Abstract
Purpose:
Maternal stress is a risk factor for adverse pregnancy outcomes (APOs). This study evaluates the associations of prenatal stress and APOs with maternal stress years after pregnancy.
Methods:
The 10-item Perceived Stress Scale (PSS) (0-40 range) was completed in the first and third trimesters, and 2-7 years after delivery among a subsample (n = 4,161) of nulliparous women enrolled at eight U.S. medical centers between 2010-2013 in a prospective, observational cohort study. Demographics, medical history, and presence of APOs (gestational diabetes (GDM), hypertensive disorders of pregnancy (HDP), preeclampsia (PE), and medically-indicated or spontaneous preterm birth (miPTB, sPTB)) were obtained. The associations of prenatal PSS and the presence of APOs with PSS scores years after delivery were estimated using multivariable linear regression.
Results:
Mean PSS scores were 12.5 (95% CI 12.3, 12.7) and 11.3 (95% CI 11.1, 11.5) in the first and third trimesters respectively and 14.9 (95% CI 14.7, 15.1) 2-7 years later, an average increase of 2.4 points (95% CI 2.2, 2.6) from the start of pregnancy. Regressing PSS scores after delivery on first-trimester PSS and PSS increase through pregnancy showed positive associations, with coefficients (95% CI) of 2.8 (2.7, 3.0) and 1.5 (1.3, 1.7) per 5-point change, respectively. Adding APO indicator variables separately showed higher PSS scores for women with HDP (0.7 (0.1, 1.3)), PE (1.3 (0.6, 2.1)), and miPTB (1.3 (0.2, 2.4)), but not those with GDM or sPTB.
Conclusions:
In this geographically and demographically diverse sample, prenatal stress and some APOs were positively associated with stress levels 2-7 years after pregnancy.
Keywords: prenatal maternal stress, adverse pregnancy outcomes, perceived stress, preterm birth, preeclampsia (5)
INTRODUCTION:
Prenatal stress has been associated with increased risk of pregnancy complications, including preterm birth (PTB), gestational diabetes (GDM) and preeclampsia (PE) (Hobel 2009, Alderdice 2012, Osbourne 2015). These complications have lasting implications, as hypertensive disorders of pregnancy (HDP; i.e., PE or gestational hypertension (GHTN)) and PTB are associated with increased risk of hypertension and cardiovascular disease in mothers (Ray 2005, Bellamy 2007, Catov 2013, Cirillo 2015, Catov 2016, Cortes 2018), while GDM is associated with increased risk of type 2 diabetes and renal and cardiovascular disease (Gunderson 2014, Beharier 2015, Appiah 2016, Lowe 2018). A few studies suggest that prenatal stress is also associated with postpartum mental health, although these reports generally have followed women only up to six months postpartum (Ngai 2015, Misri 2010). Information on the trajectories of stress from pregnancy to years after delivery is limited (Fredriksen 2017, Bayrampour 2016, Santos 2017, Mora 2009).
An additional potential consequence of adverse pregnancy outcomes (APOs) is an alteration in maternal mental health, possibly attributable to the milieu of immediate trauma and stressful sequelae of infant and maternal morbidity and caregiving. The comparatively high prevalence of post-traumatic stress disorder among women who experienced traumatic pregnancies is well-documented (Forray, 2009, Garthus, 2013, Kjeldgaard, 2018). In addition, studies specifically examining the mental health of mothers after PTB demonstrate higher stress levels and greater anxiety in the first few weeks after delivery (Misund 2014, Pichler-Stachi 2016, Helle 2016). Another study found that parents of very preterm children were more likely to experience moderate anxiety, depressive symptoms, and stress up to seven years after delivery (Treyvaud 2014). One study found that women who developed GDM were more likely to have a new diagnosis of a mood or anxiety disorder during pregnancy or within one year of delivery (Beka 2017). A systematic review found positive associations between HDP and depressive symptoms; most studies evaluated women 2 months to 2 years after delivery (Delahajje 2013). Nevertheless, the number of studies evaluating the association between APOs and maternal mental health is relatively few, those that do exist often do not distinguish between different indications for preterm delivery, and the typical follow-up is relatively short. Moreover, there is little information as to how maternal mental health during pregnancy is related to the APOs as well as to mental health many years later (Ngai 2015, Misri 2010, Fredriksen 2017, Bayrampour 2016, Santos 2017, Mora 2009).
The Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be (nuMoM2b), a cohort study of nulliparous women prospectively followed during pregnancy, with a subsample who underwent follow-up 2-7 years later as part of the Heart Health Study (HHS), provides a unique opportunity to investigate: (1) the patterns of stress during and for several years after pregnancy, and (2) the associations of stress during pregnancy and specific APOs with long-term maternal stress. We hypothesized that greater prenatal stress and the presence of APOs would be associated with greater maternal stress several years after delivery. Figure 1 provides a conceptual model of the postulated associations between stress during pregnancy, APOs, stress at follow-up, and potential confounders.
Figure 1.
