Abstract
Objective
Dental restorative composites have been extensively studied with a goal to improve material performance. However, stress induced microcracks from polymerization shrinkage, thermal and other stresses along with the low fracture toughness of methacrylate-based composites remain significant problems. Herein, the study focuses on applying a dynamic covalent chemistry (DCC)-based adaptive interface to conventional BisGMA/TEGDMA (70:30) dental resins by coupling moieties capable of thiol-thioester (TTE) DCC to the resin-filler interface as a means to induce interfacial stress relaxation and promote interfacial healing.
Methods
Silica nanoparticles (SNP) are functionalized with TTE-functionalized silanes to covalently bond the interface to the network while simultaneously facilitating relaxation of the filler-matrix interface via DCC. The functionalized particles were incorporated into the otherwise static conventional BisGMA/TEGDMA (70:30) dental resins. The role of interfacial bond exchange to enhance dental composite performance in response to shrinkage and other stresses, flexural modulus and toughness was investigated. Shrinkage stress was monitored with a tensometer coupled with FTIR spectroscopy. Flexural modulus/strength and flexural toughness were characterized in three-point bending on a universal testing machine.
Results
A reduction of 30% in shrinkage stress was achieved when interfacial TTE bond exchange was activated while not only maintaining but also enhancing mechanical properties of the composite. These enhancements include a 60% increase in Young’s modulus, 33% increase in flexural strength and 35% increase in the toughness, relative to composites unable to undergo DCC but otherwise identical in composition. Furthermore, by combining interfacial DCC with resin-based DCC, an 80% reduction of shrinkage-induced stress is observed in a thiol-ene system “equipped” with both types of DCC mechanisms relative to the composite without DCC in either the resin or at the resin–filler interface.
Significance
This behavior highlights the advantages of utilizing the DCC at the resin-filler interface as a stress-relieving mechanism that is compatible with current and future developments in the field of dental restorative materials, nearly independent of the type of resin improvements and types that will be used, as it can dramatically enhance their mechanical performance by reducing both polymerization and mechanically applied stresses throughout the composite lifetime.
Keywords: Adaptive interface, Interfacial stress relaxation, Thiol-thioester exchange, Dynamic covalent chemistries, Composites
1. Introduction
Conventional dental restorative composites are mechanically stiff, highly-crosslinked networks formulated from inorganic fillers dispersed in photocured dimethacrylate resins.1-3 Despite significant advances in composite development, premature restoration failure remains problematic. Though there are other factors such as secondary decay and hydrolytic degradation that influence the composite performance and lifetime; the often premature failure of these materials is at least in part attributed to the mechanical failure of the composite in response to stresses that arise both during and after placement.1,3-5 Typically, inorganic fillers are used in dental composite to enhance the composite’s mechanical performance including strength, wear resistance, thermal expansion coefficient and polymerization-induced volumetric shrinkage. However, high filler loadings in dental composites significantly affect their viscosity and hinder photopolymerization kinetics due to light scattering. Additionally, the inorganic filler is also a source for large stress gradients due to the modulus and thermal expansion mismatch between the filler and the resin matrix.1,3,4 This stress, along with polymerization shrinkage stress and mechanically applied stress, are believed by many to cause serious clinical issues such as microcracking at the restoration-tooth interface and microleakage, and reduce the restoration’s lifetime.1,2,4,6-8 Therefore, the successful relaxation of any and all stresses that arise or are applied to the restoration is critical but it is anticipated that stress relaxation at the particle-resin interface would be of particular importance in improving the composite performance. In one approach, several ideas have been directed towards reducing shrinkage stress of dental restoratives while maintaining all other desirable material properties while other researchers have examined the effect of the type, size distribution, loading content and surface modification of the filler on composites properties.4,7,8 Efforts have been directed to facilitate improvements in dental composites by reducing the reactive group concentration,9-11, modifying dimethacrylate monomer formulations,12,13 and exploring alternative polymerization techniques, such as thiol-ene reaction,2,14 ring-opening polymerizations,5 copper(I)-catalyzed azide-alkyne cycloadditions (CuAAC).3,15
Recently, covalent adaptable networks (CANs) such as those based on reversible addition fragmentation chain transfer (RAFT) have been successfully implemented in dense networks and as resin phase in dental composites to eliminate stresses that arise from polymerization shrinkage and external loading, by enabling dynamic bond exchange in the polymer backbone while conserving the overall covalently bonded structure (Fig. 1A). However, while RAFT is simultaneously compatible with the current light induced radical-mediated methacrylate polymerization methodologies that are clinically practiced, this approach often results in a modification of material properties such as Young’s modulus, Tg, and fracture toughness.16,17 Therefore, improving the performance of conventional dental resins by alleviating the interfacial stresses through DCC approaches that target the particle-resin interface without deteriorating the mechanical properties of the polymerized material remains an unachieved goal.
