman’s correlation test were used, as applicable. In some analyses logistic regression analyses were used. For neutrophil markers, the cut-off for positivity was determined by the 95th percentile of the healthy individuals. For outcome measures (erosive disease and joint space narrowing) the upper quartile of the RA cohort was used. GraphPad Prism and SPSS were used for the analyses. All analyses were considered statistically significant at p<0.05.
Results
Levels of calprotectin are elevated in RA patients and associated with disease activity
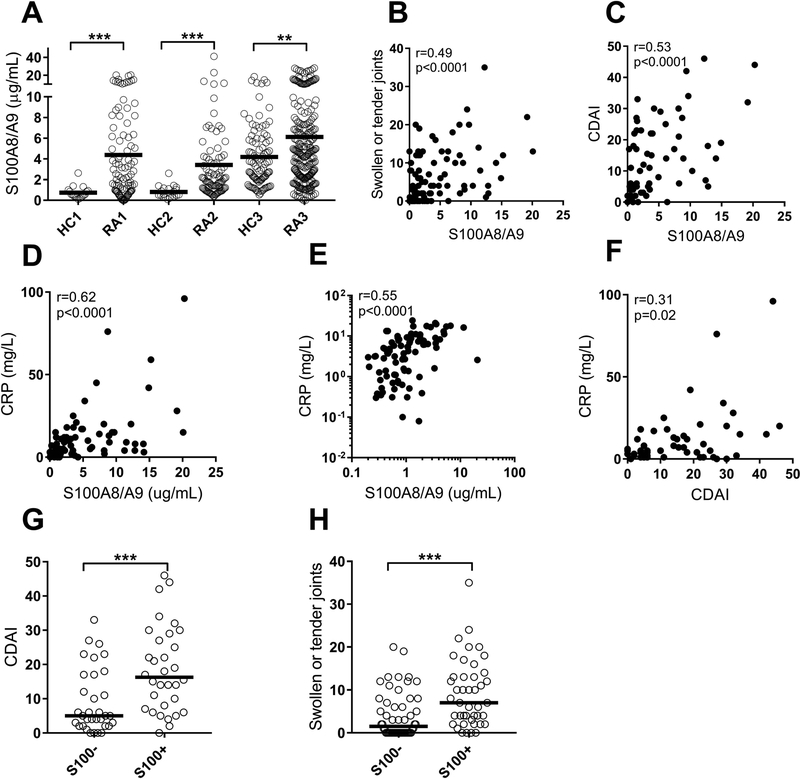
Calprotectin is an acute-phase protein known to be elevated in several inflammatory conditions including RA (20,21). Consistent with prior work, levels of calprotectin were elevated in three RA cohorts as compared to healthy individuals (p<0.001, Figure 1A). Of note, serum samples (cohort III) rendered higher baseline levels of calprotectin, compared to plasma samples (cohorts I and II), consistent with prior work from our group (27). Levels of calprotectin were not associated with any particular treatment strategy, nor with seropositivity (p=0.10, data not shown) in contrast to previous findings (21,28). Further, plasma levels of calprotectin were not associated with neutrophil count (p=0.46, data not shown). Similar to findings in SLE (29), levels of calprotectin have been shown to associate with disease activity in RA (21,30,31). Whether calprotectin would add clinical value and outperform gold standard serological markers of disease activity, including CRP, is not known. In cohort I, calprotectin was strongly correlated with markers of disease activity, including number of swollen and tender joints (r=0.49, p<0.0001, Figure 1B, 1H), CDAI (r=0.53, p<0.0001, Figure 1C, 1G), as well as CRP (r=0.62, p<0.0001, Figure 1D). The correlation with CRP was validated in Cohort II (r=0.55, p<0.0001, Figure 1E).
Figure 1. Levels of calprotectin are associated with disease activity in RA.
A) Levels of calprotectin (S100A8/A9) were analyzed in three cohorts of patients with rheumatoid arthritis (RA), and three cohorts with healthy individuals (HC). For the third cohort, HC3 and RA3, serum samples were used. B-E) Levels of calprotectin were correlated with B) numbers of swollen joints, C) disease activity as measured by CDAI, and D-E) levels of CRP in Cohort I and II, respectively. E) Correlation between CDAI and CRP. F-G) Patients with elevated levels of calprotectin (S100+) had increased F) CDAI and G) number of swollen joints. All analyses, except for Figure 1E, were done for Cohort 1. Mann-Whitney U test and Spearman correlation test were used for statistical analyses, with * p<0.05, ** p<0.01, and *** p<0.001.
