Abstract
Tributyltin (TBT) and dioxin-like polychlorinated biphenyls (PCBs) are environmental contaminants that are highly toxic to fish and co-occur in New Bedford Harbor (NBH), an estuarine Superfund site located in Massachusetts, USA. Atlantic killifish (Fundulus heteroclitus) that reside in NBH (and other highly contaminated sites along the east coast of the United States) have developed resistance to activation of the aryl hydrocarbon receptor (AHR) pathway and the toxicity of dioxin-like chemicals, such as 3,3',4,4',5-pentachlorobiphenyl, PCB126. In many biological systems, TBT disregulates adipose and bone development via the PPARγ-RXR pathway; AHR activation also disrupts adipose and bone homeostasis, potentially through molecular crosstalk between AHR and PPARγ. However, little is known about how co-exposure and the interaction of these pathways modulate the toxicological effects of these contaminants. Here, we tested the hypotheses that TBT would induce teratogenesis in killifish via activation of PPARγ and that PCB126 co-exposure would suppress PPARγ pathway activation in PCB-sensitive killifish from a reference site (Scorton Creek, SC, PCB-sensitive) but not in PCB-tolerant NBH killifish. Killifish embryos from both populations exposed to TBT (50 and 100 nM) displayed caudal fin deformities. TBT did not change the expression of pparg or its target genes related to adipogenesis (fabp11a and fabp1b) in either population. However, expression of osx/sp7, an osteoblast marker gene, and col2a1b, a chondroblast marker gene, was significantly suppressed by TBT only in SC killifish. An RXR-specific agonist, but not a PPARγ-specific agonist, induced caudal fin deformities like those observed in TBT-treated embryos. PCB126 did not induce caudal fin deformities and did not exacerbate TBT-induced fin deformities. Further, PCB126 increased expression of pparg in SC embryos and not NBH embryos, but did not change the expression of fabp1b. Taken together, these results suggest that in killifish embryos the PPARγ pathway is regulated in part by AHR, but is minimally active at least in this early life stage. In killifish, RXR activation, rather than PPARγ activation, appears to be the mechanism by which TBT induces caudal fin teratogenicity, which is not modulated by AHR responsiveness.
1. Introduction
New Bedford Harbor (NBH) is a marine Superfund site located in southeastern Massachusetts (See map in (Crawford et al., 2019)). NBH is well known for its contamination by polychlorinated biphenyls (PCBs), which were used in electronics manufacturing and discharged to the environment, leading to widespread, severe contamination throughout the harbor. Organotins were used as an antifouling agent in marine paints, potentially contaminating major fishing and shipping ports such as NBH (Pesch and Garber, 2001). We recently showed that elemental tin, a marker of organotin contamination, also is elevated in NBH (Crawford et al., 2019).
NBH is home to a number of wildlife populations, including the Atlantic killifish (“killifish,” Fundulus heteroclitus). Killifish are found in estuarine environments along the east coast of North America and have been studied extensively for their response to contaminants, most notably PCBs and polycyclic aromatic hydrocarbons (PAHs) acting through the aryl hydrocarbon receptor (AHR)(e.g., (Nacci et al., 2002; Nacci et al., 2010; Oleksiak et al., 2011; Osterberg et al., 2018; Proestou et al., 2014)). The ample genetic variation, large population sizes and small home ranges of killifish have led to the evolution of genetically distinct populations that have developed adaptations to the pollutants in the local environment (e.g., (Meyer et al., 2003; Nacci et al., 2002; Reid et al., 2016)) and have emerged as an important example of convergent evolution (Reid et al., 2016; Whitehead et al., 2012). Killifish living in PAH- or PCB-contaminated sites, including NBH, demonstrate dramatic heritable resistance, or evolved tolerance, to AHR pathway activation and to toxicity of dioxin-like PCBs (Arzuaga and Elskus, 2010; Bello et al., 2001; Nacci et al., 2002; Reid et al., 2016).
TBT is a ligand for both peroxisome proliferator activated receptor γ (PPARγ) and its DNA binding partners, the RXRs (Baker et al., 2015a; Cui et al., 2010; Grun et al., 2006; Kanayama et al., 2005; le Maire et al., 2009). In mammals, PPARγ is essential for adipogenesis, mature adipocyte function and systemic metabolic homeostasis (Gumbilai et al., 2016; He et al., 2003; Jiang et al., 2014; O'Donnell et al., 2016; Tontonoz et al., 1994; Zhang et al., 2004). Rosiglitazone, a PPARγ-activating therapeutic, and TBT induce adipogenesis in humans and rodents, in vitro and in vivo (Adams et al., 1997; Bertuloso et al., 2015; Grun et al., 2006; Kirchner et al., 2010; Miyazaki et al., 2001). Killifish, along with other fish species characterized to date, express a single form of PPARγ. Killifish express at least one form of RXRα and multiple forms of both RXRβ and RXRγ (Karchner and Hahn, unpublished analysis of XP_012719647; (Baldwin et al., 2017). Rosiglitazone and TBT induce adiposity in zebrafish larvae (Ouadah-Boussouf and Babin, 2016; Tingaud-Sequeira et al., 2011).
In mammalian models, activation of PPARγ by rosiglitazone both induces adipogenesis and suppresses bone formation (Akune et al., 2004; Lecka-Czernik et al., 1999). In vitro, TBT promotes commitment of bone marrow mesenchymal multipotent stromal cells (BM-MSCs) towards an adipocyte lineage at the expense of the osteoblast lineage (Baker et al., 2015a; Carfi et al., 2008; Kirchner et al., 2010; Koskela et al., 2012; Watt and Schlezinger, 2015; Yanik et al., 2011). In utero exposure to TBT in rodent models delays ossification, induces vertebral malformations and predisposes multipotent stromal cells to favor adipogenesis over osteogenesis (Adeeko et al., 2003; Ema et al., 1995; Kirchner et al., 2010; Tsukamoto et al., 2004). Adult TBT exposure in vivo also reduces bone formation in the cortical compartment (Watt et al., 2018).
Early life exposure to organotins (e.g. tributyltin (TBT), triphenyltin) is teratogenic in diverse fish and amphibian species. Organotins cause axial skeletal deformities, which are characterized by craniofacial deformities, bent or shortened spines and tails and failure to form fins (medaka, Oryzias latipes (Hano et al., 2007); rockfish, Sebastiscus marmoratus (Zhang et al., 2012); mahi, Coryphaena hippurus (Adema-Hannes and Shenker, 2008); zebrafish, Danio rerio (Huang et al., 2015);West African clawed frog, Xenopus tropicalis (Guo et al., 2010; Yu et al., 2011)). Further, TBT reduces osteoblast activity in scales of goldfish (Carassius auratus), nibbler (Girella punctata) and wrasse (Pseudolabrus sieboldi)(Satone et al., 2011; Suzuki et al., 2006).
Studies suggest that the AHR also regulates adipose and bone homeostasis, potentially through molecular crosstalk between AHR and PPARγ. In mammals, AHR knockout increases adiposity (Baker et al., 2015b) and AHR activation suppresses adipogenesis by downregulating PPARγ expression (Alexander et al., 1998; Arsenescu et al., 2008; Brodie et al., 1996; Gadupudi et al., 2015; Ishihara et al., 2018; van den Dungen et al., 2017). AHR has been shown to regulate bone development in a ligand-, species- and age-dependent manner (Fader et al., 2018). Following embryonic exposure, dioxin disrupts craniofacial development and suppresses ossification in zebrafish and medaka (Bums et al., 2015; Watson et al., 2017). Further, AHR2 is required for craniofacial and fin development in zebrafish (Garcia et al., 2018; Souder and Gorelick, 2019).
Collectively, there is strong evidence that TBT and PCB126 (a representative dioxin-like PCB) co-occur in urban waterways and that both target receptors that regulate adipose and bone. However, little is known about how co-exposure to common aquatic pollutants may modulate the toxicological effects of compounds acting through PPARγ-RXR and AHR pathways. Here, we tested the hypotheses that TBT would induce teratogenesis in killifish via activation of PPARγ and that differential AHR sensitivity in SC (PCB-sensitive) and NBH (PCB-tolerant) killifish would modulate PPARγ pathway responsiveness following TBT exposure. Further, we investigated the impact of co-exposure to TBT and PCB126 to interrogate how these responses change following simultaneous activation of both transcriptional pathways.
2. Methods
2.1. Chemicals and reagents
Dimethyl sulfoxide (DMSO) was purchased from American Bioanalytical (Natick, MA, USA). 3,3′,4,4′,5-pentachlorobiphenyl (PCB126, purity: ≥ 97%) was purchased from Ultra Scientific (North Kingstown, RI, USA). Tributyltin chloride (TBT, purity: 96%) and LG100268 (purity: ≥ 98%) were purchased from Sigma Aldrich (St. Louis, MO, USA). S26948 (Purity: ≥ 99%) was purchased from Tocris Bioscience (Minneapolis, MN, USA). All other reagents were from Thermo Fisher Scientific (Suwanee, GA, USA) unless noted.
2.2. Animal Care and Breeding
Reproductively active, adult killifish (F0) were collected from New Bedford Harbor (NBH, PCB-contaminated) and Scorton Creek (SC, reference) using baited traps and held in common garden laboratory conditions as described in Nacci et al. (2002). Killifish were maintained in flow-through aquaria receiving filtered ambient seawater from Narragansett Bay, Rhode Island, and fed ad libidum. F0 killifish and their offspring (F1) spawned with semi-lunar periodicity throughout the summer months (late May through August in New England) and were reared to reproductive maturity (1-2 years per generation) under the conditions described above. In order to obtain a sufficient number of embryos, a combination of non-invasive techniques (breeding baskets and manual spawning) were used to collect F2 eggs from F1 females during seasonal, semi-lunar spawning cycles between May 2016 and August 2017. Basket eggs were fertilized in situ by male killifish. Manually-spawned eggs were fertilized in vitro by expressing milt from males directly into a glass dish containing the eggs. Fertilized embryos were held at 23°C for 24 hours and were then screened microscopically to remove unfertilized or improperly developing embryos.
