Abstract
Human brain development is optimized to learn from environmental cues. The protracted development of the cortex and its connections with subcortical targets has been argued to permit more opportunity for acquiring complex behaviors. This paper uses the example of amygdala-medial prefrontal cortex circuitry development to illustrate a principle of human development - namely, that the extension of the brain’s developmental timeline allows for the (species-expected) collaboration between child and parent in co-construction of the human brain. The neurobiology underlying affective learning capitalizes on this protracted timeline to develop a rich affective repertoire in adulthood. Humans are afforded this luxuriously slow development in part by the extended period of caregiving provided by parents, and parents aid in scaffolding the process of maturation during childhood. Just as adequate caregiving is a potent effector of brain development, so is adverse caregiving, which is the largest environmental risk factor for adult mental illness. There are large individual differences in neurobiological outcomes following caregiving adversity, indicating that these pathways are probabilistic, rather than deterministic, and prolonged plasticity in human brain development may also allow for subsequent amelioration by positive experiences. The extant research indicates that the development of mental health cannot be considered without consideration of children in the context of their families.
Keywords: brain development, amygdala, medial prefrontal cortex, stress, sensitive periods, parents
Decades of research have demonstrated parents’ critical role in the healthy development of complex cognitive and affective behaviors (1–6). Caregiving is a potent effector of human development, and therefore the reach of maltreatment on behavioral and brain development can be long and significant. Early caregiving adversities, including abuse (physical, sexual, and emotional), neglect (emotional, failure to provide, and lack of supervision), and exposure to violence in the home, have been associated with altered neurodevelopment (7–9). The current paper discusses how characteristics of human brain development might foster the endurance of these links.
The Neotenous Brain.
The link between adverse caregiving and brain development is not unique to humans, and animal models have established that the link is causal (described below). However, humans do stand out, perhaps more than any other species, as a result of their neotenous brain development (see Figure 1). Originally coined to describe the retention of juvenile physical traits into adulthood (e.g., eruption of teeth, physical features) (10), neotenous development (or ‘juvenilization’) might describe human brain development as well, particularly for complex process like cognition and emotion. For example, the slow developing prefrontal cortex has demonstrated synaptic reorganization (overproduction and synapse elimination) until the third decade of life (11), well beyond what has been observed in the developmental equivalent in other mammals. Similarly, mRNA expression in the prefrontal cortex is developmentally delayed in humans relative to other primate species (12) indicating that neoteny is observed at the transcriptome level. Neoteny at the behavioral level (e.g., delayed cognition, extended play behaviors) has been described as adaptive in that it permits repeated, slow, and thus, enhanced learning opportunities (13). Scholars have speculated that there is value to this prolonged process, such that it allows for the “unprecedented opportunity for acquisition of the highest level of cognitive abilities (13).” At the same time, this characteristic of human development, which produces cognitive and affective complexity, can also increase the risk for poor outcomes following early adverse experiences.
Figure 1.
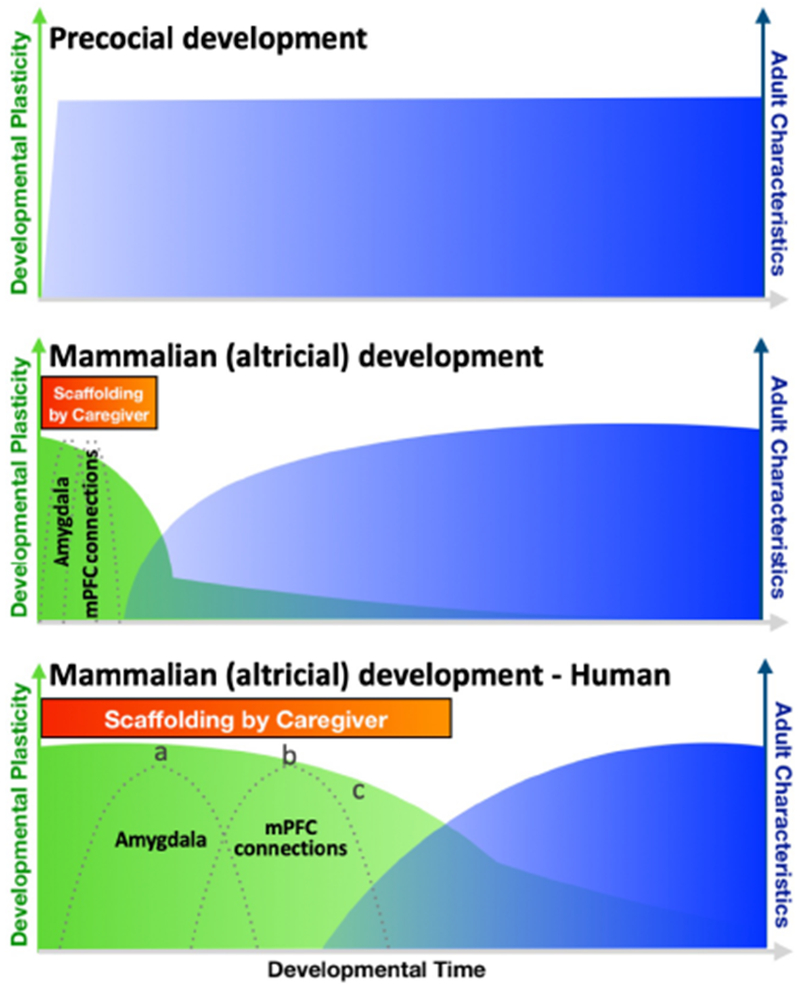
Neotenous brain development permits increased opportunity to receive caregiver input. Altricial development (bottom two panels), unlike that of precocial species (top panel), requires caregiver input, which satisfies a species-expectation that the caregiving environment will scaffold the offspring’s developing neurobiology during periods of high developmental plasticity (green) before circuitry begins to take on adult characteristics (blue). Here, the example of amygdala-medial prefrontal cortex (mPFC) circuit development is used to illustrate how an expansion, and therefore protraction, of developmental processes enables significant influence from the caregiving environment on developing neurobiology in the case of the human (bottom panel). Dotted lines are meant to represent putative sensitive periods for the amygdala and its connections with mPFC. Interventions (e.g., changes in parenting, therapy) may have differential efficacy depending on when they occur (i.e., moments a,b,c), motivating the development and use of age-specific approaches. Note: ‘Developmental Time’ on x-axis is intended to be equated across the three species-types (top, middle, and bottom).
Collectively, these papers argue that humans, more than other species, depend on learning from a very complex environment to produce rich behavioral repertoires, and a delayed onset of adult phenotypes provides more opportunities for learning these repertoires and developing strategies appropriate to the given environment. This prolonged development is comprised of multiple sensitive periods (14, 15), which are developmental moments when the environment has an especially potent and enduring impact on developing neurobiology. Moreover, the brain develops in a hierarchical fashion, where the structure and function of earlier developing regions exert maturational consequences on the structure and function of later developing regions. Referred to as ‘developmental cascades’ (16), such an organization implies that earlier occurring changes to the brain would have an impact not only on the neurobiology undergoing its sensitive period, but also on the downstream targets that receive connections from these regions (14, 17). Lesion work in non-human primates has illustrated this hierarchical organization; for example, neonatal lesions of the earlier developing amygdala and surrounding temporal lobe causes aberrant development of the prefrontal cortex (18); in other words, the prefrontal cortex might exhibit altered development despite the fact that is was never directly perturbed by the environment, by virtue of the fact that it is a developmental target of the earlier developing regions. Notably, the same lesions performed in adulthood did not produce these effects. These developmental principles of hierarchical development, sensitive periods, and prolonged development are critical tools for understanding the role of early experiences on adult functioning.
