Abstract
Tetrabromobisphenol A (TBBPA) has become a ubiquitous indoor contaminant due to its widespread use as an additive flame retardant in consumer products. Reported evidence of endocrine disruption and accumulation of TBBPA in brain tissue has raised concerns regarding its potential effects on neurodevelopment and behavior. The goal of the present study was to examine the impact of developmental TBBPA exposure, across a wide range of doses, on sexually dimorphic non-reproductive behaviors in male and female Wistar rats. We first ran a pilot study using a single TBBPA dose hypothesized to produce behavioral effects. Wistar rat dams were orally exposed using cookie treats to 0 or 0.1 mg TBBPA/kg bw daily from gestational day (GD) 9 to postnatal day (PND) 21 to assess offspring (both sexes) activity and anxiety-related behaviors. Significant effects were evident in females, with exposure increasing activity levels. Thus, this dose was used as the lowest TBBPA dose in a subsequent, larger study conducted as part of a comprehensive assessment of TBBPA toxicity. Animals were exposed to 0, 0.1, 25, or 250 mg TBBPA/kg bw daily by oral gavage starting on GD 6 through PND 90 (dosed dams GD 6 – PND 21, dosed offspring PND 22 – PND 90). Significant behavioral findings were observed for male offspring, with increased anxiety-like behavior as the primary phenotype. These findings demonstrate that exposure to environmental contaminants, like TBBPA, can have sex-specific effects on behavior highlighting the vulnerability of the developing brain.
Keywords: brain, anxiety, endocrine disruptions, sex differences, hyperactivity, neural, sexually dimorphic, neurotoxic, flame retardants, elevated plus maze, neurodevelopment
1. Introduction
Chemical flame retardants (FRs) are extensively used in a wide variety of consumer products including foam-based furniture and infant products, textiles, and electronics. Broad application has resulted in widespread human exposure (de Wit et al., 2010; Stapleton et al., 2009), raising concerns regarding the possible toxicity of these chemicals, particularly in the developing brain (Grandjean and Landrigan, 2014; Kalkbrenner et al., 2014; Landrigan et al., 2012; Masuo and Ishido, 2011; Messer, 2010; Miodovnik et al., 2011).
Tetrabromobisphenol A (TBBPA) is one of the most prevalent brominated FRs (BFR), is produced at high volumes, is the highest use BFR worldwide (Canada et al., 2012; de Wit et al., 2010; Sanders et al., 2016; Usenko et al., 2016), and has become a ubiquitous environmental contaminant (Liu et al., 2016). TBBPA is used primarily as a reactive FR in electronics such as computer boards, mobile phones, appliances, and televisions (Lilienthal et al., 2008). When used as a reactive FR, TBBPA is chemically bound to the material, minimizing its ability to migrate out of products over time. The phase out of other BFRs, especially the polybrominated diphenyl ethers (PBDEs), has led to increased use of TBBPA as an additive FR (de Wit et al., 2010), allowing it to more easily enter the environment and thereby increasing the likelihood of human exposure (Canada et al., 2012; de WLiu et al., 2016). TBBPA is used primarily as a reactive FR in electronics such as computer boards, mobile phones, appliances, and televisions (Lilienthal et al., 2008). When used as a reactive FR, TBBPA is chemically bound to the material, minimizing its ability to migrate out of products over time. The phase out of other BFRs, especially the polybrominated diphenyl ethers (PBDEs), has led to increased use of TBBPA as an additive FR (de Wit et al., 2010), allowing it to more easily enter the environment and thereby increasing the likelihood of human exposure (Canada et al., 2012; de Wit et al., 2010). TBBPA has been detected in environmental samples including air, soil, water, dust, sewage sludge, and aquatic organisms (Lilienthal et al., 2008; Liu et al., 2016; Matsukami et al., 2015; Morris et al., 2004; Ni and Zeng, 2013; Wang et al., 2015; Yang et al., 2012) in the United States and globally, demonstrating widespread use and environmental contamination. Humans are primarily exposed through a combination of ingestion, inhalation, and dermal contact with contaminated media, such as dust, or consumer products containing TBBPA (Liu et al., 2016). Detectable levels of TBBPA have been found in human serum, including maternal and cord blood serum, as well as breast milk (Abdallah and Harrad, 2011; Antignac et al., 2008; Carignan et al., 2012; Cariou et al., 2008; Liu et al., 2016; Shi et al., 2013, 2009) indicating the likelihood of fetal exposure, and newborn exposure through breast-feeding. A study of Chinese women, for example, found that TBBPA levels in human milk are about 1.21 ng/g lipid, which translates to a mean daily intake of 39.2 ng/kg body weight for a 1–6 month old nursing infant (Shi et al., 2017). Some of the highest levels ever observed in humans were obtained from cord blood serum from French newborns and ranged from 103.5 +/− 149.7 ng/g lipid (Cariou et al., 2008). In adults, TBBPA has low systemic bioavailability because, although it is rapidly absorbed from the gastrointestinal tract, it is efficiently metabolized in the liver to conjugated products, primarily TBBPA-glucuronide and TBBPA-sulfate (Schauer et al., 2006). This may not be the case for infants or fetuses because their gastrointestinal system has not fully developed (Veereman-Wauters, 1996) and hepatic competency for sulfurylation (Dawson, 2011) and glucuronidation (Coughtrie, 2015; Coughtrie et al., 1988) may be inadequate to provide protection from toxicants like TBBPA.
TBBPA is known to be hazardous to a variety of aquatic organisms, including fish and bivalves (Hu et al., 2015a, b), even at concentrations below the no observed adverse effect level (NOAEL) of 40 mg/kg bw/day for nephrotoxicity in newborn rats (Fukuda et al., 2004). Endocrine disrupting and adverse effects on survival, reproduction and development in sentinel species suggest the potential for human risk. TBBPA has been shown to be endocrine disrupting on estrogen, androgen, and thyroid signaling pathways, and a reproductive toxicant with the potential for significant effects on brain development and behavior. There is accumulating evidence demonstrating TBBPA can interact with and alter estrogen homeostasis. TBBPA has been shown to inhibit estradiol sulfurylation in vitro (Hamers et al., 2006), and crystallographic studies demonstrate that it can bind to estrogen sulfotransferase SULT1E1 with high affinity (Gosavi et al., 2013). TBBPA can also stimulate ovarian cancer cell proliferation through the G protein-coupled estrogen receptor 30 (GPR30) pathway (Hoffmann et al., 2017). A two-year oral exposure assessment conducted by the National Toxicology Program (NTP) reported an increased incidence of epithelial atypical hyperplasia in uterine tissue of Wistar Han rats exposed to 250 mg/kg/ day and tumor formation in the uterus at higher doses (NTP et al., 2014; Sanders et al., 2016). A follow-up study provided evidence of altered estrogen signaling, biosynthesis, and metabolism in the liver and uterus of rats exposed to 250 mg/kg/ day TBBPA (Sanders et al., 2016). Because sex steroids are critical for many aspects of brain organization, especially sexual differentiation (McCarthy et al., 2009; Simerly, 2002), these data provide evidence that neurodevelopment may be impacted by TBBPA exposure.
Of greatest significance for potential neurotoxicity, multiple studies have demonstrated that thyroid hormone signaling may be particularly susceptible to TBBPA exposure. TBBPA competes with thyroxine (T4) for transport by transthyretin (TTR), inhibits receptor binding of triiodothyronine (T3), and alters the expression of thyroid hormone receptors (TR) in vitro (Hamers et al., 2006; Kitamura et al., 2002; Meerts et al., 2000). TBBPA has also been shown to alter serum T4 and the expression of TR in vivo (Sanders et al., 2016). Disruption of thyroid hormone signaling is well known to result in adverse neurodevelopmental outcomes including cognitive deficits (Moog et al., 2017). Evidence of TBBPA developmental neurotoxicity in rodent models includes abnormal development of the dentate hilus, including greater numbers of reelin-expressing interneurons during post-natal development and excess neurons in adulthood (Saegusa et al., 2012). Additional studies have indicated that TBBPA can also impact aspects of learning and memory, motor activity (Nakajima et al., 2009), and auditory response (Lilienthal et al., 2008). Acute treatment of male mice with 0.1 and 5 mg/kg TBBPA altered memory-related behaviors in the contextual fear conditioning test and Y-maze as well as motor activity in the open field (Lilienthal et al., 2008). A study using Wistar rats showed that parental exposure to TBBPA throughout mating, gestation, and lactation altered auditory responses in both male and female offspring, with neuronal effects in males and cochlear effects in females (Lilienthal et al., 2008). A landmark study in male mice found that TBBPA can accumulate in the striatum at doses as low as 0.1 mg/kg bw, suggesting the potential for region-specific exposure and impacts on striatal-dependent behaviors (Nakajima et al., 2009). A principal component of the basal ganglia, the striatum has a variety of subdivisions and functions, one of which is facilitation of voluntary movement. In rodents and zebrafish, acute and developmental TBBPA exposure has been shown to adversely impact these types of behaviors including exploratory and anxiety-related behaviors (Chen et al., 2016a, b; Kim et al., 2015). There remains a paucity of data, however, regarding 1) behavioral effects following developmental exposure to more human-relevant doses, and 2) studies exploring effects by sex. Thus, the goal of the present study was to examine the impact of developmental TBBPA exposure, across a wide dose range, on sexually dimorphic non-reproductive behaviors in rats with the hypothesis that exploratory behaviors would be adversely impacted in one or both sexes. These studies were conducted as part of a broader investigation to comprehensively assess the toxicity of TBBPA across a range of tissues.
For this study we utilized a battery of behavioral tests, as we have done previously (Baldwin et al., 2017; Rebuli et al., 2015; Witchey et al., 2019) that involve voluntary movement, and thus striatal input, to assess if TBBPA can impact sexually dimorphic non-reproductive behaviors in the Wistar rat. We first ran a pilot study with a single low dose of TBBPA (~0.1 mg/kg bw) as a proof of concept and a test study to identify the lowest effective dose to be used for a second, larger study. This single dose was selected because it was the lowest dose found to accumulate in mouse striatum and induce motor effects in at least one sex (Nakajima et al., 2009). The second study was then performed as part of a larger, more comprehensive assessment of TBBPA toxicity at the NTP/ NIEHS (National Institute of Environmental Health Sciences) in partnership with NCI (National Cancer Institute) and NCSU (North Carolina State University) investigators. For this more comprehensive study, doses ranged from 0, 0.1, 25, and 250 mg/kg bw per day and contained larger sample sizes in each group. The highest dose was selected because it was found to produce adverse outcomes in the 2 year NTP carcinogenesis bioassay and a subsequent, related study (NTP et al., 2014; Sanders et al., 2016). For both studies herein, all analyses were run with sex as a biological variable to establish if there were any exposure by sex interactions.
