Abstract
Organophosphorus (OP) compounds are deadly chemicals that exert their intoxicating effects through the irreversible inhibition of acetylcholinesterase (AChE). In addition to an excess of peripheral ailments, OP intoxication induces status epilepticus (SE) which if left untreated may lead to permanent brain damage or death. Benzodiazepines are typically the primary therapies for OP-induced SE, but these drugs lose efficacy as treatment time is delayed. The CounterACT Neurotherapeutic Screening (CNS) Program was therefore established by the National Institutes of Health (NIH) to discover novel treatments that may be administered adjunctively with the currently approved medical countermeasures for OP-induced SE in a delayed treatment scenario. The CNS program utilizes in vivo EEG recordings and Fluoro-Jade B (FJB) histopathology in two established rat models of OP-induced SE, soman (GD) and diisopropylfluorophosphate (DFP), to evaluate the anticonvulsant and neuroprotectant efficacy of novel adjunct therapies when administered at 20 or 60 min after the induction of OP-induced SE. Here we report the results of multiple compounds that have previously shown anticonvulsant or neuroprotectant efficacy in other models of epilepsy or trauma. Drugs tested were ganaxolone, diazoxide, bumetanide, propylparaben, citicoline, MDL-28170, and chloroquine. EEG analysis revealed that ganaxolone demonstrated the most robust anticonvulsant activity, whereas all other drugs failed to attenuate ictal activity in both models of OP-induced SE. FJB staining demonstrated that none of the tested drugs had widespread neuroprotective abilities. Overall these data suggest that neurosteroids may represent the most promising anticonvulsant option for OP-induced SE out of the seven unique mechanisms tested here. Additionally, these results suggest that drugs that provide significant neuroprotection from OP-induced SE without some degree of anticonvulsant activity are elusive, which further highlights the necessity to continue screening novel adjunct treatments through the CNS program.
Keywords: Organophosphorus, nerve agent, diisopropylfluorophosphate, status epilepticus, anticonvulsant, neuroprotectant
Introduction
Organophosphorus (OP) compounds are highly toxic chemicals that pose serious health threats to civilian and military personnel around the world. Discovered during WWII in an attempt to synthesize novel pesticides, OP agents demonstrated potent human toxicity that was realized when pioneering researchers were hospitalized after a relatively miniscule exposure (Sidell, 1997). The shocking toxicity of these novel OP chemicals sparked years of research that resulted in the formulation of various OP nerve agents and pesticides that remain prevalent in today’s society.
The potent toxicity of OP compounds derives from the chemicals’ ability to irreversibly inhibit the enzyme acetylcholinesterase (AChE) (Costanzi et al., 2018). AChE is found in synaptic junctions throughout the central, peripheral and autonomic nervous systems, and is required to break down acetylcholine (ACh) for proper temporal control of the post-synaptic response. AChE evolved to be an amazingly efficient enzyme, with the ability to hydrolyze 25,000 ACh molecules per second (Franjesevic et al., 2018). Exposure to OP compounds causes an abrupt disruption of AChE function that results in an excessive accumulation of ACh at neuronal synapses throughout the body. Rats exposed to the OP nerve agent soman (GD) demonstrated 80% AChE inhibition just 3 min after administration, resulting in an increase in brain ACh levels that reached up to 175% of controls (McDonough and Shih, 1997; Shih and McDonough, 1997). The consequences of this OP-induced cholinergic crisis are devastating. Victims of OP intoxication commonly present with miosis, hypersecretions, vomiting, fasciculations, paralysis, convulsions (i.e., status epilepticus) and respiratory and circulatory depression (King and Aaron, 2015).
OP compounds, both nerve agents and pesticides, continue to pose serious health risks to the global population. Despite their illegality mandated by the Organization for the Prohibition of Chemical Weapons (OPCW), nerve agents continue to be used by rogue governments and terrorist organizations. OP nerve agents have been used in recent wars in Iraq-Iran and Syria and have resulted in the deaths or hospitalization of thousands of military and civilian persons (Costanzi et al., 2018). High-profile assassination attempts utilizing nerve agents have been carried out by the North Korean and Russian governments, and these actions have put a vast number of civilian bystanders at risk for exposure (Ng, 2017; Cheng, 2018; Gregory Katz, 2018). Additionally, terrorist attacks in Japan carried out by the cult Aum Shinriyko resulted in over a dozen deaths and over 5000+ victims being hospitalized (Yanagisawa et al., 2006).
While often less publicized than nerve agent exposures, the health risks posed by OP pesticides remain significant and omnipresent. Each year, over 3 million people are poisoned by OP pesticides resulting in approximately 300,000 deaths (Robb and Baker, 2019). The use of OP pesticides is tightly regulated in the United States, but similar restrictions are not found globally. Unfortunately, the majority of OP poisonings occur in developing countries where poor working conditions and suicide account for the majority of incidents. In fact, the World Health Organization (WHO) estimates that pesticide self-poisoning is the leading cause of suicide, accounting for 20% of all cases (World Health Organization (WHO), 2018). Mass civilian exposures resulting from the indirect ingestion of OP pesticides are also a very real and dangerous threat to global health. Civilian pesticide poisonings in India in 2013, and as recent as late 2018, have resulted in dozens of fatalities and hospitalizations (Gardiner Harris, 2013; Associated Press, 2018).
Treatment for OP poisoning typically involves a cocktail of therapeutics. Pretreatment with the reversible AChE inhibitor pyridostigmine has been used by militaries to spare portions of AChEs from OP inhibition (McDonough and Shih, 1997). Post-exposure treatments include the administration of a muscarinic receptor antagonist, an oxime that reactivates the AChE enzyme, and an anticonvulsant drug to treat seizures. Within the U.S., the current FDA-approved post-exposure regimen for treating OP intoxication includes atropine, pralidoxime chloride (2-PAM), and a benzodiazepine. Curtailing CNS-derived status epilepticus (SE) is a crucial goal. If left uncontrolled, OP-induced SE can produce significant widespread neural lesions that result in death or the manifestation of long-term cognitive dysfunction (Petras, 1994; McDonough et al., 1998; Chen, 2012; Wu et al., 2018). In rodent models, OP-induced SE can produce neuropathology in as little as 20 min (McDonough and Shih, 1997; Myhrer et al., 2018). Typically, benzodiazepines are the first line of treatment for OP-induced SE. While effective if administered acutely, benzodiazepines lose efficacy in a time-dependent fashion (Walton and Treiman, 1988; Towne et al., 1994; Lowenstein and Alldredge, 1998; Rice and DeLorenzo, 1999; Shih et al., 1999; Jones et al., 2002; McDonough et al., 2010; Jackson et al., 2019). In a soman model of OP intoxication, diazepam demonstrated a total loss of efficacy when administered at a 40-minute delay (Shih et al., 1999). The difficulty in treating OP-induced SE at delayed time points is predominantly twofold. First, OP-induced seizures derive purely from ACh mechanisms for a very short period of time. As time proceeds, a transition from ACh to non-ACh mechanisms occurs with seizures becoming predominantly non-ACh in nature by 40 min (McDonough and Shih, 1997). This transition results in the loss of efficacy of anticholinergics, like atropine. Second, benzodiazepines become more ineffective in a time-dependent nature. Benzodiazepine resistance in SE can arise in various ways (Deeb et al., 2012), with studies showing GABAA receptor internalization (Naylor et al., 2005) or decreased GABA sensitivity (Kapur and Coulter, 1995) as possible mechanisms. Regardless of the mechanism, this time-dependent resistance creates a need for new anticonvulsant therapies.
Consequently, the CounterACT Neurotherapeutic Screening (CNS) program was established by the Chemical Countermeasures Research Program (CCRP) at the National Institute of Allergy and Infectious Diseases (NIAID/NIH) (NIAID, 2016; NIAID, 2017, Yeung et al., 2019) with the goal of identifying novel neurotherapeutics that may additively or synergistically enhance the efficacy of the currently fielded medical countermeasures for OP-induced SE. The CNS program utilizes established rat delayed-treatment models of SE induced by OP nerve agents (soman) (Jackson et al., 2019) and OP pesticides (diisopropylfluorophosphate, DFP) (Pouliot et al., 2016) to screen for promising compounds that may be effective as an adjunct to the current standard-of-care therapies. The aim of the delayed treatment paradigm is to mimic a civilian-based, post-exposure, real-world emergency response scenario.
In this manuscript, we report the results of multiple potential anticonvulsants and neuroprotectants evaluated in the CNS program that have previously demonstrated promising results in other models of SE, epilepsy, or brain injury. Compounds tested in the CNS program that have previously demonstrated anticonvulsant efficacy include ganaxolone (Zolkowska et al., 2018; Saporito et al., 2019), diazoxide (Niaki et al., 2008; Jazayeri et al., 2013), bumetanide (Eftekhari et al., 2013; Sivakumaran and Maguire, 2016), and propylparaben (Santana-Gomez et al., 2017; Santana-Gomez et al., 2018). Tested drugs that have shown neuroprotectant efficacy in other models include citicoline (Abdolmaleki et al., 2016), MDL-28170 (Lam et al., 2017), and chloroquine (Hirata et al., 2011; Cui et al., 2015). While a majority of the results reported in this manuscript are negative data, we believe that the thorough reporting of negative results serves to enhance the reliability and validity of any positive results that are observed in our assay. Additionally, we hope data obtained from this screening program provide direction as to what classes of drugs may or may not be worth pursuing as potential adjuncts for the treatment of OP intoxication.
Materials and Methods
Drugs:
To establish preliminary proof-of-principle efficacy, the CNS program directive is to evaluate the highest tolerable dose of a drug whenever possible. The qualifying adjustments are solubility and route of administration, and often times, a lower dose is chosen based on previous literature. If doses previously described in literature are ineffective in our models, the amount of administered drug was increased as tolerated and the highest tested dose was reported in this manuscript. All test drugs or vehicles in this study were administered intraperitoneally (IP). Ganaxolone was purchased from Tocris Bioscience (Bristol, UK, cat#: 2531) and dissolved in 50% 2-hydroxypropyl-β-cyclodextrin (HPBCD) in sterile water. Ganaxolone was formulated at 10 mg/ml and given at a dose of 10 mg/kg. Diazoxide was purchased from Sigma-Aldrich (St. Louis, MO, cat#: D9035) and dissolved in 15% DMSO in “multisol” (48.5% sterile water, 40% propylene glycol, 10% ethanol, 1.5% benzyl alcohol). Diazoxide was formulated at 5 mg/ml and given at a dose of 40 mg/kg. Bumetanide was purchased from Sigma-Aldrich (St. Louis, MO, cat#: B3023) and dissolved in polyethylene glycol (PEG200). Bumetanide was formulated at 5 mg/ml and given at a dose of 4 mg/kg. Propylparaben was purchased from Sigma-Aldrich (St. Louis, MO, cat#: P53357) and was dissolved in polyethylene glycol (PEG200). Propylparaben was formulated at 500 mg/ml and given at a dose of 500 mg/kg. Citicoline was purchased from Toronto Research Chemicals (North York, ON, cat #: C508050) and dissolved in sterile saline. Citicoline was formulated at 250 mg/ml and given at a dose of 1 g/kg. MDL-28170 was purchased from AdooQ Bioscience (Irvine, CA, cat#: A15303) and dissolved in peanut oil. MDL-28170 was formulated at 10 mg/ml and given at a dose of 50 mg/kg. Chloroquine diphosphate salt (chloroquine) was purchased from Sigma-Aldrich (St. Louis, MO, cat#: C6628) and dissolved in sterile water. Chloroquine was formulated at 10 mg/ml and given at a dose of 5 mg/kg.
In the soman model, all of the following chemicals were dissolved in sterile saline. HI-6 was synthesized by Kalexsyn Medicinal Chemistry (Kalamazoo, MI) and prepared at a concentration of 250 mg/ml. Atropine methyl nitrite (AMN) was synthesized by Wedgewood Pharmacy (Swedesboro, NJ) and prepared at a concentration of 4 mg/ml. Soman was synthesized by the US Army Combat Capabilities Development Command Chemical Biological Center (Aberdeen Proving Ground, MD) and diluted to 360 μg/ml. Atropine sulfate was synthesized by Sparhawk laboratories (Lenexa, KS) and formulated at a concentration of 0.9 mg/ml. Admixed with atropine sulfate, 2-PAM from Baxter Healthcare Corporation (Deerfield, IL) was prepared at a concentration of 50 mg/ml. Midazolam (MDZ) was purchased from Akorn Pharmaceuticals (Vernon Hills, IL), where it was pre-formulated at 5 mg/ml in sterile saline.