Amygdala-mPFC Development: Neoteny, Plasticity, and Learning.
Human amygdala-medial prefrontal cortex (mPFC) circuitry is central to mature emotional behaviors including learning, attention, modulation, and prediction (19–22). We focus on this circuitry to examine principles of neoteny and development as they relate to early caregiving adversity. While several other circuits have shown correlations with early adversity, including hippocampus, amygdala, striatum, cerebellum, and cortex (e.g., 23–25), as well as alterations to the connections between these regions, the current paper is motivated to focus on amygdala-mPFC circuitry by several of its characteristics. First, amygdala-mPFC circuitry constitutes the foundation for behaviors associated with emotion regulation in adulthood (19), behaviors which are commonly affected by early life stress. Secondly, the amygdala is rich with stress hormone receptors, especially early in life, and it exhibits membrane potential characteristics early in development that make it highly reactive to stressors (26, 27). Thirdly, amygdala-mPFC circuitry is highly sensitive to environmental influences during development, as has been demonstrated by a resting-state functional magnetic resonance imaging twin study performed during childhood (28). Additionally, the hierarchical relationship between the amygdala and the mPFC provide fodder for a deeper discussion of principles of developmental cascades.
Findings from Rodents.
Evidence from several sources shows that the structural and functional connections between the amygdala and the mPFC develop very slowly across mammalian species. Using tracing methods, rodent studies have shown that fibers originating in basolateral amygdala that project to regions of the mPFC (including the anterior cingulate, paralimbic cortex, and infralimbic cortex) exhibit a continued increase in density, spine formation, and change in topography continuing into the late postweanling/juvenile periods, perhaps even extending into adulthood (29, 30), thus occurring much later than projections to other regions (e.g., thalamus, nucleus accumbens) (31). In adulthood, amygdala-mPFC connections the are strongly bidirectional, and the development of reciprocal connections from mPFC to amygdala are late occurring; initial amygdala-to-mPFC growth spurts are later followed by even later-occurring mPFC-to-amygdala connection development (32). By combining anatomical tracings with opto-genetic interrogation, it has been shown that early bursts of growth in infancy are subsequently followed by an additional burst observed in the late juvenile/early adolescent period (33). This trajectory for structural development is paralleled by an initial burst in inhibitory post-synaptic potentials (relative to excitatory) in the late infancy period, and a second burst of inhibitory tone in the late juvenile period. This juvenile period has also been shown to be the time when the dendritic trees within amygdala and mPFC exhibit their largest growth (34). Consistent with this structural growth pattern, GABA-ergic transmission in the amygdala shows continued maturation until the end of the juvenile period (35). These data suggest that the excitatory/inhibitory balance of this pathway shows a protracted development, with an increasing shift towards top-down inhibition that does not mature until adolescence. The timing of mPFC-to-amygdala synapse formation (i.e., the juvenile period (postnatal day 30), which is roughly right after weaning yet prior to puberty (36)) coincides with a normative decline in emotionality (37, 38) and the largest developmental increase in spontaneous synaptic activity of the amygdala (33). This finding is consistent with the hypothesis that strong activity in the amygdala instigates connection formation with the mPFC (17). Taken together, the rodent work has shown amygdala-mPFC circuitry constructs itself first in a bottom-up fashion (i.e., amygdala-to-mPFC); then strong excitatory activity from the amygdala temporally correlates with the beginning of the reciprocal top-down inhibitory connections that continue to strengthen into adulthood.
Findings from Humans.
Studies in humans suggest an analogous, albeit further protracted, late development of amygdala-mPFC connectivity, occurring across childhood and adolescence (Figure 1). For example, diffusion tensor imaging techniques have shown that fronto-temporal tracts, like the cingulum and uncinate fasciculus, mature later than other tracts, requiring 25 years to reach 90% of their development . By comparison, other large tracts, like those that connect occipital to temporal cortex reach 90% of their development already by 11 years old (39). Correlated activity between the amygdala and mPFC, or functional connectivity, parallels these fronto-temporal structural tracts (40). Functional connectivity can be measured during resting state (i.e., intrinsic connectivity) or during task (i.e., stimulus-elicited). Resting state studies performed during human development have shown that amygdala-mPFC functional connectivity continues to show developmental changes until early adulthood. Resting-state functional connectivity between the amygdala and mPFC is present during infancy (41), although it exhibits non-linear changes (increases in the first year, followed by decreases in the second year)(42). These early developments are followed by continued change throughout the next two decades, with some studies showing continued increases in connectivity from childhood through adulthood (43, 44) and others showing decreases across this period (45).
Amygdala-mPFC resting-state functional connectivity is developmentally predicted by stimulus-elicited functional connectivity recorded earlier during childhood (46), suggesting that the nature of early environmentally-stimulated coactivation of the amygdala and mPFC during childhood might have an enduring influence on the nature of its intrinsic connectivity later in maturity (17). This interpretation is consistent with findings from a behavioral-genetics study showing that individual differences in amygdala-mPFC intrinsic functional connectivity during development are best explained by environmental influences (28). Stimulus-elicited, or task-based, functional connectivity studies have also shown age-related changes in amygdala-mPFC connectivity throughout the first 3 decades of life (47–51), though these connectivity patterns vary as a function of the task. Many of these studies have shown that the nature of the relationship between the amygdala and mPFC differs in childhood relative to points thereafter (52, 53), and is unlikely to include “top-down” regulatory connections.
As was found in rodent models, the developmentally late onset of the regulatory connections between the mPFC and the amygdala temporally parallel elevated amygdala reactivity that attenuates with increasing age (47, 49, 50, 54, 55), which is consistent with the hypothesis that the juvenile-like lability of the amygdala is an important instigator for the formation of connections with the mPFC (17). Again similar to rodent findings, in the human these events occur during late childhood into adolescence. Behaviorally, these neurobiological transitions are paralleled by the young child exhibiting high emotional reactivity and elevated levels of developmentally-normative fears (47, 56–58), and at later ages (e.g., adolescence and adulthood), these behaviors attenuate when structural and functional connections with the mPFC correspond with better regulation of the amygdala (22, 59). Taken together, studies in humans show patterns of amygdala-mPFC development that are highly consistent with those patterns identified in the rodent - namely, human development shows strong amygdala activity and emotionality early in life, followed by adult-like amygdala-mPFC connections and associated declines in emotionality at older ages - albeit occurring at a much more protracted rate.
The Ecology of the Developing Child.
The neurodevelopmental pattern of amygdala-mPFC circuitry described thus far, in many ways, mirror the ecology of the developing child (60). During a time when parents or other caregivers are routinely available to guide children’s exploration, it may be ontogenetically unnecessary (and even inefficient) to mature this system early, since the role of the amygdala and its connections with the mPFC is to facilitate independent exploration of the environment (judging the safety and danger of encountered stimuli) and adult-like learning. That is, the parent provides significant, and perhaps sufficient, information about the affective environment and will continue to serve this role until physical independence from the parent becomes routine.
Parental modulation of early emotional learning.
Evidence for this claim comes from both rodent and human studies. In rodents, it has been shown that the dam’s presence promotes her offspring’s preference for cues associated with the dam, regardless if the stimulation is pleasurable or aversive (61). In the context of aversive cues, the dam buffers stress responsive systems (e.g., the amygdala) to paradoxically promote preference learning for her cues, and this process is the basis for attachment learning in rodents. Young children have also been shown to behaviorally prefer an aversive conditioned stimulus if acquisition occurred in the presence of their parent (62). These findings suggest a mechanism by which children learn to prefer and attach to parental cues, regardless of warmth or maltreatment (63, 64) and suggest that early emotional learning systems are constructed to allow for modification by the parent.
