Abstract
Objective:
To evaluate the degree of knee fat pad abnormalities after acute anterior cruciate ligament (ACL) tear via magnetic resonance fat pad scoring and to assess cross-sectionally its association with synovial fluid biomarkers and with early cartilage damage as quantified via T1ρ and T2 relaxation time measurements.
Design:
26 patients with acute ACL tears underwent 3T MR scanning of the injured knee prior to ACL reconstruction. The presence and degree of abnormalities of the infrapatellar (IPFP) and the suprapatellar (SPFP) fat pads were scored on MR images along with grading of effusion-synovitis and synovial proliferations. Knee cartilage composition was assessed by 3T MR T1ρ and T2 mapping in 6 knee compartments. We quantified concentrations of 20 biomarkers in synovial fluid aspirated at the time of ACL reconstruction. Spearman rank partial correlations with adjustments for age and gender were employed to evaluate correlations of MR, particularly cartilage composition and fat pad abnormalities, and biomarker data.
Results:
The degree of IPFP abnormality correlated positively with the synovial levels of the inflammatory cytokine markers IFN-γ (ρpartial=0.64, 95% CI (0.26-0.85)), IL-10 (ρpartial=0.47, 95% CI (0.04-0.75)), IL-6 (ρpartial=0.56, 95% CI (0.16-0.81)), IL-8 (ρpartial=0.49, 95% CI (0.06-0.76)), TNF-α (ρpartial=0.55, 95% CI (0.14-0.80) and of the chondrodestructive markers MMP-1 and 3 (MMP-1: ρpartial=0.57, 95% CI (0.17-0.81); MMP-3: ρpartial=0.60, 95% CI (0.21-0.83). IPFP abnormalities were significantly associated with higher T1ρ and T2 values in the trochlear cartilage (T1ρ: ρpartial=0.55, 95% CI (0.15-0.80); T2: ρpartial=0.58, 95% CI (0.18-0.81)) and with higher T2 values in the medial femoral, medial tibial as well as in patellar cartilage (0.45≤ρpartial ≤ 0.59). Correlations between SPFP abnormalities and synovial markers were not significant except for IL-6 (ρpartial=0.57, 95% CI (0.17-0.81).
Conclusions:
This exploratory study suggests that acute ACL rupture can be associated with damage to knee tissues such as the inferior fat pad of the knee. Such fat pad injury could be partially responsible for the apparent post-injury pro-inflammatory response noted in ACL-injured individuals. However, future longitudinal studies are needed to link ACL-rupture associated fat pad injury with important patient outcomes such as the development of posttraumatic osteoarthritis.
Keywords: Anterior cruciate ligament tear, post-traumatic osteoarthritis, PTOA, Inflammation, Matrix metalloproteinases, Cartilage damage, Magnetic resonance imaging, T1ρ relaxation time, T2 relaxation time
INTRODUCTION
Osteoarthritis (OA), the most prevalent form of arthritis, is a frequent cause of chronic disability in adults and most commonly affects the knee, involving the entire joint, including menisci, ligaments, subchondral bone, synovium, and periarticular muscle1. Acute trauma of the anterior cruciate ligament (ACL) is considered a major risk factor for the development of posttraumatic OA2. Despite surgical management and depending on the complexity of the injury (isolated versus combined ACL injury), ACL-injured patients have a prevalence of up to 13-50 % of developing posttraumatic OA within 10-15 years after the initial injury3,4 and have a relative risk of 3.84 to develop moderate to severe osteoarthritis within 10 year after ACL injury.5 For young and active patients, the early onset of cartilage damage is particularly devastating as it may require symptomatic and operative treatment with eventual knee replacement at an early age, thereby introducing a new set of complications and costs6,7. The exact pathomechanism underlying early cartilage damage after ACL injury has not been fully established. While proposed mechanisms include changes in tibiofemoral mechanics, concurrent injuries, or patients’ innate risk factors such as BMI and bony morphology2,8,9, there is increasing evidence that posttraumatic OA in patients with ACL injury may result from a cascade of biomechanical and biochemical changes following ACL injury10,11. In particular, sustained intraarticular inflammation is suggested to activate matrix metalloproteinases (MMPs) that in turn digest collagen and proteoglycan components of the cartilage matrix, culminating in cartilage damage associated with OA12. However, to date it is not clear what triggers and maintains this detrimental flare of inflammatory cytokines.
With respect to sources of intra-articular inflammation, the knee joint fat pads such as infrapatellar (Hoffa’s) fat pad (IPFP) and the smaller suprapatellar fat pad (SPFP), in particular the IPFP are of special interest13. While these fat pads were long neglected and disregarded as mere “space fillers”14, novel emerging evidence suggests that this adipose tissue can get entrapped during ACL injury and function as an active endocrine organ15. Specifically, it has been shown for primary OA16,17 and in animal models18 that the IPFP secretes cytokines, adipokines, and interleukins, such as interleukin (IL)-6 and tumor necrosis factor (TNF)-ɑ, that accelerate cartilage breakdown via induction of MMPs. In humans, abnormalities of the IPFP, including fat pad edema, tears and synovial proliferation, have been linked with acute ACL injury 19,20 and cartilage damage in the knee joint after ACL reconstruction (ACLR)21. However, no human study to date has investigated fat pad abnormalities and their association with the posttraumatic synovial biomarker profile in patients following ACL tears.
Quantitative magnetic resonance imaging (MRI) techniques, such as MRI T1ρ and T2 mapping, have the ability to evaluate the compositional changes in cartilage matrix22. Elevated T1ρ and T2 relaxation times have been reported in knee joints after ACL injury and ACLR, indicating the very early appearance of degenerative changes23. In fact, these non-invasive techniques can detect biochemical changes in the collagen-proteoglycan matrix prior to the occurrence of morphological changes following joint injury24 and are therefore suited to determine the early risk of OA development25. However, to date, studies are lacking investigating if, and to what extent, knee fat pad abnormalities and structural and compositional measures such as T1ρ and T2 values are correlated in patients following ACL tear. We hypothesized an association between damage of the endocrine fat pad organ intra-articularly and cartilage joint tissue abnormalities by T1rho and T2 markers mediated by fat pad inflammation. The goals of this exploratory study were: to assess approximately 2 months post ACL-injury in healthy young subjects (i) the severity of fat pad abnormalities semi-quantitatively by using a composite MR-based fat-pad synovitis knee score; (ii) to investigate the relationships between semi-quantitative fat pad scores and inflammatory synovial fluid cytokines; and (iii) to investigate the relationship between fad pad abnormalities and cartilage degradations quantified via knee cartilage MRI T1ρ and T2 mapping.
MATERIAL AND METHODS
Participants
Twenty-six young and otherwise healthy patients with acute traumatic ACL injury were recruited from the UCSF orthopedics clinics within 6 months of their injury and prior to their ACL reconstruction (ACLR) as part of an ongoing larger ACL cohort study (see for more information the publication of Amano K. et al.)26 All 26 patients agreed to a synovial fluid aspiration from their injured knee joint while undergoing general anesthesia at the time of surgery. Participants were only included if they were clinically diagnosed with a complete ACL rupture based on increased anterior-posterior laxity of the injured knee (Lachman grade>1)27 and confirmation of ACL rupture by a clinical MRI. In addition, patients had to be willing to have an ACLR, and had to be capable of undergoing the standard pre- and post-injury/operative rehabilitation. The exclusion criteria comprised a prior history of osteoarthritis, inflammatory arthritis, previous injury or surgery of either knee. Moreover, patients requiring additional surgical intervention for multiple ligamentous injuries were not eligible. Participants whose meniscus injuries needed a repair at the time of surgery were also ineligible, as they would be subjected to a different, postoperative weight bearing protocol, which could potentially affect cartilage composition. For the study, all eligible patients received the same, standardized workup: first - on average 52 days after ACL-injury - all subjects received an MRI scan of the injured knee. Next, - on average 12 days after the MRI scan - patients underwent ACL reconstruction surgery of their injured knee. At the time of the surgery, just prior to making the incision, synovial fluid was additionally aspirated from the injured knee as detailed below. The study was approved by the institutional review board and all patients gave written informed consent prior to participation
Anterior Cruciate Ligament Reconstruction Surgery
All participants underwent single-bundle ACLR with soft tissue grafts at the UCSF Department of Orthopedic Surgery using a standard surgical protocol and an anteromedial portal technique for femoral tunnel drilling. A total of 17 hamstring autografts, 1 hamstring allograft, and 8 posterior tibial allografts were implanted. Partial meniscectomy was performed in 8 participants (6 lateral only, 1 medial only, and 1 both sides). All study participants underwent a standard postoperative rehabilitation program at the UCSF Sports Medicine Clinic.