2. Methods
The ARRIVE (Animal Research: Reporting of In Vivo Experiments) Guidelines Checklist for Reporting Animal Research was used in the construction of this manuscript with all elements met (Kilkenny et al., 2010). The ARRIVE guidelines were developed in consultation with the scientific community as part of an NC3Rs (National Centre for the Replacement Refinement and Reduction of Animals in Research) initiative to improve the standard of reporting of research using animals.
2.1. Experiment 1: single dose pilot behavior study at NCSU
2.1.1. Animals
Animal care, maintenance, and experimental protocols met the standards of the Animal Welfare Act and the U.S. Department of Health and Human Services “Guide for the Care and use of Laboratory Animals” and were approved by the NCSU Institutional Animal Care and Use Committee (IACUC). A supervising veterinarian approved and monitored all procedures throughout the duration of the project. An in house colony was established from Wistar Han rats obtained from Charles River (Raleigh, NC) and bred in house as described below in humidity-and-temperature controlled rooms, each with reversed 12 h:12 h light, dark cycles (lights off at 9am) at 25 °C and 45–60% average humidity at the Biological Resource Facility at NCSU. Food and water were provided ad libitum. As in our prior studies (Patisaul et al., 2012, 2009), and in accordance with recommended practices for endocrine disrupting chemical (EDC) research (Hunt et al., 2009; Li et al., 2008; Patisaul et al., 2013; Richter et al., 2007), all animals were housed in conditions specifically designed to minimize unintended EDC exposure including use of glass water bottles, soy-free diet (Teklad 2020, Envigo Laboratories, Madison, WI), woodchip bedding and thoroughly washed polysulfone caging.
2.1.2. Dose preparation
A concentrated 10× Tetrabromobisphenol A (TBBPA; CAS# 79–94-7, 97% purity Fluka Analytical; St. Louis MO) stock was made in 100% ethanol, stirred for 6 h, and stored in amber vials at 4 °C until use. All dosing solutions were then made from aliquots of this concentrated stock by diluting them to the appropriate amount of TBBPA (30 μg) in 100% ethanol, stirred for 6 h, then stored in amber bottles for dosing. Each dosing solution (ethanol vehicle; 30 μg TBBPA) was prepared and coded in the Patisaul lab such that oral dosing and subsequent behavioral testing was performed blinded to exposure group.
2.1.3. Animal husbandry and exposure
Study animals were obtained from a cohort bred at NCSU. Adult Wistar rats (n= 41 females and 26 males) were paired and gestational day (GD) 0 was defined by the observation of a sperm plug. Of the 41 females paired 7 failed to conceive and were therefore excluded. The males were then removed and the dams housed individually. Assignment of plug-positive females to exposure groups was random, with a total of 8 litters in the control group and 5 litters in the TBBPA group. The remaining pregnant dams were used for another project (Baldwin et al., 2017).
Exposure was daily from GD 9 through postnatal day (PND) 21. Prior to dosing dams were given ¼ of a soy-free, highly palatable food treat pellet (chocolate flavored AIN-76A Rodent Diet Test Tabs, Test Diet, Richmond, IN) to habituate them to the treats. At the time of dosing, 20 μl of the vehicle (100% ethanol) or 20 μl containing 30 μg TBBPA in solution was pipetted onto ¼ of a soy-free food treat pellet, as previously reported (Patisaul et al., 2013). Consumption was monitored to ensure the dam ate the entire treat. This route of exposure was selected to minimize handling stress (which can happen with injection or gavage) (Cao et al., 2013; Patisaul et al., 2013). Dams were dosed by average weight of the females before they were impregnated (300 g), rather than by individual daily weight, to produce exposures of 0 mg/kg bw per day (vehicle controls) and approximately 0.1 mg/kg bw per day TBBPA. Litters were standardized to 10 pups (5:5 sex ratio whenever possible) on PND 1. On PND 21 pups were weaned and distributed into same sex and exposure groups (2, occasionally 3, littermates per cage) and housed in the same conditions as the dams until behavior testing (described below).
2.1.4. Behavior testing
Animals were assessed for general activity and anxiety-related behavior as adults using three validated testing paradigms (Table 1) (Beatty, 1979; Hogg, 1996; Misslin et al., 1989). Performance is sexually dimorphic in all three (Donner and Lowry, 2013), thus sex was included as a biological variable. Animals were handled via procedures that minimize husbandry and transport stress. For all tests except the running wheel (which is conducted in the home cage), animals were transported down a short hallway to a nearby testing room on a covered rolling cart and tested after a short acclimation period, as we have done previously (Baldwin et al., 2017; Hicks et al., 2016; Patisaul et al., 2013, 2012). Testing was conducted within the first 4 h of the dark cycle (between 10 AM and 2 PM), recorded using a video camera suspended over the apparatuses, and subsequently analyzed using TopScan software (Clever Sys Inc., Reston, VA) by observers blinded to exposure group. Video scoring was validated by hand by a second blinded observer using the program Stopwatch (courtesy of David A. Brown, Center for Behavioral Neuroscience, Emory University) as described previously (Hicks et al., 2016). Vaginal cytology was performed daily beginning two weeks before onset of behavioral testing to ensure that estrous cycles were normal/regular and to ensure all females were tested in the same stage of the estrous cycle, as described below: diestrus for the light/dark (L/D) box and estrus for the elevated plus maze (EPM) because that is the phase at which the sex difference in EPM activity is most pronounced (Becker et al., 2005; Patisaul et al., 2005). Fewer animals were available for L/D box testing as adults because a subset of animals were tested as juveniles for a project that was discontinued. Prior exposure to the arena as juveniles could influence their behavior if tested again as adults.
Table 1.
Summary of Experimental Design and Behavioral Tasks Utilized in the Pilot Study (Experiment 1) and Multi-Dose Study (Experiment 2)
| Doses mg/kg/day | Method of Dosing | Duration of Dosing | OF | L/D Box | EPM | Running Wheel | |
|---|---|---|---|---|---|---|---|
| Experiment 1 | 0.1 | Food Treat | GD 9 - PND 21 (Dam) | Not Included | PND 110 – 120 | PND 185 – 192 | PND 160 – 170 |
| NCSU | Ethanol Vehicle | (10-min test) | (5-min test) | (63 hours) | |||
| Experiment 2 | 0.1 | Gavage | GD 9 – PND 21 (Dam) | PND 90 & | PND 150 – 155 | PND 155 – 160 | Not Included |
| NTP/NIEHS | 25 | Sesame Oil Vehicle | PND 22 – 90 (Offspring) | PND 145 – 150 | (5-min test) | (5-min test) | |
| 250 | (30-min test) |
Adult (PND 110–120) offspring were tested first in the L/D box (control: 8♀, 14♂, TBBPA: 9♀, 5♂) and then the EPM (PND 185–192; control: 15♀, 19♂, TBBPA: 15♀, 10♂) for 10 and 5 min, respectively. L/D endpoints assessed light side exploration including latency to enter, number of entries, and time spent in the light side, which was illuminated via two 40-W clip lamps placed above the apparatus. All females were tested in diestrus for L/D endpoints.
EPM endpoints included number of open arm entries, duration in open arms, and duration in closed arms to evaluate exploratory and anxiety-like behavior. Testing was conducted under red light because it maximizes exploration and minimizes stress (Nasello et al., 1998; Pellow et al., 1985). Seven animals either lost footing or fell off the maze (3 control females and 4 control males), and thus excluded from the analyses. Performance in the EPM is sexually dimorphic with females more exploratory and less anxious, particularly in estrus (Nasello et al., 1998; Pellow et al., 1985), thus all females were tested in estrus (Patisaul et al., 2005).
Adult offspring (PND 160–170; control: 17♀, 21♂, TBBPA: 15♀, 10♂) were also tested for novelty response and activity by placing a running wheel in their home cage starting at 14:00. VitalView software (STARR Life Sciences Corp., Oakmont, PA) was used to record wheel revolutions over the course of 63 h for each cage. Once data collection was finished, revolutions over the three days were binned into 1 -h intervals for analysis. Dark phase, day 1, behavior was considered indicative of response to a novel stimulus while dark phase behavior on days 2 and 3 was considered indicative of general activity. Because the task spanned three days, females were not standardized for estrous cycle stage.
2.2. Experiment 2: multi-dose behavior study at NTP/NIEHS
2.2.1. Dose preparation
TBBPA was obtained from Sigma-Aldrich (CAS# 79–94-7, 97% purity, lot #MKBG4059 V). Stock solutions were prepared by dissolving TBBPA in ethanol (Warner-Graham Company, Cockeysville, MD) and adding appropriate volumes to known volumes of refined sesame seed oil (Jedwards International, Inc, Braintree, MA). After mixing, ethanol was removed under a stream of nitrogen with gentle stirring in a fume hood. Individual doses were made weekly and kept at room temperature in color-coded glass vials. Oral dosing and subsequent testing were performed in a blinded fashion to minimize bias.
2.2.2. Animals
All animals were treated humanely and in accordance with the approved protocols of the NIEHS Animal Care and Use Committee specific to this study. Time pregnant Wistar Han rat dams were ordered from Charles River, Raleigh, NC [Crl: WI(Han)], in which breeding occurred in the evening and females with observed copulatory plugs were indicated as pregnant and considered gestational day 0.5 (GD 0). Animals were received on GD 4 and acclimated for 2 days prior to dosing. They were fed a low phytoestrogen diet (Teklad 2919, Envigo Laboratories, Madison, WI). Pregnant dams were singly housed in polysulfone cages with Sani-Chip bedding (PJ Murphy Forest Products, Montville, NJ) and Enviro-Dri enrichment (Shepherd Specialty Papers, Watertown, TN), on ventilated racks. Animals were housed in humidity-and-temperature controlled rooms, 25 °C and 45–60% average humidity, with standard 12 h light cycles (not reversed). Food and RO/DI water were provided ad libitum.
2.2.3. Animal husbandry and exposure
Time pregnant adult Wistar rats were delivered to NIEHS on GD 4 in two blocks of 55 dams 2 weeks apart to avoid any seasonal differences in breeding outcomes. For this number of potential litters, two animal rooms were necessary so that all animals could be necropsied and observed at the same times post-lights on; rooms were maintained on staggered lights-on schedules of 7:00 am and 11:00 am for the studies described herein. Animals were acclimated to the light cycle for two days prior to dosing. All dams were weighed upon arrival and assigned to one of four specific dose groups and a room number by blocked randomization by body weight. Even representation of dose groups was housed in each room to account for identical experimental conditions. Average group body weights were compared across groups in both rooms in order to make them as consistent as possible. For allocation concealment, a random generator was then used to randomly assign groups to a TBBPA dose (each dose given a unique color code).