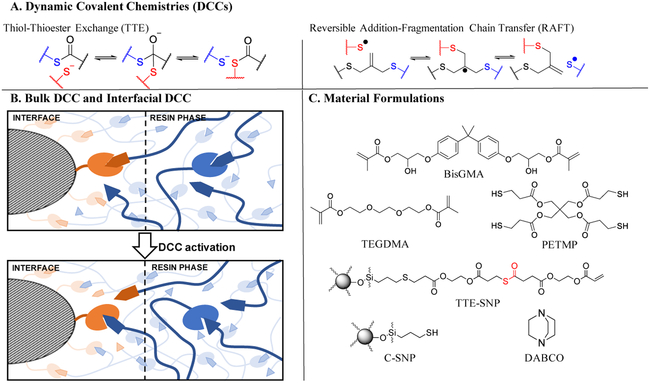
Fig. 1-.
A) Mechanisms for thiol-thioester exchange (TTE) and Reversible addition-fragmentation chain transfer (RAFT) as examples for dynamic covalent chemistries (DCCs). B) Illustration of TTE bond exchange in the resin phase and at the filler interface enables stress relaxation. As stress accumulates, TTE bond rearrangement occur between a free thiol and thioester linkage. This bond exchange enables stress relaxation without a sacrifice of crosslinking density or filler attachment to the network. C) Monomers and fillers used for this study. Resins were formulated of BisGMA/TEGDMA (70:30) mixture with 15 wt% PETMP, 1 wt% DABCO and 15 wt% of SNP, either TTE or the corresponding control. Polymerization was initiated with 1 wt% of I819 (bis(2,4,6-trimethylbenzoyl)-phenylphosphineoxide) visible light photoinitiator, and photocured with 400–500 nm visible light at 50 mW cm−2 for 5 min on each side and then postcured in an oven at 60 °C for 4 h.
To this end, a previous study has developed RAFT-containing adaptive interfaces that covalently bonded silica nanoparticles to a static thiol-ene resin and enabled bond exchange exclusively at the resin-filler interface.18 As the interfacial region between the organic resin and inorganic fillers is known to concentrate stresses and nucleate crack formation, isolating the bond exchange to occur exclusively at the interfacial region enables stress relaxation where the stress is concentrated and significantly improved the composite’s performance (Fig. 1B). Particularly, since implementation of a RAFT adaptive interface demonstrated its capability to reduce stress and improve material properties in an inert thiol-ene composite,18 here, we apply the adaptive interface concept to a conventional dental resin by introducing thiol-thioester (TTE) moieties that promote resin-filler bonding and persistent stress relaxation at the filler-resin interface, without altering the resin formulation. A fundamental difference between the RAFT and TTE DCC processes is that the RAFT-based approach is only active in facilitating bond exchange during the polymerization in the presence of the radicals that also cause polymerization. The TTE process is base or nucleophile catalyzed and as such, the bond exchange process in these composites is hypothesized to persist long after the initial polymerization is complete. As such, it is expected that interfacial stresses that arise after the polymerization, e.g., due to thermal expansion mismatch, would also be capable of relaxing. Further, the TTE approach does not require a thiyl radical to catalyze the exchange reaction and is thus compatible with multimethacrylate resin polymerizations.