In the DFP model, DFP was obtained from Battelle Memorial Institute (Columbus, OH) (Heiss et al., 2016). Pyridostigmine bromide and 2-PAM were purchased from Sigma-Aldrich (St. Louis MO). MDZ was purchased from Akorn Pharmaceuticals (Vernon Hills, IL), and AMN was purchased from Spectrum Chemicals (New Brunswick, NJ).
Animals:
In the soman model, adult, male Sprague-Dawley rats (n=96) weighing 250-300 grams prior to surgery were purchased from Charles River Laboratories (Wilmington, MA). In the DFP model, male Sprague-Dawley (n=477) rats weighing 50-75 grams were purchased from Charles River Laboratories. All rats were housed in individual cages in a temperature- and humidity-controlled environment that was on a 12 hr light: 12 hr dark cycle. Rats had ad libitum access to food and water (both models) except during experimental procedures (soman model only).
EEG Implant Surgery:
All surgical procedures used in these experiments were reviewed and approved by the Institutional Animal Care and Use Committees (IACUC) at the United States Army Medical Research Institute of Chemical Defense and the University of Utah. All procedures for these experiments were conducted in accordance with the principles stated in the Guide for the Care and Use of Laboratory Animals, and the Animal Welfare Act of 1966 (P. L. 89–544), as amended. In the soman model of OP-induced SE, surgery to implant cortical electroencephalogram (EEG) electrodes was conducted 5-7 days prior to experimental procedures. Prior to surgery, rats were administered subcutaneous meloxicam (1 mg/kg) and intradermal bupivacaine (2.5 mg/ml, 0.1 ml total volume) as analgesics. Rats were anesthetized with isoflurane (3-5% for induction, 0.5-3% for maintenance), and surgical plane was determined by the rat’s inability to respond to painful stimuli. To implant the EEG electrodes, a midline incision approximately 2.5 cm in length was made to expose the skull. Burr holes were hand drilled bilaterally above the parietal cortices as well as above the cerebellum for a reference electrode. Stainless steel screw electrodes were inserted into the burr holes and attached to a recording headset. Screw electrodes and headset were secured in place by glass ionomer cement, and the surgical incision was closed with sutures. Throughout the entire surgical process, body temperature, heart rate, respiratory rate and oxygen saturation were closely monitored. Upon completion of the surgery and recovery from anesthesia, rats were returned to their home cage.
In the DFP model, EEG recording electrodes were implanted 7 days prior to testing. To implant the electrodes, rats were anesthetized with 3% isoflurane and placed in a stereotaxic instrument. A longitudinal midline incision was made in the scalp, which was then retracted laterally to expose the skull. Next, six 500 μm holes were drilled through the skull, and small screws placed in three of the holes for anchoring the electrode headset. Then two 2-3 mm long bipolar recording electrodes (MS333-3-B, Plastics One, Roanoke, VA) were placed in two of the remaining holes on the right side of the midline, and a ground electrode was positioned through a hole on the left side of the skull. These electrodes were positioned to touch the dura for differential recording of EEG activity. The headset was then secured in place with dental cement surrounding the support screws and electrodes. Finally, the wound was closed with sutures, and rats were returned to their home cages. Electrode placement was consistent with each lab’s previous work with these two models. Each chemoconvulsant produces generalized convulsive SE that can be recorded similarly with various electrode arrangements. Some variations in our techniques, which converge on similar results, represents a strength in our approach that we feel increases the likelihood of replication in another lab.
Soman Exposure:
At approximately 0700 on the day of exposure, rats were weighed and placed into individual, Plexiglas® EEG recording chambers. Rats were connected to the EEG recording system by way of their implanted headset plug. Baseline EEG was recorded for 30 min, after which HI-6 pretreatment was administered IP. Thirty min after HI-6 pretreatment, 180 μg/kg of the organophosphorus nerve agent soman was administered subcutaneously. This dose of soman is sufficient to elicit EEG seizure activity in 100% of rats studied. One minute after soman exposure, rats were administered 2 mg/kg AMN intramuscularly (IM). AMN, in conjunction with HI-6, provides protection against systemic nerve agent toxicity that is permissive to survival until the onset of neurological symptoms. The onset of SE was determined by the appearance of repetitive spikes and sharp waves with an amplitude greater than twice that of the baseline EEG and having a sustained duration of more than 10 seconds. Twenty min after the onset of SE, rats were treated IM with 0.45 mg/kg atropine sulfate admixed with 25 mg/kg 2-PAM, 1.8 mg/kg MDZ (IM) and either vehicle or test treatment (IP). Recordings persisted for 4 hr following treatment, and at the end of this time period each animal’s EEG was evaluated. If ictal EEG activity was present at the end of the experiment, treatment was considered a failure, and the rats were euthanized so that brain tissue could be collected (as described in “Tissue Preparation and Histopathology”). If EEG recordings demonstrated an absence of epileptiform activity, rats were returned to their home cages. Twenty-four hr after treatment administration, these rats were again hooked up to the recording system, and EEG was monitored for 60 min. Following this 60-minute recording session, rats were euthanized, and brain tissue was collected for further analysis. This experimental paradigm is summarized in graphical form in Figure 1.
Figure 1.
Illustrative experimental paradigms for soman (above) and DFP (below) delayed treatment models of status epilepticus.
DFP Exposure:
In the morning on the day of the exposure, conscious, unrestrained rats were weighed (150-225 grams on the day of the exposure) and then placed into individual Plexiglas® cages. The implanted electrodes were connected to spring-covered EEG cables (Plastics One, Roanoke, VA) for recording. The protocol used a delayed treatment rodent model of OP exposure that induced SE following administration of DFP (Pouliot et al., 2016; Johnstone et al., 2019; Spampanato et al., 2019). All rats were given pyridostigmine bromide (0.026 mg/kg, IM) 30 min prior to DFP (4-6 mg/kg, SC), and AMN (2 mg/kg, IM) plus 2-PAM (25 mg/kg, IM) 1 min following DFP. These antidote compounds were necessary to decrease mortality resulting from the peripheral, potentially lethal effects of DFP, and thus to ensure survival of a sufficient number of rats for collection and evaluation of EEG. After DFP administration, EEG recordings were directly observed, and the time of the initial electrographic seizure noted. Exactly 60 min after the initial EEG seizure response, rats were administered MDZ (1.78 mg/kg, IM) and vehicle or MDZ plus the test compound (IP). EEG activity was then recorded continuously for the 24 hr following DFP administration. On the day following treatment, rats were euthanized, and brain tissue was collected as described below under “Tissue Preparation and Histopathology.” The DFP experimental paradigm is summarized in graphical form in Figure 1.
EEG Recording and Analysis:
In the soman model, EEG signals were amplified via 1902 amplifiers, digitized using a Micro1401 data acquisition interface and recorded using Spike2 software (all instruments from Cambridge Electronic Design Limited, Cambridge, England). EEG data channels were sampled at 512 Hz and filtered digitally with a 0.3 Hz high-pass filter, a 100 Hz low-pass filter, and 60 Hz notch filter. In the DFP model, EEG signals were amplified with EEG100 amplifiers (high-pass filter, 1 Hz, low-pass, 100 Hz, notch filter at 60 Hz, 5000x gain), then digitized at 500 Hz using a MP150 analog-to-digital converter, and recorded with AcqKnowledge software (BioPac Systems, Inc. Santa Barbara, CA) for subsequent analysis.
In both models, the intensity of electrographic SE was evaluated by analyzing the collected EEG following the administration of DFP or soman with two previously described quantitative algorithms. Power spectral density in the frequency range of 20-60 Hz (gamma) (Lehmkuhle et al., 2009) and spike rate (White et al., 2006) were calculated from the raw EEG recordings using custom design Python-based software developed at the University of Utah. Changes in the mean power in the gamma band and mean spike rate frequency during SE were determined by subtracting baseline levels of both variables measured during a 10-minute time bin prior to OP administration from values observed during sequential, 50% overlapping bins (5 min in the soman model, 15 min in the DFP model) during seizure activity. The mean changes in gamma power and spike rate frequency were calculated. On occasion, rats were removed from the study if poor EEG quality prevented accurate automated analysis. Additional information on the quantitative algorithms used for EEG analysis can be found in previously published manuscripts (White et al., 2006; Lehmkuhle et al., 2009).
Tissue Preparation and Histopathology:
Upon completion of EEG recording experiments in the soman model, rats were deeply anesthetized with sodium pentobarbital and transcardially perfused with saline. When rats were fully exsanguinated, 10% formalin was perfused to induce tissue fixation. Brains were subsequently removed and stored in formalin until they could be embedded in paraffin and sectioned coronally into 5 μm slices. Brain sections corresponding to 3.24 mm posterior to Bregma (George Paxinos, 2007) were slide mounted and stained for Fluoro-Jade B (FJB) positive neurons according to previously published protocols (Schmued and Hopkins, 2000). Stained brain sections were imaged using an Olympus VS120-L100-W virtual slide microscope and VS-ASW software (Olympus Corporation, Tokyo, Japan). FIJI software was used to digitally crop out regions of interest (ROI) for neuropathology analysis using the following dimensions (width × height) for each of the applicable brain regions: amygdala (1000 μm × 1000 μm), piriform cortex (200 μm × 2000 μm), thalamus (1000 μm × 1300 μm), and parietal cortex (1500 μm × 2000 μm). The hippocampus counting region consisted of the anatomical boundaries of the structure. All neuron counting analysis was conducted by treatment-blinded laboratory technicians.
In the DFP model, rats were deeply anesthetized with isoflurane and perfused with saline 24 hr after DFP injections. When rats were fully exsanguinated, 10% buffered formalin was perfused to induce tissue fixation. The formalin-fixed brains were subsequently placed in 30% sucrose for cryoprotection. Brains were then flash frozen, and 40 μm coronal sections were cut between bregma coordinates −1.8 to −6.3 mm (George Paxinos, 2007). The brain sections were mounted on glass slides and stained with FJB (Histo-chem Inc., Jefferson, AR) using previously described techniques (Schmued and Hopkins, 2000). Individual sections were imaged with a Hamamatsu Nanozoomer 2.0 HT (Olympus). An unbiased random-sampling technique was used to quantify the number of FJB positive neurons in selected brain sections as previously described (Johnstone et al., 2019). Finally, the mean number of FJB positive neurons in the 12 counted areas per brain site was calculated to estimate the neuropathological abnormalities (i.e., neuronal death). The brain regions selected for analysis were the dorsal and ventral hippocampus (CA1, CA3), hilus, amygdala, thalamus, and the parietal, piriform and entorhinal cortices. These sites were selected for this study as they have previously been shown to mediate the initiation, propagation, and/or maintenance of seizure activity (Gale, 1992; Myhrer, 2007; Myhrer et al., 2008; Apland et al., 2010; Skovira et al., 2010; Skovira et al., 2012).
Statistics:
Data were entered into GraphPad Prism 7 software (Graphpad Software, San Diego, California) for statistical analysis. Values for change in spike rate frequency and gamma power relative to baseline were compared between control and treatment groups at selected time points (1-20 hr post SE onset) using unpaired t-tests. No multiple comparison corrections were used. FJB positive neurons from each brain area of interest in the two models of OP-induced SE were averaged. Average FJB positive neurons in each of the brain regions of the two groups were individually compared using an unpaired t-test. For all statistical analysis, p<0.05 was considered to be statistically significant.
Results
1. Anticonvulsants
Ganaxolone
Ganaxolone is a synthetic 3β-methylated analog of the neurosteroid allopregnanolone. The anticonvulsant efficacy of neurosteroids has been demonstrated in multiple animal models of epilepsy (Carter et al., 1997; Biagini et al., 2010), and the ability of these steroids to potently modulate GABAA receptors makes these compounds promising drug candidates for the treatment of OP-induced SE. Ganaxolone in particular has shown encouraging efficacy for the treatment of SE; for instance, previous studies have demonstrated that both IM and IV administration of ganaxolone is beneficial for the delayed treatment of pharmacoresistant SE induced by lithium-pilocarpine (Saporito et al., 2019) and the chemical threat agent tetramethylenedisulfotetramine (TETS) (Zolkowska et al., 2018). Additionally, ganaxolone at 10 mg/kg has demonstrated efficacy against soman-induced seizures and brain injury (Reddy et al., 2015). Based off of the soman studies by Reddy et al., 2015, we tested the anticonvulsant efficacy of ganaxolone at 10 mg/kg as an adjunct to current medical countermeasures in our delayed treatment models of OP-induced SE.
