Abstract
Individuals who poorly regulate emotion exhibit premature aging and worse general health. Telomere shortening, a prognostic biomarker of physical health, is related to aging, poor immunocompetence and autonomic nervous system functioning. Cognitive reappraisal is one type of emotion regulation strategy, which involves changing one’s appraisal of an aversive situation to modify its emotional impact. Heart rate variability (HRV; i.e., oscillations in heart rate) relates to emotion regulatory processes, such that higher HRV typically reflects greater regulatory capacity. Previous research has identified a positive association between HRV and telomere length. Importantly, the association between HRV and telomere length may change depending on how often an individual uses cognitive reappraisal. One hundred and thirty-seven healthy participants completed measures of cognitive reappraisal frequency, HRV, and underwent blood draws to measure telomere length (computed with the relative ratio of telomere repeat copy number to single copy gene number) in the T cell effector population, CD8+CD28−. Cognitive reappraisal moderated the relationship between telomere length and HRV such that individuals with high cognitive reappraisal frequency had a significant positive association between HRV and telomere length, while individuals with average and less than average frequency did not exhibit this relationship. The results suggest that frequent usage of cognitive reappraisal enhances the already positive influence of HRV on chromosomal integrity in CD8+CD28− T lymphocytes. Although future research is needed to test these effects causally, these findings suggest that regularly using emotion regulation techniques may buffer the relationship between autonomic nervous system functioning and chromosomal integrity in immune cells.
Keywords: Cognitive reappraisal, Telomere length, Heart rate variability, Lymphocytes
1. Introduction
When one encounters a stressful situation, it’s often adaptive to modulate one’s emotional response to it. Emotion regulation is the ability to effectively manage and respond to emotional experiences (Gross, 1998). Individuals who are better at regulating their emotions have better mental and physical health outcomes (Aldao et al., 2010; Shahane and Denny, 2019; Verzeletti et al., 2016). For example, poor emotion regulation is associated with increased risk for premature aging, cardiovascular disease, and cancer (Appleton et al., 2014; Berna et al., 2014; Conley et al., 2016; Ebner and Fischer, 2014). Poor emotion regulation is also associated with shortened telomeres (Arenander et al., 2012). Telomeres are specific regions of DNA that form protective caps on the ends of chromosomes. Telomere length serves as a valuable prognostic biomarker of aging, general health, and disease susceptibility (Hornsby, 2007; Shammas, 2011). Accelerated telomere shortening is related to heart disease, diabetes, cancer, and osteoporosis (Shammas, 2011).
People who have difficulty managing their emotions exhibit a differential stress response compared to individuals who have better emotional control (Gross, 1998). This differential stress response impacts the autonomic nervous system, which directs heartbeat, respiration, and digestion (McCorry, 2007). Specifically, the parasympathetic branch1 of the nervous system regulates both physical and emotional responses to stress. Individuals with greater heart rate variability (i.e., HRV; the variation in time between heartbeats; Thayer et al., 2010) have greater inhibitory control over the heart, and thus are better able to physiologically and emotionally regulate during stressful experiences. Several studies demonstrated the positive relationship between HRV and emotion regulation (Mather and Thayer, 2018). Greater HRV is also associated other positive mental health outcomes including lower levels of anxiety (Chalmers et al., 2014) and depression (Agelink et al., 2002).
Multiple studies show a positive relationship between HRV and telomere length, but the mechanism explaining this relationship is unclear (Perseguini et al., 2015; Streltsova et al., 2017; Woody et al., 2017; Zalli et al., 2014). Given how emotion regulation has a strong bearing on current and future health, the association between HRV and telomere length may change as a function of emotion regulation.
1.1. Cognitive reappraisal and HRV
We focused on a particular emotion regulation strategy, cognitive reappraisal, which involves changing one’s appraisal of an aversive situation to make it feel less negative (Gross, 1998; Gross and Thompson, 2007). We chose cognitive reappraisal because it is associated with several positive health outcomes (Appleton et al., 2014; Berna et al.,2014; Conley et al., 2016; Shahane et al., 2018; Shahane and Denny, 2019).
HRV enhances functional connectivity of emotion regulation brain networks, which improves complex goal-directed thoughts and emotional well-being (Mather and Thayer, 2018). For example, women who engaged in more cognitive reappraisal showed larger increases in HRV relative to controls with no instruction to regulate (Butler et al., 2006). Further, individuals with high baseline HRV adopted reappraisal strategies more than those with low baseline HRV (Volokhov and Demaree, 2010). Thus, the relationship between cognitive reappraisal and HRV is well-characterized. However, cognitive reappraisal’s effect on the relationship between HRV and telomere length, is less apparent.
1.2. Telomere length and HRV
Telomeres prevent unnecessary recombination and nucleolytic degradation, preserving information in the genome (Hornsby, 2007; Shammas, 2011). Repeated cell divisions result in telomere shortening (Shammas, 2011), and cell division rates increase under stress (Ridout et al., 2016). Cells senesce when telomeres are shortened to a critical point. Telomere shortening in immune cells have implications for immunocompetence and inflammation (Cohen et al., 2013). The present study investigated telomere length in CD8+CD28− lymphocytes, which are of interest because they regulate host responses to infections (Cohen et al., 2013)2. Similar to the prognostic value of telomere length, greater HRV is indicative of well-being (Mather and Thayer, 2018). Men with shorter telomeres in leukocytes have blunted HRV in response to a stressor (Zalli et al., 2014), suggesting a potential disabling of their ability to emotionally regulate. Among older adults (> 60 years of age), shorter telomeres in leukocytes are associated with lower HRV (Streltsova et al., 2017). Thus, the relationship between telomere length and HRV, which indexes vagus nerve activity within the autonomic nervous system (i.e., vagal tone), is relatively well established. Little research exists, however, on the relationship between lymphocyte telomere length and resting HRV (i.e., measured without the impetus of an experimental stressor) in younger adults (< 60 years of age), and on the influence of cognitive reappraisal on this relationship.
1.3. Telomere length and cognitive reappraisal
Only one study has investigated the relationship between emotion regulation and telomere shortening. Higher telomerase3 activity, which prevents telomere shortening, is associated with adaptive emotion regulation strategies (Arenander et al., 2012). The present study in contrast, directly measures telomere length and focuses on cognitive reappraisal.
