Abstract
Corneal scarring is a major cause of blindness worldwide with few effective therapeutic options. Finding a treatment would be of tremendous public health benefit, but requires a thorough understanding of the complex interactions that underlie this phenomenon. Here, we tested the hypothesis that the large increase in expression of Semaphorin 3A (SEMA3A) in corneal wounds contributes to the development of stromal fibrosis. We first verified this increased expression in vivo, in a cat model of photorefractive keratectomy-induced corneal wounding. We then examined the impact of adding exogenous SEMA3A to cultured corneal fibroblasts, and assessed how this affected the ability of transforming growth factor-beta1 (TGF-β1) to induce their differentiation into myofibroblasts. Finally, we examined how siRNA knockdown of endogenous SEMA3A affected these same phenomena. We found exogenous SEMA3A to significantly potentiate TGF-β1’s profibrotic effects, with only a minimal contribution from cell-intrinsic SEMA3A. Our results suggest a previously unrecognized interaction between SEMA3A and TGF-β1 in the wounded cornea, and a possible contribution of SEMA3A to the regulation of tissue fibrosis and remodeling in this transparent organ.
Keywords: keratocytes, wound healing, stroma, fibroblasts, epithelium, scarring
Introduction
Corneal injury, infection and surgery lead to fibrosis and scarring, which when severe, is a primary causes of vision loss and blindness world-wide [1]. Yet clinically, there are few effective means of preventing fibrosis or treating established corneal scars [2]. This motivates ongoing scientific effort to unravel the complex cell and molecular factors that control fibrotic and scarring components of corneal wound healing. After corneal damage, stromal keratocytes are exposed to multiple growth factors and cytokines, including transforming growth factor (TGF)-β1, the strongest known pro-fibrotic agent [3, 4]. While the transparent keratocytes in and around the damaged zone undergo apoptosis, distant keratocytes transform into activated fibroblasts [5], migrating into the wound area, where exposure to TGF-β1 stimulates them to become myofibroblasts [6, 7]. Myofibroblasts - while helping to close the wound [8, 9] - are no longer transparent [10–12] and they produce and remodel the extracellular matrix (ECM) in ways that further decreases transparency [13], causing vision loss and blindness. TGF-β1 plays a key role in this process by regulating expression of multiple genes that cause myofibroblast differentiation, and control their actions [10, 14, 15]. However, the profibrotic actions of TGF-β1 can be potentiated by such molecules as platelet-derived growth factor [10, 16] and connective tissue growth factor [17–19][20].
Corneal wounding also causes levels of Semaphorin 3A (SEMA3A) to rise dramatically (10-fold) in the epithelium [21], helping to regulate epithelial healing [22]. In parallel, epidermal growth factor (EGF) released from wounded corneal epithelial cells up-regulates the expression of SEMA3A in stromal fibroblasts [23], but the role of this abundant wound healing molecule in the stroma is unknown. Here, we begin to fill this gap. The Semaphorin family [24] includes a large, diverse set of secreted, cell surface-attached, membrane-bound proteins [25]. SEMA3A is known to collapse the actin cytoskeleton and disassemble F-actin in multiple cell types [26, 27]. Given the cytoskeletal reorganization involved in the differentiation of myofibroblasts during wound healing, a logical question was whether SEMA3A contributes to this process. We first verified that SEMA3A expression is upregulated in corneal epithelial and stromal cells in our well-established, in vivo cat wound-healing model [17, 28–32]. We then assayed the expression of SEMA3A in cultured cat corneal fibroblasts before examining the effects of SEMA3A on TGF-β1-induced myofibroblast differentiation and ECM production. Finally, we knocked-down SEMA3A in cultured cat corneal fibroblasts with siRNA treatment to critically examine the residual ability of these cells to respond to TGF-β1.
Materials and methods
In vivo experiments
Animal procedures were conducted according to the NIH Guide for the Care and Use of Laboratory Animals, with protocols approved by the University of Rochester Committee on Animal Research (Assurance Number: A-3292–01). Cats were obtained from a research breeding colony managed by Liberty Research Inc. (Waverley, NY, USA). As recently described [33], photorefractive keratectomy (PRK) was performed on 6 eyes from 3 young (1–2 years old) adult, domestic, short-hair cats (felis cattus). Eyes obtained in this recent paper were studied presently at 4 weeks post-PRK, as this timepoint exhibited a large, fibrotic layer in the ablation zone; 5 additional eyes served as unoperated, normative controls.
Laser ablation:
PRK was performed by the same refractive surgeon (HH), with a commercial excimer laser (Technolas 217, Zyoptix 4.14, Bausch & Lomb Inc., Bridgewater, NJ, USA) in the center of each cat cornea, following manual debridement of the epithelium [33]. A 10D myopic ablation was performed over a 6mm optical zone, under topical (Proparacaine 0.5%, Falcon Pharmaceuticals Ltd., Fort Worth, TX, USA) and surgical anesthesia (5mg/kg Ketamine+0.04mg/kg Dexmedetomidine Hydrochloride). Immediately post-PRK, cat eyes were rinsed with balanced salt solution (BSS, Alcon Inc., Fort Worth, TX, USA) followed by a drop of antibiotics (Neomycin, Polymyxin B Sulphate, Gramicidin Ophthalmic Solution USP, Bausch & Lomb Inc., Bridgewater, NJ, USA) twice daily for 2 weeks.
