Summary :
Efficient mechanisms of central tolerance, including receptor editing and deletion, prevent highly self-reactive B cell receptors (BCRs) from populating the periphery. Despite this, modest self-reactivity persists in (and may even be actively selected into) the mature B cell repertoire. In this review, we discuss new insights into mechanisms of peripheral B cell tolerance that restrain mature B cells from mounting inappropriate responses to endogenous antigens, and place recent work into historical context. In particular, we discuss new findings that have arisen from application of a novel in vivo reporter of BCR signalling, Nur77-eGFP, expression of which scales with the degree of self-reactivity in both monoclonal and polyclonal B cell repertoires. We discuss new and historical evidence that self-reactivity is not just tolerated, but actively selected into the peripheral repertoire. We review recent progress in understanding how dual expression of the IgM and IgD BCR isotypes on mature naïve follicular B cells tunes responsiveness to endogenous antigen recognition, and discuss how this may be integrated with other features of clonal anergy. Finally, we discuss how expression of Nur77 itself couples chronic antigen stimulation with B cell tolerance.
Keywords: anergy, Nur77/Nr4a1, IgM, IgD, tolerance, B cell
1). Introduction
Classic studies of BCR transgenic mice from Nemazee, Goodnow, Weigert and colleagues revealed that immature B cells possess the capacity to edit their light chains in order to reduce self-reactivity that is inadvertently acquired through random somatic VDJ recombination, while clones that fail to do so are eliminated by deletion1–5. These mechanisms are collectively termed ‘central tolerance’ (recently reviewed by David Nemazee6), and are thought to provide a critical safeguard against overt autoimmunity. By directly cloning human BCRs, Wardemann and colleagues subsequently showed that reactivity to DNA and poly-reactivity are indeed disturbingly common among immature human B cells, and that these specificities are efficiently pruned from the maturing B cell repertoire during BM development, presumably by a combination of editing and deletion 7. Despite elimination of highly self-reactive B cell clones early during BM development, it has been argued that modest self-reactivity persists in (or is actively selected into) the mature B cell repertoire of normal mice and healthy humans. It is posited that layered mechanisms of peripheral B cell tolerance exist to restrain such B cells from mounting inappropriate responses to endogenous antigens. In this review, we discuss studies from our lab and others that have shed light on the positive and negative selection of the primary mature B cell repertoire. We discuss the teleological rationale for selecting and preserving modest self-reactivity in the primary pre-immune B cell repertoire. Finally, we review recent insights into the molecular basis of peripheral tolerance mechanisms that constrain self-reactive B cells, and position these mechanisms in the context of classic foundational work on this topic.
2). Self-reactivity in the mature B cell repertoire
Studies in humans and mice provide independent lines of evidence that some degree of self-reactivity is present in the mature, naturally occurring B cell repertoire. However, the nature, extent, and significance of this has been long-debated, in part because there has been no gold-standard definition of self-reactivity. Published studies can be broken down into those that provide either structural or functional evidence for self-reactivity (i.e. measurement of soluble Ab binding to candidate antigens, and assays of B cell responses to candidate antigens, respectively). This is a crucial distinction because the sensitivity of ELISA, immunofluorescence assay (IFA), or surface plasmon resonance (SPR) for detection of antigen-Ab binding does not faithfully capture biophysical features of interactions between membrane-associated BCRs on naïve B cells and bona fide self-antigens encountered in vivo. Membrane-associated and/or polymeric antigens can trigger BCR signaling with much greater potency than soluble monomeric or oligomeric forms and can compensate for low intrinsic binding affinity with extremely high avidity. Further, even low affinity antigen-BCR interactions, undetectable by ELISA, are biologically relevant and can be sufficient to drive normal humoral immune responses. Indeed, it has been argued that such low affinity interactions characterize most responses to foreign antigens, while only affinity-matured BCRs routinely exhibit detectable binding to monomeric antigen in vitro. Indeed, the HIV literature contains examples of germline-reverted broadly neutralizing antibodies (bNabs) that have no detectable antigen binding8,9.
Wardemann et al. reported that BCRs cloned from mature follicular (Fo) human B cells, in contrast to immature BM B cells, exhibit little DNA-reactivity and poly-reactivity (defined as binding to more than one antigen among a panel including ssDNA, dsDNA, insulin, and LPS), but a significant fraction (20%) can bind cytoplasmic antigens (assessed by immunofluorescent staining of permeabilized cells using cloned Abs)7. Very recently, Kelsoe and colleagues revisited this question (reviewed elsewhere in this volume) in a more high-throughput manner by establishing Nojima cultures of single human B cells which generate massive expansion and plasma cell differentiation, yielding high concentrations of monoclonal Abs for downstream assays10. They subsequently assessed reactivity of monoclonal IgG from these cultures against a large panel of ‘self’ and ‘foreign’ antigens using an illuminex bead-based assay. Over 1000 monoclonal Abs from 9 healthy donors were tested against about 25 candidate antigens. Both self-reactivity and poly-reactivity (defined as reactivity to both self and foreign Ag) were reduced by about 50% between transitional and mature B cell populations (and strikingly, as previously reported, this checkpoint is defective in SLE patients11). Just under 15% of mature B cells from healthy donors were reactive to at least one self-Ag in this panel, within the avidity limits of detection of this binding assay. Both conclusions are in reasonable agreement with prior work by Wardemann and colleagues despite the distinct methodologies employed7.
There are, nevertheless, at least three major questions raised by these studies; first, is self-reactivity a feature of only a minor fraction (15–20% as noted above) of the mature repertoire, or is there instead a continuous distribution of self-reactivity, some of which falls below the limits of detection of in vitro binding assays described above? Second, is the degree of self-reactivity in the mature repertoire of functional significance – are these self-antigens actually encountered and ‘sensed’ by mature B cells in vivo? Third, what are the relevant self-antigens encountered by developing B cells in vivo, and does the normal mature B cell repertoire harbor reactivity to bona fide self-antigens beyond the spectrum of specificities probed in these studies? There is certainly some evidence for the latter possibility; the self-reactive human VH4–34 heavy chain, expressed by 5–10% of the normal mature B cell repertoire, has been reported to recognize Ii antigen on RBCs and B220 on the surface of B cells 12,13. Given the vast range of potential self-antigens a B cell could recognize through its antigen receptor, how can such varied interactions be explored in an unbiased manner?
Recently, Jumaa and colleagues took a functional approach to assess self-reactivity of the mature B cell repertoire by probing antigen-induced calcium entry, rather than exclusively relying upon direct assays of antigen binding to soluble recombinant Abs 14. Interestingly, direct comparison of ELISA, SPR, FACS-based detection of antigen binding by membrane BCRs on B cells, and cell-based calcium assay, suggests that the functional calcium assay is the most sensitive of these for certain model antigens. Other differences were noted as well; soluble Abs with detectable binding to dsDNA also trigger calcium entry when expressed as IgM isotype BCRs on B cells, but fail to do so when expressed as IgG1 BCRs. Notably, the authors observe significant differences in Ag binding to previously reported polyreactive BCRs when these were expressed as soluble Ab or as cell surface BCRs. Such profound divergence between in vitro binding assays and ‘cell-based’ functional assays highlight how critical functional readouts may be in order to define what constitutes biologically relevant self-reactivity in the primary B cell repertoire. However, neither approach overcomes a primary limitation of studying self-reactivity of a diverse repertoire, namely that the identity and nature of endogenous antigens is largely unknown.
Nur77-eGFP BAC Tg reporter of antigen receptor signaling
Previous work from our lab exploiting an in vivo reporter of antigen recognition, Nur77-eGFP BAC Tg, addresses this important point (Figure 1A)15. GFP expression in this reporter line is under the control of the regulatory region of Nr4a1/ Nur77. Nr4a1–3 encode a small family of orphan nuclear hormone receptors which were originally cloned as signal-dependent primary response genes (a.k.a. immediate-early genes), and are highly upregulated by a range of mitogens, including antigen receptor stimulation 16–18. Consequently, antigen stimulation rapidly triggers reporter expression in B and T cells in vitro (Figure 1B), and GFP is also induced by infection or immunization in antigen-specific lymphocytes in vivo 15,19–21. Most strikingly, we observed a broad distribution of reporter expression in mature naïve Fo B cells in the absence of exogenous immune stimuli, and went on to show that endogenous antigen is both necessary and sufficient for such expression under steady state conditions in vivo (Figure 1C)15. We did so by taking advantage of a BCR Tg that harbors extremely high reactivity towards a foreign antigen, hen egg lysozyme (HEL). Forced expression of the IgHEL BCR Tg in reporter mice in the absence of cognate HEL antigen eliminated most GFP expression in Fo B cells, implying that endogenous antigen recognition is necessary for GFP expression. Conversely, introducing cognate antigen (soluble HEL Tg) into this genetic background was sufficient to reconstitute high GFP expression. We further showed that reporter expression was sensitive to genetic modulation of BCR signal strength via titration of the receptor-like tyrosine phosphatase CD45. These observations led us to hypothesize that Nur77-eGFP served as a functional readout of endogenous antigen encounter in vivo and might therefore represent a bona fide maker of self-reactivity that was extremely sensitive (more so than proximal biochemical events such as calcium entry) but not subject to the limitations of in vitro binding assays (e.g. ELISA, IFA, SPR). Moreover, because the reporter operates in vivo, its expression ought to reflect B cell reactivity to native conformations (and concentrations) of bona fide endogenous antigens, and importantly does not rely upon identification of such antigens. In support of this hypothesis, we observed that reporter expression among mature Fo B cells was correlated with anti-nuclear reactivity and with downregulation of IgM (but not IgD) BCR expression, a well-recognized feature of self-reactive B cells (discussed later in this review; Figures 1D, E)15,22,23. We also identified functional evidence of chronic antigen encounter – basal calcium levels were elevated in GFPHI B cells relative to GFPLO B cells15. In subsequent work, we further excluded the contribution of other immunoreceptor pathways (especially those mediated by microbial stimuli) as well as commensal flora itself to Nur77 expression in B cells under steady-state conditions 24. Although NF-κB-dependent mitogenic stimuli can drive reporter upregulation in vitro, in vivo B cell expression of Nur77-eGFP under steady state conditions is specifically regulated by antigen and BCR signaling, but not by other immunoreceptors (including CD40, MyD88-dependent TLRs, Unc93B1-dependent TLRs 3, 7 and 9, BAFF, CXCR4, and Jak-Stat-dependent cytokine receptors; Table 1). Therefore, we propose that Nur77-eGFP expression reflects endogenous antigen encounter and self-reactivity among mature Fo B cells.
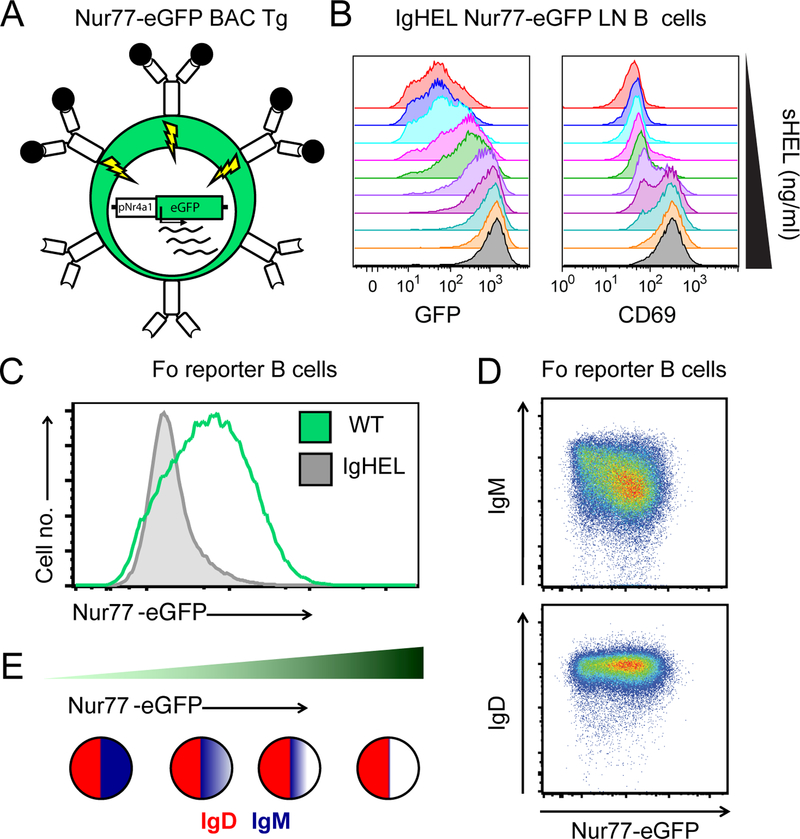
Figure 1. Nur77-eGFP BAC Tg reporter of antigen receptor signaling marks self-reactive B cells in vivo.
A. Schematic of Nur77-eGFP BAC Tg depicts eGFP transcript under the control of the regulatory region of Nr4a1. Since Nr4a1 is a primary response gene (PRG) that is rapidly transcribed in response to antigen receptor signaling, antigen encounter results in rapid GFP induction in reporter B cells.
B. IgHEL BCR Tg B cells harboring the Nur77-eGFP BAC Tg were stimulated in vitro with varying doses of soluble HEL antigen, and both GFP and CD69 expression were assessed via FACS after 24 hours.
C. Mature follicular (Fo) splenic B cells (CD23HICD93-B220+) from Nur77-eGFP BAC Tg mice with or without IgHEL BCR Tg (in the absence of cognate HEL Ag) were assessed for GFP expression via FACS immediately ex vivo. Restricting the BCR repertoire to restrict endogenous antigen recognition markedly reduces GFP expression.
D. Surface IgM and IgD BCR expression on mature Fo splenic B cells from Nur77-eGFP BAC Tg mice was assessed via FACS, revealing that IgM expression but not IgD expression is inversely correlated with GFP. Dynamic range of IgM across the repertoire is large, varying by more than an order of magnitude along a log10 scale.
E. Schematic depicts relative surface expression of IgM (blue) and IgD (red) BCRs on B cells with increasing expression of GFP (and therefore increasing self-reactivity) across the mature Fo B cell repertoire.
Table 1.