1.4. The present study
The present study aimed to investigate whether cognitive reappraisal associates with the relationship between HRV and telomere length. Neural correlates of emotion regulation affect amygdala-mediated autonomic activity and hypothalamic-pituitary-adrenal axis activity, which regulate affect, cardiovagal output, and stress responses, thereby improving immune functioning (Fagundes and Way, 2014; Thayer and Lane, 2000). Accordingly, we theorized that cognitive reappraisal affects parasympathetic neurons innervating the heart via the vagus nerve, therefore strengthening immune functioning. We expected that higher HRV would be significantly associated with longer telomeres. We hypothesized a positive association between HRV and telomere length and that one’s propensity to use reappraisal should modulate the relationship between HRV and telomere length, such that those with frequent cognitive reappraisal would exhibit a stronger positive relationship between telomere length and HRV, compared to those with less frequent reappraisal.
2. Method
2.1. Participants
We used data from the Pittsburgh Common Cold Study 3. These data were collected by the Laboratory for the Study of Stress, Immunity, and Disease (2016) at Carnegie Mellon University (Principal Investigator: Dr. Sheldon Cohen, Ph.D.). Data were downloaded at the Common Cold Project website (grant number NCCIH AT006694) www.commoncoldproject.com. Participants were recruited by newspaper advertisements to participate in a study examining the causes of the common cold. Each participant received $1000 compensation. The Carnegie Mellon University and University of Pittsburgh human subjects review boards approved all methods and all participants provided informed consent. Participants were 213 healthy individuals in Pittsburgh, Pennsylvania (42 % female; age range: 18–55 years; race: 69 % White, 23 % Black/African-American, 1 % Native American, 3 % Asian, 2 % Hispanic, 2 % Other). Of the 213 total participants, 144 participants had CD8+CD28− cell telomere length data. Of the 144 participants with CD8+CD28− cell telomere length data, 137 participants had average baseline HRV data. Thus, the analyses were conducted using these 137 participants. There were no significant differences in age, sex, or race/ethnicity between those who had telomere length measured versus those who did not.
2.2. Measures
To assess cognitive reappraisal, participants completed the Emotion Regulation Questionnaire (ERQ; Gross and John, 2003), which is a 10-item self-report measure assessing how frequently participants report regulating their emotions via reappraisal and expressive suppression rated on a 7-point Likert scale from 1 (Strongly disagree) to 7 (Strongly agree). The ERQ yields two separate summed scores for cognitive reappraisal and expressive suppression (Cronbach’s α = .79).
HRV was assessed with electrocardiogram (EKG) data. Resting HRV was assessed in four five-minute epochs. Average HRV values were computed across the four baseline epochs. Interbeat interval (IBI) sequences were extracted from EKG signals using an automated IBI extraction algorithm (Mindware Technologies, LTD Version 2.51). The sampling frequency was 250 Hz, as per Task Force recommendations (Malik, 1996). Extracted IBI records were inspected for artifacts and edited manually. High- and low-frequency power were estimated by spectral analysis of IBIs using a Fast Fourier transform algorithm (Duhamel and Vetterli, 1990). High-frequency band power was computed as a sum of the powers corresponding to peaks centered in the range of 0.12 Hz to 0.40 Hz. Low-frequency band power was computed similarly in the range of 0.04 Hz to 0.12 Hz. Very low-frequency band power was computed in the range of 0.003 Hz to 0.04 Hz. High-, low-, and very low-frequency as well as the low to high-frequency ratio was computed for each epoch automatically by Mindware analysis software.
Telomere length in lymphocytes (CD8+CD28−) was assessed in whole blood and collected into three 15 mL heparinized tubes by standard venipuncture. Lymphocyte subpopulations were separated from whole blood using the RoboSep™ automated cell separator (STEMCELL™ Technologies). CD8+ cells were separated using the RosetteSep® Human CD8 T Cell Enrichment Cocktail (15,063) and CD28− cells were separated using the EasySep® Human PE Positive Selection Kit (18,551) (Cohen et al., 2013). Standard curves and dilution factors for telomere (T) and single-copy gene (S) were calculated using Applied Biosystems SDS software to calculate a T:S ratio (O’Callaghan et al., 2008). All samples were run in duplicate (Cohen et al., 2016). Replicate values were averaged to determine a final T:S ratio (Murdock et al., 2018). Higher T:S ratios signify longer telomere length (Kroenke et al., 2011).
We included the following in the model as a priori covariates or fixed factors based on previous research related to T:S ratio and psychoneuroimmunology: age, sex, body mass index, race, education, employment, smoking status, alcohol consumption, and exercise (Fagundes et al., 2012; Murdock et al., 2018).
2.3. Procedure
Following a screening telephone interview, participants completed an in-person physical health evaluation by a study physician. Participants completed the ERQ in addition to demographic and basic health-relevant questionnaires, such as smoking status, alcohol consumption, and physical activity.
Following demographics and questionnaires, fifteen milliliter samples of whole blood were collected by standard venipuncture into three heparinized collection tubes for the telomere assay.
Participants’ resting HRV was evaluated while wearing three EKG leads on their chest using a modified lead II configuration (i.e., one at the right mid-clavicular line directly below the clavicle and one at the left and right lower margins of the ribcage in line with the midpoints of the left and right clavicles). HRV was evaluated four times (i.e., in four epochs) via IBI root mean square successive differences (RMSSD) during a resting period.
2.4. Data analysis
We first conducted bivariate correlations among the variables of interest (telomere length in CD8+CD28− cells, HRV, and cognitive reappraisal) and then investigated each of the pairwise relationships controlling for covariates. To test our moderation hypotheses, we examined the effect of cognitive reappraisal on the relationship between telomere length in CD8+CD28− cells and HRV using a general linear model (the gamlj:gamljGLM function) in the jamovi software package (Jamovi Project, 2018). CD8+CD28− cell telomere length was entered as the outcome variable, and cognitive reappraisal frequency, the average HRV of the four baseline epochs, and the product term of the two (cognitive reappraisal × HRV) were entered as the predictor variables of interest. All continuous variables were mean-centered in the general linear model. Age, sex, body mass index, race, education, employment, smoking status, alcohol consumption, and exercise were included as a priori covariates or fixed factors. We conducted simple slope analyses to further probe any moderation effects. As ancillary analyses, we tested if the main variables of interest were significantly associated with age. Further, we investigated which a priori covariates impacted telomere length, and whether the results still held when controlling for only those covariates. Next, we tested whether expressive suppression, the other subscale of the ERQ, also associated with the relationship between telomere length and HRV (See the Supplemental Materials for analysis). Lastly, as exploratory analyses, we tested if other cell types including CD4 cells, CD19 cells, CD8+CD28+ cells, and the total population of peripheral blood monocuclear leukocytes (PBML), were also associated with cognitive reappraisal and telomere length (see Supplemental Materials for these analyses). All of the analyses featured in the Supplemental Materials are discussed in detail in the discussion.