Calprotectin is superior to CRP in identifying patients with active disease
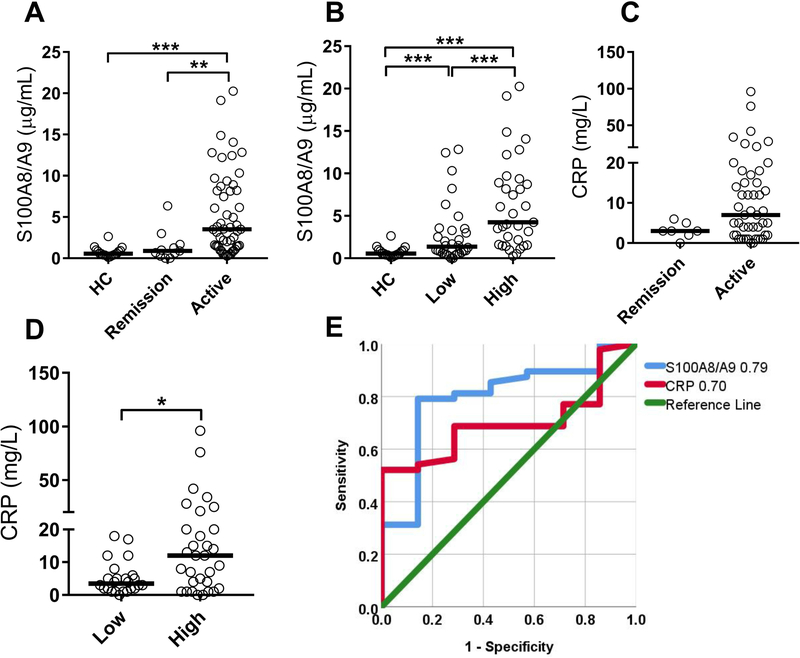
Next, we asked whether calprotectin was superior to CRP in identifying patients with active disease in RA cohort I. CRP only modestly correlated with CDAI (r=0.31, p=0.02, Figure 1F); a weaker correlation as compared to the one observed for calprotectin (Figure 1C). Levels of calprotectin could distinguish between patient in remission versus active disease (p=0.002, Figure 2A), as well as between patients with low disease activity (CDAI<11) versus patients with moderate to high disease activity (CDAI>10) (p=0.0005, Figure 2B). Patients in remission were indistinguishable from healthy individuals (Figure 2A). CRP, on the other hand, was unable to distinguish between patients in remission versus active disease (p=0.10, Figure 2C). However, CRP levels were elevated when comparing high disease activity versus low disease activity (p=0.02, Figure 2D). Using a receiver operating characteristic (ROC) curve analysis, levels of calprotectin performed better as compared to CRP in identifying active disease (AUC 0.79 and 0.70, respectively, Figure 2E). Thus, calprotectin is superior to CRP in assessing disease activity in RA.
Figure 2. Calprotectin is superior to CRP in monitoring disease activity.
A-B) Levels of calprotectin (S100A8/A9) were analyzed in plasma from healthy controls (HC) and RA patients at A) time-point of remission and active disease or B) time-point of low disease activity (CDAI<11) and high disease activity (CDAI>11). C-D) Levels of CRP were analyzed in RA patients at C) time-point of remission and active disease or D) time-point of low disease activity (CDAI<11) and high disease activity (CDAI>11). E) Receiver operator characteristic (ROC) curve assessing capacity of calprotectin and CRP to distinguish patients in remission versus active disease. All analyses were done for Cohort 1. Statistical analyses were done by Mann-Whitney U test, with * p<0.05, ** p<0.01, and *** p<0.001.
Levels of NETs are elevated in RA patients and related to disease activity
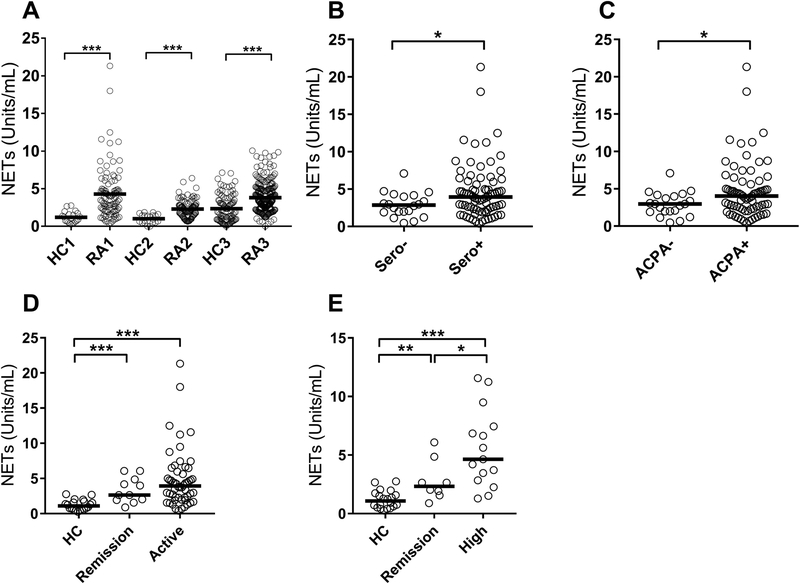
Neutrophils from RA patients are more prone to undergo NETosis than healthy controls, both spontaneously and upon activation (9,16). Serum levels of NETs and NET-derived products are elevated in RA patients and associated with disease activity (16,23,24). However, serum levels do not necessarily reflect physiological levels of NETs experienced by the RA patients, as artificial NETs are formed upon serum processing, e.g. coagulation (16). In the current study, using plasma samples (avoiding coagulation-mediated release of NETs) to assess true levels of NETs in patient circulation, we found that RA patients have markedly elevated levels of NETs as compared to healthy individuals (p<0.001 for all three cohorts, Figure 3A). In RA cohort I, levels of NETs were higher in seropositive patients (p=0.04, Figure 3B), including ACPA-positive patients (p=0.04, Figure 3C).