Synovial Fluid Collection and Biomarker Analyses
At the time of surgery, just prior to making the incision, synovial fluid was collected from the injured knee in a sterile fashion without lavage or local anesthetic. The intraarticular fluid was aspirated into a sterile container and the collected fluid was immediately centrifuged at 15,000 rpm for 30 minutes. Aliquots of the supernatant were stored at −80°C until further analysis.
Frozen synovial fluid samples were shipped to our collaborators (VK/JH/TS Biomarkers Shared Resource at Duke University) on dry ice and were analyzed using commercially available enzyme-linked immunosorbent assays (ELISA). Details of the biomarker assays and their coefficients of variance (CV) have been previously published by our group28, and are outlined in the supplemental information.
Magnetic Resonance Imaging of the Knee and Quantification of T1ρand T2 Knee Cartilage
After injury and prior to ACL reconstruction, all patients underwent 3T MRI scanning of their injured knee using the same 3 Tesla MRI scanner (GE Healthcare, Milwaukee, WI, USA) with a standard quadrature transmit/8-channel receive knee coil (Invivo, Orlando, FL, USA). Performance of MR scanner and coil were monitored regularly by a trained MR-physicist for positioning and geometric accuracy, magnetic field homogeneity, contrast and artifacts. The imaging protocol consisted of a high-resolution 3D FSE (CUBE) sequence (TR/TE = 1500/26.69 ms, field of view 16 cm, 384 x 384 matrix size, slice thickness 0.5 mm, echo train length 32), and quantitative combined T1ρ/T2 sequences (T1ρ TSL= 0/10/40/80 ms, spin-lock frequency = 500 Hz, field of view 14 cm, 256 × 128 matrix size, slice thickness 4 mm, T2 preparation TE=0/12.87/25.69/51.39ms). An in-house developed software based on Matlab (Mathworks, Natick, MA) was used to semi-automatically segment the knee cartilage in the 6 following compartments: medial femoral condyle (MFC), medial tibia (MT), lateral femoral condyle (LFC), lateral tibia (LT), trochlea (TRO) and patella (PAT). Details about the segmentation technique have been previously published29. Knee cartilage mean T1ρ and T2 relaxation times for each compartment were calculated using algorithms that have been extensively described (Fig. 1a)30.
Figure 1:
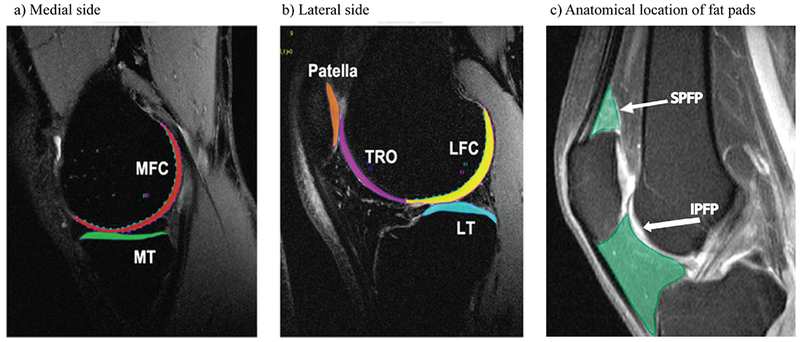
depiction of the 6 cartilage compartments used to quantify MR-based T1ρ and T2 cartilage relaxation time measurements (a and b) as well as of knee fat pads (c).
a: sagittal 3 T MR image of the medial aspect of the knee showing the cartilage of the medial femoral condyle (MFC) and of the medial tibia (MT).
b: sagittal 3 T MR image of the lateral aspect of the knee showing the cartilage of the lateral femoral condyle (LFC), the lateral tibia (LT), the trochlea (TRO) and the patella (PAT).
c: Representative sagittal MR image of the knee showing the anatomical location of the suprapatellar fat pad (SPFP) and of the infrapatellar Hoffa fat pad (IPFP). Both fat pads are highlighted in green. The arrow with the larger tip points to the suprapatellar fat pad (SPFP). The arrow with the smaller tip points towards the infrapatellar fat pad (IPFP).
MR=magnetic resonance.
Composite MRI Fat Pad and Effusion/Synovitis Score
MR images were scored by a radiologist with 4 years of experience in musculoskeletal radiology. Readings were adjudicated by a board-certified musculoskeletal radiologist (T.M.L.) with 23 years of experience. All radiologists were blinded to the demographic and clinical information. Two fat pads were evaluated: the suprapatellar fat pad (SPFP) and the infrapatellar (Hoffa’s) fat pad (IPFP) (Fig. 1b). Specifically, 4 sub-features were graded per knee as outlined in more detail in the legend to Figure 2. First, the degree of infrapatellar fat pad (IPFP) abnormality was scored on FS CUBE and non-fatsaturated proton density weighted sequences of the knee according to the Anterior Cruciate Ligament OsteoArthritis Score (ACLOAS)31 on a scale from 0 to 3 (grade 0=normal appearing fat pad, grade 1=mild hyperintense signal within the IPFP less than the cartilage signal, grade 2=moderate hyperintense signal within the IPFP similar or higher compared to cartilage signal, grade 3=severe hyperintense signal within the IPFP in the order of joint fluid). Second, the degree of suprapatellar fat pad (SPFP or quadriceps fat pad alterations) abnormality was assessed on a scale from 0 to 3 as described in figure legend 2 (Fig. 2b)32. Third, the extent of effusion-synovitis was scored from 0 (no effusion) to 3 as the anterior-posterior effusion diameter visible on sagittal fat saturated CUBE images as previously described31 and outlined in detail in the legend of Figure 2. As a fourth feature, the extent of synovial proliferation was assessed using the following 3 point-scale (Fig. 2d): grade 1 corresponded to a smooth synovium, with no proliferation or synovial bands visible; grade 2 was defined as a mild irregularity of the synovium, either focal or diffuse, and the presence of some synovial bands or small bodies; grade 3 was defined as extensive synovial thickening with irregular villo-nodular proliferation. One out of 26 patients had to be excluded from further analysis because the image quality of this subject’s MR knee scan was not sufficient to perform the MRI scoring. Therefore, a total of 25 participants were included in our final analysis from whom both- the MR fat pad scoring and synovial fluid biomarker concentrations were available. Synovial proliferations were not assessable in 8 participants, and thus 17 participants were used for analysis of synovial proliferations.
Figure 2.
