Abstract
Objective:
While the role of antiphospholipid antibodies in activating endothelial cells has been extensively studied, the impact of these antibodies on the adhesive potential of leukocytes has received less attention. Here, we investigated the extent to which antiphospholipid syndrome (APS) neutrophils adhered to resting endothelial cells under physiologic flow conditions, as well as the surface molecules required for that adhesion.
Methods:
Patients with primary APS (n=43), patients with history of venous thrombosis but negative testing for antiphospholipid antibodies (n=11), and healthy controls (n=38) were studied. Cells were introduced into a flow chamber and perfused across resting human umbilical vein endothelial cell (HUVECs). Surface adhesion molecules were quantified by flow cytometry. Neutrophil extracellular trap (NET) release (NETosis) was assessed in neutrophil-HUVEC co-cultures.
Results:
Upon perfusion of anticoagulated blood through the flow chamber, APS neutrophils demonstrated increased adhesion as compared with control neutrophils under conditions representative of either venous (n=8, p<0.05) or arterial (n=15, p<0.0001) flow. At the same time, APS neutrophils were characterized by upregulation of CD64, CEACAM1, beta-2 glycoprotein I, and activated Mac-1 on their surface (n=12–18, p<0.05 for all markers). Exposing control neutrophils to APS plasma or APS IgG resulted in increased neutrophil adhesion (n=10–11, p<0.001) and surface marker upregulation as compared with controls. A monoclonal antibody specific for activated Mac-1 reduced the adhesion of APS neutrophils in the flow-chamber assay (p<0.01). The same monoclonal antibody reduced NETosis in neutrophil-HUVEC co-cultures (p<0.01).
Conclusion:
APS neutrophils demonstrate increased adhesive potential, which is dependent upon the activated form of Mac-1. In patients, this could lower the threshold for neutrophil-endothelium interactions, NETosis, and possibly thrombotic events.
INTRODUCTION
Antiphospholipid syndrome (APS) is an autoimmune condition of unknown cause defined by the presence of circulating “antiphospholipid” antibodies (anticardiolipin, anti-beta-2-glycoprotein I/β2GPI, or lupus anticoagulant) (1). The morbidity and mortality of APS are significant, as patients carry a markedly increased risk of thrombotic events (especially stroke and deep vein thrombosis) and pregnancy loss (2). Beyond these disease-defining events, patients with APS may also develop cytopenias, heart valve damage, nephropathy, and cognitive dysfunction, among other complications (3). While it has long been recognized that circulating leukocytes play some role in the pathophysiology of APS, the impact of neutrophils has only come to light in the past few years (4). Our group and others have revealed that APS neutrophils are prone to the exaggerated release of neutrophil extracellular traps (NETs), prothrombotic tangles of DNA and microbicidal proteins released from dying neutrophils (5). At the same time, at least some APS blood does not degrade NETs normally (6). Indeed, dismantling NETs with deoxyribonuclease (7) and preventing NETosis via activation of adenosine receptors (8) have proven effective in murine models of APS. In further support of neutrophil hyperactivity in APS, our group has demonstrated that the APS neutrophil transcriptome is characterized by the upregulation of a number of meta-groups, including a cellular defense node that includes L-selectin and P-selectin glycoprotein I, amongst other adhesion molecules (9).
Beyond neutrophils, both animal models and descriptive studies of patients have demonstrated signs of smoldering endothelial activation in APS. For example, tissue factor activity is increased in carotid homogenates from antiphospholipid antibody-treated mice (10), which correlates with increased leukocyte-endothelium interplay (11). In keeping with the latter concept, antagonizing either E-selectin or P-selectin (the key selectins expressed on endothelium) is protective against thrombosis in mice; the same is true for strategies blocking the endothelial integrin ligands VCAM-1 and ICAM-1 (12, 13). One study has suggested that downregulation of endothelial nitric oxide synthase by antiphospholipid antibodies may be another important factor in increased leukocyte-endothelium interplay (14). Beyond these in vivo data, there is robust evidence in vitro that antiphospholipid antibodies can activate endothelial cells to express tissue factor and adhesion molecules (15, 16). Mechanistically, NF-κB, p38 MAPK, and Krüppel-like factors (KLFs) have all been implicated in antiphospholipid antibody-mediated activation of endothelial cells (17–19), demonstrating how antiphospholipid antibodies may co-opt pathways normally associated with more “authentic” activating stimuli.
Mac-1 is a heterodimeric beta-2 integrin especially expressed by myeloid-lineage cells. In its activated state, Mac-1 mediates cell-cell interactions by engaging a variety of surface molecules, including the endothelium-expressed glycoprotein ICAM-1. In this study, we focused not on the endothelium, but rather leukocytes (and especially neutrophils), and asked what they bring to the heterotypic adhesive interactions relevant to APS. We studied both fresh APS blood and control leukocytes conditioned with either APS plasma or APS IgG. We characterized leukocyte adhesion to resting endothelial cells under flow. We also considered key adhesion molecules, including Mac-1, on the surface of APS neutrophils and explored their role in not just adhesion, but also NETosis.
METHODS
Human subjects.
Patients were recruited from rheumatology and hematology clinics at the University of Michigan (Supplementary Tables 1-3). All 43 patients with APS fulfilled the clinical and laboratory criteria for APS established by the Sydney classification criteria (1). None of the patients met American College of Rheumatology (ACR) criteria for SLE (20). Of the patients with APS, some were classified as having “obstetric APS” if they had no prior history of vascular thrombosis, but did have APS-associated obstetric complications as defined by the Sydney criteria (≥3 unexplained, consecutive, spontaneous pregnancy losses; or ≥1 unexplained fetal deaths ≥10 weeks of gestation; or ≥1 preterm deliveries of a morphologically normal infant before 34 weeks of gestation due to severe preeclampsia, eclampsia, or features consistent with placental insufficiency) (1). Eleven patients with history of unprovoked venous thrombosis, but negative testing for antiphospholipid antibodies, were also recruited (Supplementary Table 4); many of these patients had genetic risk factors for venous thrombosis such as Factor V Leiden heterozygosity as detailed in Supplementary Table 4. Thirty-eight healthy volunteers were recruited through a posted flyer; exclusion criteria included history of a systemic autoimmune disease, active infection, and pregnancy. All 38 controls were screened for IgG anti-β2GPI and found to be negative. Blood was collected by phlebotomist venipuncture, and serum was prepared by standard methods and stored at −80°C until ready for use. IgG, IgM, and IgA anti-β2GPI, as well as IgG and IgM anticardiolipin, were determined by multiplex assay on a BioPlex 2200 System (BioRad). Lupus anticoagulant (LAC) was tested according to published guidelines (21). This study was reviewed and approved by the University of Michigan Institutional Review Board. Written informed consent was received from all participants prior to inclusion.
Preparation of human IgG.
IgG was purified from human serum with Protein G Agarose according to the manufacturer’s instructions (Pierce). Briefly, serum was diluted in IgG binding buffer and passed through a Protein G Agarose column at least five times. Elution of IgG was performed with 0.1 M glycine. The solution was neutralized with 1 M Tris, followed by overnight dialysis against PBS at 4°C. After passing through a 0.2-micron filter, IgG purity was verified by SDS-PAGE. IgG was quantified by BCA protein assay (Pierce). IgG preparations were free of endotoxin contamination as determined by a chromogenic endotoxin quantification kit (Pierce).