The thiol-thioester exchange reaction (TTE) has been introduced recently as a new class of CANs that undergo dynamic bond exchange and enable rapid stress relaxation at ambient conditions in the presence of free thiols, thioesters, and a base/nucleophile catalyst. This reaction involves the exchange of one thioester link for another, mediated by a thiolate anion that is generated from a base or nucleophile reacting with a thiol19 (Fig. 1A).
Here, silica nanoparticles functionalized with a TTE-containing silane are introduced into BisGDMA/TEGDMA (70:30) dental resins in the presence of a nucleophilic catalyst in order to induce the stress relaxation mechanism at the interface of the polymer and the filler. TTE-capable moieties undergo continuous bond cleavage and reformation reversibly, in the presence of a base/ nucleophile, leading to network relaxation and stress elimination while conserving the overall network connectivity. Material properties, including polymerization-induced shrinkage stress, flexural modulus, strength and toughness of TTE-based dental composites, are explored and compared with control composites that consist of a similar silane but without the DCC moieties.
Furthermore, as prior work has shown that combining an RAFT-interface with an RAFT-resin results in synergistic effects on mechanical properties such as strength and toughness, here, the two types of DCC approaches were combined, in the resin and at the resin-filler interface. RAFT to be radically triggered within the resin matrix during polymerization with TTE to be triggered exclusively at the interface between the silica particle and the matrix to further enhance composite performance. This approach enables the stress relaxation in both locations; within the composite resin and at the resin-filler interface and provides for long term stress relaxation throughout the entire life of the composite via long catalytic lifetime of base/nucleophile-initiated TTE reactions, while only activating the RAFT-based exchange when polymerization stresses are generated, i.e. during the light exposure. Testing on polymerization-induced shrinkage stress and post-polymerization stress relaxation is conducted and compared with control composites by eliminating the appropriate DCC functional groups or catalysts.
2. Materials and methods
2.1. Materials
2,2-Bis[4-(2-hydroxy-3-methacrylyloxypropoxy) phenyl] propane (Bis-GMA) and triethylene glycol dimethacrylate (TEGDMA) (Esstech, Essington, PA, USA) were purchased from Esstech (Essington, PA, USA) as a premixed monomer mixture in 70:30 mass ratio. Pentaerytritol tetrakis(3-mercaptopropionate) (PETMP), Triethyleneglycol-Divinylether (TEGDVE), 1,4-diazabicyclo[2.2.2]octane (DABCO), 3-chloro-2-chloromethyl-1-propene, potassium ethyl xanthogenate, ethylene diamine, and propylamine were purchased from Sigma-Aldrich. Irgacure 819 (bis(2,4,6-trimethylbenzoyl)- phenylphosphineoxide) was obtained from BASF. Schott glass (mean particle size 40 nm) untreated were generously donated by Evonik Silicas, and used as the inorganic fillers. Prior to implementation and as described later, these fillers were subsequently functionalized with thiol group for inclusion and copolymerization in the composite. All chemicals were used as received. The thioester-diacrylate20 and 2-methylene-propane-1,3-di (thioethyl vinyl ether) (MDTVE-AFT)21 were synthesized using methods reported elsewhere.