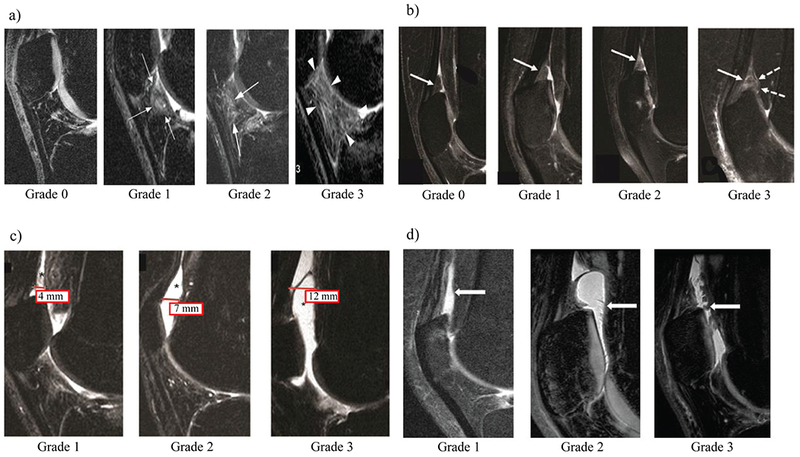
Illustration and description of the MR-based fat pad-synovitis grading scheme used in this study. Four subfeatures were scored on every knee MR scan. (a) The degree of infrapatellar Hoffa fat pad abnormality (IPFP abnormality) was scored on mid-line sagittal FS CUBE and non-fat saturated PD images according to the Anterior Cruciate Ligament OsteoArthritis Score (ACLOAS)31. White arrows point towards abnormal findings of the IPFP at each grade. Grade 0; normal appearing fat pad, only small physiologic vascular structures visible Grade 1; mild hyperintensity of the fat pad, Grade 2; moderate fat pad hyperintensity, Grade 3; severe hyperintense signal changes within the fat pad. (b) The degree of suprapatellar fat pad abnormality (SPFP abnormality) was assessed on sagittal fat-saturated intermediate weighted sequences with a small modification to a previously published grading system32. White arrows show SPFP. Grade 0 was defined by a normal appearing SPFP with isointense signal compared to the prefemoral fat pad. Grade 1 was defined by mild, hyperintense signal alterations in the SPFP compared to the prefemoral fat pad. Grade 2 was defined by moderate hyperintense signal alterations within the SPFP relative to the prefemoral fat pad signal intensity. Grade 3 SPFP was defined by severely hyperintense signal alterations within the SPFP which were accompanied by extensive fraying and/or mass effect of the fat pad. (c) The extent of effusion-synovitis was scored by assessing the anterior-posterior diameter of the joint effusion in mm on sagittal MR-images as described before. In detail, this standardized scoring system was applied to sagittal fat saturated CUBE images in the lateral compartment just mesial to the fibular head unless there was evidence of patellar subluxation: in this case, a mid-fibular head section was used. The suprapatellar recess was used as the point of reference. In detail, effusion-synovitis was graded from 0 to 3 according to the degree of capsular distension with grade 0 being equivalent to a <2 mm anterior-posterior diameter of the effusion. A joint effusion spanning ≥2 and <5 mm in the anterior-posterior (ap) diameter on the mid-slice sagittal image was graded as 1, while a joint effusion between ≥5 and <10 mm was graded as 2. Any effusion measuring equal or more than 10 mm in the ap-diameter was scored as grade 3. (d) Synovial proliferation grading scheme. White arrows point towards synovial proliferations. The presence and severity of synovial proliferations was assessed on sagittal fat saturated CUBE and non-fat saturated PD images in the suprapatellar recess and other visible areas of the joint. Grade 1 corresponded to a smooth synovium, with no proliferation or synovial bands visible; grade 2 was defined as a mild irregularity of the synovium, either focal or diffuse, and the presence of some synovial bands or small bodies; grade 3 was defined as extensive synovial thickening with irregular villo-nodular proliferation. MR = Magnetic Resonance; ACLOAS = Anterior Cruciate Ligament OsteoArthritis Scoring as published by Roemer et al31.
Statistical Analysis
The distribution of parameters was explored via visualization of histograms, Q-Q plots and Shapiro Wilk tests. Means and standard deviations of demographic and anthropometric parameters were calculated. For all biomarker assay results below the lower limit of detection (LLOD), the value 1/2 LLOD was imputed as outlined in detail by Vexler et al. 33 This imputation method was chosen over others as it is well-established and known for its simplicity and low bias.34, 33 The results between each MR grade were compared using Dunnett’s test and performed using IBM SPSS® Statistics 22 (IBM, New York, NY). Statistical significance was assumed at a level of p<0.05. In order to determine the association among synovial fluid biomarkers, the MRI fat pad grades and T1ρ and T2 values of knee cartilage, Spearman’s rank correlation analyses were performed using partial correlation coefficients adjusted for age and gender. As this study was exploratory in nature and in order to avoid overfitting given the relatively small sample size of the study, we limited the adjustments to age and gender. BMI was not controlled for given the low variation in BMI found for this population. Due to the exploratory nature of the study and as the purpose of this study was in the first line to look for potential relationships that can be studied in more depth prospectively in future work, we did not correct for multiple comparison testing, but have rather cautiously interpreted our findings. The 95% confidence intervals for the partial correlation coefficients were calculated using an implementation of the analytical method described by Ruscio (equations (4) and (7))35. Correlation analyses were performed using Matlab R2017b Statistics and Machine Learning Toolbox (Mathworks, Natick, MA). Statistical significance for partial correlation coefficients was determined when intervals did not contain zero.
Reproducibility
For all synovial biomarkers, more detailed information on assay reproducibility including a list of all intra-assay coefficients of variances can be found in the supplemental material and supplemental Table 1. With respect to reproducibility assessments of the four radiologic subfeatures intraclass correlation coefficients (ICCs) were used. Only intra-reader reproducibility was assessed as all the readings had been performed by the same reader. Overall, measures showed substantial to good agreement with intra-reader ICCs of ≥ 0.80 for the synovial proliferation subfeature, the IPFP subfeature, the SPFP subfeature32, and the effusion-synovitis subfeature31. As the reproducibility of T1rho and T2 measures had been widely validated with ICCs >0.96 and CVs of < 3 %36–39 and as the segmentations for this study were only performed by one segmentator we did not explicitly assess intra-reader reproducibility measure for this study. However, our rigorous in-house- training scheme required the segmentator to achieve in the training segmentations CVs of < 3 % for intra-reader variation before the actual segmentations for this study could be started.
RESULTS
Subject Characteristics
Included participants with ACL injury were on average 33.3 ± 8.3 years old with a mean BMI of 24.1 ± 3.5 kg/m2. Eleven participants were female and fourteen were male (Supplemental Table 2). The mean time interval between ACL injury and pre-surgical MRI was 52.3 ± 25.2 days. The mean time interval between MRI and surgery (date of synovial fluid collection) was 12.5 ±12.2 days. The time between injury and surgery (including synovial fluid collection was 64.0 ± 27.7 days. All patients had evidence of fat pad abnormalities based on an abnormality of with at least one of the four fat pad features (MR grade≥1) (Supplemental Table 3). There were no significant differences in participant characteristics between different grades of IPFP abnormalities (Supplemental Table 2).
Associations Between MRI Fat Pad/Effusion/Synovitis Features and Synovial Fluid Biomarker Concentrations and Among Synovial Fluid Biomarkers
The degree of IPFP abnormality was significantly associated with several synovial fluid cytokine biomarkers. These included IFN-γ (ρ partial= 0.64, 95% CI (0.26-0.85)), IL-10 (ρ partial= 0.47, 95% CI (0.04-0.75)), IL-6 (ρ partial= 0.56, 95% CI (0.16-0.81)), IL-8 (ρ partial= 0.49, 95% CI (0.06-0.76)), and TNF-α (ρpartial= 0.55, 95% CI (0.14-0.80) (Table 1). In addition, we observed significant correlations between the degree of IPFP abnormality and the synovial levels of cartilage degradation markers such as matrix metalloproteinases MMP-1 und MMP-3 (MMP-1: ρ partial= 0.57, 95% CI (0.17-0.81); MMP-3 (ρ partial= 0.60, 95% CI (0.21-0.83). MMP-1 and MMP-3 concentrations increased with increasing grades of severity of the IPFP abnormality (Figure 3a). With respect to severity of SPFP abnormalities, correlations with synovial markers were not statistically significant except for IL-6 (ρ partial= 0.57, 95% CI (0.17-0.81). Associations of effusion synovitis and synovial proliferation with synovial fluid markers did not achieve statistical significance.
Table 1.