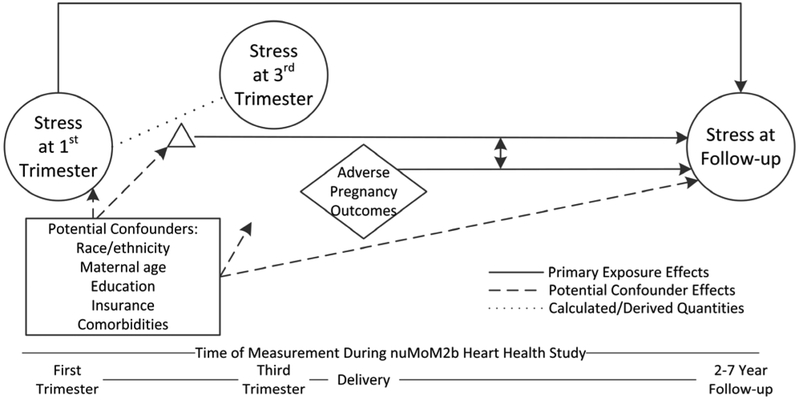
Conceptual Model Guiding our Research Questions.
MATERIALS AND METHODS:
Study Population
NuMoM2b is a geographically and demographically diverse, prospective, observational cohort study of 10,038 nulliparous pregnant women recruited from eight medical centers between October 2010 and September 2013 and followed throughout their pregnancy by research staff. Data were obtained through interviews, questionnaires, medical record review, ultrasounds, clinical measurements, and biological specimen collection during four study visits throughout pregnancy. Further details on the methods have been published elsewhere (Haas 2015). The subsequent HHS included 7,003 women, 4,508 of whom completed an in-person cardiovascular risk factor evaluation from 2 to 7 years after their nuMoM2b study pregnancy. The purpose of the HHS was to assess the association between APOs and long-term maternal cardiovascular health. Building on the resources of the nuMoM2b parent study, HHS data were collected via interviews, self-report questionnaires, physical examinations, and review of medical records. Further details on the methods have been published elsewhere (Haas 2016).
Perceived Stress Scale
The Perceived Stress Scale (PSS) was completed during nuMoM2b study visits in the first and third trimesters, and during the HHS in-person visit. The PSS is a 10-item questionnaire assessing the extent to which, during the past month, respondents judge their lives to be “unpredictable, uncontrollable or overloaded” using a 0-4 Likert scale (Cohen 1983). The scores are summed, resulting in a maximum score of 40 such that higher scores represent greater perceived stress (Cohen 1988). The PSS has been used and validated in over 17 languages and among diverse populations in both research and clinical settings, including among this study’s population (Taylor 2015, Karam 2012, Bann 2017).
APOs
For the purposes of this analysis, APOs were defined as categorical variables. GDM and HDP (PE or GHTN) were each defined as binary variables (yes or no), and PTB was ternary (miPTB, sPTB, or no PTB). Each outcome was identified by direct medical record abstraction according to pre-defined classifications (Haas 2015).
Statistical analysis
Multivariable linear regression was used to address pre-specified hypotheses regarding the association of prenatal stress and APOs with the outcome of interest – stress measured 2-7 years after first pregnancy (“HHS stress”). The initial multivariable model estimated the association between HHS stress and perceived stress during pregnancy, and considered whether that association may diminish with elapsed time since pregnancy. This model regressed HHS stress on the independent variables of first-trimester stress, the change in stress between the first and third trimesters of pregnancy, the time elapsed between third-trimester visit and the follow-up HHS visit, and the interaction between the change in stress and time elapsed.
Subsequently, to address hypotheses regarding the association between APOs and HHS stress, this model was extended by adding APOs and the interaction between APOs and the change in stress during pregnancy. The extended model was fit separately for each of the four APOs of interest (GDM, PE, HDP and PTB). Secondary analyses were conducted to adjust for potential confounders. These included race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, Asian, other), maternal age, attained education (6 levels), insurance status (commercial, government/military, or private/other), and the presence of one or more major medical comorbidities documented in the medical record at the time of pregnancy (diabetes mellitus, chronic hypertension, asthma and kidney disease).
Finally, to assess whether the observed associations between APOs and HHS stress reflect independent contributions of APOs to HHS stress, rather than appearing as an artifact of a causal pathway in which APOs mediate pre-existing stress caused by underlying illness, a mediation analysis was completed. This analysis framework (Baron 1986) requires that four criteria be met to support a claim of mediation: (1) baseline comorbidities are associated with HHS stress, (2) baseline comorbidities are associated with APO occurrence, (3) in a model with both baseline comorbidities and APOs, APOs are associated with HHS stress, and (4) in a model with both baseline comorbidities and APOs, the association between baseline comorbidities and HHS stress is nullified. These criteria were assessed using linear regression (criteria 1, 3, 4) and logistic regression (criterion 2), respectively.
Regression model assumptions were evaluated using residual plots. Results are presented as estimated regression parameters and associated 95% confidence intervals. All analyses were performed using SAS version 9.4.
RESULTS:
Sample Characteristics
Baseline demographic characteristics are presented in Table 1 for the 10,028 evaluable nuMoM2b participants and for the 4,161 women who completed all PSS surveys and who provided full covariate data. Among the HHS participants included in this analysis, 65.0% were non-Hispanic white, and 82.7% had at least some education beyond high school. These participants were demographically similar to the full cohort, with the exception of slightly higher representation of non-Hispanic white women at follow-up. Their median age upon entry into the nuMoM2b study was 27 years (min-max: 15-44). Overall, mean PSS scores were 12.5 (95% CI 12.3, 12.7) in the first trimester of pregnancy, 11.3 (95% CI 11.1, 11.5) in the third trimester of pregnancy, and 14.9 (95% CI 14.7, 15.1) 2-7 years after pregnancy. This reflects an estimated increase of 2.4 points (95% CI 2.2, 2.6) in the PSS score between the first trimester and follow-up 2-7 years later. 10.5% of the sample had PSS scores greater than 21 (the average for healthy pregnant women (Evans 2008)) at the first-trimester visit and 7.6% at the third-trimester visit. 49.9% of the sample had scores greater than 14 (the average for a population of healthy women (Cohen 1988)) at the HHS visit 2-7 years after delivery.