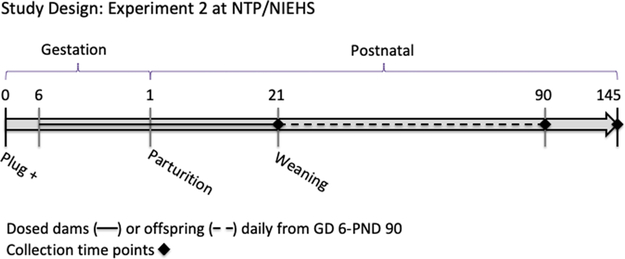
Of the 110 females, 10 were not pregnant, 8 had litters of 4 or less, 4 gave birth on GD 22, and 2 dams had complications during parturition, resulting in no viable litter. These 24 dams and any resulting offspring were humanely euthanized and not used in this study, leaving an n = 20–24 litters per dose group. TBBPA was administered orally via gavage to rats in a volume of 5 ml/kg bw to deliver 0, 0.1, 25, and 250 mg/kg bw/day. Doses were selected based on effective TBBPA doses used in previous studies conducted by the NTP (NTP et al., 2014) and the pilot behavior study conducted at NCSU (above; 0.1 mg/kg bw/d). Administration using food treats rather than gavage was tested as part of a pilot study prior to Experiment 2; however, this was not feasible due to issues with palatability at the highest dose (TBBPA 250 mg/kg) and the requirement that the treats be autoclaved. Dams were dosed daily within one hour of lights on, from GD 6 through PND 21, and pups were gavage dosed beginning PND 22 (Fig. 1), following a 90 day guideline protocol (OECD, 2018). Dams were weighed daily, and doses were adjusted based on recorded weights. All 4 TBBPA dose groups were represented in both rooms.
Fig. 1.
Study Design for Experiment 2: Developmental exposure study in Wistar Han rats. Timeline includes tissue collection time points and information regarding the dosing paradigm for the study conducted at the NIEHS. Dams were directly exposed during gestation and lactation (GD 6 - PND 21), as represented by the solid black line, while offspring were directly exposed from PND 21 – PND 90, dotted black line.
The day after birth, which occurred during the evening of GD 21, was designated PND 1. Litters were standardized to 8 (4:4 sex ratio whenever possible) on PND 4. To ensure standard litter sizes when dams had less than 8 pups, some litters included foster pups from the same dose group, which were tattooed, euthanized after weaning, and not used for any endpoints in this study. On PND 21, pups were weaned and distributed into same sex and exposure groups (2, occasionally 3, littermates per cage) and housed in the same conditions as the dams. Offspring were weighed daily until all animals of the same sex had gone through puberty. Thereafter, body weights were recorded 3 times a week. Daily doses to offspring were adjusted based on recorded weights. The overall study design for Experiment 2 is shown in Fig. 1.
2.2.4. Behavior testing
The behavioral tasks used in Experiment 2 are summarized in Table 1. Two of each behavioral apparatus, OF, EPM, and LD box arenas, were purchased from Stoelting Company (Wood Dale, IL). The EPM (Product # 60240) stood 50 cm off the ground with arms 50 cm long and 10 cm wide, two of which had 40 cm tall walls (closed arms). The LD box (Product # 63101) contained two chambers (one clear and one black) that were 40 cm wide, 40 cm long, and 35 cm high. The OF arena (Product # 60201) arrived as one large 100 × 100 cm box with a divider that yielded 4–50 × 50 cm boxes. Two animals were tested at a time in the OF arena in diagonal corners so that no animals shared a wall during testing. All lighting equipment, cameras, and video scoring equipment was the same as that used for Experiment 1, and transported from NCSU to NIEHS for these experiments.
Adult offspring were assessed, during the first 4 h of their dark cycle (between 7 and 11 pm for the first cohort and 11 pm and 3am for the second), for general activity and anxiety-related behavior similar to that described for Experiment 1. Animals were briefly transported to a nearby testing room on a covered rolling cart and tested after a short acclimation period. Vaginal cytology was initiated two weeks prior to behavioral testing to acclimate the animals to the process as well as ensure they were cycling normally. Animals were staged in order to ensure that they were all tested in the same stage of the estrous cycle: estrus for the EPM because that is the phase at which the sex difference in maze activity is most pronounced (Patisaul et al., 2005), and diestrus for the OF and L/D box.
Task order was the same for all animals and OF was run first. PND 90 was an important time point at which some of the animals were scheduled for euthanasia and tissue collection, thus this subset of animals was tested in the OF just prior to PND 90 (control: 7♀, 9♂, TBBPA 0.1: 11♀, 12♂, TBBPA 25: 10♀, 8♂, TBBPA 250: 9♀, 11♂). The following number of litters were represented in each exposure group according to sex: (control: 7♀, 9♂, TBBPA 0.1: 11♀, 11♂, TBBPA 25: 8♀, 8♂, TBBPA 250: 9♀, 11♂). Three females escaped from their OF arena, and two of the three escapees ended up in the same arena as another female test animal, thus all five animals were excluded.
All remaining animals were tested at PND 145–160 with at least a week between tasks. To help facilitate testing, the animals were adapted to reverse light. That process began on approximately PND 93 and the animals were shifted an hour a day for 10 days. Ultimately both testing cohorts were housed with 12:30am lights on and 12:30pm lights off. Test order was OF (control: 20♀, 10♂, TBBPA 0.1: 17♀, 12♂, TBBPA 25: 24♀, 9♂, TBBPA 250: 21♀, 13♂), followed by EPM (control: 22♀, 11♂, TBBPA 0.1: 19♀, 15♂, TBBPA 25: 24♀, 10♂, TBBPA 250: 23♀, 13♂), and L/D box (control: 21♀, 11♂, TBBPA 0.1: 19♀, 15♂, TBBPA 25: 24♀, 10♂, TBBPA 250: 22♀, 13♂). To maximize the number of animals tested at PND 90 some animals that were not scheduled to be euthanized at that time point were tested in the OF. These animals were thus not available for OF testing on PND 145 but were tested in the EPM and L/D box. For the OF test litter number by exposure group and sex were: (control: 13♀, 10♂, TBBPA 0.1: 13♀, 11♂, TBBPA 25: 18♀, 9♂, TBBPA 250: 13♀, 12). Two L/D box recordings (one control female and one high dose female) were unable to be scored due to technical issues. L/D litter numbers by exposure group and sex were: (control: 14♀, 11♂, TBBPA 0.1: 14♀, 13♂, TBBPA 25: 18♀, 8♂, TBBPA 250: 15♀, 12♂) and EPM litter numbers by exposure group and sex were: (control: 14♀, 11♂, TBBPA 0.1: 14♀, 13♂, TBBPA 25: 16♀, 8♂, TBBPA 250: 15♀, 12♂). Because some animals were observed to “freeze” on the open arms of the EPM within the first minute of the task, this was included as an additional endpoint and interpreted as an anxiety-like behavior.
2.2.5. Statistical analysis for all behavioral experiments
The statistical approach was designed in accordance with published guidelines for low dose endocrine disrupting chemical studies with equivalent sample sizes (Haseman et al., 2001) and similarly designed studies conducted by our lab (Baldwin et al., 2017; Rebuli et al., 2015). Potential litter effects were accounted for either in the experimental design (by testing only one animal per sex per group) or in the statistical analysis (by including litter as a co-variate). Statistical analysis was performed using Graphpad Prism version 8 (La Jolla, CA). Statistical significance was set at α ≤ 0.05.
The behavioral data were first approached by comparing the unexposed males and females using a student’s one-tailed t-test, to check for known sex differences, as we have done previously for similar studies (Rebuli et al., 2015; Witchey et al., 2019). Detection of expected sex differences was considered validation that the testing paradigm worked properly and was sufficiently robust to detect well-established sex differences. These sex differences were consistently found for animals tested in Experiment 1 (NCSU), but not always in Experiment 2 (NIEHS); possible reasons for which are explored in the discussion section.
The data for all groups were then evaluated within sex by one-way ANCOVA with exposure as the factor and litter as the co-variate. Only two endpoints were found to be significantly impacted by litter: number of entries made into the light side of the L/D box in Experiment 1 (F(1, 30) = 5.95, p ≤ 0.02) and duration in the light side of the L/D box in Experiment 2 (F(3, 126) = 3.94, p ≤ 0.05). Neither of these endpoints showed any significant effects of exposure, thus potential impact of litter was concluded to be negligible for both Experiments 1 and 2.
Animals that escaped (Experiment 2 OF PND 90) or slipped/fell off (Experiment 1 EPM) of an arena were excluded from statistical analyses, as described above. Prior to assessment of main effects, outliers (no more than one per group per endpoint; 1 from running wheel in Experiment 1, 4 from PND 145 OF in Experiment 2, 4 from LD in Experiment 2, and one from EPM in Experiment 2) were identified using Grubb’s test and removed. Significant main effects identified by one-way ANOVA were followed up with Fisher’s protected LSD as the post-hoc test. While the Fisher’s protected LSD does not provide strong family-wise error control compared with other post-hoc procedures, it was selected over more conservative options to minimize risk of Type-II error (rejecting a meaningful effect) and to be consistent with similar, prior studies (Rebuli et al., 2015). Several endpoints appeared to have a dose response even though the ANOVA did not reveal a significant effect of dose. In these situations, a Brown-Forsyth test was conducted to confirm equality of group variances, and then a test for linear trend was conducted as the post-hoc analysis. For Experiment 2, freezing behavior was analyzed via a chi-square test to assess the overall impact of exposure, and Fisher’s exact test to compare each group to the same sex controls.
Over the course of a long test, such as OF, behavior changes with experience and habituation (Bailey and Crawley, 2009; Goma and Tobena, 1978; Gould et al., 2010; Rebuli et al., 2015). Because the first 5-min of this test is when the experience is most novel for the animal, this interval provides the most salient information about anxiety-related behaviors. As the test animal habituates, it becomes less active in the arena and typically reaches a steady state by the final 5-min of this task. As a result, activity towards the end is thought to be more representative of general activity (Gould et al., 2010; Rebuli et al., 2015). Thus, OF data was analyzed using three approaches: (1) for each endpoint the data were totaled over the full 30 min testing period and compared (within sex); (2) distance traveled was analyzed by binning the data into 5-min intervals and a repeated measures ANOVA was run to assess the impact over time; and (3) area under the curve (AUC) was calculated for distance traveled in order to evaluate overall activity over the 30-min test (within sex).
Activity wheel data was binned into 1 -h intervals over the course of the dark cycle in which animals had wheel access (8 h on day 1 and 12 h on days 2 and 3). AUC was calculated for these 8- and 12 -h periods, during which we expected the animals to be most active, in order to assess sex and exposure related effects. For each light phase, AUC was calculated to assess overall activity during that period (when the animals are normally asleep). As with the other behaviors, expected sexually dimorphic responses between female and male controls were verified using a student’s one-tailed t-test. Additionally, within the unexposed controls, sex differences were compared for each 1 -h interval during the first 8 h of activity using two-way repeated measures ANOVA (sex and time as factors) and Fisher’s protected LSD The exposure group data were then analyzed within sex as described above using ANCOVA and Fisher’s protected LSD.