Associations between synovial fluid biomarker concentrations and each of the scored posttraumatic MR- knee features (the degree of infrapatellar and suprapatellar fat pad abnormalities, the extent of effusion synovitis, the extent of synovial proliferation). Associations were assessed using partial correlation coefficients (rhopartial) from Spearman’s rank correlation with adjustments for age and gender.
| Synovial fluid biomarker | Degree of IPFP abnormality | Degree of SPFP abnormality | Degree of Effusion synovitis | Degree of Synovial proliferation | ||||
|---|---|---|---|---|---|---|---|---|
| rho partial | 95% CI | rho partial | 95% CI | rho partial | 95% CI | rho partial | 95% CI | |
| Inflammatory | ||||||||
| IL-1ra (pg/ml) | 0.00 | (−0.41,0.42) | 0.14 | (−0.30,0.53) | −0.23 | (−0.59,0.21) | −0.20 | (−0.66,0.36) |
| IL-1α (pg/ml) | −0.14 | (−0.53,0.30) | 0.24 | (−0.21,0.60) | −0.33 | (−0.66,0.12) | −0.24 | (−0.68,0.33) |
| IFN-γ (pg/ml) | 0.64 | (0.26,0.85) | 0.40 | (−0.04,0.71) | 0.02 | (−0.40,0.43) | 0.30 | (−0.27,0.72) |
| IL-10 (pg/ml) | 0.47 | (0.04,0.75) | 0.37 | (−0.07,0.69) | 0.01 | (−0.41,0.42) | −0.18 | (−0.64,0.38) |
| IL-6 (pg/ml) | 0.56 | (0.16,0.81) | 0.57 | (0.17,0.81) | 0.15 | (−0.28,0.54) | 0.25 | (−0.32,0.69) |
| IL-8 (pg/ml) | 0.49 | (0.06,0.76) | 0.20 | (−0.24,0.58) | 0.06 | (−0.37,0.46) | 0.23 | (−0.34,0.67) |
| TNF-α (pg/ml) | 0.55 | (0.14,0.80) | 0.37 | (−0.07,0.69) | 0.01 | (−0.41,0.43) | 0.05 | (−0.49,0.55) |
| Cartilage degradation | ||||||||
| CTXII (ng/ml) | −0.04 | (−0.45,0.38) | 0.27 | (−0.18,0.62) | 0.10 | (−0.33,0.49) | 0.25 | (−0.32,0.68) |
| COMP (ug/ml) | −0.19 | (−0.56,0.25) | −0.04 | (−0.45,0.38) | −0.09 | (−0.49,0.34) | 0.13 | (−0.42,0.61) |
| sGAG (ug/ml) | −0.24 | (−0.60,0.20) | −0.06 | (−0.47,0.36) | −0.05 | (−0.46,0.37) | −0.10 | (−0.59,0.44) |
| MMP-1 (pg/ml) | 0.57 | (0.17,0.81) | 0.24 | (−0.20,0.60) | 0.17 | (−0.27,0.55) | 0.19 | (−0.37,0.65) |
| MMP-3 (pg/ml) | 0.60 | (0.21,0.83) | 0.19 | (−0.25,0.57) | 0.30 | (−0.14,0.64) | 0.45 | (−0.12,0.80) |
| MMP-9 (pg/ml) | 0.03 | (−0.39,0.44) | 0.05 | (−0.38,0.45) | 0.24 | (−0.20,0.60) | −0.27 | (−0.70,0.31) |
| Collagen Type II synthesis | ||||||||
| CPII (ng/ml) | 0.34 | (−0.10,0.67) | 0.17 | (−0.27,0.55) | 0.23 | (−0.22,0.59) | 0.33 | (−0.25,0.73) |
SPFP=suprapatellar fat pad; IPFP=infrapatellar Hoffa’s fat pad; IL=interleukin; IFN=interferon; TNF=tumor necrosis factor; TSG=tumor necrosis factor-stimulated gene 6 protein; NTX=N-terminal telopeptide; nM BCE=nanomoles bone collagen equivalent; CTXII=C-terminal cross linked telopeptide type II collagen; COMP=cartilage oligomeric matrix protein; sGAG= sulfated glycosaminoglycan; MMP=matrix metalloproteinases; CP II=procollagen II C-peptide.
Figure 3.
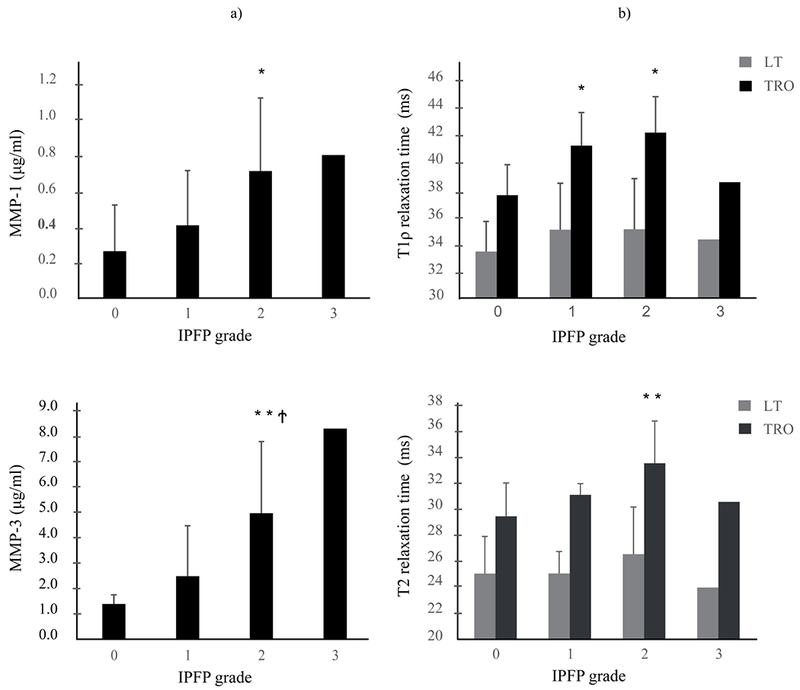
Bar graphs illustrating the relationship between levels of cartilage degradation markers MMP-1 and MMP-3 as measured in the knee joint synovial fluid and the degree of infrapatellar fat pad abnormality (IFPF) (a) and of the relationship between relaxation time measurements of MR-based cartilage compositional markers T1ρ and T2 of the lateral tibia (LT) and trochlea (TRO) and the infrapatellar fat pad (IPFP) grading (b). Concentrations of cartilage degradation markers MMP-1 and MMP-3 as measured in the synovial joint fluid are given in pg/ml (a), T1ρ and T2 cartilage relaxation time measurements are provided in ms (b). Reported are the mean values of (a) MMP-1, 3 and (b) T1ρ, T2 at each IPFP Grading with standard deviation bars.
*P<0.05, **P<0.01, versus Grade 0; †P<0.05, versus Grade 1.
MMP = matrix metalloproteinases.
Several synovial cytokines, including IL-6, -8, -10 and TNF-α were significantly positively correlated with the synovial levels of cartilage degradation markers MMP-1 and MMP-3 with ρ partial values ranging between 0.51 to 0.69 (Table 2). There were no statistically significant correlations of synovial fluid cytokines IL-1ra, -1α, -6, -8, -10, IFN-γ and TNF-α, and the cartilage degradation markers COMP, CTXII and sGAG.
Table 2.