Histology and immunohistochemistry:
after euthanasia, the corneas were excised and immersion fixed in 1% paraformaldehyde in 0.1M phosphate buffered saline (PBS), pH 7.4 for 10 minutes. They were cryoprotected in 30% sucrose in 0.1M PBS at 4°C for 2 days and serial-sectioned at 20μm using a cryostat (2800 Frigocut E, Leica Microsystems GmbH, Wetzlar, Germany), collected on gelatin-coated glass slides, and stored at −20°C until stained. Sections in the ablation center (or the geometric center of unoperated corneas) were selected for immunohistochemistry; they were co-incubated with a primary mouse monoclonal anti-α-SMA (1:100; Thermo Fisher Scientific, Waltham, MA, USA) and a feline-specific, rabbit polyclonal anti-SEMA3A antibody (1:100; YenZym Antibodies LLC., South San Francisco, CA, USA) overnight at 4°C. Some sections from each treatment group were also incubated overnight with PBS (Cellgro™, Manassas, VA, USA) containing 0.1% Triton X-100 (Sigma-Aldrich, St. Louis, MO, USA) as a negative control. Secondary antibodies (Alexa-Fluor-488 conjugated to goat anti-rabbit IgG and Alexa-Fluor-555 conjugated to goat anti-mouse IgG, both at 1:400; Invitrogen, Carlsbad, CA, USA) were applied at room temperature for 2 hours. Sections were cover-slipped with Vectashield Mounting Medium containing 4’,6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Inc., Burlingame, CA, USA).
In vitro experiments
Isolation and culture of primary cat corneal fibroblasts:
As previously described [29, 30], fresh eyeballs were obtained immediately post-mortem. The corneal epithelium and endothelium were scraped off, the stroma double-enzyme digested, then centrifuged at 3000rpm for 10 minutes, re-suspended in fibroblast growth factor (FGF)-containing medium (PromoCell GmbH, Heidelberg, Germany), counted and seeded onto culture plates (Greiner Bio-one; Kremsmünster, Austria). Passage 6 to 7 cells were used for all experiments, which were performed at least in triplicate.
SEMA3A expression:
Cat corneal fibroblasts (3×105 cells/6-cm dish in Dulbecco’s modified Eagle’s medium (DMEM)/F12 (Cellgro™, Manassas, VA, USA), 15% serum [5% fetal bovine serum (FBS; Sigma-Aldrich, St. Louis, MO, USA) + 10% newborn calf serum (NBCS; Gibco Laboratories, Gaithersburg, MD, USA)] and 1% [vol/vol] penicillin/streptomycin (Cellgro™, Manassas, VA, USA). After attachment, the serum content of the medium was decreased to 0.5% (0.25% FBS+0.25% NBCS) for 1 day to promote cellular quiescence. The cells were then treated with 1ng/ml TGF-β1 (R&D Systems Inc., Minneapolis, MN, USA) and grown for 3 days. The supernatant was then collected, centrifuged at 1000g for 5 minutes to remove detached cells and large debris and concentrated using ultra 50k Molecular Weight Cutoff (Millipore-Sigma, Burlington, MA, USA). The remaining plated cells were washed with 1× Dulbecco’s phosphate-buffered saline (dPBS; Cellgro™, Manassas, VA, USA), drained and mixed with 2× SDS loading buffer to generate whole-cell lysates. The concentrated supernatants and whole-cell lysates were processed for Western blotting. Ponceau S staining was used to verify that the same amount of supernatant was run on the gels, which were stained with a mouse monoclonal anti-α-SMA antibody (1:10,000; Thermo Fisher Scientific, Waltham, MA, USA) and a feline-specific, rabbit polyclonal anti-SEMA3A antibody (1:500; YenZym Antibodies LLC., South San Francisco, CA, USA). Levels of β-Tubulin served as a loading control, assayed using a mouse monoclonal β-Tubulin antibody (1:5000; Santa Cruz Inc., Dallas, TX, USA). Finally, 25ng recombinant human SEMA3A (R&D Systems Inc., Minneapolis, MN, USA) was used as a reference with which to contrast native SEMA3A in cat corneal stromal cells and supernatant. The membranes were scanned with a Chemi-doc machine (Bio-Rad, Hercules, CA, USA) and the resulting images were imported into Image J (NIH, USA) for measurement of relative protein expression.
Effects of exogenous SEMA3A and TGF-β1:
Quiescent corneal fibroblasts (8×104cells/well in 6-well plates) were pretreated with 100ng/ml of recombinant SEMA3A (see Supplementary Figure 1 for dose justification) for 30 minutes, with or without 1ng/ml recombinant human TGF-β1 (3-days incubation). Western blots were used to quantify the relative expression of Type 1 collagen (COL1 polyclonal antibody 1:2000; kindly provided by Dr. Larry W. Fisher, NIH, Bethesda, MD), total Fibronectin (t-FN polyclonal antibody 1:2000; Santa Cruz Inc., Dallas, TX, USA), extra-domain A-fibronectin (EDA-FN monoclonal antibody 1:2000; Santa Cruz Inc., Dallas, TX, USA) and α-SMA (as above). Levels of β-actin (mouse monoclonal antibody 1:10,000; Santa Cruz Inc., Dallas, TX, USA) were used as a loading control. Imaging and densitometry were performed as described above.
Effects of Sema3A siRNA:
Cat corneal fibroblasts (1.5×106/10mm dish) were seeded in DMEM/F12, 5% FBS+10% NBCS in the absence of penicillin/streptomycin. After 1 day, cells were transfected with Sema3A siRNA (D-058440–04, GE Healthcare Dharmacon Inc., Lafayette, CO, USA) or control siRNA (D-001810–01, GE Healthcare Dharmacon Inc., Lafayette, CO, USA). Oligofectamine (Invitrogen, Carlsbad, CA, USA) reagent was used to perform siRNA transfections as per manufacturer’s instructions. One day after transfection, the cells were harvested and re-plated at a density of 8×104/6-well plate and treated with/without 1ng/ml TGF-β1 for 3 days before harvesting and Western blots to estimate the expression of SEMA3A, α-SMA, COL1, EDA-FN and t-FN relative to those of β-actin and β-tubulin, as described above.