Human neutrophil purification.
For neutrophil preparation, blood was collected into sodium citrate tubes by standard phlebotomy techniques. The anticoagulated blood was then fractionated by density-gradient centrifugation using Ficoll-Paque Plus (GE Healthcare). Neutrophils were further purified by dextran sedimentation of the red blood cell (RBC) layer before lysing residual RBCs with 0.2% sodium chloride. Neutrophil preparations were at least 98% pure as confirmed by both flow cytometry and nuclear morphology.
In vitro flow adhesion assays.
For all flow chamber experiments, blood was collected into citrate tubes. A parallel-plate flow chamber (PPFC) with straight gaskets forming the flow channel (GlycoTech, Gaithersburg, MD) was then used for in vitro flow adhesion assays. Briefly, a single straight gasket was placed over a HUVEC monolayer cultured on a glass coverslip (22) and vacuum-sealed to the flow deck to form the bottom adhesion substrate of the chamber. For some experiments, “leukocytes” were prepared by mixing together the buffy coat and RBCs (after discarding plasma). In other cases, “neutrophils” were prepared by retrieving the neutrophil-RBC pellet that remained after Ficoll gradient separation. For these leukocyte and neutrophil experiments, cells were always brought back to their original blood volume with “flow buffer” (PBS++ with 1% BSA). 2 mL of whole blood, leukocytes, or neutrophils were introduced into the chamber from an inlet reservoir via a programmable syringe pump (KD Scientific, Holliston, MA). For “low shear” experiments, samples were perfused across the HUVEC monolayer using a laminar flow profile. The wall shear rate (WSR, γw) was fixed by adjusting the volumetric flow rate (Q) through the channel according to the equation , where h is the channel height (127 μm) and w is the channel width (0.25 cm). The h of 127 μm and WSR of 200 s−1 were chosen to approximate the flow profile within veins and venules. Low-shear samples were perfused over HUVECs for 5 minutes. For “high shear” experiments, pulsatile flow was used in the horizontal PPFC as previously described (22). Specifically, samples were perfused over HUVEC monolayers in pulsatile flow at a WSR of 1000 s−1 for 15 minutes (23–25). The flow time was chosen to ensure the same volume of blood passed through the chamber as for laminar/low-shear experiments (22). At the end of the prescribed flow time, flow buffer was added to the PPFC to flush out nonadherent cells. Ten images per sample were collected along the length of the flow chamber using a Nikon TE-2000-S inverted microscope with a digital camera (Photometrics CoolSNAP EZ with a Sony CCD sensor). Results were imaged and analyzed via NIS-Elements® analysis software and ImageJ. The adherent cells were normalized to the controls run on the same day so as to minimize variation attributable to different batches of HUVECs. For experiments involving the pretreatment, or “conditioning,” of control leukocytes, the buffy coat/RBC sample was incubated at 37°C for 1 hour with plasma, before washing again with flow buffer. For blocking experiments, anti-Mac-1 (20 μg/mL, clone CBRM1/5) antibody or isotype control were also included during the incubation.
Flow cytometry studies.
For all flow cytometry experiments, blood was collected into citrate tubes and immediately taken for further processing. Fc blocking of cells (in whole blood) was carried out using Human TruStain FcX (BioLegend), according to the manufacturer’s instructions. Subsequently, cells (still in whole blood) were stained with specific antibodies for 30 minutes on ice, followed by immediate lysis of RBCs and fixation of leukocytes using eBioscience 1-step Fix/Lyse Solution. Samples were analyzed on a LSRFortessa Cell Analyzer (BD Biosciences) and ZE5 Cell Analyzer (Bio-Rad). Further data were analyzed with FlowJo software (Tree Star). Specific primary antibodies were against: apolipoprotein H (ABS162, EMD Millipore), CD15 (W6D3, BioLegend), CD16 (3G8, BioLegend), CEACAM1 (283340, R&D systems), CD64 (10.1, BioLegend), activated LFA-1 (m24, BioLegend), activated Mac-1 (CBRM1/5, BioLegend), and CD62L (DREG-56, BioLegend). Also used were eBioscience™ Fixable Viability Dye eFluor™ 506, and secondary antibody Alexa Fluor® 680 AffiniPure Donkey Anti-Rabbit IgG (H+L) (711–625-152, Jackson ImmunoResearch). For leukocyte conditioning experiments, the sample was either spiked with increasing concentrations of APS or control IgG (10 μg/mL or 100 μg/mL) or the citrated plasma of the sample was discarded and replaced with heterologous control or APS plasma, and incubated for 1 hour at 37°C before staining and flow analysis.
TLR4 and complement inhibition.
Anticoagulated control blood was preincubated with 20 μM TLR4 inhibitor (TAK-242, Cayman Chemical) or 10 μM C5a receptor antagonist (W-54011, Cayman Chemical) for 30 minutes. The sample was then spiked with IgG as above and incubated for 1 hour at 37°C.
Quantification of NETosis.
Neutrophils were labeled with CytoTrace™ Red CMTPX (5 μM, AAT Bioquest) according to the manufacturer’s instructions and resuspended in RPMI media (Gibco) supplemented with 0.5% BSA and 0.5% fetal bovine serum (Gibco). Neutrophils (1.5 × 105/well) were then incubated in 48-well plates with a pre-established monolayer of HUVECs at 37°C. Samples were additionally treated with 100 μg/mL APS IgG or control IgG, in the presence of anti-Mac-1 (20 μg/mL, clone CBRM1/5) or isotype control. After three hours, SYTOX Green (Thermo Fisher Scientific) was added to a final concentration of 0.2 μM and incubated for an additional 10 minutes. Fluorescence was quantified at excitation and emission wavelengths of 485 nm and 520 nm, respectively, using a BioTek Cytation 5 Cell Imaging Multi-Mode Reader (BioTek). Representative images were captured by the BioTek Cytation 5 reader’s 20x objective.
Statistical Analysis.
Data analysis was with GraphPad Prism software version 7. Normally distributed data were analyzed by t testing, while skewed data were assessed by Mann–Whitney test. ANOVA with appropriate correction for multiple comparisons was also used where appropriate. For each panel of data, the specific statistical test is indicated in the figure legend. Statistical significance was defined as p <0.05.
RESULTS
APS neutrophils demonstrate increased adhesion under flow.