Intra–articular synovial fluid correlations between synovial fluid inflammatory markers and synovial fluid cartilage degeneration markers assessed by partial correlation coefficients (rho partial) obtained by Spearman’s rank correlation with adjustments for age and gender.
| Inflammatory markers |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| IL-1ra | IL-1α | IFN-γ | IL-10 | IL-6 | IL-8 | TNF-α | |||
| Cartilage degeneration markers | CTXII | rho partial | 0.15 | 0.13 | −0.16 | 0.17 | −0.09 | 0.02 | 0.02 |
| 95% CI | (−0.29,0.53) | (−0.31,0.52) | (−0.54,0.28) | (−0.27,0.55) | (−0.49,0.34) | (−0.40,0.43) | (−0.40,0.43) | ||
| COMP | rho partial | 0.01 | 0.12 | −0.19 | 0.03 | −0.20 | 0.19 | 0.10 | |
| 95% CI | (−0.40,0.43) | (−0.31,0.51) | (−0.56,0.25) | (−0.39,0.44) | (−0.57,0.24) | (−0.25,0.57) | (−0.33,0.50) | ||
| sGAG | rho partial | 0.41 | −0.16 | −0.27 | −0.23 | −0.19 | −0.12 | −0.13 | |
| 95% CI | (−0.02,0.72) | (−0.54,0.28) | (−0.62,0.17) | (−0.60,0.21) | (−0.57,0.25) | (−0.51,0.31) | (−0.52,0.30) | ||
| MMP-1 | rho partial | −0.17 | 0.15 | 0.22 | 0.69 | 0.56 | 0.62 | 0.59 | |
| 95% CI | (−0.55,0.27) | (−0.29,0.54) | (−0.22,0.59) | (0.33,0.87) | (0.16,0.81) | (0.23,0.83) | (0.20,0.82) | ||
| MMP-3 | rho partial | −0.19 | 0.13 | 0.32 | 0.57 | 0.51 | 0.61 | 0.56 | |
| 95% CI | (−0.56,0.25) | (−0.31,0.52) | (−0.12,0.66) | (0.16,0.81) | (0.09,0.77) | (0.23,0.83) | (0.16,0.80) | ||
| MMP-9 | rho partial | −0.01 | −0.01 | 0.04 | 0.10 | 0.20 | 0.16 | 0.01 | |
| 95% CI | (−0.42,0.41) | (−0.43,0.41) | (−0.38,0.45) | (−0.33,0.50) | (−0.24,0.57) | (−0.28,0.54) | (−0.41,0.42) | ||
IL=interleukin; IFN=interferon; TNF=tumor necrosis factor; CTXII=C-terminal cross linked telopeptide type II collagen; COMP=cartilage oligomeric matrix protein; sGAG=sulfated glycosaminoglycan; MMP=matrix metalloproteinases.
Associations Between Knee MR-based Fat Pad/Effusion/Synovitis Features and MR-based Cartilage T1ρ and T2 Relaxation Time Measurements
IPFP abnormalities were significantly associated with higher T1ρ values in the trochlear cartilage compartment (p partial= 0.55, 95% CI (0.15-0.80)) (Table3). With respect to T2 cartilage measurements, we found that IPFP abnormalities were significantly associated with higher T2 values in all cartilage compartments, except for the lateral femoral (LFC) and lateral tibial compartment (LT) (MFC: ρ partial= 0.51, 95% CI (0.09-0.77); MT ρ partial= 0.59, 95% CI (0.19-0.82); PAT ρ partial = 0.45, 95% CI (0.02-0.74), TRO ρ partial= 0.58, 95% CI (0.18-0.81)) (Table 3). T1ρ and T2 relaxation times were positively associated with severity of the IPFP abnormality (Figure 3b).
Table 3.
Associations between MR-based fat pad features and cartilage T1ρ and T2 values of the knee assessed by partial correlation coefficients from Spearman’s rank correlation (rho partial, ρ) with adjustments for age and gender.
| MRI fat pad features | Mean Cartilage T1ρ relaxation time values (ms) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MFC | LFC | MT | LT | PAT | TRO | |||||||
| ρ | 95% CI | ρ | 95% CI | ρ | 95% CI | ρ | 95% CI | ρ | 95% CI | ρ | 95% CI | |
| IPFP abnormality | 0.35 | (−0.09,0.68) | 0.23 | (−0.21,0.60) | 0.20 | (−0.24,0.57) | 0.24 | (−0.21,0.60) | 0.35 | (−0.09,0.68) | 0.55 | (0.15,0.80) |
| SPFP abnormality | 0.26 | (−0.18,0.62) | 0.04 | (−0.38,0.45) | 0.17 | (−0.27,0.55) | 0.05 | (−0.37,0.46) | 0.00 | (−0.42,0.41) | 0.19 | (−0.25,0.57) |
| Effusion synovitis | 0.19 | (−0.25,0.56) | 0.16 | (−0.28,0.54) | −0.01 | (−0.43,0.41) | 0.27 | (−0.17,0.62) | 0.15 | (−0.29,0.53) | 0.23 | (−0.21,0.59) |
| Synovial proliferation | 0.55 | (−0.00,0.84) | 0.34 | (−0.23,0.74) | 0.09 | (−0.46,0.58) | 0.38 | (−0.19,0.76) | 0.37 | (−0.21,0.75) | 0.22 | (−0.35,0.67) |
| MRI fat pad features | Mean cartilage T2 relaxation times values (ms) |
|||||||||||
| MFC | LFC | MT | LT | PAT | TRO | |||||||
| ρ | 95% CI | ρ | 95% CI | ρ | 95% CI | ρ | 95% CI | ρ | 95% CI | ρ | 95% CI | |
| IPFP abnormality | 0.51 | (0.09,0.77) | 0.22 | (−0.22,0.58) | 0.59 | (0.19,0.82) | 0.17 | (−0.27,0.55) | 0.45 | (0.02,0.74) | 0.58 | (0.18,0.81) |
| SPFP abnormality | 0.27 | (−0.17,0.62) | 0.05 | (−0.38,0.46) | 0.07 | (−0.36,0.47) | −0.06 | (−0.47,0.36) | 0.06 | (−0.37,0.46) | 0.14 | (−0.29,0.53) |
| Effusion synovitis | 0.16 | (−0.27,0.55) | 0.21 | (−0.23,0.58) | 0.14 | (−0.29,0.53) | 0.39 | (−0.05,0.70) | 0.37 | (−0.07,0.69) | 0.25 | (−0.19,0.61) |
| Synovial proliferation | 0.52 | (−0.04,0.83) | 0.46 | (−0.10,0.80) | 0.32 | (−0.26,0.73) | 0.54 | (−0.01,0.84) | 0.49 | (−0.08,0.82) | 0.29 | (−0.28,0.71) |
IPFP=infrapatellar Hoffa’s fat pad; SPFP=suprapatellar fat pad; MFC=medial femoral condyle; LFC=lateral femoral condyle; MT=medial tibia; LT=lateral tibia; PAT=patella; TRO=trochlea
Associations Between Knee Cartilage T1ρ and T2 Relaxation Times and Synovial Fluid Biomarkers of Cartilage Damage
MMP-1 and -3 concentrations were significantly associated with cartilage T1ρ values in the lateral tibial cartilage compartment (ρ=0.487, P=0.014 and ρ=0.399, P=0.048), and T2 values in the trochlear cartilage compartment (ρ =0.419, P=0.037 and ρ =0.476, P=0.016) respectively). However, after the effect of age and gender was partialled out, the correlations became non-significant (Table 4). With respect to the cartilage bone turnover marker Cartilage oligomeric matrix protein (COMP), we observed a negative significant association between synovial COMP levels and cartilage T2 values in the medial tibial cartilage. No significant associations were seen between synovial COMP levels and other compartmental cartilage T2 and T1rho values.
Table 4.