Figure 3. Increased levels of NETs in RA.
A) Levels of NETs (MPO-DNA complexes) were analyzed in three distinct cohorts of RA and healthy controls (HC). For the third cohort, HC3 and RA3, serum samples were used. The values are reported as Units/mL. B-C) RA patients were stratified based on B) seropositivity and C) ACPA positivity and levels of NETs assessed in the groups. D) Levels of NETs in HC, RA patients in remission (CDAI<3), and RA patients with active disease (CDAI>3). E) Levels of NETs in HC, seropositive patients in remission, as well as seropositive patients with high disease activity (CDAI>22). All analyses were done for Cohort 1. Statistical analyses were done by Mann-Whitney U test, with * p<0.05, ** p<0.01, and *** p<0.001.
We next asked whether NET levels were associated with disease activity. Levels of NETs were elevated both in remission and active disease in Cohort I (p=0.0003, and p<0.0001, respectively, Figure 3D). However, there was not a statistically significant difference in levels of NETs between patients in remission or flare (p=0.37), nor did we find a direct correlation with CDAI (r=0.16, p=0.19). Given the heterogeneity in RA, with seropositive patients having elevated levels of NETs, we next stratified patients based on seropositivity. In seropositive patients, NET positivity could distinguish between remission and active disease with a sensitivity and specificity of 68.6% and 75.0%, respectively, p=0.04. In contrast, CRP was unable to distinguish remission from active disease (sensitivity 37.8% and specificity 100%, respectively, p=0.16). Thus, NETs are increased in RA and associated with disease activity, in particular in seropositive disease.
RA patients have impaired capacity to degrade NETs
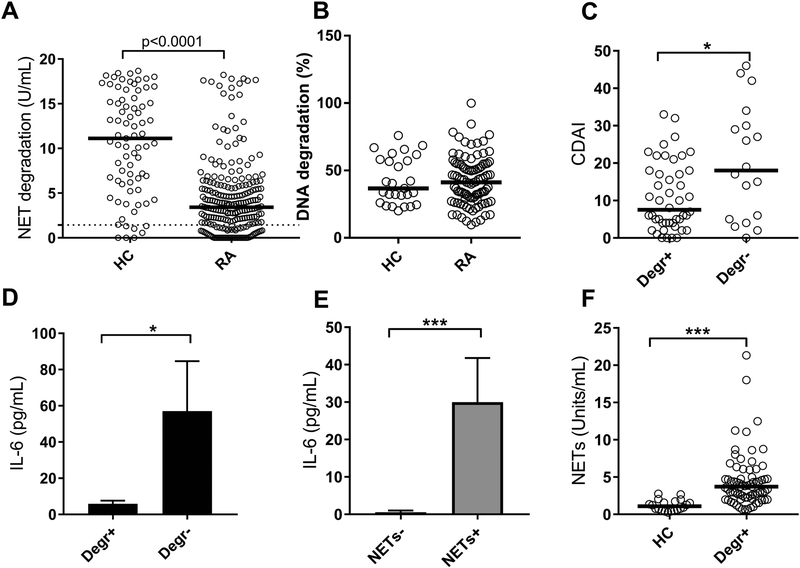
The reason for the elevated levels of NETs observed in RA is not known. Prior studies have shown evidence of NET-inducing stimuli (e.g. autoantibodies and inflammatory cytokines) in RA (9), as well as enhanced NET forming capacity of neutrophils (9,16). However, also impaired clearance could promote accumulation of NETs in the circulation. We have shown that NET degradation is impaired in SLE, and associated with disease activity and IFN induction (26,32). To determine whether NET degradation is impaired also in RA patients, the capacity of patient sera to degrade NETs was assessed using an in-house assay. As illustrated in Figure 4A, RA sera had an overall reduced ability to degrade NETs (p<0.0001, Cohort III). Of note, RA patients did not have a reduced capacity to degrade DNA (Figure 4B). These data suggest a NET-specific factor such as anti-NET antibodies, rather than reduced DNase 1 levels, contributing to the impaired degradation of NETs. Patients with low capacity to degrade NETs had increased disease activity (p=0.04, Figure 4C, Cohort I). Further, in Cohort I, reduced capacity to degrade NETs, and/or elevated levels of circulating NETs were both associated with increased inflammation, in particular IL-6 levels (p=0.03 and p=0.0002, respectively, Figures 4D–E). Unexpectedly, the ability to degrade NETs did not correlate with levels of circulating NETs (r=0.02, p=0.87). Further, even patients with sufficient NET degradation had elevated levels of NETs as compared to healthy individuals (p<0.0001, Figure 4F), suggesting that reduced NET degradation is not responsible for the accumulation of NETs in RA patients.
Figure 4. RA patients have impaired NET degradation.
A) Capacity to degrade NETs was analyzed in healthy controls (HC) and RA sera from Cohort III. The dotted line represents the cut-off for impaired NET degradation. B) Capacity to degrade isolated DNA was analyzed in HC and RA sera from Cohort I. C) Patients from Cohort I were stratified based on their capacity to degrade (Degr+) NETs or not (Degr-), and related to disease activity. D-E) Levels of serum IL-6 were related to D) NET degradation and E) circulating NET levels in Cohort I. F) Levels of NETs compared between HC and RA patients (Cohort I) with normal NET degrading capacity. Statistical analyses were done with Mann-Whitney U test, with * p<0.05, ** p<0.01, and *** p<0.001.