Regulation of Nur77/Nr4a1 in B cells
| Conclusion | Stimulus / perturbation | Pathway | Read-out | Cell type | Ref. |
|---|---|---|---|---|---|
| Does not modulate Nur77 at steady state in vivo |
CD40L−/− | CD40 | Nur77-eGFP | B cells | 24 |
| TLR7−/− | TLR7 | Nur77-eGFP | B cells | 24 | |
| Un93b13d/3d | TLR3/7/9 | Nur77-eGFP | B cells | 24 | |
| MyD88fl/flMB1Cre | MyD88 | Endogenous Nur77/Nr4a1 | PerC B1a cells, spleen | 24 | |
| Germ-free mice | MyD88/TRIF | Endogenous Nur77/Nr4a1 | Spl B cells, spleen | 24 | |
| Constit. active STAT5 | JAK/STAT | Nur77-eGFP | thymocytes | 21 | |
| Does not induce Nur77 in vitro |
BAFF | BAFFR | Nur77-eGFP | B cells | 15 |
| IL-4 | JAK/STAT | Nur77-eGFP | B cells | 24 | |
| IL-2, IL-15 | JAK/STAT | Nur77-eGFP | CD8 (IL-2, 15) CD4 (IL-2) |
159 | |
| CXCL12/SDF1 | CXCR4 | Nur77-eGFP | B cells | 24 | |
| Induces Nur77 in vitro | LPS | TLR4 / Rp150 | Nur77-eGFP | B cells | 15,24 |
| CpG | TLR9 | Nur77-eGFP | B cells | 15,24 | |
| PAM3CSK4 | TLR1/2 | Nur77-eGFP | B cells | 24 | |
| Anti-Igk; sHEL Ag | BCR | Nur77-eGFP | B cells | 15,24 | |
| Modulates Nur77 at steady state in vivo | IgHEL BCR Tg | Ag / BCR | Nur77-eGFP | B cells | 15,24 |
| IgHEL BCR Tg / sHEL | Ag / BCR | Nur77-eGFP | B cells | 15,24 | |
| Lyn−/− | BCR via ITIMs | Nur77-eGFP | B cells | 24 | |
| CD45 allelic series | BCR via SFKs | Nur77-eGFP | B cells | 15 |
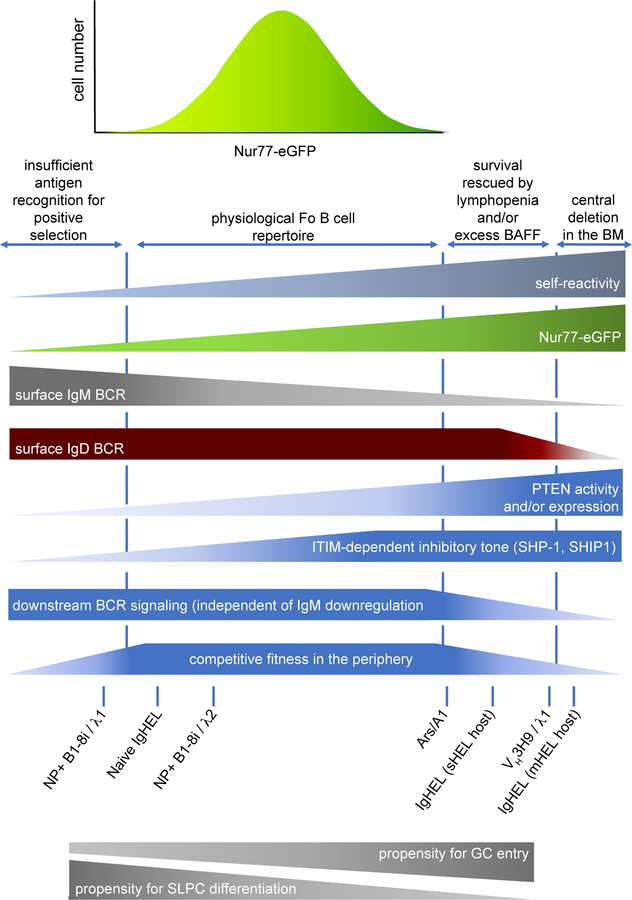
To further validate the fidelity of Nur77-eGFP as a reporter of bona fide self-reactivity in vivo, we turned to an independent, polyclonal model of B cell self-reactivity. VH3H9 is a heavy chain (H chain), originally cloned from the MRL/lpr mouse model of lupus, that biases the entire BCR repertoire towards DNA-reactivity, most strongly when paired with endogenous λ1 light chain (L chain)25. This H chain has been knocked into the endogenous BCR locus to generate site-directed (sd) VH3H9 Tg mice in which the majority of B cells express this H chain paired with endogenous L chains. This model system is extremely powerful because DNA-reactive B cells can be tracked within a polyclonal repertoire on the basis of surface λ1 L chain expression through development and beyond, and because multiple sequential tolerance mechanisms (receptor editing, deletion, anergy) are triggered and can be probed using this model25,26. We generated Nur77-eGFP reporter mice harboring the sd-VH3H9 Tg, and found that dsDNA-reactive λ1+ B cells from these mice turn on GFP reporter expression very early in development, and express much higher levels in maturity than either WT or VH3H9/κ B cells, consistent with chronic self-antigen stimulation27. Conversely, VH3H9 Tg B cells expressing Vλ3 L chains (aka λx) that are known to censor DNA-reactivity express much lower levels of GFP, as do rare ‘escapee’ B cells that express endogenous H chains. Moreover, in this polyclonal model we found that reporter expression also correlated with the degree of functional anergy. Thus, reporter expression correlates with the extent of endogenous antigen recognition under steady state conditions across a broad dynamic range, extending from non-self-reactive ‘naïve’ IgHEL BCRs (in the absence of cognate antigen) across the normal B cell repertoire, all the way to some of the most highly self-reactive BCR Tgs studied in mice. Most importantly, observations with this reporter demonstrate that the bulk of the physiologic mature Fo B cell compartment in C57Bl/6 mice harbors modest ‘self-reactivity’ (as functionally defined by reporter expression) that spans a continuum. The Nur77-eGFP reporter therefore represents a unique and very useful tool to facilitate studies of the selection and tolerance of self-reactive B cells.
3). Positive selection of the mature B cell repertoire by antigen recognition
Although modest self-reactivity of mature Fo B cells may persist because it simply falls below the threshold required to trigger central tolerance, it has also been proposed that some degree of self-reactivity is actively selected into the mature compartment and that a minimal threshold of self-reactivity (rather than just tonic BCR signaling) is actually required for optimal B cell development. However, this has been a hotly debated topic among B cell biologists, and unlike thymic development where positive selection imposes MHC restriction, the teleological rationale for positive selection of B cells by antigen is less obvious. Perhaps the clearest and most elegant evidence for antigen-dependent positive selection of B cells comes from studies of the innate-like B1a cell compartment. B1a cells have a fetal origin, are maintained by self-renewal, and exhibit an extremely restricted and highly self-reactive BCR repertoire; more than a third of the repertoire comprises only a handful of BCRs with a shared CDR3 sequence that recognizes phosphatidylcholine, an epitope exposed on the surface of dying cells 28–30. Forced expression of such BCRs via Tg models greatly expands the pool of B1a cells, and recent provocative work from Rajewsky and colleagues suggests this BCR specificity may even be sufficient to ‘convert’ B2 cells into “B1a-like” cells 29,31. Hayakawa and colleagues showed formally that Thy-1 antigen expression is necessary for Thy-1-specific (anti-Thy-1 Ab or ATA) B cells to enter the B1a compartment 32,33. By contrast, marginal zone (MZ) ATA B cell development is disfavored both at high levels of antigen and in the complete absence of antigen, but proceeds optimally at low levels of antigen, providing striking evidence not only for a sharp upper threshold for negative selection, but also a minimal degree of self-reactivity required for positive selection into this compartment 34. Dependence of B1a and MZ B cell development on strong and weak BCR signal strength respectively is likely due to such distinct thresholds for positive and negative selection 30,35. By contrast to both B1a and MZ B cells, ATA Fo B cells developed even in Thy-1 deficient mice, and IgHEL Fo B cells similarly develop in the absence of cognate soluble HEL antigen, raising questions about whether endogenous antigen recognition is necessary for Fo B cell development, or whether tonic BCR signaling is sufficient 32–34. Indeed, mixed evidence for and against antigen-dependent positive selection into the Fo compartment exists in the B cell literature 36–40. Since competition appears to play a role in this process, one challenge has been tracking development of specific B cell clones within a polyclonal repertoire 41. Another major limitation of many studies has been that the degree of cross-reactivity between BCR Tg cells and endogenous antigen (as opposed to cognate model antigen) is unknown. This has in turn made it hard to draw definitive conclusions about the minimal amount of self-reactivity and antigen-dependent signaling (as opposed to tonic BCR signaling) required for entry into mature B cell compartments, especially the Fo compartment.
Recently, we took a novel approach to address this question by capitalizing on the Nur77-eGFP reporter of endogenous antigen recognition162. B1–8i Tg mice express a fixed H chain, but a polyclonal set of endogenous L chains, approximately 5% of which are capable of binding the NP hapten42. We identified two minor B cell populations in B1–8i H chain Tg mice that each recognize the NP hapten, but differ in their L chain use and their degree of self-reactivity (as marked both by Nur77-eGFP expression and other indicators). We found that the population with less self-reactivity, NP+ Igλ1+, displays profoundly impaired entry not only into the B1a compartment, but also exhibits counter-selection during both Fo and MZ B2 cell development in the spleen. By contrast, modest self-reactivity of NP+ Igλ2+ B cells, while still counter-selected in the B1a compartment, was sufficient to drive efficient development of both MZ and Fo B cells. By titrating CD45 expression in order to genetically modulate BCR signal strength independently of antigen recognition, we established that counter-selection of NP+ Igλ1+ B cells was due to impaired positive selection rather than negative selection. Moreover, as previously noted in the ATA mode, we found that the degree of self-reactivity required for positive selection into the B1a compartment drives negative selection in the B2 compartment (see model, Figure 2) 32,33. Our data also suggested that the minimal BCR signaling threshold for entry into MZ is higher than that for entry into Fo, consistent with observations in the ATA model. Since NP+ Igλ1+ B cells express high levels of a functional BCR on their surface (as demonstrated by their efficient participation in NP-dependent immune responses), these data collectively demonstrate that purely tonic BCR signaling is not sufficient for efficient entry into any mature B cell compartment. Rather, endogenous antigen recognition is required for optimal B cell development.
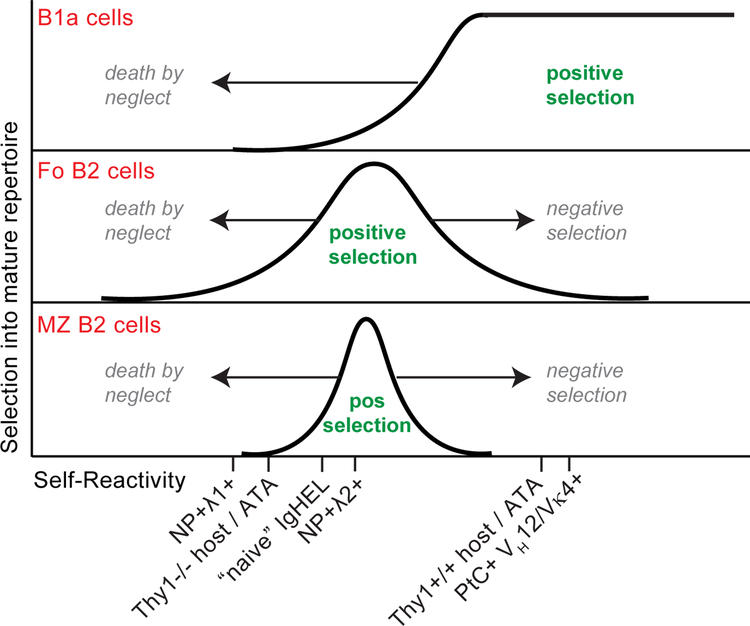
Figure 2. Model: Antigen-dependent signaling thresholds for positive and negative selection of mature B cell lineages.
Y-axis depicts efficiency of selecting individual BCRs of varying self-reactivity (x-axis) into B1a, Fo, and MZ mature B cell compartments. Area under each curve corresponds to range of BCRs that are positively selected into each compartment. Area to the left of each curve corresponds to BCRs that are counter-selected because of insufficient endogenous antigen recognition (“death by neglect”), while BCRs to the right of the curve are counter-selected because of excess self-reactivity (negative selection). Putative degree of self-reactivity and selection pressures of particular Tg BCR specificities is depicted. As previously described in the ATA model, antigen receptor signaling that normally drives negative selection of B2 cells is required for positive selection of B1 cells. Further, entry into the Fo B2 cell compartment is more permissive - at both the low and high end - than that for development of MZ B2 cells.
Where exactly does the antigen-dependent signaling threshold for optimal B2 cell development lie? To establish this, we compared the degree of Nur77-eGFP expression in these two NP-binding populations relative to the physiologic B cell repertoire, and also relative to naïve IgHEL BCR Tg cells from hosts lacking cognate HEL Ag expression. We found that cross-reactivity of the IgHEL BCR to bona fide endogenous antigens – while quite low relative to the normal B cell repertoire (see Figure 1C) – actually lies in between the two NP-binding populations that we studied. Importantly, Nur77-eGFP seems to mark a cell-intrinsic degree of BCR self-reactivity; altering the constitution of the bulk BCR repertoire via mixed bone marrow chimeras did not change the GFP expression in these clonal populations. We speculate that ATA B cells exhibit a low degree of cross-reactivity to endogenous antigens (just above that of NP+ Igλ1+ B cells) that is sufficient for Fo B cell development, but below that required for MZ B cell entry. Taken together, we can position these specificities relative to one another in terms of self-reactivity and can identify a minimal degree of self-reactivity required for efficient entry into all mature B cell compartments (Figure 2). These observations also raise provocative questions about how the extremely low degree of self-reactivity of NP-specific Igλ1+ B cells might influence their participation in typical immune responses to this model immunogen; could their behavior diverge from that of more typically self-reactive clones?
Importantly, qualitatively unique structural features of certain self-Ags (such as co-stimulatory capacity), in addition to the avidity of their interaction with BCR, may also regulate selection of specific B cell clones. For example, the profound restriction of the B1a repertoire and unique capacity of PtC-specific BCR Tgs to drive B1a fate suggests that antigen recognized by such BCRs delivers additional signals superimposed on ‘strong’ BCR stimulation28,31. Indeed, work from the Barton lab shows that expansion of PtC-specific BCRs in the B1a compartment is regulated by endosomal TLRs that recognize nucleic acids (Kreuk, L. UC Berkeley PhD thesis, 2018; Kreuk, L., B Cell Keystone conference, Dresden, 6/2018). Conversely, endosomal TLRs appear to play a role in mediating central tolerance towards DNA and RNA-reactive BCRs that deliver such TLR ligands to endosomal compartments43.
The rationale for limiting self-reactivity in mature B cells is self-evident, but why should modest endogenous antigen recognition be actively selected during B cell development? Surely, tonic BCR signaling could ensure successful pairing of edited L chains with H chains to generate a functional BCR? It has been postulated that selection of mild self-reactivity biases the BCR repertoire towards recognition of essential biological patterns displayed on microbes 44,45. Retention of some self-reactivity in the mature B cell repertoire may serve to limit ‘holes’ in the B cell repertoire generated by central tolerance mechanisms. Such holes could in turn be exploited by pathogens to evade immune recognition; in fact, deletion of self-reactive precursor BCRs is thought to pose a barrier to generation of broadly-neutralizing antibodies (bNAbs) against HIV for this reason 46,47. Conversely, relaxing the stringency of negative selection and peripheral tolerance may facilitate evolution of HIV bNAbs 48. Kelsoe and colleagues showed that many self-reactive human B cells are indeed cross-reactive to foreign antigens10. Importantly, they show that the frequency of such ‘poly-reactive’ clones declines with B cell maturation, providing direct evidence for generation of such ‘holes’ as a general consequence of tolerance checkpoints, rather than just a special case10. A related explanation is that self-reactivity ensures a mild degree of poly-reactivity among the pre-immune B cell repertoire, which may in turn serve to ensure that the primary B cell response to any given foreign antigen is polyclonal and therefore diverse. Such diversity may facilitate both competition and an efficient search of ‘mutational space’ in the germinal center, thereby optimizing affinity maturation. In contrast to deletion, clonal anergy - which is a reversible state - may be the preferred tolerance mechanism by which mature B cells maintain quiescence in the face of self-antigen recognition in order to retain self-reactivity in the mature repertoire.
4). Clonal anergy in BCR Tg models and naturally occurring self-reactive B cells
BCR Tg mouse models
If (modest) self-reactivity is positively selected into the periphery, how are such B cells restrained from mounting immune responses to self-antigen? One of the first - and still perhaps the most well-studied - genetic models of self-reactive B cells, was developed by Goodnow and colleagues, and takes advantage of the model antigen hen egg lysosome (HEL). When a BCR Tg (IgHEL) specific for HEL is introduced onto a genetic background expressing soluble HEL antigen (sHEL Tg), B cells are not eliminated through deletional tolerance as they are in the presence of membrane-associated HEL (mHEL). Rather, they make it to the periphery, but, in contrast to a background lacking cognate antigen they do not secrete high levels of anti-HEL IgM at steady state or in response to HEL-SRBC immunization 49. The functional silencing or unresponsiveness of these ‘self-reactive’ B cells is termed clonal anergy, a phenomenon that encompasses a range of independent non-deletional molecular mechanisms of peripheral B cell tolerance50. In fact, virtually every associated feature of clonal anergy was first described in this model, including attenuation of BCR signal transduction, selective IgM (but not IgD) BCR downregulation, exclusion from B cell follicles, and markedly reduced lifespan and competitive fitness in the periphery.
The most well-recognized biochemical change that characterizes ‘anergic’ B cells in the IgHEL/sHEL model is elevated basal calcium (presumably indicative of chronic antigen engagement), coupled with a marked impairment in BCR signal transduction. When stimulated with soluble HEL antigen in vitro, anergic IgHEL B cells display reduced calcium entry and impaired global protein tyrosine phosphorylation relative to naïve IgHEL B cells 51. Because anergic IgHEL B cells exhibit profound downregulation of the surface IgM (but not IgD) BCR, some degree of impaired signaling could be explained by reduced surface receptor expression. However, these phenomena can be uncoupled; after adoptive transfer into non-HEL-expressing mice, formerly anergic IgHEL B cells regain normal levels of surface IgM BCR after 36 hours but still display impaired calcium mobilization in response to antigen stimulation 51. Similarly, stimulation of anergic IgHEL B cells through IgD also produces impaired global protein phosphorylation and calcium entry despite high surface expression of IgD BCR. Importantly, all downstream signaling pathways are not equally affected; anergic IgHEL B cells exhibit phosphorylation of ERK in response to anti-IgD or HEL Ag, but such stimuli fail to drive JNK1 phosphorylation, NFκB pathway activation, or AKT activation 52,53. It is thought that these biochemical perturbations in BCR signaling contribute to functional anergy. The molecular basis for some (but not all) of these changes has been dissected in more recent studies reviewed below.