For all behavioral measures, effect size was also calculated using standardized effect size measurements as recommended by many behavioral neuroscience groups including The American Psychological Association (Fritz et al., 2012; Lakens, 2013). For ANOVA and ANCOVA, effect size was determined by calculating an Eta squared (η2), effects of which are defined as small at 0.01, medium at 0.06, and large at 0.14. In cases where a main effect did not reach statistical significance, but effect size was large or medium, an unprotected LSD was performed to further characterize group differences. Protected and unprotected LSD effect size was calculated by Cohen’s d, which characterizes effect size based on standard deviation. Effects are defined as small at d ≥ 0.2 (0.2 standard deviations or greater), medium at d ≥ 0.5, and large at ≥ 0.81 (Lakens, 2013).
3. Results
Differences in study design between Experiments 1 and 2 included aspects of animal handling and exposure, choice of behavioral tasks, and age at testing (Table 1). These differences limited direct comparisons between the two experiments, but comparisons were made when appropriate. Additionally, because it was conducted first, findings from Experiment 1 helped inform decisions about dosing and behavioral tasks to include in Experiment 2.
3.1. Experiment 1: single dose pilot behavior study at NCSU
No effect of TBBPA 0.1 exposure was found for either sex in the L/D box or EPM tasks (Table 2). However, expected sex differences in the unexposed controls were observed (Baldwin et al., 2017) for multiple endpoints including number of entries and time spent in the light compartment (p ≤ 0.009; d = 1.16; p ≤ 0.02; d = 0.93) as well as number of entries in the open arms, time spent in the open arms, and time spent in the closed arms of the EPM (p ≤ 0.04; d = 0.59; p ≤ 0.002; d = 1.06; p ≤ 0.03; d = 0.71; Table 2).
Table 2.
Summary of L/D Box and EPM Results from Experiment 1: Single Dose Pilot Behavior Study at NCSU
| Task | Endpoint | Effect of Exposure: Females | Effect of Exposure: Males | Effect of Sex |
|---|---|---|---|---|
| L/D Box | # of Entries in Light Box | 0.54 | 0.85 | 0.009 F > M |
| Duration in Light Box | 0.59 | 0.32 | 0.02 F > M | |
| Latency to Enter Light Box | 0.37 | 0.45 | 0.11 | |
| EPM | # of Open Arm Entries | 0.48 | 0.17 | 0.04 F > M |
| Time in Open Arms | 0.49 | 0.23 | 0.002 F > M | |
| Time in Closed Arms | 0.47 | 0.71 | 0.03 M > F |
All P values < 0.05 are bolded. F = Female, M = Male.
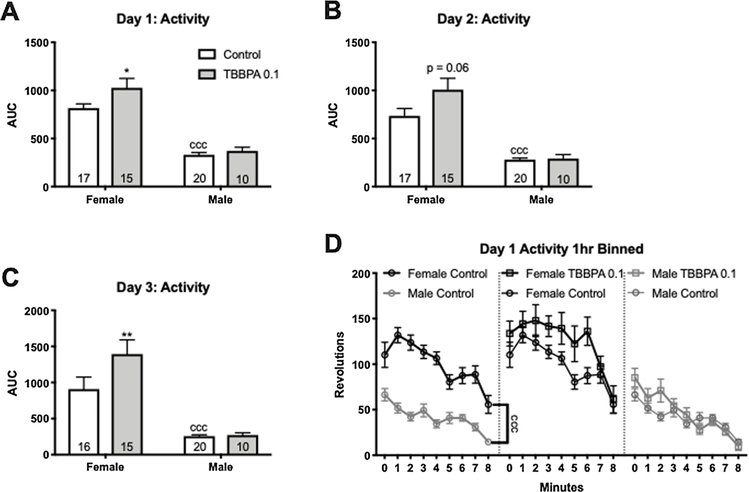
During the three days of wheel activity evaluation, there was a large sex difference in activity, with control females running significantly more than control males (p < 0.0001; d = 3.36, 2.1, and 2.1 for days 1–3 respectively; Fig. 2 A–D). No main effect of TBBPA 0.1 was found on any of the three days in male offspring. There was, however, a significant effect of exposure on female activity. TBBPA exposed females had significantly higher activity levels than control females on days 1 and 3 (p ≤ 0.05; d = 0.71; p ≤ 0.006; d = 1.05; Fig. 2A and C) and a trend for higher activity on day 2 (p = 0.06; d = 0.69; Fig. 2B). Day one activity wheel data was also binned into 1 h intervals and, as expected, control females were significantly more active than control males over the entire 8 h period (F (1, 36) = 94.76, p ≤ 0.0001; η2 = 0.41; Fig. 2D). As for the pattern of activity, running decreased over time in all groups on the first day (Fig. 2D). Throughout the task, activity during the light phase was minimal and no group differences were observed, indicating no significant impacts of TBBPA on sleep/wake cycles. Females were not staged for cycle, which likely increased inter-individual variability because females are more active in proestrous, but this variability did not meaningfully impact the overall outcome (Rodier, 1971; Steiner et al., 1981).
Fig. 2.
Effects of perinatal TBBPA exposure on running wheel activity in PND 160–170 offspring from Experiment 1. A significant difference between male and female controls was observed for all three days of wheel running (A–C; ccc p ≤ 0.001). TBBPA 0.1 (0.1 mg/kg bw/d) female activity levels were significantly increased on the first and third day of testing with activity on the second day elevated but not quite statistically significant (A and B). In males, there was no significant effect of exposure on any of the three days. Activity levels within the first 8 hs the wheels were available decreased with time, as expected (D). White bars are control groups and light gray bars are TBBPA 0.1. Graphs depict mean ± SEM (* p ≤ 0.05; ** p ≤0.01).
3.2. Experiment 2: multi-dose behavior study at NTP/NIEHS
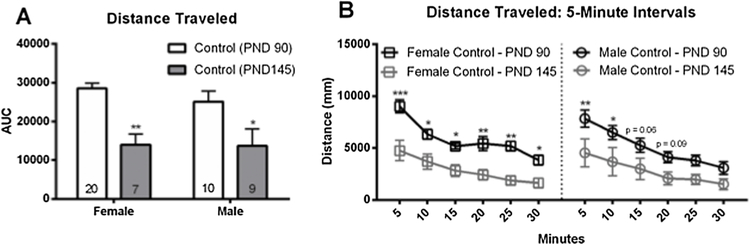
OF data in the PND 90 and PND 145 cohorts was first analyzed to test for a possible effect of age, with the intention of combining the data if none was found. Overall activity levels (shown as area under the curve; AUC) were significantly higher at PND 90 than at PND 145 for male (p ≤ 0.05; d = 1.0) and female (p ≤ 0.005; d = 1.61; Fig. 3A) offspring. When distance traveled was binned into 5-minute intervals over the 30-minute task a significant effect of age was found using a two-way repeated measures ANOVA (F (3, 42) = 4.68, p ≤ 0.007) with age and exposure as factors. PND 90 females were significantly more active than the PND 145 females over the entire 30-minute task (p ≤ 0.0002; d = 1.28, 1–5 min; p ≤ 0.02; d = 1.08, 6–10 min; p ≤ 0.04; d = 1.31, 11–15 min; p ≤ 0.008; d = 1.50, 16–20 min; p ≤ 0.004; d = 2.15, 21–25 min; p ≤ 0.05; d = 1.27, 26–30 min). PND 90 males were significantly more active than PND 145 males, but only for the first ten minutes (p ≤ 0.005; d = 0.95, 1–5 min; p ≤ 0.02; d = 0.83, 6–10 min). Notably, activity at the 15- and 20-minute time points was also suggestive of increased activity in PND 90 males (p = 0.06; d = 0.83, 11–15 min; p = 0.09; d = 1.13, 16–20 min; Fig. 3B). Because of these significant differences, PND 90 OF behavior and PND 145 OF behavior were analyzed and interpreted separately.
Fig. 3.
Differences in activity levels between PND 90 and PND 145 offspring in the open field (OF) in Experiment 2. Activity levels were significantly higher in both sexes at PND 90 than PND 145 (A). Female PND 90 offspring were significantly more active over the entire 30-minute task. Male PND 90 offspring were significantly more active for the first 10 min of the OF (B). Black hatched bars indicate control PND 90 offspring and gray hatched bars indicate control PND 145 offspring. Graphs depict mean ± SEM (* p ≤ 0.05; ** p ≤ 0.01, *** p ≤ 0.001).
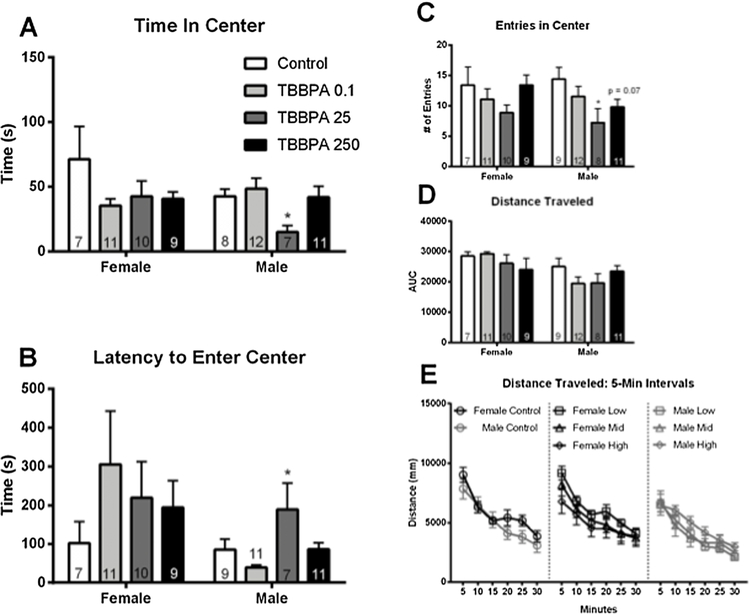
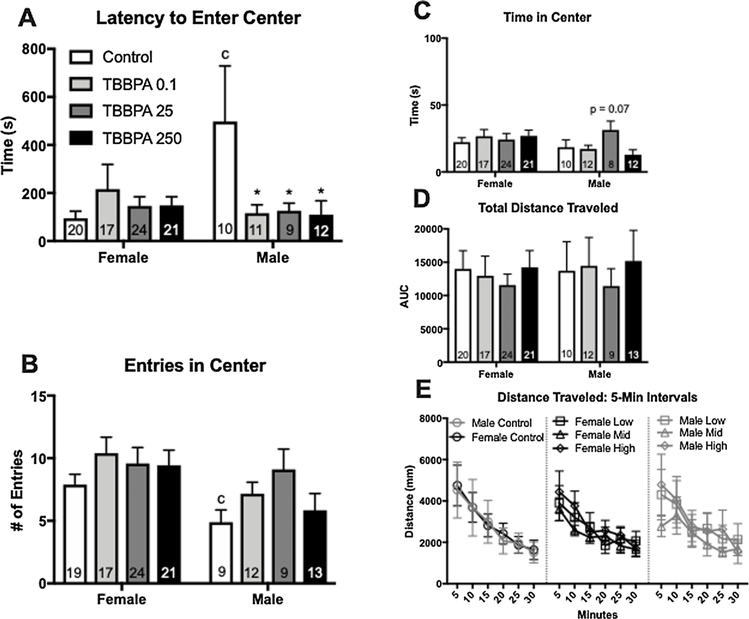
Expected sex differences were not observed in the control PND 90 animals on the OF task. In females, exposure had no effect on any aspect of the task. By contrast, exposure heightened anxiety-related behavior in males (Fig. 4A–C). A significant effect of exposure was found for time spent in the center of the OF (F (3,34) = 1.96, p ≤ 0.04; η2 = 0.22), with TBBPA 25 males spending significantly less time in the center (p ≤ 0.04; d = 1.88; Fig. 4A). Latency to enter the center of the OF arena was also significantly impacted by exposure (F (3,34) = 4.135, p ≤ 0.02; η2 = 0.26), with TBBPA 25 males taking significantly longer to explore this area (p ≤ 0.03; d = 0.75; Fig. 4B). Additional endpoints in the OF appeared to be impacted by exposure but did not reach statistical significance by ANOVA. Because effect size was large, an unprotected LSD was performed to further evaluate effect of exposure on these endpoints. Effect of exposure on number of entries made in the center of the arena did not reach statistical significance (F (3,36) = 2.682, p = 0.07; η2 = 0.18), however the unprotected LSD showed a significant reduction in the number of entries made by TBBPA 25 males compared to controls (p ≤ 0.012; d = 1.17). A similar trend was seen for TBBPA 250 males, (p = 0.07; d = 0.92; Fig. 4C). Exposure did not appear to alter activity levels in the OF as distance traveled was not significantly impacted by TBBPA (Fig. 4D and E).