The normative presence of parents and caregivers is a powerful effector of development, providing a social scaffolding for the developing child. Thus, parental presence may also be scaffolding amygdala-mPFC circuitry. Providing support for this position, parental stimuli can produce a momentary adult-like amygdala-mPFC connectivity pattern in children, a modulation that coincides with a decrease in amygdala activity (65). However, at the transition between childhood and adolescence, this circuitry changes and begins to show adult-like ‘regulatory’ connectivity patterns, regardless of parental cue presence or absence. During adolescence, parental presence may be less necessary (and also less effective) in modulating this circuitry, which has now become more adult-like (47). However, there may still be times in adolescence when the parent retains their ‘buffering’ effects, for example under conditions of risky-decision making (66).
Normative Variation in Parenting Behavior: Sensitivity and Security.
Parental care has been shown to correlate with the nature of amygdala-mPFC circuitry structure and function across a number of studies. For example, studies have shown that attachment styles, when measured in adulthood, are correlated with concurrent amygdala function (67, 68); moreover, when attachment is measured in infancy, these classifications have predicted amygdala structural differences when measured years later in childhood (e.g., 10 years old)(69) and even into adulthood (70, 71). Attachment security is thought to reflect parenting quality received (72), and these enduring associations between attachment style and amygdala development may reflect the discrete, routinized parenting behaviors experienced during childhood (73). Indeed, parenting quality itself has been correlated with amygdala and prefrontal cortex development. For example, parental sensitivity during infancy has been associated with smaller amygdala volumes (74); when measured during childhood, parental sensitivity has been shown to moderate age-related increases in amygdala-mPFC resting-state connectivity, with the suggestion that low sensitivity accelerates development of functional connections (75). In fact, early parenting behavior has predicted amygdala-mPFC circuit development across a span of years (76–79). If the links between parental care and amygdala-mPFC development are not only predictive, but also causal in humans as they have been shown to be in rodent models, these pathways not only increase the risk for internalizing problems (80, 81), but also for peer relationships (82) and future parenting behaviors (83). Taken together, these findings support the hypothesis that sensitive parenting and the associated security established in offspring are effective in influencing the nature of amygdala-mPFC circuitry function in maturity.
Caregiving Adversity.
Characterizing the link between individual differences in normative caregiving and amygdala-mPFC circuitry development provides important insights into the profound impact that caregiving adversities have on emotional development and psychopathology risk, as well as the mechanisms by which these experiences exert their effects. Mental health is dependent on adequate caregiving (4, 84), and indeed adverse caregiving is associated with increased odds for mental health problems (85–87) including externalizing and internalizing disorders that can emerge in adolescence (88), and mood, anxiety, and personality disorders that can last into old age (89). Although these mental health outcomes generally involve difficulties in emotion regulation, the specific diagnoses may reveal themselves in sex-specific ways (especially after puberty) (90), in part because of gonadal differences that emerge during this time (91), differences in amygdala development between boys and girls (92, 93), and perhaps differences in the types of maltreatment that girls may experience from boys (94). Caregiving adversity is a highly-potent stressor for the central nervous system, occurring during the brain’s most vulnerable period. This vulnerability is conferred by the numerous sensitive periods occurring throughout the first two decades of life that render neurobiological systems more or less amenable to environmental pressures in a time-specific manner (91, 95, 96), the cellular properties of the developing amygdala that increase its sensitivity and reactivity to stress, and the hierarchical nature of brain development, which can engender cascading effects of early life stress onto later developing circuits.
Findings from the non-human animal literature.
A large animal literature has established a causal role for adverse caregiving (e.g., abuse, maternal separation, exposure to maternal distress) in prematurely activating amygdala (97, 98), promoting earlier growth and myelination of amygdala cells (99, 100), amplifying amygdala excitability (101), increasing synaptic density in layer II of the infralimbic cortex (102), instantiating earlier use of mature extinction (103) and contextual fear conditioning (in male rodents) (104), and later reducing adolescent plasticity in mPFC and connectivity between amygdala and mPFC (105–107). These accelerations have been described as ontogenetic adaptations (108–110). Ontogenetic adaptations can only occur with the biological premise of developmental plasticity. In the context of developmental plasticity, activity-based processes might promote maturation of these affective circuits (as has been shown in other domains) (109, 111). Accelerating the development of amygdala-mPFC circuitry may be beneficial for young animals who have received cues of danger or abandonment in that the young animal is able to navigate stressors and threats independently to some degree (but presumably, not nearly as effectively as the adult can). That is, cues from the environment signaling inadequate caregiving may motivate a change in developmental strategies. However, accelerated development might, at the same time, truncate growth processes, thereby attenuating developmental plasticity (104), which could have deleterious consequences on later functions that depend on learning and slow growth during early sensitive periods.
Findings from the human literature.
The neotenous development of the human brain renders it vulnerable to psychosocial adversities for a prolonged period. That is, because we retain plasticity for an extended period, there is a wide window during which early life stressors can take hold. Whether early life stress produces accelerations in human brain development, or not, is not yet clear. However, there are emerging findings across studies suggestive of accelerated development. In addition to the more “mature-like” findings described above in instances of insensitive caregiving (within the normative range), extreme caregiving neglect, in the form of institutional caregiving, as well as exposure to violence (to self or other) has been associated with patterns of task-based amygdala-mPFC connectivity that more closely resemble adult patterns than child patterns (112, 113). Additionally, in the context of more normative family stressors, childhood adversity is associated with augmentation of prefrontal-subcortical circuits (114). Prenatal maternal depression has also been associated with patterns of amygdala-mPFC resting state connectivity in infants that have been interpreted as accelerations (115). Despite these initial findings, it is too early to firmly conclude that early psychosocial adversity accelerates human amygdala-mPFC development, and current findings require replication and expansion. However, if this hypothesis continues to receive support, it would call to mind Waddington’s epigenetic landscape metaphor (116), presenting multiple pathways (some more desirable than others) of development, that lead to the adult form. Accelerated development may be a preferred path under conditions of early stress, but are less desirable in the long run, perhaps because of increased risk for mental health problems.
The mechanistic pathways by which early life stress operates in humans have not yet been identified with certainty, but the large number of studies identifying correlations between adverse caregiving and altered amygdala-mPFC development is noteworthy. For example, while the amygdala in infancy does not typically seem to increase activity for emotional stimuli (117, 118), the amygdala can be recruited in infants exposed to domestic violence (119). Growing up with parental psychopathology, exposure to domestic violence, severe neglect, including institutional care in infancy, and/or physical/sexual abuse has been associated with alterations in amygdala development and connections between the amygdala and mPFC (112, 120–128). Although there is consistency across studies in that amygdala and mPFC commonly emerge as neurobiological targets of adversity, there are inconsistencies across studies in terms of the nature of the effects, particularly with regard to volume. These discrepancies may result from the unique aspects of the maltreatment experiences under investigation (e.g., threat versus deprivation of a stimulus(7); emotional/psychological harm versus physical harm, etc.). There is some promising evidence that different subtypes of maltreatment may target different developmental outcomes (129, 130). However, there are also adversity-related phenotypes that transcend subtype (e.g., 131, 132), suggesting that some behavioral domains exhibit developmental equifinality - that is, disparate adverse experiences nonetheless leading to the same final common pathway (133). At the same time, it is difficult to draw conclusions about the source of discrepancies because the importance of age is not always fully recognized; it is possible that effects of caregiving adversity change as a function of age at test or as a function of age of adversity exposure (9, 131). For example, studies that have observed larger amygdala volumes following early life adversity tend to examine children (120, 134), whereas those that identify smaller amygdala volumes tend to include adolescents (132, 135). One hypothesis by Teicher et al. (2016) (8) predicts that early life stress produces initial enlargements of amygdala volume, which then sensitize it to subsequent stressors, resulting in a volume reduction later in life. Thus, developmental changes in neurobiology should be considered seriously in studies of early adversity (e.g., 136) because findings in childhood may differ from those observed in adolescence and adulthood.