Table 1.
Baseline demographic and clinical characteristics of participants in the nuMoM2b cohort and follow-up Heart Health Study (HHS).
| nuMoM2b
cohort N=10,028 |
HHS
follow-up N=4,161 |
|||
|---|---|---|---|---|
| Baseline Characteristics | Frequency | % | Frequency | % |
| Age (years), median (min, max) | 27 (13, 45) | 27 (15, 44) | ||
| Race/Ethnicity | ||||
| Non-Hispanic White | 5,989 | 59.7% | 2,705 | 65.0% |
| Non-Hispanic Black | 1,418 | 14.1% | 502 | 12.1% |
| Hispanic | 1,700 | 17.0% | 640 | 15.4% |
| Asian | 407 | 4.1% | 122 | 2.9% |
| Other | 514 | 5.1% | 192 | 4.6% |
| Education | ||||
| Less Than High School | 816 | 8.1% | 278 | 6.7% |
| High School | 1,171 | 11.7% | 442 | 10.6% |
| Some College | 1,948 | 19.4% | 794 | 19.1% |
| Associate/Technical Degree | 1,005 | 10.0% | 468 | 11.3% |
| Bachelor’s Degree | 2,772 | 27.7% | 1,212 | 29.1% |
| Beyond Bachelor’s Degree | 2,308 | 23.0% | 967 | 23.2% |
| Insurance Status | ||||
| Commercial | 6,778 | 68.1% | 2,970 | 71.4% |
| Government/Military | 2,800 | 28.1% | 1,048 | 25.2% |
| Private/Other | 381 | 3.8% | 143 | 3.4% |
| Comorbidities* | 1,575 | 15.7% | 623 | 15.0% |
| APOs | ||||
| GDM | 396 | 4.2% | 177 | 4.3% |
| PE | 586 | 6.2% | 256 | 6.2% |
| HDP | 1,244 | 13.1% | 535 | 12.9% |
| miPTB | 338 | 3.6% | 124 | 3.0% |
| sPTB | 477 | 5.0% | 190 | 4.6% |
Missing data for full cohort as follows: education, n=8; insurance, n=69; GDM, n=616; PE and HDP, n=563; miPTB and sPTB, n=562. Percentages are calculated based on denominators that exclude participants with missing data.
nuMoM2b = Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be, HHS = Heart Health Study, APOs = adverse pregnancy outcomes, GDM = gestational diabetes mellitus, PE = preeclampsia, HDP = hypertensive disorders of pregnancy, miPTB = medically-indicated preterm birth, sPTB = spontaneous preterm birth
Denotes the self-reported presence of one or more of the following major medical comorbidities at the time of pregnancy: diabetes mellitus, chronic hypertension, asthma and kidney disease.
Association of Stress During Pregnancy with Long-Term Stress
In unadjusted regression models (data not shown), first-trimester PSS and PSS change between the first and third trimesters of pregnancy were positively associated with higher PSS scores at the HHS visit 2-7 years after pregnancy. Each 5-point increment in PSS during the first trimester corresponded to an increase of 2.8 points (95% CI 2.7, 3.0) in the HHS visit PSS, and each 5-point increase between trimesters was associated with a PSS increase of 1.5 points (95% CI 1.3, 1.7) at the HHS visit. Elapsed time between the third trimester and HHS visits contributed negligibly to stress at the HHS visit (0.2 points per elapsed year; 95% CI −0.2, 0.7). In addition, there was minimal evidence that the associations between first-trimester PSS and HHS stress, and between change in stress during pregnancy and HHS stress, diminished with elapsed time (interaction coefficient = −0.1, 95% CI −0.3, 0.1; coefficient = 0.1, 95% CI −0.1, 0.3; per elapsed year and 5-point increment in first-trimester PSS or change in stress during pregnancy, respectively). Terms related to elapsed time were thus not retained for additional models.
Association of APOs with Long-Term Stress
Independent of first-trimester stress and the change in stress during pregnancy, women who had HDP had 0.7 points higher stress at the HHS visit than those without HDP (Table 2 Model 1; 95% CI 0.1, 1.3). Women with PE also demonstrated higher stress at the HHS visit (1.3 points (95% CI 0.6, 2.1)). When considering PTB, miPTB was associated with higher stress at the HHS visit than no PTB (1.3 points (95% CI 0.2, 2.4)), but not sPTB. Also, GDM during pregnancy was not associated with stress at the HHS visit, after accounting for stress levels during pregnancy.
Table 2.