2.2. Filler Functionalization.
4 g of silica particles (Schott, OX50, 40 nm) were first taken in a glass tube and heated at 165 °C under vacuum using a Buchi heater/condenser for 3 h. The dried nanoparticles were then transferred to a 250 mL bottom rounded flask containing 200 mL of anhydrous toluene supplemented with 2 g of (3-Mercaptopropyl) trimethoxysilane prereacted for 10 min with 2 g of n-propylamine. The reaction mixture was then refluxed at 120 °C for 24 h. After sinalization of nanoparticle, the liquid suspension was centrifuged and the solid pellets collected thoroughly, and washed with toluene (3× ≈ 25 mL) and methylene chloride (3× ≈ 25 mL) in two separate washing/centrifugation cycles. The washed filler particles were dried under vacuum overnight at 70 °C. Then 2 g of the dried thiol functionalized fillers were reacted with 0.7 g thioester diacrylate in DCM in presence of 3 mL TEA base at R.T overnight. After sinalization of nanoparticle was washed with DCM (1× ≈ 25 mL), toluene (2× ≈ 25 mL) and DMSO (2× ≈ 25 mL), and dried under vacuum overnight at 70 °C. The functionalized particles were analyzed by DRIFT FT-IR spectroscopy and TGA. The mass loss difference between silanized and unfunctionalized fillers suggests successful functional group grafting on the surface of glass particles in each case. Also, the DRIFT FT-IR characterization provides evidence of silanol group disappearance around 3745 cm−1, and the appearance of the thioester group around 1700 cm−1 implying successful surface modification.
2.3. Fourier Transform Infrared Spectroscopy
An FT-IR spectrometer (Nicolet 6700) connected to a tensometer via fiber optic cables was used to monitor the real-time polymerization kinetics in concert with stress measurements. Samples were placed between two cylindrical quartz rods, and 300 mW cm−2 light was irradiated from the bottom rod using a light guide connected to a mercury lamp (Acticure 4000, EXFO) with 400–500 nm bandgap filter. The overtone signal of double bonds between 6250–6096 cm −1 was monitored during the FT-IR measurements.
2.4. Polymerization Shrinkage Stress Measurement
Shrinkage stress was measured via a tensometer using cantilever beam deflection theory (American Dental Association Health Foundation, ADAHF–PRC). A composite paste (1 mm in thickness, 6 mm in diameter) was placed between two cylindrical quartz rods, which were previously treated with a methacrylate functional silane to promote bonding at the glass surface/resin interface. A 300 mW cm−2 of light was irradiated from the bottom rod using a light guide connected to a mercury lamp (Acticure 4000, EXFO) with a 400–500 nm bandgap filter. Polymerization-induced shrinkage of sample exerted a tensile force which caused the deflection of the aluminum beam. A linear variable differential transformer was used to convert the displacement to shrinkage stress based upon beam calibration constant and cross-sectional area of the sample. For the simultaneous measurement of conversion with shrinkage stress, data were collected continuously for 10 min (n=3).
2.5. Thermogravimetric Analysis
TGA (Pyris 1, PerkinElmer) was used to analyze the functionalized silica nanoparticles. Each sample was run in a nitrogen atmosphere (20 mL min−1) from 50 to 850 °C at a heating rate of 10 °C min−1.
2.6. Three-point flexural test
Three-point bend (MTS 858 Mini Bionix II) with a strain rate of 0.1 mm min−1 and a span of 20 mm was used to obtain properties of the composites (n=5). Samples sandwiched between two glass slides and polymerization was initiated with 1 wt% of 1819, and photocured with 400–500 nm visible light at 50 mW cm−2 for 5 min on each side to ascertain uniform conversions throughout the sample thickness and then postcured in an oven at 60 °C for 4 h. Flexural tests were performed 24 hours after initial cure. Composite sample dimensions were 2/2.5/10 mm (n=5). Functional group conversion was recorded via FTIR spectra prior to and after the polymerization.