2.3. Chemical Exposures
Exposures were conducted at 23°C under 14:10 hour light:dark regimen. All exposures occurred in 20-mL glass scintillation vials beginning at 24 hours post fertilization (i.e., 1 day post fertilization (dpf)). Each vial contained one embryo in 10 mL of treatment solution. Solutions were made in 5 μM filtered ambient seawater. Embryos remained in treatment solution until 7 dpf, at which point they were transferred to individual wells of a 12-well plate containing filter paper moistened with filtered seawater. Embryo phenotype was screened microscopically at 10 dpf (further detail provided in Section 2.4).
2.3.1. Individual Chemical Exposures
Individual chemical exposures were conducted over two replicate experiments, each containing 20 embryos per treatment (total N = 40 per chemical treatment per population). Treatment groups were as follows: naïve (untreated), vehicle control (0.01% DMSO), TBT (Low: 5 nM, Medium: 50 nM and High: 100 nM), S26948 (a synthetic PPARγ agonist (Carmona et al., 2007), 1 μM), and LG100268 (a synthetic RXR agonist (Boehm et al., 1995), 200 nM). TBT concentrations were selected to establish a dose-response relationship around the EC50 in fish and mammals (~50 nM). To the best of our knowledge, S26948 and LG100268 have not been studied in teleost fish so in vitro EC50 concentrations for each therapeutic served as the basis for our dose selection (S26948: 8.83 nM in COS-7 cells transfected with human PPARγ, (Carmona et al., 2007); LG100268: 2 nM in COS-7 cells transfected with human RXRα, (Grun et al., 2006)). Since both compounds are therapeutics designed to have a short biological half-life, concentrations one to two orders of magnitude higher than the published EC50 were selected for S26948 and LG100268 given the six-day static exposure. Due to limited F2 SC embryo availability during the 2017 breeding season, LG100268 exposures were only conducted on NBH embryos.
2.3.2. Chemical Co-Exposures
Chemical co-exposures were conducted over three replicate experiments containing at least 20 embryos per treatment (total N ≥ 60 per chemical treatment per population). Chemical treatments consisted of naïve (untreated), a vehicle control (0.02% DMSO), TBT (Medium: 50 nM and High: 100 nM), PCB126 (61 pM), and TBT + PCB126 (Medium TBT + PCB126 and High TBT + PCB126). Medium and High TBT doses were selected for use in co-exposures because of their ability to consistently induce caudal fin malformation visible during 10 dpf phenotype screening. The PCB126 dose was selected based on prior studies showing that this concentration will activate the AHR pathway in SC, but not NBH, killifish embryos and produce low prevalence of cardiac teratogenicity (Nacci et al., 2002). This concentration was selected to minimize the possibility that the AHR-mediated response to PCB126 would overwhelm the effects of TBT exposure in co-exposure treatment groups.
2.4. Phenotypic Screening and Embryo Pooling
At 10 dpf, embryos were examined using a Nikon SMZ1500 stereomicroscope to identify TBT- and PCB126-mediated teratogenesis. TBT-mediated teratogenesis presented as caudal fin malformation, specifically stunted fin ray development and reduced overall fin length. The severity of caudal fin deformity was scored as 0 (normal), 1 (mild deformity – slightly shortened caudal fin), 2 (moderate deformity – shortened caudal fin, less discernable fin rays), and 3 (severe deformity – extremely short caudal fin, poorly defined or indiscernible fin rays) (Figure 1A). Some individuals scored as 3 appeared to have overall shorter tails; however, this was difficult to quantify in an intact embryo, and therefore was not considered during phenotypic scoring.
Figure 1. TBT-induced caudal fin teratogenesis.
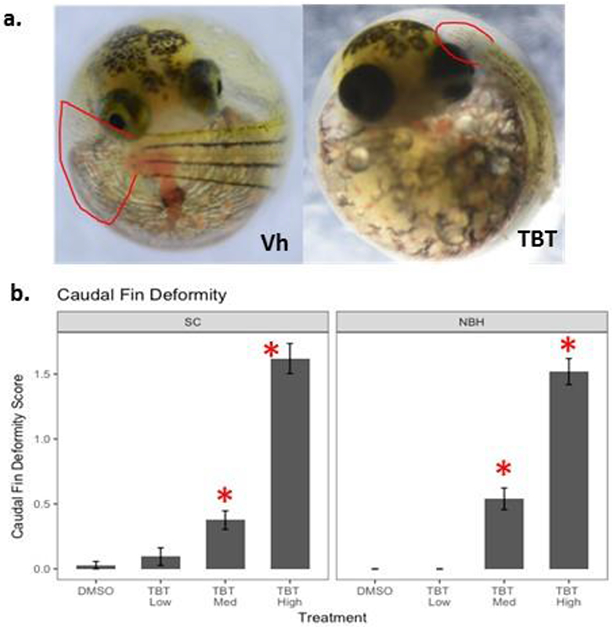
F. heteroclitus embryos were treated from 1 to 7 dpf with DMSO (0.01%, vehicle), TBT (Low: 5 nM, Medium: 50 nM and High: 100 nM) in seawater and then moved to a filter paper moistened with clean water until 10 dpf. (a) Representative images of caudal fin development. (b) Caudal fin deformity score for 10 dpf embryos. Deformity score ranged from 0 to 3, with 0 = normal and 3 = severe deformity. Data are presented as mean ± SEM. Total N≥50, screened from two replicate experiments. Two Factor ANOVA: Population p = 0.435, Treatment p <0.0001, Interaction p = 0.483. * Significantly different from DMSO in the same population (p<0.05, ANOVA, Dunnett’s)
2.5. RNA Extraction and qPCR
Following phenotypic screening, two pools of embryos (N=5 embryos per pool) from each treatment within each experiment were flash frozen for subsequent gene expression analysis. Embryos with phenotypes representative of the rest of the treatment group were selected for pools. Individual chemical exposure experiments consisted of N=4 pools per chemical treatment, whereas chemical co-exposures experiments consisted of N=6 pools per chemical treatment.
Total RNA was extracted from pooled embryos using RNA STAT-60™ (Tel-Test Inc. Friendswood, TX, USA). Genomic DNA was removed using the Aurum™ Total RNA Mini Kit (BioRad, Hercules, CA, USA). cDNA was prepared from total RNA using the GoScript™ Reverse Transcription System (Promega, Madison, WI, USA), using equal parts random and Oligo (dT)15 primers. qPCR reactions were performed using iTaq Universal SYBR® Green Supermix (BioRad) with 400 nM forward and reverse primers. Previously published primers were used for actb (Oleksiak et al., 2011), rn18s (Patel et al., 2006), ef1α (Bears et al., 2006), and cyp1a (Oleksiak et al., 2011). All new primers were designed to overlap exon junctions to prevent potential amplification of contaminating genomic DNA. Primer sequences are shown in Table 1. Thermocycling parameters were 95°C for three minutes to activate iTaq DNA polymerase, followed by 40 cycles of melting at 95°C for 15 seconds and annealing at 66°C for 60 seconds. A manually-selected threshold in the linear phase of the amplification curve was used to determine critical threshold (CT) values to standardize across multiple plates.
Table 1.
Primer information.*
| Gene | Forward (5' --> 3') | Reverse (5' --> 3') | nt | Tm (°C) |
|---|---|---|---|---|
| actb | TGGAGAAGAGCTACGAGCTCC | CCGCAGGACTCCATTCCGAG | 114 | 66a |
| rn18S | TGGTTAATTCCGATAACGAACGA | CGCCACTTGTCCCTCTAAGAA | 97 | 65 |
| ef1a | GGGAAAGGGCTCCTTCAAGT | ACGCTCGGCCTTCAGCTT | 55 | 60 |
| cyp1a | CTTTCACAATCCCACACTGCTC | GGTCTTTCCAGAGCTCTGGG | 125 | 66a |
| pparg | GGAAAGAGATGGAGACGCACAACC | CCGACGCATCGTCTGGGAACTTGG | 102 | 66 |
| fabp11a | GGCAAGCTTATTCAGAAACAGAGC | CCACGTCGCCCATTACGCATTTCGC | 103 | 66 |
| fabp1b | AGCCTTTCATGAAGGCCCTTGGTC | GTGGTGACCGTCACCTTGAAGTCG | 115 | 66 |
| col2a1a | GGTGAGACCTGCGTCTACCCAAGC | CCATCCTGAGCATAGCTGAAGTGG | 137 | 66 |
| col2a1b | GGGAGTCCTGCGTGAACCCCAAGC | CTGTCGTCACCATAACTGAAGTGG | 136 | 66 |
| osx/sp7 | CATCTGTTCTGGAGCTAGGGAATGC | TTACTGGAGAGCTGCCGGTTTTGC | 150 | 66 |
For citations, see section 2.5.
All gene expression data were normalized with the Pfaffl method (Pfaffl, 2001), using primer-specific amplification efficiency and the expression of constitutively expressed (housekeeping) genes and naïve (untreated) pooled embryo samples. Three housekeeping genes were evaluated for stability across populations and experimental replicates. Variability was greatest in actb (overall mean (standard deviation): 1.33 (0.996)), with differences between populations (SC > NBH) and experimental replicates. Expression of ef1α was less variable (0.982 (0.498)), again with SC > NBH. rn18s was most stably expressed (1.06 (0.248)), with no discernable pattern of variability between populations, experimental replicates or chemical treatments. Thus, all gene expression data presented herein are normalized to rn18S and reported as a relative expression ratio.