Association of baseline PSS, PSS change during pregnancy, and APO occurrence with PSS 2-7 years after pregnancy. Results are presented as estimated multivariable regression coefficients and 95% confidence intervals.
| Covariate | Adverse Pregnancy Outcome Included in the Model (APO) | ||||
|---|---|---|---|---|---|
| GDM | HDP | PE | sPTB* | miPTB* | |
| Model 1: Unadjusted regression coefficients. | |||||
| Intercept | 8.2 (7.8, 8.6) | 8.1 (7.7, 8.5) | 8.1 (7.7, 8.6) | 8.2 (7.7, 8.6) | |
| Baseline PSS (T1)+ | 2.8 (2.7, 3.0) | 2.8 (2.7, 3.0) | 2.8 (2.7, 3.0) | 2.8 (2.7, 3.0) | |
| PSS change (T3-T1)+ | 1.5 (1.3, 1.7) | 1.5 (1.4, 1.7) | 1.5 (1.3, 1.7) | 1.5 (1.3, 1.7) | |
| APO | 0.1 (−0.8, 1.1) | 0.7 (0.1, 1.3) | 1.3 (0.6, 2.1) | 0.2 (−0.7, 1.1) | 1.3 (0.2, 2.4) |
| PSS change X APO+ | 0.0 (−0.8, 0.8) | −0.4 (−0.9, 0.1) | −0.6 (−1.3, 0) | 0.0 (−0.7, 0.8) | −1.3 (−2.2, −0.4) |
| Model 2: Adjusted for race and baseline values of maternal age, education, insurance coverage, and comorbidities. | |||||
| Intercept | 8.7 (7.4, 10.1) | 8.7 (7.3, 10.1) | 8.7 (7.4, 10.1) | 8.7 (7.3, 10.1) | |
| Baseline PSS (T1)+ | 2.8 (2.6, 3.0) | 2.8 (2.6, 3.0) | 2.8 (2.6, 3.0) | 2.8 (2.6, 3.0) | |
| PSS change (T3-T1)+ | 1.5 (1.3, 1.7) | 1.5 (1.3, 1.7) | 1.5 (1.3, 1.7) | 1.5 (1.3, 1.7) | |
| APO | 0.2 (−0.7, 1.2) | 0.6 (0.1, 1.2) | 1.3 (0.5, 2.1) | 0.2 (−0.7, 1.1) | 1.2 (0.1, 2.3) |
| PSS change X APO+ | 0.1 (−0.8, 0.9) | −0.4 (−0.9, 0.1) | −0.5 (−1.2, 0.1) | 0.0 (−0.8, 0.8) | −1.2 (−2.1, −0.3) |
GDM = gestational diabetes mellitus, HDP = hypertensive disorders of pregnancy, PE = preeclampsia, sPTB = spontaneous preterm birth, miPTB = medically-indicated preterm birth, PSS = Perceived Stress Scale
Effects of PTB (sPTB and miPTB) were evaluated in a single model using ternary categorization of PTB. Coefficients contrast against the no PTB group.
Coefficients related to PSS at baseline (1st trimester, T1) and PSS change (3rd trimester (T3) minus T1) are provided for 5-point increments.
Adjustments for Demographic and Clinical Covariates
The associations of PSS scores in pregnancy and of APOs with PSS scores at the HHS visit remained similar even after adjusting for race/ethnicity and baseline values of age, attained education, insurance type, and the presence of medical comorbidities (Table 2 Model 2).
The association between APOs and long-term stress may reflect a causal pathway in which APOs serve as a mediator of stress due to underlying or pre-existing illness. In this scenario, an association between APOs and HHS stress would be observed due to APOs serving as a surrogate for the underlying comorbidities, rather than independently contributing to HHS stress. To explore this possibility, we evaluated the four mediation criteria described by Baron and Kenny (Baron 1986) and noted above in the Statistical Analysis, with results as follows: (1) baseline comorbidities were associated with HHS stress: participants with pre-pregnancy medical comorbidities had PSS scores 1.2 (95% CI 0.7, 1.8) points higher at the HHS visit than healthier participants (data not shown); (2) comorbidities were associated with some APOs, including HDP, PE, and miPTB, but not GDM or sPTB (Table 3, Model 1); in stress models including both APOs and comorbidities, (3) individual APOs remained associated with stress at follow-up with estimates of association similar to those previously described (Table 3, Model 2), and (4) the associations between baseline comorbidities and stress 2-7 years after delivery are preserved (Table 3, Model 2). Because result (4) violates the final criterion for mediation, we conclude that the association between APOs and stress at the HHS visit is not attributable to APOs serving a mediating role between pre-pregnancy comorbidity and stress at the HHS visit. Rather, the estimated associations reflect the independent contributions of APOs and their inseparable milieu of physical and psychological stressors.
Table 3.