About 24 hours after transfection with control siRNA or Sema3A siRNA, 1×104 cells/ml of transfected fibroblasts of each type were seeded in separate, 4-well Lab-Tek ll chamber slides (Thermo Fisher Scientific, Waltham, MA, USA). They were then treated with/without 1ng/ml TGF-β1 and cultured for another 3 days before fixing with 4% formaldehyde (Thermo Fisher Scientific, Waltham, MA, USA) for 15 minutes at room temperature. The media chambers were removed, the slides were rinsed with PBS + 5% dextrose (Sigma-Aldrich, St. Louis, MO, USA) and post-fixed with absolute acetone (Sigma-Aldrich, St. Louis, MO, USA) at −20°C for 10 minutes. After blocking with 5% normal horse serum (Sigma-Aldrich, St. Louis, MO, USA) for 30 minutes, the slides were incubated overnight at 4°C in a humid chamber with the primary mouse monoclonal α-SMA antibody described earlier (1:100). The secondary antibody was goat anti-mouse IgG (H&L) Alexa-Fluor-488 (1:200; Invitrogen, Carlsbad, CA, USA). To stain actin stress fibers, PhalloidinCruzFluor-555 conjugate (1:500; Santa Cruz Inc., Dallas, TX, USA) was added. After rinsing, slides were coverslipped with Vectashield Antifade Mounting Medium containing DAPI. Fluorescence and phase contrast images were acquired using an Olympus IX73 microscope and a DP80 Dual-Mono camera (Olympus America Inc., Center Valley, PA, USA).
Statistical analyses
In order to evaluate differences in protein expression levels on Western blots, when three or more groups were compared, inter-group differences were tested with a one- or two-way analyses of variance (ANOVA), followed by Tukey’s post-hoc tests, if appropriate. When only two groups were compared, a two-tailed Student’s t-test was performed. A probability of error of p<0.05 was considered statistically significant.
Results
Persistent increase in corneal SEMA3A expression after PRK
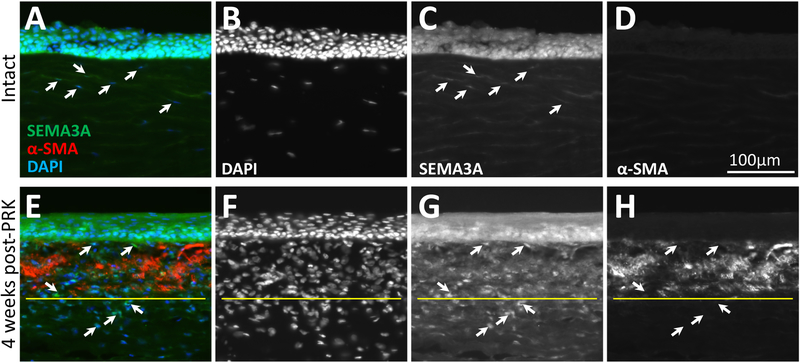
Intact, adult cat cornea exhibited its strongest staining for SEMA3A in the epithelium (Fig. 1A, C). Stromal keratocytes only stained faintly for SEMA3A (Fig. 1A, C). By 4 weeks after PRK, as previously reported [33], a strong zone of α-SMA-positive cells (Fig. 1E, H) was still evident under the healed, central corneal epithelium. The epithelium continued to stain strongly for SEMA3A, but even at this late stage post-insult, cells in the α-SMA-positive zone stained strongly for SEMA3A (Fig. 1E, G). While some were α-SMA-positive, the wound area had numerous cells - presumably fibroblasts –positive for SEMA3A but negative for α-SMA (white arrows in Figs. 1E, G, H), clustered both below and above the α-SMA-positive zones, but restricted to the ablation zone. The peripheral cornea exhibited immunostaining that was not qualitatively different from that in intact/unoperated corneas (as in Fig. 1A–D).
Figure 1. In vivo expression of SEMA3A in feline corneas.
A. Unoperated, cornea triple labelled for SEMA3A, α-SMA and DAPI. The epithelium is uppermost. Individual stains (monochrome) are shown in B-D. Keratocytes (arrowed) are faintly SEMA3A positive. Note total absence of α-SMA staining. E. Photograph of a central cat cornea 4 weeks after PRK, triple labelled as in A. Individual stains are shown in F-H. Arrows indicate α-SMA-negative cells that are strongly SEMA3A-positive. Note the zone of cellular hyper-density below the α-SMA-positive layer (demarcated with yellow line), where a significant proportion of cells [putative fibroblasts] are SEMA3A-positive.