2.6. Statistical Analysis
All exposures were repeated over the course of at least two experimental replicates. Fifteen to thirty individually exposed embryos, depending on availability in a particular spawning cycle, were included in each treatment group for each experimental replicate. For gene expression analyses, 5 embryos were pooled to produce a single biological replicate, two independent biological replicates were generated per experiment, and each experiment was performed 2 to 3 times. In the figures, data points/biological replicates generated from the same experiment are the same color. Embryo mortality never exceeded 20% in control treatments. Power calculations were conducted using G*Power (Faul et al., 2007). All other statistical analyses were performed using Microsoft R Open 3.3.2. Gene expression and phenotypic data were analyzed separately for individual and chemical co-exposures. Gene expression was evaluated using one and two factor ANOVA and t-tests to evaluate differences in mean relative expression between populations and treatment groups. All data points, including possible outliers (points falling more than 1.5 times the interquartile range below or above the first or third interquartile range, respectively), were used in analyses due to the small sample size (N≤6). Mean caudal fin deformity score and standard error were calculated for each treatment group and chemical exposure groups were compared to DMSO-exposed controls (ANOVA, Dunnett’s post-hoc comparison). Statistical significance was evaluated at α = 0.05 for all analyses.
3. Results
3.1. TBT-Induce Caudal Fin Deformity
Exposure of killifish to TBT during early embryonic development and organogenesis impaired caudal fin development (Figure 1a). The severity and prevalence of caudal fin deformities increased in a dose-dependent manner (Figure 1b). Embryos exposed to TBT-Low (5 nM) did not exhibit caudal fin teratogenesis above the background level found in control embryos (> 95% normal caudal fin phenotype). Mild to moderate caudal fin deformities were observed with exposure to TBT-Medium (50 nM) and moderate to severe deformities were observed with TBT-High (100 nM); both concentrations significantly increased the mean deformity score relative to controls. The mean deformity score did not differ significantly between SC and NBH embryos within a treatment group (ANOVA: p = 0.435; Figure 1b).
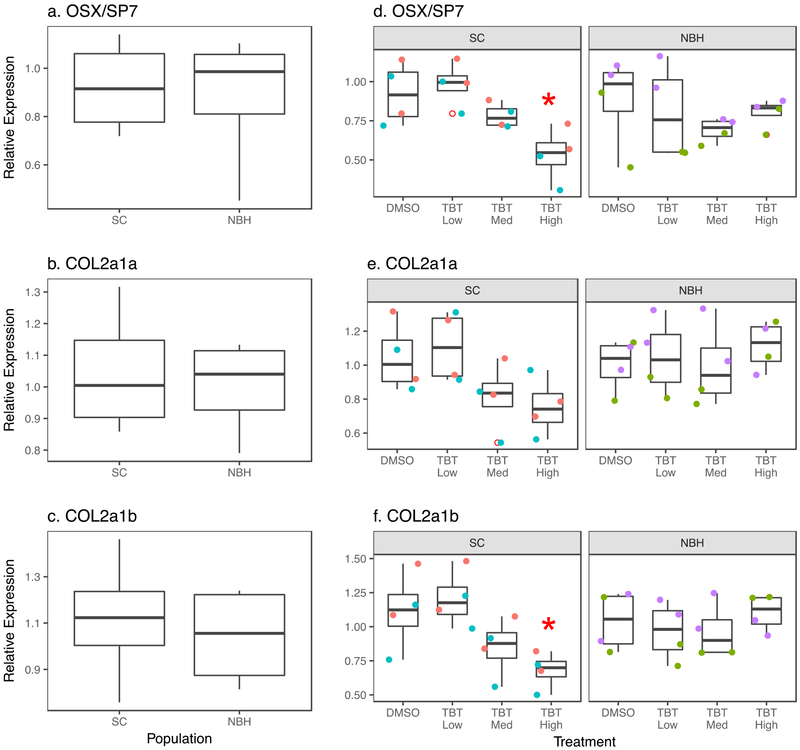
In an effort to understand the molecular targets involved in TBT-mediated caudal fin deformities, we measured the relative expression of genes known to be markers of osteoblasts (osx/sp7) and chondroblasts (col2a1a and col2a1b). No differences in basal expression of these genes were found between populations (Figure 2a-c). Following TBT exposure, osx/sp7 was suppressed in a dose-dependent manner in SC embryos and significantly suppressed in the highest TBT treatment (Figure 2d). Similar expression patterns were seen in SC embryos for col2a1a and col2a1b (Figures 2e-f), with col2a1b being significantly suppressed at the highest TBT concentration. While a trend toward suppression of osx/sp7 expression by TBT was observed in NBH embryos, there was no discernable treatment-related pattern for col2a1a and col2a1b (Figures 2d-f).
Figure 2. TBT reduces osteoblast and chondroblast gene expression in SC embryos.
F. heteroclitus embryos were treated as described in Figure 1. mRNA expression was measured by RT-qPCR in pooled embryonic killifish. Osteoblast marker gene: (a) Basal osx/sp7 expression. (d) Chemical induced osx/sp7 expression. Two Factor ANOVA: Population p = 0.876, Treatment p = 0.051, Interaction: 0.133. Chondroblast marker genes: (b) Basal col2a1a expression, (e) Chemical-induced col2a1a expression. Two Factor ANOVA: Population p = 0.131, Treatment p = 0.301, Interaction p = 0.1330. (c) Basal col2a1b expression, (f) Chemical-induced col2a1b expression. Two Factor ANOVA: Population p = 0.448, Treatment p = 0.128, Interaction: p = 0.025. Each pool consisted of 5 embryos. N=4 pools per chemical treatment, per population from two experimental replicates from SC and NBH. Data points of the same color are from biological replicates produced in the same experiment. * Significantly different from DMSO in the same population (p<0.05, ANOVA, Dunnett’s)
3.2. PPARγ-RXR/AHR Crosstalk
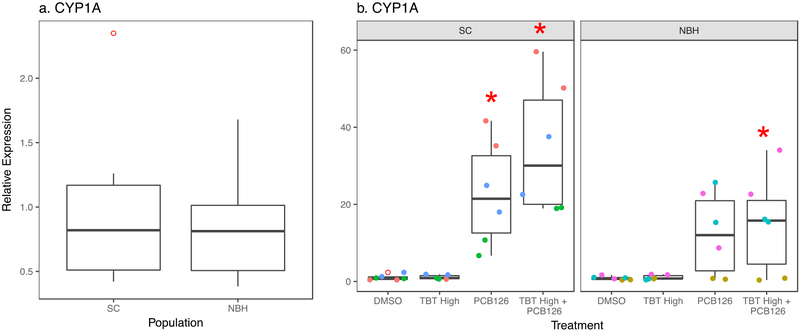
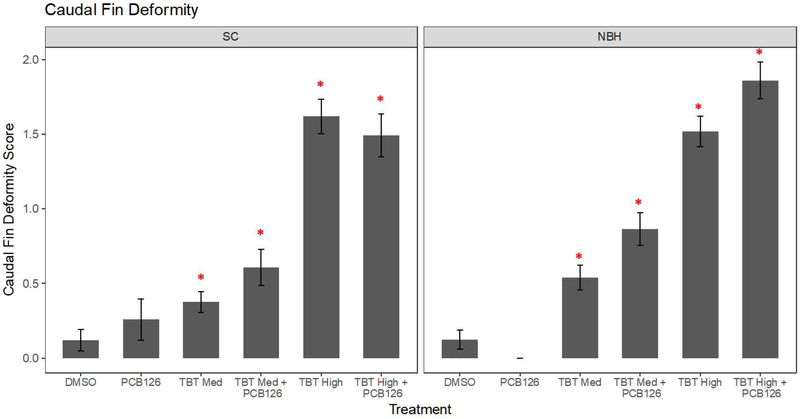
Next, we investigated whether PCB126 could modify the ability of TBT to induce fin deformities. PCB126 is well-known to induce AHR-dependent cyp1a expression and teratogenic effects (e.g., cardiac malformations in sensitive but not tolerant killifish populations (Arzuaga and Elskus, 2010; Clark et al., 2014). Basal cyp1a expression was not different between SC and NBH embryos (p = 0.672, t-test; Figure 3a). As expected, PCB126 significantly induced cyp1a in SC embryos (Figure 3b). PCB126 + TBT also induced cyp1a in NBH embryos (Figure 3c); however, “population” was a significant term in the two factor ANOVA suggesting that the induction was less in the NBH embryos. In contrast, PCB126 alone appeared to increase caudal fin deformities in SC embryos—although the increase was not statistically significant—and did not induce caudal fin deformities in NBH embryos (Figure 4). The same extent of caudal fin deformity was observed when SC and NBH embryos were exposed to TBT + PCB126 compared to TBT alone (Figure 4).
Figure 3. TBT does not alter cyp1a expression induced by PCB126.
F. heteroclitus embryos were treated from 1 to 7 dpf with DMSO (0.01%, vehicle), TBT (Medium: 50 nM and High: 100 nM) and/or PCB126 (61 pM) in seawater and then moved to a filter paper moistened with clean water until 10 dpf. mRNA expression was measured by RT-qPCR in pooled embryonic killifish. (a) Basal cyp1a expression in SC and NBH embryos. (b) Chemical-induced cyp1a expression. Two Factor ANOVA: Population p = 0.015, Treatment p < 0.001. Interaction: p = 0.190. Each pool consisted of 5 embryos. N=6 pools per chemical treatment, per population from three experimental replicates from SC and NBH. Data points of the same color are from biological replicates produced in the same experiment. * Significantly different from DMSO in the same population (p<0.05, ANOVA, Dunnett’s)
Figure 4. Low level PCB126 exposure does not induce fin teratogenesis and does not alter TBT-induced fin teratogenesis.