Heterogeneity and resilience following early adversity.
Despite these many findings linking caregiving adversities to altered amygdala-mPFC circuit development, there are large individual differences in brain development. The mental health outcomes associated with amygdala-mPFC development also exhibit significant heterogeneity (137), and many youth exhibit psychological resilience despite exposure to adversity. This heterogeneity evokes notions of developmental multifinality, which describes divergent developmental pathways for two individuals who begin with similar risk (133). Nonetheless, the sources of these individual differences require much more research. We do not yet know for certain why these individual differences exist or how to predict them. Intra-individual factors may play a role. For example, normative genetic variations have been linked with individual differences in behavioral outcomes (e.g., 138, 139). Likewise, intra-individual behaviors have also been studied; working memory skills have been shown to moderate the link between early institutional care and mental health outcomes (e.g., attention deficit hyperactivity disorder; separation anxiety symptoms)(140, 141), as have affective processing biases (142). Earlier interventions (i.e., therapy, placement in families) tend to be associated with better affective health for children and adolescents (143–147).
Heterogeneity and intervention following early adversity.
Another source of individual differences may be positive, strength promoting experiences that compete with adverse ones to ameliorate developmental outcomes after adverse experiences. For example, interventions targeting parental nurturance, sensitivity, and threatening behaviors have been shown to causally reduce child problem behavior in high-risk samples (148). This finding is particularly important since parents can be powerful buffers of stress in childhood (149). This positive effect of the parent is especially important to consider in the context of adverse environments, and it has been shown that parental presence can buffer the fear-potentiated startle of children exposed to high levels of violence (150). If human brain development is neotenous, retaining plasticity for a long time, then it might logically extend that positive experiences, even after adversity exposure, might confer additional benefits at older ages (see Figure 1, moments a,b,c). Consistent with this prediction, children and adolescents with a prior history of institutional care show steeper declines in anxiety symptoms in the future if they exhibited a dampening response to (adoptive) parental cues (“buffering”) at the initial time of testing (80). Whether or not children and adolescents exhibit this amygdala dampening in response to their parent is associated with the security they report feeling towards their (adoptive) parent at initial testing. Likewise, greater feelings of security correlate with lower internalizing problems following early institutional care (142), but not in youth with a typical caregiving background (whose scores were near floor levels). This finding suggests that while strong families are always important for emotional development, their effects may be especially visible following early adversity.
Conclusion
Human brain development is optimized to learn from environmental cues. However, this optimization also places infants and children at risk if exposed to adverse caregiving. The link between early caregiving adversity and poor mental health is not deterministic, as there is significant heterogeneity in outcome, and outcomes can change with ameliorative experiences. Nonetheless, the risk is significant. Additionally, while abusive and neglectful caregiving are potent stressors for the developing child, so is the separation of the child from his/her parent. This separation is traumatic, because children form attachments to their parents, even in the context of maltreatment. Therefore, the implications of this research for mental health include using developmentally-informed approaches to understand pathways of emotional development following early adversity, viewing stable caregiving as a basic need during development, and understanding that supporting children’s emotional development means supporting their families as well.
Acknowledgements:
This research was supported in part by grant number R01MH091864 from the National Institute of Mental Health. The content is solely the responsibility of the author and does not necessarily represent the views of funding agencies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Kernberg OF, Early ego integration and object relations. Ann N Y Acad Sci, 1972. 193: p. 233–47. [DOI] [PubMed] [Google Scholar]
- 2.Hofer MA, Early relationships as regulators of infant physiology and behavior. Acta Pediatrica, 1994. 83(s397): p. 9–18. [DOI] [PubMed] [Google Scholar]
- 3.Gewirtz JL, Baer DM, and Roth CH, A note on the similar effects of low social availability of an adult and brief social deprivation on young children’s behavior. Child Dev, 1958. 29(1): p. 149–52. [PubMed] [Google Scholar]
- 4.Bowlby J, Attachment and loss: retrospect and prospect. Am J Orthopsychiatry, 1982. 52(4): p. 664–78. [DOI] [PubMed] [Google Scholar]
- 5.Bowen M, The use of family theory in clinical practice. Compr Psychiatry, 1966. 75(5): p. 345–374. [DOI] [PubMed] [Google Scholar]
- 6.Baumrind D, Effects of Authoritative Parental Control on Child Behavior. Child Development, 1966. 37(4): p. 887–907. [Google Scholar]
- 7.McLaughlin KA, Sheridan MA, and Lambert HK, Childhood adversity and neural development: deprivation and threat as distinct dimensions of early experience. Neurosci Biobehav Rev, 2014. 47: p. 578–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teicher MH, Samson JA, Anderson CM, and Ohashi K, The effects of childhood maltreatment on brain structure, function and connectivity. Nat Rev Neurosci, 2016. 17(10): p. 652–66. [DOI] [PubMed] [Google Scholar]
- 9.Tottenham N and Sheridan MA, A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Front Hum Neurosci, 2010. 3: p. 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bufill E, Agusti J, and Blesa R, Human neoteny revisited: The case of synaptic plasticity. Am J Hum Biol, 2011. 23(6): p. 729–39. [DOI] [PubMed] [Google Scholar]
- 11.Petanjek Z, Judas M, Simic G, Rasin MR, Uylings HB, Rakic P, et al. , Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci U S A, 2011. 108(32): p. 13281–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Somel M, Franz H, Yan Z, Lorenc A, Guo S, Giger T, et al. , Transcriptional neoteny in the human brain. Proc Natl Acad Sci U S A, 2009. 106(14): p. 5743–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bjorklund DF, The role of immaturity in human development. Psychol Bull, 1997. 122(2): p. 153–69. [DOI] [PubMed] [Google Scholar]
- 14.Werker JF and Hensch TK, Critical Periods in Speech Perception: New Directions. Annual Review of Psychology, 2015. 66: p. 173–196. [DOI] [PubMed] [Google Scholar]
- 15.Nelson Iii CA, Zeanah CH, and Fox NA, How Early Experience Shapes Human Development: The Case of Psychosocial Deprivation. Neural Plasticity, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masten AS and Cicchetti D, Developmental cascades. Dev Psychopathol, 2010. 22(3): p. 491–5. [DOI] [PubMed] [Google Scholar]
- 17.Tottenham N and Gabard-Durnam LJ, The developing amygdala: a student of the world and a teacher of the cortex. Curr Opin Psychol, 2017. 17: p. 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bertolino A, Saunders RC, Mattay VS, Bachevalier J, Frank JA, and Weinberger DR, Altered development of prefrontal neurons in rhesus monkeys with neonatal mesial temporo-limbic lesions: a proton magnetic resonance spectroscopic imaging study. Cereb Cortex, 1997. 7(8): p. 740–8. [DOI] [PubMed] [Google Scholar]
- 19.Quirk GJ and Beer JS, Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Current Opinion in Neurobiology, 2006. 16(6): p. 723–7. [DOI] [PubMed] [Google Scholar]