Evaluation of the role of baseline comorbidity as a potential mediator of the association between APOs and PSS 2-7 years after pregnancy.
| Covariate | Adverse Pregnancy Outcome Included in the Model (APO) | ||||
|---|---|---|---|---|---|
| GDM | HDP | PE | sPTB* | miPTB* | |
| Model 1: Association between baseline comorbidities and APOs. Results are presented as odds ratios and Wald 95% CIs. | |||||
| Comorbidities | 1.0 (0.7, 1.6) | 1.5 (1.2, 1.8) | 1.8 (1.3, 2.5) | 1.0 (0.7, 1.5) | 1.9 (1.2, 2.8) |
| Model 2: Association of baseline comorbidities and APOs with PSS 2-7 years after pregnancy. Results are presented as estimated multivariable regression coefficients and 95% CIs. | |||||
| Intercept | 14.7 (14.5, 14.9) | 14.6 (14.3, 14.8) | 14.6 (14.4, 14.8) | 14.6 (14.4, 14.9) | |
| Comorbidities | 1.2 (0.7, 1.8) | 1.2 (0.6, 1.8) | 1.2 (0.6, 1.8) | 1.2 (0.6, 1.8) | |
| APO | 0.3 (−0.7, 1.4) | 1.1 (0.4, 1.7) | 1.9 (1.0, 2.8) | 0.5 (−0.5, 1.5) | 2.1 (0.9, 3.3) |
APOs = adverse pregnancy outcomes, PSS = Perceived Stress Scale, GDM = gestational diabetes mellitus, HDP = hypertensive disorders of pregnancy, PE = preeclampsia, sPTB = spontaneous preterm birth, miPTB = medically-indicated preterm birth
Effects of PTB (sPTB and miPTB) were evaluated in a single model using ternary categorization of PTB. Coefficients contrast against the no PTB group.
DISCUSSION:
In this analysis of data from a multi-center observational cohort study, we investigated the longitudinal relationship of stress during pregnancy and APOs to women’s stress levels 2-7 years after pregnancy. The study supported our hypothesis that greater prenatal stress, as well as certain APOs, are independently associated with higher stress levels several years later.
This study demonstrated the maintenance of perceived stress patterns during and beyond pregnancy. First-trimester stress and stress change between the first and third trimesters of pregnancy were positively associated with higher stress scores in later years. Each estimated 5-point increment in first-trimester stress predicted nearly 3 points higher stress years later, while each estimated 5-point increase in stress between trimesters was associated with 1.5 points higher stress at follow-up. The relationships with stress levels 2-7 years after pregnancy are consistent with prior studies that have observed maternal stress over shorter periods (Ngai 2015, Misri 2010, Fredriksen 2017, Bayrampour 2016, Santos 2017, Mora 2009), and extend those findings by additionally demonstrating the extent to which these relationships persist over the longer term.
Of note, mean stress levels during pregnancy (12.5 and 11.3 on the PSS) in this cohort were below what is typically viewed as clinically significant (approximately 26 points), and changes in stress were relatively small. However, our lower values in part reflect our use of the 10 versus 14 item PSS with ranges of 0-40 versus 0-56. Our finding of positive associations between baseline stress, changes in stress during pregnancy, and later stress levels nonetheless suggest that modestly higher baseline levels and increases during pregnancy could signal several future years of chronically elevated stress levels (Cohen 1988, Evans 2008, Chang 2015, Woolhouse 2014). Further studies are necessary to better understand whether it may be particularly important to provide mental health interventions to improve the long-term mental health and well-being of women with the greatest levels of perceived stress during pregnancy.
HDP, PE, and miPTB were also associated with higher stress years after pregnancy, while sPTB and GDM were not. These associations of prenatal stress and APOs with stress many years later remained even after controlling for a variety of potential confounding factors. These independent associations aligned with our hypotheses and underscore the importance of considering maternal experiences during pregnancy as part of an integrative approach to improving women’s future well-being.
To our knowledge, few studies have investigated the long-term association of distinct APOs with maternal stress several years after delivery in a large and diverse prospective cohort. The fact that only some APOs had associations with long-term stress may suggest that different perceptions of individual APOs may influence their contribution to later stress experiences. Because HDP and PE are associated with future cardiovascular health risks (Ray 2005), including risk for HDP and PE in future pregnancies which tend to be understood by women (Brown 2013, Traylor 2015), these conditions may be perceived as more concerning for long-term health and thus related to stress levels years later. Similarly, as the most common indications for miPTB are related to ischemic placental syndromes such as PE, fetal growth restriction, or placental abruption, this event also may be perceived as having a more lasting impact on maternal health and later stress levels (Ananth 2006). In contrast, GDM and sPTB may be perceived as time-limited conditions without future health implications. Although GDM is a risk factor for future development of cardiovascular or renal disease (Beharier 2015), 16% of women with GDM in one study and 47% in another underestimated their personal risk for developing type 2 diabetes, despite the vast majority of participants (90 and 89% respectively) understanding the general association between GDM and type 2 diabetes (Mukerji 2015, Kim 2007).
Predictably, the presence of comorbidities was associated with greater odds of HDP, PE, and miPTB. Pre-existing major medical conditions may have influenced the stress levels of some women both during and after pregnancy. It is important to note that while APOs may be thought of as mediators of an association between baseline comorbidities and stress years after pregnancy, the failure to meet established criteria for mediation indicated that APOs are independent factors in their association with future stress.
This study had several notable strengths including its large sample size and the relative demographic and geographic diversity of the participants. Its weaknesses include reliance on a self-report measure for indexing stress levels, and the relatively low number of women enrolled in Medicaid, the insurance program supported by federal and state governments for those with limited financial means. In the U.S., roughly 45% of births are to women enrolled in Medicaid; in this sample, approximately 25-28% were enrolled in government supported insurance, and this included insurance via the military. Our results may not generalize to women in lower socioeconomic circumstances.