3. Results and discussion
Silica nanoparticles (SNP) were synthesized from thiol-functionalized nanoparticles treated with diacrylate thioesters, that through DCC mechanisms promote resin-filler bonding and interfacial stress relaxation as illustrated in Fig. 1B. The synthesis and functionalization processes are explained further in the Experimental Section. For use as a control, SNPs were functionalized with a thiol containing silane (3-mercaptopropyl trimethoxysilane) also capable of bonding to the resin but not capable of TTE- bond exchange by following previously published methods.2 The functionalized fillers were analyzed by diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) and thermogravimetric analysis (TGA) which provided additional evidence of successful functional group attachment to the SNP surface. In the following experiments, 15 wt% SNP were dispersed into BisGMA/TEGDMA (70:30) dental resins containing 15 wt% PETMP to serve as a free thiol and 1 wt% DABCO nucleophilic catalyst, as illustrated in Fig. 1C. Polymerization was initiated with 1 wt% of I819 (bis(2,4,6-trimethylbenzoyl)-phenylphosphineoxide) visible light photoinitiator, and photocured with 400–500 nm visible light then postcured in an oven at 60 °C for 4 h, which leads to the formation of a glassy polymer network (Tg as measured in DMA ≈ 110 °C). Thermo-mechanical properties of both TTE- and control BisGMA/TEGDMA composites, such as Tg and storage modulus at 40 °C, are reported in Table 1. The TTE- and control BisGMA/TEGDMA composites showed similar Tg values, suggesting that the functional group conversion within each composites system is nearly equivalent, validating that this sample is an appropriate control.
Table 1.
Comparison of TTE- and control composite glass transition temperature (Tg), storage modulus at 40 °C, flexural modulus, flexural strength, flexural toughness from the three-point bend testing.
| Tg (°C) | Storage Modulus at 40 °C |
Modulus (GPa) |
Flexural strength (MPa) |
Flexural toughness (MJ/m3) |
|
|---|---|---|---|---|---|
| Control | 110 ± 3 | 2.4 ± 0.4 | 2.5 ± 0.1 | 90 ± 3 | 2.0 ± 0.2 |
| TE | 107 ± 1 | 2.3 ± 0.2 | 4.0 ± 0.3 | 120 ± 8 | 2.6 ± 0.3 |
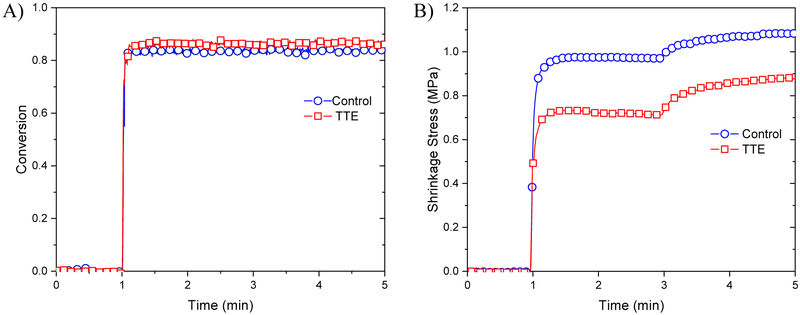
An FTIR spectrometer connected to a tensometer via fiber optic cables was utilized to investigate the effect of interfacial TTE bond exchange on bulk shrinkage stress, which is known to be one of the primary causes of failure for dental materials. Internal stress is well known to develop during the polymerization reaction due to postgelation volumetric contraction and elastic modulus evolution, which usually leads to premature failure through initiation of microcracks and interfacial debonding. The incorporation of TTE-functionalized nanoparticles within the BisGMA/TEGDMA resin resulted in a material with more than 30 % lower shrinkage stress as compared with the thioester-free nanoparticles used in the control experiment at equivalent 80 % conversion (Fig. 2A & B), while preserving the ability to relax interfacial stress for an extended period following polymerization. This outcome is due to the persistent presence of thiolate species, which enable exchange, and thus post-polymerization relaxation.
Fig. 2-.
A) Polymerization kinetics as measured by FT-IR as a function of the disappearance of C=C functional group at 6165 cm−1. B) In situ polymerization shrinkage stress for control composites (blue circle) and TTE- composites (red square) conversion using a tensometer coupled with the FTIR. All samples were placed between two quartz rods, previously treated with a methacrylate-functional silane and irradiated for 3 min at ambient temperature with 300 mW/cm2 of 400–500 nm light.