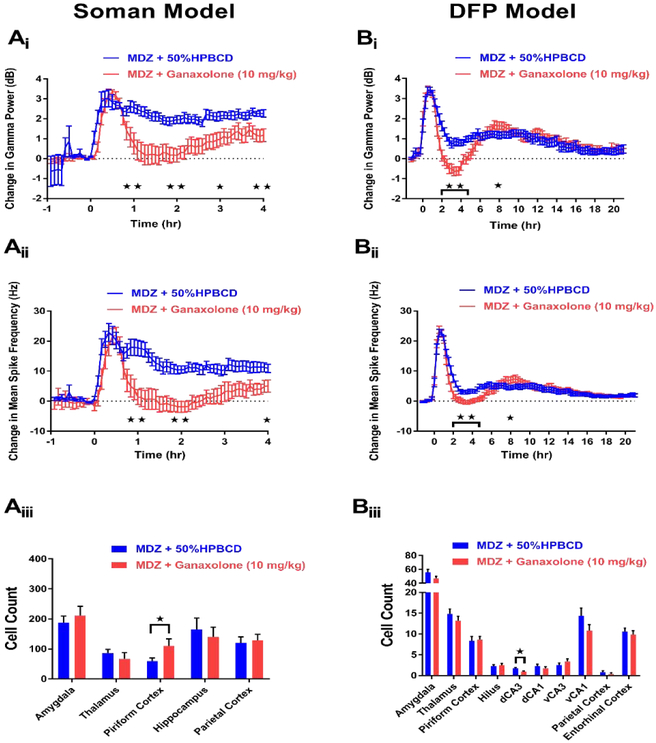
In the soman model, 9 rats were treated with midazolam (MDZ) + ganaxolone (10 mg/kg) 20 min after seizure onset. Of these rats, 0 died throughout the paradigm (Table 1), and 5 showed periods of SE termination. In rats that demonstrated SE termination, latency to seizure cessation following treatment with MDZ + ganaxolone ranged from approximately 12-77 min (average: 34 min). Only one of these rats showed continued SE termination at the end of the experimental paradigm, but this animal was found dead the subsequent day. The other 4 rats that demonstrated SE termination returned to seizing approximately 1-3.75 hr following the stop in ictal activity. A total of 13 rats received MDZ + 50% HPBCD to act as vehicle controls. Of these rats, 4 died at latencies ranging from approximately 3 min to 3 hr after treatment (Table 1). None of the remaining 9 rats that survived until the end of the paradigm demonstrated SE termination at any time point of the experiment. To further extrapolate the anticonvulsant efficacy of ganaxolone, EEG analyses to determine change in gamma power and spike rate frequency were conducted for rats that were treated with either MDZ + ganaxolone (n=9) or MDZ + 50% HPBCD (n=9). Compared to rats that received MDZ + 50% HPBCD, ganaxolone-treated rats demonstrated significant reductions in gamma power at hour time points 1-4 (Figure 2Ai). Additionally, MDZ + ganaxolone rats showed significant reductions in spike rate frequency at hour time points 1, 2 and 4 compared to controls (Figure 2Aii). Along with examining the anticonvulsant efficacy of drugs in the CNS program, FJB staining was performed in brain regions previously shown to be vulnerable to OP-induced SE (McDonough and Shih, 1997) to determine if compounds possessed neuroprotectant abilities. Compared to MDZ + vehicle-treated rats (n=11), rats that received MDZ + ganaxolone (n=7) did not show significant reductions in FJB staining in any of the five regions tested. In fact, a significant increase in FJB staining was seen in the piriform cortex of MDZ + ganaxolone-treated rats (Figure 2Aiii). These data suggest that while MDZ + ganaxolone demonstrates promising anticonvulsant efficacy in the soman model of OP-induced SE, no neuroprotection is offered by this treatment.
Table 1.
Animal mortality in each experimental group for soman and DFP models of OP-induced SE.
| Soman Model | ||
|---|---|---|
| Treatment | Animal's Treated | 4 hr Post-treatment Mortality (n) |
| MDZ + 50% HPBCD | 13 | 4/13 (31%) |
| MDZ + Ganaxolone (10 mg/kg) | 9 | 0/9 (0%) |
| MDZ + Saline | 10 | 1/10 (10%) |
| MDZ + Diazoxide (40 mg/kg) | 8 | 0/8 (0%) |
| MDZ + Bumetanide (4 mg/kg) | 8 | 0/8 (0%) |
| MDZ + Propylparaben (500 mg/kg) | 9 | 2/9 (22%) |
| MDZ + Citicoline (1 g/kg) | 10 | 0/10 (0%) |
| MDZ + MDL-28170 (50 mg/kg) | 7 | 0/7 (0%) |
| MDZ + Chloroquine (5 mg/kg) | 15 | 5/15 (33%) |
| DFP Model | ||
| Treatment | Animal's Treated | 24 hr Post-exposure Mortality (n) |
| MDZ + 50% HPBCD | 20 | 0/20 (0%) |
| MDZ + Ganaxolone (10 mg/kg) | 20 | 1/20 (5%) |
| MDZ + 15% DMSO in Multisol | 11 | 0/11 (0%) |
| MDZ + Diazoxide (40 mg/kg) | 18 | 0/18 (0%) |
| MDZ + PEG200 | 17 | 0/17(0%) |
| MDZ + Bumetanide (4 mg/kg) | 17 | 0/17(0%) |
| MDZ + Propylparaben (500 mg/kg) | 18 | 1/18 (6%) |
| MDZ + Saline | 18 | 0/18 (0%) |
| MDZ + Citicoline (1 g/kg) | 24 | 0/24 (0%) |
| MDZ + Peanut Oil | 12 | 0/12 (0%) |
| MDZ + MDL-28170 (50 mg/kg) | 14 | 0/14 (0%) |
| MDZ + Sterile Water | 15 | 0/15 (0%) |
| MDZ + Chloroquine (5 mg/kg) | 14 | 0/14 (0) |
Figure 2. MDZ + 10 mg/kg ganaxolone demonstrates anticonvulsant, but not neuroprotective, activity in both the soman (A) and DFP (B) models of SE.
Ai) Change in gamma power relative to baseline in soman-exposed rats that received MDZ + 10 mg/kg ganaxolone (red, n=9) or MDZ + 50% HPBCD in water (blue, n=9). Aii) Change in mean spike rate frequency relative to baseline in soman-exposed rats that received MDZ + 10 mg/kg ganaxolone (red, n=9) or MDZ + 50% HPBCD in water (blue, n=9). Aiii) Fluoro-Jade B staining in vulnerable brain regions in soman-exposed rats that received MDZ + 10 mg/kg ganaxolone (red, n=7) or MDZ + 50% HPBCD in water (blue, n=11). Bi) Change in gamma power relative to baseline in DFP-exposed rats that received MDZ + 10 mg/kg ganaxolone (red, n=19) or MDZ + 50% HPBCD in water (blue, n=20). Bii) Change in mean spike rate frequency relative to baseline in DFP-exposed rats that received MDZ + 10 mg/kg ganaxolone (red, n=19) or MDZ + 50% HPBCD in water (blue, n=20). Biii) Fluoro-Jade B staining in vulnerable brain regions in DFP-exposed rats that received MDZ + 10 mg/kg ganaxolone (red, n=18) or MDZ + 50% HPBCD in water (blue, n=20). Data represents mean ± SEM. Statistical significance: *p<0.05, **p<0.01
In the DFP model (Figure 1), 20 rats received treatment of MDZ + ganaxolone (10 mg/kg) 60 min after seizure onset. Of these rats, 1 died following treatment (Table 1) while none of the 20 rats administered MDZ + 50% HPBCD vehicle control died (Table 1). EEG analysis from rats of both treatment groups revealed that rats that received MDZ + ganaxolone (n=19) showed significant reductions in both gamma power and spike rate frequency at hour time points 2-5 but a slight yet significant increase in these parameters at hour 8 compared to MDZ + 50% HPBCD controls (n=20) (Figure 2Bi-ii). FJB staining results in the DFP model of OP-induced SE was analogous to results obtained in the soman model. Of all regions examined, a significant reduction in FJB was only observed in the dorsal CA3 (dCA3) region of rats that were treated with MDZ + ganaxolone (n=18) compared to controls (n=20) (Figure 2Biii). These data from the DFP model of OP-induced SE demonstrate that MDZ + ganaxolone shows acute anticonvulsant efficacy with minimal neuroprotectant capabilities.
Diazoxide
Diazoxide is a derivative of benzothiadiazine that is often used for the treatment of hypertension and hyperinsulinism (McDonald et al., 1977; Touati et al., 1998). Previous studies have demonstrated that diazoxide is able to increase seizure threshold in a nasal obstruction pentylentetrazole (PTZ) model of acute seizures. This anticonvulsant mechanism of action may arise through the opening of ATP-sensitive potassium (K-ATP) channels (Niaki et al., 2008; Roy Chowdhury et al., 2017). Additionally, other work has shown the beneficial anticonvulsant effects of 20 mg/kg diazoxide in the treatment of seizures induced by the OP pesticide dichlorvos (Jazayeri et al., 2013). In our models, diazoxide was tested at 40 mg/kg, a dose that was increased from the 20 mg/kg utilized by Jazayeri et al., 2013.
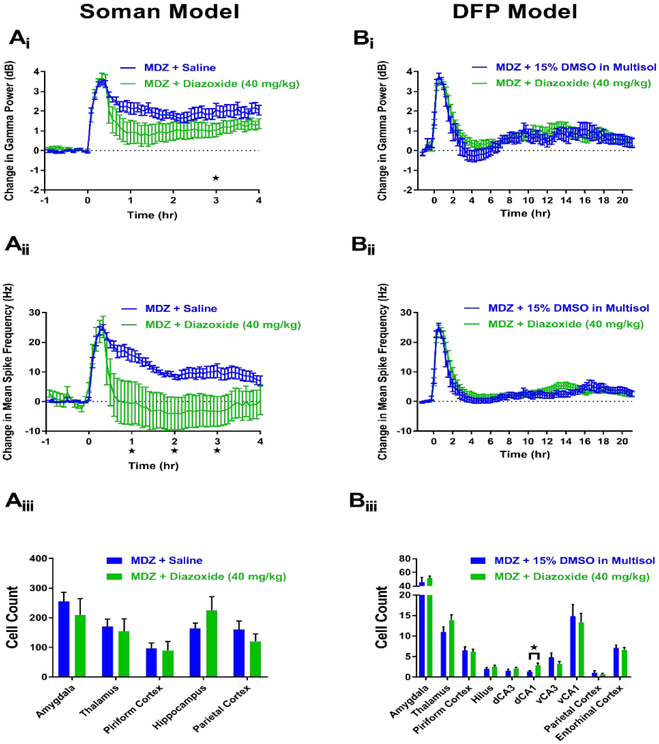
In the soman model, a total of 8 rats were treated with MDZ + diazoxide (40 mg/kg) at a delay of 20 min. Of these rats, 0 died during the experimental paradigm (Table 1) and 4 showed SE termination. In rats that demonstrated SE termination, latency to seizure cessation following treatment with MDZ + diazoxide ranged from approximately 2-86 min (average: 26 min). Three of the 4 rats that showed SE termination remained off at the end of the experimental paradigm. Of these three rats that demonstrated SE termination, 1 died before the 24 hr time point, 1 returned to SE by the 24 hr recording and 1 remained seizure free at the 24 hr recording time point. The fourth rat that demonstrated SE termination returned to seizing approximately 2.75 hr following the stop in ictal activity. A total of 10 rats received MDZ + saline and these animals acted as a control group for the remaining drugs in our study. Of these 10 rats, 1 died at the end of the experimental paradigm (Table 1), but this delayed death allowed this animal’s EEG data to be used for analysis. Of the remaining 9 rats, only 1 demonstrated SE termination approximately 2.5 hr post-treatment. This animal did not return to SE for the remainder of the 4 hr experiment but was found to be seizing at the 24 hr recording time point. EEG analysis revealed that rats treated with MDZ + diazoxide (n=8) demonstrated a significant reduction in gamma power at the 3 hr time point compared to MDZ + saline controls (n=10) (Figure 3Ai). Additionally, MDZ + diazoxide significantly decreased mean spike rate frequency at hour time points 1-3 compared to controls (Figure 3Aii). The neuroprotectant capabilities of MDZ + diazoxide were also evaluated via FJB staining in vulnerable brain regions. The results of this staining revealed that MDZ + diazoxide (n=8) does not produce significant alterations in FJB staining in any of the examined brain regions compared to MDZ + saline controls (n=17) (Figure 3Aiii).
Figure 3. MDZ + 40 mg/kg diazoxide is not neuroprotective but shows some anticonvulsant efficacy in the soman (A), but not DFP (B), model of SE.