F. heteroclitus embryos were treated as described in Figure 3. Caudal fin deformity score for 10 dpf embryos was determined from 0 to 3, with 0 = normal and 3 = severe deformity. Data are presented as mean ± SEM. Total N≥50, screened from three replicate experiments. Two Factor ANOVA: Population p = 0.161, Treatment p < 0.001, Interaction p = 0.098. * Significantly different from vehicle (p<0.05, ANOVA, Tukey’s HSD)
To determine whether chemical exposures caused changes in the PPARγ pathway and whether they differed between populations, we characterized the expression of pparg, itself, as well as two target genes previously studied in teleost fish, fabp11a and fabp1b. Fabp11a is the recognized ortholog to mammalian FABP4, a commonly used marker of the PPARγ-RXR pathway and mature adipocytes (Flynn et al., 2009; Imrie and Sadler, 2010). Fabp1b also has been shown to be a selective target of pparg in zebrafish (Laprairie et al., 2016). Basal gene expression of pparg, fabp11a, and fabp1b did not vary between SC and NBH embryos (Figure 5). Further, TBT did not induce significant changes in expression of these genes (Figure 5). In co-exposure experiments, pparg expression showed an increasing trend with PCB126 alone and significantly increased with TBT + PCB126 in SC embryos (Figure 6a). A similar trend was seen in NBH embryos although the extent of induction was less than in SC embryos, fabp1b expression was highly variable, with PCB126 potentially increasing fabp1b expression in SC embryos and decreasing fabp1b expression in NBH embryos (Figure 6b).
Figure 5. Lack of responsiveness of PPARγ pathway in killifish embryos.
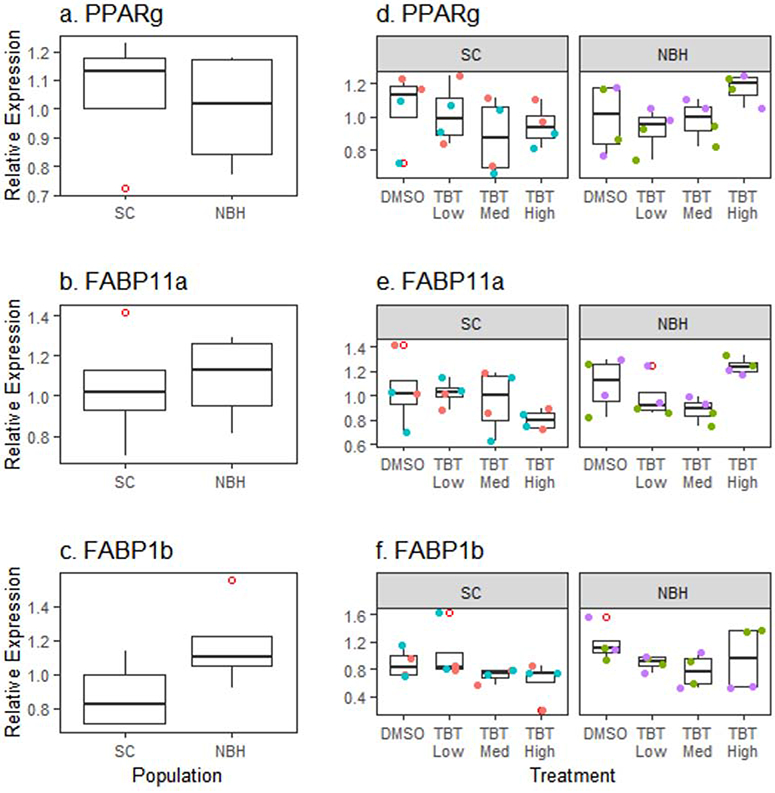
F. heteroclitus embryos were treated as described in Figure 1. mRNA expression of pparg and its target genes fabp11a and fabp1b were measured by RT-qPCR in pooled embryonic killifish. (a-c) Basal expression in SC and NBH embryos. (d-f) Expression in TBT exposed embryos. Each pool consisted of 5 embryos. N=4 pools per chemical treatment, per population from two experimental replicates from SC and NBH. Data points of the same color are from biological replicates produced in the same experiment. No statistically significant differences were detected (ANOVA).
Figure 6. PCB126 induces pparg expression only in SC embryos.
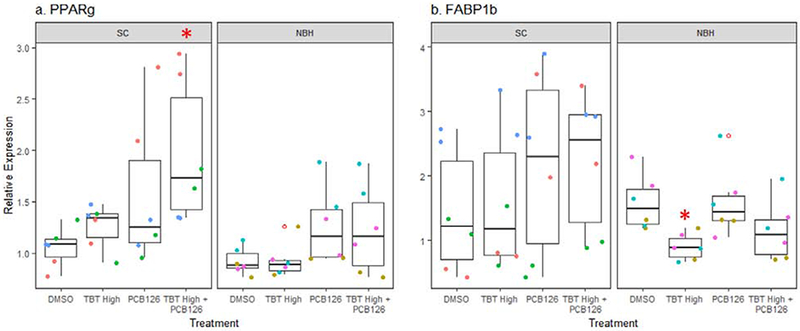
F. heteroclitus embryos were treated as described in Figure 3. mRNA expression was measured by RT-qPCR in pooled embryonic killifish. (a) Expression of pparg. Two Factor ANOVA: Population, p = 0.004. Treatment p = 0.004. Interaction p = 0.364. (b) Expression of fabp1b. Two Factor ANOVA: Population p = 0.036, Treatment p = 0.363, Interaction: p = 0.407. Each pool consisted of 5 embryos. N=6 pools per chemical treatment, per population from three experimental replicates from SC and NBH. Data points of the same color are from biological replicates produced in the same experiment. * Significantly different from DMSO in the same population (p<0.05, ANOVA, Dunnett’s)
3.2. RXR-induced caudal fin deformity
Gene expression analyses did not support a role for PPARγ activation in TBT-induced effects in killifish embryos. Furthermore, a synthetic PPARγ ligand (S26948 (Carmona et al., 2007) also did not induce PPARγ target gene expression (data not shown). Since TBT is known to bind and activate both PPARγ and RXR, we also evaluated caudal fin development in F2 killifish embryos exposed to selective therapeutic ligands for each receptor (PPARγ: S26948 and RXR: LG100268 (Boehm et al., 1995)) to determine which receptor is involved in TBT-induced caudal fin deformities. S26948 exposure did not produce caudal fin deformities in killifish embryos (Figure 7). However, the RXR ligand LG100268 induced deformities in embryos similar to those observed following TBT exposure (Figure 7).
Figure 7. An RXR ligand, but not a PPARγ ligand, induces caudal fin teratogenesis.
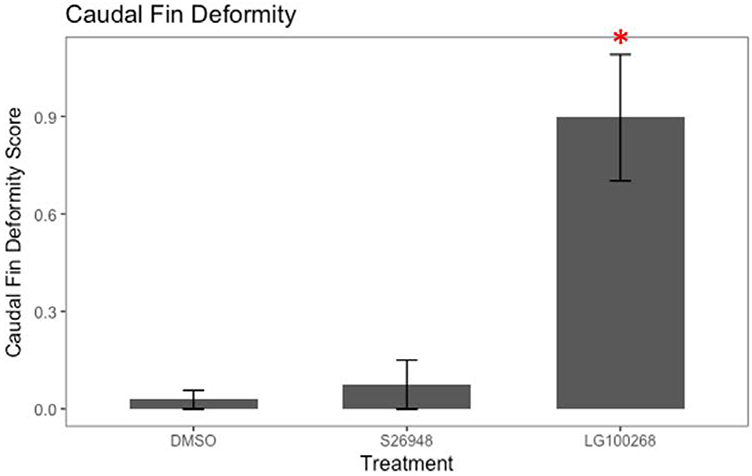
F. heteroclitus embryos were treated from 1 to 7 dpf with DMSO (0.01%, vehicle), S26948 (1 μM), and LG100268 (200 nM) in seawater and then moved to a filter paper moistened with clean water until 10 dpf. Caudal fin deformity score for 10 dpf embryos was determined from 0 to 3, with 0 = normal and 3 = severe deformity. Data are presented as mean ± SEM pooled across SC and NBH embryos. Total N≥30, screened from two replicate experiments. * Significantly different from vehicle (p<0.05, ANOVA, Dunnett’s)
4. Discussion
Killifish is a robust species, multiple populations of which have adapted to living in sites highly contaminated with PCBs and/or PAHs by developing resistance to AHR activation and AHR-mediated toxicity. Few studies have investigated how this adaptation influences the response to other chemical stressors; one has shown that there is cross-resistance to pesticides in a PAH-adapted population (Clark and Di Giulio, 2012) while another found enhanced sensitivity to the pro-oxidant tert-butylhydroquinone in a PCB-adapted population (Harbeitner et al., 2013). We sought to explore how PCB-resistance modifies the biological responses to TBT, a toxicant that is found commonly in urban waterways and likely co-occurs with PCBs in NBH (Crawford et al., 2019). Because TBT is a PPARγ ligand and AHR-PPARγ crosstalk is known in mammals, we investigated the hypothesis that TBT-induced effects on PPARγ-related endpoints would be distinct in PCB-sensitive and PCB-resistant populations. TBT induced caudal fin teratogenicity with equal potency and efficacy in SC and NBH killifish, but activation of PPARγ was not the mechanism. The PPARγ pathway is regulated, at least in part, by AHR in killifish embryos but is minimally active at this early life stage. Rather, the data support the conclusion that RXR activation is the mechanism by which TBT induces caudal fin teratogenicity.
4.1. Lack of Response of the PPARγ Pathway
Overall, the PPARγ pathway was largely unresponsive to TBT exposure in killifish embryos, regardless of population. This was surprising because TBT is widely known to stimulate adipogenesis and/or lipid accumulation in a variety of vertebrate models, including amphibians and fishes (Grun et al., 2006; Kirchner et al., 2010; Lutfi et al., 2017; Ouadah-Boussouf and Babin, 2016). However, others have shown that PPARγ activation by TBT occurs in cartilaginous fish and early diverging ray finned fishes, which split from the teleost lineage prior to the teleost genome duplication, and that PPARγ responsiveness to TBT has been lost in teleost species (Capitao et al., 2018). Cysteine 285 in the PPARγ ligand binding pocket appears to be critical for TBT to activate PPARγ, and this residue has been replaced by either tyrosine or leucine in the teleosts studied so far (Capitao et al., 2018). Even if TBT were able to activate PPARγ, it is unclear whether PPARγ is biologically active in the fish embryo.