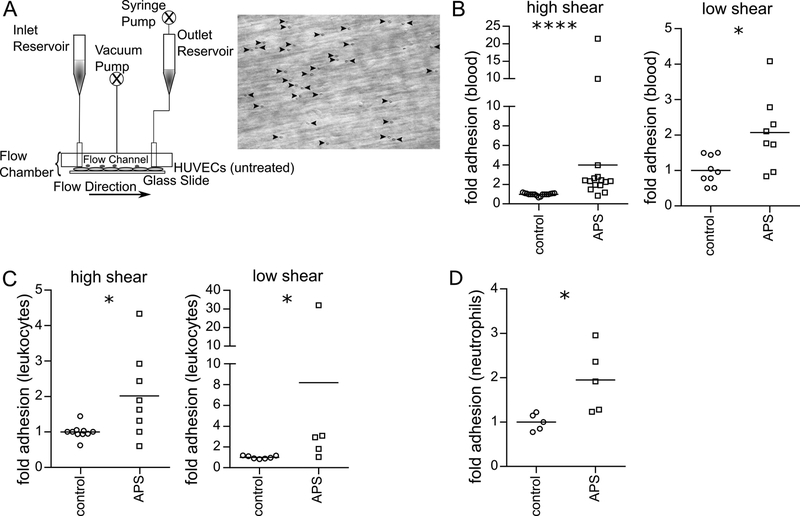
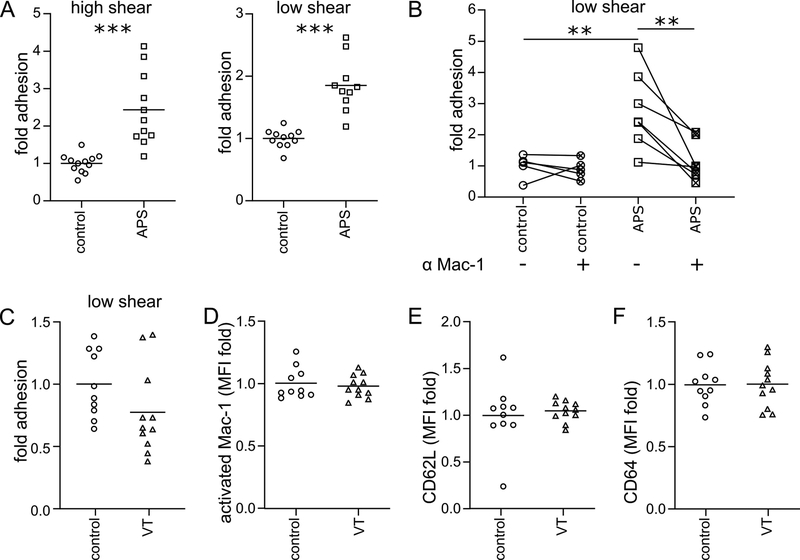
Utilizing anticoagulated whole blood collected from patients with primary APS or matched healthy volunteers (Supplemental Tables 1-3), we tested leukocyte adhesion to unactivated/resting early-passage human umbilical vein endothelial cells (HUVECs) in a parallel-plate flow chamber (PPFC) assay. Representative images of leukocyte adhesion in the PPFC assay are shown in Figure 1A. As compared with control blood, we observed increased adhesion of APS leukocytes under high-shear (1000 s−1) pulsatile flow conditions, as might be found in arteries or the arterioles (Figure 1B). Similar results were observed when blood was passed through the chamber under lower-shear (200 s−1) laminar flow, as would be found in the venous system (Figure 1B). If the increased adhesion were being driven by factors inherent to the leukocytes themselves, we reasoned that a similar phenotype would be observed if plasma (along with the cytokines and autoantibodies that might activate the HUVECs) were discarded. Indeed, isolated APS leukocytes, in the absence of plasma, still adhered in exaggerated fashion to HUVECs (under both high- and low-shear flow conditions) (Figure 1C). Finally, we removed not just plasma, but also peripheral blood mononuclear cells by spinning the blood through a density gradient. Again, we observed increased adhesion of neutrophils to HUVECs as compared with controls (Figure 1D). In summary, these data reveal that leukocytes, and specifically neutrophils, demonstrate increased adhesion to unstimulated HUVECs in the context of various flow profiles. The phenotype persisted even after plasma was discarded, consistent with an inherent role for neutrophils in the adhesive interaction.
Figure 1: APS neutrophils demonstrate increased adhesion.
Adhesion was measured under either pulsatile, high-shear (1000 s−1) conditions or laminar, low-shear (200 s−1) conditions. A, Schematic of the parallel-plate flow chamber, and a representative image from the adhesion assay. B, Anticoagulated whole blood from healthy controls or patients with APS were perfused through the flow chamber. At the end of the run, adherent cells were quantified; ****p<0.0001 (Mann Whitney test) and *p<0.05 (t test). C, Control or APS leukocytes were isolated, resuspended in flow buffer (plasma discarded), and perfused through the flow chamber; *p<0.05 (Mann Whitney test). D, Control or APS neutrophils were isolated, resuspended in flow buffer, and perfused through the flow chamber; *p<0.05 (t test).
Adhesion molecules are upregulated on the surface of APS neutrophils.
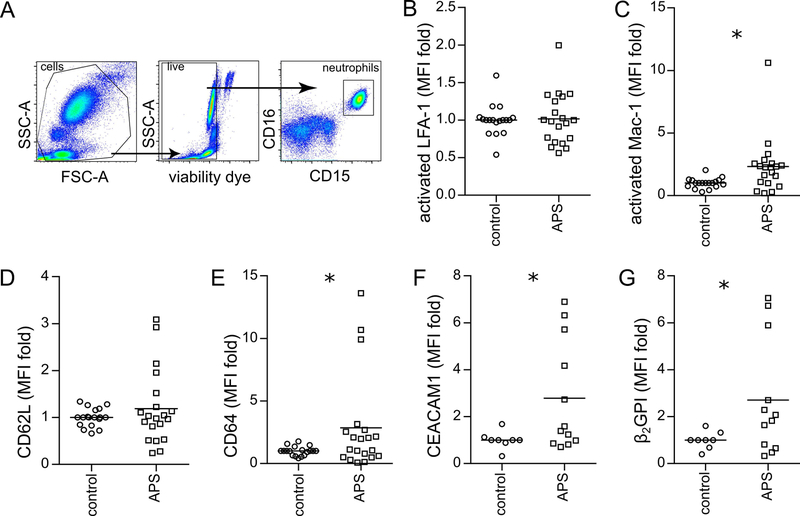
In an effort to understand what seemed to be an inherent increase in APS neutrophil adhesion, we evaluated the surface expression of various adhesion molecules on the neutrophil surface (Figure 2A). As ICAM-1 is known to be expressed even by resting HUVECs, we reasoned that beta-2 integrin family members (which are well known to interact with ICAM-1) might be upregulated on APS neutrophils, thus mediating the increased adhesion. While we observed no difference in the activated form of beta-2 integrin LFA-1 (Figure 2B), the activated form of another beta-2 integrin, Mac-1, was robustly upregulated on the surface of APS neutrophils (Figure 2C). An evaluation of other potential markers of neutrophil activation revealed no significant difference in CD62L (L-selectin), but did reveal upregulation of both CD64 and CEACAM1 (Figure 2D–F). Interestingly, autoantigen β2GPI was also present at increased levels on the surface of APS neutrophils (Figure 2G). In summary, these data demonstrate increased expression of activated Mac-1, but not activated LFA-1, on the neutrophil surface, which correlates with the upregulation of other neutrophil activation markers such as CD64 and CEACAM1.
Figure 2: Increased expression of activated Mac-1 and other adhesion molecules on APS neutrophils.
Flow cytometry was performed after treating anticoagulated whole blood with fluorescently-labeled antibodies. Mean fluorescence intensity (MFI) was normalized to controls run in the same batch. A, Schematic of gating strategy for identification of neutrophils in whole blood. B, Activated LFA-1 (not significant by t test). C, Activated Mac-1 (*p<0.05 by t test). D, CD62L (not significant by t test). E, CD64 (*p<0.05 by t test). F, CEACAM1 (*p<0.05 by t test). G, Beta-2 glycoprotein I (*p<0.05 by t test).
APS IgG-mediated upregulation of Mac-1 on neutrophils is dependent on TLR4 and complement anaphylatoxin receptors.