Importantly, many features of anergy (including IgM downregulation and attenuated BCR signal transduction) are shared by several independent self-reactive BCR Tg models, and to varying degrees among naturally occurring ‘anergic’ B cell populations (reviewed by Cambier and colleagues) 22. Nevertheless, the extent of anergy varies in proportion to the affinity and avidity of individual BCR clones for self-antigen, and the molecular mechanisms at play differ. Cambier, Wysocki, Manser and colleagues generated Ars/A1 mice harboring a heavy and light chain BCR Tg that recognizes the p-azophenylarsonate (Ars) hapten 54. However, B cells in these mice do not respond to Ars-KLH immunization, downregulate IgM expression, and display attenuated BCR signaling, suggesting that Ars/A1 B cells cross-react with an endogenous antigen that induces a state of clonal anergy. The affinity of the Ars/A1 BCR for Ars hapten is 2.36 × 10−5 M, and treatment with 10−4 M monovalent Ars-conjugated tyrosine is sufficient to compete off endogenous antigen and abolish anergy in vivo, suggesting that affinity of this BCR for cross-reactive endogenous antigen is rather low 55. Indeed, Ars/A1 B cells cross-react very weakly (2×10−3 M affinity) with ssDNA54. These measured affinities are many orders of magnitude lower than that of IgHEL for its cognate antigen (2 × 10−9 M) 49. As such, common features of both models (IgM downregulation and attenuated BCR signaling) are likely to be ‘universal’ mechanisms of peripheral B cell tolerance operative across a broad spectrum of self-reactivity. However, by contrast to the IgHEL model which requires days after transfer to a host lacking HEL antigen to restore function, clonal anergy of Ars/A1 B cells is much more rapidly reversible; within seconds of treatment with monovalent Ars/Tyr, elevated basal calcium in Ars/A1 B cells falls, and some responsiveness towards anti-IgM is restored after just 20 minutes. This suggests a vastly different rate of antigen dissociation from BCR and likely also points to distinct molecular mechanisms at play in the two models 56.
PI3K/ PTEN axis
As alluded to above, after transfer to a HEL-deficient host, anergic IgHEL B cells upregulate IgM expression after 1–4 days, and still display reduced antibody responses even 10 days after transfer 57. This suggests that some features of anergy are “hard-wired” in these cells. Indeed, anergic IgHEL B cells upregulate expression of the inositol phosphatase PTEN, which normally counteracts the activity of PI3-kinase (PI3K) to generate the second messenger PI(3,4,5)P3 in the plasma membrane (Figure 3) 53. PI(3,4,5)P3 in turn recruits PH-domain containing proteins to promote signaling downstream of the BCR via Akt, Btk, and PLCγ258. Rickert and colleagues found that deletion of PTEN in anergic IgHEL B cells completely restored antigen-dependent calcium entry, Akt signaling, competitive survival, steady state antibody secretion, and partially restored surface IgM expression53. Some of these changes may be secondary to sequestration of HEL Ag by anti-HEL Abs resulting in reduced receptor occupancy on IgHEL B cells, but it is likely that a subset are direct. Indeed, BCR-induced calcium entry is exquisitely dependent upon PI3K catalytic activity and PI(3,4,5)P3 supply, suggesting that PI(3,4,5)P3 is a limiting reagent in this pathway59.
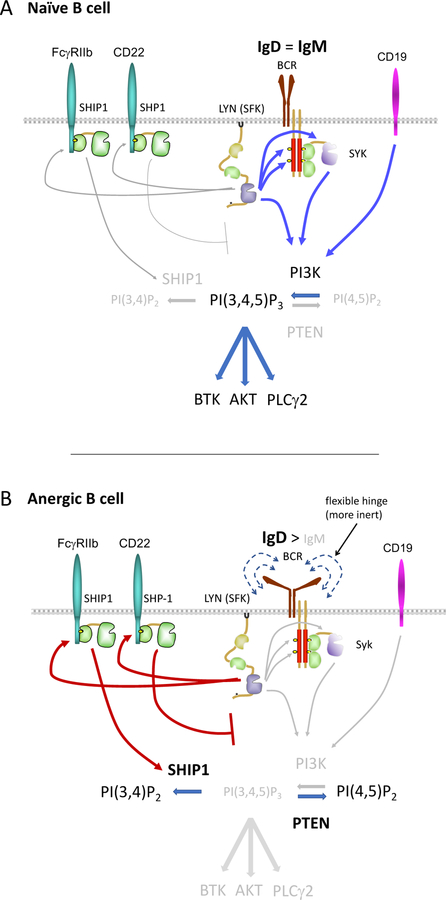
Figure 3. Model: Differences in antigen-dependent signal transduction between naïve and anergic B cells.
Schematic depicts differences in signal transduction between naïve (top) and anergic (bottom) B cells as gleaned from studies of BCR Tg models in mice. The extent of rewiring depends upon the specific BCR Tg and likely corresponds to avidity for self-antigen. Fo B cells express both IgM and IgD BCRs. While IgD expression is constant across the repertoire, IgM expression is downregulated in proportion to self-reactivity, such that the most anergic cells express much more IgD than IgM BCR on their surface. IgD BCR harbors a flexible ‘hinge’ domain that may render it less sensitive to endogenous antigens. In addition, IgD and IgM BCRs may exhibit differential association with co-stimulatory receptors (e.g. CD19) and inhibitory co-receptors (e.g. CD22). Increased engagement of inhibitory co-receptors by chronically antigen-stimulated anergic B cells results in activation of SHP-1 which represses proximal signal transduction, and of SHIP1 which hydrolyzes PI(3,4,5)P3. Increased expression of PTEN in anergic B cells similarly depletes PI(3,4,5)P3 in the plasma membrane. Collectively, reduced PI(3,4,5)P3 levels in anergic B cells impairs recruitment of PH-domain containing downstream signaling molecules to the plasma membrane and their subsequent activation, including Btk, Akt, and PLCγ2, which couple proximal BCR signaling to NF-κB, mTORC1 complex, and calcium entry respectively.
The PTEN/PI3K axis may be broadly relevant for maintaining tolerance; overexpression of CD19 (which couples BCR ligation to PI3K signaling) is sufficient to break tolerance, suggesting that the regulation of PI(3,4,5)P3 levels may play an important role in toggling between tolerant and activated B cells60,61. Inducible deletion of PTEN is also sufficient to break tolerance of Ars/A1 B cells, although unlike anergic IgHEL B cells, PTEN itself is not overexpressed in this more modestly self-reactive model 62. These findings may be generalizable to non-Tg B cells; Cambier and colleagues recently showed that PTEN expression varied continuously across the normal human B cell repertoire and correlated with increasing anergy and decreasing IgM expression 63. More recently, Shlomchik and colleagues identified PTEN overexpression in GC B cells, which serves to redirect AKT kinase activity towards negative regulators of proximal BCR signaling, thereby engaging a negative feedback loop 64. It will be exciting to explore whether a similar biochemical mechanism might be exploited by anergic B cells to suppress antigen-dependent signal transduction. However, it remains to be determined how PTEN function and expression is regulated in naturally occurring self-reactive B cells. Further, PTEN predominantly operates downstream of the most proximal segment of the BCR signaling cascade, implying additional pathways must be engaged to suppress very early events such as Syk phosphorylation 51.
ITIM-dependent inhibitory signaling
ITIM-dependent inhibitory coreceptors suppress BCR signaling through recruitment of the effector phosphatases SHP-1 and SHIP-158. SHP-1 is a tyrosine phosphatase that targets Syk and other proximal BCR signaling machinery. SHIP-1 is an inositol phosphatase that hydrolyzes PI(3,4,5)P3 to generate PI(3,4)P2, complementing the role of PTEN in suppressing PI3K-dependent signaling (Figure 3). Some inhibitory co-receptors are co-engaged with the BCR by specific autoantigens that serve as ligands for both (e.g. IgG and FcγRIIb; Sm/RNP Ags and CD72), but others like CD22 are thought to function in a more constitutive manner to regulate BCR signaling65,66. The Src family kinase Lyn serves as a molecular link to couple antigen receptor stimulation with engagement of ITIM-dependent inhibitory pathways; although other B cell SFKs are competent to mediate BCR signal transduction via ITAMs, Lyn plays a unique non-redundant role in mediating ITIM-dependent signaling67. Indeed, via Lyn activation, BCR ligation drives concomitant increases in phosphorylation of both SHP-1 and SHIP-1, ensuring graded inhibitory tone that scales with the extent of antigenic stimulation. Importantly, these pathways are robustly engaged in anergic B cells as indicated by constitutive phosphorylation of SHP-1 and SHIP-158.
Biochemical ‘anergy’ in modestly self-reactive Ars/A1 B cells requires continuous BCR occupancy and is therefore rapidly reversible in vitro56. In particular, the anergic state of these cells relies upon continuous ITIM-dependent inhibitory tone imposed by both SHIP-1 and SHP-1; Getahun, Cambier and colleagues bypassed central tolerance and showed that inducible deletion of either phosphatase in adoptively transferred mature Ars/A1 B cells leads to robust short-lived, extra-follicular plasma cell differentiation and antibody secretion 62. Importantly, such plasma cell responses are dependent upon self-antigen stimulation; analogous experiments with naïve IgHEL B cells do not trigger Ab production. Interestingly, while SHP-1 and SHIP-1 act through independent and parallel inhibitory pathways to impose anergy, SHIP-1 and PTEN exhibit cooperative roles. This confirms the significance of their common substrate PI(3,4,5)P3 as a critical gatekeeper of anergy, and suggests that PI(3,4,5)P3 concentration defines the ‘tipping point’ between tolerance and autoimmunity in peripheral B cells (Figure 3).
We previously showed that “stepping on the gas” by increasing the expression of the receptor-like tyrosine phosphatase CD45 enhances SFK priming and proximal BCR signal transduction, but ultimately results in an anergic-like state in which B cells exhibit IgM downregulation, impaired upregulation of CD86, poor survival, and fail to break tolerance, even on highly susceptible genetic backgrounds 68,69. We showed via biochemical analysis and genetic epistasis that this was due to engagement of inhibitory pathways by Lyn 69. Indeed, mice harboring a constitutively active allele of Lyn (Lynup/up) exhibit chronic engagement of ITIM-dependent signaling pathways and similarly exhibit features of B cell anergy 70. By contrast, an allele of CD45 that selectively lacks regulatory control over Lyn (and thus partially phenocopies Lyn−/− mice), selectively “disables the break” imposed by ITIM-dependent effector pathways and produces frank autoimmunity in the same genetic context 69,71. These studies suggest that it is not simply chronic antigen recognition by self-reactive B cells that eventually breaks tolerance and produces overt autoimmunity, but rather uncoupling such recognition from the inhibitory pathways normally engaged as a result of chronic stimulation. Indeed, either global or B cell-specific disruption of these ITIM-dependent pathways is sufficient to produce lupus-like autoimmunity on susceptible backgrounds61. Dependence upon these inhibitory pathways to restrain autoantibody production in mice lacking a self-reactive BCR Tg suggests that these pathways are engaged even by modestly self-reactive B cells within a physiologic repertoire.
Naturally occurring self-reactive B cells in humans
These mechanistic studies of B cell anergy rely in large part upon BCR Tg model systems in mice. How is self-reactivity handled in diverse, natural B2 cell repertoires, and by extension, which tolerance mechanisms play a relevant role in such physiologic settings? Wilson and colleagues identified a novel population of human B cells (BND) that express high surface levels of IgD but no detectable surface IgM 72. This population makes up about 2.5% of mature recirculating B cells, and importantly lacks any evidence of somatic hypermutation, suggesting that they represent naïve rather than memory B cells. About 16% of BCRs expressed by BND cells harbor dsDNA reactivity, and 75% are reactive to nuclear antigens. These specificities are markedly enriched in BND relative to the bulk mature recirculating B cell population 7,10,72. Consistent with their self-reactivity, BND cells have elevated levels of basal intracellular calcium. Despite normal surface expression of IgD, BND cells exhibit reduced tyrosine phosphorylation and calcium entry in response to both anti-IgM and anti-IgD stimulation 72. However, it is not yet clear which negative regulators are engaged in these cells to achieve attenuated signaling. Sanz and colleagues reported that IgD+IgMLO mature human B cells share many characteristics with BND cells 73. However, although signaling is dampened in response to anti-IgM, responses to anti-IgD stimulation are only modestly impaired. This suggests that rewiring of self-reactive human B cells to suppress signal transduction occurs on a spectrum and is most profound in BND cells with virtually no detectable surface IgM expression, and possibly the greatest degree of self-reactivity. It is tempting to speculate that such cells have exhausted the capacity of IgM downregulation to restrain their self-reactivity, and depend on additional mechanisms (yet to be defined) to dampen signaling. By contrast, less self-reactive B cell populations may be controlled primarily by IgM downregulation.
Naturally occurring self-reactive B cells in mice
Naturally occurring autoreactive B cells of varying phenotypes have also been described in mice. Self-reactive B cells from several Tg models, including IgHEL and Ars/A1 B cells, retain expression of the immature B cell marker CD93 (marked by the AA4.1 clone) and their surface phenotype corresponds to that of so-called splenic T3 B cells (B220+CD93+CD23+IgMLO) that can be identified in WT mice 74. T3 B cells constitute about 5–8% of splenic B cells in C57Bl/6 mice, but despite expressing a marker of immaturity, do not appear to represent a developmental intermediate 74,75. Rather, the Cambier lab has speculated that these may represent a compartment of naturally-occurring anergic B cells and termed this population ‘An1’ B cells 74. The An1/T3 population is hypo-responsive to stimulation through the IgM BCR, even when gating on equivalent IgM expression in the Fo mature compartment, is enriched for ssDNA and Smith antigen-reactive B cells, and have a short half-life 74,75. The intrinsically dampened signaling in An1 B cells suggests that they might be rewired, but the specific biochemical changes that account for this have not yet been defined.
The Nur77-eGFP reporter serves as a marker of functional self-reactivity and reveals that increasing self-reactivity is inversely correlated with surface IgM expression in the mature follicular B cell repertoire, while IgD levels remain high and invariant across the repertoire (Figures 1D, E)15. Increasing GFP expression correlates with increased basal intracellular calcium, and, similar to An1 cells and other Tg models of anergy, GFPHI Fo B cells exhibit reduced calcium entry in response to IgM ligation (Figures 4A, B)15. However, in our hands, even GFPHI B cells at the far end of the self-reactivity spectrum (top 1%) signal normally in response to IgD ligation, implying that IgM downregulation rather than downstream rewiring is the primary mechanism to attenuate BCR signaling in these cells (Figure 4C). Indeed, gating on matched levels of surface IgM confirms that this is the case (Figure 4D), and this may correspond to Tg models with lower avidity for self-antigens 22. If we gate directly on mature Fo B cells expressing low surface IgM expression (lowest 10%), we obtain very similar results – impaired responses to IgM ligation, but intact responses to stimulation through IgD (Figure 4E). However, we can identify a very small population of cells with the lowest expression of surface IgM (lowest 1%) that do exhibit impaired calcium entry in response to IgD stimulation (Figure 4F). It is tempting to speculate that these cells may correspond to human IgD+IgM- (BND) cells identified by Wilson and colleagues72. These data argue that, despite harboring functional self-reactivity across a continuum, the vast majority of the mature Fo B cell repertoire is not constrained by rewiring of BCR signal transduction. Rather, IgM downregulation may be the primary strategy employed by these cells (together with physiological ITIM-dependent signaling pathways) to maintain tolerance. As with other naturally occurring populations of anergic B cells, it remains to be determined what mechanisms constrain the small (1%) population of classically anergic Fo B cells identified on the basis of extremely low surface IgM expression, and whether these are shared with An1 and/or BND cells. It is also interesting to explore why Nur77-eGFP expression does not seem to identify these cells. One possibility is that rewiring of signal transduction markedly reduces reporter transcription.