We chose a non-thiazolidinedione synthetic PPARγ ligand (S26948) to independently test the biological activity of PPARγ in killfish embryos. S26948 also did not induce expression of a PPARγ target gene in killifish embryos. Lack of responsiveness of killifish to S26948 can be attributed any of several factors: (1) S26948 did not cross the chorion, (2) it was metabolized during the course of the six-day static exposure and three-day depuration prior to embryo preservation (i.e., S26948 may have a short biological half-life), (3) it is not a ligand for killifish pparγ, and/or (4) PPARγ signaling is minimal in the fish embryo. Adipose tissue development, which requires PPARγ activity, has not been chronicled in embryonic killifish and 10 dpf embryos may not have many, if any, differentiated adipocytes. Zebrafish begin to develop adipose tissue around 10-15 dpf (Imrie and Sadler, 2010; Minchin and Rawls, 2011), and the developmental timeframe for killifish is appreciably slower. To the best of our knowledge, S26948 has not been tested as a PPARγ ligand in teleost fish. We selected S26948 rather than the classic PPARγ positive control, rosiglitazone, given that reports do not agree as to whether rosiglitazone is an agonist of fish PPARγ (Capitao et al., 2018; Laprairie et al., 2016; Ouadah-Boussouf and Babin, 2016; Riu et al., 2011; Sanchez-Gurmaches et al., 2010; Tingaud-Sequeira et al., 2011). While timing of initiation of PPARγ activity during development is uncertain, we have also shown that S26948 did not activate the PPARγ pathway in liver of adult killifish following a three-day exposure by intraperitoneal injection (Crawford et al., 2019). Thus, it is likely that S26948 is not an agonist of killifish PPARγ.
4.2. Fin Teratogenicity
The caudal fin and caudal bone complex are recognized as skeletal regions particularly sensitive to disruption during teleost development (Fernández et al., 2008; Fernández et al., 2009; Haga et al., 2002). The skeleton and fin formation/regeneration are targets of dioxin toxicity in zebrafish (Baker et al., 2013; Zodrow and Tanguay, 2003). Further, AHR2 is required for craniofacial and fin development (Garcia et al., 2018; Souder and Gorelick, 2019). Therefore, it was surprising that PCB126, at most, modestly induced fin teratogenicity. The likely explanation is that the concentration of PCB126 we used was relatively low, approximating the EC50 for cyp1a induction, a highly sensitive AHR response in killifish embryos (Arzuaga and Elskus, 2010; Wills et al., 2010). Higher concentrations may be required to elicit fin deformities.
Our observation of TBT-induced caudal fin deformities in killifish is in line with previously published studies showing TBT-induced axial skeleton and fin abnormalities in other fish and amphibian species (Adema-Hannes and Shenker, 2008; Guo et al., 2010; Hano et al., 2007; Huang et al., 2015; Yu et al., 2011; Zhang et al., 2012). A limited number of killifish exposed to TBT in ovo were raised for approximately 12 months after hatching. Deformity of the caudal peduncle remained in one year-old fish, indicating the persistence of the phenotype (data not shown). While PPARγ activation is well known to interfere with osteogenesis (Lecka-Czernik et al., 1999), TBT did not activate PPARγ in developing killifish. However, TBT also binds and activates RXRs (le Maire et al., 2009). While PPARγ expression in zebrafish is minimal through the larval stage (Ibabe et al., 2005), RXRs are ubiquitously expressed during gastrulation and become more strongly expressed and spatially restricted by 1 dpf (Tallafuss et al., 2006; Waxman and Yelon, 2007), suggesting that RXR signaling is likely to precede PPARγ signaling in killifish embryos, as well. Guo et al. (2010) proposed that TBT-activation of RXR interferes with retinoic acid or thyroid signaling to cause skeletal and ocular deformities in Xenopus tropicalis, respectively, and TBT has been shown to interfere with thyroid signaling in Xenopus laevis (Mengeling et al., 2018). Our results show that an RXR ligand (LG100268) induced a caudal fin teratogenesis similar to that caused by TBT. Previous studies have shown a reduction in osx/sp7 expression and bone nodule formation in BM-MSCs following TBT and LG100268 exposure (Baker et al., 2015a; Watt and Schlezinger, 2015). This finding aligns with our observation of caudal fin deformities, characterized by poor cartilage and bone development. Activation of RXR, rather than PPARγ, is a likely mechanism by which TBT induces fin deformity.
Both TBT and LG100268 efficaciously induced fin deformity in SC and NBH killifish. However, expression of marker genes for osteoblast and chondroblast activity were only significantly suppressed in the SC killifish. Why the altered gene expression occurs in SC but not NBH embryos is an intriguing question. In contrast, PCB126 only modestly induced fin deformity in SC killifish alone. The difference in efficacy is likely explained by the exposure to maximally efficacious concentrations of TBT and LG100268 (100x the in vitro EC50’s) and sub-maximally efficacious concentrations of PCB126 (≈ 1x the in vivo EC50). The similar sensitivity of SC and NBH killifish to TBT- and LG100268-induced fin teratogenicity suggests that RXR signaling is not affected by adaptation to the contaminated environment. On the other hand, the likely difference in sensitivity of SC and NBH killifish to PCB126-induced fin teratogenicity are in line with previous observations of AHR’s role in skeletal development in zebrafish (Garcia et al., 2018; Souder and Gorelick, 2019).
4.3. Evidence of Crosstalk Between PPARγ and AHR Pathways
NBH killifish have evolved heritable resistance to environmental AHR agonists, such as dioxin-like PCBs, which is often measured by recalcitrance to the induction of AHR target genes, notably CYP1A (Bello et al., 2001; Nacci et al., 2002; Reid et al., 2016). As expected, PCB126 most efficaciously induced cyp1a expression in SC embryos (23-fold); however, it also significantly induced cyp1a expression in NBH embryos (12-fold). Although the reduced sensitivity of NBH fish to cyp1a induction is typically heritable through several generations (Nacci et al 2010), NBH fish are not completely insensitive; cyp1a induction of lower magnitude or requiring higher doses of inducer has been observed previously (Bello et al 2001; Oleksiak et al 2011).
In mammals, AHR activation downregulates PPARγ expression (Alexander et al., 1998; Arsenescu et al., 2008; Brodie et al., 1996; Gadupudi et al., 2015; Ishihara et al., 2018; van den Dungen et al., 2017). Thus, in fish with dysregulated AHR (i.e., NBH killifish), we hypothesized that the PPARγ pathway would be more efficaciously activated. As noted above, neither TBT nor S26948 induced expression of PPARγ target genes in SC or NBH killifish embryos. It is difficult to interpret the significance of a lack of differential responsiveness to PPARγ ligands, because this may simply result from TBT and S26948 not being ligands for killifish PPARγ. The phthalate metabolite mono-2-ethylhexyl phthalate has been shown to be an efficacious agonist of zebrafish PPARγ (Grimaldi et al., 2015) and may be better suited for testing differences in PPARγ pathway responsiveness in the two populations.
A much more intriguing result was observed in killifish treated with PCB126. Pparγ expression was significantly increased in SC embryos exposed to TBT High + PCB126 but not in NBH embryos. We also found that PCB126 induces pparg expression in liver of SC, but not NBH, killifish following adult exposure in males (Crawford et al., 2019). These results are the opposite of what has been shown in mammals, in which PCB126 reduces pparg expression and, subsequently, expression of its target genes (e.g., (Gadupudi et al., 2015)). Given the unexpected induction of pparg expression by PCB126 in SC killifish, we wondered whether this induction also occurs in other sensitive populations. We compared the PCB126-induced expression of pparg in a previously generated dataset comparing four sensitive/tolerant population pairs (Reid et al., 2016). First, pparg was identified as a gene with significant exposure-by-population interaction (Reid et al., 2016). Second, in each population pair, PCB126 increased expression of pparg to a significantly greater extent in the sensitive populations than the tolerant populations (Figure 8). The physiological significance of this observation is unknown, but it may suggest that lipid homeostatic pathways are altered in NBH killifish and potentially other tolerant killifish populations under study (Reid et al., 2016).
Figure 8. PCB126 induces expression of pparg across multiple sensitive killifish populations but not in resistant populations.
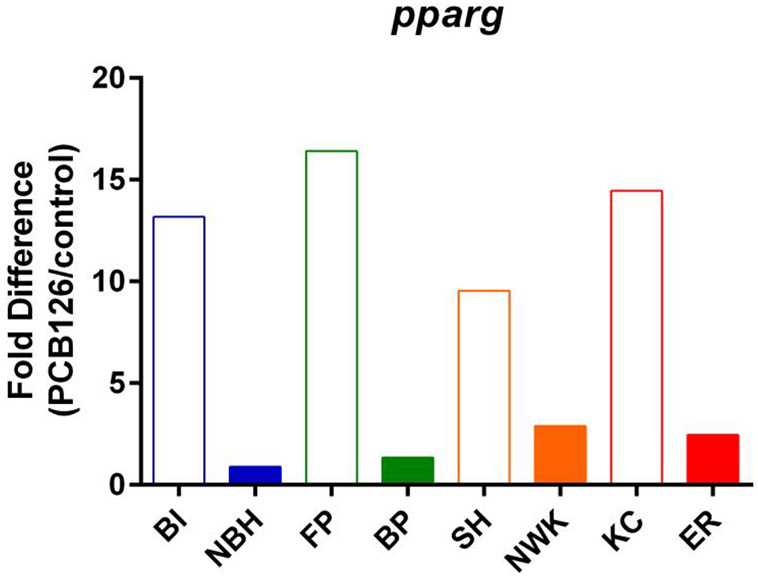
Pparg expression data are from (Reid et al., 2016). Killifish from sensitive and resistant population pairs were treated with DMSO or PCB126 (610 pM) in seawater from 1 dpf to post-organogenesis (stage 35, ~10 dpf). mRNA expression was determined by RNA-Seq. A map of the locations can be found in (Reid et al., 2016). Sensitive populations (open bars): BI = Block Island, RI. FP = Flax Pond, NY. SH = Sandy Hook, NJ. KC = Kings Creek, VA. Resistant populations (closed bars): NBH = New Bedford Harbor, MA. BP = Bridgeport, CT. NWK = Newark, NJ. ER = Elizabeth River, VA. Pparg expression following PCB126 treatment was determined by dividing the average, normalized expression level (N=3-5) in PCB126 treated embryos by that in vehicle treated embryos. Overall, there was 13.4 ± 1.5 fold induction in sensitive populations versus 1.9 ± 0.9 fold induction in resistant populations (p<0.003, Student’s t-test).