RA patients have circulating neutrophil-activating factors
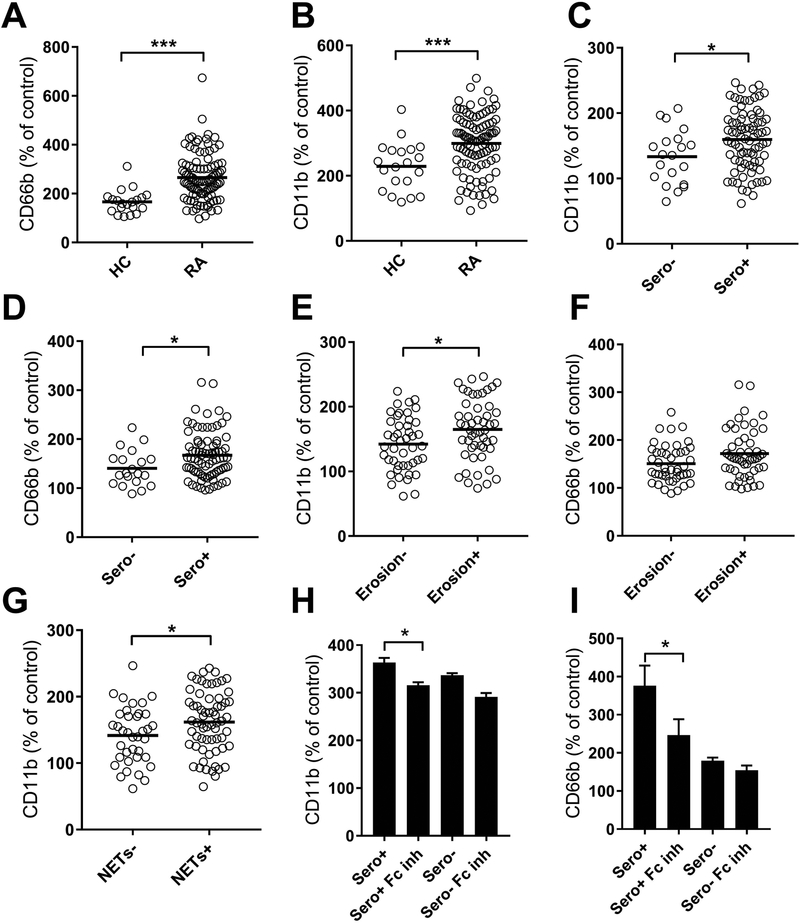
Considering the elevated levels of neutrophil-derived activation markers in peripheral blood of RA patients, we next asked whether RA patients had circulating factors acting to induce neutrophil activation. Using a functional in vitro assay to assess serum-mediated neutrophil activation, RA sera from Cohort I were found to induce marked neutrophil activation, as illustrated by CD11b and CD66b up-regulation, compared to sera from healthy individuals (p<0.0001, Figures 5A–B). Serum-mediated neutrophil activation was further increased in seropositive individuals (p=0.02, Figures 5C–D) and related to disease severity, e.g. erosive disease (Figures 5E–F). Consistent with our hypothesis, serum-mediated neutrophil activation was associated with elevated levels of circulating calprotectin (r=0.40, p<0.0001). Of note, serum-mediated neutrophil activation was also associated with elevated levels of circulating NETs in RA patients (p=0.04, Figure 5G). Given our prior studies in SLE (2,33), we expected that immune complexes would partake in the observed serum-mediated neutrophil activation. Supporting this hypothesis, blocking neutrophil FcgRIIA and FcgRIIIB, in particular in seropositive RA patients, reduced the capacity of RA sera to induce CD66b and CD11b up-regulation (p=0.02, Figures 5H–I). Of note, FcgR blockade only partially reduced the CD11b and CD66b levels, clearly suggesting that other factors, including inflammatory cytokines, may also contribute to neutrophil activation in RA.
Figure 5. Serum-mediated neutrophil activation.
Serum from healthy controls (HC) and RA patients were incubated with normal neutrophils and assessed for capacity to induce up-regulation of neutrophil activation markers A) CD66b and B) CD11b. The values are related to non-stimulated neutrophils, corresponding to 100%. C-F) RA patients were stratified based on C-D) seropositivity and E-F) erosion, and analyzed for C, E) CD11b and D, F) CD66b up-regulation. G) CD11b up-regulation stratified on presence of circulating NETs. H-I) Neutrophils were pre-incubated with anti-FcgRIIA and anti-FcgRIIIB (FcgR ab) prior to addition of RA sera and assessed for H) CD11b and I) CD66b up-regulation. Patients were stratified based on seropositivity (Sero+) or seronegativity (Sero-). All analyses were done for Cohort I. Statistical analyses were done with Mann-Whitney U test and Wilcoxon paired test, with * p<0.05, ** p<0.01, and *** p<0.001.