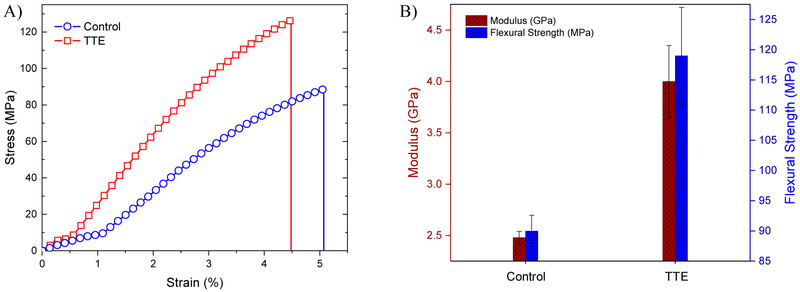
As an indication of the benefits of the continuous post polymerization stress relaxation and to investigate its effects on composite failure, mechanical properties including flexural modulus, flexural strength, and flexural toughness of both TTE- and control BisGMA/TEGDMA resins/ composites were analyzed via three-point bend tests performed on a universal testing machine (MTS) as presented in Fig. 3 and Table 1. Fig. 3A displays a representative stress–strain curve of the TTE composites in comparison with the control composite. The flexural modulus (or Young’s modulus) calculated from the initial slope of the stress–strain curve dramatically increased from approximately 2.5 GPa to 4 GPa, when TTE bond exchange was activated at the interface, leading to 33% improvement in the flexural strength value (120 ± 8 MPa) as compared with the control composites (90 ± 3 MPa), Fig. 3B and Table 1. Due to the increased elastic modulus of the TTE- composites, the composites exhibited even higher flexural toughness (2.6 ± 0.3 MJm−3), as compared with the control composites (2.0 ± 0.2 MJm−3) at equivalent strain. This improvement in flexural toughness is related to the reduction in the shrinkage stress as well as the post polymerization stress relaxation that will continue to occur over the lifetime of the composite because of the persistent catalyst, which enables stress relaxation and healing at the interface, improving the mechanical properties of the dental restorative material.
Fig. 3-.
A) Stress-strain profiles from 3-point bend test for both control composites (blue circle) and dynamic TTE-enabled interface (red square) at a displacement rate of 0.1 mm/min. B) Modulus (red columns) and flexural strength obtained from 3-point bend test for both control and TTE composites. Sample dimensions: 2 mm thick bars, 20 mm span. Resins were formulated of BisGMA/TEGDMA (70:30) mixture with 15 wt% PETMP, 1 wt% DABCO and 15 wt% of SNP, either TTE or the corresponding control. Polymerization was initiated with 1 wt% of I819, and photocured with 400–500 nm visible light at 50 mW cm−2 for 5 min on each side and then postcured in an oven at 60 °C for 4 h.
Given that RAFT has been widely studied in bulk dental materials, and following this successful demonstration of the exceptional efficiency of interface-limited TTE exchange processes in composites, here, TTE exchange is utilized in tandem with RAFT. RAFT is radically triggered within the resin matrix during the polymerization process while the TTE is triggered exclusively at the interface between the silica particle and the matrix to provide long term stress relaxation throughout the life of the composite.