Ai) Change in gamma power relative to baseline in soman-exposed rats that received MDZ + 40 mg/kg diazoxide (green, n=8) or MDZ + saline (blue, n=10). Aii) Change in mean spike rate frequency relative to baseline in soman-exposed rats that received MDZ + 40 mg/kg diazoxide (green, n=8) or MDZ + saline (blue, n=10). Aiii) Fluoro-Jade B staining in vulnerable brain regions in soman-exposed rats that received MDZ + 40 mg/kg diazoxide (green, n=8) or MDZ + saline (blue, n=17). Bi) Change in gamma power relative to baseline in DFP-exposed rats that received MDZ + 40 mg/kg diazoxide (green, n=18) or MDZ + 15% DMSO in multisol (blue, n=11). Bii) Change in mean spike rate frequency relative to baseline in DFP-exposed rats that received MDZ + 40 mg/kg diazoxide (green, n=18) or MDZ + 15% DMSO in multisol (blue, n=11). Biii) Fluoro-Jade B staining in vulnerable brain regions in DFP-exposed rats that received MDZ + 40 mg/kg diazoxide (green, n=18) or MDZ + 15% DMSO in multisol (blue, n=11). Data represents mean ± SEM. Statistical significance: *p<0.05.
In the DFP model, different anticonvulsant results were seen compared to the soman model. A total of 18 rats received MDZ + diazoxide (40 mg/kg), and of these animals, 0 died after treatment (Table 1). None of the 11 rats administered MDZ + 15% DMSO in multisol vehicle control died after treatment (Table 1). In contrast to the soman model of OP-induced SE, no significant changes in gamma power (Figure 3Bi) or mean spike rate frequency (Figure 3Bii) were abserved between MDZ + diazoxide (n=18) and MDZ + 15% DMSO in multisol (n=11) vehicle control rats at any time point in the DFP model. Similar to the soman model, there was a lack of major neuroprotection provided by MDZ + diazoxide (n=18) compared to controls (n=11). No significant decreases in FJB staining in the MDZ + diazoxide group were observed in any of the brain regions investigated, while a significant increase in staining was observed in the dorsal CA1 (dCA1) (Figure 3Biii). The data from the two models suggest that while diazoxide possesses some anticonvulsant efficacy in nerve agent-induced SE, it fails to provide major widespread neuroprotection in either of the models of OP intoxication.
Bumetanide
Bumetanide is a sulfamyl loop diuretic that is commonly used to treat heart failure. In the brain, bumetanide can block the bumetanide-sensitive sodium-potassium-chloride transporter (NKCC1) which results in a decrease in intracellular chloride concentrations. During SE, the increased activity of interneurons is thought to potentially contribute to a buildup of intracellular chloride. This, in turn, causes a reversal of the chloride gradient, resulting in a reduction in the magnitude of hyperpolarization mediated by GABA receptors, or it could even generate depolarization (Kaila et al., 2014). Previous studies using bumetanide as an anticonvulsant drug have produced mixed results. Bumetanide failed to demonstrate sufficient seizure reduction in neonatal patients suffering from hypoxic ischemic encephalopathy (Pressler et al., 2015). Conversely, bumetanide proved beneficial for a small cohort of patients suffering from temporal lobe epilepsy (Eftekhari et al., 2013) and helped combat pharmacoresistant SE in a kainic acid mouse model (Sivakumaran and Maguire, 2016). Given the potential of bumetanide to bolster the GABAergic effects of MDZ and the previous data demonstrating the drug’s efficacy against pharmacoresistant SE, bumetanide was tested for anticonvulsant activity in OP-induced SE. In our models, bumetanide was tested at 4 mg/kg, a dose that was increased from the 2 mg/kg utilized by Sivakumaran and Magure, 2016.
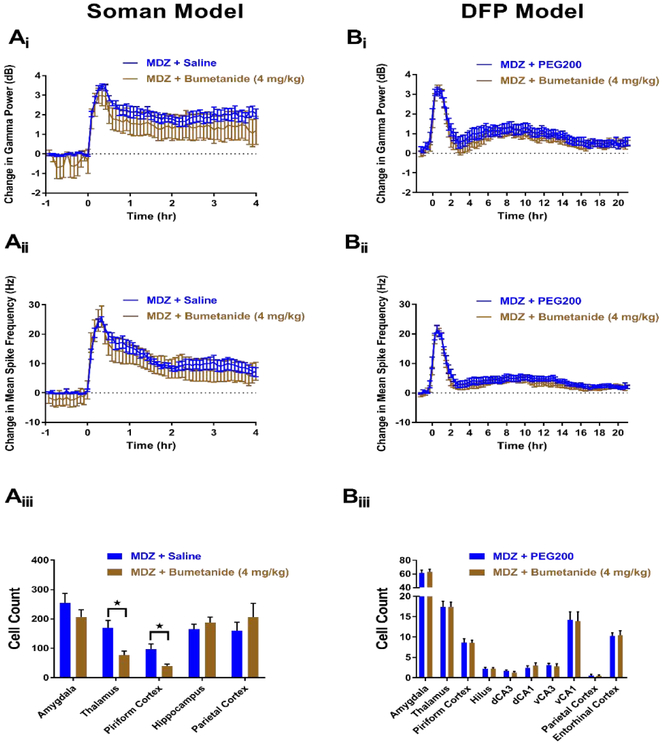
In the soman model, 8 rats were treated with MDZ + bumetanide (4 mg/kg). Of these, 0 rats died (Table 1), but none demonstrated SE termination at any time point. In addition to failing to show SE termination, rats treated with MDZ + bumetanide (n=8) did not display any significant changes in gamma power (Figure 4Ai) or mean spike rate frequency (Figure 4Aii) compared to MDZ + saline controls (n=10) at any time point throughout the experimental paradigm. FJB staining from rats that received MDZ + bumetanide (n=8) showed a significant reduction in staining in the thalamus and piriform cortex, but no other regions compared to controls (n=17) (Figure 4Aiii). Given the lack of anticonvulsant efficacy and the modest neuroprotection observed, bumetanide does not appear to be a promising adjunctive therapy in the soman model of OP-induced SE.
Figure 4. MDZ + 4 mg/kg bumetanide does not demonstrate potent anticonvulsant or neuroprotective properties in soman (A) and DFP (B) models of SE.
Ai) Change in gamma power relative to baseline in soman-exposed rats that received MDZ + 4 mg/kg bumetanide (brown, n=8) or MDZ + saline (blue, n=10). Aii) Change in mean spike rate frequency relative to baseline in soman-exposed rats that received MDZ + 4 mg/kg bumetanide (brown, n=8) or MDZ + saline (blue, n=10). Aiii) Fluoro-Jade B staining in vulnerable brain regions in soman-exposed rats that received MDZ + 4 mg/kg bumetanide (brown, n=8) or MDZ + saline (blue, n=17). Bi) Change in gamma power relative to baseline in DFP-exposed rats that received MDZ + 4 mg/kg bumetanide (brown, n=17) or MDZ + PEG200 (blue, n=17). Bii) Change in mean spike rate frequency relative to baseline in DFP-exposed rats that received MDZ + 4 mg/kg bumetanide (brown, n=17) or MDZ + PEG200 (blue, n=17). Biii) Fluoro-Jade B staining in vulnerable brain regions in DFP-exposed rats that received MDZ + 4 mg/kg bumetanide (brown, n=17) or MDZ + PEG200 (blue, n=17). Data represents mean ± SEM. Statistical significance: *p<0.05.
Similar results for MDZ + bumetanide were observed in the DFP model. A total of 17 rats were treated with bumetanide (4 mg/kg) in conjunction with MDZ. No rats in either the bumetanide or MDZ + PEG200 (n=17) vehicle control group died post-treatment (Table 1). Similar to data obtained in the soman model, MDZ + bumetanide (n=17) failed to produce an effect on gamma power (Figure 4Bi) or spike rate frequency (Figure 4Bii) at any experimental time points compared to MDZ + PEG200 controls (n=17). Additionally, FJB staining in rats who received MDZ + bumetanide (n=17) failed to demonstrate a significant reduction in FJB-labeled neurons compared to MDZ + PEG200 controls (n=17) in any other of the brain regions examined (Figure 4Biii). Data from both the soman and DFP SE models suggest that bumetanide is not a promising adjunct therapy for the treatment of OP intoxication, as the drug predominantly lacks both anticonvulsant and neuroprotective capabilities.
Propylparaben
Propylparaben is an n-propyl ester of p-hydroxybenzoic acid that is commonly used as a preservative because of its antimicrobial properties. In addition to being a widely used preservative, recent studies have suggested that propylparaben may possess anticonvulsant and neuroprotective efficacy in rodent models of SE. In a pilocarpine model of SE, propylparaben treatment following diazepam administration demonstrated anticonvulsant and neuroprotective properties compared to controls (Santana-Gomez et al., 2017). Additionally, propylparaben has been shown to augment the neuroprotective effects of levetiracetam and improve interictal EEG activity after pilocarpine-induced SE (Santana-Gomez et al., 2018). Given these data from other rodent models of SE, propylparaben was tested at 500 mg/kg in our models, a dose that was increased from the 178 mg/kg utilized by Santana-Gomez et al., 2017.
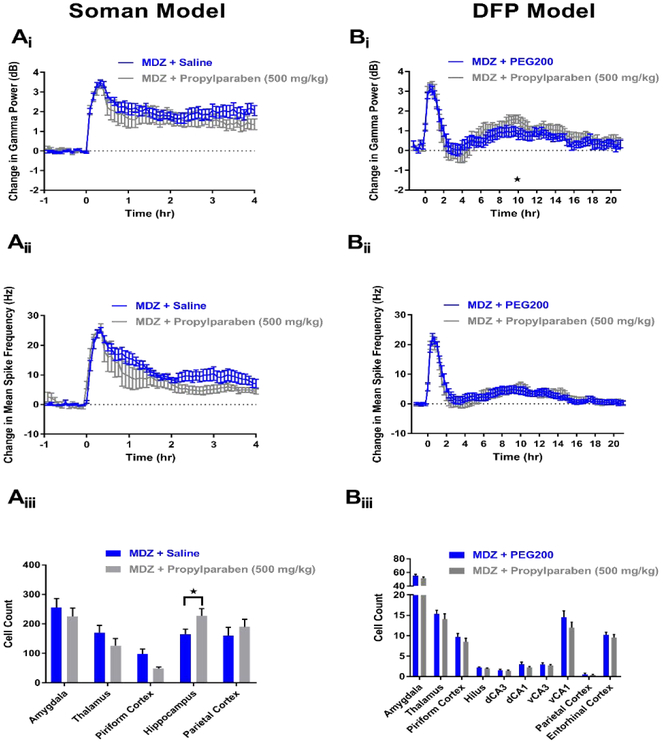
In the soman model, 9 rats received treatment of MDZ + propylparaben (500 mg/kg). Of these rats, 2 died approximately 1-4 hr post-treatment (Table 1), while the remaining 7 rats that survived failed to demonstrate SE termination at any time point of the experiment. EEG analysis from rats that received MDZ + propylparaben (n=7) did not show significant reductions in either gamma power (Figure 5Ai) or mean spike rate frequency (Figure 5Aii) compared to MDZ + saline controls (n=10). Previous studies utilizing other models of SE have suggested that propylparaben acts a neuroprotective compound. In the soman model of OP-induced SE, no decreases in FJB staining were seen in any of the brain regions studied from rats administered MDZ + propylparaben (n=7) compared to MDZ + saline controls (n=17) (Figure 5Aiii). In fact, a significant increase in staining was observed in the hippocampus of rats that received MDZ + propylparaben (Figure 5Aiii).
Figure 5. MDZ + 500 mg/kg propylparaben does not demonstrate significant anticonvulsant or neuroprotective properties in soman (A) and DFP (B) models of SE.
Ai) Change in gamma power relative to baseline in soman-exposed rats that received MDZ + 500 mg/kg propylparaben (grey, n=7) or MDZ + saline (blue, n=10). Aii) Change in mean spike rate frequency relative to baseline in soman-exposed rats that received MDZ + 500 mg/kg propylparaben (grey, n=7) or MDZ + saline (blue, n=10). Aiii) Fluoro-Jade B staining in vulnerable brain regions in soman-exposed rats that received MDZ + 500 mg/kg propylparaben (grey, n=7) or MDZ + saline (blue, n=17). Bi) Change in gamma power relative to baseline in DFP-exposed rats that received MDZ + 500 mg/kg propylparaben (grey, n=17) or MDZ + PEG200 (blue, n=17). Bii) Change in mean spike rate frequency relative to baseline in DFP-exposed rats that received MDZ + 500 mg/kg propylparaben (grey, n=17) or MDZ + PEG200 (blue, n=17). Biii) Fluoro-Jade B staining in vulnerable brain regions in DFP-exposed rats that received MDZ + 500 mg/kg propylparaben (grey, n=17) or MDZ + PEG200 (blue, n=17). Data represents mean ± SEM. Statistical significance: *p<0.05.