Cultured corneal fibroblasts and myofibroblasts synthesize and secrete SEMA3A
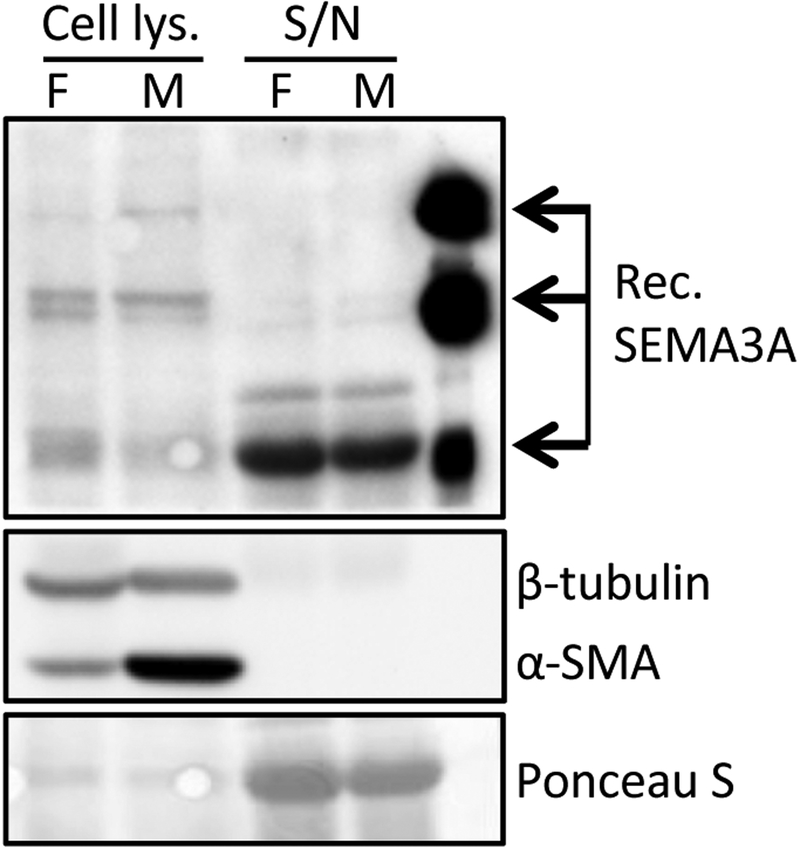
We observed three different isoforms of SEMA3A (125kDa, 95kDa and 65kDa) in fibroblast lysates (Fig. 2, lane 1) and the major secreted form of SEMA3A, the 65kDa isoform [34, 35], was dominant in their supernatant (Fig. 2, lane 3). After adding TGF-β1 to these cells, there was increased expression of α-SMA in the lysates (Fig. 2, lane 2), suggesting that fibroblasts had differentiated into myofibroblasts. Levels of all 3 isoforms of SEMA3A (125kDa, 95kDa and 65kDa) were similarly expressed in myofibroblast lysates, and their supernatant contained similar levels of the 65kDa isoform as the fibroblast supernatant. These observations confirm that fibroblasts and myofibroblasts derived from primary corneal keratocytes express detectable levels of several SEMA3A isoforms, and secrete similar amounts of 65kDa SEMA3A.
Figure 2. Basal expression of SEMA3A in cultured cat corneal fibroblasts and myofibroblasts.
Representative Western blot showing similar, relative SEMA3A expression levels in whole cell lysates and concentrated supernatants (S/N) of cat corneal fibroblasts (F) and myofibroblasts (M). Expression of α-SMA was used as an indicator of myofibroblast differentiation in the cell lysates.
SEMA3A potentiates the pro-fibrotic effects of TGF-β1 in corneal fibroblasts
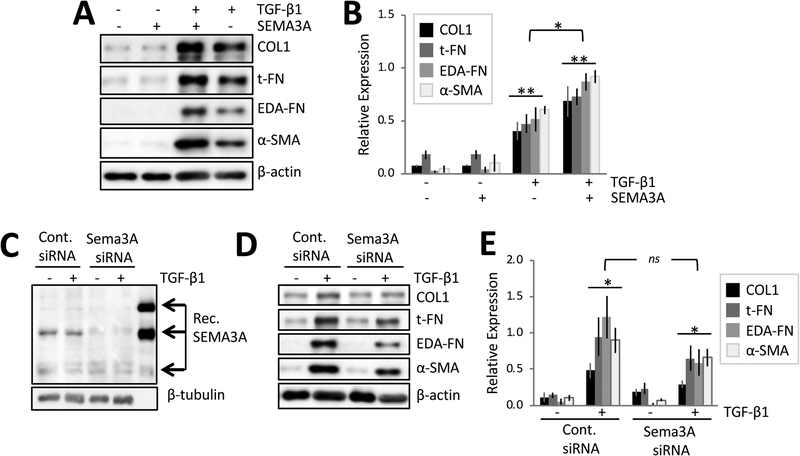
After 3 days of culture in the absence of any treatment, basal levels of EDA-FN and α-SMA in cat corneal fibroblasts were close to zero, while those of COL1 and t-FN were low but distinctly above zero (Fig. 3A, lane 1). A dose-response test for SEMA3A determined that this molecule was not, by itself, able to induce myofibroblast differentiation (morphologically-determined, data not shown) or upregulate expression of α-SMA at doses ranging from 10 to 500ng/ml (Supplementary Fig. 1). This lack of effect was replicated for 100ng/ml SEMA3A (the dose selected for present experiments) and all pro-fibrotic molecules of interest [2(treatment)x4(molecules) ANOVA with repeated measures across molecules: F(1,31)=0, p=1; Fig. 3A, lane 2; Fig. 3B]. In contrast, addition of TGF-β1 alone significantly increased relative expression of pro-fibrotic molecules compared to baseline [2(treatment)x4(molecules) ANOVA: F(1,31)=47, p=0.0005; Fig. 3A, lane 4; Fig. 3B]. However, the biggest effect occurred when fibroblasts were exposed to 100ng/ml SEMA3A + 1ng/ml TGF-β1 (Fig. 3A, lane 3; Fig. 3B); this significantly increased expression of all pro-fibrotic markers relative to baseline [2(treatment)x4(molecules) ANOVA: F(1,31)=137.67, p<0.0001], as well as over TGF-β1 alone [2(treatment)x4(molecules) ANOVA: F(1,31)=12, p=0.0134]. Combined administration of SEMA3A/TGF-β1 generated levels of pro-fibrotic molecules (mean±SD) 1.7±0.4 fold higher than following incubation with 1ng/ml TGF-β1 alone, and this was consistent across all molecules examined.