Levels of neutrophil biomarkers predict disease outcome
Calprotectin is known as an independent predictor of radiographic changes in RA (21,22). However, it is unknown whether NETs could also predict radiographic changes, the role of neutrophil biomarkers in prediction of extra-articular disease (EAD), and whether there would be added value in using a biomarker panel, rather than individual biomarkers. To investigate this, we analyzed levels of calprotectin in a longitudinal inception cohort of 250 RA patients (Cohort III) seen at follow-up a median of 8 years after RA onset. Patients with evidence of erosive disease at inception (10%) were excluded from further analysis. As a comparator, we analyzed ACPA positivity, known to predict a severe erosive disease (34). Consistent with previous literature, ACPA positivity and calprotectin were independent predictors of radiographic changes in RA (Table 1). As the two biomarkers were independently associated with radiographic change, we next asked whether combining the markers would improve the prognostic value. As illustrated in Table 1, a biomarker panel requiring positivity for both ACPA and calprotectin (ACPA-S100) was superior to the individual markers in detecting erosive disease and joint space narrowing. Patients positive for ACPA-S100 had increased odds of developing erosive disease (OR=7.5, p<0.0001) as well as joint space narrowing (OR=4.9, p=0.001) compared to calprotectin alone (OR=5.6, p=0.0002, and OR=3.5, p=0.006, respectively), as well as compared to ACPA alone (OR=6.0, p=0.002, and OR=2.0, p=0.15). Thus, the combined ACPA-S100 biomarker has improved prognostic capacity.
Table 1.
Prognostic capacity of neutrophil biomarkers
| Outcome | Erosion | Joint space narrowing | EAN | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Marker | Sens | Spec | OR | P-value | Sens | Spec | OR | P-value | Sens | Spec | OR | P-value |
| ACPA | 85.7 | 50.0 | 6.0 | 0.002 | 70.4 | 45.4 | 2.0 | 0.15 | 70.6 | 45.5 | 2.0 | 0.22 |
| S100A8/A9 | 45.2 | 87.3 | 5.6 | 0.0002 | 37.5 | 85.3 | 3.5 | 0.006 | 42.1 | 82.0 | 3.3 | 0.02 |
| NETs | 32.3 | 74.5 | 1.4 | 0.45 | 31.3 | 74.3 | 1.3 | 0.53 | 52.6 | 76.3 | 3.6 | 0.01 |
| CD66b | 32.1 | 82.3 | 2.2 | 0.10 | 29.6 | 81.4 | 1.8 | 0.22 | 41.2 | 83.7 | 3.6 | 0.02 |
| BioNeu1 | 64.5 | 58.2 | 2.5 | 0.03 | 59.4 | 56.9 | 1.9 | 0.11 | 78.9 | 57.6 | 5.1 | 0.006 |
| ACPA-S1002 | 46.4 | 89.6 | 7.5 | <0.0001 | 40.7 | 87.6 | 4.9 | 0.001 | 41.2 | 84.6 | 3.8 | 0.02 |
BioNeu is positive when a patient is positive for either S100A8/A9, NETs or CD66b.
ACPA-S100 is positive when a patient is positive for both ACPA and S100A8/A9.
Although considered an articular disease, about 50% of RA patients develop EAD, including nodules, commonly associated with increased morbidity and mortality (35). However, whether neutrophil biomarkers would be able to predict development of EAN is not known. To determine this, we measured a broad range of neutrophil markers (NETs, calprotectin, and CD66b induction) in the longitudinal RA inception cohort, and asked whether individual neutrophil biomarkers, and/or a ‘neutrophil activation signature’ (BioNeu) could predict future EAD. In our cohort, 23/165 (14%) of the RA patients had developed EAN at follow-up. All of the neutrophil markers were able to predict extra-articular nodules (Table 1). Creating a biomarker score, BioNeu, identifying patients with a neutrophil activation signature, e.g. positivity for either neutrophil activation marker, improved even more so the capacity to predict EAN development (OR=5.1, p=0.006, Table 1). Unfortunately, only one patient was recorded developing interstitial lung disease, why no further analyses could be performed for this EAD. In all, neutrophil activation at inception is an early sign of severe erosive disease as well as predictive of development of EAN.
Discussion
Neutrophils are instrumental immune cells in the RA pathogenesis, infiltrating the joint through immune complex- and complement-mediated mechanisms, partaking in release of proteolytic enzymes causing tissue damage and inflammation (36). However, even though neutrophils are known to be central in the disease pathogenesis, neutrophil biomarkers are seldom used in a clinical setting, and their clinical value as compared to current established markers, including CRP and ACPA, has not been evaluated. In the current study, we propose that a neutrophil biomarker panel, either individually or when combined with current established markers, offers significant clinical value and improves the capacity to monitor disease activity, and predict future morbidities including erosion and EAD. Further, our results highlight the essential role of neutrophils in the RA pathogenesis and support development of therapies targeting neutrophil-mediated inflammation in these patients.