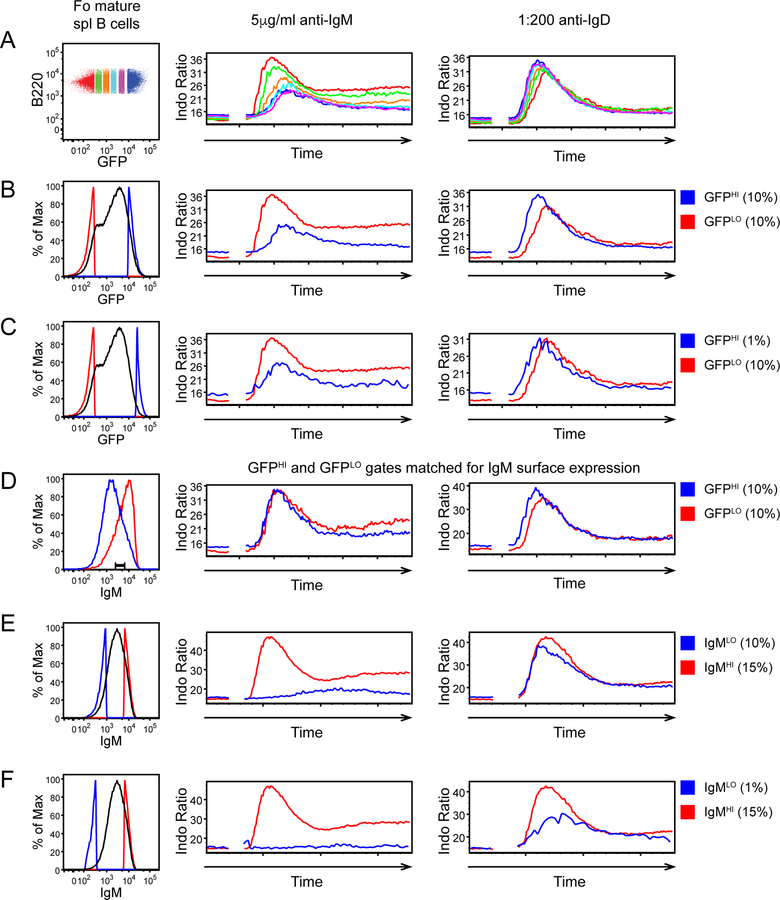
Figure 4. Most Fo B cells are constrained by IgM downregulation, while only a minor fraction exhibits rewiring of downstream signal transduction machinery.
Splenocytes were loaded with Indo-1 to detect intra-cellular calcium, and stained with B220, CD23, and CD93 to identify Fo mature B cells (B220+ CD23HI CD93-), and with anti-IgM F(ab’)1 to label surface IgM BCR without triggering a signal. After subsequent stimulation with either anti-IgM F(ab’)2 or anti-IgD, cells were collected by FACS for a total of 3 minutes. Indo-1 ratio is plotted to depict calcium entry into mature Fo B cells as a function of time. Left-hand histograms depict gates drawn either on the basis of Nur77-eGFP expression (A-C) or surface IgM expression (E, F) or both (C).
A. Fo mature B cells were gated on the basis of Nur77-eGFP along a continuum. Anti-IgM responses vary across this spectrum but IgD responses are invariant.
B, C. 10% lowest (GFPLO) and either 10% (B) or 1% (C) highest (GFPHI) gates are drawn. Anti-IgM responses are dampened in GFPHI populations, but anti-IgD responses are not, even among the B cells with 1% highest GFP expression.
D. 10% GFPLO and 10% GFPHI populations as gated in (B), were further gated to identify cells with matched surface IgM expression. This completely corrected differences in response to anti-IgM stimulation.
E, F. Fo mature B cells were gated to identify those with 15% highest surface IgM expression and either 10% (E) or 1% (F) lowest IgM. Anti-IgM responses are almost undetectable in cells with extremely low IgM, but IgD responses are intact in 10% lowest IgM gate. Only the 1% lowest IgM gate contains cells that exhibit impaired responses to IgD, suggesting intrinsically attenuated signal transduction
There may be exceptions to these general observations; Taylor, Jenkins and colleagues characterized OVA-specific B cells in a normal polyclonal repertoire in mice and show that they remain essentially ‘ignorant’ in the presence of soluble OVA 76. The most high affinity clones exhibit deletion in the presence of membrane-associate OVA, and the remainder exhibit ‘functional anergy’, but unexpectedly display completely ‘naïve’ surface phenotypes without expression of T3 markers or IgM downregulation. Importantly, OVA-specific B cells in mice – especially those that populate the periphery in mOVA hosts - have a vastly lower affinity for cognate antigen than do IgHEL B cells for HEL (more than 3 million-fold lower), and this must account for their relatively ‘naïve’ state. It would be of great interest to determine expression of Nur77-eGFP reporter in these cells in the presence and absence of mOVA in order to understand where this anergic population lies on the spectrum of self-reactivity across the physiological B cell repertoire.
Relatively intact BCR signal transduction in the vast majority of the natural mature B cell repertoire forms a striking contrast to models such as IgHEL/sHEL that rely upon PTEN upregulation to suppress downstream signal transduction. It is notable – as will be discussed in more mechanistic detail below – that anergic B cells from this double Tg model undergo extremely rapid (2–3 days) elimination in competition with non-Tg B cells77,78. Indeed, biochemical studies of these B cells were made possible by generation of a monoclonal repertoire lacking competitor B cells. Highly self-reactive specificities corresponding to ‘anergic’ IgHEL B cells may be exceedingly rare in the physiological mature B cell repertoire and do not contribute significantly to the GFPHI compartment of a physiologic B cell repertoire. Notably, several BCR Tg models with modest self-reactivity show no evidence of impaired BCR signal transduction22. It is tempting to speculate that these are more typical of clones that populate a normal B cell repertoire. We propose that the physiologic spectrum of mature Fo B cells exhibit a mild degree of self-reactivity that is adequately controlled by IgM downregulation and does not trigger biochemical rewiring of signal transduction except at far extremes of the distribution. An independent study came to similar conclusions by studying the functional properties of IgMLO and IgMHI Fo B cells in mice 79. These data collectively suggest that IgM downregulation is the primary ‘first-line’ peripheral tolerance mechanism triggered by self-antigen recognition – at least among mature Fo B cells - and additional rewiring of BCR signal transduction machinery to further dampen signaling is triggered when self-reactivity exceeds certain bounds. It is possible that these strategies are intentionally redundant and layered mechanisms, triggered when conditions (transient lymphopenia, excessive BAFF, other genetic variants in signal transduction) permit the entry and persistence of more self-reactive B cells in the mature Fo B cell repertoire. The contrast between humans and mice in this regard may reflect any of these as well as the marked difference in lifespan between our species, perhaps permitting the accumulation of more self-reactive clones in humans that must be restrained by mechanisms beyond IgM downregulation.
5). Dual expression of IgM and IgD BCRs on Fo B cells: a unique strategy to impose peripheral tolerance
Downregulation of IgM (but not IgD) BCRs is a nearly universal feature of self-reactive, anergic B cells both in BCR Tg models, and in the physiologic B cell repertoire22. Our studies of the Nur77-eGFP reporter reveal that the degree of IgM downregulation correlates with self-reactivity across the Fo B cell repertoire in mice, and suggests that this may represent a primary mechanism of peripheral B cell tolerance15. However, it has been challenging to understand how IgM downregulation could suppress responses to endogenous antigens in the face of high IgD expression since these BCR isotypes are co-expressed and, on any given B cell, contain identical antigen-binding domains. Indeed, the reason for co-expression of these two BCR isotypes on mature naïve follicular B cells has been a very long-standing puzzle in B cell biology.
IgM is the first isotype expressed on the surface of developing B cells, and it can be expressed in all known B cell lineages80. By contrast, the IgD BCR is co-expressed with IgM during a relatively narrow window of development, beginning in the late splenic transitional stages, and peaking on mature naïve Fo B cells (IgD expression is much lower on innate-like B cell lineages). Unlike other BCR isotypes, IgD is not predominantly generated through genomic class-switch recombination (although it can be in limited settings); rather, its expression on Fo B cells is the product of a developmentally-regulated alternative splicing event, and abruptly ends once naïve B cells are activated. Not only do IgM and IgD BCRs on a given B cell share an identical antigen-binding domain, they also have an identical intracellular domain that consists of three amino acids (lysine-valine-lysine), and both rely upon pairing with Igα/β for initiation of signaling, and to a more variable extent, transport to the surface of the cell 81,82. However, they differ notably in their extra-cellular constant domains; IgD uniquely harbors a long and flexible hinge c-terminal relative to the Cδ1 Ig domain 83. X-ray scattering reveals that this long hinge, along with heavy O-glycosylation, gives IgD a T-shaped structure, in contrast to the more rigid Y-shaped structure of IgM 84.
These data suggest that IgM and IgD BCRs ought to play non-redundant functions on mature naïve B cells. However, generation of two independent IgD-deficient mouse lines and one IgM-deficient mouse line in the 1990s failed to uncover such a non-redundant function for either of these BCR isotypes; B cells harboring one or the other could traverse development, enter all B cell lineages, and mount both T-independent and T-dependent immune responses 85–87. Similarly, an earlier study comparing HEL-specific IgM or IgD BCRs reported comparably efficient development, functional responses, and clonal anergy on a self-reactive genetic background 88. The only prominent difference between these two IgHEL Tg lines was that acute and chronic antigen stimulation induced receptor downregulation to quite a different extent; IgM downregulation was much more dramatic than IgD downregulation, mirroring the observations made with the dual isotype-expressing Tg line. More recently, two additional IgD-deficient mouse lines were generated by Goodnow and colleagues; one harbored deletion of ZFP318, an obligate mediator of IgD splicing, while the other (dmit) was generated via ENU mutagenesis and impaired trafficking of IgD to the cell surface 89,90. Notably, dmit mutants, by contrast to other IgD-deficient models, lost surface IgD expression without a compensatory increase in surface IgM. Collectively, these studies revealed that IgD-deficient Fo B cells, most obviously in dmit mutants, exhibit an impairment in competitive survival under steady state conditions 85,86,90. This is consistent with a well-appreciated role for tonic BCR signaling in B cell survival91–93, and in turn implies that one role for co-expression of IgD may be to deliver ‘tonic’ survival signals that preserve self-reactive IgMLO B cells in the mature Fo B cell compartment, perhaps to serve as a reservoir of protective BCR specificities that limit ‘holes’ in the repertoire. Surprisingly, no defect in competitive fitness was apparent in a recent analysis of a small family of IgD+/− patients in which IgD-sufficient and IgD-deficient B cells from the same patient could be tracked 94. However, it remains possible that the BCR repertoires of these two competing B cell populations differed and might compensate via altered antigen-dependent signaling. These studies, nevertheless, do not establish how IgM downregulation could serve as a tolerance mechanism when a signaling competent IgD BCR remains on the surface of self-reactive B cells.
Parallel lines of investigation into differences between the in vitro signaling capacity of IgM and IgD BCRs have begun to bear fruit as well. Work from Batista, Reth and colleagues have suggested that IgM and IgD are not homogeneously distributed across the plasma membrane, but exist in pre-formed and distinct “nano-clusters” 82,95–97. Differential clustering, mobility, and association of IgM and IgD BCRs with co-receptors such as CD22 and CD19 could in turn confer distinct signaling capacity despite shared use of the canonical Igα/β-dependent BCR signaling apparatus 97–99. The CD22 co-receptor deliver signals via an ITIM/LYN/SHP-1 inhibitory pathway, while CD19 is critical to drive PI3K activation, and differential association of IgM and IgD with these co-receptors on the cell surface could have important implications for the physiological roles of these BCR isotypes in B cell anergy, survival, and differentiation. Interestingly, it has been argued that IgD is associated with CD19 at rest, but IgM associates with CD19 upon stimulation 82,97. This could provide a mechanistic basis for a tonic survival function of IgD, and may also help explain why IgM downregulation is an effective strategy to suppress antigen-dependent PI(3,4,5)P3 generation, and impose tolerance. Recently, Maity and colleagues identified an obligate role for IgD expression to facilitate signaling via CXCR4, a receptor that was not previously known to interact with the BCR pathway100. This finding opens the floodgates to a host of other potential isotype-specific interactions on B cells.
Recently, Jumaa and colleagues supplied a very important insight into how direct ‘sensing’ of antigen by IgM and IgD BCRs may differ, suggesting that the flexible hinge region of IgD renders it unresponsive to monovalent antigens 101. To support this model, they took advantage of pro-B cells from so-called ‘triple-deficient’ mice (lacking Slp65, surrogate light chain L5, and RAG2) that are blocked at an early stage of development, do not express endogenous BCR, but can be transduced with model BCRs for use in signaling assays. Polyvalent forms of either NIP hapten conjugated to peptides or HEL could trigger signaling through both IgM and IgD antigen-specific BCRs introduced into this system, while monovalent variants could signal only through IgM. Moreover, deleting the hinge region from IgD or introducing it into IgM was sufficient to confer or eliminate sensitivity to monovalent antigens, respectively. More recently, this group has extended these findings to the Ramos B cell line and has made analogous observations using both human and murine BCR isotypes 102. These data suggest that self-reactive Fo B cells (IgDHI IgMLO) retain the capacity to make responses only towards multivalent antigens (e.g. immune complexes aggregated by preformed IgM Ab or via FcRs on APCs). However, recent work from Goodnow and colleagues argues against this model; isotype-specific IgHEL Tg mature Fo B cells expressing either IgD or IgM alone exhibit comparable responsiveness of both to soluble HEL Ag in vitro 90. Moreover, it was previously shown that both BCR isotypes were rendered functionally and phenotypically ‘anergic’ in the context of a host expressing soluble HEL Tg 88. Both isotypes could also mediate a similar transcriptional ‘anergy’ program in vivo, further implying comparable sensitivity to sHEL in vivo 90. Unexpectedly, IgM-only cells in the latter study induced transcription of a subset of anergy-associated genes more strongly than cells expressing both IgM and IgD BCR isotypes, suggesting that IgD “attenuates” the anergy program. Whether and how IgD could directly interfere with IgM signaling remains to be clarified.
It is currently uncertain how to reconcile the discrepancy between these reports, although conclusions may differ, at least in part, because of distinct cellular contexts, antigen doses and preparations investigated. The actual sensitivity of IgD BCRs towards monovalent antigens may in fact lie somewhere between these two extremes. Regardless, certain limitations remain: both of these key studies investigated predominantly soluble, high affinity model antigens. However, the affinity, avidity, and structural features of bona fide endogenous antigens are largely unknown and likely differ significantly from model antigens. Indeed, as noted earlier, perhaps the most well-defined naturally occurring self-reactive B cell population identified in humans, expressing the VH4–34 H chain, is argued to recognize highly abundant cell-surface antigens on RBCs and B cells 12,13. It is not yet known whether IgM and IgD BCRs might be differentially sensitive to antigen affinity. Nor is it clear how these isotypes respond to membrane-associated antigens of varying affinity and density. Yet differences in this respect are likely as it has been reported that IgM is more sensitive to high density antigens while IgD binds more tightly to low density epitopes 103.
Our laboratory became interested in understanding how IgM and IgD BCRs might differ in their responsiveness to bona fide endogenous antigens because we had observed in Nur77-eGFP reporter mice that IgM but not IgD was downregulated continuously across the entire mature Fo B cell repertoire in proportion to endogenous antigen encounter15. This strongly suggested to us that IgM BCRs might be relatively more sensitive to endogenous antigens, and as has long been speculated, that IgM downregulation could serve as an elegant and efficient tolerance mechanism if this were the case. Both the appeal and the challenge to us was to understand whether this could be the case with a diverse, naturally occurring B cell repertoire that was exposed to a complex range of bona fide endogenous antigens (that might and likely did vary tremendously from any particular model antigen studied).
We took several independent approaches to determine the sensitivity of IgM and IgD for bona fide endogenous antigens across a diverse B cell repertoire24. We introduced the Nur77-eGFP reporter into both IgM−/− and IgD−/− genetic backgrounds, and showed across multiple B cell compartments that IgD BCRs are less efficient than IgM BCRs at driving reporter GFP expression in vivo. This difference was most apparent when the BCR repertoire was either unconstrained (via BAFF overexpression), or single specificities were compared (PtC-specific B1a cells), suggesting that the selection of BCR repertoires compensate for and partially mask signaling differences in individual isotype-deficient mice. In parallel, we took advantage of allelic exclusion in IgM+/− IgD+/− mice to generate an immature B cell pool comprised of 50% IgM-only and 50% IgD-only B cells. As discussed earlier, MZ and B1a development proceed optimally in response to low and high self-reactivity, respectively 35,104. We found that IgD-only B cells outcompete IgM-only cells in the MZ compartment, but the result is reversed in the B1a compartment 24. Collectively, these observations suggest that IgM is more sensitive to bona fide endogenous antigens in vivo than IgD. Importantly, in vitro signal transduction in response to L chain cross-linking of IgM-only and IgD-only B cells revealed no corresponding defects, suggesting that ‘sensing’ of endogenous antigens, rather than differential coupling to the downstream signaling apparatus, accounted for these in vivo differences.