Fig. 4.
Effects of developmental TBBPA exposure on open field (OF) behavior in PND 90 offspring from Experiment 2. Behavior did not differ between control males and females for any of the OF measurements. In females, no effects of exposure were observed. In males, time in the center and entries into the center were significantly reduced while latency to enter the center was significantly increased in the TBBPA 25 exposure group (A–C). No significant effect of exposure was found for distance traveled (D). Distance traveled was binned into 5-minute intervals to evaluate the pattern of locomotor activity over the 30-minute test (E). No exposure – related effects were detected. White bars indicate control groups, light gray TBBPA 0.1, dark gray TBBPA 25, and black TBBPA 250. Graphs depict mean ± SEM (* p ≤ 0.05).
In the PND 145 cohort, expected sex differences in the controls were observed for number of entries and latency to enter the center of the OF arena (p ≤ 0.02; d = 0.87; p ≤ 0.01; d = 0.76 (Fig. 5A and B). As in the PND 90 females, no significant effect of exposure was found for any of the endpoints evaluated in PND 145 females. In males, main effects did not reach statistical significance but had a large effect size and were thus further analyzed. Unprotected post-hoc analysis of center latency (F (3,38) = 2.76, p = 0.07; η2= 0.17) revealed that TBBPA 0.1, TBBPA 25, and TBBPA 250 males took significantly less time to enter the center of the arena than control males (p ≤ 0.03; d= 0.73; p ≤ 0.04; d = 0.71; p ≤ 0.02; d = 0.72; Fig. 5A). Time spent in the center of the OF in males also did not reach statistical significance (F (3, 40) = 1.467, p = 0.06, η2 = 0.18), however an unprotected LSD revealed a suggestive increase in time spent in the center of the OF for TBBPA 25 males (p ≤ 0.07; d = 0.71; Fig. 5C). Exposure did not appear to alter activity levels in the OF as distance traveled was not significantly impacted by TBBPA (Fig. 5D and E).
Fig. 5.
Effects of developmental TBBPA exposure on open field (OF) behavior in PND 145 offspring from Experiment 2. As expected, a significant difference between male and female controls was observed for both latency to enter the center and number of entries made in the center of the OF (A and B; c p ≤ 0.05). However, no significant effect of sex was observed for any of the other endpoints. In females, there were no effects of exposure on any of the observed endpoints. In males, latency to enter the center was significantly reduced in all three TBBPA exposure groups (A). No significant effect of exposure was found for entries in center, time in center, or distance traveled (B–D). Distance traveled was binned into 5-minute intervals to evaluate the pattern of locomotor activity over the 30-minute test (E). No exposure – related effects were detected. White bars indicate control groups, light gray TBBPA 0.1, dark gray TBBPA 25, and black TBBPA 250. Graphs depict mean ± SEM (* p ≤ 0.05).
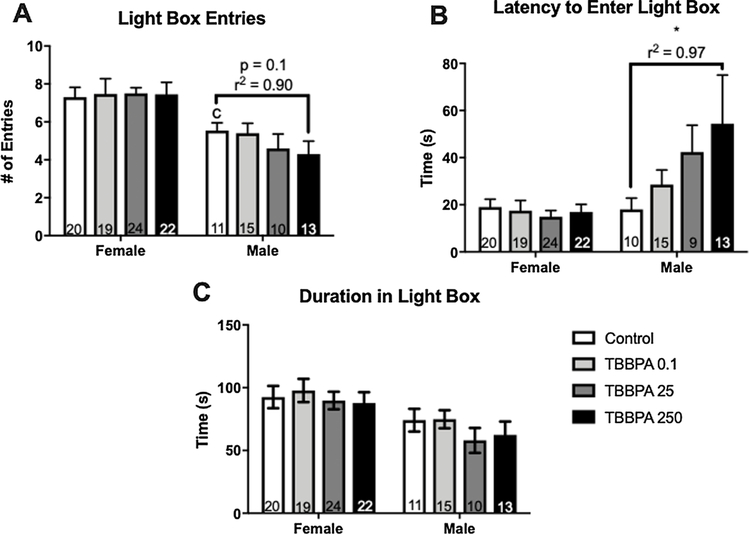
In the L/D box there was a significant effect of sex for the number of entries in the light box made by the unexposed controls (p ≤ 0.02; d = 0.92; Fig. 6A). No significant effect of sex was found for the other two endpoints, latency or duration in the light compartment, however it was suggestive for duration (p = 0.09; d = 0.53). No significant effect of exposure was found for any of the observed endpoints in either sex. In the males, however, there appeared to be a possible dose response for latency to enter the light compartment and number of entries. A test for linear trend was run and a significant trend was found for latency (F (1, 43) = 4.3, p ≤ 0.04, r2 = 0.97; Fig. 6B), but not entries (F (1, 45) = 2.7, p = 0.1, r2 = 0.90; Fig. 6A).
Fig. 6.
Effects of developmental TBBPA exposure on light dark (L/D) box behavior in Experiment 2 offspring. A significant difference between control males and females was observed for light box entries (A; c p ≤ 0.05) but not the other two endpoints. In females, exposure had no effect on any observed endpoints. In males, a suggestive linear trend was observed for number of entries in the light box (A) while a significant linear trend was observed for latency to enter the light box (B). Time spent in the light box was not significantly impacted by exposure (C). White bars indicate control groups, light gray TBBPA 0.1, dark gray TBBPA 25, and black TBBPA 250. Graphs depict mean ± SEM (* p ≤ 0.05).
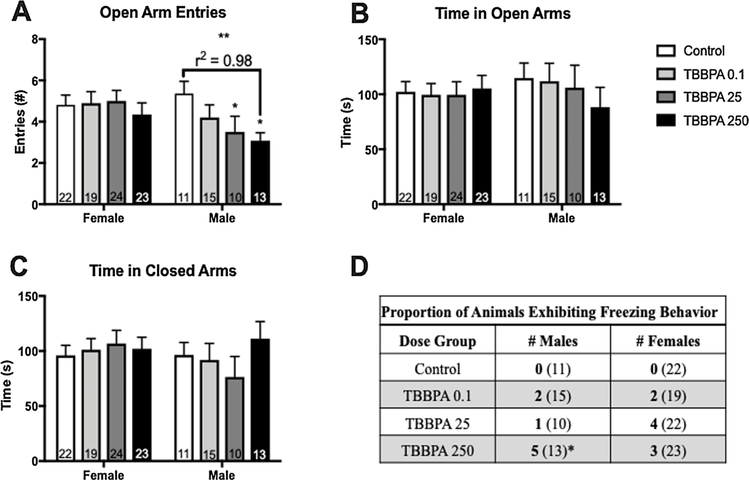
The EPM was analyzed two different ways to account for an unexpected level of freezing behavior on the open arms within the first minute of the test. Freezing was classified as the body being fully still with minimal head movement. No freezing was observed in the controls of either sex, but rats of both sexes in the TBBPA 0.1 (2♀, 2♂), TBBPA 25 (1♀, 4♂), and TBBPA 250 (5♀, 3♂) dose groups displayed this anxiety-related behavior (Fig. 7D). Freezing occurred only within the first minute of the testing and normal exploratory behavior subsequently resumed. Therefore, EPM data was first analyzed across the full five-minute test (no significant effects of sex or exposure on any outcome), and then re-analyzed excluding the first minute of the task in order to exclude the time in which freezing occurred. In the unexposed controls, no significant effect of sex was observed on any of the parameters (Fig. 7). In males, an effect of exposure was suggested for the number of entries in the open arms (F (3,45) = 2.671, p = 0.06; η2 = 0.15). An unprotected LSD test revealed that the TBBPA 25 and TBBPA 250 males made significantly fewer entries into the open arms compared to control males (p ≤ 0.05; d = 0.85; p ≤ 0.01; d = 1.34; Fig. 7A). There also appeared to be a dose response for number of entries made in the open arms by male offspring. A significant linear trend was found for number of male open arm entries (F (1, 45) = 7.9, p ≤ 0.007, r2 = 0.98), with a reduction in the number of entries observed with increasing dose (Fig. 7A). The proportion of male offspring that froze on the EPM was significantly higher for the TBBPA 250 group compared to male controls (p ≤ 0.05; Fig. 7D). Using a chi-square test for trend a significant linear trend was observed for number of males that froze on the EPM (p ≤ 0.02), with incidence of freezing increasing with dose (Fig. 7D). Closed arm behavior was not affected by exposure suggesting no change in overall activity.
Fig. 7.
Effects of developmental TBBPA exposure on elevated plus maze (EPM) behavior in Experiment 2 offspring. No significant sex differences between the control offspring were found for any EPM endpoints. No exposure-related effects were found in female offspring. In males, a significant effect of exposure was found for open arm entries in both the TBBPA 25 and 250 groups (A). A significant linear trend was also observed for open arm entries (A). No significant effect of exposure was observed for time spend in either the open arms (B) or the closed arms (C). The table (D) summarizes freezing behavior differentiated by exposure group and sex. Bolded numbers indicate the number of animals that froze during the first minute of the task, while the numbers in parentheses indicates the total number of animals tested. White bars indicate control groups, light gray TBBPA 0.1, dark gray TBBPA 25, and black TBBPA 250. Graphs depict mean ± SEM (* p ≤ 0.05; ** p ≤ 0.01).