Figure 3. SEMA3A potentiates TGF-β1’s profibrotic effects in cultured cat corneal fibroblasts.
A. Representative Western blot showing protein levels for COL1, t-FN, EDA-FN and α-SMA in cells treated with recombinant human SEMA3A with/without TGF-β1. Adding SEMA3A potentiated TGF-β1-induced increases in EDA-FN, t-FN, COL1 and α-SMA relative to TGF-β1 alone. B. Plot of relative densitometry data for EDA-FN, t-FN, COL1 and α-SMA with respect to β-actin. Data are means±SEM over 4 experiments. **ANOVA relative to baseline, p<0.0005. *ANOVA between 2 treatment groups, p<0.05. C. Representative Western blot showing SEMA3A levels in cells transfected with Sema3A siRNA or control (non-targeting) siRNA. D. Representative Western blot showing relative levels of COL1, t-FN, EDA-FN and α-SMA in fibroblasts transfected with control siRNA or Sema3A siRNA and cultured with/without TGF-β1. E. Plot of relative densitometry data for EDA-FN, t-FN, COL1 and α-SMA with respect to β-actin. Data are means±SEM over 3 experiments. *ANOVA relative to baseline, p<0.05. ns ANOVA between 2 treatment groups, p=0.227.
Sema3A effects are cell non-autonomous
Cells transfected with control siRNA expressed normal levels of the 125kDa, 95kDa and 65kDA isoforms of SEMA3A (Fig. 3C, lane 1), continuing to do so even after addition of 1ng/ml TGF-β1 (Fig. 3C, lane 2). This was consistent with our earlier result (Fig. 2). In contrast, corneal fibroblasts transfected with Sema3A siRNA showed diminished expression of the 125kDa and 95kDa isoforms of SEMA3A (Fig. 3C, lane 3) compared to cells transfected with control siRNA. This also continued to hold true after stimulation with TGF-β1 (Fig. 3C, lane 4).
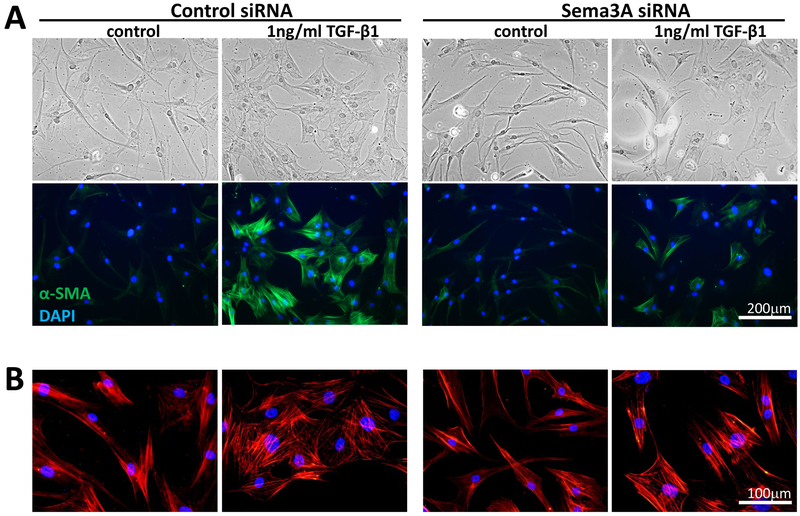
With respect to synthesis of pro-fibrotic molecules, basal levels of t-FN and COL1 were relatively unchanged by Sema3A siRNA (Fig. 3D, lanes 1 & 3), suggesting that cell-autonomous SEMA3A does not regulate their baseline expression. Addition of TGF-β1 elicited the expected increased synthesis of t-FN, COL-1, EDA-FN and α-SMA in both control (Fig. 3D, lane2) and Sema3A siRNA-transfected cells (Fig. 3D, lane 4). A 2(baseline versus TGF-β1) × 4 (molecules) repeated measures ANOVA for the control siRNA had an F(1,23)=14.68, p=0.0186. The same ANOVA for the Sema3A siRNA was also significant [F(1,23)=13.38, p=0.0216]. As such, when contrasting TGF-β1 treatment in the control versus Sema3A siRNA groups, the repeated measures ANOVA showed no main effect of treatment [F(1,23)=2.03, p=0.227]. However, there was a main effect of molecules examined [F(3,23)=10.33, p=0.00121]. Indeed, while post-hoc t-tests revealed no significant change in COL1 or total-FN expression, Sema3A siRNA treated cells expressed less EDA-FN (t2=4.47, p=0.0466) and α-SMA (t2=4.52, p=0.0456) than their control siRNA counterparts. This was confirmed by immunocytochemistry in cultured cells, where morphologically, Sema3A siRNA transfected fibroblasts maintained the ability to differentiate into myofibroblasts (Fig. 4). But although these large, multipolar cells had prominent stress fibers upon Phalloidin staining (Fig. 4B), they stained more faintly for α-SMA compared to control-siRNA-transfected cells exposed to TGF-β1 (Fig. 4A).
Figure 4. Sema3A siRNA knockdown decreases immunocytochemical expression of α-SMA in cultured corneal myofibroblasts.