These studies of IgM and IgD biology collectively support an exciting model to account for peripheral B cell quiescence despite variable engagement by endogenous antigens. Mature naïve Fo B cells downregulate surface IgM (but not IgD) in proportion to their degree of functional self-reactivity. Physical properties of IgM that render it more sensitive to endogenous antigens than IgD (rigid structure with short hinge region, and possibly unique association with distinct co-receptors) ensure that these cells remain unresponsive in the face of chronic self-antigen stimulation. Preservation of IgD expression, conversely, may sustain survival of self-reactive clones in a competitive environment (perhaps by promoting PI3K-dependent signaling via association with CD19) in order to patch ‘holes’ in the mature B cell repertoire that would otherwise arise if such clones were eliminated entirely. As discussed further below, IgD may also facilitate participation of B cells in GC-dependent immune responses upon receipt of appropriate cross-linking and co-stimulatory cues by foreign pathogens. This remarkable dual BCR isotype mechanism of peripheral tolerance may suffice to control the majority of the mature B cell repertoire, but clones that exceed the buffering capacity of this elegant system may go on to trigger a range of post-translational and even transcriptional mechanisms that further attenuate downstream signal transduction.
However, many important questions remain unanswered, including the relative responsiveness of IgM and IgD to membrane-associated and low affinity antigens, as well as the relative contribution of the IgD hinge and coreceptor association to such isotype-specific differences. Moreover, the molecular mechanism that couples chronic antigen recognition to IgM downregulation remains an open area of investigation. Prior work suggests that the cbl and cbl-b ubiquitin ligases drive Ag-dependent ubiquitination and degradation of the Igα BCR subunit, and appear to be critical for IgM downregulation in anergic IgHEL B cells, although how they are linked to antigen stimulation is not clear 105. Recent and as yet unpublished work suggests that the E3 ubiquitin ligase GRAIL may play a similar role via ubiquitination and degradation of Igα, and may be coupled to antigen recognition via PTEN (R. Nurieva, June 2018 Dresden B Cell Keystone Conference presentation; Nurieva et al. AAI abstract 2018). In conjunction, antigen-dependent upregulation of ZFP318 may favor splicing of the primary IgM/D transcript towards IgD expression in anergic B cells, and this may also be promoted by PTEN 90,102.
Although most self-reactive BCR Tg models exhibit selective downregulation of the IgM BCR, a few exceptions show reduced expression of both IgM and IgD beginning during immature stages of BM development 6. Included among these are DNA-reactive VH3H9 / λ1 B cells and an independent model (HKIR) described by Manser and colleagues in which DNA-reactive Tg B cells can also bind Ars hapten (similar to Ars/A1 model) 106. It has been suggested that this mode of BCR downregulation is associated with receptor editing, but that is not thought to be the case in the HKIR model. Rather, BCR downregulation in that model was shown to be reversible via displacement of endogenous antigen with the Ars hapten, and was associated with transcriptional repression of BCR components107. However, the precise molecular mechanism is still not clear, and likely differs from selective IgM downregulation seen in mature self-reactive B cells 6. Downregulation of both BCR isotypes clearly contributes to anergy of self-reactive Tg clones by desensitizing them to antigen, but appears to constitute a rather rare tolerance mechanism triggered in highly self-reactive clones early in development; its relative contribution to the physiological B cell repertoire may be very minor.
6). Competitive fitness of self-reactive B cells in the periphery
IgM downregulation and modulation of the downstream BCR signaling apparatus are not the only mechanisms of tolerance engaged by self-reactive B cells in the periphery. B cell clones also undergo competitive elimination in proportion to their self-reactivity; in early studies, it was noted that “anergic” IgHEL B cells turn over rapidly in vivo, and are rapidly lost after transfer into a sHEL Tg host with competing WT B cells 77,78,108. By contrast to an estimated half-life (t1/2) for naïve mature Fo B cell on the order of several weeks, the t1/2 of anergic IgHEL B cells is several days in the absence of clonal competition, and about half a day with such competition. After adoptive transfer into hosts expressing soluble HEL Ag, loss of “anergic” IgHEL B cells depends upon the presence of both competitor B cell clones and self-antigen, but is genetically separable from follicular exclusion 109,110. Interestingly, transfer of naïve IgHEL B cells into hosts expressing cognate sHEL Ag gives a nearly identical cell loss, delayed only by 24 hours relative to that of anergic IgHEL B cells, suggesting that this phenomenon is separable from other features of anergy77. After discovery of the critical B cell survival factor BAFF/BLyS, it was shown that competition for a limiting supply of this factor accounts for this phenomenon; highly self-reactive B cells are rapidly eliminated under conditions of limiting BAFF (as in a normal polyclonal B cell repertoire), while an excess supply of BAFF rescues their survival 111–114. Importantly, this phenomenon appears to exists along a continuum such that self-reactive B cells strike a balance between the degree of endogenous antigen recognition and their relative dependence upon BAFF supply.
While a limiting supply of BAFF plays a critical role in the competitive elimination of self-reactive B cells in the periphery, BAFF is not thought to regulate central deletion because immature B cells do not upregulate the BAFF receptor (BAFFR) until later in splenic stages of development. We previously showed that increasing CD45 expression in B cells (because of co-engagement of both BCR and ITIM-dependent inhibitory pathways) generates an anergic-like state characterized by a very short t1/2, profound downregulation of BAFFR, and resistance to BAFF68. This mechanism may contribute to poor survival of some anergic B cell populations. However, although anergic IgHEL B cells modestly downregulate BAFFR, they remain extremely responsive to BAFF supplementation both in vitro and in vivo. This implies the existence of another cell-intrinsic change in these anergic cells that does not render them insensitive to, but rather dependent on, BAFF.
One such change may be upregulation of the pro-apoptotic BH3-only Bcl2 family member BIM; anergic IgHEL B cells, in competitive settings, upregulate BIM in response to chronic antigen stimulation, and this may partially account for their increased dependence upon BAFF 111. Deletion of BIM rescues survival of anergic IgHEL B cells in mice expressing soluble HEL and renders these cells less dependent on BAFF for survival in vitro, but has only a modest effect on central deletion triggered by membrane-associated HEL 115,116. Importantly, deletion of BIM also indirectly overwhelms other peripheral tolerance mechanisms such that BCR signal transduction is no longer attenuated and tolerance is broken116. It is unclear whether this is a direct consequence of BIM deletion, or rather represents an indirect effect of reduced BCR occupancy as the number of surviving IgHEL B cells goes up and the supply of HEL protein is reduced by serum Ab.
Upregulation of BIM may not be the only mechanism that renders anergic B cells dependent upon BAFF for survival. We recently identified a specific function for Nur77 itself in the competitive elimination of mature Fo self-reactive B cells by taking advantage of Nr4a1−/− mice27,117. Nur77 belongs to a three-member family of orphan nuclear hormone receptors (Nur77, Nurr1, and Nor1 encoded by Nr4a1-3 respectively) that share significant structural similarities in their DNA-binding and ligand-binding domains 118. Nr4a family members are thought to function as constitutively active transcription factors with no known endogenous ligand 119. It has been argued that, in addition to their role as transcriptional regulators, Nr4a family members can also trigger apoptosis independently of DNA-binding by translocating to the mitochondria, binding directly to Bcl2, and inducing a conformational change that exposes the pro-apoptotic BH3-only domain of Bcl2 120–122. Indeed, Nur77 misexpression promotes TCR-induced apoptosis in T cell hybridomas and thymocytes, while a dominant negative construct lacking the n-terminal transactivation domain produces the opposite phenotype, implicating this family as mediators of thymic negative selection 123–125. These observations raise the possibility that Nur77 plays an analogous role in B cell tolerance.
We recently showed that Nur77 expression correlates with self-reactivity, counter-selection, and anergy among individual B cell clones from both the IgHEL and the VH3H9 models of self-reactivity 27. Although Nur77 itself was largely dispensable for receptor editing, central deletion, follicular exclusion and anergy, we discovered that Nur77 restrains the survival of mature self-reactive B cells in vitro and in vivo, particularly in settings where the B cell survival factor BAFF is limiting, and this can be rescued entirely by supplemental BAFF 27. Nur77-deficiency also results in the progressive accumulation of self-reactive B cells in the mature repertoire with age, and is sufficient to break B cell tolerance in VH3H9 Tg mice. Importantly, since Nur77 is upregulated in self-reactive B cells in proportion to the degree of chronic antigen stimulation, we argue that it serves as a direct molecular link between self-reactivity and B cell survival in the periphery; Nur77 gradually prunes B cell clones from the mature repertoire in proportion to their self-reactivity and thereby imposes a novel layer of peripheral B cell tolerance.
7). Tolerance of self-reactivity in innate-like B cells
By contrast to Fo B cells which express high levels of IgD and variable levels of IgM BCRs on their surface (that vary with self-reactivity), innate-like MZ and B1a cells express predominantly IgM and do not downregulate this receptor in proportion to their self-reactivity, suggesting that they must rely upon other tolerance strategies (unpublished data, Zikherman lab). MZ B cells are exceptionally sensitive to negative selection by strong BCR signaling, and, although there is a minimal amount of mild self-reactivity that facilitates MZ B cell development, strongly self-reactive clones are stringently excluded from that compartment (Figure 2). By contrast to MZ B cells, B1a cells are highly resistant to deletional tolerance and the threshold for positive selection of B1a cells overlaps with that for negative selection of Fo B2 cells resulting in an extremely self-reactive compartment (Figure 2). Perhaps for this reason, ITIM-dependent inhibitory tone is especially important to restrain B1a cells from activation by self-antigen; deletion of Siglec-G, CD22, SHP-1, CD5, PirB (or combinations thereof) in B1a cells results in enhanced BCR signal transduction along with concomitant expansion of the B1a compartment 126–130. In addition to this critical reliance upon inhibitory co-receptors, a number of other unique tolerance mechanisms are engaged by B1a cells. It has long been observed that B1a cells are sensitive to immobilized, but not soluble antigen, perhaps because the latter clusters BCRs and can exclude inhibitory co-receptors. A related observation is that soluble antigen stimulation of B1a cells – in contrast to B2 cells – fails to trigger downstream NF-κB signaling and proliferation, but the molecular mechanism for this is not well understood131,132.
Recently, we showed that Nur77-eGFP reporter expression was highly upregulated by chronic antigen stimulation in self-reactive B1a cells, particularly in highly self-reactive PtC-binding clones 133. Unexpectedly, we found that deletion of Nr4a1 markedly increased generation of B1a-derived natural IgM plasma cells, but did not expand the size of the B1a compartment. By contrast to ITIM-dependent inhibitory tone, Nur77 seems to regulate a distinct, downstream checkpoint by an unknown mechanism. Further delineation of this and other B1a-specific tolerance mechanisms is needed.
8). The two-signal model of B cell activation and self/non-self discrimination
In vivo B cell responses to T-dependent stimuli such as hapten-protein conjugates are characterized by an initial phase of antigen-dependent events such as entry into cell cycle, upregulation of peptide-loaded MHC-II, adhesion, and costimulatory molecules, and secretion of chemokines that collectively facilitate recruitment of T cell help, along with migration away from the B cell follicle and towards the T-B border for the purpose of accessing such help 134. Of necessity, there is a physiological delay between receipt of signal one (antigen), and acquisition of signal two in the form of T cell help (including components such as CD40L, the cytokines IL-4 and IL-21, as well as other co-stimulatory molecules on the surface of T cells). Even a “single round” of antigen stimulation is sufficient to prime B cells to seek out and respond to T cell help within a defined temporal window135,136. B cells that fail to do so may revert to a naïve-like state if the antigenic stimulus was sufficiently transient 136,137. However, B cells that receive a strong or prolonged BCR signal are ‘hard-wired’ to make a very abortive immune response, ending in apoptosis, when they fail to acquire signal two within a defined span of time 58,138. Hodgkin and colleagues have quantitatively characterized this so-called ‘death timer’, and more recently, Pierce and colleagues described the metabolic changes that accompany failure to recruit signal two 137,138. Interestingly, they identify a role for antigen-dependent chronic calcium ‘leak’ via membrane CRAC channels, and progressive mitochondrial dysfunction that can be rescued in its early stages by appropriate help, but functions as a metabolic ‘death timer’ in the absence of help. Yet the full molecular program that produces such abortive and non-productive responses to signal one (in the absence of signal two) is not completely clear.
In a number of ways, chronically antigen-stimulated self-reactive B cells exhibit behavior that resembles an abortive response to acute BCR signaling. While self-reactive B cells may not divide, in a number of models they upregulate activation markers, including MHC-II and CD86, downregulate IgM, and migrate out of the B cell follicle to the T/B border or the T cell zone itself 22. This last phenomenon is termed follicular exclusion, depends upon the chemokine receptors CXCR5 and CCR7, and has been described not only in the IgHEL model, but also in the VH3H9 and Ars/A1 models 58,78,109,139. In other words, anergic B cells exhibit antigen-induced changes that normally serve to recruit signal two in the form of T cell help. Importantly, both acute and chronic antigen stimulation triggers a program of antigen-induced cell death in B cells that can be rescued by an excess supply of the survival factor BAFF and/or a relevant costimulatory signal. Indeed, “anergic”-like phenotypes can be induced in B cells by presenting antigen (aka signal one) in the absence of signal two; transfer of naïve IgHEL B cells into sHEL-expressing hosts is sufficient to recapitulate several features of anergy following a round of rather abortive proliferation, including both IgM downregulation and markedly reduced survival 77. Conversely, provision of signal two under inappropriate circumstances can be sufficient to break B cell tolerance (e.g. Roquinsan/san mice with over-expression of co-stimulatory molecules on Tfh cells, or co-engagement of BCR and endosomal TLRs by nucleic acid-containing autoantigens) to produce frank autoimmune disease in mice and humans140,141. It is possible (perhaps likely) that the state of clonal ‘anergy’ and the abortive responses of a B cell that receives signal one without signal two exist on a continuum with one another, rely upon overlapping molecular mechanisms, and serve a similar purpose: to restrain inappropriate self-reactive B cell responses to endogenous antigens. Indeed, the two signal model of B cell activation was initially proposed by Bretscher and Cohn in part as a strategy to discriminate self from non-self 142.
Nur77 is not only upregulated in response to chronic antigen stimulation, but, along with its family members, is an immediate-early or so-called ‘primary response gene’ that is rapidly and potently (but transiently) induced by acute BCR stimulation. We found that Nur77-deficient B cells exhibit enhanced survival and proliferation relative to WT B cells in response to acute BCR stimulation, but exhibit no competitive advantage in the context of co-stimulation, in the form of either TLR stimuli or signals that mimic T cell help (unpublished data, Zikherman lab)27. We postulate that negative regulation by Nur77 of both naïve and self-reactive B cells (which exist on a continuum!) serves to confer dependence upon a second signal and thus helps maintain tolerance by restricting survival of B cells that receive signal one either acutely or chronically in the absence of signal two. The downstream effectors that mediate this role, and other molecular pathways that contribute to this important tolerance mechanism (i.e. the other components of the death timer) remain to be fully dissected, but may include upregulation of Bim by BCR engagement 111.
9). Differentiation of self-reactive B cells into short-lived plasma cells and germinal center B cells
Although the classic hallmark of clonal anergy is failure to generate rapid plasma cell responses to antigen-specific immunization, anergic B cells can – with appropriate T cell help - be recruited into the GC 90,143–145. Indeed, anergic B cells may enter the GC more efficiently than naïve B cells in some contexts145. The mechanism that underlies this preferential recruitment of self-reactive B cells towards GC fate has not been fully elucidated but may relate, in part, to differences in IgM and IgD expression; predominant IgD expression on anergic cells that downregulate IgM might serve to transmit a quantitatively (or qualitatively) distinct signal relative to IgM. Indeed, Goodnow and colleagues reported that IgD-only anergic B cells are more efficiently recruited into the GC than IgM-only anergic B cells, postulating greater survival of the former 90. Recently, Jumaa and colleagues also proposed that IgD expression enhances GC entry, although prior work suggests that such results may vary according to the immunogen studied and such assays must be controlled for varied precursor B cell frequency conferred by the distinct VDJ loci of WT and isotype-deficient animals 24,85,86,102.
We made related observations in our studies of IgM-deficient and IgD-deficient mice 24. Because Lyn−/− mice lack ITIM-dependent inhibitory tone, they develop a dramatic and spontaneous expansion of short-lived plasma cells that are not class-switched (similar to conditional deletion of PTPases in this pathway)62,67,70,146. Genetic disruption of ITIM-dependent signaling results in downregulation of the transcription factor ETS1 which suppresses PC differentiation147. This is thought to be due in part to excessive BTK-dependent signal transduction in response to endogenous antigen stimulation in the absence of inhibitory tone 147,148. Deletion of ETS1 in B cells is sufficient to drive spontaneous PC differentiation even with intact inhibitory tone and even in the absence of self-antigen stimulation, while over-expression of ETS1 can suppress such differentiation in Lyn-deficient and SHP-1-deficient B cells 147,149,150. On the Lyn−/− genetic background, we observed that IgM-only B cells spontaneously differentiate into these short-lived ‘unswitched’ plasma cells, but IgD-only B cells do not, and showed that this is associated with reduced downregulation of ETS1 by IgD-only B cells24. This implies that IgD BCRs are more inert than IgM in the face of chronic endogenous antigen stimulation, even without the safeguard of ITIM-dependent inhibitory signals. The corollary of this is that IgM downregulation on self-reactive B cells could, to some extent, compensate for loss of inhibitory tone. By contrast, in our work and in prior studies of IgM-deficient mice, IgD-only B cells are quite efficiently recruited into the GC in response to either T-D immunization (both SRBC and NP-protein conjugates, controlled for VDJ locus) or in the context of spontaneous autoimmunity in Lyn-deficient animals 24,87. This suggests that IgD does not efficiently drive short-lived plasma cell differentiation by self-reactive B cells with low IgM expression, but is sufficient for effective GC responses.