4. Discussion
This study supports and expands upon previous findings by providing evidence that developmental TBBPA exposure, even at the lowest doses tested, produced meaningful behavioral outcomes resulting in altered exploratory and anxiety-related behaviors in Wistar rats. Biological significance was evaluated using a combination of statistical significance and effect size. Behavioral effects were small in most cases but generally consistent and sex-specific, with males appearing to be more sensitive than females, and dose-related trends in males were apparent in the case of exploratory activity. Overall, males were more anxious while females appeared to be more hyperactive, albeit only in the running wheel task, which could only be conducted in Experiment 1. As part of a broader investigation to assess the toxicity of TBBPA, these findings highlight the potential for behavioral disruption and vulnerability of the brain to developmental TBBPA exposure across a wide range of doses. Although, because dosing in Experiment 1 was entirely developmental while dosing in Experiment 2 encompassed development and adulthood, it is not possible to definitively determine if developmental exposure is necessary for the induction of the effects observed.
5. Experiment 1
A prior study reported accumulation of TBBPA in the striatum and changes in behavior, including increased freezing behavior in a contextual fear conditioning paradigm, in mice following acute exposure to 0.1 mg TBBPA/kg body weight (Nakajima et al., 2009). A primary goal of Experiment 1 was to establish if this dose could produce similar effects on other striatally-influenced behaviors in rats and, if so, incorporate it in the larger, more comprehensive study (Experiment 2). No significant effects on anxiety-related behaviors were observed at this dose but running wheel behavior was heightened in the exposed females. As anticipated, profound sex differences were observed in the unexposed controls, with females showing consistently higher levels of activity than males (Sherwin, 1998), regardless of exposure. This confirms that the study was sufficiently powered to detect group differences. Sleep patterns did not appear to be disrupted in either sex, as we observed normal rhythmicity in activity with peaks just before lights on and after lights off (Benstaali et al., 2001). TBBPA 0.1 female offspring were hyperactive over the three-day testing period, including the first day, which could be specifically indicative of heightened interest in novelty. No significant changes in activity were observed in TBBPA-exposed male offspring. Notably, because male engagement with the running wheel is naturally so low, it would be difficult to detect decreased activity, an outcome that would have been consistent with the evidence of elevated anxiety-like behavior observed in males in the larger study conducted at NIEHS. This limitation emphasizes why running a battery of tests is so important when assessing possible impacts of environmental factors on behavior, including sexually dimorphic behaviors.
6. Experiment 2
Behavioral outcomes from the multi-dose study also revealed sex-specific effects on behavior; however, the data should be interpreted with some caution because expected sex-differences between male and female controls were not consistently detected. Some behavioral sex differences may be strain specific, which is not atypical for rodent strains, and best documented in mice (Bothe et al., 2005; Rebuli et al., 2015). There was also an effect of age at testing. Due to a scheduled necropsy at PND 90, some animals were tested in the OF just prior to PND 90, while others were tested at PND 145. Animals tested in the OF at PND 90 did not display any of the expected sex differences between male and female controls. Additionally, general activity levels (both sexes) were significantly higher in the PND 90 rats than the PND 145 rats. Overall, the animals tested at PND 90 behaved in a manner more similar to juvenile rats, which tend to be more active, less risk averse, and more exploratory, regardless of sex (Baldwin et al., 2017). Age-related declines in exploratory activity have been reported in rats (Doremus et al., 2006; Glenn et al., 2008), highlighting the importance of controlling for age in behavioral studies. By PND 145, expected sex differences were observed for endpoints in the OF and L/D box, although not the EPM. The two major differences between these groups, besides age, were (1) time elapsed since last TBBPA dose and testing (hours for PND 90 and weeks for PND 145), and (2) latency between last incidence of gavage stress and testing.
It is well established that environmental stress, particularly prenatal stress, can produce long-lasting changes in brain and behavior, including the disruption of brain and behavioral sex differences (Charil et al., 2010; Del Cerro et al., 2015; Weinstock, 2007). We have previously shown in newborn rats that daily dosing by gavage to rat dams can abrogate sexually dimorphic expression of estrogen and other hormone receptors in the amygdala, and hypothalamic regions critical for mediating stress responses (Cao et al., 2013). Oral gavage has been shown to elevate corticosterone levels, an effect that can last for hours (Brown et al., 2000). Oral gavage of pregnant rats alters somatic development in the offspring, including eye opening and body length, and neuromuscular development, including surface righting and forelimb grip (McDonnell-Dowling et al., 2017). Here, in Experiment 2, animals were orally dosed by gavage daily through PND 90, but not after. Thus, the younger cohort was still being dosed daily at the time of OF testing, while the older cohort was no longer being intensely handled or exposed. This is likely one of most significant reasons why behavioral outcomes were not consistent across the two cohorts. In Experiment 1, TBBPA was purposely delivered via a palatable food treat to minimize handling stress. This was not possible for Experiment 2 because of chemical taste aversion at the highest dose and the requirement that the treats be autoclaved prior to dosing, which eliminated much of the taste or palatability. Future work examining the impact of long-term EDC exposure on stress-related behaviors should account for handling, gavage, and other potential confounding stressors because they likely adversely impact replication across behavioral toxicity studies. Additionally, development of alternative dosing strategies, such as the food treats used in Experiment 1, is needed for guideline-compliant toxicity testing, particularly when considering neurodevelopmental endpoints.
Across all apparatuses, there was a robust dose-dependent anxiety-related behavioral phenotype for male offspring, with males in the TBBPA 25 dose group displaying the most consistent effects. For example, TBBPA 25 PND 90 males spent significantly less time in the center of the OF, made fewer entries into the center, and had an increased latency to enter the center of the arena. In the PND 145 OF animals, there was no evidence of heightened male anxiety. By contrast, males from all three doses TBBPA-exposed groups at this age point showed a significant reduction in latency to enter the center, which could be interpreted as decreased anxiety-like behavior. A caveat to this observation is the unexposed control male animals in this study displayed an abnormally long latency, with two males never entering the center of the OF. A potential dose response was observed for latency to enter the lit side of the L/D box and number of entries made in the lit side. A significant linear trend was found for latency to enter the light box, with latency increasing with dose and, similarly, while the linear trend analysis was not significant for number of entries made in the light box, it was suggestive that entries decreased with dose. Because the initial ANOVA was not significant, and the effects are only suggestive, these findings should be interpreted with caution but provide further evidence of potential increased anxiety-like behavior in male offspring exposed to TBBPA. A higher-powered study specifically focused on this dose range, sex, and behavioral phenotype is warranted to obtain greater resolution of this potential effect, particularly at doses of 25 mg/kg and below.
A distinct behavioral phenotype emerged when dosed animals were tested in the EPM. Originally a five-minute test, videos had to be re-analyzed to exclude the first minute of the recording due to frequent and unanticipated freezing behavior. A high proportion, ranging from 10% of TBBPA 25 males up to 38% of TBBPA 250 males froze when placed in the center of the EPM, an odd behavior which lasted for up to a minute. Such freezing behavior is not typical for this strain in our experience, and was not observed for any control animals, but occurred in exposed groups of both sexes. Exposed males appeared to be more prone to freezing as we observed a significant linear relationship between exposure and number of male offspring that froze when placed on the EPM. This finding is consistent with other evidence that exposure to TBBPA increases anxiety-like behavior, particularly in males. Most notably TBBPA 25 and TBBPA 250 males entered the open arms significantly fewer times than unexposed males, a classic indicator of heightened anxiety. No effect of exposure on closed arm behavior was observed, and there was no evidence of altered exploratory behavior or distance traveled in the OF, furthering confidence that the observed freezing effect is specifically related to anxiety. This outcome is consistent with prior findings in mice orally exposed to 0.1 or 5 mg TBBPA/kg bw as adults (Nakajima et al., 2009). These mice displayed more freezing behavior than controls in a contextual fear conditioning paradigm and more horizontal movement in the OF.
7. Conclusions
This study provides novel evidence that at doses below those previously described to induce neurodevelopmental effects in rodents (Lilienthal et al., 2008), TBBPA can have significant effects on exploratory and anxiety-related behaviors at doses within an order of magnitude of those consumed by humans (Martinez et al., 2019). Males consistently appeared to be more vulnerable to TBBPA exposure than females, displaying evidence of heightened anxiety-like behavior in a battery of tasks. Evidence of female hyperactivity found in the pilot study was not recapitulated in the full-scale study, although the running wheel task was not included in the larger study due to logistical constraints. Thus, the potential for TBBPA to induce female hyperactivity remains unclear. It also remains to be established if adverse behavioral outcomes occur in either sex in the range of 0.1 mg/kg/d, which was the lowest dose tested, because although many Experiment 2 endpoints appeared to be dose-responsive, effects at this level were not statistically significant. Notably, differences in behavior between PND 90 animals and PND 145 animals demonstrate the importance of controlling for age and external variables when assessing behavior, particularly variables that affect stress and internal dose of the chemical of interest. Differences in time between last gavage and/or differences in circulating levels of TBBPA at time of testing likely contributed to the observed differences in behavior across the two age cohorts, and the absence of expected sex differences in the PND 90 animals. Future studies should address these potential confounding factors when examining possible effects of TBBPA on brain and behavior. Collectively, these findings support the hypothesis that exposure to TBBPA may alter non-reproductive behaviors in males and females differently and will help to inform ongoing follow-up studies in tissues obtained from these animals aimed at probing potential mechanisms by which the observed behavioral phenotypes arise.
Acknowledgements
We would like to thank the Biological Resources Facility at NCSU for assistance with animal care and husbandry. We thank Hannah Harris (NIEHS) for technical assistance including dosing the animals and Rodriquez Sutton, Gordon Caviness, Spencer Bridges, and Joseph Hensley for help with animal care and husbandry at NIEHS. Additional help with behavioral testing was provided by Cassie Rhodes and we also thank David Newkirk for technical support.
Funding
NIEHS IPA Agreement with H.B.P.,P30ES025128 to NCSU,T32ES-021432to NCSU supporting K.D.R., NIEHSZ01ES102785(S.E.F., S.E.G.), and NCIZ1ABC011476 (L.S.B., G.K.).
Footnotes
Declaration of Competing Interest
The authors declare no conflict of interest.