3. Results
Table 1 contains descriptive statistics for all variables included in the models. Levene’s test for homogeneity of variance indicated no violations (p = .47). There were no outliers, which were quantitatively defined as more than three interquartile ranges from the hinges of a standard boxplot on all three predictor variables of interest (Howell, 2012).
Table 1.
Descriptive statistics for all variables included in model.
| Variable | Mean (SD) |
|---|---|
| CD8+CD28− Cell Telomere Length (T:S Ratio) | 0.587 (0.27) |
| Heart Rate Variability (RMSSD) | 58.3 (23.9) |
| Reappraisal Frequency Score | 28.9 (6.78) |
| Age (Years) | 30.5 (11.1) |
| Body Mass Index | 26.7 (5.79) |
| Employment (Hours) | 10.4 (18.4) |
| Frequency (%) or Median | |
| Sex (% Female) | 42% |
| Education (Median Years) | 15 |
| Smoking Status (% Yes) | 31% |
| Exercise (% Yes) | 85% |
| Race (% White) | 69% |
| Alcohol Consumption (Median Days per Week) | 0 |
Notes. CD8+CD28− Cell T:S ratio was the relative ratio of telomere repeat copy number to single-copy gene copy number. HRV was indexed with the average of four baseline root mean square successive differences (RMSSD). Reappraisal frequency was derived from the cognitive reappraisal frequency score on the ERQ. Age quantified in years. Body mass index measured body fat based on height and weight as per standard National Institutes of Health guidelines. Employment quantified by the number of fulltime hours worked per week. Sex quantified by male versus female frequency. Education quantified in years. Smoking status reflects whether the participant is a current smoker. Exercise defined as whether the participant engages in regular exercise at least once a week. Race quantified by asking participants how they racially identified. Alcohol consumption quantified by number of days alcohol consumed per week (weekdays and weekends).
Bivariate correlations of the continuous variables of interest were computed. In support of our first hypothesis, there was a significant positive correlation between telomere length in CD8+CD28− cells and HRV, r(135) = .19, p = .024, 95 % CI [.026, .350]. This finding is not significant (p = .41) when controlling for covariates (i.e., age, sex, body mass index, race, education, employment, smoking status, alcohol consumption, and exercise). There was no significant bivariate correlation between telomere length in CD8+CD28− cells and cognitive reappraisal frequency, r(135) = .01, p = .88, 95 % CI [−.152, .175]. When controlling for covariates, the relationship between CD8+CD28− cell telomere length and cognitive reappraisal frequency remained nonsignificant (p = .86). There was no significant bivariate correlation between cognitive reappraisal frequency and HRV, r(135) = −.04, p = .61, 95 % CI [−.18, .11]. When controlling for covariates, the relationship between cognitive reappraisal frequency and HRV remained nonsignificant (p = .46).
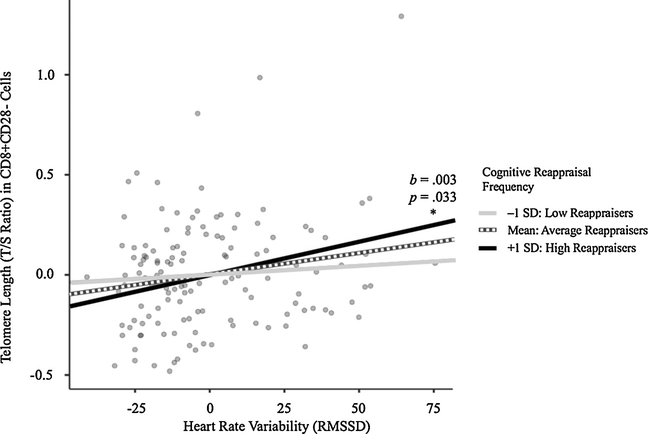
Overall, the general linear model we specified explained 10 % of the variance in CD8+CD28− cell telomere length (F(26, 110) = 1.58, p = .055). While cognitive reappraisal frequency was analyzed continuously in the general linear model, for visualization and post-hoc analysis purposes, reappraisal was defined as high reappraisal frequency (i.e., at least one standard deviation above the mean; N = 26), average reappraisal frequency (i.e., between one standard deviation below the mean and one standard deviation above the mean; N = 89), and low reappraisal frequency (i.e., at least one standard deviation below the mean; N = 22). There were no significant differences in age among the three groups, F(2, 134) = 1.68, MSE = 121, p = .19. There were no main effects of reappraisal frequency or HRV on telomere length in CD8+CD28− cells in the general linear model without the interaction term and with the interaction term included in the model. However, the cognitive reappraisal × HRV was significant in predicting CD8+CD28− cell telomere length, t(110) = 2.12, b = −4.2e−4, p = .036, as seen in Fig. 1. Table 2 contains estimates from the general linear model.
Fig. 1.
Effect of the predictor, heart rate variability (average of four baseline root mean square successive differences [RMSSD]) on the dependent variable, telomere length in CD8+CD28− cells, at different levels of the moderator, cognitive reappraisal. SD refers to standard deviation. * indicates significant slope.
Table 2.