A. Phase contrast images (top row) show a change from fibroblastic to myofibroblastic morphologies in both cells transfected with control and Sema3A siRNA, but α-SMA staining (green in bottom row) is decreased in Sema3A siRNA-treated cells. B. Phalloidin staining of different regions on the same plates as in A shows persistence of F-actin fibers and clear morphological characteristics of larger myofibroblasts in the two plates treated with TGF-β1, even when α-SMA expression was decreased, as in the Sema3A siRNA+TGF-β1 condition.
Discussion
After corneal injury, wound healing generates fibrosis, which can induce scar formation and decrease transparency of this critical ocular lens [10]. Corneal fibrosis is characterized first by transformation of keratocytes into fibroblast, thence into myofibroblast, which increase synthesis and abnormal deposition of ECM components, stimulated by a variety of growth factors and cytokines [10, 20]. TGF-β1 is the strongest-known, profibrotic growth factor, but SEMA3A is also greatly upregulated in corneal wounds [21, 22]. While SEMA3A plays key roles in axonal guidance [36, 37] cancer progression [38–40] and kidney injury [41, 42], only scant information exists about its role(s) in the wounded cornea.
In the undamaged cat cornea, epithelial cells and to a lesser degree stromal keratocytes, appeared to natively express this molecule, as previously shown in rodents [43]. Four weeks after PRK however, SEMA3A expression dramatically increased in both the epithelium and stromal cells within the wound area, again consistent with prior observations in rodent models of corneal wounding [21, 22]. SEMA3A is first synthesized as a pro-protein; differential proteolytic processing then generates multiple isoforms that can differ between species and cell types [34]. Cat corneal fibroblasts and myofibroblasts preferentially and similarly expressed the 125kDa, 95kDa and 65kDa isoforms of SEMA3A. The 65kDa isoform dominated in the supernatants, consistent with the notion that it is likely the secreted form of this molecule [34, 35]. Of relevance here, previous studies reported that expression of SEMA3A is regulated by other growth factors, including EGF [23] and hepatocyte growth factor/FGF2 [44]. Our data suggest that SEMA3A expression by stromal fibroblasts is not regulated by TGF-β1. However, keratocytes in vivo stained more faintly for SEMA3A than fibroblasts or myofibroblasts after PRK, suggesting that TGF-β1 could still influence levels of SEMA3A in stromal keratocytes.
Key to our initial question, our data show that exogenously-applied SEMA3A works synergistically with TGF-β1 to potentiate differentiation of corneal fibroblasts into myofibroblasts, and the ability of the latter to upregulate synthesis of α-SMA and extracellular matrix molecules (EDA-FN, COL1 and t-FN). This is in spite of the fact that exogenous SEMA3A alone does not seem to differentiate cat corneal fibroblasts into myofibroblasts. Consistent with this, knocking down endogenous SEMA3A with siRNA did not eliminate the cells’ ability to differentiate into myofibroblasts or synthesize profibrotic molecules. However, exogenously-added SEMA3A and TGF-β1 together significantly increased the expression of EDA-FN, t-FN, COL1 and α-SMA over levels obtained from TGF-β1 stimulation alone.
Thus, in the cornea, elevated secretion of SEMA3A after injury - likely from the epithelium [21, 22] - potentiates TGF-β1’s pro-fibrotic effects in activated fibroblasts. Although further studies are needed to determine how SEMA3A interacts with TGF-β1 to generate the synergistic effects observed in the present experiments, a possible mechanism may involve neuropilin-1, a SEMA3A receptor shown to act as a co-receptor for TGF-β1 in cancer cells [45]. Nonetheless, the present work describes a hitherto unrecognized relationship between SEMA3A and TGF-β1 in the wounded cornea, and suggests a possible contribution of cell non-autonomous SEMA3A to the regulation of tissue fibrosis and remodeling in this transparent organ. Finally, given SEMA3A’s inhibitory effect on neurite outgrowth [36, 37] and recent work showing that regenerating corneal nerves avoid corneal wound areas and the myofibroblasts they contain [33, 46] the present results also lay an interesting foundation for future investigations into roles that SEMA3A may play at the interface between regenerating neurons and fibrotic wound healing.
Supplementary Material
Highlights:
Corneal wounds cannot yet be treated effectively
SEMA3A is dramatically upregulated in corneal wounds
Exogenous SEMA3A potentiates the profibrotic effects of TGF-β1 in the stroma
Targeting SEMA3A+ TGF-β1 therapeutically could effectively control corneal fibrosis
Acknowledgments:
This research was supported by the National Eye Institute of the National Institutes of Health (R01 EY015836 and Core Grant P30 EY001319 to the Center for Visual Science) and an unrestricted grant to the University of Rochester’s Department of Ophthalmology from the Research to Prevent Blindness Foundation. The authors thank Dr. Holly Hindman, Ms. Margaret DeMagistris and Ms. Chrys Callan for performing PRK on live cats, Mrs. Tracy Bubel for tissue processing, and Mrs. Thurma McDaniel for immunocytochemical staining.
Abbreviations:
- α-SMA
alpha smooth muscle actin
- COL
collagen
- DAPI
4’,6-diamidino-2- phenylindole
- DMEM
Dulbecco’s modified Eagle’s medium
- dPBS
Dulbecco’s phosphate-buffered saline
- ECM
extracellular matrix
- EDA
extra domain A
- EGF
epidermal growth factor
- FBS
fetal bovine serum
- FGF
fibroblast growth factor
- NBCS
newborn calf serum
- PRK
photorefractive keratectomy
- SEMA3A
semaphorin 3A
- TGF-β1
transforming growth factor-beta 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no proprietary interest in any devices or pharmacologics used in this study.