CONCLUSION:
In this study of a large, geographically and demographically diverse U.S. sample, prenatal stress and some APOs were positively and independently associated with levels of stress 2-7 years after pregnancy. Routine prenatal care, with its structure of frequent patient-provider contact, offers a unique opportunity to screen for behavioral health issues and provide interventions. Moreover, clinician awareness of the psychosocial strain and potentially traumatic nature of APOs and associated provision of support services could diminish their negative impact on women’s well-being. Overall, attention to women’s mental, as well as physical, status during prenatal care could significantly improve their long-term health. As demonstrated in a systematic review of existing antenatal behavioral health interventions intended to address women’s well-being, women at greater risk for developing maternal distress benefited in the first year postpartum from interventions during pregnancy, including group prenatal care and peer or professional mentoring (Fontein-Kuipers 2014). It will be important to characterize further risk factors for prenatal and long-term stress, and risk-stratify women who would benefit from additional support during the prenatal and postnatal periods.
Acknowledgments
COMPLIANCE WITH ETHICAL STANDARDS:
Funding: This study is funded by cooperative agreement funding from the National Heart, Lung, and Blood Institute and the Eunice Kennedy Shriver National Institute of Child Health and Human Development: U10-HL119991; U10-HL119989; U10-HL120034; U10-HL119990; U10-HL120006; U10-HL119992; U10-HL120019; U10-HL119993; and U10-HL120018. Supplemental funding for this analysis was provided by the National Institutes of Health Office of Behavioral and Social Sciences Research through U10-HL119992. In addition, support was provided by the National Institutes of Health National Center for Research Resources and National Center for Advancing Translational Sciences to Clinical and Translational Science Institutes at Indiana University (UL1TR001108) and University of California, Irvine (UL1TR000153).
Abbreviations:
- APOs
adverse pregnancy outcomes
- HDP
hypertensive disorders of pregnancy
- GDM
gestational diabetes mellitus
- GHTN
gestational hypertension
- PE
preeclampsia
- PTB
preterm birth
- miPTB
medically-indicated preterm birth
- sPTB
spontaneous preterm birth
- PSS
Perceived Stress Scale
- nuMoM2b
Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be
- HHS
Heart Health Study
Footnotes
ClinicalTrials.gov Registration number NCT02231398.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure of potential conflicts of interests:
Author X has received speaker honorariums and consulting paid to Cedars-Sinai Medical Center from the American College of OB-GYN (lecture), Atlantic Health System (lecture), Abbott Diagnostics (lectures), American College of Cardiology (lectures), Cardio NAH Health Systems (lectures), Expert Exchanges (lectures), INOVA Health systems (lecture), George Washington University (lecture), Med Ed (lecture), Northwestern (young investigators grant review), Pri-Med (lectures), Oklahoma Chapter of American College of Cardiology (lecture), Renown Health System Reno (lectures), San Diego Heart Institute (lectures), Society of Vascular Medicine (lecture), St Francis Medical Center Hartford (lecture), University of Minnesota (lecture), USCF (lecture), University of Capetown (lecture), and University of Colorado (lecture). Author X has received honorarium and consulting from ACRWH (NIH advisory council), NIH-CASE (grant review study section), Springer International (book honorarium), Decision Support in Medicine LLC (book honorarium), and iRhythm (board director), and NHLBI subcontract to Research Triangle Institute (RTI) International. Author X has received personal research funding from WISE HFpEF, RWISE, Microvascular, Normal Control, FAMRI, Department of Defense, and California Institute for Precision Medicine. All other authors declare that there are no conflicts of interest.
Ethical Approval: This article does not contain any studies with animals performed by any of the authors. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent: Study procedures were approved by each center’s institutional review board prior to initiation of the study and written informed consent was obtained from all individual participants included in this study.
REFERENCES:
- Alderdice F, Lynn F, Lobel M (2012) A review and psychometric evaluation of pregnancy specific stress measures. J Psychosom Obstet Gynaecol 33(2):62–77. [DOI] [PubMed] [Google Scholar]
- Anath CV, Vintzileos AM (2006) Epidemiology of preterm birth and its clinical subtypes. J Matern Fetal Neonatal Med 12:773–782. [DOI] [PubMed] [Google Scholar]