Estimates from general linear model to predict telomere length in CD8+CD28− cells.
| Sum of Squares | df | Mean Square | F | p | η2 | ω2 | |
|---|---|---|---|---|---|---|---|
| Model | 2.696 | 26 | 0.104 | 1.58 | 0.055 | 0.272 | 0.099 |
| Sex | 0.100 | 1 | 0.100 | 1.52 | 0.22 | 0.010 | 0.003 |
| Age | 0.115 | 1 | 0.115 | 1.75 | 0.189 | 0.012 | 0.005 |
| Reappraisal Frequency | 0.003 | 1 | 0.003 | 0.05 | 0.819 | 0.000 | −0.006 |
| HRV (RMSSD) | 0.026 | 1 | 0.026 | 0.39 | 0.533 | 0.003 | −0.004 |
| Education | 0.700 | 6 | 0.117 | 1.77 | 0.111 | 0.070 | 0.031 |
| Smoking Status | 0.326 | 1 | 0.326 | 4.96 | 0.028 | 0.033 | 0.026 |
| Body Mass Index | 0.048 | 1 | 0.048 | 0.74 | 0.392 | 0.005 | −0.002 |
| Exercise | 0.097 | 1 | 0.097 | 1.48 | 0.226 | 0.010 | 0.003 |
| Employment | 0.141 | 1 | 0.141 | 2.15 | 0.145 | 0.014 | 0.008 |
| Race | 0.264 | 5 | 0.053 | 0.80 | 0.551 | 0.027 | −0.007 |
| Alcohol Consumption | 0.254 | 6 | 0.042 | 0.64 | 0.695 | 0.026 | −0.014 |
| Reappraisal Frequency*HRV | 0.296 | 1 | 0.296 | 4.50 | 0.036 | 0.030 | 0.023 |
| Residuals | 7.231 | 110 | 0.066 | ||||
| R-squared = 0.272, adjusted | |||||||
| R-squared = 0.10 |
Notes. df refers to degrees of freedom, F refers to F statistic, p refers to p-value, η2 refers to eta-squared, ω2 refers to omega-squared.
To further probe the interaction, we ran simple slope analyses, which revealed that the slope of the regression line for individuals with high reappraisal frequency was significantly greater than zero (i.e., greater HRV was significantly associated with longer telomeres), t (25) = 2.16, b = 0.003, p = .033. The slope of the regression line for individuals with low reappraisal frequency was nonsignificant, t (21)=1.23, b = −0.002, p= .22. The slope of the regression line for individuals with average reappraisal frequency was nonsignificant, t (88)=0.61, b=6.4e–4, p= .54.
Importantly, however, there was a significant difference in the slopes of the regression lines for low and high reappraisal frequency participants, t(44)=2.37, p= .022. There were no significant differences between the slopes of the average reappraisers and high reappraisers, nor between those of the average reappraisers and low reappraisers.
As an ancillary analysis, we investigated if the main variables of interest (telomere length in CD8+CD28− cells, heart rate variability, and cognitive reappraisal frequency) exhibited significant correlations with age. We found a significant bivariate correlation between telomere length in CD8+CD28− cells and age, r(135) = −.19, p= .023, 95 % CI [−.342, −.026], and a significant bivariate correlation between heart rate variability and age, r(135) = −.25, p < .001, 95 % CI [−.377, −.111]. There was no significant relationship between cognitive reappraisal frequency and age (p = .20). Next, we investigated which a priori covariates impacted telomere length, and tested whether the results still hold when controlling for only those covariates. To understand which a priori covariates impacted telomere length, we computed bivariate correlations between CD8+CD28− cell telomere length and each of the covariates that were continuous variables (Table S1). For the dichotomous variables, we computed independent t-tests (Table S2). For the categorical variable of race (i.e., more than one group), we computed an analysis of variance (Table S3). Only age and smoking status significantly impacted telomere length. When controlling for only the covariates that significantly impact telomere length in CD8+CD28− cells—age and smoking status—the significant interaction between cognitive reappraisal frequency and heart rate variability to predict telomere length in CD8+CD28− cells did not hold, t (131) = 0.84, b = 1.5e−4, p = .40.
Furthermore, we substituted expressive suppression for cognitive reappraisal (Table S4). There were no significant findings (p = .48). Lastly, we substituted telomere length in CD8+CD28− cells with telomere length in CD4 cells (Table S5), CD19 cells (Table S6), CD8+CD28+ cells (Table S7), and PBML (Table S8). When examining the suppression × HRV interaction, smoking status significantly predicted telomere length in CD8+CD28− cells (Table S4). Race significantly predicted telomere length in CD19 cells when examining the reappraisal × HRV interaction (Table S6). Age significantly predicted telomere length in CD8+CD28+ cells when examining the reappraisal × HRV interaction (Table S7). Age, BMI, alcohol consumption, and RMSSD significantly predicted telomere length in PBML when examining the reappraisal × HRV interaction (Table S8).
4. Discussion
The relationship between HRV and telomere length in CD8+CD28− cells varied depending on how frequently participants reported using cognitive reappraisal. Specifically, those who used cognitive reappraisal more frequently (one standard deviation above the mean) exhibited the expected significant positive relationship between greater HRV and longer telomeres in CD8+CD28− cells; individuals who used cognitive reappraisal the least (one standard deviation below the mean) did not exhibit the expected positive correlation between telomere length and HRV. Thus, being a frequent cognitive reappraiser appears to be protective for CD8+CD28− effector T cells.
There was a positive bivariate correlation between telomere length in CD8+CD28− cells and HRV across all subjects. This finding builds upon prior work relating shorter telomeres in PBML to low HRV (Streltsova et al., 2017). In the context of stressors, shorter telomeres in buccal cells are associated with greater reductions in HRV and men with shorter telomeres in leukocytes have blunted HRV (Woody et al., 2017; Zalli et al., 2014). In addition, research in kindergarten children found that while heart rate and cortisol reactivity were inversely related to buccal telomere length, HRV reactivity was unrelated to buccal telomere length (Kroenke et al., 2011). However, rather than in the context of a stressor, we assessed the extent to which cognitive reappraisal moderated the relationship between telomere length in CD8+CD28− cells and HRV at resting state. Thus, while the present study’s findings build upon prior work, they are also novel, as this is the first study to explicitly show a relationship between baseline HRV and telomere length in CD8+CD28− cells among younger adults (below 60 years of age). This finding, however, is not significant when controlling for covariates. Further, in contrast to prior work, we did not find a significant relationship between cognitive reappraisal frequency and heart rate variability in this data set. In addition, when controlling for only the a priori covariates that significantly impacted telomere length, the interaction does not hold. Previous literature, however, shows that telomere length is impacted by several different factors (e.g., physical activity and gender) (Arsenis et al., 2017; Starkweather et al., 2014). Although these associations were not present in the current sample, other telomere length-related studies control for these factors; accordingly, we controlled for these factors to maintain consistency across studies (e.g., Murdock et al., 2018).