Persistent ETS1 expression may not be the only mechanism diverting anergic B cells away from short-lived PC fate and towards GC entry. Brink and colleagues have shown that short-lived PC expansion is much more highly dependent upon antigen affinity than is GC entry 151,152. By virtue of predominant IgD expression and attenuated signal transduction, self-reactive anergic B cell clones may be unable to drive efficient expansion of short-lived PCs, but antigen stimulation can still generate sufficient signaling to support GC entry.
It is possible that qualitative differences in IgM- and IgD-dependent signaling in anergic and naïve B cells may also contribute to their differential propensity to support an extra-follicular short-lived PC response. For example, differential association of IgM and IgD with CD19 and CD22 or other coreceptors could drive distinct engagement of SHP-1, SHIP1, and PI3K pathways by these BCR isotypes. PI3K signaling can greatly facilitate PC differentiation, so preferential engagement of PI(3,4,5)P3 phosphatases by anergic B cells may repress PC fate to provide a second layer of restraint independently of IgM and IgD expression59.
The rationale for shunting self-reactive B cells away from PC differentiation and into the GC may be to avoid generation of autoantibodies. But what happens once self-reactive B cells enter the GC? Manser and colleagues showed that an Ars hapten-reactive BCR that was also self-reactive, could be recruited into the GC by immunization with Ars-conjugated protein, but these cells appeared to be censored in the memory compartment that emerged from the GC106. However, it was not clear how this was achieved, and moreover, these observations did not explain why we would evolve elaborate peripheral tolerance mechanisms that facilitate retention of self-reactive clones in the pre-immune B cell repertoire only to dispose of them during humoral immune responses. More recently in a series of elegant studies, Goodnow and colleagues postulated and demonstrated that self-reactive BCRs can undergo SHM and mutate away from self-reactivity in a process termed ‘clonal redemption’ (reviewed elsewhere in this volume)143–145. This phenomenon explains how self-reactive BCRs can be ‘used’ but simultaneously neutralized during a humoral immune response, and could help to explain why such clones are constrained by anergy and preserved in the peripheral repertoire, rather than deleted.
10). Tolerance in the germinal center and beyond
Not only does the host need to handle pre-existing self-reactive clones that seed the GC, but the threat of de novo self-reactivity, inadvertently acquired via random SHM, must be neutralized. Indeed, Nussenzweig and colleagues identified examples of de novo self-reactivity in the IgG+ memory compartments of healthy humans, implying that this represents a real and significant threat to B cell tolerance153. What are the cellular and molecular mechanisms of tolerance in the GC and what are its limitations?
It has long been known that soluble antigen stimulation in the absence of co-stimulation results in GC B cell apoptosis, and it has been argued that this represents a tolerance mechanism to delete self-reactive clones that inadvertently arise during the process of somatic hypermutation (SHM) and affinity maturation154–156. With a very strong BCR stimulus, such cell death occurs rapidly (within 8 hours), can be at least partially rescued by Bcl2 overexpression, but proceeds independently of Fas and CD4054–56. More recently, both the Brink and Goodnow labs elegantly demonstrated counter-selection of self-reactivity in the GC using the IgHEL model system coupled with genetic manipulation of neo-self-antigen143,157. The molecular mechanisms that underpin this selection pressure to lose self-reactivity in the GC remain to be worked out, but may include competition between autoantigen and foreign antigen for binding, and/or Ag-induced death of GC B cells triggered in the absence of T cell help. This strong counter-selection of self-reactivity in the GC, along with prior studies, collectively imply that self-reactive clones in the GC that cannot be redeemed via SHM will be deleted and points to the existence of a GC-intrinsic tolerance mechanism. The physiologic stringency and frequency of redemption and/or deletion of self-reactive B cells during and after the GC response will be important to explore further, as will the molecular mechanisms at play.
Importantly, Brink and colleagues showed that GC clones with cross-reactivity to tissue-restricted self-antigens that were not present within the GC escaped tolerance157. It was postulated that molecular mimicry between microbes and self-antigens could drive generation of high affinity autoantibodies in this way and it is clear that this is a clinically important vulnerability. Together with detection of self-reactivity in the human memory B cell compartment, these observations suggest the need for post-GC tolerance. Whether and how this might work is unknown. It is possible that some variation on tolerance mechanisms that constrain the Fo mature B cell compartment might be relevant.
In previously published work, we showed that a subset of B cells in the germinal center light zone upregulate Nur77-eGFP expression 20. Since the light zone contains FDC presenting antigen and Tfh cells, this suggests that GFPHI GC B cells may be actively signaling in response to such stimuli. As expected, BCR ligation of GC B cells in vivo could increase Nur77-eGFP (unpublished data, Zikherman lab), while in vivo treatment with the Btk inhibitor ibrutinib decreased GFP expression 20. By contrast, an anti-CD40L blocking Ab (MR1 clone) rapidly begins to dissolve the GC, but does not alter GFP expression (unpublished data, Zikherman lab). Together, these data suggest that Nur77-eGFP expression in GC B cells in vivo is dependent upon BCR signaling but independent of CD40 signaling. Since Nur77 mediates antigen-induced cell death in Fo B cells in response to either acute or chronic antigen stimulation, we hypothesize that the Nr4a family may play an analogous role in the context of the germinal center (or even post-GC memory B cells) where some mechanism must exist to restrain persistence or de novo generation of ‘rogue’ self-reactive clones. This and other molecular mechanisms at play in GC tolerance are of great interest to explore further.
11). Conclusions and future directions:
Modest self-reactivity is an essential feature of the mature Fo B cell compartment; some degree of self-reactivity is not merely tolerated, but is actively selected into and retained in the repertoire for future use. The teleological rationale for selecting a self-reactive repertoire remains uncertain. Modest self-reactivity may facilitate protective immune responses by promoting recognition of essential biological patterns shared by both host and microbe. Self-reactivity may also represent an inevitable byproduct of the quest for clonal diversity and may limit holes in the repertoire that would otherwise arise as a result of deletional tolerance mechanisms. Importantly, self-reactivity among mature B cells spans a broad continuum, and we postulate a “sliding scale” of tolerance mechanisms that maintain quiescence of individual B cell clones in the face of self-antigen encounter (Figure 5). The modes of peripheral B cell tolerance - IgM downregulation, attenuation of BCR signal transduction, and reduced survival – can be thought of as “quantitative traits” that vary continuously across the B cell repertoire. They are also inter-dependent; impairing one applies greater pressure to the others, and when this is excessive, their collective buffering capacity may be overwhelmed.
Figure 5. Model: Self-reactivity and peripheral tolerance mechanisms in mature Fo B cells exist along a continuum.
Schematic depicts, from top to bottom, distribution of functional self-reactivity in the normal mature Fo B cell repertoire as detected by Nur77-eGFP expression. At either end of this distribution lie BCR clones whose self-reactivity falls outside the thresholds for positive and negative selection. IgM but not IgD expression declines continuously with increasing self-reactivity. Among the most highly self-reactive clones (that do not typically populate the mature B cell repertoire) lie those that may downregulate both IgM and IgD BCRs. PTEN enzymatic activity and/or expression may increase continuously with increasing self-reactivity and serves to reduce PI(3,4,5)P3 levels and downstream signal transduction. Increasing engagement and activation of ITIM-dependent inhibitory phosphatases is seen as chronic antigen receptor occupancy increases. Collectively, these strategies restrain activation of B cells in response to self-antigen, but only in the most highly self-reactive B cells is downstream signal transduction rewired such that it is attenuated in response to ex vivo ligation of the IgD BCR. Examples of specific BCR transgenes with varying degrees of self-reactivity and engagement of varied tolerance mechanisms are depicted along with their putative location along this spectrum. Finally, propensity of B cells to differentiate into either short-lived PCs or to enter the GC varies such that anergic B cells are restrained from the former fate, but may have an advantage in entering the GC, where self-reactivity can be ‘redeemed’ and censored.
We propose that selective downregulation of the IgM but not the IgD BCR represents the primary layer of control to handle the pressure of irrelevant or benign antigen encounter in vivo, allowing clones to adapt to their environment while retaining functional capacity. Importantly, IgM downregulation scales continuously across the entire Fo B cell repertoire in proportion to antigen encounter. Because the IgD BCR is less sensitive to endogenous antigens, cells expressing predominantly IgD are restrained from activation and from direct extra-follicular plasma cell differentiation, but IgD expression facilitates survival and preserves (or perhaps enhances) the capacity of such clones to mount a germinal center (GC) response. Entry into the GC can then redeem self-reactivity of such clones via SHM and counter-selection, but the molecular strategies that drive such efficient counterselection and similarly constrain de novo acquisition of self-reactivity are largely unknown.
However, many components of this mechanism remain to be explored. The sensitivity of IgM and IgD BCRs to antigens ‘seen’ in different contexts is not fully defined; beyond differential sensitivity to valency, do these isotypes respond in different ways to antigens displayed on membranes? Do they exhibit different sensitivity to antigen density or affinity? These questions are important, as is the more challenging task of identifying relevant bona fide self-antigens in vivo. The molecular mechanism that couples antigen recognition to IgM downregulation also needs to be more fully elucidated.
A second layer of control seems to reside in the very wiring of proximal BCR signal transduction; antigen-induced ITAM- and ITIM-dependent signaling are coupled, such that inhibitory phosphatases are activated in proportion to BCR stimulation. Interestingly, IgM-deficient B cells that express only IgD seem to be inert enough to self-antigen stimulation that they are less dependent upon inhibitory tone supplied by Lyn. However, the vast majority of mature Fo B cells have downregulated, but not eliminated IgM expression, and such clones appear to require both layers of control. Importantly, defects at this level may play a role in genetic susceptibility to lupus, and harnessing inhibitory coreceptors to reinforce or induce antigen-specific tolerance remains a promising therapeutic avenue.
Superimposed on this is another network of negative regulatory pathways that can be engaged or even upregulated in response to overwhelming self-reactivity. The most well-characterized among these is PTEN. How PTEN expression is regulated by chronic antigen stimulation, and how dependent modestly self-reactive B cells are upon this pathway remains to be explored further. We propose that this ‘third’ layer of control (which represents the most well-recognized feature of clonal anergy, namely attenuation of BCR signal transduction) may be largely reserved for more highly self-reactive clones that exceed the buffering capacity of layers one and two.
Finally, some of the same molecular strategies that render Fo B cells dependent upon signal two also restrain survival of the most self-reactive clones in the periphery, gradually pruning these specificities from the mature repertoire. We showed recently that Nur77/Nr4a1 provides a novel link between antigen stimulation and impaired survival, and may represent an exciting new druggable target to treat autoimmunity and modulate adaptive immune responses.
The Nr4a family may play functionally analogous roles in both T and B cells in response to chronic antigen stimulation. Indeed, recent work shows that the Nr4a family plays a role in down-modulating T cell responses to chronic antigen stimulation by engaging the exhaustion program in CD8 T cells and the ‘anergy program’ in CD4 T cells that receive signal one without co-stimulation160,161. This raises the possibility that Nr4a family members collectively may play additional undiscovered roles in B cell tolerance. To unmask redundancy among this three-member family, conditional elimination of multiple family members will be necessary.
How is tolerance breached in B cell-mediated autoimmune disease? A well-recognized possibility is inappropriate provision of signal two (TLR stimulation or T cell help). An important question is whether self-reactive, anergic B cells are the source of autoantibodies and/or provide help for pathogenic T cells in various autoimmune diseases, or whether relevant specificities are in fact generated de novo in the GC. Evidence in SLE and other rituximab-sensitive diseases like GPA suggest that extra-follicular SLPCs are the origin for at least some pathogenic autoAbs 158 (discussed by Sanz and colleagues elsewhere in this volume).
This reinforces the significance of clonal anergy and its breakdown for the origins of (some) autoimmune diseases. It also suggests that targeting self-reactive clones in the periphery is a valid strategy to treat or even prevent autoimmune disease, one borne out by the development of clinical BAFF antagonists for the management of patients with SLE. We propose that depletion of self-reactive B cells may also be accomplished by synthetic agonist ligands of Nur77 (or modulators of its interaction with Bcl2), and by inhibition of PI3K signaling. The latter may serve to reinforce anergy and may selectively inhibit survival of self-reactive clones; new work from Cambier and colleagues suggests that low dose p110δ inhibition does so not only in mice with reduced PI(3,4,5)P3 phosphatase expression (as might be expected), but also in a B cell-dependent mouse model of diabetes59.
Handling of self-reactivity in the peripheral B cell repertoire remains a point of incredible vulnerability as revealed by the prevalence of B cell-driven autoimmunity, ranging from diseases characterized by pathogenic autoantibodies (such as SLE), to those in which self-reactive B cells provide inappropriate help to pathogenic T cells (such as MS and T1D). To intervene effectively in such diseases, a greater understanding of both normal tolerance and its dysregulation are essential. Continuing to explore the relevance of these pathways to human B cells will be critical to translate advances driven by the power and elegance of mouse genetics.