In the DFP model, propylparaben was inefficacious as an anticonvulsant and neuroprotectant. A total of 18 rats were administered MDZ + propylparaben (500 mg/kg), and of these rats, 1 died following treatment (Table 1). EEG analysis of rats that received MDZ + propylparaben (n=17) revealed no significant reductions in gamma power (Figure 5BI) or mean spike rate frequency (Figure 5Bii) compared to MDZ + PEG200 controls (n=17). In addition to lack of additional anticonvulsant activity, MDZ + propylparaben demonstrated a lack of neuroprotection in DFP-induced SE. Histopathology revealed no significant differences in FJB positive neurons between rats administered MDZ + propylparaben (n=17) and MDZ + PEG200 (n=17, Figure 5Biii). Overall these data from the two models suggest that propylparaben is not a promising adjunct therapy for the treatment of OP-induced SE.
2. Neuroprotectants
Citicoline
Citicoline is a dietary supplement that plays a significant role in the production of phosphatidylcholine, a process that can improve cellular membrane stability and repair (Blount et al., 2002; Fioravanti and Buckley, 2006). Through various proposed mechanisms, citicoline has been shown to possess neuroprotective properties following ischemic, hypoxic and traumatic brain injuries (Blount et al., 2002; Fioravanti and Buckley, 2006). In addition to being a neuroprotectant, a recent study demonstrated that 150 mg/kg citicoline may provide anticonvulsant activity in response to PTZ-induced seizures (Abdolmaleki et al., 2016). Given the well characterized neuroprotective benefits of citicoline and the potential for the drug to provide anticonvulsant efficacy, citicoline was tested at 1 g/kg in our models, a dose that was increased from the 150 mg/kg utilized by Abdolmaleki et al., 2016.
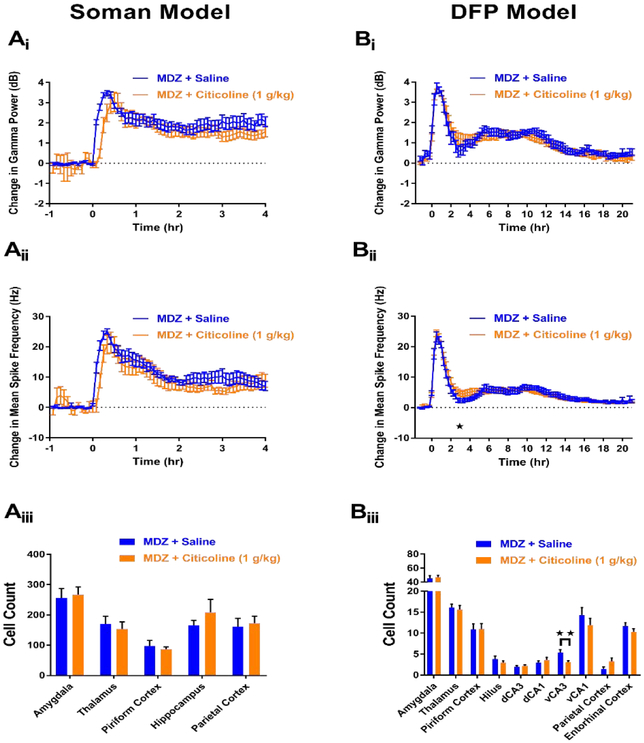
In the soman model, MDZ + citicoline (1 g/kg) was administered to 10 rats. Of these 10 rats, 0 died (Table 1), but none demonstrated SE termination at any time point throughout the experimental paradigm. EEG analysis further revealed MDZ + citicoline’s inefficacy as an anticonvulsant. No significant decreases in gamma power (Figure 6Ai) or mean spike rate frequency (6Aii) were observed at any hour time point in rats that received MDZ + citicoline (n=10) compared to MDZ + saline controls (n=10). In addition to lacking anticonvulsant efficacy, FJB staining revealed that MDZ + citicoline does not act as a neuroprotectant. No significant decreases in FJB staining were seen in any of the examined brain regions in rats that received MDZ + citicoline (n=10) compared to MDZ + saline controls (n=17) (Figure 6Aiii).
Figure 6. MDZ + 1 g/kg citicoline does not demonstrate anticonvulsant or neuroprotective properties in soman (A) and DFP (B) models of SE.
Ai) Change in gamma power relative to baseline in soman-exposed rats that received MDZ + 1 g/kg citicoline (orange, n=10) or MDZ + saline (blue, n=10). Aii) Change in mean spike rate frequency relative to baseline in soman-exposed rats that received MDZ + 1 g/kg citicoline (orange, n=10) or MDZ + saline (blue, n=10). Aiii) Fluoro-Jade B staining in vulnerable brain regions in soman-exposed rats that received MDZ + 1 g/kg citicoline (orange, n=10) or MDZ + saline (blue, n=17). Bi) Change in gamma power relative to baseline in DFP-exposed rats that received MDZ+ 1 g/kg citicoline (orange, n=24) or MDZ + saline (blue, n=18). Bii) Change in mean spike rate frequency relative to baseline in DFP-exposed rats that received MDZ + 1 g/kg citicoline (orange, n=24) or MDZ + saline (blue, n=18). Biii) Fluoro-Jade B staining in vulnerable brain regions in DFP-exposed rats that received MDZ + 1 g/kg citicoline (orange, n=24) or MDZ + saline (blue, n=18). Data represents mean ± SEM. Statistical significance: *p<0.05, **p<0.01.
In the DFP model, similar inefficacious results were observed. In the DFP model, 24 rats were treated with MDZ + citicoline (1 g/kg) and 0 rats died after treatment (Table 1). For comparison 18 rats were administered MDZ + saline vehicle control and 0 died after treatment (Table 1). EEG analysis revealed that MDZ + citicoline (n=24) caused no significant reductions in gamma power compared to MDZ + saline controls (n=18, Figure 6Bi), and mean spike rate frequency showed a significant increase at the 3 hr time point. (Figure 6Bii). FJB staining of rats that were administered MDZ + citicoline (n=24) revealed a significant reduction in staining only in the ventral CA3 (vCA3) region (Figure 6Biii) compared to MDZ + saline controls (n=18). Taken together, the results from both models demonstrate that MDZ + citicoline lacks anticonvulsant or neuroprotectant capability for the treatment of OP intoxication.
MDL-28170
MDL-28170 is a calpain I and II inhibitor that helps reduce capsaicin-mediated apoptosis. A recent study has demonstrated that 50 mg/kg MDL-28170 reduces neuronal degeneration and inflammation after pilocarpine-induced SE (Lam et al., 2017). Additionally, MDL-28170 may reduce seizure burden in rats after pilocarpine-induced SE (Lam et al., 2017). Based on this study and the innate neuroprotective properties of the drug, MDL-28170 was tested in our models at 50 mg/kg.
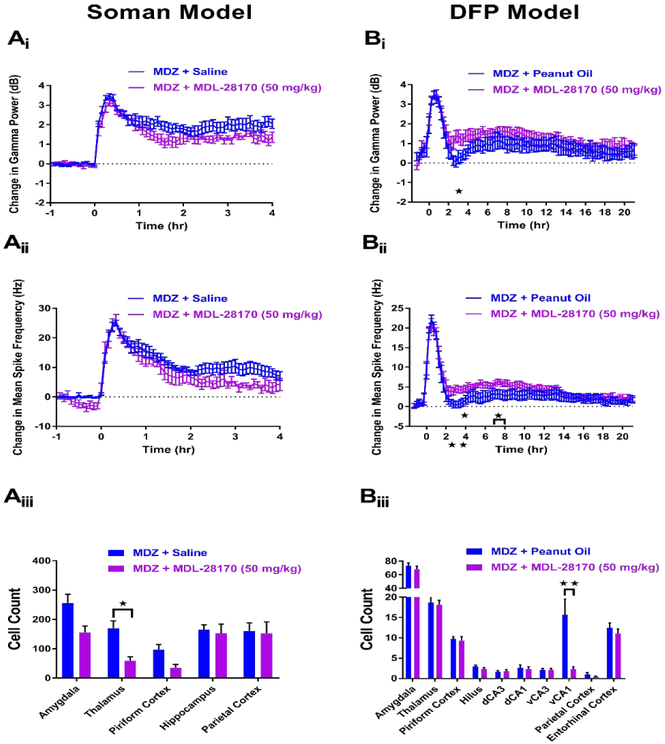
In the soman model, 7 rats received treatment of MDZ + MDL-28170 (50 mg/kg). Of these rats, 0 died (Table 1), but none demonstrated SE termination at any time point of the experimental paradigm. EEG analysis of rats that received MDZ + MDL-28170 (n=7) revealed no significant reductions in gamma power (7Ai) or mean spike rate frequency (7Aii) compared to MDZ + saline controls (n=10). Subsequent FJB staining was conducted to determine if MDZ + MDL-28170 provided neuroprotection against soman-induced SE. Significant reductions in FJB staining were seen in thalamus, but no other examined brain regions in rats that received MDZ + MDL-28170 (n=5) compared to MDZ + saline controls (n=17) (Figure 7Aiii).
Figure 7. MDZ + 50 mg/kg MDL-28170 is not an anticonvulsant in the soman (A) and DFP (B) models of SE and provides minimal neuroprotection.
Ai) Change in gamma power relative to baseline in soman-exposed rats that received MDZ + 50 mg/kg MDL-28170 (purple, n=7) or MDZ + saline (blue, n=10). Aii) Change in mean spike rate frequency relative to baseline in soman-exposed rats that received MDZ + 50 mg/kg MDL-28170 (purple, n=7) or MDZ + saline (blue, n=10). Aiii) Fluoro-Jade B staining in vulnerable brain regions in soman-exposed rats that received MDZ + 50 mg/kg MDL-28170 (purple, n=5) or MDZ + saline (blue, n=17). Bi) Change in gamma power relative to baseline in DFP-exposed rats that received MDZ + 50 mg/kg MDL-28170 (purple, n=14) or MDZ + peanut oil (blue, n=12). Bii) Change in mean spike rate frequency relative to baseline in DFP-exposed rats that received MDZ + 50 mg/kg MDL-28170 (purple, n=14) or MDZ + peanut oil (blue, n=12). Biii) Fluoro-Jade B staining in vulnerable brain regions in DFP-exposed rats that received MDZ + 50 mg/kg MDL-28170 (purple, n=14) or MDZ + peanut oil (blue, n=12). Data represents mean ± SEM. Statistical significance: *p<0.05, **p<0.01.
In the DFP model, 14 rats were treated with MDZ + MDL-28170 (50 mg/kg), and 0 rats died after treatment (Table 1). Additionally, 12 rats were administered MDZ + peanut oil vehicle control and 0 of these animals died after treatment (Table 1). EEG analysis of rats that received MDZ + MDL-28170 demonstrated a slight worsening of the anticonvulsant activity of MDZ. Compared to MDZ + peanut oil vehicle controls (n=12), rats that were administered MDZ + MDL-28170 (n=14) showed significant increases in gamma power at hour time point 3 (Figure 7Bi) and mean spike rate frequency at hour time points 3-4 and 7-8 (Figure 7Bii). In addition to lacking anticonvulsant efficacy, MDZ + MDL-28170 was not a strong neuroprotectant therapy in the DFP model of OP-induced SE. Compared to vehicle controls (n=12), significant decreases in FJB staining were only observed in the ventral CA1 (vCA1) of rats that received MDZ + MDL-28170 (n=14, Figure 7Biii). Given the lack of anticonvulsant efficacy and the minimal neuroprotection observed in both soman and DFP models, our data suggest that MDZ + MDL-28170 is not a promising treatment option for OP-induced SE.
Chloroquine
Chloroquine is an antimalarial medication that belongs to the 4-aminoquinoline class of drugs. In addition to being used to combat malaria, chloroquine has been studied as an anti-inflammatory (Oh et al., 2016) and as an adjunct therapy for cancer medications (Verbaanderd et al., 2017). The ability of chloroquine to provide neuroprotection in response to various neurological insults has also garnered attention for the drug. Studies have demonstrated that chloroquine can protect cultured neurons against glutamate-induced cell death (Hirata et al., 2011) and may provide neuroprotection in a rat model of traumatic brain injury (Cui et al., 2015). In addition to having neuroprotective properties, previous studies have demonstrated that 5 mg/kg chloroquine demonstrates anticonvulsant properties in a PTZ-induced convulsions model (N’Goumo et al., 1994). Based on the study by N’Goumo et al., 1994 and the potential neuroprotective and anticonvulsant properties of the drug, chloroquine was tested in our models at 5 mg/kg.