Associations between T1ρ and T2 values of knee cartilage and synovial fluid biomarkers of cartilage degeneration assessed by partial correlation coefficients (rho partial, ρ) obtained by Spearman’s rank correlation with adjustments for age and gender.
| Biomarkers of cartilage damage | Relaxation times of Cartilage T1ρ (ms) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MFC | LFC | MT | LT | PAT | TRO | |||||||
| ρ | 95% CI | ρ | 95% CI | ρ | 95% CI | ρ | 95% CI | ρ | 95% CI | ρ | 95% CI | |
| CTXII | −0.02 | (−0.44,0.40) | 0.24 | (−0.20,0.60) | 0.19 | (−0.25,0.57) | 0.43 | (−0.01,0.73) | 0.25 | (−0.19,0.61) | 0.09 | (−0.34,0.49) |
| COMP | −0.03 | (−0.44,0.39) | 0.11 | (−0.32,0.51) | −0.28 | (−0.63,0.16) | −0.07 | (−0.48,0.35) | −0.05 | (−0.46,0.37) | −0.36 | (−0.68,0.09) |
| sGAG | 0.08 | (−0.34,0.48) | 0.37 | (−0.07,0.69) | 0.05 | (−0.38,0.45) | −0.05 | (−0.46,0.38) | 0.09 | (−0.34,0.49) | −0.10 | (−0.50,0.33) |
| MMP-1 | −0.03 | (−0.44,0.39) | 0.08 | (−0.35,0.48) | −0.07 | (−0.47,0.36) | 0.42 | (−0.01,0.72) | 0.33 | (−0.11,0.66) | 0.25 | (−0.19,0.61) |
| MMP-3 | −0.04 | (−0.45,0.39) | 0.09 | (−0.34,0.49) | −0.15 | (−0.54,0.29) | 0.35 | (−0.09,0.67) | 0.28 | (−0.16,0.63) | 0.21 | (−0.23,0.58) |
| MMP-9 | −0.26 | (−0.61,0.19) | −0.13 | (−0.52,0.31) | −0.24 | (−0.60,0.21) | −0.09 | (−0.49,0.34) | −0.38 | (−0.70,0.06) | −0.19 | (−0.56,0.25) |
| Relaxation times of Cartilage T2 (ms) |
||||||||||||
| MFC | LFC | MT | LT | PAT | TRO | |||||||
| ρ | 95% CI | ρ | 95% CI | ρ | 95% CI | ρ | 95% CI | ρ | 95% CI | ρ | 95% CI | |
| CTXII | 0.08 | (−0.34,0.48) | 0.15 | (−0.29,0.53) | −0.14 | (−0.53,0.29) | 0.25 | (−0.20,0.61) | 0.20 | (−0.24,0.57) | −0.04 | (−0.45,0.38) |
| COMP | 0.14 | (−0.30,0.53) | 0.18 | (−0.26,0.56) | −0.48 | (−0.76,−0.05) | −0.13 | (−0.52,0.30) | 0.01 | (−0.41,0.42) | −0.20 | (−0.57,0.24) |
| sGAG | 0.17 | (−0.27,0.55) | 0.25 | (−0.19,0.61) | −0.14 | (−0.53,0.29) | 0.02 | (−0.40,0.43) | 0.10 | (−0.34,0.49) | −0.22 | (−0.58,0.23) |
| MMP-1 | 0.15 | (−0.28,0.54) | 0.20 | (−0.24,0.57) | 0.30 | (−0.14,0.64) | 0.21 | (−0.23,0.58) | 0.36 | (−0.08,0.68) | 0.41 | (−0.03,0.71) |
| MMP-3 | 0.19 | (−0.25,0.57) | 0.25 | (−0.19,0.61) | 0.19 | (−0.25,0.57) | 0.26 | (−0.18,0.62) | 0.34 | (−0.10,0.67) | 0.37 | (−0.07,0.69) |
| MMP-9 | −0.18 | (−0.56,0.26) | 0.05 | (−0.38,0.46) | −0.02 | (−0.43,0.40) | −0.18 | (−0.56,0.25) | −0.05 | (−0.46,0.37) | −0.03 | (−0.44,0.39) |
CTXII=C-terminal cross linked telopeptide type II collagen; COMP=cartilage oligomeric matrix protein; sGAG=sulfated glycosaminoglycan; MMP=matrix metalloproteinases; MFC=medial femoral condyle; LFC=lateral femoral condyle; MT=medial tibia; LT=lateral tibia; PAT=patella; TRO=trochlea.
DISCUSSION
In this study we quantified in young healthy patients following ACL tear the extent of knee fat pad abnormalities, the extent of cartilage matrix damage (T1ρ and T2 mapping) via MRI, as well as the concentrations of presurgical synovial knee fluid biomarkers and examined for the first time in humans the associations between those markers. We performed this study primarily as an exploratory correlative attempt to generate first, cross-sectional knowledge in humans on collateral joint damages and potential new candidate factors that may be associated with ACL-injury and that might be worthwhile exploring in more depth in future, prospective ACL- studies with posttraumatic OA as an outcome.
One of our main findings was that the degree of IPFP abnormality after acute ACL injury was was significantly associated not only with most of the synovial cytokine markers, but also with the expression levels of the cartilage degradation markers MMP-1 and MMP-3. Importantly, synovial fluid biomarker levels scaled with the degree of IPFP abnormality. In particular IL-6, INF-ɣ and TNF-ɑ were significantly positively associated with the degree of IPFP abnormality. Given that previous work suggests that the human IPFP contains proinflammatory cytokines such as TNF-ɑ40 and that the IPFP is able to excrete important inflammatory mediators directly into the knee joint15, our findings suggest that these biomarkers may have exuded from the IPFP in concentrations proportional to the degree of IPFP damage reflected in the IPFP MRI abnormality. However further histological work in human IPFP explant tissue harvested from ACL-torn patients have to be carried out to validate our findings.
Somewhat surprisingly, synovial fluid concentrations of the pro-inflammatory cytokines IL-6, -8 and TNF-α were significantly associated with the degree of IPFP abnormality, as were the levels of the anti-inflammatory cytokine IL-10. However, this observation could potentially be explained by the fact that the synovial fluid was collected at the time of ACL reconstruction surgery, a mean 2 months after the acute ACL-injury, and therefore might reflect a transition from a pro- to an anti-inflammatory phase after ACL injury. Based on the literature, ACL- ruptures are accompanied by an immediate pro-inflammatory response, including IL-6, -8 and TNF-α, followed by an anti-inflammatory reparative response41, including anti-inflammatory cytokines such as IL-10.
Another important finding was that we observed significant associations between the degree of IPFP abnormality and T1ρ and T2 relaxation time values of the knee cartilage, especially with the trochlear cartilage. These findings suggest that cartilage damage may occur in the short time span between acute ACL injury and surgery in patients with IPFP abnormality. In this line, one longitudinal study reported that many patients with ACL injury (17% of 111 participants) exhibit signs of patellofemoral OA as early as 1 year after ACL, with the femoral trochlea being the most affected region based on bone marrow lesions (19% of participants), cartilage lesions (31% of participants), and osteophytes (37% of participants)42. Quantitative MRI T1ρ and T2 mapping, used in this study, are more sensitive than MR-based semi-quantitative evaluation techniques for detecting the very earliest degenerative changes in knee cartilage and thereby allow for early assessment of the risk of OA development25. Our findings provide first clues that cartilage damage may occur from very early on, even within the first 1-2 months following an ACL-tear. However, further validation studies with larger sample sizes are needed to corroborate our findings.
Our study has several limitations. First, the sample size of this exploratory study was relatively small. However, despite this limited patient population we found significant correlations between the MR-based fat pad scores and the synovial fluid biomarkers. Future work should prospectively investigate the associations observed in this study. Second, we did not correct for multiple comparison testing as the purpose of this study was primarily to look for potential relationships that can be studied in more detail prospectively in future work. This might have increased the chance for a type I error. Another limitation is that participants were only eligible for this study if they had experienced an ACL tear without a concomitant meniscal injury that needed repair. This was due to the fact that patients having a combined ACL injury with a concomitant meniscus injury needing repair would have been subjected to a different weight bearing and rehabilitation regimen. As different weight bearing scenarios have been shown to alter cartilage T1 rho and T2 values of the knee,43, 44 and as the study was too small for subgroup analyses, we decided against including these patients on the cost of a reduced generalizability of our findings. However, as this study is mainly exploratory in nature we hope it will spur larger studies, in which also individuals with combined ACL-injuries (and meniscus damage needing repair) can be investigated in more detail. We also consider it as a limitation that due to the exploratory nature and the limited sample size of this study we focused exclusively on evaluating cartilage T1rho and T2 values at the -entire compartment level and did not assess correlations between biomarkers and cartilage T1 rho and T2 values at the level of cartilage subcompartments. This approach may have masked findings specific to one subcompartment, but we are hopeful that future dedicated cartilage-centered analyses will elucidate such correlations in more detail. Although contrast-enhanced MRI scans would have been preferable for synovitis grading, a second limitation is that our MRI fat pad and synovitis scores were graded on no contrast-enhanced knee MR images45. Due to possible side effects of the contrast dye, the associated costs, and the more complex handling, contrast-enhanced knee MRI scans have not been a part of large osteoarthritis epidemiological studies such as the MOST and OAI cohorts46. Instead, synovitis features are routinely scored in these larger trials on unenhanced MR images using well-validated scoring systems such as the ACLOAS score47, which we used here as well. Given this, we believe that the composite MR fat pad score that we have used for our study well depicts the amount of fat pad abnormalities. Future histologic studies are recommended to correlate the MR fat pad findings after ACL injury with corresponding histologic analysis.