Acknowledgements:
Funding sources include NIAID 5T32AI007334–28 (CT), NSF Graduate Research Fellowship 1650113 (MN), HHMI Medical Fellows Program (JH), NIAMS R01AR069520 (JZ), NIAMS K08AR059723 (JZ), Rheumatology Research Foundation (JZ)
References
- 1.Gay D, Saunders T, Camper S, Weigert M. Receptor editing: an approach by autoreactive B cells to escape tolerance. J Exp Med 1993;177(4):999–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hartley SB, Crosbie J, Brink R, Kantor AB, Basten A, Goodnow CC. Elimination from peripheral lymphoid tissues of self-reactive B lymphocytes recognizing membrane-bound antigens. Nature. 1991;353(6346):765–769. [DOI] [PubMed] [Google Scholar]
- 3.Nemazee DA, Bürki K. Clonal deletion of B lymphocytes in a transgenic mouse bearing anti-MHC class I antibody genes. Nature. 1989;337(6207):562–566. [DOI] [PubMed] [Google Scholar]
- 4.Tiegs SL, Russell DM, Nemazee D. Receptor editing in self-reactive bone marrow B cells. J Exp Med 1993;177(4):1009–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halverson R, Torres RM, Pelanda R. Receptor editing is the main mechanism of B cell tolerance toward membrane antigens. Nat Immunol 2004;5(6):645–650. [DOI] [PubMed] [Google Scholar]
- 6.Nemazee D Mechanisms of central tolerance for B cells. Nature reviews Immunology. 2017;17(5):281–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301(5638):1374–1377. [DOI] [PubMed] [Google Scholar]
- 8.Stamatatos L, Pancera M, McGuire AT. Germline-targeting immunogens. Immunol Rev 2017;275(1):203–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiao X, Chen W, Feng Y, et al. Germline-like predecessors of broadly neutralizing antibodies lack measurable binding to HIV-1 envelope glycoproteins: implications for evasion of immune responses and design of vaccine immunogens. Biochem Biophys Res Commun 2009;390(3):404–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watanabe A, Su KY, Kuraoka M, et al. Self-tolerance curtails the B cell repertoire to microbial epitopes. JCI Insight. 2019;4(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yurasov S, Wardemann H, Hammersen J, et al. Defective B cell tolerance checkpoints in systemic lupus erythematosus. J Exp Med 2005;201(5):703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cappione AJ, Pugh-Bernard AE, Anolik JH, Sanz I. Lupus IgG VH4.34 Antibodies Bind to a 220-kDa Glycoform of CD45/B220 on the Surface of Human B Lymphocytes. The Journal of Immunology. 2004;172(7):4298–4307. [DOI] [PubMed] [Google Scholar]
- 13.Cappione A, Anolik JH, Pugh-Bernard A, et al. Germinal center exclusion of autoreactive B cells is defective in human systemic lupus erythematosus. Journal of Clinical Investigation. 2005;115(11):3205–3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iype J, Datta M, Khadour A, et al. Differences in Self-Recognition between Secreted Antibody and Membrane-Bound B Cell Antigen Receptor. J Immunol 2019;202(5):1417–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zikherman J, Parameswaran R, Weiss A. Endogenous antigen tunes the responsiveness of naive B cells but not T cells. Nature. 2012;489(7414):160–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mittelstadt PR, DeFranco aL. Induction of early response genes by cross-linking membrane Ig on B lymphocytes. Journal of immunology (Baltimore, Md : 1950). 1993;150(11):4822–4832. [PubMed] [Google Scholar]
- 17.Hazel TG, Nathans D, Lau LF. A gene inducible by serum growth factors encodes a member of the steroid and thyroid hormone receptor superfamily. Proceedings of the National Academy of Sciences. 1988;85(22):8444–8448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Milbrandt J Nerve growth factor induces a gene homologous to the glucocorticoid receptor gene. Neuron. 1988;1(3):183–188. [DOI] [PubMed] [Google Scholar]
- 19.Au-Yeung BB, Zikherman J, Mueller JL, et al. A sharp T-cell antigen receptor signaling threshold for T-cell proliferation. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(35):E3679–3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mueller J, Matloubian M, Zikherman J. Cutting Edge: An In Vivo Reporter Reveals Active B Cell Receptor Signaling in the Germinal Center. The Journal of Immunology. 2015. [DOI] [PMC free article] [PubMed]
- 21.Moran AE, Holzapfel KL, Xing Y, et al. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. Journal of Experimental Medicine. 2011. [DOI] [PMC free article] [PubMed]
- 22.Cambier JC, Gauld SB, Merrell KT, Vilen BJ. B-cell anergy: from transgenic models to naturally occurring anergic B cells? Nature reviews Immunology. 2007;7(8):633–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goodnow CC, Crosbie J, Jorgensen H, Brink RA, Basten A. Induction of self-tolerance in mature peripheral B lymphocytes. Nature. 1989;342(6248):385–391. [DOI] [PubMed] [Google Scholar]
- 24.Noviski M, Mueller JL, Satterthwaite A, Garrett-Sinha LA, Brombacher F, Zikherman J. IgM and IgD B cell receptors differentially respond to endogenous antigens and control B cell fate. eLife. 2018;7:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erikson J, Radic MZ, Camper SA, Hardy RR, Carmack C, Weigert M. Expression of anti-DNA immunoglobulin transgenes in non-autoimmune mice. Nature. 1991;349(6307):331–334. [DOI] [PubMed] [Google Scholar]
- 26.Fields ML, Hondowicz BD, Wharton GN, et al. The regulation and activation of lupus-associated B cells. Immunological reviews. 2005;204:165–183. [DOI] [PubMed] [Google Scholar]
- 27.Tan C, Mueller JL, Noviski M, Huizar J, Lau D, Dubinin A, Molofsky A, Wilson PC, Zikherman J. Nur77 Links Chronic Antigen Stimulation to B Cell Tolerance by Restricting the Survival of Self-Reactive B Cells in the Periphery. J Immunol. 2019. May 15;202(10):2907–2923. doi: 10.4049/jimmunol.1801565. Epub 2019 Apr 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang Y, Wang C, Yang Q, et al. Distinct mechanisms define murine B cell lineage immunoglobulin heavy chain (IgH) repertoires. Elife. 2015;4:e09083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arnold LW, Pennell CA, McCray SK, Clarke SH. Development of B-1 cells: segregation of phosphatidyl choline-specific B cells to the B-1 population occurs after immunoglobulin gene expression. J Exp Med 1994;179(5):1585–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berland R, Wortis HH. Origins and functions of B-1 cells with notes on the role of CD5. Annu Rev Immunol 2002;20(1):253–300. [DOI] [PubMed] [Google Scholar]
- 31.Graf R, Seagal J, Otipoby KL, et al. BCR-dependent lineage plasticity in mature B cells. Science. 2019;363(6428):748–753. [DOI] [PubMed] [Google Scholar]
- 32.Hayakawa K, Asano M, Shinton SA, et al. Positive selection of natural autoreactive B cells. Science. 1999;285:113–116. [DOI] [PubMed] [Google Scholar]
- 33.Hayakawa K, Asano M, Shinton SA, et al. Positive Selection of Anti–Thy-1 Autoreactive B-1 Cells and Natural Serum Autoantibody Production Independent from Bone Marrow B Cell Development. The Journal of Experimental Medicine. 2003;197(1):87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wen L, Brill-Dashoff J, Shinton SA, Asano M, Hardy RR, Hayakawa K. Evidence of marginal-zone B cell-positive selection in spleen. Immunity. 2005;23(3):297–308. [DOI] [PubMed] [Google Scholar]
- 35.Casola S, Otipoby KL, Alimzhanov M, et al. B cell receptor signal strength determines B cell fate. Nat Immunol 2004;5(3):317–327. [DOI] [PubMed] [Google Scholar]
- 36.Bannish G, Fuentes-Panana EM, Cambier JC, Pear WS, Monroe JG. Ligand-independent signaling functions for the B lymphocyte antigen receptor and their role in positive selection during B lymphopoiesis. J Exp Med 2001;194(11):1583–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gu H, Tarlinton D, Muller W, Rajewsky K, Forster I. Most peripheral B cells in mice are ligand selected. J Exp Med 1991;173(6):1357–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levine MH, Haberman AM, Sant’Angelo DB, et al. A B-cell receptor-specific selection step governs immature to mature B cell differentiation. Proc Natl Acad Sci U S A. 2000;97(6):2743–2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosado MM, Freitas AA. The role of the B cell receptor V region in peripheral B cell survival. Eur J Immunol 1998;28(9):2685–2693. [DOI] [PubMed] [Google Scholar]
- 40.Wang H, Clarke SH. Evidence for a Ligand-Mediated Positive Selection Signal in Differentiation to a Mature B Cell. The Journal of Immunology. 2003;171(12):6381–6388. [DOI] [PubMed] [Google Scholar]
- 41.Freitas AA, Rosado MM, Viale A, Grandien A. The role of cellular competition in B cell survival and selection of B cell repertoires. Eur J Immunol 1995;25:1729–1738. [DOI] [PubMed] [Google Scholar]
- 42.Sonoda E, Pewzner-Jung Y, Schwers S, et al. B cell development under the condition of allelic inclusion. Immunity. 1997;6:225–233. [DOI] [PubMed] [Google Scholar]
- 43.Kuraoka M, Snowden PB, Nojima T, et al. BCR and Endosomal TLR Signals Synergize to Increase AID Expression and Establish Central B Cell Tolerance. Cell Rep 2017;18(7):1627–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cancro MP, Kearney JF. B cell positive selection: road map to the primary repertoire? J Immunol 2004;173(1):15–19. [DOI] [PubMed] [Google Scholar]
- 45.Zuo T, Gautam A, Wesemann DR. Affinity war: forging immunoglobulin repertoires. Current Opinion in Immunology. 2019;57:32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Verkoczy L, Diaz M, Holl TM, et al. Autoreactivity in an HIV-1 broadly reactive neutralizing antibody variable region heavy chain induces immunologic tolerance. Proc Natl Acad Sci U S A. 2010;107(1):181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kelsoe G, Haynes BF. Host controls of HIV broadly neutralizing antibody development. Immunol Rev 2017;275(1):79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schroeder KMS, Agazio A, Strauch PJ, et al. Breaching peripheral tolerance promotes the production of HIV-1-neutralizing antibodies. J Exp Med 2017;214(8):2283–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goodnow CC, Crosbie J, Adelstein S, et al. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988;334:676–682. [DOI] [PubMed] [Google Scholar]
- 50.Nossal GJ, Pike BL. Clonal anergy: persistence in tolerant mice of antigen-binding B lymphocytes incapable of responding to antigen or mitogen. Proceedings of the National Academy of Sciences. 1980;77(3):1602–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cooke MP, Heath AW, Shokat KM, et al. Immunoglobulin signal transduction guides the specificity of B cell-T cell interactions and is blocked in tolerant self-reactive B cells. The Journal of experimental medicine. 1994;179(2):425–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Healy JI, Dolmetsch RE, Timmerman LA, et al. Different nuclear signals are activated by the B cell receptor during positive versus negative signaling. Immunity. 1997;6(4):419–428. [DOI] [PubMed] [Google Scholar]
- 53.Browne CD, Del Nagro CJ, Cato MH, Dengler HS, Rickert RC. Suppression of phosphatidylinositol 3,4,5-trisphosphate production is a key determinant of B cell anergy. Immunity. 2009;31(5):749–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Benschop RJ, Aviszus K, Zhang X, Manser T, Cambier JC, Wysocki LJ. Activation and Anergy in Bone Marrow B Cells of a Novel Immunoglobulin Transgenic Mouse that Is Both Hapten Specific and Autoreactive. Immunity. 2001;14:33–43. [DOI] [PubMed] [Google Scholar]
- 55.Sharon J Structural correlates of high antibody affinity: Three engineered amino acid substitutions can increase the affinity of an anti-p-azophenylarsonate antibody 200-fold. Proc Natl Acad Sci U S A. 1990;87:4814–4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gauld SB, Benschop RJ, Merrell KT, Cambier JC. Maintenance of B cell anergy requires constant antigen receptor occupancy and signaling. Nat Immunol. 2005;6(11):1160–1167. [DOI] [PubMed] [Google Scholar]
- 57.Goodnow CC, Brink R, Adams E. Breakdown of self-tolerance in anergic B lymphocytes. Nature. 1991;352:532–536. [DOI] [PubMed] [Google Scholar]
- 58.Yarkoni Y, Getahun A, Cambier JC. Molecular underpinning of B-cell anergy. Immunol Rev 2010;237(1):249–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Franks SE, Getahun A, Cambier JC. A Precision B Cell-Targeted Therapeutic Approach to Autoimmunity Caused by Phosphatidylinositol 3-Kinase Pathway Dysregulation. J Immunol 2019. [DOI] [PMC free article] [PubMed]
- 60.Sato S, Hasegawa M, Fujimoto M, Tedder TF, Takehara K. Quantitative genetic variation in CD19 expression correlates with autoimmunity. J Immunol 2000;165(11):6635–6643. [DOI] [PubMed] [Google Scholar]
- 61.Zouali M, Sarmay G. B lymphocyte signaling pathways in systemic autoimmunity: implications for pathogenesis and treatment. Arthritis Rheum 2004;50(9):2730–2741. [DOI] [PubMed] [Google Scholar]
- 62.Getahun A, Beavers NA, Larson SR, Shlomchik MJ, Cambier JC. Continuous inhibitory signaling by both SHP-1 and SHIP-1 pathways is required to maintain unresponsiveness of anergic B cells. J Exp Med 2016;213(5):751–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smith MJ, Ford BR, Rihanek M, et al. Elevated PTEN expression maintains anergy in human B cells and reveals unexpectedly high repertoire autoreactivity. JCI Insight. 2019;4(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Luo W, Hawse W, Conter L, et al. The AKT kinase signaling network is rewired by PTEN to control proximal BCR signaling in germinal center B cells. Nat Immunol 2019. [DOI] [PMC free article] [PubMed]
- 65.Akatsu C, Shinagawa K, Numoto N, et al. CD72 negatively regulates B lymphocyte responses to the lupus-related endogenous toll-like receptor 7 ligand Sm/RNP. J Exp Med 2016;213(12):2691–2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tsubata T Ligand Recognition Determines the Role of Inhibitory B Cell Co-receptors in the Regulation of B Cell Homeostasis and Autoimmunity. Front Immunol. 2018;9:2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu Y, Harder KW, Huntington ND, Hibbs ML, Tarlinton DM. Lyn tyrosine kinase: accentuating the positive and the negative. Immunity. 2005;22(1):9–18. [DOI] [PubMed] [Google Scholar]
- 68.Zikherman J, Doan K, Parameswaran R, Raschke W, Weiss A. Quantitative differences in CD45 expression unmask functions for CD45 in B-cell development, tolerance, and survival. Proc Natl Acad Sci U S A. 2012;109(1):E3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zikherman J, Parameswaran R, Hermiston M, Weiss A. The structural wedge domain of the receptor-like tyrosine phosphatase CD45 enforces B cell tolerance by regulating substrate specificity. J Immunol 2013;190(6):2527–2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hibbs ML, Harder KW, Armes J, et al. Sustained activation of Lyn tyrosine kinase in vivo leads to autoimmunity. J Exp Med 2002;196(12):1593–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hermiston ML, Tan AL, Gupta VA, Majeti R, Weiss A. The juxtamembrane wedge negatively regulates CD45 function in B cells. Immunity. 2005;23(6):635–647. [DOI] [PubMed] [Google Scholar]
- 72.Duty JA, Szodoray P, Zheng NY, et al. Functional anergy in a subpopulation of naive B cells from healthy humans that express autoreactive immunoglobulin receptors. J Exp Med 2009;206(1):139–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Quach TD, Manjarrez-Orduno N, Adlowitz DG, et al. Anergic responses characterize a large fraction of human autoreactive naive B cells expressing low levels of surface IgM. J Immunol 2011;186(8):4640–4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Merrell KT, Benschop RJ, Gauld SB, et al. Identification of Anergic B Cells within a Wild-Type Repertoire. Immunity. 2006;25:953–962. [DOI] [PubMed] [Google Scholar]
- 75.Teague BN, Pan Y, Mudd PA, et al. Cutting edge: Transitional T3 B cells do not give rise to mature B cells, have undergone selection, and are reduced in murine lupus. J Immunol 2007;178(12):7511–7515. [DOI] [PubMed] [Google Scholar]
- 76.Taylor JJ, Martinez RJ, Titcombe PJ, et al. Deletion and anergy of polyclonal B cells specific for ubiquitous membrane-bound self-antigen. J Exp Med 2012;209(11):2065–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cyster JG, Goodnow CC. Antigen-induced exclusion from follicles and anergy are separate and complementary processes that influence peripheral B cell fate. Immunity. 1995;3(6):691–701. [DOI] [PubMed] [Google Scholar]
- 78.Cyster JG, Hartley SB, Goodnow CC. Competition for follicular niches excludes self-reactive cells from the recirculating B-cell repertoire. Nature. 1994;371(6496):389–395. [DOI] [PubMed] [Google Scholar]
- 79.Kirchenbaum GA, St Clair JB, Detanico T, Aviszus K, Wysocki LJ. Functionally responsive self-reactive B cells of low affinity express reduced levels of surface IgM. Eur J Immunol 2014;44(4):970–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gutzeit C, Chen K, Cerutti A. The enigmatic function of IgD: some answers at last. Eur J Immunol 2018;48(7):1101–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Reth M Antigen receptors in B lymphocytes. Annu Rev Immunol 1992;10:97–121. [DOI] [PubMed] [Google Scholar]
- 82.Gold MR, Reth MG. Antigen Receptor Function in the Context of the Nanoscale Organization of the B Cell Membrane. Annu Rev Immunol 2019;37:97–123. [DOI] [PubMed] [Google Scholar]
- 83.Preud’homme J, Petit I, Barra A, Morel F, Lecron J, Leliévre E. Structural and functional properties of membrane and secreted IgD. Mol Immunol 2000;37:871–887. [DOI] [PubMed] [Google Scholar]