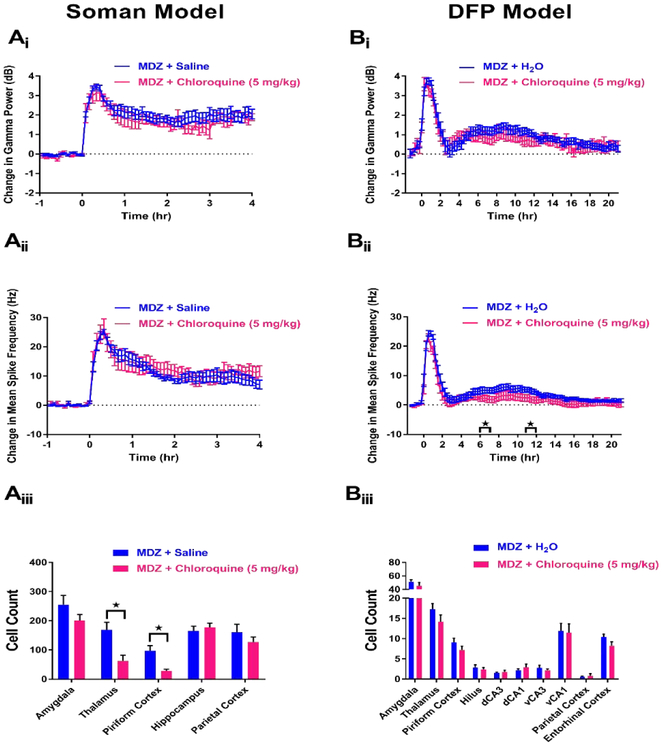
In the soman model, a total of 15 rats were treated with MDZ + chloroquine (5 mg/kg). Of these rats, 5 died (Table 1), and 0 demonstrated SE termination at any time point throughout the experiment. Additionally, one animal’s EEG had to be removed from the study because of excessive noise artifact. Analysis of EEG from the remaining rats that received MDZ + chloroquine (n=9) revealed no significant decreases in gamma power (Figure 8Ai) or mean spike rate frequency (8Aii) compared to MDZ + saline controls (n=10), suggesting that this therapy lacks anticonvulsant efficacy in the soman model. To determine if chloroquine provides neuroprotection in response to OP intoxication, FJB staining was conducted on rats from treatment and control groups. Compared to MDZ + saline controls (n=17), rats that were administered MDZ + chloroquine (n=9) showed a significant decrease in FJB staining in the thalamus and piriform cortex (Figure 8Aiii). These data suggest that while MDZ + chloroquine is not an anticonvulsant in the soman model of OP-induced SE, some slight neuroprotection may be provided by this combination of treatments.
Figure 8. MDZ + 5 mg/kg chloroquine is not an anticonvulsant but provides minor neuroprotection in the soman model (A), but not the DFP model (B) of SE.
Ai) Change in gamma power relative to baseline in soman-exposed rats that received MDZ + 5 mg/kg chloroquine (pink, n=9) or MDZ + saline (blue, n=10). Aii) Change in mean spike rate frequency relative to baseline in soman-exposed rats that received MDZ + 5 mg/kg chloroquine (pink, n=9) or MDZ + saline (blue, n=10). Aiii) Fluoro-Jade B staining in vulnerable brain regions in soman-exposed rats that received MDZ + 5 mg/kg chloroquine (pink, n=9) or MDZ + saline (blue, n=17). Bi) Change in gamma power relative to baseline in DFP-exposed rats that received MDZ + 5 mg/kg chloroquine (pink, n=14) or MDZ + sterile water (blue, n=15). Bii) Change in mean spike rate frequency relative to baseline in DFP-exposed rats that received MDZ + 5 mg/kg chloroquine (pink, n=14) or MDZ + sterile water (blue, n=15). Biii) Fluoro-Jade B staining in vulnerable brain regions in DFP-exposed rats that received MDZ + 5 mg/kg chloroquine (pink, n=13) or MDZ + sterile water (blue, n=15). Data represents mean ± SEM. Statistical significance: *p<0.05.
In the DFP model, 14 rats were administered MDZ + chloroquine (5 mg/kg), and 0 of these rats died after treatment (Table 1). For comparison 15 rats were administered MDZ + sterile water control and 0 died after treatment (Table 1). EEG analysis of rats administered MDZ + chloroquine (n=14) revealed no significant effect on gamma power at any time point of the experimental paradigm (Figure 8Bi) compared to MDZ + sterile water controls (n=15). Mean spike rate frequency analysis showed that rats who received MDZ + chloroquine had significant reductions at 6-7 hr and 11-12 hr after treatment compared to MDZ + sterile water controls (Figure 8Bii). Histopathology for rats treated with MDZ + chloroquine (n=13) demonstrated no effect on neuronal death compared MDZ + vehicle control rats (n=15) (Figure 8Biii).
Discussion
In this manuscript we have discussed the results from the CNS Program’s efforts to identify therapeutics that may be administered as potential adjuncts with the current standard-of-care medical countermeasures for the treatment of OP-induced SE. While current medical countermeasures are effective when administered acutely, OP intoxication and subsequent SE become less responsive to therapeutic intervention as treatment time is delayed. Unfortunately, in the event of a civilian mass casualty chemical emergency, the possibility of a significant delay in medical treatment is highly likely and would be dire if the scenario involves an OP compound. The drugs selected for this manuscript have all previously demonstrated anticonvulsant or neuroprotective efficacy in various models of SE or trauma. Testing a diverse range of therapeutic adjuncts will hopefully help identify which classes of drugs may be worth further exploration in future research.
The neurosteroid ganaxolone demonstrated the most promising anticonvulsant efficacy of the drugs tested in this study. Significant reductions in gamma power and spike rate were seen in both soman and DFP models of OP-induced SE, with a subset of rats showing rapid SE termination shortly after treatment. This efficacy likely resulted from the modulation of both phasic and tonic inhibition through the targeting of synaptic GABAa receptors by MDZ and extrasynaptic GABAa receptors by ganaxolone. When administered without MDZ in the soman model, however, ganaxolone was unable to terminate SE (data not shown), thereby supporting the advantage of a multimodal pharmacological approach for the treatment of OP-induced SE. Despite ganaxolone’s promising anticonvulsant efficacy, a surprisingly minimal amount of neuroprotection was seen. This lack of neuroprotection may be explained by several factors. One reason may be the slow latency to SE cessation in ganaxolone-treated rats. When ganaxolone was effective against OP-induced seizures, the average latency to SE termination was approximately 34 min post-treatment in the soman model. This time frame well surpasses the minimal seizure duration time needed to see frank neuropathology in OP-induced SE (McDonough and Shih, 1997). In addition to being slow to terminate seizures in our models, ganaxolone was not effective at maintaining SE quiescence. Four out of the 5 rats in the soman model that demonstrated SE cessation eventually returned to ictal activity. This return to SE most likely exacerbated neuronal damage and further contributed to ganaxolone’s lack of neuroprotective capabilities. Despite ganaxolone’s lack of neuroprotective abilities, neurosteroids still present a promising treatment option for OP-induced SE. Efficacious results were demonstrated with the novel neurosteroid SGE-516, which provided both anticonvulsant and neuroprotective efficacy against soman-induced SE when administered with MDZ at up to a 40 min treatment delay (Althaus et al., 2017). Additionally, the neurosteroid-like enaminone compounds, which also target δ subunit containing GABAA receptors, are another emerging class of drugs that should be further explored as these compounds demonstrate promising efficacy against OP-induced SE (Johnstone et al., 2019). Given the pharmacological properties of neurosteroids and enaminones, and their proven efficacy in other models of epilepsy and SE (Biagini et al., 2010; Rogawski et al., 2013; Reddy, 2016; Zolkowska et al., 2018; Johnstone et al., 2019; Saporito et al., 2019), efforts to develop improved derivatives of these drugs should continue.
The K-ATP channel opener diazoxide (Roy Chowdhury et al., 2017) produced conflicting anticonvulsant results in the soman and DFP models of OP-induced SE. Significant reductions in gamma power (3 hr) and mean spike rate frequency (1-3 hr) were seen in the soman, but not DFP, model of OP intoxication. One explanation for this variance could be the difference in controls between the two models. Because of the relative lack of mortality in the DFP model, the corresponding MDZ + 15% DMSO in multisol vehicle control was able to be tested simultaneously with MDZ + diazoxide. In the soman model, MRICD IACUC regulations limit the number of rats that can be exposed to nerve agent without effective treatments. These restrictions often necessitate the use of a single historical MDZ + saline control cohort instead of a true side-by-side vehicle control. The 10% ethanol component of the multisol-based vehicle used in the DFP model may have provided slight enhancement of the MDZ anticonvulsant properties that eliminated differences between diazoxide- and vehicle-treated groups. However, in the soman model, previous work has suggested that treatment with MDZ + 10% ethanol vehicles does not significantly differ from that with MDZ + saline in terms of anticonvulsant properties (data not shown). These previous data suggest that diazoxide efficacy in the soman model would be maintained if MDZ + 15% DMSO in multisol vehicle controls were tested. While anticonvulsant data were conflicting in the two models, both the soman- and DFP-induced SE paradigms demonstrated that diazoxide is not a potent neuroprotective adjunct. This result was surprising in the soman model as MDZ + diazoxide caused significant reductions in gamma power and spike rate parameters. Additionally, 4 out of 8 treated rats demonstrated SE termination for various durations throughout the experimental paradigm, and 3 out of 4 of these rats remained seizure free at the 4 hr endpoint. The lack of neuroprotection may be explained by the fact that of the 4 rats that demonstrated SE termination, one returned to seizing at the 4 hr time point, another returned to seizing at the 24 hr time point and one died overnight, leaving only one rat alive and seizure free at the time of perfusion. Diazoxide’s lack of sustained anticonvulsant efficacy may be a contributing factor to the relative absence of neuroprotection provided by the drug. Overall, the data obtained from the two models utilizing MDZ + diazoxide suggest that more research into potassium channel openers for the treatment OP-induced SE is needed. These drugs will utilize different delivery vehicles from diazoxide, which will help better elucidate whether potassium channel openers demonstrate different efficacy in soman and DFP models of SE.
The loop diuretic bumetanide proved to be a poor adjunct therapy for the treatment of OP-induced SE. No anticonvulsant efficacy was provided by MDZ + bumetanide (4 mg/kg) in either the soman or DFP model of OP intoxication. Additionally, bumetanide in conjunction with the standard medical countermeasures produced only modest neuroprotection in the soman model. The failure of bumetanide in our OP models could be explained by a few possibilities. Previous pharmacokinetic studies have demonstrated that bumetanide has poor brain penetrance (Brandt et al., 2010), though diffusion into the CNS may be increased as a result of a SE-induced increase in blood-brain barrier permeability (Gorter et al., 2015). Another issue with bumetanide is its rapid metabolism, with an average half-life of 10.4 min in adult rats (Brandt et al., 2010). These pharmacokinetic properties make bumetanide a poor treatment option for CNS-derived SE that is capable of persisting for hours. Another possible reason for the failure of bumetanide is the downregulation of NKCC1 that occurs with maturity (Payne et al., 2003). Under normal conditions, adult rat neurons display none to negligible NKCC1, though significant upregulation of the protein is seen 24 hr after SE (Brandt et al., 2010). Since our model utilizes adult rats and treatments are administered 20-60 min after SE initiation, NKCC1 upregulation is probably still minimal at the time of therapeutic intervention, meaning there is not an abundant amount of protein to target. Even if NKCC1 upregulation was rapid, a short half-life and poor brain penetrance would most likely render bumetanide ineffective.
Similar to bumetanide, the preservative propylparaben failed to demonstrate significant anticonvulsant and neuroprotective abilities in our models of OP-induced SE. Previous studies utilizing a pilocarpine model of SE demonstrated that propylparaben, in conjunction with diazepam, provided both anticonvulsant and neuroprotective efficacy (Santana-Gomez et al., 2017). The anticonvulsant properties demonstrated by propylparaben are believed to occur via the drug’s ability to inhibit voltage-gated sodium channels (Ji et al., 2004; Lara-Valderrabano et al., 2016). While sodium channel blockers are efficacious in other models of epilepsy and SE, drugs with this predominant mechanism of action have previously proved to be unsuccessful in treating OP intoxication (Shih et al., 1999). Our results utilizing MDZ + propylparaben further suggest that voltage-gated sodium channel blockers tend to be ineffective in treating OP-induced SE at delayed treatment time points.