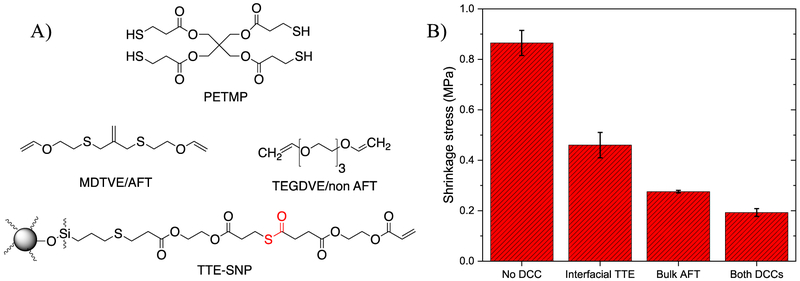
Specifically, the resin formulations presented in Fig. 4A is utilized in this study. The resin formulation is based on thiol-ene networks comprised of a divinyl AFT (2-methylene-propane-1,3-di(thioethyl vinyl ether) (MDTVE)) or non-AFT (Triethyleneglycol-Divinylether (TEGDVE)) and PETMP (added in 0.15 mol excess), with TTE based fillers that also are capable of relaxing stress in the presence of the catalyst (DABCO). Control experiments in which no stress relaxation is enabled through elimination of the RAFT functional groups or the TTE catalyst are also included.
Fig. 4-.
A) Monomers and fillers used in the formulation of the composites to examine the influence of combining RAFT resin with TTE at the SNP-polymer interface. B) Final polymerization shrinkage stress taken after 5 min reaction time at equivalent 100% conversion, as a function of the double bond conversion via tensometer in thiol-ene composites: No DCC: non-AFT resin/thioester SNPs, no catalyst; Interfacial TTE: non-AFT resin/thioester SNPs, with DABCO added; Resin based AFT only: AFT resin/thioester SNPs, no catalyst; Both DCCs: AFT resin/thioester SNP, DABCO added. Each composition contained 10 wt.% SNPs, 1 wt.% IR819, and was irradiated with 50 mW/cm2 light intensity of 400-500 nm. Two mixtures contained DABCO (1 wt.%).
To investigate the interaction of interfacial and bulk dynamic chemistries, polymerization-induced shrinkage stress was measured for each of the four formulated composites, with and without RAFT in the resin and with and without thioester exchange at the interface (Fig. 4B). As Fig. 4B shows, the composite that does not contain any exchangeable bonds exhibits the highest degree of shrinkage stress, 0.8 MPa, while around 90% shrinkage stress reduction is observed in the formulation “equipped” with both types of DCC mechanisms are activated, both throughout the network and at the particle interface. By decoupling the bond exchange to be exclusively effective at the particle interface or throughout the resin; 40% and 75% reductions in shrinkage stress were achieved, respectively. This behavior indicates a synergistic effect to dramatically reduce shrinkage stress when both interfacial and bulk dynamic chemistries are simultaneously active. As such, it is expected to lead to noticeable improvement in the mechanical properties and fracture resistance when utilized in dental restoratives.
4. Conclusion
In summary, the TTE reaction has been implemented for the first time at the resin-filler interface in conventional dental composites and was demonstrated to enable interfacial stress relaxation of these critical, highly stressed regions. This relaxation mechanism persists due to the catalytic mechanism, even in the absence of any DCC in the resin phase, which leads to significant improvement in composite performance including 30% reduction in polymerization stress, 60% improvement in flexural modulus, and 40% improvement in the flexural strength.
This approach represents a fundamental shift in dental composites by relaxing interfacial stresses while improving mechanical properties, which would be of significant clinical value for extending the lifetime of dental restorations.
Acknowledgments
The authors acknowledge financial support from the National Institutes of Health (NIH 1U01DE023777).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References.