- 84.Sun Z, Almogren A, Furtado PB, Chowdhury B, Kerr MA, Perkins SJ. Semi-extended solution structure of human myeloma immunoglobulin D determined by constrained X-ray scattering. Journal of molecular biology. 2005;353(1):155–173. [DOI] [PubMed] [Google Scholar]
- 85.Roes J, Rajewsky K. Immunoglobulin D (IgD)-deficient Mice Reveal an Auxiliary Receptor Function for IgD in Antigen-mediated Recruitment of B Cells. J Exp Med 1993;177:45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nitschke L, Kosco MH, Kohler G, Lamers MC. Immunoglobulin D-deficient mice can mount normal immune responses to thymus-independent and -dependent antigens. Proc Natl Acad Sci U S A. 1993;90:1887–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lutz C, Ledermann B, Kosco-Vilbois MH, et al. IgD can largely substitute for loss of IgM function in B cells. Nature. 1998;393:797–801. [DOI] [PubMed] [Google Scholar]
- 88.Brink R, Goodnow CC, Crosbie J, et al. Immunoglobulin M and D antigen receptors are both capable of mediating B lymphocyte activation, deletion, or anergy after interaction with specific antigen. J Exp Med 1992;176:991–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Enders A, Short A, Miosge LA, et al. Zinc-finger protein ZFP318 is essential for expression of IgD, the alternatively spliced Igh product made by mature B lymphocytes. Proc Natl Acad Sci U S A. 2014;111(12):4513–4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sabouri Z, Perotti S, Spierings E, et al. IgD attenuates the IgM-induced anergy response in transitional and mature B cells. Nat Commun 2016;7:13381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kraus M, Alimzhanov MB, Rajewsky N, Rajewsky K. Survival of Resting Mature B Lymphocytes Depends on BCR Signaling via the Igα/β Heterodimer. Cell. 2004;117:787–800. [DOI] [PubMed] [Google Scholar]
- 92.Lam KP, Kühn R, Rajewsky K. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell. 1997;90(6):1073–1083. [DOI] [PubMed] [Google Scholar]
- 93.Srinivasan L, Sasaki Y, Calado DP, et al. PI3 kinase signals BCR-dependent mature B cell survival. Cell. 2009;139(3):573–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nechvatalova J, Bartol SJW, Chovancova Z, Boon L, Vlkova M, van Zelm MC. Absence of Surface IgD Does Not Impair Naive B Cell Homeostasis or Memory B Cell Formation in IGHD Haploinsufficient Humans. J Immunol 2018;201(7):1928–1935. [DOI] [PubMed] [Google Scholar]
- 95.Maity PC, Blount A, Jumaa H, Ronneberger O, Lillemeier BF, Reth M. B cell antigen receptors of the IgM and IgD classes are clustered in different protein islands that are altered during B cell activation. Science Signaling. 2015;8(394):ra93–ra93. [DOI] [PubMed] [Google Scholar]
- 96.Mattila PK, Feest C, Depoil D, et al. The actin and tetraspanin networks organize receptor nanoclusters to regulate B cell receptor-mediated signaling. Immunity. 2013;38(3):461–474. [DOI] [PubMed] [Google Scholar]
- 97.Klasener K, Maity PC, Hobeika E, Yang J, Reth M. B cell activation involves nanoscale receptor reorganizations and inside-out signaling by Syk. Elife. 2014;3:e02069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Depoil D, Fleire S, Treanor BL, et al. CD19 is essential for B cell activation by promoting B cell receptor-antigen microcluster formation in response to membrane-bound ligand. Nature Immunology. 2008;9(1):63–72. [DOI] [PubMed] [Google Scholar]
- 99.Gasparrini F, Feest C, Bruckbauer A, et al. Nanoscale organization and dynamics of the siglec CD22 cooperate with the cytoskeleton in restraining BCR signalling. EMBO J 2016;35(3):258–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Becker M, Hobeika E, Jumaa H, Reth M, Maity PC. CXCR4 signaling and function require the expression of the IgD-class B-cell antigen receptor. Proc Natl Acad Sci U S A. 2017;114(20):5231–5236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ubelhart R, Hug E, Bach MP, et al. Responsiveness of B cells is regulated by the hinge region of IgD. Nat Immunol 2015;16(5):534–543. [DOI] [PubMed] [Google Scholar]
- 102.Setz CS, Khadour A, Renna V, et al. Pten controls B-cell responsiveness and germinal center reaction by regulating the expression of IgD BCR. EMBO J 2019. [DOI] [PMC free article] [PubMed]
- 103.Loset GA, Roux KH, Zhu P, Michaelsen TE, Sandlie I. Differential Segmental Flexibility and Reach Dictate the Antigen Binding Mode of Chimeric IgD and IgM- Implications for the Function of the B Cell Receptor. Journal of immunology. 2004;172:2925–2934. [DOI] [PubMed] [Google Scholar]
- 104.Cariappa A, Pillai S. Antigen-dependent B-cell development. Curr Opin Immunol. 2002;14:241–249. [DOI] [PubMed] [Google Scholar]
- 105.Kitaura Y, Jang IK, Wang Y, et al. Control of the B cell-intrinsic tolerance programs by ubiquitin ligases Cbl and Cbl-b. Immunity. 2007;26(5):567–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Notidis E, Heltemes L, Manser T. Dominant, hierarchical induction of peripheral tolerance during foreign antigen-driven B cell development. Immunity. 2002;17(3):317–327. [DOI] [PubMed] [Google Scholar]
- 107.Liu X, Wysocki LJ, Manser T. Autoantigen-B cell antigen receptor interactions that regulate expression of B cell antigen receptor Loci. J Immunol 2007;178(8):5035–5047. [DOI] [PubMed] [Google Scholar]
- 108.Fulcher DA, Basten A. Reduced life span of anergic self-reactive B cells in a double-transgenic model. J Exp Med 1994;179(1):125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ekland EH, Forster R, Lipp M, Cyster JG. Requirements for follicular exclusion and competitive elimination of autoantigen-binding B cells. The Journal of Immunology. 2004;172(8):4700–4708. [DOI] [PubMed] [Google Scholar]
- 110.Schmidt KN, Cyster JG. Follicular exclusion and rapid elimination of hen egg lysozyme autoantigen-binding B cells are dependent on competitor B cells, but not on T cells. The Journal of Immunology. 1999;162(1):284–291. [PubMed] [Google Scholar]
- 111.Lesley R, Xu Y, Kalled SL, et al. Reduced competitiveness of autoantigen-engaged B cells due to increased dependence on BAFF. Immunity. 2004;20(4):441–453. [DOI] [PubMed] [Google Scholar]
- 112.Thien M, Phan TG, Gardam S, et al. Excess BAFF Rescues Self-Reactive B Cells from Peripheral Deletion and Allows Them to Enter Forbidden Follicular and Marginal Zone Niches. Immunity. 2004;20(6):785–798. [DOI] [PubMed] [Google Scholar]
- 113.Hondowicz BD, Alexander ST, Quinn WJ, et al. The role of BLyS/BLyS receptors in anti-chromatin B cell regulation. International Immunology. 2007;19(4):465–475. [DOI] [PubMed] [Google Scholar]
- 114.Ota M, Duong BH, Torkamani A, et al. Regulation of the B Cell Receptor Repertoire and Self-Reactivity by BAFF. The Journal of Immunology. 2010;185(7):4128–4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Enders A, Bouillet P, Puthalakath H, Xu Y, Tarlinton DM, Strasser A. Loss of the Pro-Apoptotic BH3-only Bcl-2 Family Member Bim Inhibits BCR Stimulation–induced Apoptosis and Deletion of Autoreactive B Cells. J Exp Med 2003;198(7):1119–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Oliver PM, Vass T, Kappler J, Marrack P. Loss of the proapoptotic protein, Bim, breaks B cell anergy. J Exp Med 2006;203(3):731–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lee SL, Wesselschmidt RL, Linette GP, Kanagawa O, Russell JH, Milbrandt J. Unimpaired thymic and peripheral T cell death in mice lacking the nuclear receptor NGFI-B (Nur77). Science (New York, NY). 1995;269(5223):532–535. [DOI] [PubMed] [Google Scholar]
- 118.Maxwell MA, Muscat GE. The NR4A subgroup: immediate early response genes with pleiotropic physiological roles. Nuclear Receptor Signaling. 2006;4:e002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Winoto A, Littman DR. Nuclear hormone receptors in T lymphocytes. Cell. 2002;109 Suppl:S57–66. [DOI] [PubMed] [Google Scholar]
- 120.Lin B, Kolluri SK, Lin F, et al. Conversion of Bcl-2 from protector to killer by interaction with nuclear orphan receptor Nur77/TR3. Cell. 2004;116(4):527–540. [DOI] [PubMed] [Google Scholar]
- 121.Banta KL, Wang X, Das P, Winoto A. B cell lymphoma 2 (Bcl-2) residues essential for Bcl-2’s apoptosis-inducing interaction with Nur77/Nor-1 orphan steroid receptors. J Biol Chem 2018;293(13):4724–4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Thompson J, Winoto A. During negative selection, Nur77 family proteins translocate to mitochondria where they associate with Bcl-2 and expose its proapoptotic BH3 domain. J Exp Med 2008;205(5):1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Calnan BJ, Szychowski S, Ka-Ming Chan F, Cado D, Winoto A. A role for the orphan steroid receptor Nur77 in apoptosis accompanying antigen-induced negative selection. Immunity. 1995;3(3):273–282. [DOI] [PubMed] [Google Scholar]
- 124.Cheng LEC, Chan FKM, Cado D, Winoto A. Functional redundancy of the Nur77 and Nor-1 orphan steroid receptors in T-cell apoptosis. EMBO Journal. 1997;16(8):1865–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Woronicz JD, Calnan B, Ngo V, Winoto a. Requirement for the orphan steroid receptor Nur77 in apoptosis of T-cell hybridomas. Nature. 1994;367(6460):277–281. [DOI] [PubMed] [Google Scholar]
- 126.Hoffmann A, Kerr S, Jellusova J, et al. Siglec-G is a B1 cell-inhibitory receptor that controls expansion and calcium signaling of the B1 cell population. Nat Immunol 2007;8(7):695–704. [DOI] [PubMed] [Google Scholar]
- 127.Jellusova J, Wellmann U, Amann K, Winkler TH, Nitschke L. CD22 × Siglec-G double-deficient mice have massively increased B1 cell numbers and develop systemic autoimmunity. J Immunol 2010;184(7):3618–3627. [DOI] [PubMed] [Google Scholar]
- 128.Pao LI, Lam KP, Henderson JM, et al. B cell-specific deletion of protein-tyrosine phosphatase Shp1 promotes B-1a cell development and causes systemic autoimmunity. Immunity. 2007;27(1):35–48. [DOI] [PubMed] [Google Scholar]
- 129.Bikah G, Carey J, Ciallella JR, Tarakhovsky A, Bondada S. CD5-mediated negative regulation of antigen receptor-induced growth signals in B-1 B cells. Science. 1996;274(5294):1906–1909. [DOI] [PubMed] [Google Scholar]
- 130.Kubo T, Uchida Y, Watanabe Y, et al. Augmented TLR9-induced Btk activation in PIR-B-deficient B-1 cells provokes excessive autoantibody production and autoimmunity. J Exp Med 2009;206(9):1971–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Morris DL, Rothstein TL. Abnormal transcription factor induction through the surface immunoglobulin M receptor of B-1 lymphocytes. J Exp Med 1993;177(3):857–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Holodick NE, Rothstein TL. Atypical Response of B-1 Cells to BCR Ligation: A Speculative Model. Front Immunol. 2013;4:457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Huizar J, Tan C, Noviski M, Mueller JL, Zikherman J. Nur77 Is Upregulated in B-1a Cells by Chronic Self-Antigen Stimulation and Limits Generation of Natural IgM Plasma Cells. Immunohorizons. 2017;1(9):188–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cyster JG, Allen CDC. B Cell Responses: Cell Interaction Dynamics and Decisions. Cell. 2019;177(3):524–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Damdinsuren B, Zhang Y, Khalil A, et al. Single round of antigen receptor signaling programs naive B cells to receive T cell help. Immunity. 2010;32(3):355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Turner JS, Marthi M, Benet ZL, Grigorova I. Transiently antigen-primed B cells return to naive-like state in absence of T-cell help. Nat Commun 2017;8:15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Akkaya M, Traba J, Roesler AS, et al. Second signals rescue B cells from activation-induced mitochondrial dysfunction and death. Nat Immunol 2018;19(8):871–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Heinzel S, Marchingo JM, Horton MB, Hodgkin PD. The regulation of lymphocyte activation and proliferation. Curr Opin Immunol 2018;51:32–38. [DOI] [PubMed] [Google Scholar]
- 139.Mandik-Nayak L, Bui A, Noorchashm H, Eaton A, Erikson J. Regulation of anti-double-stranded DNA B cells in nonautoimmune mice: localization to the T-B interface of the splenic follicle. The Journal of experimental medicine. 1997;186(8):1257–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pisitkun P, Deane JA, Difilippantonio MJ, Tarasenko T, Satterthwaite AB, Bolland S. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science. 2006;312(5780):1669–1672. [DOI] [PubMed] [Google Scholar]
- 141.Vinuesa CG, Cook MC, Angelucci C, et al. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature. 2005;435(7041):452–458. [DOI] [PubMed] [Google Scholar]
- 142.Bretscher P, Cohn M. A theory of self-nonself discrimination. Science. 1970;169(3950):1042–1049. [DOI] [PubMed] [Google Scholar]
- 143.Burnett DL, Langley DB, Schofield P, et al. Germinal center antibody mutation trajectories are determined by rapid self/foreign discrimination. Science. 2018;360(6385):223–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Reed JH, Jackson J, Christ D, Goodnow CC. Clonal redemption of autoantibodies by somatic hypermutation away from self-reactivity during human immunization. J Exp Med 2016;213(7):1255–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sabouri Z, Schofield P, Horikawa K, et al. Redemption of autoantibodies on anergic B cells by variable-region glycosylation and mutation away from self-reactivity. Proc Natl Acad Sci U S A. 2014;111(25):E2567–2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nishizumi H, Taniuchi I, Yamanashi Y, et al. Impaired proliferation of peripheral B cells and indication of autoimmune disease in lyn-deficient mice. Immunity. 1995;3(5):549–560. [DOI] [PubMed] [Google Scholar]
- 147.Luo W, Mayeux J, Gutierrez T, et al. A balance between B cell receptor and inhibitory receptor signaling controls plasma cell differentiation by maintaining optimal Ets1 levels. J Immunol 2014;193(2):909–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mayeux J, Skaug B, Luo W, et al. Genetic Interaction between Lyn, Ets1, and Btk in the Control of Antibody Levels. J Immunol 2015;195(5):1955–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.John SA, Clements JL, Russell LM, Garrett-Sinha LA. Ets-1 regulates plasma cell differentiation by interfering with the activity of the transcription factor Blimp-1. J Biol Chem 2008;283(2):951–962. [DOI] [PubMed] [Google Scholar]
- 150.Russell L, John S, Cullen J, Luo W, Shlomchik MJ, Garrett-Sinha LA. Requirement for Transcription Factor Ets1 in B Cell Tolerance to Self-Antigens. J Immunol 2015;195(8):3574–3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Paus D, Phan TG, Chan TD, Gardam S, Basten A, Brink R. Antigen recognition strength regulates the choice between extrafollicular plasma cell and germinal center B cell differentiation. J Exp Med 2006;203(4):1081–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chan TD, Gatto D, Wood K, Camidge T, Basten A, Brink R. Antigen affinity controls rapid T-dependent antibody production by driving the expansion rather than the differentiation or extrafollicular migration of early plasmablasts. J Immunol 2009;183(5):3139–3149. [DOI] [PubMed] [Google Scholar]
- 153.Tiller T, Tsuiji M, Yurasov S, Velinzon K, Nussenzweig MC, Wardemann H. Autoreactivity in human IgG+ memory B cells. Immunity. 2007;26(2):205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Han S, Zheng B, Dal Porto J, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. IV. Affinity-dependent, antigen-driven B cell apoptosis in germinal centers as a mechanism for maintaining self-tolerance. J Exp Med 1995;182(6):1635–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pulendran B, Kannourakis G, Nouri S, Smith KG, Nossal GJ. Soluble antigen can cause enhanced apoptosis of germinal-centre B cells. Nature. 1995;375(6529):331–334. [DOI] [PubMed] [Google Scholar]
- 156.Shokat KM, Goodnow CC. Antigen-induced B-cell death and elimination during germinal-centre immune responses. Nature. 1995;375(6529):334–338. [DOI] [PubMed] [Google Scholar]
- 157.Chan TD, Wood K, Hermes JR, et al. Elimination of germinal-center-derived self-reactive B cells is governed by the location and concentration of self-antigen. Immunity. 2012;37(5):893–904. [DOI] [PubMed] [Google Scholar]
- 158.Hale M, Rawlings DJ, Jackson SW. The long and the short of it: insights into the cellular source of autoantibodies as revealed by B cell depletion therapy. Curr Opin Immunol 2018;55:81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Au-Yeung BB, Smith GA, Mueller JL, et al. IL-2 Modulates the TCR Signaling Threshold for CD8 but Not CD4 T Cell Proliferation on a Single-Cell Level. J Immunol 2017;198(6):2445–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chen J, López-Moyado IF, Seo H, Lio CJ, Hempleman LJ, Sekiya T, Yoshimura A, Scott-Browne JP, Rao A. NR4A transcription factors limit CAR T cell function in solid tumours. Nature. 2019. March;567(7749):530–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Liu X, Wang Y, Lu H, Li J, Yan X, Xiao M, Hao J, Alekseev A, Khong H, Chen T, Huang R, Wu J, Zhao Q, Wu Q, Xu S, Wang X, Jin W, Yu S, Wang Y, Wei L, Wang A, Zhong B, Ni L, Liu X, Nurieva R, Ye L, Tian Q, Bian XW, Dong C. Genome-wide analysis identifies NR4A1 as a key mediator of T cell dysfunction. Nature. 2019. March;567(7749):525–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Noviski M, Tan C, Huizar J, Vykunta V, Mueller JL, Zikherman J. Optimal Development of Mature B Cells Requires Recognition of Endogenous Antigens. J Immunol. 2019. July 15;203(2):418–428. [DOI] [PMC free article] [PubMed] [Google Scholar]