The measures used to index telomere length in CD8+CD28− cells, HRV, and cognitive reappraisal in the present study are reliable. Telomere shortening in immune cells have implications for immunocompetence, inflammation, and antibody responses to vaccines (Cohen et al., 2013). CD8 T cells are effector memory cells, which specifically kill damaged or virus-infected cells and are involved in the maintenance of peripheral tolerance to (auto)antigens (Cohen et al., 2013; Dai et al., 2017). CD28+ is a co-stimulatory molecule on CD4+ or CD8+ cells, which stimulates T cell activation and survival (Teijeira et al., 2019). Since the CD28− lymphocytes harbour the long living memory T cells subset, it is of particular interest to study the CD28− population with respect to telomere length playing a role in virus-specific memory and effector function, in the microenvironment of tumor cells, chronic intracellular infections, and chronic pulmonary and autoimmune diseases (Chen et al., 2018; Cohen et al., 2013; Strioga et al., 2011). Furthermore, HRV, which indexes the heart and brain interaction, was indexed with RMSSD, which quantifies beat-to-beat variance and estimates vagally mediated changes in HRV (Shaffer and Ginsberg, 2017). Each individual’s HRV measure was calculated from the average of four five-minute long epochs, which is beyond the recommended and standard convention of one five-minute long epoch; thus, this is a reliable measure of HRV. Lastly, the measure used for cognitive reappraisal has been validated in a large volume of studies showing that it is indeed a reliable marker of cognitive reappraisal frequency and health-relevant behavior (Gross, 2013; Gross and John, 2003; Shahane and Denny, 2019).
The relationships among these variables are important for several reasons. First, while there are a myriad of studies showing interrelations among the parameters of interest in the present study, no study has specifically shown the influence of cognitive reappraisal frequency (as indexed by the ERQ) on the relationship between telomere length and RMSSD in this specific subset of lymphocytes. These two particular physiological markers are strongly associated with the aging process (Perseguini et al., 2015; Shammas, 2011); thus, the present study implicates cognitive reappraisal, an indicator of cognitive flexibility, as a protective factor against morbidity and mortality. However, the exact mechanism by which telomere length and HRV are related remain unclear (Woody et al., 2017). The present study suggests that there are likely several contributing factors which are multifactorial processes defining aging driving the relationship between telomere length and HRV, including but not limited to cognitive reappraisal frequency. Other potential contributing factors include environmental and psychosocial factors, such as work-life balance, social support, positive affect, diet, and exercise (Shammas, 2011). While telomere shortening and low HRV have been implicated in aging, poor general health, morbidity, and mortality—the present study confirms that how we regulate emotion today (i.e., emotion regulation habits) may have a powerful bearing on current and future health outcomes.
A possible mechanism that may explain the present study’s findings is that emotion regulation engages functional connectivity of the amygdala and medial prefrontal cortex (Sakaki et al., 2016). Low functional connectivity of these regions may yield low amplitude oscillations in the heart, and may, therefore, be associated with decreased HRV and dysregulated vagal tone. Disrupted neurofeedback loops between the parasympathetic nervous system and the brain may impact neuro-immune interactions. For example, there may be excessive inflammation by altering activity of macrophages and other cytokine-producing cells (Lopez et al., 2018). Excessive activation of and/or dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis, a system connecting the adrenal gland located at the top of each kidney to the brain, yields greater cortisol excretion and lower HRV (Thayer et al., 2006). Telomere shortening in immune cells specifically is related to increased inflammation and elevated levels of cortisol (Eitan et al., 2014). Thus, the interactions of cognitive control tendencies (e.g., reappraisal frequency) with cardiac function, immunology, and endocrinology may explain the correlational effects in the present study. Lopez et al. (2018) propose a neuro-immuno-affective framework that outlines how emotion regulation targets in the brain (i.e., ventrolateral, dorsolateral, and ventromedial PFC) effect changes in autonomic activity via the amygdala and changes in HPA axis activity via the hypothalamus directly regulating affect, cardiovagal output, and stress responses, thereby improving immune system function. Although the findings in the present study are correlational, Lopez and colleagues’ proposed framework may explain the mechanisms underlying the present study’s findings; increased PFC and hypothalamic activity while regulating emotions, which is associated with cognitive reappraisal frequency, may affect parasympathetic neurons, which innervate the heart via the stellate ganglia and vagus nerve (Thayer and Lane, 2000), and strengthen immune functioning (Fagundes and Way, 2014). The additional aspect that is not explicitly outlined in the model but relevant to the present study, however, is how immune system activity, including but not limited to inflammation and stress, impacts telomerase activity and therefore telomere length in lymphocytes.
Despite the well-powered nature of the present study, there are a few limitations. The present findings are correlational; hence, causality cannot be established by the current data. Future experimental work should further examine these relationships by providing participants training in reappraisal longitudinally to examine changes in telomere length and HRV from baseline to post-training. Consistent with the hypothesis of a causal role for cognitive reappraisal in promoting positive health outcomes and reducing stress (Appleton et al., 2014; Shahane et al., 2018; Shahane and Denny, 2019), longitudinal training in reappraisal has been shown to reduce perceived stress over time (Denny and Ochsner, 2014), which may in tandem promote positive physiological health outcomes as well. Additionally, the present work may translate into clinical domains. Cognitive reappraisal has the potential to be used in therapeutic settings. Clinical trials for depression indicate cognitive reappraisal should be implemented into clinical practice (Troy et al., 2010). With any emotion regulation strategy, however, investigating for whom and under what circumstances reappraisal may be maladaptive in important. How an individual flexibly determines when to implement reappraisal is an important skill of its own, and worthy of future research directions (Doré et al., 2016). In addition, using telomere length as a biomarker has resulted in controversial conclusions—specifically, telomere structure depends on genetics, epigenetics, and the environment, which may be present in variable extents depending on the conditions (Danese and Lippi, 2018). Furthermore, CD8+CD28− cells are also functionally heterogeneous and the characteristics may range from enhanced cytotoxic abilities to immune regulation promotion (Mou et al., 2014). Thus, the pattern of effects should also be investigated in alternative biomarkers, such as C-reactive protein (Pepys and Hirschfield, 2003). We investigated the cognitive reappraisal × HRV interaction in other cell types (CD4, CD19, CD8+CD28+, and PBML) and found no significant reappraisal × HRV interactions (p > .05). In addition, across these cell types we found that smoking status, race, age, BMI, and alcohol consumption significantly predicted telomere length, further justifying their use as covariates as they show how these variables do indeed impact telomere length (regardless of cell type). Future research should longitudinally investigate telomere length, cognitive reappraisal, and heart rate variability to fully understand the pattern of findings over time. For example, Fagundes and colleagues have outlined a theoretical model showing the relationships among hormones, natural killer cells, macrophages, T cells, and stress (Fagundes et al., 2017), and stress is related to emotion regulation (Richardson, 2017). Stress may induce chronically high cortisol, which can sometimes lead to glucocorticoid insensitivity and allow inflammation to be in an unregulated environment (Fagundes et al., 2017, 2013). Furthermore, stress may lead to adrenocorticotropic hormone stimulation of the adrenal gland, which produces cortisol. Cortisol can impair immune system functioning, suppressing T cell, natural killer cell, and macrophage activity. Thus, in the context of the present study, perhaps emotion is related to acetylcholine release (Calandreau et al., 2006), which is related to less inflammation (Fagundes et al., 2017). In addition, some may suggest that this is simply variability in the aging process, as evidence also suggests that CD8+CD28− cell concentration declines with age and reflects replicative senescence (i.e., shortening of telomeres) (Fagnoni et al., 1996; Vallejo, 2005).