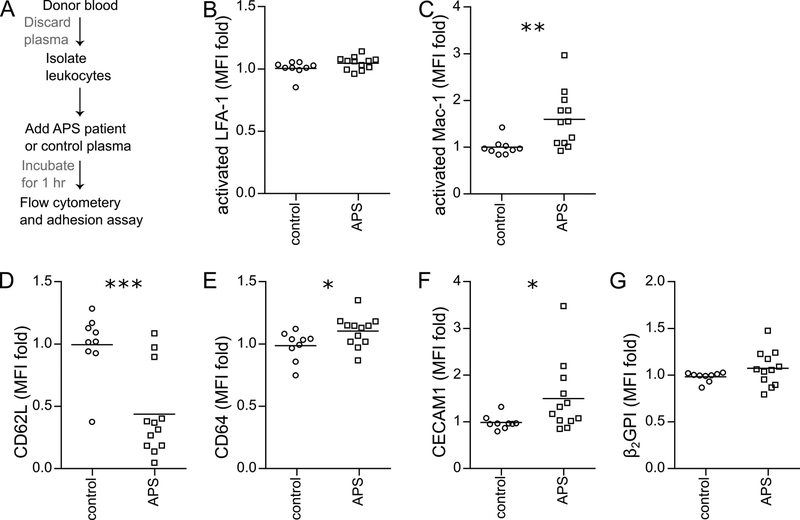
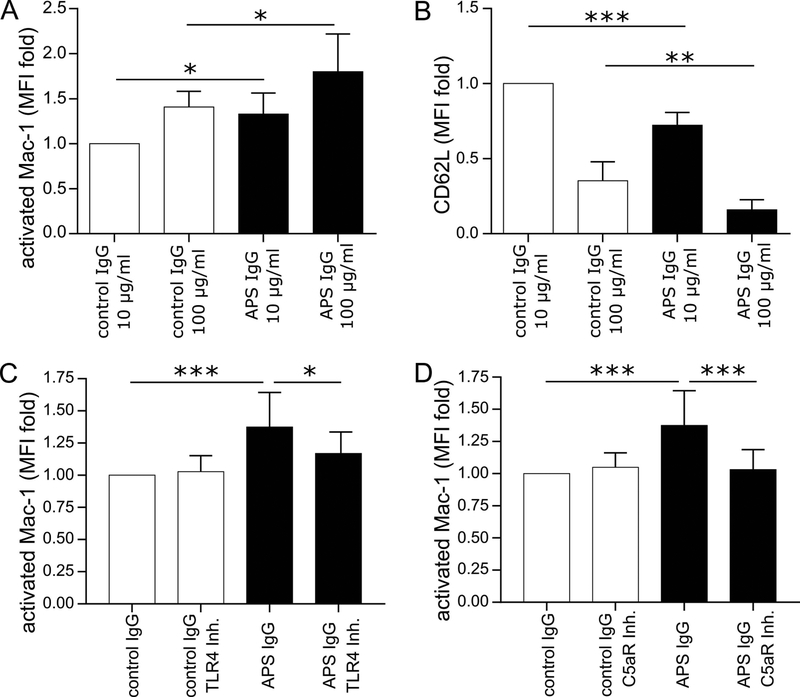
Previous work by our group has demonstrated that NETosis can be triggered from control neutrophils by incubation with either APS serum or APS IgG. Here, we explored whether adhesion molecules were also upregulated by similar treatment (Figure 3A). When we “conditioned” control blood cells with APS plasma, we did not find increased expression of activated LFA-1 on the surface of neutrophils (Figure 3B). In contrast, there was a striking increase in surface expression of activated Mac-1 (Figure 3C). We also found evidence of shedding of CD62L from neutrophils and upregulation of both CD64 and CEACAM1 (Figure 3C–E). β2GPI was measured, but was not significantly upregulated (Figure 3F). We then turned our attention to conditioning control blood with IgG purified from patients with primary APS. Under these conditions, we observed upregulation of activated Mac-1 on neutrophils (Figure 4A), along with shedding of CD62L (Figure 4B). Having previously observed that APS IgG-mediated NETosis is dependent upon Toll-like receptor 4 (TLR4), we assessed that same pathway here—now in the context of Mac-1 activation. Indeed, the TLR4-signaling inhibitor TAK-242 prevented APS IgG from upregulating activated Mac-1 on neutrophils (Figure 4C). We reasoned that we might also find a role for the complement cascade in neutrophil activation. When blood was treated with a C5a receptor-inhibitory antibody, upregulation of activated Mac-1 on neutrophils was blunted (Figure 4D). In summary, these data together indicate that control neutrophils upregulate activated Mac-1 in response to conditioning with either APS plasma or APS IgG, and that this upregulation requires TLR4 and the C5a receptor.
Figure 3: Increased expression of activated Mac-1 and other adhesion molecules when control neutrophils are conditioned with APS plasma.
Control leukocytes were conditioned with heterologous control plasma or APS plasma (A), and then incubated with fluorescently-labeled antibodies. Mean fluorescence intensity (MFI) was normalized to controls run in the same batch. B, Activated LFA-1 (not significant by t test). C, Activated Mac-1 (**p<0.01 by t test). D, CD62L (***p<0.001 by t test). E, CD64 (*p<0.05 by t test). F, CEACAM1 (*p<0.05 by Mann Whitney test). G, Beta-2 glycoprotein I (not significant by t test).
Figure 4: Exposure to purified APS IgG increases the expression of activated Mac-1 on control neutrophils in TLR4- and complement-dependent fashion.
Control leukocytes were treated with control or APS IgG as indicated. Activated Mac-1 and CD62L were quantified by flow cytometry. A, Activated Mac-1 (*p<0.05 by one-way ANOVA with correction for multiple comparisons by Holm-Sidak method; n=4 independent experiments). B, Shedding of CD62L (**p<0.01 and ****p<0.0001 by one-way ANOVA with correction for multiple comparisons by Holm-Sidak method; n=4 independent experiments). C, Control leukocytes were treated with control or APS IgG (100 μg/mL) in the presence or absence of TLR4 inhibitor. Activated Mac-1 was quantified by flow cytometry (*p<0.05 and ***p<0.001 by one-way ANOVA with correction for multiple comparisons by Holm-Sidak method; n=8 independent experiments). D, Control leukocytes were treated with control or APS IgG (100 μg/mL) in the presence or absence of C5a receptor (C5aR) inhibitor. Activated Mac-1 was quantified by flow cytometry (***p<0.001 by one-way ANOVA with correction for multiple comparisons by Holm-Sidak method; n=7 independent experiments).
Activated Mac-1 is required for increased adhesion of APS neutrophils.