4.4. Variability
On the whole, gene expression in pooled embryo samples across experimental replicates was highly variable. Given our small sample sizes (N=4 or 6 per chemical treatment per population), it was extremely difficult to detect significant differences and identify true trends in the expression of the PPARγ-pathway, osteogenic and chondrogenic genes between chemical treatments. Killifish are well known to have high genetic diversity (Reid et al., 2017). Even individuals from the F2 generation are effectively an outbred, wild population, which naturally implies greater variability among individuals than in populations of inbred or genetically identical animals, such as zebrafish or mice.
Intriguingly, the relative expression of each gene in replicate embryo pools from a given replicate experiment (two pools of N=5 embryos per pool, per experiment) tended to be clustered together compared with other embryo pools in the same treatment group from other experiments. This suggests that the specific characteristics of embryos from a given spawning cycle are more similar to one another than they are to embryos from other cycles. This kind of batch-to-batch variability has been characterized in other teleost fish species, including a meta-analysis of fathead minnow (Pimephales promelas) and zebrafish (Danio rerio), where temporally proximate batches were found to have greater transcriptomic similarity (Wang et al., 2014). Going forward, the ability to detect subtle changes in gene expression would be improved by generating at least 6 biological replicates per treatment comprised of pools of embryos from multiple independent clutches all generated in the same spawning cycle.
5. Conclusions
These studies show that TBT is a teratogen in killifish. While we hypothesized that TBT would impair bone development through activation of PPARγ, this pathway was unresponsive to TBT. Rather, the data suggest that TBT is likely acting through RXR in killifish. Thus, it is not surprising that TBT efficaciously induced caudal fin deformities in both SC and NBH killifish embryos, as the potential for PPARγ-AHR crosstalk was not at play. Interestingly, PCB126 induced pparg expression in a population dependent-manner, supporting the conclusion that PPARγ-AHR crosstalk is biologically relevant in teleosts, although not in the same manner as in mammals. Future studies are needed to identify killifish PPARγ agonists, to define how RXR activation impairs skeletal development in killifish, and to investigate the physiological and ecological significance of the effect of evolved AHR resistance on PPARγ function.
Highlights.
TBT induced caudal fin deformity in killifish embryos.
Susceptibility was the same in PCB-sensitive and PCB-tolerant killifish.
An RXR-ligand recapitulated the TBT-induced caudal fin deformity.
Co-treatment with an AHR ligand, PCB126, did not affect the caudal fin deformity.
AHR regulates PPARγ in killifish but PPARγ activity is minimal in embryos.
6. Acknowledgments
We acknowledge Ian Kirby, Joe Bishop and Ashley Bertrand for their excellent fish care, and Diana Franks for her technical expertise with gene expression analyses. This work was supported by the National Institute of Environmental Health Science (Boston University Superfund Research Program, P42ES007381; Boston University Environmental Epidemiology in Community Settings, T32ES014562).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams M, Montague CT, Prins JB, Holder JC, Smith SA, Sanders L, Digby JE, Sewter CP, Lazar MA, Chatterjee VK, O'Rahilly S, 1997. Activators of peroxisome proliferator-activated receptor gamma have depot-specific effects on human preadipocyte differentiation. J Clin Invest 100, 3149–3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adeeko A, Li D, Forsyth DS, Casey V, Cooke GM, Barthelemy J, Cyr DG, Trasler JM, Robaire B, Hales BF, 2003. Effects of in utero tributyltin chloride exposure in the rat on pregnancy outcome. Toxicol Sci 74, 407–415. [DOI] [PubMed] [Google Scholar]
- Adema-Hannes R, Shenker J, 2008. Acute lethal and teratogenic effects of tributyltin chloride and copper chloride on mahi mahi (Coryphaena hippurus) eggs and larvae. Environmental toxicology and chemistry / SETAC 27, 2131–2135. [DOI] [PubMed] [Google Scholar]
- Akune T, Ohba S, Kamekura S, Yamaguchi M, Chung UI, Kubota N, Terauchi Y, Harada Y, Azuma Y, Nakamura K, Kadowaki T, Kawaguchi H, 2004. PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest 113, 846–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander DL, Ganem LG, Fernandez-Salguero P, Gonzalez F, Jefcoate CR, 1998. Aryl hydrocarbon receptor is an inhibitory regulator of lipid synthesis and of commitment to adipogenesis. J Cell Sci 111, 3311–3322. [DOI] [PubMed] [Google Scholar]
- Arsenescu V, Arsenescu RI, King V, Swanson H, Cassis LA, 2008. Polychlorinated biphenyl-77 induces adipocyte differentiation and proinflammatory adipokines and promotes obesity and atherosclerosis. Environ Health Perspect 116, 761–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arzuaga X, Elskus A, 2010. Polluted-site killifish (Fundulus heteroclitus) embryos are resistant to organic pollutant-mediated induction of CYP1A activity, reactive oxygen species, and heart deformities. Environmental toxicology and chemistry / SETAC 29, 676–682. [DOI] [PubMed] [Google Scholar]
- Baker AH, Watt J, Huang CK, Gerstenfeld LC, Schlezinger JJ, 2015a. Tributyltin engages multiple nuclear receptor pathways and suppresses osteogenesis in bone marrow multipotent stromal cells. Chem Res Toxicol 28, 1156–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker NA, Shoemaker R, English V, Larian N, Sunkara M, Morris AJ, Walker M, Yiannikouris F, Cassis LA, 2015b. Effects of Adipocyte Aryl Hydrocarbon Receptor Deficiency on PCB-Induced Disruption of Glucose Homeostasis in Lean and Obese Mice. Environ Health Perspect 123, 944–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TR, Peterson RE, Heideman W, 2013. Early dioxin exposure causes toxic effects in adult zebrafish. Toxicol Sci 135, 241–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin WS, Boswell WT, Ginjupalli G, Litoff EJ, 2017. Annotation of the Nuclear Receptors in an Estuarine Fish species, Fundulus heteroclitus. Nucl Receptor Res 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bears H, Richards JG, Schulte PM, 2006. Arsenic exposure alters hepatic arsenic species composition and stress-mediated gene expression in the common killifish (Fundulus heteroclitus). Aquatic toxicology (Amsterdam, Netherlands) 77, 257–266. [DOI] [PubMed] [Google Scholar]
- Bello SM, Franks DG, Stegeman JJ, Hahn ME, 2001. Acquired resistance to Ah receptor agonists in a population of Atlantic killifish (Fundulus heteroclitus) inhabiting a marine superfund site: in vivo and in vitro studies on the inducibility of xenobiotic metabolizing enzymes. Toxicol Sci 60, 77–91. [DOI] [PubMed] [Google Scholar]
- Bertuloso BD, Podratz PL, Merlo E, de Araujo JF, Lima LC, de Miguel EC, de Souza LN, Gava AL, de Oliveira M, Miranda-Alves L, Carneiro MT, Nogueira CR, Graceli JB, 2015. Tributyltin chloride leads to adiposity and impairs metabolic functions in the rat liver and pancreas. Toxicol Lett 235, 45–59. [DOI] [PubMed] [Google Scholar]
- Boehm MF, Zhang L, Zhi L, McClurg MR, Berger E, Wagoner M, Mais DE, Suto CM, Davies JA, Heyman RA, et al. , 1995. Design and synthesis of potent retinoid X receptor selective ligands that induce apoptosis in leukemia cells. Journal of medicinal chemistry 38, 3146–3155. [DOI] [PubMed] [Google Scholar]
- Brodie AE, Manning VA, Hu CY, 1996. Inhibitors of preadipocyte differentiation induce COUP-TF binding to a PPAR/RXR binding sequence. Biochem Biophys Res Commun 228, 655–661. [DOI] [PubMed] [Google Scholar]
- Burns FR, Peterson RE, Heideman W, 2015. Dioxin disrupts cranial cartilage and dermal bone development in zebrafish larvae. Aquatic toxicology (Amsterdam, Netherlands) 164, 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitao AMF, Lopes-Marques MS, Ishii Y, Ruivo R, Fonseca ESS, Pascoa I, Jorge RP, Barbosa MAG, Hiromori Y, Miyagi T, Nakanishi T, Santos MM, Castro LFC, 2018. Evolutionary Exploitation of Vertebrate Peroxisome Proliferator-Activated Receptor gamma by Organotins. Environ Sci Technol 52, 13951–13959. [DOI] [PubMed] [Google Scholar]
- Carfi M, Croera C, Ferrario D, Campi V, Bowe G, Pieters R, Gribaldo L, 2008. TBTC induces adipocyte differentiation in human bone marrow long term culture. Toxicology 249, 11–18. [DOI] [PubMed] [Google Scholar]
- Carmona MC, Louche K, Lefebvre B, Pilon A, Hennuyer N, Audinot-Bouchez V, Fievet C, Torpier G, Formstecher P, Renard P, Lefebvre P, Dacquet C, Staels B, Casteilla L, Penicaud L, Consortium of the French Ministry of, R., Technology, 2007. S 26948: a new specific peroxisome proliferator activated receptor gamma modulator with potent antidiabetes and antiatherogenic effects. Diabetes 56, 2797–2808. [DOI] [PubMed] [Google Scholar]