References
- Abdallah MA, Harrad S, 2011. Tetrabromobisphenol-A, hexabromocyclododecane and its degradation products in UK human milk: relationship to external exposure. Environ. Int 37 (2), 443–448. [DOI] [PubMed] [Google Scholar]
- Antignac JP, Cariou R, Maume D, Marchand P, Monteau F, Zalko D, Berrebi A, Cravedi JP, Andre F, Le Bizec B, 2008. Exposure assessment of fetus and newborn to brominated flame retardants in France: preliminary data. Mol. Nutr. Food Res 52 (2), 258–265. [DOI] [PubMed] [Google Scholar]
- Bailey KR, Crawley JN, 2009. Anxiety-related behaviors in mice In: Buccafusco JJ (Ed.), Methods of Behavior Analysis in Neuroscience. CRC Press, Boca Raton (FL). [Google Scholar]
- Baldwin KR, Phillips AL, Horman B, Arambula SE, Rebuli ME, Stapleton HM, Patisaul HB, 2017. Sex specific placental accumulation and behavioral effects of developmental firemaster 550 exposure in wistar rats. Sci. Rep 7 (1), 7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty WW, 1979. Gonadal hormones and sex differences in nonreproductive behaviors in rodents: organizational and activational influences. Horm. Behav 12 (2), 112–163. [DOI] [PubMed] [Google Scholar]
- Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J, Young E, 2005. Strategies and methods for research on sex differences in brain and behavior. Endocrinology 146 (4), 1650–1673. [DOI] [PubMed] [Google Scholar]
- Benstaali C, Mailloux A, Bogdan A, Auzeby A, Touitou Y, 2001. Circadian rhythms of body temperature and motor activity in rodents their relationships with the light-dark cycle. Life Sci. 68 (24), 2645–2656. [DOI] [PubMed] [Google Scholar]
- Bothe GW, Bolivar VJ, Vedder MJ, Geistfeld JG, 2005. Behavioral differences among fourteen inbred mouse strains commonly used as disease models. Comp. Med 55 (4), 326–334. [PubMed] [Google Scholar]
- Brown AP, Dinger N, Levine BS, 2000. Stress produced by gavage administration in the rat. Contemp. Top. Lab. Anim. Sci 39 (1), 17–21. [PubMed] [Google Scholar]
- Canada, 2012. Risk Management Scope for Phenol, 4,4′-(1-Methylethylidene) Bis[2,6-dibromo- (Tetrabromobisphenol A), November 2012 ed. Government of Canada, Environment Canada Health Canada. [Google Scholar]
- Cao J, Rebuli ME, Rogers J, Todd KL, Leyrer SM, Ferguson SA, Patisaul HB, 2013. Prenatal bisphenol A exposure alters sex-specific estrogen receptor expression in the neonatal rat hypothalamus and amygdala. Toxicol. Sci 133 (1), 157–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carignan CC, Abdallah MA, Wu N, Heiger-Bernays W, McClean MD, Harrad S, Webster TF, 2012. Predictors of tetrabromobisphenol-A (TBBP-A) and hexabromocyclododecanes (HBCD) in milk from Boston mothers. Environ. Sci. Technol 46 (21), 12146–12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cariou R, Antignac JP, Zalko D, Berrebi A, Cravedi JP, Maume D, Marchand P, Monteau F, Riu A, Andre F, Le Bizec B, 2008. Exposure assessment of French women and their newborns to tetrabromobisphenol-A: occurrence measurements in maternal adipose tissue, serum, breast milk and cord serum. Chemosphere 73 (7), 1036–1041. [DOI] [PubMed] [Google Scholar]
- Charil A, Laplante DP, Vaillancourt C, King S, 2010. Prenatal stress and brain development. Brain Res. Rev 65 (1), 56–79. [DOI] [PubMed] [Google Scholar]
- Chen J, Tanguay RL, Simonich M, Nie S, Zhao Y, Li L, Bai C, Dong Q, Huang C, Lin K, 2016a. TBBPA chronic exposure produces sex-specific neurobehavioral and social interaction changes in adult zebrafish. Neurotoxicol. Teratol 56, 9–15. [DOI] [PubMed] [Google Scholar]
- Chen J, Tanguay RL, Xiao Y, Haggard DE, Ge X, Jia Y, Zheng Y, Dong Q, Huang C, Lin K, 2016b. TBBPA exposure during a sensitive developmental window produces neurobehavioral changes in larval zebrafish. Environ. Pollut 216, 53–63. [DOI] [PubMed] [Google Scholar]
- Coughtrie MW, 2015. Ontogeny of human conjugating enzymes. Drug Metab. Lett. 9 (2), 99–108. [DOI] [PubMed] [Google Scholar]
- Coughtrie MW, Burchell B, Leakey JE, Hume R, 1988. The inadequacy of perinatal glucuronidation: immunoblot analysis of the developmental expression of individual UDP-glucuronosyltransferase isoenzymes in rat and human liver microsomes. Mol. Pharmacol 34 (6), 729–735. [PubMed] [Google Scholar]
- Dawson PA, 2011. Sulfate in fetal development. Semin. Cell Dev. Biol. 22 (6), 653–659. [DOI] [PubMed] [Google Scholar]
- de Wit CA, Herzke D, Vorkamp K, 2010. Brominated flame retardants in the Arctic environment–trends and new candidates. Sci. Total Environ 408 (15), 2885–2918. [DOI] [PubMed] [Google Scholar]
- Del Cerro MC, Ortega E, Gomez F, Segovia S, Perez-Laso C, 2015. Environmental prenatal stress eliminates brain and maternal behavioral sex differences and alters hormone levels in female rats. Horm. Behav 73, 142–147. [DOI] [PubMed] [Google Scholar]
- Donner NC, Lowry CA, 2013. Sex differences in anxiety and emotional behavior. Pflugers Arch. 465 (5), 601–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus TL, Varlinskaya EI, Spear LP, 2006. Factor analysis of elevated plus-maze behavior in adolescent and adult rats. Pharmacol. Biochem. Behav 83 (4), 570–577. [DOI] [PubMed] [Google Scholar]
- Fritz CO, Morris PE, Richler JJ, 2012. Effect size estimates: current use, calculations, and interpretation. J. Exp. Psychol. Gen 141 (1), 2–18. [DOI] [PubMed] [Google Scholar]
- Fukuda N, Ito Y, Yamaguchi M, Mitumori K, Koizumi M, Hasegawa R, Kamata E, Ema M, 2004. Unexpected nephrotoxicity induced by tetrabromobisphenol A in newborn rats. Toxicol. Lett. 150 (2), 145–155. [DOI] [PubMed] [Google Scholar]
- Glenn MJ, Kirby ED, Gibson EM, Wong-Goodrich SJ, Mellott TJ, Blusztajn JK, Williams CL, 2008. Age-related declines in exploratory behavior and markers of hippocampal plasticity are attenuated by prenatal choline supplementation in rats. Brain Res. 1237, 110–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goma M, Tobena A, 1978. Reliability of various measures obtained in open-field test. Psychol. Rep 43 (3 Pt 2), 1123–1128. [DOI] [PubMed] [Google Scholar]
- Gosavi RA, Knudsen GA, Birnbaum LS, Pedersen LC, 2013. Mimicking of estradiol binding by flame retardants and their metabolites: a crystallographic analysis. Environ. Health Perspect 121 (10), 1194–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TDD, Kovacsics DT, C.E., 2010. The open field test In: Gould TD (Ed.), Mood and Anxiety Related Phenotypes in Mice. Humana Press. [Google Scholar]
- Grandjean P, Landrigan PJ, 2014. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 13 (3), 330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamers T, Kamstra JH, Sonneveld E, Murk AJ, Kester MH, Andersson PL, Legler J, Brouwer A, 2006. In vitro profiling of the endocrine-disrupting potency of brominated flame retardants. Toxicol. Sci 92 (1), 157–173. [DOI] [PubMed] [Google Scholar]
- Haseman JK, Bailer AJ, Kodell RL, Morris R, Portier K, 2001. Statistical issues in the analysis of low-dose endocrine disruptor data. Toxicol. Sci 61 (2), 201–210. [DOI] [PubMed] [Google Scholar]
- Hicks KD, Sullivan AW, Cao J, Sluzas E, Rebuli M, Patisaul HB, 2016. Interaction of bisphenol A (BPA) and soy phytoestrogens on sexually dimorphic sociosexual behaviors in male and female rats. Horm. Behav. 84, 121–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M, Gogola J, Kotula-Balak M, Ptak A, 2017. Stimulation of ovarian cell proliferation by tetrabromobisphenol A but not tetrachlorobisphenol A through G protein-coupled receptor 30. Toxicol. In Vitro 45 (Pt 1), 54–59. [DOI] [PubMed] [Google Scholar]
- Hogg S, 1996. A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacol. Biochem. Behav 54 (1), 21–30. [DOI] [PubMed] [Google Scholar]
- Hu F, Pan L, Cai Y, Liu T, Jin Q, 2015a. Deep sequencing of the scallop Chlamys farreri transcriptome response to tetrabromobisphenol A (TBBPA) stress. Mar. Genom 19, 31–38. [DOI] [PubMed] [Google Scholar]
- Hu F, Pan L, Xiu M, Jin Q, Wang G, Wang C, 2015b. Bioaccumulation and detoxification responses in the scallop Chlamys farreri exposed to tetrabromobisphenol A (TBBPA). Environ. Toxicol. Pharmacol 39 (3), 997–1007. [DOI] [PubMed] [Google Scholar]
- Hunt PA, Susiarjo M, Rubio C, Hassold TJ, 2009. The bisphenol A experience: a primer for the analysis of environmental effects on mammalian reproduction. Biol. Reprod 81 (5), 807–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkbrenner AE, Schmidt RJ, Penlesky AC, 2014. Environmental chemical exposures and autism spectrum disorders: a review of the epidemiological evidence. Curr. Probl. Pediatr. Adolesc. Health Care 44 (10), 277–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG, 2010. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 8 (6), e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Colon E, Chawla S, Vandenberg LN, Suvorov A, 2015. Endocrine disruptors alter social behaviors and indirectly influence social hierarchies via changes in body weight. Environ. Health 14, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura S, Jinno N, Ohta S, Kuroki H, Fujimoto N, 2002. Thyroid hormonal activity of the flame retardants tetrabromobisphenol A and tetrachlorobisphenol A. Biochem. Biophys. Res. Commun 293 (1), 554–559. [DOI] [PubMed] [Google Scholar]
- Lakens D, 2013. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front. Psychol. 4, 863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrigan PJ, Lambertini L, Birnbaum LS, 2012. A research strategy to discover the environmental causes of autism and neurodevelopmental disabilities. Environ. Health Perspect 120 (7), a258–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li AA, Baum MJ, McIntosh LJ, Day M, Liu F, Gray LE Jr., 2008. Building a scientific framework for studying hormonal effects on behavior and on the development of the sexually dimorphic nervous system. Neurotoxicology 29 (3), 504–519. [DOI] [PubMed] [Google Scholar]
- Lilienthal H, Verwer CM, van der Ven LT, Piersma AH, Vos JG, 2008. Exposure to tetrabromobisphenol A (TBBPA) in Wistar rats: neurobehavioral effects in offspring from a one-generation reproduction study. Toxicology 246 (1), 45–54. [DOI] [PubMed] [Google Scholar]