- Appiah D, Schreiner PJ, Gunderson EP, Konety SH, Jacobs DR, Nwabuo CC, Ebong IA, Whitham HK, Goff DC, Lima JA, Ku IA, Gidding SS (2016) Association of Gestational Diabetes Mellitus With Left Ventricular Structure and Function: The CARDIA Study. Diabetes care, 39(3): 400–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bann CM, Parker CB, Grobman WA, Willinger M, Simhan HN, Wing DA, Haas DM, Silver RM, Parry S, Saade GR, Wapner RJ, Elovitz MA, Miller ES, Reddy UM; NuMoM2b study (2017) Psychometric properties of stress and anxiety measures among nulliparous women. J Psychosom Obstet Gynaecol 38(1):53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron RM, Kenny DA (1986) The moderator-mediator variable distinction in social psychological research: Conceptual, strategic and statistical considerations. J Pers Social Psychol 51:1173–1182. [DOI] [PubMed] [Google Scholar]
- Bayrampour H, Tomfohr L (2016) Tough S Trajectories of Perinatal Depressive and Anxiety Symptoms in a Community Cohort. J Clin Psychiatry 77(11):1467–1473. [DOI] [PubMed] [Google Scholar]
- Beharier O, Shoham-Vardi I, Pariente G, Sergienko R, Kessous R, Baumfeld Y, Szaingurten-Solodkin I, Sheiner E (2015) Gestational diabetes mellitus is a significant risk factor for long-term maternal renal disease. J Clin Endocrinol Metab 100(4):1412–6. [DOI] [PubMed] [Google Scholar]
- Beka Q, Bowker S, Savu A, Kingston D, Johnson JA, Kaul P (2017) Development of perinatal mental illness in women with gestational diabetes mellitus: A population-based cohort study. Can J Diabetes S1499-2671(17)30278-2. [DOI] [PubMed] [Google Scholar]
- Bellamy L, Casas JP, Hingorani AD, Williams DJ (2007) Preeclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ 335(7627):974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MC, Bell R, Collins C, Waring G, Robson SC, Waugh J, Finch T (2013) Women’s perception of future risk following pregnancies complicated by preeclampsia. Hypertens Pregnancy 32:60–73. [DOI] [PubMed] [Google Scholar]
- Catov JM, Lewis CE, Lee M, Wellons MF, Gunderson EP (2013) Preterm birth and future maternal blood pressure, inflammation, and intimal-medial thickness: the CARDIA study. Hypertension 61(3):641–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catov JM, Althouse AD, Lewis CE, Harville EW, Gunderson EP (2016) Preterm delivery and metabolic syndrome in women followed from prepregnancy through 25 years later. Obstet Gynecol 127(6):1127–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HC, Yu CH, Chen SY, Chen CH (2015) The effects of music listening on psychosocial stress and maternal-fetal attachment during pregnancy. Complement Ther Med 23(4):500–515. [DOI] [PubMed] [Google Scholar]
- Cirillo PM, Cohn BA (2015) Pregnancy complications and cardiovascular disease death: 50-year follow-up of the Child Health and Development Studies pregnancy cohort. Circulation 132(13):1234–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R (1983) A global measure of perceived stress. J Health Soc Behav 24(4):385–396. [PubMed] [Google Scholar]
- Cohen S, Williamson GM (1988) Perceived stress in a probability sample of the United States In: Spacapan S, Oskamp S, editor. The Social Psychology of Health. Sage, Newbury Park, CA, pp 31–67. [Google Scholar]
- Cortés YI, Catov JM, Brooks M, Harlow SD, Isasi CR, Jackson EA, Matthews KA, Thurston RC, Barinas-Mitchell E (2017) History of Adverse Pregnancy Outcomes, Blood Pressure, and Subclinical Vascular Measures in Late Midlife: SWAN (Study of Women’s Health Across the Nation). Journal of the American Heart Association, 7(1), e007138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delahaije DH, Dirksen CD, Peeters LL, Smits LJ (2013) Anxiety and depression following preeclampsia or hemolysis, elevated liver enzymes, and low platelets syndrome. A systematic review. Acta Obstet Gynecol Scand 92(7):746–61. [DOI] [PubMed] [Google Scholar]
- Evans LM, Myers MM, Monk C (2008) Pregnant women’s cortisol is elevated with anxiety and depression — but only when comorbid. Arch Womens Ment Health 11(3):239–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontein-Kuipers YJ, Nieuwenhuijze MJ, Ausems M, Budé L, de Vries R (2014) Antenatal interventions to reduce maternal distress: a systematic review and meta-analysis of randomised trials. BJOG 121(4):389–97. [DOI] [PubMed] [Google Scholar]
- Forray A, Mayes LC, Magriples U, Epperson CN (2009) Prevalence of post-traumatic stress disorder in pregnant women with prior pregnancy complications. J Matern Fetal Neonatal Med, 22(6): 522–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredriksen E, von Soest T, Smith L, Moe V (2017) Patterns of pregnancy and postpartum depressive symptoms: Latent class trajectories and predictors. J Abnorm Psychol 126(2):173–183. [DOI] [PubMed] [Google Scholar]
- Garthus-Niegel S, von Soest T, Vollrath ME, Eberhard-Gran M (2013) The impact of subjective birth experiences on post-traumatic stress symptoms: a longitudinal study. Arch Womens Ment Health, 16(1):1–10. [DOI] [PubMed] [Google Scholar]