Neutrophils have several effector functions enabling efficient disposal of invading pathogens, including release of inflammatory mediators, such as calprotectin. Calprotectin, also known as S100A8/A9, is a heterodimer functioning as an intracellular calcium-binding protein. When released into the extracellular environment, however, it acts as an efficient danger-associated molecular pattern (DAMP), signaling through TLR4 and RAGE to induce inflammation. Further, calprotectin may facilitate extravasation of immune cells into tissue (37). We and others have reported on calprotectin being produced by several immune cells, including plasmacytoid dendritic cells (38) and platelets (27), although the majority (comprising 40% of the cytosolic content) of the calprotectin is derived from neutrophils (39). Elevated levels of calprotectin are found in many inflammatory diseases and used clinically in the diagnosis of inflammatory bowel disease (40). Also in chronic inflammatory diseases, such as RA, levels of calprotectin have been described as early as 1988, and are related to markers of inflammation, disease activity, and radiographic progression (20–22,30,31,41). In the current study, we were able to validate, and add to, previous findings. Of note, calprotectin was superior to CRP in assessing disease activity, which is a novel observation further strengthening the clinical potential of this neutrophil-derived marker. However, the main novelty, and significance, is not in the capacity of identifying patients with active disease, or the added diagnostic potential. Rather, the novel observation is that calprotectin, in combination with ACPA, may improve on the ability to identify patients prone to developing erosive disabling disease, including joint space narrowing. This is an important finding which may allow for closer monitoring and expedited and aggressive treatment of these patients to avoid disabling disease progression which may be permanent, and improve on their quality of life.
More recently, neutrophil cell death, NETosis, was proposed to be involved in the RA pathogenesis with RA-associated autoantibodies and inflammatory cytokines activating neutrophils to undergo NET formation, with subsequent induction of synovial inflammation, as well as presentation of NET-derived citrullinated peptides to antigen-specific T cells by fibroblast-like synoviocytes (9,17). Even though NETosis has been implicated in the RA pathogenesis, the role of NETs in vivo, both in mice and humans, is controversial. In mice models of RA, PAD4 inhibition, using the synthetic chemical Cl-amidine, ameliorated disease development in the CIA model (18). In stark contrast, though dependent on neutrophils for disease progression (42), PAD4 knockout did not rescue arthritis phenotype in the K/BxN serum-transfer mouse model (19). Whether the contrasting results are due to differences in the disease pathogenesis for the select mouse models, and/or due to potential off-target effects of Cl-amidine has not been determined.
In humans, early observations demonstrated elevated levels of NET-derived fragments, including cell-free DNA, or neutrophil-derived granular proteins (e.g. MPO), in the circulation of RA patients, with a few studies describing even MPO-DNA complexes as being associated with disease activity (16,23,24). However, given the marked effect of coagulation in promoting neutrophil activation and de novo NETosis (16), and the particular propensity of RA neutrophils to undergo NETosis ex vivo (9,16), these studies, assessing levels of NET in serum, do not reflect true levels of NETs seen in patients. Our investigation is the first to demonstrate true levels of NETs in plasma from RA patients, and its relation to disease activity. Our observation that levels of NETs are elevated even in clinical remission is of particular interest suggesting chronicity in the immunological and inflammatory response even when not displaying overt disease. Whether this low-grade inflammation may reflect propensity to develop atherosclerosis or other co-morbidities is not known. However, we did make the novel observation that levels of NETs predicted development of a common EAD, namely RA nodules. The underlying mechanisms for this association are not clear and in need of further investigations.
The elevated levels of circulating NETs observed in RA could be due to either increased formation of NETs and/or decreased clearance of NETs. Consistent with the first hypothesis, prior work have demonstrated increased capacity of RA neutrophils to undergo NETosis (9,16), with RA-associated autoantibodies and inflammatory cytokines driving NET formation (9,43). Similarly, we found that RA patients had circulating factors, e.g. immune complexes, promoting neutrophil activation ex vivo. Thus, there are several potential triggers of NETosis operating in RA that may account for the increased levels of circulating NETs. However, we also made the original observation of select reduced NET degradation, but not DNA degradation, in these patients, similar to what has been described by us and others in SLE (32,44). Given the presence of anti-NET antibodies in RA, including the ones targeting citrullinated histones, as well as prior work suggesting normal levels of DNase1L3 (45), we anticipate a similar mechanism as the one described in SLE (32) to also operate in RA, e.g. autoantibody-mediated blockade of DNase I. Regardless of the mechanism, NET clearance by itself did not account for the overall increase in NETosis, as RA patients with sufficient NET degradation had elevated levels of circulating NETs. As such, targeting NET formation, rather than clearance, would likely be a more beneficial therapeutic approach.
In conclusion, neutrophils are instrumental in the RA pathogenesis, reflecting key processes currently not captured efficiently in clinical settings. Our data clearly demonstrate the clinical value of neutrophil-derived biomarkers and/or panels in monitoring disease activity and predicting disease severity. Future studies are needed to validate our findings in larger cohorts, as well as evaluate whether early identification of these patients would lead to effective preventative treatment strategies.
Supplementary Material
Funding:
Supported by the Lupus Research Alliance (grant 519414), National Institute of Health (NIH; grant R01 HL117737, R01 AR39282 and N01 HD-62914) and the Institute of Translational Health Sciences (ITHS).
Footnotes
Conflict of interest: The authors declare no conflicts of interest.