References
- [1].Oliva MS, Schottman T, Gulati M, Turning the tide of corneal blindness, Indian J Ophthalmol, 60 (2012) 423–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wilson SL, El Haj AJ, Yang Y, Control of scar tissue formation in the cornea: strategies in clinical and corneal tissue engineering, J Funct Biomater, 3 (2012) 642–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Jester JV, Barry-Lane PA, Cavanagh HD, Petroll WM, Induction of alpha-smooth muscle actin expression and myofibroblast transformation in cultured corneal keratocytes, Cornea, 15 (1996) 505–516. [PubMed] [Google Scholar]
- [4].Vesaluoma M, Teppo AM, Gronhagen-Riska C, Tervo T, Release of TGF-beta 1 and VEGF in tears following photorefractive keratectomy, Curr Eye Res, 16 (1997) 19–25. [DOI] [PubMed] [Google Scholar]
- [5].Fini ME, Keratocyte and fibroblast phenotypes in the repairing cornea, Prog Retin Eye Res, 18 (1999) 529–551. [DOI] [PubMed] [Google Scholar]
- [6].Jester JV, Petroll WM, Cavanagh HD, Corneal stromal wound healing in refractive surgery: the role of myofibroblasts, Prog Retin Eye Res, 18 (1999) 311–356. [DOI] [PubMed] [Google Scholar]
- [7].West-Mays JA, Dwivedi DJ, The keratocyte: corneal stromal cell with variable repair phenotypes, Int J Biochem Cell Biol, 38 (2006) 1625–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Jester JV, Rodrigues MM, Herman IM, Characterization of avascular corneal wound healing fibroblasts. New insights into the myofibroblast, The American journal of pathology, 127 (1987) 140–148. [PMC free article] [PubMed] [Google Scholar]
- [9].Darby IA, Laverdet B, Bonte F, Desmouliere A, Fibroblasts and myofibroblasts in wound healing, Clin Cosmet Investig Dermatol, 7 (2014) 301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wilson SE, Corneal myofibroblast biology and pathobiology: generation, persistence, and transparency, Exp Eye Res, 99 (2012) 78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jester JV, Brown D, Pappa A, Vasiliou V, Myofibroblast differentiation modulates keratocyte crystallin protein expression, concentration, and cellular light scattering, Investigative ophthalmology & visual science, 53 (2012) 770–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Jester JV, Moller-Pedersen T, Huang J, Sax CM, Kays WT, Cavangh HD, Petroll WM, Piatigorsky J, The cellular basis of corneal transparency: evidence for ‘corneal crystallins’, Journal of cell science, 112 (Pt 5) (1999) 613–622. [DOI] [PubMed] [Google Scholar]
- [13].Hassell JR, Birk DE, The molecular basis of corneal transparency, Exp Eye Res, 91 (2010) 326–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Jester JV, Petroll WM, Barry PA, Cavanagh HD, Expression of alpha-smooth muscle (alpha-SM) actin during corneal stromal wound healing, Invest Ophthalmol Vis Sci, 36 (1995) 809–819. [PubMed] [Google Scholar]
- [15].Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G, Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts, J Cell Biol, 122 (1993) 103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Singh V, Barbosa FL, Torricelli AA, Santhiago MR, Wilson SE, Transforming growth factor beta and platelet-derived growth factor modulation of myofibroblast development from corneal fibroblasts in vitro, Exp Eye Res, 120 (2014) 152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jeon KI, Phipps RP, Sime PJ, Huxlin KR, Inhibitory effects of PPARgamma ligands on TGF-beta1-induced CTGF expression in cat corneal fibroblasts, Exp Eye Res, 138 (2015) 52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Grotendorst GR, Connective tissue growth factor: a mediator of TGF-beta action on fibroblasts, Cytokine Growth Factor Rev, 8 (1997) 171–179. [DOI] [PubMed] [Google Scholar]
- [19].Lipson KE, Wong C, Teng Y, Spong S, CTGF is a central mediator of tissue remodeling and fibrosis and its inhibition can reverse the process of fibrosis, Fibrogenesis & tissue repair, 5 (2012) S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ljubimov AV, Saghizadeh M, Progress in corneal wound healing, Prog Retin Eye Res, 49 (2015) 17–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cao Z, Wu HK, Bruce A, Wollenberg K, Panjwani N, Detection of differentially expressed genes in healing mouse corneas, using cDNA microarrays, Investigative ophthalmology & visual science, 43 (2002) 2897–2904. [PubMed] [Google Scholar]
- [22].Morishige N, Ko JA, Morita Y, Nishida T, Expression of semaphorin 3A in the rat corneal epithelium during wound healing, Biochemical and biophysical research communications, 395 (2010) 451–457. [DOI] [PubMed] [Google Scholar]
- [23].Ko JA, Morishige N, Yanai R, Nishida T, Up-regulation of semaphorin 3A in human corneal fibroblasts by epidermal growth factor released from cocultured human corneal epithelial cells, Biochemical and biophysical research communications, 377 (2008) 104–108. [DOI] [PubMed] [Google Scholar]
- [24].Kolodkin AL, Matthes DJ, Goodman CS, The semaphorin genes encode a family of transmembrane and secreted growth cone guidance molecules, Cell, 75 (1993) 1389–1399. [DOI] [PubMed] [Google Scholar]
- [25].Pasterkamp RJ, Kolodkin AL, Semaphorin junction: making tracks toward neural connectivity, Curr Opin Neurobiol, 13 (2003) 79–89. [DOI] [PubMed] [Google Scholar]