- Clark BW, Bone AJ, Di Giulio RT, 2014. Resistance to teratogenesis by F1 and F2 embryos of PAH-adapted Fundulus heteroclitus is strongly inherited despite reduced recalcitrance of the AHR pathway. Environ Sci Pollut Res Int 21, 13898–13908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BW, Di Giulio RT, 2012. Fundulus heteroclitus adapted to PAHs are cross-resistant to multiple insecticides. Ecotoxicology 21, 465–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford KA, Clark BW, Heiger-Bernays WJ, Karchner SI, Claus Henn BG, Griffith KN, Howes BL, Schlezinger DR, Hahn ME, Nacci DE, Schlezinger JJ, 2019. Altered lipid homeostasis in a PCB-resistant Atlantic killifish (Fundulus heteroclitus) population from New Bedford Harbor, MA, U.S.A. Aquatic toxicology (Amsterdam, Netherlands) 210, 30–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui H, Okuhira K, Ohoka N, Naito M, Kagechika H, Hirose A, Nishimaki-Mogami T, 2010. Tributyltin chloride induces ABCA1 expression and apolipoprotein A-I-mediated cellular cholesterol efflux by activating LXRalpha/RXR. Biochem Pharmacol 81, 819–824. [DOI] [PubMed] [Google Scholar]
- Ema M, Kurosaka R, Amano H, Ogawa Y, 1995. Comparative developmental toxicity of butyltin trichloride, dibutyltin dichloride and tributyltin chloride in rats. J Appl Toxicol 15, 297–302. [DOI] [PubMed] [Google Scholar]
- Fader KA, Nault R, Raehtz S, McCabe LR, Zacharewski TR, 2018. 2,3,7,8-Tetrachlorodibenzo-p-dioxin dose-dependently increases bone mass and decreases marrow adiposity in juvenile mice. Toxicol Appl Pharmacol 348, 85–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A, 2007. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39, 175–191. [DOI] [PubMed] [Google Scholar]
- Fernández I, Hontoria F, Ortiz-Delgado JB, Kotzamanis Y, Estévez A, Zambonino-Infante JL, Gisbert E, 2008. Larval performance and skeletal deformities in farmed gilthead sea bream (Sparus aurata) fed with graded levels of Vitamin A enriched rotifers (Brachionus plicatilis). Aquaculture 283, 102–115. [Google Scholar]
- Fernández I, Pimentel MS, Ortiz-Delgado JB, Hontoria F, Sarasquete C, Estévez A, Gisbert E, 2009. Effect of dietary vitamin A on Senegalese sole (Solea senegalensis) skeletogenesis and larval quality. Aquaculture 295, 250–265. [Google Scholar]
- Flynn EJ 3rd, Trent CM, Rawls JF, 2009. Ontogeny and nutritional control of adipogenesis in zebrafish (Danio rerio). Journal of lipid research 50, 1641–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadupudi G, Gourronc FA, Ludewig G, Robertson LW, Klingelhutz AJ, 2015. PCB126 inhibits adipogenesis of human preadipocytes. Toxicol In Vitro 29, 132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia GR, Bugel SM, Truong L, Spagnoli S, Tanguay RL, 2018. AHR2 required for normal behavioral responses and proper development of the skeletal and reproductive systems in zebrafish. PloS one 13, e0193484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaldi M, Boulahtouf A, Delfosse V, Thouennon E, Bourguet W, Balaguer P, 2015. Reporter cell lines to evaluate the selectivity of chemicals for human and zebrafish estrogen and peroxysome proliferator activated gamma receptors. Front Neurosci 9, 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grun F, Watanabe H, Zamanian Z, Maeda L, Arima K, Cubacha R, Gardiner DM, Kanno J, Iguchi T, Blumberg B, 2006. Endocrine-disrupting organotin compounds are potent inducers of adipogenesis in vertebrates. Mol Endocrinol 20, 2141–2155. [DOI] [PubMed] [Google Scholar]
- Gumbilai V, Ebihara K, Aizawa-Abe M, Ebihara C, Zhao M, Yamamoto Y, Mashimo T, Hosoda K, Serikawa T, Nakao K, 2016. Fat Mass Reduction With Adipocyte Hypertrophy and Insulin Resistance in Heterozygous PPARgamma Mutant Rats. Diabetes 65, 2954–2965. [DOI] [PubMed] [Google Scholar]
- Guo S, Qian L, Shi H, Barry T, Cao Q, Liu J, 2010. Effects of tributyltin (TBT) on Xenopus tropicalis embryos at environmentally relevant concentrations. Chemosphere 79, 529–533. [DOI] [PubMed] [Google Scholar]
- Haga Y, Suzuki T, Takeuchi T, 2002. Retinoic acid isomers produce malformations in postembryonic development of the Japanese flounder, Paralichthys olivaceus. Zoolog Sci 19, 1105–1112. [DOI] [PubMed] [Google Scholar]
- Hano T, Oshima Y, Kim SG, Satone H, Oba Y, Kitano T, Inoue S, Shimasaki Y, Honjo T, 2007. Tributyltin causes abnormal development in embryos of medaka, Oryzias latipes. Chemosphere 69, 927–933. [DOI] [PubMed] [Google Scholar]
- Harbeitner RC, Hahn ME, Timme-Laragy AR, 2013. Differential sensitivity to pro-oxidant exposure in two populations of killifish (Fundulus heteroclitus). Ecotoxicology 22, 387–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Barak Y, Hevener A, Olson P, Liao D, Le J, Nelson M, Ong E, Olefsky JM, Evans RM, 2003. Adipose-specific peroxisome proliferator-activated receptor gamma knockout causes insulin resistance in fat and liver but not in muscle. Proc Natl Acad Sci U S A 100, 15712–15717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Zuo Z, Zhang Y, Wang C, 2015. Toxicogenomic analysis in the combined effect of tributyltin and benzo[a]pyrene on the development of zebrafish embryos. Aquatic toxicology (Amsterdam, Netherlands) 158, 157–164. [DOI] [PubMed] [Google Scholar]
- Ibabe A, Bilbao E, Cajaraville MP, 2005. Expression of peroxisome proliferator-activated receptors in zebrafish (Danio rerio) depending on gender and developmental stage. Histochem Cell Biol 123, 75–87. [DOI] [PubMed] [Google Scholar]
- Imrie D, Sadler KC, 2010. White adipose tissue development in zebrafish is regulated by both developmental time and fish size. Dev Dyn 239, 3013–3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara Y, Tsuji M, Vogel CFA, 2018. Suppressive effects of aryl-hydrocarbon receptor repressor on adipocyte differentiation in 3T3-L1 cells. Arch Biochem Biophys 642, 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Berry DC, Tang W, Graff JM, 2014. Independent stem cell lineages regulate adipose organogenesis and adipose homeostasis. Cell Rep 9, 1007–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanayama T, Kobayashi N, Mamiya S, Nakanishi T, Nishikawa J, 2005. Organotin compounds promote adipocyte differentiation as agonists of the peroxisome proliferator-activated receptor gamma/retinoid X receptor pathway. Mol Pharmacol 67, 766–774. [DOI] [PubMed] [Google Scholar]
- Kirchner S, Kieu T, Chow C, Casey S, Blumberg B, 2010. Prenatal exposure to the environmental obesogen tributyltin predisposes multipotent stem cells to become adipocytes. Mol Endocrinol 24, 526–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koskela A, Viluksela M, Keinanen M, Tuukkanen J, Korkalainen M, 2012. Synergistic effects of tributyltin and 2,3,7,8-tetrachlorodibenzo-p-dioxin on differentiating osteoblasts and osteoclasts. Toxicol Appl Pharmacol 263, 210–217. [DOI] [PubMed] [Google Scholar]
- Laprairie RB, Denovan-Wright EM, Wright JM, 2016. Subfunctionalization of peroxisome proliferator response elements accounts for retention of duplicated fabp1 genes in zebrafish. BMC Evol Biol 16, 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- le Maire A, Grimaldi M, Roecklin D, Dagnino S, Vivat-Hannah V, Balaguer P, Bourguet W, 2009. Activation of RXR-PPAR heterodimers by organotin environmental endocrine disruptors. EMBO Rep 10, 367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecka-Czernik B, Gubrij I, Moerman EJ, Kajkenova O, Lipschitz DA, Manolagas SC, Jilka RL, 1999. Inhibition of Osf2/Cbfa1 expression and terminal osteoblast differentiation by PPARgamma2. J Cell Biochem 74, 357–371. [PubMed] [Google Scholar]
- Lutfi E, Riera-Heredia N, Cordoba M, Porte C, Gutierrez J, Capilla E, Navarro I, 2017. Tributyltin and triphenyltin exposure promotes in vitro adipogenic differentiation but alters the adipocyte phenotype in rainbow trout. Aquatic toxicology (Amsterdam, Netherlands) 188, 148–158. [DOI] [PubMed] [Google Scholar]
- Mengeling BJ, Goodson ML, Furlow JD, 2018. RXR Ligands Modulate Thyroid Hormone Signaling Competence in Young Xenopus laevis Tadpoles. Endocrinology 159, 2576–2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JN, Wassenberg DM, Karchner SI, Hahn ME, Di Giulio RT, 2003. Expression and inducibility of aryl hydrocarbon receptor pathway genes in wild-caught killifish (Fundulus heteroclitus) with different contaminant-exposure histories. Environmental toxicology and chemistry / SETAC 22, 2337–2343. [DOI] [PubMed] [Google Scholar]
- Minchin JE, Rawls JF, 2011. In vivo analysis of white adipose tissue in zebrafish. Methods in cell biology 105, 63–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki Y, Glass L, Triplitt C, Matsuda M, Cusi K, Mahankali A, Mahankali S, Mandarino LJ, DeFronzo RA, 2001. Effect of rosiglitazone on glucose and non-esterified fatty acid metabolism in Type II diabetic patients. Diabetologia 44, 2210–2219. [DOI] [PubMed] [Google Scholar]