The present study indicates that cognitive reappraisal associates with the relationship between telomere length and HRV; future work should unveil how and why this pattern exists. Additionally, we are operating under the assumption that cognitive reappraisal frequency is a proxy for cognitive reappraisal ability. Those who implement cognitive reappraisal more frequently have shown evidence of being better at cognitive reappraisal since they are naturally incorporating it more into their daily life (Gross and John, 2003). In addition, future work should explore whether these patterns are consistent in non-immune cells (i.e., buccal cells). Further, the present study included a largely non-Hispanic White sample; thus, future studies should investigate these associations in more diverse samples.
In conclusion, we demonstrated how the relationship between HRV and telomere length changes as a function of cognitive reappraisal frequency, such that individuals with high cognitive reappraisal frequency have a strong positive association between HRV and telomere length. The findings shed light on how cognitive reappraisal frequency affects two clinically-meaningful biomarkers of general health and longevity.
Supplementary Material
Acknowledgements
The data used for this article were collected at Carnegie Mellon University under the directorship of Sheldon Cohen, PhD, and were accessed via the Common Cold Project (CCP) website (www.commoncoldproject.com). The grant that supports the Common Cold Project is NCCIHAT00694. CCP data are made publicly available through a grant from primary support from the National Institute of Allergy and Infectious Diseases (R01 AI066367), as well as supplemental support from the Pennsylvania Department of Health, the National Center for Complementary and Integrative Health (RC1AT005799), the John D. and Catherine T. MacArther Foundation, and the National Institutes of Health grant awarded to the University of Pittsburgh Clinical and Translational Science Institute (UL1 RR024153 and UL1 TR000005). Preparation of this manuscript was supported by the National Heart Lung and Blood Institute1F31HL147394-01 (PI: Anoushka Shahane), 1R01HL127260-01 (PI: Christopher Fagundes), and 1F32HL146064-01 (PI: Angie LeRoy). We thank Dr. Cobi J. Heijnen for her feedback on the manuscript.
Footnotes
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi: https://doi.org/10.10167j.psyneuen.2019.104517.
Notably, the sympathetic branch equally but oppositely regulates the stress response. The parasympathetic branch of the autonomic nervous system partially regulates heart rate and HRV. During psychosocial stress, the sympathetic nervous system becomes dominant, while during safety and stability, the parasympathetic nervous system is dominant. Because neurotransmission of catecholamines (specifically, norepinephrine) is slow and because neurotransmission of acetylcholine is very fast, the parasympathetic nervous system can more rapidly impact responses (McCorry, 2007).
Specifically, cytotoxic T lymphocytes express CD8, which recognize MHC (Type I) molecules on the antigen presenting cell. Proteins on the antigen surface plug into receptor molecules called CD28 on the T cell surface (Sompayrac, 2016; Strioga et al., 2011). CD8+CD28− cells impair responsiveness of other immune cells serving as a regulatory function after repeated stimulation (Yarde et al., 2014).
Telomerase is a ribonucleoprotein DNA polymerase complex that helps maintain the integrity of telomeres by adding nucleotides to them. Greater telomerase activity is beneficial, as it increases cell growth (Hornsby, 2007).
References
- Agelink MW, Boz C, Ullrich H, Andrich J, 2002. Relationship between major depression and heart rate variability. Clinical consequences and implications for anti-depressive treatment. Psychiatry Res. 113, 139–149. [DOI] [PubMed] [Google Scholar]
- Aldao A, Nolen-Hoeksema S, Schweizer S, 2010. Emotion-regulation strategies across psychopathology: a meta-analytic review. Clin. Psychol. Rev 30, 217–237. 10.1016/j.cpr.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Appleton AA, Loucks EB, Buka SL, Kubzansky LD, 2014. Divergent associations of antecedent- and response-focused emotion regulation strategies with midlife cardiovascular disease risk. Ann. Behav. Med. Publ. Soc. Behav. Med 48, 246–255. 10.1007/s12160-014-9600-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenander J, Aschbacher K, Kurtzman L, Lin J, Prather A, Puterman E, Koslov K, Cheon J, Wolkowitz OM, Blackburn EH, Epel ES, 2012. Cell aging and resilience: associations between daily emotion regulation and increased telomerase activity. Eur. J. Psychotraumatol 3 1–1. [Google Scholar]
- Arsenis NC, You T, Ogawa EF, Tinsley GM, Zuo L, 2017. Physical activity and telomere length: impact of aging and potential mechanisms of action. Oncotarget 8, 45008–45019. 10.18632/oncotarget.16726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berna G, Ott L, Nandrino J-L, 2014. Effects of emotion regulation difficulties on the tonic and phasic cardiac autonomic response. PLoS One 9, e102971 10.1371/journal.pone.0102971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler EA, Wilhelm FH, Gross JJ, 2006. Respiratory sinus arrhythmia, emotion, and emotion regulation during social interaction. Psychophysiology 43, 612–622. 10.1111/j.1469-8986.2006.00467.x. [DOI] [PubMed] [Google Scholar]