Having found that APS plasma upregulates Mac-1 on the surface of control neutrophils, we reasoned that this upregulation might be directly responsible for increased neutrophil adhesion. Indeed, APS plasma-treated cells, but not control plasma-treated cells, demonstrated increased adhesion under both high-shear and low-shear flow conditions (Figure 5A). Furthermore, a monoclonal antibody specific for the activated form of Mac-1 effectively neutralized adhesion in the context of conditioning with APS plasma, but had no effect in the setting of control plasma (Figure 5B). To determine whether the ability of plasma to stimulate cell adhesion was unique to patients with APS or whether the phenotype might extend to any patient with history of thrombosis, we recruited 11 patients with history of unprovoked venous thrombosis but negative testing for antiphospholipid antibodies (Supplementary Table 4). As compared with healthy control plasma, plasma from the venous thrombosis (VT) cohort triggered no increase in cell adhesion (Figure 5C). Along similar lines, conditioning neutrophils with VT-cohort plasma did not alter levels of activated Mac-1 (Figure 5D), CD62L (Figure 5E), or CD64 (Figure 5F) on the neutrophil surface. Finally, we asked whether the increased cell adhesion triggered by APS plasma might be limited to patients with a history of “thrombotic APS” (i.e., at least one documented arterial, venous, or small-vessel thrombotic event). Interestingly, we observed increased adhesion whether the plasma was collected from patients with “thrombotic APS” or patients with purely “obstetric APS” (Supplemental Figure 1). In summary, these data demonstrate that antagonizing the activated form of Mac-1 is sufficient to reduce APS-relevant adhesion to levels seen in controls.
Figure 5: Increased adhesion of APS leukocytes is mediated by activated Mac-1.
A, Control leukocytes were incubated with heterologous control or APS plasma, resuspended in flow buffer, and perfused through the flow chamber. Adherent cells were quantified (***p<0.001 by t test). B, Conditions were similar to panel A, except with the addition of a blocking antibody for activated Mac-1 to some samples (**p<0.01 by one-way ANOVA with correction for multiple comparisons by Holm-Sidak method). C, Similar to panel A, control leukocytes were incubated with heterologous control plasma or plasma from patients with history of unprovoked venous thrombosis (VT) but negative antiphospholipid testing. The leukocytes were then resuspended in flow buffer and perfused through the flow chamber. Adherent cells were quantified (not significant by t test). D-F, Similar to Figure 3, control leukocytes were conditioned with heterologous control plasma or VT plasma, and then incubated with fluorescently-labeled antibodies. Mean fluorescence intensity (MFI) was normalized to controls run in the same batch. D, Activated Mac-1 (not significant by t test). E, CD62L (not significant by t test). F, CD64 (not significant by t test).
Activated Mac-1 is required for NETosis by APS neutrophils bound to endothelial cells.
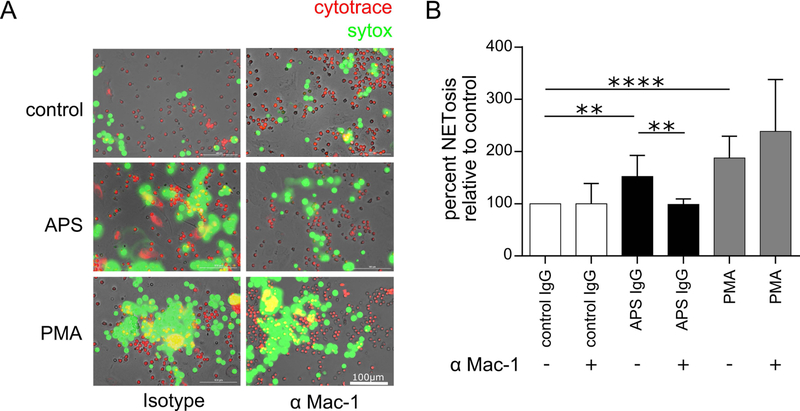
Given evidence by our group and others that NETosis proceeds most efficiently upon cell adhesion, we asked whether the aforementioned antibody targeting activated Mac-1 might mitigate NETosis. To test this, we adhered vital-dye-labeled neutrophils to resting HUVECs, and then tracked NETosis in real time via the loss of vital dye and the local release of decondensed DNA (Figure 6A). As compared with isotype treatment, the Mac-1 monoclonal antibody significantly neutralized NETosis in response to APS IgG, but not the phorbol ester PMA (Figure 6B). In summary, these data demonstrate that inhibition of the activated form of Mac-1 can neutralize NETosis, at least in the context of APS.
Figure 6: Activated Mac-1 regulates APS IgG-mediated NETosis.
Control neutrophils were plated over a monolayer of HUVECs in the presence of either activated-Mac-1 blocking antibody or isotype control. Cultures were then stimulated with control IgG (100 μg/mL), APS IgG (100 μg/mL), or phorbol myristate acetate (PMA, positive control). After 3 hours, SYTOX Green was added to the culture, and fluorescence intensity was quantified. A, In these representative images, live cells are labeled by CytoTrace™ Red and extracellular DNA (NETs) by SYTOX Green; scale bar=100 μm. B, Quantification of NETosis by fluorescence intensity of SYTOX Green (**p<0.01 and ****p<0.0001 by one-way ANOVA with correction for multiple comparisons by Holm-Sidak method; n=4 independent experiments).
DISCUSSION
While there are many studies characterizing the activated endothelium in APS (11, 15), comparatively little is known about the adhesive nature of circulating cells (26). In this study we examined the adhesive potential of APS leukocytes, and, in particular, neutrophils. We found enhanced adhesion of APS neutrophils to resting HUVECs irrespective of the flow conditions (Figure 1B–C). Notably, this functional increase in adhesion was observed in the context of upregulated adhesion molecules on the neutrophil surface, including CD64, CEACAM1, and the activated form of Mac-1 (Figure 2). These findings did not extend to patients with a history of unprovoked venous thrombosis but negative testing for antiphospholipid antibodies (Figure 5C–F), suggesting that they may be relatively unique feature of APS. Of note, all flow experiments were performed in the presence of RBCs, which are known to stabilize leukocyte adhesion, more closely modeling conditions observed in vivo (27).
In addition to thrombosis of arteries, veins, and small vessels, another hallmark of APS is pregnancy-related morbidity. On the one hand, there is evidence that obstetric complications of APS have distinct pathophysiology as compared with thrombotic APS (28); however, on the other hand, recent data have suggested that up to 60% of patients who begin with a diagnosis of “obstetric APS” will eventually develop a thrombotic event (29). While the cohort tested here was relatively enriched for patients with thrombotic complications, we identified and tested eight patients with a history of only obstetric morbidity (see definition in Methods). Interestingly, these patients with obstetric APS were indistinguishable from patients with a history of thrombotic events, in terms of neutrophil adhesion (Supplemental Figure 1). Further work in disease models will hopefully allow us to understand the extent to which activated Mac-1 may be a direct mediator of thrombotic (versus obstetric) pathophysiology.
In our previous work, we found that inhibition of TLR4 signaling could mitigate APS IgG-mediated NETosis (5). This is in addition to the work of others demonstrating that TLR4 deficiency protects mice form APS in vivo, and that neutrophil TLR4 supports phagocytosis and reactive oxygen species production by APS neutrophils (10, 30). We now show that the TLR4 inhibitor TAK-242 prevents APS IgG from upregulating activated Mac-1 on neutrophils (Figure 4C). These data would seem to support further investigation of TLR4 signaling as a potential therapeutic target in APS. Similar to the TLR4 pathway, complement contributes to neutrophil activation in many contexts. Here we show that inhibition of C5a receptor attenuates the upregulation of activated Mac-1 on APS IgG-stimulated neutrophils (Figure 4D). These data are in line with previous studies demonstrating that C5a receptor contributes to upregulation of Mac-1 (31, 32).