The neuroprotectants citicoline, MDL-28170, and chloroquine tested in our models all failed to provide widespread significant neural protection in response to OP-induced SE. The data obtained from the administration of MDZ in conjunction with citicoline, MDL-28170, and chloroquine continue to suggest that drugs that provide extensive neuroprotection in OP-induced SE without some degree of anticonvulsant activity are rare. The majority of previous studies utilizing rodent and non-human primate models of OP-induced SE demonstrate that seizure attenuation appears to be crucial for neuroprotection (Martin et al., 1985; Hayward et al., 1990; Shih et al., 2003). Continued screening of previously proven neuroprotectants will be necessary to determine if anticonvulsant efficacy is absolutely required for neuronal protection against OP-induced SE.
Here we describe our findings of the CNS program’s screening, to date, of various anticonvulsant and neuroprotective drugs in two delayed treatment models of OP-induced SE. Though we report negative data for multiple drugs, we believe the publication of these and all results are a crucial tool for steering future research in the right direction for improved treatments of OP-induced SE. Additionally, we believe our abundant reporting of negative data adds strong reliability and validity to the positive data that is produced by the CNS program. Overall, based on these findings thus far, our data encourages the further exploration of neurosteroids as promising treatment options for OP intoxication and puts an emphasis on attenuating SE in order to potentially provide crucial neuroprotection. Lastly, we hope this manuscript encourages other investigators to utilize the NIH’s CNS program (Yeung, 2019). This no cost in vivo screening program emphasizes a cooperative approach to improving how OP-induced SE is treated. Organophosphate intoxication is both complex and challenging to successfully treat, but we believe a collaborative approach to this issue via the CNS program can improve the prognosis for the thousands of people who are affected annually.
Highlights section.
The CNS program screens for novel adjunct therapies in two delayed treatment models of OP-induced status epilepticus.
Neurosteroids appear to be the most promising anticonvulsant therapy for OP-induced status epilepticus.
Significant neuroprotection against OP-induced status epilepticus seems to require some degree of anticonvulsant efficacy.
Acknowledgments
Funding
This research was supported in part by an appointment to the Postgraduate Research Participation Program at the U.S. Army Medical Research Institute of Chemical Defense administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and U.S. Army Medical Research and Development Command. Additionally, this work was also supported by an interagency agreement (AOD18013-001-00000, AOD1920-001-00000) between the NIH Office of the Director (OD) and the U.S. Army Medical Research Institute of Chemical Defense under the oversight of the Chemical Countermeasures Research Program (CCRP) within the Office of Biodefense Research (OBRS) at the National Institute of Allergy and Infectious Diseases (NIAID/NIH). Work at the University of Utah is subcontracted to the U.S. Army Medical Research Institute of Chemical Defense under Contract No. W81XWH-16-C-0140 and W81XWH-18-C-0181.
Abbreviations
- ACh
Acetylcholine
- AChE
Acetylcholinesterase
- AMN
Atropine methyl nitrate
- CNS (Program)
CounterACT Neurotherapeutic Screening
- dCA1
Dorsal CA1
- dCA3
Dorsal CA3
- DFP
Diisopropylfluorophosphate
- EEG
Electroencephalogram
- FJB
Fluoro-Jade B
- GD
Soman
- HPBCD
2-hydroxypropyl-β-cyclodextrin
- IM
Intramuscular
- IP
Intraperitoneal
- IV
Intravenous
- MDZ
Midazolam
- OP
Organophosphorus
- OPCW
Organization for the Prohibition of Chemical Weapons
- PEG
Polyethylene glycol
- PTZ
Pentylenetetrazol
- ROI
Region of interest
- SE
Status epilepticus
- TETS
Tetramethylenedisulfotetramine
- vCA1
Ventral CA1
- vCA3
Ventral CA3
- WHO
World Health Organization
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: None
Disclaimer
The views expressed in this paper are solely those of the author(s) and do not reflect the official policy of the CCRP, NIAID, NIH, HHS, USAMRICD, Department of Army, Department of Defense, or the U.S. Government. The experimental protocols for both the soman and DFP models were approved by the Animal Care and Use Committee at the United States Army Medical Research Institute of Chemical Defense, and all procedures were conducted in accordance with the principles stated in the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011), the Public Health Service Policy on Humane Care and Use of Laboratory Animals and the Animal Welfare Act of 1966 (P.L. 89-544), as amended. The surgical and experimental procedures used in the DFP studies were also reviewed and approved by the University of Utah Institutional Animal Care and Use Committee.
References
- Abdolmaleki A, Moghimi A, Ghayour MB, Rassouli MB (2016) Evaluation of neuroprotective, anticonvulsant, sedative and anxiolytic activity of citicoline in rats. European journal of pharmacology 789:275–279. [DOI] [PubMed] [Google Scholar]
- Althaus AL, McCarren HS, Alqazzaz A, Jackson C, McDonough JH, Smith CD, Hoffman E, Hammond RS, Robichaud AJ, Doherty JJ (2017) The synthetic neuroactive steroid SGE-516 reduces status epilepticus and neuronal cell death in a rat model of soman intoxication. Epilepsy & behavior : E&B 68:22–30. [DOI] [PubMed] [Google Scholar]
- Apland JP, Figueiredo TH, Qashu F, Aroniadou-Anderjaska V, Souza AP, Braga MF (2010) Higher susceptibility of the ventral versus the dorsal hippocampus and the posteroventral versus anterodorsal amygdala to soman-induced neuropathology. Neurotoxicology 31:485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Associated Press (2018, December 15) Suspected food poisoning kills 11 at Indian temple ceremony. Retrieved July 26, 2019 from https://www.apnews.com/dd1a31ec2bbc4e7faa6d277007bbf120.
- Biagini G, Panuccio G, Avoli M (2010) Neurosteroids and epilepsy. Current opinion in neurology 23:170–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blount PJ, Nguyen CD, McDeavitt JT (2002) Clinical use of cholinomimetic agents: a review. The Journal of head trauma rehabilitation 17:314–321. [DOI] [PubMed] [Google Scholar]
- Brandt C, Nozadze M, Heuchert N, Rattka M, Loscher W (2010) Disease-modifying effects of phenobarbital and the NKCC1 inhibitor bumetanide in the pilocarpine model of temporal lobe epilepsy. The Journal of neuroscience : the official journal of the Society for Neuroscience 30:8602–8612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter RB, Wood PL, Wieland S, Hawkinson JE, Belelli D, Lambert JJ, White HS, Wolf HH, Mirsadeghi S, Tahir SH, Bolger MB, Lan NC, Gee KW (1997) Characterization of the anticonvulsant properties of ganaxolone (CCD 1042; 3alpha-hydroxy-3beta-methyl-5alpha-pregnan-20-one), a selective, high-affinity, steroid modulator of the gamma-aminobutyric acid(A) receptor. The Journal of pharmacology and experimental therapeutics 280:1284–1295. [PubMed] [Google Scholar]
- Chen Y (2012) Organophosphate-induced brain damage: mechanisms, neuropsychiatric and neurological consequences, and potential therapeutic strategies. Neurotoxicology 33:391–400. [DOI] [PubMed] [Google Scholar]
- Cheng M (2018, March 12) UK says ex-spy poisoned with Soviet-developed nerve agent. Retrieved July 26, 2019 from https://www.apnews.com/1a4c95b0e6af4d70b054c8030e177b47
- Costanzi S, Machado JH, Mitchell M (2018) Nerve agents: what they are, how they work, how to counter them. ACS chemical neuroscience 9:873–885. [DOI] [PubMed] [Google Scholar]
- Cui CM, Gao JL, Cui Y, Sun LQ, Wang YC, Wang KJ, Li R, Tian YX, Cui JZ (2015) Chloroquine exerts neuroprotection following traumatic brain injury via suppression of inflammation and neuronal autophagic death. Molecular medicine reports 12:2323–2328. [DOI] [PubMed] [Google Scholar]
- Deeb TZ, Maguire J, Moss SJ (2012) Possible alterations in GABAA receptor signaling that underlie benzodiazepine-resistant seizures. Epilepsia 53 Suppl 9:79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eftekhari S, Mehvari Habibabadi J, Najafi Ziarani M, Hashemi Fesharaki SS, Gharakhani M, Mostafavi H, Joghataei MT, Beladimoghadam N, Rahimian E, Hadjighassem MR (2013) Bumetanide reduces seizure frequency in patients with temporal lobe epilepsy. Epilepsia 54:e9–12. [DOI] [PubMed] [Google Scholar]
- Fioravanti M, Buckley AE (2006) Citicoline (Cognizin) in the treatment of cognitive impairment. Clinical interventions in aging 1:247–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franjesevic AJ, Sillart SB, Beck JM, Vyas S, Callam CS, Hadad CM (2018) Resurrection and Reactivation of Acetylcholinesterase and Butyrylcholinesterase. Chemistry 25(21):5337–5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale K (1992) Subcortical structures and pathways involved in convulsive seizure generation. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society 9:264–277. [DOI] [PubMed] [Google Scholar]
- Gardiner Harris HK (2013, October 22) Two Charged in School Lunch Poisoning Case in India. Retrieved July 26, 2019 from https://www.nytimes.com/2013/10/23/world/asia/two-charged-in-school-lunch-poisoning-case-in-india.html.
- George Paxinos CW (2007) The Rat Brain in Stereotaxic Coordinates 6th Edition: Academic Press. [Google Scholar]
- Gorter JA, van Vliet EA, Aronica E (2015) Status epilepticus, blood-brain barrier disruption, inflammation, and epileptogenesis. Epilepsy & behavior : E&B 49:13–16. [DOI] [PubMed] [Google Scholar]
- Gregory Katz DK, Lawless Jill (2018, July 5) UK town faces new reality: Another nerve agent poisoning. Retrieved July 26, 2019 from https://apnews.com/12a4c10e591f456da276d9c28fa1a5d2.