- Nacci DE, Champlin D, Coiro L, McKinney R, Jayaraman S, 2002. Predicting the occurrence of genetic adaptation to dioxinlike compounds in populations of the estuarine fish Fundulus heteroclitus. Environmental toxicology and chemistry / SETAC 21, 1525–1532. [PubMed] [Google Scholar]
- Nacci DE, Champlin D, Jayaraman S, 2010. Adaptation of the Estuarine Fish Fundulus heteroclitus (Atlantic Killifish) to Polychlorinated Biphenyls (PCBs). . Estuar Coasts 33, 853–864. [Google Scholar]
- O'Donnell PE, Ye XZ, DeChellis MA, Davis VM, Duan SZ, Mortensen RM, Milstone DS, 2016. Lipodystrophy, Diabetes and Normal Serum Insulin in PPARgamma-Deficient Neonatal Mice. PloS one 11, e0160636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleksiak MF, Karchner SI, Jenny MJ, Franks DG, Welch DB, Hahn ME, 2011. Transcriptomic assessment of resistance to effects of an aryl hydrocarbon receptor (AHR) agonist in embryos of Atlantic killifish (Fundulus heteroclitus) from a marine Superfund site. BMC genomics 12, 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterberg JS, Cammen KM, Schultz TF, Clark BW, Di Giulio RT, 2018. Genome-wide scan reveals signatures of selection related to pollution adaptation in non-model estuarine Atlantic killifish (Fundulus heteroclitus). Aquatic toxicology (Amsterdam, Netherlands) 200, 73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouadah-Boussouf N, Babin PJ, 2016. Pharmacological evaluation of the mechanisms involved in increased adiposity in zebrafish triggered by the environmental contaminant tributyltin. Toxicol Appl Pharmacol 294, 32–42. [DOI] [PubMed] [Google Scholar]
- Patel MR, Scheffler BE, Wang L, Willett KL, 2006. Effects of benzo(a)pyrene exposure on killifish (Fundulus heteroclitus) aromatase activities and mRNA. Aquatic toxicology (Amsterdam, Netherlands) 77, 267–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesch CE, Garber J, 2001. Historical analysis, a valuable tool in community-based environmental protection. Mar Pollut Bull 42, 339–349. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW, 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic acids research 29, e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proestou DA, Flight P, Champlin D, Nacci D, 2014. Targeted approach to identify genetic loci associated with evolved dioxin tolerance in Atlantic killifish (Fundulus heteroclitus). BMC Evol Biol 14, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid NM, Jackson CE, Gilbert D, Minx P, Montague MJ, Hampton TH, Helfrich LW, King BL, Nacci DE, Aluru N, Karchner SI, Colboume JK, Hahn ME, Shaw JR, Oleksiak MF, Crawford DL, Warren WC, Whitehead A, 2017. The landscape of extreme genomic variation in the highly adaptable Atlantic killifish. Genome Biol Evol 9, 659–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid NM, Proestou DA, Clark BW, Warren WC, Colbourne JK, Shaw JR, Karchner SI, Hahn ME, Nacci D, Oleksiak MF, Crawford DL, Whitehead A, 2016. The genomic landscape of rapid repeated evolutionary adaptation to toxic pollution in wild fish. Science 354, 1305–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riu A, Grimaldi M, le Maire A, Bey G, Phillips K, Boulahtouf A, Perdu E, Zalko D, Bourguet W, Balaguer P, 2011. Peroxisome proliferator-activated receptor gamma Is a target for halogenated analogs of bisphenol A. Environ Health Perspect 119, 1227–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Gurmaches J, Cruz-Garcia L, Gutierrez J, Navarro I, 2010. Endocrine control of oleic acid and glucose metabolism in rainbow trout (Oncorhynchus mykiss) muscle cells in culture. Am J Physiol Regul Integr Comp Physiol 299, R562–572. [DOI] [PubMed] [Google Scholar]
- Satone H, Lee JM, Oba Y, Kusakabe T, Akahoshi E, Miki S, Suzuki N, Sasayama Y, Nassef M, Shimasaki Y, Kawabata S, Honjo T, Oshima Y, 2011. Tributyltin-binding protein type 1, a lipocalin, prevents inhibition of osteoblastic activity by tributyltin in fish scales. Aquatic toxicology (Amsterdam, Netherlands) 103, 79–84. [DOI] [PubMed] [Google Scholar]
- Souder JP, Gorelick DA, 2019. ahr2, but not ahr1a or ahr1b, is required for craniofacial and fin development and TCDD-dependent cardiotoxicity in zebrafish. Toxicol Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Tabata MJ, Kambegawa A, Srivastav AK, Shimada A, Takeda H, Kobayashi M, Wada S, Katsumata T, Hattori A, 2006. Tributyltin inhibits osteoblastic activity and disrupts calcium metabolism through an increase in plasma calcium and calcitonin levels in teleosts. Life Sci 78, 2533–2541. [DOI] [PubMed] [Google Scholar]
- Tallafuss A, Hale LA, Yan YL, Dudley L, Eisen JS, Postlethwait JH, 2006. Characterization of retinoid-X receptor genes rxra, rxrba, rxrbb and rxrg during zebrafish development. Gene Expr Patterns 6, 556–565. [DOI] [PubMed] [Google Scholar]
- Tingaud-Sequeira A, Ouadah N, Babin PJ, 2011. Zebrafish obesogenic test: a tool for screening molecules that target adiposity. Journal of lipid research 52, 1765–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tontonoz P, Hu E, Graves RA, Budavari AI, Spiegelman BM, 1994. mPPARγ2: tissue-specific regulator of an adipocyte enhancer. Genes Dev. 8, 1224–1234. [DOI] [PubMed] [Google Scholar]
- Tsukamoto Y, Ishihara Y, Miyagawa-Tomita S, Hagiwara H, 2004. Inhibition of ossification in vivo and differentiation of osteoblasts in vitro by tributyltin. Biochem Pharmacol 68, 739–746. [DOI] [PubMed] [Google Scholar]
- van den Dungen MW, Murk AJ, Kok DE, Steegenga WT, 2017. Persistent organic pollutants alter DNA methylation during human adipocyte differentiation. Toxicol In Vitro 40, 79–87. [DOI] [PubMed] [Google Scholar]
- Wang RL, Bencic DC, Garcia-Reyero N, Perkins EJ, Villeneuve DL, Ankley GT, Biales AD, 2014. Natural Variation in Fish Transcriptomes: Comparative Analysis of the Fathead Minnow (Pimephales promelas) and Zebrafish (Danio rerio). PloS one 9, e114178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson AT, Planchart A, Mattingly CJ, Winkler C, Reif DM, Kullman SW, 2017. From the Cover: Embryonic Exposure to TCDD Impacts Osteogenesis of the Axial Skeleton in Japanese medaka, Oryzias latipes. Toxicol Sci 155, 485–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt J, Baker AH, Meeks B, Pajevic PD, Morgan EF, Gerstenfeld LC, Schlezinger JJ, 2018. Tributyltin induces distinct effects on cortical and trabecular bone in female C57Bl/6J mice. J Cell Physiol 233, 7007–7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt J, Schlezinger JJ, 2015. Structurally-diverse, PPARgamma-activating environmental toxicants induce adipogenesis and suppress osteogenesis in bone marrow mesenchymal stromal cells. Toxicology 331, 66–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman JS, Yelon D (2007) Comparison of the expression patterns of newly identified zebrafish retinoic acid and retinoid X receptors. Dev Dyn 236, 587–595. [DOI] [PubMed] [Google Scholar]
- Whitehead A, Pilcher W, Champlin D, Nacci D, 2012. Common mechanism underlies repeated evolution of extreme pollution tolerance. Proc. R. Soc. B 279, 427–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills LP, Matson CW, Landon CD, Di Giulio RT, 2010. Characterization of the recalcitrant CYP1 phenotype found in Atlantic killifish (Fundulus heteroclitus) inhabiting a Superfund site on the Elizabeth River, VA. Aquatic toxicology (Amsterdam, Netherlands) 99, 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanik SC, Baker AH, Mann KK, Schlezinger JJ, 2011. Organotins are potent activators of PPAR{gamma} and adipocyte differentiation in bone marrow multipotent mesenchymal stromal cells. Toxicol Sci 122, 476–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Zhang X, Yuan J, Cao Q, Liu J, Zhu P, Shi H, 2011. Teratogenic effects of triphenyltin on embryos of amphibian (Xenopus tropicalis): a phenotypic comparison with the retinoid X and retinoic acid receptor ligands. Journal of hazardous materials 192, 1860–1868. [DOI] [PubMed] [Google Scholar]
- Zhang J, Fu M, Cui T, Xiong C, Xu K, Zhong W, Xiao Y, Floyd D, Liang J, Li E, Song Q, Chen YE, 2004. Selective disruption of PPARgamma 2 impairs the development of adipose tissue and insulin sensitivity. Proc Natl Acad Sci U S A 101, 10703–10708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zuo Z, Sun P, Wang H, Yu A, Wang C, 2012. Tributyltin exposure results in craniofacial cartilage defects in rockfish (Sebastiscus marmoratus) embryos. Mar Environ Res 77, 6–11. [DOI] [PubMed] [Google Scholar]
- Zodrow JM, Tanguay RL, 2003. 2,3,7,8-tetrachlorodibenzo-p-dioxin inhibits zebrafish caudal fin regeneration. Toxicol Sci 76, 151–161. [DOI] [PubMed] [Google Scholar]