Despite extensive CD62L shedding in control neutrophils conditioned with APS plasma (Figure 3D), CD62L shedding was not detected at a significant level in neutrophils freshly isolated from patients with APS (Figure 2D). One possibility is that the patient neutrophils have had time to upregulate CD62L expression, thereby effectively compensating for shedding. In support of this idea, CD62L was upregulated at the gene level in our recent transcriptomic profiling of APS neutrophils (9). Alternatively, it is possible that neutrophils that have shed CD62L in vivo are strongly activated to the point that they have preferentially left circulation, thereby being unavailable for our analysis. Interestingly, we also detected increased surface expression of the APS autoantigen β2GPI on the surface of APS neutrophils by flow cytometry (Figure 2G), which is in line with our recent study demonstrating a 4.5-fold increase in β2GPI gene expression in APS neutrophils (9). It should be noted that at least one group has appreciated similar β2GPI surface and expression phenotypes in circulating APS monocytes (33, 34).
Blocking experiments demonstrate that the adhesion of APS neutrophils is at least partially mediated by activated Mac-1 (Figure 5B). Interestingly, we also found that APS IgG-mediated NETosis was Mac-1 dependent (Figure 6). This latter finding is reminiscent of work by Neeli and colleagues, who found Mac-1 to be required for both hypercitrullination of histones and NETosis in response to lipopolysaccharide (LPS) stimulation (35). Interestingly, both LPS and APS IgG activate neutrophils via TLR4 (5). Collectively, the data presented here reveal a previously unknown role for activated Mac-1 in the adhesion and NETosis of APS neutrophils.
In the general population, numerous reports have suggested a link between Mac-1, neutrophils, and endothelium in thrombotic vascular diseases (36–38). For example, significant upregulation of neutrophil Mac-1 has been detected at the time of myocardial infarction and for up to one week after the event (39). In another study, neutrophils from myocardial infarction patients displayed enhanced adhesion to endothelial cells ex vivo, which could be reduced by blocking Mac-1 (40). In patients with acute ischemic stroke, there was significant upregulation of neutrophil Mac-1 immediately after the event, and persisting into the subacute phase of the stroke (41). In patients with venous thromboembolism, increased adhesive potential of neutrophils was associated with a higher rate of recurrence (42). As indicated in Supplemental Table 1, the average time from last thrombotic event to blood collection for patients with APS included in this study was some 4.5 years. One might hypothesize that upregulation of activated Mac-1 is detected only acutely (i.e., at the time of thrombosis) for persons in the general population, but continues to smolder in patients with APS, potentially consistent with the life-long anticoagulation that such patients require. To further explore this question, it will be necessary to build longitudinal APS cohorts and study them alongside cohorts from the general population.
Beyond activated Mac-1, we also observed consistent upregulation of CD64 on the surface of APS neutrophils (Figure 2E). This is somewhat reminiscent of work in patients with sickle cell disease. Sickle cell neutrophils demonstrate increased levels of CD64 and increased adhesion to endothelial cells, with some evidence that CD64 directly contributes to the adhesion (43, 44). Future studies should ask whether this surface molecule, typically thought of as an IgG receptor, might also play a role in APS neutrophil adhesion. CEACAM1 (CD66a) expression was also consistently upregulated on APS neutrophils (Figure 2F). Interestingly, there are studies to suggest that signaling through CEACAM1 (and potentially other CEACAM family members) results in activation of Mac-1 and increased adhesion to endothelial cells (45–48). At the same time, recent reports (predominantly in mice) have suggested that CEACAM1 may have inhibitory functions, protecting against neutrophil hyperactivity and neutrophil-mediated tissue damage. For example, CEACAM1 protects against ischemic stroke by inhibiting MMP9 (49, 50). CEACAM1-deficient mice also form larger carotid thrombi in a ferric chloride injury model, suggesting that CEACAM1 may inhibit arterial thrombus (51). Thus, this very interesting molecule seems to warrant further study in APS.
In conclusion, our study has uncovered a novel role for activated Mac-1 in regulating APS neutrophils and NETosis, and hints at a role for Mac-1 in APS pathophysiology. While Mac-1 can be considered as a therapeutic target in APS, mutations in CD11b are a well-recognized risk factor for lupus (52), and many, but not all, mouse studies have suggested that CD11b deficiency has the potential to exacerbate autoimmunity (52–55). Having said that, since Mac-1 binds to a variety of ligands, selective inhibition of specific Mac-1 adhesive interactions could emerge as a potential therapeutic strategy. For example, one proof-of-concept study has demonstrated that targeted inhibition of the Mac-1-CD40L interaction improved bacterial clearance and survival in a polymicrobial model of sepsis (56). Another innovative approach has involved the utilization of small-molecule Mac-1 agonists. These agonists tend to induce an intermediate-affinity conformation in Mac-1 (57), which may permit neutrophil adhesion, with less potential for endothelial damage. Indeed, a partial Mac-1 agonist not only protected MRL/lpr mice from end-organ injury, but also enhanced endothelium-dependent vasorelaxation and thereby demonstrated an overall vasoprotective effect (58). Taken together, these studies indicate that targeting Mac-1 might indeed be feasible, and emphasize the need for future research in patients with APS.
Supplementary Material
ACKNOWLEDGEMENTS
The work was supported in part by NIH R01HL134846 to JSK and NIH R01HL115138 to OE-A. JSK was also supported by career development awards from the NIH (K08AR066569), Burroughs Wellcome Fund, and the Rheumatology Research Foundation. GS was supported by the Postdoctoral Translational Scholars Program fellowship award 2UL1TR000433 from the National Center for Advancing Translational Sciences of the National Institutes of Health. WJK was supported by the National Science Foundation Graduate Research Fellowship Program.
Footnotes
AUTHORSHIP AND CONFLICT OF INTEREST DISCLOSURES
The authors have no competing interests or conflicts to disclose. GS, WJK, KG, SY, APV and ALB conducted experiments and analyzed data. GS, WJK, PLB, OE-A, and JSK designed the study. All authors participated in writing the manuscript, and gave approval before submission.
Conflict of interest: None of the authors has any financial conflict of interest to disclose.