- 20.Alexander WH and Brown JW, Medial prefrontal cortex as an action-outcome predictor. Nat Neurosci, 2011. 14(10): p. 1338–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joiner J, Piva M, Turrin C, and Chang SWC, Social learning through prediction error in the brain. NPJ Sci Learn, 2017. 2: p. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, and Casey BJ, Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol Psychiatry, 2008. 63(10): p. 927–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spinelli S, Chefer S, Suomi SJ, Higley JD, Barr CS, and Stein E, Early-life stress induces long-term morphologic changes in primate brain. Arch Gen Psychiatry, 2009. 66(6): p. 658–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bauer PM, Hanson JL, Pierson RK, Davidson RJ, and Pollak SD, Cerebellar Volume and Cognitive Functioning in Children Who Experienced Early Deprivation. Biol Psychiatry, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tottenham N and Galvan A, Stress and the adolescent brain: Amygdala-prefrontal cortex circuitry and ventral striatum as developmental targets. Neurosci Biobehav Rev, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koppensteiner P, Aizawa S, Yamada D, Kabuta T, Boehm S, Wada K, et al. , Age-dependent sensitivity to glucocorticoids in the developing mouse basolateral nucleus of the amygdala. Psychoneuroendocrinology, 2014. 46: p. 64–77. [DOI] [PubMed] [Google Scholar]
- 27.Ehrlich DE, Ryan SJ, and Rainnie DG, Postnatal development of electrophysiological properties of principal neurons in the rat basolateral amygdala. J Physiol, 2012. 590(19): p. 4819–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Achterberg M, Bakermans-Kranenburg MJ, van Ijzendoorn MH, van der Meulen M, Tottenham N, and Crone EA, Distinctive heritability patterns of subcortical-prefrontal cortex resting state connectivity in childhood: A twin study. Neuroimage, 2018. 175: p. 138–149. [DOI] [PubMed] [Google Scholar]
- 29.Cunningham MG, Bhattacharyya S, and Benes FM, Amygdalo-cortical sprouting continues into early adulthood: implications for the development of normal and abnormal function during adolescence. J Comp Neurol, 2002. 453(2): p. 116–30. [DOI] [PubMed] [Google Scholar]
- 30.Pattwell SS, Liston C, Jing D, Ninan I, Yang RR, Witztum J, et al. , Dynamic changes in neural circuitry during adolescence are associated with persistent attenuation of fear memories. Nat Commun, 2016. 7: p. 11475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bouwmeester H, Wolterink G, and van Ree JM, Neonatal development of projections from the basolateral amygdala to prefrontal, striatal, and thalamic structures in the rat. J Comp Neurol, 2002. 442(3): p. 239–49. [DOI] [PubMed] [Google Scholar]
- 32.Selleck RA, Zhang W, Samberg HD, Padival M, and Rosenkranz JA, Limited prefrontal cortical regulation over the basolateral amygdala in adolescent rats. Sci Rep, 2018. 8(1): p. 17171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arruda-Carvalho M, Wu W, Cummings KA, and Clem R, Optogenetic examination of prefrontal-amygdala synaptic development. Journal of Neuroscience, 2017. 37(11): p. 2976–2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koss WA, Belden CE, Hristov AD, and Juraska JM, Dendritic remodeling in the adolescent medial prefrontal cortex and the basolateral amygdala of male and female rats. Synapse, 2014. 68(2): p. 61–72. [DOI] [PubMed] [Google Scholar]
- 35.Ehrlich DE, Ryan SJ, Hazra R, Guo JD, and Rainnie DG, Postnatal maturation of GABAergic transmission in the rat basolateral amygdala. J Neurophysiol, 2013. 110(4): p. 926–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Curley JP, Jordan ER, Swaney WT, Izraelit A, Kammel S, and Champagne FA, The meaning of weaning: influence of the weaning period on behavioral development in mice. Dev Neurosci, 2009. 31(4): p. 318–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hefner K and Holmes A, Ontogeny of fear-, anxiety- and depression-related behavior across adolescence in C57BL/6J mice. Behav Brain Res, 2007. 176(2): p. 210–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pattwell SS, Bath KG, Casey BJ, Ninan I, and Lee FS, Selective early-acquired fear memories undergo temporary suppression during adolescence. Proc Natl Acad Sci U S A. 108(3): p. 1182–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lebel C, Walker L, Leemans A, Phillips L, and Beaulieu C, Microstructural maturation of the human brain from childhood to adulthood. Neuroimage, 2008. 40(3): p. 1044–55. [DOI] [PubMed] [Google Scholar]
- 40.Tromp DP, Grupe DW, Oathes DJ, McFarlin DR, Hernandez PJ, Kral TR, et al. , Reduced structural connectivity of a major frontolimbic pathway in generalized anxiety disorder. Arch Gen Psychiatry, 2012. 69(9): p. 925–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gabard-Durnam LJ, O’Muircheartaigh J, Dirks H, Dean DC 3rd, Tottenham N, and Deoni S, Human amygdala functional network development: A cross-sectional study from 3 months to 5years of age. Dev Cogn Neurosci, 2018. 34: p. 63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salzwedel AP, Stephens RL, Goldman BD, Lin W, Gilmore JH, and Gao W, Development of Amygdala Functional Connectivity During Infancy and Its Relationship With 4-Year Behavioral Outcomes. Biol Psychiatry Cogn Neurosci Neuroimaging, 2019. 4(1): p. 62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gabard-Durnam LJ, Flannery J, Goff B, Gee DG, Humphreys KL, Telzer E, et al. , The development of human amygdala functional connectivity at rest from 4 to 23years: A cross-sectional study. Neuroimage, 2014. 95C: p. 193–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qin S, Young CB, Supekar K, Uddin LQ, and Menon V, Immature integration and segregation of emotion-related brain circuitry in young children. Proc Natl Acad Sci U S A, 2012. 109(20): p. 7941–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jalbrzikowski M, Larsen B, Hallquist MN, Foran W, Calabro F, and Luna B, Development of White Matter Microstructure and Intrinsic Functional Connectivity Between the Amygdala and Ventromedial Prefrontal Cortex: Associations With Anxiety and Depression. Biol Psychiatry, 2017. 82(7): p. 511–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gabard-Durnam LJ, Gee DG, Goff B, Flannery J, Telzer E, Humphreys KL, et al. , Stimulus-Elicited Connectivity Influences Resting-State Connectivity Years Later in Human Development: A Prospective Study. J Neurosci, 2016. 36(17): p. 4771–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, et al. , A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. J Neurosci, 2013. 33(10): p. 4584–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perlman SB and Pelphrey KA, Developing connections for affective regulation: age-related changes in emotional brain connectivity. J Exp Child Psychol, 2011. 108(3): p. 607–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Silvers JA, Insel C, Powers A, Franz P, Helion C, Martin RE, et al. , vlPFC-vmPFC-Amygdala Interactions Underlie Age-Related Differences in Cognitive Regulation of Emotion. Cereb Cortex, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Decety J, Michalska KJ, and Kinzler KD, The contribution of emotion and cognition to moral sensitivity: a neurodevelopmental study. Cereb Cortex, 2012. 22(1): p. 209–20. [DOI] [PubMed] [Google Scholar]