n conclusion, we detected in this exploratory study in more than 3/4 of young healthy patients with acute ACL rupture varying degrees of infrapatellar fat pad abnormalities. Additionally, a higher degree of infrapatellar fat pad abnormality prior to ACL reconstruction was associated with higher synovial fluid concentrations of cytokines (IL-6, IL-8, −10, TNF-α and IFN-γ) and cartilage degradative markers (MMP-1 and -3) at the time of surgery, and was associated with early cartilage damage (especially in the trochlea) as assessed via T1ρ and T2 cartilage relaxation time measures. These findings suggest that acute trauma of the ACL can be associated with damage to endocrine active joint structures such as the infrapatellar knee joint fat pad. This could result in a potential exudation or secretion of inflammatory fat pad cytokines into the synovial fluid and be partly responsible for the apparent post-injury inflammatory response noted in ACL-injured individuals. However, future longitudinal studies are needed to link ACL-rupture associated fat pad injury with important patient outcomes such as the development of posttraumatic osteoarthritis.
Supplementary Material
Acknowledgments
Role of funding source
The study was supported funding grant funding from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health, (NIH/NIAMS P50 AR060752, NIH/NIAMS P30 066262 and R01AR064771 (TML)). In addition, this study received grant funding from the American Orthopaedic Society for Sports Medicine (AOSSM Cartilage Initiative Grant) and from the Arthroscopy Association of North America (Arthroscopy Association of North America Research Grant).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interest statement
XL reports grant funding from NIH, AOSSM, and AANA. TML reports grant funding from NIH and GE, BM and KA declare grant funding from NIH and AOSSM. BM additionally reports relevant financial activities outside the submitted work for consultancy for Stryker, Conmed and Tornier, royalties from Conmed, and pending grants. UH, KA, BJS, JLH, KM, MT, TS, VBK declare that they have no conflict of interest.
References:
- 1.Poole AR. Osteoarthritis as a whole joint disease. HSS journal. 2012;8(1):4–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dare D, Rodeo S. Mechanisms of post-traumatic osteoarthritis after injury. ACL Current rheumatology reports. 2014;16(10):448. [DOI] [PubMed] [Google Scholar]
- 3.Lohmander L, Östenberg A, Englund M, Roos H. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis & Rheumatism: Official Journal of the American College of Rheumatology. 2004;50(10):3145–3152. [DOI] [PubMed] [Google Scholar]
- 4.Øiestad BE, Engebretsen L, Storheim K, Risberg MA. Knee osteoarthritis after anterior cruciate ligament injury: a systematic review. Am J Sports Med.2009;37(7):1434–1443. doi: 10.1177/0363546509338827 [DOI] [PubMed] [Google Scholar]
- 5.Ajuied A, Wong F, Smith C, Norris M, Earnshaw P, Back D, et al. Anterior cruciate ligament injury and radiologic progression of knee osteoarthritis: a systematic review and meta-analysis. Am J Sports Med. 2014;42(9):2242–2252. doi: 10.1177/0363546513508376 [DOI] [PubMed] [Google Scholar]
- 6.Stiebel M, Miller LE, Block JE. Post-traumatic knee osteoarthritis in the young patient: therapeutic dilemmas and emerging technologies. Open access journal of sports medicine. 2014;5:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown TD, Johnston RC, Saltzman CL, Marsh JL, Buckwalter JA. Posttraumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease. Journal of orthopaedic trauma. 2006;20(10):739–744. [DOI] [PubMed] [Google Scholar]
- 8.Neogi T, Bowes MA, Niu J, De Souza KM, Vincent GR, Goggins J, et al. Magnetic resonance imaging–based three-dimensional bone shape of the knee predicts onset of knee osteoarthritis: data from the Osteoarthritis Initiative. Arthritis & Rheumatism. 2013;65(8):2048–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kannus P, Järvinen M. Age, overweight, sex, and knee instability: their relationship to the post-traumatic osteoarthrosis of the knee joint. Injury. 1988;19(2):105–108. [DOI] [PubMed] [Google Scholar]
- 10.Harkey M, Luc B, Golightly Y, Thomas A, Driban J, Hackney A, et al. Osteoarthritis-related biomarkers following anterior cruciate ligament injury and reconstruction: a systematic review. Osteoarthritis and cartilage. 2015;23(1):1–12. [DOI] [PubMed] [Google Scholar]
- 11.Bigoni M, Sacerdote P, Turati M, Franchi S, Gandolla M, Gaddi D, et al. Acute and late changes in intraarticular cytokine levels following anterior cruciate ligament injury. Journal of Orthopaedic Research. 2013;31(2):315–321. [DOI] [PubMed] [Google Scholar]
- 12.Li H, Chen C, Chen S. Posttraumatic knee osteoarthritis following anterior cruciate ligament injury: Potential biochemical mediators of degenerative alteration and specific biochemical markers. Biomedical reports. 2015;3(2):147–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ioan-Facsinay A, Kloppenburg M. An emerging player in knee osteoarthritis: the infrapatellar fat pad. Arthritis research & therapy. 2013;15(6):225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacConaill M The movements of bones and joints 3. The synovial fluid and its assistants. The Journal of bone and joint surgery British volume. 1950;32(2):244–252. [DOI] [PubMed] [Google Scholar]
- 15.Clockaerts S, Bastiaansen-Jenniskens YM, Runhaar J, Van Osch GJ, Van Offel J, Verhaar J, et al. The infrapatellar fat pad should be considered as an active osteoarthritic joint tissue: a narrative review. Osteoarthritis and Cartilage. 2010;18(7):876–882. [DOI] [PubMed] [Google Scholar]
- 16.Klein-Wieringa I, Kloppenburg M, Bastiaansen-Jenniskens Y, Yusuf E, Kwekkeboom J, El-Bannoudi H, et al. The infrapatellar fat pad of patients with osteoarthritis has an inflammatory phenotype. Annals of the rheumatic diseases. 2011;70(5):851–857. [DOI] [PubMed] [Google Scholar]
- 17.Chamuleau SAJ, Siebes M, Meuwissen M, Koch KT, Spaan JAE, Piek JJ. Association between coronary lesion severity and distal microvascular resistance in patients with coronary artery disease. American Journal of Physiology-Heart and Circulatory Physiology. 2003;285(5):H2194–H2200. doi: 10.1152/ajpheart.01021.2002 [DOI] [PubMed] [Google Scholar]
- 18.He J, Jiang Y, Alexander P, Ulici V, Zhu Y, Wu S, et al. Infrapatellar fat pad aggravates degeneration of acute traumatized cartilage: a possible role for interleukin-6. Osteoarthritis and cartilage. 2017;25(1):138–145. [DOI] [PubMed] [Google Scholar]
- 19.Draghi F, Torresi M, Urciuoli L, Gitto S. Magnetic resonance signal abnormalities within the pericruciate fat pad: a possible secondary sign for acute anterior cruciate ligament Tears. Canadian Association of Radiologists Journal. 2017;68(4):438–444. [DOI] [PubMed] [Google Scholar]
- 20.Abreu MR, Chung CB, Trudell D, Resnick D. Hoffa’s fat pad injuries and their relationship with anterior cruciate ligament tears: new observations based on MR imaging in patients and MR imaging and anatomic correlation in cadavers. Skeletal radiology. 2008;37(4):301–306. [DOI] [PubMed] [Google Scholar]
- 21.Yoon KH, Tak DH, Ko TS, Park SE, Nam J, Lee SH. Association of fibrosis in the infrapatellar fat pad and degenerative cartilage change of patellofemoral joint after anterior cruciate ligament reconstruction. The Knee. 2017;24(2):310–318. [DOI] [PubMed] [Google Scholar]