REFERENCES
- 1.Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4(2):295–306. [DOI] [PubMed] [Google Scholar]
- 2.Garcia D, Erkan D. Diagnosis and Management of the Antiphospholipid Syndrome. N Engl J Med. 2018;378(21):2010–21. [DOI] [PubMed] [Google Scholar]
- 3.Abreu MM, Danowski A, Wahl DG, Amigo MC, Tektonidou M, Pacheco MS, et al. The relevance of “non-criteria” clinical manifestations of antiphospholipid syndrome: 14th International Congress on Antiphospholipid Antibodies Technical Task Force Report on Antiphospholipid Syndrome Clinical Features. Autoimmun Rev. 2015;14(5):401–14. [DOI] [PubMed] [Google Scholar]
- 4.Rao AN, Kazzaz NM, Knight JS. Do neutrophil extracellular traps contribute to the heightened risk of thrombosis in inflammatory diseases? World J Cardiol. 2015;7(12):829–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yalavarthi S, Gould TJ, Rao AN, Mazza LF, Morris AE, Nunez-Alvarez C, et al. Release of neutrophil extracellular traps by neutrophils stimulated with antiphospholipid antibodies: a newly identified mechanism of thrombosis in the antiphospholipid syndrome. Arthritis Rheumatol. 2015;67(11):2990–3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leffler J, Stojanovich L, Shoenfeld Y, Bogdanovic G, Hesselstrand R, Blom AM. Degradation of neutrophil extracellular traps is decreased in patients with antiphospholipid syndrome. Clin Exp Rheumatol. 2014;32(1):66–70. [PubMed] [Google Scholar]
- 7.Meng H, Yalavarthi S, Kanthi Y, Mazza LF, Elfline MA, Luke CE, et al. In Vivo Role of Neutrophil Extracellular Traps in Antiphospholipid Antibody-Mediated Venous Thrombosis. Arthritis Rheumatol. 2017;69(3):655–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ali RA, Gandhi AA, Meng H, Yalavarthi S, Vreede AP, Estes SK, et al. Adenosine receptor agonism protects against NETosis and thrombosis in antiphospholipid syndrome. Nat Commun. 2019;10(1):1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knight JS, Meng H, Coit P, Yalavarthi S, Sule G, Gandhi AA, et al. Activated signature of antiphospholipid syndrome neutrophils reveals potential therapeutic target. JCI Insight. 2017;2(18). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pierangeli SS, Vega-Ostertag ME, Raschi E, Liu X, Romay-Penabad Z, De Micheli V, et al. Toll-like receptor and antiphospholipid mediated thrombosis: in vivo studies. Ann Rheum Dis. 2007;66(10):1327–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pierangeli SS, Colden-Stanfield M, Liu X, Barker JH, Anderson GL, Harris EN. Antiphospholipid antibodies from antiphospholipid syndrome patients activate endothelial cells in vitro and in vivo. Circulation. 1999;99(15):1997–2002. [DOI] [PubMed] [Google Scholar]
- 12.Pierangeli SS, Espinola RG, Liu X, Harris EN. Thrombogenic effects of antiphospholipid antibodies are mediated by intercellular cell adhesion molecule-1, vascular cell adhesion molecule-1, and P-selectin. Circulation research. 2001;88(2):245–50. [DOI] [PubMed] [Google Scholar]
- 13.Espinola RG, Liu X, Colden-Stanfield M, Hall J, Harris EN, Pierangeli SS. E-Selectin mediates pathogenic effects of antiphospholipid antibodies. Journal of thrombosis and haemostasis : JTH. 2003;1(4):843–8. [DOI] [PubMed] [Google Scholar]
- 14.Ramesh S, Morrell CN, Tarango C, Thomas GD, Yuhanna IS, Girardi G, et al. Antiphospholipid antibodies promote leukocyte-endothelial cell adhesion and thrombosis in mice by antagonizing eNOS via beta2GPI and apoER2. J Clin Invest. 2011;121(1):120–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simantov R, LaSala JM, Lo SK, Gharavi AE, Sammaritano LR, Salmon JE, et al. Activation of cultured vascular endothelial cells by antiphospholipid antibodies. J Clin Invest. 1995;96(5):2211–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Del Papa N, Guidali L, Sala A, Buccellati C, Khamashta MA, Ichikawa K, et al. Endothelial cells as target for antiphospholipid antibodies. Human polyclonal and monoclonal anti-beta 2-glycoprotein I antibodies react in vitro with endothelial cells through adherent beta 2-glycoprotein I and induce endothelial activation. Arthritis and rheumatism. 1997;40(3):551–61. [DOI] [PubMed] [Google Scholar]
- 17.Dunoyer-Geindre S, de Moerloose P, Galve-de Rochemonteix B, Reber G, Kruithof EKO. NF kappa B is an essential intermediate in the activation of endothelial cells by anti-beta(2)-glycoprotein 1 antibodies. Thrombosis and haemostasis. 2002;88(5):851–7. [PubMed] [Google Scholar]
- 18.Vega-Ostertag M, Casper K, Swerlick R, Ferrara D, Harris EN, Pierangeli SS. Involvement of p38 MAPK in the up-regulation of tissue factor on endothelial cells by antiphospholipid antibodies. Arthritis and rheumatism. 2005;52(5):1545–54. [DOI] [PubMed] [Google Scholar]
- 19.Allen KL, Hamik A, Jain MK, McCrae KR. Endothelial cell activation by antiphospholipid antibodies is modulated by Kruppel-like transcription factors. Blood. 2011;117(23):6383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis and rheumatism. 1997;40(9):1725. [DOI] [PubMed] [Google Scholar]
- 21.Pengo V, Tripodi A, Reber G, Rand JH, Ortel TL, Galli M, et al. Update of the guidelines for lupus anticoagulant detection. Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. Journal of thrombosis and haemostasis : JTH. 2009;7(10):1737–40. [DOI] [PubMed] [Google Scholar]
- 22.Charoenphol P, Huang RB, Eniola-Adefeso O. Potential role of size and hemodynamics in the efficacy of vascular-targeted spherical drug carriers. Biomaterials. 2010;31(6):1392–402. [DOI] [PubMed] [Google Scholar]
- 23.Seki J Flow pulsation and network structure in mesenteric microvasculature of rats. Am J Physiol. 1994;266(2 Pt 2):H811–21. [DOI] [PubMed] [Google Scholar]
- 24.Papaioannou TG, Stefanadis C. Vascular wall shear stress: basic principles and methods. Hellenic J Cardiol. 2005;46(1):9–15. [PubMed] [Google Scholar]
- 25.Mahler F, Muheim MH, Intaglietta M, Bollinger A, Anliker M. Blood pressure fluctuations in human nailfold capillaries. Am J Physiol. 1979;236(6):H888–93. [DOI] [PubMed] [Google Scholar]
- 26.Corban MT, Duarte-Garcia A, McBane RD, Matteson EL, Lerman LO, Lerman A. Antiphospholipid Syndrome: Role of Vascular Endothelial Cells and Implications for Risk Stratification and Targeted Therapeutics. J Am Coll Cardiol. 2017;69(18):2317–30. [DOI] [PubMed] [Google Scholar]
- 27.Abbitt KB, Nash GB. Characteristics of leucocyte adhesion directly observed in flowing whole blood in vitro. Br J Haematol. 2001;112(1):55–63. [DOI] [PubMed] [Google Scholar]
- 28.Meroni PL, Borghi MO, Grossi C, Chighizola CB, Durigutto P, Tedesco F. Obstetric and vascular antiphospholipid syndrome: same antibodies but different diseases? Nat Rev Rheumatol. 2018;14(7):433–40. [DOI] [PubMed] [Google Scholar]