- Gunderson EP, Chiang V, Pletcher MJ, Jacobs DR, Quesenberry CP, Sidney S, Lewis CE (2014) History of gestational diabetes mellitus and future risk of atherosclerosis in mid-life: the Coronary Artery Risk Development in Young Adults study. J Am Heart Assoc 3(2):e000490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas DM, Ehrenthal DB, Koch MA, Catov JM, Barnes SE, Facco F, Parker CB, Mercer BM, Bairey-Merz CN, Silver RM, Wapner RJ, Simhan HN, Hoffman MK, Grobman WA, Greenland P, Wing DA, Saade GR, Parry S, Zee PC, Reddy UM, Pemberton VL, Burwen DR; National Heart, Lung, and Blood Institute nuMoM2b Heart Health Study Network (2016) Pregnancy as a window to future cardiovascular health: Design and implementation of the nuMoM2b Heart Health Study. Am J Epidemiol 183(6):519–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas DM, Parker CB, Wing DA, Parry S, Grobman WA, Mercer BM, Simhan HN, Hoffman MK, Silver RM, Wadhwa P, Iams JD, Koch MA, Caritis SN, Wapner RJ, Esplin MS, Elovitz MA, Foroud T, Peaceman AM, Saade GR, Willinger M, Reddy UM; NuMoM2b study (2015) A description of the methods of the Nulliparous Pregnancy Outcomes Study: monitoring mothers-to-be (nuMoM2b). Am J Obstet Gynecol 212(4):539.e1–539.e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helle N, Barkmann C, Ehrhardt S, von der Wense A, Nestoriuc Y, Bindt C (2016) Postpartum anxiety and adjustment disorders in parents of infants with very low birth weight: Cross-sectional results from a controlled multicentre cohort study. J Affect Disord 194:128–34. [DOI] [PubMed] [Google Scholar]
- Hobel CJ, Goldstein A, Barrett ES (2009) Psychosocial stress and pregnancy outcome. Clin Obstet Gynecol 51(2):333–348. [DOI] [PubMed] [Google Scholar]
- Karam F, Berard A, Sheehy O, Huneau M, Briggs G, Chambers C, Einarson A, Johnson D, Kao K, Koren G, Martin B, Polifka JE, Riordan SH, Roth M, Lavigne SV, Wolfe L, the OTIS Research Committee (2012) Reliability and validity of the 4-item perceived stress scale among pregnant women: Results from the OTIS antidepressants study. Res Nurs Health 35:363–375. [DOI] [PubMed] [Google Scholar]
- Kim C, McEwen LN, Piette JD, Goewey J, Ferrara A, Walker EA (2007) Risk perception for diabetes among women with histories of gestational diabetes mellitus. Diabetes Care 30(9):2281–6. [DOI] [PubMed] [Google Scholar]
- Kjeldgaard HK, Vikanes Å, Benth JŠ, Junge C, Garthus-Niegel S, Eberhard-Gran M (2018). The association between the degree of nausea in pregnancy and subsequent posttraumatic stress. Archives of Women’s Mental Health, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe WL, Scholtens DM, Lowe LP, Kuang A, Nodzenski M, et al. (2018) Association of gestational diabetes with maternal disorders of glucose metabolism and childhood adiposity. JAMA 320(10):1005–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misri S, Kendrick K, Oberlander TF, Norris S, Tomfohr L, Zhang H, Grunau RE (2010) Antenatal depression and anxiety affect postpartum parenting stress: a longitudinal, prospective study. Can J Psychiatry 55(4):222–8. [DOI] [PubMed] [Google Scholar]
- Misund AR, Nerdrum P, Diseth TH (2014) Mental health in women experiencing preterm birth. BMC Pregnancy Childbirth 14:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora PA, Bennett IM, Elo IT, Mathew L, Coyne JC, Culhane JF (2009) Distinct trajectories of perinatal depressive symptomatology: evidence from growth mixture modeling. Am J Epidemiol 169(1):24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukerji G, Kainth S, Pendrith C, Lowe J, Feig DS, Banerjee AT, Wu W, Lipscombe LL (2016) Predictors of low diabetes risk perception in a multi-ethnic cohort of women with gestational diabetes mellitus. Diabet Med 33(10):1437–44. [DOI] [PubMed] [Google Scholar]
- Ngai FW, Ngu SF (2015) Predictors of maternal and paternal depressive symptoms at postpartum. J Psychosom Res 78(2):156–61. [DOI] [PubMed] [Google Scholar]
- Osborne LM, Monk C (2015) Perinatal depression - the fourth inflammatory morbidity of pregnancy? Theory and literature review. Psychoneuroendocrinology 38(10):1929–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichler-Stachl E, Pichler G, Baik N, Urlesberger B, Alexander A, Urlesberger P, Cheung PY, Schmölzer GM (2016) Maternal stress after preterm birth: Impact of length of antepartum hospital stay. Women Birth 29(6):105–109. [DOI] [PubMed] [Google Scholar]
- Ray JG, Vermeulen MJ, Schull MJ, Redelmeier DA (2005) Cardiovascular health after maternal placental syndrome (CHAMPS): population-based retrospective cohort study. Lancet 366(9499):1797–1803. [DOI] [PubMed] [Google Scholar]
- Santos H Jr, Tan X, Salomon R (2017) Heterogeneity in perinatal depression: how far have we come? A systematic review. Arch Womens Ment Health 20(1):11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JM (2015) Psychometric Analysis of the Ten-Item Perceived Stress Scale. Psychol Assess 2015;27(1):90–101. [DOI] [PubMed] [Google Scholar]
- Traylor J, Chandrasekaran S, Limaye M, Srinivas S, Durnwald CP (2016) Risk perception of future cardiovascular disease in women diagnosed with a hypertensive disorder of pregnancy. J Matern Fetal Neonatal Med 29(13):2067–72. [DOI] [PubMed] [Google Scholar]
- Treyvaud K, Lee KJ, Doyle LW, Anderson PJ (2014) Very preterm birth influences parental mental health and family outcomes seven years after birth. J Pediatr 164(3):515–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolhouse H, Mercuri K, Judd F, Brown SJ (2014) Antenatal mindfulness intervention to reduce depression, anxiety and stress: a pilot randomized controlled trial of the MindBabyBody program in an Australian tertiary maternity hospital. BMC Pregnancy Childbirth 14:369. [DOI] [PMC free article] [PubMed] [Google Scholar]