- Calandreau L, Trifilieff P, Mons N, Costes L, Marien M, Marighetto A, Micheau J, Jaffard R, Desmedt A, 2006. Extracellular hippocampal acetylcholine level controls amygdala function and promotes adaptive conditioned emotional response. J. Neurosci. Off. J. Soc. Neurosci 26, 13556–13566. 10.1523/JNEUROSCI.3713-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers JA, Quintana DS, Abbott MJ-A, Kemp AH, 2014. Anxiety disorders are associated with reduced heart rate variability: a meta-analysis. Front. Psychiatry 5 10.3389/fpsyt.2014.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Liu Q, Xiang AP, 2018. CD8+CD28− T cells: not only age-related cells but a subset of regulatory T cells. Cell. Mol. Immunol 15, 734 10.1038/cmi.2017.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Turner RB, Casselbrant ML, Li-Korotky H-S, Epel ES, Doyle WJ, 2013. Association between telomere length and experimentally induced upper respiratory viral infection in healthy adults. JAMA J. Am. Med. Assoc 309, 699–705. 10.1001/jama.2013.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley C, Bishop B, Anderson B, 2016. Emotions and Emotion Regulation in Breast Cancer Survivorship. Healthcare, Basel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai S-X, Gu H-X, Lin Q-Y, Wu Y-K, Wang X-Y, Huang S-Z, Xing T-S, Chen M-H, Zhang Q-F, Zheng Z-W, Sha W-H, 2017. Decreased CD8+CD28+/CD8+CD28− T cell ratio can sensitively predict poor outcome for patients with complicated Crohn disease. Medicine (Baltimore) 96, e7247 10.1097/MD.0000000000007247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese E, Lippi G, 2018. Telomere length: is the future in our “ends”? Ann. Transl. Med 6 10.21037/atm.2018.06.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny BT, Ochsner KN, 2014. Behavioral effects of longitudinal training in cognitive reappraisal. Emot. Wash. DC 14, 425–433. 10.1037/a0035276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doré BP, Silvers JA, Ochsner KN, 2016. Toward a personalized science of emotion regulation. Soc. Personal. Psychol. Compass 10, 171–187. 10.1111/spc3.12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duhamel P, Vetterli M, 1990. Fast fourier transforms: a tutorial review and a state of the art. Signal Process. 19, 259–299. 10.1016/0165-1684(90)90158-U. [DOI] [Google Scholar]
- Ebner NC, Fischer H, 2014. Emotion and aging: evidence from brain and behavior. Front. Psychol 5 10.3389/fpsyg.2014.00996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitan E, Hutchison ER, Mattson MP, 2014. Telomere shortening in neurological disorders: an abundance of unanswered questions. Trends Neurosci. 37, 256–263. 10.1016/j.tins.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagnoni FF, Vescovini R, Mazzola M, Bologna G, Nigro E, Lavagetto G, Franceschi C, Passeri M, Sansoni P, 1996. Expansion of cytotoxic CD8+CD28− T cells in healthy ageing people, including centenarians. Immunology 88, 501–507. 10.1046/j.1365-2567.1996.d01-689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagundes CP, Glaser R, Johnson SL, Andridge RR, Yang EV, Di Gregorio MP, Chen M, Lambert DR, Jewell SD, Bechtel MA, Hearne DW, Herron JB, Kiecolt-Glaser JK, 2012. Basal cell carcinoma: stressful life events and the tumor environment. Arch. Gen. Psychiatry 69, 618–626. 10.1001/archgenpsychiatry.2011.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagundes CP, Glaser R, Kiecolt-Glaser JK, 2013. Stressful early life experiences and immune dysregulation across the lifespan. Brain Behav. Immun 27, 8–12. 10.1016/j.bbi.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagundes CP, Murdock KW, Chirinos DA, Green PA, 2017. Biobehavioral pathways to Cancer incidence, progression, and quality of life. Curr. Dir. Psychol. Sci 26, 548–553. 10.1177/0963721417720958. [DOI] [Google Scholar]
- Fagundes CP, Way B, 2014. Early-life stress and adult inflammation early-life stress and adult inflammation. Curr. Dir. Psychol. Sci 23, 277–283. 10.1177/0963721414535603. [DOI] [Google Scholar]
- Gross J, 1998. Antecedent- and response-focused emotion regulation: divergent consequences for experience, expression, and physiology. J. Pers. Soc. Psychol 74, 224–237. [DOI] [PubMed] [Google Scholar]
- Gross JJ, 2013. Handbook of Emotion Regulation, 2nd ed Guilford Publications. [Google Scholar]
- Gross JJ, John OP, 2003. Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. J. Pers. Soc. Psychol 85, 348–362. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Thompson RA, 2007. Emotion regulation: conceptual foundations Handbook of Emotion Regulation. The Guilford Press, New York, NY, US, pp. 3–24. [Google Scholar]
- Hornsby PJ, 2007. Telomerase and the aging process. Exp. Gerontol 42, 575–581. 10.1016/j.exger.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke CH, Epel E, Adler N, Bush NR, Obradovic J, Lin J, Blackburn E, Stamperdahl JL, Boyce WT, 2011. Autonomic and adrenocortical reactivity and buccal cell telomere length in kindergarten children. Psychosom. Med 73, 533–540. 10.1097/PSY.0b013e318229acfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez RB, Denny BT, Fagundes CP, 2018. Neural mechanisms of emotion regulation and their role in endocrine and immune functioning: a review with implications for treatment of affective disorders. Neurosci. Biobehav. Rev 95, 508–514. 10.1016/j.neubiorev.2018.10.019. [DOI] [PubMed] [Google Scholar]
- Malik M, 1996. Heart rate variability. Ann. Noninvasive Electrocardiol 1, 151–181. 10.1111/j.1542-474X.1996.tb00275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Thayer JF, 2018. How heart rate variability affects emotion regulation brain networks. Curr. Opin. Behav. Sci., Emotion-cognition interactions 19, 98–104. 10.1016/j.cobeha.2017.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCorry LK, 2007. Physiology of the autonomic nervous system. Am. J. Pharm. Educ 71 Mindware Technologies, LTD V 2.51 n.d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou D, Espinosa J, Lo DJ, Kirk AD, 2014. CD28 negative T cells: is their loss our gain? Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg 14, 2460–2466. 10.1111/ajt.12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdock KW, Seiler A, Chirinos DA, Garcini LM, Acebo SL, Cohen S, Fagundes CP, 2018. Low childhood subjective social status and telomere length in adulthood: the role of attachment orientations. Dev. Psychobiol 60, 340–346. 10.1002/dev.21601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Callaghan N, Dhillon V, Thomas P, Fenech M, 2008. A quantitative real-time PCR method for absolute telomere length. BioTechniques 44, 807–809. 10.2144/000112761. [DOI] [PubMed] [Google Scholar]