- 1.Schneider LFJ, Cavalcante LM, Silikas N. Shrinkage Stresses Generated during Resin-Composite Applications: A Review. J Dent Biomech. 2010; 1(1):131630–131630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Podgórski M, Becka E, Claudino M, Shah PK, Stansbury JW, Bowman CN. Ester-free thiol – ene dental restoratives — Part A : Dent Mater. 2015;31(11):1255–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moszner N, Salz U. Recent Developments of New Components for Dental Adhesives and Composites. Macromol Mater Eng 2007,. 2007;292:245–271. [Google Scholar]
- 4.Cramer NB, Stansbury JW, Bowman CN. Recent Advances and Developments in Composite Dental Restorative Materials. J Dent Res. 2011;90(4):402–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ms S, Auj Y, Physicomechanical SA. Physicomechanical evaluation of low-shrinkage dental nanocomposites based on silsesquioxane cores. 2007:230–238. [DOI] [PubMed] [Google Scholar]
- 6.Drummond JL. Degradation, fatigue and failure of resin dental composite materials materials. J Dent Res. 2009;87(8):710–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah PK, Stansbury JW. Role of filler and functional group conversion in the evolution of properties in polymeric dental restoratives. Dent Mater. 2014;30(5):586–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonalves F, Azevedo CLN, Ferracane JL, Braga RR. BisGMA/TEGDMA ratio and filler content effects on shrinkage stress. Dent Mater. 2011;27(6):520–526. [DOI] [PubMed] [Google Scholar]
- 9.Liu J, Howard GD, Lewis SH, Barros MD, Stansbury JW. A study of shrinkage stress reduction and mechanical properties of nanogel-modified resin systems. Eur Polym J. 2012;48(11):1819–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moraes RR, Garcia JW, Barros MD, et al. Control of polymerization shrinkage and stress in nanogel-modified monomer and composite materials. Dent Mater. 2011;27(6):509–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carioscia JA, Lu H, Stanbury JW, Bowman CN. Thiol-ene oligomers as dental restorative materials. 2005:1137–1143. [DOI] [PubMed] [Google Scholar]
- 12.Klee JE, Schneider C, Ho D, Burgath A, Frey H, Mu R. Hyperbranched Polyesters and their Application in Dental Composites : Monomers for Low Shrinking Composites. 2001;354(June 2000):346–354. [Google Scholar]
- 13.Chung C, Kim J, Kim M, Kim K, Kim K. Development of a new photocurable composite resin with reduced curing shrinkage. 2002;18:174–178. [DOI] [PubMed] [Google Scholar]
- 14.Park HY, Kloxin CJ, Abuelyaman AS, Oxman JD, Bowman CN. Stress Relaxation via Addition–Fragmentation Chain Transfer in High T g , High Conversion Methacrylate-Based Systems. Macromolecules. 45(14):5640–5646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song HB, Sowan N, Shah PK, et al. Reduced shrinkage stress via photo-initiated copper(I)-catalyzed cycloaddition polymerizations of azide-alkyne resins. Dental Materials. June 25, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park HY, Kloxin CJ, Abuelyaman AS, Oxman JD, Bowman CN. Stress Relaxation via Addition–Fragmentation Chain Transfer in High T g , High Conversion Methacrylate-Based Systems. Macromolecules. 45(14):5640–5646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park HY, Kloxin CJ, Fordney MF, Bowman CN. Stress reduction and T g enhancement in ternary Thiol-Yne-methacrylate systems via addition-fragmentation chain transfer. Macromolecules. 2012;45(14):5647–5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sowan N, Cox LM, Shah PK, Song HB, Stansbury JW, Bowman CN. Dynamic Covalent Chemistry at Interfaces: Development of Tougher, Healable Composites through Stress Relaxation at the Resin-Silica Nanoparticles Interface. Adv Mater Interfaces. 2018;1800511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Worrell BT, McBride MK, Lyon GB, et al. Bistable and photoswitchable states of matter. Nat Commun. 2018;9(1):2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Podgórski M, Worrell BT, Sinha J, Mcbride MK, Bowman CN. Thermal Metamorphosis in (Meth)acrylate Photopolymers: Stress Relaxation, Reshaping, and Second-Stage Reaction′. Macromolecules. 2019;52:8114–8123. [Google Scholar]
- 21.Kloxin CJ, Scott TF, Bowman CN. Stress Relaxation via Addition–Fragmentation Chain Transfer in a Thiol-ene Photopolymerization. Macromolecules. 2009;42(7):2551–2556. [DOI] [PMC free article] [PubMed] [Google Scholar]