References
- 1.Kaplan MJ & Radic M Neutrophil extracellular traps: double-edged swords of innate immunity. J Immunol 2012;189:2689–2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lood C, Blanco LP, Purmalek MM, Carmona-Rivera C, De Ravin SS, Smith CK, et al. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat Med 2016;22:146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lood C & Hughes GC Neutrophil extracellular traps as a potential source of autoantigen in cocaine-associated autoimmunity. Rheumatology (Oxford) 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kolaczkowska E & Kubes P Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 2013;13:159–175. [DOI] [PubMed] [Google Scholar]
- 5.Nathan C Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol 2006;6:173–182. [DOI] [PubMed] [Google Scholar]
- 6.Pham CT Neutrophil serine proteases: specific regulators of inflammation. Nat Rev Immunol 2006;6:541–550. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Romo GS, Caielli S, Vega B, Connolly J, Allantaz F, Xu Z, et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Transl Med 2011;3:73ra20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villanueva E, Yalavarthi S, Berthier CC, Hodgin JB, Khandpur R, Lin AM, et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol 2011;187:538–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, Gizinski A, Yalavarthi S, Knight JS, et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med 2013;5:178ra140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wright HL, Moots RJ & Edwards SW The multifactorial role of neutrophils in rheumatoid arthritis. Nat Rev Rheumatol 2014;10:593–601. [DOI] [PubMed] [Google Scholar]
- 11.Elkon KB Poking holes in rheumatoid joints. Sci Transl Med 2013;5:209fs239. [DOI] [PubMed] [Google Scholar]
- 12.Romero V, Fert-Bober J, Nigrovic PA, Darrah E, Haque UJ, Lee DM, et al. Immune-mediated pore-forming pathways induce cellular hypercitrullination and generate citrullinated autoantigens in rheumatoid arthritis. Sci Transl Med 2013;5:209ra150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corsiero E, Bombardieri M, Carlotti E, Pratesi F, Robinson W, Migliorini P, et al. Single cell cloning and recombinant monoclonal antibodies generation from RA synovial B cells reveal frequent targeting of citrullinated histones of NETs. Ann Rheum Dis 2016;75:1866–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dwivedi N, Upadhyay J, Neeli I, Khan S, Pattanaik D, Myers L, et al. Felty’s syndrome autoantibodies bind to deiminated histones and neutrophil extracellular chromatin traps. Arthritis Rheum 2012;64:982–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pratesi F, Dioni I, Tommasi C, Alcaro MC, Paolini I, Barbetti F, et al. Antibodies from patients with rheumatoid arthritis target citrullinated histone 4 contained in neutrophils extracellular traps. Ann Rheum Dis 2014;73:1414–1422. [DOI] [PubMed] [Google Scholar]
- 16.Sur Chowdhury C, Giaglis S, Walker UA, Buser A, Hahn S & Hasler P Enhanced neutrophil extracellular trap generation in rheumatoid arthritis: analysis of underlying signal transduction pathways and potential diagnostic utility. Arthritis Res Ther 2014;16:R122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carmona-Rivera C, Carlucci PM, Moore E, Lingampalli N, Uchtenhagen H, James E, et al. Synovial fibroblast-neutrophil interactions promote pathogenic adaptive immunity in rheumatoid arthritis. Sci Immunol 2017;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papadaki G, Kambas K, Choulaki C, Vlachou K, Drakos E, Bertsias G, et al. Neutrophil extracellular traps exacerbate Th1-mediated autoimmune responses in rheumatoid arthritis by promoting DC maturation. Eur J Immunol 2016;46:2542–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rohrbach AS, Hemmers S, Arandjelovic S, Corr M & Mowen KA PAD4 is not essential for disease in the K/BxN murine autoantibody-mediated model of arthritis. Arthritis Res Ther 2012;14:R104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuruto R, Nozawa R, Takeishi K, Arai K, Yokota T & Takasaki Y Myeloid calcium binding proteins: expression in the differentiated HL-60 cells and detection in sera of patients with connective tissue diseases. J Biochem 1990;108:650–653. [DOI] [PubMed] [Google Scholar]
- 21.Abildtrup M, Kingsley GH & Scott DL Calprotectin as a biomarker for rheumatoid arthritis: a systematic review. J Rheumatol 2015;42:760–770. [DOI] [PubMed] [Google Scholar]
- 22.Hammer HB, Odegard S, Syversen SW, Landewe R, van der Heijde D, Uhlig T, et al. Calprotectin (a major S100 leucocyte protein) predicts 10-year radiographic progression in patients with rheumatoid arthritis. Ann Rheum Dis 2010;69:150–154. [DOI] [PubMed] [Google Scholar]
- 23.Kaneko C, Kobayashi T, Ito S, Sugita N, Murasawa A, Nakazono K, et al. Circulating levels of carbamylated protein and neutrophil extracellular traps are associated with periodontitis severity in patients with rheumatoid arthritis: A pilot case-control study. PLoS One 2018;13:e0192365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perez-Sanchez C, Ruiz-Limon P, Aguirre MA, Jimenez-Gomez Y, Arias-de la Rosa I, Abalos-Aguilera MC, et al. Diagnostic potential of NETosis-derived products for disease activity, atherosclerosis and therapeutic effectiveness in Rheumatoid Arthritis patients. J Autoimmun 2017;82:31–40. [DOI] [PubMed] [Google Scholar]