- 29.de Jesus GR, Sciascia S, Andrade D, Barbhaiya M, Tektonidou M, Banzato A, et al. Factors associated with first thrombosis in patients presenting with obstetric antiphospholipid syndrome (APS) in the APS Alliance for Clinical Trials and International Networking Clinical Database and Repository: a retrospective study. BJOG. 2019;126(5):656–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gladigau G, Haselmayer P, Scharrer I, Munder M, Prinz N, Lackner K, et al. A role for Toll-like receptor mediated signals in neutrophils in the pathogenesis of the anti-phospholipid syndrome. PLoS One. 2012;7(7):e42176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyabe Y, Miyabe C, Murooka TT, Kim EY, Newton GA, Kim ND, et al. Complement C5a receptor is the key initiator of neutrophil adhesion igniting immune complex-induced arthritis. Sci Immunol. 2017;2(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bekker P, Dairaghi D, Seitz L, Leleti M, Wang Y, Ertl L, et al. Characterization of Pharmacologic and Pharmacokinetic Properties of CCX168, a Potent and Selective Orally Administered Complement 5a Receptor Inhibitor, Based on Preclinical Evaluation and Randomized Phase 1 Clinical Study. Plos One. 2016;11(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caronti B, Calderaro C, Alessandri C, Conti F, Tinghino R, Palladini G, et al. Beta2-glycoprotein I (beta2-GPI) mRNA is expressed by several cell types involved in anti-phospholipid syndrome-related tissue damage. Clin Exp Immunol. 1999;115(1):214–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Conti F, Sorice M, Circella A, Alessandri C, Pittoni V, Caronti B, et al. Beta-2-glycoprotein I expression on monocytes is increased in anti-phospholipid antibody syndrome and correlates with tissue factor expression. Clin Exp Immunol. 2003;132(3):509–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neeli I, Dwivedi N, Khan S, Radic M. Regulation of extracellular chromatin release from neutrophils. J Innate Immun. 2009;1(3):194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Libby P, Lichtman AH, Hansson GK. Immune effector mechanisms implicated in atherosclerosis: from mice to humans. Immunity. 2013;38(6):1092–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doring Y, Drechsler M, Soehnlein O, Weber C. Neutrophils in atherosclerosis: from mice to man. Arterioscler Thromb Vasc Biol. 2015;35(2):288–95. [DOI] [PubMed] [Google Scholar]
- 38.Jickling GC, Liu D, Ander BP, Stamova B, Zhan X, Sharp FR. Targeting neutrophils in ischemic stroke: translational insights from experimental studies. J Cereb Blood Flow Metab. 2015;35(6):888–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meisel SR, Shapiro H, Radnay J, Neuman Y, Khaskia AR, Gruener N, et al. Increased expression of neutrophil and monocyte adhesion molecules LFA-1 and Mac-1 and their ligand ICAM-1 and VLA-4 throughout the acute phase of myocardial infarction: possible implications for leukocyte aggregation and microvascular plugging. J Am Coll Cardiol. 1998;31(1):120–5. [DOI] [PubMed] [Google Scholar]
- 40.Han L, Shen X, Pan L, Lin S, Liu X, Deng Y, et al. Aminobenzoic acid hydrazide, a myeloperoxidase inhibitor, alters the adhesive properties of neutrophils isolated from acute myocardial infarction patients. Heart Vessels. 2012;27(5):468–74. [DOI] [PubMed] [Google Scholar]
- 41.Tsai NW, Chang WN, Shaw CF, Jan CR, Huang CR, Chen SD, et al. The value of leukocyte adhesion molecules in patients after ischemic stroke. J Neurol. 2009;256(8):1296–302. [DOI] [PubMed] [Google Scholar]
- 42.Zapponi KC, Mazetto BM, Bittar LF, Barnabe A, Santiago-Bassora FD, De Paula EV, et al. Increased adhesive properties of neutrophils and inflammatory markers in venous thromboembolism patients with residual vein occlusion and high D-dimer levels. Thromb Res. 2014;133(5):736–42. [DOI] [PubMed] [Google Scholar]
- 43.Fadlon E, Vordermeier S, Pearson TC, Mire-Sluis AR, Dumonde DC, Phillips J, et al. Blood polymorphonuclear leukocytes from the majority of sickle cell patients in the crisis phase of the disease show enhanced adhesion to vascular endothelium and increased expression of CD64. Blood. 1998;91(1):266–74. [PubMed] [Google Scholar]
- 44.Lard LR, Mul FP, de Haas M, Roos D, Duits AJ. Neutrophil activation in sickle cell disease. J Leukoc Biol. 1999;66(3):411–5. [DOI] [PubMed] [Google Scholar]
- 45.Stocks SC, Ruchaud-Sparagano MH, Kerr MA, Grunert F, Haslett C, Dransfield I. CD66: role in the regulation of neutrophil effector function. Eur J Immunol. 1996;26(12):2924–32. [DOI] [PubMed] [Google Scholar]
- 46.Skubitz KM, Campbell KD, Skubitz AP. CD66a, CD66b, CD66c, and CD66d each independently stimulate neutrophils. J Leukoc Biol. 1996;60(1):106–17. [DOI] [PubMed] [Google Scholar]
- 47.Skubitz KM, Skubitz AP. Two new synthetic peptides from the N-domain of CEACAM1 (CD66a) stimulate neutrophil adhesion to endothelial cells. Biopolymers. 2011;96(1):25–31. [DOI] [PubMed] [Google Scholar]
- 48.Skubitz KM, Skubitz AP. Interdependency of CEACAM-1, −3, −6, and −8 induced human neutrophil adhesion to endothelial cells. J Transl Med. 2008;6:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ludewig P, Sedlacik J, Gelderblom M, Bernreuther C, Korkusuz Y, Wagener C, et al. Carcinoembryonic antigen-related cell adhesion molecule 1 inhibits MMP-9-mediated blood-brain-barrier breakdown in a mouse model for ischemic stroke. Circ Res. 2013;113(8):1013–22. [DOI] [PubMed] [Google Scholar]
- 50.Sobey CG, Drummond GR. CEACAM1: an adhesion molecule that limits blood-brain barrier damage by neutrophils after stroke. Circ Res. 2013;113(8):952–3. [DOI] [PubMed] [Google Scholar]
- 51.Wong C, Liu Y, Yip J, Chand R, Wee JL, Oates L, et al. CEACAM1 negatively regulates platelet-collagen interactions and thrombus growth in vitro and in vivo. Blood. 2009;113(8):1818–28. [DOI] [PubMed] [Google Scholar]
- 52.Khan SQ, Khan I, Gupta V. CD11b Activity Modulates Pathogenesis of Lupus Nephritis. Front Med (Lausanne). 2018;5:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosetti F, Mayadas TN. The many faces of Mac-1 in autoimmune disease. Immunol Rev. 2016;269(1):175–93. [DOI] [PubMed] [Google Scholar]
- 54.Tang T, Rosenkranz A, Assmann KJ, Goodman MJ, Gutierrez-Ramos JC, Carroll MC, et al. A role for Mac-1 (CDIIb/CD18) in immune complex-stimulated neutrophil function in vivo: Mac-1 deficiency abrogates sustained Fcgamma receptor-dependent neutrophil adhesion and complement-dependent proteinuria in acute glomerulonephritis. J Exp Med. 1997;186(11):1853–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kevil CG, Hicks MJ, He X, Zhang J, Ballantyne CM, Raman C, et al. Loss of LFA-1, but not Mac-1, protects MRL/MpJ-Fas(lpr) mice from autoimmune disease. Am J Pathol. 2004;165(2):609–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wolf D, Anto-Michel N, Blankenbach H, Wiedemann A, Buscher K, Hohmann JD, et al. A ligand-specific blockade of the integrin Mac-1 selectively targets pathologic inflammation while maintaining protective host-defense. Nat Commun. 2018;9(1):525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maiguel D, Faridi MH, Wei C, Kuwano Y, Balla KM, Hernandez D, et al. Small molecule-mediated activation of the integrin CD11b/CD18 reduces inflammatory disease. Sci Signal. 2011;4(189):ra57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Faridi MH, Khan SQ, Zhao W, Lee HW, Altintas MM, Zhang K, et al. CD11b activation suppresses TLR-dependent inflammation and autoimmunity in systemic lupus erythematosus. J Clin Invest. 2017;127(4):1271–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.