- 51.Wu M, Kujawa A, Lu LH, Fitzgerald DA, Klumpp H, Fitzgerald KD, et al. , Age-related changes in amygdala-frontal connectivity during emotional face processing from childhood into young adulthood. Hum Brain Mapp, 2016. 37(5): p. 1684–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ducharme S, Albaugh MD, Hudziak JJ, Botteron KN, Nguyen TV, Truong C, et al. , Anxious/depressed symptoms are linked to right ventromedial prefrontal cortical thickness maturation in healthy children and young adults. Cereb Cortex, 2014. 24(11): p. 2941–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dougherty LR, Blankenship SL, Spechler PA, Padmala S, and Pessoa L, An fMRI Pilot Study of Cognitive Reappraisal in Children: Divergent Effects on Brain and Behavior. J Psychopathol Behav Assess, 2015. 37(4): p. 634–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Swartz JR, Carrasco M, Wiggins JL, Thomason ME, and Monk CS, Age-related changes in the structure and function of prefrontal cortex-amygdala circuitry in children and adolescents: a multi-modal imaging approach. Neuroimage, 2014. 86: p. 212–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vink M, Derks JM, Hoogendam JM, Hillegers M, and Kahn RS, Functional differences in emotion processing during adolescence and early adulthood. Neuroimage, 2014. 91: p. 70–6. [DOI] [PubMed] [Google Scholar]
- 56.Tottenham N, Phuong J, Flannery J, Gabard-Durnam L, and Goff B, A negativity bias for ambiguous facial-expression valence during childhood: converging evidence from behavior and facial corrugator muscle responses. Emotion, 2013. 13(1): p. 92–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shechner T, Hong M, Britton JC, Pine DS, and Fox NA, Fear conditioning and extinction across development: evidence from human studies and animal models. Biol Psychol, 2014. 100: p. 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gullone E, The development of normal fear: a century of research. Clin Psychol Rev, 2000. 20(4): p. 429–51. [DOI] [PubMed] [Google Scholar]
- 59.Hein TC, Mattson WI, Dotterer HL, Mitchell C, Lopez-Duran N, Thomason ME, et al. , Amygdala habituation and uncinate fasciculus connectivity in adolescence: A multi-modal approach. Neuroimage, 2018. 183: p. 617–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Callaghan B, Meyer H, Opendak M, Van Tieghem M, Harmon C, Li A, et al. , Using A Developmental Ecology Framework to Align Fear Neurobiology Across Species. Annual Review of Clinical Psychology, 2019. 15: p. 345–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Landers MS and Sullivan RM, The development and neurobiology of infant attachment and fear. Dev Neurosci, 2012. 34(2–3): p. 101–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tottenham N, Shapiro M, Caldera C, Flannery J, and Sullivan RM, Parental presence switches avoidance to attraction learning in children. . Nature Human Behaviour, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ainsworth MD and Bell SM, Attachment, exploration, and separation: illustrated by the behavior of one-year-olds in a strange situation. Child Dev, 1970. 41(1): p. 49–67. [PubMed] [Google Scholar]
- 64.Stronach EP, Toth SL, Rogosch F, Oshri A, Manly JT, and Cicchetti D, Child maltreatment, attachment security, and internal representations of mother and mother-child relationships. Child Maltreat, 2011. 16(2): p. 137–45. [DOI] [PubMed] [Google Scholar]
- 65.Gee DG, Gabard-Durnam L, Telzer EH, Humphreys KL, Goff B, Shapiro M, et al. , Maternal buffering of human amygdala-prefrontal circuitry during childhood but not during adolescence. Psychol Sci, 2014. 25(11): p. 2067–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Telzer EH, Ichien NT, and Qu Y, Mothers know best: redirecting adolescent reward sensitivity toward safe behavior during risk taking. Soc Cogn Affect Neurosci, 2015. 10(10): p. 1383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lemche E, Giampietro VP, Surguladze SA, Amaro EJ, Andrew CM, Williams SC, et al. , Human attachment security is mediated by the amygdala: evidence from combined fMRI and psychophysiological measures. Hum Brain Mapp, 2006. 27(8): p. 623–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vrticka P, Andersson F, Grandjean D, Sander D, and Vuilleumier P, Individual attachment style modulates human amygdala and striatum activation during social appraisal. PLoS One, 2008. 3(8): p. e2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bernier A, Dégeilh F, Leblanc É, Daneault V, Bailey HN, and Beauchamp MH, Mother-Infant Interaction and Child Brain Morphology: A Multidimensional Approach to Maternal Sensitivity. Infancy, 2019. 24(2): p. 120–138. [DOI] [PubMed] [Google Scholar]
- 70.Lyons-Ruth K, Pechtel P, Yoon SA, Anderson CM, and Teicher MH, Disorganized attachment in infancy predicts greater amygdala volume in adulthood. Behav Brain Res, 2016. 308: p. 83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moutsiana C, Johnstone T, Murray L, Fearon P, Cooper PJ, Pliatsikas C, et al. , Insecure attachment during infancy predicts greater amygdala volumes in early adulthood. J Child Psychol Psychiatry, 2015. 56(5): p. 540–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bowlby J, Attachment and Loss. 1969, New York: Basic Books. [Google Scholar]
- 73.Callaghan BL and Tottenham N, The Neuro-Environmental Loop of Plasticity: A Cross-Species Analysis of Parental Effects on Emotion Circuitry Development Following Typical and Adverse Caregiving. Neuropsychopharmacology, 2016. 41(1): p. 163–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rifkin-Graboi A, Kong L, Sim LW, Sanmugam S, Broekman BF, Chen H, et al. , Maternal sensitivity, infant limbic structure volume and functional connectivity: a preliminary study. Transl Psychiatry, 2015. 5: p. e668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thijssen S, Muetzel RL, Bakermans-Kranenburg MJ, Jaddoe VW, Tiemeier H, Verhulst FC, et al. , Insensitive parenting may accelerate the development of the amygdala-medial prefrontal cortex circuit. Dev Psychopathol, 2017. 29(2): p. 505–518. [DOI] [PubMed] [Google Scholar]
- 76.Romund L, Raufelder D, Flemming E, Lorenz RC, Pelz P, Gleich T, et al. , Maternal parenting behavior and emotion processing in adolescents-An JMRI study. Biol Psychol, 2016. 120: p. 120–125. [DOI] [PubMed] [Google Scholar]
- 77.Kopala-Sibley DC, Cyr M, Finsaas MC, Orawe J, Huang A, Tottenham N, et al. , Early Childhood Parenting Predicts Late Childhood Brain Functional Connectivity During Emotion Perception and Reward Processing. Child Dev, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Aupperle RL, Morris AS, Silk JS, Criss MM, Judah MR, Eagleton SG, et al. , Neural responses to maternal praise and criticism: Relationship to depression and anxiety symptoms in high-risk adolescent girls. Neuroimage Clin, 2016. 11: p. 548–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Whittle S, Simmons JG, Dennison M, Vijayakumar N, Schwartz O, Yap MB, et al. , Positive parenting predicts the development of adolescent brain structure: a longitudinal study. Dev Cogn Neurosci, 2014. 8: p. 7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Callaghan B, Gee DG, Gabard-Durnam L, and Tottenham N, Decreased amygdala reactivity to parent cues protects against anxiety following early adversity: an examination across 3-years. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Burghy CA, Stodola DE, Ruttle PL, Molloy EK, Armstrong JM, Oler JA, et al. , Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nat Neurosci, 2012. 15(12): p. 1736–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tan PZ, Lee KH, Dahl RE, Nelson EE, Stroud LJ, Siegle GJ, et al. , Associations between maternal negative affect and adolescent’s neural response to peer evaluation. Dev Cogn Neurosci, 2014. 8: p. 28–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim P, Leckman JF, Mayes LC, Newman MA, Feldman R, and Swain JE, Perceived quality of maternal care in childhood and structure and function of mothers’ brain. Dev Sci, 2010. 13(4): p. 662–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ainsworth MD, Object relations, dependency, and attachment: a theoretical review of the infant-mother relationship. Child Development, 1969. 40(4): p. 969–1025. [PubMed] [Google Scholar]
- 85.Cicchetti D and Toth SL, Child maltreatment. Annu Rev Clin Psychol, 2005. 1: p. 409–38. [DOI] [PubMed] [Google Scholar]
- 86.Zeanah CH, Egger HL, Smyke AT, Nelson CA, Fox NA, Marshall PJ, et al. , Institutional rearing and psychiatric disorders in Romanian preschool children. Am J. Psychiatry, 2009. 166(7): p. 777–85. [DOI] [PubMed] [Google Scholar]