- Pepys MB, Hirschfield GM, 2003. C-reactive protein: a critical update. J. Clin. Invest 111, 1805–1812. 10.1172/JCI200318921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perseguini NM, Verlengia R, Milan JC, Minatel V, Rehder-Santos P, Takahashi ACM, Santana-Lemos BA, Calado RT, Filho PF, Porta A, Catai AM, 2015. Cardiac autonomic modulation, C-reactive protein or telomere length: which of these variables has greater importance to aging? Int. J. Cardiol 178, 79–81. 10.1016/j.ijcard.2014.10.123. [DOI] [PubMed] [Google Scholar]
- Richardson CME, 2017. Emotion regulation in the context of daily stress: impact on daily affect. Personal. Individ. Differ 112, 150–156. 10.1016/j.paid.2017.02.058. [DOI] [Google Scholar]
- Ridout KK, Ridout SJ, Price LH, Sen S, Tyrka AR, 2016. Depression and telomere length: a meta-analysis. J. Affect. Disord 191, 237–247. 10.1016/j.jad.2015.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaki M, Yoo HJ, Nga L, Lee T-H, Thayer JF, Mather M, 2016. Heart rate variability is associated with amygdala functional connectivity with MPFC across younger and older adults. NeuroImage 139, 44–52. 10.1016/j.neuroimage.2016.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer F, Ginsberg JP, 2017. An overview of heart rate variability metrics and norms. Front. Public Health 5 10.3389/fpubh.2017.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahane AD, Denny BT, 2019. Predicting emotional health indicators from linguistic evidence of psychological distancing. Stress Health. 10.1002/smi.2855. [DOI] [PubMed] [Google Scholar]
- Shahane AD, Lopez RB, Denny BT, 2018. Implicit reappraisal as an emotional buffer: reappraisal-related neural activity moderates the relationship between inattention and perceived stress during exposure to negative stimuli. Cogn. Affect. Behav. Neurosci 10.3758/s13415-018-00676-x. [DOI] [PubMed] [Google Scholar]
- Shammas MA, 2011. Telomeres, lifestyle, cancer, and aging. Curr. Opin. Clin. Nutr. Metab. Care 14, 28–34. 10.1097/MCO.0b013e32834121b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sompayrac L, 2016. How the Immune System Works, 6th edition, 5th ed. John Wiley & Sons, Ltd, Oxford, UK. [Google Scholar]
- Starkweather AR, Alhaeeri AA, Montpetit A, Brumelle J, Filler K, Montpetit M, Mohanraj L, Lyon DE, Jackson-Cook CK, 2014. An integrative review of factors associated with telomere length and implications for biobehavioral research. Nurs. Res 63, 36–50. 10.1097/NNR.0000000000000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streltsova LI, Tkacheva ON, Plokhova EV, Akasheva DU, Strajesko I, Dudinskaya E, Boytsov S, 2017. Age-related changes in heart rate variability and their relation with leucocyte telomere length. Cardiovasc. Ther. Prev 10.15829/1728-8800-2017-1-54-60. [DOI] [Google Scholar]
- Strioga M, Pasukoniene V, Characiejus D, 2011. CD8+CD28− and CD8+CD57+ T cells and their role in health and disease. Immunology 134, 17–32. 10.1111/j.1365-2567.2011.03470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teijeira A, Garasa S, Etxeberria I, Gato-Canas M, Melero I, Delgoffe GM, 2019. Metabolic consequences of T-cell costimulation in anticancer immunity. Cancer Immunol. Res 7, 1564–1569. 10.1158/2326-6066.CIR-19-0115. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Hall M, Sollers JJ, Fischer JE, 2006. Alcohol use, urinary cortisol, and heart rate variability in apparently healthy men: evidence for impaired inhibitory control of the HPA axis in heavy drinkers. Int. J. Psychophysiol. Off. J. Int. Organ. Psychophysiol 59, 244–250. 10.1016/j.ijpsycho.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD, 2000. A model of neurovisceral integration in emotion regulation and dysregulation. J. Affect. Disord., Arousal in Anxiety 61, 201–216. 10.1016/S0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- Troy AS, Wilhelm FH, Shallcross AJ, Mauss IB, 2010. Seeing the silver lining: cognitive reappraisal ability moderates the relationship between stress and depressive symptoms. Emot. Wash. DC 10, 783–795. 10.1037/a0020262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejo AN, 2005. CD28 extinction in human T cells: altered functions and the program of T-cell senescence. Immunol. Rev 205, 158–169. 10.1111/j.0105-2896.2005.00256.x. [DOI] [PubMed] [Google Scholar]
- Verzeletti C, Zammuner VL, Galli C, Agnoli S, 2016. Emotion regulation strategies and psychosocial well-being in adolescence. Cogent Psychol. 3, 1199294 10.1080/23311908.2016.1199294. [DOI] [Google Scholar]
- Volokhov RN, Demaree HA, 2010. Spontaneous emotion regulation to positive and negative stimuli. Brain Cogn. 73, 1–6. 10.1016/j.bandc.2009.10.015. [DOI] [PubMed] [Google Scholar]
- Woody A, Hamilton K, Livitz IE, Figueroa WS, Zoccola PM, 2017. Buccal telomere length and its associations with cortisol, heart rate variability, heart rate, and blood pressure responses to an acute social evaluative stressor in college students. Stress Amst. Neth 20, 249–257. 10.1080/10253890.2017.1328494. [DOI] [PubMed] [Google Scholar]
- Yarde DN, Lorenzo-Arteaga K, Corley KP, Cabrera M, Sarvetnick NE, 2014. CD28−CD8+ T cells are significantly reduced and correlate with disease duration in juveniles with type 1 diabetes. Hum. Immunol 75, 1069–1074. 10.1016/j.humimm.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalli A, Carvalho LA, Lin J, Hamer M, Erusalimsky JD, Blackburn EH, Steptoe A, 2014. Shorter telomeres with high telomerase activity are associated with raised allostatic load and impoverished psychosocial resources. Proc. Natl. Acad. Sci. U. S. A 111, 4519–4524. 10.1073/pnas.1322145111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.