- [26].Brown JA, Bridgman PC, Disruption of the cytoskeleton during Semaphorin 3A induced growth cone collapse correlates with differences in actin organization and associated binding proteins, Dev Neurobiol, 69 (2009) 633–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hung RJ, Terman JR, Extracellular inhibitors, repellents, and semaphorin/plexin/MICAL-mediated actin filament disassembly, Cytoskeleton (Hoboken), 68 (2011) 415–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Jeon KI, Phipps RP, Sime PJ, Huxlin KR, Antifibrotic Actions of Peroxisome Proliferator-Activated Receptor gamma Ligands in Corneal Fibroblasts Are Mediated by beta-Catenin-Regulated Pathways, The American journal of pathology, 187 (2017) 1660–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Jeon KI, Kulkarni A, Woeller CF, Phipps RP, Sime PJ, Hindman HB, Huxlin KR, Inhibitory effects of PPARgamma ligands on TGF-beta1-induced corneal myofibroblast transformation, The American journal of pathology, 184 (2014) 1429–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Huxlin KR, Hindman HB, Jeon KI, Buhren J, MacRae S, DeMagistris M, Ciufo D, Sime PJ, Phipps RP, Topical rosiglitazone is an effective anti-scarring agent in the cornea, PLoS One, 8 (2013) e70785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Buhren J, Nagy L, Swanton JN, Kenner S, MacRae S, Phipps RP, Huxlin KR, Optical effects of anti-TGFbeta treatment after photorefractive keratectomy in a cat model, Investigative ophthalmology & visual science, 50 (2009) 634–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Nagy LJ, MacRae S, Yoon G, Wyble M, Wang J, Cox I, Huxlin KR, Photorefractive keratectomy in the cat eye: biological and optical outcomes, J Cataract Refract Surg, 33 (2007) 1051–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Jeon KI, Hindman HB, Bubel T, McDaniel T, DeMagistris M, Callan C, Huxlin KR, Corneal myofibroblasts inhibit regenerating nerves during wound healing, Sci Rep, 8 (2018) 12945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Adams RH, Lohrum M, Klostermann A, Betz H, Puschel AW, The chemorepulsive activity of secreted semaphorins is regulated by furin-dependent proteolytic processing, EMBO J, 16 (1997) 6077–6086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].De Wit J, De Winter F, Klooster J, Verhaagen J, Semaphorin 3A displays a punctate distribution on the surface of neuronal cells and interacts with proteoglycans in the extracellular matrix, Mol Cell Neurosci, 29 (2005) 40–55. [DOI] [PubMed] [Google Scholar]
- [36].Raper JA, Semaphorins and their receptors in vertebrates and invertebrates, Curr Opin Neurobiol, 10 (2000) 88–94. [DOI] [PubMed] [Google Scholar]
- [37].Luo Y, Raible D, Raper JA, Collapsin: a protein in brain that induces the collapse and paralysis of neuronal growth cones, Cell, 75 (1993) 217–227. [DOI] [PubMed] [Google Scholar]
- [38].Chedotal A, Chemotropic axon guidance molecules in tumorigenesis, Prog Exp Tumor Res, 39 (2007) 78–90. [DOI] [PubMed] [Google Scholar]
- [39].Capparuccia L, Tamagnone L, Semaphorin signaling in cancer cells and in cells of the tumor microenvironment--two sides of a coin, Journal of cell science, 122 (2009) 1723–1736. [DOI] [PubMed] [Google Scholar]
- [40].Hu ZQ, Zhou SL, Zhou ZJ, Luo CB, Chen EB, Zhan H, Wang PC, Dai Z, Zhou J, Fan J, Huang XW, Overexpression of semaphorin 3A promotes tumor progression and predicts poor prognosis in hepatocellular carcinoma after curative resection, Oncotarget, 7 (2016) 51733–51746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Jayakumar C, Ranganathan P, Devarajan P, Krawczeski CD, Looney S, Ramesh G, Semaphorin 3A is a new early diagnostic biomarker of experimental and pediatric acute kidney injury, PLoS One, 8 (2013) e58446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lewandowska L, Matuszkiewicz-Rowinska J, Jayakumar C, Oldakowska-Jedynak U, Looney S, Galas M, Dutkiewicz M, Krawczyk M, Ramesh G, Netrin-1 and semaphorin 3A predict the development of acute kidney injury in liver transplant patients, PLoS One, 9 (2014) e107898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Morishige N, Ko JA, Liu Y, Chikama T, Nishida T, Localization of semaphorin 3A in the rat cornea, Exp Eye Res, 86 (2008) 669–674. [DOI] [PubMed] [Google Scholar]
- [44].Do MK, Shimizu N, Suzuki T, Ohtsubo H, Mizunoya W, Nakamura M, Sawano S, Furuse M, Ikeuchi Y, Anderson JE, Tatsumi R, Transmembrane proteoglycans syndecan-2, 4, receptor candidates for the impact of HGF and FGF2 on semaphorin 3A expression in early-differentiated myoblasts, Physiol Rep, 3 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Glinka Y, Stoilova S, Mohammed N, Prud’homme GJ, Neuropilin-1 exerts co-receptor function for TGF-beta-1 on the membrane of cancer cells and enhances responses to both latent and active TGF-beta, Carcinogenesis, 32 (2011) 613–621. [DOI] [PubMed] [Google Scholar]
- [46].Hindman HB, DeMagistris M, Callan C, McDaniel T, Bubel T, Huxlin KR, Impact of topical anti-fibrotics on corneal nerve regeneration in vivo, Exp Eye Res, 181 (2019) 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.