- 87.Kessler RC, McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky AM, et al. , Childhood adversities and adult psychopathology in the WHO World Mental Health Surveys. Br J Psychiatry, 2010. 197(5): p. 378–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Oshri A, Rogosch FA, Burnette ML, and Cicchetti D, Developmental pathways to adolescent cannabis abuse and dependence: child maltreatment, emerging personality, and internalizing versus externalizing psychopathology. Psychol Addict Behav, 2011. 25(4): p. 634–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Raposo SM, Mackenzie CS, Henriksen CA, and Afifi TO, Time Does Not Heal All Wounds: Older Adults Who Experienced Childhood Adversities Have Higher Odds of Mood, Anxiety, and Personality Disorders. Am J Geriatr Psychiatry, 2013. [DOI] [PubMed] [Google Scholar]
- 90.Bale TL and Epperson CN, Sex differences and stress across the lifespan. Nat Neurosci, 2015. 18(10): p. 1413–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Andersen SL and Teicher MH, Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci, 2008. 31(4): p. 183–91. [DOI] [PubMed] [Google Scholar]
- 92.Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, et al. , Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4–18 years. Journal of Comparative Neurology, 1996. 366(2): p. 223–230. [DOI] [PubMed] [Google Scholar]
- 93.Bramen JE, Hranilovich JA, Dahl RE, Forbes EE, Chen J, Toga AW, et al. , Puberty influences medial temporal lobe and cortical gray matter maturation differently in boys than girls matched for sexual maturity. Cereb Cortex, 2011. 21(3): p. 636–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Trickett PK, Kim K, and Prindle J, Variations in emotional abuse experiences among multiply maltreated young adolescents and relations with developmental outcomes. Child Abuse Negl, 2011. 35(10): p. 876–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lupien SJ, McEwen BS, Gunnar MR, and Heim C, Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience, 2009. 10(6): p. 434–445 %@ 1471–003X. [DOI] [PubMed] [Google Scholar]
- 96.Tottenham N, The importance of early experiences for neuro-affective development. Curr Top Behav Neurosci, 2014. 16: p. 109–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Moriceau S, Shionoya K, Jakubs K, and Sullivan RM, Early-life stress disrupts attachment learning: the role of amygdala corticosterone, locus ceruleus corticotropin releasing hormone, and olfactory bulb norepinephrine. J Neurosci, 2009. 29(50): p. 15745–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Debiec J and Sullivan RM, Intergenerational transmission of emotional trauma through amygdala-dependent mother-to-infant transfer of specific fear. Proc Natl Acad Sci U S A, 2014. 111(33): p. 12222–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ono M, Kikusui T, Sasaki N, Ichikawa M, Mori Y, and Murakami-Murofushi K, Early weaning induces anxiety and precocious myelination in the anterior part of the basolateral amygdala of male Balb/c mice. Neuroscience, 2008. 156(4): p. 1103–10. [DOI] [PubMed] [Google Scholar]
- 100.Guadagno A, Wong TP, and Walker CD, Morphological and functional changes in the preweaning basolateral amygdala induced by early chronic stress associate with anxiety and fear behavior in adult male, but not female rats. Prog Neuropsychopharmacol Biol Psychiatry, 2018. 81: p. 25–37. [DOI] [PubMed] [Google Scholar]
- 101.Malter Cohen M, Jing D, Yang RR, Tottenham N, Lee FS, and Casey BJ, Early-life stress has persistent effects on amygdala function and development in mice and humans. Proc Natl Acad Sci U S A, 2013. 110(45): p. 18274–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ovtscharoff W Jr. and Braun K, Maternal separation and social isolation modulate the postnatal development of synaptic composition in the infralimbic cortex of Octodon degus. Neuroscience, 2001. 104(1): p. 33–40. [DOI] [PubMed] [Google Scholar]
- 103.Callaghan BL and Richardson R, Maternal separation results in early emergence of adult-like fear and extinction learning in infant rats. Behav Neurosci, 2011. 125(1): p. 20–8. [DOI] [PubMed] [Google Scholar]
- 104.Bath KG, Manzano-Nieves G, and Goodwill H, Early life stress accelerates behavioral and neural maturation of the hippocampus in male mice. Horm Behav, 2016. 82: p. 64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chocyk A, Bobula B, Dudys D, Przyborowska A, Majcher-Maslanka I, Hess G, et al. , Early-life stress affects the structural and functional plasticity of the medial prefrontal cortex in adolescent rats. Eur J Neurosci, 2013. 38(1): p. 2089–107. [DOI] [PubMed] [Google Scholar]
- 106.Yan CG, Rincon-Cortes M, Raineki C, Sarro E, Colcombe S, Guilfoyle DN, et al. , Aberrant development of intrinsic brain activity in a rat model of caregiver maltreatment of offspring. Transl Psychiatry, 2017. 7(1): p. e1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Walker CD, Bath KG, Joels M, Korosi A, Larauche M, Lucassen PJ, et al. , Chronic early life stress induced by limited bedding and nesting (LBN) material in rodents: critical considerations of methodology, outcomes and translational potential. Stress, 2017. 20(5): p. 421–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Callaghan BL and Richardson R, Early experiences and the development of emotional learning systems in rats. Biol Mood Anxiety Disord, 2013. 3: p. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Callaghan BL and Tottenham N, The Stress Acceleration Hypothesis: effects of early-life adversity on emotion circuits and behavior. Current Opinion in Behavioral Sciences, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Oppenheim RW, Metamorphosis and adaptation in the behavior of developing organisms. Dev Psychobiol, 1980. 13(4): p. 353–6. [DOI] [PubMed] [Google Scholar]
- 111.Travaglia A, Bisaz R, Sweet ES, Blitzer RD, and Alberini CM, Infantile amnesia reflects a developmental critical period for hippocampal learning. Nat Neurosci, 2016. 19(9): p. 1225–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, et al. , Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc Natl Acad Sci U S A, 2013. 110(39): p. 15638–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wolf RC and Herringa RJ, Prefrontal-Amygdala Dysregulation to Threat in Pediatric Posttraumatic Stress Disorder. Neuropsychopharmacology, 2016. 41(3): p. 822–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Herringa RJ, Burghy CA, Stodola DE, Fox ME, Davidson RJ, and Essex MJ, Enhanced prefrontal-amygdala connectivity following childhood adversity as a protective mechanism against internalizing in adolescence. Biol Psychiatry Cogn Neurosci Neuroimaging, 2016. 1(4): p. 326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Posner J, Cha J, Roy AK, Peterson BS, Bansal R, Gustafsson HC, et al. , Alterations in amygdala-prefrontal circuits in infants exposed to prenatal maternal depression. Transl Psychiatry, 2016. 6(11): p. e935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Goldberg AD, Allis CD, and Bernstein E, Epigenetics: a landscape takes shape. Cell, 2007. 128(4): p. 635–8. [DOI] [PubMed] [Google Scholar]
- 117.Blasi A, Mercure E, Lloyd-Fox S, Thomson A, Brammer M, Sauter D, et al. , Early specialization for voice and emotion processing in the infant brain. Curr Biol, 2011. 21(14): p. 1220–4. [DOI] [PubMed] [Google Scholar]
- 118.Dehaene-Lambertz G, Montavont A, Jobert A, Allirol L, Dubois J, Hertz-Pannier L, et al. , Language or music, mother or Mozart? Structural and environmental influences on infants’ language networks. Brain Lang, 2010. 114(2): p. 53–65. [DOI] [PubMed] [Google Scholar]