- Liu K, Li J, Yan S, Zhang W, Li Y, Han D, 2016. A review of status of tetrabromobisphenol A (TBBPA) in China. Chemosphere 148, 8–20. [DOI] [PubMed] [Google Scholar]
- Martinez MA, Castro I, Rovira J, Ares S, Rodriguez JM, Cunha SC, Casal S, Fernandes JO, Schuhmacher M, Nadal M, 2019. Early-life intake of major trace elements, bisphenol A, tetrabromobisphenol A and fatty acids: comparing human milk and commercial infant formulas. Environ. Res 169, 246–255. [DOI] [PubMed] [Google Scholar]
- Masuo Y, Ishido M, 2011. Neurotoxicity of endocrine disruptors: possible involvement in brain development and neurodegeneration. J. Toxicol. Environ. Health B Crit. Rev 14 (5–7), 346–369. [DOI] [PubMed] [Google Scholar]
- Matsukami H, Tue NM, Suzuki G, Someya M, Tuyen le H, Viet PH, Takahashi S, Tanabe S, Takigami H, 2015. Flame retardant emission from e-waste recycling operation in northern Vietnam: environmental occurrence of emerging organophosphorus esters used as alternatives for PBDEs. Sci. Total Environ 514, 492–499. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Wright CL, Schwarz JM, 2009. New tricks by an old dogma: mechanisms of the Organizational/Activational Hypothesis of steroid-mediated sexual differentiation of brain and behavior. Horm. Behav 55 (5), 655–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell-Dowling K, Kleefeld S, Kelly JP, 2017. Consequences of oral gavage during gestation and lactation on rat dams and the neurodevelopment and behavior of their offspring. J. Am. Assoc. Lab. Anim. Sci 56 (1), 79–83. [PMC free article] [PubMed] [Google Scholar]
- Meerts IA, van Zanden JJ, Luijks EA, van Leeuwen-Bol I, Marsh G, Jakobsson E, Bergman A, Brouwer A, 2000. Potent competitive interactions of some brominated flame retardants and related compounds with human transthyretin in vitro. Toxicol. Sci 56 (1), 95–104. [DOI] [PubMed] [Google Scholar]
- Messer A, 2010. Mini-review: polybrominated diphenyl ether (PBDE) flame retardants as potential autism risk factors. Physiol. Behav 100 (3), 245–249. [DOI] [PubMed] [Google Scholar]
- Miodovnik A, Engel SM, Zhu C, Ye X, Soorya LV, Silva MJ, Calafat AM, Wolff MS, 2011. Endocrine disruptors and childhood social impairment. Neurotoxicology 32 (2), 261–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misslin R, Belzung C, Vogel E, 1989. Behavioural validation of a light/dark choice procedure for testing anti-anxiety agents. Behav. Processes 18 (1–3), 119–132. [DOI] [PubMed] [Google Scholar]
- Moog NK, Entringer S, Heim C, Wadhwa PD, Kathmann N, Buss C, 2017. Influence of maternal thyroid hormones during gestation on fetal brain development. Neuroscience 342, 68–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris S, Allchin CR, Zegers BN, Haftka JJ, Boon JP, Belpaire C, Leonards PE, Van Leeuwen SP, De Boer J, 2004. Distribution and fate of HBCD and TBBPA brominated flame retardants in North Sea estuaries and aquatic food webs. Environ. Sci. Technol 38 (21), 5497–5504. [DOI] [PubMed] [Google Scholar]
- Nakajima A, Saigusa D, Tetsu N, Yamakuni T, Tomioka Y, Hishinuma T, 2009. Neurobehavioral effects of tetrabromobisphenol A, a brominated flame retardant, in mice. Toxicol. Lett 189 (1), 78–83. [DOI] [PubMed] [Google Scholar]
- Nasello AG, Machado C, Bastos JF, Felicio LF, 1998. Sudden darkness induces a high activity-low anxiety state in male and female rats. Physiol. Behav. 63 (3), 451–454. [DOI] [PubMed] [Google Scholar]
- Ni HG, Zeng H, 2013. HBCD and TBBPA in particulate phase of indoor air in Shenzhen. China. Sci Total Environ 458–460, 15–19. [DOI] [PubMed] [Google Scholar]
- NTP, 2014. NTP (National Toxicology Program) Technical Report on the Toxicology Studies of Tetrabromobisphenol A (CAS NO. 79–94-7) in F344/NTac Rats and B6C3F1mice and Toxicology and Carcingogenesis Studies of Tetrabromobisphenol a in Wistar Han [Crl:WI9Han)] Rats and B6C3F1 Mice (gavage Studies) NTP TR 587. NIH. [Google Scholar]
- OECD, 2018. Test No. 408: Repeated Dose 90-Day Oral Toxicity Study in Rodents, OECD Guidelines for the Testing of Chemicals, Section 4. [Google Scholar]
- Patisaul HB, Blum A, Luskin JR, Wilson ME, 2005. Dietary soy supplements produce opposite effects on anxiety in intact male and female rats in the elevated plus-maze. Behav. Neurosci 119 (2), 587–594. [DOI] [PubMed] [Google Scholar]
- Patisaul HB, Roberts SC, Mabrey N, McCaffrey KA, Gear RB, Braun J, Belcher SM, Stapleton HM, 2013. Accumulation and endocrine disrupting effects of the flame retardant mixture firemaster((R)) 550 in rats: an exploratory assessment. J. Biochem. Mol. Toxicol 27 (2), 124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patisaul HB, Sullivan AW, Radford ME, Walker DM, Adewale HB, Winnik B, Coughlin JL, Buckley B, Gore AC, 2012. Anxiogenic effects of developmental bisphenol a exposure are associated with gene expression changes in the juvenile rat amygdala and mitigated by soy. PLoS One 7 (9), e43890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patisaul HB, Todd KL, Mickens JA, Adewale HB, 2009. Impact of neonatal exposure to the ERalpha agonist PPT, bisphenol-A or phytoestrogens on hypothalamic kisspeptin fiber density in male and female rats. Neurotoxicology 30 (3), 350–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M, 1985. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J. Neurosci. Methods 14 (3), 149–167. [DOI] [PubMed] [Google Scholar]
- Rebuli ME, Camacho L, Adonay ME, Reif DM, Aylor DL, Patisaul HB, 2015. Impact of low-dose oral exposure to bisphenol a (BPA) on juvenile and adult rat exploratory and anxiety behavior: a CLARITY-BPA consortium study. Toxicol. Sci 148 (2), 341–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter CA, Birnbaum LS, Farabollini F, Newbold RR, Rubin BS, Talsness CE, Vandenbergh JG, Walser-Kuntz DR, vom Saal FS, 2007. In vivo effects of bisphenol A in laboratory rodent studies. Reprod. Toxicol 24 (2), 199–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier WI 3rd, 1971. Progesterone-estrogen interactions in the control of activity-wheel running in the female rat. J. Comp. Physiol. Psychol 74 (3), 365–373. [DOI] [PubMed] [Google Scholar]
- Saegusa Y, Fujimoto H, Woo GH, Ohishi T, Wang L, Mitsumori K, Nishikawa A, Shibutani M, 2012. Transient aberration of neuronal development in the hippocampal dentate gyrus after developmental exposure to brominated flame retardants in rats. Arch. Toxicol 86 (9), 1431–1442. [DOI] [PubMed] [Google Scholar]
- Sanders JM, Coulter SJ, Knudsen GA, Dunnick JK, Kissling GE, Birnbaum LS, 2016. Disruption of estrogen homeostasis as a mechanism for uterine toxicity in Wistar Han rats treated with tetrabromobisphenol A. Toxicol. Appl. Pharmacol 298, 31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer UM, Volkel W, Dekant W, 2006. Toxicokinetics of tetrabromobisphenol a in humans and rats after oral administration. Toxicol. Sci 91 (1), 49–58. [DOI] [PubMed] [Google Scholar]
- Sherwin CM, 1998. Voluntary wheel running: a review and novel interpretation. Anim. Behav 56 (1), 11–27. [DOI] [PubMed] [Google Scholar]
- Shi Z, Jiao Y, Hu Y, Sun Z, Zhou X, Feng J, Li J, Wu Y, 2013. Levels of tetrabromobisphenol A, hexabromocyclododecanes and polybrominated diphenyl ethers in human milk from the general population in Beijing, China. Sci. Total Environ 452–453, 10–18. [DOI] [PubMed] [Google Scholar]
- Shi Z, Zhang L, Zhao Y, Sun Z, Zhou X, Li J, Wu Y, 2017. A national survey of tetrabromobisphenol-A, hexabromocyclododecane and decabrominated diphenyl ether in human milk from China: occurrence and exposure assessment. Sci. Total Environ. 599–600, 237–245. [DOI] [PubMed] [Google Scholar]
- Shi ZX, Wu YN, Li JG, Zhao YF, Feng JF, 2009. Dietary exposure assessment of Chinese adults and nursing infants to tetrabromobisphenol-A and hexabromocyclododecanes: occurrence measurements in foods and human milk. Environ. Sci. Technol 43 (12), 4314–4319. [DOI] [PubMed] [Google Scholar]
- Simerly RB, 2002. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu. Rev. Neurosci 25, 507–536. [DOI] [PubMed] [Google Scholar]
- Stapleton HM, Klosterhaus S, Eagle S, Fuh J, Meeker JD, Blum A, Webster TF, 2009. Detection of organophosphate flame retardants in furniture foam and U.S. House dust. Environ. Sci. Technol 43 (19), 7490–7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner M, Katz RJ, Baldrighi G, Carroll BJ, 1981. Motivated behavior and the estrous cycle in rats. Psychoneuroendocrinology 6 (1), 81–90. [DOI] [PubMed] [Google Scholar]
- Usenko CY, Abel EL, Hopkins A, Martinez G, Tijerina J, Kudela M, Norris N, Joudeh L, Bruce ED, 2016. Evaluation of common use brominated flame retardant (BFR) toxicity using a zebrafish embryo model. Toxics 4 (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veereman-Wauters G, 1996. Neonatal gut development and postnatal adaptation. Eur. J. Pediatr 155 (8), 627–632. [DOI] [PubMed] [Google Scholar]
- Wang J, Liu L, Wang J, Pan B, Fu X, Zhang G, Zhang L, Lin K, 2015. Distribution of metals and brominated flame retardants (BFRs) in sediments, soils and plants from an informal e-waste dismantling site, South China. Environ. Sci. Pollut. Res. Int 22 (2), 1020–1033. [DOI] [PubMed] [Google Scholar]
- Weinstock M, 2007. Gender differences in the effects of prenatal stress on brain development and behaviour. Neurochem. Res 32 (10), 1730–1740. [DOI] [PubMed] [Google Scholar]
- Witchey SK, Fuchs J, Patisaul HB, 2019. Perinatal bisphenol A (BPA) exposure alters brain oxytocin receptor (OTR) expression in a sex- and region- specific manner: a CLARITY-BPA consortium follow-up study. Neurotoxicology 74, 139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Wang S, Liu H, Yan Z, 2012. Tetrabromobisphenol A: tissue distribution in fish, and seasonal variation in water and sediment of Lake Chaohu, China. Environ. Sci. Pollut. Res. Int 19 (9), 4090–4096. [DOI] [PubMed] [Google Scholar]