- Hayward IJ, Wall HG, Jaax NK, Wade JV, Marlow DD, Nold JB (1990) Decreased brain pathology in organophosphate-exposed rhesus monkeys following benzodiazepine therapy. Journal of the neurological sciences 98:99–106. [DOI] [PubMed] [Google Scholar]
- Heiss DR, Zehnder DW 2nd, Jett DA, Platoff GE Jr., Yeung DT, Brewer BN (2016) Synthesis and storage stability of diisopropylfluorophosphate. Journal of chemistry 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata Y, Yamamoto H, Atta MS, Mahmoud S, Oh-hashi K, Kiuchi K (2011) Chloroquine inhibits glutamate-induced death of a neuronal cell line by reducing reactive oxygen species through sigma-1 receptor. Journal of neurochemistry 119:839–847. [DOI] [PubMed] [Google Scholar]
- Jackson C, Ardinger C, Winter KM, McDonough JH, McCarren HS (2019) Validating a model of benzodiazepine refractory nerve agent-induced status epilepticus by evaluating the anticonvulsant and neuroprotective effects of scopolamine, memantine, and phenobarbital. Journal of pharmacological and toxicological methods 97:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazayeri A, Zolfaghari S, Ostadhadi S (2013) Anticonvulsant effect of diazoxide against dichlorvos-induced seizures in mice. The scientific world journal 2013:697305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji X, Xu Z, Criswell HE, Boysen PG (2004) Propylparaben inhibits voltage-dependent sodium channels and protects cardiomyocytes from ischemia-reperfusion injury. Life sciences 74:3043–3052. [DOI] [PubMed] [Google Scholar]
- Johnstone TBC, McCarren HS, Spampanato J, Dudek FE, McDonough JH, Hogenkamp D, Gee KW (2019) Enaminone modulators of extrasynaptic alpha4beta3delta gamma-aminobutyric acid A receptors reverse electrographic status epilepticus in the rat after acute organophosphorus poisoning. Frontiers in pharmacology 10:560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DM, Esmaeil N, Maren S, Macdonald RL (2002) Characterization of pharmacoresistance to benzodiazepines in the rat Li-pilocarpine model of status epilepticus. Epilepsy research 50:301–312. [DOI] [PubMed] [Google Scholar]
- Kaila K, Ruusuvuori E, Seja P, Voipio J, Puskarjov M (2014) GABA actions and ionic plasticity in epilepsy. Current opinion in neurobiology 26:34–41. [DOI] [PubMed] [Google Scholar]
- Kapur J, Coulter DA (1995) Experimental status epilepticus alters gamma-aminobutyric acid type A receptor function in CA1 pyramidal neurons. Annals of neurology 38:893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AM, Aaron CK (2015) Organophosphate and carbamate poisoning. Emergency medicine clinics of North America 33:133–151. [DOI] [PubMed] [Google Scholar]
- Lam PM, Carlsen J, Gonzalez MI (2017) A calpain inhibitor ameliorates seizure burden in an experimental model of temporal lobe epilepsy. Neurobiology of disease 102:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara-Valderrabano L, Rocha L, Galvan EJ (2016) Propylparaben reduces the excitability of hippocampal neurons by blocking sodium channels. Neurotoxicology 57:183–193. [DOI] [PubMed] [Google Scholar]
- Lehmkuhle MJ, Thomson KE, Scheerlinck P, Pouliot W, Greger B, Dudek FE (2009) A simple quantitative method for analyzing electrographic status epilepticus in rats. Journal of neurophysiology 101:1660–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstein DH, Alldredge BK (1998) Status epilepticus. The New England journal of medicine 338:970–976. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Doebler JA, Shih TM, Anthony A (1985) Protective effect of diazepam pretreatment on soman-induced brain lesion formation. Brain research 325:287–289. [DOI] [PubMed] [Google Scholar]
- McDonald WJ, Smith G, Woods JW, Perry HM, Danielson BD (1977) Intravenous diazoxide therapy in hypertensive crisis. The American journal of cardiology 40:409–415. [DOI] [PubMed] [Google Scholar]
- McDonough JH, McMonagle JD, Shih TM (2010) Time-dependent reduction in the anticonvulsant effectiveness of diazepam against soman-induced seizures in guinea pigs. Drug and chemical toxicology 33:279–283. [DOI] [PubMed] [Google Scholar]
- McDonough JH Jr., Shih TM (1997) Neuropharmacological mechanisms of nerve agent-induced seizure and neuropathology. Neuroscience and biobehavioral reviews 21:559–579. [DOI] [PubMed] [Google Scholar]
- McDonough JH Jr., Clark TR, Slone TW Jr., Zoeffel D, Brown K, Kim S, Smith CD (1998) Neural lesions in the rat and their relationship to EEG delta activity following seizures induced by the nerve agent soman. Neurotoxicology 19:381–391. [PubMed] [Google Scholar]
- Myhrer T (2007) Neuronal structures involved in the induction and propagation of seizures caused by nerve agents: implications for medical treatment. Toxicology 239:1–14. [DOI] [PubMed] [Google Scholar]
- Myhrer T, Enger S, Aas P (2008) Anticonvulsant efficacy of drugs with cholinergic and/or glutamatergic antagonism microinfused into area tempestas of rats exposed to soman. Neurochemical research 33:348–354. [DOI] [PubMed] [Google Scholar]
- Myhrer T, Mariussen E, Aas P (2018) Development of neuropathology following soman poisoning and medical countermeasures. Neurotoxicology 65:144–165. [DOI] [PubMed] [Google Scholar]
- National Institute of Allergy and Infectious Disease (2017) The NIH medical research program directed against chemical threats: 2017 report on research progress and future directions. Retrieved July 26, 2019 from https://www.niaid.nih.gov/sites/default/files/NIHChemicalPlan.pdf.
- National Institute of Allergy and Infectious Disease (2016) Chemical Countermeasures Research Program. Retrieved July 26, 2019 from https://www.niaid.nih.gov/research/chemical-countermeasures-program.
- Naylor DE, Liu H, Wasterlain CG (2005) Trafficking of GABA(A) receptors, loss of inhibition, and a mechanism for pharmacoresistance in status epilepticus. The Journal of neuroscience : the official journal of the Society for Neuroscience 25:7724–7733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng E (2017, October 3) Post-mortem: VX poison killed brother of North Korean leader. Retrieved July 26, 2019 from https://apnews.com/90e425dbaf1e44d1ba77e2eea890fc67.
- N'Gouemo P, Ben Attia M, Belaidi M (1994) Effects of chloroquine on pentylenetetrazol-induced convulsions in mice. Pharmacological research 30:99–103. [DOI] [PubMed] [Google Scholar]
- Niaki SE, Shafaroodi H, Ghasemi M, Shakiba B, Fakhimi A, Dehpour AR (2008) Mouth breathing increases the pentylenetetrazole-induced seizure threshold in mice: a role for ATP-sensitive potassium channels. Epilepsy & behavior : E&B 13:284–289. [DOI] [PubMed] [Google Scholar]
- Oh S, Shin JH, Jang EJ, Won HY, Kim HK, Jeong MG, Kim KS, Hwang ES (2016) Anti-inflammatory activity of chloroquine and amodiaquine through p21-mediated suppression of T cell proliferation and Th1 cell differentiation. Biochemical and biophysical research communications 474:345–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne JA, Rivera C, Voipio J, Kaila K (2003) Cation-chloride co-transporters in neuronal communication, development and trauma. Trends in neurosciences 26:199–206. [DOI] [PubMed] [Google Scholar]
- Petras JM (1994) Neurology and neuropathology of soman-induced brain injury: an overview. Journal of the experimental analysis of behavior 61:319–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouliot W, Bealer SL, Roach B, Dudek FE (2016) A rodent model of human organophosphate exposure producing status epilepticus and neuropathology. Neurotoxicology 56:196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressler RM, Boylan GB, Marlow N, Blennow M, Chiron C, Cross JH, de Vries LS, Hallberg B, Hellstrom-Westas L, Jullien V, Livingstone V, Mangum B, Murphy B, Murray D, Pons G, Rennie J, Swarte R, Toet MC, Vanhatalo S, Zohar S (2015) Bumetanide for the treatment of seizures in newborn babies with hypoxic ischaemic encephalopathy (NEMO): an open-label, dose finding, and feasibility phase 1/2 trial. The Lancet neurology 14:469–477. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Kuruba R, and Wu X (2015) Efficacy of neurosteroid therapy against soman-induced seizures and brain injury. Presented at the 9th Annual NIH Countermeasures Against Chemical Threats Network Research Symposium June 15–17, 2015 New York, NY. [Google Scholar]
- Reddy DS (2016) Neurosteroids for the potential protection of humans against organophosphate toxicity. Annals of the New York Academy of Sciences 1378:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice AC, DeLorenzo RJ (1999) N-methyl-D-aspartate receptor activation regulates refractoriness of status epilepticus to diazepam. Neuroscience 93:117–123. [DOI] [PubMed] [Google Scholar]
- Robb EL, Baker MB (2019, March 2) Organophosphate Toxicity. In: StatPearls. Retrieved July 26, 2019 from https://www.ncbi.nlm.nih.gov/books/NBK470430/.
- Rogawski MA, Loya CM, Reddy K, Zolkowska D, Lossin C (2013) Neuroactive steroids for the treatment of status epilepticus. Epilepsia 54 Suppl 6:93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy Chowdhury U, Dosa PI, Fautsch MP (2017) ATP sensitive potassium channel openers: A new class of ocular hypotensive agents. Experimental eye research 158:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santana-Gomez CE, Valle-Dorado MG, Dominguez-Valentin AE, Hernandez-Moreno A, Orozco-Suarez S, Rocha L (2018) Neuroprotective effects of levetiracetam, both alone and combined with propylparaben, in the long-term consequences induced by lithium-pilocarpine status epilepticus. Neurochemistry international 120:224–232. [DOI] [PubMed] [Google Scholar]
- Santana-Gomez CE, Orozco-Suarez SA, Talevi A, Bruno-Blanch L, Magdaleno-Madrigal VM, Fernandez-Mas R, Rocha L (2017) Propylparaben applied after pilocarpine-induced status epilepticus modifies hippocampal excitability and glutamate release in rats. Neurotoxicology 59:110–120. [DOI] [PubMed] [Google Scholar]
- Saporito MS, Gruner JA, DiCamillo A, Hinchliffe R, Barker-Haliski M, White HS (2019) Intravenously administered ganaxolone blocks diazepam-resistant lithium-pilocarpine-induced status epilepticus in rats: comparison with allopregnanolone. The Journal of pharmacology and experimental therapeutics 368:326–337. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Hopkins KJ (2000) Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain research 874:123–130. [DOI] [PubMed] [Google Scholar]
- Shih T, McDonough JH Jr., Koplovitz I (1999) Anticonvulsants for soman-induced seizure activity. Journal of biomedical science 6:86–96. [DOI] [PubMed] [Google Scholar]
- Shih TM, McDonough JH Jr. (1997) Neurochemical mechanisms in soman-induced seizures. Journal of applied toxicology : JAT 17:255–264. [DOI] [PubMed] [Google Scholar]
- Shih TM, Duniho SM, McDonough JH (2003) Control of nerve agent-induced seizures is critical for neuroprotection and survival. Toxicology and applied pharmacology 188:69–80. [DOI] [PubMed] [Google Scholar]
- Sidell FR (1997) Nerve Agents. In: Medical Aspects of Chemical and Biological Warfare (Zajtchuk R, ed), pp 129–181. Bethesda, Maryland: Office of the Surgeon General. [Google Scholar]
- Sivakumaran S, Maguire J (2016) Bumetanide reduces seizure progression and the development of pharmacoresistant status epilepticus. Epilepsia 57:222–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skovira JW, McDonough JH, Shih TM (2010) Protection against sarin-induced seizures in rats by direct brain microinjection of scopolamine, midazolam or MK-801. Journal of molecular neuroscience : MN 40:56–62. [DOI] [PubMed] [Google Scholar]
- Skovira JW, Shih TM, McDonough JH (2012) Neuropharmacological specificity of brain structures involved in soman-induced seizures. Neurotoxicology 33:463–468. [DOI] [PubMed] [Google Scholar]
- Spampanato J, Pouliot W, Bealer SL, Roach B, Dudek FE (2019) Antiseizure and neuroprotective effects of delayed treatment with midazolam in a rodent model of organophosphate exposure. Epilepsia 60(7): 1387–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touati G, Poggi-Travert F, Ogier de Baulny H, Rahier J, Brunelle F, Nihoul-Fekete C, Czernichow P, Saudubray JM (1998) Long-term treatment of persistent hyperinsulinaemic hypoglycaemia of infancy with diazoxide: a retrospective review of 77 cases and analysis of efficacy-predicting criteria. European journal of pediatrics 157:628–633. [DOI] [PubMed] [Google Scholar]
- Towne AR, Pellock JM, Ko D, DeLorenzo RJ (1994) Determinants of mortality in status epilepticus. Epilepsia 35:27–34. [DOI] [PubMed] [Google Scholar]
- Verbaanderd C, Maes H, Schaaf MB, Sukhatme VP, Pantziarka P, Sukhatme V, Agostinis P, Bouche G (2017) Repurposing drugs in oncology (ReDO)-chloroquine and hydroxychloroquine as anti-cancer agents. Ecancermedicalscience 11:781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton NY, Treiman DM (1988) Response of status epilepticus induced by lithium and pilocarpine to treatment with diazepam. Experimental neurology 101:267–275. [DOI] [PubMed] [Google Scholar]
- White AM, Williams PA, Ferraro DJ, Clark S, Kadam SD, Dudek FE, Staley KJ (2006) Efficient unsupervised algorithms for the detection of seizures in continuous EEG recordings from rats after brain injury. Journal of neuroscience methods 152:255–266. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) (2018, August 24) Suicide. Retrieved July 26, 2019 from https://www.who.int/news-room/fact-sheets/detail/suicide
- Wu X, Kuruba R, Reddy DS (2018) Midazolam-resistant seizures and brain injury after acute intoxication of diisopropylfluorophosphate, an organophosphate pesticide and durrogate for nerve agents. The Journal of pharmacology and experimental therapeutics 367:302–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa N, Morita H, Nakajima T (2006) Sarin experiences in Japan: acute toxicity and long-term effects. Journal of the neurological sciences 249:76–85. [DOI] [PubMed] [Google Scholar]
- Yeung D (2019) CounterACT neurotherapeutic screening (CNS) program. Retrieved July 26, 2019 from https://www.ninds.nih.gov/sites/default/files/CNS_program_0.pdf.
- Yeung DT, Platoff GE Jr, Harper JR and Jett DA (2019) An Overview of the NIAID/NIH Chemical Medical Countermeasures Product Research and Development Program, in Chemical Warfare Agents: Biomedical and Psychological Effects, Medical Countermeasures, and Emergency Response, 3 ed., Lukey BJ, Roman JA Jr and Salem H, Eds., pp. 615–626. Boca Raton, CRC Press. [Google Scholar]
- Zolkowska D, Wu CY, Rogawski MA (2018) Intramuscular allopregnanolone and ganaxolone in a mouse model of treatment-resistant status epilepticus. Epilepsia 59 Suppl 2:220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]