- 119.Graham AM, Fisher PA, and Pfeifer JH, What sleeping babies hear: a functional MRI study of interparental conflict and infants’ emotion processing. Psychol Sci, 2013. 24(5): p. 782–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lupien SJ, Parent S, Evans AC, Tremblay RE, Zelazo PD, Corbo V, et al. , Larger amygdala but no change in hippocampal volume in 10-year-old children exposed to maternal depressive symptomatology since birth. Proc Natl Acad Sci U S A, 2011. 108(34): p. 14324–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.McLaughlin KA, Sheridan MA, Gold AL, Duys A, Lambert HK, Peverill M, et al. , Maltreatment Exposure, Brain Structure, and Fear Conditioning in Children and Adolescents. Neuropsychopharmacology, 2016. 41(8): p. 1956–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Garrett AS, Carrion V, Kletter H, Karchemskiy A, Weems CF, and Reiss A, Brain activation to facial expressions in youth with PTSD symptoms. Depress Anxiety, 2012. 29(5): p. 449–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Marusak HA, Martin KR, Etkin A, and Thomason ME, Childhood trauma exposure disrupts the automatic regulation of emotional processing. Neuropsychopharmacology, 2015. 40(5): p. 1250–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.De Bellis MD and Hooper SR, Neural substrates for processing task-irrelevant emotional distracters in maltreated adolescents with depressive disorders: a pilot study. J Trauma Stress, 2012. 25(2): p. 198–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.McCrory EJ, De Brito SA, Kelly PA, Bird G, Sebastian CL, Mechelli A, et al. , Amygdala activation in maltreated children during pre-attentive emotional processing. Br J Psychiatry, 2013. 202(4): p. 269–76. [DOI] [PubMed] [Google Scholar]
- 126.Maheu FS, Dozier M, Guyer AE, Mandell D, Peloso E, Poeth K, et al. , A preliminary study of medial temporal lobe function in youths with a history of caregiver deprivation and emotional neglect. Cogn Affect Behav Neurosci, 2010. 10(1): p. 34–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tottenham N, Hare TA, Millner A, Gilhooly T, Zevin JD, and Casey BJ, Elevated Amygdala Response to Faces Following Early Deprivation. Developmental Science, 2011. 14(2): p. 190–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Roth MC, Humphreys KL, King LS, and Gotlib IH, Self-reported neglect, amygdala volume, and symptoms of anxiety in adolescent boys. Child Abuse Negl, 2018. 80: p. 80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sumner JA, Colich NL, Uddin M, Armstrong D, and McLaughlin KA, Early Experiences of Threat, but Not Deprivation, Are Associated With Accelerated Biological Aging in Children and Adolescents. Biol Psychiatry, 2019. 85(3): p. 268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dennison MJ, Rosen ML, Sambrook KA, Jenness JL, Sheridan MA, and McLaughlin KA, Differential Associations of Distinct Forms of Childhood Adversity With Neurobehavioral Measures of Reward Processing: A Developmental Pathway to Depression. Child Dev, 2019. 90(1): p. e96–e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pechtel P, Lyons-Ruth K, Anderson CM, and Teicher MH, Sensitive periods of amygdala development: the role of maltreatment in preadolescence. Neuroimage, 2014. 97: p. 236–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hanson JL, Nacewicz BM, Sutterer MJ, Cayo AA, Schaefer SM, Rudolph KD, et al. , Behavioral Problems After Early Life Stress: Contributions of the Hippocampus and Amygdala. Biol Psychiatry, 2015. 77(4): p. 314–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cicchetti D and Rogosch FA, Equifinality and multifinality in developmental psychopathology. Development and Psychopathology, 1996. 8(4): p. 597–600. [Google Scholar]
- 134.Tottenham N, Hare TA, Quinn BT, McCarry T, Nurse M, Gilhooly T, et al. , Prolonged institutional rearing is associated with atypically large amygdala volume and emotion regulation difficulties. Developmental Science, 2010. 13(1): p. 46–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hodel AS, Hunt RH, Cowell RA, Van Den Heuvel SE, Gunnar MR, and Thomas KM, Duration of early adversity and structural brain development in post-institutionalized adolescents. Neuroimage, 2015. 105: p. 112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Flannery JE, Gabard-Durnam LJ, Shapiro M, Goff B, Caldera C, Louie J, et al. , Diurnal cortisol after early institutional care-Age matters. Dev Cogn Neurosci, 2017. 25: p. 160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tottenham N, Risk and developmental heterogeneity in previously institutionalized children. J Adolesc Health, 2012. 51(2 Suppl): p. S29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gunnar MR, Wenner JA, Thomas KM, Glatt CE, McKenna MC, and Clark AG, The brain-derived neurotrophic factor Val66Met polymorphism moderates early deprivation effects on attention problems. Dev Psychopathol, 2012. 24(4): p. 1215–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Drury SS, Gleason MM, Theall KP, Smyke AT, Nelson CA, Fox NA, et al. , Genetic sensitivity to the caregiving context: The influence of 5httlpr and BDNF val66met on indiscriminate social behavior. Physiol Behav, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Alba L, Flannery J, Shapiro M, and Tottenham N, Working Memory Moderates the Association Between Early Institutional Care and Separation Anxiety Symptoms in Late Childhood and Adolescence. Development and Psychopathology, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tibu F, Sheridan MA, McLaughlin KA, Nelson CA, Fox NA, and Zeanah CH, Disruptions of working memory and inhibition mediate the association between exposure to institutionalization and symptoms of attention deficit hyperactivity disorder. Psychol Med, 2016. 46(3): p. 529–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Vantieghem MR, Gabard-Durnam L, Goff B, Flannery J, Humphreys KL, Telzer EH, et al. , Positive valence bias and parent-child relationship security moderate the association between early institutional caregiving and internalizing symptoms. Dev Psychopathol, 2017. 29(2): p. 519–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Green SA, Goff B, Gee DG, Gabard-Durnam L, Flannery J, Telzer EH, et al. , Discrimination of amygdala response predicts future separation anxiety in youth with early deprivation. J Child Psychol Psychiatry, 2016. 57(10): p. 1135–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fox NA, Almas AN, Degnan KA, Nelson CA, and Zeanah CH, The effects of severe psychosocial deprivation and foster care intervention on cognitive development at 8 years of age: findings from the Bucharest Early Intervention Project. J Child Psychol Psychiatry, 2011. 52(9): p. 919–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Loman MM, Wiik KL, Frenn KA, Pollak SD, and Gunnar MR, Postinstitutionalized children’s development: growth, cognitive, and language outcomes. J Dev Behav Pediatr, 2009. 30(5): p. 426–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Castle J, Groothues C, Bredenkamp D, Beckett C, O’Connor T, and Rutter M, Effects of qualities of early institutional care on cognitive attainment. E.R.A. Study Team. English and Romanian Adoptees. Am J Orthopsychiatry, 1999. 69(4): p. 424–37. [DOI] [PubMed] [Google Scholar]
- 147.Nelson CA, Zeanah CH, Fox NA, Marshall PJ, Smyke AT, and Guthrie D, Cognitive recovery in socially deprived young children: the Bucharest Early Intervention Project. Science, 2007. 318(5858): p. 1937–1940. [DOI] [PubMed] [Google Scholar]
- 148.Lind T, Bernard K, Ross E, and Dozier M, Intervention effects on negative affect of CPS-referred children: results of a randomized clinical trial. Child Abuse Negl, 2014. 38(9): p. 1459–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hostinar CE, Sullivan RM, and Gunnar MR, Psychobiological mechanisms underlying the social buffering of the hypothalamic-pituitary-adrenocortical axis: a review of animal models and human studies across development. Psychol Bull, 2014. 140(1): p. 256–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.van Rooij SJ, Cross D, Stevens JS, Vance LA, Kim YJ, Bradley B, et al. , Maternal buffering of fear-potentiated startle in children and adolescents with trauma exposure. Soc Neurosci, 2017. 12(1): p. 22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]