- 22.Akella SV, Reddy Regatte R, Gougoutas AJ, Borthakur A, Shapiro EM, Kneeland JB, et al. Proteoglycan-induced changes in T1ρD relaxation of articular cartilage at 4T. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine. 2001;46(3):419–423. [DOI] [PubMed] [Google Scholar]
- 23.Li X, Kuo D, Theologis A, Carballido-Gamio J, Stehling C, Link TM, et al. Cartilage in anterior cruciate ligament–reconstructed knees: MR imaging T1ρ and T2—initial experience with 1-year follow-up. Radiology. 2011;258(2):505–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X, Cheng J, Lin K, Saadat E, Bolbos RI, Jobke B, et al. Quantitative MRI using T1ρ and T2 in human osteoarthritic cartilage specimens: correlation with biochemical measurements and histology. Magnetic resonance imaging. 2011;29(3):324–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zarins Z, Bolbos R, Pialat J, Link T, Li X, Souza R, et al. Cartilage and meniscus assessment using Tlrho and T2 measurements in healthy subjects and patients with osteoarthritis. Osteoarthritis and cartilage. 2010;18(11): 1408–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amano K, Huebner JL, Stabler TV, Tanaka M, McCulloch CE, Lobach I, et al. Synovial fluid profile at the time of anterior cruciate ligament reconstruction and its association with cartilage matrix composition 3 years after surgery. The American journal of sports medicine. 2018;46(4):890–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gurtler R, Stine R, Torg J. Lachman test revisited. Contemporary orthopaedics. 1990;20(2):145–154. [PubMed] [Google Scholar]
- 28.Amano K, Huebner JL, Stabler TV, Tanaka M, McCulloch CE, Lobach I, et al. Synovial fluid profile at the time of anterior cruciate ligament reconstruction and its association with cartilage matrix composition 3 years after surgery. The American journal of sports medicine. 2018;46(4):890–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gong J, Pedoia V, Facchetti L, Link TM, Ma CB, Li X. Bone marrow edema-like lesions (BMELs) are associated with higher T1ρ and T2 values of cartilage in anterior cruciate ligament (ACL)-reconstructed knees: a longitudinal study. Quantitative imaging in medicine and surgery. 2016;6(6):661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li X, Han ET, Busse RF, Majumdar S. In vivo T1ρ mapping in cartilage using 3D magnetization-prepared angle-modulated partitioned k-space spoiled gradient echo snapshots (3D MAPSS). Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine. 2008;59(2):298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roemer FW, Frobell R, Lohmander LS, Niu J, Guermazi A. Anterior Cruciate Ligament OsteoArthritis Score (ACLOAS): longitudinal MRI-based whole joint assessment of anterior cruciate ligament injury. Osteoarthritis and cartilage. 2014;22(5):668–682. [DOI] [PubMed] [Google Scholar]
- 32.Schwaiger BJ, Wamba JM, Gersing AS, Nevitt MC, Facchetti L, McCulloch CE, et al. Hyperintense signal alteration in the suprapatellar fat pad on MRI is associated with degeneration of the patellofemoral joint over 48 months: data from the osteoarthritis initiative. Skeletal radiology. 2018;47(3):329–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vexler A, Tao G, Chen X. A toolkit for clinical statisticians to fix problems based on biomarker measurements subject to instrumental limitations: from repeated measurement techniques to a hybrid pooled-unpooled design. Methods Mol Biol. 2015;1208:439–460. doi: 10.1007/978-1-4939-1441-8_31 [DOI] [PubMed] [Google Scholar]
- 34.Adams SB, Setton LA, Bell RD, Easley ME, Huebner JL, Stabler T, et al. Inflammatory cytokines and matrix metalloproteinases in the synovial fluid after intra-articular ankle fracture. Foot & ankle international. 2015;36(11):1264–1271. [DOI] [PubMed] [Google Scholar]
- 35.Ruscio J Constructing confidence intervals for Spearman’s rank correlation with ordinal data: a simulation study comparing analytic and bootstrap methods. Journal of Modern Applied Statistical Methods. 2008;7(2):7. [Google Scholar]
- 36.Li X, Pedoia V, Kumar D, Rivoire J, Wyatt C, Lansdown D, et al. Cartilage T1ρ and T2 relaxation times: longitudinal reproducibility and variations using different coils, MR systems and sites. Osteoarthritis and cartilage. 2015;23(12):2214–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gupta R, Virayavanich W, Kuo D, Su F, Link T, Ma B, et al. MR T1ρ quantification of cartilage focal lesions in acutely injured knees: correlation with arthroscopic evaluation. Magnetic resonance imaging. 2014;32(10):1290–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wise BL, Niu J, Guermazi A, Liu F, Heilmeier U, Ku E, et al. Magnetic resonance imaging lesions are more severe and cartilage T2 relaxation time measurements are higher in isolated lateral compartment radiographic knee osteoarthritis than in isolated medial compartment disease–data from the Osteoarthritis Initiative. Osteoarthritis and cartilage. 2017;25(1):85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chanchek N, Gersing AS, Schwaiger BJ, Nevitt MC, Neumann J, Joseph GB, et al. Association of diabetes mellitus and biochemical knee cartilage composition assessed by T2 relaxation time measurements: data from the osteoarthritis initiative. Journal of Magnetic Resonance Imaging. 2018;47(2):380–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ushiyama T, Chano T, Inoue K, Matsusue Y. Cytokine production in the infrapatellar fat pad: another source of cytokines in knee synovial fluids. Annals of the rheumatic diseases. 2003;62(2):108–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lieberthal J, Sambamurthy N, Scanzello CR. Inflammation in joint injury and post-traumatic osteoarthritis. Osteoarthritis and cartilage. 2015;23(11):1825–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Culvenor AG, Collins NJ, Guermazi A, Cook JL, Vicenzino B, Khan KM, et al. Early knee osteoarthritis is evident one year following anterior cruciate ligament reconstruction: a magnetic resonance imaging evaluation. Arthritis & Rheumatology. 2015;67(4):946–955. [DOI] [PubMed] [Google Scholar]
- 43.Nishii T, Kuroda K, Matsuoka Y, Sahara T, Yoshikawa H. Change in knee cartilage T2 in response to mechanical loading. J Magn Reson Imaging. 2008;28(1):175–180. doi: 10.1002/jmri.21418 [DOI] [PubMed] [Google Scholar]
- 44.Souza RB, Baum T, Wu S, Feeley BT, Kadel N, Li X, et al. Effects of unloading on knee articular cartilage T1rho and T2 magnetic resonance imaging relaxation times: a case series. J Orthop Sports Phys Ther. 2012;42(6):511–520. doi: 10.2519/jospt.2012.3975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Lange-Brokaar B, Ioan-Facsinay A, Yusuf E, Visser A, Kroon H, Andersen S, et al. Degree of synovitis on MRI by comprehensive whole knee semi-quantitative scoring method correlates with histologic and macroscopic features of synovial tissue inflammation in knee osteoarthritis. Osteoarthritis and cartilage. 2014;22(10):1606–1613. [DOI] [PubMed] [Google Scholar]
- 46.Roemer FW, Guermazi A, Felson DT, Niu J, Nevitt MC, Crema MD, et al. Presence of MRI-detected joint effusion and synovitis increases the risk of cartilage loss in knees without osteoarthritis at 30-month follow-up: the MOST study. Annals of the rheumatic diseases. 2011;70(10):1804–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roemer FW, Guermazi A, Zhang Y, Yang M, Hunter DJ, Crema MD, et al. Hoffa’s fat pad: evaluation on unenhanced MR images as a measure of patellofemoral synovitis in osteoarthritis. American Journal of Roentgenology. 2009;192(6):1696–1700. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