- 25.Knipp M & Vasak M A colorimetric 96-well microtiter plate assay for the determination of enzymatically formed citrulline. Anal Biochem 2000;286:257–264. [DOI] [PubMed] [Google Scholar]
- 26.Leffler J, Gullstrand B, Jonsen A, Nilsson JA, Martin M, Blom AM, et al. Degradation of neutrophil extracellular traps co-varies with disease activity in patients with systemic lupus erythematosus. Arthritis Res Ther 2013;15:R84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lood C, Tyden H, Gullstrand B, Jonsen A, Kallberg E, Morgelin M, et al. Platelet-Derived S100A8/A9 and Cardiovascular Disease in Systemic Lupus Erythematosus. Arthritis Rheumatol 2016;68:1970–1980. [DOI] [PubMed] [Google Scholar]
- 28.Hammer HB, Haavardsholm EA & Kvien TK Calprotectin (a major leucocyte protein) is associated with the levels of anti-CCP and rheumatoid factor in a longitudinal study of patients with very early rheumatoid arthritis. Scand J Rheumatol 2008;37:179–182. [DOI] [PubMed] [Google Scholar]
- 29.Tyden H, Lood C, Gullstrand B, Jonsen A, Ivars F, Leanderson T, et al. Pro-inflammatory S100 proteins are associated with glomerulonephritis and anti-dsDNA antibodies in systemic lupus erythematosus. Lupus 2017;26:139–149. [DOI] [PubMed] [Google Scholar]
- 30.Hurnakova J, Zavada J, Hanova P, Hulejova H, Klein M, Mann H, et al. Serum calprotectin (S100A8/9): an independent predictor of ultrasound synovitis in patients with rheumatoid arthritis. Arthritis Res Ther 2015;17:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hurnakova J, Hulejova H, Zavada J, Hanova P, Komarc M, Mann H, et al. Relationship between serum calprotectin (S100A8/9) and clinical, laboratory and ultrasound parameters of disease activity in rheumatoid arthritis: A large cohort study. PLoS One 2017;12:e0183420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leffler J, Martin M, Gullstrand B, Tyden H, Lood C, Truedsson L, et al. Neutrophil extracellular traps that are not degraded in systemic lupus erythematosus activate complement exacerbating the disease. J Immunol 2012;188:3522–3531. [DOI] [PubMed] [Google Scholar]
- 33.Lood C, Arve S, Ledbetter J & Elkon KB TLR7/8 activation in neutrophils impairs immune complex phagocytosis through shedding of FcgRIIA. J Exp Med 2017;214:2103–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jilani AA & Mackworth-Young CG The role of citrullinated protein antibodies in predicting erosive disease in rheumatoid arthritis: a systematic literature review and meta-analysis. Int J Rheumatol 2015;2015:728610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Das S & Padhan P An Overview of the Extraarticular Involvement in Rheumatoid Arthritis and its Management. J Pharmacol Pharmacother 2017;8:81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paoliello-Paschoalato AB, Marchi LF, de Andrade MF, Kabeya LM, Donadi EA & Lucisano-Valim YM Fcgamma and Complement Receptors and Complement Proteins in Neutrophil Activation in Rheumatoid Arthritis: Contribution to Pathogenesis and Progression and Modulation by Natural Products. Evid Based Complement Alternat Med 2015;2015:429878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schiopu A & Cotoi OS S100A8 and S100A9: DAMPs at the crossroads between innate immunity, traditional risk factors, and cardiovascular disease. Mediators Inflamm 2013;2013:828354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lood C, Stenstrom M, Tyden H, Gullstrand B, Kallberg E, Leanderson T, et al. Protein synthesis of the pro-inflammatory S100A8/A9 complex in plasmacytoid dendritic cells and cell surface S100A8/A9 on leukocyte subpopulations in systemic lupus erythematosus. Arthritis Res Ther 2011;13:R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teigelkamp S, Bhardwaj RS, Roth J, Meinardus-Hager G, Karas M & Sorg C Calcium-dependent complex assembly of the myeloic differentiation proteins MRP-8 and MRP-14. J Biol Chem 1991;266:13462–13467. [PubMed] [Google Scholar]
- 40.Walsham NE & Sherwood RA Fecal calprotectin in inflammatory bowel disease. Clin Exp Gastroenterol 2016;9:21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berntzen HB, Munthe E & Fagerhol MK The major leukocyte protein L1 as an indicator of inflammatory joint disease. Scand J Rheumatol Suppl 1988;76:251–256. [DOI] [PubMed] [Google Scholar]
- 42.Wipke BT & Allen PM Essential role of neutrophils in the initiation and progression of a murine model of rheumatoid arthritis. J Immunol 2001;167:1601–1608. [DOI] [PubMed] [Google Scholar]
- 43.Aleyd E, Al M, Tuk CW, van der Laken CJ & van Egmond M IgA Complexes in Plasma and Synovial Fluid of Patients with Rheumatoid Arthritis Induce Neutrophil Extracellular Traps via FcalphaRI. J Immunol 2016;197:4552–4559. [DOI] [PubMed] [Google Scholar]
- 44.Hakkim A, Furnrohr BG, Amann K, Laube B, Abed UA, Brinkmann V, et al. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci U S A 2010;107:9813–9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao Q, Yang C, Wang J, Li Y & Yang P Serum level of DNase1l3 in patients with dermatomyositis/polymyositis, systemic lupus erythematosus and rheumatoid arthritis, and its association with disease activity. Clin Exp Med 2017;17:459–465. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.